ABSTRACT
Steroidal 17-carbonyl reduction is crucial to the production of natural bioactive steroid medicines, and boldenone (BD) is one of the important C-17-hydroxylated steroids. Although efforts have been made to produce BD through biotransformation, the challenges of the complex transformation process, high substrate costs, and low catalytic efficiencies have yet to be mastered. Phytosterol (PS) is the most widely accepted substrate for the production of steroid medicines due to its similar foundational structure and ubiquitous sources. 17β-Hydroxysteroid dehydrogenase (17βHSD) and its native electron donor play significant roles in the 17β-carbonyl reduction reaction of steroids. In this study, we bridged 17βHSD with a cofactor regeneration strategy in Mycobacterium neoaurum to establish a one-step biocatalytic carbonyl reduction strategy for the efficient biosynthesis of BD from PS for the first time. After investigating different intracellular electron transfer strategies, we rationally designed the engineered strain with the coexpression of 17βhsd and the glucose-6-phosphate dehydrogenase (G6PDH) gene in M. neoaurum. With the establishment of an intracellular cofactor regeneration strategy, the ratio of [NADPH]/[NADP+] was maintained at a relatively high level, the yield of BD increased from 17% (in MNR M3M-ayr1S.c) to 78% (in MNR M3M-ayr1&g6p with glucose supplementation), and the productivity was increased by 6.5-fold. Furthermore, under optimal glucose supplementation conditions, the yield of BD reached 82%, which is the highest yield reported for transformation from PS in one step. This study demonstrated an excellent strategy for the production of many other valuable carbonyl reduction steroidal products from natural inexpensive raw materials.
IMPORTANCE Steroid C-17-carbonyl reduction is one of the important transformations for the production of valuable steroidal medicines or intermediates for the further synthesis of steroidal medicines, but it remains a challenge through either chemical or biological synthesis. Phytosterol can be obtained from low-cost residues of waste natural materials, and it is preferred as the economical and applicable substrate for steroid medicine production by Mycobacterium. This study explored a green and efficient one-step biocatalytic carbonyl reduction strategy for the direct conversion of phytosterol to C-17-hydroxylated steroids by bridging 17β-hydroxysteroid dehydrogenase with a cofactor regeneration strategy in Mycobacterium neoaurum. This work has practical value for the production of many valuable hydroxylated steroids from natural inexpensive raw materials.
KEYWORDS: C-17-hydroxylated steroids, phytosterol, cofactor regeneration, 17β-hydroxysteroid dehydrogenase, glucose-6-phosphate dehydrogenase
INTRODUCTION
C-17-hydroxylated steroids belong to a family of natural steroids with important medicinal value and are useful precursors for the synthesis of other high-performance steroid medicines (1, 2). Boldenone (BD) (androsta-1,4-dien-17-ol-3-one), a protein anabolic hormone, is one of the important C-17-hydroxylated steroids, which can support nitrogen retention, stimulate erythropoietin release, and promote protein synthesis. BD used to be mainly produced from androst-4-ene-3,17-dione (AD) by chemical synthesis (3), but the high-cost processes and severe experimental conditions inhibited its development. Research in this area has grown rapidly over the past decades, but the challenge of a green and efficient approach for C-17-carbonyl reduction of steroids has yet to be mastered. With the constant development of green sustainable microbial transformation technologies, the production of BD through biotransformation has gained much research interest. In a previous study, BD was achieved from AD via Arthrobacter simplex and recombinant Pichia pastoris ordered biotransformations (4). Besides, many other microorganisms, such as Mucor racemosus, Nostoc muscorum, and Arthrobacter oxydans, could also complete the 17β-carbonyl reduction reaction of steroids (5–8). Despite these efforts, the application of these biological methods in C-17-hydroxylated synthesis remains underutilized due to limitations in substrate scope and catalytic efficiencies.
The conversion of inexpensive and biorenewable raw materials into value-added bioproducts has always been one of the focal issues highlighted in research (9–11). Phytosterol (PS) is ubiquitous in waste natural material such as the residue from vegetable oil extraction, filter cake in the sugarcane industry, and waste products in the paper industry (12). It is composed of a cyclopentane perhydrophenanthrene sterol ring structure and a C-17 side chain (9), which has a sterol ring structure similar to that of BD. In view of their similar foundational structures, BD could be produced from PS through side chain degradation and 17β-carbonyl reduction successively. Mycobacteria are rich in mycolic acids and can catabolize natural sterols as carbon and energy sources. Previous studies showed that PS could be degraded by Mycobacterium neoaurum effectively (9, 13, 14). Some important intermediates that can be used as ideal precursors to synthesize valuable steroidal pharmaceuticals could be accumulated by mycobacteria when their sterol metabolism pathway was interrupted (15). Studies on the 17β-carbonyl reduction reaction of steroids by several Mycobacterium species mutants proved fruitful, and other attempts at the 17β-carbonyl reduction reaction of corn oil phytosterols by Pseudomonas aeruginosa were also performed (1, 16–18), while their low transformation efficiencies still cannot meet the demand of industrial production. A stepwise biotransformation strategy was established through bridging M. neoaurum with P. pastoris to further improve the yield of BD, but its transformation process was complex (9). Therefore, a concise and efficient one-step biotransformation strategy should be designed and established.
Until now, there have been many studies on the degradation of PS by M. neoaurum, but studies on its 17β-carbonyl reduction products remain to be strengthened. Traditionally, BD was produced from androst-1,4-diene-3,17-dione (ADD) through 17β-carbonyl reduction. In fact, ADD can be produced from PS by Mycobacterium sp. (13, 14). To improve the purity of ADD, an efficient C-1,2-dehydrogenase should be substituted for the conversion of AD to ADD (19). In addition, 17β-hydroxysteroid dehydrogenase (17βHSD) is another key enzyme in the production of BD. Many observations suggested that M. neoaurum does not contain an evident 17β-carbonyl reduction from C-17-one to C-17-alcohol (15, 20). To strengthen the 17β-carbonyl reduction of natural steroids, the key enzyme 17βHSD should be overexpressed in M. neoaurum. Although improvements have been realized in overexpressing the key enzyme in engineered strains (21–23), efficient 17β-carbonyl reduction of natural steroids remains one of the most challenging tasks in the field of steroid medicine production. Generally, the 17βHSDs of fungi tend to catalyze reduction, and the bacterial counterpart shifts toward oxidation (5, 22, 24). Therefore, in our study, the 17βHSD screened from the fungal genome should be overexpressed in M. neoaurum to enhance the 17β-carbonyl reduction reaction.
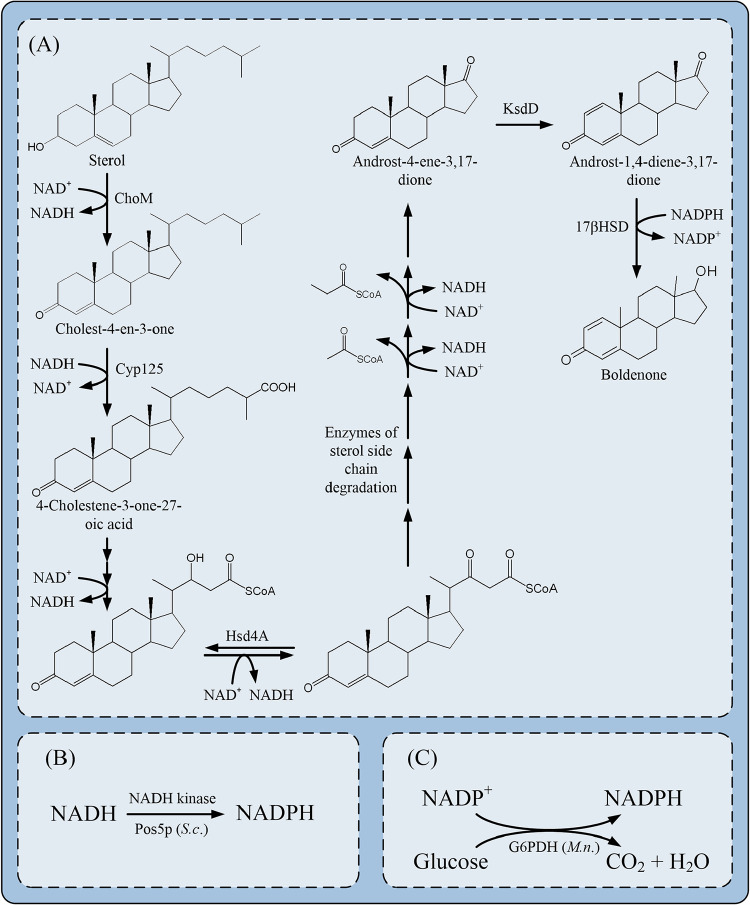
An efficient 17β-carbonyl reduction strategy was motivated by combining 17βHSD with its native electron donor. Until now, glucose dehydrogenase (GDH) (25, 26), formate dehydrogenase (FDH) (27), NADH kinase (Pos5) (28), and glucose-6-phosphate dehydrogenase (G6PDH) (29) have been successfully used for cofactor regeneration in many studies. During the side chain degradation of PS, an excess amount of NADH accumulates, indicating that the direct conversion of NADH to NADPH may be an available way to increase the intracellular NADPH concentration and thereby promote the 17β-carbonyl reduction reaction of steroids (Fig. 1A). Pos5, an NADH kinase that can catalyze the direct phosphorylation of the extra NADH to NADPH, is the major source of mitochondrial NADPH regeneration in Saccharomyces cerevisiae (Fig. 1B) (28). On the other hand, the pos5 gene without a mitochondrial targeting sequence prefers NADH over NAD+ as a cytosolic NADH kinase. Efficient NADPH-regenerating systems have been constructed in recombinant strains by Pos5p to produce GDP-l-fucose, ε-caprolactone, lysine, l-malate, and protopanaxadiol (30–33). Besides, the expression of the G6PDH gene, which is the limiting enzyme in the oxidative pentose phosphate pathway, is a representative method for NADPH regeneration (Fig. 1C). G6PDH catalyzes the oxidation of glucose-6-phosphate to 6-phosphogluconolactone with the concomitant reduction of NADP+ to NADPH. As one of the most important enzymes for cellular NADPH regeneration, the overexpression of G6PDH has been widely used to supplement NADPH in metabolic engineering in vivo. The expression of the G6PDH gene in Bacillus subtilis RH33 resulted in a significant enhancement of riboflavin production (34). The yields of 1-(R)-phenylethanol and poly-γ-glutamic acid were improved by overexpressing G6PDH efficiently (35, 36). Taken together, an efficient and stable NADPH regeneration strategy should be selected and established in M. neoaurum to promote the production of BD.
FIG 1.
Side chain degradation of PS in M. neoaurum (M.n.) (A) and enzymes for NADPH regeneration (B and C). S.c., S. cerevisiae.
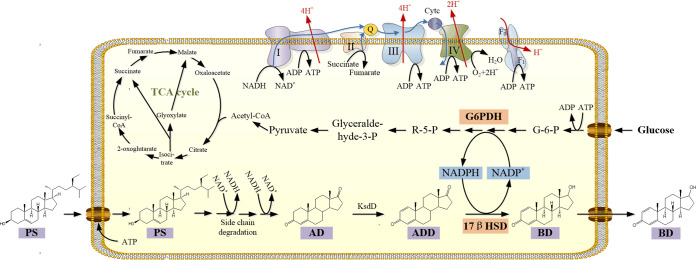
Here, we demonstrate a strategy for addressing this challenge that relies on the conversion of inexpensive and biorenewable raw materials and bridges a hydroxylase with the cofactor regeneration strategy with the ultimate goal of achieving a green and efficient one-step biotransformation strategy in the field of steroid 17β-carbonyl reduction. As shown in Fig. 2, after entering the cell, PS will undergo side chain elimination, C-1,2 dehydrogenation, and 17β-carbonyl reduction successively. The sequence of the key enzyme 17βHSD, which has been screened in our previous study (9), was optimized for expression in M. neoaurum. To establish an efficient NADPH regeneration strategy, the effects of endogenous G6PDH from M. neoaurum and exogenous Pos5p from S. cerevisiae, which were coexpressed with 17βHSD in M. neoaurum, were also investigated. This is the first report on the integration of 17βHSD with a cofactor regeneration strategy for efficient BD production from PS in M. neoaurum. This biotransformation strategy also provides what is perhaps an ideal platform for the production of many valuable steroids from natural inexpensive raw materials.
FIG 2.
Strategy for biotransformation integrated with cofactor NADPH regeneration for BD production from PS. A total of 5.0 g/liter PS was transformed by 70 g/liter resting cells of MNR M3M recombinants with HP-β-CD at a molar ratio of 1:3 and 30 g/liter initial glucose supplementation. TCA, tricarboxylic acid; Cytc, cytochrome c.
RESULTS
Screening and optimization of 17βhsd in M. neoaurum.
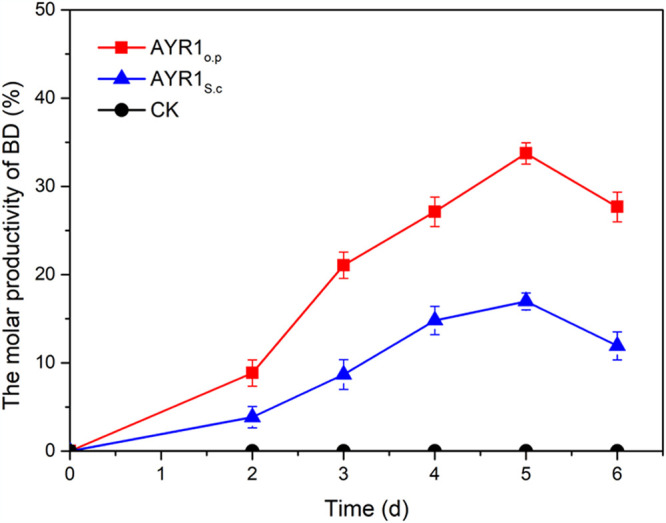
17βHSD is the key enzyme for the 17β-carbonyl reduction of steroids. In our previous study, three potential 17βHSDs, namely, 17βHSD from Cyberlindnera jadinii (AYR1C.j), 17βHSD from S. cerevisiae (AYR1S.c), and 17βHSD from Cochliobolus lunatus (17β-HSDcl), were screened from the GenBank database (9). In the present study, the three 17βHSD genes were individually expressed with the M. neoaurum expression vector pMV261 in MNR M3M. Next, 3.0 g/liter PS was added at the beginning and transformed by growing cells of MNR M3M recombinants with hydroxypropyl-β-cyclodextrin (HP-β-CD) at a molar ratio of 1:3 for 6 days. As shown in Table 1, the recombinant strain MNR M3M-ayr1S.c displayed relatively higher catalytic activity than the others. To make 17β-carbonyl reduction more efficient, the ayr1S.c gene sequence was codon optimized according to the codon preference of M. neoaurum (ayr1op) and expressed by pMV261 in MNR M3M, resulting in recombinant strain MNR M3M-ayr1op. Compared with MNR M3M-ayr1S.c, the catalytic activity of MNR M3M-ayr1op increased from 16.9% to 33.7% (increased by 17%) in 5 days (Fig. 3). Besides, the initial conversion rate of MNR M3M-ayr1op was also enhanced from 0.06 g/liter/day to 0.13 g/liter/day (enhanced by 2.3-fold) compared with MNR M3M-ayr1S.c. These results indicated that recombinant MNR M3M-ayr1op, which showed efficient 17β-carbonyl reduction of steroids in MNR M3M, was more suitable for the production of BD.
TABLE 1.
Conversion of PS by MNR M3M overexpressing different recombinant 17βHSDs
| Strain | Mean yield of BD (%) ± SD | Avg yield rate of BD (mg/liter/day) ± SD |
|---|---|---|
| MNR M3M-ayr1S.c | 16.99 ± 0.83 | 70.44 ± 0.09 |
| MNR M3M-ayr1C.j | 8.32 ± 0.27 | 34.48 ± 0.02 |
| MNR M3M-17βHSDcl | 0.55 ± 0.19 | 2.28 ± 0.01 |
| MNR M3M | 0 | 0 |
FIG 3.
Effects of Ayr1S.c codon optimization on BD production. A total of 3.0 g/liter PS was added at the beginning and transformed by growing cells of MNR M3M recombinants with HP-β-CD at a molar ratio of 1:3 for 6 days. All assays were performed in triplicate, with three independent measurements. Standard deviations of data from biological replicates are represented by error bars.
Introduction of the NADPH regeneration strategy in M. neoaurum.
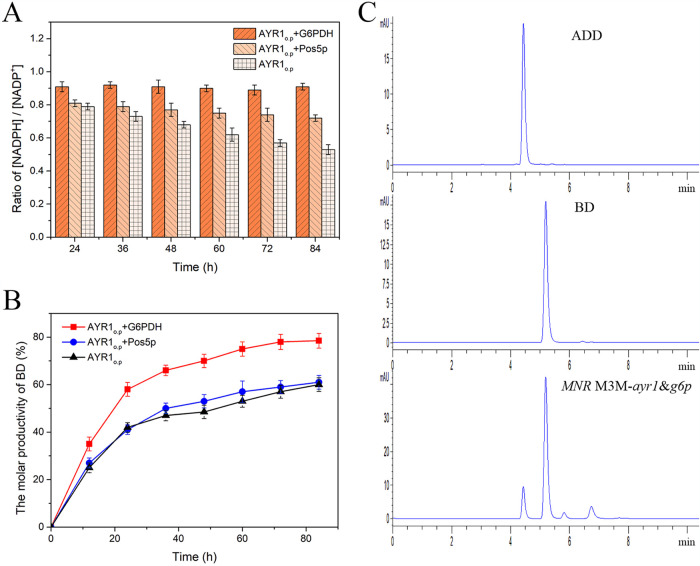
17βHSD from S. cerevisiae is an NADPH-dependent enzyme, and it is crucial to establish an efficient cofactor regeneration strategy for the production of BD. Overexpression of Pos5p from S. cerevisiae and of G6PDH from M. neoaurum are two alternative approaches to promote cofactor regeneration. Therefore, the effects of the coexpression of pos5p and g6pdh with ayr1op, resulting in two recombinant strains (MNR M3M-ayr1&p5p and MNR M3M-ayr1&g6p, respectively), were investigated. As shown in Fig. 4A, both NADPH regeneration strategies displayed higher NADPH concentrations than the control after coexpression. However, the yield of BD did not show a significant improvement in MNR M3M-ayr1&p5p (Fig. 4B).
FIG 4.
Intracellular [NADPH]/[NADP+] ratios (A) and BD yields (B) of different MNR M3M recombinants and HPLC analysis of PS transformation (C). A total of 3.0 g/liter PS was transformed by 70 g/liter resting cells of MNR M3M recombinants with HP-β-CD at a molar ratio of 1:3 for 84 h. All assays were performed in triplicate, with three independent measurements. Standard deviations of data from biological replicates are represented by error bars.
Compared with MNR M3M-ayr1&p5p, the recombinant strain MNR M3M-ayr1&g6p increased BD production significantly, and the yield of BD was improved by 18% in 84 h compared with the control (Fig. 4B). With the coexpression of g6pdh in MNR M3M-ayr1&g6p, an intracellular NADPH regeneration strategy was established (Fig. 2), the ratio of [NADPH]/[NADP+] was maintained at a relatively high level, the 17β-carbonyl reduction of steroids was promoted effectively, and productivity was increased by 6.5-fold compared with the initial productivity (Fig. 4B), which indicated that G6PDH from M. neoaurum was more suitable for the production of BD in M. neoaurum.
Effects of transformation conditions on the production of BD.
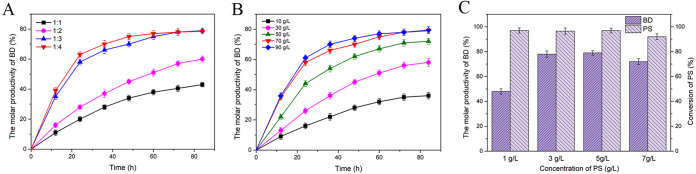
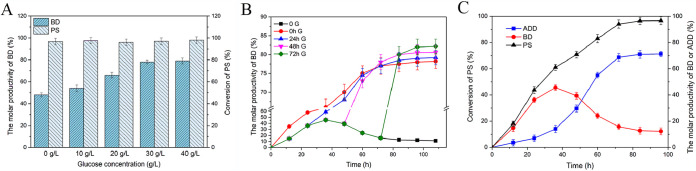
The low aqueous solubility of steroid substrates and the inhibition or toxicity of both the substrate and product severely inhibited the transformation of PS. HP-β-CD is a cyclic oligosaccharide that can form inclusion complexes with low-aqueous-solubility organic molecules to improve their water solubility and bioavailability. Besides, treatment of mycobacteria with HP-β-CD relieves the inhibitory effect of steroids on the electron transfer chain and cell growth (37). Given its strong complexing ability and good detoxification ability, HP-β-CD is the optimal medium for enhancing the transformation of PS (38). To improve the yield of BD from PS, the effects of different molar ratios of PS to HP-β-CD on the biotransformation process were investigated. As shown in Fig. 5A, HP-β-CD significantly enhanced the yield of BD. With increasing molar ratios of HP-β-CD, the yield of BD was improved, and maximum BD production was obtained when the molar ratio of PS to HP-β-CD was 1:3. But when the molar ratio was >1:3, the yield of BD did not continue to increase. These results indicated that the molar ratio of 1:3 was optimal for the production of BD.
FIG 5.
Effects of HP-β-CD molar ratio (A), biomass (B), and substrate concentration (C) on BD production. PS (1.0 g/liter, 3.0 g/liter, 5.0 g/liter, and 7.0 g/liter) was transformed by resting cells of MNR M3M recombinants (10 g/liter, 30 g/liter, 50 g/liter, 70 g/liter, and 90 g/liter) with HP-β-CD (molar ratios of 1:1, 1:2, 1:3, and 1:4) for 84 h. All assays were performed in triplicate, with three independent measurements. Standard deviations of data from biological replicates are represented by error bars.
Besides, the biomass of recombinant strain MNR M3M-ayr1&g6p also greatly affected the production of BD. As shown in Fig. 5B, a high yield of BD was achieved with the increased biomass used in the transformation. When 70 g/liter MNR M3M-ayr1&g6p wet cells was used in the system, the yield of BD reached the maximum but did not increase further when the biomass was over 70 g/liter. Except for the inhibition of substrate transfer by high-aggregation-level strains (23), the oxidation of C-17-hydroxy steroids in M. neoaurum is another important inhibition factor that cannot be ignored in the transformation and had also been reported in previous research (9).
To further improve the yield of BD, the substrate concentrations during transformation were also determined. The results are shown in Fig. 5C: the yield of BD was low when the concentration of PS was <3 g/liter. When the concentration of PS was 3 g/liter, the yield of BD was enhanced by 30% and did not show an obvious improvement when the concentration of PS reached 5 g/liter. However, the yield of BD exhibited a slight decline when the concentration of PS was >5 g/liter. It had been reported that the excessive accumulation of the intermediate product ADD may affect the respiratory chain and cell membrane of strains (39–41). Therefore, an appropriate substrate concentration was important for the production of BD.
Effects of glucose supplementation on the production of BD.
In comparison, fungal 17βHSDs prefer NADPH as the cofactor (22). Glucose supplementation can increase the intracellular ratio of [NADPH]/[NADP+], but the ratio declines with the consumption of glucose (42). In this study, glucose was also added to the biotransformation system as a cosubstrate for the regeneration of NADPH. As shown in Fig. 6A, the yield of BD was obviously improved after supplementation with glucose, and the maximum yield of 78% was obtained with supplementation with 30 g/liter glucose, which was a 30% improvement from the glucose-free control. When the glucose concentration continued to increase, the yield of BD was not further improved. Besides, the time of supplementation of glucose also had an effect on the yield of BD (Fig. 6B). When glucose was added to the system at the beginning, the yield of BD was 78%, whereas when glucose was added after 72 h, the yield of BD reached a maximum of 82%, which was a 4% improvement compared with the beginning-supplementation control, and it did not further increase when the addition time continued to be delayed. Additionally, the initial specific rate was improved from 5.08 μmol/g cells/h with the original glucose supplement to 9.32 μmol/g cells/h with the 72-h glucose supplement. As shown in Fig. 6C, when PS was transformed without glucose, the accumulation of BD increased at the beginning and then decreased quickly with the accumulation of ADD, indicating that the intracellular reducing power was insufficient. Therefore, an appropriate amount of glucose supplementation at a suitable transformation period is conducive to the production of BD.
FIG 6.
Effects of glucose supplementation concentration (A), supplementation time (B), and transformation without glucose (C) on BD production. A total of 5.0 g/liter PS was transformed by 70 g/liter resting cells of MNR M3M recombinants with HP-β-CD at a molar ratio of 1:3 for 96 h. All assays were performed in triplicate, with three independent measurements. Standard deviations of data from biological replicates are represented by error bars.
DISCUSSION
Boldenone is one of the important hydroxylation steroidal compounds, which are rare in natural content, complicated to extract, and difficult for chemical synthesis. Despite the effectiveness in the C-17 modification of steroids, modification with a hydroxyl group in steroids through C=O carbonyl reduction remains a challenge. Recently, 17β-carbonyl reduction steroids have been produced through the side chain degradation of some sterols (cholesterol or phytosterol) by Mycobacterium, but their low production efficiency, inefficient 17βHSD, and requirement for electron-donating cofactors are the main barriers limiting the development of engineered strains for industrial production. In our previous study, an ordered biotransformation method was proposed, employing A. simplex and recombinant P. pastoris to produce BD from AD (4). In fact, AD can be produced from PS by Mycobacterium sp. (9, 13, 14). Based on that research, this study expressed a fungal 17βHSD from S. cerevisiae in M. neoaurum for the production of BD coupled with G6PDH. The yield of BD reached 82%, which is the highest yield reported for transformation from PS in one step. The results indicated that the development of engineered strains that can produce other high-value-added steroids from sterols by one-step biosynthesis will be an interesting topic for future study.
17βHSD from S. cerevisiae is an NADPH-dependent enzyme, and the improvement of the intracellular cofactor NADPH content could efficiently promote biological transformation. Glucose is an important material and energy source for living organisms, and exogenously added glucose can participate in the metabolic systems with NADH and NADPH generation at the same time. In order to accumulate a large amount of NADPH, a cofactor NADPH regeneration strategy should be designed and established in M. neoaurum. The bioprocess of phytosterol-to-BD production involves three processes: sterol side chain elimination, steroid core oxidation, and 17β-carbonyl reduction. Sterol side chain elimination is initiated by cholesterol oxidase (41), which is followed by further oxidation to a C-17-keto steroid intermediate with the release of propionyl-CoA and acetyl-CoA (Fig. 1) (43). As postulated, 21 molecules of NADH and 10 molecules of reduced flavin adenine dinucleotide (FADH2) are formed with the full removal of the side chain for one molecule of β-sitosterol (44). Therefore, the generation of NADPH from NADH would be an expected cofactor regeneration strategy. In fact, there are three NADH kinase isozymes. Utr1 and Yef1 are cytosolic NADH kinases and not critical for the supply of cytosolic NADPH, while the mitochondrial NADH kinase Pos5 is critical for mitochondrial NADPH supplementation. In this study, the effects of Pos5p from S. cerevisiae and G6PDH from M. neoaurum, which were coexpressed with AYR1op, were investigated. As shown in Fig. 4, although both Pos5p and G6PDH were beneficial for intracellular NADPH regeneration, the coexpression of g6pdh was more effective than that of pos5p for BD biosynthesis in recombinant M. neoaurum. With the generation of NADPH from NADH during the side chain degradation of PS in MNR M3M-ayr1&p5p, the formation of NAD+ was also promoted. The oxidation of BD was enhanced by Hsd4A, which was one of the key enzymes in the side chain degradation of PS and could catalyze the oxidation of C-17-hydroxy steroids (Fig. 1). Therefore, although NADPH accumulated and side chain degradation of PS was promoted, the yield of BD declined at the same time, which was consistent with previous reports (45). In fact, the intracellular redox cofactors of microorganisms are in a dynamic equilibrium process. The cofactor requirements of industrial microorganisms vary during different fermentation stages. Although the production of many steroids requires a large amount of NADPH, the increase of the NADPH/NADP+ ratio could lead to deficiencies in ATP and NADH simultaneously, which could seriously affect the growth of the strains. In view of these results, it is of great significance to explore a cofactor balance system in the whole cell for improving the biotransformation efficiency of steroidal compounds.
Steroidal compounds are hydrophobic lipid compounds. Naturally, sterols can cross mycobacteria by direct contact between sterol particles and the cell envelope (46). But its low transfer efficiency severely limits the biotransformation of sterols. With its excellent water solubility, strong complexing ability, and good biocompatibility, HP-β-CD is an effective medium for enhancing the transfer efficiency of steroidal compounds (47). HP-β-CD not only forms inclusion complexes with steroids but also increases cell membrane permeability by extracting lipophilic components from cytomembranes (47) and subsequently improves the yield of BD (Fig. 5A). ADD, the C-1,2 dehydrogenation product of AD, is the precursor for the production of BD (Fig. 1). It was reported that the production of ADD from PS by M. neoaurum was always accompanied by small amounts of AD, which could affect the yield of BD (9). However, the ratio of ADD/AD is increased in the presence of HP-β-CD, as HP-β-CD affects the expression of KsdD, which is involved in the steroid catabolic pathway of mycobacteria (37). As shown in Fig. 6C, more accumulation of ADD was conducive to the production of BD, indicating that HP-β-CD was good for the production of BD. Next, we investigated the influence of the recombinant strain biomass on the bioconversion of PS to BD. The yield of BD did not increase further when the biomass was over 70 g/liter (Fig. 5B). In an investigation of the effect of a whole-cell biocatalyst on the reduction of cortisone, further increases of the biocatalyst did not enhance the conversion rate (48). Shao et al. also found that a high biomass has a negative effect on product formation (23), which was consistent with our results. When the biomass was enhanced, the cell aggregation level was high, and the cell respiratory function and uptake of the substrate were inhibited (49), which subsequently hampered the production of BD. Besides, in our study, BD could be oxidized by Hsd4A, a key enzyme in the side chain degradation of PS in M. neoaurum. When the engineered M. neoaurum biomass was enhanced, the oxidase also accumulated, which significantly prevented the accumulation of BD (9). On the other hand, the oxidation of C-17-hydroxy steroids in M. neoaurum was also not conducive to the accumulation of BD when the concentration of PS was <3 g/liter (Fig. 5C). The elimination of the oxidation of BD without affecting the side chain degradation of PS is an urgent problem to be solved in our next step.
In general, the biotransformation process from PS to BD undergoes side chain degradation and the 17β-carbonyl reduction reaction successively. To establish an efficient strategy for BD production from PS, the effect of glucose supplementation was also investigated. As shown in Fig. 6A, the maximum yield of BD was obtained with 30 g/liter glucose supplementation. This result may be caused by the ratio of [NADPH]/[NADP+], which had already been maintained at a relatively high level by 30 g/liter glucose supplementation. G6PDH is the limiting enzyme in the oxidative pentose phosphate pathway, which is a representative pathway for NADPH regeneration, while BD is produced by 17βHSD and its cofactor NADPH. After the supplementation of glucose at the beginning, glucose metabolism was enhanced by the coexpression of g6pdh and ayr1op and resulted in enhanced NADPH accumulation, which promoted the 17β-carbonyl reduction reaction by 17βHSD, subsequently resulting in enhanced BD production. Besides, the time of supplementation with glucose was another crucial factor in the production of BD. As shown in Fig. 6C, when PS was transformed without glucose, the accumulation of the intermediate product ADD reached the maximum in 72 h. This result may be due to the oxidation of M. neoaurum, which significantly prevented the accumulation of BD. As previously reported, when BD was transformed by M. neoaurum directly, BD decreased rapidly, and ADD continued to accumulate at the same time (9, 50, 51). This indicated that the large accumulation of ADD was beneficial for BD production. When adequate glucose was supplemented in 72 h, a considerable amount of NADPH was generated through the intracellular NADPH regeneration strategy, which promoted the 17β-carbonyl reduction of ADD that had largely accumulated, thereby enhancing the yield of BD.
Steroidal compounds with hydroxyl groups play an important role in improving drug activity and the metabolism of exogenous compounds. Its complicated extraction process, difficult chemical synthesis, and other problems are not conducive to the development of innovative drugs. Microbial transformation technologies could effectively solve the above-mentioned problems. This study established the efficient 17β-carbonyl reduction of steroids successfully by introducing the NADPH regeneration strategy to MNR M3M-ayr1&g6p, in which 17βhsd and g6pdh were coexpressed. In order to further improve the yield of BD, the effects of glucose supplementation conditions were also analyzed. After the optimization of transformation conditions, the highest yield of BD produced by PS transformation was up to 82%, which was produced in one step by recombinant M. neoaurum coexpressing 17βhsd and g6pdh for the first time. This study provides a promising and green biotransformation strategy for the production of many valuable 17β-carbonyl reduction steroidal products from natural inexpensive raw materials.
MATERIALS AND METHODS
Strains, plasmids, and reagents.
M. neoaurum TCCC 11028 MNR M3ΔksdD::ksdDMNR (MNR M3M), described previously by Xie et al. (19), was obtained from the Tianjin University of Science and Technology Culture Collection Center (TCCC) (China) and cultured as described previously (47). Escherichia coli DH5α (TransGen Biotech, China) was used as the host strain for cloning. S. cerevisiae CICC32315 used in this study was conserved in our laboratory. The E. coli-M. neoaurum shuttle vector pMV261 was a gift from W. R. Jacobs, Jr. (Howard Hughes Medical Institute). Kanamycin (50 μg/ml) (Solarbio, China) was used for plasmid selection and maintenance. PS (98.4% purity) was obtained from Cofco Tech Bioengineering Co. Ltd. (China). 4-Androstene-3,17-dione (AD), androst-1,4-diene-3,17-dione (ADD) (Sigma-Aldrich Co., USA), and BD standards (Heowns, USA) were used in this study. Hydroxypropyl-β-cyclodextrin (HP-β-CD) (with a 31.7% degree of substitution and an average relative molecular mass of 1,523) was procured from Xi’an Deli Biology and Chemical Industry Co. Ltd. (China).
Construction and transformation of recombinant plasmids in M. neoaurum.
A list of strains, plasmids, and primers used in this study is shown in Table 2. According to previous reports and the complete cDNA sequences of M. neoaurum, the gene sequences of ayr1S.c (GenBank gene identifier 854682), ayr1C.j (GenBank gene identifier 30991180), 17β-hsdcl (GenBank accession number AF069518) (9), and g6pdh (NCBI Protein accession no. AHC23815.1) were amplified by employing a PCR technique with the primer pairs ayr1S.c-F/ayr1S.c-R, ayr1C.j-F/ayr1C.j-R, 17β-hsdcl-F/17β-hsdcl-R, and g6p-F/g6p-R (Table 2), respectively. The amplified genes were then subcloned into BamHI/HindIII-digested sites of the E. coli-M. neoaurum shuttle vector pMV261 to form pMV261-ayr1S.c, pMV261-ayr1C.j, pMV261-17β-hsdcl, and pMV261-g6pdh. Next, MNR M3M electrocompetent cells were transformed with pMV261-ayr1S.c, pMV261-ayr1C.j, and pMV261-17β-hsdcl to generate the recombinant strains MNR M3M-ayr1S.c, MNR M3M-ayr1C.j, and MNR M3M-17β-hsdcl, respectively (52). According to previous reports (9, 28), the gene sequences of ayr1S.c and NADH kinase (GenBank gene identifier 855913) were optimized for ayr1op and pos5p for expression in M. neoaurum and then synthesized by Genewiz Biotechnology (China), and the insertion of the respective genes into the plasmids was conducted as described above. Next, MNR M3M electrocompetent cells were transformed with pMV261-ayr1op to generate the recombinant strain MNR M3M-ayr1op.
TABLE 2.
Bacterial strains, plasmids, and primers used in this studya
| Strain, plasmid, or primer | Significant property(ies) or sequence (5′–3′) | Reference, source, or purpose |
|---|---|---|
| Strains | ||
| M. neoaurum TCCC 11028 MNR M3ΔksdD::ksdDMNR (MNR M3M) | MNR M3, with ksdD deletion and replacement by ksdDMNR from MNR | 19 |
| MNR M3M-ayr1S.c | MNR M3M containing plasmid pMV261-ayr1S.c | This work |
| MNR M3M-ayr1C.j | MNR M3M containing plasmid pMV261-ayr1C.j | This work |
| MNR M3M-17β-hsdcl | MNR M3M containing plasmid pMV261-17β-hsdcl | This work |
| MNR M3M-ayr1op | MNR M3M containing plasmid pMV261-ayr1op | This work |
| MNR M3M-ayr1&g6p | MNR M3M containing plasmid pMV261-ayr1op+g6pdh | This work |
| MNR M3M-ayr1&p5p | MNR M3M containing plasmid pMV261-ayr1op+pos5p | This work |
| Plasmids | ||
| pMV261 | Shuttle vector of M. neoaurum and E. coli carrying the heat shock hsp60 promoter; Kanr | Gift from W. R. Jacobs, Jr. (Howard Hughes Medical Institute) |
| pMV261-ayr1S.c | pMV261 containing the ayr1S.c gene from S. cerevisiae; hsp60 Kanr | This work |
| pMV261-ayr1C.j | pMV261 containing the ayr1C.j gene from Cyberlindnera jadinii; hsp60 Kanr | This work |
| pMV261-17β-hsdcl | pMV261 containing the 17β-hsdcl gene from Cochliobolus lunatus; hsp60 Kanr | This work |
| pMV261-ayr1op | pMV261 containing the ayr1op gene optimized for expression in M. neoaurum; hsp60 Kanr | This work |
| pMV261-g6pdh | pMV261 containing the g6pdh gene from M. neoaurum; hsp60 Kanr | This work |
| pMV261-pos5p | pMV261 containing the pos5p gene from S. cerevisiae optimized for expression in M. neoaurum; hsp60 Kanr | This work |
| pMV261-ayr1op+g6pdh | pMV261 containing the ayr1op and g6pdh genes; hsp60 Kanr | This work |
| pMV261-ayr1op+pos5p | pMV261 containing the ayr1op and pos5p genes; hsp60 Kanr | This work |
| Primers | ||
| ayr1S.c-F | CGCGGATCCAATGTCGGAGTTACAGTCACAACCTA | ayr1S.c amplification |
| ayr1S.c-R | CCCAAGCTTCTAATCGTCCTTATTCTTCTGTTTCG | ayr1S.c amplification |
| ayr1C.j-F | CGCGGATCCAATGCCAGATCTCAGTACTCGTCAA | ayr1C.j amplification |
| ayr1C.j-R | CCCAAGCTTTCATTCGAGGTGCAAGTCAACA | ayr1C.j amplification |
| 17β-hsdcl-F | CGCGGATCCAATGCCACACGTAGAGAACGCA | 17β-hsdcl amplification |
| 17β-hsdcl-R | CCCAAGCTTTTATGCGGCACCACCATCTAG | 17β-hsdcl amplification |
| ayr1op-F | CGCGGATCCAATGTCCGAACTGCAGTCGCAGCCGA | ayr1op amplification |
| ayr1op-R | CCCAAGCTTTCAGTCGTCCTTATTCTTCTGCTTC | ayr1op amplification |
| p5p-F | CGCGGATCCATCGACCCTCGATTCGCACTC | p5p amplification |
| p5p-R | CCCAAGCTTTTAGTCATTATCGGTCTGCC | p5p amplification |
| g6p-F | CGCGGATCCAGTGCCCCGCCGAGACCTGAG | g6p amplification |
| g6p-R | CCCAAGCTTTCAGTCATCCGGCAGGTGCC | g6p amplification |
| g6p-SD-F (p5p-SD-F) | CCCAAGCTTTAAGTAGCGGGGTTGCCGTCACC | g6p (p5p) and SD amplification |
The restriction enzyme sites are underlined, and the “A” in boldface type was used to avoid a frameshift mutation.
To overexpress ayr1op and g6pdh or ayr1op and pos5p in M. neoaurum, primer pairs g6p-SD-F (containing a Shine-Dalgarno [SD] sequence for ribosome binding)/g6p-R and p5p-SD-F/p5p-R (Table 2) were used to amplify the g6pdh and pos5p fragments, respectively. These new g6pdh and pos5p fragments were digested using solely HindIII and then inserted into the HindIII site of plasmid pMV261-ayr1op to form the coexpression plasmids pMV261-ayr1op+g6pdh and pMV261-ayr1op+pos5p, respectively. Next, MNR M3M electrocompetent cells were transformed with pMV261-ayr1op+g6pdh and pMV261-ayr1op+pos5p to generate the recombinant strains MNR M3M-ayr1&g6p and MNR M3M-ayr1&p5p, respectively.
Microbial cultivation and transformation of PS by M. neoaurum.
The recombinant MNR M3M strains were cultivated in two stages: 36 h for the seed culture and 48 h for the induction culture. Seed medium and induction medium were prepared as described previously (47). Induction medium contained 0.5 g/liter PS, and whole cells were grown in 250-ml Erlenmeyer flasks containing 50 ml induction medium and shaken using a rotary shaker (140 rpm) at 30°C with an 8% (vol/vol) inoculum of the seed culture. The catalytic activity of the recombinant MNR M3M strain is defined as the molar yield of BD produced by the complete transformation of 3 g/liter PS by growing induction cells at 30°C. After the induction culture, the cells were harvested by centrifugation at 5,000 × g for 10 min at 4°C and washed three times with KH2PO4-NaOH buffers (50 mM, pH 7.2). Next, 70 g/liter cells was used for whole-cell biotransformation with 3 g/liter PS and HP-β-CD added at a molar ratio of 1:3, and 30 g/liter glucose was added as a cosubstrate at the beginning. The transformation was conducted at 30°C at 200 rpm and lasted 48 h. All experiments were performed in triplicate.
Next, whole cells (10 g/liter, 30 g/liter, 50 g/liter, 70 g/liter, and 90 g/liter [wet cell weight]) were resuspended with KH2PO4-NaOH buffers, and 1.0 g/liter, 3.0 g/liter, 5.0 g/liter, or 7.0 g/liter PS was added with HP-β-CD at a molar ratio of 1:1, 1:2, 1:3, or 1:4. Besides, the concentrations (10 g/liter, 20 g/liter, 30 g/liter, and 40 g/liter) and times (24 h, 48 h, 72 h, and 96 h) of glucose supplemented as a cosubstrate were also investigated. The transformation was conducted at 30°C at 200 rpm and lasted 48 h. All experiments were performed in triplicate.
Analytical methods.
The intracellular ratios of [NADPH]/[NADP+] were analyzed according to the manufacturer’s instructions for the [NADPH]/[NADP+] ratio assay kit (Solarbio, China). The intracellular ratios of [NADH]/[NAD+] were determined as described previously (53). The product-on-substrate yields (moles of BD per mole of PS converted) were analyzed by a high-performance liquid chromatography (HPLC) system, which was used to determine the concentrations of AD, ADD, and BD (38). One-milliliter samples were withdrawn periodically and extracted with isovolumetric ethyl acetate twice during bioconversion. The extracted samples were analyzed by reversed-phase HPLC with a Zorbax Eclipse Plus C18 column (5 μm, 4.6 by 150 mm) at a 254-nm UV detection wavelength at 30°C. The mobile phase was composed of methanol and water (70:30, vol/vol) with a flow rate of 1.0 ml/min (9). All experiments were performed in triplicate.
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China, Synthetic Biology Research (no. 2019YFA0905300); the National Natural Science Foundation of China (21978221); the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-KJGG-001-08); and the Innovative Research Team of Tianjin Municipal Education Commission (TD13-5013), Natural Science Foundation of Tianjin (18JCYBJC24700).
Contributor Information
Yanbing Shen, Email: shenyb@tust.edu.cn.
Min Wang, Email: minw@tust.edu.cn.
Maia Kivisaar, University of Tartu.
REFERENCES
- 1.Eisa M, El-Refai H, Amin M. 2016. Single step biotransformation of corn oil phytosterols to boldenone by a newly isolated Pseudomonas aeruginosa. Biotechnol Rep (Amst) 11:36–43. 10.1016/j.btre.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammed HH, Badawi ME, El-Tarabany MS, Rania M. 2016. Effects of boldenone undecylenate on growth performance, maintenance behaviour, reproductive hormones and carcass traits of growing rabbits. Pol J Vet Sci 19:245–251. 10.1515/pjvs-2016-0031. [DOI] [PubMed] [Google Scholar]
- 3.Florey KG. February 1959. Method of preparing 1-dehydrotestosterone and esters thereof. US patent 2,875,216.
- 4.Tang R, Shen Y, Wang M, Zhou H, Zhao Y. 2019. Highly efficient synthesis of boldenone from androst-4-ene-3,17-dione by Arthrobacter simplex and Pichia pastoris ordered biotransformation. Bioprocess Biosyst Eng 42:933–940. 10.1007/s00449-019-02092-y. [DOI] [PubMed] [Google Scholar]
- 5.Donova MV, Egorova OV, Nikolayeva VM. 2005. Steroid 17β-reduction by microorganisms—a review. Process Biochem 40:2253–2262. 10.1016/j.procbio.2004.09.025. [DOI] [Google Scholar]
- 6.Faramarzi MA, Yazdi MT, Ghostinroudi H, Amini M, Ghasemi Y, Jahandar H, Arabi H. 2006. Nostoc muscorum: a regioselective biocatalyst for 17-carbonyl reduction of androst-4-en-3,17-dione and androst-1,4-dien-3,17-dione. Ann Microbiol 56:253–256. 10.1007/BF03175014. [DOI] [Google Scholar]
- 7.Faramarzi MA, Zolfaghary N, Yazdi MT, Adrangi S, Rastegar H, Amini M, Badiee M. 2009. Microbial conversion of androst-1,4-dien-3,17-dione by Mucor racemosus to hydroxysteroid-1,4-dien-3-one derivatives. J Chem Technol Biotechnol 84:1021–1025. 10.1002/jctb.2128. [DOI] [Google Scholar]
- 8.Sarmah U, Roy MK, Singh HD. 1989. Steroid transformation by a strain of Arthrobacter oxydans incapable of steroid ring degradation. J Basic Microbiol 29:85–92. 10.1002/jobm.3620290206. [DOI] [Google Scholar]
- 9.Tang R, Shen Y, Xia M, Tu L, Luo J, Geng Y, Gao T, Zhou H, Zhao Y, Wang M. 2019. A highly efficient step-wise biotransformation strategy for direct conversion of phytosterol to boldenone. Bioresour Technol 283:242–250. 10.1016/j.biortech.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Shen X, Jain R, Wang J, Yuan Q, Yan Y. 2017. Establishing a novel biosynthetic pathway for the production of 3,4-dihydroxybutyric acid from xylose in Escherichia coli. Metab Eng 41:39–45. 10.1016/j.ymben.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 11.He K, Sun H, Song H. 2018. Engineering phytosterol transport system in Mycobacterium sp. strain MS136 enhances production of 9a-hydroxy-4-androstene-3,17-dione. Biotechnol Lett 40:673–678. 10.1007/s10529-018-2520-9. [DOI] [PubMed] [Google Scholar]
- 12.Donova MV, Dovbnya DV, Sukhodolskaya GV, Khomutov SM, Nikolayeva VM, Kwon I, Han K. 2005. Microbial conversion of sterol-containing soybean oil production waste. J Chem Technol Biotechnol 80:55–60. 10.1002/jctb.1156. [DOI] [Google Scholar]
- 13.Wei W, Wang FQ, Fan SY, Wei DZ. 2010. Inactivation and augmentation of the primary 3-ketosteroid-1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstene-3,17-dione. Appl Environ Microbiol 76:4578–4582. 10.1128/AEM.00448-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao K, Xu LQ, Wang FQ, Wei DZ. 2014. Characterization and engineering of 3-ketosteroid-Δ1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4-androstene-3,17-dione through the catabolism of sterols. Metab Eng 24:181–191. 10.1016/j.ymben.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Xiong L, Liu H, Xu L, Sun W, Wang F, Wei D. 2017. Improving the production of 22-hydroxy-23,24-bisnorchol-4-ene-3-one from sterols in Mycobacterium neoaurum by increasing cell permeability and modifying multiple genes. Microb Cell Fact 16:89. 10.1186/s12934-017-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrego S, Espinosa EE, Martı E, Fonseca M. 2000. Conversion of cholesterol to testosterone by Mycobacterium sp. MB-3638. Rev Cienc Biol 31:17–20. [Google Scholar]
- 17.Egorova OV, Nikolayeva VM, Sukhodolskaya GV, Donova MV. 2009. Transformation of C19-steroids and testosterone production by sterol-transforming strains of Mycobacterium sp. J Mol Catal B Enzym 57:198–203. 10.1016/j.molcatb.2008.09.003. [DOI] [Google Scholar]
- 18.Lo CK, Pan CP, Liu WH. 2002. Production of testosterone from phytosterol using a single-step microbial transformation by a mutant of Mycobacterium sp. J Ind Microbiol Biotechnol 28:280–283. 10.1038/sj/jim/7000243. [DOI] [PubMed] [Google Scholar]
- 19.Xie RL, Shen YB, Qin N, Wang YB, Su LQ, Wang M. 2015. Genetic differences in ksdD influence on the ADD/AD ratio of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 42:507–513. 10.1007/s10295-014-1577-2. [DOI] [PubMed] [Google Scholar]
- 20.Xiong L-B, Sun W-J, Liu Y-J, Wang F-Q, Wei D-Z. 2017. Enhancement of 9a-hydroxy-4-androstene-3,17-dione production from soybean phytosterols by deficiency of a regulated intramembrane proteolysis metalloprotease in Mycobacterium neoaurum. J Agric Food Chem 65:10520–10525. 10.1021/acs.jafc.7b03766. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Wang F, Lin L, Yao K, Wei D. 2012. Characterization and application of fusidane antibiotic biosynethsis enzyme 3-ketosteroid-Δ1-dehydrogenase in steroid transformation. Appl Microbiol Biotechnol 96:133–142. 10.1007/s00253-011-3855-5. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Cabezon L, Galan B, Garcia JL. 2017. Engineering Mycobacterium smegmatis for testosterone production. Microb Biotechnol 10:151–161. 10.1111/1751-7915.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao M, Zhang X, Rao Z, Xu M, Yang T, Li H, Xu Z, Yang S. 2016. Efficient testosterone production by engineered Pichia pastoris co-expressing human 17β-hydroxysteroid dehydrogenase type 3 and Saccharomyces cerevisiae glucose 6-phosphate dehydrogenase with NADPH regeneration. Green Chem 18:1774–1784. 10.1039/C5GC02353J. [DOI] [Google Scholar]
- 24.Cabrera JE, Paz JLP, Genti-Raimondi S. 2000. Steroid-inducible transcription of the 3β/17β-hydroxysteroid dehydrogenase gene (3β/17β-hsd) in Comamonas testosteroni. J Steroid Biochem Mol Biol 73:147–152. 10.1016/S0960-0760(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka M, Rohani LP, Yamamoto K, Wada M, Kawabata H, Kita K, Yanase H, Shimizu S. 1997. Enzymatic production of ethyl (R)-4-chloro-3-hydroxybutanoate: asymmetric reduction of ethyl 4-chloro-3-oxobutanoate by an Escherichia coli transformant expressing the aldehyde reductase gene from yeast. Appl Microbiol Biotechnol 48:699–703. 10.1007/s002530051118. [DOI] [PubMed] [Google Scholar]
- 26.Kataoka M, Kita K, Wada M, Yasohara Y, Hasegawa J, Shimizu S. 2003. Novel bioreduction system for the production of chiral alcohols. Appl Microbiol Biotechnol 62:437–445. 10.1007/s00253-003-1347-y. [DOI] [PubMed] [Google Scholar]
- 27.Bäumchen C, Roth AH, Biedendieck R, Malten M, Follmann M, Sahm H, Bringer-Meyer S, Jahn D. 2007. d-Mannitol production by resting state whole cell biotransformation of d-fructose by heterologous mannitol and formate dehydrogenase gene expression in Bacillus megaterium. Biotechnol J 2:1408–1416. 10.1002/biot.200700055. [DOI] [PubMed] [Google Scholar]
- 28.Pain J, Balamurali MM, Dancis A, Pain D. 2010. Mitochondrial NADH kinase, Pos5p, is required for efficient iron-sulfur cluster biogenesis in Saccharomyces cerevisiae. J Biol Chem 285:39409–39424. 10.1074/jbc.M110.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon DH, Kim MD, Lee TH, Oh YJ, Ryu YW, Seo JH. 2006. Elevation of glucose 6-phosphate dehydrogenase activity increases xylitol production in recombinant Saccharomyces cerevisiae. J Mol Catal B Enzym 43:86–89. 10.1016/j.molcatb.2006.06.014. [DOI] [Google Scholar]
- 30.Dong X, Chen X, Qian Y, Wang Y, Wang L, Qiao W, Liu L. 2017. Metabolic engineering of Escherichia coli W3110 to produce L-malate. Biotechnol Bioeng 114:656–664. 10.1002/bit.26190. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Caiyin Q, Zhao F, Wu Y, Wenyu L. 2018. Engineering Saccharomyces cerevisiae for enhanced production of protopanaxadiol with cofermentation of glucose and xylose. J Agric Food Chem 66:12009–12016. 10.1021/acs.jafc.8b04916. [DOI] [PubMed] [Google Scholar]
- 32.Lee WH, Kim JW, Park EH, Han NS, Kim MD, Seo JH. 2013. Effects of NADH kinase on NADPH-dependent biotransformation processes in Escherichia coli. Appl Microbiol Biotechnol 97:1561–1569. 10.1007/s00253-012-4431-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Wang X, Lu X, Ma C, Chen K, Ouyang P. 2019. Methanol fermentation increases the production of NAD(P)H-dependent chemicals in synthetic methylotrophic Escherichia coli. Biotechnol Biofuels 12:17. 10.1186/s13068-019-1356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Chen T, Ma X, Shen Z, Zhao X. 2011. Enhancement of riboflavin production with Bacillus subtilis by expression and site-directed mutagenesis of zwf and gnd gene from Corynebacterium glutamicum. Bioresour Technol 102:3934–3940. 10.1016/j.biortech.2010.11.120. [DOI] [PubMed] [Google Scholar]
- 35.Cai D, He P, Lu X, Zhu C, Zhu J, Zhan Y, Wang Q, Wen Z, Chen S. 2017. A novel approach to improve poly-γ-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Sci Rep 7:43404. 10.1038/srep43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauter M, Prokoph A, Kasprzak J, Becker K, Baronian K, Bode R, Kunze G, Vorbrodt HM. 2015. Coexpression of Lactobacillus brevis ADH with GDH or G6PDH in Arxula adeninivorans for the synthesis of 1-(R)-phenylethanol. Appl Microbiol Biotechnol 99:4723–4733. 10.1007/s00253-014-6297-z. [DOI] [PubMed] [Google Scholar]
- 37.Su L, Xu S, Shen Y, Xia M, Ren X, Wang L, Shang Z, Wang M. 2020. The sterol carrier hydroxypropyl-β-cyclodextrin enhances metabolism of phytosterols by Mycobacterium neoaurum. Appl Environ Microbiol 86:e00441-20. 10.1128/AEM.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen YB, Wang M, Wang YL, Luo JM. 2009. Effect of hydroxypropyl-β-cyclodextrin on the side-chain bioconversion of phytosterols by Mycobacterium sp. NRRL B-3683. Chem Eng Chin Univ 23:440–444. [Google Scholar]
- 39.Donova MV. 2007. Transformation of steroids by actinobacteria: a review. Appl Biochem Microbiol 43:1–14. 10.1134/S0003683807010012. [DOI] [PubMed] [Google Scholar]
- 40.Malaviya A, Gomes J. 2008. Androstenedione production by biotransformation of phytosterols. Bioresour Technol 99:6725–6737. 10.1016/j.biortech.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 41.Yao K, Wang FQ, Zhang HC, Wei DZ. 2013. Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng 15:75–87. 10.1016/j.ymben.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Guevara G, Flores YO, Fernandez de las Heras L, Perera J, Llorens JMN. 2019. Metabolic engineering of Rhodococcus ruber Chol-4: a cell factory for testosterone production. PLoS One 14:e0220492. 10.1371/journal.pone.0220492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García JL, Uhía I, Galán B. 2012. Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb Biotechnol 5:679–699. 10.1111/j.1751-7915.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szentirmai A. 1990. Microbial physiology of side chain degradation of sterols. J Ind Microbiol Biotechnol 6:101–115. [Google Scholar]
- 45.Xu LQ, Liu YJ, Yao K, Liu HH, Tao XY, Wang FQ, Wei DZ. 2016. Unraveling and engineering the production of 23,24-bisnorcholenic steroids in sterol metabolism. Sci Rep 6:21928. 10.1038/srep21928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donova MV, Nikolayeva VM, Dovbnya DV, Gulevskaya SA, Suzina NE. 2007. Methyl-β-cyclodextrin alters growth, activity and cell envelope features of sterol transforming mycobacteria. Microbiology (Reading) 153:1981–1992. 10.1099/mic.0.2006/001636-0. [DOI] [PubMed] [Google Scholar]
- 47.Shen YB, Wang M, Li HN, Wang YB, Luo JM. 2012. Influence of hydroxypropyl-β-cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 39:1253–1259. 10.1007/s10295-012-1130-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang D, Zhang R, Zhang J, Chen L, Zhao C, Dong W, Zhao Q, Wu Q, Zhu D. 2014. Engineering a hydroxysteroid dehydrogenase to improve its soluble expression for the asymmetric reduction of cortisone to 11β-hydrocortisone. Appl Microbiol Biotechnol 98:8879–8886. 10.1007/s00253-014-5967-1. [DOI] [PubMed] [Google Scholar]
- 49.Borrego S, Niubó E, Ancheta O, Espinosa ME. 2000. Study of the microbial aggregation in Mycobacterium using image analysis and electron microscopy. Tissue Cell 32:494–500. 10.1016/S0040-8166(00)80005-1. [DOI] [PubMed] [Google Scholar]
- 50.Dlugovitzky DG, Fontela MS, Martinel Lamas DJ, Valdez RA, Romano MC. 2015. Mycobacterium smegmatis synthesizes in vitro androgens and estrogens from different steroid precursors. Can J Microbiol 61:451–455. 10.1139/cjm-2015-0025. [DOI] [PubMed] [Google Scholar]
- 51.Liu WH, Lo CK. 1997. Production of testosterone from cholesterol using a single-step microbial transformation of Mycobacterium sp. J Ind Microbiol Biotechnol 19:269–272. 10.1038/sj.jim.2900456. [DOI] [PubMed] [Google Scholar]
- 52.Papavinasasundaram KG, Colston MJ, Davis EO. 1998. Construction and complementation of a recA deletion mutant of Mycobacterium smegmatis reveals that the intein in Mycobacterium tuberculosis recA does not affect RecA function. Mol Microbiol 30:525–534. 10.1046/j.1365-2958.1998.01083.x. [DOI] [PubMed] [Google Scholar]
- 53.Su L, Shen Y, Zhang W, Gao T, Shang Z, Wang M. 2017. Cofactor engineering to regulate NAD+/NADH ratio with its application to phytosterols biotransformation. Microb Cell Fact 16:182. 10.1186/s12934-017-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]