Abstract
Natural deep eutectic solvents (NADESs) are considered as green solvents, and due to their promising sustainability, they have been applied in many research fields. In this study, the main goal is to use various NADES systems to replace the traditional solvents used in conservation and restoration to remove varnish layers in a painting. The toxicity of traditional solvents, such as toluene or acetone, is well known in the chemistry field. To replace them, it is important to understand the intrinsic physicochemical properties of a solvent that may act as a substitute. Polarity and solubility are proposed as the best parameters required for this study. The Nile red probe was used to confirm the similarity between the polarity of deep eutectic systems (DESs) and traditional solvents. According to their polarities and Hansen solubility parameters, it is possible to predict the best solvents to solubilize the natural resin varnishes. Besides this, some arithmetic models can also be applied to estimate the critical or thermodynamic properties, which are useful tools to predict the behavior of these solvents. We have further proven the possibility of dissolving natural varnishes such as dammar or mastic in hydrophobic DESs, such as menthol + lauric acid, menthol + decanoic acid, or menthol + thymol.
Keywords: painting, varnishes, NADES, group contribution, thermodynamic properties, Hansen solubility parameters, Teas chart
Short abstract
Replacing volatile organic solvents in art conservation and restoration by natural deep eutectic solvents to prevent conservators from exposure to harsh chemicals.
Introduction
Dammar and mastic are two examples of resins that originated from the trees of the family Dipterocarpaceae and plants of genus Pistacia of the family Anacardiaceae, respectively, and are the most studied varnishes.1 The studies carried out since the 1960s about their properties such as composition, solubility, and aging time made them a model substance. Both have a low molecular weight, belong to the triterpenoid class composed of a ring system of 30 carbons (a cycle isoprenoid) and a complex mixture of oxidized functional groups that are present even when the resins are fresh and, in many cases, which are responsible for the presence of acidic functional groups in an aged resin. Also, the polymeric fraction of those resins improves the physical and optical properties by forming a coating layer over the paint film that protects and creates a uniform surface to work on.2−4 This layered structure often gives important information necessary for the removability/solubility tests, depending on the type of resin.
Deep eutectic systems (DESs), first described by Abbott in 2003,5 are currently one of the new fields of study of green technologies, besides ionic liquids (ILs). They are formed by a mixture of two or more organic compounds that are typically solid but can also be a liquid; at a particular molar ratio, it leads to a decrease of the melting point by the hydrogen bonding interaction and becomes liquid at room temperature.6−8 Natural deep eutectic solvents (NADESs) are formed when the compounds that constitute the DES are, principally, primary metabolites such as sugar, amino acids, and organic acids. Although the mechanism of DES/NADES formation is not yet well understood, these systems demonstrate that they fulfill most of the principles of green chemistry (e.g., low volatility and toxicity, biodegradability, biocompatibility, easy production, high purity, and so forth).9−14 The characterization of NADES involves the determination of their physicochemical properties such as polarity, viscosity, solubility, density, hydrophilicity, and freezing point,15,16 which are of extreme relevance to scaling up of the technology.
The main goal of this work is to use some of these systems to replace volatile organic solvents such as toluene, acetone, propanol, and so forth, commonly used in conservation and restoration to remove varnish layers of natural resins such as mastic and dammar.
Materials and Methods
dl-Menthol (Men) (≥95% purity, CAS: 89-78-1), borneol (Bor) (97.0%, CAS: 464-45-9), thymol (Thy) (≥98.5 purity, CAS: 89-83-8), myristic acid (Myr) (≥98.0% purity, CAS: 544-63-8), lactic acid (Lac) (≥85.0% purity, CAS: 50-21-5), lauric acid (Lau) (≥98.0% purity, CAS: 0143-07-07), betaine (Bet) (≥99.0% purity, CAS: 107-43-7), iso-octane (≥99% purity, CAS: 540-84-1), acetone (≥95% purity, CAS: 67-64-1), toluene(99.8% purity, CAS: 108-88-3), ethanol (96% purity, CAS: 64-17-5), methanol (99.8% purity, CAS: 67-56-1), o-xylene (97% purity, CAS: 95-47-6), 2-propanol (≥95% purity, CAS: 67-63-0), Nile red (98.0% purity, CAS: 7385-67-3), and decanoic acid (Dec) (≥98% purity, CAS: 334-48-5) were supplied by Sigma Aldrich. dl-Malic acid (Mal) (extra pure, CAS: 6915-15-7) was obtained from Scharlau. Shellsol A (CAS: 64742-95-6), Shellsol D40 (CAS: 64742-48-9), Shellsol T (CAS: 90622-57-4), turpentine (CAS: 8006-64-2), white spirit (CAS:110-54-3), mastic (CAS: 61789-92-2), and dammar (CAS: 9000-16-2) were obtained from Kremer.
NADES Preparation
Different components were weighed and mixed with simultaneous stirring at 65 °C until a clear viscous liquid was formed. The preparation of DES was done according to Table 1.
Table 1. List of All the NADESs Studied and Their Visual Aspect Observed at Room Temperature.
| DES | visual aspect |
|---|---|
| Men + Lau (2:1)12 | transparent liquid |
| Men + Lau (4:1)12 | transparent liquid |
| Men + Lau (8:1)12 | white solid |
| Men + Myr (4:1) | white solid |
| Men + Myr (8:1) | white solid |
| Men + Dec (2:1) | transparent liquid |
| Men + Dec (7:2) | transparent liquid |
| Men + Dec (1:3) | white solid |
| Men + Mal (8:1) | white solid |
| Men + Thy (1:1)17 | transparent liquid |
| Men + Thy (2:1) | transparent liquid |
| Men + Thy (4:1) | transparent liquid |
| Men + Thy (8:1) | white solid |
| Men + Bor (7:2) | transparent liquid |
| Thy + Bor (1:1)17 | white solid |
| Lac + Bet + Dec (2:1:0.25) | white solid |
| Lac + Bet + Dec (2:1:0.35) | white solid |
| Lac + Bet + Dec (5:1:0.1) | white solid |
| Men + Lau + Dec (2:1:1) | transparent liquid |
| Men + Lau + Dec (4:1:1) | transparent liquid |
Determination of the Water Content
The water content of all the systems was measured using the instrumental equipment 831 Karl Fischer coulometer with a generator electrode (Metrohm) and a moisture analyzer DAB (Kern).
Varnish Preparation
Both varnishes were prepared following the same process, that is, dissolving the resin gums in turpentine, in a ratio of 1:2 (w/v), while stirring at 35 °C. After preparing the varnishes, 10 glass slides were brushed with a thick coat of dammar, while another 10 glass slides were brushed with mastic varnish. The glass slides have 26 × 76 mm and were left to dry for a week at room temperature.
Determination of Physicochemical Properties
Polarity
The polarity of all the systems and solvents was determined with the solvatochromic method using Nile red, previously dissolved in ethanol, and then measured with a dilution of 1:200 of Nile red in a DES. This assay was performed in triplicate. The absorbance of the solutions was measured using a UV–visible spectroscopy (Thermo Scientific GENESYS 50 for the DES systems and Multimode plate reader VICTOR NIVO for the traditional solvents) in the wavelength range of 400–800 nm.
Contact Angle
The measurement of the contact angle of NADESs was carried out using a KSV CAM-100 goniometer of KSV Instruments Ltd, configuring the number and interval of frames to, respectively, 60 and 1000 ms. The process consists of dropping a drop of solvent on the surface of the substrate and immediately starting data recording. Here, 24 glass slides varnished with dammar and mastic varnishes (12 for each) were used as the substrate, and each test was performed at least three times. The listed results correspond to the mean ± standard deviation.
Viscosity and Density
Viscosity and density were determined using the Anton Paar equipment, model SVM 3001, an automated rotational Stabinger viscometer–densimeter. For all the systems, the range of the temperature scan selected was 20–60 °C. Each system was measured in triplicate, and the tabulated results shown represent their mean ± standard deviation.
Estimation of Critical and Thermodynamic Properties
The precise models for estimating the thermodynamic properties of DES systems were made using the genetic programing. In the published models, the critical temperature (Tc), critical volume (Vc), and acentric factor (ω) are chosen as the input data for the equations proposed. Some equations showed in this work are simplified comparative to the original ones.18
Results and Discussion
Polarity
The traditional varnishes, namely, dammar and mastic resin, are composed of polar (such as hydroxylic or carboxylic acids) or hydrophobic groups. Thus, DESs were selected according to the structural features of their components, which resulted in polarities similar to those of the traditional solvents or some of their mixtures used for varnish removal.
To evaluate the solvent’s polarity, Nile red dye was chosen. Nile red has been used as a substantial lipophilic dye for fat stains; it exhibits large shifts at the wavelength of the maximum absorbance with solvent polarity and because of its unique stability in extremely acidic media and capacity to not lose molar absorptivity in the presence of acids as Reichardt’s dye does, it has been used commonly as an alternative to Reichardt dye.19,20
Using the same solvatochromic method, the interaction of probes/solvents can be measured by UV/visible spectroscopy.21 The solvatochromic absorption bands using Nile red were similar in all samples—a single typical spectral band around 400–600 nm with a wavelength corresponding to the maximum absorption.
The maximum wavelength (λmax) of each sample allowed the calculation of the polarity parameter (ENR) using the following equation22
| 1 |
where the ENR (kcal·mol–1) is the energy of transition and λmax (nm) is the maximum wavelength of the Nile red dye dissolved in NADESs. Because the more polar solvents cause Nile red to be absorbed at higher wavelengths, according to eq 1, these solvents will have lower ENR values. This shift in the λmax of Nile red by this type of solvent allows the calculation of the relative polarity of the solvent of interest.23
The normalization for both (traditional solvents and NADESs) was made using the values from Table 2, considering water as the reference for the more polar solvent [ENRN (water) = 1.0] and iso-octane the reference for a more nonpolar solvent [ENR (iso-octane) = 0.0], as shown below in eq 2. This normalization is based on the Reichardt method,23 adapting the polarity extremes for the data set of this study.
| 2 |
Table 2. Maximum Wavelength of Absorption, ENR, and Normalized ENR Values of Traditional Solvents and Their Mixturesa.
| solvents | λmax | ENR | ENRN | % water |
|---|---|---|---|---|
| water | 585.32 | 48.85 ± 0.17 | 1.000 | |
| Lac + Bet + Dec (5:1:0.1) | 574.33 | 49.78 ± 0.05 | 0.903 ± 0.010 | N/D |
| Thy + Bor (1:1) | 562.33 | 50.84 ± 0.14 | 0.792 ± 0.010 | N/D |
| Men + Thy (1:1) | 561.67 | 50.90 ± 0.05 | 0.786 ± 0.010 | 0.157 ± 0.020 |
| methanol | 559.50 | 51.10 ± 0.05 | 0.765 ± 0.010 | 22,265 ± 0.210 |
| Lac + Bet + Dec (2:1:0.35) | 555.00 | 51.52 ± 0.09 | 0.722 ± 0.010 | N/D |
| Men + Thy (2:1) | 553.33 | 51.67 ± 0.11 | 0.706 ± 0.010 | 0.127 ± 0.020 |
| Lac + Bet + Dec (2:1:0.25) | 553.33 | 51.67 ± 0.22 | 0.706 ± 0.020 | N/D |
| ethanol | 552.33 | 51.76 ± 0.14 | 0.696 ± 0.010 | <0.001 |
| isopropanol | 549.67 | 52.02 ± 0.07 | 0.670 ± 0.010 | 0.216 ± 0.020 |
| Men + Thy (4:1) | 549.00 | 52.08 ± 0.10 | 0.663 ± 0.010 | 0.133 ± 0.010 |
| Men + Mal (8:1) | 547.33 | 52.24 ± 0.11 | 0.646 ± 0.010 | N/D |
| Men + Thy (8:1) | 545.67 | 52.40 ± 0.06 | 0.630 ± 0.010 | N/D |
| iso-octane/isopropanol (1:9) | 542.00 | 52.75 ± 0.00 | 0.593 ± 0.010 | 0.513 ± 0.120 |
| iso-octane/isopropanol (2:8) | 541.67 | 52.78 ± 0.06 | 0.589 ± 0.010 | N/D |
| iso-octane/isopropanol (3:7) | 541.67 | 52.78 ± 0.06 | 0.589 ± 0.010 | 0.814 ± 0.090 |
| Men + Bor (7:2) | 540.33 | 52.91 ± 0.06 | 0.576 ± 0.010 | 0.158 ± 0.030 |
| Men + Lau (4:1) | 539.57 | 52.99 ± 0.07 | 0.568 ± 0.010 | 0.166 ± 0.020 |
| acetone | 539.50 | 53.00 ± 0.08 | 0.567 ± 0.010 | <0.2 |
| Men + Lau (8:1) | 538.43 | 53.01 ± 0.07 | 0.556 ± 0.000 | N/D |
| Men + Dec (2:1) | 538.00 | 53.14 ± 0.00 | 0.552 ± 0.000 | 0.203 ± 0.020 |
| Men + Myr (4:1) | 537.67 | 53.18 ± 0.06 | 0.548 ± 0.010 | N/D |
| iso-octane/iso-propanol (4:6) | 537.33 | 53.21 ± 0.11 | 0.545 ± 0.010 | 0.459 ± 0.020 |
| Men + Lau + Dec (4:1:1) | 537.00 | 53.24 ± 0.01 | 0.542 ± 0.010 | 0.088 ± 0.010 |
| Men + Dec (7:2) | 536.67 | 53.28 ± 0.11 | 0.538 ± 0.010 | 0.140 ± 0.010 |
| Men + Lau (2:1) | 535.33 | 53.41 ± 0.06 | 0.524 ± 0.010 | 0.067 ± 0.010 |
| Men + Lau + Dec (2:1:1) | 535.00 | 53.44 ± 0.10 | 0.521 ± 0.010 | 0.144 ± 0.020 |
| iso-octane/isopropanol (5:5) | 535.00 | 53.44 ± 0.10 | 0.521 ± 0.010 | 0.465 ± 0.020 |
| Men + Dec (1:3) | 532.00 | 53.74 ± 0.00 | 0.489 ± 0.000 | N/D |
| iso-octane/isopropanol (6:4) | 530.67 | 53.88 ± 0.06 | 0.475 ± 0.010 | 0.494 ± 0.140 |
| toluene | 526.00 | 54.36 ± 0.00 | 0.425 ± 0.000 | 0.049 ± 0.000 |
| iso-octane/isopropanol (7:3) | 525.67 | 54.39 ± 0.06 | 0.422 ± 0.010 | 0.361 ± 0.210 |
| Shellsol A | 525.33 | 54.42 ± 0.06 | 0.418 ± 0.010 | 0.112 ± 0.000 |
| xylene | 524.67 | 54.49 ± 0.06 | 0.411 ± 0.010 | 0.110 ± 0.000 |
| turpentine | 518.67 | 55.09± 0.06 | 0.345 ± 0.010 | N/D |
| iso-octane/isopropanol (8:2) | 518.00 | 55.55 ± 0.06 | 0.300 ± 0.010 | 0.208 ± 0.190 |
| white spirit (about 16% aromatic) | 516.67 | 55.36 ± 0.03 | 0.323 ± 0.010 | 0.051 ± 0.000 |
| iso-octane/isopropanol (9:1) | 514.67 | 55.67 ± 0.06 | 0.300 ± 0.010 | 0.168 ± 0.020 |
| Shellsol D40 | 494.50 | 57.82 ± 0.15 | 0.064 ± 0.020 | 0.008 ± 0.000 |
| Shellsol T (0% aromatic) | 494.00 | 57.88 ± 0.00 | 0.058 ± 0.000 | 0.008 ± 0.000 |
| iso-octane | 489.33 | 58.43 ± 0.03 | 0.000 | 0.006 ± 0.000 |
N/D—not determined.
The traditional solvents used to remove varnish layers can be a pure solvent or a mixture of solvents. Mixing two organic compounds with different solubilities (e.g., aliphatic hydrocarbons with weak alcohols) may minimize the risk of swelling and impregnation in the lower layers of the painting.24 Not only the polarity of systems but also there are more empirical features that are taken into consideration by the conservators to make the best decision on the solvent or combination of solvents to be used. The polarity of the traditional solvents used to remove varnish layers was determined following the same procedure used for NADESs, and the results are shown in Table 2, with the polarity decreasing from the top to the bottom of the table.
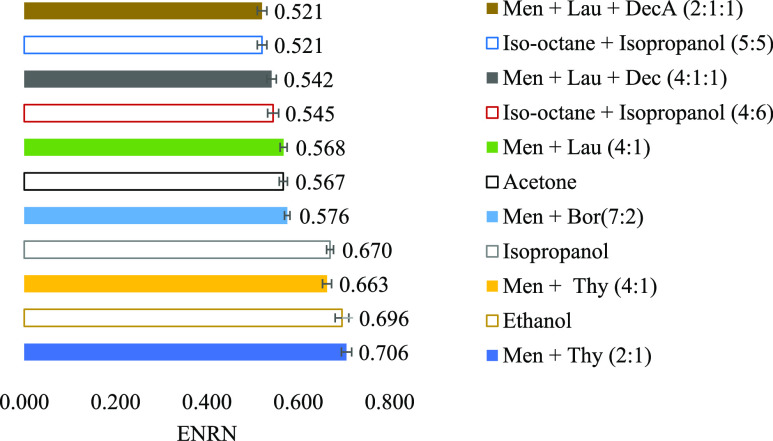
Observing the polarity values presented above in Table 2, it is possible to compare and organize some of them by a similarity order. The graphic in Figure 1 shows that some organic solvents such as acetone, isopropanol, ethanol, iso-octane/isopropanol (5:5), and iso-octane/isopropanol (4:6), may be replaced with systems such as Men + Lau (4:1) or Men + Bor (7:2), Men + Thy (4:1), Men + Thy (2:1), Men + Lau + Dec (2:1:1), and Men + Lau + Dec (4:1:1) respectively, considering the polarity parameter (ENRN). This provides indications of which of the NADES systems could be used in the replacement of the conventional solvents if only polarity was to be considered.
Figure 1.
Polarity comparison between traditional solvents (colorless graphic bars) and some NADES systems. This graphic shows some solvents which can, possibly, be replaced by a specific NADES. For comparison, normalized values were used.
Water contents of the solvents are also listed in Table 2. These values correspond to an average of at least three measurements. Because it may interfere with the polarity of the samples and become another important factor that needs to be considered, the water present in the systems was strictly controlled by Karl Fisher, and the risk is minimized.
The components of NADES in this study contain hydroxylic or carboxylic groups. These groups can facilitate the formation of hydrogen bonds and, hypothetically, when they are interacting, they may expel water molecules out of the system. For this reason, the percentage of water present is low (less than 0.3%) and the influence on the system’s polarity is very limited.
Removability/Solubility Test
Through the polarity evolution it is possible to select some promising systems which can be used to test the ability of NADES to remove the varnish layer. The dry varnish on the glass blade surface was cleaned using a cotton swab containing the NADES. If successful, the glass will produce an effect such as the one shown in Figure 2.
Figure 2.
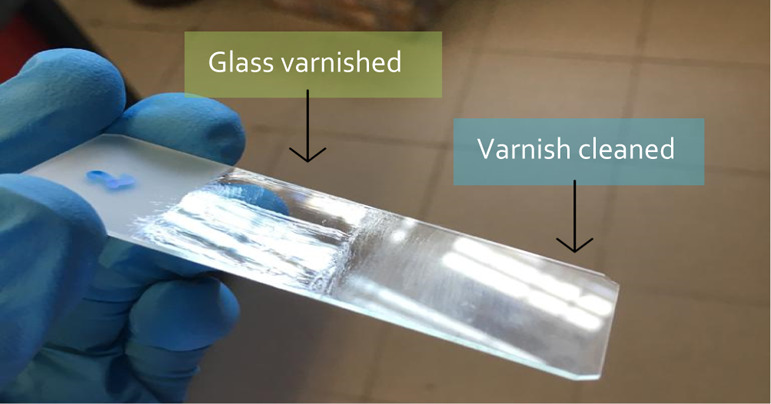
Glass slice containing dammar varnish, which was cleaned using the Men + Lau (4:1) system.
Figures 3 and 4 illustrate the investigated glass slides with mastic and dammar varnishes, after cleaning with different DESs, respectively. According to these figures, it is possible to successfully remove both varnishes with the selected eutectic systems. Glass slides with mastic varnishes 1–7 were cleaned using, respectively, with Men + Lau (4:1), Men + Lau + Dec (4:1:1), Men + Lau + Dec (2:1:1), Men + Thy (2:1), Men + Thy (4:1), Men + Bor (7:2), and Men + Dec (4:1). While glass slides with dammar varnishes 1–6 were cleaned using Men + Lau (4:1), Men + Lau + Dec (4:1:1), Men + Lau + Dec (2:1:1), Men + Thy (2:1), Men + Thy (4:1), and Men + bor (7:2), respectively.
Figure 3.
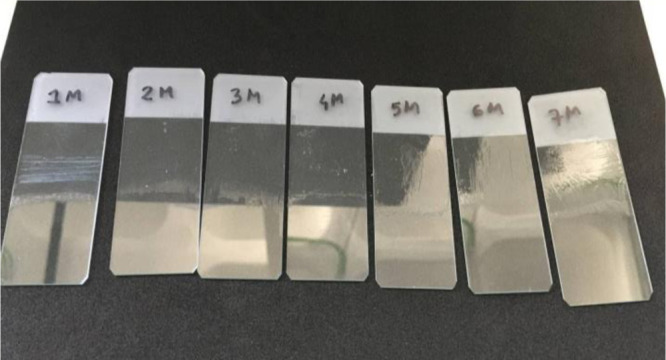
Glass slides with mastic varnish, after cleaning (the bottom half of the glass slide) with NADESs: (1) Men + Lau (4:1), (2) Men + Lau + Dec (4:1:1), (3) Men + Lau + Dec (2:1:1), (4) Men + Thy (2:1), (5) Men + Thy (4:1), (6) Men + Bor (7:2), and (7) Men + Dec (4:1).
Figure 4.

Glass slides with dammar varnish, after cleaning (the bottom half of the glass slide) with NADESs: 1. Men + Lau (4:1), 2. Men + Lau + Dec (4:1:1), 3. Men + Lau + Dec (2:1:1), 4. Men + Thy (2:1), 5. Men + Thy (4:1), and 6. Men + bor (7:2).
The dammar resin has proved to be soluble at least in 1:2 (w/v) DESs—same proportion as it is solubilized in turpentine. To quantify the solubility degree of dammar, three different eutectic systems that are relatively less polar were selected (Figure 5), namely, Men + Dec (1:3), Men + Lau (2:1), and Men + Lau (4:1). It was observed that dissolving consecutively a certain amount of dammar resin in 3 mL of NADES, there is a maximum quantity until a glue coat is formed: 1.73, 1.5, and 1.53 g for 1, 2, and 3, respectively, as shown in Figure 5.
Figure 5.

Dammar resin dissolved in different NADESs: (1) Men + Dec (1:3); (2) Men + Lau (2:1), and (3) Men + Lau (4:1).
The same amount of mastic resin is dissolved in the systems as described above in Figure 5. However, the viscosity of the products that formed was relatively low; this is because although the solubility behavior of dammar appears to be similar to mastic, when fresh, its polymeric fraction makes this kind of resin not completely soluble in alcohol-based solvents, such as ethanol. On the other hand, mastic is slightly more polar than dammar.2 These effects were observed during the cleaning process; while dammar-coated slides are more susceptible to solubilization with more hydrophobic systems, relatively more polar systems are able to better solubilize the mastic.
Teas Chart
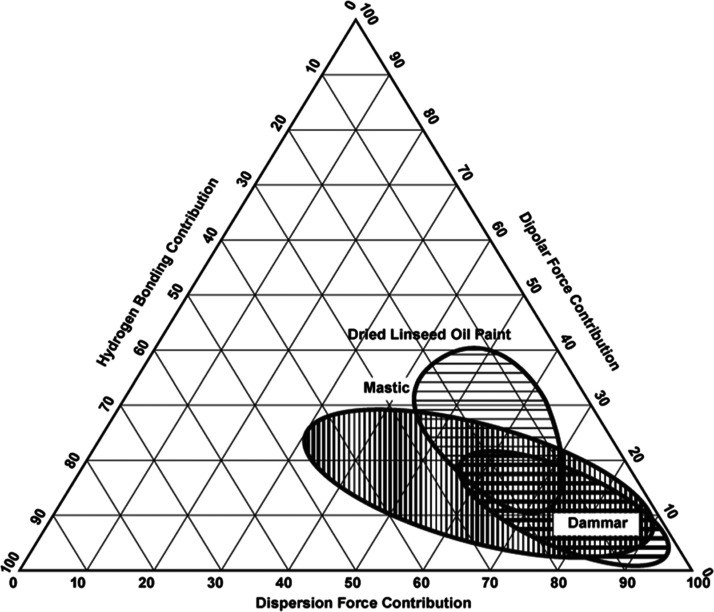
A Teas chart is a useful tool often used by the conservators to locate the solubility region of various solvents. It is based on a triangular graphic that uses the three Hansen’s solubility parameters (δd—dispersion forces; δp—polar forces; and δh—hydrogen bonding) to describe solvents power.25 The mapped “solubility regions” of fresh dammar and mastic, presented in Chart 1, help select which NADESs are less likely to damage the oil paint and then be used to remove the varnish.
Chart 1. Teas Plot for the Estimated Solubility of Mastic, Dammar, and Dried Linseed Oil Painta.
a Ref: https://www.naturalpigments.com/artist-materials/resin-mediums-damar-maroger/.
Estimation of Solubility Parameters
Hansen’s solubility parameters (δd, δp, and δh) of the most promising DES and traditional solvents listed in Table 2 were estimated using the group contribution method of Hoftyzer–Van Krevelen’s26 (see more in the Supporting Information). Once known, these parameters can be used to calculate the three fractional solubility parameters presented in eqs 3–5
| 3 |
| 4 |
| 5 |
where Fd, FP, and Fh correspond to the relative contributions to the cohesiveness of the substance of three types of intermolecular forces: dispersion, polar, and hydrogen bonding, respectively.27 These parameters were calculated and are listed in Table 3. Here, DES and any other solvent with a similar polarity are presented in the same color. When locating the approximate position of each compound on the Teas plot in Chart 1, it is possible to observe that both DES and traditional solvents lie in the “solubility region” of mastic; however, with respect to dammar, the values, both for DES and traditional solvents, are outside of its region. Although this seems to contradict the experimental results presented in Figure 5, literature only gives us the position of a very small number of solvents because most of the solvents used to clean are hydrophobic or ternary mixtures of compounds such as acetone, diacetone, alcohol, and mineral spirits.27−29 In fact, dammar is considered better than mastic, as a picture varnish, due to its resistance to yellowing, optical qualities, and lower polarity.28 Because hydrogen bonding is the predominate intermolecular force in the eutectic system formation, it is expected that there is a high contribution of Fh (and Fp) and, consequently, a low contribution to Fd. This means that the DES’s position can be oriented to the “solubility region” of dammar (more to the right of Fd), if its hydrophobic character increased. To solve this problem, regarding the more commonly used solvents, many authors proposed the use of binary mixtures, such as acetone/mineral spirits, propan-2-ol/butanone and propan-2-ol/iso-octane, progressively varying their proportions.4,25 Mixing solvents with different solubility behaviors has the advantage of allowing the adjustment or refinement of the solvent power and selecting which one can be used.
Table 3. Hansen’s and Hildebrand’s Total Solubility Parameters of the Set Solvents with Similar Polarity Calculated (in MPa1/2) Using Hoftyzer and Kreveleńs Method and Their Respective Fractional Solubility Parameters.
| solvents | δd | δp | δh | δt | Fd | Fp | Fh |
|---|---|---|---|---|---|---|---|
| Men + Lau + Dec (2:1:1) | 17.0 | 2.4 | 8.6 | 19.2 | 61.7 | 8.5 | 30.8 |
| iso-octane/isopropanol (5:5) | 9.3 | 2.1 | 5.1 | 15.5 | 56.4 | 12.5 | 31.0 |
| Men + Lau + Dec (4:1:1) | 17.3 | 2.5 | 9.3 | 19.8 | 59.5 | 8.7 | 31.8 |
| iso-octane/isopropanol (4:6) | 10.1 | 2.1 | 5.1 | 16.2 | 51.9 | 13.8 | 34.2 |
| Men + Lau (4:1) | 17.6 | 2.6 | 9.7 | 20.2 | 58.8 | 8.7 | 32.4 |
| acetone | 15.3 | 10.4 | 5.2 | 19.2 | 49.5 | 33.7 | 16.9 |
| Men + Bor (7:2) | 18.5 | 2.9 | 10.8 | 21.6 | 57.3 | 9.1 | 33.6 |
| isopropanol | 14.8 | 6.5 | 16.2 | 22.9 | 39.4 | 17.4 | 43.1 |
| Men + Thy (4:1) | 18.0 | 2.9 | 10.8 | 21.2 | 56.8 | 9.2 | 34.0 |
| ethanol | 15.4 | 8.6 | 18.5 | 25.6 | 36.3 | 20.2 | 43.6 |
| Men + Thy (2:1) | 18.0 | 3.0 | 10.9 | 21.3 | 56.8 | 9.3 | 34.1 |
By analyzing the graph in Chart 1, it is also possible to observe that a solvent with values of Fd, FP, and Fh in the ranges of 60–70, 10–20, and 10–25, respectively, will solubilize any of those resins. However, the important issue here is the knowledge of the solubility region for the oil paint. If the oil paint is too sensitive to the solvent, upon contact, it can lead to softening, swelling, and consequent removal of the exposed pigments.4 Another tool often used to classify the swelling degree or miscibility of solvents is the Hildebrand total solubility parameter (δt).26 It is based on Hansen’s solubility parameters (δd, δp, and δh, in MPa1/2), which can be calculated using the following equation
| 6 |
According to Stolow,24,30 an organic solvent will produce the greatest degrees of swelling, if its value of δt is in the range of 18–22.5 MPa1/2. As can be seen in Table 3, the values of all the DESs are in this range. However, it is important to clarify that the Hildebrand parameter is not the best tool to describe the eutectic system behavior, due to the low accuracy regarding polar or hydrogen bonding solvents.31−33
Aging Varnish
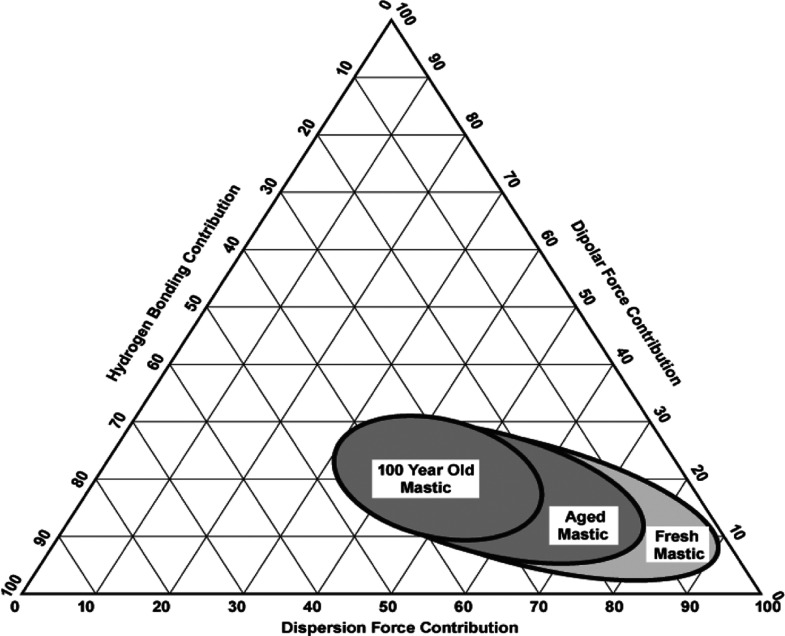
Aging is the main factor responsible for the change in varnish solubility, which is caused by many natural processes (e.g., photo-oxidation by the light influence). It leads to the increase of hydrophilic and acidity characters of the varnish and consequently the increase of strongly polar functional groups such as hydroxyls and carboxylic groups.3,34 It causes a significant increase of the values of Fh and Fp and decrease of the Fd values. In fact, Feller1 points out that a value of Fd above 68 can lead to the swelling of the oil paint.25 What is noticeable is that, once aged, the varnish will no longer be soluble in certain solvents previously used. In these cases, Teas chart parameters provide very relevant information for the possible mixtures that could be tested. As can be seen in Chart 2, solvents more polar are required during the aging process because it is well known that, once aged, the varnish often is no longer soluble in various solvents previously used.
Chart 2. Teas Plot for the Estimated Change in the Solubility of Mastic as a Function of Light Aginga.
a Ref: https://www.naturalpigments.com/artist-materials/resin-mediums-damar-maroger/.
Physical Properties: Contact Angle, Viscosity, and Density
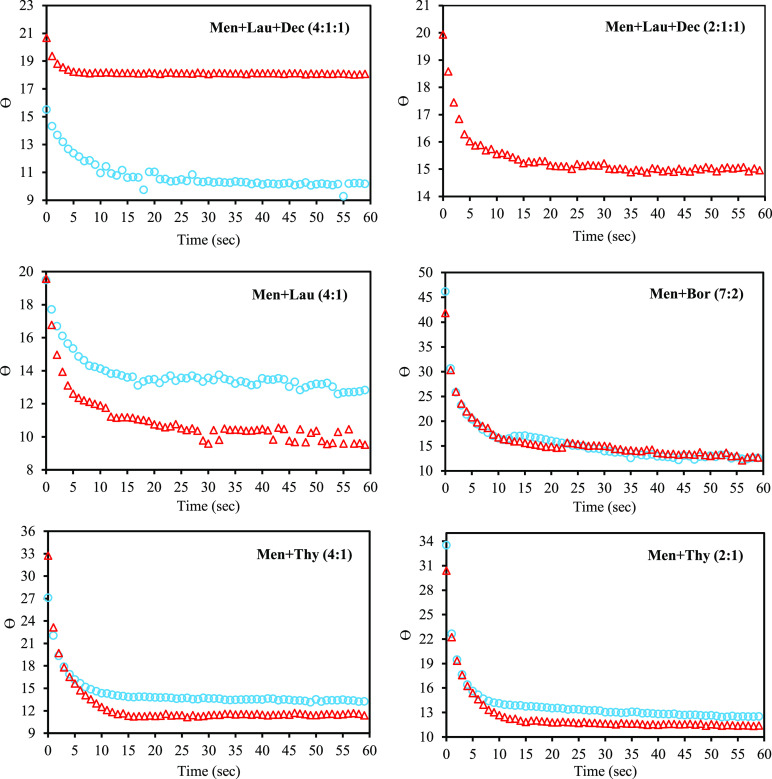
Besides polarity, other important physical properties are also relevant to this study. Measuring the contact angle, it confirmed the solubilization of both types of varnishes by NADESs and traditional solvents; however, compared to traditional solvents, where the drop is rapidly absorbed upon contact with the surface of the varnishes, NADES drops are slowly absorbed by the varnish. On the other hand, by analyzing the presented behaviors in Figure 6, it is obvious that the contact angle of the six NADESs presented are very low (<20°). This effect is commonly associated with a substrate with a high surface energy, which more easily attracts the drop, leading to a good wetting.35,36 According to Fox–Zisman,37 a low contact angle surface reveals a greater interaction between the drop and the investigated surface, comparative to the internal force of the drop. It may be a significant advantage for conservators because it allows them to have more control on the removability/solubility tests.
Figure 6.
Measured contact angles of investigated NADESs on surfaces varnished with varnishes based on natural mastic and dammar resins (mastic O and dammar Δ).
Another interesting observation regarding the contact angle that was presented by NADESs with the same components, but with different molar ratios [Men + Dec + Lau (2:1:1) and Men + Dec + Lau (4:1:1), as well as Men + Thy (4:1) and Men + Thy (2:1)], is that the less polar (<ENRN) system is more attracted by a dammar varnished surface. Also, regarding the angle stability on the surface of the dammar varnish, the NADES, Men + Lau + Dec (4:1:1) showed a good angle stability after 5 s; conversely, Men + Lau + Dec (2:1:1) showed angle stability after 15 s, and finally Men + Thy (4:1), Men + Thy (2:1), and Men + Bor (7:2) showed it after 10 s. On the other hand, with a mastic varnish as the substrate, Men + Dec + Lau (4:1:1) was shown to stabilize at 30 s, while Men + Thy (4:1), Men + Thy (2:1), and Men + Bor (7:2) stabilized after 10 s. The average angles of NADES on mastic and damar are presented in Table 4. It was not possible to measure the contact angle of the system Men + Lau + Dec (2:1:1) on mastic because this system, compared to the others, is quickly absorbed by the substrate. Also, in the case of Men + Lau (4:1), it is very difficult to confirm the real angle on both varnishes because, as it can be seen in Figure 6, there is a higher variability over time for this system.
Table 4. Measured Experimental Contact Angles, Polarities, and Viscosities of the Investigated NADESs in This Study.
| θaverage |
viscosity (mPa·s) | |||
|---|---|---|---|---|
| NADES | mastic | dammar | ENRN | at 293.15 K |
| Men + Lau + Dec (4:1:1) | 10.79 ± 1.12 | 18.22 ± 0.37 | 0.542 ± 0.010 | 30.11 ± 0.07 |
| Men + Lau + Dec (2:1:1) | not defined | 15.39 ± 0.86 | 0.521 ± 0.010 | 23.24 ± 0.04 |
| Men + Lau (4:1) | 13.77 ± 1.20 | 11.01 ± 1.72 | 0.568 ± 0.010 | 42.50 ± 0.62 |
| Men + Bor (7:2) | 15.77 ± 5.20 | 15.90 ± 4.69 | 0.576 ± 0.010 | 150.69 ± 0.33 |
| Men + Thy (4:1) | 14.34 ± 2.25 | 12.61 ± 3.39 | 0.663 ± 0.010 | 79.69 ± 0.18 |
| Men + Thy (2:1) | 13.94 ± 3.06 | 12.68 ± 3.03 | 0.706 ± 0.010 | 68.94 ± 0.16 |
Table 4 also includes the experimental data on the viscosity of the systems. Regarding the presented NADES viscosities, it can be observed that the three most viscous systems [Men + Bor (7:2), Men + Thy (4:1), and Men + Thy (2:1)] are also the three systems with the characteristic behaviors mentioned above. They show a similar contact angle on the two different investigated varnishes. However, for the Men + Lau (4:1) and Men + Lau + Dec (4:1:1) systems, there is a significant gap between the angles formed with the two investigated varnished surfaces. Also, in order to have a more comprehensive investigation on the physical properties of studied NADESs, their values of density and viscosity have been measured over a wide temperature range of 293.15–333.15 K and are presented in Tables S1 and S2 of the Supporting Information. The information of densities and viscosities for each NADES can be useful for choosing the most appropriate NADES according to the conditions and aims of each application. Because, in addition to the important properties of polarity and contact angle, density and especially viscosity should be considered as important operational properties for using NADES as new solvents. The interesting point of the measured densities of Table S2 is smaller density values with respect to water densities under the same conditions. Also, according to Table S1, two NADESs of Men + Lau + Dec (2:1:1) and Men + Lau + Dec (4:1:1) showed significantly lower viscosity values with respect to the other investigated NADESs.
Estimation of Other Physical Properties
The group contribution models were first used to estimate the thermodynamic behavior of mixtures containing ILs, as they cannot be experimentally determined because the degradation process starts when the temperature approaches its normal boiling point.38 Because the DES systems are, to some extent, similar to ILs, it is possible to apply the same group contribution models to the estimation of critical properties. DESs have been recently introduced; therefore, most of their thermodynamic properties are still unknown. Our group has studied and screened the properties of the NADESs listed in the literature in order to propose correlations to estimate the physical properties of the DESs with high accuracy, avoiding the need for complex and time-consuming experiments. These methods are, therefore, very useful, allowing the development of a correlation with the properties which are experimentally measured.39 For that, with only the knowledge of the molecular structure and the molecular weight of a compound, it is possible to estimate the critical properties of a certain compound. The accuracy of the estimated properties was reported in the literature and tested using the densities of the liquids. This has often been used by some authors in the study of ILs.38,40 The evaluation of the critical and thermodynamic properties of NADESs and traditional solvents was carried out using the modified Lydersen–Joback–Reid group contribution41,42 and the Lee–Kesler mixing rules,38 as proposed by Knapp et al.44 and are listed in Table 5. Details of the calculations of characterization properties of the investigated NADESs are presented in the Supporting Information.
Table 5. Critical and Thermodynamic Properties of the Studied Solvents Using Correlative Modelsa.
| solvents | Mw (g/mol) | Tc (K) | Pc (bar) | Vc (cm3/mL) | ω | ρ (g/cm3) | Cp (J/mol·k) | σ (mN/m) |
|---|---|---|---|---|---|---|---|---|
| Men + Lau + Dec (2:1:1) | 171.28 | 826.18 | 20.09 | 720.05 | 0.940 | 1.108 | 470.75 | 10.49 |
| iso-octane/isopropanol (5:5) | 87.16 | 537.22 | 34.01 | 326.5 | 0.420 | 0.954 | 172.91 | 31.2 |
| Men + Lau + Dec (4:1:1) | 166.28 | 688.65 | 22.95 | 637.38 | 0.796 | 1.112 | 378.42 | 10.48 |
| iso-octane/isopropanol (4:6) | 81.75 | 534.50 | 36.25 | 309.64 | 0.446 | 0.965 | 154.18 | 31.27 |
| Men + Lau (4:1) | 165.07 | 735.56 | 27.82 | 529.48 | 0.584 | 1.101 | 309.56 | 11.88 |
| acetone | 58.08b | 508.10b | 47.00b | 209.00b | 0.304b | 0.785c | 124.50d | 23.10e |
| Men + Bor (7:2) | 155.82 | 786.05 | 34.51 | 472.82 | 0.48 | 1.162 | 297.86 | 22.62 |
| isopropanol | 60.09b | 508.30b | 47.62b | 220.00b | 0.665b | 0.785c | 143.69d | 24.00e |
| Men + Thy (4:1) | 155.06 | 700.53 | 31.95 | 449.46 | 0.517 | 1.002 | 297.57 | 24.97 |
| ethanol | 46.06b | 513.92b | 61.48b | 167.00b | 0.649b | 0.789c | 114.90d | 21.82e |
| Men + Thy (2:1) | 154.25 | 687.98 | 32.87 | 428.18 | 0.523 | 1.098 | 296.52 | 27.97 |
These parameters were estimated at 298.15 K.
Critical properties, ref (45).
Density, ref (46).
Heat capacity, ref (47).
Surface tension, ref.: https://www.engineeringtoolbox.com/surface-tension-d_962.html.
Properties such as density, heat capacity, and surface tension are specific for each compound and help to better characterize and describe them. Compared to many other systems tested in the literature, the values presented are in the same range (see more details in the Supporting Information). Although density is a basic physical property of any chemical compound, these data are still unavailable for DES. This problem also applies to the relative surface tension data. Because the cleaning method consists of applying the solvent onto the paint surface, it is crucial to know what the surface tension is, and depending on the value of surface tension, it is possible to predict the capacity of absorption of DESs for the paint layers. In fact, penetration is one of the big challenges, when thinking about DESs as alternative solvents, because they do not evaporate. However, the viscosity of a DES can be an advantage, as it allows for a certain working time before penetration. Regarding the calculated surface tension of NADESs listed in Table 5, we notice that they present relatively low values of surface tension when compared to the traditional solvents. Surface tension also depends on the nature of the varnish surface (hydrophilic or hydrophobic), and because multiple layers of varnish (from the surface to the pictorial layer) can have different degrees of aging, this will affect the absorption of a DES. Theoretically, the older the layer the better it will be dissolved with a DES.
Conclusions
In order to replace the traditional solvents by the DES in the field of conservation and restoration, it is crucial to understand their physicochemical properties. Because of the complexity of NADES, there are many parameters which affect the solubility strength. Surely, polarity is just one of those parameters for NADES characterization, as it can reflect the solubility of compounds. In the study of solvents commonly used in conservation, the polarity parameter was a good tool, allowing the identification of some similar properties between the two systems. Some of these DESs were then used as solvents to dissolve natural resin varnishes, proving the higher dissolving capacity of these systems. In this study, it was observed that when fresh, dammar and mastic are soluble in a wide range of compounds. However, knowing that the solubility of these resins changes over time, more experiments should be done. Nevertheless, through the contribution groups, it is possible to predict the behavior of a DES in the Teas chart. The Teas chart complemented this study because it showed theoretically that DESs can be used to remove the mastic varnish, even when aged. Nevertheless, further studies should be carried out regarding the dammar varnish and its “solubility region”. The empirical three-dimensional method, although with some inaccuracies, if interpreted too rigidly, has the advantage of allowing the observation of the effects of solvents as well as their mixtures and establish where it lies on the Teas plot. Further, it allows one to determine the solvents that clean satisfactorily and swell the oil paint the least. Furthermore, determination of properties such as density, surface tension, heat capacity, and critical properties may be essential for a better understanding of these solvents, as well their behavior under certain conditions. For future works, it is crucial to test the acidity and/or solubility of the aged resin varnish samples removed using some of these selected DES systems and understand their effectiveness as well as limitations when used in the context of cleaning the varnish of an oil painting.
Acknowledgments
This work received funding from ERC-2016-CoG 725034 and was supported by the Associate Laboratory for Green Chemistry-LAQV, financed by national funds from FCT/MCTES (UIDB/50006/2020).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.1c04591.
Experimental densities and viscosities of investigated NADESs, calculation steps for the characterization of NADESs, and detailed procedure for the calculation of solubility parameters (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Feller R. L. Dammar and mastic varnishes—hardness, brittleness, and change in weight upon drying. Stud. Conserv. 1958, 3, 162–174. 10.1179/sic.1958.024. [DOI] [Google Scholar]
- Phenix A.; Wolbers R.. Removal of varnish: organic solvents as cleaning agents. Conservation Easel Paint Joyce Hill Stoner Rebecca Rushf; Routledge: New York, 2012; pp 524–563. [Google Scholar]
- Van der Doelen G. A.; van den Berg K. J.; Boon J. J.; Shibayama N.; De La Rie E. R.; Genuit W. J. L. Analysis of fresh triterpenoid resins and aged triterpenoid varnishes by high-performance liquid chromatography–atmospheric pressure chemical ionisation (tandem) mass spectrometry. J. Chromatogr. A 1998, 809, 21–37. 10.1016/S0021-9673(98)00186-1. [DOI] [Google Scholar]
- Carlyle L.The Artist’s Assistant: Oil Painting Instruction Manuals and Handbooks in Britain 1800–1900, with Reference to Selected Eighteenth-Century Sources; Archetype Publications Ltd: London, 2001; Vol. 228. [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. 10.1039/B210714G. [DOI] [PubMed] [Google Scholar]
- Guthrie F. On eutexia. Proc. Phys. Soc. Lond. 1884, 6, 124. 10.1088/1478-7814/6/1/312. [DOI] [Google Scholar]
- Seddon K. R. Room-temperature ionic liquids: neoteric solvents for clean catalysis. Kinet. Catal. 1995, 37, 693–697. 10.1002/chin.199709207. [DOI] [Google Scholar]
- Rogers R. D.; Seddon K. R. Ionic liquids--solvents of the future?. Science 2003, 302, 792–793. 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez A.; Edler K. J.; Arnold T.; Alba Venero D.; Jackson A. J. Protein conformation in pure and hydrated deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 8667–8670. 10.1039/C7CP00459A. [DOI] [PubMed] [Google Scholar]
- Paiva A.; Craveiro R.; Aroso I.; Martins M.; Reis R. L.; Duarte A. R. C. Natural deep eutectic solvents - Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. 10.1021/sc500096j. [DOI] [Google Scholar]
- Yang Z.Toxicity and biodegradability of deep eutectic solvents and natural deep eutectic solvents. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley, 2019; pp 43–60. [Google Scholar]
- Silva J. M.; Pereira C. V.; Mano F.; et al. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. 10.1021/acsabm.9b00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayyan M.; Hashim M. A.; Al-Saadi M. A.; Hayyan A.; AlNashef I. M.; Mirghani M. E. S. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. 10.1016/j.chemosphere.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Hayyan M.; Looi C. Y.; Hayyan A.; Wong W. F.; Hashim M. A. In Vitro and in Vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS One 2015, 10, e0117934 10.1371/journal.pone.0117934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.; An Y.; Row K. H. Emerging applications of (micro) extraction phase from hydrophilic to hydrophobic deep eutectic solvents: opportunities and trends. TrAC, Trends Anal. Chem. 2021, 136, 116187. 10.1016/j.trac.2021.116187. [DOI] [Google Scholar]
- Craveiro R.; Aroso I.; Flammia V.; et al. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. 10.1016/j.molliq.2016.01.038. [DOI] [Google Scholar]
- Martins M. A. R.; Silva L. P.; Schaeffer N.; et al. Greener Terpene-Terpene Eutectic Mixtures as Hydrophobic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 17414–17423. 10.1021/acssuschemeng.9b04614. [DOI] [Google Scholar]
- Haghbakhsh R.; Bardool R.; Bakhtyari A.; Duarte A. R. C.; Raeissi S. Simple and global correlation for the densities of deep eutectic solvents. J. Mol. Liq. 2019, 296, 111830. 10.1016/j.molliq.2019.111830. [DOI] [Google Scholar]
- Reichardt C. Empirical Parameters of Solvent Polarity as Linear Free-Energy Relationships. Angew Chem. Int. Ed. Engl. 1979, 18, 98–110. 10.1002/anie.197900981. [DOI] [Google Scholar]
- Deye J. F.; Berger T. A.; Anderson A. G. Nile Red as a Solvatochromic Dye for Measuring Solvent Strength in Normal Liquids and Mixtures of Normal Liquids with Supercritical and Near Critical Fluids. Anal. Chem. 1990, 62, 615–622. 10.1021/ac00205a015. [DOI] [Google Scholar]
- Ranjkesh A.; Hagh Parast M.; Park J.-S.; Choi J.-C.; Zakerhamidi M. S.; Kim H.-R. Determination of polarity parameters for liquid crystals using solvatochromic method in anisotropic and isotropic phases. Liq. Cryst. 2017, 44, 695–704. 10.1080/02678292.2016.1233584. [DOI] [Google Scholar]
- Jessop P. G.; Jessop D. A.; Fu D.; Phan L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. 10.1039/c2gc16670d. [DOI] [Google Scholar]
- Reichardt C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. 10.1021/cr00032a005. [DOI] [Google Scholar]
- Phenix A.; Sutherland K. The cleaning of paintings: effects of organic solvents on oil paint films. Stud. Conserv. 2001, 46, 47–60. 10.1179/sic.2001.46.supplement-1.47. [DOI] [Google Scholar]
- Hedley G. Solubility parameters and varnish removal: a survey. Conserv 1980, 4, 12–18. 10.1080/01410096.1980.9994931. [DOI] [Google Scholar]
- Van Krevelen D. W.; Te Nijenhuis K.. Properties of Polymers; Elsevier, 2009. [Google Scholar]
- Hansen C. M.Hansen Solubility Parameters: A User's Handbook, Second Edition (2nd ed.), CRC Press, 2007, ISBN: 9781420006834, 10.1201/9781420006834. [DOI] [Google Scholar]
- Hill J.Conservation of Easel Paintings; Routledge, 2013. [Google Scholar]
- Rao N. S.; Schumacher G.; Rao N. S.; Schumacher G.. Thermodynamic Properties of Polymers. Design Formulas for Plastics Engineers; Carl Hanser Verlag GmbH, 2004; pp 35–41. [Google Scholar]
- Feller R. L.; Curran M. Changes in solubility and removability of varnish resins with age. Bull. Am. Inst. Conserv. 1975, 15, 17–48. 10.1179/019713675806156654. [DOI] [Google Scholar]
- Venkatram S.; Kim C.; Chandrasekaran A.; Ramprasad R. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194. 10.1021/acs.jcim.9b00656. [DOI] [PubMed] [Google Scholar]
- Salehi H. S.; Ramdin M.; Moultos O. A.; Vlugt T. J. H. Computing solubility parameters of deep eutectic solvents from Molecular Dynamics simulations. Fluid Phase Equilib. 2019, 497, 10–18. 10.1016/j.fluid.2019.05.022. [DOI] [Google Scholar]
- Lee S. H.; Lee S. B. The Hildebrand solubility parameters, cohesive energy densities and internal energies of 1-alkyl-3-methylimidazolium-based room temperature ionic liquids. Chem. Commun. 2005, 3469–3471. 10.1039/b503740a. [DOI] [PubMed] [Google Scholar]
- Dietemann P.; Higgitt C.; Kälin M.; Edelmann M. J.; Knochenmuss R.; Zenobi R. Aging and yellowing of triterpenoid resin varnishes - Influence of aging conditions and resin composition. J. Cult. Herit. 2009, 10, 30–40. 10.1016/j.culher.2008.04.007. [DOI] [Google Scholar]
- Marshall K. L.; Didovets O.; Saulnier D. Contact-angle measurements as a means of probing the surface alignment characteristics of liquid crystal materials on photoalignment layers. Liq. Cryst. 2014, 9182, 91820J. 10.1117/12.2062074. [DOI] [Google Scholar]
- Bracco G.; Holst B.. Surface Science Techniques; Springer, 2013; Vol. 51. [Google Scholar]
- Zisman W. A.Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution; American Chemical Society, 1964; pp 1–51. [Google Scholar]
- Valderrama J. O.; Robles P. A. Critical properties, normal boiling temperatures, and acentric factors of fifty ionic liquids. Ind. Eng. Chem. Res. 2007, 46, 1338–1344. 10.1021/ie0603058. [DOI] [Google Scholar]
- Haghbakhsh R.; Taherzadeh M.; Duarte A. R. C.; Raeissi S. A general model for the surface tensions of deep eutectic solvents. J. Mol. Liq. 2020, 307, 112972. 10.1016/j.molliq.2020.112972. [DOI] [Google Scholar]
- Valderrama J. O.; Abu-Sharkh B. F. Generalized rackett-type correlations to predict the density of saturated liquids and petroleum fractions. Fluid Phase Equilib. 1989, 51, 87–100. 10.1016/0378-3812(89)80356-5. [DOI] [Google Scholar]
- Lydersen A. L.“Estimation of Critical Properties of Organic Compounds” University of Wisconsin, College of Engineering, Engineering Experimental Station Report 3: Madison, Wisconsin, U.S.A., 1955.
- Joback K. G.; Reid R. C. Estimation of Pure Component Properties from Group Contribution. Chem. Eng. Commun. 1987, 57, 233–243. 10.1080/00986448708960487. [DOI] [Google Scholar]
- Knapp H.; Doring R.; Oellrich L.; Plocker U.; Prausnitz J. M.. Vapor-Liquid Equilibria for Mixtures of Low Boiling Substances (Ternary Systems). Dechema Chemistry Data Series, Vol. VI, Part 2, 3 und 4. VonH. Knapp, S. ZeckundR. Langhorst. DECHEMA, Frankfurt/M. 1989. 3. 418 S., geb., DM 742; Wiley Online Library, 1990. [Google Scholar]
- Reid R. C.; Sherwood T. K.; Street R. E.. The Properties of Gases and Liquids; McGraw Hill Book Co., 1959; Vol. 12, p 38. [Google Scholar]
- Joshi D. R.; Adhikari N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. 10.9734/jpri/2019/v28i330203. [DOI] [Google Scholar]
- Domalski E. S.; Hearing E. D. Heat Capacities and Entropies of Organic Compounds in the Condensed Phase Volume II. J. Phys. Chem. Ref. Data 1990, 19, 881–1047. 10.1063/1.555876. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.