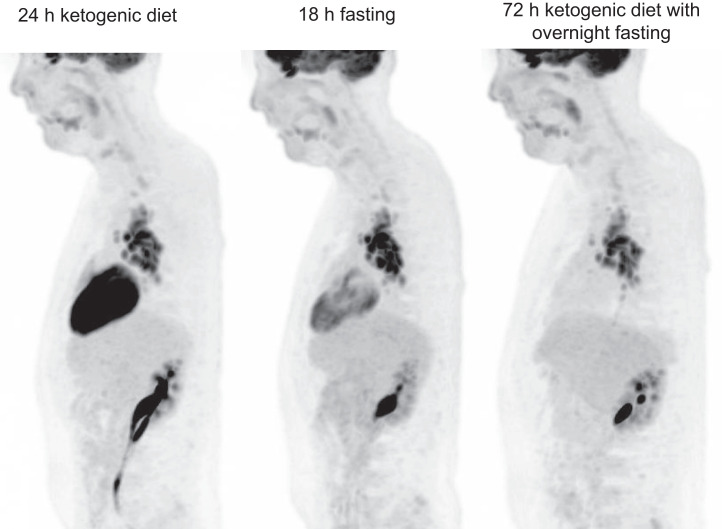
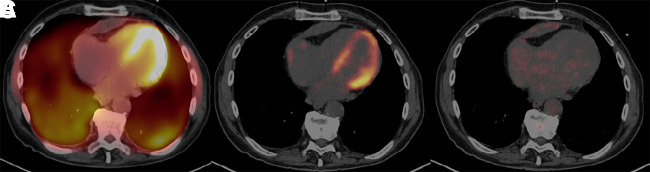
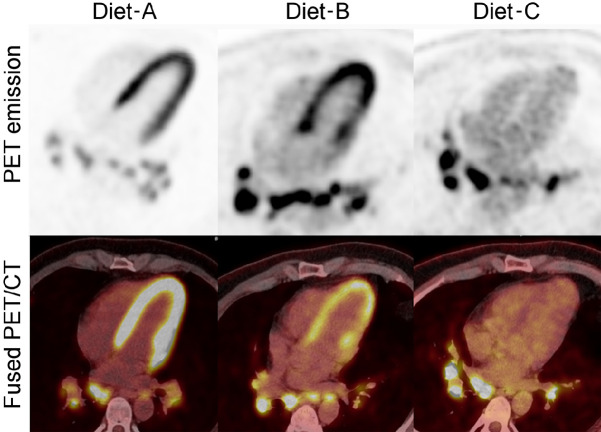
Visual Abstract
Keywords: cardiac sarcoidosis, PET/CT, myocardial suppression, ketogenic diet, fasting
Abstract
A definitive dietary preparation recommendation is not possible based on literature on the achievement of myocardial suppression for diagnosis of cardiac sarcoidosis (CS) with 18F-FDG PET/CT. Our goal is to compare 3 different dietary preparations in achievement of the best myocardial suppression and CS diagnosis. Methods: We retrospectively reviewed and compared 3 dietary preparations used at our institution. Three different diets were applied from March 2014 to December 2019: a 24-h ketogenic diet with overnight fasting (n = 94); 18-h fasting (n = 44); and 72-h daytime ketogenic diet with 3-d overnight fasting (n = 98). The interpretation of initial reports was recorded, and an independent radiologist (observer) retrospectively reevaluated each case regarding CS diagnosis (negative, positive, indeterminant) and myocardial suppression (complete, failed, partial). Interobserver agreement was analyzed. We measured SUVmax from blood pool, liver, and the most suppressed normal myocardium. Results: We identified superior myocardial suppression with the 72-h preparation, indicated by higher blood pool-to-myocardium and liver-to-myocardium ratios (P < 0.001). Myocardial suppression rates for the 72-h ketogenic diet, 24-h ketogenic diet, and 18-h fasting preparations were as follows: complete myocardial suppression, 96.9%, 68.1%, and 52.3%, respectively; failed myocardial suppression, 0%, 23.4%, and 25%, respectively; and partial myocardial suppression, 3.1%, 8.5%, and 22.7%, respectively (P < 0.001). The 72-h preparation had significantly fewer indeterminant and positive examinations. CS diagnosis rates for 72-h ketogenic diet, 24-h ketogenic diet, and 18-h fasting preparations were negative, 82.7%, 52.1%, and 27.3%, respectively; indeterminant, 2.0%, 24.5%, and 40.9%, respectively; and positive, 15.3%, 23.4%, and 31.8%, respectively (P < 0.001). A high agreement was present with the observer and the report (κ = 0.88). Conclusion: A 72-h daytime ketogenic diet with 3-d overnight fasting achieved substantially superior myocardial suppression versus a 24-h ketogenic diet with overnight fasting and an 18 h-fasting using 18F-FDG PET/CT. This 72-h preparation results in significantly fewer indeterminant and potentially false-positive CS results.
The diagnosis of cardiac sarcoidosis (CS) is challenging. Clinical presentation may vary from asymptomatic to ventricular tachycardia, high-grade atrioventricular block, or heart failure (1,2). The gold standard for diagnosis is endomyocardial biopsy. However, it is invasive, is prone to false-negatives from sampling errors, and has low sensitivity for CS when compared with autopsy (3). Therefore, noninvasive imaging methods, cardiac MRI and cardiac 18F-FDG PET/CT (cPET/CT), are frequently used when screening or symptoms indicate cardiac involvement. Although both modalities have limitations, they ultimately proved complementary as they measure different pathologic processes (3–8).
The major limitation of cPET/CT relates to the physiologic myocardial glucose metabolism. A normal healthy myocardium uses a combination of free fatty acids and glucose for energy in normal conditions. Thus, differentiation of myocardial inflammation from normal physiologic myocardial activity requires the suppression of physiologic myocardial uptake. This is achieved by switching the myocardial metabolism from glucose to free fatty acid consumption (9). Several different dietary and pharmacologic modifications have been proposed to achieve this goal. And yet, no standardized method exists, with variation in protocols among institutions (3,4,7,9–16). Most recently, the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and American Society of Nuclear Cardiology (ASNC) jointly reported a consensus guideline for appropriate dietary modification to assess CS, including the following 2 options (3). First, the patient consumes at least 2 high-fat (>35 g), low-carbohydrate (<3 g) meals the day before the study and fasts for at least 4–12 h. Second, the patient fasts for more than 18 h before the study. Despite these attempts, a recent large literature review concluded that “a definitive dietary preparation recommendation is not possible based on current evidence; however, scan readability does seem to be improved when preparation includes a reduced carbohydrate intake” (10).
Since 2012, we used 3 different dietary modifications for patients with suspected CS, to suppress background myocardial 18F-FDG metabolism. The first 2 methods were similar to the above-mentioned consensus guideline. The third method, a new dietary protocol, included a 72-h prolonged ketogenic diet with 3-d overnight fasting. Our purpose was to compare the effect of 3 different dietary modifications on cPET/CT in patients with suspicion of CS.
MATERIALS AND METHODS
Patient Selection and Diets
This Health Insurance Portability and Accountability Act–compliant retrospective study was approved by the institutional review board (STUDY00005814), and the need for written informed consent was waived. All dedicated cPET/CTs for CS from January 2012 to December 2019, obtained at the University of Minnesota, either for initial diagnosis or follow-up for assessing treatment response were collected in a PACS folder. Three different dietary preparations were used during this time. Diet-A (24-h ketogenic diet) was applied from January 2012 to February 2018, Diet-B (18-h fasting) from March 2018 to September 2018, and Diet-C (72-h ketogenic diet with 3-d overnight fasting) from September 2018 to December 2019 (Table 1).
TABLE 1.
Simplified Copy of Dietary Instruction Given for Each Patient in a Specific Diet Group
| Diet | Description | Allowed foods | Forbidden foods |
|---|---|---|---|
| A | High-fat, low-carbohydrate diet beginning 24 h before the study. Nothing by mouth, except water and oral pills, 6 h before the examination. |
Beverages: club soda, black coffee, tea (without sugar), or water. Seasonings: anything that does not contain sugar. Fats/oils: butter, margarine, or vegetable oils. Eggs. Meat: chicken, turkey, fish, beef, or pork (watch closely for sugars in processed meats), meat-only sausages, and nonsweetened bacon. Shellfish: any nonprocessed shellfish. Vegetables: alfalfa sprouts, artichokes, asparagus, bean sprouts, broccoli, Brussels sprouts, cabbage, celery, cucumber, eggplant, any lettuce |
No carbohydrate or sugars. Anything with glucose, fructose, sucrose, or lactose. No artificial sweeteners. No fruit or fruit juices. No diet or regular sodas or sports drinks. No alcohol. No dairy products such as milk, cheese, cream, or yogurt. No pastas, breads, cereals, rice, bagels, crackers, or muffins. No breading on any food. No candy, cookies, or cake. No gum, mints, or cough drops. No condiments such as ketchup and mustard with sugar in it. No starchy vegetables such as corn, peas, carrots, most legumes, grains, and potatoes. |
| B | 18 h of fasting before the study. Nothing by mouth, except water and oral pills, for 18 h before the study. |
||
| C | High-fat, low-carbohydrate ketogenic diet beginning 72 h before the study. Nothing by mouth, except water and oral pills, for 3 overnight fasts before the exam. First 2 nights from 08:00 pm until at least 08:00 am the next morning. The night before the test from 08:00 pm until the time of the test. For diabetics, consider avoiding prolonged fasting, preferably obtain the examination at noon hours after ketogenic breakfast + morning insulin, followed by 6-h fasting. |
Beverages: club soda, black coffee, tea (without sugar), or water, Seasonings: anything that does not contain sugar. Fats/oils: butter, margarine, or vegetable oils. Eggs. Meat: chicken, turkey, fish, beef, or pork (watch closely for sugars in processed meats), meat-only sausages, and nonsweetened bacon. Shellfish: any nonprocessed shellfish |
No carbohydrate or sugars. Anything with glucose, fructose, sucrose, or lactose. No artificial sweeteners. No fruit or fruit juices. No diet or regular sodas or sports drinks. No alcohol. No dairy products such as milk, cheese, cream, or yogurt. No pastas, breads, cereals, rice, bagels, crackers, or muffins. No breading on any food. No candy, cookies, or cake. No gum, mints, or cough drops. No condiments such as ketchup and mustard with sugar in it. Vegetables are not permitted. No beans or nuts. |
Our goal was to compare an equal number of examinations for each diet. However, Diet-B was applied for a limited time, therefore there are fewer cPET/CTs in this group than in the Diet-A and Diet-C groups. We identified 98 studies for Diet-C. Therefore, the latest 98 studies using Diet-A going back from February 2018 were selected. However, 4 were excluded later because of the lack of image processing using the PET/CT viewer. In the end, a total of 236 cPET/CTs from 160 unique patients were included for analysis (Diet-A: 94 studies in 70 patients; Diet-B: 44 studies in 39 patients; Diet-C: 98 studies in 86 patients). Of the 160 subjects, 128 (80%) had only 1 unique diet and 32 (20%) had more than one type of diet.
PET/CT Acquisition
Apart from the dietary modifications, smoking for 6 h and exercise for 24 h were restricted before cPET/CT. If patients had diabetes, blood glucose level should be under 200 mg/dL and insulin was restricted for 6 h before the study. cPET/CT was rescheduled if the above-mentioned criteria were not met.
All cPET/CTs were obtained with Biograph mCT 64 PET/CT scanners (Siemens). All examinations included 13N-ammonia perfusion and 18F-FDG metabolism studies, excluding follow-ups, as 13N-ammonia study was not performed routinely for follow-ups. A 10-min 13N-ammonia study was performed using 740 MBq of 13N-ammonia with 128 × 128 resolution. Then, patients were taken to a separate room for 18F-FDG injection, and 18F-FDG studies were performed 60 min after 555 MBq of 18F-FDG administration with 256 × 256 resolution.
Data Collection
A research fellow obtained the following information from the electronic medical records: examination date, patient age, sex, body mass index, the dose of administered 13N-ammonia and 18F-FDG, fingertip blood glucose level immediately before cPET/CT, diet type, dietary compliance, and whether a repeated examination was performed due to dietary noncompliance. All cPET/CTs were initially reported by 2 independent nuclear radiologists. The reports were retrospectively reviewed by the research fellow and categorized into 3 categories regarding the presence of CS—active inflammation from CS = positive, no active inflammation from CS = negative, and indeterminant—and regarding the myocardial suppression—complete suppression, partial suppression, and failed suppression.
Image Analysis
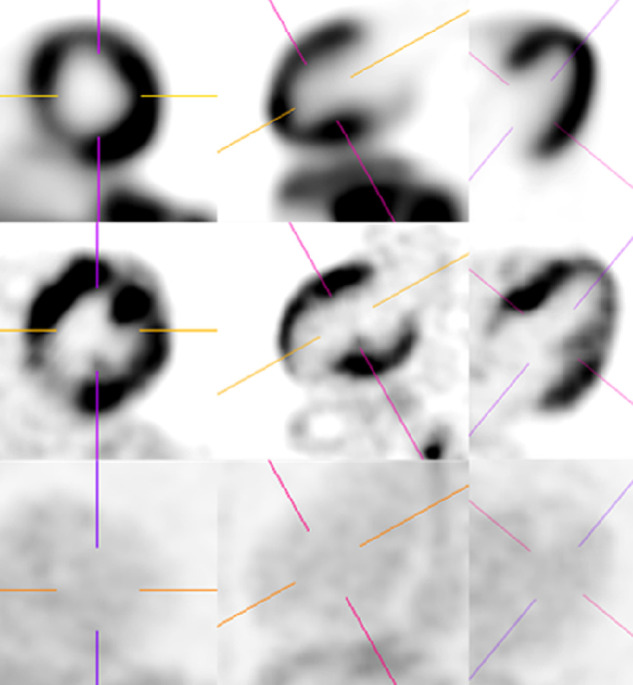
All included cases were retrospectively evaluated by a separate nuclear radiologist (the observer). Qualitative and quantitative assessment was performed using SyngoVia MMoncology and MIcardiology applications. Each examination was reformatted in the oblique axial/coronal/sagittal planes according to the anatomic cardiac orientation (Fig. 1) using the PET emission and CT images. Particularly, in those cases in which the myocardium was heterogeneous or completely suppressed, the anatomic orientation of the heart was adjusted based on CT appearance. Quantitatively, bloodpool-maxSUV (from the mediastinal descending thoracic aorta), liver-maxSUV (from the right hepatic lobe), myocardium-maxSUV (from the most suppressed portion of the left ventricle), and lesion-maxSUV (from the most 18F-FDG–avid portion of the myocardium if active CS was suspected) were measured. Bloodpool-maxSUV/myocardium-maxSUV and liver-maxSUV/myocardium-maxSUV ratios were calculated.
FIGURE 1.
A 62-y-old woman with inferior wall motion abnormality, arrythmia, and an old outside PET/CT report raising suspicion of CS. Prior endomyocardial biopsy was negative; there was no pathologic or imaging proof of sarcoidosis elsewhere in the body. On the basis of clinical findings, patient was considered as presumably having CS and referred for PET/CT. First PET/CT was obtained with Diet-B (top: 13N-ammonia; middle: 18F-FDG/PET), which was reported as active CS with complete myocardial suppression. However, the observer evaluated as indeterminant secondary to partial suppression. Patient subsequently received steroids, and a follow-up study was performed with Diet-C (bottom). This time, both report and observer agreed that there was complete suppression with no active CS. While it is possible that this presumable CS case might have responded to treatment, it is also possible that the initial interpretation was incorrect, and patient might have received unnecessary treatment.
Qualitatively, the presence of myocardial suppression was visually categorized in 3 groups: if no physiologic uptake was perceived, it was categorized as complete suppression; if there was heterogeneous uptake without typical mismatch pattern and appearance was not suggestive of early active disease, it was categorized as partial suppression; and if there was diffuse uptake, it was categorized as failed suppression. The presence of CS was evaluated qualitatively according to 2 criteria based on literature. The first analysis consisted of 6 categories, per the SNMMI–ASNC guideline: 1, normal; 2, diffuse nonspecific; 3, focal uptake without mismatch on 13N-ammonia representing early active disease; 4, focal uptake with mismatch pattern representing active disease; 5, focal uptake with mismatch pattern, mixed with non-18F-FDG–avid perfusion defects, representing mixed scar and inflammation; 6, non-18F-FDG–avid perfusion defect, representing scar (3). The second analysis consisted of 4 categories according to Lu et al.: 1, normal; 2, ringlike diffuse uptake at the base, considered negative for CS; 3. focal uptake considered positive for CS; 4, diffuse myocardial uptake considered indeterminant (7). To compare the interobserver variability between the radiology report and the observer, these analyses were recategorized, accordingly, the SNMMI–ASNC classification—category 1 + 6 = negative; category 2 = indeterminant; category 3 + 4 + 5 = positive; and the Lu classification—1 + 2 = negative; 3 = positive; 4 = indeterminant. The observer also evaluated the presence of systemic active sarcoidosis based on PET/CT, categorized in 2 groups, negative and positive.
Statistical Evaluation
Descriptive statistics were used to compare the 3 diet groups on several clinical and demographic measures. All variables were summarized on a per-study, as opposed to a per-subject, basis. χ2 tests were used to statistically compare the 3 groups on categoric measures, whereas 1-way ANOVA tests were used for the continuous measures. Weighted Cohen’s κ was used to evaluate the interrater reliability of myocardial suppression and presence of active CS between the report and the observer and recategorization of the SNMMI–ASNC and Lu classifications. Linear mixed-effect regression models were also used to compare the myocardial glucose suppression between the diet groups while accounting for the within-subject effects and repeated measurements. The myocardial suppression response variable was treated as an ordinal variable, where failed = 0, partially suppressed = 1, and completely suppressed = 2. P values of less than 0.05 were used to determine statistical significance. R software (version 3.6.0; R Foundation) was used for analyses.
RESULTS
Patients demonstrated high compliance with the applied diets without statistically significant difference (Diet-A: 89/94 [94.7%], Diet-B: 41/44 [93.2%], Diet-C: 96/98 [99%], P = 0.292). Noncompliance led to 2 of 5 repeated exams in the Diet-A group, 3 of 3 in the Diet-B group, and 1 of 2 in the Diet-C group. Below-mentioned analyses included the cases with dietary noncompliance. A repeated analysis excluding those with dietary noncompliance (not provided) did not show any meaningful difference.
Quantitative measurements are presented in Table 2. No difference was detected between the groups regarding sex, body mass index, applied amount of 13N-ammonia and 18F-FDG . Regarding age, there was statistically significant but clinically insignificant difference between the groups. No difference was found between the groups regarding the liver uptake. Regarding the blood-pool uptake, there was statistically significant difference between the groups, although this difference was not clinically or radiologically significant. Most importantly, there was significantly lower myocardial uptake in the Diet-C group than in the other diet groups. Of equal importance, the blood-pool/myocardium and liver/myocardium maxSUV ratios were significantly higher in the Diet-C group, indicating a better myocardial suppression relative to background references. It is also noteworthy that lesion uptake was similar in all 3. Mean fingertip glucose was also significantly lower in the Diet-C group than in the other groups. Although there was no patient with a fingertip glucose < 60 mg/dL in the Diet-A and Diet-B groups, fingertip glucose was < 60 mg/dL in 6 patients using Diet-C at the time of 18F-FDG injection, with the lowest being 43. None of these patients demonstrated any clinical signs of hypoglycemia; thus, it was decided to continue the PET/CT acquisition with attention to hypoglycemia symptoms. These patients tolerated the entire PET/CT well with no complication related to hypoglycemia.
TABLE 2.
Quantitative Comparison of Diets
| Variable | Diet-A (n = 94) | Diet-B (n = 44) | Diet-C (n = 98) | Total (n = 236) | P |
|---|---|---|---|---|---|
| Age (y) | 0.018 | ||||
| Mean (SD) | 55.9 (11.4) | 58.3 (10.2) | 60.6 (11.6) | 58.3 (11.4) | |
| Range | 26–83 | 26–73 | 24–81 | 24–83 | |
| Sex | 0.570 | ||||
| Male | 29 (30.9%) | 14 (31.8%) | 37 (37.8%) | 80 (33.9%) | |
| Female | 65 (69.1%) | 30 (68.2%) | 61 (62.2%) | 156 (66.1%) | |
| Body mass index | 0.380 | ||||
| Missing | 3 | 2 | 6 | 11 | |
| Mean (SD) | 31.5 (6.3) | 30.1 (6.1) | 31.6 (6.5) | 31.3 (6.3) | |
| Range | 17.2–47.2 | 17.9–46.1 | 18.3–52 | 17.2–52 | |
| 13N-ammonia (MBq) | 0.620 | ||||
| Not present. | 17 | 15 | 31 | 63 | |
| Mean (SD) | 717.8 (140.6) | 740 (103.6) | 736.3 (103.6) | 728.9 (122.1) | |
| Range | 266.4–899.1 | 373.7–891.7 | 92.5–854.7 | 92.5–899.1 | |
| 18F-FDG (MBq) | 0.355 | ||||
| Mean (SD) | 555 (44.4) | 558.7 (37) | 566.1 (33.3) | 562.4 (40.7) | |
| Range | 388.5–658.6 | 469.9–610.5 | 451.4–636.4 | 388.5–658.6 | |
| Blood glucose (mg/dL) | 0.032 | ||||
| Mean (SD) | 99.5 (21) | 99.4 (17.6) | 91.6 (25.7) | 96.2 (22.8) | |
| Range | 60–196 | 77–150 | 43–202 | 43–202 | |
| Blood pool–SUVmax | 0.005 | ||||
| Mean (SD) | 2.6 (0.6) | 2.6 (0.5) | 2.9 (0.6) | 2.7 (0.6) | |
| Range | 1.3–3.9 | 1.4–3.6 | 1.5–4 | 1.3–4 | |
| Liver–SUVmax | 0.462 | ||||
| Mean (SD) | 3.6 (0.9) | 3.7 (0.8) | 3.8 (0.8) | 3.7 (0.8) | |
| Range | 1.3–6.7 | 2.1–5.8 | 1.9–6.5 | 1.3–6.7 | |
| Myocardium–SUVmax | <0.001 | ||||
| Mean (SD) | 3.5 (3.5) | 4.4 (3.7) | 2.0 (0.8) | 3 (2.9) | |
| Range | 1.2–22 | 0.9–16.2 | 0.8–7.4 | 0.8–22 | |
| Blood pool/myocardium | <0.001 | ||||
| Mean (SD) | 1.1 (0.5) | 0.9 (0.5) | 1.6 (0.4) | 1.3 (0.5) | |
| Range | 0.1–2.2 | 0.1–2 | 0.4–3.1 | 0.1–3.1 | |
| Liver/myocardium | <0.001 | ||||
| Mean (SD) | 1.6 (0.8) | 1.4 (0.8) | 2.1 (0.6) | 1.8 (0.8) | |
| Range | 0.2–3.9 | 0.2–3.9 | 0.5–4.1 | 0.2–4.1 | |
| Lesion–SUVmax | 0.858 | ||||
| Mean (SD) | 7.4 (4.1) | 7 (3.1) | 7.8 (3.5) | 7.4 (3.6) | |
| Range | 3.2–19 | 4.2–15.3 | 4–15.1 | 3.2–19 |
Table 3 shows qualitative comparison of the groups. Regarding the myocardial suppression, the observer found statistically superior myocardial suppression with Diet-C, with which the complete suppression rate reached 96.9% with 0% failed and 3.1% partial suppression. This included the cases with dietary noncompliance. These rates were similar for Diet-C according to the radiology report; however, the radiology report indicated higher rates of complete myocardial suppression and lower rates of partial and failed suppression in Diet-A and Diet-B. There was borderline statistical insignificance among the groups regarding myocardial suppression according to the original report (P = 0.052). A comparison of each individual diet using the mixed-effects model per subject also showed significant myocardial suppression with Diet-C compared with Diet-A (effect: 0.51, CI: 0.32–0.69, P = 0.000) and Diet-B (effect: 0.67, CI: 0.44–0.90, P = 0.000). This was not significant when Diet-A was compared with Diet-B (effect: 0.16, CI: −0.07–0.40, P = 0.169).
Table 3.
Qualitative Comparison of Diets Regarding Myocardial Suppression and Diagnosis According to Initial Radiology Report and Observer
| Qualitative variables | Diet-A (n = 94) | Diet-B (n = 44) | Diet-C (n = 98) | Total (n = 236) | P |
|---|---|---|---|---|---|
| Myocardial suppression according to radiology report | 0.052 | ||||
| Failed suppression | 9 (9.6%) | 7 (15.9%) | 3 (3.1%) | 19 (8.1%) | |
| Partial suppression | 2 (2.1%) | 2 (4.5%) | 1 (1%) | 5 (2.1%) | |
| Complete suppression | 83 (88.3%) | 35 (79.5%) | 94 (95.9%) | 212 (89.8%) | |
| Myocardial suppression according to observer | <0.001 | ||||
| Failed suppression | 22 (23.4%) | 11 (25.0%) | 0 (0%) | 33 (14%) | |
| Partial suppression | 8 (8.5%) | 10 (22.7%) | 3 (3.1%) | 21 (8.9%) | |
| Complete suppression | 64 (68.1%) | 23 (52.3%) | 95 (96.9%) | 182 (77.1%) | |
| Diagnosis per radiology report | <0.001 | ||||
| Negative | 49 (52.1%) | 15 (34.1%) | 82 (83.7%) | 146 (61.9%) | |
| Indeterminant | 4 (4.3%) | 6 (13.6%) | 1 (1%) | 11 (4.7%) | |
| Positive | 41 (43.6%) | 23 (52.3%) | 15 (15.3%) | 79 (33.5%) | |
| Diagnosis per observer (recategorized according to SNMMI–ASNC) | <0.001 | ||||
| Negative | 49 (52.1%) | 12 (27.3%) | 81 (82.7%) | 142 (60.2%) | |
| Indeterminant | 23 (24.5%) | 18 (40.9%) | 2 (2%) | 43 (18.2%) | |
| Positive | 22 (23.4%) | 14 (31.8%) | 15 (15.3%) | 51 (21.6%) | |
| Diagnosis per observer (recategorized according to Lu) | <0.001 | ||||
| Negative | 53 (56.4%) | 18 (40.9%) | 81 (82.7%) | 152 (64.4%) | |
| Indeterminant | 19 (20.2%) | 12 (27.3%) | 2 (2%) | 33 (14%) | |
| Positive | 22 (23.4%) | 14 (31.8%) | 15 (15.3%) | 51 (21.6%) | |
| Diagnosis per observer (SNMMI–ASNC) | <0.001 | ||||
| Normal | 40 (42.6%) | 10 (22.7%) | 62 (63.3%) | 112 (47.5%) | |
| Diffuse nonspecific | 23 (24.5%) | 18 (40.9%) | 2 (2%) | 43 (18.2%) | |
| Early active disease | 8 (8.5%) | 2 (4.5%) | 4 (4.1%) | 14 (5.9%) | |
| Active disease | 6 (6.4%) | 9 (20.5%) | 5 (5.1%) | 20 (8.5%) | |
| Mixed scar/active disease | 8 (8.5%) | 3 (6.8%) | 6 (6.1%) | 17 (7.2%) | |
| Scar | 9 (9.6%) | 2 (4.5%) | 19 (19.4%) | 30 (12.7%) | |
| Diagnosis per observer (Lu) | <0.001 | ||||
| Normal | 49 (52.1%) | 12 (27.3%) | 81 (82.7%) | 142 (60.2%) | |
| Negative | 4 (4.3%) | 6 (13.6%) | 0 (0%) | 10 (4.2%) | |
| Positive | 22 (23.4%) | 14 (31.8%) | 15 (15.3%) | 51 (21.6%) | |
| Indeterminant | 19 (20.2%) | 12 (27.3%) | 2 (2%) | 33 (14.0%) | |
| Systemic sarcoidosis | 0.414 | ||||
| Negative | 55 (58.5%) | 30 (68.2%) | 65 (66.3%) | 150 (63.6%) | |
| Positive | 39 (41.5%) | 14 (31.8%) | 33 (33.7%) | 86 (36.4%) |
Regarding the assessment of myocardial suppression, there was moderate agreement between the observer and the radiology report (Table 4). This agreement was mostly valid in the cases of complete suppression; however, the number of failed and partial suppression cases were fewer in the radiology reports than in the observer’s evaluation.
Table 4.
Correlation of Myocardial Suppression According to Initial Radiology Report and Observer
| Observer | |||||
|---|---|---|---|---|---|
| Radiology report | Failed (n = 33) | Partial (n = 21) | Complete (n = 182) | Total (n = 236) | |
| Failed | 12 (36.4%) | 1 (4.8%) | 6 (3.3%) | 19 (8.1%) | |
| Partial | 2 (6.1%) | 3 (14.3%) | 0 (0%) | 5 (2.1%) | |
| Complete | 19 (57.6%) | 17 (81%) | 176 (96.7%) | 212 (89.8%) |
κ = 0.41.
There was no statistical difference among the groups regarding the presence of systemic active sarcoidosis on whole-body PET/CT. The observer found more “normal” and “scar” cases in the Diet-C group, with markedly decreased amount of “diffuse nonspecific” cases, compared with the Diet-A and Diet-B groups using the SNMMI–ASNC assessment (Table 3). According to the Lu assessment, the groups were also statistically different: Diet-C had more “normal” cases with decreased number of “positive“ and “indeterminant” cases than did Diet-A and Diet-B. After recategorization of these assessments into 3 categories as “positive,” “negative,” and “indeterminant,” the Diet-C group had significantly more negative, fewer positive, and less indeterminant active CS diagnosis than did Diet-A and Diet-B (Table 3). Notably, this recategorization demonstrated near-complete agreement (Table 5). A similar observation was also found according to the radiology report, showing a high degree of agreement with the observer (Table 6). The numbers of the observer and the radiology report were remarkably similar for all diets when the diagnosis was negative (Table 3). The numbers of indeterminant and positive cases by the observer and the radiology report were also similar when Diet-C was used but remarkably different when Diet-A and Diet-B were used. Some examples are shown in Figures 1–4.
Table 5.
Correlation of Observer’s Diagnosis According to Recategorized SNMMI--ASNC and Lu Classification
| Recategorization according to SNMMI–ASNC | |||||
|---|---|---|---|---|---|
| Recategorization according to Lu Recategorization according to Lu | Negative (n = 142) | Indeterminant (n = 43) | Positive (n = 51) | Total (n = 236) | |
| Negative | 142 (100%) | 10 (23.3%) | 0 (0%) | 152 (64.4%) | |
| Indeterminant | 0 (0%) | 33 (76.7%) | 0 (0%) | 33 (14.0%) | |
| Positive | 0 (0%) | 0 (0%) | 51 (100%) | 51 (21.6%) |
κ = 0.97.
TABLE 6.
Correlation of Initial Radiology Report with Observer According to Recategorized Diagnosis Based on SNMMI–ASNC and Lu Classification
| Radiology report | |||||
|---|---|---|---|---|---|
| Negative (n = 146) | Indeterminant (n = 11) | Positive (n = 79) | Total (n = 236) | ||
| Observer | Recategorization according to SNMMI–ASNC* | ||||
| Negative | 141 (96.6%) | 0 (0%) | 1 (1.3%) | 142 (60.2%) | |
| Indeterminant | 3 (2.1%) | 11 (100%) | 29 (36.7%) | 43 (18.2%) | |
| Positive | 2 (1.4%) | 0 (0%) | 49 (62%) | 51 (21.6%) | |
| Recategorization according to Lu† | |||||
| Negative | 142 (97.3%) | 2 (18.2) | 8 (10.1%) | 152 (64.4%) | |
| Indeterminant | 2 (1.4%) | 9 (81.8%) | 22 (27.8%) | 33 (14%) | |
| Positive | 2 (1.4%) | 0 (0%) | 49 (62%) | 51 (21.6%) | |
κ = 0.88.
κ = 0.82.
FIGURE 2.
A 73-y-old man with chronic pericarditis and suspicion of CS. First examination was obtained with Diet-B: 13N-ammonia (A) and 18F-FDG (B). Initially, this examination was interpreted as incomplete myocardial suppression and indeterminant CS, in agreement with the observer. Repeated 18F-FDG study with Diet-C (C) showed complete myocardial suppression with negative CS (the observer and the report were in agreement).
FIGURE 3.
A 35-y-old man with systemic sarcoidosis. The first examination was obtained with Diet-A. Because of incomplete myocardial suppression, examination was repeated 2 d later with Diet-B. Results of this second examination showed partial myocardial suppression in lateral wall with indeterminant result. Patient underwent follow-up MRI (not shown here), which was negative for CS at that time. A third follow-up with Diet-C showed complete myocardial suppression. Note the persistence of hypermetabolic mediastinal lymph nodes in all 3 examinations.
FIGURE 4.
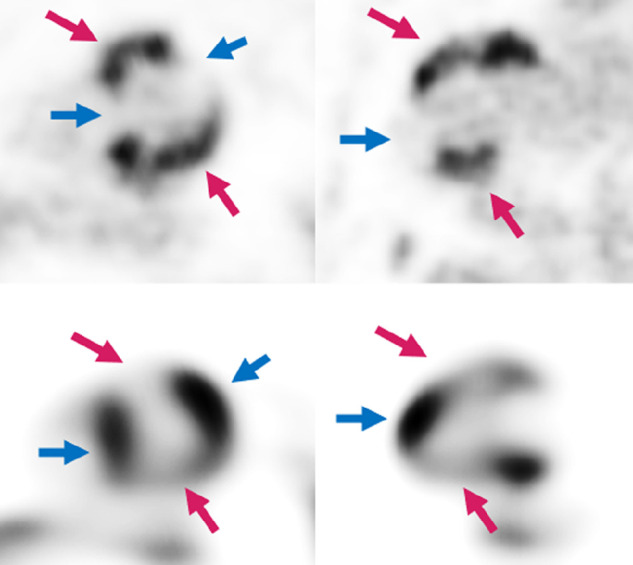
A 65-y-old man with new arrythmia and cardiac MRI (not shown), suggestive of sarcoidosis. Patient was referred for PET/CT obtained with Diet-C. Top row is 18F-FDG and lower row is 13N-ammonia. Both report and observer called this examination as active CS with complete myocardial suppression. Note mismatch pattern at regions with active CS (pink arrows) and how myocardium is well suppressed at normally perfused areas (blue arrows).
DISCUSSION
Since 2012, we used diets (Diet-A and Diet-B) similar to those recommended by the SNMMI–ASNC guidelines. However, during multidiscipline conferences what we had determined as active inflammation was not correlating with either clinical findings or MRI in many cases. Therefore, we questioned our ability of myocardial suppression and decided to change the diet protocol. A new 72-h ketogenic diet during the day, with overnight fasting on 3 consecutive days (Diet-C), markedly improved myocardial suppression rates, and this new protocol was quantitatively and qualitatively superior to the other previously used dietary modifications, including the 24-h ketogenic diet with 6-h fasting (Diet-A) and 18-h fasting (Diet-B). The new regimen also resulted in better interobserver agreement. When we examined the radiology reports with the 2 older dietary regimens, there were higher rates of positive diagnoses, but the observer was not in agreement, calling most of these positive cases indeterminant, due to the possibility of potential inadequate myocardial suppression and associated false-positive results. It is possible that many of these cases with Diet-A and Diet-B were overcalled as positive secondary to incomplete suppression, in particular, the previously described “focal on diffuse” imaging patterns obtained with these diets were probably the reason why the radiologists had reported these exams as “positive,” which is a common problem in prior study design (17,18). But that was not the case when Diet-C was used; excellent myocardial suppression was achieved with increased certainty in the diagnosis and increased agreement between the observer and report.
Our findings are supported by findings in the preclinical setting. Clement et al. demonstrated that marked myocardial background suppression was achieved with better determination of the myocarditis in 18F-FDG PET/CT in mice with extension of ketogenic diet up to 7 d as opposed to 18-h fasting. Each mouse was given a standard diet with calculated calories and nutrients, and PET/CT findings were correlated with pathologic confirmation. This animal study proves that extension of ketogenic diet results in better myocardial suppression (13). However, 7-d dietary restrictions could be difficult for certain patients in terms of dietary compliance and our 3-d dietary restriction seems more pragmatic in a clinical setting.
It is well-known that people often experience ketogenic flu in the first few days of a ketogenic diet (19). The durations of these symptoms indicate that physiologic response to ketogenic diet takes several days. On the basis of our data, the body needs greater than 24 h to fully switch to ketogenic metabolism in the myocardium. When we first started this new dietary regimen, we were not aware of the study by Lu et al., which compared 2 groups (7). The first group contained PET/CTs from 12 patients with preparation using a 24-h ketogenic diet with a ketogenic breakfast 4 h before PET/CT; the second group contained 193 PET/CTs from 185 patients with preparation using a 72-h ketogenic diet with a ketogenic breakfast 4 h before PET/CT. According to the results of Lu et al. (7), 7 of 193 (3.6%) indeterminant, 167 of 193 (86.6%) negative, and 19 of 193 (9.8%) positive CS diagnoses were made with the 72-h ketogenic diet, whereas 5 of 12 (41.7%) indeterminant, 6 of 12 (50%) negative, and 1 of 12 (8.3%) positive CS diagnoses were made with the 24-h ketogenic diet. When we applied the same classification method, our diagnostic rates with the 72-h ketogenic diet demonstrated similar rates whereas the 24-h ketogenic diet group showed different results. Perhaps, our results could be more robust for 24-h ketogenic diet, since we used a larger sample size (94 vs. 12). However, there are differences in the 72-h ketogenic diet method. A ketogenic breakfast 4 h before 18F-FDG injection was used in the study by Lu et al. On the contrary, we additionally applied the principles of intermittent fasting, including at least 12 h of overnight fasting for 3 consecutive days and fasting after 8 pm until the 18F-FDG injection for the PET/CT examination (which is always after 8 am). Despite that difference, our findings are in line with the results of Lu et al. It should also be emphasized that our dietary modification was very well tolerated by our patients and the number of cases with noncompliance were the lowest with this diet, although not significantly different than other regimens. With the Diet-C, we observed low serum glucose levels < 60 mg/dL in 6 patients. Despite that, none of these patients demonstrated any clinical signs of hypoglycemia and tolerated the entire PET/CT examination without any complication. However, cases of diabetes can be challenging, and prolonged fasting could be omitted for those with diabetes to avoid potential hypoglycemia; examination can be performed in the afternoon after a ketogenic breakfast along with morning insulin administration, followed by 6 h of fasting.
There is no consistency in the diagnosis of CS with PET/CT based on prior literature. We think the most reliable current classification is the SNMMI–ASNC consensus guideline (3), which allows a more standard and well-defined approach. That was the primary reason why we used this method for classification in our study. However, the largest diet study in this topic was performed by Lu et al., and they used a classification containing 4 groups based on prior literature (7). According to their study, the group with “ringlike diffuse uptake at the base” is not described in the SNMMI–ASNC consensus guideline. We think the “ringlike diffuse uptake at the base” pattern is more representative of partial myocardial suppression, and in our entire cohort we had 10 observations of this type, without classical mismatch, which were more compatible with “diffuse nonspecific” according to the SNMMI–ASNC classification; that is the main reason for the discrepancy shown in the Tables 3 and 5.
Our study has several strengths. To our knowledge, this is the first study that compares 3 different dietary modifications with a relatively large and equal number of examinations in each group. Two of these dietary modification strategies are mainly applied and recommended by current guidelines and the third one, which performs better, has not been used in the clinical setting despite preclinical evidence to support its use. The results of this new kind of diet are reported for the first time in the literature, excluding the paper by Lu et al., which used a similar but slightly different type of diet (7). Even though the paper by Lu et al. has the largest study cohort (7,17), the compared groups did not contain a comparably similar number of patients as our study. Interobserver variability regarding the myocardial suppression was determined and CS diagnosis was made based on 2 different scoring systems, one proposed by Blankstein et al. and the SNMMI–ASNC guideline and the other one proposed by Lu et al. (3,4,7). The agreement of these 2 scoring systems is also studied for the first time.
We acknowledge several limitations of our study. This is a retrospective study. However, our findings are valuable because existing strategies have limitations and our findings have the potential to change clinical practice and may improve the understanding of CS and cardiac metabolism. Second, we did not give a standard calculated diet to each patient, which is possible for mice, as demonstrated by Clement et al. (13), but impractical for clinical usage and real-life scenarios in humans. Third, the diagnosis made in the current study is not confirmed by a gold standard, as there is no existing practical gold standard, or by clinical correlation, as clinical diagnosis was retrospectively diverted by the initial PET/CT report. This could be only performed in a longitudinal prospective study. However, at our institution, all potential CS cases are reviewed in a monthly multidisciplinary meeting comprising physicians with expertise in nuclear medicine, cardiology, cardiac electrophysiology, cardiac MRI, pulmonology, and sarcoidosis.
CONCLUSION
It appears that the SNMMI–ASNC diet recommendations are inadequate to suppress the background myocardial glucose uptake, leading to many indeterminant and potentially false-positive results. A 72-h ketogenic diet with overnight fasting before cPET/CTs is well tolerated and superior to other dietary preparations, providing excellent physiologic myocardial glucose metabolism suppression and markedly decreasing the number of indeterminant results and potentially false-positive results for active CS diagnosis.
DISCLOSURE
Jerry Froelich receives grant support from Siemens Healthineers for a project unrelated to the present article. For Nathan Rubin, research reported in this publication was supported by NIH grant P30 CA77598 using the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota, and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1-TR002494. (The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.) Rebecca Cogswell serves on the advisory board of Abbott Lab Hearmate-3 and her husband is employed by Medtronic, unrelated to the present article. Henri Roukuz is a consultant for Boston Scientific, receiving grants from Medtronic unrelated to the present article. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank Marie Steinberger, RN, for teaching us about the ketogenic diet and recommending to us that the cardiac metabolism might also take longer than 24 h for the physiology to adjust to the ketogenic diet. We also thank PET/CT technologists Todd Kes and Cassandra Koldenhoven, for their excellent coaching of dietary preparations and the high quality of examinations.
KEY POINTS.
QUESTION: Which dietary modification is better to suppress physiologic myocardial 18F-FDG metabolism to diagnose active inflammation from CS?
PERTINENT FINDINGS: Three dietary preparations, 24-h ketogenic diet with overnight fasting, 18 h-fasting and 72-h ketogenic diet with 3-d overnight fasting, were retrospectively compared regarding their ability to suppress the physiologic myocardial 18F-FDG uptake and diagnose CS with PET/CT. The latter diet demonstrated markedly improved myocardial suppression, higher interobserver agreement, and fewer indeterminant/positive CS diagnoses.
IMPLICATIONS FOR PATIENT CARE: A 72-h ketogenic diet with overnight fasting can be applied instead of the current SNMMI–ASNC dietary recommendations, as this new diet is well tolerated and improves the CS diagnosis by eliminating indeterminant results and false-positives.
REFERENCES
- 1. Mankad P, Mitchell B, Birnie D, Kron J. Cardiac sarcoidosis. Curr Cardiol Rep. 2019;21:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med. 2017;58:1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang IC, Bang JI, Yoon YE, Lee WW. Myocardial positron emission tomography for evaluation of cardiac sarcoidosis: specialized protocols for better diagnosis. J Cardiovasc Imaging. 2020;28:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lebasnier A, Legallois D, Bienvenu B, et al. Diagnostic value of quantitative assessment of cardiac 18F-fluoro-2-deoxyglucose uptake in suspected cardiac sarcoidosis. Ann Nucl Med. 2018;32:319–327. [DOI] [PubMed] [Google Scholar]
- 7. Lu Y, Grant C, Xie K, Sweiss NJ. Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and very-low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. Clin Nucl Med. 2017;42:88–94. [DOI] [PubMed] [Google Scholar]
- 8. Sgard B, Brillet PY, Bouvry D, et al. Evaluation of FDG PET combined with cardiac MRI for the diagnosis and therapeutic monitoring of cardiac sarcoidosis. Clin Radiol. 2019;74:81e9–81e18. [DOI] [PubMed] [Google Scholar]
- 9. Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR. 2008;190:W151-6. [DOI] [PubMed] [Google Scholar]
- 10. Atterton-Evans V, Turner J, Vivanti A, Robertson T. Variances of dietary preparation for suppression of physiological 18F-FDG myocardial uptake in the presence of cardiac sarcoidosis: a systematic review. J Nucl Cardiol. 2020;27:481–489. [DOI] [PubMed] [Google Scholar]
- 11. Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: a randomized controlled trial. J Nucl Cardiol. 2010;17:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christopoulos G, Jouni H, Acharya GA, et al. Suppressing physiologic 18-fluorodeoxyglucose uptake in patients undergoing positron emission tomography for cardiac sarcoidosis: the effect of a structured patient preparation protocol. J Nucl Cardiol. 2021;28:661–671. [DOI] [PubMed] [Google Scholar]
- 13. Clément A, Boutley H, Poussier S, et al. A 1-week extension of a ketogenic diet provides a further decrease in myocardial 18F-FDG uptake and a high detectability of myocarditis with FDG-PET. J Nucl Cardiol. 2020;27:612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manabe O, Yoshinaga K, Ohira H, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial 18F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol. 2016;23:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osborne MT, Hulten EA, Murthy VL, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2017;24:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soussan M, Brillet PY, Nunes H, et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:120–127. [DOI] [PubMed] [Google Scholar]
- 17. Lu Y, Sweiss NJ, Macapinlac HA. Prognostic impact of FDG-PET/CT images in patients with suspected cardiac sarcoidosis: optimal patient preparation, not complex unconventional analytics, is key to meaningful data. JACC Cardiovasc Imaging. 2019;12:217–219. [DOI] [PubMed] [Google Scholar]
- 18. Lu Y, Patel DC, Sweiss N. Using and interpreting 18 F-FDG PET/CT images in patients referred for assessment of cardiac sarcoidosis: the devil is in the details [letter]. J Nucl Med. 2017;58:2039. [DOI] [PubMed] [Google Scholar]
- 19. Bostock ECS, Kirkby KC, Taylor BV, Hawrelak JA. Consumer reports of “keto flu” associated with the ketogenic diet. Front Nutr. 2020;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]