Visual Abstract
Keywords: prostate cancer, PSMA, PET/CT, 68Ga-THP PSMA, phase II clinical trial
Abstract
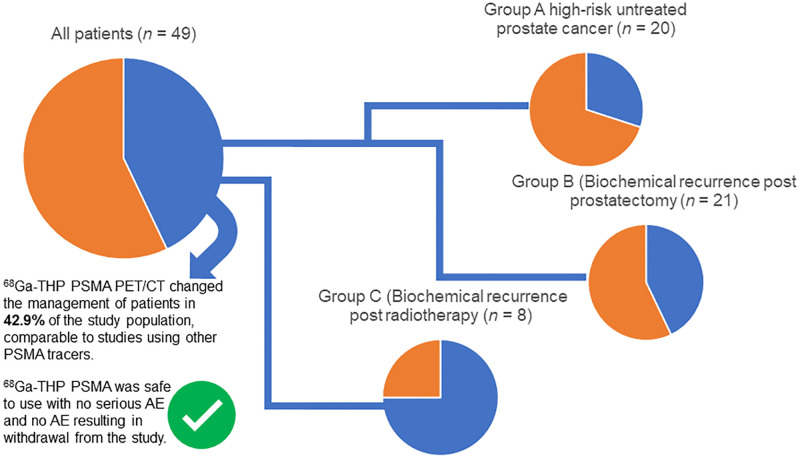
Our objectives were to assess the safety and clinical impact of a novel, kit-based formulation of 68Ga-tris(hydroxypyridinone) (68Ga-THP) prostate-specific membrane antigen (PSMA) for PET/CT in guiding the management of patients with prostate cancer. Methods: Patients were prospectively recruited to group A (high-risk untreated prostate cancer; Gleason score ≥ 4 + 3, or prostate-specific antigen (PSA) level > 20 ng/mL or clinical stage > T2c), group B (biochemical recurrence and eligible for salvage treatment after radical prostatectomy with 2 consecutive rises in PSA with a 3-mo interval between reads and a final PSA level > 0.1 ng/mL or a PSA level ≥ 0.5 ng/mL), or group C (biochemical recurrence with radical curative radiotherapy or brachytherapy at least 3 mo before enrollment, and an increase in PSA level > 2.0 ng/mL above the nadir level after radiotherapy or brachytherapy). Patients underwent evaluation with PET/CT 60 min after intravenous administration of 160 ± 30 MBq of 68Ga-THP PSMA. Safety was assessed through vital signs, cardiovascular profile, serum hematology, biochemistry, urinalysis, PSA, and adverse events (AEs). A change in management was reported when the predefined clinical management of the patient was altered as a result of the 68Ga-THP PSMA PET/CT findings. Results: Forty-nine patients were evaluated with PET/CT: 20 in group A, 21 in group B, and 8 in group C. No patients experienced serious AEs, discontinued the study because of AEs, or died during the study. Two patients had treatment-emergent AEs attributed to 68Ga-THP PSMA (pruritus in one patient and a rash at the intravenous catheter site in another). A management change secondary to the PET/CT findings occurred in 42.9% of all patients: 30% in group A, 42.9% in group B, and 75% in group C. Conclusion: 68Ga-THP PSMA was safe to use, with no serious AEs and no AEs resulting in withdrawal from the study. 68Ga-THP PSMA PET/CT changed the management of 42.9% of the study population, comparable to studies using other PSMA tracers. These data form the basis of a planned phase III study of 68Ga-THP PSMA in patients with prostate cancer.
Prostate-specific membrane antigen (PSMA) imaging with PET/CT has undergone rapid clinical acceptance across a variety of centers throughout the world within several years of first-in-humans studies (1). There is growing evidence of superiority over typical standard-of-care imaging such as CT and bone scanning, as well as over other PET tracers such as choline, but market authorization for use is still lacking (2–4).
Despite the growing number of articles published, relatively few studies have been performed in a clinical trial format. There is also limited systematically collected data on the safety of PSMA PET/CT (5).
There are several types of 68Ga-PSMA PET tracer being used worldwide (6). The greatest use has been with 68Ga-HBED PSMA, but other types include PSMA I&T and PSMA 617. The most common method of producing 68Ga PSMA tracers for clinical use includes a manual/semiautomated multistep process requiring heating and strict pH conditions to facilitate a multistep process that can take approximately 25 min to produce a dose (7). Cold-kit formulations such as 68Ga-tris(hydroxypyridinone) (68Ga-THP) PSMA involve a single-step manufacturing process without the need for heating, requiring approximately 5 min to produce a dose (8).
68Ga-THP PSMA has been investigated in a phase I trial that evaluated 14 patients (9). Of these, 8 patients went on to have prostatectomy, and the tracer was able to identify tumor that was positive for PSMA expression on histopathology. In addition, in 6 patients who had a positive 68Ga-HBED PSMA PET/CT result, 68Ga-THP PSMA was able to demonstrate concordance in number of metastases. Some initial safety data were collected, with no adverse events (AEs) reported. Despite lower absolute uptake, the tumor-to-liver uptake ratio was similar to that of 68Ga-HBED PSMA (9).
The aims of this study were to evaluate PSMA PET/CT in 3 clinical settings using a cold-kit formulation: 68Ga-THP PSMA. The primary objective was to assess the safety of 68Ga-THP PSMA in prostate cancer, and the secondary objective was to evaluate clinical impact by assessing whether the 68Ga-THP PSMA PET/CT findings brought about a change in the management of patients with prostate cancer.
MATERIALS AND METHODS
This study was a prospective open-label phase II clinical trial in patients with newly diagnosed and recurrent prostate cancer (ClinicalTrials.gov identifier NCT03617588). The Institutional Review Board (Research and Ethics Committee) approved this study (reference 18/LO/0370), and all subjects gave written informed consent. The primary endpoint was evaluation of the safety of PSMA PET/CT (specifically AEs related to 68Ga-THP PSMA use), and the secondary endpoint was evaluation of a change in management plan after PET/CT.
Patients were identified from clinic and tumor board meetings from the primary site and from 3 other hospitals in London.
Patients
Inclusion Criteria
The study group consisted of men older than 18 y old who were diagnosed with prostate cancer, had an Eastern Oncology Group performance status of 0–2 (10), and had not received hormone therapy related to prostate cancer within the previous 3 mo. The following criteria divided the study cohort into 3 distinct groups based on clinical setting.
Group A (new diagnosis, high risk) included men with newly diagnosed (histopathologically proven) prostate cancer of Gleason grade 4 + 3 or above, a prostate-specific antigen (PSA) level of more than 20 ng/mL, or a clinical stage greater than T2c and potentially operable disease.
Regarding group B (biochemical recurrence [BCR] after radical prostatectomy), the initial criterion for inclusion in the study was any patient with 3 rises in PSA after radical prostatectomy. This was amended to include a first diagnosis of BCR with 2 consecutive rises in PSA with a 3-mo interval between reads and a final PSA level of more than 0.1 ng/mL or a PSA level of more than 0.5 ng/mL at the time of recruitment.
Group C (BCR after radiotherapy) included men who had a first diagnosis of BCR with previous radical curative therapy with radiotherapy or brachytherapy at least 3 mo before enrollment, and with BCR based on an increase in PSA level to more than 2.0 ng/mL above the nadir level after radiotherapy or brachytherapy.
Exclusion Criteria
The exclusion criteria were receiving another investigational medical product from 1 mo before to 1 mo after administration of 68Ga-THP PSMA, known hypersensitivity to 68Ga-THP PSMA or any of its constituents, or an estimated glomerular filtration rate of less than 20 mL/min/1.73 m2.
Protocol
Patients were recruited from June 2018 to July 2019 and underwent 4 visits as outlined in Table 1.
TABLE 1.
Study Protocol Demonstrating Data Collected at Each of 4 Patient Visits
| Event | Visit 1 prescan data (≤4 wk before scan) | Visit 2 (day of scan) | Visit 3, by telephone (next working day) | Visit 4, outpatient (∼2 wk after scan)* |
|---|---|---|---|---|
| Eligibility confirmation | X | |||
| Informed consent | X | |||
| Demographics | X | |||
| Medical history, including prostate cancer treatment history and imaging history | X | X | ||
| Physical examination† | X | X | X | |
| ECOG performance status | X | X | ||
| Concomitant medications | X | X | X | X |
| Management plan‡ | X | X | ||
| Study registration | X | |||
| Serum full blood count, urea, electrolytes, liver function tests, PSA | X | X | ||
| Urinalysis | X | X | ||
| Cardiovascular profile (electrocardiography) | X | X§ | X | |
| Vital signs | X | X‖ | X | |
| 68Ga-THP PSMA PET/CT administration and imaging | X | |||
| AEs | X | X | X |
Window of 0–6 wk was permitted, depending on local clinical practice.
Comprised height (visit 1 only), weight, body surface area, and description of external signs of cancer.
It was recognized that revised management plan might be decided on before outpatient appointment, and this was permitted. Deciding management plan outside of visit did not constitute protocol deviation.
Electrocardiography: visit 2, approximately 1 h before scan.
Vital signs: visit 2, before scan, during injection, after scan (for 2 h after injection), and before discharge.
ECOG = Eastern Cooperative Oncology Group.
Safety
AEs, regardless of suspected relationship to study treatment, were recorded throughout the study, from the 68Ga-THP PSMA administration until 30 d after the administration of 68Ga-THP PSMA. All AEs were followed up until resolution or until visit 4. Common terminology criteria grading was used for AEs (11).
Any related serious AEs that occurred at any time after 30 d after the administration of 68Ga-THP PSMA were reported.
Safety was assessed by means of physical examination, vital signs, cardiovascular profile (including 12-lead electrocardiography), performance status, and laboratory evaluations (hematology, biochemistry, and urinalysis).
68Ga-THP PSMA PET/CT
Radiopharmaceutical production of the 68Ga-THP PSMA was in accordance with good manufacturing practices and used a kit-based formulation, by a method previously described (8).
No patient preparation was required before the scan. The target administered activity of 68Ga-THP PSMA was 250 MBq ± 20% intravenously via a vein in the right upper limb.
PET/CT was performed on a GE Healthcare Discovery 710 time-of-flight scanner with Q.Clear reconstruction algorithm.
Imaging Evaluation
All images were reviewed on GE Healthcare Advantage workstations by 2 nuclear medicine radiologists/physicians in consensus, both of whom had over 3 y of experience in reporting PSMA PET/CT findings and had undergone training that included a review of 50 68Ga-THP PSMA PET/CT studies performed at another site.
Criteria for a Positive Scan
Focal areas of tracer uptake that were greater than background activity and could not be explained by physiologic activity were attributed to prostate cancer or prostate cancer metastases.
Criteria for a Negative Scan
A negative scan was defined as one lacking any uptake that was higher than background activity and attributable to prostate cancer or prostate cancer metastases.
Disease distribution was classified as being in the prostate (or prostate bed), pelvic lymph nodes, extrapelvic lymph nodes, bones, or other sites, such as the liver parenchyma. The reviewing nuclear medicine radiologist was permitted to request additional imaging to clarify equivocal areas or potential synchronous diseases. Image reviewers were not masked to any existing standard-of-care imaging that had occurred (e.g., bone scanning, CT, or MRI) and were aware of all clinical details, including PSA value.
Criteria for a Management Plan
Management plans were created by the primary clinician who was evaluating the patient (urologist or oncologist). The initial management plan relied on standard-of-care clinical and imaging details as per local, national, and international guidelines. The revised management plan took all of the above factors into account, along with the additional information from the 68Ga-THP PSMA PET/CT study. A change in management was defined as a change that was influenced by, or altered directly in response to, the findings of the 68Ga-THP PSMA PET/CT study. A change that was primarily the result of non-PET factors, such as comorbidities, was not counted as a change in management.
Statistics
The sample size was based on the degree of uncertainty in estimating the percentage of patients with a change in management, as measured by the CI. Because of the phase of the study, relatively wide CIs were allowed.
For group A, previous literature suggested that approximately 25% of patients would have a change in management (12). Assuming a 95% confidence level, 20 patients would be sufficient to estimate the secondary outcome that was within ±20% of the population value.
For groups B and C, previous literature suggested that approximately 45% would have a change in management (13). With a 95% confidence level, 40 patients in the 2 groups combined would be sufficient to estimate the outcome that was within ±15% of the population value. Two-sided 95% and 80% Clopper–Pearson exact CIs were calculated.
Validation
Validation included a combination of histology correlation (22 patients) and, when available, follow-up of PSA levels beyond trial participation (31 patients).
RESULTS
Patients
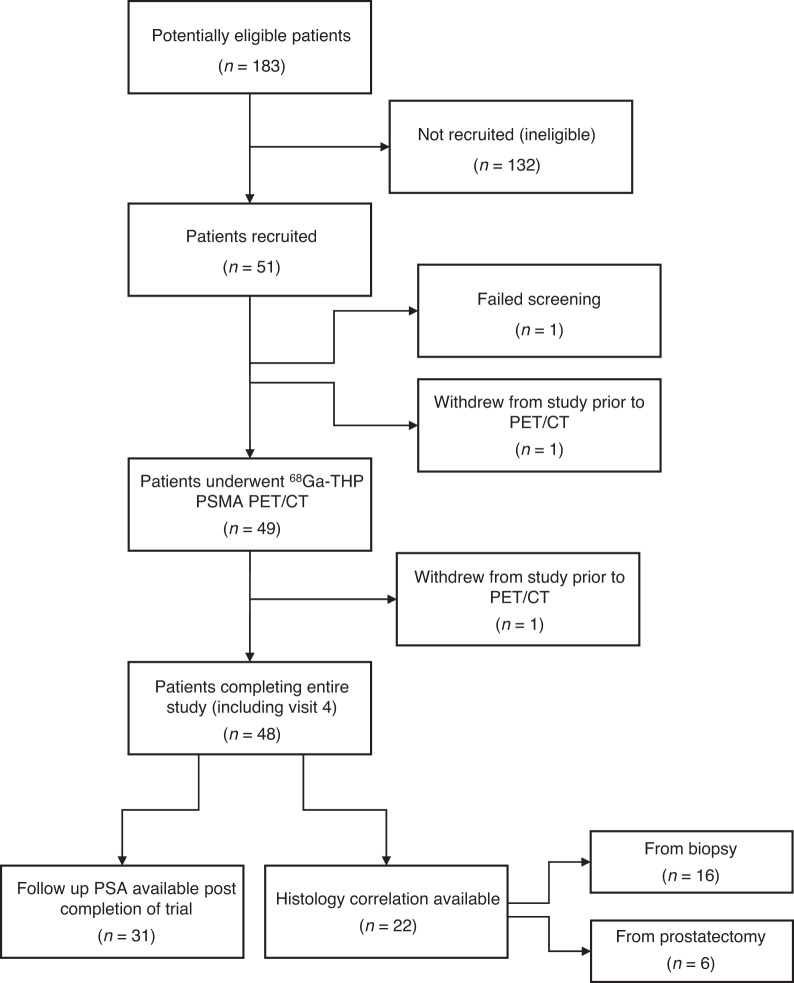
Forty-nine patients underwent evaluation with 68Ga-THP PSMA PET/CT, and 48 patients completed all visits, including the final safety visit. The median age of recruited patients was 67 y (range, 43–80 y).
Figure 1outlines the number of patients at each stage of the trial, and Table 2 shows patient demographics and clinical characteristics.
FIGURE 1.
Number of patients in each stage of trial.
TABLE 2.
Demographic and Baseline Characteristics in Group A (New Diagnosis, High-Risk) and in Groups B and C (Combined Recurrence After Radical Prostatectomy and After Radiotherapy)
| Characteristic | Newly diagnosed (group A) | BCR (groups B and C) | Total |
|---|---|---|---|
| Patients (n) | 20 | 29 | 49 |
| Age | |||
| Median | 68.5 | 66.0 | 67.0 |
| Minimum | 49 | 43 | 43 |
| Maximum | 76 | 80 | 80 |
| Race (n)* | |||
| White | 13 (65.0%) | 21 (72.4%) | 34 (69.4%) |
| Afro-Caribbean | 7 (35.0%) | 5 (17.2%) | 12 (24.5%) |
| Asian | 0 (0.0%) | 3 (10.3%) | 3 (6.1%) |
| ECOG performance status (n) | |||
| 0 | 19 (95.0%) | 29 (100.0%) | 48 (98.0%) |
| 1 | 1 (5.0%) | 0 (0.0%) | 1 (2.0%) |
| PSA before scan (μg/L) | 20 | 29 | 49 |
| Mean | 25.2 | 1.6 | 11.2 |
| SD | 27.00 | 2.32 | 20.70 |
| Median | 13.8 | 0.4 | 4.2 |
| Range | 5–90 | 0–10 | 0–90 |
| Initial Gleason score | |||
| ≤3 + 4 | 1 (5.0%) | 15 (51.7%) | 16 (32.7%) |
| ≥4 + 3 | 19 (95.0%) | 14 (48.3%) | 33 (67.3%) |
Prostate cancer aggressiveness can vary according to race.
Safety
No serious AEs were reported, no patients were withdrawn from the trial because of AEs, and no deaths were reported.68Ga-THP PSMA was well tolerated.
Two patients (4.1%) had treatment-emergent AEs that were considered related to 68Ga-THP PSMA PET/CT. These were grade 1 pruritus (duration, 22 d; 1 patient in group A) and a grade 1 catheter site rash (duration, 4 d; 1 patient in group B), according to the common terminology criteria for AEs. An additional 9 treatment-emergent AEs were reported, not related to 68Ga-THP administration.
No clinically significant variations in serum hematology, clinical chemistry, or urinalysis parameters were detected, and no AEs were identified from these parameters.
There were no clinically significant changes in vital signs for any patients during the study. No AEs were recorded from electrocardiography findings.
Imaging Findings
All imaging was of diagnostic quality. In 2 patients, image interpretation was more challenging because of a high body mass index, because the injected activity was at the lower end of the acceptable range, and because of the arms-down position in a patient who could not elevate the arms above the head.
A minor halo artifact was frequently encountered, but the images could be reviewed without scatter correction to reduce the effect of this artifact.
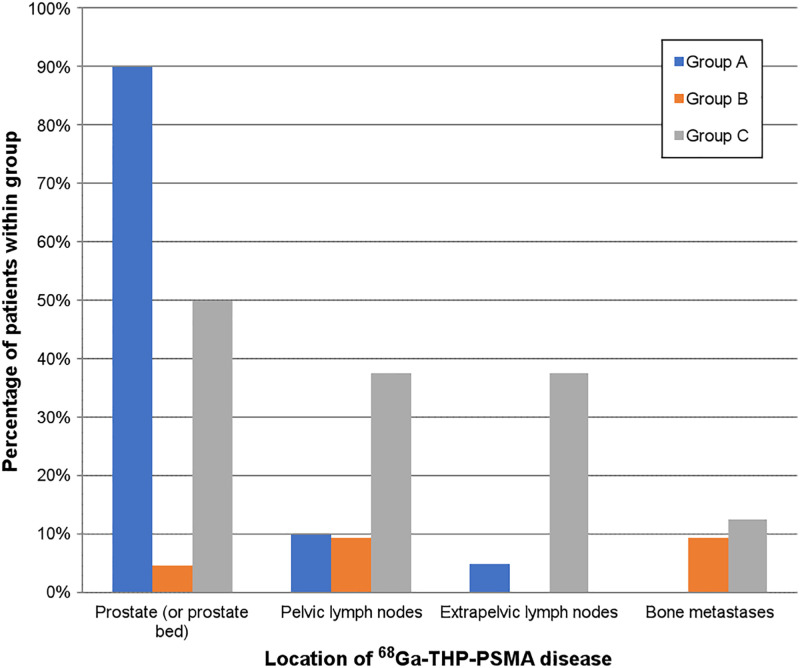
68Ga-THP PSMA PET/CT was positive in 27 patients (55.1%). Most patients in group A had focal uptake in the prostate gland. In 2 of these patients, focal increased uptake in the prostate was difficult to localize on PET/CT, leading to a positivity rate of 90% in this group. Fifteen patients had prostate disease only, 1 had prostate–plus–seminal vesicle involvement, 1 had prostate–plus–pelvic lymph node involvement, and 1 had prostate involvement plus involvement of the lymph nodes below and above the diaphragm (Fig. 2).
FIGURE 2.
Locations of 68Ga-THP uptake positive for tumor in groups A–C.
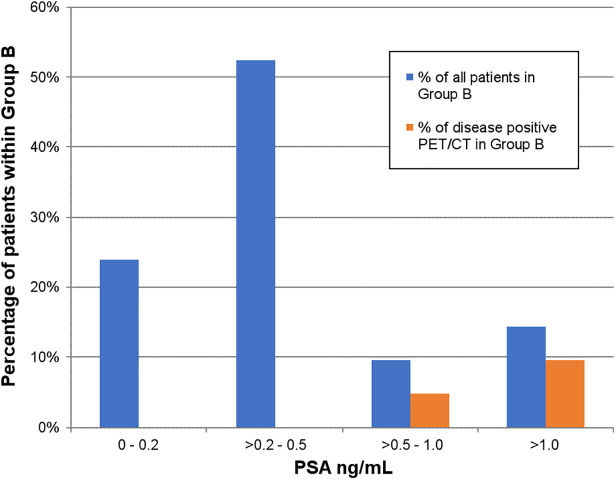
Only 3 of 21 patients in group B were positive for disease (14.3%). One had uptake in the prostatectomy bed, 1 had prostate disease and pelvic lymph node involvement (PSA, 1.77 ng/mL), 1 had pelvic lymph node and bone metastases (PSA, 0.6 ng/mL), and 1 had bone metastases only (PSA, 2.54 ng/mL).
Positive scans in this group were demonstrated only when PSA at the time of the scan was more than 0.5 ng/mL (Figs. 2 and 3).
FIGURE 3.
Proportion of patients in group B and proportion of positive scans in this group, stratified according to serum PSA level.
Patients in group C tended to have a higher proportion of positive scans than did patients in group B (6/8, 75%). Disease was identified in the treated prostate in 50% of the patients in this group, and disease was identified in a relatively greater proportion of lymph nodes (pelvic and extrapelvic) and bones in this group than in groups A and B. 68Ga-THP was positive in the prostate alone in 3 patients; in the prostate and lymph nodes above and below the diaphragm in 1 patient; in the pelvic and retroperitoneal lymph nodes in 1 patient; and in the pelvic, extrapelvic, and bone in 1 patient (Fig. 2).
In 3 patients, additional imaging was requested. The first request was for a 68Ga-HBED PSMA study for short-interval follow-up of a small-volume mildly avid pelvic node; this node was negative on 68Ga-HBED PSMA. The second request was for MRI of the liver to evaluate solitary focal uptake in the liver; this study revealed imaging features typical of a hemangioma. The third request was for MRI of the spine to evaluate focal uptake in the L5 vertebral body; this study showed no metastases, and the uptake at L5 was deemed to be secondary to degenerative change.
Patients demonstrated the typical physiologic distribution of this tracer, which has been described previously (9).
In some patients, areas of uptake were relatively mild in the prostate, and manipulation of the window setting was required to evaluate further. Requirement of manipulation due to mild uptake was also noted during training with 68Ga-THP PSMA cases before the trial, suggesting that both background and tumor activity may be less than in patients being evaluated with 68Ga-HBED PSMA.
Change in Management
There were 21 changes from the original management plan in total across all 3 groups. Of these, 6 occurred in group A (30%), 8 in group B (42.9%), and 6 in group C (75%), giving a 51.7% change in management in groups B and C combined (Table 3).
TABLE 3.
Scan Positivity and Management Change per Patient Group
| Group | Positive | Intermodality change | Intramodality change | Other (imaging/short-interval follow-up) | Total management change per group |
|---|---|---|---|---|---|
| A | 18 (90%) | 4 (RP to RT [1], RT to RP [1], RT to RT with field change [1], RP to hormones [1]) | 1 (RP with lymph node dissection to RP alone) | 1 (spine MRI) | 30.0% |
| B | 3 (14.3%) | 6 (RT to surveillance [4], RT to hormones [2]) | 1 (RT to RT with field change) | 2 (liver MRI [1], short-interval repeat PET/CT [1]) | 42.9% |
| C | 6 (75%) | 6 (salvage RP/focal therapy of prostate to hormones/chemotherapy [3], hormones to template biopsy for consideration of salvage RP/focal therapy [2], salvage RP/focal therapy of prostate to surveillance [1]) | 0 | 0 | 75.0% |
| Total | 27 (55.1%) | 16 | 2 | 3 | 42.9% |
RP = radical prostatectomy; RT = radiotherapy.
Most of the changes throughout the 3 groups were intermodality (16/21)—for example, from prostatectomy to radiotherapy, or from radical or salvage local treatment to systemic treatment. A change from salvage treatment of the prostate bed to surveillance was also considered an intermodality change.
A minority of changes were intramodality—that is, a change from prostatectomy with lymph node dissection to prostatectomy alone or an adjustment of the radiotherapy fields to include lymph nodes.
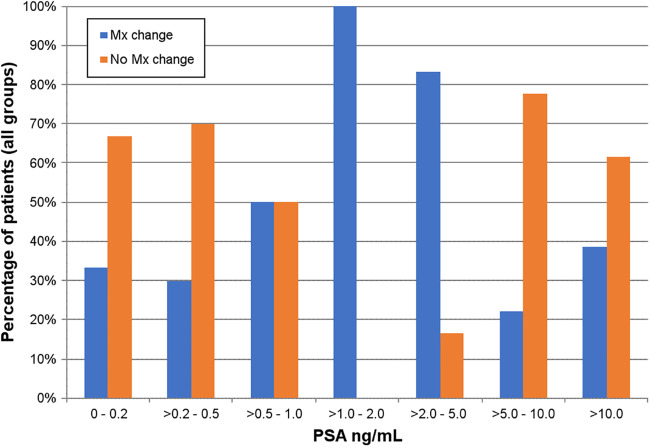
Management changes occurred at all PSA levels (Fig. 4).
FIGURE 4.
Proportion of patients with management (Mx) change (all groups) stratified according to PSA level.
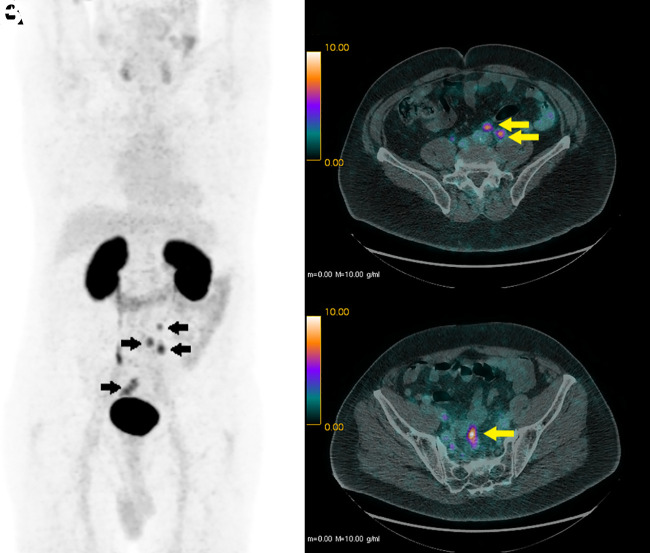
An example of a positive 68Ga-THP PSMA PET/CT finding with a resulting change in management is shown in Figure 5.
FIGURE 5.
68Ga-THP PSMA PET/CT in 62-y-old man in group C. (A) Maximum-intensity projection, with arrows showing 68Ga-THP PSMA–avid lymph nodes in pelvis and retroperitoneum. (B) Axial PET/CT image, with arrows showing some small-volume retroperitoneal lymph nodes. (C) Axial PET/CT image, with arrow showing some small-volume pelvic nodal disease. Patient’s management changed from potential salvage options (prostatectomy or focal therapy) to systemic treatment with hormones.
Validation
In all 22 patients with a histopathologic reference standard (6 from prostatectomy and 16 from biopsy) within 3 mo of the date of the 68Ga-THP PSMA PET/CT study, disease within the prostate identified on 68Ga-THP PSMA PET/CT could be correlated with a site of disease on histology. In all patients for whom follow-up PSA measurements were available after trial participation (14 with a management change and 17 without), PSA was reduced.
In the 2 group A patients for whom disease was difficult to identify, prostatectomy was performed on one and revealed pT2 Gleason 4 + 3 acinar adenocarcinoma with a tumor volume of 0.2 cm2, and a pretrial biopsy in the other demonstrated a Gleason 4 + 5 tumor with a maximum length of 7 mm (involving 40% of the core), which was ultimately treated with radiotherapy.
DISCUSSION
This was the first phase II clinical trial using 68Ga-THP PSMA (a kit-based formulation) and showed a lack of serious AEs and a lack of patients discontinuing the study due to AEs. The confirmed safety profile when reviewing serum hematology, chemistry, urinalysis, vital signs, and electrocardiography further fortified the safety profile of this tracer. These were all important findings when considering a phase III study with this tracer.
The lack of serious AEs is concordant with published data on safety using other PSMA agents (68Ga-PSMA-11, 18F-PSMA-11, and 18F DCFPyL) (5,14,15). The 68Ga PSMA-11 data showed no clinically reported AEs. With 18F-PSMA-11, no clinically relevant changes in vital parameters were observed and no patients reported any side effects. The DCFPyL study showed no serious AEs, and no AEs related to heart rate or blood pressure were seen. One patient reported 2 AEs that were classified as unlikely to be attributable to the radiotracer, and another patient had a fall in platelet count attributed to treatment for prostate cancer.
This study demonstrated that clinically significant levels of management change occurred after 68Ga-THP PSMA PET/CT in high-risk patients and in those with BCR (42.9% change in management across all groups).
In the primary high-risk group, a change in management occurred in 30% of patients. This finding is comparable to the findings from a recent study performed with the same tracer; that study showed that management changed in 24% of high-risk patients (12). The management change rate in high-risk patients in a randomized multicenter study using 68Ga-HBED PSMA was 28% (4,12).
In the BCR groups combined, a management change occurred in 51.7% of patients. This was comparable to the findings of the metaanalysis of studies performed with PSMA in the recurrence setting by Han et al. and slightly higher than the proportion of management change we described previously using 68Ga-HBED PSMA (13).
There were fewer changes in the postprostatectomy group (42.9%) than in the postradiotherapy group (75%). This may in part have been due to the large proportion of patients scanned at very low PSA levels in group B; 23.8% of patients in this group had a PSA of 0.1–0.2 ng/mL and 52.4% had a PSA of 0.2–0.5 ng/mL and an associated low number of positive scans. The low PSA levels at the time of scanning in the postprostatectomy group was driven by the definition of BCR in this group, reflecting clinicians’ threshold to consider offering early salvage radiotherapy. In the BCR group evaluated by Kulkarni et al., there were no positive scans in the population with a PSA of less than 0.5 ng/mL, and that study reported higher rates of management change at higher PSA levels (12). Derlin et al. did demonstrate positive findings at very low PSA levels: 20.0% for a PSA value of more than 0.2 to less than 0.5 ng/mL, and 22.2% for a PSA value of 0.01–0.2 ng/mL (16). This finding is in contrast to the findings from other published data using 68Ga-HBED PSMA, which showed 38% of scans to be positive when PSA was less than 0.5 ng/mL in BCR (17). Others have also demonstrated higher positive rates at very low PSA levels, and this is one of the key strengths of PSMA over other PET tracer such as 18F/11C-choline. However, our previous study using 68Ga-HBED PSMA demonstrated a positive rate of 15.8% in a PSA range of 0.2–0.5 ng/mL (18). As a possible explanation, many studies have not excluded hormone use before the scan (which would increase PSMA upregulation/scan positivity), and another important consideration is that 68Ga-THP PSMA has faster renal clearance of THP and potentially lower affinity than 68Ga-HBED PSMA (12). Both PSMA tracers would need to be investigated in the same population with low PSA levels or at least in a matched-pair analysis to evaluate further.
The higher rate of management change in the postradiotherapy BCR group (group C) may have been in part due to the greater proportion of positive PET/CT results in this group, as well as the greater number of available options to treat these patients, including salvage prostatectomy, salvage focal therapy, further salvage radiotherapy/brachytherapy, systemic therapies, or watchful waiting.
The cold-kit manufacturing method of this tracer has several potential benefits if permitted to enter mainstream clinical use. A short manufacturing time is helpful in the context of a short–half-life radiotracer, and less complex radiopharmacy production may allow more patients to be scanned with produced doses.
This study had some limitations. The endpoint chosen for this trial—management change—has been used in multiple studies evaluating PSMA PET/CT (19–21). In a well-designed clinical trial, use of the same endpoint as in other studies allows for potential progression of PSMA tracers toward regulatory approval; although a survival or progression endpoint is more objective, it may take years to be reached. There remains a lack of evidence that a management change improves survival or another surrogate endpoint. However, the increased accuracy of disease detection implies more personalized and appropriate management for the stage of disease and reduction of the risks and side effects of ineffective treatments.
There is a lack of full validation within this and many studies on PSMA PET/CT (19–21). Although histology was available in some patients, mainly those with recent biopsy and those who underwent prostatectomy soon after the PET/CT, histology was not available in all patients and other sites of disease, such as lymph nodes, were not sampled to provide systematic validation. Such sampling is often not feasible, particularly in the BCR setting. Diagnostic accuracy assessment was not within the scope of this phase II trial but is planned to be evaluated in a larger phase III study.
The study design involved recording the intended management plan after and on the basis of 68Ga-THP PSMA PET/CT, and factors related to clinician–patient consultations may have led to an executed management different from that recorded in the study.
In one patient for whom lymph node dissection was not performed, the lack of uptake in nodes partly informed the decision not to proceed with lymphadenectomy, even though the clinician and patient were aware that a negative scan did not exclude disease entirely. This example touches on the complex issue of PSMA-guided targeted therapy and the need for robust histopathologic studies to confirm the limits of disease detection within nodes.
CONCLUSION
68Ga-THP PSMA (kit formulation) was well tolerated and safe to use, with no serious AEs and no AEs resulting in withdrawal from this phase II study. 68Ga-THP PSMA PET/CT changes the management of prostate cancer patients in BCR (42.9%) and, to a lesser degree, in primary high-risk disease. The levels of management change described in this prospective trial are comparable to those found by others who have evaluated PSMA in these prospective and retrospective clinical settings. These data form the basis of a planned phase III study of 68Ga-THP PSMA in patients with prostate cancer.
DISCLOSURE
Asim Afaq is supported by the UCL NIHR Experimental Cancer Medicine Centre and receives research support funds from Theragnostics Ltd. (funding of a research radiographer), is an expert reader for Theragnostics Ltd./Pharmatrace, and receives speaker honorarium/travel support from Astellas, Curium, Janssen, and GE. Heather Payne is supported by the UCLH/UCL Comprehensive Biomedical Research Centre and attended and received honoraria for advisory boards, received travel expenses for medical meetings, and served as a consultant for AstraZeneca, Astellas, Janssen, Sanofi Aventis, Ferring Bayer, and Novartis. Marie Meagher is a research radiographer funded by Theragnostics Ltd.. Michael Ferris is an employee of Theragnostics Ltd. Greg Mullen is an employee and shareholder of Theragnostics Ltd. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We are grateful to all patients involved in this trial and all staff who facilitated this trial, including Robert Shortman, Luke Hoy, and Raymond Endozo.
KEY POINTS.
QUESTION: Is 68Ga-THP PSMA PET/CT safe to use, and does it change the management of patients with prostate cancer?
PERTINENT FINDINGS: This phase II, open-label prospective clinical trial showed 68Ga-THP PSMA PET/CT to be well tolerated and safe to use. Management was changed in 30% of patients with untreated high-risk disease and in 51.7% of patients with recurrent disease.
IMPLICATIONS FOR PATIENT CARE: These data show the potential utility of 68Ga-THP PSMA in untreated and recurrent disease, and the safety data serve as the basis for a planned phase III study.
REFERENCES
- 1. Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–495. [DOI] [PubMed] [Google Scholar]
- 2. Afaq A, Ell PJ, Bomanji JB. Is it time to fund routine NHS usage of PSMA PET-CT? Nucl Med Commun. 2019;40:975–979. [DOI] [PubMed] [Google Scholar]
- 3. Miyahira AK, Pienta KJ, Babich JW, et al. Meeting report from the Prostate Cancer Foundation PSMA theranostics state of the science meeting. Prostate. 2020;80:1273–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen JB, Zacho HD, Haberkorn U, et al. A comprehensive safety evaluation of 68Ga-labeled ligand prostate-specific membrane antigen 11 PET/CT in prostate cancer: the results of 2 prospective, multicenter trials. Clin Nucl Med. 2017;42:520–524. [DOI] [PubMed] [Google Scholar]
- 6. Czarniecki M, Mena E, Lindenberg L, et al. Keeping up with the prostate-specific membrane antigens (PSMAs): an introduction to a new class of positron emission tomography (PET) imaging agents. Transl Androl Urol. 2018;7:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eder M, Neels O, Müller M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel). 2014;7:779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young JD, Abbate V, Imberti C, et al. 68Ga-THP-PSMA: A PET imaging agent for prostate cancer offering rapid, room-temperature, 1-step kit-based radiolabeling. J Nucl Med. 2017;58:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofman MS, Eu P, Jackson P, et al. Cold kit for prostate-specific membrane antigen (PSMA) PET imaging: phase 1 study of 68Ga-tris(hydroxypyridinone)-PSMA PET/CT in patients with prostate cancer. J Nucl Med. 2018;59:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ECOG performance status. ECOG-ACRIN Cancer Research Group website. https://ecog-acrin.org/resources/ecog-performance-status. Accessed July 7, 2021.
- 11. NCI Common Terminology Criteria for Adverse Events. National Institutes of Health website. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_60. Updated September 21, 2020. Accessed July 7, 2021..
- 12. Kulkarni M, Hughes S, Mallia A, et al. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. [DOI] [PubMed] [Google Scholar]
- 14. Piron S, De Man K, Van Laeken N, et al. Radiation dosimetry and biodistribution of 18F-PSMA-11 for PET imaging of prostate cancer. J Nucl Med. 2019;60:1736–1742. [DOI] [PubMed] [Google Scholar]
- 15. Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Derlin T, Schmuck S, Juhl C, et al. PSA-stratified detection rates for [68Ga]THP-PSMA, a novel probe for rapid kit-based 68Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:913–922. [DOI] [PubMed] [Google Scholar]
- 17. Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afaq A, Alahmed S, Chen SH, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. [DOI] [PubMed] [Google Scholar]
- 19. Fendler WP, Ferdinandus J, Czernin J, et al. Impact of 68Ga-PSMA-11 PET on the management of recurrent prostate cancer in a prospective single-arm clinical trial. J Nucl Med. 2020;61:1793–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonni I, Eiber M, Fendler WP, et al. Impact of 68Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: a prospective single-center study. J Nucl Med. 2020;61:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hope TA, Aggarwal R, Chee B, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]