Visual Abstract
Keywords: hepatocellular carcinoma, macrovascular invasion, transarterial radioembolization, systemic treatment
Abstract
Systemic therapy remains the recommended first-line treatment for hepatocellular carcinoma (HCC) with macrovascular invasion (MVI). Transarterial radioembolization (TARE) is a promising alternative treatment, given its potential to impart a superior quality of life. The aims of this study were, first, to characterize trends and correlates for TARE as a first-line treatment for HCC patients with MVI in the United States and, second, to compare survival after TARE versus systemic therapy. Methods: We used the U.S. National Cancer Database to identify patients with T3BN0M0 HCC during 2010–2017. We performed multivariable logistic regression to identify factors associated with use of TARE versus systemic therapy and Cox proportional-hazards regression to identify factors associated with overall survival. Results: Of 11,259 patients with T3BN0M0 HCC, 1,454 (12.9%) and 3,915 (34.7%) were treated with TARE and systemic therapy, respectively. The proportion of patients who received TARE increased from 13.0% in 2010 to 37.0% in 2017. Older age, white race, and receiving care at an academic cancer program were associated with receipt of TARE, whereas lack of insurance, higher model-for-end-stage-liver-disease score, Charlson comorbidity index of at least 3, and Northeast region were associated with receipt of systemic therapy. TARE was associated with reduced mortality compared with systemic therapy (adjusted hazard ratio, 0.74; 95% CI, 0.68–0.80), with consistent results observed in propensity-weighted analysis and across all examined subgroups. Conclusion: Use of TARE as first-line therapy for HCC with MVI has increased in the United States. Patient characteristics, region, and medical center type affected the use of TARE. TARE was associated with reduced mortality compared with systemic therapy for HCC patients with MVI.
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and typically occurs in the setting of chronic liver disease. It is among the leading causes of cancer incidence and mortality globally (1). Macrovascular invasion (MVI) of the portal vein or hepatic vein is one of the defining features of advanced-stage HCC (2). Traditionally, systemic therapy using the molecular targeted agent sorafenib has been the only treatment that increased median survival and time to progression in these patients (3,4). Recent advances in systemic therapy include the approval of lenvatinib and, more recently, the combination of atezolizumab and bevacizumab (5,6).
Transarterial radioembolization (TARE) with 90Y microspheres is a form of locoregional therapy in HCC patients that can be provided safely to patients with portal vein invasion and has been shown to achieve a better time to progression than conventional transarterial chemoembolization (7). TARE has been proposed as an alternative therapy for HCC patients with MVI, given the potential for response and downstaging (8). Several retrospective studies (9–11) and randomized controlled trials (12,13) in HCC patients with MVI demonstrated that TARE was associated with overall survival (OS) comparable to, and treatment-related adverse events lower than, those with systemic therapy. Although professional society guidelines continue to endorse systemic treatment as the first-line therapy for HCC with MVI (14,15), TARE has been widely adopted in clinical practice (16).
Trends in the use of TARE and comparisons of its effectiveness with that of systemic therapy in real-world clinical practice have not been well characterized. Therefore, the aim of this study was to characterize—for HCC with MVI in the United States—temporal trends in the use of TARE, factors associated with the use of TARE, and OS after TARE as a first-line treatment compared with systemic treatment.
MATERIALS AND METHODS
Database
The National Cancer Database (NCDB) is a large, nationwide clinical oncology database jointly sponsored by the American College of Surgeons and the American Cancer Society. The NCDB contains hospital registry data from more than 1,500 Commission on Cancer–accredited facilities in the United States, representing more than 70% of newly diagnosed cancer cases and 34 million historical records.
Patients and Variables
All patients who were diagnosed with tumor stage T3BN0M0 HCC between January 2010 and December 2017 were identified from the NCDB. The diagnosis of HCC was based on oncology code C22.0 of the International Classification of Diseases, third revision, and histology codes 8170–8175. T3BN0M0 HCC was defined as tumor involving a major branch of a large vein of the liver without lymph node involvement or extrahepatic metastasis. Patients for whom treatment information was missing or who did not receive TARE or systemic treatment were excluded.
TARE was defined using the variable “phase I radiation treatment modality,” which records the radiation modality administered during the first phase of radiation treatment delivered during the first course of cancer treatment. Patients with any of the following codes were considered to have received TARE: brachytherapy not otherwise specified; brachytherapy, intracavitary, low dose rate; brachytherapy, intracavitary, high dose rate; brachytherapy, interstitial, low dose rate; brachytherapy, interstitial, high dose rate; or radioisotopes not otherwise specified. Systemic therapy was defined using the variable “chemotherapy” or “immunotherapy,” which record the type of chemotherapy or immunotherapy administered as the first-course treatment at any facility.
Patient demographics, socioeconomic status, medical comorbidities, treatment facility, and treatment region were extracted from the NCDB. Demographic information included patient`s age, sex, and race/ethnicity. Socioeconomic status was characterized using insurance coverage, median income, educational attainment, and living environment. Patient medical comorbidities were described in terms of the Charlson/Deyo comorbidity index (0, 1, 2, ≥3). Liver and HCC-specific clinical data, including model-for-end-stage-liver-disease (MELD) score, method of diagnosis, tumor burden, and α-fetoprotein (AFP) level, were captured for all patients. Treating facilities were classified into 4 categories: academic (>500 new cancer diagnoses annually and at least 4 postgraduate training programs), comprehensive community (>500 new cancer diagnoses annually), integrated network (no minimum caseload, joint venture with multiple facilities, at least one of which is a hospital and participates in the Commission on Cancer–accredited cancer program), and community (100–500 new cancer diagnoses annually). The facilities were also categorized according to their geographic region within the United States (Northeast, Midwest, South, West).
Statistical Analysis
Bivariate comparison of TARE versus systemic treatment for continuous and categoric variables was performed using the Welch t test, Wilcoxon–Mann–Whitney test, or Pearson χ2 test when appropriate. Univariate and multivariable logistic regression was used to identify factors associated with use of TARE versus systemic therapy. Kaplan–Meier analysis was used to estimate survival probabilities, and the log-rank test was used to compare Kaplan–Meier curves. Time to event was defined as the time from HCC diagnosis to last follow-up or death. Furthermore, univariate and multivariable Cox proportional-hazards regression was used to identify factors associated with OS. To adjust for potential confounders, propensity score matching and inverse-probability-of-treatment–weighted analyses were performed (17). Propensity score–matched cohorts were constructed by performing a 1:1 match using a caliper of 0.20 and the nearest-neighbor method (18). A multivariable logistic regression model was used to construct the propensity scores, including age, sex, race, ethnicity, insurance, comorbidity, AFP level, MELD score, facility type, and geographic region. Inverse-probability-of-treatment–weighted analysis was based on the propensity scores and included in the Cox proportional-hazards regression model as case weights. The proportional-hazards assumption among all survival models was assessed by the scaled Schoenfeld residuals and by the goodness-of-fit test as proposed by Grambsch and Therneau (19). To account for missing data in the NCDB, the chained-equation approach for multiple imputations was used before performing regression analyses (20). All statistical analyses were performed using R statistical software (version 4.0.3; R Foundation) with 2-sided tests and a significance level of 0.05.
RESULTS
Patient Characteristics
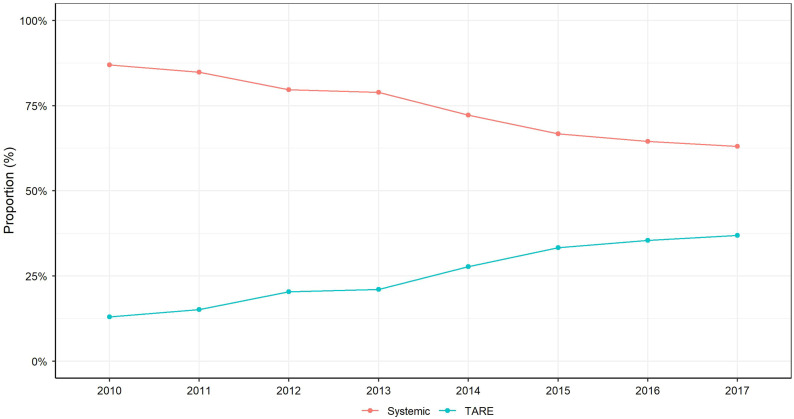
Of 11,259 patients diagnosed with T3BN0M0 HCC during 2010–2017, 1,454 (12.9%) and 3,915 (34.8%) were treated with TARE and systemic therapy, respectively, and included in the study (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). The proportion of patients receiving TARE increased from 13.0% in 2010 to 37.0% in 2017 (Fig. 1). The median age of patients was 63.0 y, with 80.7% being male (Table 1). The cohort was 64% non-Hispanic white, 16.3% black, and 10.2% Hispanic. Over half the patients had government (Medicare or Medicaid) insurance coverage, although 34.5% had private insurance and 5.4% of patients were uninsured. Over three fourths (76.0%) had a comorbidity score of 0–1, and the median MELD score was 11. The median tumor diameter was 7.1 cm, and 85% had an elevated AFP level at diagnosis. Nearly two thirds (61.7%) of patients were treated at an academic center, 22.2% at a comprehensive community cancer center, and 12.2% at an integrated network.
FIGURE 1.
Proportion of patients who received TARE vs. systemic treatment for HCC with MVI between 2010 and 2017.
TABLE 1.
Clinical Features of Patients Before and After Propensity Score Matching, Part 1
| Before propensity score matching | After propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | All (n = 5,369) | Systemic (n = 3,915) | TARE (n = 1,454) | P | All (n = 1,136) | Systemic (n = 568) | TARE (n = 568) | P |
| Demographics | ||||||||
| Age (y) | 63.0 ± 9.73 | 62.6 ± 9.84 | 64.2 ± 9.33 | <0.001 | 63.9 ± 9.13 | 63.7 ± 9.49 | 64.1 ± 8.75 | 0.420 |
| Sex | 0.720 | 0.880 | ||||||
| Male | 4,331 (80.7%) | 3,153 (80.5%) | 1,178 (81.0%) | 917 (80.7%) | 460 (81.0%) | 457 (80.5%) | ||
| Female | 1,038 (19.3%) | 762 (19.5%) | 276 (19.0%) | 219 (19.3%) | 108 (19.0%) | 111 (19.5%) | ||
| Race | <0.001 | 0.587 | ||||||
| White | 3,347 (64.0%) | 2,356 (61.8%) | 991 (70.0%) | 781 (68.8%) | 385 (67.8%) | 396 (69.7%) | ||
| Hispanic | 536 (10.2%) | 426 (11.2%) | 110 (7.77%) | 87 (7.66%) | 47 (8.27%) | 40 (7.04%) | ||
| Black | 851 (16.3%) | 660 (17.3%) | 191 (13.5%) | 168 (14.8%) | 81 (14.3%) | 87 (15.3%) | ||
| Asian + others | 496 (9.48%) | 373 (9.78%) | 123 (8.69%) | 100 (8.80%) | 55 (9.68%) | 45 (7.92%) | ||
| Socioeconomic factors | ||||||||
| Insurance status | <0.001 | 0.933 | ||||||
| Uninsured | 285 (5.42%) | 247 (6.46%) | 38 (2.65%) | 39 (3.43%) | 21 (3.70%) | 18 (3.17%) | ||
| Private | 1,815 (34.5%) | 1,319 (34.5%) | 496 (34.5%) | 392 (34.5%) | 197 (34.7%) | 195 (34.3%) | ||
| Medicaid/Medicare | 3,072 (58.4%) | 2,209 (57.8%) | 863 (60.1%) | 683 (60.1%) | 340 (59.9%) | 343 (60.4%) | ||
| Other | 88 (1.67%) | 49 (1.28%) | 39 (2.72%) | 22 (1.94%) | 10 (1.76%) | 12 (2.11%) | ||
| Median income | 0.025 | 0.716 | ||||||
| <$40,227 | 1,168 (23.7%) | 882 (24.5%) | 286 (21.6%) | 246 (21.7%) | 125 (22.0%) | 121 (21.3%) | ||
| $40,227–$50,353 | 1,103 (22.4%) | 825 (22.9%) | 278 (21.0%) | 268 (23.6%) | 135 (23.8%) | 133 (23.4%) | ||
| $50,354–$63,332 | 1,113 (22.6%) | 790 (21.9%) | 323 (24.4%) | 279 (24.6%) | 145 (25.5%) | 134 (23.6%) | ||
| $63,333+ | 1,540 (31.3%) | 1,103 (30.6%) | 437 (33.0%) | 343 (30.2%) | 163 (28.7%) | 180 (31.7%) | ||
| Without high school diploma | 0.003 | 0.099 | ||||||
| ≥17.6% | 1,345 (27.3%) | 1,023 (28.4%) | 322 (24.3%) | 269 (23.7%) | 152 (26.8%) | 117 (20.6%) | ||
| 10.9%–17.5% | 1,360 (27.6%) | 1,007 (27.9%) | 353 (26.6%) | 310 (27.3%) | 152 (26.8%) | 158 (27.8%) | ||
| 6.3%–10.8% | 1,305 (26.5%) | 934 (25.9%) | 371 (28.0%) | 338 (29.8%) | 162 (28.5%) | 176 (31.0%) | ||
| <6.3% | 922 (18.7%) | 641 (17.8%) | 281 (21.2%) | 219 (19.3%) | 102 (18.0%) | 117 (20.6%) | ||
| Urban/rural | 0.373 | 0.885 | ||||||
| Metro | 4,554 (87.1%) | 3,338 (87.5%) | 1,216 (86.1%) | 974 (85.7%) | 489 (86.1%) | 485 (85.4%) | ||
| Urban | 600 (11.5%) | 424 (11.1%) | 176 (12.5%) | 142 (12.5%) | 70 (12.3%) | 72 (12.7%) | ||
| Rural | 73 (1.40%) | 52 (1.36%) | 21 (1.49%) | 20 (1.76%) | 9 (1.58%) | 11 (1.94%) | ||
Available in only 24% of patients.
Data are number followed by percentage in parentheses, except for MELD and tumor size (median followed by interquartile range) and age (mean ± SD).
Factors Associated with Receipt of TARE
In multivariable logistic regression analysis (Table 2), independent predictors of receiving TARE included older age (odds ratio [OR], 1.17; 95% CI, 1.09–1.27), having private insurance (OR, 2.04; 95% CI, 1.38–2.95) or Medicaid/Medicare (OR, 1.89; 95% CI, 1.28–2.72) versus being uninsured, and treatment in the Midwest (OR, 1.64; 95% CI, 1.36–2.08), South (OR, 1.27; 95% CI, 1.07–1.56), and West (OR, 1.74; 95% CI, 1.42–2.20) regions versus Northeast region. Factors associated with decreased odds of TARE included Hispanic ethnicity (OR, 0.69; 95% CI, 0.54–0.88) or Asian/other (OR, 0.74; 95% CI, 0.60–0.97) versus white race/ethnicity, receiving treatment in a community cancer program (OR, 0.42; 95% CI, 0.27–0.68) or comprehensive community cancer program (OR, 0.73; 95% CI, 0.61–0.86) versus an academic program, Charlson index of at least 3 versus 0 or 1 (OR, 0.82; 95% CI, 0.68–0.98), and higher MELD score (OR, 0.76;95% CI, 0.66–0.80).
TABLE 2.
Factors Predicting TARE Treatment Among Patients with T3BN0M0 HCC
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | P | HR | P | |
| Age (10-y change) | 1.194 (1.122–1.272) | <0.001 | 1.174 (1.091–1.267) | <0.001 |
| Sex, male (reference) | (reference) | (reference) | ||
| Sex, female | 0.969 (0.831–1.129) | 0.691 | 0.957 (0.802–1.122) | 0.607 |
| Race, white (reference) | (reference) | (reference) | ||
| Race, Hispanic | 0.615 (0.496–0.768) | <0.001 | 0.685 (0.536–0.883) | 0.003 |
| Black | 0.688 (0.576–0.817) | <0.001 | 0.829 (0.675–1.013) | 0.070 |
| Race, Asian + others | 0.796 (0.664–1.013) | 0.034 | 0.735 (0.604–0.973) | 0.012 |
| Uninsured (reference) | (reference) | (reference) | ||
| Private insurance | 2.362 (1.648–3.318) | <0.001 | 2.038 (1.376–2.950) | <0.001 |
| Medicaid/Medicare insurance | 2.470 (1.736–3.452) | <0.001 | 1.889 (1.276–2.716) | <0.001 |
| Other insurance | 5.004 (2.913–8.547) | <0.001 | 3.225 (1.730–5.869) | <0.001 |
| Median income, <$40,227 (reference) | (reference) | (reference) | ||
| Median income, $40,227–$50,353 | 1.039 (0.850–1.221) | 0.680 | 0.943 (0.742–1.144) | 0.599 |
| Median income, $50,354–$63,332 | 1.252 (1.061–1.511) | 0.013 | 1.095 (0.889–1.394) | 0.430 |
| Median income, $63,333+ | 1.202 (1.025–1.429) | 0.030 | 0.980 (0.761–1.258) | 0.872 |
| Without high school diploma, ≥17.6% (reference) | (reference) | (reference) | ||
| Without high school diploma, 10.9%–17.5% | 1.100 (0.949–1.323) | 0.262 | 0.976 (0.808–1.203) | 0.811 |
| Without high school diploma, 6.3%–10.8% | 1.246 (1.057–1.471) | 0.009 | 1.024 (0.815–1.264) | 0.829 |
| Without high school diploma, <6.3% | 1.377 (1.151–1.652) | <0.001 | 1.181 (0.896–1.511) | 0.212 |
| Metro (reference) | (reference) | (reference) | ||
| Urban | 1.125 (0.941–1.363) | 0.212 | 1.161 (0.939–1.444) | 0.174 |
| Rural | 1.085 (0.626–1.740) | 0.753 | 1.134 (0.608–1.901) | 0.663 |
| Facility, academic (reference) | (reference) | (reference) | ||
| Facility, community cancer program | 0.346 (0.231–0.534) | <0.001 | 0.424 (0.274–0.676) | <0.001 |
| Facility, comprehensive community cancer program | 0.762 (0.656–0.892) | <0.001 | 0.729 (0.612–0.864) | <0.001 |
| Facility, integrated network | 1.003 (0.826–1.196) | 0.973 | 1.034 (0.845–1.274) | 0.747 |
| Region, Northeast (reference) | (reference) | (reference) | ||
| Region, Midwest | 1.651 (1.390–2.017) | <0.001 | 1.640 (1.364–2.076) | <0.001 |
| Region, South | 1.110 (0.951–1.333) | 0.225 | 1.268 (1.068–1.562) | 0.014 |
| Region, West | 1.556 (1.287–1.906) | <0.001 | 1.740 (1.418–2.198) | <0.001 |
| Charlson index, 0 or 1 (reference) | (reference) | (reference) | ||
| Charlson index, 2 | 1.087 (0.870–1.352) | 0.457 | 1.117 (0.873–1.416) | 0.371 |
| Charlson index, 3 | 0.787 (0.662–0.934) | 0.006 | 0.821 (0.676–0.984) | 0.039 |
| AFP, normal (reference) | (reference) | (reference) | ||
| AFP, elevated | 0.992 (0.841–1.182) | 0.928 | 1.123 (0.947–1.373) | 0.223 |
| MELD (10-unit change) | 0.736 (0.660–0.785) | <0.001 | 0.756 (0.663–0.801) | <0.001 |
| Tumor size | 0.993 (0.983–1.002) | 0.136 | 0.992 (0.982–1.001) | 0.101 |
Data in parentheses are 95% CIs.
Factors Associated with OS
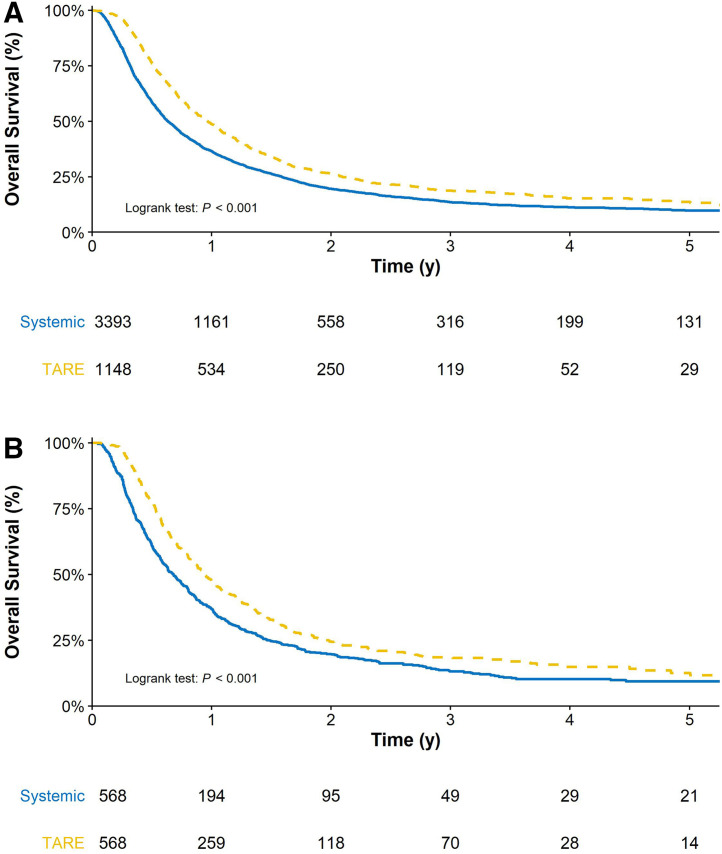
Over a median follow-up of 8.18 mo, the median OS for the entire cohort was 8.64 mo. One- and 3-y survival estimates were 37.3% and 9.6%, respectively. Patients who received TARE had a higher OS than patients who received systemic treatment at 1 y (46.5% vs. 34.2%), 2 y (21.8% vs. 16.4%), and 3 y (10.4% vs. 9.3%) (Fig. 2). After propensity score matching, patients who received TARE continued to demonstrate a higher OS than those who received systemic treatment at 1 y (45.6% vs. 34.2%), 2 y (20.8% vs. 16.7%), and 3 y (12.3% vs. 8.6%) (Fig. 2B).
FIGURE 2.
OS estimates of patients treated with TARE vs. systemic treatment before propensity score matching and after propensity score matching (B).
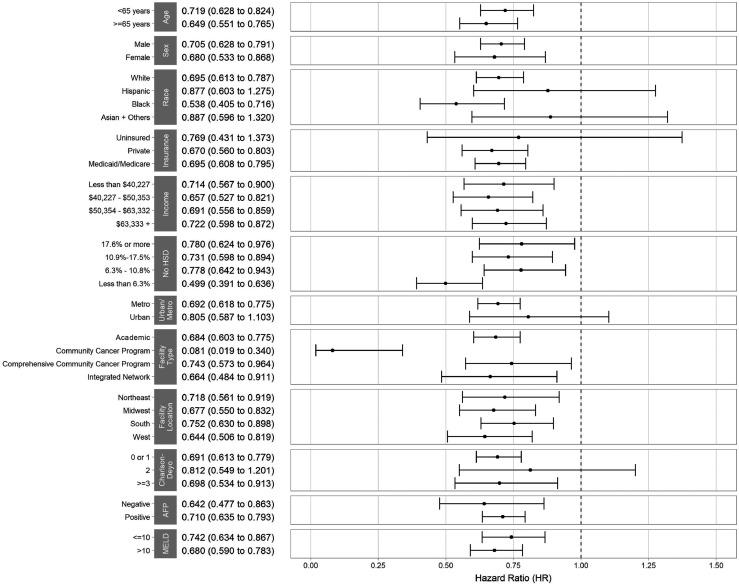
In multivariable Cox regression analysis, receipt of TARE was independently associated with reduced mortality (hazard ratio [HR], 0.74; 95% CI, 0.68–0.80). Results were consistent in propensity score matching (HR, 0.72; 95% CI, 0.63–0.82) and inverse-probability-of-treatment–weighted (HR, 0.74; 95% CI, 0.66–0.83) analyses. Similarly, results were consistent across examined subgroup analyses (Fig. 3). Other factors associated with reduced mortality included female sex (HR, 0.91; 95% CI, 0.84–0.99) and Hispanic ethnicity (HR, 0.85; 95% CI, 0.75–0.96) (Table 3). Treatment at a community cancer program (HR, 1.38; 95% CI, 1.16–1.63), comprehensive community cancer program (HR, 1.23; 95% CI, 1.14–1.34) or integrated network (HR, 1.15; 95% CI, 1.04–1.28); receiving care in the Midwest versus Northeast regions (HR, 1.17; 95% CI, 1.06–1.29); elevated AFP level (HR, 1.36; 95% CI, 1.22–1.51); and higher MELD (HR, 1.11; 95% CI, 1.06–1.17) were independently associated with shorter OS.
FIGURE 3.
Comparison of survival in various subgroup of patients treated with TARE vs. systemic treatment. HSD = high school degree.
TABLE 3.
Factors Associated with OS Among Patients with T3BN0M0 HCC
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | P | HR | P | |
| Age (10-y change) | 1.022 (0.990–1.056) | 0.184 | 1.035 (0.998–1.072) | 0.063 |
| Sex, male (reference) | (reference) | (reference) | ||
| Sex, female | 0.925 (0.852–1.003) | 0.060 | 0.912 (0.840–0.990) | 0.029 |
| Race, white (reference) | (reference) | (reference) | ||
| Race, Hispanic | 0.830 (0.741–0.930) | 0.001 | 0.850 (0.754–0.959) | 0.008 |
| Race, black | 0.947 (0.865–1.036) | 0.232 | 0.933 (0.846–1.029) | 0.164 |
| Race, Asian + others | 0.887 (0.791–0.994) | 0.040 | 0.894 (0.793–1.008) | 0.066 |
| Uninsured (reference) | (reference) | (reference) | ||
| Private insurance | 0.909 (0.787–1.051) | 0.198 | 0.920 (0.793–1.067) | 0.269 |
| Medicaid/Medicare insurance | 0.970 (0.843–1.116) | 0.672 | 0.956 (0.827–1.106) | 0.547 |
| Other insurance | 0.877 (0.659–1.167) | 0.368 | 0.912 (0.685–1.215) | 0.530 |
| Median income, <$40,227 (reference) | (reference) | (reference) | ||
| Median income, $40,227–$50,353 | 1.024 (0.927–1.131) | 0.638 | 0.978 (0.878–1.089) | 0.682 |
| Median income, $50,354–$63,332 | 0.923 (0.836–1.018) | 0.107 | 0.884 (0.790–0.989) | 0.032 |
| Median income, $63,333+ | 0.991 (0.907–1.083) | 0.841 | 0.949 (0.839–1.073) | 0.404 |
| Without high school degree, ≥17.6% (reference) | (reference) | (reference) | ||
| Without high school degree, 10.9%–17.5% | 1.086 (0.993–1.189) | 0.072 | 1.082 (0.981–1.193) | 0.114 |
| Without high school degree, 6.3%–10.8% | 1.098 (1.005–1.199) | 0.038 | 1.117 (1.003–1.244) | 0.044 |
| Without high school degree, <6.3% | 1.088 (0.985–1.202) | 0.097 | 1.131 (0.988–1.294) | 0.073 |
| Metro (reference) | (reference) | (reference) | ||
| Urban | 1.134 (1.025–1.255) | 0.014 | 1.106 (0.992–1.233) | 0.070 |
| Rural | 1.276 (0.982–1.657) | 0.068 | 1.171 (0.892–1.537) | 0.255 |
| Facility, academic (reference) | (reference) | (reference) | ||
| Facility, community cancer program | 1.552 (1.311–1.838) | <0.001 | 1.376 (1.159–1.634) | <0.001 |
| Facility, comprehensive community cancer program | 1.247 (1.149–1.352) | <0.001 | 1.234 (1.135–1.342) | <0.001 |
| Facility, integrated network | 1.164 (1.055–1.285) | 0.003 | 1.153 (1.043–1.275) | 0.005 |
| Region, Northeast (reference) | (reference) | (reference) | ||
| Region, Midwest | 1.179 (1.069–1.301) | <0.001 | 1.167 (1.055–1.292) | 0.003 |
| Region, South | 1.013 (0.929–1.105) | 0.763 | 0.963 (0.879–1.054) | 0.414 |
| Region, West | 1.000 (0.900–1.111) | 0.996 | 0.987 (0.883–1.102) | 0.813 |
| Charlson index 0 or 1 (reference) | (reference) | (reference) | ||
| Charlson index 2 | 0.994 (0.878–1.126) | 0.928 | 0.979 (0.863–1.109) | 0.735 |
| Charlson index 3 | 1.082 (0.989–1.184) | 0.087 | 1.073 (0.979–1.177) | 0.131 |
| AFP, normal (reference) | (reference) | (reference) | ||
| AFP, elevated | 1.330 (1.202–1.473) | <0.001 | 1.356 (1.220–1.506) | <0.001 |
| MELD score (10-unit change) | 1.134 (1.084–1.186) | <0.001 | 1.113 (1.061–1.167) | <0.001 |
| Treatment, systemic (reference) | (reference) | (reference) | ||
| Treatment, TARE | 0.745 (0.691–0.803) | <0.001 | 0.739 (0.684–0.798) | <0.001 |
Data in parentheses are 95% CIs.
DISCUSSION
Our study highlighted several important findings regarding patients with T3BN0M0 HCC treated with TARE or systemic therapy in the United States. Although systemic therapy continues to be the most common first-line treatment for patients diagnosed with T3BN0M0 HCC, the proportion of patients receiving TARE nearly tripled from 13.0% to 37.0% between 2010 and 2017. Second, we found significant variation in the receipt of TARE versus systemic therapy according to race/ethnicity, socioeconomic status, treating facility type, and geographic region. Lastly, treatment with TARE was associated with significantly improved survival compared with systemic treatment.
TARE’s role in the treatment of HCC has evolved over several decades. After establishment of TARE’s efficacy and safety profiles, its long-term treatment outcomes were examined across HCC tumor stages (21–23). Recent clinical trials have evaluated TARE’s relative efficacy and tolerability compared with sorafenib in patients with advanced HCC. The SARAH trial found no significant difference in median OS between patients who received TARE and patients who received sorafenib; however, tolerability and quality of life were significantly better in the TARE group (12). Similarly, the SIRveNIB trial also reported no significant difference in median OS but fewer patients in the TARE group experiencing adverse effects of a grade of at least 3 (13). A recent metaanalysis of these 2 comparative trials (SARAH and SIRveNIB) plus the SORAMIC study, in which TARE was followed by sorafenib, showed that median OS with TARE was noninferior to that with sorafenib (HR, 0.91; 95% CI, 0.78–1.05), with significantly lower rates of severe adverse effects (28.9% vs. 43.3%, P < 0.01) (24).
Although the phase 3 trials did not reach their primary endpoints of difference in OS, it should be noted that both trials reported the dose of injected radiation but did not measure the actual radiation dose delivered to the tumor, as the latter has been shown to predict treatment response (25). The recently published phase 2 DOSISPHERE-01 trial attempted to address this issue by comparing the efficacy of a personalized versus standard dosimetry approach in 60 patients with locally advanced HCC (26). The authors found a significant difference between the 2 groups, with 71% of patients in the personalized dosimetry group compared with 36% of patients in the standard dosimetry group having objective responses (P = 0.0074) (26). Therefore, additional trials incorporating personalized dosimetry may help better elucidate outcomes of patients treated with TARE (27).
The findings of our study have important implications in the context of the above studies evaluating TARE’s role in patients with advanced HCC. Decades of research have shown that TARE is a safe and effective form of treatment for patients with advanced HCC with or without MVI. Moreover, multiple studies have consistently shown that TARE is better tolerated, with fewer serious adverse effects and higher quality of life, than sorafenib. Given such advantages, it is not surprising that the overall proportion of patients with advanced HCC receiving TARE over systemic treatment has significantly increased between 2010 and 2017 despite the society guidelines not yet formally endorsing TARE as first-line therapy. We have found significant variation in the likelihood of receiving TARE over systemic treatment according to race/ethnicity, socioeconomic status, treatment region, and treating facility type. The historically underserved nonwhite racial/ethnic groups and those of lower socioeconomic status had a lower likelihood of receiving TARE. Patients treated in community cancer centers also had a lower likelihood of receiving TARE than did patients treated in academic institutions, reflecting the relative lack of access to advanced interventional procedures in the community. The recently published inaugural AACR cancer disparities progress report highlights adverse differences in numerous measures of cancer burden, access to care, and outcomes among various population groups in the United States and emphasizes the need for more collaboration between the various stakeholders and more cancer health disparity research (28).
Our results must be interpreted in light of the recent advances in immunotherapy for treatment of HCC. The IMbrave150 study, a global, multicenter, open-label, phase 3 randomized trial, reported significantly improved OS in patients with unresectable HCC receiving atezolizumab plus bevacizumab than in patients receiving sorafenib (9). As our study period was from 2010 to 2017, patients received systemic treatment when sorafenib was the first-line treatment. Therefore, it would be misleading to conclude from our study that TARE is superior to all forms of systemic treatment. Instead, the results of our study and recent developments in immunotherapy for HCC highlight the exciting new possibility of combining TARE and immunotherapy for patients with HCC. Besides its locoregional antitumor efficacy, ionizing radiation from TARE may induce immune-mediated antitumor responses distant from the targeted area (29). There is a growing body of evidence supporting the ability of ionizing radiation to activate an immune response via releasing a flood of tumor-associated antigens into the circulation (30), facilitating tumor antigen manifestation to T cells (31), and modulating the tumor microenvironment for improved recognition and killing by CD8-positive T cells (32). Such findings suggest that immune checkpoint blockade may further enhance the immune responses caused by TARE and synergistically achieve improved antitumor effects, and there are several ongoing clinical trials to address this question (33).
Our study also has several limitations related to its design. First, this was a retrospective study of a large cancer-focused database and some pertinent data such as the exact type of systemic treatment and what patients received as second-line therapy. Second, we did not have data on the degree of portal vein invasion, which has been shown to correlate with OS.
CONCLUSION
TARE is associated with improved OS compared with systemic therapy in HCC patients with MVI. Although we noted increasing use for HCC patients with MVI, there continues to be notable variation in its use across the United States. In light of improved systemic therapy options for advanced HCC, continued studies are needed to evaluate the role of TARE, including in combination with immunooncology agents.
DISCLOSURE
Ju Dong Yang’s research is supported by the American College of Gastroenterology Junior Faculty Development Award, the Department of Defense Peer Reviewed Cancer Research Program Career Development Award (CA191051), the Cedars-Sinai Clinical Scholar Award, and the Huiying Foundation and provides a consulting service for Exact Sciences and Gilead. Amit Singal’s research is funded by National Institutes of Health R01 MD12565, and he has been on advisory boards and served as a consultant for Genentech, Bayer, Eisai, BMS, Exelixis, AstraZeneca, and Target RWE. No other potential conflict of interest relevant to this article was reported.
KEY POINTS.
QUESTION: What are the use trend and outcome of TARE in comparison to systemic treatment for HCC patients with MVI?
PERTINENT FINDINGS: In a retrospective cohort study of 5,369 HCC patients with MVI from the U.S. NCDB between 2010 and 2017, use of TARE increased rapidly and was independently associated with improved survival compared with systemic treatment.
IMPLICATIONS FOR PATIENT CARE: TARE might be an effective treatment for HCC patients with MVI, and additional studies evaluating TARE’s role in combination with the newer immunooncology agents are needed.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer. 2017;6:360–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. [DOI] [PubMed] [Google Scholar]
- 4. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 7. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151:1155–1163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. [DOI] [PubMed] [Google Scholar]
- 9. Cho YY, Lee M, Kim HC, et al. Radioembolization is a safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: a propensity score analysis. PLoS One. 2016;11:e0154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de la Torre MA, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int. 2016;36:1206–1212. [DOI] [PubMed] [Google Scholar]
- 11. Edeline J, Crouzet L, Campillo-Gimenez B, et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016;43:635–643. [DOI] [PubMed] [Google Scholar]
- 12. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–1636. [DOI] [PubMed] [Google Scholar]
- 13. Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36:1913–1921. [DOI] [PubMed] [Google Scholar]
- 14. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 15. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 16. Tohme S, Bou Samra P, Kaltenmeier C, Chidi AP, Varley PR, Tsung A. Radioembolization for hepatocellular carcinoma: a nationwide 10-year experience. J Vasc Interv Radiol. 2018;29:912–919.e2. [DOI] [PubMed] [Google Scholar]
- 17. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 20. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 21. Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. [DOI] [PubMed] [Google Scholar]
- 22. Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. [DOI] [PubMed] [Google Scholar]
- 23. Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. [DOI] [PubMed] [Google Scholar]
- 24. Venerito M, Pech M, Canbay A, et al. NEMESIS: noninferiority, individual-patient metaanalysis of selective internal radiation therapy with 90Y resin microspheres versus sorafenib in advanced hepatocellular carcinoma. J Nucl Med. 2020;61:1736–1742. [DOI] [PubMed] [Google Scholar]
- 25. Kappadath SC, Mikell J, Balagopal A, Baladandayuthapani V, Kaseb A, Mahvash A. Hepatocellular carcinoma tumor dose response after 90Y-radioembolization with glass microspheres using 90Y-SPECT/CT-based voxel dosimetry. Int J Radiat Oncol Biol Phys. 2018;102:451–461. [DOI] [PubMed] [Google Scholar]
- 26. Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17–29. [DOI] [PubMed] [Google Scholar]
- 27. Lewandowski RJ, Salem R. Radioembolisation with personalised dosimetry: improving outcomes for patients with advanced hepatocellular carcinoma. Lancet Gastroenterol Hepatol. 2021;6:2–3. [DOI] [PubMed] [Google Scholar]
- 28. Cancer disparities progress report 2020. American Association for Cancer Research website. https://cancerprogressreport.aacr.org/wp-content/uploads/sites/2/2020/09/AACR_CDPR_2020.pdf. Published 2020. Accessed July 12, 2021.
- 29. Mole RH. Whole body irradiation: radiobiology or medicine? Br J Radiol. 1953;26:234–241. [DOI] [PubMed] [Google Scholar]
- 30. Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Curr Opin Oncol. 2009;21:71–76. [DOI] [PubMed] [Google Scholar]
- 31. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. [DOI] [PubMed] [Google Scholar]
- 33. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]