Abstract
Background:
Prescription drug monitoring programs (PDMPs) are critical for pharmacists to identify risky opioid medication use. We performed an independent evaluation of the PDMP-based Narcotic Score (NS) metric.
Methods:
This study was a one-time, cross-sectional health assessment within 19 pharmacies from a national chain among adults picking-up opioid medications. The NS metric is a 3-digit composite indicator. The WHO Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) was the gold-standard to which the NS metric was compared. Machine learning determined optimal risk thresholds; Receiver Operating Characteristic curves and Spearman (P) and Kappa (K) coefficients analyzed concurrent validity. Regression analyses evaluated participant characteristics associated with misclassification.
Results:
The NS metric showed fair concurrent validity (area under the curve≥0.70; K=0.35; P=0.37, p<0.001). The ASSIST and NS metric categorized 37% of participants as low-risk (i.e., not needing screening/intervention) and 32.3% as moderate/high-risk (i.e., needing screening/intervention). Further, 17.2% were categorized as low ASSIST risk but moderate/high NS metric risk, termed false positives. These reported disability (OR=3.12), poor general health (OR=0.66), and/or greater pain severity/interference (OR=1.12/1.09; all p<0.05; i.e., needing unmanaged-pain screening/intervention). A total of 13.4% were categorized as moderate/high ASSIST risk but low NS metric risk, termed false negatives. These reported greater overdose history (OR=1.24) and/or substance use (OR=1.81–12.66; all p<0.05).
Conclusions:
The NS metric could serve as a useful initial universal prescription opioid-risk screener given its: 1) low-burden (i.e., no direct assessment); 2) high accuracy (86.5%) of actionable data identifying low-risk patients and those needing opioid use/unmanaged pain screening/intervention; and 3) broad availability.
Keywords: Community pharmacy, prescription drug monitoring program, risky opioid use
1.1. Introduction
Community pharmacies are underutilized service settings to address the opioid epidemic. Community pharmacies commonly include chain, grocery, and independent settings. Despite recent national declines in opioid prescribing (IQVIA Institute, 2018), 9.7 million Americans in 2019 reported misusing a prescribed opioid (SAMHSA., 2020), and more than 36% of those misusing opioid medications obtained them by filling a prescription (SAMHSA., 2020). Community pharmacies are nearly ubiquitous across the US given more than 93% of Americans live 5 miles or less (Chain Drug Stores, 2011) of the more than 60,000 locations (CDC, 2013). A major limitation for community pharmacies addressing the opioid epidemic has been their inability to access patient health information, such as is routinely available within electronic health records (Roberts et al., 2019).
The prescription drug monitoring program (PDMP) is the most commonly available and useful clinical tool for pharmacists to identify possible opioid misuse (Ali et al., 2017; Young et al., 2017). The PDMP captures patient-level prescription dispensing information to inform monitoring, dispensing decisions, and possible intervention. Appriss Health is the largest PDMP platform vendor in the US and facilitates PDMP data sharing within 52 PDMPs, captures 400 million monthly transactions, and serves more than 30,000 pharmacies (Appriss Health, 2021). PDMP programs, such as the Appriss platform, have demonstrated mixed results for improving opioid safety, with some studies demonstrating reductions in opioid prescribing (Ali et al., 2017; Bao et al., 2016; Dowell et al., 2016; Kreiner et al., 2017; Lin et al., 2017; Manasco et al., 2016; Young et al., 2017), but with unclear effects on illicit substance use outcomes (Ali et al., 2017), including rates of opioid-related overdose (Nam et al., 2017; Patrick et al., 2016; Paulozzi et al., 2011). PDMP output information available to pharmacists and prescribers often presents un-summarized lists of patient fill data, which is of limited clinical utility, thus, requiring users to rely on “best judgment” when providing patient care and referrals.
Appriss Health has developed and preliminarily tested an opioid risk measure, the Narcotic Score (NS) metric, to identify potential risk for unintentional fatal opioid overdose (Huizenga et al., 2016). However, the NS metric has not been tooled to have clinically actionable risk threshold scores, nor has the validity of the NS metric been evaluated in relation to gold-standard clinical metrics of risky opioid use. Possessing a nationally scalable clinical metric of risky opioid use could support community pharmacists’ decision-making to address opioid-related safety for patients. For instance, a risk metric anchored to specific action steps based in clinically validated risk thresholds could promote appropriate responses by pharmacists for needed care of patients. Such a tool could have an important impact for increasing the clinical utility of PDMPs across the US—thus possibly reducing the mixed effects noted above that have been heretofore shown in the literature for these data systems.
The objective of this paper is to present results of an independent evaluation of the NS metric that identified clinically useful risk thresholds as well as concurrent validity using a gold-standard metric (ClinicalTrials.gov identifier: NCT03936985). This paper follows reporting guidelines set forth in the STrengthening the Reporting of OBservational studies in Epidemiology checklist for cross-sectional studies. This study was funded by the National Institute on Drug Abuse and by the NIH Helping to End Addiction Long-TermSM Initiative. Appriss Health only provided NS metric scores to study investigators (detailed below) and took no part in the design, conduct, or analyses reported herein.
2.1. Material and Methods
2.1.1. Design, Sites, and Participants
This study was a one-time, cross-sectional, self-administered, health survey among adult patients currently being dispensed opioid medications from 19 pharmacies sites in Ohio and Indiana within a large national US chain from November 2019 to October 2020. Sites were selected by corporate pharmacy partners based on a convenience sampling approach and their judgement that the locations would be feasible for study implementation (had at least 300 patients filling opioid medications within a 6-month period) as well as generally accessible by car to research staff supporting the project. Participant recruitment followed a convenience sampling method and was initiated at point-of-dispensing with pharmacy staff charged with offering study information to all patients or caregivers picking up opioid medications. Interested patients were provided a computer tablet wherein they could enter their contact information that, once submitted, generated an automatic email that included a brief study overview and link to the online consent document. Patients unable to submit contact information on the tablet within the pharmacy or surrogates were given a study flyer with information on how patients could initiate the survey from personal devices. After completing informed consent, patients were directed to complete a study eligibility self-screening assessment.
Individuals were included in the study if they were 18 years of age and older, English speaking, and not receiving current cancer treatment (self-reported). Participants were excluded if they were solely filling buprenorphine or buprenorphine combination products (i.e., patients receiving opioid use disorder treatment with no other opioid medication use); had previously completed the survey (verified by study staff examining identifying information following health assessment submission); or had self-reported current involvement with the criminal justice system. Those meeting all study inclusion/exclusion criteria were advanced to the study survey. Participants who completed the survey were provided with a $50 gift card. This project was approved by the University of Cincinnati and University of Utah Institutional Review Boards.
2.1.2. Measures
Primary measures.
The primary outcome variable for this survey was the NS metric. The NS metric is a continuous indicator on a 000–999 scale, with the last digit representing the number of active opioid prescriptions (those with more than 9 prescriptions coded as 9) and the first two numbers representing a deterministic composite risk score comprised of component indicators, including opioid dosages, numbers of prescribers/pharmacies associated with opioid prescriptions/fills, overlapping opioid prescriptions, and benzodiazepine prescriptions—well-known indicators associated with opioid-related adverse events (Cochran et al., 2017; Huizenga et al., 2016; Sullivan et al., 2010). Higher scores indicate increased risk for adverse opioid-related outcomes (e.g., misuse). The NS metric was provided for each participant to the study team by Appriss Health. This process involved the study team sharing identifying information from participants with Appriss Health (name, address, date of birth, time of survey completion, and pharmacy location where their opioid was filled). Appriss Health in turn identified the specific participants within the Ohio and Indiana PDMP program data, and patient NS scores were returned, wherein quality assurance checks by the research team ensured accuracy of the data.
All other study assessments were completed online as self-report at the pharmacy location or a convenient location for participants. The survey contained 39–52 questions (dependent on number of substances used by participants). The World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) was used as the gold-standard to which the NS metric was compared. The ASSIST has been found to have criterion, construct, concurrent, and discriminant validity (Humeniuk and Ali, 2006). A priori risk threshold identification and validation was conducted using the ASSIST prescription opioids subscale, developed/validated by McNeely, et al. (McNeely et al., 2016a) Responses were calculated into three discrete ASSIST risk categories—low (no intervention recommended), moderate (brief intervention recommended), and high (referral to treatment recommend) (Humeniuk and Ali, 2006).
Substance use characteristics.
We likewise utilized the additional ASSIST subscales that included street opioids, cannabis, sedatives, cocaine, tobacco, alcohol, methamphetamine (e.g., “crystal meth”), hallucinogens, prescription stimulants (e.g., Adderall and Ritalin), and inhalants (Humeniuk and Ali, 2006) to characterize the population. Lifetime overdose frequency of any drug was assessed using the overdose frequency item from the criterion-valid Overdose Experiences, Self and Witnessed—Drug instrument (Fernandez et al., 2019). This instrument provides a definition of overdose and asks, “How many times in your life has this kind of situation happened to you?” with response options of 0 through 5, or 6 or more.
Physical and mental health characteristics.
Pain was assessed using the Brief Pain Inventory, a well-validated, reliable instrument consisting of a 4-item pain severity subscale and a 7-item pain interference subscale (Keller et al., 2014), with continuous scores of 0–10 and higher scores indicating worse pain severity or pain interference. General health status was measured using a 1-item subscale from the construct-valid Short Form-12 (Luo et al., 2003). Depression was captured using the 2-item criterion-valid Patient Health Questionnaire (PHQ)-2, with a score of 3 considered as the optimal cut-point for depressive disorders (Kroenke et al., 2003).
Morphine milligram equivalents.
Total patient morphine milligram equivalent (MME) dosage over the past 180 days from the PDMP record was also included in the study dataset. Request for these data, linkage, and quality assurance followed the same processes as the NS metric detailed above.
Demographics.
Participant demographics evaluated herein included age (years), sex (male vs. female), ethnicity (Hispanic vs. non-Hispanic), race (White vs. other [“other” analyzed herein given limited sample size amongst subgroups], see Limitations), marital status (married, divorced, widowed, separated, never married, and member of an unmarried couple), employment status (full-time, part-time, temporary leave, looking for work, retired, disabled, homemaker, and student), and insurance status (insured vs. not insured).
2.1.3. Power
Power estimates were based on the allocation ratio of the national rate of prescription opioid use disorder among those prescribed opioid medications in the last year (2.1%; (Han et al., 2017). We calculated an array of sample sizes with α=0.05 and a 0.70 (“fair”) area under the curve (AUC) value (Youngstrom, 2014), with a conservative null hypothesis assumption of 0.5 for discrimination power (Obuchowski, 2005). Our validation sample possessed >80% power to detect an AUC ≥0.70.
2.1.4. A Priori Analyses
A receiver operating characteristics (ROC) analysis was conducted to assess the overall discriminating ability of the NS metric with the ASSIST. AUC ROC curve values were calculated, and the following scale was used for evaluation: <0.70=poor, ≥0.70=fair, ≥0.80=good ≥0.9=excellent (Youngstrom, 2014).
The risk threshold was selected using a machine learning method. Participants were randomly split into training and validation datasets, stratified by ASSIST risk levels. A grid search cross-validation approach was used to select the risk threshold with a training set, in which the optimal value that gave the lowest average misclassification rate was selected. Following value selection, we validated selected risk threshold value using the independent validation set.
The concurrent validity of the NS metric was evaluated by Cohen’s Kappa (K) coefficient (Cohen, 1960; Landis and Koch, 1977) and Spearman (P) correlation (Kendall, 1970) using the independent validation set, with the ASSIST as the standard. Strength of agreement for the K coefficient was labeled as: 0–0.2=slight, 0.21–0.4=fair, 0.41–0.6=moderate, 0.61–0.8=substantial, and 0.81–1=near perfect (Landis and Koch, 1977). Strength of agreement for the P correlation was labeled as: 0–0.29=low, 0.3–0.49=moderate, 0.5–1=high degree (Schober et al., 2018).
2.1.5. Secondary Analyses
In order to describe misclassified individuals who were positive for opioid misuse risk on the NS metric but had low-risk on the ASSIST (i.e., false positives) and conversely those who were low on the NS metric but were moderate or high-risk on the ASSIST (i.e., false negatives), we created a confusion matrix and conducted univariate logistic regression analyses. These univariate analyses assessed the association between false positive/negative designation and participant demographic and health characteristics in the full dataset. In addition, we also conducted a ROC analysis with MME and the ASSIST as a sensitivity analysis for comparison to the NS metric.
3.1. Results
3.1.1. Participant Demographics and Behavioral/Health Characteristics
A total of 1,464 patients from the 19 pharmacies completed the survey and had sufficient data in the PDMP system to generate the NS metric (see Appendix 1 for detailed enrollment chart). Table 1 shows participants were on average 49.6 years of age, with most being White (93%) and female (62.2%). The largest proportion of participants reported currently being employed (35.2%), with the largest portion of those not employed having a disability (22.5%). Most participants reported having health insurance (94.3%). On the ASSIST, 54.1% were identified as having recent history of low-risk opioid medication use, 43.6% had moderate-risk use, and 2.3% had high-risk use. A total of 10% of patients reported an illicit or prescription drug overdose in their lifetime, and roughly 20% of respondents screened positive for depression.
Table 1.
Participant Characteristics (N=1,464)
| Variable | N (%a) |
|---|---|
| Ageb | 49.6 (14.8) |
| Femalec | 911 (62.2) |
| Not Hispanic/Latinx | 1447 (98.8) |
| White | 1361 (93.0) |
| Marital status | |
| Married | 797 (54.5) |
| Divorced | 244 (16.7) |
| Widowed | 72 (4.9) |
| Separated | 53 (3.6) |
| Never married | 213 (14.6) |
| Member unmarried couple | 77 (5.3) |
| Employment | |
| Full-time | 515 (35.2) |
| Part-time | 79 (5.4) |
| Temp leave | 72 (4.92) |
| Looking for work | 45 (3.1) |
| Retired | 208 (14.2) |
| Disabled | 329 (22.5) |
| Homemaker | 64 (4.4) |
| Student | 16 (1.1) |
| Insured | 1380 (94.3) |
| Overdose Experiences, Self and Witnessed—Drug instrument | |
| No lifetime overdose | 1318 (90.0) |
| 1 overdose | 56 (3.8) |
| 2 overdoses | 42 (2.9) |
| 3 overdoses | 13 (0.9) |
| 4 overdoses | 4 (0.3) |
| 5 overdoses | 8 (0.6) |
| 6 + overdoses | 17 (1.2) |
| Short Form12 general health | |
| Poor | 148 (10.1) |
| Fair | 447 (30.5) |
| Good | 565 (38.6) |
| Very good | 246 (16.8) |
| Excellent | 54 (3.7) |
| Patient Health Questionnaire 2 (depression) | 286 (19.9) |
| Brief Pain Inventory | |
| Pain severityb | 4.85(2.2) |
| Pain interferenceb | 4.84(2.8) |
| Alcohol, Smoking, and Substance Involvement Screening Test | |
| Prescription opioids | |
| Low | 772 (54.1) |
| Moderate | 623 (43.6) |
| High | 33 (2.3) |
| Street opioids | |
| Low | 1430 (98.7) |
| Moderate | 13 (0.9) |
| High | 6 (0.4) |
| Cannabis | |
| Low | 1274 (88.5) |
| Moderate | 158 (10.9) |
| High | 8 (0.6) |
| Sedatives | |
| Low | 1199 (82.9) |
| Moderate | 238 (16.5) |
| High | 10 (0.7) |
| Cocaine | |
| Low | 1417 (97.9) |
| Moderate | 30 (2.1) |
| High | 1 (0.1) |
| Tobacco | |
| Low | 894 (62.3) |
| Moderate | 495 (34.5) |
| High | 45 (3.1) |
| Alcohol | |
| Low | 1300 (90.5) |
| Moderate | 115 (8.0) |
| High | 21 (1.5) |
| Methamphetamine | |
| Low | 1426 (98.5) |
| Moderate | 18 (1.2) |
| High | 3 (0.2) |
| Hallucinogensd | |
| Low | 1428 (99.4) |
| Moderate | 9 (0.6) |
| Prescription stimulantsd | |
| Low | 1387 (95.7) |
| Moderate | 62 (4.3) |
| Inhalantsc | |
| Low | 1453 (99.9) |
| Moderate | 2 (0.1) |
Categories may not total 1,464 due to “prefer not to answer” responses.
Mean (SD) used in place of N (%).
“none of these describe me” analyzed as male due to limited sample size of subgroup (n=1).
No high-risk use was reported.
3.1.2. Overall Discriminating Validity of NS Metric
ROC analyses of the NS metric discriminating high- compared to moderate-risk for prescription opioid use on the ASSIST showed fair discrimination (AUC=0.70, standard error [SE]=0.05, 95% confidence interval [CI]=0.59, 0.80), Figure 1. ROC analyses of the NS metric discriminating moderate- compared to low-risk for prescription opioid use on the ASSIST also showed fair discrimination (AUC=0.74, SE=0.01, 95% CI=0.71, 0.76), Figure 1.
Figure 1.
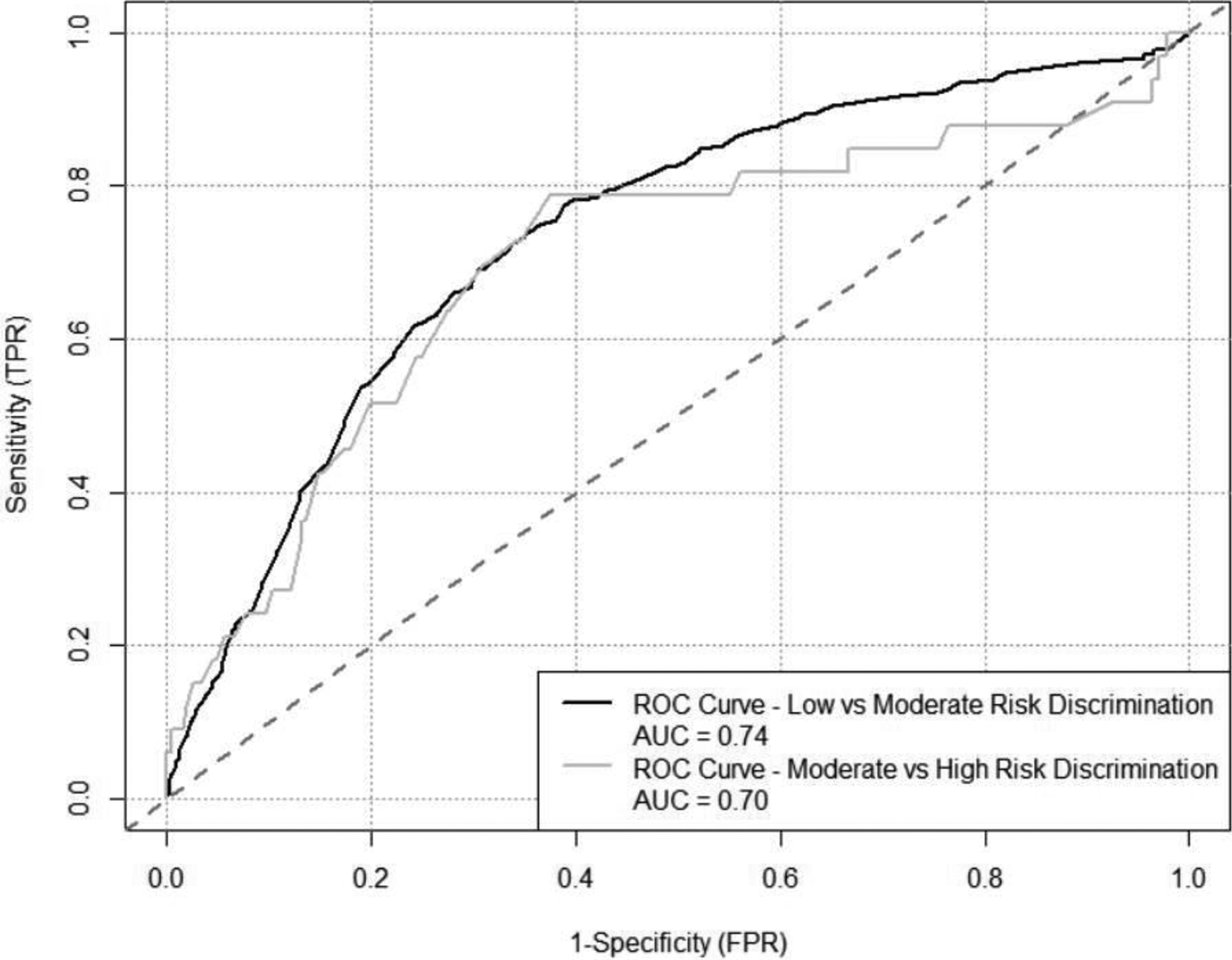
ROC Curve for Narcotic Score Discriminating High-risk vs. Moderate-risk and Moderate-risk vs. Low-risk Opioid Use Patients Validation Sample
3.1.3. Identified Risk Threshold Scores and Concurrent Validity
The identified risk threshold for the NS metric for high- vs. moderate-risk was a score of 602 and for moderate- vs. low-risk was a score of 291, selected based on the training dataset. The agreement between the established risk thresholds with the ASSIST was evaluated with the independent validation set and assessed as fair (K=0.35) and moderate (P=0.37; p<0.001).
The confusion matrix (Table 2) shows the largest proportions of the NS metric risk threshold scores corresponded to the relevant ASSIST risk thresholds, with 37% mapping to low NS metric and ASSIST risk (NS metric ≤291), 30.2% mapping to moderate NS metric and ASSIST risk (NS metric>291 to ≤602), and 0.1% mapping to high NS metric and ASSIST risk (NS metric >602).
Table 2.
Confusion Matrix for Narcotic Score Metric vs. Prescription Opioid Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) Risk Levels (N=1,464)
| Actual Risk Levels Defined by ASSIST | |||
|---|---|---|---|
| Predicted Risk Levels Defined by Narcotic Score Metric | Low Risk |
Moderate Risk |
High Risk |
| Low Risk Level | 527 (37%) | 189 (13.3%) | 2 (0.1%) |
| Moderate Risk Level | 243 (17.1%) | 430 (30.2%) | 24 (1.7%) |
| High-risk Level | 2 (0.1%) | 4 (0.3%) | 2 (0.1%) |
Table shows 1423 responses due to exclusion of “prefer not to answer” responses on the ASSIST questionnaire
3.1.4. False Positive/Negative
A total of 17.2% of participants were classified as false positives and 13.4% as false negatives. Table 3 shows participants more likely to be designated as false positive: were retired (odds ratio [OR]=3.40, 95% CI= 2.20, 5.26), had disabilities (OR=3.12, 95% CI= 2.10, 4.63), had >1 employment source (OR=2.50, 95% CI=1.27, 4.93), or were on temporary work leave (OR=2.04, 95% CI=1.02, 4.06). Also, false positive participants: had patterns of lower likelihood for substance use (OR=0.16–0.58, p<0.05), were more likely to be widows (OR=2.65, 95% CI=1.56, 4.52), had poorer general health (OR=0.66, 95% CI=0.56, 0.76), and had increased levels of pain severity (OR=1.12, 95% CI=1.05, 1.19) and pain interference (OR=1.09, 95% CI=1.04, 1.15). Additional descriptive analyses (results not shown) of pain among false positive patients showed 28.4% (n=58) with mild, 43.1% with moderate (n=88), and 28.4% (n=58) with high pain severity and 33.8% (n=69) with mild, 25% with moderate (n=51), and 41.2% (n=84) with high pain interference.
Table 3.
Univariate logistic regression for false negative and false positive classification by Narcotic Scoresa (N=1,464)
| False Positive | False Negative | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Age | 1.03 (1.02, 1.04) | <0.01 | 0.98 (0.98, 0.99) | <0.01 |
| Marital status | ||||
| Married | 1 (ref) | 1 (ref) | ||
| Divorced | 1.28 (0.88, 1.87) | 0.19 | 1.01 (0.67, 1.50) | 0.97 |
| Widowed | 2.65 (1.56, 4.52) | <0.01 | 0.25 (0.08, 0.80) | 0.02 |
| Separated | 1.28 (0.63, 2.63) | 0.49 | 1.00 (0.46, 2.18) | 0.99 |
| Never married | 0.80 (0.51, 1.26) | 0.34 | 1.23 (0.82, 1.83) | 0.32 |
| Member unmarried couple | 0.83 (0.41, 1.65) | 0.59 | 0.94 (0.48, 1.83) | 0.85 |
| White race | 0.82 (0.48, 1.41) | 0.48 | 0.60 (0.36, 0.99) | 0.05 |
| Non-Hispanic | 1.14 (0.24, 5.31) | 0.87 | .b | . |
| Employment | ||||
| Full-time | 1 (ref) | 1 (ref) | ||
| Part-time | 1.15 (0.52, 2.53) | 0.73 | 1.36 (0.75, 2.47) | 0.31 |
| Temp leave | 2.04 (1.02, 4.06) | 0.04 | 1.53 (0.84, 2.80) | 0.17 |
| Looking for work | 1.57 (0.63, 3.90) | 0.33 | 2.18 (1.10, 4.32) | 0.03 |
| Retired | 3.40 (2.20, 5.26) | <0.01 | 0.67 (0.41, 1.09) | 0.11 |
| Disabled | 3.12 (2.10, 4.63) | <0.01 | 0.58 (0.37, 0.89) | 0.01 |
| Keeping house | 2.35 (1.17, 4.72) | 0.02 | 1.79 (0.97, 3.30) | 0.06 |
| Student | 0.68 (0.09, 5.26) | 0.71 | 0.36 (0.05, 2.74) | 0.32 |
| Other | 3.08 (1.55, 6.15) | <0.01 | 0.41 (0.15, 1.17) | 0.10 |
| Multiple reported | 2.50 (1.27, 4.93) | 0.01 | 0.96 (0.47, 1.95) | 0.90 |
| Insured | 0.93 (0.49, 1.75) | 0.81 | 0.55 (0.31, 0.99) | 0.05 |
| Overdose history | 0.81 (0.65, 1.01) | 0.06 | 1.24 (1.09, 1.41) | <0.01 |
| Poor general health | 0.66 (0.56, 0.76) | <0.01 | 1.09 (0.94, 1.26) | 0.27 |
| Depression | 0.99 (0.91, 1.07) | 0.77 | 1.04 (0.96, 1.13) | 0.28 |
| Pain severity | 1.12 (1.05, 1.19) | <0.01 | 0.99 (0.93, 1.05) | 0.71 |
| Pain interference | 1.09 (1.04, 1.15) | <0.01 | 0.99 (0.94, 1.04) | 0.71 |
| ASSISTc Illicit/prescription drug use | ||||
| Prescription opioids | . | . | 12.66 (8.41, 19.07) | <0.01 |
| Street opioids | 0.32 (0.05, 1.93) | 0.21 | 3.15 (1.60, 6.20) | <0.01 |
| Cannabis | 0.47 (0.27, 0.79) | 0.01 | 1.81 (1.26, 2.61) | <0.01 |
| Sedatives | 0.37 (0.23, 0.60) | <0.01 | 1.87 (1.36, 2.57) | <0.01 |
| Cocaine | 1.16 (0.49, 2.77) | 0.74 | 3.32 (1.62, 6.80) | <0.01 |
| Tobacco | 1.17 (0.91, 1.50) | 0.21 | 1.11 (0.86, 1.44) | 0.42 |
| Alcohol | 0.58 (0.35, .95) | 0.03 | 2.03 (1.46, 2.83) | <0.01 |
| Methamphetamine | 0.27 (0.04, 1.84) | 0.18 | 5.92 (2.63, 13.31) | <0.01 |
| Hallucinogens | . | . | 11.67 (2.89, 47.0) | <0.01 |
| Prescription stimulants | 0.16 (0.04, 0.67) | 0.01 | 3.17(1.83, 5.48) | <0.01 |
| Inhalants | . | . | . | . |
False negative =low Narcotic Scores and moderate/high ASSIST scores. False positive=moderate/high Narcotic Scores and low ASSIST scores.
“.” represents associations that could not be estimated due to limited sample size or indicators that perfectly predicted the outcome.
Alcohol, Smoking, and Substance Involvement Screening Test.
Examining false negatives, those looking for work (OR=2.18, 95% CI=1.10, 4.32), with history of drug overdose (OR=1.24, 95% CI=1.09, 1.41) were among those with the highest odds for misclassification. There was a consistent pattern of increased odds (OR=1.81–12.66, p<0.05) for illicit/prescription drug use among those with false negative reports.
3.1.5. Sensitivity Analyses
ROC analyses of MME discriminating high- compared to moderate-risk for prescription opioid use on the ASSIST showed poor discrimination (AUC=0.65, 95% CI=0.54,0.75). ROC analyses of MME discriminating moderate- compared to low-risk for prescription opioid use on the ASSIST showed fair discrimination (AUC=0.70, 95% CI=0.68,0.73).
4.1. Discussion
This study sought to identify risk thresholds and validate a national opioid use risk metric currently deployed by the largest US PDMP vendor, the NS metric, which to date, had largely undefined clinical utility. Results demonstrated the NS metric is a screening tool with fair discriminative accuracy and fair to moderate concurrent validity for detecting recent risky prescription opioid use as measured by the WHO ASSIST. Totaling those correctly identified with low or elevated opioid risk (69.4%) and false positives (17.2.%; i.e., high opioid utilization without risky use reported—likely needing additional pain screening), the NS metric provides a high level of accuracy (86.5%) of clinically actionable information, with superior performance compared typical measures of opioid risk, such as MME (MME compared to NS metric: high vs. moderate-risk 0.65 vs 0.70 and moderate vs. low-risk of 0.70 vs. 0.74, respectively). These findings suggest the NS metric could play a valuable role as a clinically useful “universal screen” due to its wide availability to community pharmacists and its low burden relative to other potential “quick screens,” given it relies upon passive assessment. It is important to note this study was powered based on the national prevalence of prescription opioid use disorder, 2.1% (Han et al., 2017). In our study sample, we identified a similar rate of high-risk prescription opioid use of 2.3% among our participants—which provides additional confidence for the external validity of the sample.
False positive misclassifications appear to be among those with disability, unmanaged pain, poor general health, and work status-related issues. In clinical workflow, false positive classifications would likely need to be clarified/resolved through subsequent patient screening after the pharmacist receives the risk score notification. For instance, future research should investigate if the NS metric is detecting higher levels of opioid use among those with significant unmanaged pain potentially related to work status or disability. Ruling out false positive risk possibly driven by unmanaged pain, with tools like the Brief Pain Inventory (Krebs et al., 2009), could aid pharmacists to effectively triage these patients to non-opioid or complementary pain management resources and referral (U.S. Department of Health and Human Services, 2019), including evidence-based online programs (Winhusen et al., 2021) that could reduce pharmacy staff and patient burden related to office-based care. Such a model could be implemented within a clinical decision support tool and follow a pattern, such as is depicted in Figure 2. The NS metric triggers opioid safety information distribution for low-risk patients. Through passive PDMP screening (i.e. not requiring staff effort), eliminating the need to screen low-risk patients is a clinically significant advantage of the NS metric. Given the tens-of-thousands of patients filling opioids in community pharmacies annually, universal active patient screening is likely impractical within an already very busy practice environment (Kaplan et al., 2021). The NS metric can substantially decrease unnecessary disruptions to workflow and potential patient inconvenience by excluding approximately 50% of low-risk patients from staff-to-patient screening—thus significantly increasing the feasibility of screening in community pharmacies. The NS metric likewise triggers confirmatory screening for moderate and high-risk patients, with those who present as low-risk on confirmatory screening (false positives) receiving auxiliary pain screening, and moderate/high-risk patients (true positives) receiving intervention, warm handoff referral, and naloxone dispensation. The approach of brief universal screening followed by confirmatory assessment is a long-established and recommended standard practice for patients with substance use in healthcare settings (NIAAA, 2007; Smith et al., 2010). Cut-points previously established for the Brief Pain Inventory (0–4=mild pain, 5–6= moderate pain, 7–10=high pain; (Palos et al., 2006) might be utilized to categorize patients by pain level, with low pain patients being continually monitored within the PDMP while moderate and high pain patients could receive non-opioid or complementary pain management resources and referral (U.S. Department of Health and Human Services, 2019). For such a tool, implementation science-based training, monitoring, and follow up with pharmacy professionals could be useful to ensure these tools are effectively received/employed (Ducharme et al., 2016).
Figure 2.
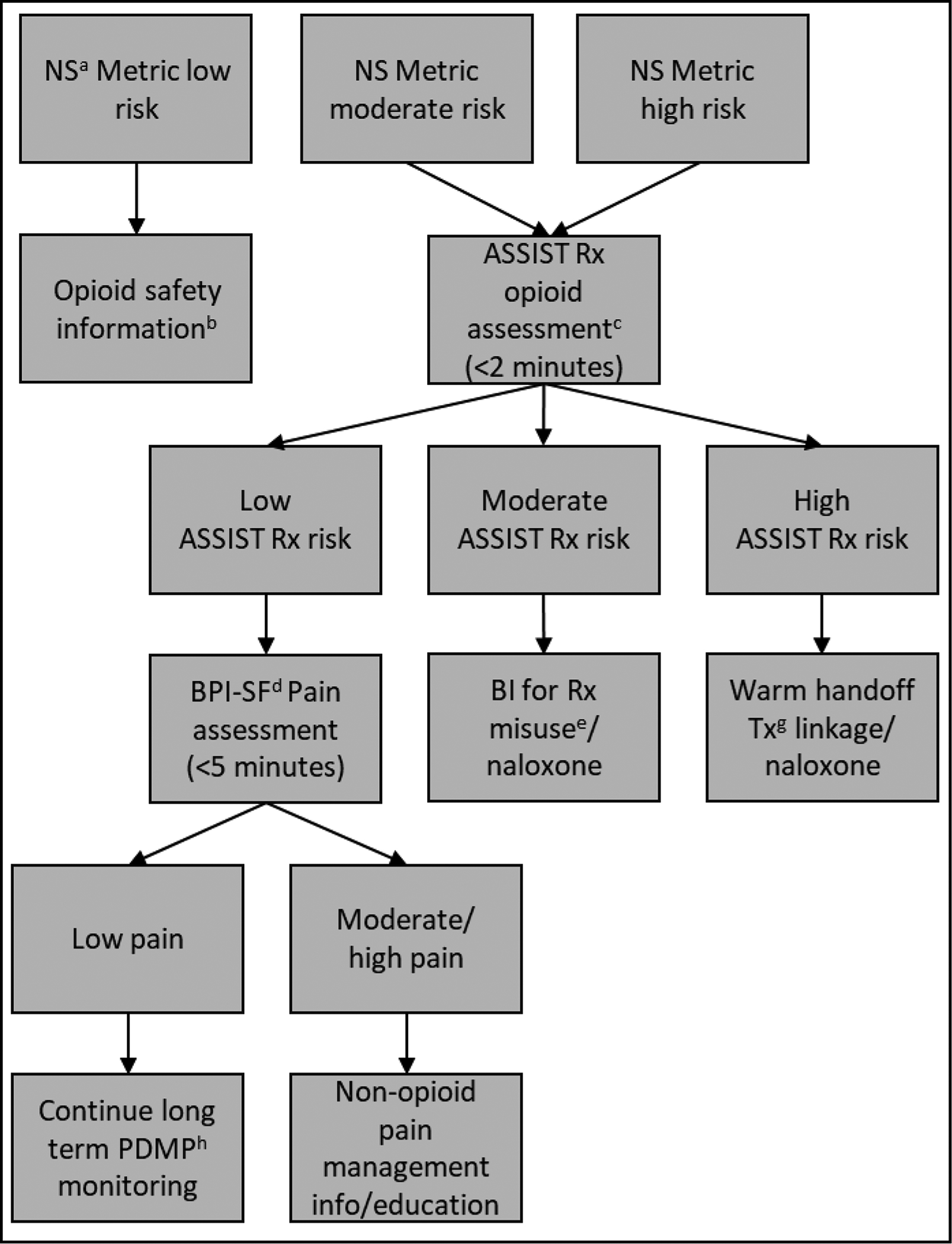
Prescription Drug Monitoring Clinical Decision Support Tool for Prescription Opioid Risk: Flow Chart
a Narcotic score. BThis flow chart only applies to Rx opioid risk. Non-Rx opioid substance screening may be feasible under other circumstances. CWHO Alcohol, Smoking, and Substance Involvement Screening Test prescription opioid use risk assessment. d Brief Pain Inventory-Short Form. e Brief intervention for prescription misuse. f Brief intervention for treatment linkage. e Treatment. h Prescription Drug Monitoring Program.
Implementation of such a clinical decision support tool would come with benefits and challenges for community pharmacy settings. Benefits likely include empowering pharmacists with clinically actionable data produced through passive screening (i.e. not requiring staff time) to engage patients with risky opioid use, which resources are limited within the field of pharmacy practice (Martin et al., 2021). Further, given possibility of implementing such a tool within a PDMP platform that transcends systems and state boundaries, this approach has the potential for significant scalability. In contrast, tool implementation would need support from leadership within community pharmacy settings given the potential draw on staff time for training and actual utilization—and likely hands-on learning to prepare for patient interactions as well as pre-recorded training information on how to use the tool. Further, to increase implementation feasibility, the ability to utilize less costly staff, such as pharmacy technicians, to coordinate or possibly deliver screenings and to make contacts with patient referral agencies (e.g. in anticipation of or actual referral to) may help to offset more costly pharmacist staff time, who likely would be delivering actual interventions. To this important point of cost and feasibility, the ability of the NS metric to eliminate screening need for low-risk patients greatly facilitates feasibility. Further, implementation science approaches for integrating such tools would be critical to staging success and sustainability (Kirk et al., 2016).
Conversely, false negative designation appears to point to a possible limitation to the NS metric given misclassification among individuals with regular substance use involvement and drug overdose history. The inability of the NS metric to detect use of non-opioid substances is not surprising and may not be problematic in that community pharmacy may not be an optimal venue to screen for and intervene with non-opioid substance misuse. Alternatively, this shortcoming of the NS metric may suggest a need for pharmacists to specifically screen patients for other substance use as a supplement to the PDMP. The possible heightened overdose risk within this subpopulation is concerning. It is important for pharmacists to monitor other metrics for detecting unintentional fatal opioid overdose risk (e.g. overdose risk metrics developed Appriss Health). An additional possible avenue for detection of risk could be to explore with data mining or machine learning approaches additional variables available within the PDMP platform or within pharmacy records that could signal opioid-related risk not detected by the NS metric—and devise/test methods for implementation into screening workflow. Nevertheless, specifically examining the true low-risk categorized participants vs. the total low-risk categorized group (i.e. sensitivity) showed a rate of 73.4%. Previous studies of widely used measures of opioid medication misuse have generally similar rates for their identified risk cutoffs. For instance, for the Current Opioid Misuse Measure, true misuse risk/total identified as misusing was 77% (Butler et al., 2007). For the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) Tool, true problem use/total identified problem use identified was 71%, and true high-risk use/total identified high-risk use was 48% (McNeely et al., 2016b). For the Prescription Opioid Misuse Measure, true possible misuse risk/total identified possible misuse risk was 82% (Knisely et al., 2008). These facts, in conjunction with the fair level of discrimination (≥0.70) and ability to identify elevated risk and need for confirmatory screening, suggest the NS metric may have a role as a low-burden universal initial screening for opioid medication risk in community pharmacy.
4.1.1. Limitations
While this study possesses many strengths, results herein should be considered in relation to its limitations. Except for the NS metric that is based on opioid dispensation data, other assessments herein were self-reported. Future studies seeking to increase objectivity may choose to collect health record-based or biological samples. Nevertheless, statistical analyses presented herein demonstrated expected relationships between self-reported data and the NS metric, which indicate the validity of participant responses. In addition, given the frequency of concomitant medication use among those misusing opioid medications (Ferries et al., 2017), capturing and exploring use of other medications may add insight into the behavioral profiles of patients identified as at risk in this study. An additional limitation is, despite its robust size, the sample was somewhat homogenous in terms of racial/ethnic distribution (i.e., comprised mostly of White participants, typical of the broader demographics of prescription opioid use (Friedman et al., 2019; Muench et al., 2020) as well as had a larger proportion of females vs. males. Next steps in validation must work to examine racial/ethnic and sex performance differences. Furthermore, while an important feature of this study was it recruited patients from geographically diverse settings, analyses were not conducted by rural vs. urban settings outside of the Midwest, and findings may only be applicable to states/regions wherein data were collected. Next steps should examine rural/urban performance differences in other areas of the US given disparities often noted for the rural impact of the opioid epidemic (Monnat and Rigg, 2016; Thomas et al., 2019). Additionally, PDMP programs do not capture methadone for opioid use disorder treatment given this medication is dispensed through specialty treatment programs. Only methadone for pain management is captured, which associated MMEs are included in the NS metric risk calculation for patients. Future research should actively seek to capture and understand how methadone treatment impacts the risk/benefit profile of community pharmacy patients dispensed opioid medications for pain. Finally, we acknowledge detected rates of substance use among our population, such as street opioids, may be higher herein than the general population (SAMHSA., 2020), which may be explained partially by the regular opioid medication use among the sampled population (NIDA, 2018).
5.1. Conclusion
This study demonstrated the NS metric may serve as a useful broad-based universal screen for risky opioid medication use among community pharmacy patients. Further research should seek to implement the identified threshold scores for the NS metric within a clinical decision support tool with PDMP platforms. Such a platform could include additional screening and guidance on how to provide brief intervention, warm handoff, or naloxone dispensation/training. Such steps in large-scale screening and intervention for risky opioid medication use may stand to make important advancements for protecting patient health and addressing the opioid epidemic.
Supplementary Material
Funding:
This study was supported by the National Institute on Drug Abuse (UG1DA013732; UG1DA049444) and by the NIH Helping to End Addiction Long-TermSM (HEAL) Initiative. Dr. Ghitza was substantially involved in UG1DA013732; UG1DA049444, consistent with his role as Scientific Officer. He had no substantial involvement in the other cited grants. The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Funding:
This work was supported by the National Institutes of Health [NIDA: UG1DA013732; UG1DA049444].
Footnotes
Conflicts: None
6.1 References
- Ali MM, Dowd WN, Classen T, Mutter R, Novak SP, 2017. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: Evidence from the National Survey of Drug Use and Health. Addict Behav 69, 65–77. [DOI] [PubMed] [Google Scholar]
- Appriss Health, 2021. Who We Are. https://apprisshealth.com/about/overview/. (Accessed March 20 2021).
- Bao Y, Pan Y, Taylor A, Radakrishnan S, Luo F, Pincus HA, Schackman BR, 2016. Prescription Drug Monitoring Programs Are Associated With Sustained Reductions In Opioid Prescribing By Physicians. Health Aff 35(6), 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN, 2007. Development and validation of the Current Opioid Misuse Measure. Pain 130(1), 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2013. Select features of state pharmacist collaborative practice laws. US Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Chain Drug Stores, N., 2011. 2011–2012 Chain Pharmacy Industry Profile. National Association of Chain Drug Stores, Alexandria, VA. [Google Scholar]
- Cochran G, Gordon AJ, Lo-Ciganic WH, Gellad WF, Frazier W, Lobo C, Chang CH, Zheng P, Donohue JM, 2017. An Examination of Claims-based Predictors of Overdose from a Large Medicaid Program. Med Care 55(3), 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1960. A Coefficient of Agreement for Nominal Scales. Educ Psychol Meas 20(1), 37–46. [Google Scholar]
- Dowell D, Zhang K, Noonan RK, Hockenberry JM, 2016. Mandatory Provider Review And Pain Clinic Laws Reduce The Amounts Of Opioids Prescribed And Overdose Death Rates. Health Aff 35(10), 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Chandler RK, Harris AHS, 2016. Implementing Effective Substance Abuse Treatments in General Medical Settings: Mapping the Research Terrain. J Subst Abuse Treat 60, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AC, Bush C, Bonar EE, Blow FC, Walton MA, Bohnert ASB, 2019. Alcohol and Drug Overdose and the Influence of Pain Conditions in an Addiction Treatment Sample. J Addict Med 13(1), 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferries EA, Gilson AM, Aparasu RR, Chen H, Johnson ML, Fleming ML, 2017. The Prevalence of and Factors Associated With Receiving Concurrent Controlled Substance Prescriptions. Subst Use Misuse, 1–7. [DOI] [PubMed] [Google Scholar]
- Friedman J, Kim D, Schneberk T, Bourgois P, Shin M, Celious A, Schriger DL, 2019. Assessment of Racial/Ethnic and Income Disparities in the Prescription of Opioids and Other Controlled Medications in California. JAMA Intern Med 179(4), 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM, 2017. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 167(5), 293–301. [DOI] [PubMed] [Google Scholar]
- Huizenga J, Breneman B, Patel V, Speights D, 2016. NARxCHECK® Score as a Predictor of Unintentional Overdose Death. Appriss, Louisville, KY. [Google Scholar]
- Humeniuk R, Ali R, 2006. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and Pilot Brief Intervention: A Technical Report of Phase II Findings of the WHO ASSIST Project. WHO, Geneva. [Google Scholar]
- IQVIA Institute, I., 2018. Medicine Use and Spending in the U.S. A Review of 2017 and Outlook to 2022, Reports. IQVIA Institute, iqvia.com. [Google Scholar]
- Kaplan A, Nguyen V, Godie M, 2021. Overworked, understaffed: Pharmacists say industry in crisis puts patient safety at risk, NBC News. NBC Universal Television. [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS, 2014. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 20(5), 309–319. [DOI] [PubMed] [Google Scholar]
- Kendall MG, 1970. Rank Correlation Methods Griffin, London. [Google Scholar]
- Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L, 2016. A systematic review of the use of the Consolidated Framework for Implementation Research. Implement Sci 11(1), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely JS, Wunsch MJ, Cropsey KL, Campbell ED, 2008. Prescription Opioid Misuse Index: A brief questionnaire to assess misuse. J Subst Abuse Treat 35(4), 380–386. [DOI] [PubMed] [Google Scholar]
- Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Asch SM, Kroenke K, 2009. Development and Initial Validation of the PEG, a Three-item Scale Assessing Pain Intensity and Interference. J Gen Intern Med 24(6), 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner PW, Strickler GK, Undurraga EA, Torres ME, Nikitin RV, Rogers A, 2017. Validation of prescriber risk indicators obtained from prescription drug monitoring program data. Drug Alcohol Depend 173 Suppl 1, S31–s38. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, 2003. The Patient Health Questionnaire-2: Validity of a Two-Item Depression Screener. Med Care 41(11), 1284–1292. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG, 1977. The measurement of observer agreement for categorical data. Biometrics 33(1), 159–174. [PubMed] [Google Scholar]
- Lin DH, Lucas E, Murimi IB, Jackson K, Baier M, Frattaroli S, Gielen AC, Moyo P, Simoni-Wastila L, Alexander GC, 2017. Physician attitudes and experiences with Maryland’s prescription drug monitoring program (PDMP). Addiction 112(2), 311–319. [DOI] [PubMed] [Google Scholar]
- Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, Hey L, 2003. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine 28(15), 1739–1745. [DOI] [PubMed] [Google Scholar]
- Manasco AT, Griggs C, Leeds R, Langlois BK, Breaud AH, Mitchell PM, Weiner SG, 2016. Characteristics of state prescription drug monitoring programs: a state-by-state survey. Pharmacoepidemiol Drug Saf 25(7), 847–851. [DOI] [PubMed] [Google Scholar]
- Martin HD, Modi SS, Feldman SS, 2021. Barriers and facilitators to PDMP IS Success in the US: A systematic review. Drug Alcohol Depend 219, 108460. [DOI] [PubMed] [Google Scholar]
- McNeely J, Strauss SM, Rotrosen J, Ramautar A, Gourevitch MN, 2016a. Validation of an audio computer-assisted self-interview (ACASI) version of the alcohol, smoking and substance involvement screening test (ASSIST) in primary care patients. Addiction 111(2), 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely J, Wu LT, Subramaniam G, Sharma G, Cathers LA, Svikis D, Sleiter L, Russell L, Nordeck C, Sharma A, O’Grady KE, Bouk LB, Cushing C, King J, Wahle A, Schwartz RP, 2016b. Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) Tool for Substance Use Screening in Primary Care Patients. Ann Intern Med 165(10), 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat SM, Rigg KK, 2016. Examining Rural/Urban Differences in Prescription Opioid Misuse Among US Adolescents. J Rural Health 32(2), 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench J, Fankhauser K, Voss RW, Huguet N, Hartung DM, O’Malley J, Bailey SR, Cowburn S, Wright D, Barker G, Ukhanova M, Chamine I, 2020. Assessment of Opioid Prescribing Patterns in a Large Network of US Community Health Centers, 2009 to 2018. JAMA Netw Open 3(9), e2013431–e2013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YH, Shea DG, Shi Y, Moran JR, 2017. State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care 23(5), 297–303. [PubMed] [Google Scholar]
- NIAAA, 2007. Helping patients who drink too much: A clinicians guide. National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- NIDA, 2018. Prescription Opioids and Heroin Research Report. National Institute on Drug Abuse, Bethesda, MD. [Google Scholar]
- Obuchowski N, 2005. Fundamentals of Clinical Research for Radiologists. AJR Am J Roentgenol 184, 364–372.15671347 [Google Scholar]
- Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS, 2006. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J. Pain 7(1), 49–56. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Fry CE, Jones TF, Buntin MB, 2016. Implementation Of Prescription Drug Monitoring Programs Associated With Reductions In Opioid-Related Death Rates. Health Aff 35(7), 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Kilbourne EM, Desai HA, 2011. Prescription Drug Monitoring Programs and Death Rates from Drug Overdose. Pain Med 12(5), 747–754. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Reeves K, Divine H, 2019. Community pharmacists’ lack of access to health records and its impact on targeted MTM interventions. J Am Pharm Assoc 59(4s), S81–s84. [DOI] [PubMed] [Google Scholar]
- SAMHSA., 2020. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Rockville, MD. [Google Scholar]
- Schober P, Boer C, Schwarte LA, 2018. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg 126(5), 1763–1768. [DOI] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R, 2010. A single-question screening test for drug use in primary care. Arch Intern Med 170(13), 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Fan M-Y, Devries A, Brennan Braden J, Martin BC, 2010. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain 150(2), 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N, van de Ven K, Mulrooney KJD, 2019. The impact of rurality on opioid-related harms: A systematic review of qualitative research. Int J Drug Policy, 102607. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2019. Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. U.S. Department of Health and Human Services, Washinton DC. [Google Scholar]
- Winhusen T, Wilson M, Dolor RJ, Theobald J, Lewis D, Regan SL, Vonder Meulen MB, 2021. Design considerations for a remote randomized multi-site clinical trial evaluating an e-health self-management program for chronic pain patients receiving opioid therapy. Contemp Clin Trials 101, 106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LD, Kreiner PW, Panas L, 2017. Unsolicited Reporting to Prescribers of Opioid Analgesics by a State Prescription Drug Monitoring Program: An Observational Study with Matched Comparison Group. Pain Med. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, 2014. A Primer on Receiver Operating Characteristic Analysis and Diagnostic Efficiency Statistics for Pediatric Psychology: We Are Ready to ROC. J Pediatr Psychol 39(2), 204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


