ABSTRACT
The mechanisms controlling entry into and exit from the death phase in the bacterial life cycle remain unclear. Although bacterial growth studies in batch cultures traditionally focus on the first three phases during incubation, two additional phases, the death phase and the long-term stationary phase, are less understood. Although there are a number of stressors that arise during long-term batch culture, including nutrient depletion and the accumulation of metabolic toxins such as reactive oxidative species, their roles in cell death are not well-defined. By manipulating the environmental conditions of Escherichia coli incubated in long-term batch culture through chemical and mechanical means, we investigated the role of volatile metabolic toxins in modulating the onset of the death phase. Here, we demonstrate that with the introduction of substrates with high binding affinities for volatile compounds, toxic by-products of normal cell metabolism, into the headspace of batch cultures, cells display a prolonged stationary phase and delayed entry into the death phase. The addition of these substrates allows cultures to maintain a high cell density for hours to days longer than cultures incubated under standard growth conditions. A similar effect is observed when the gaseous headspace in culture flasks is continuously replaced with sterile air, mechanically preventing the accumulation of metabolic by-products in batch cultures. We establish that toxic compound(s) are produced during the exponential phase, demonstrate that buildup of toxic by-products influence entry into the death phase, and present a novel tool for improving high-density growth in batch culture that may be used in future research or industrial or biotechnology applications.
IMPORTANCE Bacteria, such as Escherichia coli, are routinely used in the production of biomaterials because of their efficient and sustainable capacity for synthesis of bioproducts. Industrial applications of microbial synthesis typically utilize cells in the stationary phase, when cultures have the greatest density of viable cells. By manipulating culture conditions to delay the transition from the stationary phase to the death phase, we can prolong the stationary phase on a scale of hours to days, thereby maintaining the maximum density of cells that would otherwise quickly decline. Characterization of the mechanisms that control entry into the death phase for the model organism E. coli not only deepens our understanding of the bacterial life cycle but also presents an opportunity to enhance current protocols for batch culture growth and explore similar effects in a variety of widely used bacterial strains.
KEYWORDS: culture viability, long-term survival, stationary phase
INTRODUCTION
Escherichia coli incubated in long-term batch culture exhibit five phases: lag phase, exponential or logarithmic phase, stationary phase, death phase, and long-term stationary phase (1, 2). Although most research studies emphasize the first three phases, we focus here on the fourth: the death phase. When culturing E. coli in laboratory settings, it is common practice to adjust the volume of batch cultures according to the desired output (3–6), meaning that greater volumes are used to grow more cells. It is generally assumed that all cell cultures experience approximately the same growth conditions and stressors, regardless of vessel shape and volume (3) or growth medium composition (7). However, previous work from our group has shown that changes in culture volume alone can have a direct impact on cell viability, and we observe reproducible differences in cell yield and long-term viability depending on culture vessel conditions using different growth media (3).
Although we have previously described the physiological changes that arise from differences in culture vessels and speculated that these changes are likely caused by the various intensities of metabolic stressors, including oxygen tension and nutrient depletion (3), here we investigate how these stressors influence entry into successive phases. Because laboratory and industrial applications of microbial growth frequently utilize cells in the stationary phase, when cultures maintain their highest density and may exhibit higher production rates (8–10), understanding why and how cells remain in the stationary phase or enter the death phase is of particular importance. The mechanisms that mark the end of stationary phase and initiate death phase are not well-defined; however, factors including the accumulation of toxic metabolites, oxidative stress, and nutrient starvation appear to play a significant role (2, 3, 11–15). Although a number of studies attribute the cause of death to oxidative stress (11–13), this is clearly not the only mechanism (2, 15). For example, while anaerobic cultures do not accumulate reactive oxidative species (ROS) (7), cells still enter the death phase after several days in batch culture (3).
We previously demonstrated that cell death is not related to oxidative stress alone (3) and hypothesized that accumulation of volatile metabolites outgassed during normal cell metabolism in rich media, such as the production of ammonia following amino acid catabolism, may be toxic to cells. During normal cell metabolism, batch cultures of bacteria produce a variety of organic compounds (such as hydrocarbons, ketones, alcohols, acids, sulfur or nitrogen compounds, and terpenes) and inorganic compounds (such as nitric oxide, hydrogen sulfide, ammonia, and hydrogen cyanide) that can become toxic if they accumulate. Although research in this area is continuing to develop (16–22), metabolic compounds are known to influence bacterial growth and response to environmental changes and was recently reviewed by Audrain et al. (23).
To explore methods of by-product removal, we identified materials traditionally used as “scrubbers” in bioprocessing and filtration, such as activated charcoal, zeolite, and silica gel, to adsorb undesired compounds through chemical adhesion of ions or molecules (24–29). Although industry practice commonly uses activated charcoal and zeolite filters through a liquid bypass, these porous materials, similar to silica gel, are also capable of binding gaseous compounds through adsorption or ion exchange (24, 30–33). The adsorptive properties of these three substrates have been extensively studied for ammonium (29, 31, 34–36) and are exploited as a chemical mean to remove by-products. In E. coli batch culture, the volatile form of ammonium, ammonia, is released as a metabolite.
In addition to exploring chemical means of by-product removal, following the assumption that toxic metabolites are outgassed and collect in the headspace of E. coli batch cultures, we tested the effect of removal of volatile compound (VC) buildup by mechanical means. Using an air pump to continuously drive sterilized air into vessels, the gaseous headspace is entirely replaced every few seconds, effectively flushing out any toxins that would otherwise collect in the headspace. Aeration is known to stimulate growth of several facultative and obligate aerobic bacterial species in both laboratory and industrial settings (37–42).
In this study, we hypothesize that the accumulation of VC toxic by-products, such as ammonia, contributes to the transition into the death phase and that removal of these toxins could prolong the stationary phase and maintain the maximum density of cells beyond what is observed under standard growth conditions. Here, we show how chemical and mechanical manipulation of the growth environment effectively modulates entry into the death phase by preventing the accumulation of toxic VCs.
RESULTS
Adsorptive substrates in the headspace of culture flasks result in greater cell yields and prolonged time in the stationary phase.
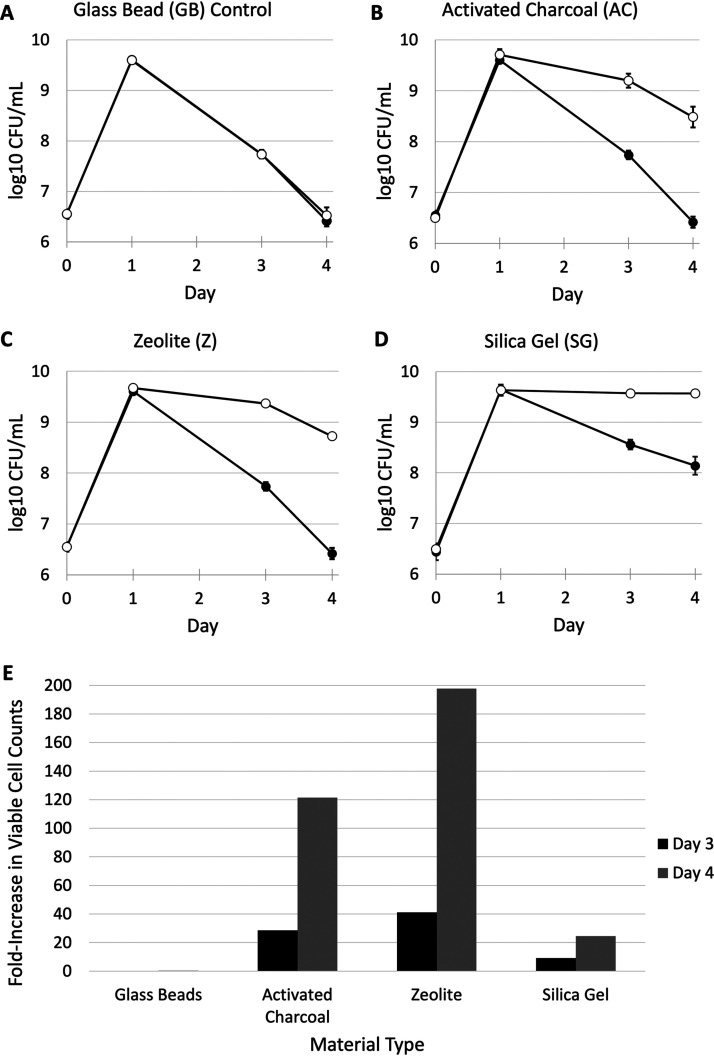
To investigate whether the presence of adsorptive substrates in batch cultures prolongs stationary phase and delays entry into the death phase, we introduced adsorptive substrates capable of chemically binding gaseous VCs inside the headspace of culture vessels. Sterile paper teabags filled with 2 g of activated charcoal (AC), zeolite (Z), silica gel (SG), or nonadsorptive glass beads (GB) as a control were placed in the necks of 125-ml Erlenmeyer flasks containing 12.5 ml LB medium. The cultures were incubated for at least 3 days, unless otherwise noted. We observed that addition of each of the adsorptive substrates leads to increased cell density; however, each material affects cell growth patterns differently. Viable cell counts of all overnight cultures approached ∼1010 CFU/ml after 1 day of incubation (Fig. 1). The addition of glass beads, which served as a negative control, had no effect on viable cell counts (Fig. 1A). However, the addition of activated charcoal or zeolite to cultures led to greater cell densities after 3 to 4 days of incubation (∼30- and ∼120-fold increase, respectively, for activated charcoal; ∼40- and ∼200-fold increase, respectively, for zeolite) (Fig. 1B and C). Similarly, the addition of silica gel also led to greater cell densities, with counts consistently above 109 CFU/ml throughout the incubation period. Although cultures containing activated charcoal or zeolite demonstrate higher cell yields compared to untreated controls, the addition of silica gel extends the stationary phase most substantially (∼100-fold increase after 5 days of incubation) (Fig. 1E). Given the demonstrated effects, it is likely that the accumulation of toxic metabolites, which may be adsorbed by the activated charcoal or zeolite, plays a key role in entry into the death phase.
FIG 1.
Survival dynamics of E. coli in 125-ml flasks with control (closed circles) and experimental substrates (open circles). (A) 2 g glass bead (GB) (negative control). (B) 2 g activated charcoal (AC). (C) 2 g zeolite (Z). (D) 2 g silica gel (SG). (E) Fold increase in viable cell counts versus control cultures on days 3 and 4. Graphs represent averages of three biological replicates (n = 3). The error bars represent standard deviations, *, P < 0.05 using Welch’s t test. For some data points, the standard deviation is so small that the error bars are covered by the data point.
Adsorptive substrates delay the onset of cell death in different growth media and culture volumes.
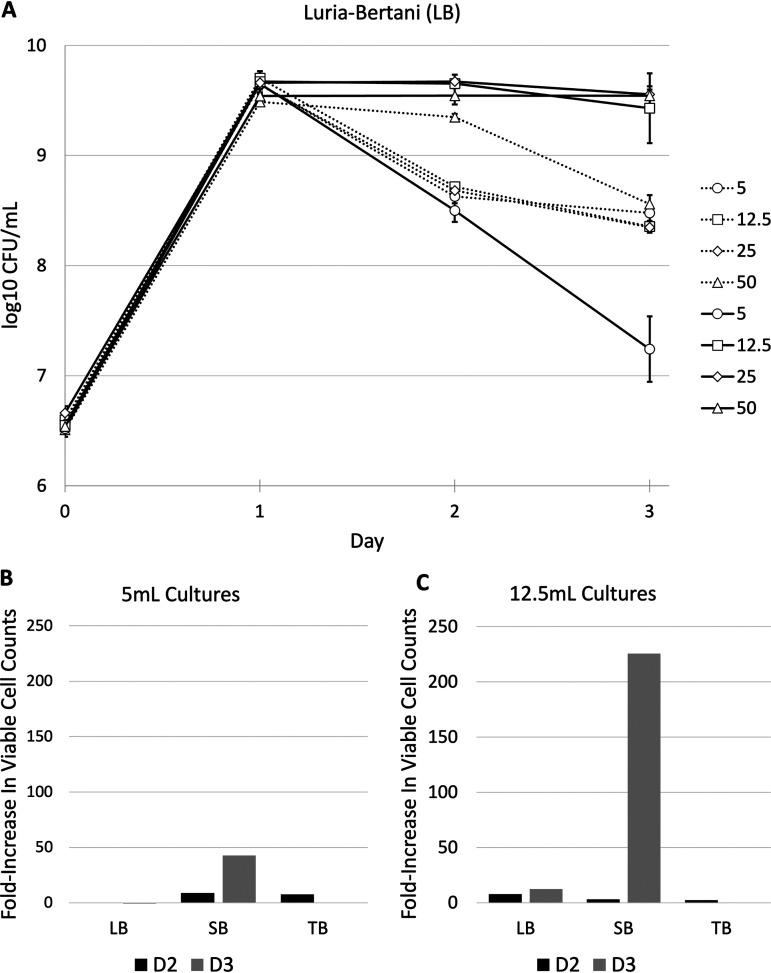
To test the efficacy of adsorptive substrates maintaining high cell density, we tested the effect of zeolite across a range of culture volumes using three different growth media: Luria-Bertani/lysogeny broth (LB), super broth (SB), and terrific broth (TB). In all three media, the presence of zeolite maintained higher cell densities compared to control cultures (Fig. 2). All cultures were inoculated at an initial density of ∼5 × 106 CFU/ml and approach a maximum cell density of ∼1 × 1010 CFU/ml after 1 day of incubation (hereafter referred to as “day 1”). In LB medium, 12.5- and 25-ml cultures with added zeolite demonstrate ∼10-fold greater cell counts than those without the substrate by day 2 (Fig. 2A). Cell densities were also greater in 50-ml LB cultures with added zeolite; however, this effect did not occur until day 3 because death is also delayed in 50-ml cultures without the addition of zeolite (3). The addition of zeolite did not lead to increased cell counts in 5-ml cultures grown in LB (Fig. 2B). When the effect of zeolite was tested using other media, 5-ml cultures grown in SB demonstrated an ∼10- and ∼45-fold increase on days 2 and 3, respectively (Fig. 2B), whereas 5-ml cultures incubated in TB demonstrate an ∼10-fold increase on day 2. For 12.5-ml cultures, increased cell density was observed in all media on day 2 (∼9-, ∼4-, and ∼3-fold increases in LB, SB, and TB, respectively) and only in LB or SB media on day 3 (∼12- and ∼230-fold increases, respectively) (Fig. 2C).
FIG 2.
Survival dynamics of E. coli in 125-ml flasks in three different growth media across a range of volumes. (A) LB cultures with (solid shapes) and without (open shapes) 2 g zeolite in 5-, 12.5-, 25-, and 50-ml cultures. (B and C) Fold increase of viable cell counts of cultures grown in LB, SB, or TB with 2 g of zeolite compared to those without in 5- and 12.5-ml cultures. No data were collected for TB day 3. The graphs represent averages of data from three biological replicates. The error bars represent standard deviations. *, P < 0.05 using Welch’s t test. For some data points, the standard deviation is so small that the error bars are covered by the data point. D2, day 2; D3, day 3.
Timing of zeolite addition or removal affects survival.
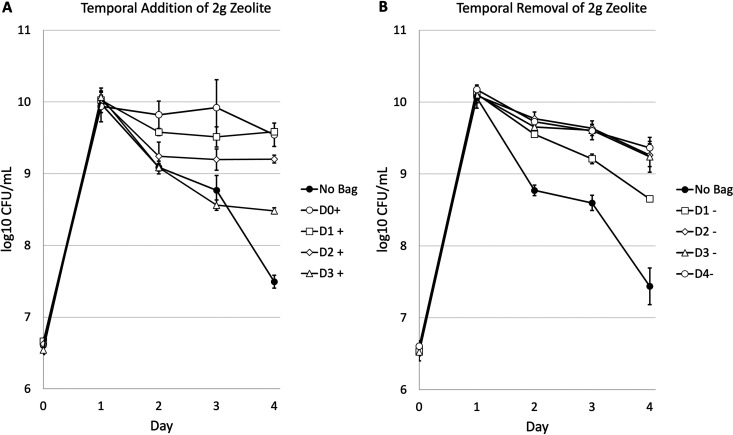
To determine the time period during which adsorbents have a beneficial effect on cell viability, 2 g of zeolite was temporarily added or removed over 4 days of batch culture incubation (Fig. 3). Zeolite was either added on day 0, 1, 2, or 3 of incubation (Fig. 3A) or removed on day 1, 2, 3, or 4 of incubation (Fig. 3B). Upon the addition of zeolite, the cultures were able to significantly reduce or completely halt the loss of viability normally observed during death phase. Cultures with zeolite added on day 1 (D1+ in Fig. 3) show an ∼2.5-fold decrease in the number of viable cells the day after addition from ∼1 × 1010 to ∼3.8 × 109 CFU/ml. However, this cell density was maintained through day 4 of growth, resulting in an overall 120-fold increase in long-term viable cell yield compared to cultures without zeolite. The addition of zeolite on days 2 (D2+) or 3 (D3+) halted the decline in viable cell counts, maintaining high cell densities for the remainder of the experiment, with final cell yields of ∼50- and ∼10-fold greater than the untreated control cultures, respectively. Compared to cultures with zeolite added at the time of inoculation (D0+), cultures receiving zeolite on subsequent days demonstrate lower cell yields throughout the rest of the experiment.
FIG 3.
Survival dynamics of E. coli in 125 ml flasks in 12.5-ml LB with temporal addition or removal of 2 g zeolite. (A) D0+, addition on day 0; D1+, addition on day 1; D2+, addition on day 2; D3+, addition on day 3. (B) D1-, removal on day 1; D2-, removal on day 2; D3-, removal on day 3; D4-, removal on day 4. The graphs represent averages of data from three biological replicates. The error bars represent standard deviations. For some data points, the standard deviation is so small that the error bars are covered by the data point.
Batch cultures where zeolite was removed on day 2 (D2-) or day 3 (D3-) of incubation maintained a greater number of viable cells, similar to cultures in which zeolite was not removed until the end of the trial (D4-), resulting in an ∼60-fold increase in viable cell counts on day 4 compared to the control cultures to which zeolite was never added.
Zeolite and silica gel confer distinct effects on cell survival.
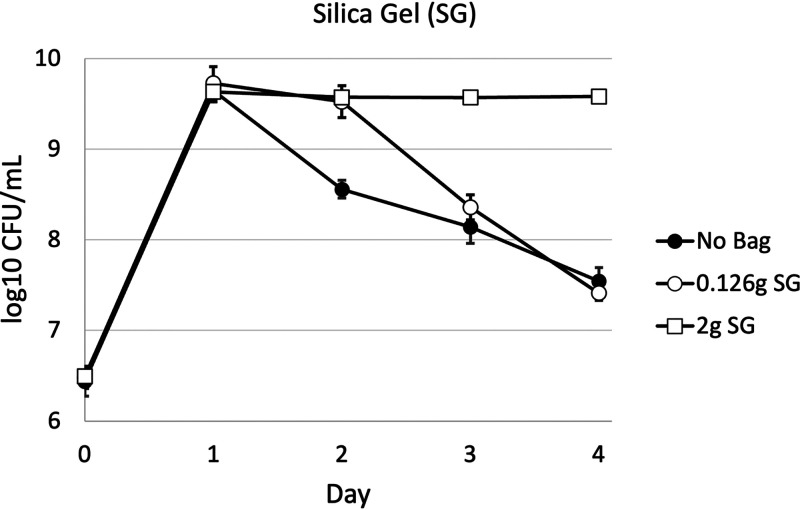
When considering the adsorptive capacity of zeolite and silica gel, equal weights of the two compounds do not demonstrate equivalent chemical properties because they bind volatile compounds at different rates or, in other words, have different binding capacities. To investigate the efficiency of silica gel versus zeolite as an absorbent for volatile compounds, we compared our standard amount of silica gel to what would be considered an “equivalent” amount zeolite (e.g., 0.126 g silica gel), calculated by determining their relative sorption capacities with respect to ammonium (29). Various amounts of silica gel (0, 0.126, and 2g) were introduced into the necks of 125-ml Erlenmeyer flasks containing 12.5 ml of LB medium. All cultures were inoculated at an initial density of ∼5 × 106 CFU/ml and reached a maximum cell density of ∼1 × 1010 CFU/ml by day 1. Cultures containing 2 g of silica gel maintain their maximum cell density for 4 days, achieving an ∼100-fold greater cell density on day 4 compared to cultures without added silica gel (Fig. 4). Cultures with 0.126 g of silica gel exhibited a moderate effect through day 2 with viable counts ∼10-fold greater than control cultures. After day 2, cultures with the lesser amount of silica gel performed similarly to control cultures. Compared to 0.126 g of silica gel, the positive growth effect of a binding capacity equivalent amount of zeolite (2 g) outlasted that of silica gel by at least 1 day (Fig. 3C and 4).
FIG 4.
Survival dynamics of E. coli in 125-ml flasks with or without 0.126 and 2 g of silica gel (SG) beads. The graphs represent averages of data from three biological replicates. The error bars represent standard deviations. For some data points, the standard deviation is so small that any error bars are covered by the data point.
Displacement of air in flask headspace prolongs stationary phase.
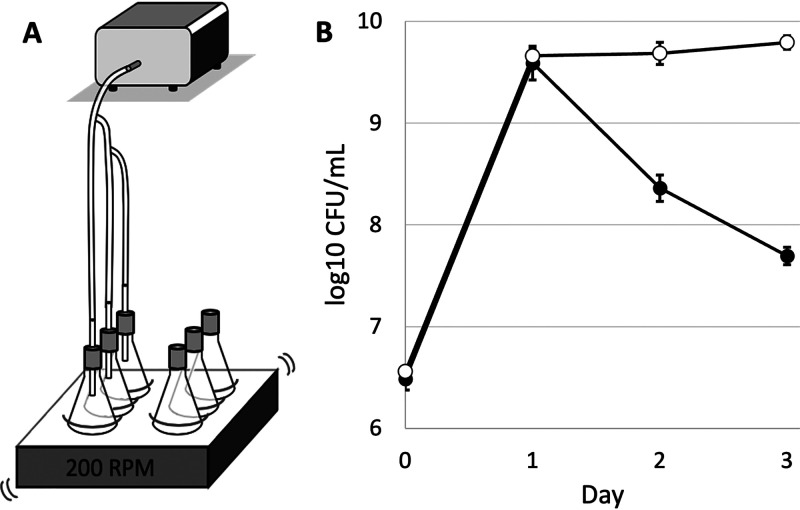
In an effort to demonstrate that the accumulation of VCs in the culture vessel headspace was, in fact, directly contributing to the timing of entry into the death phase, we designed a mechanism that continually replaces the headspace with fresh air, thereby removing toxic VCs outgassed into the headspace (Fig. 5A). By pumping in sterile air at a rate of 0.375 liters per minute (LPM), the headspace air was entirely displaced every 20 s. Cultures with and without airflow had similar outgrowths, with initial cell densities of ∼5 × 106 CFU/ml and reaching ∼1 × 1010 CFU/ml on day 1. High viable cell counts in cultures with airflow were maintained through day 3 (Fig. 5B), whereas those from the cultures without airflow demonstrated a typical death curve.
FIG 5.
Survival dynamics of E. coli grown in 125-ml flasks with and without air pumping. (A) Schematic for air pump mechanism. (B) Unfilled circles represent counts for culture flasks connected to air pump, and filled circles represent no air pump. The graphs represent averages of data from three biological replicates. The error bars represent standard deviations. For some data points, the standard deviation is so small that the error bars are covered by the data point.
DISCUSSION
Chemical and mechanical manipulation of the headspace in culture vessels allows E. coli to prolong the stationary phase and maintain high cell yield. We observed that the introduction of the adsorptive substrates activated charcoal, zeolite, and silica gel, all capable of binding a variety of VCs to effectively remove them from culture vessel headspace, leads to increased cell culture viability after the logarithmic growth phase, as well as delayed entry into the death phase. Metabolite production in E. coli has been well-characterized, and there are several candidate VCs that may contribute to cell death in batch culture (23). For example, a number of alcohols produced during normal metabolism are known to be toxic and inhibit growth by destabilizing cell membranes (43–46). Similarly, ketones have long been thought to inhibit cell growth possibly by limiting the oxygen available to cells (47).
Indoles produced by the tryptophanase enzyme degrading tryptophan rapidly accumulate in the early log and lag phases of E. coli and can be toxic at high concentrations by disrupting the membrane and generating a superoxide (48–50). Although indole concentrations may not reach toxic levels during the stationary phase in E. coli batch cultures (48), accumulation in the headspace of batch cultures grown beyond stationary phase, like those in this study, may indeed contribute to cell death. A recent study has shown that sulfur-containing volatiles, such as dimethyl trisulfide, can significantly inhibit growth, possibly by the disruption of quorum sensing, but also notes that growth inhibition effects are likely caused by a blend of volatiles working together (51).
In addition to the compounds described above, bacteria grown in rich media almost always metabolize amino acids as sources of both carbon and energy and produce soluble ammonium and gaseous ammonia as a result of excess nitrogen metabolism (3). At high concentrations, ammonium can have a detrimental effect on growth of E. coli, and the rise in pH due to ammonia can affect the availability of nutrients in the environment (21, 52). Because the adsorptive substrates tested in this study have known binding affinities for ammonium compounds, we hypothesize that the ammonia produced during cell metabolism is a possible candidate influencing entry into the death phase. Studies on the use of adsorptive substrates in liquid and gaseous filtration systems have highlighted their ability to bind and remove gaseous NH3 or liquid NH4+ (27, 29, 31–36). For these reasons, we hypothesize that ammonia is likely one of the primary VCs that leads to toxic effects on the cell.
Further support for ammonia’s contribution to the transition into the death phase is the observation that increased culture density is correlated with the known binding capacities of these three absorbents for ammonium. Zeolite, with an ammonium sorption capacity of ∼6.3 mg/g, has a reproducibly greater positive effect on cell density from days 3 to 5 in batch culture than activated charcoal, which has a sorption capacity of 5.3 mg/g (53). The addition of silica gel, whose sorption capacity is ∼16 times greater than that of zeolite at 100 mg/g (29), results in consistently higher cell densities throughout day 4, emulating the effects of a prolonged stationary phase (Fig. 1B to D). However, our results comparing ammonium binding efficiency of zeolite to silica gel suggest that ammonia is not the only VC contributing to cell death (Fig. 3C and 4). By comparing what would be considered amounts of zeolite and silica gel that equate to equivalent levels of ammonium binding capacity (2 and 0.126 g, respectively) (29) and observing that the addition of these amounts of each substrate produces distinct effects on cell growth and survival patterns, we hypothesize that a variety of VCs together contribute to cell death. Future studies will determine the relative concentrations of VCs such as indoles, ketones, and ammonia accumulated in the headspace of long-term batch cultures and in adsorptive substrates placed in the headspace to better understand this effect.
By comparing various masses of silica gel used, we observed that the positive growth effect of greater amounts of silica gel (2 g) outlasts that of the smaller amount (0.126 g) by 1 day (Fig. 4). This demonstrates an upper limit on binding capacity, indicating that absorbent substrates can become saturated with VCs outgassed into the headspace of culture vessels and lose their efficacy. Together, these observations strongly support a model in which, as more volatile compounds are bound, cellular survival improves.
The time-course experiment with the addition or removal of zeolite from long-term batch cultures allowed us to determine the temporal effect of toxic VCs on E. coli cells. Cultures with zeolite added on day 1 (Fig. 3) show a slight decrease in the number of viable cells on day 2, indicating that the role of zeolite in delaying death phase is most prominent between the logarithmic and stationary phases of incubation. In the presence of zeolite, toxic metabolites such as ammonia are likely removed from the headspace of the culture vessel, and the decrease in viable cell counts normally observed during the transition to death phase is delayed. This suggests that removal of the toxic compound(s) maintains cell viability but does not revive populations that have already died. The removal of zeolite after 1, 2, or 3 days of incubation enabled cultures to maintain high cell densities similar to cultures that retained zeolite in the headspace of the vessel for the duration of the experiment. These growth and survival patterns indicate that the majority of toxic compound(s) are produced during exponential phase and then adsorbed before cells begin to lose viability at the end of stationary phase.
To further investigate our hypothesis that toxic compounds outgassed into the headspace contribute to cell death, we created a mechanism that would allow us to replace the air in the headspace, removing these compounds (Fig. 5B). The air pumping mechanism allows the headspace to be entirely replaced every 20 s, thereby flushing out any toxic metabolites that may have collected during incubation. We demonstrate that constantly replacing the headspace significantly increases relative cell yield over time (Fig. 5A). This experiment serves as a proof of concept, demonstrating that removal of volatile compounds that collect in the headspace during normal cell growth not only leads to maintenance of high cell density but also delays the death phase—ultimately supporting our hypothesis that volatile compounds produced during normal cell metabolism are outgassed into the headspace and contribute to cell death.
Ultimately, this study helps elucidate the mechanisms that control entry into the death phase and opens the possibility of exploring how we may improve current protocols for batch culture incubation. In our analysis of various growth environments, we have determined that each culture condition has particular advantages and disadvantages that must be considered both as a research tool and for industrial applications. Although both zeolite and activated charcoal lead to higher cell density, they easily degrade to dust that can fall into the culture, causing contamination. Air replacement, while also leading to higher-density cultures, accelerates culture evaporation. Therefore, silica gel appears to be the better adsorptive for both research and, possibly, industrial applications, because in addition to the applications described here, it is already a well-known substrate that is accessible, does not cause culture evaporation, and has a lower likelihood of culture contamination via degradation. Furthermore, the addition of silica gel through a tablet or capsule would not require major modification of batch-culture vessels.
Our results suggest that the accumulation of toxin(s) into the headspace of a culture vessel during normal bacterial cell metabolism is correlated with the timing of entry into the death phase. By manipulating the growth environment through the use of adsorptive substrates or headspace air replacement, we are able to prolong the stationary phase and delay entry into the death phase. Despite the extensive research on ammonia and ammonium adsorption by activated charcoal, zeolite, and silica gel and the knowledge that ammonia is released during normal cell metabolism of amino acids in rich media, we cannot conclude that ammonia is the sole contributor to the toxic effects observed. Analysis of the chemical composition of headspace gasses or saturated substrates using various biochemical techniques will further illuminate which VCs accumulate and consequently may cause cells to enter the death phase.
MATERIALS AND METHODS
Bacterial strain and incubation conditions.
E. coli K-12 lineage strain PFM2, derived from MG1655 (54) with an added kanamycin resistance cassette, was used in this study. Cultures were initiated by transferring cells directly from a frozen 20% glycerol stock into 5 ml of Luria-Bertani/lysogeny broth (Lennox) medium (LB) (Difco) in 18- by 150-mm borosilicate test tubes and incubated overnight at 37°C with aeration in a TC-7 rolling drum (New Brunswick Scientific, Edison, NJ). The cells were then diluted into LB, super broth (SB) (Difco), or terrific broth (TB) (Difco) at a 1:1,000 (vol/vol) ratio, with 50 μg/ml kanamycin and transferred into 125-ml Erlenmeyer flasks to initiate experiments. The cultures were incubated at 37°C in flasks on a shaking platform at 200 rpm throughout each experiment.
Placement of adsorptive substrates in headspace of culture vessels.
Adsorptive substrates were added to culture vessels by placing weighed portions in sterile, paper tea bags. Empty tea bags were sterilized in 95% ethanol for 20 min, drained, and then dried overnight under sterile conditions. The following adsorptive substrates were used: activated charcoal pellets (329428, Sigma-Aldrich), impregnated zeolite blend (Z687375, Sigma-Aldrich), or silica gel granules 3-8 mesh desiccant (BT213560, VWR Scientific). Activated charcoal and zeolite were sterilized by autoclaving at 121°C. Silica gel granules were packed under sterile conditions by the manufacturer. Substrates were then weighed out and placed into the teabags, which were then secured in the neck of each flask, leaving enough space between the bags and the culture medium to avoid wetting of the bags. Unless otherwise stated, 2g of each material was used in each bag. For the temporal addition and removal of zeolite-containing bags, bags for individual culture vessels were either removed on day 1, 2, 3, or 4 or inserted on day 0, 1, 2, or 3.
Headspace air replacement in culture vessels.
For the headspace air replacement experiment, a Top Fin AIR-8000 aquarium air pump with 1/8-in. (inner diameter) laboratory tubing (VWR Scientific) was connected through a 0.2-μm sterile syringe filter (VWR Scientific) to sterile glass tubes running through metal caps of 125-ml Erlenmeyer flasks. The air pump was used on its lowest setting with an approximate pumping rate of 0.375 liters per minute (LPM).
Calculating viable cell counts during incubation.
Cell growth yield and survival was monitored by performing serial dilutions on cells sampled periodically from the cultures, followed by plating on LB agar (55). The limit of detection was >1 × 103 CFU/ml for all experiments (55).
ACKNOWLEDGMENTS
This work was supported in part by the U.S. Army Research Office (grants W911NF1010444 and W911NF1210321 to S.E.F.). We are grateful to Lacey Westphal for helpful discussions.
Contributor Information
Steven E. Finkel, Email: sfinkel@usc.edu.
Robert M. Kelly, North Carolina State University
REFERENCES
- 1.Finkel SE. 2006. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat Rev Microbiol 4:113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]
- 2.Pletnev P, Osterman I, Sergiev P, Bogdanov A, Dontsova O. 2015. Survival guide: Escherichia coli in the stationary phase. Acta Naturae 7:22–33. doi: 10.32607/20758251-2015-7-4-22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kram K, Finkel S. 2014. Culture volume and vessel affect long-term survival, mutation frequency, and oxidative stress in E. coli. Appl Environ Microbiol 80:1732–1738. doi: 10.1128/AEM.03150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sambrook J. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 5.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 6.Karen E, Roger B. 2002. Growth in liquid media. Curr Protoc Mol Biol 59:1.2.1–1.2.2. [DOI] [PubMed] [Google Scholar]
- 7.Kram K, Finkel S. 2015. Rich medium composition affects Escherichia coli survival, glycation, and mutation frequency during long-term batch culture. Appl Environ Microbiol 81:4442–4450. doi: 10.1128/AEM.00722-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salminen S, von Wright A. 2004. Lactic acid bacteria: microbiological and functional aspects, 3rd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- 9.Hermann T. 2003. Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172. doi: 10.1016/s0168-1656(03)00149-4. [DOI] [PubMed] [Google Scholar]
- 10.Eggeling L, Sahm H. 2009. Amino acid production. p 150–158. In Schaechter M (ed), Encyclopedia of microbiology, 3rd ed. Academic Press, Oxford, England. doi: 10.1016/B978-012373944-5.00129-2. [DOI] [Google Scholar]
- 11.Kolter R, Siegele DA, Tormo A. 1993. The stationary phase of the bacterial life cycle. Annu Rev Microbiol 47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 12.Nyström T. 2001. Not quite dead enough: on bacterial life, culturability, senescence, and death. Arch Microbiol 176:159–164. doi: 10.1007/s002030100314. [DOI] [PubMed] [Google Scholar]
- 13.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyström T. 2004. Stationary-phase physiology. Annu Rev Microbiol 58:161–181. doi: 10.1146/annurev.micro.58.030603.123818. [DOI] [PubMed] [Google Scholar]
- 15.Fredriksson Å, Nyström T. 2006. Conditional and replicative senescence in Escherichia coli. Curr Opin Microbiol 9:612–618. doi: 10.1016/j.mib.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Garbeva P, Liu X, Klein Gunnewiek PJA, Clocchiatti A, Hundscheid MPJ, Wang X, de Boer W. 2020. Volatile-mediated antagonism of soil bacterial communities against fungi. Environ Microbiol 22:1025–1035. doi: 10.1111/1462-2920.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilocca B, Cao A, Migheli Q. 2020. Scent of a killer: microbial volatilome and its role in the biological control of plant pathogens. Front Microbiol 11:41. doi: 10.3389/fmicb.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz-Bohm K, Martín-Sánchez L, Garbeva P. 2017. Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front Microbiol 8:2484. doi: 10.3389/fmicb.2017.02484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmassry MM, Piechulla B. 2020. Volatilomes of bacterial infections in humans. Front Neurosci 14:257. doi: 10.3389/fnins.2020.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kai M. 2020. Diversity and distribution of volatile secondary metabolites throughout Bacillus subtilis isolates. Front Microbiol 11:559. doi: 10.3389/fmicb.2020.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SE, Pham CA, Zambri MP, McKillip J, Carlson EE, Elliot MA. 2019. Streptomyces volatile compounds influence exploration and microbial community dynamics by altering iron availability. mBio 10:e00171-19. doi: 10.1128/mBio.00171-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kai M, Effmert U, Lemfack MC, Piechulla B. 2018. Interspecific formation of the antimicrobial volatile schleiferon. Sci Rep 8:16852. doi: 10.1038/s41598-018-35341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Audrain B, Farag MA, Ryu C-M, Ghigo J-M. 2015. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol Rev 39:222–233. doi: 10.1093/femsre/fuu013. [DOI] [PubMed] [Google Scholar]
- 24.Hark EF. February 1989. Water purification process. US patent 4808287A.
- 25.Doumas JJ, Molof AH, Raymond GH, Wikstrom L. April 1980. Process and apparatus for treating drinking water. US patent 4198296.
- 26.Chang W-S, Hong S-W, Park J. 2002. Effect of zeolite media for the treatment of textile wastewater in a biological aerated filter. Process Biochem 37:693–698. doi: 10.1016/S0032-9592(01)00258-8. [DOI] [Google Scholar]
- 27.Li M, Zhu X, Zhu F, Ren G, Cao G, Song L. 2011. Application of modified zeolite for ammonium removal from drinking water. Desalination 271:295–300. doi: 10.1016/j.desal.2010.12.047. [DOI] [Google Scholar]
- 28.Levy E. July 1996. Process for filtering water prior to carbonation. US patent 5538746.
- 29.Ro SK, Lima MI, Reddy BG, Jackson AM, Gao B. 2015. Removing gaseous NH3 using biochar as an adsorbent. Agriculture 5:991–1002. doi: 10.3390/agriculture5040991. [DOI] [Google Scholar]
- 30.Strazhesko DN, Strelko VB, Belyakov VN, Rubanik SC. 1974. Mechanism of cation exchange on silica gels. J Chromatogr A 102:191–195. doi: 10.1016/S0021-9673(01)85446-7. [DOI] [Google Scholar]
- 31.Annelie H. 2001. Ion exchange of ammonium in zeolites: a literature review. J Environ Eng 127:673–681. [Google Scholar]
- 32.Nordell E, Hansson AB, Karlsson M. 2013. Zeolites relieves inhibitory stress from high concentrations of long chain fatty acids. Waste Manag 33:2659–2663. doi: 10.1016/j.wasman.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Kuo S, Pedram EO, Hines AL. 1985. Analysis of ammonia adsorption on silica gel using the modified potential theory. J Chem Eng Data 30:330–332. doi: 10.1021/je00041a029. [DOI] [Google Scholar]
- 34.Wang J, Jin W, Guo H, Wang X, Liu J. 2015. Experimental study on ammonia nitrogen adsorption performance of zeolite powder. Chem Eng Trans 46:79–84. [Google Scholar]
- 35.Franus W, Wdowin M. 2011. Removal of ammonium ions by selected natural and synthetic zeolites. Gospod Surowcami Miner 26:133–148. [Google Scholar]
- 36.Asilian H, Mortazavi S, Kazemian H, Phaghiehzadeh S, Shahtaheri S, Salem M. 2004. Removal of ammonia from air, using three Iranian natural zeolites. Iran J Public Health 33:45–51. [Google Scholar]
- 37.Duetz WA, Rüedi L, Hermann R, O'Connor K, Büchs J, Witholt B. 2000. Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl Environ Microbiol 66:2641–2646. doi: 10.1128/AEM.66.6.2641-2646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen LA. 1940. The influence of aeration on bacterial growth and on associated chemical changes. Proc Soc Agric Bacteriol 3:14–18. doi: 10.1111/j.1365-2672.1940.tb03924.x. [DOI] [Google Scholar]
- 39.McDaniel L, Bailey E, Zimmerli A. 1965. Effect of oxygen supply rates on growth of Escherichia coli. Appl Microbiol 13:109–114. doi: 10.1128/am.13.1.109-114.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn RK. 1954. Agitation–aeration in the laboratory and in industry. Bacteriol Rev 18:254–274. doi: 10.1128/br.18.4.254-274.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenberger RF, Kogut M. 1958. The influence of growth rate and aeration on the respiratory and cytochrome system of a fluorescent pseudomonad grown in continuous culture. J Gen Microbiol 19:228–243. doi: 10.1099/00221287-19-2-228. [DOI] [PubMed] [Google Scholar]
- 42.Winslow CE, Walker HH, Sutermeister M. 1932. The influence of aeration and of sodium chloride upon the growth curve of bacteria in various media. J Bacteriol 24:185–208. doi: 10.1128/jb.24.3.185-208.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Qureshi N. 2009. How microbes tolerate ethanol and butanol. N Biotechnol 26:117–121. doi: 10.1016/j.nbt.2009.06.984. [DOI] [PubMed] [Google Scholar]
- 44.Sattayawat P, Yunus IS, Jones PR. 2020. Bioderivatization as a concept for renewable production of chemicals that are toxic or poorly soluble in the liquid phase. Proc Natl Acad Sci USA 117:1404–1413. doi: 10.1073/pnas.1914069117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh SC, Wang JH, Lai YC, Su CY, Lee KT. 2018. Production of 1-dodecanol, 1-tetradecanol, and 1,12-dodecanediol through whole-cell biotransformation in Escherichia coli. Appl Environ Microbiol 84:e01806-17. doi: 10.1128/AEM.01806-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilbanks B, Trinh CT. 2017. Comprehensive characterization of toxicity of fermentative metabolites on microbial growth. Biotechnol Biofuels 10:262. doi: 10.1186/s13068-017-0952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potezny N, Atkinson ER, Rofe AM, Conyers RA. 1981. The inhibition of bacterial cell growth by ketone bodies. Aust J Exp Biol Med Sci 59:639–649. doi: 10.1038/icb.1981.57. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Ding X, Rather PN. 2001. Indole can act as an extracellular signal in Escherichia coli. J Bacteriol 183:4210–4216. doi: 10.1128/JB.183.14.4210-4216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong Q, Cheng F, Liang J, Wang X, Chen Y, Fang X, Hu L, Hang Y. 2019. Profiles of volatile indole emitted by Escherichia coli based on CDI-MS. Sci Rep 9:13139. doi: 10.1038/s41598-019-49436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garbe TR, Kobayashi M, Yukawa H. 2000. Indole-inducible proteins in bacteria suggest membrane and oxidant toxicity. Arch Microbiol 173:78–82. doi: 10.1007/s002030050012. [DOI] [PubMed] [Google Scholar]
- 51.Tyc O, Zweers H, de Boer W, Garbeva P. 2015. Volatiles in inter-specific bacterial interactions. Front Microbiol 6:1412. doi: 10.3389/fmicb.2015.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller T, Walter B, Wirtz A, Burkovski A. 2006. Ammonium toxicity in bacteria. Curr Microbiol 52:400–406. doi: 10.1007/s00284-005-0370-x. [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves M, Sánchez-García L, Oliveira-Jardim E, Silvestre-Albero J, Rodríguez-Reinoso F. 2011. Ammonia removal using activated carbons: effect of the surface chemistry in dry and moist conditions. Environ Sci Technol 45:10605–10610. doi: 10.1021/es203093v. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Popodi E, Tang H, Foster PL. 2012. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc Natl Acad Sci USA 109:E2774–E2783. doi: 10.1073/pnas.1210309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraigsley AM, Finkel SE. 2009. Adaptive evolution in single species bacterial biofilms. FEMS Microbiol Lett 293:135–140. doi: 10.1111/j.1574-6968.2009.01526.x. [DOI] [PubMed] [Google Scholar]