Abstract
Objective:
To test the hypothesis that admission hemoglobin (Hb) levels are associated with outcome in primary, non-traumatic intracerebral hemorrhage (ICH).
Design:
Individual patient data meta-analysis of three studies of ICH.
Setting:
Two randomized clinical trials and one multi-ethnic observational study.
Patients:
Patients with spontaneous, non-traumatic ICH.
Interventions:
None.
Measurements and Main Results:
Our exposure of interest was admission Hb levels and the primary outcome was 3-month post-ICH dichotomized modified Rankin Scale (mRS) (0–3 versus 4–6). Intermediate outcomes were admission hematoma volume and hematoma expansion defined as 6 mL or 33% increase in hemorrhage size on repeat CT. A total of 4,172 ICH patients were included in the study (mean age 63 [SD 14]; female sex 1,668 [40%]). Each additional g/dL of admission Hb was associated with 14% (OR 0.86, 95%CI 0.82–0.91) and 7% (OR 0.93, 95%CI 0.88–0.98) reductions in the risk of poor outcome in unadjusted and adjusted analyses, respectively. Dose-response analyses indicated a linear relationship between admission Hb levels and poor outcome across the entire evaluated range (test-for-trend p<0.001). No consistent associations were found between admission Hb levels and hematoma volume or hematoma expansion.
Conclusions:
Higher Hb levels are associated with better outcome in ICH. Further research is needed to evaluate admission Hb levels as both a therapeutic target and predictor of outcome.
Keywords: Cerebral Hemorrhage, Hemoglobin A, Anemia, Glasgow Coma Scale, Meta-Analysis, Prognosis
INTRODUCTION
Spontaneous, non-traumatic intracerebral hemorrhage (ICH) is a devastating condition with high morbidity and mortality1 and few therapeutic interventions available2. One strategy to accelerate the development of new therapies is to study biological pathways routinely evaluated in the acute setting that already have safe and effective interventions available3,4.
Low admission hemoglobin levels (Hb) are associated with worse outcomes in acute coronary syndrome5 and ischemic stroke6 and may also play a role in hemorrhagic stroke. A few small, single-center studies have found associations between low admission Hb7–10 and anemia9,11–16 and increased morbidity and mortality in ICH. Proposed mechanisms that would explain this relationship with worse outcome include impaired cerebral oxygen delivery17 and coagulopathy18,19 leading to ICH expansion10, a powerful predictor of poor outcome20. While promising, the existing evidence is limited by the small sample size of existing studies.
We therefore aimed to evaluate the relationship between admission Hb levels and outcome in patients with ICH across multiple studies of this condition, all with excellent ascertainment quality for the collected data. We hypothesize that higher admission Hb levels are associated with better outcomes in these patients. We conducted an individual participant data (IPD) meta-analysis of over 4,000 ICH patients enrolled in the Antihypertensive Treatment of Acute Cerebral Hemorrhage 2 (ATACH-2) clinical trial21, the Factor Seven for Acute Hemorrhagic Stroke (FAST) clinical trial22, and the Ethnic/Racial Variation in Intracerebral Hemorrhage (ERICH) observational study23.
MATERIAL AND METHODS
Study Design
We performed an IPD meta-analysis24 across the ATACH-2, FAST and ERICH studies. Details about the design and inclusion/exclusion criteria of these studies are available elsewhere21–23. Briefly, ATACH-2 (NCT01176565) randomized 1,000 ICH patients with elevated systolic blood pressure (>180 mmHg) presenting within 4.5 hours of symptom onset to intensive or standard blood-pressure lowering treatment. FAST (NCT00127283) randomized 841 ICH patients presenting within 4 hours of symptom onset to receive recombinant activated factor 7 or placebo. ERICH (NCT01202864) enrolled 3,000 ICH patients, including equal proportions of whites, blacks and Hispanics.
Standard Protocol Approvals, Registrations, and Patient Consents
Each study included was approved by the appropriate Institutional Review Boards and written informed consent was obtained from each participant or each participant’s legal representative.
Definition of ICH
All studies enrolled patients with a primary, non-traumatic ICH, defined as an acute neurological deficit with associated new intraparenchymal blood documented on neuroimaging. ICH cases secondary to tumors, vascular malformations or hemorrhagic conversion of an ischemic stroke were excluded.
Exposure and outcome
Our exposure of interest was baseline Hb levels, as measured on routine admission laboratories. Hb measurements were converted to mg/dL. Patients missing admission Hb levels or 3-month outcome data were excluded. Our primary outcome was functional outcome 3 months after the ICH, as measured by the modified Rankin scale (mRS). The mRS is a 7-point ordinal scale with 0 corresponding to full recovery and 6 corresponding to death25.
Neuroimaging analysis
Baseline and follow-up head CT scans were obtained in all 3 studies. The determination of ICH location, ICH volume and IVH presence were performed centrally by blinded investigators in each study. ICH volumes were measured using manual or semi-automatic segmentation of the parenchymal hematoma. ICH location was classified as lobar or nonlobar, as the former is mainly linked to cerebral amyloid angiopathy and the latter to long-standing hypertension26. Nonlobar hemorrhages included those compromising the thalami or basal ganglia, those located below the tentorium and primary intraventricular hemorrhages (IVH). Hematoma expansion was defined as 6 mL or 33% increase in hemorrhage size on a follow-up scan (24 hours after the initiation of treatment in ATACH-2 and FAST trials, and first available repeat neuroimaging study in ERICH).
Statistical Methods
Categorical variables are presented as count (percentage [%]) and continuous variables as mean (standard deviation [SD]) or median (interquartile range [IQR]), as appropriate.
Exposure and outcome modeling.
We modeled admission Hb levels as a continuous variable and functional outcome as a dichotomous variable, defining good outcome as mRS 0–3 and poor outcome as 4–6.25,27 In sensitivity analyses, we dichotomized Hb values as anemia (<12 mg/dL for women and <13 mg/dL for men) or no anemia. For analysis involving intermediate neuroimaging endpoints, we modeled ICH volume as a continuous variable and ICH expansion as a dichotomous variable.
Association testing.
The primary analyses involved a one-stage IPD meta-analysis, implementing multivariable, mixed-effects logistic regression modeling to test for association between admission Hb levels and outcome. As a secondary analysis, we conducted a two-stage IPD meta-analysis, fitting multivariable logistic regression models for each study separately, with subsequent pooling of study-specific results using fixed-effects and random-effects (with inverse-variance weighting) meta-analyses. Multivariable models included universal confounders (gender, race), the components of the ICH score (age, ICH location, ICH volume, presence of IVH, Glasgow Coma Scale) and anticoagulation (only for ERICH study). We used tau2, Q and I2. to assess heterogeneity. In sensitivity analyses, we (1) adjusted for the trial intervention when analyzing ATACH-2 and FAST data, (2) excluded anticoagulated participants from ERICH, (3) included participants from clinical trials only, (3) included only participants with pre-stroke mRS compatible with independence, (4) analyzed only participants with Hb levels outside the usual transfusion threshold (7 mg/dL), and (5) dichotomized outcome as mRS of 0–2 vs 3–6. In stratified analyses, we evaluated lobar and non-lobar hemorrhages separately. Finally, we performed secondary analysis looking at Hb measurements at intermediate time points (24, 48 and 72 hours after randomization), and the change between baseline and 72-hour in ATACH-2, the only study with these data available.
Association testing for intermediate neuroimaging endpoints.
We used the same analytical approach to test for association between admission Hb levels and admission hematoma volume and hematoma expansion and further tested if the relationship between Hb levels and ICH volume changed after log-transforming ICH volume.
Dose-response analyses.
We evaluated the shape of the dose-response curve between admission Hb levels and ICH outcome by estimating the unadjusted risk of poor outcome across strata of admission Hb levels. To further assess this dose-response curve, we calculated the predicted probabilities of poor outcome based on our regression models across strata of admission Hb levels using 5-fold cross-validation and used generalized additive models to explore potential ceiling effects.
Data availability
Anonymized data from the ATACH-2 and ERICH studies are publicly available via the National Institute of Neurological Disorders and Stroke Archive of Clinical Research Datasets. Anonymized data from the FAST trial is accessible upon reasonable request from the study’s sponsor.
RESULTS
A total of 4,841 ICH patients were enrolled in ATACH-2, FAST and ERICH. After excluding 669 patients who had missing data for admission Hb levels or outcome, a total of 4,172 ICH patients (mean age 63 [SD 14], 1,668 [40%] were female) were included in the study, including 905 from ATACH-2, 2,521 from ERICH and 746 from FAST (Table 1). There were 976 (23%) lobar and 3167 (76%) nonlobar hemorrhages (29 with missing location). The mean admission Hb was 13.8 (1.9) g/dL when considering all studies together, and 14.3 (1.7), 14.0 (1.6) and 13.6 (2.0), respectively, when evaluating each study separately (Table 2). Poor outcomes were observed in 1,940 (47%) patients when considering all studies, and 346 (38%), 359 (48%) and 1235 (49%), respectively, when evaluating each study separately (Table 2). Among all participants, 770 (18.5%) died.
Table 1.
Demographic characteristics by study.
| Variable | Overall (n=4,172) | ATACH-2 (n=905) | ERICH n=2521) | FAST (n=746) |
|---|---|---|---|---|
| Age (years), mean (SD) | 62.56 (13.69) | 62.23 (13.03) | 62.10 (14.14) | 64.57 (12.71) |
| Female, n (%) | 1,668 (40.0) | 342 (37.8) | 1047 (41.5) | 279 (37.4) |
| White race, n (%) | 1080 (31.5) | 239 (26.4) | 841 (33.4) | N/A |
| Hypertension, n (%) | 3101 (76.4) | 716 (81.2) | 1883 (75.0) | 502 (75.3) |
| Diabetes, n (%) | 889 (21.7) | 179 (19.8) | 625 (24.8) | 85 (12.7) |
| Hyperlipidemia, n (%) | 967 (24.2) | 217 (25.5) | 671 (27.0) | 79 (11.8) |
| Atrial fibrillation, n (%) | 316 (7.9) | 32 (3.6) | 256 (10.5) | 28 (4.2) |
| Ever smoker, n (%) | 1423 (37.1) | 399 (44.1) | 1009 (44.5) | 15 (2.2) |
| Anticoagulation, n (%) | 287 (6.9) | 0 (0.0) | 287 (11.4) | 0 (0.0) |
| Lobar ICH, n (%) | 976 (23.4) | 104 (11.5) | 778 (30.9) | 94 (13.1) |
| IVH present, n (%) | 1574 (38.6) | 234 (26.4) | 1069 (43.6) | 271 (36.7) |
Abbreviations: ATACH-2=Antihypertensive Treatment of Acute Cerebral Hemorrhage-II trial. ERICH=Ethnic/Racial Variations of Intracerebral Hemorrhage study. FAST=Recombinant Factor VIIa in Acute Intracerebral Hemorrhage trial. ICH=Intracerebral hemorrhage.
Table 2.
Exposure and outcomes.
| Variable | Overall (n=4,172) | ATACH-2 (n=905) | ERICH (n=2521) | FAST (n=746) |
|---|---|---|---|---|
| Hemoglobin (g/dL), mean (SD) | 13.84 (1.91) | 14.26 (1.73) | 13.63 (2.02) | 14.03 (1.62) |
| ICH volume (mL), mean (SD) * | 19.99 (23.88) | 13.70 (12.05) | 21.39 (26.14) | 22.88 (25.35) |
| Hematoma expansion, n (%) | 781 (24.0) | 195 (24.3) | 370 (21.5) | 216 (29.9) |
| Poor outcome † , n (%) | 1940 (46.5) | 346 (38.2) | 1235 (49.0) | 359 (48.1) |
| Death, n (%) | 770 (18.5) | 66 (7.3) | 554 (22.0) | 150 (20.1) |
Abbreviations: ATACH-2=Antihypertensive Treatment of Acute Cerebral Hemorrhage-II trial. ERICH=Ethnic/Racial Variations of Intracerebral Hemorrhage study. FAST=Recombinant Factor VIIa in Acute Intracerebral Hemorrhage trial. ICH=Intracerebral hemorrhage.
Missing data for baseline ICH volume = 92.
Poor outcome defined as modified Rankin Scale ≥ 4 at 3-month follow-up.
Admission hemoglobin levels and ICH outcome
We found a significant association between higher admission Hb levels and lower risk of poor outcome (Table 3). The primary analysis combining IPD across studies and implementing multivariable mixed-effects logistic regression indicated that each additional g/dL of admission Hb was associated with a 7% decrease in risk of poor outcome (OR 0.93, 95%CI 0.88–0.98; p=0.006). These results remained unchanged when implementing two-stage meta-analysis using either fixed-effects (OR 0.93, 95%CI 0.89–0.97; p=0.0007) or random-effects (OR 0.92, 95%CI 0.86–0.99; p=0.038) approaches. Importantly, there was no heterogeneity across study-specific estimates (tau2 = 0.0003; I2 = 0.0%, Q=0.85 [p=0.65]). Sensitivity analyses including the trial intervention in ATACH-2 and FAST did not change the results nor did the exclusion of anticoagulated participants from ERICH (OR 0.93, 95%CI 0.89–0.97; p<0.001). Additionally, analysis including only participants from clinical trials, only those with pre-stroke mRS compatible with independence, or only those outside the usual transfusion threshold yielded consistent results, as did dichotomizing mRS as 0–2 vs 3–6. Analyses stratified by hemorrhage location yielded similar results in nonlobar hemorrhages but did not reach statistical significance in lobar ICH (Table 3). Finally, we performed secondary analysis using Hb measurements at 24 hr, 48 hr and 72 hr in the ATACH-2 study. Hb levels at all these three time points were associated with outcome (24hr p=0.05 and 48hr and 72hr both p<0.001). A positive change in Hb levels was also associated with outcome (OR 0.73; 95%CI 0.62–0.84; p<0.001).
Table 3.
Hemoglobin association with outcome (mRS ≥4) at 3-month after ICH.
| Study or Analysis Strategy | All patients (N=4,172) | Lobar (N=976) | Non-Lobar (N=3,167) | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Study-specific results | ||||||
| ATACH-2 | 0.88 (0.79–0.99) | 0.028 | 0.68 (0.47 – 0.98) | 0.04 | 0.90 (0.80 – 1.01) | 0.09 |
| ERICH | 0.93 (0.88–0.98) | 0.01 | 0.94 (0.85 – 1.04) | 0.23 | 0.92 (0.86 – 0.98) | 0.01 |
| FAST | 0.95 (0.84–1.07) | 0.37 | 1.01 (0.69 – 1.49) | 0.95 | 0.92 (0.81 – 1.05) | 0.21 |
| Combined analyses | ||||||
| One-stage IPD meta-analysis | 0.93 (0.88–0.98) | 0.006 | 0.93 (0.75 – 1.14) | 0.47 | 0.92 (0.87 – 0.98) | 0.007 |
| Two-stage IPD meta-analysis (Fixed Effects) | 0.93 (0.89–0.97) | 0.0007 | 0.93 (0.85 – 1.02) | 0.10 | 0.92 (0.87 – 0.97) | 0.001 |
| Two-stage IPD meta-analysis (Random Effects) | 0.92 (0.86–0.99) | 0.039 | 0.89 (0.56 – 1.40) | 0.37 | 0.92 (0.90 – 0.94) | 0.003 |
Abbreviations: ATACH-2=Antihypertensive Treatment of Acute Cerebral Hemorrhage-II trial. ERICH=Ethnic/Racial Variations of Intracerebral Hemorrhage study. FAST=Recombinant Factor VIIa in Acute Intracerebral Haemorrhage trial. OR=Odds Ratio. 95%CI= 95% confidence interval. IPD=Individual participant data.
Missing data for ICH location = 29. Models adjusted by age, sex, race, ICH location (except in stratified analyses by location), ICH volume, presence of IVH, Glasgow Coma Scale and, in ERICH, anticoagulation use. Heterogeneity (all patients): tau2 = 0.0003, I2 = 0.0%, Q=0.85 (p=0.65). Heterogeneity (lobar): tau2 = 0.0216, I2 = 33.2%, Q=2.99 (p=0.22). Heterogeneity (nonlobar): tau2 = < 0.0001, I2 = 0.0%, Q=0.07 (p=0.97).
Anemia on admission and ICH outcome
The results outlined above remained consistent when categorizing ICH patients as anemic or non-anemic. The primary analysis combining IPD across studies indicated that anemic versus non-anemic patients had a 49% higher risk of poor outcome (OR 1.49; 95%CI 1.17–1.89; p=0.001). These results remained unchanged when implementing two-stage meta-analysis using either fixed-effects (OR 1.54, 95%CI 1.26–1.88; p<0.0001) or random-effects (OR 1.54; 95%CI 1.21–1.95; p=0.015). There was no heterogeneity (tau2=0.0025; I2=0.0%, Q=0.55 [p=0.76]).
Dose-response analysis
To evaluate whether the association between admission Hb levels and poor outcome was only driven by anemia, we evaluated how this association changed across different strata of the exposure. We found a consistent linear relationship between admission Hb levels and ICH outcome across a wide range of evaluated values. We observed this linear correlation when both estimating unadjusted risks of poor outcomes across different strata of admission Hb levels (test-for-trend p<0.0001, Figure 1), and formally testing the association between Hb levels and the predicted risks from the adjusted regression model (p<0.0001). No ceiling effect was identified.
Figure 1.
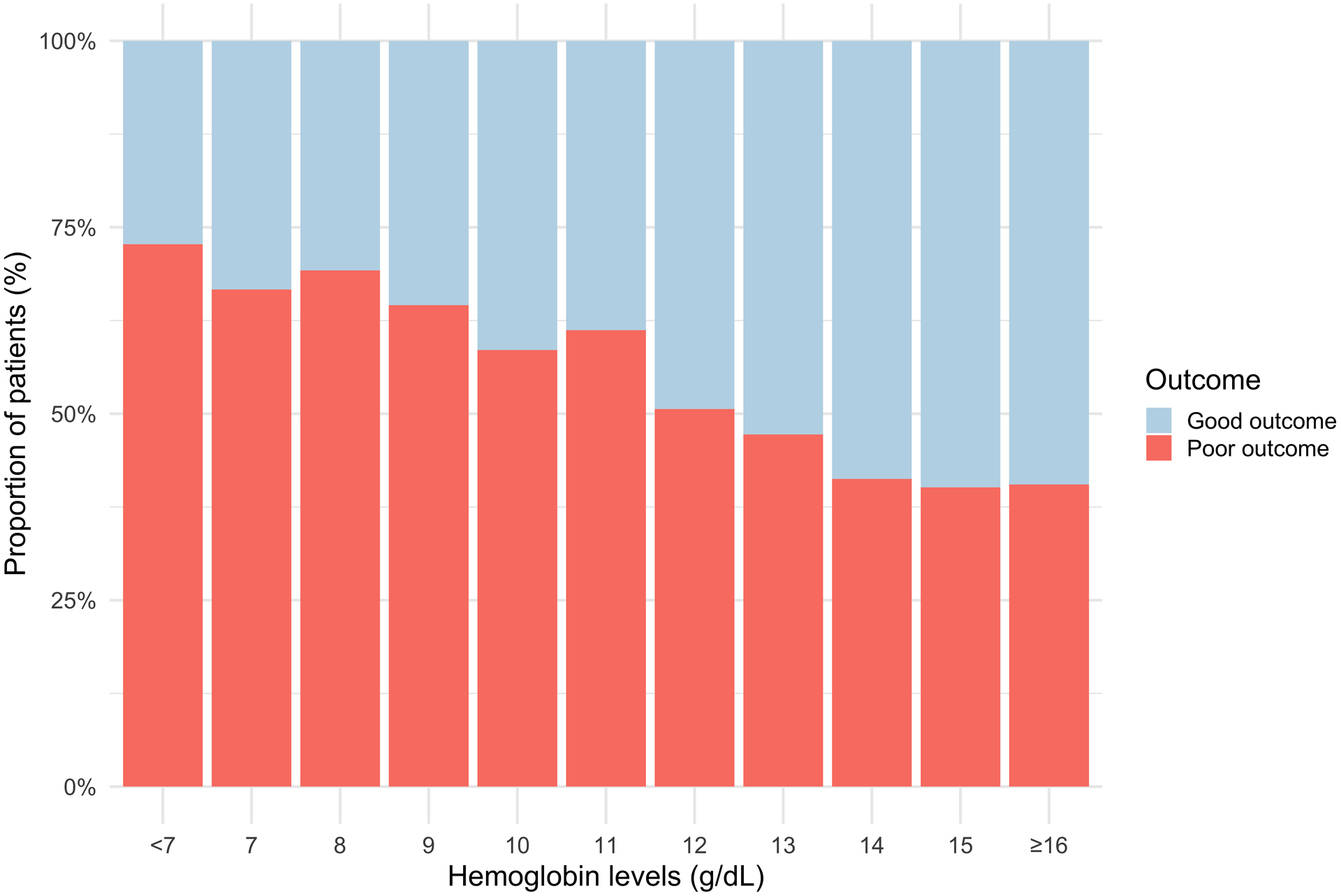
Dose response analysis.
Proportion of patients with poor outcome in each hemoglobin level subgroup.
Admission hemoglobin levels and ICH volume and expansion
We evaluated whether the observed association between admission Hb levels and outcomes was mediated by admission hematoma volume and hematoma expansion. Mean time to follow-up CT in ERICH was 24.1 hours (SD 40). For this analysis, we excluded patients with primary intraventricular hemorrhages (e.g., those without any intraparenchymal blood) and those with missing data for hematoma expansion. These exclusions led to an effective sample size of 4,035 ICH cases for the analysis of admission hematoma volume and 3,215 for the analysis of hematoma expansion. The primary analysis did not find an association between admission Hb levels and these neuroimaging markers (Table 4). Sensitivity analysis using log-transformed ICH volume as outcome did not change the results. Study-specific analyses did reveal an inverse association between admission Hb levels and ICH volume in ERICH (beta=−1.09; 95%CI −1.60 to −0.58, p<0.0001). We did not find significant associations for hematoma expansion. Sensitivity analysis including time from symptom onset to CT did not significantly change the results.
Table 4.
Hemoglobin association with baseline ICH volume and ICH expansion.
| Study or Analysis Strategy | ICH volume | ICH expansion | ||
|---|---|---|---|---|
| Beta (95%CI) | P value | OR (95%CI) | P value | |
| Study-specific results | ||||
| ATACH-2 | 0.14 (−0.35, 0.62) | 0.58 | 0.98 (0.88–1.09) | 0.745 |
| ERICH | −1.09 (−1.60, −0.58) | <0.0001 | 0.94 (0.88–1.01) | 0.077 |
| FAST | 0.24 (−0.86, 1.35) | 0.67 | 1.03 (0.92–1.16) | 0.58 |
| Meta-analysis | ||||
| One-stage IPD meta-analysis | −0.35 (−1.24, 0.55) | 0.45 | 0.99 (0.92–1.05) | 0.71 |
| Two-stage IPD meta-analysis (Fixed Effects) | −0.38 (−0.72, −0.05) | 0.026 | 0.97 (0.92–1.02) | 0.20 |
| Two-stage IPD meta-analysis (Random Effects) | −0.30 (−2.17, 1.58) | 0.57 | 0.97 (0.87–1.09) | 0.40 |
Abbreviations: ATACH-2=Antihypertensive Treatment of Acute Cerebral Hemorrhage-II trial. ERICH=Ethnic/Racial Variations of Intracerebral Hemorrhage study. FAST=Recombinant Factor VIIa in Acute Intracerebral Hemorrhage trial. OR=Odds Ratio. 95%CI= 95% confidence interval. IPD=Individual participant data. ICH volume models: Models adjusted by age, sex, race, ICH location and anticoagulation use (only in ERICH). Heterogeneity: tau2 = 0.4236, I2 = 84.5% (54.0%−94.8%), Q=12.93 (p=0.0016). ICH expansion models: Models adjusted by age, sex, race, ICH location, baseline ICH volume, treatment (in ATACH and FAST) and anticoagulation use (only in ERICH). Heterogeneity: tau2 = 0.0008, I2 = 0.0% (0.0%−89.2%), Q=1.92 (p=0.38).
DISCUSSION
We report the results of a large observational study evaluating whether admission Hb levels are associated with functional outcome in spontaneous, non-traumatic ICH. We pooled IPD from ERICH, a multi-ethnic prospective observational study of ICH, and ATACH-2 and FAST, two landmark clinical trials that evaluated aggressive blood pressure reduction and activated Factor VII, respectively, in this condition. We found that higher admission Hb levels were associated with a lower risk of poor outcome in ICH, with consistent effect estimates across the included studies. We also found that this association held across a wide range of evaluated Hb levels and that it was stronger for nonlobar hemorrhages. We did not find a consistent association between admission Hb levels and admission hematoma volume or hematoma expansion.
Our findings confirm the existing evidence for an association between admission Hb levels and functional outcome in ICH. A small, single-center study found that low Hb levels were associated with worse functional outcome, and this association was mediated by a higher risk of hematoma expansion.10 While promising, these results were limited by the small sample size and single-center design of the study. A meta-analysis on this topic based on summary statistics (not using IPD) including 7 studies showed that anemia at admission (defined as Hb <12 g/dL for women and <13 g/dL for men) was associated with higher mortality and worse outcome.13 These results were limited by the high meta-analytic heterogeneity of the pooled estimates and the limited modeling strategy of the exposure.
By addressing the limitations outlined above, our study provides important new evidence linking admission Hb levels to functional outcome in ICH. We combined IPD from three well-phenotyped studies of ICH to achieve the power necessary to appropriately evaluate the hypothesis of interest. In addition, the availability of admission Hb levels allowed us to model the exposure as a continuous variable and assess whether the observed associations held across different strata of this variable. We found a linear association between hemoglobin levels and outcome. However, visual inspection of Figure 1 shows that the proportion of patients with poor outcome stabilizes around 14 mg/dL. Further, in stratified analyses we found that the relationship held for non-lobar but not for lobar ICH. One possible explanation is that smaller changes in brain oxygenation could have more significant consequences in patients with nonlobar ICH. Follow-up studies should evaluate whether the known biological differences across these subtypes play a role in explaining our findings.
Unlike prior studies, we did not find a consistent association between admission Hb levels and hematoma volume or expansion, two possible mediators of the observed association with outcome. Possible explanations for the null results for hematoma volume are the relatively low hematoma volume seen in ATACH-2, and the exclusion of anticoagulated patients from both clinical trials. Regarding hematoma expansion, the inclusion of interventional trials whose therapeutic effect was aimed at reducing hematoma expansion could bias these results towards the null. Notably, we did find an association between admission Hb levels and hematoma volume and a trend towards a protective effect of higher baseline Hb levels on hematoma expansion in ERICH, results that emphasize the need for further research in this area, with focus on possible mediating mechanisms.
There is significant experience with the utilization of blood products in the hyperacute, acute and subacute periods of acute brain injury, including subarachnoid hemorrhage and traumatic brain injury. However, the efficacy of such interventions has been variable in the literature. A small retrospective study investigating packed red blood cells transfusions in ICH concluded that this interventions was safe and was associated with improved survival at 30 days in multivariable analyses adjusting for ICH score variables,28 but a more recent study found worse outcomes in patients receiving transfusions.29 Combined with prior reports, this study suggests that Hb levels may be a potential target for clinical interventions, but further research is needed to establish a causal association. Alternatively, low hemoglobin levels could be a mediator of poor outcomes in patients with other comorbidities.
Our study has a number of limitations. Being an exploratory analysis of existing studies, the presented results could correspond to false-positive associations caused by multiple testing. Furthermore, the overrepresentation of nonlobar ICH, explained by different factors depending on the study, resulted in a lower number of lobar hemorrhages; therefore, the lack of statistical significance of the analysis focused on lobar ICH should be interpreted with caution, as it could be due to limited statistical power. Additionally, the three studies had a different distribution of outcomes, as ATACH-2 had significantly better outcomes. Moreover, the lack of data on transfusions and the exclusion criteria of both randomized clinical trials may limit the generalizability of our results. However, the strength of the association and consistency of the estimates across the included studies and analytical strategies provides significant reassurance against this scenario. Even if replicated across different cohorts, the observational nature of our study precludes the possibility of making any causal inferences. The observed association could be the result of bias caused by confounding by underlying serious comorbidities (like cancer) that both lower Hb levels and increase the risk of poor outcome. Pre-clinical studies using animal models, genetic analyses and randomized clinical trials are needed to determine whether the association between admission Hb levels and outcome in ICH represents a causal relationship. Even in the absence of a causal link justifying therapeutic interventions, admission Hb levels would still hold significant value as a very early biomarker for poor outcome in this condition. These early biomarkers of future prognosis particularly helpful to appropriately select patients for clinical trials.
CONCLUSIONS
In conclusion, we report a large IPD meta-analysis that pooled data from the ATACH-2, FAST and ERICH studies to examine the relationship between admission Hb levels and functional outcome after ICH. We found that higher admission Hb levels were associated with better outcomes and that this association was stronger for non-lobar hemorrhages. Further research is needed to evaluate admission Hb level as both a therapeutic target and early predictor of outcome.
ACKNOWLEDGMENTS
We would like to that the patients that agreed to participate in the evaluated studies. We would also like to thank the National Institute of Neurological Disorders and Stroke and Novo Nordisk for making the data necessary for this work available.
Sources of support
Audrey C. Leasure supported by the NIH (R03NS112859). Lauren H. Sansing is supported by the NIH (R01NS095993, R01NS097728, U01NS113445). Hooman Kamel is supported by the NIH (R01NS097443, U01NS095869, R01HL144541, U01NS106513). Santosh B. Murthy is supported by the NIH (K23NS105948) and the Leon Levy Foundation. Kevin N. Sheth is supported by the NIH (U24NS107136, U24NS107215, R01NR018335, U01NS106513, R03NS112859) and the American Heart Association (18TPA34170180,17CSA33550004). Guido Falcone is supported by the National Institute on Aging (K76AG59992), the National Institute of Neurological Disorders and Stroke (R03NS112859), the American Heart Association (18IDDG34280056), a Yale Pepper Scholar Award (P30AG021342), and the Neurocritical Care Society Research Fellowship.
The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Copyright Form Disclosure: Drs. Acosta, Sansing, Langefeld, Woo, Sheth, and Falcone received support for article research from the National Institutes of Health (NIH). Drs. Acosta and Falcone disclosed work for hire. Drs. Langefeld and Woo’s institutions received funding from the NIH. Dr. Kamel serves as a PI for the NIH-funded ARCADIA trial (National Institute of Nuerological Disoders and Stroke U01NS095869) which receives in-kind study drug from the BMS-Pfizer Alliance for Eliquis® and ancillary study support from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Mayer recieved funding from Idorsia, MaxQ AI, Bayer, Brain Cool, Biogen, and Nestle. Dr. Sheth’s institution received funding from Biogen, Novartis, and Bard, and he recieved funding from Hyperfine and Zoll. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Gross BA, Jankowitz BT, Friedlander RM. Cerebral Intraparenchymal Hemorrhage: A Review. JAMA - J Am Med Assoc. 2019;321(13):1295–1303. doi: 10.1001/jama.2019.2413 [DOI] [PubMed] [Google Scholar]
- 2.Cordonnier C, Demchuk A, Ziai W, et al. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392(10154):1257–1268. doi: 10.1016/S0140-6736(18)31878-6 [DOI] [PubMed] [Google Scholar]
- 3.Mei H, Xia T, Feng G, et al. Opportunities in systems biology to discover mechanisms and repurpose drugs for CNS diseases. Drug Discov Today. 2012;17(21–22):1208–1216. doi: 10.1016/j.drudis.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 4.Fagan SC. Drug repurposing for drug development in stroke. Pharmacotherapy. 2010;30(7 PART 2). doi: 10.1592/phco.30.pt2.51S [DOI] [PubMed] [Google Scholar]
- 5.Stucchi M, Cantoni S, Piccinelli E, et al. Anemia and acute coronary syndrome: current perspectives. Vasc Health Risk Manag. 2018:14–109. doi: 10.2147/VHRM.S140951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellert L, Schrader F, Ringleb P, et al. The impact of low hemoglobin levels and transfusion on critical care patients with severe ischemic stroke. STroke: RelevAnt Impact of HemoGlobin, Hematocrit and Transfusion (STRAIGHT)-an observational study. J Crit Care. 2014;29(2):236–240. doi: 10.1016/j.jcrc.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Chang TR, Boehme AK, Aysenne A, et al. Nadir hemoglobin is associated with poor outcome from intracerebral hemorrhage. Springerplus. 2013;2(1):1–5. doi: 10.1186/2193-1801-2-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diedler J, Sykora M, Hahn P, et al. Low hemoglobin is associated with poor functional outcome after non-traumatic, supratentorial intracerebral hemorrhage. Crit Care. 2010;14(2):0–7. doi: 10.1186/cc8961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlas RS, Honney K, Loke YK, et al. Impact of Hemoglobin Levels and Anemia on Mortality in Acute Stroke: Analysis of UK Regional Registry Data, Systematic Review, and Meta-Analysis. J Am Heart Assoc. 2016;5(8):1–16. doi: 10.1161/JAHA.115.003019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roh DJ, Albers DJ, Magid-Bernstein J, et al. Low hemoglobin and hematoma expansion after intracerebral hemorrhage. Neurology. 2019;93(4):e372–e380. doi: 10.1212/wnl.0000000000007820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Zhou T, Li Y, et al. Anemia increases the mortality risk in patients with stroke: A meta-analysis of cohort studies. Sci Rep. 2016;6:1–8. doi: 10.1038/srep26636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuramatsu JB, Gerner ST, Lücking H, et al. Anemia is an independent prognostic factor in intracerebral hemorrhage: An observational cohort study. Crit Care. 2013;17(4):R148. doi: 10.1186/cc12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Pan X, Wei C, et al. Associations of anemia with outcomes in patients with spontaneous intracerebral hemorrhage: A meta-analysis. Front Neurol. 2019;10(APR):1–9. doi: 10.3389/fneur.2019.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng YJ, Liu GF, Liu LP, et al. Anemia on admission increases the risk of mortality at 6 months and 1 year in hemorrhagic stroke patients in China. J Stroke Cerebrovasc Dis. 2014;23(6):1500–1505. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 15.Bussière M, Gupta M, Sharma M, et al. Anaemia on admission is associated with more severe intracerebral haemorrhage and worse outcomes. Int J Stroke. 2015;10(3):382–387. doi: 10.1111/j.1747-4949.2012.00951.x [DOI] [PubMed] [Google Scholar]
- 16.Kumar MA, Rost NS, Snider RW, et al. Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med. 2009;37(4):1442–1447. doi: 10.1097/CCM.0b013e31819ced3a [DOI] [PubMed] [Google Scholar]
- 17.Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13(3):1–22. doi: 10.1186/cc7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livio M, Marchesi D, Remuzzi G, et al. Uraemic Bleeding: Role of Anaemia and Beneficial Effect of Red Cell Transfusions. Lancet. 1982;320(8306):1013–1015. doi: 10.1016/S0140-6736(82)90050-2 [DOI] [PubMed] [Google Scholar]
- 19.Roeloffzen WWH, Kluin-Nelemans HC, Bosman L, et al. Effects of red blood cells on hemostasis. Transfusion. 2010;50(7):1536–1544. doi: 10.1111/j.1537-2995.2010.02586.x [DOI] [PubMed] [Google Scholar]
- 20.Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology. 2011;76(14):1238–1244. doi: 10.1212/WNL.0b013e3182143317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi AI, Palesch YY, Barsan WG, et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N Engl J Med. 2016;375(11):1033–1043. doi: 10.1056/NEJMoa1603460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–2137. doi: 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]
- 23.Woo D, Rosand J, Kidwell C, et al. The ethnic/racial variations of intracerebral hemorrhage (ERICH) study protocol. Stroke. 2013;44(10):120–125. doi: 10.1161/STROKEAHA.113.002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tierney JF, Vale C, Riley R, et al. Individual participant data (IPD) metaanalyses of randomised controlled trials: Uidance on their use. PLoS Med. 2015;12(7):1–16. doi: 10.1371/journal.pmed.1001855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broderick JP, Adeoye O, Elm J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke. 2017;48(7):2007–2012. doi: 10.1161/STROKEAHA.117.017866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falcone GJ, Biffi A, Brouwers HB, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013;70(8):988–994. doi: 10.1001/jamaneurol.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamford JM, Sandercock PAG, Wariow CP, et al. Interobserver agreement for the assessment of handicap in stroke patients: To the editor. Stroke. 1989;20(6):828. doi: 10.1161/01.STR.20.6.828 [DOI] [PubMed] [Google Scholar]
- 28.Sheth KN, Gilson AJ, Chang Y, et al. Packed red blood cell transfusion and decreased mortality in intracerebral hemorrhage. Neurosurgery. 2011;68(5):1286–1292. doi: 10.1227/NEU.0b013e31820cccb2 [DOI] [PubMed] [Google Scholar]
- 29.Roh DJ, Carvalho Poyraz F, Magid-Bernstein J, et al. Red Blood Cell Transfusions and Outcomes After Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(12):1–8. doi: 10.1016/j.jstrokecerebrovasdis.2020.105317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the ATACH-2 and ERICH studies are publicly available via the National Institute of Neurological Disorders and Stroke Archive of Clinical Research Datasets. Anonymized data from the FAST trial is accessible upon reasonable request from the study’s sponsor.


