Abstract
Background:
As transmasculine persons utilize androgen gender-affirming hormone therapy as a part of transition, guidance has been lacking on the effects of therapy on the ovaries, especially for those who may desire retention.
Aim:
To describe the ovarian histopathology of transmasculine persons on testosterone therapy following oophorectomy at the time of hysterectomy preformed for gender affirmation.
Methods:
This was a multicenter case series of transmasculine patients on testosterone therapy who underwent hysterectomy with oophorectomy for gender affirmation between 1/2015 and 2/2017 at 5 tertiary care referral centers. Patients were identified by their current procedural and ICD codes.
Outcomes:
Pre-, perioperative and pathologic data were queried from the electronic medical record (EMR) and ovarian tissue descriptions from pathology reports were grouped into the following classifications: 1) simple/follicular cysts; 2) polycystic ovaries; 3) complex cysts; 4) endometriomas; 5) other masses; 6) atrophy; and 7) normal.
Results:
85 patients were included in the study. At the time of oophorectomy, the mean age and body mass index (BMI) of the cohort were 30.4 ± 8.4 years and 30.2 ± 7.3 kg/m2, and the average interval from initiation of testosterone to oophorectomy was 36 .3 ± 37.9 months. On examination of ovarian histopathology 49.4% (42) of specimens were found to have follicular/simple cysts, 5.9% (5) were polycystic, and 38.8% (33) had normal pathology. For those specimens with volume documented (n=41), the median volume was 9.6 (range 1.5–82.5) cm3. There was no association between the duration of testosterone therapy or BMI and the presence of cysts on the ovaries.
Clinical Implications:
The results of this study find benign histopathology in ovaries of a large cohort of transmasculine persons on testosterone which should be included when counseling patients on ovarian retention, as transmasculine patients may choose to retain their ovaries while on testosterone for a variety of reasons (including no desire to undergo surgery, desire for backup sex steroids, and potential use for future fertility),.
Strengths & Limitations:
This is a large multicenter study seeking to address the uncertainty in present counseling surrounding ovarian conservation in transmasculine persons on testosterone. This study is limited by its retrospective nature and inability to address ovarian function after testosterone discontinuance.
Conclusions:
In this cohort of transmasculine patients on testosterone therapy undergoing hysterectomy with oophorectomy for gender affirmation, ovarian histopathology is benign in all the specimens.
Keywords: transmasculine, transmale, transgender man, fertility, ovarian pathology, ovarian histology, testosterone, polycystic ovaries, female to male
Introduction
As a part of transition, many transmasculine persons (transgender men and gender nonbinary persons assigned female at birth) utilize androgen gender-affirming hormone therapy.1 Reproductive health counseling regarding the ovary for transmasculine persons has historically centered on fertility preservation counseling prior to the initiation of hormones, as present guidelines note that little is known regarding the effect of this therapy on the potential for future biologic children2,3. These guidelines have also recommended total hysterectomy and bilateral salpingo-oophorectomy due in part to concerns that transmasculine persons are unlikely to undergo cancer screening and there is some concern for increased risk of endometrial cancer after testosterone therapy (there has also been theoretical belief that removal of ovaries would aid in virilization). 2,4 Additionally numerous transmasculine persons seek gender affirming hysterectomies, including as a prerequisite for genital affirmation surgeries, and these hysterectomies have often included oophorectomy by default. 1,5–8
With rapid evolution of transgender reproductive health however, a more diverse picture has emerged regarding ovarian utilization and retention. While historically there were concerns that testosterone could negatively impact ovarian function and long-term fertility, a growing body of evidence suggests that testosterone may not suppress ovarian function to the level previously thought. Research has shown ovulation may not be completely suppressed on testosterone; persons have also had successful reports of ovulation induction as well as pregnancies after testosterone usage suggesting fertility after discontinuation of testosterone may not be as greatly impaired as previously suggested.9–11 Studies also show that while low rates of transmasculine youth desire genetically linked offspring or utilization of fertility preservation, the number who desire genetically related children increases in older populations. Additionally, no evidence has arisen to show increased ovarian cancer rates in this population. Testosterone gender-affirming hormone therapy is also known to well suppress endogenous estrogen and as such no present guideline recommends removal of ovaries to add in the virializing process..9,12,2,13–15 Additionally, while some transmasculine persons may desire hysterectomy, not all do, and as such many may retain ovaries which are exposed to testosterone. Lastly, there is evidence that access to lifelong testosterone gender affirming therapy, while desired by many, is not guaranteed due to cost, health care access, insurance coverage, and that the ovaries could serve as a backup source of sex steroids. 1,8,16,17
Due to the above data, there is growing call to reconsider the focus of early fertility preservation counseling and removal of ovaries at time of hysterectomy, and to expand it to include counseling on longer ovarian retention with potential for future use. With this call emerges a need to better understand the expected changes ovaries will undergo while on testosterone, to inform counseling of patients who are considering retention. A common barrier identified by those reporting a desire for pregnancy or biologic children in their future, including after initiating testosterone, is a lack of counseling on the effects of long-term testosterone on the ovaries.9,17–19 As such better counseling is needed, so that patients can make informed choices regarding ovarian retention with potential for future utilization. This includes understanding the expected histopathologic changes, such as cyst or mass formation or atrophy, as aspects beyond fertility-centered outcomes are presently minimally addressed in the literature regarding the ovaries on gender affirming hormones.
The objective of this study is to describe the characteristics of ovarian histopathology after the initiation of testosterone in a large cohort of transmasculine persons. Such information may further assist in guiding provider counseling of transmasculine patients with regards to the ovary for retention while on testosterone. Our hypothesis was that ovaries would have normal or benign pathology which would allow patients to consider ovarian retention while on testosterone gender affirming hormone therapy.
Methods
This study is a retrospective, multicenter cohort study at five tertiary care referral centers, approved by the Institutional Review Boards at the University of Kansas School of Medicine (Kansas City, KS), the University of South Florida (Tampa, Florida), MedStar Washington Hospital Center (Washington, District of Columbia), Cleveland Clinic (Cleveland, Ohio), and MetroHealth Medical Center (Cleveland, Ohio).
Methodology was as previously described in our publication on uterine pathology.20 Briefly, all transmasculine patients age 18 years and older who underwent hysterectomy with oophorectomy following administration of exogenous testosterone from 1/2015–12/2017 were included. Patients were identified by their Current Procedural Terminology codes and their International Classification of Diseases (ICD) 9 and 10 codes. Once patients were identified, the system-wide electronic medical record was queried for demographics, pre- and perioperative data. Preoperative data included medical, surgical, obstetric, gynecologic history, concomitant medication use and body mass index (BMI), as well as most recent total testosterone and estradiol levels prior to surgery. Length of testosterone therapy prior to surgery was recorded as number of months of therapy. Perioperative data included pre and postoperative diagnosis and any surgical complications.
Pathologic data for all ovarian specimens were obtained from pathologic reports from each institution. The size and weight of the ovarian tissue were recorded as well as ovarian tissue description, with descriptions provided by reviewing pathologist grouped into the following classifications: 1) simple/follicular cysts; 2) polycystic appearing ovaries (PCAO), defined by reviewing pathologist)21; 3) complex cysts; 4) endometriomas; 5) other masses (defined as solid masses on the ovary); 6) atrophy (defined by reviewing pathologist)21; and 7) normal. When available, pre-operative pelvic ultrasound reports, performed while the patient was on testosterone, were reviewed for assessment of radiologic ovarian findings.
Chart abstraction was performed by the study team. Study data were collected and managed using REDCap electronic data capture hosted at the University of Kansas Medical Center and MedStar Washington Hospital Center.22,23
Statistical analysis consisted of descriptive statistics to tabulate means, ranges, standard deviations, and 95% confidence intervals. Using IBM SPSS v24, Mann Whitney U tests were utilized to assess for differences in duration of testosterone use or BMI between those who had cysts and those who did not, logarithmic regression was used to assess for the influence of duration of testosterone on development of PCAO on pathology.24 Analysis of covariance (ANCOVA) was used to assess the relationship between time on testosterone and mean ovarian volume, when controlling for BMI and age. In the case of ANCOVA testosterone duration was grouped; the grouping and rationale are as described in our previous publication on uterine pathology and listed in Table 1.20
Table 1:
Testosterone grouping for statistical analysis
| Groupings | Rationale | |
|---|---|---|
| Testosterone duration | 0–1 years >1 – 2 years >2 – 4 years >4 years |
Most patients achieve amenorrhea within 1 year of testosterone gender affirming hormone therapy.2 Virilization effects of testosterone can take 2–5 years to reach maximum effect.3 |
Pelusi C, Costantino A, Martelli V. Effects of three different testosterone formulations in female-to-male transsexual persons. J Sex Med 2014; 11(12):3002–11
Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int J Transgenderism. 2012;13(4):165–232
Results
During the study period, 94 patients underwent hysterectomy, and of these patients, 85 had concomitant oophorectomy (84 (98.8%) bilateral, 1 (1.2%) unilateral), which were thus included in this analysis. Complete histopathologic data and length of testosterone therapy was available for all patients.
Patient characteristics are summarized in Table 2. The mean age of patients at time of oophorectomy was 30.4 ± 8.4 years, and 31.2% of patients were 25 years or younger. The majority of patients were nulliparous (78.8%, n=67) and 23 (27.1%) had a documented history of menstrual irregularities including polycystic ovarian syndrome (PCOS) or abnormal uterine bleeding prior to initiation of testosterone. No patients in this cohort underwent puberty suppression with gonadotropin releasing hormone (GnRH) analogs prior to starting testosterone therapy.
Table 2:
Demographics
| Variable | N (%) |
|---|---|
|
| |
| Age (mean +/− STD) | 30.4 +/− 8.4 |
|
| |
| Race | |
| Hispanic | 0 (0) |
| Non-Hispanic White | 39 (45.9) |
| Non-Hispanic Black | 5 (5.9) |
| Asian Pacific Islander | 0 (0) |
| American Indian | 0 (0) |
| Other | 0 (0) |
| Missing = 41 | |
|
| |
| BMI (kg/m2) | |
| <18.5 | 2 (2.4) |
| 18.5–25.0 | 22 (25.9) |
| 25.1 – 30.0 | 21 (24.7) |
| 30.1–40.0 | 32 (37.6) |
| >40.0 | 8 (9.4) |
|
| |
| Comorbidities | |
| Mental Health Diagnosis | 27 (31.8) |
| Hypothyroid | 10 (11.8) |
| Endometriosis | 7 (8.2) |
| Hypertension | 6 (7.1) |
| Hyperlipidemia | 6 (7.1) |
| Asthma | 5 (5.9) |
| Type II Diabetes | 5 (5.9) |
|
| |
| Duration of Testosterone Use | |
| 0–1 years | 22 (25.9) |
| >1 – 2 years | 27 (31.8) |
| >2 – 4 years | 16 (18.8) |
| >4 years | 20 (23.5) |
|
| |
| Type of Testosterone Used | |
| Intramuscular (IM) Cypionate | 72 (84.7) |
| Subcutaneous Cypionate | 9 (10.6) |
| IM Sustanon | 1 (1.2) |
| Transdermal | 2 (2.4) |
|
| |
| Concomitant Hormonal Use | |
| Combined oral contraceptives | 9 (10.6) |
| Depo-medroxyprogesterone | 7 (8.2) |
| Levonorgestrel 52mg | 2 (2.4) |
| Intrauterine device | 1 (1.2) |
| Missing = 76 | |
|
| |
| Ovarian Histopathology | |
| Atrophic | 3 (3.5) |
| Simple Cysts | 42 (49.4) |
| Polycystic | 5 (5.9) |
| Complex Cysts | 0 (0) |
| Endometriomas | 4 (4.7) |
| Ovarian Mass | 1 (1.2) |
| Normal Ovaries | 33 (38.8) |
The mean interval from initiation of testosterone gender affirming hormone therapy to oophorectomy was 36.3 ± 37.9 months. Types of testosterone used are listed in Table 2. Within a year prior to surgery, 45 patients had documented testosterone levels. The mean level was 567.7 ± 341.5 ng/dL. Estradiol levels within a year prior to surgery were available in 30 patients. The mean level was 45.3 ± 29.8 pg/mL.
Of the 22 patients with documented ultrasounds prior to hysterectomy, the most common (36.4% (8)) finding was normal appearance, with ovarian morphology listed in Table 3 along with their associated histopathologic findings.
Table 3:
Ovarian Classification on Ultrasound and Correlative Pathologic Findings
| Ovarian Histopathology* | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Classification (n=22) | N (%) Total |
Atrophic | Simple Cysts | Polycystic | Ovarian Mass | Normal Ovaries |
|
| ||||||
| Normal Ovaries | 8 (36.4) | 1 | 1 | 6 | ||
| Atrophic | 5 (22.7) | 1 | 3 | 1 | ||
| Multifollicular | 4 (18.2) | 1 | 1 | 2 | ||
| PCAO | 2 (9.1) | 2 | ||||
| Not Visualized | 3 (13.6) | 1 | 2 | |||
Only histopathology from those who had US performed is included
Of the 85 ovarian pathology specimens, histopathology was documented as normal in 33 (38.8%), simple/follicular cysts in 42 (49.4%), PCAO in 5 (5.9%), endometriomas in 4 (4.7%), and benign fibroma in 1 (1.2%). There were no complex cysts documented. There were 3 patients (3.8%) with atrophic ovaries documented on pathology (none of whom had ovarian volume documented); their ages ranged from 37–41 years old. Three of these specimens had more than one histopathological finding documented (two with both endometriomas and simple cysts, one with atrophy and simple cysts). There was no difference in distribution of duration of testosterone usage (U=1.33, p=0.19) , or BMI (U=−0.95, p=0.34) between those who did and did not have ovarian cysts. Duration of testosterone was additionally not found to significantly predict PCAO on pathology (OR = 0.995 (95% CI 0.965–1.025) p=0.701).
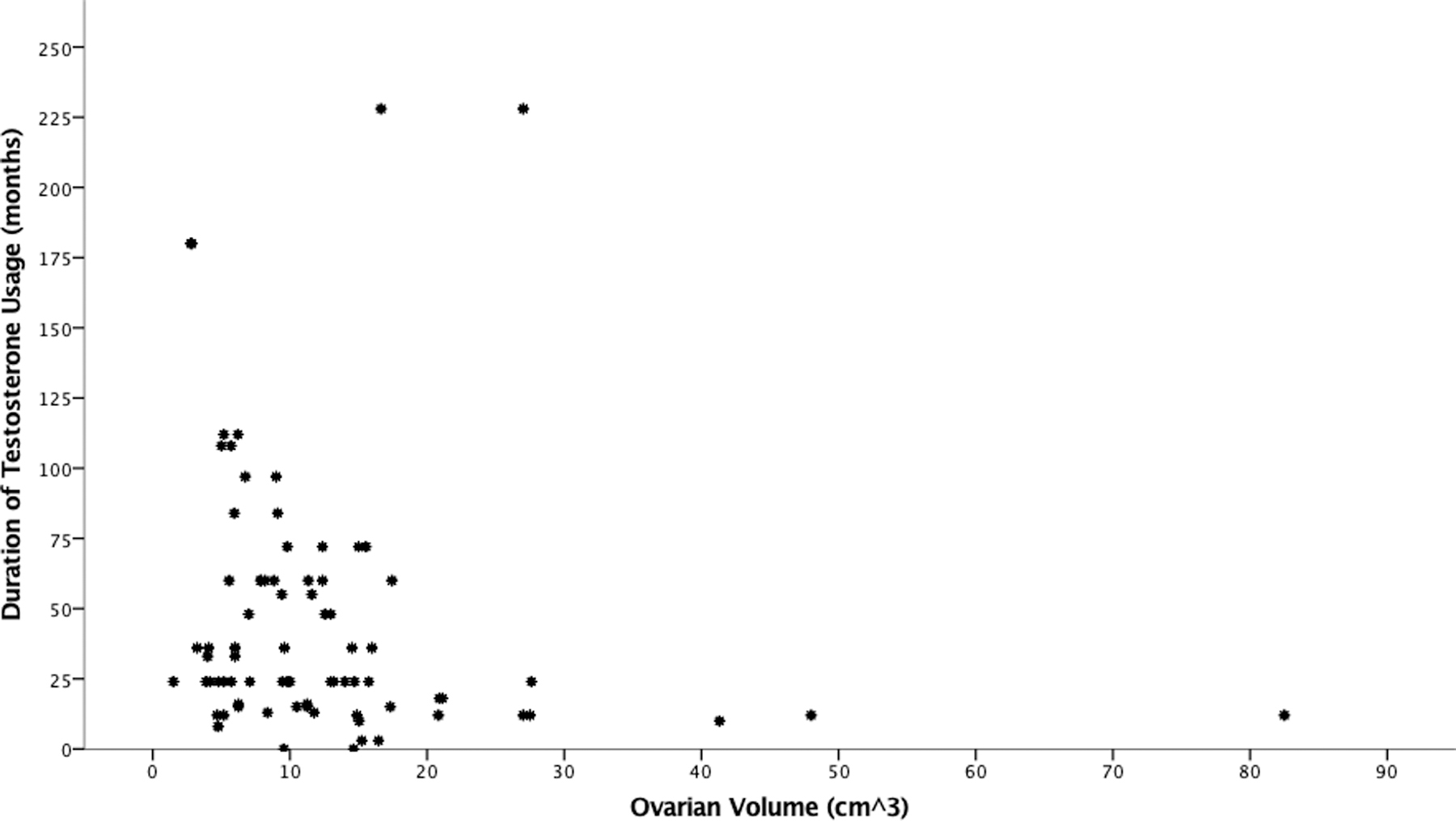
41 patients had ovarian volume documented (with one person having a unilateral oophorectomy for a total of 81 ovaries). The median was 9.6cm3 (Range 1.5–82.5). Of those ovaries that did not have any masses or simple cysts, 24 (33.3%) were greater than 10cm3. Ovarian volume compared to duration on testosterone is seen in Figure 1. An analysis of covariance (ANCOVA) was used to assess whether increasing duration of testosterone is associated with decreasing ovarian volume when controlling for differences in BMI and age. Prior to the ANCOVA analysis increasing duration of testosterone was statistically significantly associated with decreasing ovarian volume (F (3,37)=3.5160, p=0.024). Mean+/− standard deviation of each groups volume was as follows: 0–1 year (n=8, 22.0 ± 19.6cm3), >1–2 years (n=13, 11.0 ± 5.6cm3), >2–4 years (n=7, 8.2 ± 4.2cm3), >4 years (n=13, 9.8 ± 4.7cm3). A tukey post hoc analysis found increase in duration from 0–1 year to >2–4 and >4 years to have significant decreases in volume (mean differences −13.8cm3, p=0.041 and −12.2cm3, p=0.037 respectively). When controlling for BMI and Age, the difference in ovarian volumes between the groups remained significant (mean values: 21.8 ± 3.5 cm3, 10.1 ± 2.8 cm3, 9.8 ± 3.9 cm3, 9.9 ± 2.7 cm3; F(3,37)=3.801, p=0.04).
Figure 1:
Ovarian Volume compared to Duration of Testosterone Usage
Discussion
Ovaries are commonly removed at the time of hysterectomy for gender affirmation in transmasculine persons on testosterone, and current guidelines do not discourage this practice.15,25,26 This practice is related to numerous beliefs, including that ovaries are no longer needed with the use of exogenous sex steroids, that fertility is impaired by testosterone, as well as that there may be cancer risk and these individuals would fail to undergo cancer screening. 2,15,27 Given emerging data that shows the ability to utilize ovaries after undergoing gender affirming hormone therapy, and the knowledge that not all transmasculine persons may have lifelong access to testosterone or desire to undergo hysterectomy and oophorectomy, it is important to provide counseling on the expected ovarian changes while on gender affirming hormone therapy for those who desire to retain them.
This study reveals that ovarian pathology remains benign while on testosterone. In general, ovaries in this cohort were within range for reproductive age ovaries and noted to have benign spectrum of pathology including folliculogenesis, and simple cysts. None of the ovaries had pathology which would have required oophorectomy in a reproductive age cisgender female.28,29 A significant inverse correlation was noted between duration of testosterone use and ovarian volume. Additionally, four patients in our cohort had endometriomas, which would be unusual in persons who had complete ovarian suppression, further supporting persistent activity.30 There were no neoplasms noted. Lastly, three of the older patients (37–41) had pathologically atrophic ovaries. While these persons are on the older end of our cohort they are still young to have atrophic ovaries as they are younger than common ages of menopause, and all of them had durations of testosterone use below the mean (12–36 months).31,32
Past studies, the majority with small numbers, have reported diverse morphologic changes of ovarian tissue in this population, summarized in Table 4. Multicystic and PCAO appear to be the predominate histopathology in certain studies (68.4–100%) while others focused on larger numbers of atretic follicles.12,25,33–36 Some reported increased thickness of the tunica albuginea and collagenous layer as well as stromal hyperplasia, similar to those with PCOS. 12,34–36 In addition to these morphologic changes, other studies have focused on follicular development given its involvement in fertility. Lierman et al noted the majority of cumulus-oocyte-complexes harvested from oophorectomies of persons undergoing gender affirming surgery after testosterone gender affirming hormone therapy had normal early follicles, spindle structure and chromosomal alignment, but had minimal secondary antral follicles which was suggestive of minimal follicular maturation while on testosterone.26.While our cohort did not address follicular maturation, numerous functional cysts were found suggestive of ovarian activity.
Table 4:
Comparison of studies on ovarian histopathology of transmasculine persons on testosterone
| Study Title | Type | Number of subjects | Age of Subjects (years) FN1 | Duration of Testosterone Use (months) FN1,FN2 | Ovarian Volume (cm3) FN3 | Number of Follicles FN4 | Follicular Histology | Stromal Histology | Additional findings |
|---|---|---|---|---|---|---|---|---|---|
| Futterweit et al (JCEM 1986) | Retrospective analysis of histologic specimens after hystrectomy-salpingoopherectomy (HSO) | 19 | 26.8 ± 6.6 (20–45) | 37.2 ± 25.2 (12–120) | 89.5%: multiple cystic follicles 68.4%: histological features consistent with polycystic ovarian morphology (PCOM) |
68.4% thickened collagenized cortex 84.2% stromal hyperplasia 26.3% scattered luteinized stromal cells 36.8% luteinized theca |
42.1% hirsute, 26.3% abnormal uterine bleeding (AUB) prior to testosterone | ||
| Amirikia (Fert and Steril 1986) | Prospective analysis of histologic specimens after HSO | 10 | 29.4 ± 1.6 FN5 | 35 ± 20.2 (14–84) | 7.9 ± 1.2 | primordial: 9.9 ± 2.3 per 100 power field atretic: 2.6 ± 0.5 cystic: 2.0 ± 0.4 |
0% theca hyperplasia | 2 subjects reported AUB prior to testosterone |
|
| Spinder et al (JCEM 1989) | Retrospective analysis of histologic specimens after HSO | 26 | 26 (19–37) FN6 | 17.6± 6 ( 9–36) | 69.2%: multicystic 69.2%: consistent with PCOM | 96.2%: collagenization of tunica 80.8%: stromal hyperplasia 69.2%: luteinized theca |
|||
| Pache et al (Histopath 1991) | Retrospective analysis of histologic specimens after HSO | 17 | 25 (18–35) FN6 | 21 (11–72) FN6 | 7.6±3.6 | antral, cystic, atretic: 27 ± 13 atretic only: 17 ± 11 |
96.6%: primordial follicles 62.1%: healthy antral follies 100%: multiple cystic atretic follicles |
100%: stromal hyperplasia 41.4%: clustered luteinized stromal cells 96.6%: thickened collagenized cortex 100%: theca hyperplasia “Many” leutenized theca |
|
| Grynberg et al (Repro Biomed Online 2010) | Retrospective analysis of histologic specimens after bilateral mastectomy and HSO | 112 | 28.9 ± 0.9 FN5 | 44.4 ± 7.2 FN5 | Right: 10.9 ± 0.68 Left: 10.1 ± 0.66 |
Antral, cystic and atretic: 16.5 ± 0.62 | 79.5%: consistent with PCOM | Stromal hyperplasia | 12% had AUB prior to testosterone |
| Ikeda et al (Hum Repro 2012) | Retrospective analysis of histologic specimens after HSO | 11 | 33.2 ± 6.0 (26–42) | 70 ± 55 (17–164) | primordial, primary preantral and early antral and aetretic: 14.16 per cm2 | Aetretic: High density primodial, primary, pre-antral and early antral: equivocal to persons without testosterone exposure |
90.9% stromal hyperplasia 72.7% leutenized theca |
2 had PCOM prior to testosterone (didn’t meet clinical criteria for polycystic ovarian syndrome (PCOS) | |
| Loverro Et al (Tiawan J of obs and Gyn 2016) | Prospective analysis of histologic specimens after HSO | 12 | 28.1 ± 6.6 (20–32) | 31.9 ± 14.3 | 83.3% multifolicular | ||||
| Lierman et al (JARG 2017 | Prospective analysis of cumulus-oocyte-complex MII stage oocytes following HSO | 16 (680 COCs, 38.1% survived MII after IVM) | 24.1 ± 6.1 FN5 | 12.5 ± 4.9 FN5 | normal spindle shape + chromosome alignment 92.2% of vitrified group 87.1% of non-cryopreserved |
||||
| Khalifa et al (IJ Gyn Path 2018) | Retrospective analysis of histologic specimens after HSO | 23 | 29 ± 7.7 (20–46) | 19–288 FN7 | All follicles (excluding secondary) mean 0.7/mm2 (Range 1.5–32.5) |
100% multiple follicular cysts | one had congenital adrenal hyperplasia 2 had a teratoma 2 had AUB |
||
| Grimstad Et al (current study) | Retrospective analysis of histologic specimens after HSO | 85 | 30.4± 8.4 (18–53) | 36.3 ± 37.9 (1–228) | Median 9.6cm3 (1.5–82.5) | 49.4%: simple cysts 5.9%: PCOM 4.7%: endometriomas 3.5%: atrophy 1.2%: benign fibroma FN8 |
27.1%, had AUB +/− PCOS |
Footnotes
unless noted data is presented as mean ± standard deviation (range)
some studies used mix of years/weeks; all were converted to months
unless noted data is presented as mean ± standard deviation (SD)
unless noted data is presented as Mean ± SD per ovary
range not available
SD not available
No mean or SD available. Range includes one person who did not have a bilateral salpingoopherecotmy
3 of these specimens had more than one histology documented (two with both endometriomas and simple cysts, one with atrophy and simple cysts
While past studies have suggested PCAO morphology from testosterone, our study found a significant inverse correlation between duration of testosterone use and ovarian volume. However, one third of the ovaries without pathology had ovarian volume greater than 10 cc, which is consistent with PCAO. The large percentage of enlarged volume of ovaries may reflect early polycystic changes with exposure to testosterone with progressive decrease in volume with prolonged exposure. Only a few of the prior mentioned studies reported on volumetric changes, and despite differences in documented mean volumes (7.6–10.9 cm3), all fell within range for reproductive age cisgender females.5,36,37 The two largest of all of these studies, Gyrnberg et al and our own, did find volumes which trended closer to the cutoff for PCOS (10cm3). This is not surprising given the androgenic aspects of PCOS, as well as some data which suggests increased incidence of PCOS in transmasculine persons.38,39 It should be noted that PCAO in the setting of hyperandrogenism has not been associated with an increase in cancer risk, and reversibility of fertility impairment has been noted; in both persons with PCOS and congenital adrenal hyperplasia (CAH) decreasing hyperandrogenemia is associated with increasing pregnancy potential.40,41,42 While there are limitations in comparing hyperandrogenism in PCOS, CAH and transmasculine persons on testosterone (including type of androgen, source, and timing and duration of exposure, as well as variations in other reproductive hormones in these conditions) the aggregate evidence supports persistence of benign pathology in ovarian tissue exposed to hyperandrogenism.42–49
Recommendations to reevaluate routine oophorectomy in transmasculine persons have already begun to emerge.6 This is due to a number of reasons. First, access to gender affirming hormone therapy, even for those who plan to be on it for life, is not guaranteed due to a number of variables including trans health clinician prevalence, geographic limitations and insurance coverage.1 If retained, these ovaries may protect individuals from the effects of hypogonadism on bone and heart if the individual is unable to obtain testosterone therapy for a prolonged period of time without significant feminization .1 Ovarian conservation does not appear to significantly impede virilization, and most visualized virilization effects are permanent and will not be undone by testosterone discontinuance and estrogen reactivation.15 Second, in the literature on transmasculine persons or other hyperandrogenic conditions, there does not appear to be an increased incidence of ovarian cancer and to date there is no method of screening for ovarian cancer.50,51 As prophylactic oophorectomy is not practiced for average risk cisgender females of reproductive age, counseling on this should be reconsidered in transmasculine persons. Of note, in this cohort, individuals who chose to only undergo hysterectomy without oophorectomy, salpingectomy was performed for protection from malignancy.
Furthermore, the fertility intentions of transmasculine patients are diverse. Current literature estimates nearly half of transgender and gender nonbinary adults express a desire for biological children, some of whom may desire to have biologically related children after initiation of testosterone.9,11,52,53 As desires around family building can change over the course of a person’s life, ovarian conservation offers transmasculine patients an additional option should they be unable to access, or have yet to decide upon the utilization of fertility preservation. Additionally, fertility preservation techniques (e.g. oocyte and embryo preservation, or ovarian tissue cryopreservation) are invasive, carry risk, and have yet to become universally accessible, in part due to cost. Ovarian tissue cryopreservation is additionally still experimental and to date all pregnancies achieved with this technique have required autotransplantation of the tissue.54–57 Ovarian conservation, even if hysterectomy is performed, offers patients the opportunity to make fertility decisions after the initiation of testosterone. 9,17–19 The majority of our findings did not show pathology which would permanently limit ovarian utilization for future pregnancy. Histologic atrophy was seen in three patients who were younger than the expected age range for atrophy in cisgender women which might suggest that those on testosterone should be counseled on the possibility that atrophy could occur earlier than expected postmenopausal ages.32 These patients were all on testosterone for less than the mean duration of our cohort, thus increasing testosterone duration did not appear to linked to risk of atrophy; further studies will be needed to better understand risk factors which may lead to atrophy on testosterone.
A limitation of a number of the studies of transmasculine persons, including our own, is the lack of control data. This includes control data comparing to age matched controls (as ovaries are typically left in situ during benign hysterectomies per guidelines, and age matched oncology patients cannot be used as a control due to increased risk for ovarian pathology) as well as control data comparing the changes seen in ovarian morphology and function on testosterone to individual baseline data prior to testosterone use (e.g. we cannot identify if the PCAO in our cohort were also polycystic prior to testosterone).50 Two small studies have suggested increased incidence in PCOS (36.4–50%) in the transmasculine population prior to testosterone, reinforcing the need for serial longitudinal analysis of interval changes utilizing ultrasound or minimally invasive techniques such as biopsy.38,58 Indication bias is another limitation as these patients all presented for gender affirming hysterectomies, not for alternative complaints (e.g. pain) and thus were more likely to have normal ovaries. Additional limitations of our study include the retrospective nature, missing data, the fact that histopathology was evaluated by a variety of pathologists and inability to ascertain fertility potential based on pathology alone.
Strengths of this study include its large multicenter nature, diverse range of exposure to testosterone, and inclusion of serum, radiographic and pathologic data.
Current literature has shown that transgender and gender diverse persons may desire to retain their ovaries for future use after the initiation of gender affirming hormone therapy, including for fertility (both for oocyte retrieval or personal-pregnancy) and possible backup sex steroids, which requires clinicians to be able to counsel on expected ovarian changes on gender affirming hormone therapy.9,17 Emerging evidence in cisgender women support additional benefits to ovarian retention.50 Based on this cohort, the majority pathologic descriptions of physiologic follicles, simple cysts, and PCAO in this large retrospective cohort suggests both ongoing physiologic activity and no evidence of increased malignant pathology despite long-term exogenous testosterone exposure.
Acknowledgements:
The authors would like to acknowledge Dr. Monica Gaddis for assistance with statistical analysis as well as Dr. Lauri Hochberg for her guidance and ultrasound expertise.
Findings presented at:
1. 32nd Annual National Association for Pediatric and Adolescent Gynecology Clinical Meeting (April 12–14, 2018, West Palm Beach, FL)
2. 2017 University of California, San Francisco National Transgender Health Summit (November 11–12, 2017, Oakland, CA)
3. 35th Annual Conference on Lesbian, Gay, Bisexual, and Transgender Health by the Gay and Lesbian Medical Association (September 13–16, 2017, Philadelphia, PA)
4. 2017 Philadelphia Trans Health Conference (September 7–9, 2017, Philadelphia, PA)
5. 2nd Annual University of Kansas Department of Obstetrics and Gynecology Research Forum (May 15th, 2017, Kansas City, KS)
Footnotes
Disclosure: The authors report no conflicts of interest or financial support.
Twitter: A large multi-site cohort of transmasculine persons reveals ovarian pathology is benign on testosterone gender affirming hormone therapy.
Handles: @Frances.Grimstad, @kylieerrr, @Gomezlobopagdoc, @CFerrandoMD
Bibliography
- 1.James SE, Herman JL, Keisling M, Mottet L, Anafi M. The Report of the 2015 US Transgender Survey. Washington, D.C.: National Center for Transgender Equality; 2016. [Google Scholar]
- 2.Coleman E, Bockting W, Botzer M, et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. International Journal of Transgenderism. 2012;13(4):165–232. [Google Scholar]
- 3.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al. Endocrine Treatment of Transsexual Persons:An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(9):3132–3154. [DOI] [PubMed] [Google Scholar]
- 4.Unger CA. Care of the transgender patient: the role of the gynecologist. American Journal of Obstetrics & Gynecology. 2014;210(1):16–26. [DOI] [PubMed] [Google Scholar]
- 5.Grynberg M, Fanchin R, Dubost G, et al. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reprod Biomed Online. 2010;20(4):553–558. [DOI] [PubMed] [Google Scholar]
- 6.Reilly ZP, Fruhauf TF, Martin SJ. Barriers to Evidence-Based Transgender Care: Knowledge Gaps in Gender-Affirming Hysterectomy and Oophorectomy. Obstet Gynecol. 2019;134(4):714–717. [DOI] [PubMed] [Google Scholar]
- 7.Obedin-Maliver J, Light A, de Haan G, Jackson RA. Feasibility of Vaginal Hysterectomy for Female-to-Male Transgender Men. Obstetrics & Gynecology. 2017;129(3):457. [DOI] [PubMed] [Google Scholar]
- 8.Grimstad F, Boskey E. Empowering transmasculine youth by enhancing reproductive health counseling in the primary care setting. Journal of Adolescent Health. Accepted Manuscript 2020. [DOI] [PubMed]
- 9.Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127. [DOI] [PubMed] [Google Scholar]
- 10.Adeleye AJ, Cedars MI, Smith J, Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet. August 2019. [DOI] [PMC free article] [PubMed]
- 11.Leung A, Sakkas D, Pang S, Thornton K, Resetkova N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril. October 2019. [DOI] [PubMed]
- 12.Ikeda K, Baba T, Noguchi H, et al. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28(2):453–461. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch MB, Bhakri V, Kubicek K. Effects of Cross-Sex Hormone Treatment on Transgender Women and Men. Obstet Gynecol. 2015;125(3):605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UCSF Transgender Care, Department of Family and Community Medicine, University of California San Francisco. Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People. 2nd ed. Deutsch MB, ed; 2016. transcare.ucsf.edu/guidelines.
- 15.Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. [DOI] [PubMed] [Google Scholar]
- 16.White Hughto JM, Rose AJ, Pachankis JE, Reisner SL. Barriers to Gender Transition-Related Healthcare: Identifying Underserved Transgender Adults in Massachusetts. Transgender Health. 2017;2(1):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Matson M, Macapagal K, et al. Attitudes Toward Fertility and Reproductive Health Among Transgender and Gender-Nonconforming Adolescents. J Adolesc Health. 2018;63(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingo E, Ingraham N, Roberts SCM. Reproductive Health Care Priorities and Barriers to Effective Care for LGBTQ People Assigned Female at Birth: A Qualitative Study. Womens Health Issues. 2018;28(4):350–357. [DOI] [PubMed] [Google Scholar]
- 19.von Doussa H, Power J, Riggs D. Imagining parenthood: the possibilities and experiences of parenthood among transgender people. Cult Health Sex. 2015;17(9):1119–1131. [DOI] [PubMed] [Google Scholar]
- 20.Grimstad FW, Fowler KG, New EP, et al. Uterine pathology in transmasculine persons on testosterone: a retrospective multicenter case series. Am J Obstet Gynecol. December 2018. [DOI] [PubMed]
- 21.Mills SE. Histology for Pathologists. 4th ed.. Philadelphia: Wolters Kluwer; 2012. [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SPSS Inc. SPSS Statistics for Mac. Armonk, NY: IBM Corp.; 2017. [Google Scholar]
- 25.Khalifa MA, Toyama A, Klein ME, Santiago V. Histologic Features of Hysterectomy Specimens From Female-Male Transgender Individuals. Int J Gynecol Pathol. September 2018. [DOI] [PubMed]
- 26.Lierman S, Tilleman K, Braeckmans K, et al. Fertility preservation for trans men: frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J Assist Reprod Genet. 2017;34(11):1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger CA. Care of the transgender patient: a survey of gynecologists’ current knowledge and practice. J Womens Health (Larchmt). 2015;24(2):114–118. [DOI] [PubMed] [Google Scholar]
- 28.Alammari R, Lightfoot M, Hur H-C. Impact of Cystectomy on Ovarian Reserve: Review of the Literature. J Minim Invasive Gynecol. 2017;24(2):247–257. [DOI] [PubMed] [Google Scholar]
- 29.Emans SJ, Laufer MR. Emans, Laufer, Goldstein’s Pediatric and Adolescent Gynecology. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 30.Rana N, Thomas S, Rotman C, Dmowski WP. Decrease in the size of ovarian endometriomas during ovarian suppression in stage IV endometriosis. Role of preoperative medical treatment. J Reprod Med. 1996;41(6):384–392. [PubMed] [Google Scholar]
- 31.Laszczyńska M, Brodowska A, Starczewski A, Masiuk M, Brodowski J. Human postmenopausal ovary--hormonally inactive fibrous connective tissue or more? Histol Histopathol. 2008;23(2):219–226. [DOI] [PubMed] [Google Scholar]
- 32.McKinlay SM. The normal menopause transition: an overview. Maturitas. 1996;23(2):137–145. [DOI] [PubMed] [Google Scholar]
- 33.Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691. [DOI] [PubMed] [Google Scholar]
- 34.Spinder T, Spijkstra JJ, van den Tweel JG, et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab. 1989;69(1):151–157. [DOI] [PubMed] [Google Scholar]
- 35.Futterweit W, Deligdisch L. Histopathological effects of exogenously administered testosterone in 19 female to male transsexuals. J Clin Endocrinol Metab. 1986;62(1):16–21. [DOI] [PubMed] [Google Scholar]
- 36.Pache TD, Chadha S, Gooren LJ, et al. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19(5):445–452. [DOI] [PubMed] [Google Scholar]
- 37.Amirikia H, Savoy-Moore RT, Sundareson AS, Moghissi KS. The effects of long-term androgen treatment on the ovary. Fertil Steril. 1986;45(2):202–208. [DOI] [PubMed] [Google Scholar]
- 38.Becerra-Fernández A, Pérez-López G, Román MM, et al. Prevalence of hyperandrogenism and polycystic ovary syndrome in female to male transsexuals. Endocrinol Nutr. 2014;61(7):351–358. [DOI] [PubMed] [Google Scholar]
- 39.Balen AH, Laven JSE, Tan S-L, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9(6):505–514. [DOI] [PubMed] [Google Scholar]
- 40.Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo). 2015;70(11):765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 42.Reichman DE, White PC, New MI, Rosenwaks Z. Fertility in patients with congenital adrenal hyperplasia. Fertility and Sterility. 2014;101(2):301–309. [DOI] [PubMed] [Google Scholar]
- 43.Hugues J-N, Durnerin IC. Impact of androgens on fertility - physiological, clinical and therapeutic aspects. Reprod Biomed Online. 2005;11(5):570–580. [DOI] [PubMed] [Google Scholar]
- 44.Pache TD, Hop WC, de Jong FH, et al. 17 beta-Oestradiol, androstenedione and inhibin levels in fluid from individual follicles of normal and polycystic ovaries, and in ovaries from androgen treated female to male transsexuals. Clin Endocrinol (Oxf). 1992;36(6):565–571. [DOI] [PubMed] [Google Scholar]
- 45.Caanen MR, Soleman RS, Kuijper EAM, et al. Antimüllerian hormone levels decrease in female-to-male transsexuals using testosterone as cross-sex therapy. Fertility and Sterility. 2015;103(5):1340–1345. [DOI] [PubMed] [Google Scholar]
- 46.Barnes RB, Rosenfield RL, Ehrmann DA, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79(5):1328–1333. [DOI] [PubMed] [Google Scholar]
- 47.Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463. [DOI] [PubMed] [Google Scholar]
- 48.Barbieri RL, Evans S, Kistner RW. Danazol in the treatment of endometriosis: analysis of 100 cases with a 4-year follow-up. Fertil Steril. 1982;37(6):737–746. [DOI] [PubMed] [Google Scholar]
- 49.Tei C, Miyazaki T, Kuji N, Tanaka M, Sueoka K, Yoshimura Y. Effect of danazol on the pregnancy rate in patients with unsuccessful in vitro fertilization-embryo transfer. J Reprod Med. 1998;43(6):541–546. [PubMed] [Google Scholar]
- 50.Evans EC, Matteson KA, Orejuela FJ, et al. Salpingo-oophorectomy at the Time of Benign Hysterectomy: A Systematic Review. Obstet Gynecol. 2016;128(3):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106(2):219–226. [DOI] [PubMed] [Google Scholar]
- 52.De Sutter P, Verschoor A, Hotimsky A, Kira K. The desire to have children and the preservation of fertility in transsexual women: A survey. International Journal of Transgenderism. 2002;6(3):No Pagination Specified-No Pagination Specified. [Google Scholar]
- 53.Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487. [DOI] [PubMed] [Google Scholar]
- 54.Duncan FE, Zelinski M, Gunn AH, et al. Ovarian Tissue Transport to Expand Access to Fertility Preservation: from Animals to Clinical Practice. Reproduction. 2016;152(6):R201–R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Insogna IG, Ginsburg ES. Infertility, Inequality, and How Lack of Insurance Coverage Compromises Reproductive Autonomy. AMA Journal of Ethics. 2018;20(12):1152–1159. [DOI] [PubMed] [Google Scholar]
- 56.Neblett MF, Hipp HS. Fertility Considerations in Transgender Persons. Endocrinol Metab Clin North Am. 2019;48(2):391–402. [DOI] [PubMed] [Google Scholar]
- 57.Nahata L, Chen D, Moravek MB, et al. Understudied and Under-Reported: Fertility Issues in Transgender Youth—A Narrative Review. The Journal of Pediatrics. 2019;205:265–271. [DOI] [PubMed] [Google Scholar]
- 58.. Balen AH, Schachter ME, Montgomery D, Reid RW, Jacobs HS. Polycystic ovaries are a common finding in untreated female to male transsexuals. Clinical Endocrinology. 1993;38(3):325–329. [DOI] [PubMed] [Google Scholar]


