Abstract
Objective:
To evaluate changes in airway volumes and respiratory performance in patients undergoing rapid maxillary expansion and determine whether any correlations exist between the morphological and respiratory functional modifications induced by rapid maxillary expansion and pretreatment airway stenosis.
Materials and Methods:
Fifteen patients (11 females and 4 males; mean age, 7.5 ± 0.3 years) were enrolled in the study. Each patient underwent cone beam computed tomography and polysomnography examination before rapid maxillary expansion and after the removal of the maxillary expander 12 months later. The airway regions were segmented and the volumes were computed.
Results:
The upper, middle, and lower airway volumes were significantly increased 2305 mm3, 1144 mm3, and 1915 mm3, respectively. Similarly, oxygen saturation was increased (+5.3%) and the apnea/hypopnea index was improved (−4.2 events). All the observed modifications were statistically significant (P < .05). Baseline middle and lower airway volume showed a significant negative correlation with the oxygen saturation modification.
Conclusions:
The results of this study showed that when rapid maxillary expansion is performed in subjects having posterior crossbite, oxygen saturation is improved. The improvement is greater in subjects having more reduced middle and lower airway volumes.
Keywords: Maxillary expansion, Airway, Polysomnography, Oxygen saturation, Computed tomography
INTRODUCTION
Rapid maxillary expansion (RME) is a commonly used orthodontic technique to manage skeletal transverse maxillary discrepancy. The original procedure was first proposed by Angell.1 Haas2,3 highlighted the benefits of this treatment, still widely used among clinicians.
Because of the proximity of the hard palate to the nasal cavity, RME was frequently advocated for expanding the nasal airway.4,5 Several authors4–7 have investigated the advantages of RME in improving nasal airflow in patients having nasal stenosis.
Airway changes induced by RME treatment have been studied by means of functional examinations such as rhinomanometry8 and acoustic rhinometry.9,10 These studies showed a significant decrease in nasal airway resistance with consequent improvement of nasal breathing.8–10 Another investigation11 reported up to a 45% increase in nasal cross-sectional areas after expansion. In spite of this evidence and considering the V-shaped pattern of the midpalatal suture,4,5 increasing respiratory performance as the sole purpose of the procedure has been considered an insufficient indication for RME.11 Recently, the reduction in radiation dose obtained with cone beam computed tomography (CBCT) and low-dose multislice computed tomography has allowed the development of PC software capable of computing nasal airway volume. These methods have been used by several authors to better estimate the effects of RME on nasal airway changes.12–21
Polysomnography (PSG) is considered the gold standard for diagnosing such conditions as obstructive sleep apnea (OSA)22 and sleep bruxism.23 It provides various quantitative parameters to evaluate respiratory function such as oxygen saturation (SpO2) and apnea/hypopnea index (AHI).
Morphological modification of the airway space does not necessarily imply greater respiratory performance or vice versa. Although several studies have investigated such relationships, results are sometimes contradictory or conflicting.20–23,24 Moreover, no investigation has so far evaluated the relationship between the initial amount of airway stenosis and the functional nasal improvement produced by RME treatment.
The aim of the present longitudinal study was therefore to evaluate changes in airway volume and respiratory performance in patients undergoing RME and to determine whether any correlation exists between the functional and morphological effects of RME and the airway dimension.
MATERIALS AND METHODS
Population and Study Design
This study followed a prospective longitudinal design and enrolled subjects treated at the Department of Orthodontics of the University of Insubria, Varese, Italy. A signed informed consent was obtained from the parents of the patients prior to treatment. The protocol was reviewed and approved by the Ethics Committee (University of Insubria, Varese, Italy; Approval No. 5184) and the procedures followed adhered to the World Medical Organization Declaration of Helsinki.
Inclusion criteria of the sample group: no history of previous orthodontic treatment, apparently good general health according to the medical history, constricted maxillary arches with the presence of unilateral or bilateral posterior crossbite, early mixed dentition with stage 1 or 2 cervical vertebral maturation25 as assessed on lateral cephalograms derived from CBCT recordings collected as detailed below, and maxillary and mandibular first molars fully erupted.
Fifteen subjects (11 females and 4 males; mean age, 7.5 ± 0.3 years) were enrolled in the study, recruited from November 2011 to June 2012. Each patient underwent CBCT and PSG examination at baseline, prior to RME and immediately after removal of the maxillary expander 12 months later.
Rapid Maxillary Expansion Therapy and PSG Examination
All subjects were fitted with a Haas-type maxillary expander with a 10-mm screw (A167-1439, Forestadent, Pforzheim, Germany) banded on the maxillary second deciduous molars using glass ionomer cement (Multi-Cure, 3M-Unitek, Monrovia, Calif) in accordance with the manufacturer's instructions. The screw of the palatal expander was initially turned twice (0.45-mm initial transverse activation). Afterward, parents of the patients were instructed to turn it once per day (0.225-mm activation per day). Maxillary expansion was continued until dental overcorrection was obtained: when the lingual cusps of the maxillary first molars occluded onto the buccal cusps of the mandibular first molars. The screw was then locked with a light-cured composite (Premise Flowable; Kerr Corp, Orange, Calif); the expander remained on the teeth as a passive retainer over the following months. During this period, none of the patients underwent any further orthodontic treatment, and the expander was removed 12 months after insertion. The PSG examination (Embletta; Embla, Thornton, Colo) was performed before and after RME. During this examination, SpO2 was collected as the primary outcome; AHI was collected as the secondary outcome.
Image Recording and Postprocessing
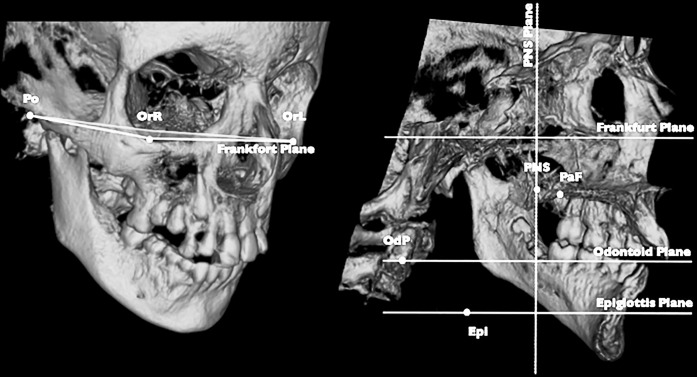
The CBCT scans (i-CAT; Imaging Sciences International, Hatfield, Pa) were performed in the seated position (120 KV, 3.8 mA, 30 s), with the patient's head in the natural head position, which was evaluated by a trained operator before scanning.26 The operator reproduced the head position while the patient was in the machine and made sure that the position was maintained and performed the scan. During the examination, patients were asked to occlude their teeth in maximum intercuspation; the exam was executed at the end of the patient's exhalation phase. These procedures allowed us to obtain a reproducible head position during the CBCT scans.27 The DICOM files were processed in Mimics software (version 10.11, Materialise Medical Co, Leuven, Belgium). A set of landmarks and planes was defined (Figure 1).
Figure 1.
3D landmarks and planes. Po: porion right and left; Or: orbitale right and left; PNS: posterior nasal spine; PaF: palatal foramen right and left; OdP: middle point of odontoid process of second cervical vertebra; Epi: top of the epiglottis. Frankfort plane: plane passing through PoR-PoL-OrR-OrL; PNS plane: projection on PNS of plane perpendicular to Frankfort passing through PoR-PoL; odontoid plane: passing through OdP and parallel to Frankfort; epiglottis plane: passing through top of the epiglottis and parallel to Frankfort.
Planes were used to obtain a reproducible head position and to define airway compartments as follows: (1) upper, between the edges of the nasal bones and ethmoid bone from nares to the PNS plane; (2) middle, between the PNS plane and odontoid plane; and (3) mandibular, between the odontoid plane and epiglottis plane (Figure 2). Moreover, the distance between the contralateral palatal foramens was used to assess the magnitude of the orthopedic maxillary expansion. The airway regions were thus segmented using a threshold-based procedure manually executed by an expert operator (A.Z.) and corrected slice by slice. Segmented airway spaces and landmarks were then exported respectively in stereolithographic and iges files. For subsequent processing in Rhinoceros Software (Robert McNeel & Associates, Seattle, Wash), a logarithmic sequence, specifically written for this purpose, automatically computed volumes for each of the airway regions.
Figure 2.
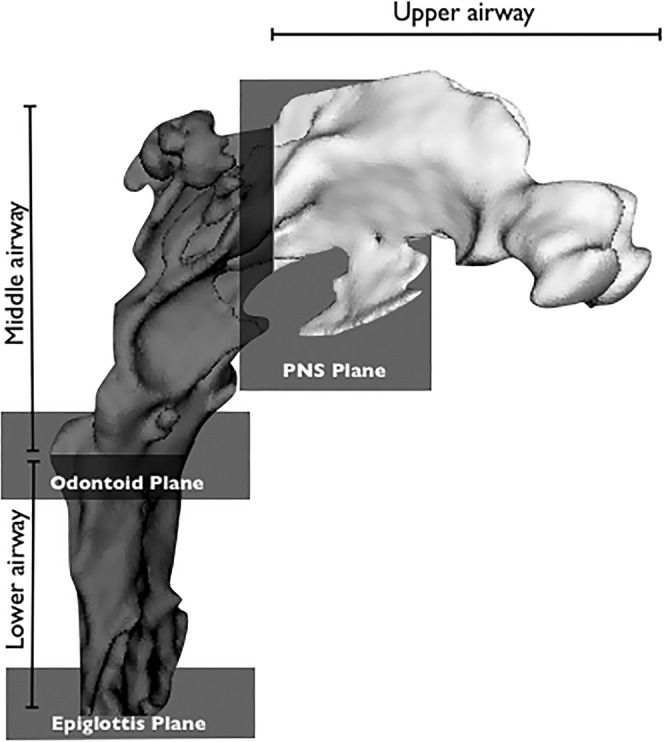
Airway compartments. Upper airway from nares to PNS plane; middle airway from PNS Plane to odontoid plane; lower airway from odontoid plane to epiglottis plane.
A sample size of at least 10 subjects was necessary to detect an effect size (ES) coefficient28 of 1.0 for each recorded parameter between the time points, with an alpha set at 0.05 and a power of 0.8. The ES coefficient is the ratio of the difference between the recordings of the two groups, divided by the within-subject SD. An effect size of at least 0.8 is considered a large effect,28 while a value of at least 1.0 indicates good diagnostic potential.29
With the aim of quantifying the full method error of the recordings of both these palatal parameters, the method of moments (MME) variance estimator was used.30 Therefore, the mean error and 95% CIs between the repeated recordings were calculated using the MME variance estimator and expressed as percentages.31 The MME variance estimator has the advantages of not being affected by any unknown bias, ie, systematic errors, between pairs of measurements.30
Data Analysis
SPSS software, version 13.0 (SPSS Inc, Chicago, Ill) was used to perform the statistical analysis. Parametric methods were used after we tested the existence of the assumptions through the Shapiro-Wilk test and Levene's test for normality of the distributions and equality of the variances between the time points, respectively. A paired sample t-test was employed to assess the significance of the difference of each parameter between the time points.
A Pearson rho correlation coefficient was employed to evaluate the strength of the relationship between the baseline volumes of each airway compartment and the absolute changes in SpO2 and AHI events. Finally, multiple backward linear regressions were used to estimate association of the baseline volumes of each airway compartment (explanatory variables) with either the SpO2 or AHI events changes. Age and sex were also entered in the models as covariates. A P value less than .05 was used in rejecting the null hypothesis.
RESULTS
Method errors as mean (95% CI) was 0.3 mm (0.1 mm–0.5 mm) for the interpalatal foramen distance and 179.6 mm3 (84.2 mm3–298.9 mm3) for total airway volume dimension.
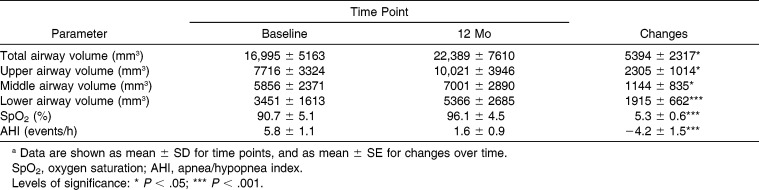
The interpalatal foramen distance was 26.6 ± 2.9 mm and 29.1 ± 3.0 mm at baseline and 1 year, respectively. Total airway volume was 16,995 ± 5163 mm3 and 22,389 ± 7610 mm3 at baseline and after treatment, respectively. Other descriptive statistics for each recorded parameter is summarized in Table 1. The upper, middle, and lower airway volumes underwent mean increases of 2305 mm3, 1144 mm3, and 1915 mm3, respectively. Similarly, SpO2 and AHI underwent improvements of 5.3% and −4.2 events, respectively. All the improvements were statistically significant (P < .05).
Table 1.
Airway Compartment Volumes and Respiratory Functional Parameters Recorded at Each Time Point (n = 15)a
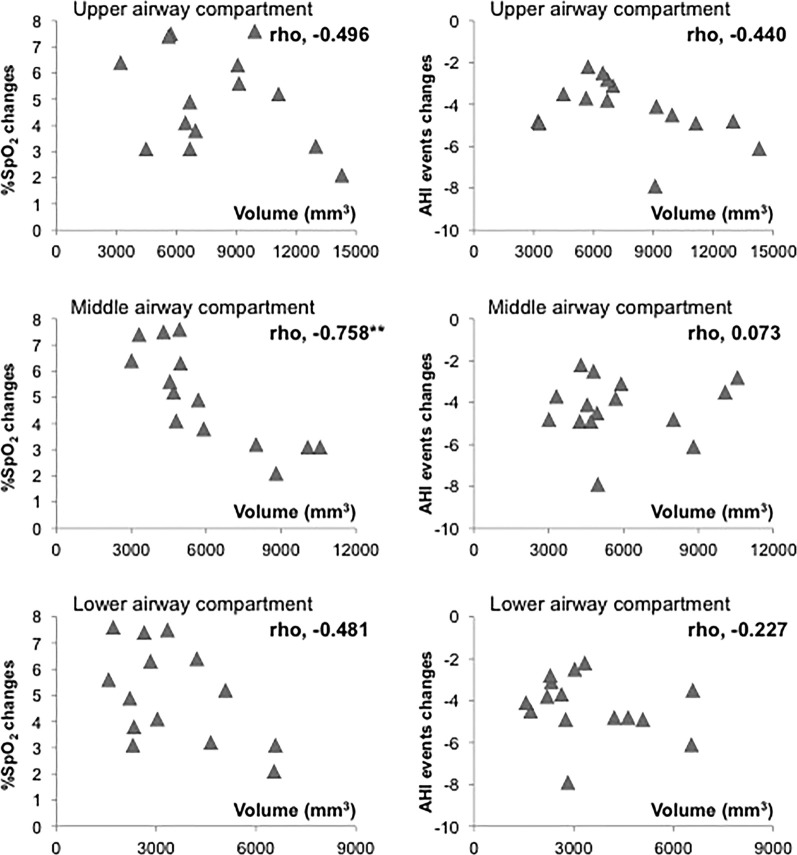
Results of the bivariate correlations between the baseline volume of each airway compartment with the SpO2 and AHI absolute changes are shown in Figure 3. Generally, negative correlations were seen, even though only the baseline middle airway compartment volume reached a significant level of negative correlation with the SpO2 changes (rho = −0.758, P < .01).
Figure 3.
Bivariate correlations between the baseline volume of each airway compartment with the SpO2 and AHI absolute changes (n = 15). Correlations between parameters are shown as rho coefficients. Level of significance: ** P < .01
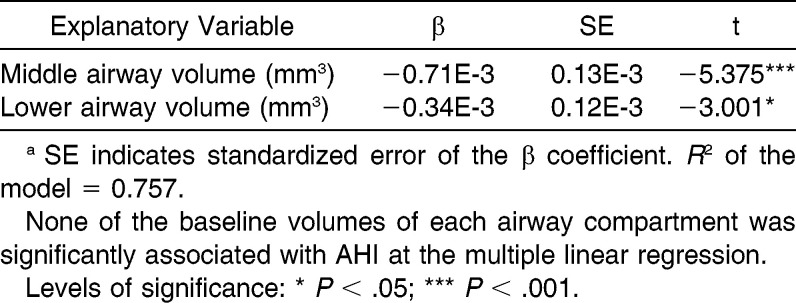
Among the multiple backward logistic regressions, no explanatory variable recorded at baseline was significantly associated with the changes in AHI events. On the contrary, baseline middle and lower airway volumes showed a significant negative association with the SpO2 changes (Table 2, P < .05).
Table 2.
Results of Multiple Backward Linear Regression to Estimate Association of the Baseline Volumes of Each Airway Compartment with SpO2 Changes (n = 15)a
DISCUSSION
To the best of our knowledge, this is the first study demonstrating a significant correlation between baseline middle and lower airway volumes and SpO2 changes obtained as a consequence of RME in subjects having posterior crossbite. The multiple backward logistic regressions showed that the more the subjects presented a reduced nasal volume in the middle and lower compartments, the more they would benefit from RME in terms of improved SpO2, with the middle compartment being better correlated than the lower (β values, 0.71E-3 and −0.34E-3, respectively). Interestingly, the combination of baseline middle and lower airway volumes explained up to 75.7% of the overall SpO2 changes after treatment. This result might suggest the importance of not only nasal obstruction, as shown by other authors,24 but also the contingent stenosis of the middle and lower pharynx wherein tonsil and adenoid hypertrophy might play an important role in reducing the airway lumen.
The regressions model showed no association of the baseline airway compartments with sex, age, or AHI variations (Table 2). Evaluating AHI as a secondary outcome, we found in the sample group an improvement in the index with a reduction in apneic events of 4.2 per hour. These findings support those stating that RME is effective in improving respiratory function. There is some evidence in the literature showing that RME improves respiratory function in OSA patients, thus reducing the AHI.22
The tested sample had value of 5.8 ± 1.1 events/hour for AHI at baseline, which might be considered slightly higher than physiological standards. Diagnosis of OSA was excluded during the subjects' enrollment since none of the subjects presented any of the symptoms or signs to indicate a positive diagnosis of PSG.32
RME treatment might positively affect nasal function by enlarging the hard and the soft nasal airway tissues. It is known that RME produces a functional improvement of the breathing pattern in patients with nasal obstruction or stenosis.24 Previous studies12–15,19,20,24 divided the airway into various compartments evaluating volume before and after RME to better clarify the effects of treatment. Several authors17,19,20 have reported a significant enlargement of the nasal cavity and nasopharynx, in agreement with the present results, but no significant increase in the other investigated airway compartments, such as the oropharynx and hypopharynx, suggesting that effects on the upper airway were local as a result of soft tissue adaptation farther from the midpalatal suture.19
Nevertheless, differences in compartmental segmentation were noticeable between the present method and the above-mentioned studies, which might explain the different outcomes. Indeed, according to the present results, not only the upper and nasal airways but also the middle and lower airway compartments underwent significant volume increases. Such increases were greater for the upper compartment, ie, the nasal cavity, and slightly lower for the middle and lower compartments. On the contrary, Zhao et al.13 reported no significant changes in airway volumes after RME. However, despite the inclusion of a control group, the retrospective nature of that study should be taken into account.
According to the results of the present study, there was a good response from the respiratory mucosal tissue, which seemed to follow the bony expansion, even though this evidence is still questioned, especially in long-term, follow-up studies.33
Lack of a control group should be considered a limitation of the present study as well as the difficulty of standardizing CBCT acquisition,34 such as repositioning of the tongue (due to dental changes35) and the mandible36 (as a result of the clinical procedure). Threshold-based segmentation of the airway may not be easy to standardize, although we believe that the present method errors are acceptable. Combining morphological recording with functional respiratory analysis is therefore recommended.
CONCLUSIONS
RME treatment induced significant increases in upper, middle, and lower airway compartment volume.
Lower baseline airway volumes of the middle and lower compartments were associated with greater increases in SpO2, but not in AHI changes.
REFERENCES
- 1.Angell EC. Treatment of irregularities of the permanent or adult teeth. Dent Cosmos. 1860;1:540–544. [Google Scholar]
- 2.Haas AJ. Rapid expansion of the maxillary dental arch and nasal cavity by opening the midpalatal suture. Angle Orthod. 1961;31:73–90. [Google Scholar]
- 3.Haas AJ. Long-term posttreatment evaluation of rapid palatal expansion. Angle Orthod. 1980;50:189–217. doi: 10.1043/0003-3219(1980)050<0189:LPEORP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Wertz RA. Changes in nasal airflow incident to rapid maxillary expansion. Angle Orthod. 1968;38:1–11. doi: 10.1043/0003-3219(1968)038<0001:CINAIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Wertz RA. Skeletal and dental changes accompanying rapid midpalatal suture opening. Am J Orthod. 1970;58:41–66. doi: 10.1016/0002-9416(70)90127-2. [DOI] [PubMed] [Google Scholar]
- 6.Brown GVI. The application of orthodontic principles to nasal disease. Iowa State Dent Soc Trans. 1902:67–79. [Google Scholar]
- 7.White BC, Woodside DG, Cole P. The effect of rapid maxillary expansion on nasal airway resistance. J Otolaryngol. 1989;18:137–143. [PubMed] [Google Scholar]
- 8.Monini S, Malagola C, Villa MP, et al. Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch Otolaryngol Head Neck Surg. 2009;135:22–27. doi: 10.1001/archoto.2008.521. [DOI] [PubMed] [Google Scholar]
- 9.De Felippe NLO, Bhushan N, Da Silveira AC, Viana G, Smith B. Long-term effects of orthodontic therapy on the maxillary dental arch and nasal cavity. Am J Orthod Dentofacial Orthop. 2009;136:490.e1–e8. doi: 10.1016/j.ajodo.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Compadretti GC, Tasca I, Bonetti GA. Nasal airway measurements in children treated by rapid maxillary expansion. Am J Rhinology. 2006;20:385–393. doi: 10.2500/ajr.2006.20.2881. [DOI] [PubMed] [Google Scholar]
- 11.Warren DW, Hershey HG, Turvey TA, Hinton VA, Hairfield WM. The nasal airway following maxillary expansion. Am J Orthod Dentofacial Orthop. 1987;91:111–6. doi: 10.1016/0889-5406(87)90467-7. [DOI] [PubMed] [Google Scholar]
- 12.Haralambidis A, Ari-Demirkaya A, Acar A, Küçükkeleş N, Ateş M, Ozkaya S. Morphologic changes of the nasal cavity induced by rapid maxillary expansion: a study on 3-dimensional computed tomography models. Am J Orthod Dentofacial Orthop. 2009;136:815–821. doi: 10.1016/j.ajodo.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Nguyen M, Gohl E, Mah JK, Sameshima G, Enciso R. Oropharyngeal airway changes after rapid palatal expansion evaluated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2010;137:S71–S78. doi: 10.1016/j.ajodo.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Görgülü S, Gokce SM, Olmez H, Sagdic D, Ors F. Nasal cavity volume changes after rapid maxillary expansion in adolescents evaluated with 3-dimensional simulation and modeling programs. Am J Orthod Dentofacial Orthop. 2011;140:633–640. doi: 10.1016/j.ajodo.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro AN, de Paiva JB, Rino-Neto J, Illipronti-Filho E, Trivino T, Fantini SM. Upper airway expansion after rapid maxillary expansion evaluated with cone beam computed tomography. Angle Orthod. 2012;82:458–463. doi: 10.2319/030411-157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martina R, Cioffi I, Farella M, et al. Transverse changes determined by rapid and slow maxillary expansion—a low-dose CT-based randomized controlled trial. Orthod Craniofac Res. 2012;15:159–168. doi: 10.1111/j.1601-6343.2012.01543.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith T, Ghoneima A, Stewart K, et al. Three-dimensional computed tomography analysis of airway volume changes after rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2012;141:618–626. doi: 10.1016/j.ajodo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Cordasco G, Nucera R, Fastuca R, et al. Effects of orthopedic maxillary expansion on nasal cavity size in growing subjects: a low dose computer tomography clinical trial. Int J Pediatr Otorhinolaryngol. 2012;76:1547–1551. doi: 10.1016/j.ijporl.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Chang Y, Koenig LJ, Pruszynski JE, Bradley TG, Bosio JA, Liu D. Dimensional changes of upper airway after rapid maxillary expansion: a prospective cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013;143:462–470. doi: 10.1016/j.ajodo.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 20.El H, Palomo JM. Three-dimensional evaluation of upper airway following rapid maxillary expansion. A CBCT study. Angle Orthod. 2014;84:265–273. doi: 10.2319/012313-71.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caprioglio A, Meneghel M, Fastuca R, Zecca PA, Nucera R, Nosetti L. Rapid maxillary expansion in growing patients: correspondence between 3-dimensional airway changes and polysomnography. Int J Pediatr Otorhinolaryngol. 2014;78:23–27. doi: 10.1016/j.ijporl.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Villa MP, Rizzoli A, Miano S, Malagola C. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath. 2011;15:179–184. doi: 10.1007/s11325-011-0505-1. [DOI] [PubMed] [Google Scholar]
- 23.International Classification of Sleep Disorders (ICSD3) Diagnostic and Coding Manual (3rd ed) Darien, IL: American Academy of Sleep Medicine; 2014. American Academy of Sleep Medicine. [Google Scholar]
- 24.Iwasaki T, Saitoh I, Takemoto Y, et al. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: a cone-beam computed tomography study. Am J Orthod Dentofacial Orthop. 2013 Feb;143(2):235–245. doi: 10.1016/j.ajodo.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Baccetti T, Franchi L, McNamara JA. The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin Orthod. 2005;11:119–129. [Google Scholar]
- 26.Cooke MS1, Wei SH. The reproducibility of natural head posture: a methodological study. Am J Orthod Dentofacial Orthop. 1988;93:280–288. doi: 10.1016/0889-5406(88)90157-6. [DOI] [PubMed] [Google Scholar]
- 27.Weber DW, Fallis DW, Packer MD. Three-dimensional reproducibility of natural head position. Am J Orthod Dentofacial Orthop. 2013;143:738–744. doi: 10.1016/j.ajodo.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Perinetti G, Contardo L. Posturography as a diagnostic aid in dentistry: a systematic review. J Oral Rehabil. 2009;36:922–936. doi: 10.1111/j.1365-2842.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 30.Springate SD. The effect of sample size and bias on the reliability of estimates of error: a comparative study of Dahlberg's formula. Eur J Orthod. 2012;34:158–163. doi: 10.1093/ejo/cjr010. [DOI] [PubMed] [Google Scholar]
- 31.Perinetti G, Marsi L, Castaldo A, Contardo L. Is postural platform suited to study correlations between the masticatory system and body posture? A study of repeatability and a meta-analysis of reported variations. Prog Orthod. 2012;13:273–280. doi: 10.1016/j.pio.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto MA, Itikawa CE, Valera FC, Faria G, Anselmo-Lima WT. Long-term effects of rapid maxillary expansion on nasal area and nasal airway resistance. Am J Rhinol Allergy. 2010;24:161–165. doi: 10.2500/ajra.2010.24.3440. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro AN, de Paiva JB, Rino-Neto J, Illipronti-Filho E, Trivino T, Fantini SM. Upper airway expansion after rapid maxillary expansion evaluated with cone beam computed tomography. Angle Orthod. 2012;82:458–463. doi: 10.2319/030411-157.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ugolini A, Cerruto C, Di Vece L, et al. Dental arch response to Haas-type rapid maxillary expansion anchored to deciduous vs permanent molars: a multicentric randomized controlled trial. Angle Orthod. 2014 doi: 10.2319/041114-269.1. Ahead of print. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fastuca R, Zecca P, Caprioglio A. Role of mandibular displacement and airway size in improving breathing after rapid maxillary expansion. Progr in Orth. 2014;15:40. doi: 10.1186/s40510-014-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]