Abstract
OBJECTIVE: Disruption of intracellular Ca2+ homeostasis via excessive and pathological Ca2+ release from the endoplasmic reticulum (ER) and/or sarcoplasmic reticulum (SR) through ryanodine receptor (RyRs) Ca2+ channels play a critical role in the pathology of systemic inflammatory response syndrome (SIRS) and associated multiple organ dysfunction syndrome (MODS) in sepsis or septic shock. Dantrolene, a potent inhibitor of RyRs, is expected to ameliorate SIRS and MODS and decrease mortality in sepsis or septic shock patients. This review summarized the potential mechanisms of therapeutic effects of dantrolene in sepsis or septic shock at molecular, cell, and organ levels and provided suggestions and strategies for future clinical studies.
Keywords: Bacteria, Endotoxin, Virus, SARS-CoV-2, COVID-19, Infection, Calcium, Apoptosis, Systemic inflammation response syndrome (SIRS), Multiple organ dysfunction syndrome (MODS), Mortality
Introduction
Sepsis is defined as infected patients with an increase of ≥2 Sequential Organ Failure Score (SOFA) points, while septic shock is defined as refractory hypotension requiring vasopressors with concurrent hyperlactemia (>2 mmol/L) despite adequate fluid resuscitation1. Sepsis is the number one cause of both admission and mortality in critically ill patients in intensive care units (ICU)2–4. It affects 1.5 million patients in the US alone critically each year and leads to 250,000 deaths5. It cost public health care in the US alone 23.7 billion dollars in 20135,6. The incidence of sepsis has steadily increased during the last several decades4. Although the mortality of sepsis has decreased in recent years, it is still high – between 15–20%5. Despite the fact that the diagnosis of sepsis and septic shock has improved over the years1, new effective drugs for the treatment of sepsis or septic shock are still urgently needed. Besides new drug development based on an increased understanding of the pathological mechanisms of sepsis or septic shock, old drugs already approved by the Food and Drug Administration (FDA) can provide faster supplies of effective drugs and quicker clinical studies7.
Increasing evidence suggests that pathological Ca2+ release from the endoplasmic reticulum (ER) and/or sarcoplasmic reticulum (SR) via ryanodine receptor (RyRs) Ca2+ channels plays a critical role in systemic inflammation response syndrome (SIRS)8,9 and associated multiple organ dysfunction syndrome (MODS)10 in sepsis and septic shock. We proposed that dantrolene, an antagonist of RyRs, can be repurposed as an effective treatment for this disease11–19. This review summarized the potential mechanisms of dantrolene’s therapeutic effects in sepsis at molecular, cell, and organ levels and provided suggestions and strategies for future clinical studies.
Disruption of intracellular Ca2+ homeostasis as a primary pathology in sepsis
Sepsis is commonly triggered by pathogen-associated molecular patterns (PAMP), such as bacterial endotoxin lipopolysaccharide (LPS) or SARS-CoV-2 virus, or damage-associated molecular patterns (DAMP), such as fibronectins, small fragments of hyaluronan, and even saturated fatty acids in response to cellular damage20,21. Infection is considered a primary cause of sepsis and associated septic shock1. Increasing evidence suggests that intracellular Ca2+ dysregulation caused by abnormal Ca2+ release from the ER/SR via over activation of RyRs plays a critical role in sepsis pathology and contributes to excessive pathological inflammation termed SIRS or “cytokine syndrome” as well as associated MODS and mortality13,22–31. As demonstrated in Figure 1, we have commonly seen PAMP (e.g., LPS or SARS-CoV-2 virus spike (S) protein), bind to Toll-Like Receptor 4 (TLR-4) and then activate the nuclear factor kappa light chain enhancer of activated B cells (NF-κB) to increase nuclear transcription and cytosol production of proinflammation cytokine proteins, such as tumor necrosis factor-alpha (TNF-α)21,32,33. TNF-α is able to increase the opening of Ca2+ channels on the ER membrane known as InsP3 receptors (InsP3R)34, which in turn release Ca2+ from the ER into cytosol and trigger Ca2+ influx from extracellular space into cytosol via store-operated calcium channels (SOCCs) on the plasma membrane35–37. On the other hand, activation of TLR-4 also increases the endogenous agonist of ryanodine receptor (RyRs) Ca2+ channels on the ER membrane, Cyclic ADP-ribose (cADPR), and results in activation of RyRs8,38, amplifying the Ca2+ release from the ER/SR and elevation of cytosol Ca2+ concentration ([Ca2+]c), which further activates RyRs and leads to Ca2+ induced Ca2+ release (CICR)39. Excessive and abnormal Ca2+ release from the ER/SR via RyRs will cause ER/SR Ca2+ depletion and ER/SR stress40. It also overloads mitochondria with Ca2+ and results in increased production of reactive oxygen species (ROS)41 and/or reactive nitrate species (RNS)42, further amplifying opening of RyRs43,44. Mitochondrial Ca2+ overloading and associated damage results in impaired ATP production45, autophagy dysfunction46, and apoptosis47. A combination of ER/SR stress and mitochondrial damage eventually leads to cell and/or organ damage, which results in clinical symptoms of sepsis and multiple organ damage syndrome (MODS), and mortality (Figure 1). Additionally, the elevation of [Ca2+]c activates inducible nitric oxide synthase (iNOS) and increases the production of nitric oxide (NO), contributing to pathological vasodilation and associated septic shock, MODS, and Mortality10. Dantrolene, an FDA approved drug for the treatment of malignant hyperthermia, inhibits RyRs channel opening and ameliorates the pathological decrease of ER/SR Ca2+ and the abnormal increase of cytosol and mitochondria Ca2+ concentrations as well as associated sepsis/septic shock pathology (Figure 1)14,45. Thus, dantrolene is expected to be an effective drug to treat MODS and reduce mortality during sepsis or septic shock13,14,18.
Figure 1.
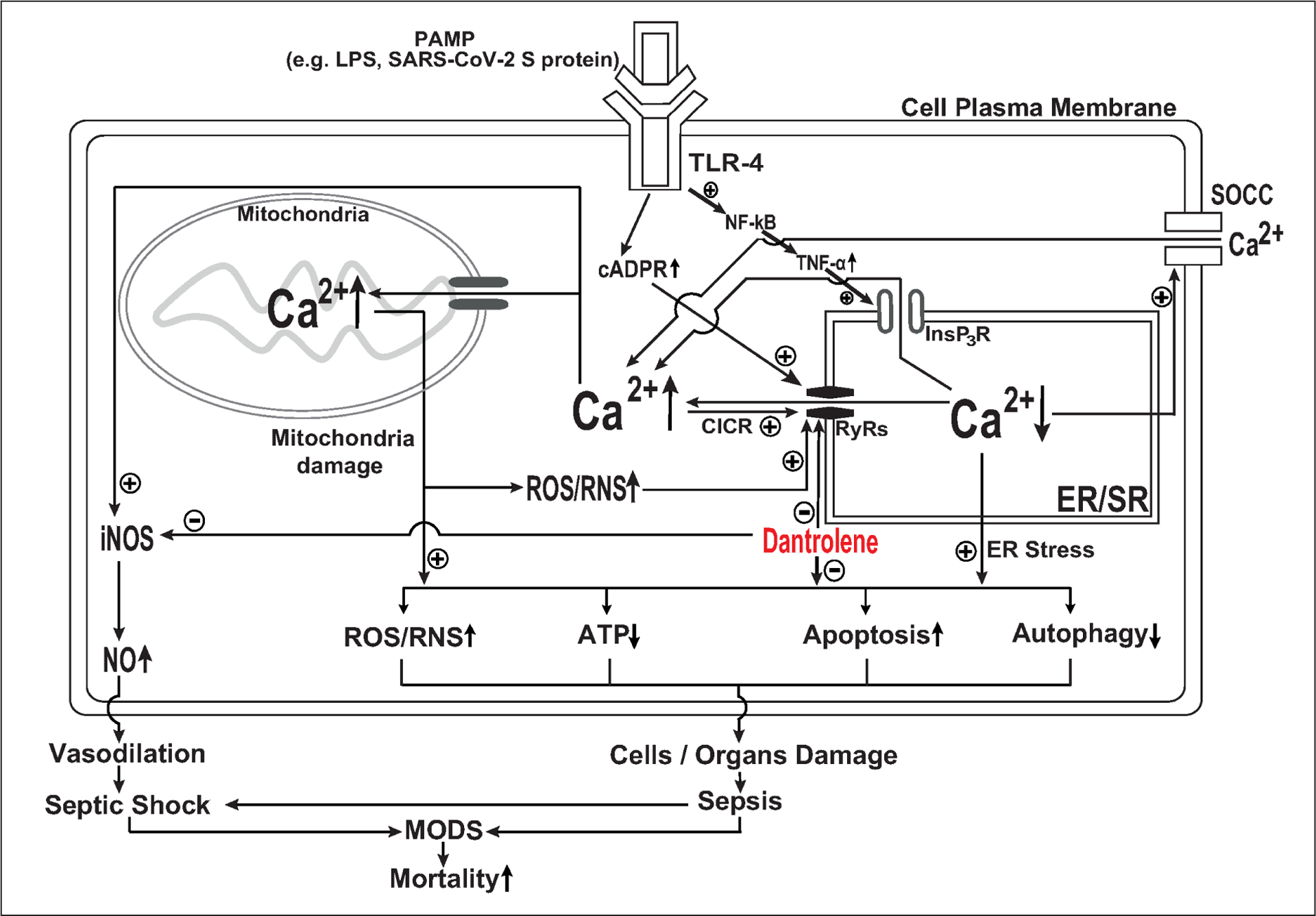
Mechanisms of Dantrolene treatment of sepsis or septic shock: PAMP: pathogen-associated molecular patterns (PAMP), LPS: lipopolysaccharide (LPS), TLR-4: Toll-like receptor-4, NF-κB: activate nuclear factor kappa light chain enhancer of activated B cells, cADPR: Cyclic ADP-ribose, TNF-α: tumor necrosis factor-alpha, ER: endoplasmic reticulum, SR: sarcoplasmic reticulum, InsP3R: InsP3 receptors, SOCC: store operated Ca2+ channels, RyRs: ryanodine receptors, CICR: Ca2+ induced Ca2+ release, ROS: reactive oxygen species, RNS: reactive nitrate species, iNOS: inducible nitric oxide synthase (iNOS), NO: nitric oxide, MODS: multiple organ damage syndrome.
Dantrolene inhibits SIRS during sepsis
Bacterial endotoxins and/or viruses activate TLR-4 and increase cytokine release as a defense mechanism to protect cell damage from invading PAMP (Figure 1). However, excessive and pathological cytokine release results in SIRS or cytokine storms, damaging cells/organs and leading to MODS48–51. Pathological inflammatory response with abnormally elevated levels of cytokines (IL-1β, IL-6, IL-8, MCP-1, IP-10, TNFα, IFN-γ, etc.) has been observed in sepsis patients and results in SIRS3,13. Therefore, amelioration of SIRS and associated MODS is considered a strategy to reduce the severity of sepsis or septic shock and associated mortality. As demonstrated in figure 1, excessive Ca2+ release from the ER/SR via RyRs and associated ER/SR stress play an important role in cytokine release and SIRS. Thus, inhibition of RyRs is expected to ameliorate SIRS during sepsis13,24,25,27. Dantrolene, a Food and Drug Administration (FDA) approved drug for the treatment of malignant hyperthermia, inhibits RyRs channel opening and ameliorates the pathological decrease of ER/SR Ca2+ as well as the increase of cytosol and mitochondria Ca2+ concentrations52–54. Thus, it is expected to ameliorate SIRS or “cytokine storms”.
Considering the important role of ER/SR stress caused by depletion of ER/SR Ca2+ via over activation of RyRs in the vicious cycle of activation of TLR-4 and the production of proinflammation cytokines (Figure 1), inhibition of RyRs and amelioration of ER/SR stress may break the vicious cycle of pathological production of proinflammation cytokines and associated SIRS. Dantrolene has been demonstrated to suppress plasma and tissue concentrations of IL-655, IL-856, IL-1β, TNF-α13,22, and IFN-γ24–27. Dantrolene also significantly reduced mortality in sepsis animal models with SIRS13,15,17,18,57,58, and showed no significant side effects or toxicity in a sepsis animal model11. On the other hand, dantrolene promoted protective antiinflammation cytokine, IL-10, in sepsis animal models24. The combined effects of dantrolene on suppressing pathological cytokine and promoting protective cytokine release make it a good candidate to balance host inflammation response towards a beneficial effect during sepsis.
Among the proinflammation cytokines, TNF-α plays a critical role in disrupting intracellular Ca2+ homeostasis. TNF-α activates InsP3R34 and then RyRs, promoting overloading of Ca2+ in mitochondria and increasing production of ROS/RNS, further activating RyRs and pathological Ca2+ release from the ER/SR (Fig. 1). Because RyRs have a Ca2+ channel conductance much higher than InsP3R, it is considered a primary Ca2+ channel, which releases Ca2+ from the ER/SR into cytosol, especially during pathological stresses. In accordance, dantrolene, via its inhibition of RyRs, demonstrates inhibition of TNF-α and associated SIRS or “cytokines storms” in various kinds of animal models13,22,26. Dantrolene has also been proposed to inhibit excessive and pathological inflammation triggered by the SARS-CoV-2 virus (Fig. 1) and has been repurposed to treat COVID-19 patients53,59.
Dantrolene ameliorates MODS during sepsis or septic shock
MODS is commonly seen in sepsis, especially in septic shock patients. The mortality of sepsis patients has been closely associated with SIRS and MODS – higher numbers of organ injury (OI) result in increased mortality60–62. Clinical variables reflecting the severity of MODS, such as sequential organ failure assessment (SOFA) and quick SOFA (q SOFA), have been used to predict mortality in sepsis patients49. Clearly, an important strategy to reduce mortality in sepsis patients is the reduction of MODS10. Disruption of intracellular Ca2+ homeostasis, especially the pathological decrease of ER but increase of cytosol and mitochondria Ca2+ concentrations (Figure 1), plays a critical role in cell and then organ damage in sepsis. Inhibition or amelioration of the Ca2+ dysregulation is expected to minimize the cell and organ damage and dysfunction9,12,15,18,63–65. Furthermore, as abnormal and pathological Ca2+ release from the ER/SR via over activation of RyRs plays an important role in proinflammation cytokine release, ER/SR stresses, and mitochondria damage, and associated multiple pathology pathways, dantrolene is expected to inhibit these pathologies as well as associated cell or organ damage and dysfunction by inhibiting Ca2+ dysregulation in sepsis (Figure 1).
Dantrolene is thought to ameliorate MODS, and therefore mortality in sepsis or septic shock patients via the following proposed multiple mechanisms13,53,64. 1) Inhibition of cell damage induced by multiple pathologies resulting from excessive Ca2+ release from the ER/SR via over activation of RyRs in sepsis patients (Figure 1, mitochondria and ER damage66–69, excessive reactive oxygen species (ROS)70 or nitrate species (RNS), reduction of ATP production71, impaired autophagy72–74, apoptosis75,76). Dantrolene protected cells against oxidative stress by elevating the levels of GSH and GSH/GSSG77,78. Dantrolene lowered mitochondrial superoxide, ROS and associated mitochondria damage79. Dantrolene has been reported to promote autophagy activity by augmenting autophagy induction74,80 and, therefore, potentially ameliorating autophagy impairment in sepsis. 2) Inhibition of hypoxia damage in various organs. Severe hypoxia and associated hypoxia damage is frequently seen in sepsis patients with lung damage and acute respiratory distress syndrome (ARDS) and is a significant contributing factor to cell and multiple organ injury81. While abnormal and excessive Ca2+ release from the ER/SR via over activation of RyRs plays an important role in hypoxia-mediated cell/organ damage53,82, dantrolene has been demonstrated to be protective against hypoxia-induced cell/organ damage43,54,83–85. Dantrolene inhibited multiple organ damage, including lung43 heart28,77, brain83, liver, and kidney damage28. Thus, dantrolene is expected to reduce MODS in critically ill COVID-19 patients53 and in sepsis or septic shock patients. 3) Inhibition of ischemia damage in various organs. Septic shock, a severe form of sepsis, is commonly seen in sepsis patients. Ischemia in various organs during septic shock contributes to MODS significantly in these patients. Excessive and abnormal Ca2+ release from the ER/SR via RyRs overactivation and elevation of [Ca2+]c activates iNOS and abnormally increases NO production, which in turn causes vasodilation and hypotension during septic shock. Dantrolene has been demonstrated to inhibit iNOS and the production of NO in a sepsis animal model86. It is well known that pathological Ca2+ release from the ER/SR via RyRs plays an important role in ischemia-mediated cell and organ damage25,83,87,88. Accordingly, dantrolene, as a potent inhibitor of RyRs89,90, protected ischemia damage in multiple organs, including CNS83,88,91,92, heart93, liver26, kidney28, lung43, and muscle94 damage. Pawar et al19 demonstrated the effectiveness of dantrolene in treating high fever in sepsis patients19.
Practical consideration of using dantrolene in sepsis patients
As dantrolene is a clinically available drug to treat patients suffering from malignant hyperthermia, neuroleptic malignant syndrome, etc., considerable experience regarding its methods of use and side effects has been achieved95–97. For future clinical studies of dantrolene treatment of sepsis patients, we propose the following practical points for designing clinical trials. 1) In-hospital vs. critically ill sepsis patients on ventilators: Despite the muscle relaxant effects of dantrolene and common clinical features of respiratory failure in sepsis patients, critically ill patients on ventilators can be recruited for initial clinical studies without concern for breathing difficulty due to the potential muscle relaxant effects of dantrolene. This is, in part, because the muscle relaxant effects of dantrolene are much weaker than those of muscle relaxants normally used during anesthesia practice (e.g., vecuronium), and typically do not affect patient breathing after a long-term use via oral administration95. The early use of dantrolene even before intubation and mechanical ventilation may provide early treatment of sepsis. Additionally, sepsis patients on ventilators typically receive muscle relaxants (e.g., vecuronium, etc.). So, the muscle relaxant effects of dantrolene may become a confounding factor for data analysis in clinical studies. However, a significant effect is not expected due to its weaker muscle relaxant effects at commonly used doses. 2) Ryanodex vs. traditional dantrolene: Because traditional dantrolene requires a high volume of solvent (20 mg/60 ml), which is not suitable for sepsis patients commonly suffering from acute respiratory distress syndrome (ARDS), Ryanodex, which requires a much lower volume of solvent (250 mg/5 ml), is expected to be a more flexible and less restricted drug to treat sepsis patients. 3) Dantrolene administration routes, dose, and duration: considering the disturbance of gastrointestinal function in sepsis patients, intravenous administration is preferred over oral administration. Compared to oral administration, an intravenous approach provides more reliable absorption and stable plasma concentration, minimizing the variance of therapeutic efficacy among studied patients. Long-term dantrolene administration via intranasal approach increased brain concentrations and durations without significant side effects in animal studies98,99. Intranasal administration of dantrolene can be considered for those sepsis or septic shock patients with significant CNS damage and symptoms. Treatment of sepsis patients with respiratory failure on ventilators is not as emergent as for malignant hyperthermia. Thus, we propose treatments at a relatively lower dose, but for a longer duration. Krause et al95 suggested that a dose of less than 400 mg/day of dantrolene will be relatively safe and will not cause liver dysfunction. Also, continuous intravenous infusion of dantrolene for up to 7 days was tolerable in patients as long as the total dose was within the recommended safe margin100. On the other hand, an intravenous bolus injection of dantrolene every 6 hours can be considered if IV access is a limitation, as long as the total daily doses do not cause liver toxicity. 4) Safety monitoring: as the primary side effect of dantrolene is liver toxicity, AST and ALT should be monitored daily, and dantrolene treatment should be stopped if AST/ALT is abnormally elevated. Other commonly observed side effects, such as muscle weakness, CNS depression, and/or gastrointestinal symptoms should be carefully monitored.
Dantrolene as a potential drug for the treatment of COVID-19 patients
As an infectious pathogen, SARS-CoV-2 virus is able to cause sepsis or septic shock in COVID-19 patients101. We have previously repurposed dantrolene to treat COVID-19 patients53. Dantrolene treats COVID-19 patients via the following proposed mechanisms53: 1) Inhibition of upper stream pathology in disrupted Ca2+ homeostasis induced by over activation of RyRs; 2) Inhibition of host cell infection and replication of SARS-CoV-2 virus and reduction of viral load; 3) Inhibition of SARS-CoV-2 virus-mediated cell damage by amelioration of downstream pathologies mediated by Ca2+ dysregulation, such as mitochondrial damage, ER stresses, and associated ROS toxicity, energy failure (reduced ATP production), excess inflammation, impaired autophagy, and apoptosis, etc.; 4) Inhibition of SARS-CoV-2 virus-mediated MODS and associated patient mortality.
A new case report demonstrated that intravenous dantrolene (20 mg, 4 times/day) for three days followed by oral dantrolene (50 mg, three times/day) in a young critically ill COVID-19 patient with MODS on ventilator treatment was associated with a decrease of body temperature, creatine kinase (CK), myoglobin, C-reactive protein (CRP), ferritin levels, quick extubation, and contributed to overall full recovery59.
Conclusions
Dantrolene has been repurposed to treat sepsis or septic shock and COVID-19 patients by ameliorating ER stress and mitochondrial damage as well as associated multiple pathologies including SIRS through inhibiting excessive and pathological Ca2+ release from the ER/SR via RyRs. Dantrolene is expected to minimize MODS and reduce mortality in sepsis or septic shock and COVID-19 patients, pending future extensive and well-designed clinical studies.
Funding
The research project was supported by grants to HW from National Institute of Health (NIH), National Institute on Aging (R01AG061447) and National Institute of Health (NIH), National Institute of Aging (NIA) 3R01AG061447-03S1.
Footnotes
Competing Interests
Drs. Huafeng Wei and Ge Liang are listed as inventors of a US provisional patent application entitled “Intranasal Administration of Dantrolene for Treatment of Alzheimer’s Disease” filed on June 28, 2019 (Serial number 62/868,820) by the University of Pennsylvania Trustee, Philadelphia, PA, USA. The provisional patent application is also part of the research collaboration agreement between the University of Pennsylvania and Eagle Pharmaceutical Company, which produces and sells a new formula of dantrolene (Ryanodex) for the treatment of malignant hyperthermia. Other authors declare no conflict of interest.
References
- 1).Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Genga KR, Russell JA. Update of Sepsis in the Intensive Care Unit. J Innate Immun 2017; 9: 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Purcarea A, Sovaila S. Sepsis, a 2020 review for the internist. Rom J Intern Med 2020; 58: 129–137. [DOI] [PubMed] [Google Scholar]
- 4).Rhee C, Klompas M. Sepsis trends: increasing incidence and decreasing mortality, or changing denominator? J Thorac Dis 2020; 12(Suppl 1): S89–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Liu AC, Patel K, Vunikili RD, Johnson KW, Abdu F, Belman SK, Glicksberg BS, Tandale P, Fontanez R, Mathew OK, Kasarskis A, Mukherjee P, Subramanian L, Dudley JT, Shameer K. Sepsis in the era of data-driven medicine: personalizing risks, diagnoses, treatments and prognoses. Brief Bioinform 2020; 21: 1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Rockville (MD), 2006. [PubMed] [Google Scholar]
- 7).Kim HI, Park S. Sepsis: early recognition and optimized treatment. Tuberc Respir Dis (Seoul) 2019; 82: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Franco L, Bodrato N, Moreschi I, Usai C, Bruz-zone S, Scarf i S, Zocchi E, De Flora A. Cyclic ADP-ribose is a second messenger in the lipopolysaccharide-stimulated activation of murine N9 microglial cell line. J Neurochem 2006; 99: 165–176. [DOI] [PubMed] [Google Scholar]
- 9).Yang J, Zhang R, Jiang X, Lv J, Li Y, Ye H, Liu W, Wang G, Zhang C, Zheng N, Dong M, Wang Y, Chen P, Santosh K, Jiang Y, Liu J. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca(2+) leakage promote cardiac contractile dysfunction in sepsis. J Biol Chem 2018; 293: 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Armstrong BA, Betzold RD, May AK. Sepsis and septic shock strategies. Surg Clin North Am 2017; 97: 1339–1379. [DOI] [PubMed] [Google Scholar]
- 11).Beebe DS, Belani KG, Tuohy SE, Sweeney MF, Gillingham K, Komanduri V, Palahniuk RJ. Is dantrolene safe to administer in sepsis? The effect of dantrolene after endotoxin administration in dogs and rats. Anesth Analg 1991; 73: 289–294. [DOI] [PubMed] [Google Scholar]
- 12).Celes MR, Malvestio LM, Suadicani SO, Prado CM, Figueiredo MJ, Campos EC, Freitas AC, Spray DC, Tanowitz HB, da Silva JS, Rossi MA. Disruption of calcium homeostasis in cardiomyocytes underlies cardiac structural and functional changes in severe sepsis. PLoS One 2013; 8: e68809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Fischer DR, Sun X, Williams AB, Gang G, Pritts TA, James JH, Molloy M, Fischer JE, Paul RJ, Hasselgren PO. Dantrolene reduces serum TNFalpha and corticosterone levels and muscle calcium, calpain gene expression, and protein breakdown in septic rats. Shock 2001; 15: 200–207. [DOI] [PubMed] [Google Scholar]
- 14).Hassoun SM, Marechal X, Montaigne D, Bouazza Y, Decoster B, Lancel S, Neviere R. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med 2008; 36: 2590–2596. [DOI] [PubMed] [Google Scholar]
- 15).Hotchkiss RS, Karl IE. Dantrolene ameliorates the metabolic hallmarks of sepsis in rats and improves survival in a mouse model of endotoxemia. Proc Natl Acad Sci U S A 1994; 91: 3039–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Qiu YW, Chen D, Xu MY, Li ST. Beneficial effects of dantrolene on sepsis-induced diaphragmatic dysfunction are associated with downregulation of high-mobility group box 1 and calpain-caspase-3 proteolytic pathway. J Surg Res 2016; 200: 637–647. [DOI] [PubMed] [Google Scholar]
- 17).Williams AB, Decourten-Myers GM, Fischer JE, Luo G, Sun X, Hasselgren PO. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J 1999; 13: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 18).Wray CJ, Sun X, Gang GI, Hasselgren PO. Dantrolene downregulates the gene expression and activity of the ubiquitin-proteasome proteolytic pathway in septic skeletal muscle. J Surg Res 2002; 104: 82–87. [DOI] [PubMed] [Google Scholar]
- 19).Pawar SC RH, Adamson R, LaRosa JA, Chamberlain R. Dantrolene in the treatment of refractory hyperthermic conditions in critical care: a multicenter retrospective study. Open J Anesthesiol 2015; 5: 63–71. [Google Scholar]
- 20).Cen X, Liu S, Cheng K. The role of Toll-Like Receptor in inflammation and tumor immunity. Front Pharmacol 2018; 9: 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 2017; 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Hotchkiss RS, Osborne DF, Lappas GD, Karl IE. Calcium antagonists decrease plasma and tissue concentrations of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 alpha in a mouse model of endotoxin. Shock 1995; 3: 337–342. [PubMed] [Google Scholar]
- 23).Conrad DM, Hanniman EA, Watson CL, Mader JS, Hoskin DW. Ryanodine receptor signaling is required for anti-CD3-induced T cell proliferation, interleukin-2 synthesis, and interleukin-2 receptor signaling. J Cell Biochem 2004; 92: 387–399. [DOI] [PubMed] [Google Scholar]
- 24).Hasko G, Szabo C, Nemeth ZH, Lendvai B, Vizi ES. Modulation by dantrolene of endotoxin-induced interleukin-10, tumour necrosis factor-alpha and nitric oxide production in vivo and in vitro. Br J Pharmacol 1998; 124: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Lin HP, Zheng YQ, Zhou ZP, Wang GX, Guo PF. Ryanodine receptor antagonism alleviates skeletal muscle ischemia reperfusion injury by modulating TNF-alpha and IL-10. Clin Hemorheol Microcirc 2018; 70: 51–58. [DOI] [PubMed] [Google Scholar]
- 26).Lopez-Neblina F, Toledo-Pereyra LH, Toledo AH, Walsh J. Ryanodine receptor antagonism protects the ischemic liver and modulates TNF-alpha and IL-10. J Surg Res 2007; 140: 121–128. [DOI] [PubMed] [Google Scholar]
- 27).Nemeth ZH, Hasko G, Szabo C, Salzman AL, Vizi ES. Calcium channel blockers and dantrolene differentially regulate the production of inter-leukin-12 and interferon-gamma in endotoxemic mice. Brain Res Bull 1998; 46: 257–261. [DOI] [PubMed] [Google Scholar]
- 28).Boys JA, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Effects of dantrolene on ischemia-reperfusion injury in animal models: a review of outcomes in heart, brain, liver, and kidney. J Investig Med 2010; 58: 875–882. [DOI] [PubMed] [Google Scholar]
- 29).Kajii T, Kobayashi S, Shiba S, Fujii S, Tamitani M, Kohno M, Nakamura Y, Nanno T, Kato T, Okuda S, Uchinoumi H, Oda T, Yamamoto T, Yano M. Dantrolene prevents ventricular tachycardia by stabilizing the ryanodine receptor in pressure-overload induced failing hearts. Biochem Biophys Res Commun 2020; 521: 57–63. [DOI] [PubMed] [Google Scholar]
- 30).Tamitani M, Yamamoto T, Yamamoto N, Fujisawa K, Tanaka S, Nakamura Y, Uchinoumi H, Oda T, Okuda S, Takami T, Kobayashi S, Sakaida I, Yano M. Dantrolene prevents hepatic steatosis by reducing cytoplasmic Ca(2+) level and ER stress. Biochem Biophys Rep 2020; 23: 100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Wu CC, Yen MH. Beneficial effects of dantrolene on lipopolysaccharide-induced haemodynamic alterations in rats and mortality in mice. Eur J Pharmacol 1997; 327: 17–24. [DOI] [PubMed] [Google Scholar]
- 32).Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol 2020; 92: 2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Brandao SCS, Ramos JOX, Dompieri LT, Godoi E, Figueiredo JL, Sarinho ESC, Chelvanambi S, Aikawa M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev 2020; S1359–6101: 30205–30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, Bhattacharya S, Bhattacharya J. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest 2011; 121: 1986–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Beringer A, Gouriou Y, Lavocat F, Ovize M, Miossec P. Blockade of store-operated calcium entry reduces IL-17/TNF cytokine-induced inflammatory response in human myoblasts. Front Immunol 2018; 9: 3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP(3)-induced Ca2+ release. FASEB J 2002; 16: 1145–1150. [DOI] [PubMed] [Google Scholar]
- 37).Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 2007; 42: 173–182. [DOI] [PubMed] [Google Scholar]
- 38).Bastide B, Snoeckx K, Mounier Y. ADP-ribose stimulates the calcium release channel RyR1 in skeletal muscle of rat. Biochem Biophys Res Commun 2002; 296: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 39).Verkhratsky A, Shmigol A. Calcium-induced calcium release in neurones. Cell Calcium 1996; 19: 1–14. [DOI] [PubMed] [Google Scholar]
- 40).Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 2011; 3: a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).SanMartin CD, Veloso P, Adasme T, Lobos P, Bruna B, Galaz J, García A, Hartel S, Hidalgo C, Paula-Lima AC. RyR2-mediated Ca2+ release and mitochondrial ROS generation partake in the synaptic dysfunction caused by amyloid beta peptide oligomers. Front Mol Neurosci 2017; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Cali T, Ottolini D, Brini M. Mitochondrial Ca2+ and neurodegeneration. Cell Calcium 2012; 52: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Du W, Frazier M, McMahon TJ, Eu JP. Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest 2005; 128: 556S–558S. [DOI] [PubMed] [Google Scholar]
- 44).Mikami Y, Kanemaru K, Okubo Y, Nakaune T, Suzuki J, Shibata K, Sugiyama H, Koyama R, Murayama T, Ito A, Yamazawa T, Ikegaya Y, Sakurai T, Saito N, Kakizawa S, Iino M. Nitric oxide-induced activation of the type 1 ryanodine receptor is critical for epileptic seizure-induced neuronal cell death. EBioMedicine 2016; 11: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Seidlmayer LK, Kuhn J, Berbner A, Arias-Loza PA, Williams T, Kaspar M, Czolbe M, Kwong JQ, Molkentin JD, Heinze KG, Dedkova EN, Ritter O. Inositol 1,4,5-trisphosphate-mediated sarcoplasmic reticulum-mitochondrial crosstalk influences adenosine triphosphate production via mitochondrial Ca2+ uptake through the mitochondrial ryanodine receptor in cardiac myocytes. Cardiovasc Res 2016; 112: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, Smaili SS. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal 2014; 21: 123–137. [DOI] [PubMed] [Google Scholar]
- 47).Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4: 552–565. [DOI] [PubMed] [Google Scholar]
- 48).Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth 2010; 2: 161–175. [PMC free article] [PubMed] [Google Scholar]
- 49).Chakraborty RK, Burns B. Systemic Inflammatory Response Syndrome. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 50).Kaml GJ, Davis KA. Surgical critical care for the patient with sepsis and multiple organ dysfunction. Anesthesiol Clin 2016; 34: 681–696. [DOI] [PubMed] [Google Scholar]
- 51).Sun X, Wang T, Cai D, Hu Z, Chen J, Liao H, Zhi L, Wei H, Zhang Z, Qiu Y, Wang J, Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev 2020; 53: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Sun L, Wei H. Ryanodine receptors: a potential treatment target in various neurodegenerative disease. Cell Mol Neurobiol 2020. August 24. DOI: 10.1007/s10571-020-00936-w. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Jiang B, Liang S, Liang G, Wei H. Could dantrolene be explored as a repurposed drug to treat COVID-19 patients by restoring intracellular calcium homeostasis? Eur Rev Med Pharmacol Sci 2020; 24: 10228–10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg 2010; 111: 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Ducreux S, Zorzato F, Müller C, Sewry C, Muntoni F, Quinlivan R, Restagno G, Girard T, Treves S. Effect of ryanodine receptor mutations on interleukin-6 release and intracellular calcium homeostasis in human myotubes from malignant hyperthermia-susceptible individuals and patients affected by central core disease. J Biol Chem 2004; 279: 43838–43846. [DOI] [PubMed] [Google Scholar]
- 56).Hisatsune J, Nakayama M, Isomoto H, Kurazono H, Mukaida N, Mukhopadhyay AK, Azuma T, Yamaoka Y, Sap J, Yamasaki E, Yahiro K, Moss J, Hirayama T.Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-kappaB activation. J Immunol 2008; 180: 5017–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Cameron EM, Zhuang J, Menconi MJ, Phipps R, Fink MP. Dantrolene, an inhibitor of intracellular calcium release, fails to increase survival in a rat model of intra-abdominal sepsis. Crit Care Med 1996; 24: 1537–1542. [DOI] [PubMed] [Google Scholar]
- 58).Wray CJ, Sun XY, Gang GI, Hasselgren PO. Dantrolene downregulates the gene expression and activity of the ubiquitin-proteasome proteolytic pathway in septic skeletal muscle. J Surg Res 2002; 104: 82–87. [DOI] [PubMed] [Google Scholar]
- 59).Chiba N, Matsuzaki M, Mawatari T, Mizuochi M, Sakurai A, Kinoshita K. Beneficial effects of dantrolene in the treatment of rhabdomyolysis as a potential late complication associated with COVID-19: a case report. Eur J Med Res 2020; 26: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, Jabaley CS, Carpenter D, Kaplow R, Hernandez-Romieu AC, Adelman MW, Martin GS, Coopersmith CM, Murphy DJ. ICU and ventilator mortality among critically ill adults with Coronavirus disease 2019. Crit Care Med 2020; 48: e799–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Iwasaki M, Saito J, Zhao H, Sakamoto A, Hirota K, Ma D. Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: molecular mechanisms and implications. Inflammation 2021; 44: 13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol 2020; 51: 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Dada LA, Sznajder JI. Mitochondrial Ca(2)+ and ROS take center stage to orchestrate TNF-alpha-mediated inflammatory responses. J Clin Invest 2011; 121: 1683–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Hotchkiss RS, Bowling WM, Karl IE, Osborne DF, Flye MW. Calcium antagonists inhibit oxidative burst and nitrite formation in lipopolysaccharide-stimulated rat peritoneal macrophages. Shock 1997; 8: 170–178. [DOI] [PubMed] [Google Scholar]
- 65).Huang Y, Wang G, Peng T. Calpain activation and organ failure in sepsis: molecular insights and therapeutic perspectives. Shock 2020. October 9. DOI: 10.1097/SHK.0000000000001679. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 66).Bauer TM, Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res 2020; 126: 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 2004; 11: 372–380. [DOI] [PubMed] [Google Scholar]
- 68).Murasawa S, Iuchi K, Sato S, Noguchi-Yachide T, Sodeoka M, Yokomatsu T, Dodo K, Hashimoto Y, Aoyama H. Small-molecular inhibitors of Ca2+-induced mitochondrial permeability transition (MPT) derived from muscle relaxant dantrolene. Bioorgan Med Chem 2012; 20: 6384–6493. [DOI] [PubMed] [Google Scholar]
- 69).Li F, Hayashi T, Jin G, Deguchi K, Nagotani S, Nagano I, Shoji M, Chan PH, Abe K. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res 2005; 1048: 59–68. [DOI] [PubMed] [Google Scholar]
- 70).Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res 2006; 3: 327–337. [DOI] [PubMed] [Google Scholar]
- 71).Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 2010; 142: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sci 2008; 65: 3640–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Vervliet T, Pintelon I, Welkenhuyzen K, Bootman MD, Bannai H, Mikoshiba K, Martinet W, Nadif Kasri N, Parys JB, Bultynck G. Basal ryanodine receptor activity suppresses autophagic flux. Biochem Pharmacol 2017; 132: 133–142. [DOI] [PubMed] [Google Scholar]
- 74).Wang Y, Liang G, Liang S, Mund R, Shi Y, Wei H. Dantrolene ameliorates impaired neurogenesis and synaptogenesis in induced pluripotent stem cell lines derived from patients with Alzheimer’s disease. Anesthesiology 2020; 132: 1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003; 22: 8608–8618. [DOI] [PubMed] [Google Scholar]
- 76).Yano T, Nakayama R, Imaizumi T, Terasaki H, Ushijima K. Dantrolene ameliorates delayed cell death and concomitant DNA fragmentation in the rat hippocampal CA1 neurons subjected to mild ischemia. Resuscitation 2001; 50: 117–125. [DOI] [PubMed] [Google Scholar]
- 77).Todorova VK, Siegel ER, Kaufmann Y, Kumarapeli A, Owen A, Wei JY, Makhoul I, Klimberg VS. Dantrolene attenuates cardiotoxicity of Doxorubicin without reducing its antitumor efficacy in a breast cancer model. Transl Oncol 2020; 13: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Keles I, Bozkurt MF, Aglamis E, Fidan AF, Ceylan C, Karalar M, Coban S, Denk B, Buyukokuroglu ME. Protective effects of dantrolene and methylprednisolone against spinal cord injury-induced early oxidative damage in rabbit bladder: a comparative experimental study. Adv Clin Exp Med 2019; 28: 1697–1704. [DOI] [PubMed] [Google Scholar]
- 79).Godai K, Takahashi K, Kashiwagi Y, Liu CH, Yi H, Liu S, Dong C, Lubarsky DA, Hao S. Ryanodine receptor to mitochondrial reactive oxygen species pathway plays an important role in chronic human immunodeficiency virus gp120MN-induced neuropathic pain in rats. Anesth Analg 2019; 129: 276–286. [DOI] [PubMed] [Google Scholar]
- 80).Chung KM, Jeong EJ, Park H, An HK, Yu SW. Mediation of autophagic cell death by type 3 Ryanodine Receptor (RyR3) in adult hippocampal neural stem cells. Front Cell Neurosci 2016; 10: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Jiang B, Wei H. Oxygen therapy strategies and techniques to treat hypoxia in COVID-19 patients. Eur Rev Med Pharmacol Sci 2020; 24: 10239–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Lu F, Tian Z, Zhang W, Zhao Y, Bai S, Ren H, Chen H, Yu X, Wang J, Wang L, Li H, Pan Z, Tian Y, Yang B, Wang R, Xu C. Calcium-sensing receptors induce apoptosis in rat cardiomyocytes via the endo(sarco)plasmic reticulum pathway during hypoxia/reoxygenation. Basic Clin Pharmacol Toxicol 2010; 106: 396–405. [DOI] [PubMed] [Google Scholar]
- 83).Gwak M, Park P, Kim K, Lim K, Jeong S, Baek C, Lee J. The effects of dantrolene on hypoxic-ischemic injury in the neonatal rat brain. Anesth Analg 2008; 106: 227–233. [DOI] [PubMed] [Google Scholar]
- 84).Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 2005; 125: 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Katchman AN, Hershkowitz N. Early anoxia-induced vesicular glutamate release results from mobilization of calcium from intracellular stores. J Neurophysiol 1993; 70: 1–7. [DOI] [PubMed] [Google Scholar]
- 86).Li CY, Chou TC, Wu CC, Wong CS, Ho ST, Yen MH, Ding YA. Dantrolene inhibits nitric oxide synthase in rat alveolar macrophages treated with lipopolysaccharide and interferon-gamma. Can J Anaesth 1998; 45: 246–252. [DOI] [PubMed] [Google Scholar]
- 87).Benveniste H, Jorgensen MB, Diemer NH, Hansen AJ. Calcium accumulation by glutamate receptor activation is involved in hippocampal cell-damage after ischemia. Acta Neurol Scand 1988; 78: 529–536. [DOI] [PubMed] [Google Scholar]
- 88).Zhang L, Andou Y, Masuda S, Mitani A, Kataoka K. Dantrolene protects against ischemic, delayed neuronal death in gerbil brain. Neurosci Lett 1993; 158: 105–108. [DOI] [PubMed] [Google Scholar]
- 89).Liou B, Peng Y, Li R, Inskeep V, Zhang W, Quinn B, Dasgupta N, Blackwood R, Setchell KD, Fleming S, Grabowski GA, Marshall J, Sun Y. Modulating ryanodine receptors with dantrolene attenuates neuronopathic phenotype in Gaucher disease mice. Hum Mol Genet 2016; 25: 5126–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Oo YW, Gomez-Hurtado N, Walweel K, van Helden DF, Imtiaz MS, Knollmann BC, Laver DR. Essential role of calmodulin in RyR inhibition by Dantrolene. Mol Pharmacol 2015; 88: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Wei HF, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochemistry 1996; 67: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 92).Kocogullari CU, Emmiler M, Cemek M, Sahin O, Aslan A, Ayva E, Tur L, Buyukokuroglu ME, Demirkan I, Cekirdekci A. Can dantrolene protect spinal cord against ischemia/reperfusion injury? An experimental study. Thorac Cardiovasc Surg 2008; 56: 406–411. [DOI] [PubMed] [Google Scholar]
- 93).Preckel B, Schlack W, Comfere T, Thamer V. Effect of dantrolene in an in vivo and in vitro model of myocardial reperfusion injury. Acta Anaesthesiol Scand 2000; 44: 194–201. [DOI] [PubMed] [Google Scholar]
- 94).Klenerman L, Lowe NM, Miller I, Fryer PR, Green CJ, Jackson MJ. Dantrolene sodium protects against experimental ischemia and reperfusion damage to skeletal muscle. Acta Orthop Scand 1995; 66: 352–358. [DOI] [PubMed] [Google Scholar]
- 95).Krause T, Gerbershagen MU, Fiege M, Weiss-horn R, Wappler F. Dantrolene--A review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004; 59: 364–373. [DOI] [PubMed] [Google Scholar]
- 96).Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care 2009; 10: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Ward A, Chaffman MO, Sorkin EM. Dantrolene--a review of its pharmacodynamic and pharmaco-kinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs 1986; 32: 130–168. [DOI] [PubMed] [Google Scholar]
- 98).Shi Y, Zhang L, Gao X, Zhang J, Ben Abou M, Liang G, Meng Q, Hepner A, Eckenhoff MF, Wei H. Intranasal Dantrolene as a disease-modifying drug in Alzheimer 5XFAD mice. J Alzheimers Dis 2020; 76: 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Wang J, Shi Y, Yu S, Wang Y, Meng Q, Liang G, Eckenhoff MF, Wei H. Intranasal administration of dantrolene increased brain concentration and duration. PLoS One 2020; 15: e0229156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Muehlschlegel S, Carandang R, Hall W, Kini N, Izzy S, Garland B, Ouillette C, van der Bom IM, Flood TF, Gounis MJ, Weaver JP, Barton B, Wakhloo AK. Dantrolene for cerebral vasospasm after subarachnoid haemorrhage: a randomised double blind placebo-controlled safety trial. J Neurol Neurosurg Psychiatry 2015; 86: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 101).Alhazzani W. Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving sepsis campaign: guidelines on the management of critically Ill adults with Coronavirus disease 2019 (COVID-19). Crit Care Med 2020; 48: e440–e469. [DOI] [PMC free article] [PubMed] [Google Scholar]


