Abstract
This review article incorporates information from the 4th Global Meningococcal Initiative summit meeting. Since the introduction of stringent COVID-19 infection control and lockdown measures globally in 2020, there has been an impact on IMD prevalence, surveillance, and vaccination compliance. Incidence rates and associated mortality fell across various regions during 2020. A reduction in vaccine uptake during 2020 remains a concern globally. In addition, several Neisseria meningitidis clonal complexes, particularly CC4821 and CC11, continue to exhibit resistance to antibiotics, with resistance to ciprofloxacin or beta-lactams mainly linked to modifications of gyrA or penA alleles, respectively. Beta-lactamase acquisition was also reported through horizontal gene transfer (blaROB-1) involving other bacterial species. Despite the challenges over the past year, progress has also been made on meningococcal vaccine development, with several pentavalent (serogroups ABCWY and ACWYX) vaccines currently being studied in late-stage clinical trial programmes.
Keywords: COVID-19, Coronavirus, Pandemic, Vaccination, Neisseria meningitidis, Invasive meningococcal disease, Serogroup, Antibiotic resistance, Sexual transmission
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has brought significant changes to society, from global and nation-specific efforts to control the respiratory spread of the virus (masks, social distancing, quarantine and lockdown measures) to the re-structuring of healthcare systems. In redirecting medical resources, the response to the pandemic has affected the surveillance, diagnosis and vaccination programmes for a range of vaccine-preventable diseases, including invasive meningococcal disease (IMD).
The 4th Summit Meeting of the Global Meningococcal Initiative (GMI) sought to: (i) highlight the impact of the COVID-19 pandemic and lockdown on the epidemiology and vaccine schedules of meningococcus; (ii) highlight the growing trend of resistance of IMD to antibiotic treatments; and (iii) explore the future of broad coverage meningococcal vaccines. Other topics were also discussed, including potential sexual transmission of Neisseria meningitidis.
This meeting is the latest in a series of summits organised by the GMI, where a multidisciplinary group of scientists, public health officials and healthcare professionals come together to raise awareness of IMD.1 , 2
The impact of the COVID-19 pandemic on the epidemiology, surveillance, and vaccination schedules of IMD
IMD epidemiology
Since the onset of the COVID-19 pandemic, the average number of IMD cases has decreased relative to previous years. For example, confirmed IMD cases in France decreased significantly during 16 March–15 May 2020 (23 cases) compared with the same period in 2018 (73) and 2019 (68). The reduction in cases in 2020 coincided with the introduction of stringent COVID-19 control and national lockdown measures.3 Moreover, lockdown may have impacted the transmission of hyperinvasive isolates associated with specific meningococcal serogroups in France. In particular, cases associated with serogroup W (clonal complex: CC11) were high at the start of 2020 prior to lockdown implementation,3 but, during the initial lockdown, cases associated with this serogroup significantly decreased (3 cases compared with 14 and 21 cases within the same period in 2018 and 2019, respectively).3 Despite decreases in these hyperinvasive lineage isolates, there was an indication that in 2020 SARS-CoV-2 infections preceded some IMD cases (n = 4), particularly in the elderly (median age 71 years). However, no confirmatory SARS-CoV-2 testing was available for these cases. In addition, the cases presented with meningococcal pneumonia; it is possible that SARS-CoV-2 was suspected based on this meningococcal presentation rather than because of a separate, preceding infection.3
As shown in Table 1 , declines in meningococcal disease during 2020 have also been observed in other regions across the world. In Brazil, the number of confirmed IMD cases in 2020 was 357 compared with 1021 the previous year and 1131 in 2018 (a 65% reduction in the number of cases from 2019).4 In Chile, there was a 90% reduction in IMD cases in 2020 compared with 2019.5 A similar pattern was observed in Mexico, with only 12 IMD cases reported in 2020 versus 48 cases in 2019.6
Table 1.
Number of cases and incidence rate of IMD 2018–2020, by country.
| 2018 |
2019 |
2020 |
||||
|---|---|---|---|---|---|---|
| Country | Number of cases | Incidence | Number of cases | Incidence | Number of cases | Incidence |
| Spain77 | 346 | 0.74 | 400 | 0.86 | 266 | 0.56 |
| France3 | 202 | 176 | 129 | |||
| South Africa7,8 | 111 | 46 | ||||
| New Zealand78 | 120 | 139 | 2.3 | |||
| China | 109 | 132 | ||||
| Brazil4 | 1131 | 0.55 | 1021 | 0.49 | 357 | 0.17 |
| Chile5 | 76 | 69 | 6 | |||
| Mexico6 | 30 | 48 | 12 | |||
| United States79 | 0.1 | 371 | 0.11 | |||
Although rates of IMD have been falling in South Africa over the past decade, there was a substantial reduction in cases between 2019 and 2020 (111 and 46 cases, respectively; Table 1).7 , 8 This decrease was also evident across all reported serogroups (B, C, W and Y, as well as unknown).
There has also been a stark decline in IMD cases in China. The incidence rate of IMD in 2020 decreased by 58% compared with the average incidence rate between 2017 and 2019 (number of cases in 2020: 53; number of cases in 2019: 132 [unpublished data]; Table 1). A similar trend was observed in Russia, with the IMD incidence rate decreasing by over 50% between 2019 and 2020 (0.6 and 0.26 cases/100,000, respectively).
Since IMD is notifiable in many countries and the disease is severe enough to warrant hospitalisation in most people, it is unlikely that poor surveillance measures/lack of notification accounted for the decline of IMD in 2020. This is particularly unlikely in countries where IMD surveillance is well established. Overall, the data suggest that the observed reduction in hyperinvasive meningococcal transmission was more likely a result of COVID-19 control measures.
A recent analysis of invasive disease cases associated with Streptococcus pneumoniae, Haemophilus influenzae, and N. meningitidis was conducted in 26 countries during the period Jan 1, 2018–May 31, 2020. Case data associated with the non-respiratory pathogen Streptococcus agalactiae were also collected. All countries experienced a large reduction in cases due to the respiratory pathogens between Jan 1, 2020 and May 31, 2020. However, no reduction was observed with Streptococcus agalactiae indicating that the decline in respiratory diseases during this period was more likely due to limited transmission than declines in surveillance sensitivity.9
Despite these overall declines, meningococcal outbreaks were identified in 2020 through surveillance efforts. In the sub-Saharan meningitis belt, the national Ministries of Health have conducted surveillance and case reporting throughout 2020, supported by the World Health Organization (WHO), the United Nations Children's Fund (UNICEF), the Africa Centres for Disease Control and Prevention (Africa CDC), the Global Alliance for Vaccines and Immunisation (Gavi), the US Centers for Disease Control and Prevention (CDC), and other partners. During the 2020 epidemic season (December–June) in the sub-Saharan African meningitis belt, there was a meningococcal serogroup C (MenC) outbreak identified in Benin and a meningococcal serogroup X (MenX) outbreak in Ghana.10 However, a number of countries in sub-Saharan Africa have not reported IMD data over the last 18 months.11
IMD vaccination during the COVID-19 pandemic
Many countries suspended immunisation programmes temporarily during the early stages of the pandemic to minimise the risk of transmitting SARS-CoV-2. In the first five months of the global pandemic, 27 countries postponed planned measles or measles-containing vaccine campaigns; seven countries temporarily stopped inactivated polio vaccinations; seven countries suspended tetanus–diphtheria immunisation; and five countries suspended oral cholera vaccinations.12
In many other countries, although vaccination programmes did not stop, vaccine uptake slowed due to containment measures (parents were uncertain about whether vaccinations were taking place and experienced difficulties in booking appointments with widespread school closures).13 Unpublished data from an online survey conducted in 8 countries (United States, United Kingdom, Italy, France, Germany, Argentina, Brazil and Australia) indicate that around 50% of parents delayed or cancelled a scheduled meningococcal vaccination appointment for their child during the COVID-19 pandemic. These parents selected lockdown regulations (63%) and concerns surrounding contracting SARS-CoV-2 (33%) as the main reasons for this vaccination disruption.14 This effect is also highlighted in England where the number of first Hexavalent vaccine doses, which are given on the same visit as MenB, fell when social distancing measures were enforced in March 2020. It is possible that messaging surrounding the continuation of routine immunisation programmes may have been disrupted by guidance surrounding the prevention of SARS-CoV-2 transmission.15
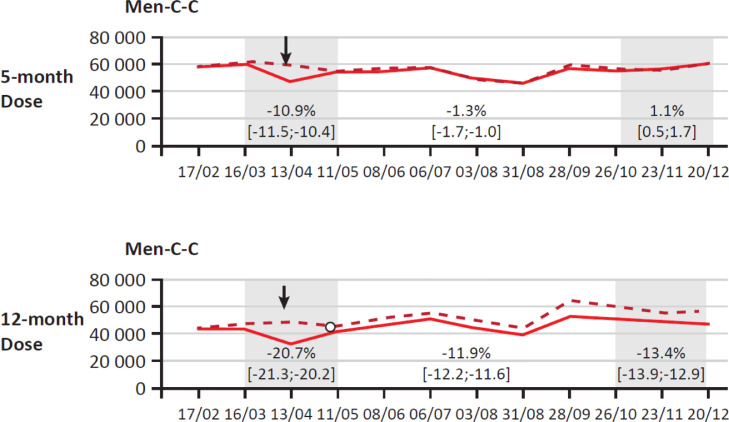
MenC conjugate vaccine uptake was affected in France during the period March 16–May 20, 2020. Uptake of both the 5-month and 12-month dose was significantly reduced during this time (−11% and −21% respectively) (Fig. 1 ).16 Following communications focussed on informing parents of the importance of meningococcal vaccination, coverage with the initial 5-month dose returned to pre-pandemic levels.16 However, the reduction in uptake for the 12-month booster with MenC persisted; during the period October 26–December 20, 2020 booster dose uptake was significantly lower than in the same period in 2019 (−14%).16
Fig. 1.
MenC vaccine uptake (at 5 months and 12 months old) in France during the COVID-19 pandemic between February and December 2020. [Solid line denotes actual use compared with expected use as a dotted line].
A recent publication aimed to quantify the impact of the COVID-19 pandemic on routine immunisation programmes in South-East Asia and the Western Pacific. In total, 95% (18/19) of the countries included in the study reported vaccination disruption between the start of the pandemic and June 01, 2020. In 13 countries, delivery of meningococcal A vaccines (MenA or MenACWY) was impacted. By the end of the study, it was noted that coverage rates for MenACWY vaccination had not yet returned to pre-COVID-19 levels.17
Immunisation practices have also been disrupted in other regions, including Africa. During the pandemic, healthcare disruption, misinformation on vaccination, stay-at-home orders, as well as the suspension of transport services became the predominant barriers to immunisation.18 However, the short-term disruption of meningococcal serogroup A (MenA) vaccination in the African meningitis belt had no immediate effect on disease owing to the persistence of direct and indirect benefits from past MenA mass-vaccination campaigns for 1- to 29-year-olds.19 In some countries MenA vaccination was expanded despite the pandemic.20
There is some evidence to suggest that meningococcal vaccine uptake has been lower in South America because of the COVID-19 pandemic. In Brazil, for MenC vaccination, there was 96% coverage in 2014.21 However, since 2014, coverage rates for MenC vaccination have declined, falling to 78% in 2020.22
Meningococcal antibiotic resistance
Each year, antibiotic-resistant diseases are responsible for at least 700,000 deaths globally; a figure that is set to increase.23 A sustained effort is required to contain this global public health crisis. However, the current COVID-19 pandemic is an unprecedented global challenge that has understandably dominated healthcare systems and economies globally over the past two years. Subsequently, there may be a number of indirect implications of COVID-19 on existing public health threats. In the case of antibiotic resistance, the COVID-19 pandemic may be exacerbating such issues. Broad-spectrum antibiotics are frequently and pre-emptively prescribed for patients with SARS-CoV-2 to limit bacterial co-infection or secondary infection.24 However, such practices may lead to antibiotic overuse and result in the promotion of resistance.25 It is therefore important to track how antibiotic resistance is impacting treatments prescribed for life-threatening diseases, such as IMD, and how it has evolved over recent years.
Ciprofloxacin resistance and the role of commensal Neisseria species
There is ongoing concern regarding the increasing prevalence of antibiotic-resistant N. meningitidis strains, particularly in the Asia–Pacific region.
The susceptibility to a range of antibiotics of N. meningitidis isolates (N = 538, isolated from 2005 to 2019) collected from across China has recently been characterised.26 All isolates collected were susceptible to ceftriaxone, meropenem, azithromycin, chloramphenicol and rifampicin. However, the average ciprofloxacin susceptibility rate across all serogroups was 24.9%.21 The majority (98.7%) of MenA isolates and 88.8% of MenC isolates were not susceptible to ciprofloxacin (see Table 2 ). Specifically, MenA:CC5 and MenB/C:CC4821 are both clonal lineages that exhibit a high degree of ciprofloxacin resistance in China.22 In addition, isolates of a ‘Chinese-strain sub-lineage’ of MenW:CC11 are ciprofloxacin-resistant while those in the other subcluster are susceptible.27
Table 2.
Susceptibility to six antibiotics versus meningococcal serogroups in China.
| Serogroup | No. of strains |
Antibiotic-susceptible rate (%) |
|||||
|---|---|---|---|---|---|---|---|
| SMZ | Ciprofloxacin | Penicillin | Ampicillin | Ceftriaxone | Minocycline | ||
| A | 78.0 | 0.0 | 1.3 | 97.4 | 96.2 | 100.0 | 75.6 |
| B | 135.0 | 0.7 | 41.5 | 76.3 | 80.0 | 93.3 | 99.3 |
| C | 116.0 | 1.7 | 11.2 | 85.3 | 89.7 | 99.1 | 95.7 |
| W | 92.0 | 62.0 | 30.4 | 88.0 | 92.4 | 97.8 | 100.0 |
| NG | 90.0 | 0.0 | 30.0 | 80.0 | 83.3 | 97.8 | 98.9 |
| Others | 27.0 | 0.0 | 33.3 | 92.6 | 92.6 | 100.0 | 100.0 |
| Total | 538.0 | 11.2 | 24.9 | 84.8 | 87.7 | 97.4 | 95.2 |
Since 2004, all ciprofloxacin-resistant isolates of the various clonal complexes in China contained a T91I mutation in the gyrA gene, with more genetic diversity for gyrA compared with susceptible strains.28 Ciprofloxacin-susceptible strains did not contain the T91l mutation.23 This T91l mutation appeared to have arisen in 2004.29 Ciprofloxacin-resistant N. meningitidis strains are not exclusive to China, with resistant isolates also being identified in Africa, Europe, South America, the USA and Canada.30, 31, 32, 33, 34, 35
The origin of quinolone resistance in N. meningitidis in China has been recently studied. Through genomic analysis of N. meningitidis (N = 198) and commensal Neisseria (N = 293) isolates, antibiotic resistance in N. meningitidis isolates was linked to horizontal gene transfer of gyrA alleles;36 N. lactamica and N. subflava were identified as donors.36 Transformation of a N. meningitidis isolate with gyrA alleles from these donors increased the minimum inhibitory concentration (MIC) for ciprofloxacin of the N. meningitidis isolates between 0.004 µg/mL to a range of between 0.125 and 0.19 µg/mL.36
The link between antibiotic-resistant N. meningitidis and Haemophilus influenzae
The ROB-1 β-lactamase gene confers resistance against β-lactam antibiotics in Haemophilus influenzae.37 A penicillin-resistant N. meningitidis strain (serogroup Y), which expressed beta-lactamase, was recently observed in Canada and France (an isolate collected in each country in 2016 and 2017, respectively).38 , 39 Both strains were ST-3587 (CC23) and possessed a ROB-1 β-lactamase gene (blaROB-1), but were susceptible to third-generation cephalosporins.38 , 39
In early 2020, two β-lactamase-producing MenY isolates were detected in the United States; however, in addition to containing blaROB-1, these isolates were also resistant to ciprofloxacin40 A call for MenY isolates from across the USA led to the finding of 33 β-lactamase positive isolates (2013–2020); 11 were also ciprofloxacin-resistant (2019–2020). Twenty-two of the 33 cases were recovered from Hispanic individuals. All 22 isolates containing the blaROB-1 gene were of the clonal complex CC23 (19 were ST-3587; two were ST-15379 and one was ST-13034).40 The 11 ciprofloxacin-resistant isolates were all MenY ST-3587 (CC23).40 Based on these findings, the US CDC has encouraged healthcare professionals to ascertain the susceptibility of isolates to penicillin and ampicillin before using these antibiotics to treat a case of meningococcal disease.
ST-3587 MenY isolates with both the blaROB-1 and the T91I gyrA mutations have also been collected from El Salvador and were associated with an outbreak of meningococcal disease (2017–2019).41 Again, all isolates were of the clonal complex CC23 (ST-3587) and contained the blaROB-1 and T91I gyrA mutations.
Additional N. meningitidis isolates containing the blaROB-1 gene have been identified, with seven isolates collected from Mexico (between 2016 and 2019) and three more from Europe (collected between 2017 and 2018 in England, Sweden and Germany). All 10 of these isolates were also MenY (all CC23; ST-3587), and phylogenetic analysis showed that these isolates were closely related to the isolates identified in the US.41
The susceptibility of N. meningitidis to third-generation cephalosporins
Antibiotic resistance amongst N. meningitidis isolates is not solely associated with ciprofloxacin or penicillin. In 2006, ceftriaxone-non-susceptible MenA isolates were identified in India using a latex agglutination kit.42 Two of the isolates were also highly resistant to ciprofloxacin (MICs >32 µg/mL). Given the very high MICs cited as part of the study and the paucity of detailed information, there remain questions about the accuracy of these results.
Further evidence of cephalosporin-resistant N. meningitidis was reported from France during 2012–2015.43 In 2012, 27% of invasive isolates received at the National Reference Centre for Meningococci (n = 95/357) exhibited penicillin G MICs between 0.125 and 0.5 µg/mL,43 with the isolates containing a number of penA allele mutations and low MICs for cefotaxime, indicating susceptibility to this antibiotic. Over the next three years, 2% of all Penl invasive isolates (n = 25) (i.e., those with intermediate susceptibility to penicillin G) were found to harbour a new altered penA allele (penA327). The allele was identical to the penAXXXIV allele in Neisseria gonorrhoeae, which is associated with reduced susceptibility to third generation cephalosporins (MIC of 2 µg/mL).43 , 44 The 25 isolates identified also demonstrated a 10-fold increase in MIC to cefotaxime (mean MIC (2013–2015) = 0.087 [95% CI: 0.076, 0.099]; 2012 isolates: mean MIC = 0.005 [95% CI: 0.004, 0.005]).
More recent cases of cefotaxime- and penicillin-resistant N. meningitidis have been reported in Costa Rica.42, 43, 44 From June 2019 to January 2020, two MenY cases were identified in adults, but with no apparent epidemiological connection. Both isolates contained the pen327 allele and were ST-13,517 (CC167).45, 46, 47 Although both isolates were susceptible to ceftriaxone, they exhibited high MICs for penicillin and cefotaxime (0.5 µg/mL and 0.25 µg/mL, respectively).
Globally, third-generation cephalosporin-resistant N. meningitidis remains relatively uncommon. However, given the importance of these antibiotics in IMD therapy and chemoprophylaxis, future phenotypic and genomic surveillance is important.
Penicillin resistance
Reduced susceptibility/resistance to penicillin is frequently associated with modifications in penA. The modifications in these alleles result in changes within a region of the penicillin binding protein 2 (PBP2) that is homologous to the active site of PBP2x from S. pneumoniae, with changes in five amino acids.48 Studies have elucidated the nature of binding between PBP2 and radioactive penicillin.48 High amounts of radioactive penicillin G are required to bind with altered PBP2 isolates with higher MICs. This indicates a reduced affinity of altered PBP2 for penicillin G. These changes in the five amino acids also impact the function of PBP2 that catalyses the reaction of D,D-transpeptidation in peptidoglycan, which is a structure within the cell wall.48 A modified structure of meningococcal peptidoglycan is characterised in the isolates with reduced susceptibility to penicillin.48
Altered penA alleles are frequently associated with penicillin resistance and can be broadly categorised into two discrete types: ‘mosaic or altered’ with an MIC ≥0.125 µg/mL and ‘wildtype’ (MIC <0.125 µg/mL).42 In PubMLST (Public Database for Molecular Typing and Microbial Genome Diversity), the most frequently identified alleles are harboured by a genetically diverse set of isolates.49 From recent data in the Republic of Ireland, the frequency of isolates with high MICs and mosaic alleles has increased since the late 1990s, especially amongst MenB strains.50 A similar increase has been reported in Australia.51 However, given the isolate diversity associated with these alleles, there remains no significant evidence of clonal expansion or horizontal spread of a specific penA allele.
Broad coverage vaccines against Neisseria species
An update on MenABCWY pentavalent meningococcal vaccines
There are currently two serogroup A, B, C, W, Y pentavalent vaccines in development; the first of which is a combination of a MenB vaccine formulation (composed of a factor H binding protein [fHbp]) (MenB-FHbp) and a quadrivalent MenACWY vaccine conjugated to a tetanus toxoid carrier protein (MenACWY-TT).52
Several studies have assessed the immunogenicity and safety of this combination vaccine. A recent Phase II study assessed immunogenicity outcomes for the pentavalent vaccine (administered in a single dose or two separate doses) compared with a co-administered MenB-FHbp (two or three doses) and MenACWY-TT (single dose) combination in adolescents and young adults (10–25 years).53 Human serum bactericidal antibody (hSBA) assays were utilised to assess protective immune responses against serogroups A, C, W and Y, as well as against four MenB test heterologus strains (fHbp variants A22, A56, B24, B44).53 There were similar immunogenic responses across serogroups A, C, W, Y and B, irrespective of whether the participant was quadrivalent-naïve or -experienced.50 There was also a higher proportion of participants with a ≥ 4-fold increase in hSBA titre one month after doses 1 and 2 for the MenABCWY vaccine compared with the MenACWY-CRM197 formulation for serogroups A, C, W and Y; this pattern was also identified for serogroup B.53
The other pentavalent ABCWY vaccine currently in a clinical trial is a combination of the four-component MenB vaccine (4CMenB) and a quadrivalent MenACWY vaccine conjugated to Corynebacterium diphtheriae protein (CRM197) protein,52 a form of diphtheria toxin, with no toxin activity.54 The 4CMenB component includes three recombinant antigens (Neisseria adhesin A [NadA], neisserial heparin binding antigen [NHBA] and fHbp) and a PorA-containing outer membrane vesicle derived from a New Zealand MenB strain.52
A Phase II study was conducted amongst 495 adolescents in Panama, Colombia and Chile comparing different dosing schedules for the MenACWY-CRM197 + 4CMenB formulation versus the MenACWY-CRM197 vaccine alone, with immunogenicity being assessed using hSBA.55 The two dose pentavalent vaccine schedule induced an improved immunogenic response against MenACWY, with a greater proportion of patients achieving hSBA titers ≥5 compared with a single dose of MenACWY-CRM197.55 In addition, those who received two doses of the pentavalent vaccine exhibited a better response against MenB antigens (≥68% of participants achieved hSBA titres ≥5 against each of the MenB test strains) compared with one dose.55
Antibody persistence was also analysed as part of the trial.56 MenACWY antibody concentrations waned in all groups over a 4-year period following vaccination, but remained higher than pre-vaccination levels. The percentage of those with hSBA titres ≥8 against MenA, MenC, MenW and MenY was higher in the groups that had previously received a MenACWY vaccine across all time-points (1 and 4 months post final dose).56 Response persistence varied by serogroup between those who had previously received two doses of the MenACWY + 4CMenB vaccine and those who had previously received a single MenACWY dose.56 For MenC, a higher proportion of participants had a hSBA ≥8 in the MenACWY + 4CMenB group compared with the MenACWY group at 4 years following vaccination. However, the reverse trend was observed for serogroups A and Y.56
As part of the persistence study, the percentage of participants with hSBA titres ≥5 was higher in those who had received three doses of the MenACWY + 4CMenB vaccine (two priming doses [2 months apart] and booster [6 months after first dose]) versus non-primed individuals (either one dose of MenACWY-CRM197 and two MenACWY + 4CMenB doses or two MenACWY + 4CMenB doses) for all seven serogroup B strains investigated.56
The breadth of coverage (BoC) of the MenACWY + 4CMenB vaccine has also been investigated as part of a recent study.57 BoC is defined as 1-relative risk (RR) x 100% (RR corresponds to the ratio between the percentage of isolates for which sera are seronegative at a hSBA titre threshold of 1:4 against the selected strains in the MenABCWY vs the control group).57 The trial was conducted in US adolescents between 10 and 18 years with participants receiving either three doses of the MenACWY + 4CMenB vaccine at 0, 2 and 6 months or a single dose of the MenACWY-CRM197 vaccine.57 A 4CMenB control group was not included as part of the study. One month following vaccination, the BoC for MenACWY + 4CMenB across 110 MenB strains, randomly-selected from a repository of US IMD strains causing invasive disease in the US, was 67% (95% CI: 65, 69) after two doses,57 and this increased to 71% (95% CI: 69, 73) after three doses.57 However, the BoC decreased to 44% (95% CI: 41, 47) and 51% (95% CI: 48, 55) 4 months after vaccination with two or three doses of MenACWY + 4CMenB, respectively.57
Development of a pentavalent MenACWXY vaccine for Africa
MenA has virtually disappeared from the sub-Saharan meningitis belt following an extensive vaccination campaign amongst 1–29 year olds followed by routine immunisation of children with MenAfriVac®, a vaccine specifically developed for the region to protect against MenA disease. 59 Efforts in sub-Saharan Africa are now addressing residual outbreaks caused by serogroups C, W and X. Currently licensed quadrivalent meningococcal conjugate vaccines remain too expensive for Africa and do not offer coverage against MenX strains.58
Efforts have been directed towards developing and testing a pentavalent (MenACWXY) conjugate vaccine formulation (NmCV-5).59 The vaccine is freeze-dried with the polysaccharides being conjugated to either of two different carrier proteins. MenA and MenX polysaccharides are conjugated to tetanus toxoid (a high yield conjugate, which is highly immunogenic) and MenC, MenW and MenY polysaccharides are conjugated to recombinant CRM197.
Initial Phase I and II safety and immunogenicity studies in the US and Africa are now complete amongst people aged 18–45 years and 12–16 months, respectively.54 , 60 , 61 Data from the Phase II study in Africa (N = 375) demonstrated that NmCV-5 is well tolerated and immunogenic across all five serogroups.54 The study indicated that SBA concentrations were similar or higher with a single dose of the NmCV-5 vaccine compared with two doses of Menactra (MenACWY conjugated to diphtheria toxoid).54 There was no discernible improvement in the immune responses with the addition of aluminium phosphate as adjuvant to the NmCV-5 formulation.54 Further data indicate that NmCV-5 boosts both anti-tetanus toxoid and anti-diphtheria toxoid (DT) responses ∼10 fold (manuscript in preparation). However, Menactra (DT carrier) boosts DT responses to a somewhat higher extent than NmCV-5 (CRM197 carrier).
Following this Phase II programme there are now several ongoing or planned Phase III studies to further assess the immunogenicity and safety of NmCV-5. The first is an observer-blind, randomised, controlled study amongst healthy individuals (2–29 years) in Mali and The Gambia, with 168-day follow-up post-vaccination.62 A total of 1800 participants were randomised to receive either NmCV-5 or Menactra as part of the trial. The primary objective is to demonstrate non-inferiority of NmCV-5 to Menactra in terms of SBA assay (using rabbit complement, rSBA) sero-response or geometric mean titres (GMTs) to MenA, MenC, MenY and MenW. A second Phase III study in people aged 18–85 years in India is complete and serology studies are ongoing.
The third Phase III study will be conducted in young infants (at 9 and 15 months of age) in Mali, with a total follow-up of 168 days post-vaccination, and will compare the immunogenicity of NmCV-5 to Nimenrix (Men-ACWY conjugated to TT; Pfizer Europe). A total of 1200 participants will be randomised as part of the trial. Similar to the other Phase III studies, the primary objective is to demonstrate non-inferiority of NmCV-5 in terms of rSBA titers (≥8).
Meningococcal disease and sexual transmission
Social and physical distancing measures enforced during the COVID-19 pandemic may have indirectly influenced the incidence of sexually transmitted diseases due to altered sexual risk behaviours.63 In many countries, stay-at-home lockdown orders may have limited the spread of such diseases through decreased social contact. However, the redirection of public health resources toward COVID-19 may have reduced access to testing and diagnosis, which could also impact transmission dynamics.64 As such, efforts must be made to increase our understanding of the current and future epidemiological behaviour of sexually-transmitted diseases, which could return to pre-COVID patterns as society reopens.
In recent years, N. meningitidis has been isolated from genitourinary and anal specimens of patients. A N. meningitidis urethritis outbreak in Columbus, Ohio, US, starting in January 201566 was initially presumed to be a N. gonorrhoeae outbreak. Of 117 cases reported in 2015–2016, a majority (98%) occurred in heterosexual men (3–36 cases per month amongst heterosexual men) and 83% (n = 98) amongst African-Americans. The most prevalent risk factor was oral sex (performing or receiving) with a female in the past year (97% [N = 113]).66 Further N. meningitidis urethritis outbreaks were identified in eastern and midwestern US states such as Pennsylvania, Georgia, Indiana and Michigan.66, 67, 68
These outbreaks were caused by the US N. meningitidis urethritis clade (US_NmUC), which belongs to CC11 lineage 11.2/ET-15 lineage and has a fine type of PorA P1.5–1, 10–8; FetA F3–6; PorB 2–2.65 The isolates also express a unique fHbp allele. The corresponding fHbp peptide (peptide 896) is a member of variant group 1 (subfamily B) and can enhance the resistance of strains to complement-mediated killing.65 The allele is recognised by antibodies generated by the fHbp antigen in currently licensed MenB vaccines.69
Vaccines covering both meningococci and gonococci
There remains the question of whether a vaccine could protect against both meningococci and gonococci. However, gonococcal vaccine development has remained challenging: no correlate of protection against the disease has been defined and natural infection with gonorrhoea does not induce a protective immune response.
Although meningococcal vaccines have not been directly assessed for protection against gonococcal disease, indirect evidence has suggested that cross-protection does occur. A MenB outbreak in New Zealand necessitated the development of an outer membrane vesicle (OMV) vaccine based on a New Zealand strain of MenB (MeNZB).70 , 71 A subsequent MeNZB vaccination programme sought to vaccinate all those <20 years, with one million individuals vaccinated during the period 2004 to 2006. Vaccine effectiveness (VE) was moderate at 73% (95% confidence intervals [CI]: 52–85) against strain-specific disease and 67% (95% CI: 57–76) against all circulating meningococcal strains.72 Following this period, there appeared to be a gradual decrease in the number of gonorrhoea cases.73
To understand the relationship between the MeNZB vaccine and gonorrhoea, a case control study was conducted in adolescents (born between 1984 and 1998) who attended sexual health clinics with gonorrhoea, chlamydia, or both and had been eligible for the MeNZB vaccine.63 The adjusted estimate for VE against confirmed cases of gonorrhoea amongst adolescents and adults (15–30 years) based on the odds ratio was 31% (95% CI: 21, 39).74
A similar epidemiological relationship between meningococcal vaccination and gonorrhoea cases has been identified in Cuba. A tailor-made meningococcal vaccine in Cuba, VA-MENGOC-BC (MBV), was produced and introduced in Cuba in the late 1980s to reduce case numbers in an epidemic, with young children being vaccinated during this time.75 There was a reduction in the incidence of gonorrhoea following the vaccination campaigns. Although there was a similar and concurrent reduction in syphilis during this period, syphilis cases have again begun to rise, while gonorrhoea cases have remained low.76 A recent clinical trial has analysed serum, saliva and oropharyngeal swab samples (N = 92) from young adults (both Neisseria carriers and non-carriers) who received the VA-MENGOC-BC (MBV) vaccine during infancy and a booster during the trial.68 Immunological responses against MenB were boosted with a further dose and Neisseria carriers who received a booster dose also exhibited anti-gonococcal salivary IgA and serum IgG responses. It was posited that anti-gonococcal mucosal antibody responses may have resulted in the reduced incidence of gonorrhoea in the epidemiological data.76
Conclusions
The onset of 2020 brought about a new set of challenges for IMD surveillance, treatment and prevention. Despite this, IMD cases appeared to be declining over the course of 2020 in multiple countries, likely due to stringent infection control and lockdown measures minimising close contacts and limiting social gatherings that would normally facilitate meningococcal transmission. However, the reduction in childhood vaccination in some countries in 2020 may facilitate the future surging of bacterial diseases; promotion of catch-up vaccination could help compensate for the impact of the pandemic on routine vaccination.
Despite these challenges, catch-up IMD vaccination programmes and enhanced surveillance efforts have continued in many countries. Surveillance and control of meningococcal disease remains robust across various countries. However, antibiotic-resistant N. meningitidis strains remain an ongoing concern across the world, with some isolates exhibiting resistance to both ciprofloxacin and β-lactam antibiotics. Such cases have led to enhanced public health measures in the US.
Equally, concerns regarding the prevalence and outbreaks of IMD associated with specific serogroups has led to the development of pentavalent MenABCWY vaccines. With MenA in decline in sub-Saharan Africa, efforts are now focused on producing a pentavalent vaccine that can address residual outbreaks associated with MenC, MenX and MenW.
Authors’ contributions
Authors discussed and agreed to the manuscript's objectives and contributed throughout to the development and editing of it. All authors read and approved the final manuscript.
Conflicts of Interest
M. Alderson has received grant funding for development of a meningococcal vaccine from the UK Foreign, Commonwealth & Development Office (formerly Department for Internal Development).
R. Borrow, J. Lucidarme and X. Bai perform contract work on behalf of Public Health England for GSK, PATH, Sanofi Pasteur and Pfizer.
D. Caugant, P.D. Arkwright have nothing to disclose.
E.C. Dinleyici performs contract work for the Eskisehir Osmangazi University funded by GSK, Sanofi Pasteur and Pfizer.
J. Galajeva has nothing to disclose.
L.H. Harrison has served as a consultant to GSK, Pfizer, Merck, and Sanofi Pasteur.
S. Meiring has received grant funding for a meningococcal carriage study by Sanofi Pasteur.
M.A.P. Sáfadi reports research grants and personal fees for advisory boards from GSK, Pfizer, and Sanofi.
V. Smith represents Meningitis Research Foundation, which receives grants from Sanofi Pasteur, GSK, Pfizer and Serum Institute.
D. Stephens receives grant funding (AI127863 AI148576) from the US NIH for studies of N. meningitidis and N. gonorrhoeae.
M.K. Taha performs contract work for the Institut Pasteur funded by GSK, Pfizer and Sanofi Pasteur. M.K. Taha has a patent NZ630133A Patent with GSK “;Vaccines for serogroup X meningococcus”; issued.
J.A. Vázquez performs contract work for the Institute of Health Carlos III funded by GSK and Pfizer and he has received personal fees from GSK, Pfizer and Sanofi Pasteur.
B. Zhu has nothing to disclose.
L.A. McNamara has nothing to disclose.
A. Skoczyńska has performed contract work for the National Medicines Institute funded by Pfizer and has received personal fees from Pfizer and Sanofi Pasteur.
Acknowledgments
Acknowledgments
The authors were assisted in the preparation of the manuscript by Matt Gunther, a medical writer at Ashfield Health, a company that is part of UDG Healthcare. Medical writing support was funded by Sanofi Pasteur.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Min Aye A.M., Bai X., Borrow R., Bory S., Carlos J., Caugant D.A., et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): the global meningococcal initiative. J Infect. 2020;81:698–711. doi: 10.1016/j.jinf.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Bai X., Borrow R., Bukovski S., Caugant D.A., Culic D., Delic S., et al. Prevention and control of meningococcal disease: updates from the global meningococcal initiative in eastern Europe. J Infect. 2019;79:528–541. doi: 10.1016/j.jinf.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Taha M.-.K., Deghmane A.-.E. Impact of COVID-19 pandemic and the lockdown on invasive meningococcal disease. BMC Res Notes. 2020;13:399. doi: 10.1186/s13104-020-05241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health of Brazil, 2020; http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/meninbr.def (Last accessed, May 2021).
- 5.Cuban Ministry of Health, Institute of Public Health, 2020; https://www.ispch.cl/wp-content/uploads/2021/02/Informe-Neisseria-meningitidis-SE-1-53-2020-v1-1.pdf (Last accessed, May 2021).
- 6.Mexican Ministry of Health, 2020; https://saludpublica.mx/index.php/spm/article/view/11725 (Last accessed, May 2021).
- 7.National Institute for Communicable Diseases. Annual Surveillance Review; https://www.nicd.ac.za/wp-content/uploads/2021/02/GERMS-Annual-Review-2019_.pdf (Last accessed, May 2021).
- 8.PubMLST database; https://pubmlst.org/static/iris/(Last accessed, May 2021).
- 9.Brueggemann A.B., Jansen van Rensburg M.J., Shaw D., McCarthy N.D., Jolley K.A., Maiden M.C.J., et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;6:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjorlolo S., Egbenya D.-.L. A twin disaster: addressing the COVID-19 pandemic and a cerebrospinal meningitis outbreak simultaneously in a low-resource country. Glob Health Action. 2020;13 doi: 10.1080/16549716.2020.1795963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Meningitis Bulletins; https://www.menafrinet.org/who-meningitis-bulletins (Last accessed, August 2021).
- 12.WHO; https://www.who.int/news/item/22-05-2020-at-least-80-million-children-under-one-at-risk-of-diseases-such-as-diphtheria-measles-and-polio-as-covid-19-disrupts-routine-vaccination-efforts-warn-gavi-who-and-unicef (Last accessed, May 2021).
- 13.Dinleyici E.C., Borrow R., Palazzi Safadi M.A., van Damme P., Munoz F.M. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccin Immunother. 2021;17:400–407. doi: 10.1080/21645515.2020.1804776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GSK press release 2021; https://www.gsk.com/en-gb/media/press-releases/half-of-parents-surveyed-either-cancelled-or-delayed-their-child-s-scheduled-meningitis-vaccination-during-the-covid-19-pandemic-gsk-survey-shows-1/(Last accessed, November 2021)
- 15.Public Health England. Health Protection Report, 2020; https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/933545/hpr2120_chldhd-vc_wk43.pdf (Last accessed, November 2021)
- 16.Taine M., Offredo L., Drouin J., Toubiana J., Weill A., Zureik M., et al. Mandatory infant vaccinations in France during the COVID-19 pandemic in 2020. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.666848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris R.C., Chen Y., Cote P., Ardillion A., Vievera M.C., Ong-Lim A., et al. Lancet Reg Health West Pac. 2021;10 doi: 10.1016/j.lanwpc.2021.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO; https://www.who.int/news/item/15-07-2020-who-and-unicef-warn-of-a-decline-in-vaccinations-during-covid-19 (Last accessed, June 2021)
- 19.Gaythorpe K.A.M., Abbas K., Huber J., Karachaliou A., Thakkar N., Woodruff K., et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. Elife. 2021;10:e67023. doi: 10.7554/eLife.67023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO, Weekly Epidemiological record August 2021; {
- 21.Nunes A.A., De Jesus Lopes De Abreu A., Cintra O., Cintra M.A.C.T., Barbosa Coelho E., Castro De Barros E.N. Meningococcal disease epidemiology in Brazil (2005-2018) and impact of MenC vaccination. Vaccine. 2021;39:605–616. doi: 10.1016/j.vaccine.2020.11.067. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health of Brazil; http://pni.datasus.gov.br (Last accessed, May 2021)
- 23.WHO. Report to the Secretary-General of the United Nations, 2019; https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdfsfvrsn=5b424d7_6 (Last accessed, November 2021)
- 24.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strathdee A.S., Davies S.C. and Marcelin J.R. Lancet 2020;396:1050–53 [DOI] [PMC free article] [PubMed]
- 26.X.u. Li, et al. Chin J Prev Med2021; 55: doi:10.3760/cma.j.cn112150-20200922-01227
- 27.Zhu B., Lucidarme J., Bai X., guo P., Zhang A., Borrow R., Gao W., Xu L., Gai Y., Shao Z. Comparative genomic analyses of Chinese serogroup W ST-11 complex Neisseria meningitidis isolates. J Infect. 2020;80:54–60. doi: 10.1016/j.jinf.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhu B., Fan Y., Xu Z., Xu L., Du P., Gao Y., et al. Genetic diversity and clonal characteristics of ciprofloxacin-resistant meningococcal strains in China. J Med Microbiol. 2014;63:1411–1418. doi: 10.1099/jmm.0.078600-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao P., Xu L., Zhang A., Zhu B., Shao Z. Evolutionary analysis of gyrA gene from Neisseria meningitidis bacterial strains of clonal complex 4821 collected in China between 1978 and 2016. BMC Microbiol. 2020;20:71. doi: 10.1186/s12866-020-01751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang R.S.W., Law D.K.S., Deng S., Hoang L. Ciprofloxacin-resistant Neisseria meningitidis in Canada: likely imported strains. Can J Microbiol. 2017;63:265–268. doi: 10.1139/cjm-2016-0716. [DOI] [PubMed] [Google Scholar]
- 31.du Plessis M., de Gouveia L., Skosana H., Thomas J., Blumberg L., Klugman K.P., et al. Invasive Neisseria meningitidis with decreased susceptibility to fluoroquinolones in South Africa, 2009. J Antimicrobiol Chemo. 2010;65:2258–2260. doi: 10.1093/jac/dkq291. [DOI] [PubMed] [Google Scholar]
- 32.Hong E., Thulin Hedberg S., Abad R., Fazio C., Enriquez R., Deghmane A.-.E., et al. Target gene sequencing to define the susceptibility of Neisseria meningitidis to ciprofloxacin. Antimicrob Agents Chemother. 2013;57:1961–1964. doi: 10.1128/AAC.02184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorhouet-Pereira C., Efron A., Gagetti P., Faccone D., Regueira M., Corso A., et al. Phenotypic and genotypic characteristics of Neisseria meningitidis disease-causing strains in Argentina, 2010. PLoS ONE. 2013;8:e58065. doi: 10.1371/journal.pone.0058065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorla M.C., Cassiolato A.P., Watanabe Pinhata J.M., de Moraes C., Corso A., Gagetti P., et al. Emergence of resistance to ciprofloxacin in Neisseria meningitidis in Brazil. J Med Mircobiol. 2018;67:286–288. doi: 10.1099/jmm.0.000685. [DOI] [PubMed] [Google Scholar]
- 35.Wu H.M., Harcourt B.H., Hatcher C.P., Wei S.C., Novak R.T., Wang X., et al. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009;360:886–892. doi: 10.1056/NEJMoa0806414. [DOI] [PubMed] [Google Scholar]
- 36.Chen M., Zhang C., Zhang X., Chen M. Meningococcal quinolone resistance originated from several commensal Neisseria species. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01494-19. e01494–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlowsky J.A., Verma G., Zhanel G.G., Hoban D.J. Presence of ROB-1 beta-lactamase correlates with cefaclor resistance among recent isolates of Haemophilus influenzae. J Antimicrob Chemother. 2000;45:871–875. doi: 10.1093/jac/45.6.871. [DOI] [PubMed] [Google Scholar]
- 38.Tsang R.S.W., Ahmad T., Jamison F.B., Tyrell G.J. WGS analysis of a penicillin-resistant Neisseria meningitidis strain containing a chromosomal ROB-1 β-lactamase gene. J Antimicrob Chemother. 2019;74:22–28. doi: 10.1093/jac/dky391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong E., Deghmane A.-.E., Taha M.-.K. Acquisition of Beta-Lactamase by Neisseria meningitidis through Possible Horizontal Gene Transfer. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00831-18. e00831–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara L.A., Potts C., Blain A.E., Retchless A.C., Reese N., Swint S., et al. Detection of ciprofloxacin-resistant, β-lactamase-producing neisseria meningitidis Serogroup Y Isolates - United States, 2019-2020. MMWR Morb Mortal Wkly Rep. 2020;69:735–739. doi: 10.15585/mmwr.mm6924a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potts C.C., Retchless A.C., McNamara L.A., Marasini D., Reese N., Swint S., et al. Acquisition of ciprofloxacin resistance among an expanding clade of β-lactamase positive, serogroup Y Neisseria meningitidis in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab358. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manchanda V., Bhalla P. Emergence of non-ceftriaxone-susceptible Neisseria meningitidis in India. J Clin Microbiol. 2006;44:4290–4291. doi: 10.1128/JCM.01903-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deghmane A.-.E., Hong E., Taha M.-.K. Emergence of meningococci with reduced susceptibility to third-generation cephalosporins. J Antimicrob Chemother. 2017;72:95–98. doi: 10.1093/jac/dkw400. [DOI] [PubMed] [Google Scholar]
- 44.Ohnishi M., Saika T., Hoshina S., Iwasaku K., Nakayama S.-.I., Watanabe H., et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148–149. doi: 10.3201/eid1701.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chanto G. INCIENSA; 2016. Red Nacional de Laboratorios de Bacterología. http://www.inciensa.sa.cr (Last accessed, May 2021) [Google Scholar]
- 46.PubMLST database; https://pubmlst.org (Last accessed, May 2021).
- 47.PubMLST database; https://pubmlst.org (Last accessed, May 2021).
- 48.Antignac A., Boneca I.G., Rouselle J.-.C., Namane A., Carlier J.-.P., Vazquez J.S., et al. Correlation between alterations of the penicillin-binding protein 2 and modifications of the peptidoglycan structure in Neisseria meningitidis with reduced susceptibility to penicillin G. J Biol Chem. 2003;278:31529–31535. doi: 10.1074/jbc.M304607200. [DOI] [PubMed] [Google Scholar]
- 49.PubMLST database; https://pubmlst.org/(Last accessed, May 2021).
- 50.Bennett D.E., Meyler K.L., Cafferkey M.T., Cunney R.J. Antibiotic susceptibility and molecular analysis of invasive Neisseria meningitidis recovered in the Republic of Ireland, 1996 to 2016. Eur J Clin Microbiol Infect Dis. 2021;40:1127–1136. doi: 10.1007/s10096-020-04114-0. [DOI] [PubMed] [Google Scholar]
- 51.Lahra M.M., Hogan T.R. Australian Meningococcal Surveillance Programme annual report, 2019. Commun Dis Intell. 2020;17:44. doi: 10.33321/cdi.2020.44.62. [DOI] [PubMed] [Google Scholar]
- 52.Pizza M., Bekkat-Berkani R. Rappuoli. Vaccines against meningococcal diseases. Microorganisms. 2020;8:1521. doi: 10.3390/microorganisms8101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson J. et al. IDWeek 2020, October 21–25, 2020; virtual event.
- 54.Alderson M.R., LaForce F.M., Meulen A.S.-T., Hwang A., Preziosi M.-.P., Klugman K.P. Eliminating meningococcal epidemics from the African meningitis belt: the case for advanced prevention and control using next-generation meningococcal conjugate vaccines. J Infect Dis. 2019;220:S274–S278. doi: 10.1093/infdis/jiz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saez-Llorens X., Aguilera Vaca D.C., Abarca K., Maho E., Grana M.G., Heijnen E., et al. Immunogenicity and safety of investigational vaccine formulations against meningococcal serogroups A, B, C, W, and Y in healthy adolescents. Hum Vaccin Immunother. 2015;11:1507. doi: 10.1080/21645515.2015.1029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saez-Llorens X., Beltran-Rodriguez J., Novo Pizarro J.M., Mensi I., Keshavan P., Toneatto D.Hum Vaccin Immunother2018;14:1161. doi: 10.1080/21645515.2018.1457595. [DOI] [PMC free article] [PubMed]
- 57.Welsch J.A., Senders S., Essink B., Klein T., Smolenov I., Pedotti P., et al. Breadth of coverage against a panel of 110 invasive disease isolates, immunogenicity and safety for 2 and 3 doses of an investigational MenABCWY vaccine in US adolescents - Results from a randomized, controlled, observer-blind phase II study. Vaccine. 2018;36:5309. doi: 10.1016/j.vaccine.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Mustapha M.M., Harrison L.H. Vaccine prevention of meningococcal disease in Africa: major advances, remaining challenges. Hum Vaccin Immunother. 2018;14:1107–1115. doi: 10.1080/21645515.2017.1412020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meningitis Vaccine Project Closure Conference, 2016; https://www.who.int/immunization/research/meetings_workshops/Kulkarni_LaForce_MVPconf16.pdf?ua=1 (last access, May 2021).
- 60.Chen W.H., Neuzil K.M., Boyce C.R., Pasetti M., Reymann M.K., Martellet L., et al. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect Dis. 2018;18:1088–1096. doi: 10.1016/S1473-3099(18)30400-6. [DOI] [PubMed] [Google Scholar]
- 61.Tapia M.D., Soe S.O., Naficy A., Diallo F., Haidara F.C., Chaudhari A., et al. Meningococcal serogroup ACWYX conjugate vaccine in Malian toddlers. N Engl J Med. 2021;384:2115–2123. doi: 10.1056/NEJMoa2013615. [DOI] [PubMed] [Google Scholar]
- 62.Clinicaltrials.gov (NCT03964012); https://clinicaltrials.gov/ct2/show/NCT03964012 (Last accessed, May 2021)
- 63.Rodriguez I., Hernandez Y. Sexually transmitted diseases during the COVID-19 pandemic: a focus on syphilis and gonorrhoea in Cuba. Public Health Pract. 2021:2. doi: 10.1016/j.puhip.2020.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sentis A., Prats-Uribe A., Lopex-Corbeto, Montoro-Fernandez M., Nomah D.K., Garcia de Olalla P., et al. The impact of the COVID-19 pandemic on sexually transmitted infections surveillance data: incidence drop or artefact? BMC Public Health. 2021;21:1637. doi: 10.1186/s12889-021-11630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Retchless A.C., Kretz C.B., Chang H.Y., Bazan J.A., Abrams A.J., Norris Turner A., et al. Expansion of a urethritis-associated Neisseria meningitidis clade in the United States with concurrent acquisition of N. gonorrhoeae alleles. BMC Genomics. 2018;19:176. doi: 10.1186/s12864-018-4560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bazan J.A., Norris Turner A., Kirkcaldy R.D., Retchless A.C., Kretz C.B., Briere E., et al. Large cluster of Neisseria meningitidis urethritis in Columbus, Ohio, 2015. Clin Infect Dis. 2017;65:92–99. doi: 10.1093/cid/cix215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toh E., Gangaiah D., Batteiger B.E., Williams J.A., Arno J.A., Tai A., et al. Neisseria meningitidis ST11 complex isolates associated with Nongonococcal urethritis, Indiana, USA, 2015–2016. Emerg Infect Dis. 2017;23:336–339. doi: 10.3201/eid2302.161434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bazan J.A., Peterson A.S., Kirkcaldy R.D., Briere E.C., Maierhofer C., Norris Turner A., et al. Notes from the field: increase in Neisseria meningitidis-associated urethritis among men at two sentinel clinics - Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:550–552. doi: 10.15585/mmwr.mm6521a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trumenba SmPC; https://www.medicines.org.uk/emc/product/2670/smpc#gref (Last accessed, August 2021).
- 70.Arnold R., Galloway Y., McNicholas A., O'Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100–7106. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 71.Lopez L., et al. ESR Surveillance Report. 2013 https://surv.esr.cri.nz/PDF_surveillance/MeningococcalDisease/2012/2012AnnualRpt.pdf (Last accessed, May 2021) [Google Scholar]
- 72.Kelly C., Arnold R., Galloway Y., O'Hallahan J. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Amer J Epidemiol. 2007;166:817–823. doi: 10.1093/aje/kwm147. [DOI] [PubMed] [Google Scholar]
- 73.The Institute of Environmental Science and Research Ltd Sexually transmitted infections in New Zealand. Annual Surveillance Report. 2013 [Google Scholar]
- 74.Petousis–Harris H., Paynter J., Morgan J., Saxton P., McArdle B., Goodyear-Smith F., et al. Lancet. 2017;390:1603–1610. doi: 10.1016/S0140-6736(17)31449-6. [DOI] [PubMed] [Google Scholar]
- 75.Pérez O., del Campo J., Cuello M., Gonzalez E., Osmir C., Llanes C., et al. Enfoques mucosales en vacunologia de Neisseria. Vaccimonitor. 2009;18:53–55. [Google Scholar]
- 76.Reyes Diaz L.M., de SJB Lastre Gonzalez M., Cuello M., Sierra-Gonzalez V.G., Pupo R.R., Lantero M.I., et al. VA-MENGOC-BC vaccination induces serum and mucosal anti neisseria gonorrhoeae immune responses and reduces the incidence of gonorrhea. Pediatr Infect Dis J. 2021;40:375–381. doi: 10.1097/INF.000000000000304. [DOI] [PubMed] [Google Scholar]
- 77.National Surveillance Network, 2020; https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Boletines/Documents/Boletin_Epidemiologico_en_red/boletines%20en%20red%202020/IS_Nº42-201013-WEB.pdf (Last accessed, November 2021)
- 78.ESR. Invasive Meningococcal Disease Report, 2019; https://surv.esr.cri.nz/PDF_surveillance/MeningococcalDisease/2019/MeningococcalDisease_Q4_2019.pdf (Last accessed, November 2021)
- 79.CDC. Meningococcal surveillance, 2019; https://www.cdc.gov/meningococcal/surveillance/index.html#f1 (Last accessed, November 2021)