Significance
Using state-of-the-art biologging technology, we document the occurrence of fevers in wild vervet monkeys and demonstrate that fevers coincide with overt sickness behavior. In so doing, we demonstrate a hidden cost of sociality: Febrile animals were twice as likely to receive aggression from their group mates and were six times more likely to be wounded following the onset of a fever. Sick animals were targeted when least able to fight back, potentially improving the attacker’s social status and further reducing a sick animal’s survival prospects. Understanding disease transmission dynamics requires greater attention to the ways in which social structure can change as a result of infection and how such shifts can influence future patterns of transmission.
Keywords: aggression, body temperature, disease, fever, sickness behavior
Abstract
Fevers are considered an adaptive response by the host to infection. For gregarious animals, however, fever and the associated sickness behaviors may signal a temporary loss of capacity, offering other group members competitive opportunities. We implanted wild vervet monkeys (Chlorocebus pygerythrus) with miniature data loggers to obtain continuous measurements of core body temperature. We detected 128 fevers in 43 monkeys, totaling 776 fever-days over a 6-year period. Fevers were characterized by a persistent elevation in mean and minimum 24-h body temperature of at least 0.5 °C. Corresponding behavioral data indicated that febrile monkeys spent more time resting and less time feeding, consistent with the known sickness behaviors of lethargy and anorexia, respectively. We found no evidence that fevers influenced the time individuals spent socializing with conspecifics, suggesting social transmission of infection within a group is likely. Notably, febrile monkeys were targeted with twice as much aggression from their conspecifics and were six times more likely to become injured compared to afebrile monkeys. Our results suggest that sickness behavior, together with its agonistic consequences, can carry meaningful costs for highly gregarious mammals. The degree to which social factors modulate the welfare of infected animals is an important aspect to consider when attempting to understand the ecological implications of disease.
Evolutionary studies of animal behavior generally focus on the adaptive value of typical behavior in healthy animals. It has long been recognized, however, that sick animals can also provide insights into the behavioral determinants of survival and adaptation, particularly in the face of environmental challenges (1). The occurrence of fevers in a diverse range of animals (1) suggests that, despite the significant metabolic costs associated with the maintenance of elevated body temperatures (2), the fever response is an evolutionarily conserved strategy, acting principally to fight off infectious pathogens or other noninfectious irritants (3).
The behavioral responses that accompany fevers are similarly considered to be complementary and beneficial (1, 4, 5) rather than maladaptive byproducts of an inability to cope with infection (6). Collectively referred to as “sickness behavior,” responses such as lethargy, anorexia, loss of body weight, sleepiness, and the cessation of grooming can all help alleviate the body’s increased metabolic demand when fighting infection (1, 7). Importantly, however, the survival benefits of sickness behavior must be traded against costs incurred such as increased risk of predation, a reduction in social engagement and reproductive opportunities, or reduced territorial defense (8). For gregarious species experiencing local competition for resources (9), detectable evidence of sickness behavior may offer group members the opportunity to gain competitive advantage through the targeted aggression of debilitated rivals. Yet, to date there is no record of increased aggression toward infected conspecifics. Birds (10) and rats (11) reduced aggressive behavior when sickness behaviors were artificially induced, and wild mongoose showed no change in agonistic behavior when sick (12). Cues of sickness, however, may allow conspecifics to identify individuals less likely to engage in aggressive encounters. Such detection, coupled with reduced rates of aggression, could explain why male house finches preferentially feed near infected conspecifics (13). Humans detect sick individuals using both visual and olfactory cues (14), and there is evidence to suggest that some nonhuman primates use olfactory cues to detect conspecifics infected with parasites (15). However, it remains unclear to what extent nonhuman primates can detect sickness itself and whether this affects the behavior of conspecifics toward sick individuals (7, 16, 17).
Here, we use continuous measurements of core body temperature and corresponding behavioral data to identify naturally occurring fevers in wild vervet monkeys (Chlorocebus pygerythrus) and to test the prediction that monkeys would spend more time resting (lethargy) and less time foraging (anorexia) when febrile. On the assumption that sickness behavior can be detected, if it occurs, by conspecifics, we then assessed the possibility that conspecifics reduced their social engagement with febrile individuals and vice versa. Finally, we tested the prediction that, if the weakened status of a febrile monkey can be detected, then they would receive more conspecific aggression and that a reduced capacity to mount a viable behavioral defense would lead to an increased likelihood of injury.
Results
Overall, we collected concurrent body temperature and behavioral data from 59 monkeys across 1,264 calendar days (n = 16,997 “monkey-days”). This sample included 412 monkey-days on which a monkey was febrile (Fig. 1), 5,622 monkey-days that involved aggression, and 216 monkey-days when injuries occurred. Mean 24-h body temperatures were on average 0.7 °C higher on fever-days (38.7 ± 0.5 °C) compared to nonfever days (38.0 ± 0.3 °C), maximum 24 h body temperatures were on average 0.5 °C higher during fever-days (39.9 ± 0.5 °C) compared to nonfever days (39.4 ± 0.4 °C), and minimum 24-h body temperatures were on average 0.7 °C higher during fever-days (37.5 ± 0.6 °C) compared to nonfever days (36.8 ± 0.5 °C).
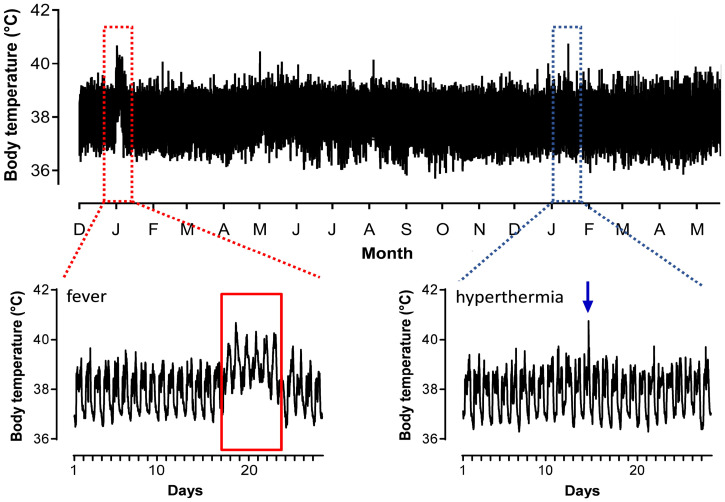
Fig. 1.
An illustration of a sustained upward shift in vervet monkey body temperature associated with a 6-d fever (red) and a relatively transient spike in body temperature associated with hyperthermia (blue). The data plotted represent 5-min recordings of body temperature from a single male vervet monkey across an 18-mo period (Upper) that encapsulates febrile (Lower Left) and hyperthermic (Lower Right) body temperature patterns.
Fevers had a meaningful positive effect on time spent resting and traveling, a meaningful negative effect on time spent feeding, and no meaningful effect on time spent socializing or the giving or receiving of grooming (Table 1 and SI Appendix, Fig. S1). Of the 412 monkey-days on which a monkey was febrile, 39% involved aggression and 8% resulted in newly acquired injuries. Across the remaining monkey-days on which a monkey was afebrile, 33% involved aggression and 1% involved injuries. Fevers had a meaningful positive effect on the likelihood of receiving aggression or becoming injured (Table 1, Fig. 2, and SI Appendix, Fig. S1). Our models predict that febrile monkeys were twice as likely to receive aggression and six times more likely to get injured compared to afebrile monkeys.
Table 1.
Results of the GLMMs estimating the effect of fevers on sickness behavior, aggression received, and injury
| Model | Estimate ± error fever (n/y) | Probability of direction % fever (n/y) | Estimate ± error sex (female/male) | Probability of direction % sex (female/male) | Model probability predictions* | R2 marginal | R2 conditional | |
| Febrile | Afebrile | |||||||
| Resting, SI Appendix, Table S1 |
0.08 ± 0.03 | 99.88 | 0.17 ± 0.04 | 100 | 0.35 | 0.32 | 0.41 | 0.59 |
| Feeding, SI Appendix, Table S2 |
−0.17 ± 0.03 | 100 | −0.06 ± 0.04 | 95.28 | 0.25 | 0.29 | 0.36 | 0.51 |
| Traveling, SI Appendix, Table S3 |
0.07 ± 0.03 | 97.92 | 0.08 ± 0.04 | 98.52 | 0.26 | 0.24 | 0.34 | 0.43 |
| Socializing, SI Appendix, Table S4 |
−0.04 ± 0.06 | 70.80 | −0.88 ± 0.08 | 100 | 0.10 | 0.09 | 0.19 | 0.40 |
| Grooming given, SI Appendix, Table S5 |
−0.02 ± 0.09 | 57.57 | −1.47 ± 0.12 | 100 | 0.06 | 0.06 | 0.16 | 0.36 |
| Grooming received, SI Appendix, Table S6 |
−0.02 ± 0.09 | 59.15 | −0.37 ± 0.09 | 100 | 0.03 | 0.03 | 0.05 | 0.15 |
| Aggression received, SI Appendix, Table S7 |
0.33 ± 0.12 | 99.68 | −0.64 ± 0.40 | 94.86 | 0.02 | 0.01 | 0.01 | 0.16 |
| Injured, SI Appendix, Table S8 |
1.99 ± 0.23 | 100 | 0.31 ± 0.33 | 83.28 | 0.03 | 0.005 | 0.003 | 0.03 |
Calculated for the reference sex category (females) using the following equations: 1) activity Poisson GLMMs: febrile probability = exponentiate (intercept estimate + fever estimate), afebrile probability = exponentiate (intercept estimate); and 2) aggression and injury Bernoulli GLMMs: febrile probability = inverse logit (intercept estimate + fever estimate), afebrile probability = inverse logit (intercept estimate).
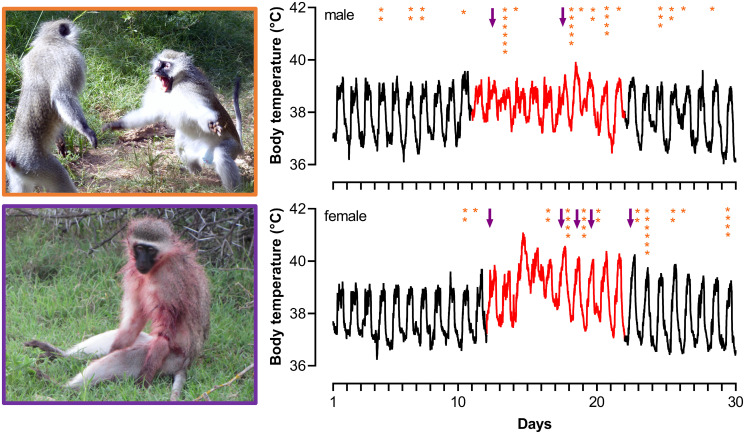
Fig. 2.
Illustration of the number of aggressions received (orange asterisks) and the timing of injury (purple arrows) across two fever periods (red lines). The data plotted represent 5-min recordings of body temperature from a single male (Upper Right) and female (Lower Right) vervet monkey. Photographs of male–male aggression (Upper Left, image credit: C. Young, NTU Psychology, Nottingham Trent University, Nottingham, UK) and an injured febrile male (Lower Left, image credit: R. Blersch, Department of Psychology, Lethbridge University, Lethbridge, AB, Canada).
Males were more likely to be the victims of aggression when they were febrile than were females (Table 2). Across all aggressions directed toward afebrile victims, 69% of the victims were male and 31% were female. Across all aggressions directed toward febrile victims, 80% of the victims were male and 20% were female. Whether the victim of aggression was febrile or not did not predict the sex of the aggressor, the sex combination of the aggressive dyad, whether the aggression was targeted up or down the dominance hierarchy, or the distance in dominance rank between opponents.
Table 2.
Results of the GLMMs estimating the effect of fevers on the sex and dominance status of the victims and aggressors
| Model | Estimate ± error febrile victim (n/y) | Probability of direction % fever (n/y) | Model probability predictions* | R2 marginal | R2 conditional | |
| Febrile | Afebrile | |||||
| Aggressor sex (female/male), SI Appendix, Table S9 |
−0.17 ± 0.19 | 81.42 | 0.52 | 0.56 | 0.002 | 0.42 |
| Victim sex (female/male), SI Appendix, Table S10 |
1.22 ± 0.41 | 99.85 | 0.97 | 0.89 | 0.005 | 0.74 |
| Aggression dyad sex combination (mixed/same), SI Appendix, Table S11 |
0.02 ± 0.29 | 52.14 | 0.77 | 0.77 | 0.001 | 0.54 |
| Direction of aggression (down/up hierarchy), SI Appendix, Table S12 |
−0.42 ± 0.59 | 76.80 | 0.06 | 0.08 | 0.001 | 0.86 |
| Rank difference, SI Appendix, Table S13 |
−0.03 ± 0.02 | 93.51 | — | — | 0.001 | 0.693 |
Febrile probability = inverse logit (intercept estimate + febrile victim estimate), afebrile probability = inverse logit (intercept estimate).
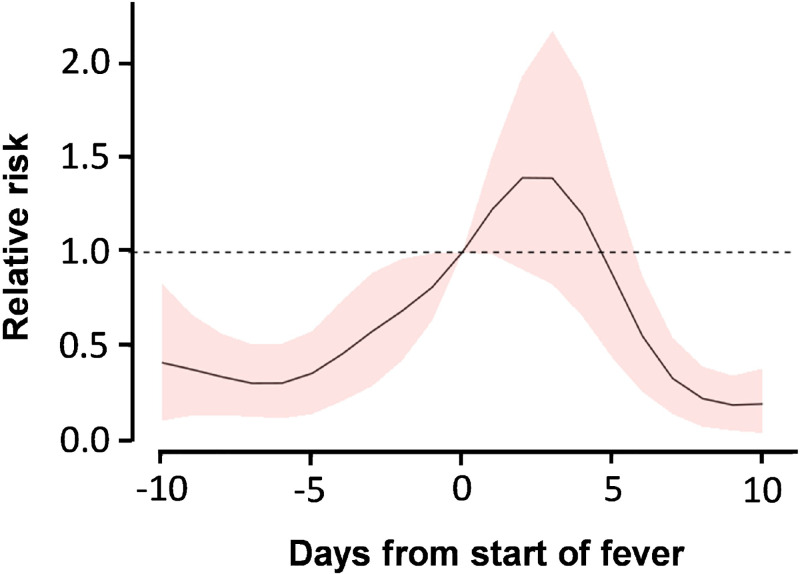
When we compared the relative risk of injury to the day on which a fever started, we found that the risk of injury was much lower 10 days prior to the onset of a fever (risk = 0.41, 95% CI: 0.10 to 0.83), reached a maximum 2 days after the onset of the fever (risk = 1.40, 95% CI: 0.91 to 1.94), and returned to the prefever level by 10 days after the onset of fever (risk = 0.19, 95% CI: 0.04 to 0.38, R2 marginal = 0.05, R2 conditional = 0.09, SI Appendix, Table S14 and Fig. 3). That is, monkeys were more likely to get injured in the days closer to the onset of a fever, with the maximum probability of injury being two days after the onset of a fever. This result suggests that injuries were more likely when a monkey already had an established fever and were not the cause of the fever itself.
Fig. 3.
Estimated trends for the estimated relative risk of injury (probability of injury/probability of injury at onset of fever) over a 21-d time window centered on the start of a fever event. The colored fill is truncated to indicate the 95% credibility interval.
Discussion
Wild vervet monkeys displayed fevers and sickness behaviors in the form of anorexia and lethargy. Sickness behaviors were facultative, such that monkeys still traveled with the troop and engaged in social activities, including grooming. Febrile monkeys, however, were twice as likely to be targeted with aggression from other troop members and were six times more likely to get injured, compared to afebrile monkeys.
Monkeys spent less time feeding when febrile, which is consistent with the expected sickness behavior of anorexia (1, 8, 18) and observations of other sick primates (16, 19). At first glance, anorexia might seem to be a counterintuitive response, given the increase in metabolic rate associated with fevers and immune function (2). However, anorexia serves to reduce the body’s overall expenditure of energy and can limit the available nutrients that would otherwise allow a pathogen to proliferate (1, 8). Monkeys spent more time resting when febrile, which suggests signs of lethargy, but also spent more time traveling and showed no change in time socializing. Although it might seem reasonable to suggest that a reduction in travel and social time would further help to reduce overall energetic expenditure, the cessation of these activities may not represent a viable option for highly gregarious and mobile species. Traveling with the group provides protection from predators (20) and between-group competitiveness for resources (21) and facilitates other social benefits that might affect individual fitness (22–25). The increase in traveling time when febrile could also reflect lethargy and the slower pace of a febrile monkey when traveling with the group. Alternatively, it is possible that harassment by conspecifics results in febrile individuals being more frequently displaced from one place to another or that increased stress from harassment may activate overall arousal and hence time spent traveling (26). Such flexibility in the demonstration of sickness behavior supports the view that such behavior reflects a motivational reorganization of behavioral priorities that can be shaped by both environmental and social context (18, 27, 28).
The interaction between sociality and sickness behaviors can be complex (29). Sick animals may either withdraw (30, 31) or interact more frequently with others (32, 33), depending on the nature of the relationship and the social context. For example, it may be disadvantageous to engage with threatening individuals while sick but advantageous to associate with an ally who may afford protection (34). It has previously been shown that vervet monkeys infested with gastrointestinal parasites continued to engage in allogrooming but tended to have fewer social interactions compared to healthy monkeys (16). Here, we found that there was no influence of fever on either grooming given or received by febrile animals, confirming both that febrile monkeys did not reduce the amount of effort placed into grooming, and that group members did not alter their affiliative behavior toward those with fevers. Collectively, the behavioral response of our vervet monkeys to infection suggests that disease transmission within a group is likely, and an important avenue of future inquiry is to identify the social predictors of fevers and disease transmission.
If the stakes are high enough, it may pay conspecific bystanders to capitalize on the weakened status of a sick individual and target them with aggression. This concept offers an intriguing variation on the argument that acts of aggression between animals often appear random and unprovoked (35). Our suggestion here is that, in at least some instances, aggressive attacks may not be as random as they appear to a human observer. Instead, these attacks may represent the seizing of an opportunity to target animals who have a reduced ability to respond, potentially improving the attacking animal’s status in addition to potentially exerting a negative effect on their opponent’s fitness and survival prospects. Males were increasingly more likely to be the targets of aggression when they were febrile than were females. One explanation may be that as males are the migratory sex, male competition is more pronounced than it is for females (36), and thus, the targeting of sick males who lack stable coalitions (24) is more advantageous than it would be toward more socially integrated philopatric females.
Humans detect sick individuals using both visual and olfactory cues (14), and there is evidence to suggest that nonhuman primates may also use olfactory cues to detect conspecifics infected with parasites (15). However, it remains unclear to what extent nonhuman primates can detect sickness itself and whether detecting sickness affects the behavior of conspecifics toward sick individuals (7, 16, 17). A more parsimonious explanation, therefore, is that conspecifics detect and act upon the sickness behaviors of febrile individuals (such as lethargy), thus providing a counterargument for the adaptive value of sickness behavior (4). An alternative, but not mutually exclusive, explanation for the increased aggression toward febrile monkeys is that febrile monkeys may allow aggression to escalate uncharacteristically because they misread social cues. Indeed, inflammation impairs social cognition and the ability to identify emotional states in others (37) and may also slow behavioral transition rates (12).
We can offer some additional, albeit anecdotal, evidence in support of the hypothesis that targeted aggression toward febrile individuals can have important social consequences (38). In 2015, we observed a rank reversal between the first- and second-ranked females in one of our study troops (39). Such wins up the hierarchy are rare for females in our population (40), and, in line with our suggestion here, this event coincided with the alpha female presenting with a fever. Over a 7-d fever period, we witnessed 12 instances of aggression against the alpha female and four injuries (Fig. 2). Over the next 3 mo, the alpha and beta females engaged in 92 dominance interactions, 98% of which were won by the beta female, thus consolidating the rank reversal between these two females.
Parasites and viral pathogens are ubiquitous in primate populations, and we know that they can drive devastating mortality rates (7, 17). Indeed, this is where most attention has been focused to date, with respect to understanding transmission dynamics within and between groups, and its implications for conservation efforts, zoonotic disease prevention, and human health (1, 8, 41). Our ability to tie fevers to sickness behavior, combined with a greater risk of attack and injury, suggests that social behavior represents more than just a route for disease spread. Changes to social structure may also be a consequence of infection, over and above the demographic changes associated with disease-related mortality. Such effects may thus compound the effects of disease for gregarious animals, as mortality and dominance turnovers can lead to drastic shifts in the overall tenor and behavioral profile of social groups (38, 42). The degree to which social factors modulate the welfare of infected animals is an important aspect to consider when attempting to understand the ecological implications of disease. That knowledge will become increasingly relevant with the increase in disease prevalence predicted under climate change (43).
Methods
Data Collection.
Data were collected between January 2012 and May 2018 from three groups (River Bend Mob [RBM], River Side Troop [RST], and Picnic Troop [PT]) of wild vervet monkeys in South Africa. Monkeys were fully habituated to the presence of researchers and were individually identifiable (22). We surgically implanted a subset of our adult monkey population (n = 59: 30 females and 29 males) with miniature temperature-sensitive data loggers (2012-2013: mlog T1C, Sigma Delta Technologies, resolution = 0.06 °C; 2013 to 2018: DST centi-T loggers, Star-Oddi, resolution = 0.03 °C), which recorded core body temperature at 5-min intervals. For full details of the capture and surgery procedure, see McFarland et al. (23). Capture and surgical procedures were approved by the University of the Witwatersrand Animal Ethics Research Committee (2010/41/04; 2015/04/14B).
The International Union of Physiological Sciences (IUPS) defines a fever as “an elevation of the set-point of body temperature… [which is] actively established and defended” (44). By contrast, hyperthermia is a rise in body temperature “not accompanied by supportive changes in thermoeffector activities” (44). Based on these definitions, we classified a fever as a >0.5 °C increase in a monkey’s mean and minimum 24-h body temperature above their overall mean and minimum body temperature lasting a minimum of two consecutive days (Fig. 1) (45). This classification allowed us to exclude instances of short-term hyperthermia (Fig. 1). Our definition of a fever identified a sample that includes both low- and high-grade fevers (46). Because of the rarity of high-grade fevers (a >1.5 °C increase in mean and minimum 24-h body temperature: <1% of our fever sample), we were unable to run the analyses on these different grades of fever separately. However, any effect of a fever on sickness behavior or aggression can therefore be considered conservative.
When fevers were separated by fewer than 7 d, monkeys were considered to be “febrile” across this period for the purpose of our analysis. Although these intermediary days are not defined as febrile through body temperature elevation, we considered these intermediary days to be a period of continued vulnerability in regard to reduced capacity and sickness behavior and the potential to be targeted with aggression. In total, we collected 34,353 monkey-days of continuous body temperature measurement from 59 monkeys (30 females, 29 males) over the 6-year study and detected 128 fevers in 43 monkeys totaling 776 fever-days. Fevers lasted between 2 and 20 d, and fever episodes, including intermediary days, lasted up to 46 d. Vervet monkeys, including those in our population, have been shown to host a number of gastrointestinal parasites (16, 47) and viruses (48, 49), some of which have been linked to sickness behavior and possible social transmission. Given gastrointestinal parasites are largely considered to be nonpathogenic, fevers identified in our study are most likely to be the result of viral or bacterial infections (46).
Instantaneous scan data (22) were collected daily at 30-min intervals across daylight hours from all adults that could be located within a 10-min period. The activity of each scanned monkey was recorded as resting, feeding, traveling, socializing (i.e., allogrooming, playing, or mating), or other (22). The identities of the actor and recipient were recorded during allogrooming (hereafter grooming) and ad libitum occurrences of aggression (40). Decided dyadic agonistic interactions exchanged between adults (RBM = 6,627, RST = 8,194, and PT = 7,046) were used to determine dominance ranks using standardized David’s scores (50). Yearly scores were calculated separately for each group and standardized to facilitate comparisons between groups and years (40). Newly acquired injuries were recorded during daily censuses. Injuries from predation attempts or accidents were exceptionally rare, and monkeys were twice as likely to get injured on days they received aggression [SI Appendix, Table S15; see also (36)]. Injuries were therefore assumed to be the result of conspecific aggression. Behavioral data collection protocols were approved by the University of Lethbridge (Animal Welfare Protocols 0702/1505).
Statistical Analysis.
We ran a series of Bayesian generalized linear mixed models (GLMMs) and a generalized additive model (GAM), using the “brms” package (51) in R 3.5.0 (52). We ran four chains for 2,000 or 4,000 iterations, after 1,000 warmup iterations, to confirm convergence. All models converged (R-hat < 1.01), and effective sample size exceeded 400 [i.e., >number of chains × 100 (51)]. We set weakly informative priors centered on zero [i.e., normal (0,1)] for the main effects and used the posterior predictive check function to assess model fit, and to confirm the suitability of our choice of priors and likelihood model distributions (53). We used the “bayestestR” package (54) to generate probability of direction (pd) estimates for the fixed effects. These estimates indicate the certainty of the direction (negative or positive) of an effect and are interpretively helpful because they are closely correlated with commonly used frequentist P values (54), with pd ∼97.5%, pd ∼99.5%, and pd ∼99.95% corresponding to what Colquhoun (55) considered to indicate weak (P < 0.05), moderate (P < 0.01), and strong (P < 0.001) evidence of effects, respectively. We used the ‘r2_bayes’ function to calculate marginal and conditional R2 values for the fixed effect and whole models, respectively (56). Full-model results are provided in the supplementary material (SI Appendix, Tables S1–S15).
We ran six GLMMs specifying a Poisson distribution, entering the daily count of our six scanned activities (i.e., resting, feeding, traveling, socializing, grooming given, and grooming received), in turn, as the outcome variable and the total number of subject scans collected each day as the offset variable. We ran two GLMMs specifying a Bernoulli distribution, entering aggression received (no/yes) or injury (no/yes), in turn, as the outcome variable. In all eight GLMMs, we entered fever-day (no/yes) as the predictor variable. We also entered monkey sex (female/male) as a predictor variable to control for sex differences in activity patterns and aggressive behavior (22, 40). We entered date ID and monkey ID as crossed random intercepts to deal with repeated measures and to control for seasonal variation in activity patterns, aggressive behavior, and injury (22, 36, 40). The inclusion of fever episode ID as an additional random intercept did not improve the models or change the magnitude or direction of effects and was therefore not included in our final models.
We ran four GLMMs specifying a Bernoulli distribution, entering aggressor sex (female/male), victim sex (male/female), sex combination of the aggression dyad (mixed/same), and aggression direction (down/up the dominance hierarchy), in turn, as the outcome variable. We also ran a GLMM specifying a Gaussian distribution, entering absolute rank difference between aggressive opponents as the outcome variable. In these five GLMMs, we entered whether the victim of aggression was febrile or not as the predictor variable and date ID, victim ID, and/or aggressor ID as crossed random intercepts.
Given that injuries may be either the cause or a consequence of fevers, we used a 21-d GAM time-window approach (57), centered over the onset of a fever, to confirm whether injuries were more likely to occur before or after the onset of a fever. We ran a GAM specifying a Bernoulli distribution, entering injury (no/yes) as the outcome variable. We entered day from the start of the fever, monkey sex, and a circular spline for day of year as predictor variables and monkey ID as a random intercept.
Acknowledgments
We are grateful to the Tompkins family for permission to work on the Samara Private Game Reserve, our team of verveteers and veterinarians for assistance with data collection and surgery, and two anonymous reviewers for helpful suggestions that improved this manuscript. This research was funded by faculty research grants from the University of the Witwatersrand; a Claude Leon Fellowship awarded to R.M.; Natural Sciences and Engineering Research Council of Canada Discovery grants to S.P.H. and L.B.; a Canada Research Chair award to L.B.; National Research Foundation of South Africa grants to A.F., R.H., S.P.H., and Duncan Mitchell; a Carnegie Corporation of New York grant to A.F.; and a Harry Oppenheimer Fellowship to Duncan Mitchell.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107881118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Hart B. L., Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 (1988). [DOI] [PubMed] [Google Scholar]
- 2.Martin L. B., Scheuerlein A., Wikelski M., Linking physiological and fitness costs of immune activity: Increased immune activity elevates energy expenditure of house sparrows. Proc. Biol. Sci. 270, 153–158 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harden L. M., Kent S., Pittman Q. J., Roth J., Fever and sickness behavior: Friend or foe? Brain Behav. Immun. 50, 322–333 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Hart B. L., Behavioral adaptations to pathogens and parasites: Five strategies. Neurosci. Biobehav. Rev. 14, 273–294 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R., Kelley K. W., Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 21, 153–160 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulin R., “Adaptive” changes in the behaviour of parasitized animals: A critical review. Int. J. Parasitol. 25, 1371–1383 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Nunn C., Altizer S., Altizer S. M., Infectious Diseases in Primates: Behavior, Ecology and Evolution (Oxford University Press, 2006). [Google Scholar]
- 8.Adelman J. S., Martin L. B., Vertebrate sickness behaviors: Adaptive and integrated neuroendocrine immune responses. Integr. Comp. Biol. 49, 202–214 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Stockley P., Campbell A., Female competition and aggression: Interdisciplinary perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moyers S. C., Kosarski K. B., Adelman J. S., Hawley D. M., Interactions between social behaviour and the acute phase immune response in house finches. Behaviour 152, 2039–2058 (2015). [Google Scholar]
- 11.Cirulli F., De Acetis L., Alleva E., Behavioral effects of peripheral interleukin-1 administration in adult CD-1 mice: Specific inhibition of the offensive components of intermale agonistic behavior. Brain Res. 791, 308–312 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Fairbanks B. M., Hawley D. M., Alexander K. A., No evidence for avoidance of visibly diseased conspecifics in the highly social banded mongoose (Mungos mungo). Behav. Ecol. Sociobiol. 69, 371–381 (2015). [Google Scholar]
- 13.Bouwman K. M., Hawley D. M., Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol. Lett. 6, 462–465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regenbogen C., et al., Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl. Acad. Sci. U.S.A. 114, 6400–6405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirotte C., et al., Mandrills use olfaction to socially avoid parasitized conspecifics. Sci. Adv. 3, e1601721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman C. A., et al., Social behaviours and networks of vervet monkeys are influenced by gastrointestinal parasites. PLoS One 11, e0161113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rushmore J., Bisanzio D., Gillespie T. R., Making new connections: Insights from primate-parasite networks. Trends Parasitol. 33, 547–560 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lopes P. C., Adelman J., Wingfield J. C., Bentley G. E., Social context modulates sickness behavior. Behav. Ecol. Sociobiol. 66, 1421–1428 (2012). [Google Scholar]
- 19.Ghai R. R., Fugere V., Chapman C. A., Goldberg T. L., Davies T. J., Sickness behaviour associated with non-lethal infections in wild primates. Proc. R. Soc. B. Bio. Sci. 282, 20151436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs N., Bonnell T., Dostie M., Barrett L., Henzi S. P., Working the crowd: Sociable vervets benefit by reducing exposure to risk. Behav. Ecol. 27, 988–994 (2016). [Google Scholar]
- 21.Arseneau-Robar T. J. M., Taucher A. L., Schnider A. B., van Schaik C. P., Willems E. P., Intra-and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Anim. Behav. 123, 129–137 (2017). [Google Scholar]
- 22.McFarland R., Barrett L., Boner R., Freeman N. J., Henzi S. P., Behavioral flexibility of vervet monkeys in response to climatic and social variability. Am. J. Phys. Anthropol. 154, 357–364 (2014). [DOI] [PubMed] [Google Scholar]
- 23.McFarland R., et al., Social integration confers thermal benefits in a gregarious primate. J. Anim. Ecol. 84, 871–878 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Freeman N. J., Young C., Barrett L., Henzi S. P., Coalition formation by male vervet monkeys (Chlorocebus pygerythrus) in South Africa. Ethology 122, 45–52 (2016). [Google Scholar]
- 25.Young C., et al., Climate induced stress and mortality in vervet monkeys. R. Soc. Open Sci. 6, 191078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katafuchi T., et al., Prolonged effects of polyriboinosinic:polyribocytidylic acid on spontaneous running wheel activity and brain interferon-α mRNA in rats: A model for immunologically induced fatigue. Neuroscience 120, 837–845 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Aubert A., Sickness and behaviour in animals: A motivational perspective. Neurosci. Biobehav. Rev. 23, 1029–1036 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Lopes P. C., When is it socially acceptable to feel sick? Proc. Biol. Sci. 281, 20140218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennessy M. B., Deak T., Schiml P. A., Sociality and sickness: Have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav. Immun. 37, 15–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley K. W., et al., Cytokine-induced sickness behavior. Brain Behav. Immun. 17 (suppl. 1), S112–S118 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Weber N., et al., Badger social networks correlate with tuberculosis infection. Curr. Biol. 23, R915–R916 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Loehle C., Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335 (1995). [Google Scholar]
- 33.Willette A. A., Lubach G. R., Coe C. L., Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain Behav. Immun. 21, 807–815 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moieni M., Eisenberger N. I., Effects of inflammation on social processes and implications for health. Ann. N. Y. Acad. Sci. 1428, 5–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silk J. B., Practice random acts of aggression and senseless acts of intimidation: The logic of status contests in social groups. Evol. Anthropol. 11, 221–225 (2002). [Google Scholar]
- 36.Henzi S. P., Lucas J. W., Observations on the Inter-troop movement of adult vervet monkeys (Cercopithecus aethiops). Folia Primatol. (Basel) 33, 220–235 (1980). [DOI] [PubMed] [Google Scholar]
- 37.Moieni M., Irwin M. R., Jevtic I., Breen E. C., Eisenberger N. I., Inflammation impairs social cognitive processing: A randomized controlled trial of endotoxin. Brain Behav. Immun. 48, 132–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohn D. W. H., Gabanyi I., Kinoshita D., de Sá-Rocha L. C., Lipopolysaccharide administration in the dominant mouse destabilizes social hierarchy. Behav. Processes 91, 54–60 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Young C., et al., Faecal glucocorticoid metabolite monitoring as a measure of physiological stress in captive and wild vervet monkeys. Gen. Comp. Endocrinol. 253, 53–59 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Young C., McFarland R., Barrett L., Henzi S. P., Formidable females and the power trajectories of socially integrated male vervet monkeys. Anim. Behav. 125, 61–67 (2017). [Google Scholar]
- 41.Hawley D. M., Altizer S. M., Disease ecology meets ecological immunology: Understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25, 48–60 (2011). [Google Scholar]
- 42.Sapolsky R. M., Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418 (2004). [Google Scholar]
- 43.Altizer S., Ostfeld R. S., Johnson P. T., Kutz S., Harvell C. D., Climate change and infectious diseases: From evidence to a predictive framework. Science 341, 514–519 (2013). [DOI] [PubMed] [Google Scholar]
- 44.IUPS Thermal Commission, Glossary of terms for thermal physiology. Jpn. J. Physiol. 51, 245–280 (2001). [Google Scholar]
- 45.Hetem R. S., et al., Fever and sickness behavior during an opportunistic infection in a free-living antelope, the greater kudu (Tragelaphus strepsiceros). Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R246–R254 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Ogoina D., Fever, fever patterns and diseases called ‘fever’—A review. J. Infect. Public Health 4, 108–124 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Blersch R., et al., Gastrointestinal parasites of vervet monkeys (Chlorocebus pygerythrus) in a high latitude, semi-arid region of South Africa. J. Parasitol. 105, 630–637 (2019). [PubMed] [Google Scholar]
- 48.Ma D., et al.; International Vervet Research Consortium, SIVagm infection in wild African green monkeys from South Africa: Epidemiology, natural history, and evolutionary considerations. PLoS Pathog. 9, e1003011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey A. L., et al., Arteriviruses, pegiviruses, and lentiviruses are common among wild African monkeys. J. Virol. 90, 6724–6737 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vries H., Stevens J. M., Vervaecke H., Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592 (2006). [Google Scholar]
- 51.Bürkner P.-C., Brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017). [Google Scholar]
- 52.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). [Google Scholar]
- 53.McElreath R., Statistical Rethinking: A Bayesian Course With Examples in R and Stan (CRC Press, 2020). [Google Scholar]
- 54.Makowski D., Ben-Shachar M. S., Lüdecke D., Bayestest R., Describing effects and their uncertainty, existence and significance within the Bayesian framework. J. Open Source Softw. 4, 1541 (2019). [Google Scholar]
- 55.Colquhoun D., An investigation of the false discovery rate and the misinterpretation of p-values. R. Soc. Open Sci. 1, 140216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakagawa S., Schielzeth H., A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods Ecol. Evol. 4, 133–142 (2013). [Google Scholar]
- 57.Wood S. N., Generalized Additive Models: An Introduction with R (CRC Press, 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article and/or supporting information.