Abstract
Introduction
Immunomodulators, including dexamethasone (DEX), have been recommended by the Infectious Disease Society of America (IDSA) to treat moderate, severe, and critical COVID-19. Tocilizumab (TCZ) was added to the treatment recommendations based on recent data from two large randomized controlled trials and its potential synergistic effect with DEX.
Method
We included adult patients admitted from June until October 2020 with a PCR confirmed SARS-CoV-2 infection. 135 patients with severe to critical COVID-19 and received TCZ and/or corticosteroid or DEX were retrospectively evaluated and followed until hospital discharge or death.
Results
The cohort was divided into two different groups of patients; TCZ group received TCZ ± corticosteroid, N = 100 and DEX group received DEX, N = 35. Groups were analyzed for hospital mortality. The rate of hospital mortality was 36% in TCZ and 37% in the DEX group, p = 0.91. Age of 60 years and above was associated with higher mortality rate with OR = 1.030 and 95% CI = (1.004, 1.057). More than 50% of patients required MV in both groups. Development of bacterial or fungal infection post immunomodulator were similar in TCZ and DEX groups, 29% vs. 31.4%.
Conclusion
Our study revealed that age of 60 years and above is the only factor associated with higher mortality rate regardless of the type of immunomodulator therapy. Findings of this study also revealed the lack of synergistic effect between TCZ and DEX on the hospital mortality.
Keywords: Severe COVID-19, Tocilizumab, Dexamethasone
Introduction
SARS-COV19 is the etiology of the newly emerged Coronavirus Disease (COVID-19) in December 2019 [1]. It belongs to the ribonucleic acid (RNA) viruses family that caused severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) years ago [1]. SARS-CoV2 has been found to affect different organs in the human body, including vital organs and the immune system [2]. Some clinical manifestations associated with COVID-19 are fever, cough, dyspnea, respiratory failure, gastrointestinal symptoms, including nausea, vomiting, and diarrhea [3]. The molecular basis of SARS-COV19 and cellular entry mechanism have been studied and believed to be started with the interaction between the virus and the angiotensin-Converting enzyme 2 (ACE2) receptor on host cell via S protein [4]. ACE2 receptors are expressed in a variety of organs such as respiratory tract, mucosal epithelial cells, lung alveolar cells, arterial and venous endothelial cells but also in other tissues, including the gastrointestinal tract, accounting for the mild enteritis that sometimes presents in patients with COVID-19 [4].
Patients who suffer from a severe decline in their clinical course during COVID-19 are believed to have three phases [5,6]. First, the early phase where viral replication suppresses immune response via inhibition of nuclear factor-kappa light chain, therefore delaying interferon production. The second, pulmonary phase, where hypoxia developed secondary to the cellular damage caused by ACE-2 receptors, induces activation of the immune system. Third, the hyperinflammatory phase starts nine to twelve days after the onset of disease where acute respiratory distress syndrome, cytokine storm, and septic shock appear. Interleukins levels (ILs) are high at this stage due to the activation of T lymphocytes and other innate immunity cells. Alveolar membrane integrity is affected negatively, which progresses into increased alveolar permeability resulting in pulmonary edema [5,6]. IDSA has recommended immunomodulators including steroids such as dexamethasone (DEX) to treat moderate, severe, and critical COVID-19 [7]. Data from the UK-RECOVERY trial showed mortality benefits associated with a low dose of DEX in patients on mechanical ventilation (MV) and those who required oxygen supplementation therapy [8]. Another potential therapeutic option is IL-6 inhibitors such as Tocilizumab (TCZ). IL-6 plays crucial role in COVID-19 in which believed that drives cytokine storm associated with progressive COVID-19 disease [6]. Earlier, retrospective data including those from China suggests the effectiveness of TCZ for COVID-19 patients with cytokine storm [9]. Cumulative data showed that TCZ could be an attractive therapeutic option for patients with COVID-19 and elevated IL-6 at risk of cytokine storm [10,11]. Mortality benefit associated with TCZ utilization was explored in few studies and found to be improved in some patients [12]. Interestingly, some prospective, randomized controlled trials (RCTs) are contradicting results from anecdotal retrospective data. Results from three different prospective randomized controlled trials indicate no additional effect of TCZ on the disease progression or death when compared to standard of care (SOC) [[13], [14], [15]]. Roche Ltd, TCZ’s manufacturer have conducted two double blinded RCTs, COVACTA and EMPACTA that both did not show TCZ efficacy in reducing mortality [[16], [17], [18]]. The largest RCTs that studied the clinical outcomes associated with TCZ are RECOVERY and REMAP-CAP trials. Both trials prove that TCZ is associated with 28- or 90-day mortality benefit and decreased need for invasive mechanical ventilation in patients with severe COVID-19 [19,20].
In this study, we were aiming to evaluate the impact of TCZ therapy with or without corticosteroid versus DEX monotherapy on the hospital mortality for patients with severe to critical COVID-19 pneumonia.
Methodology
Study design
King Abdulaziz Medical City (KAMC) is a tertiary teaching hospital in Riyadh, Saudi Arabia, with 1200 beds capacity. We retrospectively included adult patients admitted to KAMC from June, 1st 2020, until October, 1st 2020, with a PCR positive for COVID-19 and diagnosed with severe or critical COVID-19. A patient must have received at least one dose of TCZ to be considered illegible for the TCZ arm. Since corticosteroid was part of our local COVID-19 treatment protocol after RECOVERY trial findings, we were confident that those patients admitted to the ICU directly have had corticosteroid prescribed starting from June 2020. TCZ prescribing pattern has changed in our institution in response to the conflicting data available and the findings from RCTs published by that time. So we decided to include patients with severe to critical COVID-19 who had not received TCZ but corticosteroid mainly DEX from August 1st to October, 1st 2020.
Definitions
Severe COVID-19
Patient with positive PCR for COVID-19 and had two or more of the following: Respiratory rate ≥30/min with blood oxygen saturation ≤94% at room air requiring non-invasive oxygen therapy (high flow nasal cannula-HFNC, face mask-FM, continuous positive airway pressure-CPAP); PaO2/FiO2 ratio <300; lung infiltrates >50% of the lung field on chest X-ray within 24−48 h of hospital admission [21,22].
Critical COVID-19
Patient with positive PCR for COVID-19 and received invasive mechanical ventilation [22].
Obesity
Defined as patient with body mass index (BMI) =/>30 kg/m2.
Immunocompromised status
Defined as patients with any of the following: age >70 years of age; diabetes mellitus (DM); receiving immunosuppressive therapy; active malignancy.
Data collection
Baseline characteristics were collected from patients’ electronic records, including demographic data (age, weight, and body mass index), medical history- including comorbidities (heart disease, diabetes mellitus-DM, hypertension-HTN, respiratory diseases, rheumatological diseases), laboratory markers before administering TCZ or corticosteroid (ferritin, C reactive protein-CRP, lactate dehydrogenase-LDH, interleukin 6-IL-6, D-dimer, procalcitonin-PCT, lymphocytes, and platelet count), respiratory status and chest Xray changes before administering TCZ or corticosteroids; TCZ dose administered and frequency of dosing; type of oxygen therapy on day-1, day-3, day7, and day-14 post-TCZ or corticosteroid. The number of patients who received corticosteroids (DEX and methylprednisolone- MP), dose, and duration of corticosteroid therapy. The number of patients who went to the intensive care unit (ICU) after administering TCZ or corticosteroid and days spent in ICU. The number of patients who developed infection and septic shock within 7–14 days after TCZ or corticosteroid therapy.
Outcomes
The study's primary outcome was the effect of TCZ therapy with or without corticosteroids compared to DEX alone on the hospital mortality. The secondary efficacy outcomes included need for invasive mechanical ventilation, days on mechanical ventilation, and hospital length of stay. Development of septic shock and/or bacterial or fungal infection within 7–14 days after starting immunomodulator were the safety outcomes of interest.
Statistical analysis
Categorical variables were expressed as percentages and compared between groups using the Chi-square test or Fisher’s exact test based on the data distribution. Continuous variables were presented as the means and standard deviation. The Student’s t-test or Wilcoxon/Kruskal-Wallis tests were used to compare the groups for continuous variables based on the data distribution. Further, we used multivariate logistic regression analysis to model the mortality as an outcome. We included patients whether received TCZ, DEX, or TCZ and DEX, patients with obesity, DM, heart disease, immunocompromised status, HTN, baseline CRP, and patient’s age and sex as predictors with backward elimination and p-value = 0.5 for entry and 0.05 to stay in the model.
Results
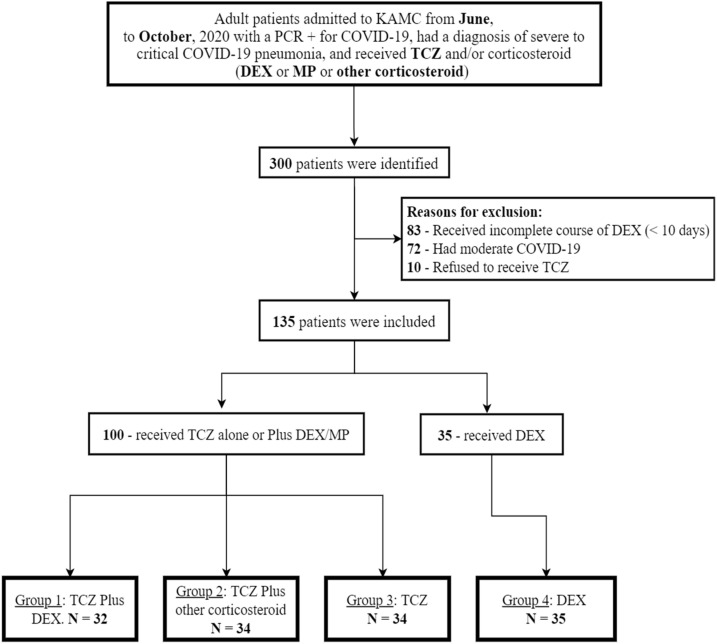
A total of 300 patients were identified during the study period in which 135 patients had been randomly included in the analysis to formulate two major groups, which have been further characterized into four groups. See Patients Inclusion Flow Chart (Fig. 1 ).
Fig. 1.
Patients’ allocation chart.
Two major groups, including those who received TCZ with or without corticosteroid (TCZ group), N = 100 and who received DEX alone (DEX group), N = 35. Then, grouped into four different groups; G1- received TCZ and DEX, N = 32; G2- received TCZ and other corticosteroids, N = 34; G3- received TCZ alone, N = 34; G4- received DEX, N = 35. In the TCZ group, patients who received corticosteroid accounted for 66/100 (66%) for which 32 had DEX and 30 had MP. The rest had oral prednisolone and intravenous hydrocortisone. Moreover, patients in the DEX group received DEX monotherapy as the only immunomodulator prescribed. For more descriptive details, see Table 1 .
Table 1.
Patients’ characteristics.
| Number of patients | N | TCZ (TCZ ± corticosteroid) | DEX | P value |
|---|---|---|---|---|
| 100 | 35 | |||
| Baseline characteristics | ||||
| Age, Mean (SD) | Years | 60 (13.59) | 63.0 (17.59) | 0.0129* |
| Male sex | N (%) | 87 (87) | 23 (65.7) | 0.0053** |
| Body mass index | Kg/m2 | 31.2 (9.31) | 31.9 (14.21) | 0.4738* |
| Obese | N (%) | 45 (45) | 15 (42.9) | 0.8262** |
| Hypertension | N (%) | 47 (47) | 24 (68.6) | 0.0278** |
| Diabetes mellitus | N (%) | 44 (44) | 26 (74.3) | 0.0020** |
| Heart Disease | N (%) | 9 (9) | 11 (31.4) | 0.0013** |
| Immunocompromised | N (%) | 44 (44.0) | 19 (54.3) | 0.2938** |
| Onset of symptoms to administration of TCZ or DEX | Days (SD) | 7 (5.47) | 7.9 (7.28) | 0.9899* |
| Laboratory parameters- pre TCZ or DEX dose | ||||
| C-reactive protein (CRP) | mg/dL | 249.8 (177.9) | 116.2 (88.38) | <0.0001* |
| Lactate dehydrogenase (LDH) | U/L | 645.0 (213.17) | 497.2 (199) | 0.0004* |
| Ferritin | ng/mL | 2231.9 (2305.26) | 782 (902.75) | <0.0001* |
| Procalcitonin (PCT) | ng/mL | 1.7 (4.69) | 0.7 (1.08) | 0.0888* |
| Platelets | 109/L | 293 (105) | 257(153.3) | 0.218* |
| Lymphocytes | 109/L | 1.0 (0.58) | 1.3 (0.86) | 0.0997* |
| D-dimer | mcg/mL | 6.5 (10.8) | 4.6 (9.15) | 0.2131* |
| Clinical status before administering TCZ or DEX | ||||
| Rapid deterioration in respiratory function | N (%) | 95 (95) | 26 (74.3) | 0.0016*** |
| Persistent fever | N (%) | 68 (68) | 7 (20) | <0.0001** |
| Infiltrates on CXR/chest CT | N (%) | 94 (94) | 34 (97.1) | 0.6764*** |
| ICU stay | N (%) | 65 (65) | 13 (37.1) | 0.0041** |
| Type of oxygen support 24 h pre dose | MV, N (%) | 39 (39) | 6 (17.1) | <0.0001*** |
| NIV, N (%) | 61 (61) | 29 (82.9) | ||
| Corticosteroids and TCZ administration information | ||||
| TCZ dose | Mean (SD) | 625.6 (155.35) | – | |
| Frequency of TCZ | 1, N (%) | 100 (100) | – | |
| 2, N (%) | 13 (13) | – | ||
| 3, N (%) | 3 (3) | – | ||
| Received corticosteroid | N (%) | 66 (66) | 35 (100) | <0.0001** |
| Duration of corticosteroid | Mean (SD) | 10 (5.34) | 9 (2.44) | 0.7421* |
| Corticosteroid type | Dexamethasone | 32 (48.5) | 35 (100) | |
| Hydrocortisone | 3 (4.5) | – | ||
| Methylprednisolone | 30 (45.5) | – | ||
| Prednisolone | 1 (1.5) | – | ||
| Other treatment modalities | ||||
| Favipravir | N (%) | 3 (3) | 1 (2.9) | 1.0000*** |
| Lopinavir/ritonavir | N (%) | 2 (2) | 7 (20) | 0.0011*** |
| Convalescent plasma | N (%) | 3 (3) | NA | 0.5679*** |
| Hydroxychloroquine (HCQ) | N (%) | 12 (12) | 1 (2.9) | 0.1827*** |
| Final disposition for patients requiring ICU admission/transferal | ||||
| ICU admission after TCZ or DEX | N (%) | 20 (20) | 17 (48.6) | 0.0011** |
| ICU discharge alive for patients who went to ICU after receiving TCZ or DEX | N (%) | 14 (70) | 10 (58.8) | 0.4779** |
| ICU length of stay | Mean (SD) | 9.4 (5.62) | 8.8 (6.97) | 0.8305** |
| ARDS | N (%) | 65 (65) | 29 (82.9) | 0.0480** |
| Total number of pts required Mechanical ventilation | N (%) | 63 (63) | 18 (51.4) | 0.2291** |
| Septic shock after Dex or TCZ | N (%) | 21 (21) | 7 (20) | 0.9001** |
Wilcoxon rank sum test is used to calculate the p-value.
Chi-square test is used to calculate the p-value.
Fisher exact test is used to calculate the P-value.
Baseline characteristics of patients in TCZ ± corticosteroid and DEX group
A total of 100 patients with an average age of 60 years where male gender constitute 87%. The most frequently encountered comorbidities were hypertension, followed by diabetes mellitus and heart disease. Most patients were found to be immunocompromised (44%) and obese (45%) as per study definition. The mean time from onset of symptoms to administration of immunomodulator therapy was 7 days (Table 1).
In the DEX group, the average age was 63 years where 65.7% of patients were male. Baseline comorbidities were hypertension, diabetes mellitus, heart disease, and obesity, respectively 68.6%, 74.3%, 31.4%, and 43%. Half of patients were immunocompromised and had a mean of 8 days between onset of symptoms and administration of immunomodulator (Table 1).
Clinical status before administering immunomodulators
Inflammatory markers were markedly elevated in the TCZ group, particularly CRP, LDH, and ferritin. Lymphocyte count was 1.3 × 109/L in the DEX group versus 1 × 109/L in the TCZ group. The majority of patients in TCZ had rapid deterioration in their respiratory function and persistent fever 95% vs. 74% and 68% vs. 20% compared to the DEX group. All patients had infiltrates in chest X-ray consistent with pneumonia. Patients who required direct ICU admission or transfer were 65% vs. 37% in TCZ and DEX groups, respectively. Nearly a third of the patients were on MV already in the TCZ group compared to 17% in the DEX group (Table 1).
Treatment modalities accompanied immunomodulator
Only 13% had received a second dose of TCZ with a mean dose of 625 mg. Patients received corticosteroid for a mean duration of 10 days vs. 9 days in TCZ and DEX, respectively. The most frequently used combination therapy was HCQ, followed by lopinavir/ritonavir, favipiravir, and convalescent plasma (Table 1).
Final disposition of patients requiring ICU admission/transfer
More patients required ICU transferal after DEX administration in DEX group 17/35 (48.6%) vs. 20/100 (20%) in TCZ ± corticosteroid. A total of 63/100 (63%) and 18/35 (51.4%) in TCZ and DEX groups required invasive mechanical ventilation, respectively.
Outcomes of interest
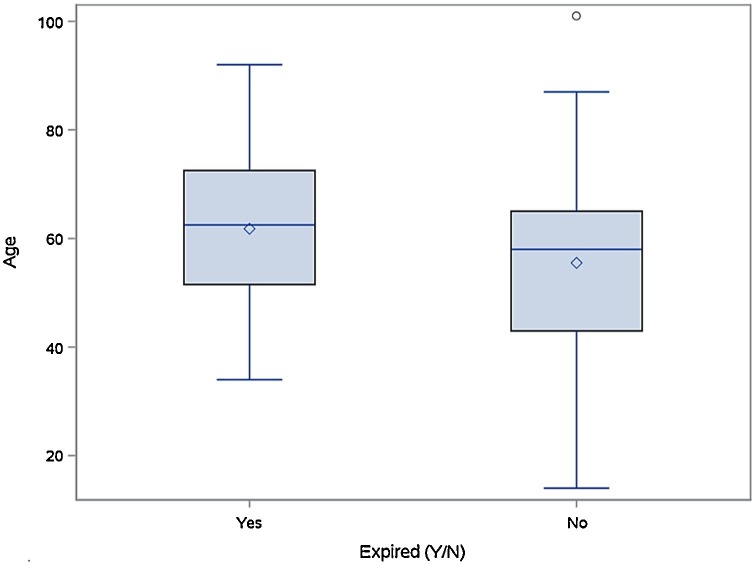
The rate of hospital mortality was 36% in TCZ and 37% in the DEX group, p = 0.91. The result of univariate analysis of mortality is provided in supplementary material Table 1. Patient’s age was the only significant predictor of mortality with OR = 1.030 and 95% CI = (1.004, 1.057). Fig. 2 shows the distribution of age between the two groups.
Fig. 2.
Relation between hospital mortality and age.
After receiving immunomodulator therapy, the number of patients who required mechanical ventilation was 24/63 (38%) and 12/35 (34%) in TCZ and DEX, respectively. Twenty-eight in TCZ and five patients in DEX were extubated with a mean duration on mechanical ventilation of 12.8 days and 6.4 days, respectively. The mean hospital length of stay was 16.7 days in TCZ compared to 18.5 days in DEX. The rates of bacterial or fungal infection development within 7 days or after were 29% vs. 31.4% in the TCZ and the DEX groups (Table 2 ).
Table 2.
Outcomes of interest.
| Outcomes of interest | |||
|---|---|---|---|
| TCZ (TCZ ± corticosteroid) | DEX | ||
| Primary outcome | |||
| Hospital mortality | N (%) | 35 (36) | 13 (37) |
| Secondary outcomes | |||
| Number of patients required MV after TCZ or DEX | N/total (%) | 24/63 (38) | 12/18 (34) |
| Days on MV | Mean (SD) | 12.8 (6.87) | 6.4 (2.07) |
| Hospital length of stay | Mean (SD) | 16.7 (11.69) | 18.5 (12.98) |
| Infection developed =/>7 days after TCZ or DEX | N (%) | 29 (29) | 11 (31.4) |
Sub-group analysis based on immunomodulator received
G1- received TCZ and DEX, N = 32; G2- received TCZ and other corticosteroids, mostly methylprednisolone (MP), N = 34; G3- received TCZ alone, N = 34; G4- received DEX, N = 35. Patients in G3 were younger compared to other groups with a mean age of 53 years. The majority of patients in all groups were male. Patients with diabetes mellitus constitute more than 50% of all groups except in G3. The mean of symptoms onset to hospital presentation was 5 days except in G4, 7.5 days. All inflammatory markers, including PCR, LDH, and ferritin, were significantly high in all groups except in G4. Lymphocyte count was 1.3 × 109/L in G4, which is higher when compared to other groups. More details in Table 3 .
Table 3.
Characteristics and outcomes of subgroups.
| G1 N = 32 (TCZ + DEX) | G2 N = 34 (TCZ + other corticosteroid) | G3 N = 34 (TCZ) | G4 N = 35 (DEX) | Total N = 135 | ||
|---|---|---|---|---|---|---|
| Age | Mean (SD) | 59.2 ± 11.39 | 55.6 ± 13.72 | 53.1 ± 15.00 | 63.0 ± 17.59 | 57.7 ± 14.99 |
| Sex | Male, N (%) | 30 (93.8%) | 29 (85.3%) | 28 (82.4%) | 23 (65.7%) | 110 (81.5%) |
| Obesity | N (%) | 8 (25.0%) | 16 (47%) | 21 (61.8%) | 15 (42.9%) | 60 (44.4%) |
| HTN | N (%) | 14 (43.8%) | 20 (58.8%) | 13 (38.2%) | 24 (68.6%) | 71 (52.6%) |
| DM | N (%) | 18 (56.3%) | 14 (41.2%) | 12 (35.3%) | 26 (74.3%) | 70 (51.9%) |
| Heart disease | N (%) | 4 (12.5%) | 3 (8.8%) | 2 (5.9%) | 11 (31.4%) | 20 (14.8%) |
| Immunocompromised | N (%) | 17 (53%) | 17 (50%) | 10 (29.4%) | 19 (54.3%) | 63 (46.7%) |
| Onset of symptoms, days | Mean (SD) | 5.0 ± 3.99 | 5.0 ± 5.50 | 5.2 ± 2.69 | 7.5 ± 5.89 | 5.7 ± 4.72 |
| Days from symptoms onset to administration | Mean (SD) | 7.3 ± 6.5 | 6.5 ± 5.80 | 7.1 ± 3.98 | 7.9 ± 7.28 | 7.2 ± 5.97 |
| CRP | Mean (SD) | 206.8 ± 157 | 299.2 ± 192 | 239.4 ± 175 | 116.2 ± 88.4 | 215.6 ± 170 |
| LDH | Mean (SD) | 652.8 ± 207 | 658.2 ± 201 | 624.4 ± 235 | 497.2 ± 200 | 606.9 ± 219 |
| Ferritin | Mean (SD) | 2752.6 ± 2565 | 1671.5 ± 1187 | 2290.9 ± 2809 | 782.4 ± 903 | 1878.1 ± 2143 |
| PCT | Mean (SD) | 1.0 ± 1.70 | 3.1 ± 7.51 | 0.9 ± 1.58 | 0.7 ± 1.08 | 1.4 ± 4.06 |
| Lymphocytes | Mean (SD) | 1.0 ± 0.52 | 0.9 ± 0.65 | 1.2 ± 0.55 | 1.3 ± 0.86 | 1.1 ± 0.68 |
| ICU admission post immunomodulatory | N (%) | 8 (25.0%) | 7 (20.6%) | 5 (14.7%) | 17 (48.6%) | 37 (27.4%) |
| Septic shock after immunomodulator | N (%) | 10 (31.3%) | 8 (23.5%) | 3 (8.8%) | 7 (20.0%) | 28 (20.7%) |
| Number of pt receiving MV before immunomodulatory | N (%) | 11 (34.4%) | 16 (47.1%) | 12 (35.3%) | 6 (17.1%) | 45 (33.3%) |
| Total no. of pt required MV | N (%) | 22 (68.8%) | 23 (67.6%) | 18 (52.9%) | 18 (51.4%) | 81 (60%) |
| Days on MV | Mean (SD) | 15.0 ± 9.55 | 10.3 ± 3.44 | 11.7 ± 3.43 | 6.4 ± 2.07 | 11.8 ± 6.77 |
| Hospital length of stay | Mean (SD) | 23.9 ± 15 | 14.3 ± 5.9 | 12.5 ± 9.4 | 18.5 ± 13 | 17.2 ± 12 |
| Hospital mortality | N (%) | 11 (35.5%) | 15 (46.9%) | 9 (26.5%) | 13 (37.1%) | 48 (36.4%) |
The need for ICU transfer was higher in G4, with almost 48% of patients. Development of septic shock ranges between 31% in G1 to 8% in G3. The percentage of patients who required mechanical ventilation was highest in G1 = 68.8% compared to 51% in G 4. Days on mechanical ventilation were shorter in G4 compared to other groups. On the other hand, the mean hospital length of stay was more than 15 days, with the most prolonged duration was 23.9 days in G1. Hospital mortality rates were similar among different groups, with the lowest rate of 26.5% in G3.
Discussion
In the present study, we found the prescribing pattern of TCZ at KAMC consistent with the current recommendations by IDSA [7]. TCZ was prescribed for patients who experienced rapid deterioration of their respiratory status, including increased oxygen requirements or the need for mechanical ventilation along with elevated inflammatory markers (CRP, LDH, and ferritin) and the presence of infiltrates in chest X-rays. TCZ dose ranges between 4−8 mg/kg with a maximum dose of 800 mg, and a second dose was administered for only 13%. This implies the uncertainty among our clinicians on the potential benefits and harms associated with repeated doses of TCZ at that time.
Additional findings of this study have revealed the lack of any differences in hospital mortality associated with combining TCZ with a corticosteroid, particularly DEX compared to DEX monotherapy. Hospital mortality was statistically indifferent between the TCZ arm and DEX arm (36% vs. 37%, p = 0.9). The hospital mortality rate in the TCZ arm in our study is relatively higher at 36% compared to RECOVERY and REMAP-CAP clinical trials (31% and 27%), respectively [19,20]. The clinical efficacy of TCZ has been tested in many prospective RCTs. The first published RCT has included adult patients with COVID-19 pneumonia with a PaO2/FiO2 ratio between 200 and 300 mm Hg. Results indicate no additional effect of TCZ in terms of disease progression compared to standard of care (SOC). Notably, this study was an open-label where it seems that patients presented in the later stage of their disease course with no corticosteroid prescribed [14]. Hermine et al. study, another RCT where TCZ did not reduce World Health Organization-Clinical Progression Scale (WHO-CPS) scores lower than 5 at day 4 but might have reduced the risk of non-invasive ventilation (NIV), MV, or death by day 14 [13]. Additionally, post-hoc subgroup analysis (for corticosteroids, including DEX) showed that the effect of TCZ was boosted when combined with corticosteroids (HR, 0.38; 90% CI, 0.13–1.11) [13]. In Stone’s et al. study, 161 patients received TCZ and compared to 82 patients in the placebo group. No statistically significant differences were found between groups in respect to death, need for intubation, and disease progression [15]. Roche Ltd, TCZ’s manufacturer have conducted two double-blinded RCTs, COVACTA and EMPACTA [16]. The COVACTA study, reported no mortality difference at day 28 between patients who received TCZ and SOC [17]. The EMPACTA study showed efficacy in reduction of mechanical ventilation or death by day 28 with no improvement in survival rate [18]. RECOVERY and REMAP-CAP were the largest RCTs that prove the favorable clinical outcomes associated with TCZ [19,20].
The additive effect of TCZ and corticosteroid was clearly seen in RECOVERY trial based on prespecified analysis of hospital mortality for patients who received TCZ and corticosteroid vs. corticosteroid monotherapy (29% vs. 35% with RR = 0.79 and 95% CI: 0.70–0.89, p = 0.01) [20]. Interestingly, the lack of synergistic effect of TCZ + DEX compared to DEX on the hospital mortality was evident in our study. Age of 60 years or above seems to be the only factor that associated with hospital morality regardless of which immunomodulator received in present study. The rate of ICU transferal was lower in the TCZ group than the DEX group after administering immunomodulator (20% vs. 48.6%), which is consistent with Snow and Chen et al. [23,24]. Contradictory to existing data, the impact of TCZ on the need for MV was negative compared to DEX (24/63 (38%) vs. 12/18 (34%) and data from RECOVERY (265/1754 (15%) vs. 343/1800 (19%); 95% CI: 0.79 (0.69–0.92); p = 0.0019) [20]. The rate of bacterial or fungal infection was similar in both groups, suggesting that TCZ plus DEX is not necessarily associated with a significant increase in the risk of infection.
This cohort is at higher risk for sampling and selection biases secondary to the retrospective nature of this study; however, it is presenting real-world data on this group of COVID-19 patients. The small number of participants might have had impacted the ability to find out the statistical difference when comparing outcomes between groups.
Conclusion
In conclusion, the present study did not show any additional survival benefit with TCZ ± corticosteroid compared to DEX. This could highlight and emphasize the importance of exploring the results from RCTs that suggested the potential benefit of this combination.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.11.017.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Drosten C., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 2.Wan Y., et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge X.Y., et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian W., et al. Immune suppression in the early stage of COVID-19 disease. Nat Commun. 2020;11(1):5859. doi: 10.1038/s41467-020-19706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khadke S., et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J. 2020;17(1):154. doi: 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhimraj A., et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. Apr 27:ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group R.C., et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo P., et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassoun A., et al. Utilizing tocilizumab for the treatment of cytokine release syndrome in COVID-19. J Clin Virol. 2020;128:104443. doi: 10.1016/j.jcv.2020.104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M., et al. Tocilizumab for treating COVID-19: a systemic review and meta-analysis of retrospective studies. Eur J Clin Pharmacol. 2021;77(3):311–319. doi: 10.1007/s00228-020-03017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermine O., et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvarani C., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone J.H., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parr J.B. Time to reassess tocilizumab’s role in COVID-19 pneumonia. JAMA Intern Med. 2021;181(1):12–15. doi: 10.1001/jamainternmed.2020.6557. [DOI] [PubMed] [Google Scholar]
- 17.Rosas I.O., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salama C., et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Investigators R.-C., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 23.Snow T.A.C., et al. Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials. Intensive Care Med. 2021;47(6):641–652. doi: 10.1007/s00134-021-06416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C.X., et al. Systematic review and meta-analysis of tocilizumab in persons with coronavirus disease-2019 (COVID-19) Leukemia. 2021;35(6):1661–1670. doi: 10.1038/s41375-021-01264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.