Abstract
Objective:
To identify genetic and environmental factors contributing to hypodontia and microdontia by using Korean twin family data.
Materials and Methods:
A total of 1267 individuals (525 men and 742 women; 180 monozygotic twins [MZ] and 43 dizygotic twins [DZ] from 282 families) underwent an oral examination as part of the Healthy Twin Study in Korea. Dental anomalies classified as hypodontia or microdontia were diagnosed using radiographs and clinical examinations. In order to estimate genetic contributions to dental anomalies, we estimated the pairwise concordance rate (PCR), recurrence risk ratio (RRR), and heritability (h2).
Results:
The prevalence of hypodontia and microdontia was 3.55% and 3.00%, respectively. MZ had the highest PCR and RRR (13.0–15.3). The PCR and RRR values for both anomalies were much higher for DZ (5.0–11.9) than for siblings (1.4–2.6), despite the fact that DZ pairs and sibling pairs share 50% genetic identity. Further genetic analysis revealed both an additive genetic effect (0.38 when hypodontia and microdontia were pooled) and a strong “twin effect” (0.52 when hypodontia and microdontia were pooled).
Conclusions:
This twin-based study revealed that the formation of dental anomalies is affected by both genetic and environmental factors, and that the impact of these factors varies according to the specific dental anomaly.
Keywords: Microdontia, Hypodontia, Korean twins, Genetics
INTRODUCTION
Dental anomalies cause malocclusions by disturbing normal dental development.1 Developmental dental anomalies such as hypodontia and microdontia are not uncommon in many populations, but little is known about their underlying causes.2,3
The prevalence rates of these anomalies varies across populations; previous studies have reported a prevalence as low as 2.3% and as high as 11.3%.4–6 Substantial differences in frequency may at least partly be explained by study design, and a population-based study is more likely to determine the true frequency of these anomalies.7–10
Twins can be either identical or fraternal. Identical twins, or monozygotic twins (MZ), share 100% of their genetic information, whereas fraternal twins, or dizygotic twins (DZ), result from two egg cells each fertilized by a different sperm. Genetically, DZ are normal siblings, sharing approximately 50% of their genetic information.
The Healthy Twin Study of Korea has recruited families representing the general population of Korea.11 Recruitment of both MZ and DZ as well as nontwin siblings has endowed the study with the ability to perform high-resolution analysis of the contribution of genetic and environmental parameters to phenotypes of interest.
Because hypodontia and microdontia are the most common types of dental anomalies,12–14 we assessed genetic contributions to these phenotypes by calculating the pairwise concordance rate (PCR), recurrence risk ratio (RRR), and heritability (h2); we assessed environmental contributions by evaluating the degree of shared environmental effects.
MATERIALS AND METHODS
Study Population
A total of 1267 individuals (525 men, 742 women, ranging in age from 17 to 81 years) who underwent dental examination as part of the Healthy Twin Study in Korea, participated in this study. The Healthy Twin Study, a nationwide, twin-family cohort study in Korea, recruited Korean adult twins and their family members who had no ascertained preexisting syndromic diseases.11,15 The study population comprised 282 families with different types of relationships: 360 MZ (180 pairs), 87 DZ (42 pairs of twins and one pair of triplets), 304 nontwin siblings, and 278 spouses (139 pairs). A zygosity questionnaire and discriminating algorithms were used to determine MZ and DZ.16
Diagnosis of Dental Anomalies
Dental anomalies were diagnosed using radiographs, clinical examinations, and medical and dental histories. We investigated hypodontia, which was defined as congenital absence of at least one permanent tooth or tooth germ (third molars excluded).7,17 We also investigated microdontia excluding third molars, which commonly affected the maxillary lateral incisor (incisal width mesiodistally narrower than the cervical width, including the peg lateral).18,19 When teeth were missing, only those cases with positive history of microdontia or hypodontia were counted. All examinees had cephalograms and panoramic radiographs taken; when clinically diagnosed microdontia or hypodontia was consistent with radiographic findings, we made the final diagnosis of microdontia/hypodontia.
Statistical Analysis
We examined the incidence of hypodontia, microdontia, and pooled cases according to sex, age, and zygosity status. PCR and RRR were estimated based on the type of family relationship: MZ or DZ twin pairs, siblings, parent-offspring, or spouse pairs.20,21 We calculated the age and sex-standardized prevalence of each dental anomaly based on those of the study subjects.
Two different models were implemented to explain the variance in dental anomalies. Our first model was a polygenic model that included only additive genetic effects (A) and random errors (E). The second model included “shared environmental effects” (C) and comprised common environmental effects (C) that were not explained by covariates. Thus, for example, an AE model would be one that included both additive genetic effects and random errors. The symbol h2 was defined as the proportion of phenotypic variance explained by additive genetic effects.22,23 A liability threshold model was implemented to analyze single discrete traits (affected or unaffected). This model assumes a certain genetic threshold according to population prevalence; when genetic risk is greater than the threshold, a specific disease is manifested.24,25 We chose the best fitting model based on likelihood estimators.
Ethics Statement
The study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB 2005-08-113-027). Informed consent was received from all subjects.
RESULTS
Demographics
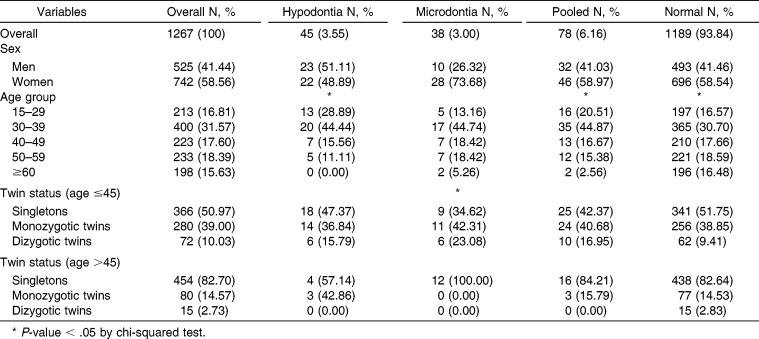
Overall demographic data are shown in Table 1. Among 1267 individuals, 45 were diagnosed with hypodontia and 38 with microdontia. A total of 78 individuals had at least one of the two dental anomalies. The prevalence was 4.38% in men and 2.96% in women for hypodontia, and 1.90% in men and 3.77% in women for microdontia. The mean age (standard deviation) of participants was 43.04 ± 14.15 years (range, 17–81). There was a significant decrease in the prevalence of hypodontia (P = .0001) and pooled hypodontia/microdontia (P = .0006) according to age. Microdontia did not show meaningful trends according to age (P = .21). The distribution of dental anomalies according to relationship types (MZ, DZ, or other family members) was analyzed in two age groups (older or younger than 45 years) in order to reduce age effects.
Table 1.
Prevalence of Hypodontia and Microdontia According to Demographic Characteristics
Pairwise Concordance Rate and Recurrence Risk Ratio
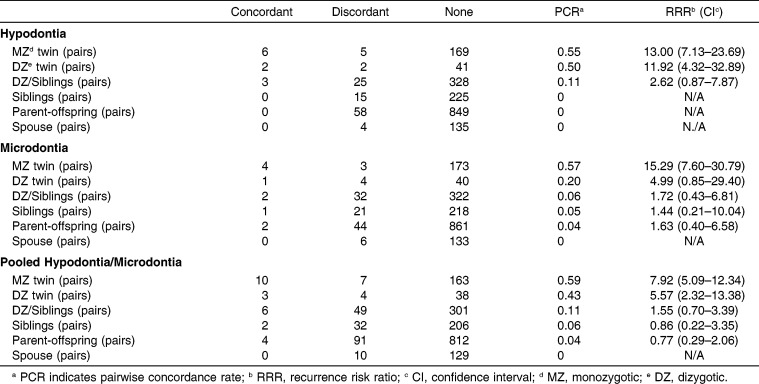
PCR and RRR values are reported in Table 2.
Table 2.
Pairwise Concordance and Recurrence Risk Ratios
Hypodontia
Six pairs of MZ had concordant hypodontia. Five pairs of MZ had discordant hypodontia. The PCR and RRR of hypodontia in MZ were 0.55 and 13.00 (95% confidence intervals, 7.13–23.69), respectively; this means that if one twin had hypodontia, the cotwin had a 13-fold increased risk of hypodontia. Among DZ, there were two concordant pairs and two discordant pairs; accordingly, the PCR was 0.50. The RRR of hypodontia in DZ was 11.92 (4.32–32.89), which was slightly lower than that of MZ. When we pooled DZ and nontwin siblings, both of whom share the same degree of genetic influences, there were three concordant and 25 discordant pairs, which led to a PCR of 0.11 and an RRR of 2.62 (range, 0.87–7.87). The last concordant pair was between one DZ twin and another sibling. There were no concordant pairs among parent-offspring or spouse pairs, so RRR could not be estimated for these familial groupings.
Microdontia
MZ showed highly aggregated patterns of microdontia manifestation. There were four concordant and three discordant pairs, resulting in a PCR of 0.57 and an RRR of 15.29 (range, 7.60–30.79); DZ had a PCR of 0.20 and an RRR of 4.99 (0.85–29.40), calculated from one concordant and four discordant pairs. Pooling the DZ and siblings yielded two concordant pairs and 32 discordant pairs, leading to a PCR of 0.06 and an RRR of 1.72 (0.43–6.81). There was one concordant pair and 21 discordant pairs of nontwin siblings, resulting in a PCR of 0.05 and an RRR of 1.44 (range, 0.21–10.04). There were two concordant and 44 discordant parent-offspring pairs, with a PCR of 0.04 and an RRR of 1.63 (0.40–6.58). No concordant pairs were found between spouses.
Pooled Hypodontia/Microdontia
When we pooled hypodontia and microdontia, we found 10 concordant and seven discordant pairs among MZ; the PCR was 0.59 and the RRR was 7.92 (5.09–12.34). For DZ, we observed three concordant and four discordant pairs, resulting in a PCR of 0.43 and an RRR of 5.57 (2.32–13.38). Pooled DZ and siblings and parent-offspring pairs resulted in PCRs of 0.11, 0.06, and 0.04, respectively, corresponding to RRRs of 1.55, 0.86, and 0.77. No concordant pairs were detected among MZ spouse pairs.
Heritability Estimates
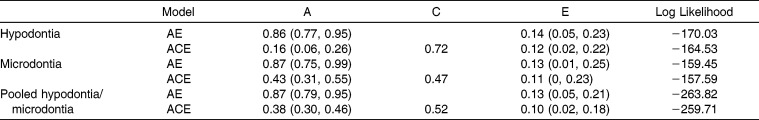
The h2 estimates of hypodontia, microdontia, and presence of either anomaly are presented in Table 3. In the AE model, the h2 estimates of hypodontia, microdontia, and pooled hypodontia or microdontia were 0.86, 0.87, and 0.87, respectively. In the ACE model, the h2 estimates were 0.16, 0.43, and 0.38 for hypodontia, microdontia, and pooled hypodontia or microdontia, respectively. Twin common environmental effect estimates were 0.72, 0.47, and 0.52 for hypodontia, microdontia, and pooled hypodontia or microdontia, respectively. The ACE model with twin common environmental effects had a lower log likelihood ratio than did the AE model, indicating that the ACE model had a better fit.
Table 3.
Estimates of Additive Genetic (A), Shared Environmental With a Twin (C), and Nonshared Environmental (E) Influences
DISCUSSION
Prevalence
Several studies have reported the prevalence of dental anomalies in different populations.7,8,26–28 Differences in prevalence among populations have been attributed to racial differences, different sampling techniques, and different diagnostic criteria. In east Asian populations, including Koreans, one of the distinct characteristics of dental anomalies is a higher prevalence of mandibular incisor hypodontia than in other populations.7,8,17,26,27
The prevalence of hypodontia (3.55%, Table 1) is consistent with previous studies (2.3% to 11.3%).4–6 A higher prevalence of dental anomalies has been reported in studies that have examined orthodontic patients vs those that have examined the general population.4–9,17,26,27,29 Because the twin study population in this study was community based, the prevalence of hypodontia that we calculated is likely to be an accurate estimate of the prevalence of hypodontia in the Korean general population, and is therefore much lower than that reported in previous studies that examined orthodontic patients.17,27
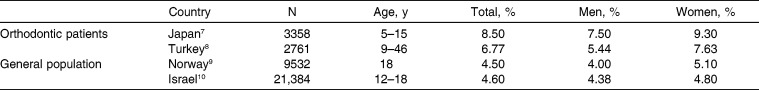
The prevalence of hypodontia in orthodontic patients and the general population are reported in Table 4.7–10 In adolescents, the prevalence of hypodontia was 8.50% for orthodontic patients and 4.50% for the general population. The wider age range of Turkish orthodontic patients sampled (9–46 years) is the most likely explanation for the lower prevalence of hypodontia in this population compared with that of Japanese orthodontic patients (5–15 years). In our study, the prevalence of hypodontia decreased with increasing age. This may be due to orthodontic or prosthetic treatment for missing teeth.
Table 4.
Prevalence of Hypodontia
Genes and Environment
A higher PCR and RRR in MZ than in other relatives, such as DZ, nontwin siblings, parent-offspring pairs, and spouses, suggests a strong genetic contribution to dental anomalies (Table 2). A close relationship between hypodontia and microdontia has been reported, and genetics plays an important role in dental development.2,3,5,6,14,18,30,31 However, the details differ based on the type of dental anomaly.
Overall, PCR, RRR, and h2 estimation revealed genetic contributions to dental anomalies. PCR and RRR decreased when comparing MZ with spouses; h2 estimates also supported a considerable genetic contribution to overall dental anomalies.
PCR and RRR of hypodontia in MZ and DZ were similar, implying a twin common environmental effect as well as a genetic effect (Table 2). The h2 estimates of hypodontia also indicate a strong contribution from the twin common environment (Table 3). The h2 estimates showed that the twin common environmental effect (0.72) was stronger than the additive genetic effect (0.16; Table 3). Because DZ, nontwin siblings, and parent-offspring pairs share an average of 50% of their genetic information, the difference between PCR and RRR (Table 2) of DZ twin pairs and other relative pairs indicates environmental as well as genetic contributions to hypodontia.
Strong genetic and twinning effects were evident when the two dental anomalies were pooled. PCR and RRR values of MZ and DZ were much higher than those of other first-degree relative types. In addition, MZ had slightly higher PCR and RRR values than did DZ (Table 2). RRR values of MZ and DZ for pooled anomalies were more stable than separate analysis (Table 2). In the h2 estimation, the additive genetic effect was 0.38, which was larger than that for hypodontia, but smaller than that for microdontia. The twin common environmental effect was 0.52, which was larger than that of microdontia, but smaller than that of hypodontia (Table 3). Therefore, both genetic and twin common environmental effects contribute to the presence of these dental anomalies, although the contribution of a twin common environment was larger than that of the genetic contribution.
Genetic and Environmental Influences in Twins and Siblings
Our results are consistent with those of previous studies reporting that dental anomalies have a high h2 estimate; if one twin in a pair is affected by a dental anomaly, the risk of the other having that anomaly is increased.32,33 Nontwin siblings share half their genetic information with each other, as do DZ; however, concordance and RRR values for nontwin siblings were much lower than for DZ. Conventional genetic theory assumes that the degree of environmental sharing is the same between DZ and nontwin siblings. However, one of our most important findings is the difference between DZ and siblings. In Table 3, official testing for twinning effects (ie, the influence of being a twin, whether MZ or DZ) was 0.72, exceeding that of additive genetic effects (0.16). The marked difference between twin effects and shared sibling effects indicates that the influence of very early environmental effects, such as intrauterine effects or factors in early infancy, are not usually shared by siblings.
CONCLUSIONS
Both genetic and environmental factors contribute to the development of dental anomalies.
Although hypodontia and microdontia showed some differences in genetic vs environmental effects, pooled analysis showed consistent results.
Pooled analysis for microdontia/hypodontia suggests strong shared environmental effects only between twins that are not seen in siblings, indicating the possible importance of early life influences (such as intrauterine environment or nutrition in early infancy).
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Foundation of Korea (2012K2A1A2032536) and the Global Research Network Program (2011-220-E00006). We would like to thank all twin families for their participation and also Eung Min Kim and Won Hee Cho (Department of Orthodontics, Institute of Oral Health Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea) for assistance with data collection and analysis.
REFERENCES
- 1.Wisth PJ, Thunold K, Boe OE. Frequency of hypodontia in relation to tooth size and dental arch width. Acta Odontol Scand. 1974;32:201–206. doi: 10.3109/00016357409002548. [DOI] [PubMed] [Google Scholar]
- 2.Militi D, Militi A, Cutrupi MC, et al. Genetic basis of non syndromic hypodontia: a DNA investigation performed on three couples of MZ twins about PAX9 mutation. Eur J Paediatr Dent. 2011;12:21–24. [PubMed] [Google Scholar]
- 3.Vieira AR, D'Souza RN, Mues G, et al. Candidate gene studies in hypodontia suggest role for FGF3. Eur Arch Paediatr Dent. 2013;14:405–410. doi: 10.1007/s40368-013-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thilander B, Myrberg N. The prevalence of malocclusion in Swedish schoolchildren. Scand J Dent Res. 1973;81:12–21. doi: 10.1111/j.1600-0722.1973.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 5.Larmour CJ, Mossey PA, Thind BS, Forgie AH, Stirrups DR. Hypodontia—a retrospective review of prevalence and etiology. Part I. Quintessence Int. 2005;36:263–270. [PubMed] [Google Scholar]
- 6.Pandey P, Ansari AA, Choudhary K, Saxena A. Familial aggregation of maxillary lateral incisor agenesis (MLIA) BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-007846. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo T, Ozoe R, Kubota M, Akiyama M, Shimooka S. A survey of hypodontia in Japanese orthodontic patients. Am J Orthod Dentofacial Orthop. 2006;129:29–35. doi: 10.1016/j.ajodo.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Topkara A, Sari Z. Prevalence and distribution of hypodontia in a Turkish orthodontic patient population: results from a large academic cohort. Eur J Paediatr Dent. 2011;12:123–127. [PubMed] [Google Scholar]
- 9.Nordgarden H, Jensen JL, Storhaug K. Reported prevalence of congenitally missing teeth in two Norwegian counties. Community Dent Health. 2002;19:258–261. [PubMed] [Google Scholar]
- 10.Eidelman E, Chosack A, Rosenzweig KA. Hypodontia: prevalence amongst Jewish populations of different origin. Am J Phys Anthropol. 1973;39:129–133. doi: 10.1002/ajpa.1330390113. [DOI] [PubMed] [Google Scholar]
- 11.Sung J, Cho SI, Lee K, et al. Healthy Twin: a twin-family study of Korea—protocols and current status. Twin Res Hum Genet. 2006;9:844–848. doi: 10.1375/183242706779462822. [DOI] [PubMed] [Google Scholar]
- 12.Garib DG, Alencar BM, Lauris JR, Baccetti T. Agenesis of maxillary lateral incisors and associated dental anomalies. Am J Orthod Dentofacial Orthop. 2010;137:732 e1–e6; discussion 732–733. doi: 10.1016/j.ajodo.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Peck S, Peck L, Kataja M. Prevalence of tooth agenesis and peg-shaped maxillary lateral incisor associated with palatally displaced canine (PDC) anomaly. Am J Orthod Dentofacial Orthop. 1996;110:441–443. doi: 10.1016/s0889-5406(96)70048-3. [DOI] [PubMed] [Google Scholar]
- 14.Alvesalo L, Portin P. The inheritance pattern of missing, peg-shaped, and strongly mesio-distally reduced upper lateral incisors. Acta Odontol Scand. 1969;27:563–575. doi: 10.3109/00016356909026309. [DOI] [PubMed] [Google Scholar]
- 15.Gombojav B, Song YM, Lee K, et al. The Healthy Twin Study, Korea updates: resources for omics and genome epidemiology studies. Twin Res Hum Genet. 2013;16:241–245. doi: 10.1017/thg.2012.130. [DOI] [PubMed] [Google Scholar]
- 16.Song YM, Lee D, Lee MK, et al. Validity of the zygosity questionnaire and characteristicsof zygosity-misdiagnosed twin pairs in the Healthy Twin Study of Korea. Twin Res Hum Genet. 2010;13:223–230. doi: 10.1375/twin.13.3.223. [DOI] [PubMed] [Google Scholar]
- 17.Kim YH, Kim SH, Baek SH. Consideration of clinically related dental anomalies: prevalence and association. J Korean Dent Sci. 2010;3:17–24. [Google Scholar]
- 18.Baccetti T. A controlled study of associated dental anomalies. Angle Orthod. 1998;68:267–274. doi: 10.1043/0003-3219(1998)068<0267:ACSOAD>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Backman B, Wahlin YB. Variations in number and morphology of permanent teeth in 7-year-old Swedish children. Int J Paediatr Dent. 2001;11:11–17. doi: 10.1046/j.1365-263x.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 20.McGue M. When assessing twin concordance, use the probandwise not the pairwise rate. Schizophr Bull. 1992;18:171–176. doi: 10.1093/schbul/18.2.171. [DOI] [PubMed] [Google Scholar]
- 21.Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. Am J Hum Genet. 1990;46:229–241. [PMC free article] [PubMed] [Google Scholar]
- 22.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 23.Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trait loci. Adv Genet. 2001;42:151–181. doi: 10.1016/s0065-2660(01)42021-9. [DOI] [PubMed] [Google Scholar]
- 24.Sung J, Lee K, Song YM. Heritabilities of the metabolic syndrome phenotypes and related factors in Korean twins. J Clin Endocrinol Metab. 2009;94:4946–4952. doi: 10.1210/jc.2009-1268. [DOI] [PubMed] [Google Scholar]
- 25.Duggirala R, Williams JT, Williams-Blangero S, Blangero J. A variance component approach to dichotomous trait linkage analysis using a threshold model. Genet Epidemiol. 1997;14:987–992. doi: 10.1002/(SICI)1098-2272(1997)14:6<987::AID-GEPI71>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Goya HA, Tanaka S, Maeda T, Akimoto Y. An orthopantomographic study of hypodontia in permanent teeth of Japanese pediatric patients. J Oral Sci. 2008;50:143–150. doi: 10.2334/josnusd.50.143. [DOI] [PubMed] [Google Scholar]
- 27.Kim YH. Investigation of hypodontia as clinically related dental anomaly: prevalence and characteristics. ISRN Dent. 2011:246135. doi: 10.5402/2011/246135. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endo T, Ozoe R, Yoshino S, Shimooka S. Hypodontia patterns and variations in craniofacial morphology in Japanese orthodontic patients. Angle Orthod. 2006;76:996–1003. doi: 10.2319/082905-303. [DOI] [PubMed] [Google Scholar]
- 29.Altug-Atac AT, Erdem D. Prevalence and distribution of dental anomalies in orthodontic patients. Am J Orthod Dentofacial Orthop. 2007;131:510–514. doi: 10.1016/j.ajodo.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Wallace C, Clayton D, Fine P. Estimating the relative recurrence risk ratio for leprosy in Karonga District, Malawi. Lepr Rev. 2003;74:133–140. [PubMed] [Google Scholar]
- 31.Basdra EK, Kiokpasoglou MN, Komposch G. Congenital tooth anomalies and malocclusions: a genetic link. Eur J Orthod. 2001;23:145–151. doi: 10.1093/ejo/23.2.145. [DOI] [PubMed] [Google Scholar]
- 32.Lapter M, Slaj M, Skrinjaric I, Muretic Z. Inheritance of hypodontia in twins. Coll Antropol. 1998;22:291–298. [PubMed] [Google Scholar]
- 33.Markovic M. Hypodontia in twins. Swed Dent J Suppl. 1982;15:153–162. [PubMed] [Google Scholar]