Abstract
Background
Major depressive disorder (MDD) is highly debilitating, difficult to treat, has a high rate of recurrence, and negatively impacts the individual and society as a whole. One potential treatment for MDD is n‐3 polyunsaturated fatty acids (n‐3PUFAs), also known as omega‐3 oils, naturally found in fatty fish, some other seafood, and some nuts and seeds. Various lines of evidence suggest a role for n‐3PUFAs in MDD, but the evidence is far from conclusive. Reviews and meta‐analyses clearly demonstrate heterogeneity between studies. Investigations of heterogeneity suggest different effects of n‐3PUFAs, depending on the severity of depressive symptoms, where no effects of n‐3PUFAs are found in studies of individuals with mild depressive symptomology, but possible benefit may be suggested in studies of individuals with more severe depressive symptomology. Hence it is important to establish their effectiveness in treating MDD. This review updates and incorporates an earlier review with the same research objective (Appleton 2015).
Objectives
To assess the effects of n‐3 polyunsaturated fatty acids (also known as omega‐3 fatty acids) versus a comparator (e.g. placebo, antidepressant treatment, standard care, no treatment, wait‐list control) for major depressive disorder (MDD) in adults.
Search methods
We searched the Cochrane Central Register of Controlled trials (CENTRAL), Ovid MEDLINE, Embase and PsycINFO together with trial registries and grey literature sources (to 9 January 2021). We checked reference lists and contacted authors of included studies for additional information when necessary.
Selection criteria
We included studies in the review if they: used a randomised controlled trial design; provided n‐3PUFAs as an intervention; used a comparator; measured depressive symptomology as an outcome; and were conducted in adults with MDD. Primary outcomes were depressive symptomology (continuous data collected using a validated rating scale) and adverse events. Secondary outcomes were depressive symptomology (dichotomous data on remission and response), quality of life, and non‐completion of studies.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane. We assessed the certainty of the evidence using GRADE criteria.
Main results
The review includes 35 relevant studies: 34 studies involving a total of 1924 participants investigated the impact of n‐3PUFA supplementation compared to placebo, and one study involving 40 participants investigated the impact of n‐3PUFA supplementation compared to antidepressant treatment.
For the placebo comparison, n‐3PUFA supplementation resulted in a small to modest benefit for depressive symptomology, compared to placebo: standardised mean difference (SMD) (random‐effects model) −0.40 (95% confidence interval (CI) −0.64 to −0.16; 33 studies, 1848 participants; very low‐certainty evidence), but this effect is unlikely to be clinically meaningful. An SMD of 0.40 represents a difference between groups in scores on the HDRS (17‐item) of approximately 2.5 points (95% CI 1.0 to 4.0), where the minimal clinically important change score on this scale is 3.0 points. The confidence intervals include both a possible clinically important effect and a possible negligible effect, and there is considerable heterogeneity between studies. Sensitivity analyses, funnel plot inspection and comparison of our results with those of large well‐conducted trials also suggest that this effect estimate may be biased towards a positive finding for n‐3PUFAs. Although the numbers of individuals experiencing adverse events were similar in intervention and placebo groups (odds ratio (OR) 1.27, 95% CI 0.99 to 1.64; 24 studies, 1503 participants; very low‐certainty evidence), the confidence intervals include a small decrease to a modest increase in adverse events with n‐3PUFAs. There was no evidence for a difference between n‐3PUFA and placebo groups in remission rates (OR 1.13, 95% CI 0.74 to 1.72; 8 studies, 609 participants, low‐certainty evidence), response rates (OR 1.20, 95% CI 0.80 to 1.79; 17 studies, 794 participants; low‐certainty evidence), quality of life (SMD −0.38 (95% CI −0.82 to 0.06), 12 studies, 476 participants, very low‐certainty evidence), or trial non‐completion (OR 0.92, 95% CI 0.70 to 1.22; 29 studies, 1777 participants, very low‐certainty evidence). The evidence on which these results are based was also very limited, highly heterogeneous, and potentially biased.
Only one study, involving 40 participants, was available for the antidepressant comparison. This study found no differences between treatment with n‐3PUFAs and treatment with antidepressants in depressive symptomology (mean difference (MD) −0.70, 95% CI −5.88 to 4.48), rates of response to treatment (OR 1.23, 95% CI 0.35 to 4.31), or trial non‐completion (OR 1.00, 95% CI 0.21 to 4.71). Confidence intervals are however very wide in all analyses, and do not rule out important beneficial or detrimental effects of n‐3PUFAs compared to antidepressants. Adverse events were not reported in a manner suitable for analysis, and rates of depression remission and quality of life were not reported.
Authors' conclusions
At present, we do not have sufficient high‐certainty evidence to determine the effects of n‐3PUFAs as a treatment for MDD. Our primary analyses may suggest a small‐to‐modest, non‐clinically beneficial effect of n‐3PUFAs on depressive symptomology compared to placebo; however the estimate is imprecise, and we judged the certainty of the evidence on which this result is based to be low to very low. Our data may also suggest similar rates of adverse events and trial non‐completion in n‐3PUFA and placebo groups, but again our estimates are very imprecise. Effects of n‐3PUFAs compared to antidepressants are very imprecise and uncertain. More complete evidence is required for both the potential positive and negative effects of n‐3PUFAs for MDD.
Plain language summary
Omega‐3 fatty acids for depression in adults
Why is this review important?
Major depressive disorder (MDD) is characterised by depressed mood or a markedly decreased pleasure or interest in all activities, or both. It has negative impacts on the individual and on society, often over the long term. One possible treatment for MDD is n‐3 polyunsaturated fatty acids (n‐3PUFAs), also known as omega‐3 oils, naturally found in fatty fish, in some other seafood and in some nuts and seeds. Various lines of evidence suggest that n‐3PUFAs may impact on depressive symptoms, but a lot of studies have different findings, making it difficult to draw conclusions.
Who will be interested in this review?
Health professionals, including general practitioners, mental health and psychiatric specialists; individuals with MDD; and the people around them.
What questions does this review aim to answer?
Do n‐3PUFAs, compared to an alternative, have an effect on depressive symptoms, negative side effects, rates of recovery, quality of life, and rates of study non‐completion, in individuals with MDD?
Which studies were included in the review?
This review is an update of earlier work (Appleton 2015), using the same methods. We searched scientific databases for all randomised controlled trials in adults with MDD, where individuals received either n‐3PUFAs or an alternative, that were completed up to January 2021.
We have included 35 relevant studies: 34 of them involving 1924 people compared the effects of n‐3PUFAs with those of placebo, and one study involving 40 people compared the effects of n‐3PUFAs with those of antidepressants. All studies were of direct relevance to our review, but we considered the certainty of the evidence to be low to very low.
What does the evidence from the review tell us?
At present, we do not have enough high quality evidence to determine the effects of n‐3PUFAs as a treatment for MDD. We found a small‐to‐modest positive effect of n‐3PUFAs compared to placebo, but the size of this effect is unlikely to be meaningful to people with MDD, and we considered the evidence to be of low or very low certainty, with many differences between studies. There was also insufficient high quality evidence to determine the effects of n‐3PUFAs on negative side effects or numbers not completing studies.
What should happen next?
We need more evidence, particularly to explain the differences between study findings, e.g. by looking at individuals who may or may not benefit from n‐3PUFAs. Future studies should also compare n‐3PUFAs with usual antidepressant treatment, and investigate the way these treatments may work.
Summary of findings
Summary of findings 1. n3PUFAs compared to placebo for depression in adults.
| n3PUFAs compared to placebo for depression in adults | ||||||
| Patient or population: Adult patients with major depressive disorder (MDD) Settings: Clinical and community settings Intervention: n3PUFAs Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | N3PUFAs | |||||
| Depressive symptomology (continuous) HDRS where possible; higher scores indicate greater symptomology Follow‐up: 4 ‐ 16 weeks | The mean depressive symptomology (continuous) in the intervention groups was 0.40 standard deviations lower (0.64 to 0.16 lower). This represents a small to modest difference between groups, equivalent to a HDRS depressive symptomology score of 2.5 (1.0 ‐ 4.0) | 1848 (33 studies) | ⊕⊝⊝⊝ very lowa,b,c,d,e | SMD ‐0.40 (‐0.64 to 0.16) | ||
| Adverse events Study reports Follow‐up: 0 ‐ 16 weeks | Study population | OR 1.27 (0.99 to 1.64) | 1503 (24 studies) | ⊕⊝⊝⊝ very lowc,d,e,f,g | ‐ | |
| 452 per 1000 | 512 per 1000 (450 to 575) | |||||
| Moderate | ||||||
| 250 per 1000 | 297 per 1000 (248 to 353) | |||||
| Depressive symptomology (dichotomous ‐ remission) Depressive symptomology rating scale as used by authors Follow‐up: 4‐16 weeks | Study population | OR 1.13 (0.74 to 1.72) | 609 (8 studies) | ⊕⊕⊝⊝ lowc,d,f,g,h,i | ‐ | |
| 329 per 1000 | 356 per 1000 (266 to 457) | |||||
| Moderate | ||||||
| 174 per 1000 | 192 per 1000 (135 to 266) | |||||
| Depressive symptomology (dichotomous ‐ response) Depressive symptomology rating scale as used by authors Follow‐up: 4‐16 weeks | Study population | OR 1.20 (0.80 to 1.79) | 794 (17 studies) | ⊕⊕⊝⊝ lowc,d,f,g,h,i | ‐ | |
| 445 per 1000 | 490 per 1000 (391 to 589) | |||||
| Moderate | ||||||
| 235 per 1000 | 269 per 1000 (197 to 355) | |||||
| Quality of life Validated quality of life scales as used by authors, CGI (7‐point scale) where possible, higher scores indicate poorer quality of life Follow‐up: 4 ‐ 16 weeks | The mean quality of life in the intervention groups was 0.38 standard deviations lower (0.82 lower to 0.06 higher). This represents a small to modest difference between groups, equivalent to a CGI score of 0.38 (95% CI 0.06 to 0.82) | 476 (12 studies) | ⊕⊝⊝⊝ very lowc,d,f,g,i,j | SMD ‐0.38 (‐0.82 to 0.06) | ||
| Trial non‐completion Study reports Follow‐up: 0 ‐ 16 weeks | Study population | OR 0.92 (0.70 to 1.22) | 1777 (29 studies) | ⊕⊝⊝⊝ very lowc,d,e,f,g | ‐ | |
| 162 per 1000 | 151 per 1000 (119 to 191) | |||||
| Moderate | ||||||
| 200 per 1000 | 187 per 1000 (149 to 234) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aQuality of the evidence downgraded by one level for study limitations. Judgements of high risk of bias in all studies, and different effects when comparing analyses including only those studies with judgements of low risk of selection bias (allocation concealment), performance bias (blinding of participants and personnel), or attrition bias (incomplete outcome data), and analyses including all studies. bQuality of the evidence downgraded by one level for inconsistency. Evidence of high heterogeneity between studies. Heterogeniety is not well explained by the subgroup analyses. cNo serious concerns regarding indirectness. All evidence used is directly relevant to the research question dQuality of the evidence downgraded by one level for imprecision. Moderate to wide confidence intervals eQuality of the evidence downgraded by one level for publication bias. Strong suspicion of publication bias based on visual inspection of the funnel plot. fQuality of the evidence downgraded by one level for study limitations. Judgements of high risk of bias in all studies included in this analysis gNo serious concerns regarding inconsistency. Limited evidence of heterogeneity between studies hSelected studies only were available to be included in this analysis iFunnel plots were not created for this analysis due to low numbers of studies involved. jQuality of the evidence downgraded by one level for inconsistency. High heterogeneity between studies.
Background
Description of the condition
Major depressive disorder (MDD) is characterised by: depressed mood; markedly diminished pleasure or interest in all activities; significant weight loss or weight gain, or decrease or increase in appetite; insomnia or hypersomnia; psychomotor agitation or retardation; fatigue or lethargy; feelings of worthlessness or inappropriate guilt; disruptions to concentration and decision‐making; and recurrent thoughts of death (APA 2013). Diagnosis is achieved by: the presence of four or more symptoms (as above), plus depressed mood or markedly diminished pleasure or interest in all activities, for a consecutive period of two weeks; significant distress or impairment in functioning as a result of symptoms; and an inability to attribute symptoms to the physiological effects of a substance or another medical condition (APA 2013). MDD can be highly debilitating; can affect all areas of an individual's life; can be difficult to treat, with a high rate of recurrence; and often exists in combination with other conditions and disorders, such as cardiovascular disease and anxiety disorders (APA 2013). Recent estimates from the Global Burden of Disease Study 2017 (GBD 2018) suggest global prevalence rates for MDD of 163 million cases in 2017, and global incidence rates of 242 million cases, resulting in 33 million years lived with disability (YLDs) globally, an increase of 12.6% since 2007. In 2017, depressive disorders were the third leading cause of YLDs globally, with a 14.3% increase in the number of all‐age YLDs since 2007 (GBD 2018). Given this increasing trend, there is an urgent need for effective treatments and strategies for prevention.
Description of the intervention
One suggested potential treatment for MDD is n‐3 polyunsaturated fatty acids (n‐3PUFAs), also known as omega‐3 fatty acids.
n‐3PUFAs are a family of polyunsaturated fatty acids, named as such because of the positioning of the first double carbon bond on the third atom from the methyl end of the acyl chain. All members of the family are derived from parent fatty acid 18:3n‐3 (alpha‐linolenic acid (ALA)), via desaturation and elongation. ALA, however, can not be synthesised by humans, and thus must be obtained from the diet (Haag 2003; Ruxton 2005). Longer‐chain n‐3PUFAs can be formed in humans, but biological conversion is slow and inefficient, making diet an important source for these fatty acids too (Ma 1995). Dietary sources of ALA include certain nuts and seeds, such as walnuts, flaxseed and rapeseed (canola) oil. Dietary sources of the longer n‐3PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) include fatty fish, some white fish, shellfish and other seafood such as seaweed, and certain eggs and animal products, depending on the animal's diet (BNF 1999; James 2000; Ruxton 2005; Simopolous 1999).
Links between n‐3PUFAs and MDD were suggested following recognition of a reduction in the dietary consumption of n‐3PUFAs in recent decades and an increase in depressive conditions (Simopolous 1999). Coupled with the reduction in n‐3PUFA intakes, intakes of n‐6 long chain polyunsaturated fatty acids (n‐6PUFAs) have also increased. Closely related to n‐3PUFAs, n‐6PUFAs (named from the positioning of the first double bond on the sixth carbon atom from the methyl end of the acyl chain) are derived from parent essential fatty acid 18:2n‐6 (linoleic acid (LA)), and for synthesis, share the same desaturases and elongases as n‐3PUFAs. n‐3PUFAs and n‐6PUFAs thus compete for synthesis from their parent fatty acids. Dietary sources of LA and n‐6PUFAs include plant and vegetable seeds and oils, as found in margarines and many processed foods (James 2000; Simopolous 1999). Our traditional diet is thought to have contained approximately equal amounts of energy from n‐3PUFAs and n‐6PUFAs (Simopolous 1999). By comparison, a current western diet is estimated to contain approximately five to 20 times more energy from n‐6PUFAs than from n‐3PUFAs (Gregory 2000; Simopolous 1999).
Early work investigating population consumption levels of n‐3PUFAs and n‐3PUFA‐rich foods, such as fish, suggested links with population levels of MDD and various psychiatric conditions (Hibbeln 1998; Noaghiul 2003; Peet 2004), and studies since have found similar associations. Within countries, n‐3PUFA intakes have been negatively associated with depressive illness (e.g. Silvers 2002; Tanskanen 2001). In clinical studies, low levels of n‐3PUFAs have been found in individuals diagnosed with MDD (e.g. Edwards 1998; Peet 1998) and depressive disorders (e.g. Garland 2007), and in individuals reporting high levels of depressed mood (e.g. Mamalakis 2002; Mamalakis 2006), compared to controls. Continuous relationships between n‐3PUFA status and depressive symptoms have also been found (e.g. Edwards 1998). In randomised controlled trials (RCTs), beneficial effects of supplementation with n‐3PUFAs compared to placebo have been reported for MDD (e.g. Nemets 2002; Su 2003) and depressive disorders (e.g. Frangou 2006; Stoll 1999).
How the intervention might work
The positive effects of n‐3PUFAs on depressive illness are thought to occur as a result of changes to cell membrane structure and function, impacting particularly on cell communication, neurotransmitter activities and inflammatory processes (Haag 2003; James 2000; Ruxton 2005). Further details are provided in Appendix 1. Disrupted and abnormal cell signalling, neurotransmitter system activities and inflammatory processes have all been implicated in MDD (Parker 2006b; Stahl 2008).
Why it is important to do this review
n‐3PUFAs are known to be important in brain development and function, and have been linked to depression in a variety of studies (see Appendix 2). Not all studies, however, report beneficial effects (see Appendix 2), and reviews and meta‐analyses clearly demonstrate variability between studies (e.g. Appleton 2006; Appleton 2008b; Appleton 2010; Lin 2007; Parker 2006b; Smith 2011; Stahl 2008). Early meta‐analyses revealed some small benefit of n‐3PUFAs for depressive disorders (Appleton 2006; Lin 2007), but investigations of the heterogeneity also suggested different effects of n‐3PUFAs, depending primarily on severity of depressive symptoms at baseline (Appleton 2010). Sensitivity analyses based on severity of depressive symptoms at baseline suggest no benefits of n‐3PUFAs for individuals with mild depressive symptoms or without a diagnosis of depression, but provide some evidence of benefits in individuals with severe depressive symptoms or with depressive diagnoses (Appleton 2010). These findings suggest a possible benefit of n‐3PUFAs for MDD.
Many reviews investigating a role for n‐3PUFAs in depressive disorders have now been conducted (e.g. Appleton 2006; Appleton 2010; Bae 2018; Bai 2020; Chambergo‐Michilot 2021; Grosso 2014; Liao 2019; Lin 2012; Martins 2011; Schefft 2017), and reviews of reviews are also available (Firth 2019; Haller 2019; Nasir 2019). Our earlier review (Appleton 2015) suggested a small‐to‐modest non‐clinically beneficial effect of n‐3PUFAs compared to placebo, and many other reviews suggest similar effect sizes. Many reviews, however, use a very broad definition of depression to include a variety of depressive disorders and conditions, and/or include studies that vary in severity of depressive symptomology to include studies in individuals with mild depression to MDD. Definitions of MDD also vary. Many reviews also focus on specific populations, e.g. older adults, individuals with adjunctive therapy. This review considers solely major or unipolar depressive disorder. This review also focuses on adults, regardless of comorbid conditions and therapeutic status.
Some reviews also consider a range of nutritional or complementary therapies for MDD and other depressive disorders (e.g. Firth 2019; Haller 2019; Schefft 2017), and reviews of other treatments for MDD and other depressive disorders are also available. A recent search of the Cochrane Library revealed 678 completed reviews or reviews in progress on treating or preventing depression. Most of these reviews investigate pharmacological (e.g. antidepressant) or psychological (e.g. cognitive behavioural therapy) treatments for depressive conditions, or depressive components of other conditions, e.g. overweight, or focus on specific clinical populations, e.g. people with stroke or people with diabetes mellitus. Ten of these reviews include mention of n‐3PUFAs. Our earlier review (Appleton 2015) focused solely on n‐3PUFAs as a treatment for MDD, while all other reviews include n‐3PUFAs for the treatment or prevention of other depressive or psychiatric conditions, and one review includes n‐3PUFAs as a comparator for treatment with pharmacological antidepressant fluoxetine for overweight / obesity (Serralde‐Zuñiga 2019).
Objectives
To assess the effects of n‐3 polyunsaturated fatty acids (n‐3PUFAs) (also known as omega‐3 fatty acids) versus a comparator (e.g. placebo, antidepressant treatment, standard care, no treatment, wait‐list control) for major depressive disorder in adults.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) were eligible, as we consider this to be the best research design for assessing the efficacy of an intervention. We included all suitable RCTs, regardless of quality, but we also recorded measures of risk of bias. Cross‐over and cluster‐RCTs were eligible for inclusion. We excluded observational and case‐control studies.
Types of participants
Participant characteristics
Trials involving adults (18 years and over) were suitable for inclusion. We included trials regardless of other participant demographics (e.g. gender, country of residence). If a trial included children and adults, we included only the data from the adult subgroup, and only if the subgroup was defined in publications, either through mention of a subgroup as part of the method, in details of the Participant characteristics, or through the use of subgroup analyses. If data from both children and adults were mixed, we did not include these trials or these data.
Diagnosis
Our primary interest was in trials that enrolled participants with a diagnosis of major or unipolar depressive disorder. We therefore included trials that specified the study of "major" or "unipolar" depressive disorder, given by a trained professional, using a recognized diagnostic schedule. We recognize, however, that not all participants with debilitating depressive symptomology will have a formal diagnosis, and that the language used to report such diagnoses may vary by culture and era. To ensure no trials were missed, we also considered trials that included individuals with a diagnosis of "depression" or "depressive disorder", given by a trained professional, using a recognised diagnostic schedule, where antidepressant treatment was considered appropriate and where an alternative depressive disorder was not specified; and we considered trials that used a validated rating scale to specify high levels of depressive symptomology. Where MDD was defined using a validated rating scale, we used established cut‐off values to describe MDD. These cut‐off values were: Beck Depression Inventory (BDI) (Beck 1987): 17 or more of 63; Geriatric Depression Screening Scale (GDS) (Yesvage 1983): 9 or more of 15; Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983): 15 or more of 21; Hamilton Depression Rating Scale (HDRS) (Hamilton 1960): 17 or more of 54; Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979): 30 or more of 60; Patient Health Questionnaire (PHQ9) (Kroenke 2001): 15 or more of 27. Acceptable exceptions were where authors used an alternative cut‐off value and classified this explicitly as MDD or equivalent to a depressive diagnosis. Similarly, if these cut‐off values were used specifically to classify "mild" or "moderate" depression, these trials were considered unsuitable for inclusion in the review. These exceptions were made to account for differences between cultures or eras in appropriate cut‐off values for MDD. Cut‐offs were identified in advance of data extraction to reduce bias and ensure consistency between data extractors. If trials reported a diagnosis and use of a cut‐off value on a rating scale, either diagnosis or rating score were considered acceptable to warrant inclusion; we did not require both a diagnosis and a score above a cut‐off for individuals in these trials. The use of differing methods to define MDD was intended to allow the capture and inclusion of all trials which investigate a debilitating level of depressive symptomology, regardless of the specific language or diagnostic methods used. Alternative diagnoses of dysthymia were also considered, due to the similarity between diagnoses of MDD and dysthymia (APA 2013).
We excluded trials that enrolled participants with a primary diagnosis of an alternative depressive disorder, e.g. bipolar disorder, postpartum depression (APA 2013), or any other psychiatric condition. We excluded trials that describe a diagnosis of MDD that was given only during or in relation to pregnancy. We also excluded trials that specifically stated study of "mild" or "moderate" depression.
Trials were considered if the overall research population had a suitable depressive diagnosis, or if a subgroup of the overall population with a suitable depressive diagnosis were identified. If a subgroup was used, we included only the data from the subgroup in the review, and only if the subgroup was defined in publications, either through mention of a subgroup as part of the method, in details of the Participant Characteristics, or through the use of subgroup analyses. If data from individuals both with and without a suitable depressive diagnosis were mixed, we did not include these trials or these data.
Comorbidities
We included trials regardless of the inclusion of participants with other comorbid conditions (physical conditions, e.g. congestive heart disease, or psychiatric conditions, e.g. anxiety). The inclusion of trials involving participants with comorbid conditions was due to the high likelihood of existing comorbidities in the MDD population (APA 2013), and a desire to make the review as generalisable as possible. We investigated effects due to existing comorbidities in subgroup analyses.
Adjunctive therapy
We also included trials regardless of participant use of adjunctive therapy. We included trials that recruited participants with concomitant adjunctive therapy due to the high likelihood of adjunctive therapy use in the MDD population (APA 2013), and a desire to make the review as generalisable as possible. We recorded adjunctive therapies as part of the review, and also investigated adjunctive therapy use in subgroup analyses.
Setting
We included trials regardless of setting, provided they used a clinical assessment or depressive rating score, as above.
Types of interventions
Experimental intervention
We included trials if they used an exposure of n‐3PUFAs as the sole or as an adjunctive therapy. We included trials regardless of: the type and source of n‐3PUFA provided (pure ALA, EPA, DHA or any combination of these, fish, flaxseed, rapeseed, etc.); the dose of n‐3PUFA or duration of supplementation; and the mode of provision (i.e. supplement capsules, supplemented foods). We kept records of these differences, and used sensitivity analyses to investigate effects based on n‐3PUFA type, and duration of supplementation. We included trials if details of the type of n‐3PUFA, dose, and ratio were not available, as mechanisms for action remain unknown. Where trials included adjunctive therapy, these studies were included only if the adjunctive therapy did not systematically differ between experimental and comparator groups, i.e. trials were included if n‐3PUFAs were provided in addition to usual medication, but trials were not included if n‐3PUFAs were provided alongside other bioactive agents and neither n‐3PUFAs nor the bioactive agents were provided as the comparator. We accepted trials with a 'lead‐in' phase to allow for spontaneous remission or placebo responding in participants, and recorded use of the 'lead‐in' phase.
Comparator intervention
We included trials regardless of the comparator used, but there had to be a comparator. We counted wait‐list controls, no treatment or standard care as possible comparators, provided randomisation and the completion of outcomes also occurred, as required for a randomised controlled trial. We recorded all comparators. We conducted separate analyses, depending on the comparator used, to allow clear combination of like with like.
Types of outcome measures
We included trials that met the above criteria, regardless of whether they reported on all of the following outcomes.
Primary outcomes
1. Depressive symptomology (continuous data): We assessed depressive symptomology using any continuous validated measure. The most commonly‐used validated rating scales are the Beck Depression Inventory (BDI) (Beck 1987), the Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979), and the Hamilton Depression Rating Scale (HDRS) (Hamilton 1960), but we also included trials using other scales.
2. Adverse events: We recorded measures of adverse events where possible. We recorded the number and type (e.g. gastrointestinal, psychiatric) of adverse events experienced, as reported in trials. We used the number of individuals experiencing adverse events, rather than the number of events, in analyses where possible. Where adverse events were not reported, we recorded this.
Secondary outcomes
3. Depressive symptomology (dichotomous data): We also assessed depressive symptomology using remission or response (improvement) as assessed using clinical diagnoses by a trained professional or a validated rating scale, where provided.
4. Quality of life (continuous data): We assessed quality of life using any continuous validated measure.
5. Trial non‐completion: We recorded the number of individuals leaving each trial early, and the reasons for this.
Timing of outcome assessment
Where trials used multiple time points, we used only data from the longest follow‐up period for analyses. Previous work suggests that effects are likely to increase over time (Calder 2003; Ruxton 2005). In all trials, depressive and quality‐of‐life outcomes were assessed at prespecified time points (as detailed in the Characteristics of included studies tables), while adverse events and trial withdrawal were considered possible at any time following randomisation.
Search methods for identification of studies
We identified suitable trials for inclusion by searching databases, international trial registers and published review articles, and by contacting authors of published trials.
Electronic searches
We ran searches on the following bibliographic databases using relevant keywords, subject headings (controlled vocabularies) and search syntax appropriate to each resource (Appendix 3).
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR) (all available years).
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 1) in the Cochrane Library (searched 9 January 2021).
MEDLINE Ovid (2015 to 9 January 2021).
Embase Ovid (2015 to 9 January 2021).
PsycINFO Ovid (2015 to 9 January 2021).
Searches of the main bibilographic databases were conducted for the previous version of this review via the CCMDCTR (all years to May 2015) (Appendix 4). An additional search of CINAHL was conducted to May 2013 only.
We also searched international trial registries via the World Health Organization’s trials portal (ICTRP) and ClinicalTrials.gov, to identify unpublished or ongoing trials.
There were no restrictions by date, language or publication status applied to the overall searches. We ran our most recent database searches on 9 January 2021.
Searching other resources
We checked the reference lists of all included trials and relevant reviews to identify additional trials missed from the electronic searches. We also contacted authors of included trials for information on unpublished or ongoing trials, or to request additional trial data.
Data collection and analysis
We downloaded search results into Covidence (Covidence 2021). We downloaded selected trials into Review Manager 5.4 (RevMan 2020). We detail the number of search results at each stage of the search and selection process in the Results section.
Selection of studies
Two review authors (from PV, SD or RP) independently screened the titles and abstracts of all trials identified by the search, and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the potentially‐relevant full‐text trial reports/publications, and two review authors (PV, RP) independently screened the full text, identified trials for inclusion, and recorded reasons for exclusion of the ineligible trials. We resolved disagreements through discussion or consultation with a third review author (KA). We identified and excluded duplicate records, and we collated multiple reports that related to the same trial, so that each trial rather than each report was the unit of interest in the review. We included in the list and obtained titles or abstracts which were potentially relevant, but where relevance was not clear. We obtained and translated articles in foreign languages. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram.
Data extraction and management
We used a data collection form to extract all trial characteristics and outcome data. We developed the form specifically for this work, and piloted it on two trials in the review, prior to use for all trials. Two review authors (from KA, PV, HS or RP) extracted the following characteristics and outcome data from included trials:
Methods: research design, total duration of trial, details of any 'lead‐in' period, use of several trial centres, trial location, trial setting, and date of trial.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria, and exclusion criteria, withdrawals.
Interventions: intervention, comparator, concomitant therapies, and comorbidities.
Outcomes: primary and secondary outcomes, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Data were extracted for each relevant study from each trial. We defined 'a study' as any single comparison between n‐3PUFAs and comparator, thus a trial may contain one or more independent studies. Data were extracted separately for each independent study. Where multiple reports of the same trial were available, we extracted data from all reports. We resolved discordances by independent extraction and then by discussion with a third review author. We also contacted corresponding authors directly for relevant information.
We have noted data that were not usable for analyses in the Characteristics of included studies tables (Notes section). Two review authors (KA, PV) transferred all data into the Review Manager 5.4 file (RevMan 2020), and double‐checked that we had entered data correctly by comparing the data presented in the review with the trial reports.
Main comparisons
n‐3PUFAs versus comparator. Analyses are conducted by comparator type.
Assessment of risk of bias in included studies
Three review authors (from KA, PV, HS or RP) independently assessed the risks of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. We assessed the risks of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as high, low or unclear risk, using the criteria provided in Appendix 5, and have provided a supporting statement from the trial reports together with a justification for our judgement in each risk of bias table. Direct quotes from publications are given in quotation marks. Comments without quotation marks represent our summaries of the available evidence. The criteria for judging risk of bias were agreed in advance of our earlier review (Appleton 2015) by select review authors, following some experience of the literature, but prior to formal data extraction. We have summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we have noted this in the risk of bias table. We have taken account when considering treatment effects of the risk of bias for the studies that contribute to each outcome.
Measures of treatment effect
Continuous data
We recorded depressive symptomology and quality of life using all scales as used in each study, after ensuring comparable direction. We conducted analyses of data from only one scale per study. For depressive symptomology, we used the scale most commonly used in all studies (the HDRS: Hamilton 1960), where possible. All depression scales are orientated to higher scores demonstrating increased depressive symptomology. For quality of life, we used the scale most commonly used in all studies reporting quality of life (the CGI: Guy 1976), where possible. The CGI is orientated such that higher scores demonstrate poorer functioning. Where quality of life was measured using a measure where higher scores denote better quality of life, scores were reversed for analysis.
We collected continuous data in the form of N, mean, and standard deviation per intervention group at baseline and at the end of each intervention, as required for meta‐analysis. If data were only provided in other forms, e.g. as medians, change from baseline, we contacted trial authors and requested appropriate data.
We analysed continuous data as a standardised mean difference (SMD) with a 95% confidence interval (CI). We undertook meta‐analyses only where this was meaningful, i.e. where treatments, participants and the underlying clinical question were similar enough for pooling to make sense. Where multiple arms were reported in a single trial, we included only the relevant arms in each analysis.
Dichotomous data
Data on adverse events were reported by the number of individuals experiencing these events, as opposed to the number of events, where possible. We collected dichotomous data in the form of N per intervention group. We analysed dichotomous data as Mantel‐Haenszel odds ratios (ORs) with 95% CIs. We also recorded reasons where possible.
We recorded depressive remission and response as provided.
Data on trial non‐completion were reported as the number of individuals failing to complete each trial, and reasons given for non‐completion.
Unit of analysis issues
Cross‐over RCTs
No cross‐over RCTs were included.
Cluster‐RCTs
No cluster‐RCTs were included.
RCTs with multiple treatment groups
Where trials used multiple treatment groups, we treated each group independently. We treated each comparison with n‐3PUFAs as an independent study and included them in all appropriate analyses. Where trials used multiple treatment groups, we used the same comparator for all treatment groups, and split the data from comparison groups across treatment groups, as equally as possible for analysis. Where insufficient numbers required numbers of individuals with events either to be rounded up or rounded down, the number of individuals was rounded to err on the side of no effect as opposed to an effect. Assuming individuals took part in only one treatment/comparator group, groups are independent. No studies involved individuals in more than one treatment or comparison group.
Dealing with missing data
We contacted investigators in order to verify key study characteristics and obtain missing numerical outcome data where possible. We documented correspondence with trialists. We used intention‐to‐treat (ITT) data where possible. We extracted data from per‐protocol populations and included them if ITT data were not available.
Where we could not obtain standard deviations from trial authors, we imputed them by using standard deviation data from all other trials using the same measure in the review (Furukawa 2006).
Assessment of heterogeneity
We undertook meta‐analysis where treatments, participants and the underlying clinical question were similar enough for pooling to make sense, i.e. where n‐3PUFAs were used as a treatment, where participants had a diagnosis of major/unipolar depressive disorder (or equivalent), and where n‐3PUFAs were implemented as a treatment for major/unipolar depressive disorder. Main analyses include all studies to allow sufficient numbers of studies for analyses to be meaningful, and were conducted using a random‐effects model and Hedges' adjusted g, to allow consideration of the likely heterogeneity between studies (Deeks 2001; Egger 2001; Sterne 2001). We also applied a fixed‐effect model as sensitivity analyses to investigate bias as a result of systematic differences between large and small studies that can be exacerbated by the use of a random‐effects model (Deeks 2001; Egger 2001; Sterne 2001). Large differences between the results of our primary analyses using random‐ and fixed‐effect models would suggest using caution when interpreting results.
We investigated heterogeneity using the I2 statistic (Higgins 2002; Higgins 2003). We reported I2 statistics and grouped the I2 statistic into four bands for interpretation, as recommended in the Cochrane Handbook (Higgins 2011). These bands were:
0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity.
We identified a priori possible sources of heterogeneity, to include the comparator used, publication bias, the presence or absence of comorbid conditions (physical and psychiatric), use of n‐3PUFAs as a sole or adjunctive therapy, and the risk of selection bias, performance bias and attrition bias. Following our previous review (Appleton 2015), we also identified several additional potential sources of heterogeneity to include: the enrolment of participants with a specified 'major' or 'unipolar' depression diagnosis; the use of EPA specifically as a treatment; the inclusion of ALA in placebo capsules; treatment duration for 12 weeks or more; the use of data from per‐protocol analyses; the use of imputed standard deviations from other studies in analyses; and the consideration of multiple comparison groups from the same trial as individual studies.
We investigated heterogeneity between studies based on the presence/absence of comorbidities and the presence/absence of adjunctive therapy using subgroup analyses. We investigated all other potential sources of heterogeneity (with the exception of publication bias) using sensitivity analyses.
Assessment of reporting biases
We investigated publication bias using funnel plot asymmetry (Sterne 2001). It should be noted that publication bias is one of several possible causes of asymmetry in funnel plots.
Data synthesis
We combined studies reporting mean and standard deviation data using meta‐analysis (Sterne 2001).
For continuous data, we calculated the standardised mean effect for all studies using Hedges' adjusted g (Deeks 2001). Hedges’ adjusted g is a formulation of effect size used in the SMD method that includes an adjustment to correct for small sample bias (Deeks 2001). Studies were weighted using the inverse‐variance method. We used random‐effects models primarily to estimate the SMDs for all analyses (Deeks 2001; Egger 2001; Sterne 2001). The random‐effects model assumes non‐identical effects in different studies, and can be preferable to a fixed‐effect model where heterogeneity between studies is high and unexplained. We also applied a fixed‐effect model as sensitivity analyses. Effect sizes are provided as means and standard deviations, and are related to specific scales to allow understanding by clinicians and practitioners.
For dichotomous data, we used the Mantel‐Haenszel method, and calculated effect sizes as odds ratios.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses investigating effects of n‐3PUFAs on MDD in:
Studies involving individuals with comorbid conditions, studies involving individuals without comorbid conditions, and studies involving a mix of individuals both with and without comorbid conditions. This analysis demonstrates effects due to participant characteristics which may affect treatment recommendations and outcomes. We conducted analyses using the same methods as for the main analyses, using: (i) studies in which participants were clearly identified as having comorbid conditions; (ii) studies in which participants were clearly identified as being without comorbid conditions (based on inclusion and exclusion criteria); and (iii) studies where participants with and without comorbid conditions were mixed, or where the presence or absence of comorbid conditions was not clear.
Studies involving individuals receiving adjunctive therapies, studies involving individuals not receiving adjunctive therapies, and studies involving a mix of individuals both receiving and not receiving adjunctive therapies. This analysis demonstrates effects due to participant characteristics which may affect treatment recommendations and outcomes. Analyses were conducted using the same methods as for the main analyses, using (i) studies in which participants were clearly identified as receiving adjunctive therapies; (ii) studies in which participants were clearly identified as not receiving adjunctive therapies (based on inclusion and exclusion criteria); and (iii) studies where participants receiving and not receiving adjunctive therapies were mixed, or where the presence or absence of adjunctive therapy use was not clear. For the purpose of these analyses, adjunctive therapy included antidepressants, psychotherapy, and any other therapies that may affect mood.
We conducted subgroup analyses only for the n‐3PUFA versus placebo comparison, and only for the primary outcomes.
Sensitivity analysis
We conducted sensitivity analyses to investigate the impact of various aspects of study methodology. These analyses investigated the effects of:
Including all studies versus only studies that we judged to be at low risk of bias. These analyses demonstrate the importance of the use of only those studies at low risk of bias, and the levels of confidence and caution that should be exercised in considering the analyses of all studies. We defined low risk of bias as in the Cochrane Handbook (Higgins 2011), using (i) selection bias, measured using allocation concealment; (ii) performance bias, using blinding of participants and personnel; (iii) attrition bias, using incomplete outcome data. We conducted separate analyses, one for each risk of bias domain. We chose these domains as the ones most likely to impact on RCTs investigating subjective outcomes such as depressive symptomology. We conducted separate analyses using the same methods as for the main analyses.
Using a fixed‐effect model as opposed to a random‐effects model. The random‐effects model was used for all main analyses. We conducted fixed‐effect analyses using the same data as for the main analyses.
Including all studies versus only those studies that enrolled individuals with a specified diagnosis of "major" or "unipolar" depressive disorder, or a subgroup of these individuals. Not all studies specified use of these diagnoses, but used alternative methods to describe a population where high depressive symptomology was debilitating. We conducted analyses using the same methods as for the main analyses.
Including all studies versus only those studies that used a treatment that was solely or predominantly EPA. Some reviews of n‐3PUFAs in depressive disorders have suggested a benefit from supplementation solely with EPA or predominantly with EPA (Firth 2019; Grosso 2014; Liao 2019), and some molecular evidence may support this hypothesis (Kalkman 2021). We conducted analyses using the same methods as for the main analyses.
Including all studies versus only those that do not use an oil in the placebo capsules that also contains n‐3PUFAs. We found some studies that used a placebo capsule containing ALA (parent n‐3PUFA of EPA and DHA) and these studies were included in the review due to low conversion rates of ALA to longer chain fatty acids in humans (Ma 1995). We conducted analyses using the same methods as for the main analyses.
Including all studies versus only those studies that provided supplementation for 12 weeks or more. While effects of supplementation are likely to increase with time, full incorporation into tissues has been suggested to take three to six months in humans (depending on n‐3PUFA type and dose) (Arterburn 2006; Marangoni 1993), thus maximal effects may not be achieved until after this period. We conducted analyses using the same methods as for the main analyses.
Including all studies versus only those studies that provided ITT data for analysis. We conducted analyses using the same methods as for the main analyses.
Including all studies versus only those that did not involve data imputation. Standard deviation data were unavailable for some studies, and we imputed them to allow inclusion of these studies in our main analyses. We conducted analyses using the same methods as for the main analyses.
Including all studies as described versus the inclusion of all trials that were split for analysis as complete trials. Several trials used multiple treatments, and so were split for our primary analyses (as described above) to allow accurate description of all studies as required for subgroup analyses, and to allow consistency between all studies. We combined trials that we had split for the main analyses. We pooled data and conducted analyses using the same methods as for the main analyses.
We conducted sensitivity analyses only for the n‐3PUFA versus placebo comparison. We applied the sensitivity analyses using a fixed‐effect model to all outcomes for completeness, but restricted all other sensitivity analyses to test only our primary outcomes.
Summary of findings and assessment of the certainty of the evidence
We have provided a summary of findings table, as recommended in the Cochrane Handbook (Higgins 2011). This summary of findings table is for the comparison of n‐3PUFAs with placebo, and includes all primary and secondary outcomes: depressive symptomology (continuous), adverse events, depressive symptomology (dichotomous remission and response), quality of life, and trial non‐completion. We assessed the certainty of evidence for all outcomes using the GRADE system. This considers within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
This updated review includes 28 trials with a total of 1944 participants. With the addition of the updated searches to the earlier searches, we identified a total of 2748 records of potential relevance to our review through our searches. Following the removal of duplicates, 1930 remained. Screening by title and abstract resulted in the removal of a further 1492 records, to result in the consideration of 438 full‐text papers. Of these, 113 records were found to relate to RCTs of relevance to our review, while 325 records were excluded. Records were excluded at this stage because they did not: refer to an RCT, involve individuals or a subgroup of individuals with MDD, involve adults, test n‐3PUFAs, involve a comparator, or they did not include depressive outcomes. We included trials in the review only if they met our eligibility criteria. Reasons for exclusion of select studies throughout the screening process are provided in Appendix 6. This appendix is intended to clarify our study selection criteria, and includes studies that either required discussion among the team, or are found in other reviews in this area, or both. Records that related to trials that are currently 'ongoing' and currently 'awaiting classification' remain in the review at this stage, but may be excluded once full details of these trials become available. We provide full details of the search results in the PRISMA flow diagram (Figure 1). The updated searches resulted in the inclusion of eight trials in addition to the 20 trials included in the previous review.
1.
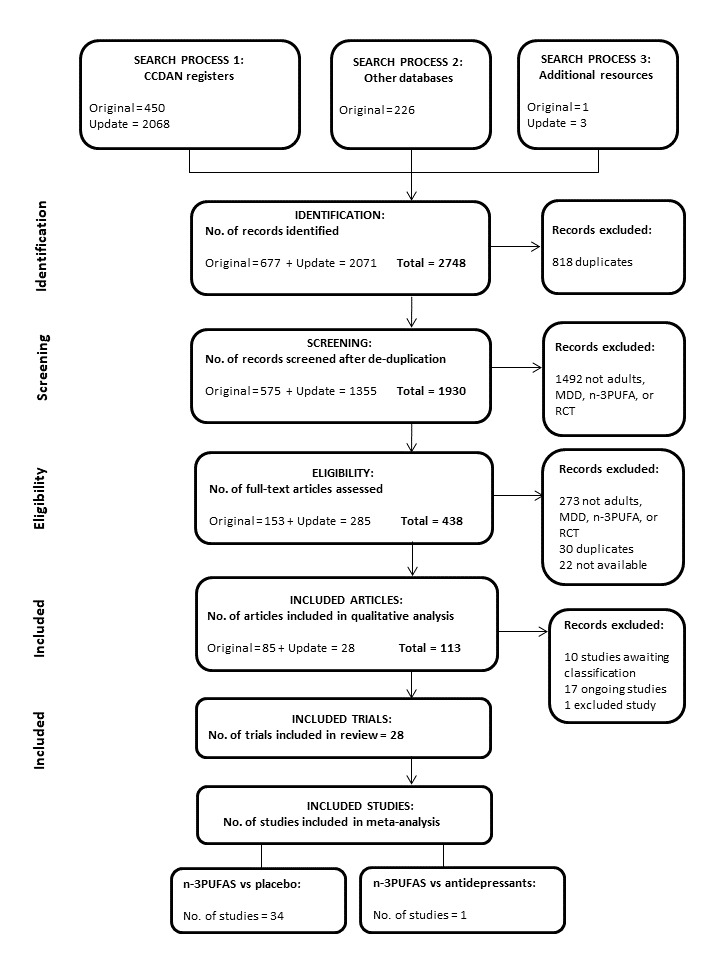
Of the 28 trials, Lucas 2009 and Mazereeuw 2016 involve individuals both with and without MDD, so we have included only the subgroup of individuals with MDD in our review. The Coryell 2005 trial includes tests of two doses of n‐3PUFA (approximately 1 g/d, and approximately 2 g/d); the Da Silva 2005 trial involves individuals who were randomised depending on antidepressant status (antidepressants use / no antidepressant use) at trial entry; the Jazayeri 2008 trial involves two separate comparator groups (placebo / antidepressant); the Jiang trial includes tests of an EPA+DHA treatment and an EPA‐only treatment, the Mischoulon 2015 trial includes tests of an enriched EPA treatment and an enriched DHA treatment; and the Peet 2002 trial includes tests of three doses of n‐3PUFA (1 g/d, 2 g/d, and 4 g/d). In these six trials, all groups were independent, and we have considered each as a separate study. This has resulted in the inclusion in our review of 35 independent studies (Bot 2010; Carney 2009; Carney 2020; Chang 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gharekhani 2014; Gonzalez 2011; Grenyer 2007; Jahangard 2018; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Lespérance 2011; Lucas 2009; Marangell 2003; Masoumi 2016; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005; Su 2003). Nine of these independent studies were additions to the previous review (Carney 2020; Chang 2020; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Masoumi 2016; Mazereeuw 2016; Shinto 2016).
Published data were available for all, apart from one (Kamath 2017) independent study for our primary outcome measure of depressive symptomology, and for 27 studies for our outcome of adverse events. We sought additional data, additional details or clarification from all corresponding authors. Of these, we were unable to contact Alfonso Gonzalez (corresponding author for Gonzalez 2011) or Lauren Marangell (corresponding author for Marangell 2003). The email addresses provided for these individuals did not work, and subsequent web‐based and telephone‐based searches were not fruitful. We contacted, but did not receive responses from Kuan‐Pin Su (corresponding author for Chang 2020), Serge Brand (corresponding author for Jahangard 2018), Jayesh Kamath (corresponding author for Kamath 2017), and Samira Tavakolian (corresponding author for Masoumi 2016). We received responses from all other corresponding authors. Where additional information was provided by authors, we have detailed this in the Characteristics of included studies tables.
Included studies
We provide full characteristics of the 35 independent studies in the Characteristics of included studies tables. We found considerable differences between studies in all aspects of study methodology. Full detail of the differences in each aspect of study methodology are given below. We used data from all studies in all analyses where possible. Published data were missing from analyses due only to insufficient detail, e.g. Da Silva (AD) 2005 and Da Silva (nAD) 2005 report 31 participants and two withdrawals, but fail to provide initial group allocation for the two withdrawals, rendering these data unsuitable for use in analyses.
Design
All studies included in the review were from RCTs involving parallel groups randomised to receive either n‐3PUFAs or a comparator.
Sample sizes
The studies included 1944 participants. Studies varied in sample size, although most studies were small. The number of participants included in each study were as follows: 5 (Kamath 2017), 11 (across both Coryell (1g/d) 2005 and Coryell (2g/d) 2005), 20 (Gonzalez 2011; Nemets 2002), 21 (Mazereeuw 2016), 25 (Bot 2010), 28 (Su 2003), 29 (Lucas 2009), 31 (across Da Silva (AD) 2005 and Da Silva (nAD) 2005), 35 (Park 2015), 36 (Marangell 2003), 39 (Shinto 2016), 41 (Mischoulon 2009), 42 (Gertsik 2012), 46 (Rondanelli 2010), 50 (Jahangard 2018), 54 (Gharekhani 2014), 59 (Chang 2020), 60 (across Jazayeri (v placebo) 2008 and Jazayeri (v AD) 2008) (Masoumi 2016), 70 (across Peet (1g/d) 2002; Peet (2g/d) 2002; and Peet (4g/d) 2002), 77 (Silvers 2005), 83 (Grenyer 2007), 108 (across Jiang (EPA+DHA) 2018 and Jiang (EPA only) 2018), 122 (Carney 2009), 144 (Carney 2020), 196 (across Mischoulon (DHA) 2015; and Mischoulon (EPA) 2015) and 432 (Lespérance 2011). In all trials, intervention and comparator groups were composed of approximately equal numbers.
Setting
Participants were recruited from hospitals, clinics and associated University settings (Bot 2010; Carney 2009; Carney 2020; Chang 2020; Gharekhani 2014; Grenyer 2007; Jahangard 2018; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Masoumi 2016; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015; Shinto 2016; Su 2003); and from community settings (Da Silva (AD) 2005; Da Silva (nAD) 2005; Lucas 2009). Some studies used recruitment methods to capture individuals from both clinical and community settings (Coryell (1g/d) 2005; Coryell (2g/d) 2005; Gertsik 2012; Lespérance 2011; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Silvers 2005). One study was based in a residential nursing home (Rondanelli 2010). Three studies did not report recruitment setting (Gonzalez 2011, Marangell 2003, Nemets 2002).
Studies were undertaken in the USA (Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Gertsik 2012; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Shinto 2016), Iran (Gharekhani 2014; Jahangard 2018; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Masoumi 2016), Canada (Lespérance 2011; Lucas 2009; Mazereeuw 2016), Taiwan (Chang 2020; Su 2003), Australia (Grenyer 2007), Brazil (Da Silva (AD) 2005; Da Silva (nAD) 2005), Italy (Rondanelli 2010), Korea (Park 2015), the Netherlands (Bot 2010), New Zealand (Silvers 2005), the United Kingdom (Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002) and Venezuela (Gonzalez 2011). Country of study was not reported for Marangell 2003 or Nemets 2002. These authors are based in the USA and Israel respectively.
Participants
This review relates only to MDD in adults, so all the included studies involved adults. One study used a local definition of adults (16+ years), and has been included (Gharekhani 2014). Mean ages ranged from a mean of 29 years (across Coryell (1g/d) 2005 and Coryell (2g/d) 2005) to a mean of 84 years (Rondanelli 2010). Most participants in all studies were women, with the exception of six (Carney 2009; Carney 2020; Chang 2020; Gharekhani 2014; Jahangard 2018; Mazereeuw 2016). Percentages of women ranged from 52% (Bot 2010) to 92% (Shinto 2016), and three studies involved only women (Lucas 2009; Masoumi 2016; Rondanelli 2010). In the studies with a majority of men, the percentages of men ranged from 56% (Gharekhani 2014) to 68% (Jahangard 2018). Distribution of gender was not reported in four studies (Gertsik 2012; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002).
Most studies reported the inclusion of participants based on a diagnosis of "major" or "unipolar" depressive disorder or depressive episode. Six studies enrolled participants with a depressive disorder or depressive episode, as defined by a psychiatrist (without explicit use of the terms "major" or "unipolar") (Gharekhani 2014; Masoumi 2016; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Silvers 2005), and in all these studies antidepressant treatment was considered appropriate. One study included participants with a rating on the BDI of 16 or more, where this was justified as equivalent to a diagnosis of clinical depression in the study population (Gharekhani 2014). One study enrolled individuals with "major depressive disorder", and also detailed mild to moderate symptomology (Shinto 2016); one study enrolled individuals with "major depressive disorder" using a cut‐off value on the HDRS lower than our predefined value, and also detailed mild to severe symptomology (Chang 2020); one study enrolled individuals with a "current depressive episode", and also detailed mild to severe symptomology (Silvers 2005); three studies enrolled individuals with "depression" and included consideration of a cut‐off value on the HDRS lower than our predefined value (Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002); and one study enrolled individuals with "depression" and included consideration of a cut‐off value on the BDI lower than our predefined value (Masoumi 2016). These studies were all included in the review, because additional diagnostic criteria and treatment criteria were met. One study enrolled individuals with diagnoses of MDD or "dysthymia", as assessed by a psychiatrist, and used a cut‐off score of more than 10 on the GDS (Rondanelli 2010). This study was included in the review, considering the similarity in these depressive diagnoses, and the presence of high depressive symptomology. Limited details were also available for one study (Kamath 2017), but this study is titled 'A randomized, double‐blind placebo‐controlled study evaluating the efficacy of omega 3 fatty acid augmentation of desvenlafaxine for the treatment of major depressive disorder in patients with medical illness', and antidepressant treatment was considered appropriate; this study was included.
Twelve studies included individuals from populations with physical comorbidities: diabetes (Bot 2010), cardiovascular disease or risk of cardiovascular disease (Carney 2009; Carney 2020; Chang 2020; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Mazereeuw 2016), diabetes, cardiovascular disease or cancer (Kamath 2017), end‐stage renal disease (Gharekhani 2014), multiple sclerosis (Shinto 2016) and Parkinson's disease (Da Silva (AD) 2005; Da Silva (nAD) 2005). The individuals in studies by Carney 2020; Da Silva (AD) 2005; Da Silva (nAD) 2005, Kamath 2017 and Mazereeuw 2016 may also have had psychiatric comorbidities. Four studies included individuals with no comorbidities (based on exclusion criteria) (Jahangard 2018; Marangell 2003; Mischoulon 2009; Su 2003). Seven studies included individuals with no physical comorbidities, but some/possible psychiatric comorbidities (Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Lucas 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015), while three studies included individuals with no psychiatric comorbidities, but some/possible physical comorbidities (Gertsik 2012; Gonzalez 2011; Rondanelli 2010), and six studies included individuals with some/possible physical and psychiatric comorbidities (Coryell (1g/d) 2005; Coryell (2g/d) 2005; Grenyer 2007; Lespérance 2011; Masoumi 2016; Silvers 2005). The trial by Peet 2002 (Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002) reports no comorbidities, but also does not report excluding individuals with physical or psychiatric comorbidities.
Studies included individuals who were all receiving adjunctive therapy for depression at the time of the trial, either having started in advance of trial entry or given as part of the trial (Bot 2010; Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Gertsik 2012; Gonzalez 2011; Jahangard 2018; Jazayeri (v placebo) 2008; Kamath 2017; Masoumi 2016; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Shinto 2016), individuals who were not receiving adjunctive therapy (Chang 2020; Da Silva (nAD) 2005; Gharekhani 2014; Jazayeri (v AD) 2008; Lucas 2009; Marangell 2003; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015), and a mix of individuals receiving and not receiving adjunctive therapy (Grenyer 2007; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lespérance 2011; Mazereeuw 2016; Mischoulon 2009; Nemets 2002; Rondanelli 2010; Silvers 2005; Su 2003). Adjunctive therapy took the form of antidepressant medication in all studies, with the exception of Mischoulon 2009, and included psychotherapy (Lespérance 2011; Mischoulon 2009; Silvers 2005). In Rondanelli 2010, antidepressants were not taken, but participants were permitted to take benzodiazepines, which may have impacted mood.
Interventions
Studies used either a sole EPA intervention, at doses of 1 g/d (Bot 2010; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Mischoulon 2009; Peet (1g/d) 2002), 2 g/d (Carney 2020; Jiang (EPA only) 2018; Nemets 2002; Peet (2g/d) 2002), 3 g/d (Gonzalez 2011), or 4 g/d (Peet (4g/d) 2002); a sole DHA intervention at a dose of 2 g/d (Marangell 2003); or EPA/DHA combinations, at doses of 1.14 g/d (EPA:DHA ‐ 740:400) (Coryell (1g/d) 2005), 1.2 g/d (EPA:DHA – 720:480) (Da Silva (AD) 2005; Da Silva (nAD) 2005), 1.2 g/d (EPA:DHA –1050:150) (Lespérance 2011; Lucas 2009), 1.8 g/d (EPA:DHA ‐ 1080:720) (Gharekhani 2014), 1.88 g/d (EPA:DHA – 930:750) (Carney 2009), 2 g/d (EPA:DHA ‐ 2:1) (Jiang (EPA+DHA) 2018), 2.28 g/d (EPA:DHA ‐ 1480:800) (Coryell (2g/d) 2005), 2.76 g/d (EPA:DHA – 0.56:2.2) (Grenyer 2007), 3 g/d (EPA:DHA ‐ 2:1) (Chang 2020), 3 g/d (EPA:DHA – 600:2400) (Silvers 2005), 3.3 g/d (EPA:DHA ‐ 1.95:1.35) (Shinto 2016), 5.22 g/d (EPA:DHA ‐ 3420:1800) (Park 2015) or 6.6 g/d (EPA:DHA – 4400:2200) (Su 2003). Five studies used an intervention consisting of EPA, DHA and other n‐3PUFAs, at doses of 1.224 g/d (EPA:DHA:other ‐ 180:900:144) (Mischoulon (DHA) 2015), 1.436 g/d (EPA:DHA:other ‐ 1060:274:102) (Mischoulon (EPA) 2015), 1.9 g/d (EPA:DHA:other ‐ 1.2:0.6:0.1) (Mazereeuw 2016), 2.4 g/d (EPA:DHA:other – 1800:400:200) (Gertsik 2012) or 3.13 g/d (EPA:DHA:other – 1670:830:630) (Rondanelli 2010). Three studies did not specify the n‐3PUFAs provided (Jahangard 2018; Kamath 2017; Masoumi 2016).
All studies used a placebo comparator, with the exception of Jazayeri (v AD) 2008, which compared n‐3PUFAs with antidepressants. Different placebos were used: oil (Coryell (1g/d) 2005; Coryell (2g/d) 2005), rapeseed oil (Jazayeri (v placebo) 2008), rapeseed oil plus medium‐chain triglycerides (Bot 2010), corn oil (Carney 2009; Carney 2020; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018), corn oil / soybean oil blend (50:50) (Mazereeuw 2016), olive oil (Gertsik 2012; Grenyer 2007; Silvers 2005; Su 2003), mineral oil (Da Silva (AD) 2005; Da Silva (nAD) 2005), paraffin oil (Gharekhani 2014; Mischoulon 2009; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010), safflower oil plus oleic acid (Park 2015), soybean oil (Chang 2020; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015), soybean oil plus 1% fish oil (Shinto 2016); sunflower oil plus 2% fish oil (Lespérance 2011; Lucas 2009). We included studies using rapeseed oil and soybean oil as a comparator, due to likely effects as a result of longer n‐3PUFAs and the reported low conversion rates of ALA to longer n‐3PUFAs (Ma 1995). The oil used in the Coryell 2005 studies also contained some ALA (6%). Six studies did not report the placebo used (Gonzalez 2011; Jahangard 2018; Kamath 2017; Marangell 2003; Masoumi 2016; Nemets 2002). In all cases where dose was reported, the placebo was given in a similar dose to the intervention.
Treatment duration for each trial was as follows: four weeks (Nemets 2002), one month (Masoumi 2016), six weeks (Coryell (1g/d) 2005; Coryell (2g/d) 2005; Marangell 2003), eight weeks (Gertsik 2012; Gonzalez 2011; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Lespérance 2011; Lucas 2009; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Rondanelli 2010; Su 2003), 10 weeks (Carney 2009; Carney 2020), 12 weeks (Bot 2010; Chang 2020; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Mazereeuw 2016; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Silvers 2005), 3 months ( Da Silva (AD) 2005; Da Silva (nAD) 2005; Shinto 2016), and 4 months (Gharekhani 2014; Grenyer 2007). For all studies, analyses were conducted on measurements taken at the end of treatment.
In the trial where n‐3PUFAs were compared with antidepressants (Jazayeri (v AD) 2008), n‐3PUFAs were given using EPA only, at a dose of 1 g/d, and compared with 20 mg/d fluoxetine (antidepressant).
Outcomes
Primary outcomes
Depressive symptomology (continuous data): Depressive symptomology was measured using continuous scales in all studies, at both baseline and study end. Most studies used the Hamilton Depression Rating Scale (HDRS) (Hamilton 1960) (including the HDRS‐short form (Reynolds 1995)), the Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979), and/or the Beck Depression Inventory (BDI) (Beck 1987), but other measures including the Inventory of Depressive Symptomology Self Report (IDS‐SR) (Trivedi 2004) (Lespérance 2011), the Hopkins Symptom Checklist Depression Scale (HSCL) (Williams 2004) (Lucas 2009), and the Geriatric Depression Scale (GDS) (Yesvage 1983) (Rondanelli 2010) were also used. In almost all studies, depressive symptomology scores were also collected at additional time points between baseline and study end.
Adverse events: Number of individuals experiencing adverse events were reported or provided for 27 studies (Bot 2010; Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gharekhani 2014; Grenyer 2007; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Lespérance 2011; Lucas 2009; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005; Su 2003). In some studies only the number of individuals experiencing serious adverse events (Bot 2010; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Gertsik 2012), clinically relevant adverse events (Nemets 2002) or emerging or worsening adverse events (Mischoulon (DHA) 2015; Mischoulon (EPA) 2015) were provided, and three studies reported only the number of individuals experiencing adverse events reported by at least 5% of participants (Bot 2010; Gertsik 2012; Lespérance 2011). Four studies reported the number of adverse events rather than the number of individuals experiencing them (Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Marangell 2003; Mazereeuw 2016). Three studies did not measure or report adverse events (Chang 2020; Jahangard 2018; Masoumi 2016). Six studies did not report adverse events fully, clearly or in detail (Carney 2009; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gonzalez 2011; Grenyer 2007; Lespérance 2011). Many studies also reported types of adverse events experienced. Most adverse events were gastrointestinal, although psychological and other physical events were also reported. We included data on adverse events in analyses, provided that the number of individuals reporting adverse events was reported in the n‐3PUFA and placebo group using the same definition of adverse events (serious adverse events, etc.).
Secondary outcomes
Depressive symptomology (dichotomous data): Depressive symptomology in dichotomous terms was reported in 20 studies (Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gonzalez 2011; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Marangell 2003; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010), and could be calculated for two studies (Nemets 2002; Shinto 2016). We used these data to provide rates of remission or response, or both. As determined by original authors, "remission" was defined as an end point score within the no/low depression range on the scale used (score 7 or less on the HDRS (Gertsik 2012; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015), score 8 or less on the BDI (Carney 2009; Carney 2020; Shinto 2016), score less than 5 on the BDI‐II (Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018), score less than 11 on the GDS (Rondanelli 2010)), and "response" was defined as a 50% improvement in depression scale score.
Quality of life: Quality of life was measured in 18 studies, using a range of validated scales: Clinical Global Impression (CGI) (Guy 1976) (Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Lucas 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015), Patient Global Impression (PGI) (Guy 1976) (Gertsik 2012), Global Assessment of Functioning Scale (GAF) (Diguer 1993), (Grenyer 2007; Marangell 2003), Psychological General Well‐being Schedule (PGWB) (Dupuy 1984) (Lucas 2009), the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) (Endicott 1993) (Mischoulon 2009), the Short Form Health Survey (SF‐36 or SF‐12) (Ware 1993) (Gharekhani 2014; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Mazereeuw 2016; Rondanelli 2010; Shinto 2016) and Likert scales (Grenyer 2007). We considered these scales to assess quality of life, although some of them were used as secondary measures of depression in some studies. For the CGI and PGI, higher scores denote poorer quality of life. For the GAF, PGWB, QLESQ, SF‐36 and SF‐12, higher scores denote better quality of life.
Trial non‐completion: All studies reported numbers of individuals who did not complete, with the exception of Rondanelli 2010, where no details are provided but full data sets are available for all participants, so we presume all participants completed the trial, and Chang 2020, where it was not clear at what stage participants withdrew. For all other studies, figures ranged from 0% (Coryell (1g/d) 2005; Coryell (2g/d) 2005; Jahangard 2018; Masoumi 2016) to 50% (Gonzalez 2011). Many studies provided reasons for withdrawal.
Excluded studies
Our searches identified only one trial registration that we have classified as an excluded study based on Cochrane criteria (Characteristics of excluded studies). This trial registration (NCT00963196) details a trial that appears to meet our inclusion criteria, but the study was withdrawn prior to participant enrolment.
Ongoing studies
Seventeen RCTs investigating n‐3PUFAs versus a comparator in adults with MDD are currently ongoing. We provide details of these in the tables of Characteristics of ongoing studies. Details are based on trial registrations, associated publications, and some contact with authors. We have included all potentially relevant studies, to allow subsequent updates of the review to be as inclusive as possible. Some of the studies that are currently included as ongoing studies may be excluded from updates of the review once study details become clearer following completion and publication. Only subgroups of participants in some studies may also be included in subsequent updates, depending on inclusion/exclusion criteria. Some trials, for example, focus on adolescents, but include individuals aged up to 25 years (Amminger 2013), and while most respondents in this trial may not be relevant to our review, it may be possible to include a subset of individuals aged over 18 years, depending on randomisation procedures and subgroup specification. We tried to contact all authors for ongoing studies, and received responses from the authors for three of these (Marriott 2016; Rapaport 2015; Sahoo 2016). Publications from these trials relevant to our review were in progress at the time of writing.
Studies awaiting classification
Ten trials are currently awaiting classification. Details of these are provided in the tables of Characteristics of studies awaiting classification. These search results comprise one conference abstract (Kwak 2013), and nine trial registrations. We cannot yet include the conference abstract, as we have not so far been able to obtain enough information on this study to be sure that it is relevant to our review. We have been unable to contact the author. The nine trial registrations relate to trials that are now described on trial register websites as "completed". We have emailed all contact authors for further information to allow clarification. Contact emails are either unavailable (EUCTR2006‐004949‐41‐IT; NCT00816322), have been returned undelivered (Lima 2006; Murck 2002; Murck 2003; Murck 2004; Naqvi 2008) or have elicited no response (Bafghi 2011; Su 2005).
Risk of bias in included studies
Details of the risk of bias judgements for each study are given in the tables of Characteristics of included studies, and we present graphical representations of the overall risk of bias in included studies in Figure 2 and Figure 3. We judged the risks of bias to be very variable between studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged 28 of the 35 studies to be at low risk of bias for random sequence generation (Bot 2010; Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gharekhani 2014; Grenyer 2007; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lespérance 2011; Lucas 2009; Masoumi 2016; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005; Su 2003). In most of these studies, randomisation was undertaken using a computer‐generated random‐number generator, but drawing lots (Da Silva (AD) 2005; Da Silva (nAD) 2005) and a random‐number table (Nemets 2002; Rondanelli 2010) were also used. For the remaining seven studies (Chang 2020; Gertsik 2012; Gonzalez 2011; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Kamath 2017; Marangell 2003), insufficient details were provided, resulting in a judgement of unclear risk of bias.
Allocation concealment
We judged 22 studies to be at low risk of bias for allocation concealment (Bot 2010; Carney 2020; Grenyer 2007; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lespérance 2011; Lucas 2009; Masoumi 2016; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005; Su 2003). In these studies, allocation concealment was ensured by individuals outside the main research team conducting the allocation, or by using sequential numbering that had been prepared by individuals outside the main research team. We judged two studies to be at high risk of bias (Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008), following comments from the author that the randomisation sequence was not concealed from researchers. For all other studies (Carney 2009; Chang 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gharekhani 2014; Gonzalez 2011; Kamath 2017; Marangell 2003), insufficient details were provided, leading to a judgement of unclear risk of bias.
Blinding
Blinding of participants and personnel;
We judged eight studies to be at low risk of bias for blinding of study participants and personnel to treatment allocation (Bot 2010; Carney 2020; Lespérance 2011; Lucas 2009; Nemets 2002; Rondanelli 2010; Shinto 2016; Silvers 2005). In these studies, blinding was undertaken by adding a small amount of fish oil to the comparator treatment to control for fishy aftertaste or adding flavours to both treatments to mask a fishy aftertaste, or both, and following investigation, blinding was found to be successful. We judged 15 studies at high risk of bias (Carney 2009; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gharekhani 2014; Grenyer 2007; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Mischoulon 2009; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002). In these studies, there were no reports of attempting to mask the fishy taste of the intervention, despite good descriptions of the placebo otherwise, or fishy odour was specifically identified as an adverse event, and there were no assessments to check successful concealment. In one study, most participants correctly guessed their allocation (Grenyer 2007). We judged eight studies to be at unclear risk of bias (Chang 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Gonzalez 2011; Jahangard 2018; Kamath 2017; Marangell 2003; Masoumi 2016) due to no report of attempts to mask a fishy taste, but no clear description of other aspects of the placebo. We judged a further four studies to be at unclear risk of bias (Mazereeuw 2016; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Su 2003) because flavour was added to the capsules to mask a fishy taste, but there was no assessment to check the success of this precaution.
Blinding of outcome assessment
We judged the blinding of outcome assessments depending on the individuals making the assessment (participant, researcher, clinician) and the blinding of those persons, as detailed in the blinding of participants and personnel. Thus, we rated participant‐rated measures at a low risk of bias if we considered participants to be successfully blinded to treatment allocation, at unclear risk of bias if blinding was unclear, and at high risk of bias if we considered participants not to be successfully blinded. We treated personnel‐rated measures in a similar fashion. In all cases, we used study reports of the individuals making the assessment if possible, or used standard assessments if details were not specified, e.g. in standard practice, the BDI is a self‐report instrument for completion by patients. Where multiple outcome measures were used and these were given different judgements of risk of bias, we took the key risk of bias judgement to be the one applicable to the outcome measure we used in our analyses.
Mood:
We judged 23 studies to be at low risk of bias, following ratings of adequate blinding of those making the assessments or following adequate blinding of those making the mood assessment used in our analyses (Bot 2010; Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Grenyer 2007; Jahangard 2018; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Lespérance 2011; Lucas 2009; Mazereeuw 2016; Mischoulon 2009; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005). We rated 11 studies at unclear risk of bias, where it was unclear who had made the assessment or whether those individuals were successfully blinded (Chang 2020; Gertsik 2012; Gonzalez 2011; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Marangell 2003; Masoumi 2016; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Su 2003). We judged one study to be at high risk of bias, where there was a high risk of bias in the blinding of those making the mood assessment (Gharekhani 2014).
Adverse events:
We rated nine studies at low risk of bias following judgements of adequate blinding of those making the assessments (Bot 2010; Carney 2020; Lespérance 2011; Lucas 2009; Mazereeuw 2016; Nemets 2002; Rondanelli 2010; Shinto 2016; Silvers 2005). We judged 11 studies to be at high risk of bias, where assessments were made by those at high risk of performance bias due to inadequate blinding (Gharekhani 2014; Grenyer 2007; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Mischoulon 2009; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002). We rated 12 studies at unclear risk of bias, where it was not apparent who had made the assessment or if those individuals were successfully blinded (Carney 2009; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gonzalez 2011; Kamath 2017; Marangell 2003; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Su 2003). Three studies were also given an unclear rating for risk of bias because adverse events were not assessed (Chang 2020; Jahangard 2018; Masoumi 2016).
Incomplete outcome data
Mood:
We rated outcome data for mood as complete if there were no missing outcome data; or if: analyses were conducted using intention‐to‐treat (ITT) data, where ITT was defined as including all those randomised; data were missing for less than 10% of the total randomised population; reasons for missing outcome data were unlikely to be related to true outcome; the difference in missing data between intervention and comparator group was not more than 10% of the total randomised population; and the missing data were not unbalanced between intervention and comparator groups in numbers and reasons.
We rated nine studies at low risk of bias for publication or provision of ITT data (as above) (Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Jahangard 2018; Lucas 2009; Masoumi 2016; Nemets 2002; Rondanelli 2010). We judged 24 studies to be at high risk of bias due to the unavailability of ITT data (Bot 2010; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gonzalez 2011; Marangell 2003; Mazereeuw 2016; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Silvers 2005), or publication or provision of ITT data but a higher than 10% dropout rate (Gharekhani 2014; Grenyer 2007; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lespérance 2011; Mischoulon 2009; Shinto 2016; Su 2003). One study was rated at unclear risk of bias due to an unclear description of when participants withdrew, meaning that it was unclear if the completer analyses presented were ITT (Chang 2020). One study did not report mood data (Kamath 2017), but the trial registration specified no intentions to summarise data if fewer than 10 data points were available. This study also included a high dropout rate, so was given a judgement of high risk.
Adverse events:
We judged outcome data for adverse events to be complete if all adverse events were clearly reported, data were missing for less than 10% of the total randomised population, and the difference in missing data between intervention and comparator group was not more than 10% of the total randomised population; and outcome data were judged to be incomplete if all adverse events were clearly not reported, if data were missing for 10% of the total randomised population or more, and the difference in missing data between intervention and comparator groups was more than 10% of the total randomised population. We rated three studies at low risk of bias, due to clear complete reporting of all adverse events (Carney 2020; Lucas 2009; Rondanelli 2010). We judged 16 studies to be at high risk of bias due to clear reporting of all adverse events but a higher than 10% dropout rate (Gharekhani 2014; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Shinto 2016; Silvers 2005; Su 2003) and eight studies to be at high risk of bias due to clear incomplete reporting of all adverse events and/or high dropout rate (Bot 2010; Gertsik 2012; Gonzalez 2011; Grenyer 2007; Lespérance 2011; Marangell 2003; Mazereeuw 2016; Nemets 2002). We judged five studies at unclear risk of bias where adverse events were not clearly reported (Carney 2009; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005). Three studies were also judged at unclear risk of bias because adverse events were not assessed (Chang 2020; Jahangard 2018; Masoumi 2016).
Selective reporting
We judged 16 studies to be at low risk of bias for selective reporting, where reported outcomes have been checked against protocols or where authors have informed us that all planned outcomes have been reported (Bot 2010; Carney 2009; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gharekhani 2014; Grenyer 2007; Lespérance 2011; Lucas 2009; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Silvers 2005; Su 2003). Judgements of high risk of reporting bias were given to seven studies where all outcomes have not (yet) been reported (Gertsik 2012; Jazayeri (v placebo) 2008; Jazayeri (v AD) 2008; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015), to two studies where adverse events were not assessed but would be expected (Chang 2020; Masoumi 2016), one study where all outcomes have not (yet) been reported and adverse events were not assessed (Jahangard 2018) and to two unpublished studies (Coryell (1g/d) 2005; Coryell (2g/d) 2005). We judged three studies at high risk of bias where the reporting of some data was unclear or incomplete (Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Shinto 2016). We judged two studies at unclear risk of bias where protocols were not available and authors have not confirmed complete reporting (Gonzalez 2011; Marangell 2003). We judged one study at unclear risk of bias where data were not provided, but where insufficient data were gained for summarising (Kamath 2017), and one study where all prespecified outcomes were reported, plus some additional outcomes, but these outcomes were not our primary or secondary outcome measures (Carney 2020).
Other potential sources of bias
All studies, with the exception of 10, appeared to be free from other sources of bias ‐ Park 2015 received a rating of high risk of bias for reporting a significant imbalance in all measures of mood and quality of life between intervention and comparator groups at baseline, Kamath 2017 received a rating of high risk of bias due to early study termination due to low recruitment and resources, and we found discrepancies between the protocol and published paper for eight studies resulting in a judgement of unclear risk of bias (Carney 2020; Chang 2020; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Masoumi 2016; Mazereeuw 2016; Shinto 2016).
Effects of interventions
See: Table 1
Comparison 1: n‐3PUFAs versus placebo
Thrity‐four independent studies involving 1924 individuals contribute to this comparison (Bot 2010; Carney 2009; Carney 2020; Chang 2020; Coryell (1g/d) 2005; Coryell (1g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gharekhani 2014; Gonzalez 2011; Grenyer 2007; Jahangard 2018; Jazayeri (v placebo) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Lespérance 2011; Lucas 2009; Marangell 2003; Masoumi 2016; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005; Su 2003); see also Table 1.
Primary outcomes
1.1 Depressive symptomology (continuous data)
Thirty‐three studies provided continuous data on depressive symptomology in 1848 individuals, and were included in analyses. Analyses were based on HDRS scores for 22 studies (Carney 2009; Carney 2020; Chang 2020; Gertsik 2012; Gonzalez 2011; Grenyer 2007; Jazayeri (v placebo) 2008; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lucas 2009; Marangell 2003; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Silvers 2005; Su 2003), on MADRS score for eight studies (Bot 2010; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005, Jahangard 2018; Lespérance 2011; Shinto 2016), BDI score for two studies (Gharekhani 2014; Masoumi 2016), and GDS score for one study (Rondanelli 2010).
n‐3PUFAs were more effective than placebo: standardised mean difference (SMD) −0.40 (95% confidence interval (CI) −0.64 to −0.16) (see Analysis 1.1, Figure 4, Figure 5), but effect sizes are small to modest, and there was substantial evidence of heterogeneity between studies (I2 = 81%). Confidence intervals also range between a very small and a modest effect size, and suggest a possible clinically important effect at their upper end. Using GRADE criteria, we judged the certainty of the evidence to be very low. A standardised mean difference of 0.40 represents a difference between groups in scores on the HDRS (17‐item) of approximately 2.5 points (95% CI 1.0 to 4.0).
1.1. Analysis.

Comparison 1: n‐3PUFAs vs placebo, Outcome 1: Depressive symptomology (continuous)
4.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.1 Depressive symptomology (continuous).
5.

Funnel plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.1 Depressive symptomology (continuous).
1.2 Adverse events
The number of individuals experiencing adverse events did not differ between n‐3PUFA and placebo groups: OR 1.27 (95% CI 0.99 to 1.64); 24 studies, 1503 participants (see Analysis 1.2, Figure 6, Figure 7). Confidence intervals however are wide, and suggest that effects could range from a decrease of 1% to an increase of 64% in adverse events in n‐3PUFA groups, compared with placebo. Using GRADE criteria, we judged the certainty of the evidence to be very low. There was no evidence of heterogeneity (I2 = 2%).
1.2. Analysis.

Comparison 1: n‐3PUFAs vs placebo, Outcome 2: Adverse events
6.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.2 Adverse events.
7.

Funnel plot of comparison: 1 n‐3PUFAs vs Placebo, outcome: 1.2 Adverse events.
Secondary outcomes
1.3 Depressive symptomology (dichotomous data)
There was no evidence of a difference in remission rates following supplementation with n‐3PUFAs compared with placebo: OR 1.13 (95% CI 0.74 to 1.72); 8 studies, 609 participants (see Analysis 1.3, Figure 8), but confidence intervals are very wide. Confidence intervals suggest a possible effect ranging from a 26% reduction in remission rates with n‐3PUFAs compared with placebo, to a 72% increase in remission rates. Using GRADE criteria, we judged the certainty of the evidence to be low. There was little evidence of heterogeneity (I2 = 24%).
1.3. Analysis.

Comparison 1: n‐3PUFAs vs placebo, Outcome 3: Depressive symptomology (dichotomous ‐ remission)
8.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.3 Depressive symptomology (dichotomous data): remission.
There was no evidence of a difference in response rates following supplementation with n‐3PUFAs compared with placebo: OR 1.20 (95% CI 0.80 to 1.79); 17 studies, 794 participants (see Analysis 1.4, Figure 9), but confidence intervals are again very wide. Confidence intervals suggest a possible effect ranging from a 20% reduction in response rates with n‐3PUFAs compared with placebo, to a 79% increase in response rates. Using GRADE criteria, we judged the certainty of the evidence to be low. There was little evidence of heterogeneity (I2 = 27%).
1.4. Analysis.

Comparison 1: n‐3PUFAs vs placebo, Outcome 4: Depressive symptomology (dichotomous ‐ response)
9.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.4 Depressive symptomology (dichotomous data): response.
1.4 Quality of life
Continuous data on quality of life were available in 476 participants from 12 studies (Da Silva (AD) 2005; Da Silva (nAD) 2005; Gharekhani 2014; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lucas 2009; Marangell 2003; Mazereeuw 2016; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015; Rondanelli 2010). We conducted analyses on data from the CGI (Da Silva (AD) 2005; Da Silva (nAD) 2005; Lucas 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015), the GAF (Marangell 2003), the full SF‐36 (Mazereeuw 2016) and the SF‐36 (mental health summary scale) (Gharekhani 2014; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Rondanelli 2010). We reversed scores for the GAF and SF‐36, so that in all scales a higher score denotes poorer quality of life.
There was no strong evidence of a difference in quality of life between n‐3PUFA and placebo groups: SMD −0.38 (95% CI −0.82 to 0.06). Confidence intervals ranged between a negligible and a large effect size, suggesting both a possible absence of effect at the lower end, and a possible important effect at the upper end. Using the CGI, these effect sizes are equivalent to a difference in scores of approximately −0.38 (95% CI −0.82 to 0.06) on a seven‐point scale. Using GRADE criteria, we judged the certainty of the evidence to be very low, and there was considerable evidence of heterogeneity between studies (I2 = 79%) (see Analysis 1.5, Figure 10).
1.5. Analysis.

Comparison 1: n‐3PUFAs vs placebo, Outcome 5: Quality of life
10.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.5 Quality of life.
1.5 Trial non‐completion
Rates for trial non‐completion were similar in n‐3PUFA and placebo groups: OR 0.92 (95% CI 0.70 to 1.22); 29 studies, 1777 participants (see Analysis 1.6, Figure 11, Figure 12), but again confidence intervals are wide, and suggest that effects could range from a reduction of 30% to an increase in study withdrawals of 22% in n‐3PUFA groups, compared to placebo. Using GRADE criteria, we judged the certainty of the evidence to be very low. There was no evidence of heterogeneity (I2 = 0%).
1.6. Analysis.

Comparison 1: n‐3PUFAs vs placebo, Outcome 6: Trial non‐completion
11.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 1.6 Trial non‐completion.
12.

Funnel plot of comparison: 1 n‐3PUFAs vs Placebo, outcome: 1.6 Trial non‐completion.
Comparison 2: n‐3PUFAs versus antidepressants
Data were only available from one study for this comparison, involving 40 participants (Jazayeri (v AD) 2008).
Primary outcomes
2.1 Depressive symptomology (continuous data)
Depressive symptomology based on the HDRS was similar in the n‐3PUFA and antidepressant groups: MD (HDRS (24 item)) −0.70 (95% CI −5.88 to 4.48); 1 study, 40 participants (see Analysis 2.1). Confidence intervals are however very wide, and do not rule out a modest benefit or detriment of n‐3PUFAs, compared to antidepressants.
2.1. Analysis.

Comparison 2: n‐3PUFAs vs antidepressant, Outcome 1: Depressive symptomology (continuous)
2.2 Adverse events
Adverse events were only reported in terms of the number of events experienced, as opposed to the number of individuals experiencing at least one event.
Secondary outcomes
2.3 Depressive symptomology (dichotomous data)
Response rates were similar in n‐3PUFA and antidepressant groups: OR 1.23 (95% CI 0.35 to 4.31); 1 study, 40 participants (see Analysis 2.2), but confidence intervals are very wide, and do not rule out an important benefit or detriment of n‐3PUFAs, compared to antidepressants. Remission rates were not reported.
2.2. Analysis.

Comparison 2: n‐3PUFAs vs antidepressant, Outcome 2: Depressive symptomology (dichotomous ‐ response)
2.4 Quality of life
Quality of life was not reported in this study.
2.5 Trial non‐completion
Rates for non‐completion were similar in n‐3PUFA and antidepressant groups: OR 1.00 (95% CI 0.21 to 4.71); 1 study, 40 participants (see Analysis 2.3). Confidence intervals however are again very wide, and do not rule out important effects in either direction.
2.3. Analysis.

Comparison 2: n‐3PUFAs vs antidepressant, Outcome 3: Trial non‐completion
Subgroup analyses
We conducted subgroup analyses only for the n‐3PUFA versus placebo comparison, and only for the primary outcomes, but the number of studies and the number of participants are low. There were insufficient numbers of studies and participants for subgroup analyses to be conducted for other outcomes or for the n‐3PUFA versus antidepressant comparison.
3. Subgroup analyses based on comorbidities
There was a suggestion of greater effect sizes (n‐3PUFAs compared to placebo) in depressive symptomology (continuous) in studies including individuals without comorbid conditions: SMD −1.67 (95% CI −2.98 to −0.37); 4 studies, 154 participants, compared to studies including individuals with comorbid conditions: SMD −0.11 (95% CI −0.39 to 0.18); 11 studies, 594 participants, and studies with a mix of individuals with and without comorbid conditions: SMD −0.30 (95% CI −0.59 to −0.00); 18 studies, 1100 participants (see Analysis 3.1, Figure 13). The number of studies and the number of individuals in each subgroup however were small, particularly in the subgroup of studies including individuals without comorbid conditions (4 studies, 154 participants). Confidence intervals are also very wide, suggesting that effects could range from strong effects to those that are negligible, and the evidence of heterogeneity within each subgroup was high (I2 = 59% to 91%). There was no statistical evidence of a difference between subgroups (P = 0.06), and the evidence of heterogeneity between subgroups was high (I2 = 65%).
3.1. Analysis.

Comparison 3: Subgroup analyses ‐ n‐3PUFAs vs placebo ‐ analyses based on comorbidities, Outcome 1: Depressive symptomology (continuous)
13.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 3.1 Depressive symptomology (continuous): Sub‐groups based on presence / absence of comorbidities.
Rates of adverse events were similar across the subgroups. Analysis of studies including individuals with comorbid conditions: OR 1.28 (95% CI 0.59 to 2.74); 8 studies, 497 participants, studies including individuals without comorbid conditions: OR 0.82 (95% CI 0.19 to 3.50); 2 studies, 69 participants, and studies with a mix of individuals with and without comorbid conditions: OR 1.34 (95% CI 1.00 to 1.80); 14 studies, 937 participants, indicated no statistical evidence of a difference between subgroups (P = 0.80), and no evidence of heterogeneity between subgroups (I2 = 0%) (see Analysis 3.2). Heterogeneity was high in the subgroup of studies including individuals with comorbid conditions (I2 = 56%), but low in the other two subgroups (I2 = 0%), and confidence intervals are again very wide, suggesting possible effects that could range between a reduction in events with n‐3PUFAs of 81% to an increase in events of 250%.
3.2. Analysis.

Comparison 3: Subgroup analyses ‐ n‐3PUFAs vs placebo ‐ analyses based on comorbidities, Outcome 2: Adverse events
4. Analyses based on adjunctive therapy
The effect of n‐3PUFAs compared to placebo on depressive symptomology (continuous) in studies with a mix of individuals receiving and not receiving adjunctive therapy was SMD −0.24 (95% CI −0.55 to 0.07); 10 studies, 832 participants, in studies with individuals not receiving adjunctive therapy was SMD −0.29 (95% CI −0.73 to 0.15); 7 studies, 367 participants, and in studies only including individuals receiving adjunctive therapy was SMD −0.55 (95% CI −1.04 to −0.06); 16 studies, 649 participants. There was no statistical evidence of a difference between subgroups (P = 0.58) (see Analysis 4.1, Figure 14). However, the number of studies and the number of individuals in each subgroup were small, and confidence intervals are very wide, suggesting that effects could range from a large to a small effect of n‐3PUFAs, compared with placebo. There was no evidence of heterogeneity between subgroups (I2 = 0%), and evidence of heterogeneity was high in all subgroups (I2 = 69% to 87%).
4.1. Analysis.

Comparison 4: Subgroup analyses: n‐3PUFAs vs placebo ‐ analyses based on adjunctive therapy, Outcome 1: Depressive symptomology (continuous)
14.

Forest plot of comparison: 1 n‐3PUFAs vs placebo, outcome: 4.1 Depressive symptomology (continuous): Sub‐groups based on use / non‐use of adjunctive therapies.
Rates of adverse events were similar across the subgroups. Analysis of studies with a mix of individuals receiving and not receiving adjunctive therapy: OR 1.24 (95% CI 0.89 to 1.71); 8 studies, 752 participants, studies including individuals not receiving adjunctive therapy: OR 2.04 (95% CI 1.03 to 4.03); 4 studies, 259 participants, and studies including individuals receiving adjunctive therapy: OR 1.06 (95% CI 0.60 to 1.88); 12 studies, 492 participants, indicated that there was no statistical evidence of a difference between subgroups (P = 0.33) and limited evidence of heterogeneity between subgroups (I2 = 10.9%), see Analysis 4.2. However, confidence intervals are very wide and suggest possible effects ranging from a reduction in adverse events with n‐3PUFAs to a large increase in adverse events, compared with placebo. Evidence of heterogeneity was low in all subgroups (I2 = 0% to 23%).
4.2. Analysis.

Comparison 4: Subgroup analyses: n‐3PUFAs vs placebo ‐ analyses based on adjunctive therapy, Outcome 2: Adverse events
Sensitivity analyses
We conducted sensitivity analyses only for the n‐3PUFA versus placebo comparison. The sensitivity analyses using a fixed‐effect model were for all outcomes, while all other sensitivity analyses were conducted only for our primary outcome measures.
5. Low risk of bias
5.1 Selection bias
The results of analyses (random‐effects model) using only the studies that we judged to be at low risk of selection bias based on allocation concealment assessment (Bot 2010; Carney 2020; Grenyer 2007; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lespérance 2011; Lucas 2009; Masoumi 2016; Mazereeuw 2016; Mischoulon 2009; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Nemets 2002; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Shinto 2016; Silvers 2005; Su 2003) were:
Depressive symptomology (continuous data): SMD −0.36 (95% CI −0.67 to −0.05); I2 = 85%; 22 studies, 1449 participants.
Adverse events: OR 1.33 (95% CI 1.02 to 1.72); I2 = 0%; 18 studies, 1269 participants.
These analyses demonstrate a statistical difference between n‐3PUFA and placebo groups in depressive symptomology and adverse events, but confidence intervals are wide and suggest both a possible clinically important benefit of n‐3PUFAs and a negligible effect in depressive symptomology, with a possible increase in adverse events from 2% to 72%, with n‐3PUFAs compared to placebo. Effect sizes in depressive symptomology are slightly smaller than those in analyses of all studies, and effect sizes in adverse events are slightly larger than those found in the analyses of all studies.
5.2 Performance bias
The results of all analyses using only the studies that we judged to be at low risk of performance bias based on blinding of participants and personnel assessment (Bot 2010; Carney 2020; Lespérance 2011; Lucas 2009; Nemets 2002; Rondanelli 2010; Shinto 2016; Silvers 2005) were:
Depressive symptomology (continuous data): SMD 0.00 (95% CI −0.28 to 0.29); I2 = 62%; 8 studies, 793 participants.
Adverse events: OR 1.15 (95% CI 0.71 to 1.86); I2 = 30%; 8 studies, 812 participants.
These analyses demonstrate no differences between n‐3PUFA and placebo groups in depressive symptomology or adverse events, but confidence intervals are wide and suggest both a possible benefit of n3PUFAs and a detrimental effect of n‐3PUFAs in depressive symptomology, with a range of effects in adverse events from a possible reduction of 29% to a possible increase in adverse events of 86%, for n‐3PUFAs compared to placebo.
5.3 Attrition bias
The results of all analyses using only the studies that we judged to be at low risk of attrition bias, based on assessment of incomplete outcome data for depressive symptomology (Carney 2009; Carney 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Jahangard 2018; Lucas 2009; Masoumi 2016; Nemets 2002; Rondanelli 2010), and based on incomplete outcome data for adverse events (Carney 2020; Lucas 2009; Rondanelli 2010) were:
Depressive symptomology (continuous data): SMD −0.75 (95% CI −1.46 to 0.07); I2 = 93%; 9 studies, 482 participants.
Adverse events: OR 1.71 (95% CI 0.88 to 3.32); I2 = 0%; 3 studies, 219 participants.
These analyses demonstrate no differences between n‐3PUFA and placebo groups in depressive symptomology or adverse events, but confidence intervals are very wide and suggest both a possible clinically important benefit of n‐3PUFAs and a negligible effect in depressive symptomology, with a range of effects in adverse events from a possible reduction of 12% to a possible increase in adverse events of 232% for n‐3PUFAs compared with placebo. Effect sizes in both depressive symptomology and adverse events are also larger than in analyses of all studies, but confidence intervals are also wider and heterogeneity is higher in the analyses for depressive symptomology.
6. Fixed‐effect models
The results of all analyses using a fixed‐effect model were:
Depressive symptomology (continuous data): SMD −0.21 (95% CI −0.31 to −0.11); 33 studies, 1848 participants.
Adverse events: OR 1.29 (95% CI 1.02 to 1.63); 24 studies, 1503 participants.
Depressive symptomology (dichotomous data) ‐ remission: OR 1.09 (95% CI 0.77 to 1.55); 8 studies, 609 participants.
Depressive symptomology (dichotomous data) ‐ response: OR 1.15 (95% CI 0.85 to 1.56); 17 studies, 794 participants.
Quality of life: SMD −0.26 (95% CI −0.46 to −0.07); 12 studies, 476 participants.
Trial non‐completion: OR 0.93 (95% CI 0.71 to 1.21); 29 studies, 1777 participants.
Results are similar to those achieved using a random‐effects model, although effect sizes are noticeably smaller for measures of depressive symptomology and quality of life. Effect sizes in depressive symptomology are half the size using a fixed‐effect model compared to using a random‐effects model. Differences between n‐3PUFA and placebo groups in adverse events and in quality of life also become statistically significant. Supplementation with n‐3PUFAs results in increased adverse events and increased quality of life compared with supplementation with placebo.
Reporting bias
The funnel plot for the main analysis of depressive symptomology (continuous) is presented in Figure 5. This figure demonstrates considerable asymmetry, suggesting possible publication bias in this outcome.
Funnel plots for adverse events and trial non‐completion demonstrate some asymmetry. These figures also suggest possible publication bias in these outcomes (Figure 7 and Figure 12 respectively).
Sensitivity analyses based on study methodology
7. Enrolment of individuals with a specified diagnosis of major or unipolar depressive disorder
Seven studies enrolled individuals without a depressive diagnosis that was specified as 'major' or 'unipolar' depressive disorder (Gharekhani 2014; Masoumi 2016; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Rondanelli 2010; Silvers 2005).
The results of analyses without these studies were:
Depressive symptomology (continuous data): SMD −0.31 (95% CI −0.55 to −0.06); I2 = 77%; 26 studies, 1560 participants.
Adverse events: OR 1.30 (95% CI 0.96 to 1.76); I2 = 11%; 18 studies, 1257 participants.
These analyses demonstrate a small‐to‐modest benefit for n‐3PUFAs compared to placebo for depressive symptomology and no differences in adverse events, although confidence intervals again suggest an effect size for depressive symptomology that ranges from small to clinically important, and both a possible small decrease and a large increase in adverse events, for n‐3PUFAs compared to placebo. The overall effect size for depressive symptomology is smaller than that for all studies.
8. Use of a treatment that was solely or predominantly eicosapentaenoic acid (EPA)
8.1. Use of a treatment that was solely EPA
Ten studies used an intervention that was solely EPA (Bot 2010; Carney 2020; Gonzalez 2011; Jazayeri (v placebo) 2008; Jiang (EPA only) 2018; Mischoulon 2009; Nemets 2002; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002).
The results of analyses using only these studies were:
Depressive symptomology (continuous data): SMD −0.18 (95% CI −0.44 to 0.09); I2 = 27%; 10 studies, 401 participants.
Adverse events: OR 1.54 (95% CI 0.89 to 2.67); I2 = 0%; 8 studies, 353 participants.
These analyses demonstrate no differences between n‐3PUFAs and placebo for depressive symptomology or adverse events, although confidence intervals again suggest an effect size for depressive symptomology that ranges from small to clinically important, and both a possible reduction and an increase in adverse events, for n‐3PUFAs compared to placebo. The overall effect size for depressive symptomology is smaller than that for all studies, and the evidence of heterogeneity is also smaller.
8.2. Use of a treatment that was predominantly EPA
Seventeen studies used an intervention that was predominantly EPA (Carney 2009; Chang 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gertsik 2012; Gharekhani 2014; Jiang (EPA+DHA) 2018; Lespérance 2011; Lucas 2009; Mazereeuw 2016; Mischoulon (EPA) 2015; Park 2015; Rondanelli 2010; Shinto 2016; Su 2003).
The results of analyses using only these studies were:
Depressive symptomology (continuous data): SMD −0.26 (95% CI −0.52 to −0.01); I2 = 65%; 17 studies, 1073 participants.
Adverse events: OR 1.20 (95% CI 0.77 to 1.87); I2 = 34%; 13 studies, 982 participants.
These analyses demonstrate a small benefit for n‐3PUFAs compared to placebo for depressive symptomology and no differences between groups for adverse events, although confidence intervals again suggest an effect size for depressive symptomology that ranges from small to clinically important, and both a possible reduction and an increase in adverse events, for n‐3PUFAs compared to placebo. The overall effect size for depressive symptomology is smaller than that for all studies, and the evidence of heterogeneity is also smaller.
9. Inclusion of ALA in placebo capsules
Nine studies used a placebo containing ALA (an n‐3PUFA) as a comparison (Bot 2010; Chang 2020; Coryell (1g/d) 2005; Coryell (2g/d) 2005; Jazayeri (v placebo) 2008; Mazereeuw 2016; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Shinto 2016).
The results of analyses without these studies were:
Depressive symptomology (continuous data): SMD −0.58 (95% CI −0.89 to −0.27); I2 = 85%; 24 studies, 1483 participants.
Adverse events: OR 1.25 (95% CI 0.85 to 1.83); I2 = 0%; 18 studies, 1252 participants.
These analyses demonstrate a modest benefit for n‐3PUFAs compared to placebo for depressive symptomology and no differences between groups for adverse events, although confidence intervals again suggest an effect size for depressive symptomology that ranges from small to clinically important, and both a possible reduction and an increase in adverse events, for n‐3PUFAs compared to placebo. The overall effect size for depressive symptomology is larger than that for all studies, but the evidence of heterogeneity between studies is also slightly higher.
10. Treatment duration for 12 weeks or more
Seventeen studies provided supplementation for 12 weeks or more (Bot 2010; Chang 2020; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gharekhani 2014; Grenyer 2007; Jahangard 2018; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Kamath 2017; Mazereeuw 2016; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Shinto 2016; Silvers 2005).
The results of analyses using only these studies were:
Depressive symptomology (continuous data): SMD −0.38 (95% CI −0.79 to 0.03); I2 = 81%; 16 studies, 614 participants.
Adverse events: OR 1.26 (95% CI 0.70 to 2.28); I2 = 23%; 11 studies, 412 participants.
These analyses demonstrate no differences between n‐3PUFAs and placebo for depressive symptomology or adverse events. Overall effect sizes are similar to those found in our main analyses, but confidence intervals are wider, and again suggest an effect size for depressive symptomology that ranges from very small to clinically important, and both a possible reduction and an increase in adverse events, for n‐3PUFAs compared to placebo.
11. Use of data from per protocol analyses
We could not obtain clear ITT data (either from publications or from authors) for 14 studies (Bot 2010; Chang 2020; Da Silva (AD) 2005; Da Silva (nAD) 2005; Gonzalez 2011; Marangell 2003; Mazereeuw 2016; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Park 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002; Silvers 2005).
The results of analyses without these studies were:
Depressive symptomology (continuous data): SMD −0.53 (95% CI −0.88 to −0.18); I2 = 87%; 19 studies, 1347 participants.
Adverse events: OR 1.23 (95% CI 0.82 to 1.85); I2 = 31%; 16 studies, 1121 participants.
These analyses demonstrate a modest benefit for n‐3PUFAs compared to placebo for depressive symptomology and no differences between groups for adverse events, although confidence intervals again suggest an effect size for depressive symptomology that ranges from small to clinically important, and both a possible reduction and an increase in adverse events, for n‐3PUFAs compared to placebo. The overall effect size for depressive symptomology is larger than that for all studies, but the evidence of heterogeneity between studies is also slightly higher.
12. Use of imputed standard deviations from other studies in analyses
We imputed standard deviations for six studies (Chang 2020; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002).
The results of analyses without these studies were:
Depressive symptomology (continuous data): SMD −0.46 (95% CI −0.75 to −0.17); I2 = 84%; 27 studies, 1543 participants.
Adverse events: OR 1.22 (95% CI 0.87 to 1.72); I2 = 20%; 19 studies, 1258 participants.
These analyses demonstrate a modest benefit for n‐3PUFAs compared to placebo for depressive symptomology and no differences between groups for adverse events, although confidence intervals again suggest an effect size for depressive symptomology that ranges from small to clinically important, and both a possible reduction and an increase in adverse events, for n‐3PUFAs compared to placebo. The overall effect size for depressive symptomology is larger than that for all studies, but the evidence of heterogeneity between studies is also slightly higher.
13. Consideration of multiple comparison groups from the same study as individual studies
Five trials used multiple treatment groups that we considered in our primary analyses as independent studies (Coryell (1g/d) 2005; Coryell (2g/d) 2005; Da Silva (AD) 2005; Da Silva (nAD) 2005; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015; Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002). Use of these studies as independent groups may magnify between‐study heterogeneity, although data for all of these studies have been provided for each group separately, so that combining groups results in standard deviations that are estimations based on pooling calculations.
The results of analyses using combined as opposed to split studies were:
Depressive symptomology (continuous data): SMD −0.43 (95% CI −0.70 to −0.17); I2 = 84%; 27 studies, 1848 participants.
Adverse events: OR 1.26 (95% CI 0.94 to 1.71); I2 = 19%; 19 studies, 1503 participants.
These results are very comparable to those conducted using all studies as independent studies.
Discussion
Summary of main results
Our update searches resulted in the addition of nine independent studies (from eight trials) involving 486 participants published since 2015, to the 26 studies (20 trials), 1458 participants, of our earlier review (Appleton 2015). Thus, the updated review includes 35 studies (from 28 trials) comparing the impact of n‐3PUFAs on major depressive disorder (MDD) to two different comparators. Thirty‐four studies involving 1924 participants investigated the impact of n‐3PUFAs in MDD compared to placebo, and one study involving 40 participants investigated the impact of n‐3PUFAs in MDD compared to antidepressant treatment.
For the comparison with placebo, we provide a summary of findings table (Table 1).
Our primary outcomes were depressive symptomology assessed using a continuous measure, and adverse events. Mean depressive symptomology in n‐3PUFA groups was lower than in placebo groups following treatment, with a small‐to‐modest effect size representing a difference between groups in scores on the HDRS (17‐item) of approximately 2.5 (1.0 to 4.0 respectively). NICE guidelines (NICE 2004) have previously suggested a reduction in HDRS score of 3 or more to be clinically meaningful, thus the clinical significance of our effect size is small. The confidence intervals do not exclude a clinically meaningful effect, but also include a negligible effect at the lower end. Furthermore, the completeness and certainty of the evidence was very low (see below).
Numbers of individuals experiencing adverse events were similar between intervention and placebo groups, although assessments of adverse events were suitable for analysis in only 24 of the 34 studies. Our confidence intervals suggest that effects could range from a very small decrease to a modest increase in adverse events in n‐3PUFA groups compared with placebo. Furthermore, the completeness and certainty of the evidence providing these results were also very low.
Rates of depression remission and response were also similar following n‐3PUFA supplementation compared to placebo, but confidence intervals again suggest a range of possible effects from a small reduction in remission and response rates to a large increase. Quality of life was similar in n‐3PUFA compared with placebo groups, although our confidence intervals again suggest both a possible negligible effect and a possible clinically important benefit of n‐3PUFAs compared to placebo. Rates of trial non‐completion were also similar between intervention and placebo groups, although our confidence intervals again suggest possible effects that could range from a small reduction to a modest increase in trial withdrawals in n‐3PUFA groups compared with placebo.
There was only one study involving 40 participants for the comparison with antidepressants. This study found no differences between treatment with n‐3PUFAs and treatment with antidepressants in depressive symptomology, rates of response to treatment, or non‐completion. Adverse events were not reported in a manner suitable for analysis, and rates of depression remission and quality of life were not reported.
Overall completeness and applicability of evidence
The evidence for both comparisons and for all outcomes is limited and highly heterogeneous, resulting in findings that are imprecise and potentially biased.
Firstly, for the comparison with placebo, the evidence comes from 34 studies, involving only 1924 participants and with only 1848 participants contributing to our main analysis. While data are available from all except for one small study for the analyses on our primary outcome of depressive symptomology, only small numbers of studies contributed to some of our outcomes.
The studies available were highly heterogeneous. All studies were directly relevant to our research question, but we found considerable differences in all aspects of study methodology. Studies differed in the type of participants involved, the interventions used, the comparators used, the duration of supplementation, and the range and measurement of outcomes assessed.
Most available studies were also small. Over half of all participants derive from only five trials: Carney 2009 (122 participants), Carney 2020 (144 participants), Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018 (108 participants), Lespérance 2011 (432 participants), and Mischoulon (DHA) 2015; Mischoulon (EPA) 2015 (196 participants). We judged these trials to be at low risk of bias on most measures, but the contribution of these five trials to our overall outcomes was high, even using a random‐effects model, and the outcomes of our meta‐analyses reflect the outcomes of these specific trials. All five trials found a negligible mean difference between n‐3PUFA and placebo groups following supplementation in all outcomes assessed. Given the proportion of data in our analyses that is provided from these few trials, biases or methodologically specific outcomes in these trials may have contributed to our overall result.
The funnel plot for depressive symptomology also suggests an absence of small‐to‐medium studies showing null findings. This asymmetry suggests probable publication bias, and that our analyses and overall effect size estimates may be biased towards a positive finding for n‐3PUFAs compared to the true situation. Sensitivity analyses using a fixed‐effect model also demonstrated a smaller standardised mean difference between n‐3PUFA and placebo groups than that found using a random‐effects model, suggesting a positive influence from small positive studies in our main analyses.
Sensitivity analyses using only the studies that we judged to be at low risk of bias also suggest bias in our main analyses towards a positive finding for n‐3PUFAs. Many studies were judged to be at high risk of bias in various domains. Analyses using only studies that we judged to be at low risk of selection bias and performance bias report smaller effect sizes than those found in our main analyses, and confidence intervals include the possibility of no differences between groups. This evidence, alongside that of the funnel plot and the findings using a fixed‐effects model, suggest that the true effect of n‐3PUFAs is likely to be smaller than that reported in our main analyses. Analyses using only the studies judged to be at low risk of attrition bias report larger effect sizes in depressive symptomology than we found in our main analyses, but confidence intervals are very wide, include the possibility of a negligible effect, and heterogeneity between studies is considerable.
Imprecise effect size estimates were found for all outcomes. In all analyses, possible effects range from negligible (and in some analyses from negative) effects to important clinical benefits. While this imprecision does not rule out clinically relevant effects, considerable caution must be used in interpreting all effect size estimates. Further evidence, in the form of adequately‐powered well‐designed trials, is clearly required before firm conclusions can be drawn.
Findings in our primary outcome of depressive symptomology and our secondary outcome of quality of life also demonstrate considerable evidence of heterogeneity. Subgroup analyses investigated possible sources, based on the inclusion of individuals with/without comorbid conditions and the inclusion of individuals using/not using adjunctive therapy, but we found little explanation for this heterogeneity. There is some evidence of different effects depending on the presence or absence of comorbid conditions, but there is considerable overlap in effect size estimates for the different subgroups, with considerable evidence of heterogeneity, and the strongest evidence for an effect stems from a subgroup including only four studies. Limited explanation was also gained from the analyses on adjunctive therapy, where we found few differences between subgroups, although findings again are far from conclusive.
Sensitivity analyses also investigated impacts of other aspects of study methodology. Analyses investigating the effects of diagnosis, and treatment solely or predominantly with EPA revealed a lesser beneficial effect of n‐3PUFAs compared to placebo than we found in our main analyses, with increased precision. This increased precision suggests a more consistent effect in these studies, supporting smaller effects of n‐3PUFAs on depressive symptomology or adverse events in those with a specified "major" or "unipolar" depressive diagnosis and in those receiving an EPA only or predominantly EPA treatment. The possibility of different effects depending of n‐3PUFA type has been suggested in the literature (Martins 2011; Ross 2007; Sublette 2011), although much of this speculation is based on post‐hoc observation, and the two trials that directly compare treatments using different n‐3PUFAs find no differences between treatments (Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015). Effects size estimates do increase with the removal of studies that use placebos containing ALA. This analysis suggests that ALA may confer some impacts on depressive symptomology similar to those of the longer chain n‐3PUFAs EPA and DHA, but few studies were available for assessment and heterogeneity between study findings remains substantial. Investigation of effects in only the studies using treatment schedules of 12 weeks or more resulted in comparable effect sizes to those found in our main analyses, with reduced precision. Opportunity for confounding may be increased with longer treatment schedules, but these findings may also suggest bias in our main analyses towards a positive finding for n‐3PUFAs. Effect size estimates for depressive symptomology increase in analyses involving only the studies providing ITT data, and in analyses involving only the studies where SDs are reported, but confidence intervals and heterogeneity again also increase. These increased effect sizes suggest greater benefits of n‐3PUFAs compared with placebo in studies providing complete data sets, an effect that may demonstrate both the spontaneous remission in depressive symptoms often found in this field and the added benefit of receiving treatment. Likely apparent effects of n‐3PUFAs as a result of spontaneous remission and/or a placebo response have been suggested elsewhere (Kirsch 2019; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015, Nasir 2019), and spontaneous remission, a placebo response, or both, may also explain our effects in studies with a low risk of attrition bias. Given the possibility of study withdrawal due to improvements as well as detriments in depressive symptomology, the direction of the differences in the results between analyses using ITT populations and analyses using per‐protocol populations, or based on attrition bias, may be uninformative in this research field. Sensitivity analyses investigating the use of complete trials, as opposed to individual studies, reveal few differences in findings from those in our main analyses, but again substantial heterogeneity remains.
The remaining high heterogeneity in our sensitivity analyses suggests that other differences between studies may also contribute to study findings. Notably our subgroup analyses and sensitivity analyses explain very little of the heterogeneity found. Discussion of potential mechanisms of action for n‐3PUFAs suggest different responses to n‐3PUFAs based on individual characteristics, such as n‐3PUFA metabolism and inflammation status (Kalkman 2021; Nasir 2019; Rapaport 2016), but these effects will be difficult to tease apart in aggregate analyses, such as meta‐analyses. We have no further hypotheses to explain additional heterogeneity, even following consideration of those studies demonstrating strong effects versus those demonstrating no effects. Some studies do report clear positive effects for n‐3PUFAs (Jahangard 2018, Masoumi 2016, Su 2003), but similarities between these studies and differences from other studies are unclear.
Only one study was available for the comparison with antidepressant treatment. This study was small, with 20 participants randomised to each treatment arm, and 20% of participants in each arm did not complete the study. Adverse events were reported by the number of events rather than the number of individuals experiencing them, remission rates were not reported, but response rates and trial non‐completion data were supplied.
Quality of the evidence
The certainty of the evidence for both comparisons for all outcomes is low to very low. Our judgements of certainty according to GRADE are given in Table 1.
For the placebo comparison, for our primary outcome of depressive symptomology, we considered the certainty of evidence to be very low. The body of evidence was composed of limited, predominantly small studies, within which there was substantial evidence of heterogeneity that remains unexplained. Furthermore, most of the contributing studies include judgements of high risk of bias in at least one of the domains assessed, and sensitivity analyses reveal different findings in analyses using all studies and analyses using only studies judged to be at low risk of selection bias, performance bias or attrition bias. In analyses using only studies judged to be at low risk of selection bias and performance bias, we found limited impacts of n‐3PUFAs on depressive symptomology, compared to placebo. We found similar results from the five large trials mentioned earlier (Carney 2009; Carney 2020; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018; Lespérance 2011; Mischoulon (DHA) 2015; Mischoulon (EPA) 2015). In analyses using only studies judged to be at low risk of attrition bias, we found a modest benefit of n‐3PUFAs, but confidence intervals are very wide and heterogeneity is considerable. This high level of unexplained heterogeneity in all our analyses of depressive symptomology suggests considerable need for caution. Sensitivity analyses using fixed‐effect models also demonstrate smaller effects of n‐3PUFAs on depressive symptomology compared to our main analyses. These findings suggest that the positive effect of n‐3PUFAs in our main analyses is a consequence predominantly of the inclusion of small studies that may be at high risk of bias. The true effect size estimate is thus likely to be smaller than that provided by our main analyses.
For our second primary outcome of adverse events, the certainty of evidence was very low. There was limited evidence of heterogeneity between studies in this analysis, but confidence intervals are wide, suggesting a range of possible effects; data were only available from selected studies, and visual inspection of the funnel plot suggests probable publication bias. Analyses using only studies judged to be at low risk of selection bias, low risk of performance bias, or low risk of attrition bias also suggest some effects differing from those presented in our main analyses.
For our secondary outcomes of depression remission and response rates, the certainty of the evidence was low. Heterogeneity between studies was low, but confidence intervals are very wide, suggesting a broad range of possible effects, selected studies only were available for these analyses, and we judged various elements of the included studies to be at high risk of bias.
For our secondary outcome of quality of life, the certainty of the evidence was very low. Selected studies only were available for these analyses, confidence intervals are very wide, suggesting a broad range of possible effects, there was considerable heterogeneity, and we judged various elements of the included studies to be at high risk of bias.
For our secondary outcome of trial non‐completion, the certainty of the evidence was very low. Heterogeneity between studies was low, but not all studies were available for this analysis, confidence intervals are again wide, suggesting a range of possible effects, and visual inspection of the funnel plot suggests a probability of publication bias.
For the antidepressant comparison, the certainty of the evidence was low. Evidence for this analysis came from only one study. We judged this study to be at high risk of bias for allocation concealment, because the randomisation sequence was not concealed from researchers; for performance bias, because no steps were reported to mask the fishy taste of the intervention or check concealment; for attrition bias, due to a 20% dropout rate; and for reporting bias, because some outcomes have not yet been published. We judged data on depressive symptomology to be at low risk of bias due to good blinding of study personnel, but we judged data on adverse events to be at high risk of detection bias because these were reported by participants, and adequate blinding was unclear. We considered the risk of attrition bias for adverse events to be high, because while all adverse events were reported, dropout from the study was higher than 10%.
Inconsistency in reporting for both comparisons was obvious. Depressive symptomology was frequently reported and analysed using non‐ITT data (assuming a definition of ITT based on number randomised). Adverse events were reported in a variety of ways: by individual and event for all events, by individual for all events, by individual for only serious, likely or frequent events, by event type for all events, by event type only for serious, likely related or frequent events, or a combination of these. Adverse events were included for analysis (based on the number of individuals) in 24 of the 35 studies. Our secondary outcomes of remission and response in depressive symptomology and quality of life were not well‐reported. Numbers of participants who did not complete each trial were well‐reported.
Potential biases in the review process
The findings of this review are likely to be biased, due to the evidence available to contribute to analyses. Only a limited number of studies were available for assessing all outcomes for both comparisons, only a few studies were available for assessing some outcomes, and there was a high relative weighting in all analyses for the placebo comparison from five large trials.
The review process also may have been biased. Our searches were more likely to detect articles published in English and in mainstream journals. We tried to minimise this bias by including translated articles, but translations were only undertaken for full articles that we selected for inclusion based on titles and abstracts. Reporting was also poor for some studies, and we received no responses from some authors, so that the information and data available to us were incomplete. We found poor reporting specifically in the definition of MDD used in some studies, resulting in discussions over study inclusion. Studies that we excluded from the review based on our inclusion/exclusion criteria are included in Appendix 6, to clarify our decision‐making in this respect. We made judgements of risk of bias according to predefined rules, but the information required to make these judgements was also often poorly reported, and our correspondence with authors was again incomplete. Judgements of risk of bias, however, were completed following all data collection, so did not impact on the review process. We relied on authors of existing relevant studies or trial registrations for information on unpublished studies. Our searches covered relevant conference‐based publications, but we made no further attempts to identify unpublished literature.
Our analyses may also be biased due to the limited available data from some studies, and the compromises necessary to ensure sufficient data for analyses to be conducted. Reliance on available data (even from authors), meant that only a few studies could contribute to certain analyses. Remission and response rates were not assessed in all studies, but could have been calculated had raw data been available. Most studies did not assess quality of life. Studies were combined regardless of differences in various aspects of study methodology. Our sensitivity analyses revealed limited effects as a result of these differences, but caution should be applied. The number of analyses conducted and inconsistencies in findings may also limit the strength of conclusions that can be drawn.
Agreements and disagreements with other studies or reviews
Many reviews investigating a role for n‐3PUFAs in depressive disorders have now been conducted (Bae 2018; Bai 2020; Chambergo‐Michilot 2021; Grosso 2014; Liao 2019; Lin 2012; Martins 2011; Schefft 2017), and reviews of reviews are also available (Firth 2019; Haller 2019; Nasir 2019). These reviews typically use a very broad definition of depression to consider studies of individuals with a variety of depressive disorders and conditions, and/or include studies that vary in severity of depressive symptomology to include studies in individuals with mild and moderate depression. Early reviews tended to use a broader working definition of depressive symptomology, to allow inclusion of adequate studies for analyses, but more recent reviews have used tighter inclusion criteria. Definitions of MDD however, are found to vary. Many reviews also focus on specific populations, e.g. older adults (Bae 2018), individuals with adjunctive therapy (Chambergo‐Michilot 2021).
Of the recent reviews, Liao 2019 investigates n‐3PUFAs in MDD using a research question very similar to ours. This review includes 26 trials, 19 of which are also included in our review, while six trials were excluded from our review because they specify the study of mild‐to‐moderate depression, and one trial is a duplicate analysis of an already included study. This review also lacks two large recent trials that are included in our review (Carney 2020; Jiang (EPA+DHA) 2018; Jiang (EPA only) 2018), and two medium‐sized studies which we have included (Jahangard 2018; Masoumi 2016). Despite these differences, the effect size reported by Liao 2019 is similar to ours; they report a benefit of n‐3PUFAs compared with placebo for MDD of SMD −0.28 (95% CI −0.47 to −0.09), I2 = 75%.
Bai 2020 includes consideration of n‐3PUFAs as a treatment for MDD, as part of a review on the treatment of MDD with anti‐inflammatory agents. This review includes 13 of the trials we have included, plus an additional three trials which we excluded on the grounds that individuals in these trials have depressive disorders other than MDD, such as perinatal depression, and one duplicate analysis of an already included study. This review also reports a beneficial effect of n‐3PUFAs for MDD compared with placebo of SMD −0.35 (95% CI −0.60 to −0.09), 12 studies, 746 participants, I2 = 61%.
A review of complementary therapies by Schefft 2017 also found some evidence for a benefit from n‐3PUFAs as an adjunct to antidepressant treatment. Analyses revealed an effect size of SMD −0.48 (95% CI −0.84 to −0.11); 10 studies, 402 participants, which was further strengthened by the removal of four studies on individuals with comorbidities (SMD −0.70 (95% CI −0.81 to −0.09)). These analyses considered a subset of the studies included in our analyses, plus an additional study that we excluded, because the individuals in this study did not have a diagnosis of MDD and the study reported an interest in mild‐to‐moderate depression (Mozaffari‐Khosravi 2013).
Older reviews, including our earlier review, also report similar combined effect size estimates representing a beneficial effect of n‐3PUFAs compared to placebo for MDD: (Appleton 2015): SMD (depressive symptomology) −0.30 (95% CI −0.10 to −0.50); 25 studies, 1373 participants, very low‐certainty evidence; OR (adverse events) 1.24 (95% CI 0.95 to 1.62); 19 studies, 1207 participants, very low‐certainty evidence; Grosso 2014: SMD (depressive symptomology) −0.38 (95% CI −0.18 to −0.59); I2 = 55%; Lin 2012: SMD (depressive symptomology) −0.29 (95% CI −0.10 to −0.48); 11 studies; and Martins 2011: SMD (depressive symptomology) −0.45 (95% CI −0.75 to −0.15); 14 studies.
Of the reviews of reviews, Nasir 2019 provides a comprehensive overview of 15 meta‐analyses investigating the impacts of n‐3PUFAs as a treatment for depressive disorders. They report combined effect size estimates ranging from SMD −0.10 (95% CI −0.02 to −0.17) to SMD −0.65 (95% CI −0.18 to −1.12), with effects reduced to negligible when publication bias was statistically accounted for: SMD −0.01 (95% CI 0.13 to −0.15) and SMD −0.06 (95% CI 0.08 to −0.21). They conclude that "Meta‐analyses of randomised controlled trials of omega‐3 fatty acids for depression are inconclusive, with strong evidence of publication bias, sizable heterogeneity between included studies, and substantial methodological shortcomings in included trials" (abstract), such that "recommending the use of omega‐3 fatty acids in adulthood psychiatric conditions should be revisited" (abstract).
Firth 2019 includes four reviews of n‐3PUFAs as a nutritional treatment for MDD: Schefft 2017, Grosso 2014, Mocking 2016, which provides a combined effect size estimate of SMD −0.40 (95% CI −0.11 to −0.68); 13 studies, and Hallahan 2016 that provides combined effect size estimates of G −0.61 (95% CI −0.38 to −0.85); I2 = 61%, for EPA treatment in individuals with a depressive diagnosis, and G −0.03, SE 0.10 for DHA treatment in individuals with a depressive diagnosis, compared with placebo. The conclusions reflect these later reviews to suggest "small‐to‐moderate positive effects from high‐EPA formulas in clinical depression generally, as well as an adjunctive to SSRIs in MDD", despite criticisms of some of the reviews (Bastiaansen 2016). This review also concluded that the nutritional intervention for mental health disorders with the strongest evidential support was omega‐3 fatty acids, but does not include any of our previous reviews (Appleton 2006; Appleton 2010; Appleton 2015), despite reported searches of the Cochrane Library.
The review of reviews by Haller 2019 includes only our previous review on n‐3PUFAs (Appleton 2015) as a complementary treatment for MDD, and concludes that of all the treatments considered, sufficient evidence of benefit was only available to support the use of St John's Wort.
Conclusions from the reviews of reviews are very different. In the individual reviews, findings are more similar. Consistent findings from several meta‐analyses, despite the inclusion and exclusion of different trials, may suggest a consistent effect of n‐3PUFAs versus placebo, but the consistency is more likely in the limited evidence available for investigating these effects. All reviews are based on the same very limited pool of studies, and report wide confidence intervals and so a wide range of possible effects, substantial heterogeneity between studies, and a high probability of publication bias.
Our effect size estimate is also comparable to some degree with that suggested by meta‐analyses of the effects of antidepressants for MDD, compared to placebo. Kirsch 2008 reports a weighted mean difference using the HDRS between antidepressant and placebo groups in 35 US Food and Drug Administration (FDA)‐registered studies of 1.8 HDRS scores, SMD −0.32 (95% CI −0.25 to −0.40). Turner 2008 reports a mean weighted effect size of −0.37 (95% CI −0.33 to −0.41) from published and −0.15 (95% CI −0.08 to −0.22) from unpublished US FDA‐registered studies. Fountoulakis 2013, in an analysis of meta‐analyses, confirms a SMD of −0.32 (95% CI −0.25 to −0.40), as the result from the most appropriate analysis of the Kirsch 2008 data set. More recent analyses confirm these results; Cipriani 2018, in a meta‐analysis of 432 studies, report an SMD of −0.30 (95% CI −0.26 to −0.34) for antidepressants compared to placebo, a consistent effect that was also supported by Bayesian analyses (Volkmann 2020). Confidence intervals are tighter for the analyses of antidepressants than was found in our analyses, suggesting greater precision in these effect size estimates, but comparable small‐to‐modest effect size estimates both in our findings and in those of reviews of antidepressants should not be taken as evidence in support of n‐3PUFAs. The small size of the overall effect estimate for both n‐3PUFAs and antidepressants should argue not for a favourable comparison of n‐3PUFAs with antidepressants, but for increased demand for more effective treatments for depressive symptomology from elsewhere. Similar calls are found elsewhere (Volkmann 2020).
[Note: To allow comparability between studies and consistency in this section, all results have been reported where a negative effect size demonstrates a benefit (reduced depressive symptomology) of n‐3PUFAs / antidepressants compared with placebo, and a positive effect size demonstrates a detriment of n‐3PUFAs / antidepressants; the direction of the reporting may differ in the original publications, depending on the way in which the analyses were conducted].
Authors' conclusions
Implications for practice.
At present, we do not have sufficient high‐certainty evidence to determine the effects of n‐3PUFAs as a treatment for MDD. Our primary analyses suggest a small‐to‐modest, non‐clinically beneficial effect of n‐3PUFAs on depressive symptomology compared to placebo, although the effect size estimate is imprecise, and the certainty of the evidence on which this result is based is low to very low. Sensitivity analyses, funnel plot inspection and comparison of our results with those of large well‐conducted trials also suggest that this effect size estimate is likely to be biased towards a positive finding for n‐3PUFAs, and that the true effect is likely to be smaller. The one study in our review that directly compares n‐3PUFAs and antidepressants finds comparable benefit, but the certainty of the evidence here is very low. Our data suggest similar rates of adverse events and numbers not completing trials in n‐3PUFA and placebo groups. The data on adverse events and trial non‐completion are again of very low certainty, but given the high rates of adverse events associated with some antidepressants, n‐3PUFAs may offer an alternative treatment of possible benefit and reduced side effects. However, whether all possible negative side effects are studied in trials is questionable, and dropouts as a result of lack of improvement testify to the negative side effects of false hope. Failure to seek or administer conventional treatment, as a result of treatment with n‐3PUFAs, may also represent an opportunity cost. We need more evidence, and particularly more complete evidence, for both the positive and negative effects of n‐3PUFAs for MDD.
Implications for research.
More adequately‐powered well‐designed studies are required to increase the evidence base, and explore particularly the heterogeneity found between studies investigating the impact of n‐3PUFAs on depressive symptomology. Many studies are currently underway, but studies that compare n‐3PUFAs with usual antidepressant treatment, and studies to investigate differing effects depending on individual characteristics and study methodology are important. Our review suggests similar effects for n‐3PUFAs and antidepressant treatment for depressive symptomology, but benefits of n‐3PUFAs in terms of adverse events, compliance and patient acceptability are often provided by practitioners. Studies that compare n‐3PUFAs with antidepressant treatment for all possible outcomes are required. Long‐term benefits, long‐term acceptability and long‐term compliance are rarely considered, and neither is cost effectiveness. Studies comparing individuals, different treatments and treatments of differing durations are also needed. Studies do find positive effects, and identification of those who are likely to benefit, or the particular treatments of beneficial impact would be of value. Studies of adequate supplementation duration, while also considering likely confounding, adverse events and acceptability are particularly needed. Mechanistic studies are also preferentially required. Hypotheses investigating different effects depending on participant type or study methodology should be based on proposed mechanisms to increase efficacy, as opposed to post‐hoc comparisons of individual studies. Future research should target the elucidation of mechanisms both for the development and treatment of MDD, and should identify the possible actions in these pathways for n‐3PUFAs. Precise and complete trial reporting would also be of value.
What's new
| Date | Event | Description |
|---|---|---|
| 22 November 2021 | New citation required but conclusions have not changed | The review has been updated. |
| 22 November 2021 | New search has been performed | This is an update of an existing Cochrane Review (Appleton 2015). We used the same methods as in the previous review with some refinements, as detailed in the section on 'Differences between protocol and review'. The update includes 9 independent studies (from 8 randomised controlled trials) published since 2015, in addition to the 26 independent studies (from 20 trials) included in the previous review. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 9 December 2016 | Amended | It has recently transpired that the MADRS scores used in our analyses for the trial by Bot et al (2010) were reversed between intervention and placebo groups. These data resulted from correspondence with the authors of this trial and they have recently confirmed an error. Reversal of the data for these groups changes our results minimally, does not change our interpretation of our results and does not change our conclusions. Reversal of these data results in the following changes to the results of the following analyses: 1.1: n‐3PUFAs vs placebo: depressive symptomology (continuous data)(Figures 4,5): original published result – SMD = ‐0.32 (95% CI ‐0.52 to ‐0.12), I2 = 58%; revised result ‐ SMD = ‐0.30 (95% CI ‐0.50 to ‐0.10), I2 = 59%. This result represents a difference between groups in scores on the HDRS (17‐item) of approximately 2.1 points (95% CI 0.7 to 3.5). 3: n‐3PUFAs vs placebo: depressive symptomology (continuous data) subgroup analyses based on presence/absence of comorbidities (Figure 13): Individuals with comorbidities: original published result ‐ SMD = ‐0.65 (95% CI ‐1.28 to ‐0.02), I2 = 74%; revised result ‐ SMD = ‐0.54 (95% CI ‐1.21 to 0.12), I2 = 77%. Statistical evidence of a difference between subgroups remains (p = 0.04), and evidence of heterogeneity between subgroups remains high (I2 = 68%). 4: n‐3PUFAs vs placebo: depressive symptomology (continuous data) subgroup analyses based on presence/absence of adjunctive therapies (Figure 14): Individuals receiving adjunctive therapy: original published result ‐ SMD = ‐0.21 (95% CI ‐0.42 to 0.01), I2 = 0%; revised result ‐ SMD = ‐0.16 (95% CI ‐0.38 to 0.05), I2 = 0%. No statistical evidence of a difference between subgroups remains (p = 0.48), and no evidence of heterogeneity between subgroups remains (I2 = 0%). 5.1: n‐3PUFAs vs placebo: depressive symptomology (continuous data) sensitivity analyses based on selection bias: original published result ‐ SMD = ‐0.21 (95% CI ‐0.45 to 0.03), I2 = 59%; revised result ‐ SMD = ‐0.18 (95% CI ‐0.42 to 0.06), I2 = 60%. 5.2: n‐3PUFAs vs placebo: depressive symptomology (continuous data) sensitivity analyses based on performance bias: original published result ‐ SMD = ‐0.14 (95% CI ‐0.55 to 0.26), I2 = 69%; revised result ‐ SMD = ‐0.07 (95% CI ‐0.48 to 0.35), I2 = 70%. 6: n‐3PUFAs vs placebo: depressive symptomology (continuous data) sensitivity analyses using fixed effects models: original published result ‐ SMD = ‐0.20 (95% CI ‐0.31 to ‐0.09); revised result ‐ SMD = ‐0.19 (95% CI ‐0.30 to ‐0.08). 7.1: n‐3PUFAs vs placebo: depressive symptomology (continuous data) sensitivity analyses based on use of a treatment that was solely EPA: original published result ‐ SMD = ‐0.45 (95% CI ‐0.74 to ‐0.15), I2 = 0%; revised result ‐ SMD = ‐0.37 (95% CI ‐0.66 to ‐0.08), I2 = 0%. 11: n‐3PUFAs vs placebo: depressive symptomology (continuous data) sensitivity analyses based on consideration of multiple comparison groups from the same study as individual studies: original published result ‐ SMD = ‐0.34 (95% CI ‐0.56 to ‐0.12), I2 = 67%; revised result ‐ SMD = ‐0.32 (95% CI ‐0.54 to ‐0.10), I2 = 68%. |
| 1 May 2014 | New citation required and major changes | This protocol replaces the withdrawn protocol Silvers 2009 (withdrawn). |
Acknowledgements
This work is supported by Bournemouth University, UK; the University of Bristol, UK; and the NIHR Biomedical Research Unit in Nutrition, Diet and Lifestyle supported by University Hospitals NHS Foundation Trust and the University of Bristol, UK.
We are grateful to Nick Meader, Centre for Reviews and Dissemination, University of York who assisted with translation to determine eligibility of two studies written in Japanese. Nick Meader is Deputy Co‐ordinating Editor of Cochrane Common Mental Disorders but was not involved in the editorial process or decision making for this article.
Cochrane Common Mental Disorders (CCMD) supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, Senior Editor, Cochrane Mental Health and Neuroscience Network, and Imperial College London; Nuala Livingstone, Associate Editor, Cochrane Mental Health and Neuroscience Network.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Jessica Hendon, CCMD, Centre for Reviews and Dissemination, University of York
Peer‐reviewers (provided comments and recommended an editorial decision): Alice V Stanton, Schools of Medicine, Pharmacy & Biomolecular Sciences, Royal College of Surgeons in Ireland, and Devenish Nutrition, Ireland (clinical/content review); Maria Isabel Costa, Universidade Fernando Pessoa (consumer review); Eleonora Uphoff*, CCMD, Centre for Reviews and Dissemination, University of York (methods review).
Copy Editor (copy‐editing and production): Kate Cahill, Cochrane Tobacco Addiction Group, Nuffield Department of Primary Care Health Sciences, University of Oxford
* Eleonora Uphoff is part of the CCMD Editorial Base, and provided peer‐review comments on this article, but Eleonora Uphoff was not otherwise involved in the editorial process or decision making for this article.
The authors and the CCMD Editorial Team are grateful to the peer reviewers for their time and comments. They would also like to thank Cochrane Copy Edit Support for the team's help.
Cochrane Group funding acknowledgement: The UK National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health and Social Care.
Appendices
Appendix 1. How the intervention might work
The positive effects of n‐3PUFAs on depressive illness are thought to occur as a result of integration into the cell membrane phospholipid bilayer, resulting in changes in structure and function (Haag 2003; James 2000; Ruxton 2005). Incorporation into the cell membrane can influence the physical state of the membrane, resulting in increased fluidity and permeability (Ehringer 1990; Hirashima 2004; Tappia 1997), possibly aiding cross‐cell membrane transport and communication (Haag 2003).
Secondly, n‐3PUFAs are also thought to have effects on surrounding molecules and cell functions via enzyme activity of direct involvement in various neurotransmitter pathways (Haag 2003; James 2000; Ruxton 2005; Stahl 2008). Supplementation with n‐3PUFAs has been found to result in increased serotonergic and dopaminergic activity; and decreased concentrations of noradrenalin (Chalon 2006; De la Presa Owens 1999; Hamazaki 2005; Sawazaki 1999; Yao 2004), and n‐3PUFA‐deficient diets have been associated with reduced receptor density and disruptions to neurotransmitter activity in serotonergic (De la Presa Owens 1999; Delion 1994; Delion 1996; McNamara 2006), dopaminergic (Chalon 2006; De la Presa Owens 1999; Delion 1994; Delion 1996; McNamara 2006; Takeuchi 2002), and adrenergic systems (Takeuchi 2002) compared to controls.
Thirdly, n‐3PUFAs are thought to have effects on surrounding molecules and cell functions via enzyme activity which results in the release of fatty acids from the phospholipid bilayer to form a number of anti‐inflammatory eicosanoids, prostaglandins, and leukotrienes (Calder 2003; James 2000; Ruxton 2005; Stahl 2008). Supplementation with n‐3PUFAs has been found to result in reduced production of inflammatory cytokines ‐ tumour necrosis factor alpha (TNFa), interleukin 1B, interleukin‐6, C‐reactive protein and serum amyloid A (Calder 2003; Caughey 1996; James 2000; Rallidis 2003).
Disruptions to and abnormal cell signalling, neurotransmitter system activities and inflammatory processes have all been implicated in MDD (Parker 2006b; Stahl 2008). Recent work focuses specifically on a role for inflammatory processes in depressive disorders, and the possible interaction of immune processes, neurotransmitter pathways and cell signalling activities, via inflammation (Husted 2016; Miller 2016; Raison 2013). Meta‐analyses demonstrate increased levels of circulating inflammatory cytokines in individuals with MDD compared to healthy controls (Goldsmith 2016; Köhler 2017) and associations between improved inflammatory profiles and improvements in depressive symptoms (Goldsmith 2016). Inhibition of the inflammatory cytokine TNFa has also been reported to reduce depressive symptoms in those with high circulating inflammatory markers (Raison 2013). Rapaport 2016 further reports improvements in the depressive symptoms of individuals with specific combinations of inflammatory markers in response to n‐3PUFA (specifically EPA) treatment. Importantly, however, not all individuals with MDD are found to benefit from anti‐inflammatory treatments (Goldsmith 2016; Raison 2013; Rapaport 2016), and adverse consequences following inflammatory treatments in those without inflammation have been suggested (Miller 2016).
A comprehensive review of these interconnected mechanisms in relation to the actions of n‐3PUFAs is provided by Kalkman 2021.
Appendix 2. Why it is important to do this review
n‐3PUFAs have been linked to depression in a variety of epidemiological studies (Hibbeln 1998; Noaghiul 2003; Peet 2004; Silvers 2002; Tanskanen 2001); clinical studies (Edwards 1998; Garland 2007; Mamalakis 2002; Mamalakis 2006; Peet 1998); and RCTs (Frangou 2006; Nemets 2002; Stoll 1999; Su 2003).
However, several epidemiological studies have found no association between n‐3PUFA intake and depressive illness (e.g. Appleton 2007; Frangou 2006; Hakkarainen 2004; Miyake 2006; Stoll 1999; Su 2003). Clinical studies have reported no differences in n‐3PUFA levels between individuals diagnosed with MDD and controls (e.g. Browne 2006; Mamalakis 2004) and no clear associations (Appleton 2008a). Several RCTs have also reported no effects of supplementation on MDD (e.g. Grenyer 2007; Silvers 2005), depressive illness (e.g. Keck 2006) or depressed mood (e.g. Rogers 2008).
Reviews in this area clearly demonstrate considerable variability between studies (e.g. Appleton 2006; Appleton 2008b; Appleton 2010; Lin 2007; Parker 2006b; Smith 2011; Stahl 2008). Meta‐analyses also report considerable heterogeneity between studies ( Appleton 2006; Appleton 2010; Lin 2007). Meta‐analyses reveal some small benefit of n‐3PUFAs for depressive disorders (Appleton 2006; Lin 2007), but investigations of the considerable heterogeneity also suggest differential effects of n‐3PUFAs dependent primarily on severity of depressive symptoms at baseline (Appleton 2010). Sensitivity analyses based on severity of depressive symptoms at baseline suggest no benefits of n‐3PUFAs for individuals with mild depressive symptoms or without diagnosis of depression, but also provide some evidence of benefits in individuals with severe depressive symptoms or with depressive diagnoses (Appleton 2010). These findings suggest a possible benefit of n‐3PUFAs for MDD.
Appendix 3. Database search strategies
Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
In January 2021 we updated the search of the now archived CCMDCTR using the following terms: ((affective next disorder*) or (affective next symptom*) or mental* or mood* or depress* or dysthymi*) AND (omega3* or omega‐3* or (fatty acid*) or PUFA or n‐3PUFA* or n3PUFA* or ((n3 or n‐3 or w3 or w‐3) near/3 polyunsaturat*) or ((n3 or n‐3 or w3 or w‐3) near/3 oil*) or (fish* near/2 oil*) or (cod near/2 oil*) or dha or docosahex* or eicosapent* or epa or ethyl‐eicosapent* or ethyleicosapent* or alphalinolen* or alpha‐linolen* or linolenate* or linolenic*)
This register is current to June 2016 only.
***************************
Cochrane Library (Issue 1 of 12, 2021)
#1 ((affective next disorder*) or (affective next symptom*) or mental* or mood* or depress* or dysthymi*):ti,ab,kw (140093)
#2 (omega3* or omega‐3* or (fatty next acid*) or PUFA or n‐3PUFA* or n3PUFA* or ((n3 or n‐3 or w3 or w‐3) near/3 polyunsaturat*) or ((n3 or n‐3 or w3 or w‐3) near/3 oil*) or (fish* near/2 oil*) or (cod near/2 oil*) or dha or docosahex* or eicosapent* or epa or ethyl‐eicosapent* or ethyleicosapent* or alphalinolen* or alpha‐linolen* or linolenate* or linolenic*):ti,ab,kw (21389)
#3 (#1 and #2) (1453) (1429 Trials; 23 Reviews; 1 Protocol)
Limit 2015‐ 2021 (974 Trials records; 13 Reviews; 1 Protocol)
***************************
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to January 07, 2021> Date limited 2015 onwards Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 depression/ (123065)
2 depressive disorder/ (73445)
3 depressive disorder, major/ (30871)
4 dysthymic disorder/ (1146)
5 depressive disorder, treatment‐resistant/ (1402)
6 mental disorders/ (164814)
7 mood disorders/ (14535)
8 depress*.tw,kf. (479630)
9 dysthymi*.tw,kf. (3202)
10 (affective disorder* or affective symptom*).tw,kf. (19089)
11 (mood disorder* or mental health).tw,kf. (179455)
12 or/1‐11 (773302)
13 fatty acids, omega‐3/ (13672)
14 docosahexaenoic acids/ (8955)
15 eicosapentaenoic acid/ (6280)
16 fish oils/ (7779)
17 cod liver oil/ (539)
18 alpha‐linolenic acid/ (2968)
19 (omega3* or omega 3*).tw,kf. (17231)
20 fatty acid*.tw,kf. (226541)
21 (PUFA or n‐3PUFA* or n3PUFA*).tw,kf. (12585)
22 ((n3 or n‐3 or w3 or w‐3) adj3 polyunsaturat*).tw,kf. (5688)
23 ((n3 or n‐3 or w3 or w‐3) adj3 oil*).tw,kf. (877)
24 (fish* adj2 oil*).tw,kf. (11476)
25 (cod adj2 oil*).tw,kf. (978)
26 (dha or docosahex* or eicosapent* or epa or ethyl‐eicosapent* or ethyleicosapent*).tw,kf. (35576)
27 (alphalinolen* or alpha‐linolen*).tw,kf. (5455)
28 (linolenate* or linolenic*).tw,kf. (11661)
29 or/13‐28 (256289)
30 12 and 29 (4736)
31 controlled clinical trial.pt. (94005)
32 randomized controlled trial.pt. (520386)
33 clinical trials as topic/ (194184)
34 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf. (669557)
35 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or crossover or cross‐over or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kf. (590321)
36 placebo.ab,ti,kf. (220520)
37 trial.ti. (233030)
38 (control* adj3 group*).ab. (558810)
39 (control* and (trial or study or group*) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,hw. (26812)
40 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf. (178382)
41 double‐blind method/ or random allocation/ or single‐blind method/ (284314)
42 or/31‐41 (1785871)
43 exp animals/ not humans.sh. (4774680)
44 42 not 43 (1550427)
45 30 and 44 (708)
46 (2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).yr,ed,ez,dc. (8135625)
47 45 and 46 (352)
***************************
Ovid APA PsycInfo <1806 to January Week 1 2021> Date limited 2015 onwards Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp major depression/ (135234)
2 "depression (emotion)"/ (25797)
3 *mental disorders/ (68984)
4 *affective disorders/ (11094)
5 (depress* or dysthymi* or affective disorder* or affective symptom* or mood disorder* or mental health).tw,id. (492840)
6 or/1‐5 (532555)
7 fatty acids/ (2855)
8 (fatty acid* or omega3* or omega 3* or PUFA or n‐3PUFA* or n3PUFA*).tw,id. (4959)
9 ((n3 or n‐3 or w3 or w‐3) adj3 (oil* or polyunsaturat*)).tw,id. (269)
10 ((cod or fish*) adj2 oil*).tw,id. (354)
11 (dha or docosahex* or eicosapent* or epa or ethyl‐eicosapent* or ethyleicosapent* or alphalinolen* or alpha‐linolen* or linolenate* or linolenic*).tw,id. (1628)
12 or/7‐11 (6122)
13 6 and 12 (1092)
14 clinical trials.sh. (11837)
15 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. (90443)
16 (RCT or at random or (random* adj3 (administ* or allocat* or assign* or class* or control* or crossover or cross‐over or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,id. (106831)
17 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. (30190)
18 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. (26826)
19 trial.ti. (31856)
20 placebo.ti,ab,id,hw. (40979)
21 treatment outcome.md. (21136)
22 treatment effectiveness evaluation.sh. (25219)
23 mental health program evaluation.sh. (2154)
24 or/14‐23 (203001)
25 13 and 24 (247)
26 (2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).yr,an. (1089957)
27 25 and 26 (106)
***************************
Ovid Embase <1980 to 2021 Week 01> Date limited 2015 onwards Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 *mood disorder/ (9106)
2 exp depression/ (482958)
3 (depress* or dysthymi* or affective disorder* or affective symptom* or mood disorder* or mental health).tw,kw. (791144)
4 or/1‐3 (933706)
5 exp unsaturated fatty acid/ (151393)
6 fish oil/ or cod liver oil/ (18235)
7 (fatty acid* or omega3* or omega 3* or PUFA or n‐3PUFA* or n3PUFA*).tw,kw. (258383)
8 ((n3 or n‐3 or w3 or w‐3) adj3 (oil* or polyunsaturat*)).tw,kw. (7786)
9 ((fish* or cod) adj2 oil*).tw,kw. (15581)
10 (dha or docosahex* or eicosapent* or epa or ethyl‐eicosapent* or ethyleicosapent*).tw,kw. (43081)
11 (alphalinolen* or alpha‐linolen* or linolenate* or linolenic*).tw,kw. (12980)
12 or/5‐11 (357826)
13 4 and 12 (7938)
14 randomized controlled trial/ (636916)
15 randomization.de. (89593)
16 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. (255231)
17 *clinical trial/ (17184)
18 placebo.de. (348188)
19 placebo.ti,ab. (312798)
20 trial.ti. (313199)
21 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. (962193)
22 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or control* or crossover or cross‐over or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kw. (807737)
23 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. (311484)
24 (control* and (study or group?) and (waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kw,hw. (42776)
25 or/14‐24 (1777414)
26 ((animal or nonhuman) not (human and (animal or nonhuman))).de. (5746950)
27 25 not 26 (1614146)
28 13 and 27 (1454)
29 (2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021*).yr,dp,dc. (10494040)
30 28 and 29 (526)
***************************
Cumulative Index to Nursing and Allied Health (CINAHL) (all years to May 2013 only)
S1 (MH "Depression") S2 (MH "Depression, Reactive") S3 (MH "Dysthymic Disorder") S4 (MH "Affective Disorders") S5 (MH "Affective Symptoms") S6 (depress* or dysthymi* or “adjustment disorder*” or “affective disorder*” or “affective symptom*” or “mood disorder*”) S7 (S1 or S2 or S3 or S4 or S5 or S6 or S7) S8 (MH “FISH OILS”) S9 (MH “FATTY ACIDS, OMEGA‐3”) S10 (MH “DOCOSAHEXAENOIC ACIDS”) S11 (MH “EICOSAPENTAENOIC ACID”) S12 (AB ( (DHA or Docosahex* or Eicosapent* or EPA or “fatty acid*” or fish* or linolenic or omega‐3 or n‐3 or w‐3 or PUFA* or “cod liver oil” or “cod‐liver oil”) ) OR TI ( (DHA or Docosahex* or Eicosapent* or EPA or “fatty acid*” or fish* or linolenic or omega‐3 or n‐3 or w‐3 or PUFA* or “cod liver oil” or “cod‐liver oil”))) S13 (S8 or S9 or S10 or S11 or s12) S14 (MH "Clinical Trials+") S15 (PT Clinical trial) S16 (TX clini* n‐3 (trial* or study or studies)) S17 (TX ((singl* N1 blind*) or (singl* N1 mask*)) or TX ((doubl* N1 blind*) or (doubl* N1 mask*)) or TX ((tripl* N1 blind*) or (tripl* N1 mask*))) S18 (TX random* n‐3 control*) S19 (MH "Random Assignment") S20 (TX random and (allocat* or assign*)) S21 (TX placebo*) S22 (TX (waitlist* or (wait* and list*)) and (control* or group)) S23 (TX "treatment as usual" or TI TAU or AB TAU) S24 (TX (control* n‐3 (trial* or study or studies or group*))) S25 (MH "Quantitative Studies") S26 (S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25) S27 (S7 and S13 and s26)
***************************
ClinicalTrials.gov
#1 (omega3 OR omega‐3) AND depression | First posted from 06/01/2015 to 01/09/2021
Synonyms automatically applied: (omega3 or n‐3 fatty acids or n‐3 PUFA or n3 polyunsaturated fatty acid) and (depression or depressive disorders or depressed or depressive illness or depressive state or decreased mood or depressing or depressive neurosis or depressivity or feeling blue or feeling down or low mood or melancholic or melancholy or miserable) n=58
#2 (eicosapentaenoic acid and depression) AND depression | First posted from 06/01/2015 to 01/09/2021
Synonyms automatically applied: (omega 3 fatty acid or docosahexaenoic acid or acid eicosapentaenoic or ICOSAPENT or alpha‐linolenic acid or eicosapentanoic acid or Ethyl‐EPA or icosapentaenoic acid or miraxion) and (depression or depressive disorders or depressed or depressive illness or depressive state or decreased mood or depressing or depressive neurosis or depressivity or feeling blue or feeling down or low mood or melancholic or melancholy or miserable) n=23
#3 (docosahexaenoic acid and depression) AND depression | First posted from 06/01/2015 to 01/09/2021
Synonyms automatically applied: (omega 3 fatty acid or eicosapentaenoic acid or alpha‐linolenic acid or docosahexaenoate or docosahexenoic acids) and (depression or depressive disorders or depressed or depressive illness or depressive state or decreased mood or depressing or depressive neurosis or depressivity or feeling blue or feeling down or low mood or melancholic or melancholy or miserable) n=24
#4 (#1 or #2 or #3) n=59
***************************
Appendix 4. Cochrane Common Mental Disorders Controlled Trials Register (CCMDCTR)
Cochrane Common Mental Disorders (CCMD) maintains two archived clinical trials registers at its editorial base in York, UK: a references register and a studies‐based register. The CCMDCTR‐References Register contains over 40,000 reports of RCTs in depression, anxiety and neurosis. Approximately 50% of these references have been tagged to individual, coded trials. The coded trials are held in the CCMDCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary; (please contact the CCMD Information Specialists for further details). Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to 2016), Embase (1974 to 2016) and PsycINFO (1967 to 2016); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registers via the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCMD's generic search strategies (used to identify RCTs) can be found on the Group's website, (cmd.cochrane.org/specialised-register), with an example of the core MEDLINE search (used to inform the register) listed below. The CCMDCTR is current to June 2016 only
Core search strategy used to inform the Cochrane Common Mental Disorders Group's Specialised Register: OVID MEDLINE (to June 2016)
A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ 2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf. 3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.) 4. (1 and 2 and 3) Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
****************************************************************************
The CCMDCTR (Studies and References Registers) was searched for the previous published version of this review in May 2015 using the following search terms:
(depress* or dysthymi* or “affective disorder*” or “affective symptom*” or “mood disorder*” or "mental health") AND (dha or docosahex* or eicosapent* or epa or “fatty acid*” or *fish* or *linolenic* or *omega* or n‐3 or w‐3 or *PUFA* or “cod liver oil”)
****************************************************************************
Appendix 5. Risk of bias Assessment Tool
Risk of Bias Assessment Tool
SEQUENCE GENERATION
|
LOW RISK The investigators describe a random component in the sequence generation process such as: · Referring to a random number table; Using a computer random number generator; Coin tossing; Shuffling cards or envelopes; Throwing dice; Drawing of lots/slips; Minimization*. *Minimization may be implemented without a random element, and this is considered to be equivalent to being random |
|
HIGH RISK The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · Sequence generated by odd or even date of birth · Sequence generated by some rule based on date (or day) of admission · Sequence generated by some rule based on hospital or clinic record number. Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example: · Allocation by judgement of the clinician · Allocation by preference of the participant · Allocation based on the results of a laboratory test or a series of tests · Allocation by availability of the intervention. |
|
UNCLEAR RISK Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’. |
ALLOCATION CONCEALMENT
|
LOW RISK Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · Central allocation (including telephone, web‐based, and pharmacy‐controlled, randomization) · Sequentially numbered drug containers of identical appearance · Sequentially numbered, opaque, sealed envelopes – all 3 features of the envelopes must be described |
|
HIGH RISK Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · Using an open random allocation schedule (e.g. a list of random numbers) · Assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered) · Alternation or rotation · Date of birth · Case record number · Any other explicitly unconcealed procedure. |
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
BLINDING OF PARTICIPANTS AND PERSONNEL
|
LOW RISK · Some assessment of blinding at follow‐up, and blinding found to be successful · Flavours of both intervention and control treatments masked by flavour · Small amount of fish oil added to placebo |
|
HIGH RISK · Either participants or study personnel were not blinded. · Participants guessed allocation · No attempts to mask / counter fish oil taste / smell, despite clear description of other aspects of intervention and placebo. |
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Low’ or ‘High Risk’ |
BLINDING OF OUTCOME ASSESSORS
|
LOW RISK · Methods of blinding of outcome assessors described sufficiently and deemed adequate |
|
HIGH RISK Any one of the following: · Methods of blinding of outcome assessors described sufficiently but deemed inadequate · No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding |
|
UNCLEAR RISK · Insufficient information to permit judgement of ‘Low’ or ‘High Risk’ |
Where more than one outcome measure is used, overall score will be based on the one used in our analyses.
INCOMPLETE OUTCOME DATA= only relevant to data after randomisation
|
LOW RISK Any one of the following: · No missing outcome data · ITT analysis (includes all those randomized) · Missing outcome data less than 10% of the total randomised population · Missing outcome data for mood less than 5% of the total randomised population · Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias) · Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups · Difference in missing data between the groups not greater than 10% · i.e. intervention group of 120 has 6 drop out (5% of trial arm) and control group of 100 has 2 drop out (2% of trial arm): difference in missing data is 3% therefore LOW RISK · Difference in missing data for mood between the groups not greater than 5% |
|
HIGH RISK Any one of the following: · Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups · Difference in missing data between the groups greater than 10% · i.e. intervention group of 120 has 6 drop out (5% of trial arm) and control group of 100 has 26 drop out (26% of trial arm): difference in missing data is 21% therefore HIGH RISK · Difference in missing data for mood between the groups greater than 5% · Overall missing data greater than 10% of the total randomised population · Overall missing data for mood greater than 5% of the total randomised population · Stated as ‘intention‐to‐treat analysis’ but doesn’t use this · Analysed using ‘per protocol’ analyses · ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization |
|
UNCLEAR RISK Any one of the following: · Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomized not stated, no reasons for missing data provided) · Dropouts not mentioned |
SELECTIVE OUTCOME REPORTING
|
LOW RISK Any of the following: · The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way – use trial registration number if available to locate protocol · The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). · The study protocol is not available but authors state that all outcomes are reported. |
|
HIGH RISK Any one of the following: · Not all of the study’s pre‐specified primary and secondary outcomes have been reported · Pre‐specified in methods section · Or pre‐specified in protocol · One or more primary or secondary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified · One or more reported primary or secondary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect) · One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis – any data excluded from the analysis despite the data being available (i.e. so the reviewers decided not to include it in the meta‐analysis) · The study report fails to include results for a key outcome that would be expected to have been reported for such a study Outcomes refer in all cases to all study outcomes. |
|
UNCLEAR RISK Insufficient information to permit judgement of ‘Yes’ or ‘No’. · No protocol is available, and no contact can be gained with authors. |
OTHER BIAS
|
LOW RISK The study appears to be free of other sources of bias. |
|
HIGH RISK · Stopped early due to some data‐dependent process (including a formal‐stopping rule) · Significant baseline imbalance for mood outcomes |
|
UNCLEAR RISK There may be a risk of bias, but there is either: · Insufficient information to assess whether an important risk of bias exists · Insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 6. Select studies excluded based on study inclusion / exclusion criteria
Studies that were identified during our searches and then excluded from the review, with the rationale for exclusion
| Study | Aspect of Study Design | Details |
| Studies identified in our database searches | ||
| Abedi 2014 | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| Ginty 2015 | Mild – moderate depressive condition | Participants had no clinical diagnosis and study uses a cut‐off value on the BDI of 10. Exclusion criteria included taking medication for depression. |
| Keshavarz 2018 (Mostafavi 2014) | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression (protocol) |
| Mazaherioun 2018 | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| NEURAPRO series (Amminger and colleagues) | Alternative psychiatric condition | Study of schizophrenia, psychosis and personality disorders |
| Opiyo 2019 | Alternative depressive condition | Study of depression during / surrounding pregnancy |
| Parletta 2019 (Parletta 2014) | Confounded Intervention | The n‐3PUFA intervention has additional active components that are not also provided to the control group |
| Sharifan 2017 | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| Sarris 2019 | Confounded intervention | The n‐3PUFA intervention has additional active components that are not also provided to the control group |
| Su 2014 | Alternative depressive condition | Study of medication‐induced (IFN‐alpha‐induced) depression |
| Su 2016 | Alternative depressive condition | Study of medication‐induced (IFN‐alpha‐induced) depression |
| Su 2018 | No comparator | Study of two different n‐3PUFAs, no non‐n‐3PUFA comparator |
| Tu 2020 | No comparator | Study of two different n‐3PUFAs in MDD participants, no non‐n‐3PUFA comparator |
| Yang 2019 | No comparator | Study of three different n‐3PUFA combinations, no non‐n‐3PUFA comparator |
| Studies included in our earlier review (Appleton 2015) | ||
| Gabbay 2018 (Gabbay 2006) | Adolescent population | Non‐adult study population |
| Ravi 2016 (Khalili 2014) | Mild – moderate depressive condition | Participants had no clinical diagnosis. Study uses a cut‐off value on the BDI‐II‐Persian of 16, but this is stated to include consideration of mild‐moderate depression. BDI‐II‐Persian is not validated to diagnose MDD. Protocol states consideration of mild, moderate and severe depressive episodes, and exclusion criteria include receiving drugs that affect mood including antidepressants. |
| Rees 2008 (Rees 2006) | Alternative depressive condition | Study of depression during / surrounding pregnancy |
|
Tayama 2019 (Tayama 2014) |
Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| Studies included in other reviews | ||
| Antypa 2012 | Previous depressive condition | Study of individuals considered to have recovered from a depressive disorder (mild symptoms only) |
| Frangou 2006 | Alternative depressive condition | Study of bipolar disorder |
| Freeman 2008 | Alternative depressive condition | Study of depression during / surrounding pregnancy |
| Giltay 2011 | No depressive condition | Study of healthy individuals |
| Hallahan 2007 | Alternative depressive condition | Study investigates recurrent self‐harm |
| Jacka 2017 | Confounded intervention | The n‐3PUFA intervention has additional active components that are not also provided to the control group |
| Khajehnasiri 2015 | Mild – moderate depressive condition | No psychiatric diagnosis, and study uses a BDI cut‐off value of 10 or more |
| Mozaffari‐Khosravi 2013 | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| Nemets 2006 | Child population | Non‐adult study population |
| Rogers 2008 | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| Sinn 2012 | No depressive diagnosis | Study of individuals with a non‐depressive health condition |
| Stoll 1999 | Alternative depressive condition | Study of bipolar disorder |
| Su 2008 | Alternative depressive condition | Study of depression during / surrounding pregnancy |
| Tajalizadekhoob 2011 | Mild – moderate depressive condition | Study specifically aims to investigate mild – moderate depression |
| Van de Rest 2008 | No depressive condition | Study of healthy individuals |
Data and analyses
Comparison 1. n‐3PUFAs vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Depressive symptomology (continuous) | 33 | 1848 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.64, ‐0.16] |
| 1.2 Adverse events | 24 | 1503 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.99, 1.64] |
| 1.3 Depressive symptomology (dichotomous ‐ remission) | 8 | 609 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.74, 1.72] |
| 1.4 Depressive symptomology (dichotomous ‐ response) | 17 | 794 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.79] |
| 1.5 Quality of life | 12 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.82, 0.06] |
| 1.6 Trial non‐completion | 29 | 1777 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.22] |
Comparison 2. n‐3PUFAs vs antidepressant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Depressive symptomology (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.2 Depressive symptomology (dichotomous ‐ response) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3 Trial non‐completion | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Subgroup analyses ‐ n‐3PUFAs vs placebo ‐ analyses based on comorbidities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Depressive symptomology (continuous) | 33 | 1848 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.64, ‐0.16] |
| 3.1.1 Individuals with comorbidites | 11 | 594 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.39, 0.18] |
| 3.1.2 Individuals with/without comorbidities (mixed) | 18 | 1100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.59, ‐0.00] |
| 3.1.3 Individuals without comorbidities | 4 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐2.98, ‐0.37] |
| 3.2 Adverse events | 24 | 1503 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.99, 1.64] |
| 3.2.1 Individuals with comorbidities | 8 | 497 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.59, 2.74] |
| 3.2.2 Individuals with/without comorbidities (mixed) | 14 | 937 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [1.00, 1.80] |
| 3.2.3 Individuals without comorbidities | 2 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.19, 3.50] |
Comparison 4. Subgroup analyses: n‐3PUFAs vs placebo ‐ analyses based on adjunctive therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Depressive symptomology (continuous) | 33 | 1848 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.64, ‐0.16] |
| 4.1.1 Individuals receiving adjunctive therapy | 16 | 649 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.04, ‐0.06] |
| 4.1.2 Individuals receiving/not receiving adjunctive therapy (mixed) | 10 | 832 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.55, 0.07] |
| 4.1.3 Individuals not receiving adjunctive therapy | 7 | 367 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.73, 0.15] |
| 4.2 Adverse events | 24 | 1503 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.99, 1.64] |
| 4.2.1 Individuals receiving adjunctive therapy | 12 | 492 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.60, 1.88] |
| 4.2.2 Individuals receiving/not receiving adjunctive therapy | 8 | 752 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.89, 1.71] |
| 4.2.3 Individuals not receiving adjunctive therapy | 4 | 259 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [1.03, 4.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bot 2010.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 25 participants had a mean age = 54 yrs (SD = 11), 13 women, 12 men, recruited via VU University Medical Centre diabetes outpatient clinic (Amsterdam, NL), advertisements on websites, newspapers and magazines. Participants were recruited between April 2006 and May 2007, the trial was performed between June 2006 and July 2007 Comorbidities: Diabetes Type 1 and 2 in all participants, no other comorbidities Adjunctive therapy: Yes for all participants (usual antidepressants) Inclusion Criteria: aged 18 ‐ 75 years, diagnosed with diabetes (Type 1 or 2, or use of insulin or oral hypoglycaemic agents), on antidepressant medication for at least 2 months, met criteria for MDD using Composite International Diagnositic Interview Exclusion criteria: serious co‐morbid disease, using fish oil supplementation or consuming more than 3 servings of fish/week, alcohol or drug abuse, suicidal ideation, or allergic to fish, fish products or rapeseed oil |
|
| Interventions |
Intervention: E‐EPA (1 g/d, including mixed tocopherols), 2 x 500 mg capsules per day, plus ongoing therapy Comparator: Rapeseed oil + medium chain triglycerides (1 g/d, including mixed tocopherols), 2 x 500 mg capsules per day, plus ongoing therapy Treatment received for 12 weeks |
|
| Outcomes |
Primary: MADRS measured at baseline, 1, 3, 5, 7, 9 and 12 weeks; Adverse Events Secondary: Trial non‐completion |
|
| Notes |
Funded by Dutch Diabetes Research Foundation and Minami Nutrition, Belgium Supplements provided by Minami Nutrition, Belgium Conflicts of interest: CoI declared by one author Compliance: EPA levels in red blood cell (RBC) phospholipids Depressed mood (continuous): Analysis conducted on MADRS scores at 12 weeks, published per protocol data Adverse events: Adverse events reported in the analyses do not include side effects. 1 individual in the intervention group experienced an allergic reaction. Side effects were not split according to group: no side effects in 8 individuals, prevalent side effects were stomach ache (n = 10), belching (n = 7), nausea (n = 6), diarrhoea (n = 5). Values in the analysis are for adverse events. Trial non‐completion: Intervention group = 2 (1 allergic reaction, 1 loss to follow‐up), Comparator group = 0. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation occurred with computer‐generated random number, performed by an employee of the pharmacy of VU University Medical Centre" (P.2, Mocking 2012) |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a pharmacy employee "who was not involved in data collection or analysis" (P.2, Mocking 2012) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants and researchers were blinded to treatment allocation until completion of data collection." Identical packaging sent out by the pharmacy. Participants instructed not to chew to avoid fishy taste. (P.283 Bot 2010, P.2 Mocking 2012), but no report of masking the fishy taste. "concealment appeared to be successful." ‐ 33% in both groups correctly guessed treatment when questioned. (P.285, Bot 2010) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | MADRS: "Research nurse and researchers were blinded toward treatment allocation until completion of data collection" (P.283, Bot 2010, P.2, Mocking 2012) |
| Blinding of outcome assessment (Adverse Events) | Low risk | Adverse events were assessed by nurses based on participant report. Participants did not guess their treatment group (P.285, Bot 2010) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | MADRS: 1 person in intervention group lost to follow‐up, analysis not ITT (although stated as ITT) (P.284, Bot 2010) |
| Incomplete outcome data (Adverse Events) | High risk | Adverse events: AEs reported but only the prevalent side effects (P.285, Bot 2010) |
| Selective reporting (reporting bias) | Low risk | All depression outcomes reported (Protocol included in Mocking 2012) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Carney 2009.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 10 weeks Pre‐randomisation: Paticipants were given a 2½ ‐ 3½ week supply of sertraline (25 mg/ day) plus placebo for 2 weeks then reassessed for depression, compliance and tolerance to medication |
|
| Participants |
Participants: 122 participants with a mean age = 58.3 years, 41 women, recruited from cardiology practices in St Louis, Missouri, US and from cardiac diagnostic labs affiliated with Washington University School of Medicine, USA. They were informed of the study via physicians, study staff or pamphlets. Patients were recruited to the study between May 2005 and December 2008. Comorbidities: CHD in all participants, no psychiatric comorbidities Adjunctive therapy: Yes for all participants ‐ sertraline (50 mg/d) Inclusion criteria: score ≥ 16 BDI‐II, DSM‐IV criteria for current MDE (using SCID), CHD as documented by > 50% stenosis in at least 1 major coronary artery, a history of revascularisation or hospitalisation for an acute coronary syndrome; continued to meet DSM criteria, score ≥ 16 BDI‐II, reported no serious adverse events, and took both drugs ≥ 85% of days during pre‐randomisation Exclusion criteria: Cognitive impairment, comorbid psychiatric disorders, psychosis, high risk of suicide or current substance abuse, an acute coronary syndrome within the previous 2 months, a left ventricular ejection fraction of less than 30%, advanced malignancy or physical inability to participate, use of antidepressants, anticonvulsants, lithium, or n‐3PUFA supplements, sensitivity to sertraline or n‐3PUFA or physician/patient refusal |
|
| Interventions |
Intervention: EPA/DHA combination (2 g/d ethyl esters, providing EPA 930 mg, DHA 750 mg), 2 capsules per day, plus 50 mg/d sertraline Comparator: Corn oil (2 g/d), 2 capsules per day, plus 50 mg/d sertraline Treatment received for 10 weeks |
|
| Outcomes |
Primary: BDI‐II (21‐item), HDRS (17‐item) both assessed weekly for 10 weeks, Adverse events Secondary: Response, Remission based on BDI, Trial non‐completion |
|
| Notes |
Funded by National Heart Lung and Blood institute, US Supplements provided by GlaxoSmithKline Inc, antidepressants provided by Pfizer Inc. Conflicts of interest: CoIs declared by 2 authors Compliance: RBC membrane levels of EPA and DHA assessed before and after treatment. Capsule counts at each study visit Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 10 weeks, using published ITT data Adverse events: Adverse effects reported as a percentage rather than number of participants reporting 1 new symptom in the 10‐week trial; Intervention group = 63% adverse effects (19% symptoms previously associated with high doses of n‐3PUFAs), Control group = 73% adverse effects (22% symptoms previously associated with high doses of n‐3PUFAs). 14 adverse events, but details do not add up to 14. Values in the analysis are for adverse effects. Trial non‐completion: Intervention = 3 (1 = health problems related to treatment, 1 = withdrew consent, 1 = wanted other treatment); Comparator = 4 (2 = health problems related to treatment, 1 = withdrew consent, 1 = wanted other treatment) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A SAS permuted‐block randomisation allocation programme" (P.1652, Carney 2009) |
| Allocation concealment (selection bias) | Unclear risk | "The group assignments were concealed in sealed envelopes and opened at enrolment" (P.1652, Carney 2009), not clear if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and nurses were blinded and an identical placebo was used (P.1652, Carney 2009). There was no attempt to mask the fishy taste and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BDI (self‐reported scale) ‐ HIGH HDRS professional rating scale ‐ study psychiatrists and nurses were blinded ‐ LOW |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | Adverse events ‐ unclear whether these were reported by clinicians or participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT on BDI and HDRS |
| Incomplete outcome data (Adverse Events) | Unclear risk | AEs were not clearly reported |
| Selective reporting (reporting bias) | Low risk | All major outcomes were reported (additional information from authors) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Carney 2020.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 10 weeks | |
| Participants |
Participants: 144 participants (Intervention group: 71 participants, mean age 58.5 years (SD 9.6), 45 men, 26 women; Comparator group: 73 participants, mean age 60.5 years (SD 9.3), 43 men, 30 women), recruited from secondary care cardiology practices between May 2014 and June 2018 in USA Comorbidities: Evidence of, or at risk of, stable coronary artery disease (CAD ‐ history of myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or at least a 50% stenosis in 1 or more major coronary artery) in all participants, some with hypertension (92%), diabetes (47%) and obesity (reported as BMI, % not reported). Possible psychiatric comorbidities Adjunctive therapy: Yes, 50 mg/d of sertraline for all participants Inclusion criteria: Aged 25 years and older, diagnosed with coronary artery disease (CAD ‐ history of myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or at least a 50% stenosis in 1 or more major coronary artery), or 2 or more major cardiac risk factors; met criteria for MDD using DSM‐5; and a BDI‐II score of 17 or more Exclusion criteria: Moderate to severe cognitive impairment; or another major Axis I diagnosis other than an anxiety disorder, or a high risk of suicide; were not expected to survive 1 year; had a known sensitivity to sertraline or omega‐3, or an allergy to fish oil or shellfish; or were currently taking an antidepressant, lithium, or omega‐3 supplements |
|
| Interventions |
Intervention: EPA 2000 mg/d (4 capsules of 500 mg/d) Comparator: Placebo corn oil capsules identical in appearance to intervention capsules (4 capsules each day) Treatment received for 10 weeks |
|
| Outcomes |
Primary: BDI‐II (21‐item), HDRS (17‐item), assessed at baseline and 10 weeks; PHQ‐9, assessed at baseline and every week until week 10; adverse events Secondary: Depression remission (BDI‐II ≤ 8); Depression response > 50% improvement; Trial non‐completion |
|
| Notes |
Supported by: Grant from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland Supplements provided by: Atrium Innovations Inc. provided the EPA and matching placebo capsules Conflicts of interest: Dr. Carney or a member of his family is a stockholder in Pfizer Inc. Dr. Harris is the President of OmegaQuant Analytics, LLC. Compliance: RBC membrane %EPA, capsule counts Depressed mood (continuous): Analyses conducted on HDRS (17 item), at 10 weeks, ITT data Adverse events: Intervention group: 17 individuals (confirmed by authors), 60 events: 35 gastrointestinal, 11 neurological, 14 hospital admissions. Comparator group: 10 individuals (confirmed by authors), 57 events: 33 gastrointestinal, 14 neurological, 10 hospital admissions Depression remission = BDI‐II ≤ 8 Depression response > 50% improvement on BDI‐II Trial non‐completion: 1 participant from each treatment arm did not complete the trial, reasons not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group allocation was determined by a SAS permuted block random allocation program", p.3 |
| Allocation concealment (selection bias) | Low risk | "The assignments were coded and concealed to ensure that the double‐blind was maintained.", p.3 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants ‐ "placebo capsules were identical", p. 3., and "The difference between actual and suspected group assignment was not significant (estimated kappa [κ] = 0.16; 95%CI −0.03, 0.36).", p.6 Personnel ‐ "Only the pharmacist, who had no direct contact with the patients or study staff, was unblinded to group assignment during the trial.", p.3 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Only the pharmacist, who had no direct contact with the patients or study staff, was unblinded to group assignment during the trial. ", p. 3 |
| Blinding of outcome assessment (Adverse Events) | Low risk | "Only the pharmacist, who had no direct contact with the patients or study staff, was unblinded to group assignment during the trial. ", p. 3 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, Figure 1, confirmed by authors |
| Incomplete outcome data (Adverse Events) | Low risk | Adverse events reported separately for each treatment arm, p6 ‐ p7, confirmed by authors |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes reported, but some additional outcomes (Beck Anxiety Inventory) reported too |
| Other bias | Unclear risk | Discrepancies between the trial registration and paper |
Chang 2020.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 59 participants (Intervention group: 30 participants, mean age 61.1 years (SD 9.14), 18 men, 12 women; Comparator group: 29 participants, mean age 61.93 years (SD 8.95), 20 men, 9 women), recruited from secondary care setting between January 2016 to March 2017 in Taiwan Comorbidities: Myocardial infarction or coronary artery disease in all participants, no psychiatric comorbidities Adjunctive therapy: No for all participants Inclusion criteria: Patients were diagnosed with either a stable myocardial infarction or coronary artery disease, where there was no acute coronary event within recent 2 months nor a left ventricular ejection fraction (LVEF) that was ≥ 30%; received a diagnosis of MDD with Mini‐ Neuropsychiatric Interview (MINI), had a HDRS‐21 score ≥ 8, and provided consent to participate in the study Exclusion criteria: Patients comorbid with other psychiatric disorders, cognitive impairment such as dementia, currently comorbid substance use disorder (including alcohol, opioid, or amphetamine), with high suicide risk, end‐stage cancers, or physical inability to participate were excluded |
|
| Interventions |
Intervention: 2 g per day of EPA and 1 g per day of DHA Comparator: Placebo; soybean oil 3 g per day Treatment received for 12 weeks |
|
| Outcomes |
Primary: BDI‐II (21‐item), HDRS‐21, at baseline, 1, 2, 4, 8, 12 wks Secondary: none |
|
| Notes |
Supported by: The authors of this work were supported by the following grants: MOST 106‐2314‐B‐039‐027‐MY3; 106‐2314‐B‐038‐049; 106‐2314‐B039‐031; 106‐2314‐B‐039‐035; 105‐2918‐I‐039‐001; 104‐2314‐B‐039‐ 022‐MY2, and 104‐2314‐B‐039‐050‐MY3 from the Ministry of Science and Technology, Taiwan; NHRI‐EX106‐10528NI from the National Health Research Institutes, Taiwan; and CRS‐106‐063, DMR‐107‐202, DMR‐107‐204, DMR‐107‐091, DRM‐107‐097, DRM‐108‐091 and Chinese Medicine Research Center from the China Medical University, Taiwan Supplements provided by: Not reported Conflicts of interest: The authors state that there are no conflicts of interest Compliance: n‐3PUFA changes in RBC membranes, at baseline and 12 weeks Depressed mood (continuous): Analyses conducted on HDRS (21‐item) at 12 weeks. Completer analysis (not clear if this is also ITT analysis). SDs imputed from other studies using the HDRS‐21 Adverse events: Not assessed. Trial non‐completion: Not reported clearly; 66 participants were recruited and 59 completed, but it is unclear whether participants dropped out before or after randomisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail provided |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Laboratory measures (Blood Sample section) were conducted on coded samples by investigators who were blind to the patients’ information". P. 16. No other blinding mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Laboratory measures (Blood Sample section) were conducted on coded samples by investigators who were blind to the patients’ information". P. 16. No other blinding mentioned |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many were randomised. “66 patients were eligible and asked to participate. Seven patients declined to participate due to inconvenience to follow the study protocol. Fifty‐nine patients completed the study and were included in the analysis”, p. 15. Different numbers are also reported in an earlier conference abstract ‐ "Sixty‐one patients completed the study, where 32 were randomized to n‐3 PUFAs group (...) and 29 were randomized to placebo group (...)", p. S11; and the trial registration "actual enrolment: 60 participants". |
| Incomplete outcome data (Adverse Events) | Unclear risk | AEs not assessed |
| Selective reporting (reporting bias) | High risk | AEs not assessed, but would be expected in this field |
| Other bias | Unclear risk | Discrepancies between protocol and paper ‐ AEs are included in the protocol |
Coryell (1g/d) 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomised parallel‐arm trial for 6 weeks, following 4‐week open‐label trial of escitalopram (10 mg/d) to prospectively identify SSRI non‐responders (< 50% improvement) for augmentation | |
| Participants |
Participants: 11 participants with a mean age of 28.8 (SD 9.3, range 18 ‐ 48) years, 9 women and 2 men (split across Coryell (1g/d) 2005 and Coryell (2g/d) 2005). Participants recruited via clinician referrals and advertisements, in Iowa City, USA, dates ‐ not reported. Comorbidities: Possible physical and/or psychiatric comorbidities Adjunctive therapy: Yes for all participants ‐ escitalopram Inclusion: Aged 18 ‐ 55 years; current diagnosis of MDD; meets DSM‐IV criteria Antidepressant for no more than 3 days within the past month or antidepressant for at least the past month with no change in type or dose Exclusion: More than 2 adequate antidepressant trials in the current episode; meets DSM‐IV criteria for substance dependence in the past year; substance abuse within the past month; meets DSM‐IV criteria for an eating disorder in the past year; allergy to fish; bleeding disorder/taking warfarin; omega‐3 supplements for 3 or more days in the past 4‐month period; known to be pregnant; taking medications known to produce affective symptoms; history of non‐response to escitalopram/Lexapro |
|
| Interventions |
Intervention: EPA/DHA combination (740 mg EPA/d + 400 mg DHA/d), 2 capsules, plus 2 placebo capsules Comparator: 4 placebo capsules (oil, 6% ALA ‐ email from trialist) All participants receive 4 capsules with either 0 or 2 capsules containing EPA/DHA. Treatment was received for 6 weeks |
|
| Outcomes |
Primary: MADRS scores, measurements at 6 weeks. Adverse events Secondary: HDRS, Response based on 50% improvement based on MADRS and HDRS |
|
| Notes |
Supplements provided by Ocean Nutrition Canada Ltd. Conflicts of interest: None Compliance: Capsule counts at each study visit Depressed mood (continuous): Analysis conducted on MADRS scores at 6 weeks, using unpublished ITT data (missing data for HDRS). Placebo group split across 2 intervention groups (1 g/d = 2 participants, 2 g/d = 2 participants). Adverse events: Data on serious and non‐serious adverse events were collected. No serious AEs were reported, but no data on non‐serious adverse events were available. Values in the analysis are for serious adverse events Trial non‐completion: No withdrawals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, simple randomisation (email correspondence from trialist) |
| Allocation concealment (selection bias) | Unclear risk | Researcher was blind to allocation, research nurse was not blind to allocation. Both had contact with participants and unclear who allocated participants (email correspondence from trialist) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Researcher was blinded, research nurse was not blinded, and both had contact with participants. Participants were stated as 'blinded', but no details of blinding of taste. Possible attempts to check blinding, but no data available (email correspondence from trialist) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments made by researcher, and researcher was blinded (email correspondence from trialist) |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | Outcome assessments made by participants, and unclear if they were blinded (email correspondence from trialist) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete MADRS outcome data, and ITT analysis (email correspondence from trialist) |
| Incomplete outcome data (Adverse Events) | Unclear risk | No serious AEs were reported, but data on non‐serious adverse events are not available (email correspondence from trialist) |
| Selective reporting (reporting bias) | High risk | Data not published (email correspondence from trialist) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Coryell (2g/d) 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomised parallel‐arm trial for 6 weeks, following 4‐week open‐label trial of escitalopram (10 mg/d) to prospectively identify SSRI non‐responders (< 50% improvement) for augmentation | |
| Participants |
Participants: 11 participants with a mean age of 28.8 (SD 9.3, range 18 ‐ 48) years, 9 women and 2 men (split across Coryell (1g/d) 2005 and Coryell (2g/d) 2005). Participants recruited via clinician referrals and advertisements, in Iowa City, USA, dates ‐ not reported. Comorbidities: Possible physical and/or psychiatric comorbidities Adjunctive therapy: Yes for all participants ‐ escitalopram Inclusion: Aged 18 ‐ 55 years; current diagnosis of MDD; meets DSM‐IV criteria Antidepressant for no more than 3 days within the past month or antidepressant for at least the past month with no change in type or dose Exclusion: More than 2 adequate antidepressant trials in the current episode; meets DSM‐IV criteria for substance dependence in the past year; substance abuse within the past month; meets DSM‐IV criteria for an eating disorder in the past year; allergy to fish; bleeding disorder/taking warfarin; omega‐3 supplements for 3 or more days in the past 4‐month period; known to be pregnant; taking medications known to produce affective symptoms; history of non‐response to escitalopram/Lexapro |
|
| Interventions |
Intervention: EPA/DHA combination (1480 mg EPA/d + 800 mg DHA/d), 4 capsules Comparator: Placebo capsules, 4 capsules (oil, 6% ALA ‐ email from trialist) All participants receive 4 capsules with either 0 or 4 capsules containing EPA/DHA Treatment was received for 6 weeks |
|
| Outcomes |
Primary: MADRS scores, measurements at 6 weeks, Adverse events Secondary: HDRS, Response based on 50% improvement based on MADRS and HDRS |
|
| Notes |
Supplements provided by Ocean Nutrition Canada Ltd. Conflicts of interest: None Compliance: Capsule counts at each study visit Depressed mood (continuous): Analysis conducted on MADRS scores at 6 weeks, using unpublished ITT data (missing data for HDRS). Placebo group split across 2 intervention groups (1 g/d = 2 participants, 2 g/d = 2 participants) Adverse events: Data on serious and non‐serious adverse events were collected. No serious AEs were reported, but no data on non‐serious adverse events were available. Values in the analysis are for serious adverse events Trial non‐completion: No withdrawals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, simple randomisation (email correspondence from trialist) |
| Allocation concealment (selection bias) | Unclear risk | Researcher was blind to allocation, research nurse was not blind to allocation. Both had contact with participants and unclear who allocated participants (email correspondence from trialist) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Researcher was blinded, research nurse was not blinded, and both had contact with participants. Participants were stated as 'blinded', but no details of blinding of taste. Possible attempts to check blinding, but no data available (email correspondence from trialist) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments made by researcher, and researcher was blinded (email correspondence from trialist) |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | Outcome assessments made by participants, and unclear if they were blinded (email correspondence from trialist) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete MADRS outcome data, and ITT analysis (email correspondence from trialist) |
| Incomplete outcome data (Adverse Events) | Unclear risk | No serious AEs were reported, but data on non‐serious adverse events are not available (email correspondence from trialist) |
| Selective reporting (reporting bias) | High risk | Data not published (email correspondence from trialist) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Da Silva (AD) 2005.
| Study characteristics | ||
| Methods | Pilot randomised controlled parallel‐arm trial, 12 weeks Participants split across Da Silva (AD) 2005 and Da Silva (nAD) 2005, depending on antidepressant use, prior to randomisation |
|
| Participants |
Participants: 31 participants, with a mean age = 64.4 (range 49 ‐ 78) years, 58% women (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005). Participants were selected from Association of Patients with Parkinson's disease of Paraná, Curitiba, Brazil, dates ‐ not reported. Comorbidities: Parkinson's disease (PD), other possible comorbidities Adjunctive therapy: Yes for all participants: antidepressants Inclusion criteria: Parkinsons disease, DSM‐IV criteria for MDD (MINI plus, and a SCID), score < 2.5 Hoehn & Yahr scale for PD (Hoehn 1967), no signs of dementia (MMSE) (Folstein 1975), UPDRS assessment (Taylor 2005), taking medication for depression for at least 1 yr or refused to take medication Exclusion criteria: initiated antidepressant use after diagnosis, cognitive and memory declines, drug/alcohol dependent. Any participant who presented with an alteration of PD (above 0.5 point on Hoehn and Yahr scale) after 3 months was also excluded |
|
| Interventions |
Intervention: EPA/DHA combination (720 mg/d EPA, 480 mg/d DHA, plus tocopherols), 4 capsules, plus ongoing therapy Comparator: Mineral oil, 4 capsules/d, plus ongoing therapy Treatment received for 3 months |
|
| Outcomes |
Primary: MADRS, BDI assessed at baseline and 12 weeks, Adverse events Secondary: Response based on MADRS, CGI assessed at baseline and 12 weeks, Trial non‐completion |
|
| Notes |
No funding reported. Supplements provided by Herbarium Foundation for Health and Research Conflicts of interest: None declared Compliance: RBC membrane levels of EPA and DHA assessed before and after treatment Depressed mood (continuous): Analysis conducted on MADRS scores at 12 weeks, per protocol data provided by authors Adverse events: 2 individuals reported adverse events ‐ 1 GI, 1 other physical (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005 ‐ group not reported). Values could not be included in analysis Response (50% improvement in MADRS score) ‐ Intervention group = 42%, comparator group = 6% (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005 ‐ group not reported). Values could not be included in analysis. Quality of Life: Analysis conducted on CGI Trial non‐completion: 2 individuals withdrew (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005 ‐ group not reported) (1 collateral effects, 1 worsening health status). Values could not be included in analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by drawing (additional information from the author) |
| Allocation concealment (selection bias) | Unclear risk | "Identification of the groups and separation of the respective capsules were carried out in the _ laboratory at University Federal do Parana" (P.353) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither researcher nor participants knew which substance was given (identical placebo). Not reported if the fishy taste was disguised, and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | MADRS and BDI (both evaluated by trained psychologist blinded to allocation) (P.353) |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | Adverse events ‐ unclear whether these were reported by clinicians or participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data not ITT |
| Incomplete outcome data (Adverse Events) | Unclear risk | AEs were not clearly reported |
| Selective reporting (reporting bias) | Low risk | All depression data reported (additional information from author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Da Silva (nAD) 2005.
| Study characteristics | ||
| Methods | Pilot randomised controlled parallel‐arm trial, 12 weeks Participants split across Da Silva (AD) 2005 and Da Silva (nAD) 2005, depending on antidepressant use, prior to randomisation |
|
| Participants |
Participants: 31 participants, with a mean age = 64.4 (range 49 ‐ 78) years, 58% women (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005). Participants were selected from Association of Patients with Parkinson's disease of Paraná, Curitiba, Brazil, dates ‐ not reported. Comorbidities: Parkinson's disease (PD), other possible comorbidities Adjunctive therapy: No for all participants Inclusion criteria: Parkinsons disease, DSM‐IV criteria for MDD (MINI plus, and a SCID), score < 2.5 Hoehn & Yahr scale for PD (Hoehn 1967), no signs of dementia (MMSE) (Folstein 1975), UPDRS assessment (Taylor 2005), taking medication for depression for at least 1 yr or refused to take medication Exclusion criteria: initiated antidepressant use after diagnosis, cognitive and memory declines, drug/alcohol dependent. Any participant who presented with an alteration of PD (above 0.5 point on Hoehn and Yahr scale) after 3 months was also excluded |
|
| Interventions |
Intervention: EPA/DHA combination (720 mg/d EPA, 480 mg/d DHA, plus tocopherols), 4 capsules Comparator: Mineral oil, 4 capsules/d Treatment received for 3 months |
|
| Outcomes |
Primary: MADRS, BDI assessed at baseline and 12 weeks, Adverse events Secondary: Response based on MADRS, CGI assessed at baseline and 12 weeks, Trial non‐completion |
|
| Notes |
No funding reported. Supplements provided by Herbarium Foundation for Health and Research Conflicts of interest: None declared Compliance: RBC membrane levels of EPA and DHA assessed before and after treatment Depressed mood (continuous): Analysis conducted on MADRS scores at 12 weeks, per protocol data provided by authors Adverse events: 2 individuals reported adverse events ‐ 1 GI, 1 other physical (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005 ‐ group not reported). Values could not be included in analysis Response (50% improvement in MADRS score) ‐ Intervention group = 42%, comparator group = 6% (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005 ‐ group not reported). Values could not be included in analysis Quality of Life: Analysis conducted on CGI Trial non‐completion: 2 individuals withdrew (split across Da Silva (AD) 2005 and Da Silva (nAD) 2005 ‐ group not reported) Values could not be included in analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by drawing (additional information from the author) |
| Allocation concealment (selection bias) | Unclear risk | "Identification of the groups and separation of the respective capsules were carried out in the _ laboratory at University Federal do Parana" (P.353) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither researcher or participants knew which substance was given (identical placebo). Not reported if the fishy taste was disguised, and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | MADRS and BDI (both evaluated by trained psychologist blinded to allocation) (P.353) |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | Adverse events ‐ unclear whether these were reported by clinicians or participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data not ITT |
| Incomplete outcome data (Adverse Events) | Unclear risk | AEs were not clearly reported |
| Selective reporting (reporting bias) | Low risk | All depression data reported (additional information from author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias. |
Gertsik 2012.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks Pre‐randomisation 1 week placebo run‐in phase |
|
| Participants |
Participants: 42 participants, mean age = 40.5 years (SD = 10.2). Distribution of gender was not reported. Recruited via local advertisements or physician referral from the Greater Los Angeles area with preliminary telephone screening, dates ‐ not reported. Comorbidities: No psychiatric comorbidities, possible physical comorbidities Adjunctive therapy: Yes for all participants, citalopram (20 mg/d) with possible increase in dose after 4 weeks Inclusion criteria: aged 18 ‐ 65 years; DSM IV criteria for MDD via the SCID, score > 17 on HDRS (21‐item), contraception use in women of childbearing age; still qualifying for inclusion after 1 week run‐in Exclusion criteria: psychiatric disorders including psychotic depression and bipolar disorders, current drug/alcohol abuse/dependence or history of such in past 6 months, unstable medical or neurological conditions likely to interfere with treatment, history of allergy to citalopram or n‐3PUFA, finfish or shellfish, history of failure to respond to citalopram, history of seizure disorder, pregnancy, need for concomitant psychotropic medication including other antidepressants, active suicidal ideation or safety concerns, exposure to fluoxetine or MAOIs in previous 2 months, anticoagulant therapy, dietary intake of > 3 g n‐3PUFAs/day at baseline |
|
| Interventions |
Intervention: EPA/DHA combination (EPA = 1800 mg/d, DHA= 400 mg/d, other n‐3PUFAs = 200mg/d), 2 capsules, twice daily with meals, plus citalopram (20 mg/d) Comparator: Olive oil (4 g/d), 2 capsules, twice daily with meals, plus citalopram (20 mg/d) Treatment received for 8 weeks |
|
| Outcomes |
Primary: HDRS (21‐item), BDI, MADRS ‐ all assessed at baseline, randomisation, 2, 4, 6 and 8 weeks; Adverse events Seconday: Remission and response based on HDRS; CGI, PGI; Trial non‐completion |
|
| Notes |
Funded by NIH National Centre for Complementary and Alternative Medicine & National Centre for Research Resources, USA Supplements provided by Nordic Naturals Conflicts of Interest: CoIs declared by 2 authors Compliance: Capsule counts and assessment of citalopram blood levels Depressed mood (continuous): Analysis conducted on HDRS (21‐item) scores at 8 weeks, ITT data taken from published graph Adverse events: Only reported for completers. No significant adverse events. Only frequently‐reported adverse effects were reported: Intervention group = 6 (all GI), comparator group = 4 (all GI). Less than 5% of participants in either group reported other adverse events, e.g. headache, sedation or sexual dysfunction. Numbers reported in the analysis relate to frequently‐reported adverse effects Depression Remission / Response: based on HDRS. Quality of life: CGI, PGI, data not reported. Trial non‐completion: Intervention group = 3 (2 undisclosed exclusion criteria, 1 lost to follow‐up), comparator group = 7 (2 lack of efficacy, 5 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Block randomised by sex to receive citalopram. Half of the subjects also received omega‐3 and the other half received placebo" but method of sequence generation not reported (P.62) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was described as "masked" but not clear who was blinded (P.61). It was unclear if the fishy taste was disguised and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | HDRS, MADRS and BDI ‐ all unclear ‐ The study was described as "masked" but it was unclear who was blinded (P.61). |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs ‐ measured by Treatment Emergent Symptoms Scale (Guy 1976). The study was described as "masked" but not clear who was blinded (P.61) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS, MADRS, BDI ‐ Analysis not ITT (ITT data provided separately) and > 10% missing data |
| Incomplete outcome data (Adverse Events) | High risk | AEs ‐ only reported for completers, and > 10% missing data |
| Selective reporting (reporting bias) | High risk | No protocol to check for additional outcome measures. CGI, PGI not reported |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Gharekhani 2014.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 4 months | |
| Participants |
Participants: 54 participants. Details reported only for completers ‐ mean age by group (Intervention = 56.8 (SD = 13.09) years; Comparator = 57.2 (SD = 15.19) years), 20 women, 25 men. Participants recruited from haemodialysis (HD) units of 2 teaching hospitals affiliated with Tehran University of Medical Sciences, Iran, dates ‐ not reported. Comorbidities: end‐stage renal disease, no psychiatric comorbidities Adjunctive treatment: no, for all participants Inclusion criteria: Adult patients who had been treated with HD for at least 3 months Exclusion criteria: BDI < 16, pregnancy, malabsorption syndrome, malignancy, inflammatory or infectious diseases, hypothyroidism, medical or surgical illness in recent 3 months, haemoglobinopathies, asthma, chronic obstructive pulmonary disease, coagulopathies, known psychiatric disorders, lack of tolerance or hypersensitivity to fish products as well as those who were receiving corticosteroid, non‐steroidal anti‐inflammatory drugs, omega‐3 fatty acids in the previous 3 months, anticoagulants including warfarin, immunomodulator or immunosuppressive were excluded |
|
| Interventions |
Intervention: EPA/DHA combination (1080 mg/d EPA: 720 mg/d DHA). 2 capsules, 3 x daily with meals Comparator: Placebo (paraffin oil), 2 capsules, 3 x daily with meals Treatment for 4 months |
|
| Outcomes |
Primary: BDI, Adverse events Secondary: SF‐36 (mental health component summary); Trial non‐completion BDI and SF‐36 assessed at baseline and 4 months whilst undergoing haemodialysis |
|
| Notes |
Funded: Tehran University of Medical Sciences (grant No: 17020) Conflicts of Interest: None reported Compliance: Pill counts Depressed mood (continuous): Analysis conducted on BDI at 4 months, ITT data provided by authors Adverse events: All adverse events reported (side effects). Intervention group = 8 (all GI), comparator group = 0 Quality of life: SF‐36 means and SDs, data provided by authors. Mental health summary scale used in analyses. Trial non‐completion: Intervention group = 2 (1 non‐compliance, 1 surgery), comparator group = 7 (1 hospitalisation, 1 undergoing renal transplantation, 2 discomfort from taking large capsule, 1 death due to CHD, 2 changing HD centre) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study used a 9‐block permuted randomisation procedure to allocate participants randomly into 2 groups. Each block contained an equal number of omega‐3 and control group selections, with the order of the blocks permuted. Random numbers to allocate blocks and randomise group selection were generated using Microsoft Office Excel software (P.656, correspondence from authors) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masking of fishy taste and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | BDI (self report) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs (self report) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | BDI ‐ ITT data provided by authors, but > 10% dropout |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported (correspondence from authors), but > 10% dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence from authors) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Gonzalez 2011.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks | |
| Participants |
Participants: 20 participants. 10 completing participants were a mean age = 38.77 years (SD = 10.74, range 30 ‐ 54), 8 women, dates ‐ not reported. Comorbidities: none reported, possible physical comorbidities Adjunctive treatment: Yes for all participants, fluoxetine (20 mg/d) Inclusion criteria: aged 18 ‐ 60 years; diagnosis of MDD (assessed by SCID‐ID), single or recurrent episode according to DSM‐IV‐TR criteria Exclusion criteria: not on antidepressants for at least 1 month prior to first blood sample collection, other psychiatric conditions; fish allergy; coagulopathies or taking aspirin |
|
| Interventions |
Intervention: EPA (3 g/d), 3 capsules, plus fluoxetine (20 mg/d) Comparator: Placebo, 3 capsules, plus fluoxetine (20 mg/d) |
|
| Outcomes |
Primary: HDRS assessed at baseline, 2, 4, 6 and 8 weeks Secondary: Response based on HDRS; Trial non‐completion Treatment for 8 weeks |
|
| Notes |
No funding reported. Conflicts of Interest: not reported. Compliance: not reported Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 8 weeks, calculated from published per protocol data Adverse events: AEs not reported fully or clearly. Values could not be included in analyses Trial non‐completion: 10 people withdrew (due to collateral effects, development of medical diseases, and stopped attending the psychiatric clinic), but group allocation unclear. Data are not clearly reported for one individual |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised on a double‐blind basis" but method of sequence generation not reported (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" (abstract) not reported who was blind to treatment. It was unclear if the fishy taste was disguised, and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | HDRS ‐ unclear whether outcome assessor was blind to treatment |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs ‐ unclear whether outcome assessor was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS ‐ not ITT, > 10% dropout (50%) |
| Incomplete outcome data (Adverse Events) | High risk | AEs ‐ "10 did not continue the study for several reasons. Among these, treatment withdrawal was due to collateral effects, development of medical diseases..." (translation) (P.74). > 10% dropout |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to check for additional outcome measures |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Grenyer 2007.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 16 weeks | |
| Participants |
Participants: 83 outpatients from Northfields Clinics, University of Wollongong, Australia, mean age 45.3 (range 18 ‐ 70) years, 51 women, dates ‐ not reported. Comorbidities: Yes in some participants: anxiety (54%), personality disorder (57%) Adjunctive therapy: Yes in some participants: 74% currently taking therapeutic doses of antidepressants Inclusion criteria: aged 18 ‐ 75 years, SCID DSM‐IV primary diagnosis of MDD, HDRS > 16 Exclusion criteria: serious medical condition, non‐consent for venipuncture, comorbid substance abuse, psychotic, bipolar, OCD or eating disorder |
|
| Interventions |
Intervention: EPA/DHA combination (tuna fish oil providing 2.2 g/d DHA, 0.56 g/d EPA, plus 80 mg vit E), 8 x 1 g capsules, plus ongoing therapy Comparator: Olive oil, 8 g/d, 8 x 1 g capsules per day, plus ongoing therapy Treatment received for 16 weeks |
|
| Outcomes |
Primary: HDRS, BDI ‐ baseline, 3 week intervals until 16 weeks, Adverse events Secondary: GAF, Likert scales of aches/pains, energy, fatigue, sleep, appetite; Trial non‐completion |
|
| Notes |
Funded by Clover Corporation Plc, Australia, University of Wollongong, Australia, and the Australian Research Council Supplements provided by Clover Corporation Plc, Australia Conflict of Interest: Not reported Compliance: Fortnightly capsule counts, EPA and DHA in RBC membranes, plasma cholesterol and alpha‐tocopherol at baseline, 6 weeks and 16 weeks Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 16 weeks, ITT data provided by authors Adverse events: Only prespecified adverse events are reported. ~⅓ of sample noticed changes in stools due to capsules across both groups. Only significant differences between groups also reported (belching, noticeable aftertaste in the mouth and breath), but no values. Data could not be included in analyses Quality of life: GAF measured, but no data available Trial non‐completion: Interventon group = 8, comparator group = 15. Reasons ‐ 8 time/commitment, 4 moved away, 3 hospitalised, 2 time constraints, 6 lost to follow‐up (reasons not split by group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "urn randomisation balanced _ on _ prognostic factors of age, sex, therapy, HDRS score" (P.1394) Randomisation was undertaken by a person unconnected with the study in a different location, who used a computer randomisation programme. Researchers gave them the blocking variables and the allocation was emailed back (correspondence with author) |
| Allocation concealment (selection bias) | Low risk | "Randomisation and capsule packing performed externally" (P.1394) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, clinicians and researchers were blind to allocation. Identical placebo and capsules odourless, however when checked the majority ("90% fish oil group, 64% placebo group) of participants correctly guessed their assignment" (P.1395) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS ‐ clinician‐rated: physicians blinded to allocation (LOW) BDI ‐ self report (HIGH) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs ‐ participant‐rated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis conducted but 28% dropped out |
| Incomplete outcome data (Adverse Events) | High risk | AEs not clearly reported, plus > 10% drop out. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Jahangard 2018.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 50 participants: (Intevrention group: 25 participants, mean age 41.28 years (SD 11.56), 17 men, 8 women; Comparator group: 25 participants, mean age 43.64 years (SD 11.29), 17 men, 8 women), recruited from secondary care setting in Iran, dates ‐ not reported. Comorbidities: No physical or psychiatric comorbidities Adjunctive therapy: Sertraline, at therapeutic doses, for all participants Inclusion criteria: 1. Age between 18 and 65 years; 2. Current state of MDD, as ascertained by a trained psychiatrist or clinical psychologist based on the DSM 5 criteria; 3. willing and able to comply with the study conditions (following a clinical interview; completing self‐rating questionnaires; adherence to the medication regimen); 4. Intake of sertraline at therapeutic dosages and as prescribed by a psychiatrist; 5. Signed written informed consent. 6. Interrupting possible further treatments such as neuromodulation, sports/exercising, psychotherapy, and intake of further medications such as nonsteroidal antiinflammatory drug (NSAID), anxiolytics, and hypnotics Exclusion criteria: 1. Current comorbid psychiatric disorders such as substance use disorder, signs of psychotic disorders, post‐traumatic stress disorder, bipolar disorders, as ascertained by a psychiatric interview; 2. Current suicidally, as ascertained by a trained psychiatrist or clinical psychologist and based on a structured and clinical psychiatric interview; 3. Female patients; pregnant or willing to get pregnant during the study; breast feeding. 4. Chronic diseases such as diabetes mellitus, hypertension or epilepsy, as ascertained by a thorough medical interview and from medical records |
|
| Interventions |
Intervention: Omega‐3 fatty acids ‐ not otherwise specified, 1000 mg/day, 1 capsule Comparator: Placebo ‐ not otherwise specified, identical to intervention in shape, weight, colour and scent Treatment received for 12 weeks |
|
| Outcomes |
Primary: MADRS, BDI‐II (21 item), at baseline, 6 and 12 weeks Secondary: Trial non‐completion |
|
| Notes | Study is the doctoral thesis of Ali Sadeghi (doctoral thesis number at the Hamadan University of Medical Sciences, Hamadan, Iran: 9605313486), and was performed without external funding Supplements provided by: Not reported Conflicts of interest: None declared Compliance: Flow diagram indicates "no erratic use or withdrawal", method to assess erratic use is not reported Depressed mood (continuous): Analyses conducted on MADRS score at 12 weeks, ITT data as published Adverse events: Not assessed Trial non‐completion: No participants withdrew |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization occurred with the software randomization.com", p 50 |
| Allocation concealment (selection bias) | Low risk | "a psychologist not otherwise involved in the study assigned participants to the two study conditions", p 50 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "participants were blind to the study condition assignment", p. 50, "the placebo was identical to the O3PUFA capsule in shape, weight, colour ad scent", p. 52. No test of blinding was undertaken. Blinding of personnel is unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "experts (trained psychiatrists and clinical psychologists) blind to participants' rated participants' symptoms of depression", p. 50 |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used |
| Incomplete outcome data (Adverse Events) | Unclear risk | AEs not assessed |
| Selective reporting (reporting bias) | High risk | AEs not assessed and would be expected. Not all outcomes in protocol are reported (social competencies, physical activity, cognitive performance). Some outcomes not given in the protocol are reported (anxiety sensitivity, intolerance of uncertainty, emotion regulation) |
| Other bias | Unclear risk | Discrepancies between protocol and paper |
Jazayeri (v AD) 2008.
| Study characteristics | ||
| Methods | Randomised controlled parallel 3‐arm trial, 8 weeks | |
| Participants |
Participants: 60 outpatients from the Roozbeh Psychiatry Hospital, Tehran, Iran (split across Jazayeri (v placebo) 2008 and Jazayeri (v AD) 2008)). 48 participants completing the study had a mean age = 34.8 years, 33 women, dates ‐ not reported. Comorbidities: No physical comorbidities, possible psychiatric comorbidities Adjunctive therapy: No for all participants Inclusion criteria: Aged 20 ‐ 59 years, DSM‐IV criteria for MDD (SCID), no psychotic features, scoring > 15 HDRS (24‐item), medication‐free for at least 6 weeks Exclusion criteria: comorbid psychiatric diagnosis (other than dysthymia and anxiety), significant medical illness established by medical history, physical examination or laboratory tests, suicidal thoughts, substance abuse, history of hypomanic/manic/mixed episode, pregnancy and lactation, consumption of n‐3PUFAs in the previous year and dietary intake of > 1 serving of fish per week, use of non‐steroid anti‐inflammatory drugs and other drugs 2 weeks before or during the intervention. |
|
| Interventions |
Intervention: E‐EPA (1.1 g/d providing 1 g/d pure EPA, plus 11 mg vitamin E), 2 x 550 mg capsules, plus 20 mg/d fluoxetine placebo (starch and avicel) (EPA group) Comparator: Rapeseed oil (1.1 g/d, plus 11 mg vitamin E), 2 x 550 mg capsules, plus 20 mg/d fluoxetine (Fluoxetine group) Treatment received for 8 weeks |
|
| Outcomes |
Primary: HDRS (24‐item) ‐ baseline, 2, 4, 6, 8 weeks; Adverse events Secondary: Response based on HDRS; Trial non‐completion |
|
| Notes |
Supported by Vice Chancellor for Research, Tehran University of Medical Sciences, Iran Supplements provided by Minami Nutrition, Belgium Conflicts of Interest: not reported Compliance: Capsule counts Depressed mood (continuous): Analysis conducted on HDRS (24‐item) scores at 8 weeks, ITT data provided by authors Adverse events: Number of events reported rather than number of participants experiencing events. Intervention group = 5 adverse events (3 GI, 1 psychological, 1 other physical), Comparator group = 28 adverse events (6 GI, 10 psychological, 12 other physical). Data could not be included in analyses. Trial non‐completion: Intervention group = 4 (1 developing suicidal ideation, 1 non‐compliance, 2 lost to follow‐up), Comparator group = 4 (1 drowsiness, 1 non‐compliance, 2 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prearranged block randomisation" (P.193) but unclear how sequence was generated Permuted‐block randomisation (correspondence with authors) |
| Allocation concealment (selection bias) | High risk | The randomisation sequence was not concealed from researchers (correspondence with authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Double dummy technique used to blind participants"; however, no steps taken to mask fish taste and no assessment to check concealment (P.194 ‐ 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS ‐ physicians blind to treatment allocation |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs ‐ participant‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS ‐ ITT data provided by authors, > 10% dropout. |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported (Table 4, p.196), but > 10% dropout. |
| Selective reporting (reporting bias) | High risk | Some outcomes not yet published (correspondence with authors) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Jazayeri (v placebo) 2008.
| Study characteristics | ||
| Methods | Randomised controlled parallel 3‐arm trial, 8 weeks | |
| Participants |
Participants: 60 outpatients from the Roozbeh Psychiatry Hospital, Tehran, Iran (split across Jazayeri (v placebo) 2008 and Jazayeri (v AD) 2008)). 48 participants completing the study had a mean age = 34.8 years, 33 women, dates ‐ not reported. Comorbidities: No physical comorbidities, possible psychiatric comorbidities Adjunctive therapy: Yes, fluoxetine as part of the study Inclusion criteria: Aged 20 ‐ 59 years, DSM‐IV criteria for MDD (SCID), no psychotic features, scoring > 15 HDRS (24‐item), medication‐free for at least 6 weeks Exclusion criteria: comorbid psychiatric diagnosis (other than dysthymia and anxiety), significant medical illness established by medical history, physical examination or laboratory tests, suicidal thoughts, substance abuse, history of hypomanic/manic/mixed episode, pregnancy and lactation, consumption of n‐3PUFAs in the previous year and dietary intake of > 1 serving of fish per week, use of non‐steroid anti‐inflammatory drugs and other drugs 2 weeks before or during the intervention. |
|
| Interventions |
Intervention: E‐EPA (1.1 g/d providing 1 g/d pure EPA, plus 11 mg vitamin E), 2 x 550 mg capsules, plus 20 mg/d fluoxetine (Fluoxetine + EPA combination group) Comparator: Rapeseed oil (1.1 g/d, plus 11 mg vit E), 2 x 550 mg capsules, plus 20 mg/d fluoxetine (Fluoxetine group) Treatment received for 8 weeks |
|
| Outcomes |
Primary: HDRS (24‐item) ‐ baseline, 2, 4, 6, 8 weeks; Adverse events Secondary: Response based on HDRS; Trial non‐completion |
|
| Notes |
Supported by Vice Chancellor for Research, Tehran University of Medical Sciences, Iran Supplements provided by Minami Nutrition, Belgium Conflicts of Interest: not reported Compliance: Capsule counts Depressed mood (continuous): Analysis conducted on HDRS (24‐item) scores at 8 weeks, ITT data provided by authors Adverse events: Number of events reported rather than number of participants experiencing events. Intervention group = 20 adverse events (6 GI, 4 psychological, 10 other physical), Comparator group = 28 adverse events (6 GI, 10 psychological, 12 other physical). Data could not be included in analyses. Trial non‐completion: Intervention group = 4 (1 steatorrhoea, 1 physical conditions, 1 non‐compliance, 1 lost to follow‐up), Comparator group = 4 (1 drowsiness, 1 non‐compliance, 2 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prearranged block randomisation" (P.193) but unclear how sequence was generated Permuted‐block randomisation (correspondence with authors) |
| Allocation concealment (selection bias) | High risk | The randomisation sequence was not concealed from researchers (correspondence with authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Double dummy technique used to blind participants", however no steps taken to mask fish taste and no assessment to check concealment (P.194 ‐ 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS ‐ physicians blind to treatment allocation |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs ‐ participant‐reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS ‐ ITT data provided by authors, > 10% dropout |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported (Table 4, p.196), but > 10% dropout. |
| Selective reporting (reporting bias) | High risk | Some outcomes not yet published (correspondence with authors). |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Jiang (EPA+DHA) 2018.
| Study characteristics | ||
| Methods | Randomised controlled, multicentre, parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 108 participants in total (EPA+DHA Intervention group: 36 participants, mean age 57.73 years (SD 16.14), 21 men, 15 women; Comparator group: 36 participants, mean age 57.91 years (SD 11.68), 13 men, 23 women), recruited from primary and secondary care settings between June 2014 and May 2016 in USA Comorbidities: Congestive heart failure in all participants, no psychiatric comorbidities Adjunctive therapy: Intervention group: Antidepressant use in 14 (38.9%) participants (SSRIs in 8 participants); Comparator group: Antidepressant use in 15 (41.7%) participants (SSRIs in 10 participants) Inclusion criteria: > 18 years of age, clinical diagnosis of Coronary Heart Failure with NYHA functional class II or greater; DSM‐IV diagnosis of MDD; and HDRS ≥ 18 Exclusion criteria: Significant cognitive impairment (MMSE ≤ 23), alcohol / drug dependency within 90 days, physical disability, life‐threatening comorbidity with likely 50% mortality, suicidal ideations, presence of psychoses, bipolar disorder, or severe personality disorders, or both, current use of antipsychotic medications, or psychotropic medications, except SSRI or benzodiazepine or both, pregnancy or lactating, hypersensitivity or intolerance of n‐3, or use for ≥ study dose for more than 3 months, treatment with ECT or TMS, hypo / hyperthyroidism, treatment with any investigational agent 1 month before, acute coronary syndrome, vascularisation procedure within past month, planned cardiac surgery within 3 months |
|
| Interventions |
Intervention: 500 mg EPA:DHA (2:1) per capsule and 4 capsules a day, unless intolerant when dose was reduced, 1 capsule minimum Comparator: Corn oil, capsules matched to intervention, identical in colour and smell Treatment received for 12 weeks |
|
| Outcomes |
Primary: HDRS, BDI‐II (21‐item), at baseline and 12 weeks, Adverse events Secondary: Depression remission based on BDI‐II, QoL: SF‐36 at baseline and 12 weeks, Trial non‐completion |
|
| Notes |
Supported by the National Institute of Mental Health collaborative R34 mechanism (NIMH 1R34MH097034) Supplements provided by: Ocean Nutrition Canada Ltd. Conflicts of interest: Dr. Harris is the owner of OmegaQuant Analytics, LLC, which performed the omega‐3 assays for this study free of charge. Dr. O’Connor has received funding from Actelion Pharmaceuticals Ltd., Amgen Inc., Biscardia LLC, Faculty Connection, GE Healthcare, Ikaria, Novella Clinical Inc., Pfizer Inc., Pozen, and Roche Diagnostics; serves as a consultant for Novartis, HeartWare, ResMed, Johnson & Johnson, Gilead, Critical Diagnostics, BG Medicine, Otsuka, Astellas, Cytokinetics, and Capricor; and holds stock or stock options in Neurotronik/ Interventional Autonomics Corporation. Compliance: RBC membrane ‐ omega 3 index, %EPA, %DHA, %DPA, pill counts Depressed mood (continuous): Analyses conducted on HDRS scores at 12 weeks, ITT data. Adverse events: 20 individuals in intervention group (3 gastrointestinal, 15 other specific physical complaint, 12 other); 15 individuals in control group (2 gastrointestinal, 9 other specific physical complaint, 4 other) Depression remission: Data not provided in a form that could be used Quality of Life: Analyses conducted on SF‐36 emotional wellbeing scale at 12 weeks, completer data only Trial non‐completion: 8 participants from each treatment arm failed to complete (Intervention group: 1 was not reported, 7 violated the protocol; Control group: 4 were not reported, 4 violated the protocol). Reasons for early withdrawal from the trial were not split by group, but included: medication side effects (n = 2), patient felt too sick to continue (n = 2), lost to follow‐up (n = 1), protocol violation (n = 1), transportation issues (n = 1), and death (n = 1). 1 patient could tolerate only 1 capsule daily, and 18 patients took 2 capsules daily; the rest of the participants took 4 capsules daily |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization schedule was generated separately for each site in blocks of 12", p. 836 |
| Allocation concealment (selection bias) | Low risk | "Schedule was generated by an external statistician who was not involved with the study and sent directly to the study pharmacist", p.836 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The placebo and omega‐3 capsules were identical in colour and smell" p.836, but there was no test of this. Blinding of personnel not reported. Fishy odour was reported as a specified adverse event |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)", trial registration, but no further detail provided |
| Blinding of outcome assessment (Adverse Events) | High risk | There was "formal face‐to‐face training of study personnel on providing formal psychiatric diagnostic interviews, administering study instruments, and participants’ safety monitoring;" p. 834 ‐ 5, but AEs include "fishy odor" p.839 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis used for depression outcomes, but higher than 10% withdrawals. High risk of bias for QoL ‐ as only completer data are provided |
| Incomplete outcome data (Adverse Events) | High risk | All adverse events reported in table 4, but higher than 10% withdrawals |
| Selective reporting (reporting bias) | High risk | Data on depression remission are unclear. Remission and QoL outcomes are reported, but were not prespecified. Many other outcomes are also reported that were not prespecified |
| Other bias | Unclear risk | Discrepancies between protocol and paper |
Jiang (EPA only) 2018.
| Study characteristics | ||
| Methods | Randomised controlled, multicentre, parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 108 participants in total (EPA only Intervention group: 36 participants, mean age 58.1 years (SD 10.16), 16 men, 20 women; Comparator group: 36 participants, mean age 57.91 years (SD 11.68), 13 men, 23 women), recruited from primary and secondary care settings between June 2014 and May 2016 in USA Comorbidities: Congestive heart failure in all participants, no psychiatric comorbidities Adjunctive therapy: Intervention group: Antidepressant use in 16 (44.4%) participants (SSRIs in 12 participants); Comparator group: Antidepressant use 15 (41.7%) participants (SSRI in 10 participants) Inclusion criteria: > 18 years of age, clinical diagnosis of Coronary Heart Failure with NYHA functional class II or greater; DSM‐IV diagnosis of MDD; and HDRS ≥ 18 Exclusion criteria: Significant cognitive impairment (MMSE ≤ 23), alcohol / drug dependency within 90 days, physical disability, life‐threatening comorbidity with likely 50% mortality, suicidal ideations, presence of psychoses, bipolar disorder, and/or severe personality disorders, current use of antipsychotic medications, or psychotropic medications, except SSRI and/or benzodiazepine, pregnancy or lactating, hypersensitivity or intolerance of n‐3, or use for ≥ study dose for > 3 months, treatment with ECT or TMS, hypo / hyperthyroidism, treatment with any investigational agent 1 month before, acute coronary syndrome, vascularisation procedure within past month, planned cardiac surgery within 3 months |
|
| Interventions |
Intervention: 500 mg EPA per capsule and 4 capsules a day, unless intolerant when dose was reduced, 1 capsule minimum Comparator: Corn oil, capsules matched to intervention, identical in colour and smell Treatment received for 12 weeks |
|
| Outcomes |
Primary: HDRS, BDI‐II (21‐item), at baseline and 12 weeks, Adverse events Secondary: Depression remission based on BDI‐II, QoL: SF‐36 at baseline and 12 weeks, Trial non‐completion |
|
| Notes |
Supported by the National Institute of Mental Health collaborative R34 mechanism (NIMH 1R34MH097034). Supplements provided by: Ocean Nutrition Canada Ltd. Conflicts of interest: Dr. Harris is the owner of OmegaQuant Analytics, LLC, which performed the omega‐3 assays for this study free of charge. Dr. O’Connor has received funding from Actelion Pharmaceuticals Ltd., Amgen Inc., Biscardia LLC, Faculty Connection, GE Healthcare, Ikaria, Novella Clinical Inc., Pfizer Inc., Pozen, and Roche Diagnostics; serves as a consultant for Novartis, HeartWare, ResMed, Johnson & Johnson, Gilead, Critical Diagnostics, BG Medicine, Otsuka, Astellas, Cytokinetics, and Capricor; and holds stock or stock options in Neurotronik/ Interventional Autonomics Corporation. Compliance: RBC membrane ‐ omega 3 index, %EPA, %DHA, %DPA, pill counts Depressed mood (continuous): Analyses conducted on HDRS scores at 12 weeks, ITT data. Adverse events: Intervention group: 20 individuals (2 gastrointestinal, 11 other specific physical complaint, 10 other); Comparator group: 15 individuals (2 gastrointestinal, 9 other specific physical complaint, 4 other). Depression remission: Data not provided in a form that could be used. Quality of Life: Analyses conducted on SF‐36 emotional wellbeing scale at 12 weeks, completer data only. Trial non‐completion: 12 participants from the intervention arm failed to complete (3 not reported why, 9 violated the protocol); 8 participants from the comparator group (4 not reported why, 4 violated the protocol). Reasons for early withdrawal from the trial were not split by group, but included: medication side effects (n = 2), patient felt too sick to continue (n = 2), lost to follow‐up (n = 1), protocol violation (n = 1), transportation issues (n = 1), and death (n = 1). 1 patient could tolerate only 1 capsule daily, and 18 patients took 2 capsules daily; the rest of the participants took 4 capsules daily |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization schedule was generated separately for each site in blocks of 12", p. 836 |
| Allocation concealment (selection bias) | Low risk | "Schedule was generated by an external statistician who was not involved with the study and sent directly to the study pharmacist", p. 836 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The placebo and omega‐3 capsules were identical in colour and smell" p. 836, but there was no test of this. Blinding of personnel not reported. Fishy odour was reported as a specified adverse event |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)", trial registration, but no further detail provided. |
| Blinding of outcome assessment (Adverse Events) | High risk | There was "formal face‐to‐face training of study personnel on providing formal psychiatric diagnostic interviews, administering study instruments, and participants’ safety monitoring;" p. 834 ‐ 5, but AEs include "fishy odor" p. 839 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis used for depression outcomes, but higher than 10% withdrawals. High risk of bias for QoL ‐ as only completer data are provided |
| Incomplete outcome data (Adverse Events) | High risk | All adverse events reported in table 4, but higher than 10% withdrawals |
| Selective reporting (reporting bias) | High risk | Data on depression remission are unclear. Remission and QoL outcomes are reported, but were not prespecified. Many other outcomes are also reported that were not prespecified |
| Other bias | Unclear risk | Discrepancies between protocol and paper |
Kamath 2017.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 5 participants (Intervention group: age = 53 ‐ 66 years, 1 man, 1 woman; Comparator: age = 53 ‐ 66 years, 1 man, 2 women), recruited from February 2013 – June 2016, through the University of Conneticut Health Center, USA Comorbidities: cardiovascular disease, diabetes or cancer in all participants, possible anxiety‐related comorbidities Adjunctive therapy: Antidepressants (desvenlafaxine 50mg/day) in all participants Inclusion criteria: 18 years of age or older; diagnosed with depression and have cardiovascular disease, diabetes or cancer; able to provide written informed consent prior to initiation of any study‐related procedures; able to understand and comply with the requirements of the study. Exclusion criteria: Hospitalised patients or psychotherapy for depression begun within 4 weeks; patients with medically reversible causes of depression (e.g. hypothyroidism); patients with significant comorbid symptoms (e.g. pain, insomnia) that have a direct causal relation to depressive and anxiety symptoms with these comorbid symptoms dominating the clinical scenario; patients will be enrolled in the study if these comorbid symptoms merely coexist with depressive and anxiety symptoms and are not dominating the clinical scenario as judged by the study investigator; patients with an identifiable diagnosis of substance abuse or dependence within 6 months prior to evaluation (except those in full remission, or those with caffeine or nicotine dependence) as defined by DSM‐IV criteria; patients with any clinically significant unstable or inadequately treated comorbid medical condition which, in the opinion of the investigator, would make the patient unsuited for the study; patients with currently active or with significant history of other clinically significant psychiatric disorders such as bipolar disorder, schizophrenia etc. Pregnant patients, breastfeeding or those planning to become pregnant during the study; any other condition, which, in the opinion of the investigator, would make the patient, unsuited for enrolment in the study, including known or suspect history of allergy to fish oil, fish or desvenlafaxine |
|
| Interventions |
Intervention: Omega 3 FA supplement (not otherwise specified) (range 2.4 gm/day ‐ 4.8 gm day) Comparator: Placebo for omega 3 FA supplement (not otherwise specified) Treatment received for 12 weeks |
|
| Outcomes |
Primary: MADRS, HADS ‐ baseline and 12 weeks, Adverse events Secondary: SF‐12 ‐ baseline and 12 weeks, Trial non‐completion |
|
| Notes |
Sponsored by: University of Connecticut Health Center, Farmington, Connecticut, United States, 06030 Supplements provided by: Not reported Conflicts of interest: Not reported Compliance: Not reported Depressed mood (continuous): no data available. Adverse events: Intervention group: 1 individual (gastrointestinal); Comparator group: 1 individual (memory‐related) Quality of Life: no data available Trial non‐completion: 1 participant from each study arm failed to complete Study terminated (Lack of recruitment and no resources). Due to very low enrolment in both arms, only descriptive analyses were completed for both arms |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study reported as “triple masked (participant, investigator, outcomes assessor)”, trial registration, but no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study reported as “triple masked (participant, investigator, outcomes assessor)”, trial registration, but no further details provided |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | Study reported as “triple masked (participant, investigator, outcomes assessor)”, trial registration, but no further details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data provided, but “data were not intended to be summarized if there were fewer than 10 participants”, trial registration, higher than 10% withdrawals |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, trial registration, but higher than 10% withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No MADRS, HADS or QoL data provided, but “data were not intended to be summarized if there were fewer than 10 participants”, trial registration |
| Other bias | High risk | "Study terminated due to low recruitment and lack of resources", trial registration |
Lespérance 2011.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks | |
| Participants |
Participants: 432 outpatients with mean age = 46.0 (SD = 12.4) years, 68.5% women, were recruited via adverts, physician referrals and caseloads of study investigators from 8 academic and psychiatric clinics in Canada. The study ran from Oct 2005 to Jan 2009. Comorbidities: Yes in some participants: anxiety disorders (52.8%), possible physical comorbidities Adjunctive therapy: Yes for some participants: 40.3% antidepressants at baseline, 14.8% undergoing psychotherapy, 27.1% regularly used at least 1 other psychotropic medication Inclusion criteria: aged 18 years and over, met diagnostic criteria for MDE (MINI 5), score ≥ 27 IDS‐SR, clinically significant depressive symptoms for ≥ 4 weeks, if taking antidepressants ‐ to have been at maximum dosage for > 4 weeks, or if not on antidepressants to have been intolerant for ≥ 2 previous antidepressants or refused to take them despite medical advice Exclusion criteria: known allergy or intolerance to fish/sunflower oil, taken > 14 g of n‐3PUFA supplements during past 4 weeks, diagnosis of alcohol/drug abuse/dependency during past 12 months or bipolar disorder (MINI), significant suicidal risk based on clinical judgement, history of MI, pancreatic insufficiency or coagulation diseases, regularly taking drugs or herbs with antiplatelet or anticoagulant properties, non‐menopausal pregnant women or those not taking contraception |
|
| Interventions |
Intervention: EPA/DHA combination (EPA = 1050 mg/d, DHA = 150 mg/d), 3 x capsules daily, plus ongoing therapy Comparator: Sunflower oil + 2% fish oil (to help blind), 3 x capsules daily, plus ongoing therapy Treatment received for 8 weeks |
|
| Outcomes |
Primary: IDS‐SR, MADRS at baseline, 1, 2, 4 and 8 weeks, Adverse events Secondary: Trial non‐completion |
|
| Notes |
Funded by Isodis Natura and Foundation Du Centre Hospitalier de l'Universite de Montreal and the CRCHUM Supplements provided by Isodis Natura Conflicts of Interest: CoIs declared by 3 authors Compliance: Reported in results, but method of assessment not reported Depressed mood (continuous): Analysis conducted on MADRS scores at 8 weeks, unadjusted ITT data provided by authors. Adverse events: Adverse events only gained from completers, only includes events reported by ≥ 5% population. Serious adverse events reported by event not by individual. Serious adverse events reported: Intervention group = 7 (3 physical, 4 psychological), Comparator group = 4 (4 physical). Number of participants with non‐serious adverse events: Intervention group = 322 events in 161 participants (215 GI, 107 other), Comparator group = 294 events in 148 participants (181 GI, 113 other). Data in the analysis are for non‐serious adverse events Trial non‐completion: Intervention = 30 (reasons not reported), Comparator = 27 (reasons not reported) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, "randomly permuted blocks of 2 and 4, _ stratified by study site and baseline antidepressant use/non‐use." (P.1056 and correspondence from author) |
| Allocation concealment (selection bias) | Low risk | "Group assignment _ using sequentially‐numbered containers", generated by co‐ordinating centre. "Only technician preparing containers had access to randomisation codes" (P.1056) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study research personnel and participants were blinded. 2% fish oil was added to placebo to control for fishy aftertaste. James' blinding index used to check blinding of treatment allocation (P. 1056) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | IDS‐SR and MADRS both low ‐ study psychiatrists, personnel were blinded |
| Blinding of outcome assessment (Adverse Events) | Low risk | AEs ‐ participant‐assessed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis and although similar dropouts in each group, > 10% dropout |
| Incomplete outcome data (Adverse Events) | High risk | AEs ‐ only reported AEs reported by > 5% of participants, > 10% dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias. |
Lucas 2009.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks. Subgroup analysis of 29 women with MDD (randomisation stratified according to MDD diagnosis) | |
| Participants |
Participants: 29 postmenopausal women with mean age = 49.6 years were recruited from the general population in Quebec, Canada through newspaper, radio and television advertisements, and flyers posted in clinics and by clinicians. Participants were recruited from March 2005 to November 2006, study ran until February 2007 Comorbidities: No physical comorbidities, possible psychiatric comorbidities Adjunctive therapy: No for all participants Inclusion criteria: aged 40 ‐ 55 years, postmenopausal, score ≤ 72 on the PGWB and score < 26 on the HDRS (21‐item) Exclusion criteria: score ≥ 26 on the 21‐item HDRS, physical conditions known to affect mental health, substance abuse/dependence, high consumption of fish (> 3 serving per week), fish allergies, past or current schizophrenia or bipolar disorder, risk of suicide or homicide, postmenopausal for more than 5 years, use of St John's Wort, antidepressants, hormone replacement therapy or fish oil supplements in previous 3 months, use of anticoagulants |
|
| Interventions |
Intervention: EPA/DHA combination (1.5 g/d ethyl esters, providing 1050 mg/d EPA, 150 mg/d DHA), 3 capsules daily Comparator: Sunflower oil (1.5 g/d, plus 0.2% regular fish oil [18% EPA/12% DHA]), 3 capsules daily Treatment received for 8 weeks |
|
| Outcomes |
Primary: 21‐item HDRS, 20‐item HSCL (Williams 2004) measured at baseline, 4 and 8 weeks. Adverse events Secondary: PGWB, CGI, Trial non‐completion |
|
| Notes |
Supported by Laval University, Canada Supplements provided by Isodus Natura, Belgium Conflicts of Interest: CoIs declared by one author Compliance: Capsule counts, and RBC membrane analysis Depressed mood (continuous): Analysis conducted on HDRS (21‐item) scores at 8 weeks, ITT data provided by authors Adverse events: Adverse events reported by event, not by individuals. Only includes events reported by ≥ 5% population. Adverse events are not published separately for the subgroup. Adverse events (number of individuals) in the analysis were provided by the authors Quality of life: CGI data used in the analysis Trial non‐completion: Intervention group = 1 (lack of efficacy), comparator group = 2 (adverse events) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified by history of major depressive episode". Computer‐generated stratified randomisation lists prepared by a statistician. (P.642) |
| Allocation concealment (selection bias) | Low risk | "Researchers responsible for seeing participants allocated next available entry number." Statistician gave randomisation list to pharmacy who packaged capsules. (P.642) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants, investigators and staff were blind to treatment assignment until the last participants completed study" (P.642) Capsules were obtained directly from the pharmacist Matching placebo with added fish for aftertaste "There was no difference in the number of people guessing their allocation correctly." (P.645) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS/CGS/HSCL/PGWB ‐ all low. "Participants, investigators and staff were blind to treatment assignment until the last participants completed study" (P. 642) |
| Blinding of outcome assessment (Adverse Events) | Low risk | AEs ‐ reported by participants who were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis ‐ additional information from authors |
| Incomplete outcome data (Adverse Events) | Low risk | All AEs reported ‐ information provided by the authors |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Marangell 2003.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 6 weeks | |
| Participants |
Participants: 36 participants. 35 participants completing the study had a mean age = 47.3 years, 28 women, location and dates ‐ not reported. Comorbidities: No Adjunctive therapy: No Inclusion criteria: aged 18 ‐ 65 years, met DSM‐IV criteria for MDD without psychotic features (assessed by SCID), score ≥ 12 on the MADRS and score ≥ 17 on the 28‐item HDRS, medication‐free for ≥ 2 weeks prior to enrolment, dietary intake of ≤ 1 serving of fish per week Exclusion criteria: physical conditions or psychiatric comorbidities, treatment resistance |
|
| Interventions |
Intervention: DHA (2 g/d) Comparator: placebo (2 g/d) Treatment received for 6 weeks |
|
| Outcomes |
Primary: MADRS, HDRS (28‐item) measured at baseline, 2 and 6 weeks; Adverse events Secondary: Response based on MADRS; GAF; Trial non‐completion |
|
| Notes |
Funded by Martek Biosciences Corporation, USA Conflicts of Interest: not reported Compliance: RBC DHA levels Depressed mood (continuous): Analysis conducted on HDRS (28‐item) scores at 6 weeks, per protocol data as published Adverse events: Number of events reported rather than number of participants with at least 1 adverse event. Intervention group = 25 events (19 GI, 6 other physical), comparator group = 5 (1 GI, 4 other physical). Data could not be included in analyses Trial non‐completion: 1 participant withdrew (group allocation unclear) (reason not reported). Data could not be included in analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported other than "double blind" specified in title. It was unclear if the fishy taste was disguised and no assessment to check concealment. It was unclear whether or not the placebo was identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | MADRS/HDRS ‐ unclear whether assessor was blinded to treatment |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs ‐ unclear whether assessor was blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | MADRS/HDRS ‐ not ITT analysis |
| Incomplete outcome data (Adverse Events) | High risk | Not all AEs reported ‐ "AEs included ..." P.997. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to check prespecified outcome measures |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Masoumi 2016.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 1 month | |
| Participants |
Participants: 60 participants (Intervention group: 30 participants, mean age 55.17 years (SD 7.33), 30 women. Comparator group: 30 participants, mean age 55.67 years (SD 6.06), 30 women), recruited from primary care setting in Iran, from October 2013 – July 2014 Comorbidities: Possible physical or psychiatric comorbidities, or both Adjunctive therapy: Citalopram (20 mg/d), for all participants Inclusion criteria: Age range 45 ‐ 65 years, history of at least 12 months of amenorrhoea, depression criteria according to the DSM‐IV measure, earning a score higher than 10 on the Beck’s Depression Inventory (BDI), confirmation of depression by the psychiatrist, having no history of hysterectomy, oophorectomy or radiation therapy, receiving no antidepressant medication during the past 6 months, having no sensitivity to herbs, not diabetic or with no cardiovascular disease, and a negative history of hormone therapy Exclusion criteria: Not consenting to the study, a depression score higher than 30 at follow‐ups and any known drug side effects |
|
| Interventions |
Intervention: Omega‐3 ‐ not otherwise specified, 1g/day Comparator: Placebo ‐ not otherwise specified, daily Treatment received for 1 month |
|
| Outcomes |
Primary: BDI‐II (21 item), at baseline, 1, 2, and 4 weeks Secondary: Trial non‐completion |
|
| Notes |
Supported by the research deputy of the Hamadan University of Medical Sciences Supplements provided by: The omega‐3 drug was manufactured at the International Agensis in America and prepared by Poorateb pharmaceutical companies in Iran Conflicts of interest: None declared Compliance: No details on compliance are reported Depressive symptomology (continuous): Analyses conducted on BDI‐II score at 1 month. ITT data used Adverse events: Not assessed Trial non‐completion: No participants withdrew from either arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated in the two groups using a permuted block randomization technique, ... by a researcher who did not participate in sampling", p. 2 |
| Allocation concealment (selection bias) | Low risk | "Both medications were prepared in similar shapes and were coded by the pharmacist according to the allocation sequence." p. 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Neither the patients nor the physician, and the data analyser were aware of the type of intervention.", p.2. "Both medications were prepared in similar shapes", p. 2. but there was not test of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants complete the measure, and unclear if this blinding was successful. "Neither the patients nor the physician, and the data analyser were aware of the type of intervention.", p. 2. "Both medications were prepared in similar shapes", p. 2. but there was no test of blinding |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs not assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses conducted |
| Incomplete outcome data (Adverse Events) | Unclear risk | AEs not assessed |
| Selective reporting (reporting bias) | High risk | AEs not assessed and would be expected |
| Other bias | Unclear risk | Discrepancies between the protocol and paper ‐ some AEs (complications with drug) are included in the protocol |
Mazereeuw 2016.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks. Subgroup analysis of 21 individuals with MDD (data supplied by authors) | |
| Participants |
Participants: 21 participants (Intervention group: 10 participants, mean age 60.7 years (SD 9.8), 6 men, 4 women. Comparator group: 11 participants, mean age 60.1 years (SD 8.0), 7 men, 4 women), recruited from secondary care setting in Canada between August 2010 and February 2014 Comorbidities: Evidence of stable coronary artery disease (history of myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or at least a 50% stenosis in 1 or more major coronary arteries) in all participants. Possible psychiatric comorbidities Adjunctive therapy: Existing therapy permitted in some participants ("Antidepressant use was permitted if used at a stable dose for at least 3 months before the trial", 2016, p.437) Inclusion criteria: Aged 45 to 80 years with stable coronary artery disease (history of myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty or at least a 50% stenosis in 1 or more major coronary arteries) and has the ability to speak and understand English Exclusion criteria: Excluded patients were those with a significant acute medical illness, a clinically significant cognitive impairment (Standardized Mini‐Mental State Examination [sMMSE] score < 24), a neurological condition, unstable angina (Canadian Cardiovascular Society class 4), ventricular tachycardia or an implantable cardioverter defibrillator, or both, or a high risk of mortality (Killip class > II); who were currently abusing ethanol or other substances; women of childbearing potential, or allergic or hyper‐sensitive to fish; or who have contraindications to soybean/corn oil or a pre‐existing bleeding disorder, history of electroconvulsive therapy, suicidal ideation or a history of suicidal ideation/attempts (determined during SCID‐I at screening/baseline visits); severe depression, defined by Hamilton Depression Rating score > 23; Current or history of psychotic episode or personality disorder |
|
| Interventions |
Intervention: 1.9 ‐ g n‐3PUFA daily (1.2 g EPA, 0.6 g DHA, with 0.1 g other n‐3PUFA), 3 capsules daily, in an ethyl‐ester form Comparator: 3 g capsules daily of 50/50 soybean/corn oil blend containing < 0.1 ‐ g n‐3PUFA with negligible EPA and DHA Treatment received for 12 weeks. |
|
| Outcomes |
Primary: HDRS (17‐item), BDI‐II (21‐item) at baseline, 4, 8, 12 weeks, Adverse events Secondary: Quality of life using SF‐36, Trial non‐completion |
|
| Notes |
Supported by the Ontario Mental Health Foundation, Canadian Institutes of Health Research (MOP 114913). Supplements provided by Ocean Nutrition Canada (Dartmouth, NS). Conflicts of interest: The authors declare no conflicts of interest Compliance: Plasma EPA+DHA concentrations, capsule counts Depressed mood (continuous): Analyses conducted on HDRS‐17 at 12 weeks, as provided by authors. Completers' data only provided Adverse events: 10 events reported in each study arm. Data from completers provided by authors. No data on individuals, so data could not be included in analyses Quality of life: SF‐36 data provided by authors. Completers data only Trial non‐completion: 4 participants dropped out from the intervention group and 2 from the control group. Reasons not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A block randomisation code was independently computer generated at [outside location]", 2016, p. 437 |
| Allocation concealment (selection bias) | Low risk | "Kits with study medication were consecutively pre‐packaged as per the randomization sequence [..] and were administered in order by study personnel", 2016, p.437 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants: "The n‐3 PUFA and placebo capsules were similar in appearance (dark brown) and taste (lemon‐lime flavoring)", 2016, p.437, but no test of blinding. "All study personnel remained blind to treatment allocation until the database was locked.', 2016, p.437 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study personnel remained blind to treatment allocation until the database was locked.", 2016, p.437 |
| Blinding of outcome assessment (Adverse Events) | Low risk | "All study personnel remained blind to treatment allocation until the database was locked.", 2016, p.437 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Subgroup data provided for completers only. High withdrawals also |
| Incomplete outcome data (Adverse Events) | High risk | Subgroup data provided by authors, but for completers only. High withdrawals also |
| Selective reporting (reporting bias) | High risk | SF‐36 data not reported in text; subgroup analyses are not prespecified in the protocol; additional outcomes reported in the text |
| Other bias | Unclear risk | Discrepancies between protocol and paper |
Mischoulon (DHA) 2015.
| Study characteristics | ||
| Methods | Multicentre parallel design randomised controlled trial, 8 weeks | |
| Participants |
Participants: 196 participants (split across Mischoulon (DHA) 2015 and Mischoulon (EPA) 2015): 177 participants considered evaluable (provided 1 post‐baseline assessment); Mean age 45.8 (SD 12.5) years, 59.3% women (n 105), 40.7% men (n 72). Participants recruited at Massachusetts General Hospital and Cedars‐Sinai Medical Center through advertisements and referrals from outpatient programmes, from May 2006 to June 2011 Comorbidities: anxiety disorders/dysthymia in some participants, no serious/unstable physical comorbidities Adjunctive therapy: no, for all participants Inclusion criteria: A diagnosis of MDD per the SCID‐I/P), a CGI‐S score ≥ 3, and a baseline 17‐item HDRS‐17 score ≥ 15 Exclusion criteria: pregnancy or women of childbearing potential who were not using a medically‐accepted means of contraception; suicidality or homicidality; serious or unstable medical illness; current or past history of organic mental disorders, substance use disorders, any psychotic disorders, and bipolar disorder; history of multiple adverse drug reactions or allergy to the study compounds; concurrent use of psychotropic medications, systematic corticosteroid or steroid antagonists, anticoagulants, or immunosuppressant agents; electroconvulsive therapy during the current episode; any trial of ≥ 6 weeks with citalopram 40 mg/d or equivalent antidepressant during the current episode (to select a less refractory sample that would be more likely to respond to treatment); history of use of 1 g/d of n‐3 supplements; history of a bleeding disorder; psychotherapy; smoking 10 cigarettes per day; vitamin E supplementation > 400 IU; menstruating individuals unable to have baseline and post‐treatment blood drawn during the follicular phase; and individuals unable to refrain from nonsteroidal anti‐inflammatory use for > 72 hours prior to blood work. People with a CGI‐I score of 1 or 2 (i.e. “much improved” or “very much improved”) during the baseline visit (1 week after the screen visit) were excluded from the study |
|
| Interventions |
Intervention: 1000 mg DHA enriched mix (consisting of 45 mg EPA / 225 mg DHA (EPA:DHA 1:5), plus 10% docosapentaenoic acid (DPA, n‐3), 2% heneicosapentaenoic acid (HPA, n‐3), 1% stearidonic acid (SDA, n‐3), 1% eicosatetraenoic acid (ETA, n‐3), 0.4% α‐linolenic acid (ALA, n‐3), 1% arachidonic acid (AA, n‐6), 0.5% linoleic acid (LA, n‐6), and 20% unspecified fatty acids) per soft‐gel capsule. 4 DHA enriched capsules (plus EPA arm placebo capsules) every morning Comparator: 980 mg soybean oil per capsule (formed of 53.6% LA, 7.1% ALA, 0.1% myristic acid, 11% palmitic acid, 4% stearic acid, 0.2% palmitoleic acid, and 24% oleic acid), 4 capsules every morning (plus EPA arm placebo capsules) Treatment for 8 weeks |
|
| Outcomes |
Primary: HDRS (17‐item), QIDS‐SR16, every 2 weeks for 8 weeks, Adverse events (PRISE scale) Secondary: Depression remission and response; CGI (Scale), CGI (Improvement), WBS (Ryff 1995), QLESQ, every 2 weeks for 8 weeks, Trial non‐completion |
|
| Notes |
Supported by NIH Grant Supplements provided by Nordic Naturals Conflicts of Interest: CoIs reported for several authors Compliance: NR Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 8 weeks, published modified ITT (at least 1 post‐baseline assessment) data used for analyses, end outcome scores calculated from change data, SDs imputed from other studies using the HDRS (17‐item). Placebo group split across 2 intervention groups (DHA = 29 participants, EPA = 30 participants) Adverse events: Adverse events reported by individuals, 20 ‐ 30% of participants endorsed some baseline PRISE physical or depressive symptoms. The following participants experienced emerging or worsening adverse events: Intervention = 40 of 56, Comparator = 33 of 60 (correspondence from author). Values included in the analysis are for emerging or worsening AEs Depression remission defined as final HDRS (17‐item) score ≤ 7; Depression response defined as improvement ≥ 50% in HDRS (17‐item). Quality of life: CGI scale data used in analyses Trial non‐completion: Intervention group = 15 (2 insufficient time/energy, 5 lost to follow‐up, 3 violated protocol, 2 family emergency, 3 NR), comparator group = 12 (1 health problems related to treatment, 1 scheduling issues, 3 lost to follow‐up, 3 violated protocol, 4 NR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A fixed‐block size of 30 participants (MGH) or a randomly‐permuted block size between 6 and 15 participants (CSMC)." (P. 55) |
| Allocation concealment (selection bias) | Low risk | "Only blind treatment codes, co‐ordinated between both site pharmacies, were noted on randomisation lists provided to study staff." (P. 55) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Flavours added to mask taste but no check to assess blinding (correspondence from authors) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported for mood scale (P. 55) |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs rated by participants (P. 55) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Mood scales ‐ not ITT and > 10% dropout ‐ P. 55, and correspondence from authors |
| Incomplete outcome data (Adverse Events) | High risk | All reported (correspondence from authors), but > 10% withdrawals |
| Selective reporting (reporting bias) | High risk | Well‐being scale and n‐3PUFA blood levels still to be reported (correspondence from authors) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Mischoulon (EPA) 2015.
| Study characteristics | ||
| Methods | Multicentre parallel design randomised controlled trial, 8 weeks | |
| Participants |
Participants: 196 participants (split across Mischoulon (DHA) 2015 and Mischoulon (EPA) 2015): 177 participants considered evaluable (provided 1 post‐baseline assessment); Mean age 45.8 (SD 12.5) years, 59.3% women (n 105), 40.7% men (n 72). Participants recruited at Massachusetts General Hospital and Cedars‐Sinai Medical Center through advertisements and referrals from outpatient programmes, from May 2006 to June 2011 Comorbidities: anxiety disorders/dysthymia in some participants, no serious/unstable physical comorbidities Adjunctive therapy: no, for all participants Inclusion criteria: A diagnosis of MDD per the SCID‐I/P), a CGI‐S score ≥ 3, and a baseline 17‐item HDRS‐17 score ≥ 15 Exclusion criteria: pregnancy or women of childbearing potential who were not using a medically‐accepted means of contraception; suicidality or homicidality; serious or unstable medical illness; current or past history of organic mental disorders, substance use disorders, any psychotic disorders, and bipolar disorder; history of multiple adverse drug reactions or allergy to the study compounds; concurrent use of psychotropic medications, systematic corticosteroid or steroid antagonists, anticoagulants, or immunosuppressant agents; electroconvulsive therapy during the current episode; any trial of ≥ 6 weeks with citalopram 40 mg/d or equivalent antidepressant during the current episode (to select a less refractory sample that would be more likely to respond to treatment); history of use of 1 g/d of n‐3 supplements; history of a bleeding disorder; psychotherapy; smoking 10 cigarettes per day; vitamin E supplementation > 400 IU; menstruating individuals unable to have baseline and post‐treatment blood drawn during the follicular phase; and individuals unable to refrain from nonsteroidal anti‐inflammatory use for > 72 hours prior to blood work. People with a CGI‐I score of 1 or 2 (i.e. “much improved” or “very much improved”) during the baseline visit (1 week after the screen visit) were excluded from the study |
|
| Interventions |
Intervention: 1000 mg EPA enriched mix (consisting of 530 mg EPA / 137 mg DHA per soft gel (EPA:DHA 4:1), plus 7% stearidonic acid (SDA, n‐3), 1% heneicosapentaenoic acid (HPA, n‐3), 1% docosapentaenoic acid (DPA, n‐3), 1% eicosatetraenoic acid (ETA, n‐3), 0.2% α‐linolenic acid (ALA, n‐3), 3% arachidonic acid (AA, n‐6), 0.2% linoleic acid (LA, n‐6), and 10% – 11% unspecified fatty acids) per soft‐gel capsule. 2 EPA enriched capsules (plus DHA arm placebo capsules) every morning Comparator: 980 mg soybean oil per capsule (formed of 53.6% LA, 7.1% ALA, 0.1% myristic acid, 11% palmitic acid, 4% stearic acid, 0.2% palmitoleic acid, and 24% oleic acid), 2 capsules every morning (plus DHA arm placebo capsules) Treatment for 8 weeks |
|
| Outcomes |
Primary: HDRS (17‐item), QIDS‐SR16, every 2 weeks for 8 weeks, Adverse events (PRISE) Secondary: Depression remission and response; CGI‐S, CGI‐I, WBS (Ryff 1995), QLESQ, every 2 weeks for 8 weeks, Trial non‐completion |
|
| Notes |
Supported by NIH Grant Supplements provided by Nordic Naturals Conflicts of Interest: CoIs reported for several authors Compliance: NR Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 8 weeks, published modified ITT (at least 1 post‐baseline assessment) data used for analyses, end outcome scores calculated from change data, SDs imputed from other studies using the HDRS (17‐item). Placebo group split across 2 intervention groups (DHA = 29 participants, EPA = 30 participants) Adverse events: Adverse events reported by individuals, 20 ‐ 30% of participants endorsed some baseline PRISE physical or depressive symptoms. The following participants experienced emerging or worsening adverse events: Intervention = 39 of 60, Comparator = 33 of 60 (correspondence from author). Values included in the analysis are for emerging or worsening AEs Depression remission defined as final HDRS (17‐item) score ≤ 7; Depression response defined as improvement ≥ 50% in HDRS (17‐item). Quality of life: CGI scale data used in analyses Trial non‐completion: Intervention group = 15 (2 insufficient time/energy, 1 increased depression, 1 dizziness, 5 lost to follow‐up, 2 violated protocol, 1 moved away, 3 NR), comparator group = 12 (1 health problems related to treatment, 1 scheduling issues, 3 lost to follow‐up, 3 violated protocol, 4 NR) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A fixed‐block size of 30 participants (MGH) or a randomly‐permuted block size between 6 and 15 participants (CSMC)." (P. 55) |
| Allocation concealment (selection bias) | Low risk | "Only blind treatment codes, co‐ordinated between both site pharmacies, were noted on randomisation lists provided to study staff." (P. 55) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Flavours added to mask taste but no check to assess blinding (author correspondence) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self‐reported for mood scales (P. 55) |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs rated by participants (P. 55) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Mood scales ‐ not ITT and > 10% dropout ‐ P. 55, and correspondence from author |
| Incomplete outcome data (Adverse Events) | High risk | All reported (correspondence from author), but > 10% withdrawals. |
| Selective reporting (reporting bias) | High risk | Well‐being scale and n‐3PUFA blood levels still to be reported (correspondence from author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Mischoulon 2009.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks | |
| Participants |
Participants: After 57 participants were randomised at a screening visit, 41 completed a baseline visit and entered into the study; mean age 43 years (SD = 13), 63% women. These participants were recruited via advertisements and referrals to the Massachussets General Hospital Depression Clinical and Research Programme, from Jan 2003 to June 2006. Comorbidities: No Adjunctive therapy: Yes in some participants: concurrent psychotherapy if receiving therapy prior to enrolment Inclusion criteria: aged 18 ‐ 80 years, DSM‐IV diagnosis of MDD (using SCID‐IP), score ≥ 18 on the 17‐item HDRS and ≥ 3 on the CGI‐SI scale, ability to provide informed written consent, free from antidepressant, antipsychotic or mood‐stabilisation medication Exclusion criteria: unstable medical conditions, psychiatric or psychotic comorbidities, current serious suicide or homicidal risk, substance abuse, currently taking n‐3PUFA supplements, history of adverse drug reactions or allergy to study drugs, pregnancy or no use of medically‐approved contraception among women of child‐bearing potential, breastfeeding, failure to respond to ≥ 1 antidepressant trial, history of unstable seizure disorder, history of electroconvulsive therapy in previous 6 months, anticoagulant use |
|
| Interventions |
Intervention: E‐EPA (1 g/d, plus 0.2% alpha tocopherol), 2 x 500 mg capsules twice daily or both at once Comparator: Paraffin oil (1 g/d, plus 0.2% alpha tocopherol), 2 x 500 mg capsules twice daily or both at once Treatment received for 8 weeks |
|
| Outcomes |
Primary: HDRS (17‐item) measured every 2 weeks for 8 weeks; Adverse events Secondary: Remission and response based on HDRS; QLESQ; Trial non‐completion |
|
| Notes |
Funded by the National Center for Complementary and Alternative Medicine, NIH, USA Supplements provided by Amarin Neuroscience Ltd, UK Conflicts of Interest: CoIs declared from many authors. Compliance: Capsule counts at each visit; Plasma n‐3PUFA levels measured Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 8 weeks, ITT data provided by authors Adverse events: Adverse events were reported in 7 individuals ‐ Intervention group = 2 (2 GI), comparator group = 5 (5 GI) Quality of life: QLESQ ‐ data not reported. Trial non‐completion: Intervention group = 6 (1 non‐response, 1 commuting, 4 lost to follow‐up), comparator group = 11 (2 non‐response, 1 feeling better, 8 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation performed by research pharmacy using www.randomization.com" (P.1637) |
| Allocation concealment (selection bias) | Low risk | Assigned medications were coded and sent to treatment team by research pharmacy (P.1637) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Study clinicians and participants remained blind to assignment for duration of study" (P.1637). It was unclear if the fishy taste was disguised and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS ‐ "Study clinicians remained blind to assignment for duration of study" (P. 1637) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs rated by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS ‐ ITT numbers obtained through correspondence with author, but >10% dropout |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, but >10% dropout |
| Selective reporting (reporting bias) | High risk | All primary outcome measures reported (correspondence with author). QLESQ was a planned outcome and measured but not analysed or reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Nemets 2002.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 4 weeks | |
| Participants |
Participants: 20 participants with mean age = 53.4 (SD = 11.7, range 28 ‐ 73) years, 17 women, location adn dates ‐ not reported. Comorbidities: Yes in some participants: 1 participant had comorbid OCD Adjunctive therapy: Yes in some participants: all with the exception of 1 participant Inclusion criteria: recurrent MDD (according to DSM‐IV criteria) from ≥ 2 clinical interviews with ≥ 2 specialist psychiatrists spaced at least 1 week apart, aged 18 ‐ 75 years, no unstable medical disease, no psychotic or psychiatric comorbidities other than panic disorder, dysthymic disorder or OCD, no substance abuse |
|
| Interventions |
Intervention: E‐EPA (2 g/d), 2 x 500 mg capsules, twice daily, plus ongoing therapy Placebo: placebo, 2 x 500 mg capsules, twice daily, plus ongoing therapy Treatment received for 4 weeks |
|
| Outcomes |
Primary: HDRS (24‐item) measured at baseline and weekly for 4 weeks; Adverse events Secondary: Response based on HDRS, Trial non‐completion |
|
| Notes |
Funding: not reported Supplements provided by Laxdale Ltd., UK. Conflicts of Interest: not reported Compliance: not reported Depressed mood (continuous): Analysis conducted on HDRS (24‐item) scores at 4 weeks, ITT data calculated from publication Adverse events: Only clinically relevant adverse events were investigated, none found. Values in the analysis are for clinically relevant AEs Trial non‐completion: Intervention group = 0, comparator group = 1 (symptoms worsened) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised according to a random‐number table (correspondence with author) |
| Allocation concealment (selection bias) | Low risk | Senior investigator generated random‐number table and was in a different building to senior clinician. Senior clinician was not aware of the randomisation sequence. (Correspondence with author) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Intervention and placebo capsules were matching (P. 477), although no attempt to match taste. "No participants reported fishy sensations when asked specifically, and debriefing recorded a completely random guess rate by participant and clinician" (P. 478) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS ‐ assessors blind to treatment assignment |
| Blinding of outcome assessment (Adverse Events) | Low risk | AEs ‐ participant‐rated, participants blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | HDRS ‐ 1 participant dropped out, but possible to conduct ITT analysis using LOCF |
| Incomplete outcome data (Adverse Events) | High risk | Only clinically relevant AEs reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Park 2015.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 35 participants, mean age only reported by group (Intervention 43.5 (SD 3.72) years; comparator 39.41 (SD 3.58) years); 27 women, 8 men. Participants recruited from Hanyang University Hospital, Korea, from 2010 to 2013 Comorbidities: None reported, possible psychiatric comorbidities Adjunctive therapy: Yes, usual care and antidepressant medications in all participants Inclusion criteria: CES‐D‐K (Cho 1998) score > 24, confirmed by psychiatrist according to DMS‐IV Exclusion criteria: pregnant, lactating, < 18 / > 65 years old, taking supplements containing n‐3PUFAs, medical comorbidity (CV disease, dementia), chronic depression lasting > 2 years or treatment‐resistant depression, other primary psychiatric disorders (bipolar or schizophrenia) |
|
| Interventions |
Intervention: E‐EPA/DHA combination (EPA 3420 mg/d, DHA 1800 mg/d), 3 capsules daily Comparator: safflower oil and oleic acid (3g), 3 capsules daily Treatment for 12 weeks |
|
| Outcomes |
Primary: HDRS (17‐item), CES‐D‐K measured at baseline, 4, 8, 12 weeks; Adverse events Secondary: CGI, CGI‐IS, Trial non‐completion |
|
| Notes |
Funded by the Korean Research Foundation Supplements provided by DSM Nutritional Products, Switzerland Conflicts of Interest: None declared, however Dr Y Park is a founder of Omega Quant Asia (a laboratory specialising in fatty acid analysis) Compliance: Plasma n‐3PUFA levels measured Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 12 weeks, data using modified ITT (at least 1 post‐baseline visit) provided by authors Adverse events: Adverse events were reported in 4 individuals: Intervention group 3 (3 fishy eructation), comparator group 1 (1 fishy eructation) Quality of life: Analysis conducted on CGI (scale) Trial non‐completion: Intervention group = 6 (1 rejected blood sampling, 5 participant decision), comparator group = 5 (1 rejected blood sampling, 4 participant decision) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Independent statistician, _ computer‐generated randomisation scheme allowing for randomisation blocks" (P. 143) |
| Allocation concealment (selection bias) | Low risk | "Sequentially‐numbered containers with either n‐3PUFAs or placebo were randomly assigned to participants. Identity codes were concealed in sequentially‐numbered opaque envelopes managed by the study investigators" (P. 143) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempt to mask flavour or check blinding (P. 142) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS scores were "measured by psychiatrist who was blinded to treatment groups" (P. 142) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs ‐ participant‐rated (participants not blinded effectively) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS ‐ > 10% missing in the overall sample and not ITT analysis |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, P 144, but > 10% withdrawals. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence from authors) |
| Other bias | High risk | Significant baseline imbalance for mood disorders (P. 144) |
Peet (1g/d) 2002.
| Study characteristics | ||
| Methods | Randomised controlled multicentre parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 70 participants with a mean age of 44.7 years were recruited by family physicians in the UK who had an interest in depression and experience in conducting clinical trials (split across Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002), dates ‐ not reported. Comorbidities: none reported, but possible physical and/or psychiatric comorbidities Adjunctive therapy: Yes in all participants: antidepressants Inclusion criteria: aged 18 ‐ 70 years, score ≥ 15 on the 17‐item HDRS despite ongoing treatment with a standard antidepressant at an adequate dose. |
|
| Interventions |
Intervention: E‐EPA (1 g/d + 3 g/d placebo), 4 x 500 mg capsules, twice daily Comparator: liquid paraffin (4 g/d), 4 x 500 mg capsules, twice daily Treatment received for 12 weeks |
|
| Outcomes |
Primary: HDRS (17‐item), MADRS, BDI were all measured at baseline, 4, 8 and 12 weeks; Adverse events Secondary: Response based on HDRS, MADRS and BDI; Trial non‐completion |
|
| Notes |
Funding: not reported Conflicts of Interest: CoIs declared by one author. Other author works for Laxdale Ltd., UK. Compliance: Capsule counts Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 12 weeks, published ITT data (although 1 participant from the placebo group is missing from these data). Placebo group split across all 3 intervention groups (1 g/d = 5 participants, 2 g/d = 6 participants, 4 g/d = 6 participants), SDs calculated from all other studies also using the HDRS (17‐item) Adverse events: Intervention group: 18 events experienced by 9 participants (7 GI, 4 psychological, 7 other physical), comparator group: 23 events experienced by 10 participants (4 GI, 2 psychological, 17 other physical) Trial non‐completion: Intervention groups (2 per group, reasons not separated by group 1 g/d, 2g/d, 4 g/d) = 6 (3 withdrew consent, 1 lack of efficacy, 1 violated protocol, 1 adverse event), comparator group = 4 (1 withdrew consent, 1 violated protocol, 1 adverse event, 1 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by PCI clinical services computer" (P. 914) |
| Allocation concealment (selection bias) | Low risk | "Capsules were packed and coded by PCI clinical services." Participants were randomly allocated on entry to study. "PCI Clinical Services had no involvement with the rest of the trial." (P. 914) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants took the same number of capsules, placebo and intervention capsules were identical in appearance. Participants, researchers and assessors blind to treatment allocation. (P. 914) It was unclear if they disguised the fishy taste and no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS/MADRS ‐ assessors blind to treatment allocation (LOW) BDI ‐ participant‐rated (HIGH) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs assessed by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS/MADRS/BDI ‐ Not ITT analysis (only 17 participants used in the analysis of placebo group), plus >10% withdrawals |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, but >10% withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence from author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Peet (2g/d) 2002.
| Study characteristics | ||
| Methods | Randomised controlled multicentre parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 70 participants with a mean age of 44.7 years were recruited by family physicians in the UK who had an interest in depression and experience in conducting clinical trials (split across Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002), dates ‐ not reported. Comorbidities: none reported, but possible physical and/or psychiatric comorbidities Adjunctive therapy: Yes in all participants: antidepressants Inclusion criteria: aged 18 ‐ 70 years, score ≥ 15 on the 17‐item HDRS despite ongoing treatment with a standard antidepressant at an adequate dose. |
|
| Interventions |
Intervention: E‐EPA (2 g/d + 2 g/d placebo), 4 x 500 mg capsules, twice daily Comparator: liquid paraffin (4 g/d), 4 x 500 mg capsules, twice daily Treatment received for 12 weeks |
|
| Outcomes |
Primary: HDRS (17‐item), MADRS, BDI were all measured at baseline, 4, 8 and 12 weeks; Adverse events Secondary: Response based on HDRS, MADRS and BDI; Trial non‐completion |
|
| Notes |
Funding: not reported Conflicts of Interest: CoIs declared by one author. Other author works for Laxdale Ltd., UK. Compliance: Capsule counts Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 12 weeks, published ITT data (although 1 participant from the placebo group is missing from these data). Placebo group split across all 3 intervention groups (1 g/d = 5 participants, 2 g/d = 6 participants, 4 g/d = 6 participants), SDs calculated from all other studies also using the HDRS (17‐item) Adverse events: Intervention group: 24 events experienced by 13 participants (8 GI, 2 psychological, 14 other physical), comparator group: 23 events experienced by 10 participants (4 GI, 2 psychological, 17 other physical) Trial non‐completion: Intervention groups (2 per group, reasons not separated by group 1 g/d, 2g/d, 4 g/d) = 6 (3 withdrew consent, 1 lack of efficacy, 1 violated protocol, 1 adverse event), comparator group = 4 (1 withdrew consent, 1 violated protocol, 1 adverse event, 1 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by PCI clinical services computer" (P. 914) |
| Allocation concealment (selection bias) | Low risk | "Capsules were packed and coded by PCI clinical services." Participants were randomly allocated on entry to study. "PCI Clinical Services had no involvement with the rest of the trial." (P. 914). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants took the same number of capsules, placebo and intervention capsules were identical in appearance. Participants, researchers and assessors blind to treatment allocation. (P. 914) It was unclear if they disguised the fishy taste and no assessment to check concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS/MADRS ‐ assessors blind to treatment allocation (LOW) BDI ‐ participant‐rated (HIGH) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs assessed by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS/MADRS/BDI ‐ Not ITT analysis (only 17 participants used in the analysis of placebo group), plus >10% withdrawals |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, but >10% withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence from author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Peet (4g/d) 2002.
| Study characteristics | ||
| Methods | Randomised controlled multicentre parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 70 participants with a mean age of 44.7 years were recruited by family physicians in the UK who had an interest in depression and experience in conducting clinical trials (split across Peet (1g/d) 2002; Peet (2g/d) 2002; Peet (4g/d) 2002), dates ‐ not reported. Comorbidities: none reported, but possible physical and/or psychiatric comorbidities Adjunctive therapy: Yes in all participants: antidepressants Inclusion criteria: aged 18 ‐ 70 years, score ≥ 15 on the 17‐item HDRS despite ongoing treatment with a standard antidepressant at an adequate dose. |
|
| Interventions |
Intervention: E‐EPA (4 g/d), 4 x 500 mg capsules, twice daily Comparator: liquid paraffin (4 g/d), 4 x 500 mg capsules, twice daily Treatment received for 12 weeks |
|
| Outcomes |
Primary: HDRS (17‐item), MADRS, BDI were all measured at baseline, 4, 8 and 12 weeks; Adverse events Secondary: Response based on HDRS, MADRS and BDI; Trial non‐completion |
|
| Notes |
Funding: not reported Conflicts of Interest: CoIs declared by one author. Other author works for Laxdale Ltd., UK. Compliance: Capsule counts Depressed mood (continuous): Analysis conducted on HDRS (17‐item) scores at 12 weeks, published ITT data (although 1 participant from the placebo group is missing from these data). Placebo group split across all 3 intervention groups (1 g/d = 5 participants, 2 g/d = 6 participants, 4 g/d = 6 participants), SDs calculated from all other studies also using the HDRS (17‐item) Adverse events: Intervention group: 15 events experienced by 10 participants (5 GI, 0 psychological, 10 other physical), comparator group: 23 events experienced by 10 participants (4 GI, 2 psychological, 17 other physical) Trial non‐completion: Intervention groups (2 per group, reasons not separated by group 1 g/d, 2g/d, 4 g/d) = 6 (3 withdrew consent, 1 lack of efficacy, 1 violated protocol, 1 adverse event), comparator group = 4 (1 withdrew consent, 1 violated protocol, 1 adverse event, 1 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by PCI clinical services computer" (P. 914) |
| Allocation concealment (selection bias) | Low risk | "Capsules were packed and coded by PCI clinical services." Participants were randomly allocated on entry to study. "PCI Clinical Services had no involvement with the rest of the trial." (P. 914) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants took the same number of capsules, placebo and intervention capsules were identical in appearance. Participants, researchers and assessors blind to treatment allocation. (P. 914) It was unclear if they disguised the fishy taste and no assessment to check concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS/MADRS ‐ assessors blind to treatment allocation (LOW) BDI ‐ participant‐rated (HIGH) |
| Blinding of outcome assessment (Adverse Events) | High risk | AEs assessed by participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS/MADRS/BDI ‐ Not ITT analysis (only 17 participants used in the analysis of placebo group), plus >10% withdrawals |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, but >10% withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence from author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Rondanelli 2010.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks | |
| Participants |
Participants: 46 women with a mean age of 83.9 years, resident in a nursing home in Pavia, Italy for ≥ 3 months. Data were gathered between January 2006 and December 2007. Comorbidities: No psychiatric comorbidities, arthritis in some individuals Adjunctive therapy: No antidepressants, possible use of other therapies Inclusion criteria: aged 65 ‐ 95 years, BMI of 19 ‐ 30 kg/m2, score > 10 on the GDS, MMSE score > 24, met DSM‐IV criteria for MDD or dysthymia, as assessed by senior psychiatrist Exclusion criteria: presence of clinically uncontrolled organic disease or clinically relevant lab abnormalities, any psychotic or psychiatric comorbidities, including suicidal ideation, current use of psychotropic drugs other than benzodiazepines. Ongoing pharmacological treatment for physical conditions, at the time of enrolment, was maintained during the study |
|
| Interventions |
Intervention: EPA/DHA combination (3.13 g/d ‐ EPA = 1.67 g/d, DHA = 0.83 g/d, other n‐3PUFAs = 0.63 g/d) Comparator: Paraffin oil (2.5 g/d) Treatment received for 8 weeks |
|
| Outcomes |
Primary: GDS was measured before and after treatment at week 0 and week 8. Adverse events Secondary: Remission and response based on GDS; SF‐36 (mental health summary score); Trial non‐completion |
|
| Notes |
Funded by Regione Lomdardia, Italy Intervention provided by Also SpA Div. Also‐Enervit, Zelbio (Co), Italy. Conflicts of Interest: None declared. Compliance: EPA and DHA levels in RBC membranes Depressed mood (continuous): Analysis conducted on GDS scores at 8 weeks, published ITT data Adverse events: No serious adverse events reported. Minor adverse events: Intervention group = 6 (6 GI), comparator group = 6 (5 GI, 1 other physical). Values in the analysis are for minor events Quality of life: Analyses conducted on SF‐36 mental health summary score Trial non‐completion: Not mentioned, but full data sets provided for all participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Bottles _ for each treatment group were assigned a participant number according to a coded (AB) block randomisation table prepared by an independent statistician." (P. 57 / 58.) |
| Allocation concealment (selection bias) | Low risk | "As participants were enrolled they were assigned a progressive participant number." "Investigators were blinded to the randomisation table, the code assignments and the procedure." (P. 58) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators were blinded to the randomisation table, the code assignments and the procedure." (P. 58). Bottles of oily preparation were identical for each treatment group and lemon flavour was added to both oils. No participants complained about "a fish smell or eructation or made any comment about the contents of the supplement or perception of being in 1 of the 2 groups." (P. 60) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | GDS ‐ Investigators and participants blind to treatment |
| Blinding of outcome assessment (Adverse Events) | Low risk | AEs ‐ investigators and participants blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | GDS ‐ ITT analysis |
| Incomplete outcome data (Adverse Events) | Low risk | All AEs are reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Shinto 2016.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 3 months | |
| Participants |
Participants: 39 participants (Intervention group: 21 participants, mean age 50.7 years (SD 11.6), 2 men, 19 women; Comparator group: 18 participants, mean age 51.9 years (SD 10.0), 1 man, 17 women), recruited from secondary care setting in USA between July 2005 and June 2009 Comorbidities: Multiple sclerosis in all participants, no psychiatric comorbidities Adjunctive therapy: Yes for all participants. Inclusion criteria state "stable antidepressant medication for 3 months, stable multiple sclerosis medication for 6 months". Inclusion criteria: 18 ‐ 85 years, diagnosed with multiple sclerosis by McDonald criteria, diagnosed with MDD by DSM IV, BDI score 10 ‐ 30, stable antidepressant medication for 3 months, stable multiple sclerosis medication for 6 months Exclusion criteria: BDI over 30, MADRS over 30, MS exacerbation or corticosteroid therapy within 1 month of enrolment, MMSE ≤ 24, pregnancy, current or past history of significant ventricular arrhythmia, fish oil or cod liver oil supplementation in last 30 days, > 1 6‐ounce serving per week of fish or seafood in last 30 days, Suicidal thoughts, other psychological disorders |
|
| Interventions |
Intervention: 1.95 g EPA, 1.35 g DHA / day, provided in 6 capsules. Each capsule had 0.325 g EPA, 0.225 g DHA (0.64 g n‐3PUFAs). Comparator: Soybean oil with 1% fish oil for taste and smell, 6 capsules per day Treatment received for 3 months. |
|
| Outcomes |
Primary: MADRS, BDI‐II at baseline, 1 and 3 months, Adverse events Secondary: Depression remission and response, Quality of life using SF‐36, all at baseline, 1 and 3 months, Trial non‐completion |
|
| Notes |
Supported by: NIH/National Center for Complemenary and Alternative Medicine K23AT002155; NIH/National Center for Advancing Translational Research UL1TR000128. Supplements provided by: Nordic Naturals, Watsonville, CA. Conflicts of interest: None declared Compliance: RBC DHA% total fatty acids/EPA% total fatty acids, capsule counts Depressed mood (continuous): Analyses conducted on MADRS score at 3 months. Data set provided with the published paper. ITT data calculated using last observation carried forward Adverse events: Intervention group: 11 individuals (2 gastrointestinal, 7 other specific physical complaints); Comparator group: 16 individuals (2 gastrointestinal, 15 other specific physical events). Data provided as published in the text of the paper Depression remission: Calculated from full data set published with the paper, using BDI‐II score of 8 or less Depression response: Calculated from full data set published with the paper, using BDI‐II score reduction of more than 50% Quality of Life: Analyses conducted on SF‐36 mental health scale. Adjusted data from completers only, as published in the text of the paper Attached dataset is insufficiently detailed for use. Data are not suitable for use in analyses Trial non‐completion: 6 from intervention group failed to complete (2 lost to follow‐up, 2 health problems unrelated to treatment, 1 insufficient time or energy, 1 stopped their usual antidepressant and therefore violated the protocol); 2 from control group (both health problems unrelated to treatment) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomization list", (p. 3) |
| Allocation concealment (selection bias) | Low risk | "Participants were randomized … by an independent pharmacist ... using a computer‐generated randomization list" (p. 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo capsules were flavoured to taste and smell similar to the fish oil capsules", p. 3., "When asked about treatment assignment at the end of the study the majority reported no knowledge of treatment assignment, research staff (100%), placebo subjects (75%), and omega‐3 FA subjects (80%)", (p. 8) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Correspondence from authors ‐ "Study personnel trained and certified in MADRS conducted interview for MADRS score (primary outcome). BDI and QOL self‐reported." |
| Blinding of outcome assessment (Adverse Events) | Low risk | Adverse events reported by participants, (p. 4) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Full data set is provided, but higher than 10% withdrawals. Published QoL data is for completers only and insufficient detail in the full data set provided to allow use |
| Incomplete outcome data (Adverse Events) | High risk | Adverse events reported in table 3, but higher than 10% withdrawals |
| Selective reporting (reporting bias) | High risk | Not all time points reported in the main text. Full data set provided, but data sheet is incomplete for some outcomes (AEs) and insufficient detail for some outcomes (QoL) |
| Other bias | Unclear risk | Discrepancies between the protocol, paper and additional data set |
Silvers 2005.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 12 weeks | |
| Participants |
Participants: 77 participants with a mean age = 38.8 years, 41 women, recruited through a Community Mental Health Service, general practices and advertisements in community newspapers in New Zealand. Participants were recruited between July 2000 and September 2001. Comorbidities: possible physical and psychiatric comorbidities Adjunctive therapy: Yes for some participants: 61 participants taking antidepressants, 21 participants receiving psychotherapy Inclusion criteria: current depressive episode, aged 18 ‐ 65 years, stable medication for ≥ 2 months prior to enrolment, willing to provide blood samples and, if female, premenopausal with a normal menstrual cycle, available for the length of the study Exclusion criteria: any psychotic or psychiatric comorbidities other than anxiety disorders, currently taking n‐3PUFA supplements, allergy to seafood or objection to taking fish‐/olive oil‐based products, blood clotting disorders or use of anticoagulants, any unstable medical conditions or conditions likely to affect gastrointestinal absorption |
|
| Interventions |
Intervention: EPA/DHA combination (8 g/d DHA enriched tuna oil providing 0.6 g/d EPA, 2.4 g/d DHA, 80 mg vitamin E), 4 x 1 g capsules, twice daily, plus ongoing therapy Comparator: Olive oil (8 g/d) 4 x 1 g capsules, twice daily, plus ongoing therapy Treatment received for 12 weeks |
|
| Outcomes |
Primary: HDRS Short Form (9‐item) (score of > 10 represents severe depression) and BDI‐II were measured at baseline and weeks 2, 4, 8 and 12. Adverse events Secondary: Trial non‐completion |
|
| Notes |
Funded by Foundation for Research, Science and Technology, New Zealand. Supplements provided by Clover Corporation Plc, Australia Conflicts of Interest: No CoIs declared. Compliance: RBC membrane EPA and DHA levels measured, participants completing exit interview asked about compliance Depressed mood (continuous): Analysis conducted on HDRS (9‐item) scores at 12 weeks, per protocol data obtained from authors Adverse events: Intervention group ‐ 20 events in 14 participants (11 GI, 7 other physical, 2 not reported); comparator group ‐ 16 events in 14 participants (8 GI, 2 psychological, 5 other physical, 1 not reported) Trial non‐completion: Intervention group: 16 (2 withdrew before baseline, 9 discontinued intervention, 1 head trauma, 1 physical disorder, 2 scored < 6 on HDRS at week 0, 1 not reported); comparator group 16 (2 withdrew before baseline, 5 discontinued intervention, 1 head trauma, 3 personality disorders, 1 bipolar disorder, 4 scored < 6 on HDRS at week 0) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation "according to a prearranged computer‐generated code" (P. 212) |
| Allocation concealment (selection bias) | Low risk | "Randomisation sequence generated by statistician not directly involved in the study" (P. 212). "Allocation sequence was concealed from both participants and the research psychologists" (P. 213) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules looked identical, fish smell and taste were minimal. Participants were told only that both oils were natural and aftertaste might be experienced. "No evidence that participants guessed their treatment allocation (P = 0.804)" (P. 215) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | HDRS/BDI ‐ both researchers and participants blind to treatment allocation |
| Blinding of outcome assessment (Adverse Events) | Low risk | AEs ‐ participant‐rated, participants blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS/BDI ‐ analysis conducted on only those providing 1 follow‐up (not ITT), and > 10% dropout |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, but > 10% withdrawals. |
| Selective reporting (reporting bias) | Low risk | All relevant outcome measures reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
Su 2003.
| Study characteristics | ||
| Methods | Randomised controlled parallel‐arm trial, 8 weeks Pre‐randomisation: all participants received single‐blind placebo capsules for 1 week, those with a ≥ 20% decrease in HDRS score (placebo responders) were excluded |
|
| Participants |
Participants: 28 outpatients referred by Taipei Medical University‐Wan Fang Hospital. 22 participants completing the trial had a mean age = 38.4 years, 18 women, dates ‐ not reported. Comorbidities: No Adjunctive therapy: Yes in some, if participants on stable medication at enrolment Inclusion criteria: aged 18 ‐ 60 years, diagnosis with DSM‐IV MDD and no other comorbid Axis I or Axis II psychiatric disorder, rated > 18 on the HDRS (21‐item), stable medication or psychotherapy for 4 weeks before enrolment, physically healthy under evaluations of medical history, physical examinations, and laboratory tests and competent to understand the study and give written informed consent Exclusion criteria: Participants receiving antipsychotics or mood stabilizers, ≥ 20% decrease in HDRS score (placebo responders) following pre‐randomisation |
|
| Interventions |
Intervention: EPA/DHA combination (6.6 g/d ‐ 4.4 g/d EPA and 2.2 g/d DHA, plus tocopherols and tertiary‐butyl hydroquinone), 5 capsules, twice daily Comparator: Olive oil ethyl esters (plus tocopherols and tertiary‐butyl hydroquinone), 5 capsules, twice daily Treatment received for 8 weeks |
|
| Outcomes |
Primary: HDRS (21‐item) measured at ‐1, 0, 2, 4, 6 and 8 weeks. Adverse events Secondary: Trial non‐completion |
|
| Notes |
Funded by National Science Council, and China Chemical and Pharmaceutical Company, Taiwan Supplements provided by China Chemical and Pharmaceutical Company, Taiwan Conflicts of Interest: not reported Compliance: EPA and DHA levels from RBCs Depressed mood (continuous): Analysis conducted on HDRS (21‐item) scores at 8 weeks, ITT data provided by authors Adverse events: Intervention group = 1 GI, 1 psychological; comparator group = 1 other physical Trial non‐completion: Intervention group = 2 (1 non‐compliance, 1 lost to follow‐up), comparator group = 4 (1 non‐compliance, 3 lost to follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number sheet generated in Excel (correspondence with author) |
| Allocation concealment (selection bias) | Low risk | Packages were consecutively numbered according to randomisation schedule by an independent nutritionist (correspondence with author) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Orange flavour was added to the capsules, which were identical to blind the participants (P.268). However there was no assessment to check concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | HDRS ‐ unclear whether assessors were blind to treatment allocation |
| Blinding of outcome assessment (Adverse Events) | Unclear risk | AEs ‐ unclear whether participants were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HDRS ‐ ITT analysis obtained from author, but higher than 10% dropout |
| Incomplete outcome data (Adverse Events) | High risk | All AEs reported, but higher than 10% dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (correspondence with author) |
| Other bias | Low risk | Study appeared to be free from other sources of bias |
BDI: Beck depression inventory CES‐D‐K: Center for Epidemiological Studies depression scale Korean version CGI: clinical global impression CHD: coronary heart disease DHA: docosahexaenoic acid DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth edition EPA: eicosapentaenoic acid GAF: global assessment of functioning GDS: geriatric depression scale GI: gastrointestinal HSCL: Hopkins symptom checklist depression scale ITT: intention‐to‐treat HDRS: Hamilton depression rating scale LOCF: last observation carried forward MADRS: Montgomery‐Asberg depression rating scale MAOI: monoamine oxidase inhibitor MDD: major depressive disorder MDE: major depressive episode MMSE: mini mental state examination OCD: Obsessive‐compulsive disorder PGWB: psychological general well being RBC: red blood cell QLESQ: quality of life enjoyment and satisfaction questionnaire SCID: structured clinical interview (depression) SD: standard deviation SSRI: selective serotonin reuptake inhibiting UPDRS: Unified Parkinson disease rating scale WBS: well‐being scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT00963196 | Study record indicates study withdrawn prior to enrolment |
Characteristics of studies awaiting classification [ordered by study ID]
Bafghi 2011.
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | Participants: Aged between 18‐75 years; a Beck Depression Inventory II score of 10‐28; a Hamilton Rating Scale for Depression score of 8‐18; no changes in doses or types of antidepressant medications within 4 weeks prior to the study entry; diagnose of mild to moderate depression with structured clinical interview by a psychiatrist according to DSM‐IV‐TR criteria. Total sample size: 81 participants |
| Interventions | Intrevention: Intervention arm 1: EPA 1000 mg/day (2 oral capsules) for 12 weeks Intervention arm 2: DHA 930 mg/day (2 oral capsules) for 12 weeks Comparator: Coconut Oil 1000 mg/day (2 oral capsules) for 12 weeks |
| Outcomes | Primary: Depressive symptomology (continuous score) HDRS, at weeks 0, 6, and 12 Secondary: Depression remission HDRS, at 12 weeks |
| Notes | Irct201010054873N |
EUCTR2006‐004949‐41‐IT.
| Methods | Randomised placebo‐controlled double‐blind trial |
| Participants | Adults aged between 18 and 65, affected by MDD or recurrent depressive disorder according to DSM‐IV‐TR and the HDRS |
| Interventions | Intervention: fish oil 30 EPA/DHA plus SSRI Comparator: placebo, plus SSRI |
| Outcomes | Primary: Improvement in HDRS and CGI score |
| Notes | No contact details |
Kwak 2013.
| Methods | 12‐week, parallel‐group, double‐blind addition of choline alfoscerate or E‐EPA to ongoing antidepressant therapy |
| Participants | Adults aged over 60 years with depression |
| Interventions | Intervention: E‐EPA 2 g/d plus usual treatment Comparator: Choline alfoscerate 800 mg/d plus usual treatment |
| Outcomes | Primary: Executive function: Controlled Oral Word Association Test; Korean Stroop Color‐Word Test; Trail Making Test part B Depressive symptoms: Korean Geriatric depression scale (K‐GDS); Quick Inventory of Depressive Symptomology‐Self Report (QIDSSR) |
| Notes | Unsure if an RCT and unsure of MDD diagnosis ‐ no correspondence from author |
Lima 2006.
| Methods | Randomised placebo‐controlled trial |
| Participants | Adults age 18 ‐ 60 years with major depressive episode, according to DSM‐IV criteria |
| Interventions | Intervention: Fluoxetine (oral) 20 mg/day plus omega‐3 (oral) 900 mg/day Comparator: Fluoxetine (oral) 20 mg/day plus placebo |
| Outcomes | Primary: 1. Response to differential treatment at 2, 4 and 6 weeks 2. Magnitude of the response at 2, 4 and 6 weeks 3. Biochemical analyses on blood samples at 0 and 6 weeks: Neurotransmitters in plasma; Isolation of lymphocytes; Neurotransmitters in lymphocytes; Detection of tryptophan hydroxylase; Folate levels; Homocysteine levels; Vitamin B12 levels. 4. In participants who took omega‐3, brain‐derived neurotrophic factor (BDNF) in serum and lymphocytes. Secondary: Correlation between response to antidepressant and biochemical measurements |
| Notes | No response from PI |
Murck 2002.
| Methods | Randomised placebo‐controlled trial, 12 weeks |
| Participants | Participants: Provided written informed consent; HDRS score of 14 or more; Treatment for =8 weeks with one or more standard antidepressants, with no change in antidepressant dosage or drug for at least 4 weeks; likely to be maintained on this treatment and dosage for the duration of the study; Diagnosis of major depressive disorder (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM IV]); Male or female, of any race, aged 18‐65. Actual enrolment reported as January 2002 ‐ December 2002, study completed December 2003. |
| Interventions | Interventions: 1 g, 2 g or 4 g ethyl‐EPA/day Comparator: Placebo Treatment duration: 12 weeks. |
| Outcomes | Primary: Depression |
| Notes | ISRCTN44366049, no response from PI |
Murck 2003.
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants: Provided written informed consent; Diagnosis of major depressive disorder (Diagnostic and Statistical Manual of Mental Disorders, Fourth edition [DSM‐IV]); Score of between and including 16 and 25 on the HDRS; No treatment with any antidepressant medication (including St John's Wort) in the last 12 weeks from the date of Visit 0 (screen); Male or female of any race aged 18‐75. Actual enrolment reported as January 2003 ‐ January 2004, study completed December 2003. |
| Interventions | Interventions: 0.5 g, 1 g, or 2 g ethyl‐EPA/day Comparator: Placebo Treatment duration: 12 weeks. |
| Outcomes | Primary: Depression |
| Notes | ISRCTN63565713, no response from PI |
Murck 2004.
| Methods | Multicentre, double‐blind, randomised, parallel‐group, placebo‐controlled trial |
| Participants | Participants: Adults aged 18 ‐ 75 with: Score of ≥ 16 on the HDRS; Treatment for ≥ 8 weeks with 1 or more standard antidepressants, at stable dose for ≥ 3 weeks; Currently receiving at least the minimum therapeutic dose of 1 or more standard antidepressants, as defined in the BNF; Diagnosis of major depressive disorder (DSM‐IV). |
| Interventions | Intervention: 1 g/d ethyl EPA Comparator: Placebo |
| Outcomes | Not reported |
| Notes | No response from PI |
Naqvi 2008.
| Methods | Allocation: Randomised Endpoint Classification: Efficacy Study Intervention Model: Parallel Assignment Masking: Double‐blind (participant, caregiver, investigator, outcomes assessor) Primary Purpose: treatment |
| Participants | Adolescents between the ages of 13 and 21 currently under standard care treatment at the Child Division of the Department of Psychiatry at Cedars‐Sinai Medical Center Diagnosed with MDD using the DSM‐IV diagnostic criteria |
| Interventions | Intervention: Cognitive behaviour therapy in combination with omega‐3 fatty acid supplements Comparator: Cognitive behaviour therapy in combination with placebo |
| Outcomes | Primary: CDI, HDRS, both 8 times for an average of 8 weeks |
| Notes | No working contact details |
NCT00816322.
| Methods | Allocation: Randomised Endpoint classification: Safety/efficacy study Intervention Model: Parallel Assignment Masking: Double‐blind (participant, caregiver, investigator, outcomes assessor) Primary Purpose: treatment |
| Participants | Adults aged between 18 and 65 years meeting DSM‐IV criteria for MDD |
| Interventions | Intervention: Omega‐3 fatty acids Comparator: placebo |
| Outcomes | Primary: HDRS Secondary: BDI; adverse effects; recurrence rate |
| Notes | No response from PI |
Su 2005.
| Methods | Allocation: Randomised Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Double‐blind (participant, caregiver, investigator, outcomes assessor) Primary Purpose: treatment |
| Participants | Adults aged 18 ‐ 65 years meeting DSM‐IV criteria for MDD |
| Interventions | Intervention: DHA/EPA (1.6 ~ 2.8 g/d (5 capsules)) Comparator: placebo (5 g/d (5 capsules)) |
| Outcomes | Primary: HDRS Secondary: BDI; Adverse events |
| Notes | No response from PI |
BDI: Beck depression inventory BNF: British National Formulary CDI: Children's depression inventory CGI: Clinical global impression DHA: docosahexaenoic acid DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth edition EPA: eicosapentaenoic acid HDRS: Hamilton depression rating scale MADRS: Montgomery‐Asberg Depression Rating Scale MDD: major depressive disorder MMSE: Mini‐Mental State Examination SSRI: selective serotonin reuptake inhibiting
Characteristics of ongoing studies [ordered by study ID]
Amminger 2013.
| Study name | Youth Depression Alleviation: A randomised controlled trial of omega‐3 fatty acids (fish oil) for major depressive disorder in young people (YoDA‐F) |
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants aged 15 ‐ 25 years, seeking help for psychological distress A score between 11 and 20 on the QIDS‐A17‐C at first contact with the service AND after 1 week (plus 1 ‐ 5 days if the client is unable to attend earlier) at the second assessment, or at 2 subsequent (weekly) follow‐up assessments A diagnosis of MDD using the SCID‐I/P |
| Interventions | Intervention: Cognitive behavioural case management plus 4 capsules of marine fish oil per day (providing approximately 840 mg of EPA, approximately 560 mg of DHA, and approximately 5 mg of Vitamin E) Comparator: Cognitive behavioural case management plus 4 capsules of placebo per day (approximately 700 mg paraffin oil) |
| Outcomes | Primary: Change in depressive symptoms as assessed by QIDS‐A17‐C between baseline and 12 weeks Secondary: Change in depressive symptoms as assessed by QIDS‐A17‐C between baseline and 26 weeks, Remission rate at 12 and 26 week follow‐up, Changes to symptomology and psychosocial functioning assessed across a range of domains assessed at baseline and weeks 4, 8, 12, and 26 |
| Starting date | February 2014 |
| Contact information | G Paul Amminger, Orygen Youth Health Research Centre |
| Notes | ACTRN12613001352796 |
Belmaker 2007.
| Study name | Folic acid and omega ‐3 fatty acid supplementation in depressed older adults |
| Methods | Randomised placebo‐controlled trial |
| Participants | Age 65+ (year of birth ‐1942 or below), with depression (as defined by the DSM‐IV diagnostic criteria for depression: major or minor depression or dysthymia). Actual enrolment reported as 15 participants (May 2007 ‐ Oct 2008) |
| Interventions | Intervention: Omega‐3 (2000 mg per day: active docosahexaenoic acid (DHA) and eicosapentanoic acid (EPA), proportion 1:1), (with vitamin B12 (1000 mcg per day)), tested in isolation and in combination with Folic acid (1600 mcg per day). Placebo: Omega‐3 and folic acid placebos (4 arms) |
| Outcomes | Primary: Severity of depressive symptoms, assessed at baseline, 4, 8, and 12 weeks Secondary: Cognitive status, nutritional status, possible side effects, assessed at baseline, 4, 8 and 12 weeks |
| Starting date | May 2007 |
| Contact information | Prof. RH Belmaker, Beersheva Mental Health Center, Israel |
| Notes | Clinical Trials: ID: NCT00480207 |
Chen 2017.
| Study name | Fish oil as adjunct treatment for major depressive disorder |
| Methods | Randomised placebo‐controlled trial, 6 months |
| Participants | Participants: Able to provide informed consent; Men or women aged 18 ‐ 50 years; a primary psychiatric diagnosis of major depressive disorder (MDD), by Diagnostic and Statistical Manual‐5th ed (DSM‐5) using the MINI; HAMD total score ≥ 21; no significantly modification of their diet from the time they sign consent to the end of study participation Aiming to enrol 120 participants |
| Interventions | Intervention: Fish oil, 1 g/day (containing EPA 1440 mg, DHA 960 mg) (4 capsules, taken with meals) Placebo: Soybean oil, 1 g/day (4 capsules, taken with meals). |
| Outcomes | Primary: HDRS at weeks 0, 4, 12, 24 and 48 Secondary: CGI, HAM‐A, BDI and SAS at weeks 0, 4, 12, 24 and 48 |
| Starting date | October 2017 |
| Contact information | Jindong Chen, MD, Central South University, China |
| Notes | Clinical Trials: ID: NCT03295708 |
Chiu 2010.
| Study name | Fish oil supplementation in late‐life depression |
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants: Aged 60 years old or over; a previous diagnosis of major depressive disorder according to the Chinese version of Structured Clinical Interview for DSM IV‐TR Axis‐I Disorder; depressive symptoms were stable for at least three consecutive weeks and the 17‐item Hamilton Depression Rating Scale score less or equal to 10; capacity to provide informed consent. Actual enrolment reported as 89 participants (May 2007 to Sept 2010) |
| Interventions | Intervention: 600mg EPA + 400 mg DHA + tertiary butyl hydroquinone 0.2mg/g and tocopherols 2 mg/g / capsule, 3 capsules/day Placebo: identical capsules of olive oil, 3 capsules/day |
| Outcomes | Primary: Recurrence of depression (defined as DSM‐IV‐TR diagnosis, a score of 3 or more on the suicide scale of the HDRS‐17 item, or hospitalisation due to depression), at weeks 8, 16, 24, 32, 40 and 48. Secondary: Cognitive function at week 48. |
| Starting date | May 2007 |
| Contact information | Chih‐Chiang Chiu, MD, Department of Psychiatry, Taipei City Psychiatric Center, Taipei City Hospital, Taiwan |
| Notes | Clinical Trials: ID: NCT01235533 |
Fang 2019.
| Study name | Study on the effect of PRKCB1 modulating inflammatory factors and the role for developing major depressive disorder |
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants: Drug‐naïve or medication‐free for no less than 4 weeks; 18 ‐ 60 years old, Han nationality;
junior high school diploma or above; meeting the criteria of major depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐IV‐TR; Scored 17 or higher on the Hamilton's Depression Scale with 17 items (HAMD‐17) and scored 2 or higher for the 2nd item (depressive mood);
Written informed consent has been obtained Aiming to enrol 350 participants |
| Interventions | Intervention: EPA ‐ 180 mg, DHA ‐ 120 mg, plus escitalopram ‐ 10 ‐ 20 mg/d Comparator: escitalopram ‐ 10 ‐ 20 mg/d |
| Outcomes | Primary: remission of acute phase (HDRS score of 7 or less) at week 12, remission of consolidate or maintenance phase (HDRS score of 7 or less) at 12 months |
| Starting date | January 2019 |
| Contact information | Yiru Fang Fang, MD. PhD. Shanghai Mental Health Center, China |
| Notes | Clinical Trials: ID: NCT03899194 |
Howe 2008.
| Study name | Omega‐3 fatty acid supplementation for symptoms of depression in patients with cardiovascular disease |
| Methods | Randomised controlled trial, parallel, blinded |
| Participants | Adults aged between 18 ‐ 75 years with: (a) angiographically‐documented coronary artery disease, defined as > 50% stenosis in an epicardial coronary artery on selective coronary angiography (b) comorbid depression as determined by a score of ≥ 16 on the CES‐D scale |
| Interventions | Intervention: 4 x 1 g/d capsules of EPA‐rich fish oil for 6 months (each capsule will contain 500 mg EPA and 25 mg DHA) Comparator: 4 x 1 g/d capsules of soybean/corn oil for 6 months (each capsule will contain 500 mg soybean oil and 500 mg corn oil) |
| Outcomes | Primary: HDRS Secondary: SF‐36; SAQ; flow mediated dilatation in the brachial artery; Changes in cerebral blood flow measured by transcranial Doppler ultrasound Measurements taken at baseline, 3 months (HDRS, SF‐36, SAQ) and 6 months |
| Starting date | November 2008 |
| Contact information | Professor Peter Howe, Nutritional Physiology Research Centre, University of South Australia |
| Notes |
Marriott 2016.
| Study name | The better resiliency among veterans with omega‐3's study (BRAVO) |
| Methods | Randomised placebo‐controlled trial, 6 months |
| Participants | Participants: Veteran or non‐veteran identified as being at risk for suicide and presently under the care of a mental healthcare provider (a release of information from his/her mental healthcare provider is required.); age 18 ‐ 90; within the participant's medical history, either a suicide attempt in the last 6 months, or a suicide attempt during the adult lifetime AND current diagnosis of an episode of depression as diagnosed on the Mini International Neuropsychiatric Interview (MINI), or an inpatient admission with suicide risk in the last 6 months, or an inpatient admission with suicide risk during the adult lifetime AND current diagnosis of an episode of depression as diagnosed on the Mini International Neuropsychiatric Interview (MINI), or positive suicidal behaviour or ideation based on a psychiatrist‐ administered Columbia‐Suicide Severity Rating Scale (C‐SSRS) and psychiatrist review of participant medical history and physical, or a score of 0 or greater on the Implicit Associations Test‐Suicide (IAT‐S), or > or 9 on the Beck Hopelessness Scale (BHS) and psychiatrist review of participant medical history and physical; participant can safely eat walnuts, pecans, almonds, peanuts and all other nuts, apples, peaches, pears, pomegranates, aronia, jackfruit, and passion fruit, the herb rosemary, and the fish salmon, trout and cod, drink and eat food that contain whey or milk protein, or both, willingness to drink the juice boxes 3 times each day for 6 months, have a stable residence with adequate space to store the juice, and capacity to provide written informed consent. Additional inclusion criteria for Depressive Symptoms sub‐analysis: enrolment in the primary study of suicide risk reduction; a Beck Depression Inventory ≥ 30; a diagnosis of a depressive disorder Actual enrolment reported as 125 participants (March 2014 ‐ August 2016) |
| Interventions | Intervention: 550 mg EPA + 550 mg DHA, provided as a fruit juice/smoothie, 3 times a day, to result in a dose of 1650 mg EPA and 1650 mg DHA / day Comparator: Fruit juice including 1100 mg macadamia oil, 3 times a day |
| Outcomes | Primary: Suicadal behaviours and thinking, at months 1, 3, 5 and 6 Secondary: Symptoms associated with suicidal risk, associated with negative affect, PTSD and cognitive functioning, at months 1, 3, 5 and 6 |
| Starting date | March 2014 |
| Contact information | Bernadette Marriott, PhD, Medical University of South Carolina, USA |
| Notes | Clinical Trials: ID: NCT01901887 |
Nakano 2014.
| Study name | Augmentation of omega‐3 fatty acid with antidepressants for major depressive disorder: a double‐blind, randomised controlled trial |
| Methods | Randomised, double‐blind, parallel‐groups, controlled trial |
| Participants | Adults aged between 20 ‐ 65 years old with a MDE, where: the person did not receive any antidepressant drugs for major depression, has a HDRS (17‐item) score, the major depressive episode is the focus of the treatment and the treating physician has judged escitalopram to be the appropriate first‐line drug, and is a native Japanese speaker |
| Interventions | Intervention: Omega‐3 polyunsaturated fatty acid Comparator: placebo |
| Outcomes | Primary: HDRS, at 12 weeks Secondary: MADRS; BDI; QIDS‐J; CGI‐S; RS‐14; Serum BDNF, proBDNF, MMP‐9, fatty acid level; Plasma IL‐6 |
| Starting date | April 2014 |
| Contact information | Wakako Nakano, University of Occupational and Environmental Health Department of Psychiatry |
| Notes | No working contact details |
Parker 2006a.
| Study name | A study of omega‐3 as an augmentor of antidepressant treatment for major depression |
| Methods | Allocation: randomised Endpoint classification: Safety/efficacy study Intervention model: Parallel assignment Masking: Double‐blind Primary purpose: treatment |
| Participants | Adults aged between 18 and 65 years presenting with a first or new episode of DSM‐IV non‐psychotic MDD warranting treatment with antidepressant mediation |
| Interventions | Intervention: Omega‐3 (fish oil) Comparator: placebo (paraffin oil) |
| Outcomes | Primary: Change from pretreatment score on Depression Rating scale at 4 weeks Secondary: Daily mood rating; weekly measure of depression; weekly measure of anxiety; weekly measure of functional status |
| Starting date | February 2006 |
| Contact information | Catherine Owen, University of New South Wales |
| Notes |
Piperoglou 2014.
| Study name | Adjunctive natural low dose docosahexaenoic acid (DHA) omega‐3 in a 16 week random double‐blind placebo controlled (RDBPC) cross‐over withdrawal study in a group of chronic, psychiatric out‐patients with anxiety and mood disorders |
| Methods | Randomised controlled, double‐blind, cross‐over trial Following the open‐label phase (first 4 weeks of the study) there will be 2 double‐blind cross‐over phases, each of 8 weeks duration, where the participant will first take DHA omega‐3 then look‐alike placebo capsule containing safflower oil, or placebo then DHA omega‐3. In the final 4 weeks phase all participants receive DHA omega‐3 |
| Participants | Adults aged 20 ‐ 70 who are: 1. Outpatients with chronic anxiety and/or depressive symptoms 2. Patients currently taking DHA (NeuroSpark) capsules for at least 3 months prior to study entry |
| Interventions | Intervention: Natural low‐dose docosahexaenoic acid (DHA) omega‐3 (NeuroSpark) 130 ‐ 390 mg per day in addition to standard psychiatric treatments Comparator: safflower oil capsules Treatment given for 16 weeks |
| Outcomes | Primary: HAM‐A, HDRS, LSEQ, Fatigue questionnaire Secondary: Change from baseline in cognitive function; levels of metabolites of Arachidonic acid (AA); cytokines (e.g. TNF‐alpha and others), inflammatory markers (CRP), RBC membrane PUFA analyses to measure PUFA levels Measurements taken at weeks 0, 4, 12, 20 and 24 (various measures at each time point) |
| Starting date | May 2014 |
| Contact information | Michael Piperoglou, University of Melbourne |
| Notes |
Rapaport 2015.
| Study name | Omega‐3 fatty acids for major depressive disorder with high inflammation: a personalized approach |
| Methods | Randomised placebo‐controlled trial, 12 weeks |
| Participants | Participants: able to provide informed consent. Men or women aged 18 ‐ 80 years. A primary psychiatric diagnosis of major depressive disorder (MDD), by Diagnostic and Statistical Manual‐5th ed (DSM‐5) using the Mini International Neuropsychiatric Interview (MINI v.7.0). Screening and baseline visit Inventory of Depressive Symptoms, Clinician‐rated (IDS‐C30) total score ≥ 25. Currently overweight at screening, defined as BMI > 25 kg/m2 Screening visit high‐sensitivity C‐reactive protein concentration ≥ 3 mg/L. Willing to not significantly modify their diet from the time they sign consent through the end of study participation Actual enrolment reported as 61 participants |
| Interventions | Intervention: 1 g/d, 2g/d, 4g/d EPA, for 12 weeks. Comparator: Soybean oil All participants consume 4 x 1 g capsules/d that are intervention, placebo or a combination |
| Outcomes | Primary: IDS‐C30 Depression score at 12 weeks, Change in IDS‐C30 Depression score from baseline, Plasma concentrations of inflammatory markers Secondary: Plasma concentrations of inflammatory markers, and markers of gene expression for inflammatory markers |
| Starting date | December 2015 |
| Contact information | David Mischoulon, MD, PhD, Principal Investigator, Massachusetts General Hospital |
| Notes | Clinical Trials: ID: NCT02553915 |
Sahoo 2016.
| Study name | Effect of omega‐3 fatty acids versus 5‐hydroxytryptophan as add on therapy to sertraline in controlling suicidal ideation in patients with depression: A comparative study |
| Methods | Randomised placebo‐controlled trial, 8 weeks |
| Participants | Participants: Patients presenting with ICD‐10 depressive episode for the first time, HAM‐D score of 15 or more on 17‐item version, Age between 18 and 65 years, Patient or his/her relative willing to give written informed consent prior to enrolment in the study Actual enrolment is reported as 70 participants (October 2014 ‐ August 2015) |
| Interventions | Intervention: Omega‐3 oil ‐ 1 g/d, plus sertraline 50 mg/d, for 8 weeks Comparator: Sertraline 50 mg/d only, for 8 weeks |
| Outcomes | Primary: Suicidal ideation (Becks Suicidal Ideation Scale), at 0, 4 and 8 weeks Secondary: HDRS, BDI, CGI, and WHOQOL‐BREF |
| Starting date | October 2014 |
| Contact information | Jyoti Prakesh Sahoo, Pt. B. D. Sharma PGIMS, Rohtak, Haryana, India |
| Notes |
Smith 2010.
| Study name | An 8‐week randomised, double‐blind, placebo controlled trial investigating the role of adjunctive bioactive lipids specifically; docosahexaenoic acid (DHA) versus eicosapentaenoic acid (EPA) in Major Depressive Disorder ‐ with a 6 week open label extension of DHA in patients aged 18‐65 years. |
| Methods | 8 week randomised, double‐blind, placebo‐controlled trial |
| Participants | Adults aged between 18 ‐ 65 years diagnosed with a MDE |
| Interventions | Arm 1: DHA (2 tablets (260 mg/day)) Arm 2: EPA (2 tablets or 360 mg/day) Arm 3: Sunflower oil (2 tablets or 2000 mg/day) In addition and where possible patient’s background antidepressant medication will remain as a fixed dose for the 8 week study period |
| Outcomes | Primary: HDRS, change from baseline at 8 weeks Secondary: BDNF levels, change from baseline at 8 weeks |
| Starting date | October 2010 |
| Contact information | Deirdre Smith, The Professorial Research Unit, University of Melbourne |
| Notes |
Tabasi 2020.
| Study name | Determining the effectiveness of transcranial direct current stimulation (tDCS) and omega‐3 supplementation on executive functions, food craving, and depressive symptoms in patients with depression and overweight |
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants: Women with depression (Beck Depression Score above 14), overweight or obese (BMI ≥ 25) and age group 18 to 60 years |
| Interventions | Intervention: Intervention group 1: 3 sessions of transcranial direct current stimulation (tDCS), plus omega‐3 supplement (5 cc daily, containing 736 mg (EPA) And 460 mg (DHA)) Intervention group 2: 3 sessions of transcranial direct current stimulation (tDCS), plus omega‐3 placebo supplement (5 cc daily, soybean oil) Intervention group 3: 3 sessions of sham transcranial direct current stimulation (tDCS), plus omega‐3 supplement (5 cc daily, containing 736 mg of eicosapentaenoic acid (EPA) and 460 mg docosahexaenoic acid (DHA)).0 mg DHA) Comparator: 3 sessions of sham transcranial direct current stimulation (tDCS), and the placebo of omega‐3 (5 cc daily, soybean oil) |
| Outcomes | Primary: BDI, at baseline, 14 and 28 days Secondary: Executive function (Simple Strop computer software and Wisconsin card computer test), Individual cravings score in the Summary Food Trait Questionnaire (FCQ‐T), body weight and body fat percentage, appetite score (Simple Appetite Questionnaire (SNAQ) at baseline, 14 and 28 days |
| Starting date | December 2019 |
| Contact information | Seyed Ali Mostafavi, Tehran University of Medical Sciences, Iran |
| Notes | Irct20200716048117N |
Tanna 2020.
| Study name | Role of omega 3 fatty acid in etiopathogenesis of depression and trial of two drugs: flax seed oil and Ashwagnadharishta in its management |
| Methods | Randomised placebo‐controlled trial |
| Participants | Participants: Patients fulfilling the Diagnostic criteria DSM IV of major depressive disorder and age above 15 and below 70 years will be included in the present study |
| Interventions | Intervention: Flaxseed 10m l 2x day mixed with food intervention; and Flaxseed 10 ml 2x day mixed with food intervention with Ashwagandharishta 25 ml 2x day mixed with equal amount of water after lunch and dinner Comparator: Ashwagandharishta 25 ml 2x day mixed with equal amount of water after lunch and dinner (3 arms) |
| Outcomes | Primary: MADRS, BDI, HDRS, at 0 and 60 days Secondary: HAM‐A, DASS 21, at 0 and 60 days |
| Starting date | May 2011 |
| Contact information | Dr Ila Tanna, Department of Rog Nidan & kaya chikitsa, Gujurat, India |
| Notes | CTRI/2020/10/028383 |
Yao 2005.
| Study name | Decreasing risk of coronary artery disease in schizophrenia by omega‐3 fatty acid supplementation (CAD) |
| Methods | Allocation: Randomised Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Double‐blind (participant, caregiver, investigator, outcomes assessor) Primary Purpose: treatment |
| Participants | Adults aged 18 or over meeting: DSM‐IV criteria for schizophrenia (or schizoaffective disorder), major depression, or bipolar (depressed phase) disorder who are treated with antipsychotic, antidepressant or antimanic drugs and a lipid‐lowering drug (statin) for 2 months or longer |
| Interventions | Intervention: EPA (2 g in 4 x 500 mg soft gels daily) + antipsychotic drug (doctor's choice) treatment for baseline, 1 month, 2 months and 4 months duration Comparator: Placebo (soy bean oil, 2 g in 4 x 500 mg soft gels daily) + antipsychotic drug (doctor's choice) treatment for baseline, 1 month, 2 months and 4 months duration |
| Outcomes | Primary: To assess whether EPA supplementation can lead to improvement in further reducing CAD risk profile Secondary: To test whether EPA supplementation can simultaneously improve the psychiatric status of patients with schizophrenia |
| Starting date | September 2005 |
| Contact information | Jeffrey Yao, University of Pittsburgh and VA Pittsburgh Healthcare System |
| Notes | No working contact details |
Yousef 2018.
| Study name | Role of omega‐3 polyunsaturated fatty acid in the management of major depressive disorder |
| Methods | Randomised placebo‐controlled trial, 12 weeks |
| Participants | Participants: Patients aged 20 to 40 years, who were already diagnosed with depression and taking antidepressant treatment Actual enrolment reported as 70 participants (May 2017 to August 2017) |
| Interventions | Intervention: Omega‐3 (EPA 300mg, and 200mg DHA)/day, 1 capsule/day, for 12 weeks Comparator: Corn Oil (500 mg corn oil), 1 capsule/day, for 12 weeks |
| Outcomes | Primary: BDI |
| Starting date | May 2017 |
| Contact information | Naiza Yousef, Nutritionist, Allama Iqbal Open University Islamabad, India |
| Notes | Clinical Trials: ID: NCT03732378 |
BDI: Beck depression inventory BDNF: Brain‐derived neurotropic factor CAD: coronary artery disease CES‐D: Center for Epidemiologic Studies – Depression CGI: Clinical global impression DASS: Depression anxiety stress scale CDRS‐R: children's depression rating scale ‐ revised DHA: docosahexaenoic acid DSM‐IV/‐TR: DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth edition / Fourth edition revised EPA: eicosapentaenoic acid HADS: Hospital anxiety and depression scale HAM‐A: Hamilton Anxiety Scale HDRS: Hamilton depression rating scale
IDS‐C30: Inventory of Depressive Symptomatology‐30 item‐Clinician Rated LSEQ: Leeds sleep evaluation questionnaire MDD: major depressive disorder MDE: major depressive episode PANAS: Positive And Negative Affect Scale QIDS‐A17‐C: Quick inventory for depressive symptomatology ‐ adolescent version
SAS: Self‐Rating Anxiety Scale SAQ: Seattle Angina Questionnaire SCID‐IP: Structured Clinical Interview for DSM‐IV Axis I Disorders, patient version SDQ: Simple dietary questionnaire
Differences between protocol and review
The following differences between protocol and final updated review have arisen, for the reasons provided:
Protocol: "Only studies involving adults (18 years and over) will be included". Review: One study involving adults (16 years and over) is included (Gharekhani 2014). Age 16 years is the definition of adult in the country in which this study was undertaken.
Refinement to the Protocol: Protocol: "We only included studies that enrolled participants with a primary diagnosis of major or unipolar depressive disorder, from a trained professional or using a validated rating scale". Review: 'Our primary interest was in studies that enrolled participants with a diagnosis of major or unipolar depressive disorder, thus, we included studies that specified the study of "major" or "unipolar" depressive disorder, given by a trained professional, using a recognized diagnostic schedule. We recognize however, that not all participants with debilitating depressive symptomology will have a formal diagnosis, and that the language used to report such diagnoses may vary by culture and era. To ensure no studies were missed, we also considered studies that included individuals with a diagnosis of "depression" or "depressive disorder", given by a trained professional, using a recognized diagnostic schedule, where antidepressant treatment was considered appropriate and where an alternative depressive disorder was not specified, including "mild" or "moderate" depression; and studies that used a validated rating scale to specify high levels of depressive symptomology. Where MDD was defined using a validated rating scale, we used established cut‐off values to describe MDD. These cut‐off values were: Beck Depression Inventory (BDI) (Beck 1987): 17 or more of 63; Geriatric Depression Screening Scale (GDS) short (Yesvage 1983): 5 or more of 15; Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983): 15 or more of 21; Hamilton Depression Rating Scale (HDRS) (Hamilton 1960): 17 or more of 54; the Montgomery‐Asberg Depression Rating Scale (MADRS) (Montgomery 1979): 30 or more of 60; Patient Health Questionnaire (PHQ9) (Kroenke 2001): 15 or more of 27. Acceptable exceptions were where authors used an alternative cut‐off value and classified this explicitly as MDD or equivalent to a diagnosis. Similarly, if these cut‐off values were used to specifically classify "mild" or "moderate" depression, these studies were considered unsuitable for inclusion in the review. These exceptions were made to account for differences between cultures or eras in appropriate cut‐off values for MDD. Cut‐offs were identified in advance of data extraction, to reduce bias and ensure consistency between data extractors.
Refinement to the Protocol: Protocol: "We only included studies that enrolled participants with a primary diagnosis of major or unipolar depressive disorder, from a trained professional or using a validated rating scale". Review: ‘If studies reported a diagnosis and use of a cut‐off on a rating scale, either diagnosis or rating score were considered acceptable to warrant study inclusion; we did not require both a diagnosis and a score above a cut‐off for individuals in these studies’. This detail provided comparable study inclusion criteria for all identified studies.
Refinement to the Protocol: Protocol: "We only included studies that enrolled participants with a primary diagnosis of major or unipolar depressive disorder, from a trained professional or using a validated rating scale". Review: ‘We also excluded studies that specifically stated study of "mild" or "moderate" depression. Where severity of depression was unclear or contradicted standard cut‐off values, we used the authors' definition of level of depression, to account for differences between countries or cultures in appropriate cut‐off values for MDD.
Protocol: ‘We will include a subgroup only if the subgroup is defined and distinguished prior to randomisation’. Review: ‘We will include a subgroup only if the subgroup was defined in publications, either through mention of a subgroup as part of the method, in details of the Participant Characteristics, or through the use of subgroup analyses’. Where distinction prior to randomisation was not clear, alternative methods to identify a defined subgroup were used.
Protocol: "Studies will be included regardless of participant medication". Review: Studies were included regardless of participant medication and other treatments for depressive symptomology, so we have stated "Studies were included regardless of participant use of adjunctive therapy".
Protocol: "Experimental intervention: Studies will be included regardless of source of n‐3PUFA provided ..., but records of differences will be made". Review: Records of differences based on source of n‐3PUFA provided were made and have been investigated in sensitivity analyses. We conducted sensitivity analyses following the publication of a number of similar comparisons since the conception of this review, and following reviewers' comments.
Refinement to the Protocol: Protocol: "We also included studies regardless of participant use of adjunctive therapy". Review: ‘Where studies included adjunctive therapy, these studies were included only if the adjunctive therapy did not systematically differ between experimental and comparator groups, i.e. studies were included if n‐3PUFAs were provided in addition to usual medication, but studies were not included if n‐3PUFAs were provided alongside other bioactive agents in the experimental group, and neither n‐3PUFAs nor the bioactive agents were provided to comparator’. This distinction was made to allow consideration only of true investigations of n‐3PUFAs.
Protocol: "Secondary outcomes include: Failure to complete". "Secondary outcomes include: Trial non‐completion". This terminology was changed to remove any suggestion of failure.
Protocol: "Where studies use multiple time points, data will be tabulated for all outcomes at all time points where assessments have been made, but only those of longest follow‐up will be included in statistical analyses". Review: Data for all time points have not been tabulated. This has not been done due to the variety of time points used across studies, and the difficulty and low value of comparing across varied time points.
Protocol: "Complementary searches will be conducted .... in BIOSIS Citation Index (1969 to date), and Web of Science (1900 to date)". Review: These searches were not completed. We decided that due to the topic of the review, searches in Biosis and Web of Science would be very unlikely to reveal additional studies.
Protocol: "We will assess the risk of bias according to the following domains. 1. Random sequence generation, 2. Allocation concealment, 3. Blinding of participants and personnel, 4. Blinding of outcome assessment, 5. Incomplete outcome data, 6. Selective outcome reporting, 7. Other bias". Review: We have made assessments of outcome data (blinding of outcome assessment, and incomplete outcome data) separately for each primary outcome. This was done because different judgements could be given to different outcome assessments for some studies, depending on methods of measurement, and it was meaningless to try and combine these.
Protocol: "Data from subgroups of little relevance to the research question, e.g. groups of men and women, will be recorded as reported, and subsequently combined for analysis". Review: Data have not been presented separately for subgroups of little relevance to the research question, because we found none.
Protocol: "Adverse effects and failure to complete data will not be statistically summarised". Review: We have statistically summarised data on adverse effects and failure to complete, where data were available. We did this because of the amount of data available and the value of these statistical summaries.
Protocol: Subgroup analyses will be conducted "using only studies in which participants are clearly identified as having comorbid conditions, and using only studies in which participants are clearly identified as being without comorbid conditions. Studies where participants with and without comorbid conditions were mixed, and studies that do not clearly identify whether participants have comorbid conditions or not, will not be included in this analysis". Review: We have conducted subgroup analyses based on comorbidities using all studies. We did this to allow investigation of effects of comorbidities in the whole data set.
Protocol: Subgroup analyses will be conducted "using only studies in which participants are clearly identified as receiving adjunct therapy, and using only studies in which participants are clearly identified as not receiving adjunct therapy. Studies where participants with adjunct therapies are mixed, and studies that do not clearly identify whether participants are receiving or not receiving adjunct therapies will not be included in this analysis". Review: We have conducted subgroup analyses based on adjunctive therapy using all studies. We did this to allow investigation of effects of adjunctive therapy in the whole data set. For these analyses, We have defined adjunctive therapy as including psychotherapy as well as antidepressant medication, and we have limited it to adjunctive therapies for depression.
Protocol: Sensitivity analyses on risk of bias will be conducted where "low risk of bias will be defined as in the Cochrane Handbook (Higgins 2011)". Review: We have further defined low risk of bias as "using (i) selection bias, measured using allocation concealment; (ii) performance bias, using blinding of participants and personnel; (iii) attrition bias, using incomplete outcome data. We conducted three separate analyses, one for each type of bias".
We conducted sensitivity analyses that we had not proposed in the protocol. These analyses investigated possible methodological sources of heterogeneity that became apparent during the review or the write‐up processes, or both. These sensitivity analyses are identified in the review as 'sensitivity analyses investigating aspects of study methodology'. These are to be distinguished from our preplanned sensitivity analyses. We applied the sensitivity analyses using a fixed‐effect model to all outcomes for completeness, but restricted all other sensitivity analyses to testing only our primary outcomes.
Planned methods not used in the review
Protocol: Unit of analysis issues: Cross‐over RCTs: We will include only the first study phase of cross‐over RCTs in analyses. We think cross‐over RCTs are unlikely to be used in this field. Cluster‐RCTs: We will include cluster‐RCTs in primary analyses, where the cluster will act as the unit of investigation. We think cluster‐RCTs are unlikely to be used in this field. Review: We have not used these methods because we did not find any cross‐over or cluster‐RCTs during our searches. The statements in the protocol will be applied where appropriate in future updates of the review.
Contributions of authors
For this update, PV, SD and/or RP (two review authors) screened all articles identified by searches, and KA, PV, HS and/or RP (two review authors) extracted data from all eligible studies. All review authors collectively resolved disagreements. KA entered all data into Review Manager 5, and PV checked all entered data. KA conducted all analyses, and wrote up the review. All authors checked and subsequently revised this draft.
Sources of support
Internal sources
-
Bournemouth University, UK
Researcher time
-
University of Bristol, UK
Researcher time
External sources
-
National Institute for Health Research, NIHR, UK
SD and RC contribution to this review update is supported by Cochrane Infrastructure funding to the Common Mental Disorders Cochrane Review Group.
Declarations of interest
KA: None known PV: None known HS: None known SD: Is an Information Specialist for Cochrane Common Mental Disorders but was not involved in the editorial approval process for this review. AN: None known RC: Leads and has responsibility for Cochrane Common Mental Disorders, which has supported parts of the review process and is largely funded by a grant from the National Institute for Health Research (NIHR) in the UK. RC was not involved in the editorial process for this review. RP: None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bot 2010 {published data only}30877831
- Bot M, Pouwer F, Assies J, Jansen E, Beekman AT, De Jonge P. Supplementation with eicosapentaenoic omega-3 fatty acid does not influence serum brain-derived neurotrophic factor in diabetes mellitus patients with major depression: a randomized controlled pilot study. Neuropsychobiology 2011;63(4):219-23. [DOI] [PubMed] [Google Scholar]
- Bot M, Pouwer F, Assies J, Jansen E, Diamant M, Snoek FJ, et al. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study [ISRCTN30877831; NTR624]. Journal of Affective Disorders 2010;126(1-2):282-6. [DOI] [PubMed] [Google Scholar]
- Mocking RJ, Assies J, Bot M, Jansen E, Schene AH, Pouwer F. Biological effects of add-on eicosapentaenoic acid supplementation in diabetes mellitus and co-morbid depression: a randomized controlled trial. PloS ONE 2012;7(11):e49431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwer F. Addition of eicosapentaenoic acid to maintenance anti-depressant therapy in diabetes patients with major depressive disorder: a double-blind, placebo-controlled pilot study. www.controlled-trials.com/ISRCTN30877831 (Accessed 21 August 2014).
Carney 2009 {published data only}
- Anonymous. News breaks. Nutrition Today 2009;44(6):234-5. [Google Scholar]
- Anonymous. Use of omega-3 with treatment for depression in heart disease patients may not provide benefit. Nurse Prescribing 2009;7(11):523-4. [Google Scholar]
- Bot M, Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, et al. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. Journal of Psychosomatic Research 2011;71(1):13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer MS, Harris WS. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: A randomized controlled trial [NCT00116857]. JAMA 2009;302(15):1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Steinmeyer BC. 'Omega-3 fatty acids for CHD with depression': Reply to comment. JAMA 2010;303(9):836. [DOI] [PubMed] [Google Scholar]
- Carney RM, Steinmeyer BC, Freedland KE, Rubin EH, Rich MW, Harris WS. Baseline Blood Levels of Omega-3 and Depression Remission: A secondary analysis of data from a placebo-controlled trial of omega-3 supplements. J Clin Psychiatry 2016;77:e138-e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM. Omega-3 fatty acids to improve depression and reduce cardiovascular risk factors. ClinicalTrials.gov/show/NCT00116857 (Accessed 21 August 2014).
- D'Anci KE. Nutrition updates. Nutrition Reviews 2010;68(1):71-3. [DOI] [PubMed] [Google Scholar]
- Roest AM, Carney RM, Stein PK, Freedland KE, Meyer H, Steinmeyer BC, et al. Obstructive sleep apnea/hypopnea syndrome and poor response to sertraline in patients with coronary heart disease. Journal of Clinical Psychiatry 2012;73(1):31-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 2020 {published and unpublished data}
- Anon. EPA with antidepressant did not improve treatment efficacy in cardiac patients. Brown University Psychopharmacology Update 2019;Sept.
- Carney RM, Freedland KE, Rubin EH, Rich MW, Steinmeyer BC, Harris WS. A randomized placebo-controlled trial of Omega-3 and sertraline in depressed patients with or at risk for coronary heart disease. Journal of Clinical Psychiatry 2019;80(4):19m12742. [PMID: doi: 10.4088/JCP.19m12742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02021669. Omega-3 for depression and other cardiac risk factors - 2 (Omega-3(2)). clinicaltrials.gov/ct2/show/NCT02021669 (first received 27 December 2013).
Chang 2020 {published and unpublished data}
- Chang JP, Chang SS, Chen HT, Su KP, Pariante CM. A double blind-placebo controlled trial of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in patients with cardiovascular diseases (CVDs) and comorbid major depressive disorder (MDD). Nutritional Neuroscience 2018;21:S11. [Google Scholar]
- Chang JP, Chang SS, Yang HT, Chen HT, Chien YC, Yang B, et al. Omega-3 polyunsaturated fatty acids in cardiovascular diseases comorbid major depressive disorder - Results from a randomized controlled trial. Brain, Behavior, and Immunity 2020;85:14-20. [DOI] [PubMed] [Google Scholar]
- NCT03072823. Omega-3 polyunsaturated fatty acids on major depressive disorder in patients with cardiovascular diseases. NCT03072823 (first received 17 March 2017).
Coryell (1g/d) 2005 {unpublished data only}
- Coryell WH. Essential fatty acids for major depression. ClinicalTrials.gov/show/NCT00256412 (First received 21 November 2005).
- Fiedorowicz JG, Hale N, Spector AA, Coryell WH. Neuroticism but not omega-3 fatty acid levels correlate with early responsiveness to escitalopram. Annals of Clinical Psychiatry 2010;22(3):157-63. [PMC free article] [PubMed] [Google Scholar]
Coryell (2g/d) 2005 {unpublished data only}
- Coryell WH. Essential fatty acids for major depression. ClinicalTrials.gov/show/NCT00256412 (Accessed 22/07/2014).
- Fiedorowicz JG, Hale N, Spector AA, Coryell WH. Neuroticism but not omega-3 fatty acid levels correlate with early responsiveness to escitalopram. Annals of Clinical Psychiatry 2010;22(3):157-63. [PMC free article] [PubMed] [Google Scholar]
Da Silva (AD) 2005 {published and unpublished data}
- Da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, et al. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. Journal of Affective Disorders 2008;111(2-3):351-9. [DOI] [PubMed] [Google Scholar]
Da Silva (nAD) 2005 {published data only}
- Da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, et al. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. Journal of Affective Disorders 2008;111(2-3):351-9. [DOI] [PubMed] [Google Scholar]
Gertsik 2012 {published data only}
- Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. Journal of Clinical Psychopharmacology 2012;32(1):61-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsik L. PUFA augmentation in treatment of major depression. ClinicalTrials.gov/show/NCT00067301 (First received 15 August 2003).
Gharekhani 2014 {published and unpublished data}
- Dashti-Khavidaki S, Gharekhani A, Khatami M-R, Miri E-S, Khalili H, Razeghi E, et al. Effects of omega-3 fatty acids on depression and quality of life in maintenance hemodialysis patients. American Journal of Therapeutics 2014;21(4):275-87. [DOI] [PubMed] [Google Scholar]
- Gharekhani A, Khatami MR, Dashti-Khavidaki S, Razeghi E, Abdollahi A, Hashemi-Nazari SS, et al. Potential effects of omega-3 fatty acids on anemia and inflammatory markers in maintenance hemodialysis patients. DARU 2014;22(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharekhani A, Khatami M-R, Dashti-Khavidaki S, Razeghi E, Noorbala A-A, Hashemi-Nazari S-S, et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. European Journal of Clinical Pharmacology 2014;70(6):655-65. [DOI] [PubMed] [Google Scholar]
- IRCT201202203043N5. Evaluating therapeutic effects of omega-3 fatty acids on anemia and nutritional status in hemodialysis patients with depression. en.irct.ir/trial/3051 (first received 10 April 2012).
Gonzalez 2011 {published data only}
- Gonzalez A, Mata S, Sanchez P, Gonzalez D, Urbina M, Fazzino F, et al. Omega-3 fatty acids as adjunctive of antidepressant therapy and its effects on brain-derived neurotrophic factor in serum, monocytes and lymphocytes. Archivos Venezolanos de Farmacologia y Terapeutica 2011;30(4):72-8. [Google Scholar]
Grenyer 2007 {published data only}
- Grenyer BF, Crowe T, Meyer B, Owen AJ, Grigonis-Deane EM, Caputi P, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Progress in Neuro-psychopharmacology & Biological Psychiatry 2007;31(7):1393-6. [DOI] [PubMed] [Google Scholar]
- Meyer BJ, Grenyer BF, Crowe T, Owen AJ, Grigonis-Deane EM, Howe PR. Improvement of major depression is associated with increased erythrocyte DHA. Lipids 2013;48(9):863-8. [DOI] [PubMed] [Google Scholar]
- Rotblatt M, Grenyer BFS. Fish oil not effective for chronic refractory depression. Focus on Alternative & Complementary Therapies 2008;13(1):30-1. [Google Scholar]
Jahangard 2018 {published data only}Irct201705249014N
- Irct201705249014N. Effect of omega-3-fatty acids versus placebo on clinical symptoms of patients with major depressive disorder. Irct201705249014N.
- Jahangard L, Sadeghi A, Ahmadpanah M, Holsboer-Trachsler E, Sadeghi Bahmani D, Haghighi M, et al. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders - results from a double-blind, randomized and placebo-controlled clinical trial. Journal of Psychiatric Research 2018;107:48-56. [DOI] [PubMed] [Google Scholar]
Jazayeri (v AD) 2008 {published data only}
- Jazayeri S, Keshavarz SA, Tehrani-Doost M, Djalali M, Hosseini M, Amini H, et al. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Research 2010;178(1):112-5. [DOI] [PubMed] [Google Scholar]
- Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Australian and New Zealand Journal of Psychiatry 2008;42(3):192-8. [DOI] [PubMed] [Google Scholar]
Jazayeri (v placebo) 2008 {published data only}
- Jazayeri S, Keshavarz SA, Tehrani-Doost M, Djalali M, Hosseini M, Amini H, et al. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Research 2010;178(1):112-5. [DOI] [PubMed] [Google Scholar]
- Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Australian and New Zealand Journal of Psychiatry 2008;42(3):192-8. [DOI] [PubMed] [Google Scholar]
Jiang (EPA+DHA) 2018 {published data only}
- Jiang W, Whellan DJ, Adams KF, Babyak MA, Boyle SH, Wilson JL, et al. Long-chain omega-3 fatty acid supplements in depressed heart failure patients: results of the OCEAN trial. JACC Heart Failure 2018;6(10):833-43. [DOI] [PubMed] [Google Scholar]
- NCT02057406. Omega 3 for treatment of depression in patients with heart failure (OCEAN). clinicaltrials.gov/ct2/show/NCT02057406 (first received 7 February 2014).
Jiang (EPA only) 2018 {published data only}
- Jiang W, Whellan DJ, Adams KF, Babyak MA, Boyle SH, Wilson JL, et al. Long-chain omega-3 fatty acid supplements in depressed heart failure patients: results of the OCEAN trial. JACC Heart Failure 2018;6:833-43. [DOI] [PubMed] [Google Scholar]
- Jiang W. Omega 3 for treatment of depression in patients with heart failure (OCEAN). ClinicalTrials.gov/show/NCT02057406 (first received 7 February 2014).
Kamath 2017 {published data only}
- Kamath J. Omega 3 FA supplements as augmentation in the treatment of depression. ClinicalTrials.gov/show/NCT01803711 (first received 04 March 2013).
Lespérance 2011 {published data only}47431149
- Lespérance F, Frasure-Smith N, St-André E, Turecki G, Lesperance P, Wisniewski SR. The efficacy of omega-3 supplementation for major depression: A randomized controlled trial [ISRCTN47431149]. Journal of Clinical Psychiatry 2011;72(8):1054-62. [DOI] [PubMed] [Google Scholar]
- Lespérance F. Double-blind, placebo-controlled, randomised trial of eicosapentaenoic acid (EPA) for major depression. www.controlled-trials.com/ISRCTN47431149/ (Accessed 21 August 2014).
Lucas 2009 {published data only}69617477
- Lucas M, Asselin G, Mérette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. American Journal of Clinical Nutrition 2009;89(2):641-51. [DOI] [PubMed] [Google Scholar]
- Lucas M. Double-blind, placebo-controlled, randomised, clinical trial of eicosapentaenoic acid in the treatment of mood disorders among middle-aged women. www.controlled-trials.com/ISRCTN69617477 (Accessed 21 August 2014).
Marangell 2003 {published data only}
- Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HF, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. American Journal of Psychiatry 2003;160(5):996-8. [DOI] [PubMed] [Google Scholar]
- Marangell LB, Zboyan HA, Cress KK, Benisek D, Arterburn L. A double-blind, placebo-controlled study of docosahexaenoic acid (DHA) in the treatment of depression. In: 4th Congress of the International Society for the Study of Lipids and Fatty Acids, Tsukuba, Japan. 2000:105.
Masoumi 2016 {published data only}IRCT2013052113405N1
- IRCT2013052113405N1. Comperssion with the combined effects of omega _3 and citalopram for depression during menopause in postmenopausal women referred to health centers in Hamadan city in 1391: a double-blind clinical trial. IRCT2013052113405N1 (first received 22 July 2013).
- Masoumi SZ, Kazemi F, Tavakolian S, Rahimi A, Oshvandi K, Soltanian A, et al. Effect of citalopram in combination with omega-3 on depression in post-menopausal women: a triple blind randomized controlled trial. Journal of Clinical and Diagnostic Research: JCDR 2016;10(10):QC01-QC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazereeuw 2016 {published and unpublished data}
- Lanctôt K, Hermann N. Treating depression in coronary artery disease with omega-3 fatty acids. ClinicalTrials.gov/show/NCT00981383 (first received 22 September 2009).
- Mazereeuw G, Herrmann N, Andreazza AC, Scola G, Ma DW, Oh PI, et al. Oxidative stress predicts depressive symptom changes with omega-3 fatty acid treatment in coronary artery disease patients. Brain, Behavior, and Immunity 2017;60:136-41. [DOI] [PubMed] [Google Scholar]
- Mazereeuw G, Herrmann N, Oh PI, Ma DW, Wang CT, Kiss A, et al. Omega-3 fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease: analyses from a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology 2016;36(5):436-44. [PMID: 10.1097/JCP.0000000000000565] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereeuw G. Omega-3 fatty acids and depressive symptoms in coronary artery disease. In: Dissertation Abstracts International: Section B: The Sciences and Engineering. Vol. 77. 2017:8-B(E).
Mischoulon (DHA) 2015 {published and unpublished data}
- Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead B, Fehling K, Martinson M, et al. Clinical predictors of response to omega-3 fatty acids in major depressive disorder [abstract]. In: Biological Psychiatry [abstracts of the 70th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP; 2015 May 14 - 16;Toronto, ON Canada]. 2015:147S.
- Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA, et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. Journal of Clinical Psychiatry 2015;76(1):54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D. Depression, inflammation, and omega-3 fatty acids. Neuropsychopharmacology 2015;Suppl 1:S46. [Google Scholar]
- Mischoulon D. Omega-3 fatty acids for treatment of major depression: differential effects of EPA and DHA, and associated biochemical and immune parameters. ClinicalTrials.gov/ct2/show/NCT00361374 (first received 08 August 2006).
- Rapaport MH, Mischoulon D. Omega-3 fatty acids for treatment of major depression: differential effects of EPA and DHA, and associated biochemical and immune parameters. ClinicalTrials.gov/ct2/show/NCT00517036 (first received 16 August 2007).
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Molecular Psychiatry 2016;21(1):71-9. [DOI] [PMC free article] [PubMed]
- Rapaport MH, Schettler P, Pace TW, Kinkead B, Nierenberg AA, Mischoulon D. Inflammation, depression and N-3 fatty acids: a case of personalized medicine abstract. In: Neuropsychopharmacology [abstracts of the 52nd Annual Meeting of the American College of Neuropsychopharmacology, ACNP; 2013 Dec 8 - 12; Hollywood, FL United States]. 2013:S353-S4.
- Rapaport MH. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder [abstract]. In: Biological Psychiatry [abstracts of the 70th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP; 2015 May 14 - 16; Toronto, ON Canada]. 2015:146S-7S.
Mischoulon (EPA) 2015 {published and unpublished data}
- Mischoulon, D. Depression, inflammation, and omega-3 fatty acids. Neuropsychopharmacology 2015;suppl 1:S46. [Google Scholar]
- Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead B, Fehling K, Martinson M, et al. Clinical predictors of response to omega-3 fatty acids in major depressive disorder [abstract]. In: Biological Psychiatry [abstracts of the 70th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP; 2015 May 14 - 16;Toronto, ON Canada]. 2015:147S.
- Mischoulon D, Nierenberg AA, Schettler PJ, Kinkead BL, Fehling K, Martinson MA, et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. Journal of Clinical Psychiatry 2015;76(1):54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D. Omega-3 fatty acids for treatment of major depression: differential effects of EPA and DHA, and associated biochemical and immune parameters. ClinicalTrials.gov/ct2/show/NCT00361374 (first received 08 August 2006).
- Rapaport MH, Mischoulon D. Omega-3 fatty acids for treatment of major depression: differential effects of EPA and DHA, and associated biochemical and immune parameters. ClinicalTrials.gov/ct2/show/NCT00517036 (first received 16 August 2007).
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Molecular Psychiatry 2016;21:71-9. [DOI] [PMC free article] [PubMed]
- Rapaport MH, Schettler P, Pace TW, Kinkead B, Nierenberg AA, Mischoulon D. Inflammation, depression and N-3 fatty acids: a case of personalized medicine abstract. In: Neuropsychopharmacology [abstracts of the 52nd Annual Meeting of the American College of Neuropsychopharmacology, ACNP; 2013 Dec 8 - 12; Hollywood, FL United States]. 2013:S353-S4.
- Rapaport MH. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder [abstract]. In: Biological Psychiatry [abstracts of the 70th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry, SOBP; 2015 May 14 - 16; Toronto, ON Canada]. 2015:146S-7S.
Mischoulon 2009 {published data only}
- Anonymous. Omega-3 fatty acid effective as monotherapy for MDD. Brown University Psychopharmacology Update 2009;20(12):3-4. [Google Scholar]
- Mischoulon D, Papakostas GI, Dording CM, Farabaugh AH, Sonawalla SB, Agoston AM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder [NCT00096798]. Journal of Clinical Psychiatry 2009;70(12):1636-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D. Ethyl eicosapentanoic acid (Ethyl-EPA) for treating major depression. ClinicalTrials.gov/show/NCT00096798 (first received 16 November 2004).
Nemets 2002 {published data only}
- Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. American Journal of Psychiatry 2002;159(3):477-9. [DOI] [PubMed] [Google Scholar]
- Nemets B, Stahl Z, Belmaker RH. Omega-3 fatty acid treatment of depressive breakthrough during unipolar maintenance. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):149. [Google Scholar]
- Stahl Z, Nemets B, Belmaker RH. Omega-3 fatty acid treatment of depressive breakthrough during unipolar maintenance. European Neuropsychopharmacology 2002;12(Suppl 3):S178. [Google Scholar]
Park 2015 {published and unpublished data}
- Park Y, Park Y-S, Kim SH, Oh DH, Park Y-C. Supplementation of n-3 polyunsaturated fatty acids for major depressive disorder: A randomized, double-blind, 12-week, placebo-controlled trial in Korea. Annals of Nutrition & Metabolism 2015;66(2-3):141-8. [DOI] [PubMed] [Google Scholar]
- Park Y. Omega-3 index as a risk factor for depression in Korean and effect of Omega-3 fatty acids on Korean patients with depression [KCT0001206]. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=4315 (Accessed 25th June 2015).
Peet (1g/d) 2002 {published and unpublished data}
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Archives of General Psychiatry 2002;59(10):913-9. [DOI] [PubMed] [Google Scholar]
Peet (2g/d) 2002 {published and unpublished data}
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs.. Archives of General Psychiatry 2002;59(10):913-9. [DOI] [PubMed] [Google Scholar]
Peet (4g/d) 2002 {published and unpublished data}
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs.. Archives of General Psychiatry 2002;59(10):913-9. [DOI] [PubMed] [Google Scholar]
Rondanelli 2010 {published data only}
- Rizzo AM, Corsetto PA, Montorfano G, Opizzi A, Faliva M, Giacosa A, et al. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutrition Journal 2012;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. Journal of the American College of Nutrition 2010;29(1):55-64. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, et al. Long chain omega 3 polyunsaturated fatty acids supplementation in the treatment of elderly depression: effects on depressive symptoms, on phospholipids fatty acids profile and on health-related quality of life. Journal of Nutrition, Health & Aging 2011;15(1):37-44. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Opizzi A, Faliva M, Mozzoni M, Antoniello N, Cazzola R, et al. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutritional Neuroscience 2012;15:46-54. [DOI] [PubMed] [Google Scholar]
Shinto 2016 {published and unpublished data}
- Shinto L, Marracci G, Mohr DC, Bumgarner L, Murchison C, Senders A, Bourdette D. Omega-3 fatty acids for depression in multiple sclerosis: a randomized pilot study. PLoS ONE 2016;11:e0147195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto L. Fish oil for the treatment of depression in patients with multiple sclerosis. ClinicalTrials.gov/show/NCT00122954 (first received 22 July 2005).
Silvers 2005 {published data only}
- Anonymous. Adjunctive fish oil for depression. Nurses' Drug Alert 2005;29(3):22-3. [Google Scholar]
- Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2005;72(3):211-8. [DOI] [PubMed] [Google Scholar]
Su 2003 {published data only}
- Su KP, Huang SY, Chiu CC, Shen WW. Corrigendum to "Omega-3 fatty acids in major depressive disorder A preliminary double-blind, placebo-controlled trial" [Eur Neuropsychopharmacol. 13 (2003) 267-71]. European Neuropsychopharmacology 2004;14(2):173. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. European Neuropsychopharmacology 2003;13(4):267-71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
NCT00963196 {published data only}
- NCT00963196. Study of supplementation of antidepressants with fish oil to improve time to clinical response (SADFAT). clinicaltrials.gov/ct2/show/NCT00963196 (first received 21 August 2009).
References to studies awaiting assessment
Bafghi 2011 {published data only}Irct201010054873N
- Irct201010054873N. Comparison of the effect of eicosapentaenoic acid and docosahexaenoic acid on the treatment of depression. Irct201010054873N.
EUCTR2006‐004949‐41‐IT {published data only}
- EUCTR2006-004949-41-IT. Randomized double-blind study to evaluate the adjuvant effect of polyunsaturated fatty acids omega-3 in therapy with SSRI paroxetine mesylate in unipolar mood depression and recurrent depression. www.ClinicalTrialsregister.eu/ctr-search/trial/2006-004949-41/IT (first received 11 September 2007).
Kwak 2013 {published data only}
- Kwak KP, Lee KH. A comparative study of addition therapy of choline alfoscerate and omega 3 fatty acid in older depressed patients with or without executive dysfunction. International Psychogeriatrics 2014;25:S75-192. [Google Scholar]
Lima 2006 {published data only}ISRCTN06123818
- Lima L. A randomised study on the antidepressive effect of fluoxetine and folic acid, as possible augmenter, and the SYNThesis of serotonin (5-HT) in lymphocytes prior and after treatment. isrctn.org/ISRCTN06123818 (Accessed 26th April 2021).
Murck 2002 {published data only}ISRCTN44366049
- ISRCTN44366049. A multicentre, double-blind, randomised, parallel group, placebo-controlled, dose ranging pilot study of ethyl eicosapentaenoate (EPA) as adjunct therapy in patients who remain depressed following treatment with standard antidepressant therapy. ISRCTN44366049.
Murck 2003 {published data only}ISRCTN63565713
- ISRCTN63565713. A multicentre, double-blind, randomised, parallel group, placebo-controlled trial of LAX-101 (ethyl eicosapentaenoate [EPA]) in patients with a new or recurrent episode of depression. ISRCTN63565713.
Murck 2004 {published data only}38354847
- ISRCTN38354847. A multicentre, double-blind, randomised, parallel group, placebo-controlled trial of LAX-101 (ethyl eicosapentaenoate [EPA]) as adjunct therapy in patients who remain depressed following treatment with standard antidepressant therapy. ISRCTN38354847.
- Murck H. A multicentre, double-blind, randomised, parallel group, placebo-controlled trial of Lax-101 (ethyl eicosapentaenoate) as adjunct therapy in patients who remain depressed following treatment with standard antidepressant therapy. National Research Register 2004.
Naqvi 2008 {published data only}
- Naqvi S. Treating adolescent depression with fish oils. ClinicalTrials.gov/show/NCT00658476 (first received 15 April 2008).
NCT00816322 {published data only}
- NCT00816322. The effect of fish oil in major depressive disorder. ClinicalTrials.gov/show/NCT00816322 (Accessed 28th April 2021).
Su 2005 {published data only}
- Su KP. The effect of fish oil in major depressive disorder. ClinicalTrials.gov/show/NCT01371383 (first received 10 June 2011).
References to ongoing studies
Amminger 2013 {published data only}
- Amminger GP, Rice S. Youth Depression Alleviation: a randomised controlled trial of omega-3 fatty acids (fish oil) for major depressive disorder in young people (YoDA-F). www.anzctr.org.au/ACTRN12613001352796.aspx (received 06 December 2013).
- Rice SM, Hickie IB, Yung AR, Mackinnon A, Berk M, Davey C, et al. Youth depression alleviation: the Fish Oil Youth Depression Study (YoDA-F): A randomized, double-blind, placebo-controlled treatment trial. Early Intervention in Psychiatry 2016;10:290-9. [DOI] [PubMed]
Belmaker 2007 {published data only}
- NCT00480207. Folic acid and omega -3 fatty acid supplementation in depressed older adults. NCT00480207 (first received 30 May 2007).
Chen 2017 {published data only}
- NCT03295708. Fish oil as adjunct treatment for major depressive disorder. NCT03295708 (first received 28 September 2017).
Chiu 2010 {published data only}
- NCT01235533. Fish oil supplementation in late-life depression. NCT01235533 (first received 05 November 2010).
Fang 2019 {published data only}
- NCT03899194. Study on the effect of PRKCB1 modulating inflammatory factors and the role for developing major depressive disorder. NCT03899194 (first received 02 April 2019).
Howe 2008 {published data only}
- Howe P. Fish oil treatment for depression in cardiovascular disease. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82950 (first received 29 September 2008).
Marriott 2016 {published data only}
- Marriott BP, Hibbeln JR, Killeen TK, Magruder KM, Holes-Lewis K, Tolliver BK, et al. Design and methods for the Better Resiliency Among Veterans and non-Veterans with Omega-3's (BRAVO) study: a double blind, placebo-controlled trial of omega-3 fatty acid supplementation among adult individuals at risk of suicide. Contemporary Clinical Trials 2016;47:325-3. [DOI] [PubMed] [Google Scholar]
- Marriott BP. The Better Resiliency Among Veterans With Omega-3's (BRAVO) Study. NCT01901887 (first received 17 July 2013).
- Tolliver B, Marriott B, Hibbeln J, Magruder K, Marzolf A, Hamner M, et al. Comparison of the implicit association test with established clinical rating scales in suicide risk assessment: Baseline data from the better resiliency among veterans and non-veterans with omega-3 s (BRAVO) study. Neuropsychopharmacology 2016;41(Supplement 1):S487-8. [Google Scholar]
Nakano 2014 {published data only}
- Nakano W. Augmentation of omega-3 fatty acid with antidepressants for major depressive disorder: a double-blind, randomized controlled trial. apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN-UMIN000013525 (Accessed 26th April 2021).
Parker 2006a {published data only}
- Parker GB. A study of omega-3 as an augmentor of antidepressant treatment for major depression. ClinicalTrials.gov/show/NCT00289484 (first received 09 February 2006).
Piperoglou 2014 {published data only}
- Piperoglou M. Adjunctive natural low dose docosahexaenoic acid (DHA) omega-3 in a 16 week random double-blind placebo controlled (RDBPC) cross-over withdrawal study in a group of chronic, psychiatric out-patients with anxiety and mood disorders. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365804 (first received 21 March 2014).
Rapaport 2015 {published data only}
- Lamon-Fava S, So J, Mischoulon D, Ziegler TR, Dunlop BW, Kinkead B, et al. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2021;164:102219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport M, Mischoulon D, Dunlop B, Nierenberg A, Kinkead B, Lamon-Fava S, et al. A molecular rationale for n-3 PUFA augmentation of antidepressant action. Neuropsychopharmacology 2019;44(Supplement):434. [Google Scholar]
- Rapaport MH. Omega-3 fatty acids for major depressive disorder with high inflammation: a personalized approach. NCT02553915 (first received 18 September 2015).
Sahoo 2016 {published data only}
- Sahoo JP, Singh J, Khurana H. Effect of omega-3 fatty acids versus 5-hydroxytryptophan as add on therapy to sertraline in controlling suicidal ideation in patients with depression: a comparative study. International Journal of Pharmacological Research 2016;6:152-6. [Google Scholar]
- Singh J, Sahoo JP, Khurana H, Singh P. Effect of omega-3 fatty acids vs 5-hydroxytryptophan as add on therapy in controlling suicidal ideation. Indian Journal of Physiology and Pharmacology 2015;Suppl 1:131. [Google Scholar]
Smith 2010 {published data only}
- Smith D. An 8 week randomised, double-blind, placebo controlled trial investigating the role of adjunctive bioactive lipids specifically; docosahexaenoic acid (DHA) versus eicosapentaenoic acid (EPA) in major depressive disorder - with a 6 week open label extension of DHA in patients aged 18 - 65 years. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000710022 (first received 20 August 2010).
Tabasi 2020 {published data only}Irct20200716048117N
- Irct20200716048117N. The effect of transcranial direct current stimulation (tDCS) and omega-3 supplementation on depression and weight. Irct20200716048117N.
Tanna 2020 {published data only}CTRI/2020/10/028383
- CTRI/2020/10/028383. Role of omega 3 fatty acid in etiopathogenesis of depression and trial of two drugs: flax seed oil and Ashwagnadharishta in its management. CTRI/2020/10/028383.
Yao 2005 {published data only}
- Yao JK. Coronary artery disease (CAD) risk in schizophrenia: effect of omega-3 fatty acid supplementation. ClinicalTrials.gov/show/NCT00167310 (first received 14 September 2005).
Yousef 2018 {published data only}
- NCT03732378. Role of omega-3 polyunsaturated fatty acid in the management of major depressive disorder. NCT03732378 (first received 06 November 2018).
Additional references
Abedi 2014
- Abedi P. The impact of omega-3 fatty acids on depression of menopausal women: a randomized double-blind clinical trial. In: Climacteric. Vol. 17. 2014:63.
Antypa 2012
- Antypa N, Smelt AH, Strengholt A, Van der Does AJ. Effects of omega-3 fatty acid supplementation on mood and emotional information processing in recovered depressed individuals. Journal of Psychopharmacology 2012;26:738–43. [DOI] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Appleton 2006
- Appleton KM, Hayward RC, Gunnell D, Peters TJ, Rogers PJ, Kessler, D, et al. Effects of n3 long chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. American Journal of Clinical Nutrition 2006;84(6):1308-16. [DOI] [PubMed] [Google Scholar]
Appleton 2007
- Appleton KM, Peters TJ, Hayward RC, Heatherley SV, McNaughton SA, Rogers PJ, et al. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Social Psychiatry and Psychiatric Epidemiology 2007;42(2):100-4. [DOI] [PubMed] [Google Scholar]
Appleton 2008a
- Appleton KM, Gunnell D, Peters TJ, Ness AR, Kessler D, Rogers PJ. No clear evidence of an associations between plasma concentrations of n-3 long chain polyunsaturated fatty acids and depressed mood in a non-clinical population. Prostaglandins, Leukotrienes and Essential Fatty Acids 2008;78(6):337-42. [DOI] [PubMed] [Google Scholar]
Appleton 2008b
- Appleton KM, Rogers PJ, Ness AR. Is there a role for n-3 long-chain polyunsaturated fatty acids in the regulation of mood and behaviour? A review of the evidence to date from epidemiological studies, clinical studies and intervention trials. Nutrition Research Reviews 2008;21(1):13-41. [DOI] [PubMed] [Google Scholar]
Appleton 2010
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. American Journal of Clinical Nutrition 2010;91(3):757-70. [DOI] [PubMed] [Google Scholar]
Arterburn 2006
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. American Journal of Clinical Nutrition 2006;83(6 suppl):1467S-76S. [DOI] [PubMed] [Google Scholar]
Bae 2018
- Bae JH, Kim G. Systematic review and meta-analysis of omega-3-fatty acids in elderly patients with depression. Nutrition Research 2018;50:1-9. [DOI] [PubMed] [Google Scholar]
Bai 2020
- Bai S, Guo W, Feng Y, Deng H, Li G, Nie H, et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. Journal of Neurology, Neurosurgery & Psychiatry 2020;91(1):21–32. [DOI] [PubMed] [Google Scholar]
Bastiaansen 2016
- Bastiaansen JA, Munafò MR, Appleton KM, Oldehinkel AJ. The efficacy of fish oil supplements in the treatment of depression: food for thought. Translational Psychiatry 2016;6(12):e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1987
- Beck AT, Steer RA. Beck Depression Inventory Manual. San Antonio, Texas: Psychological Corporation, 1987. [Google Scholar]
BNF 1999
- British Nutrition Foundation. BNF Briefing paper: n-3 fatty acids and health. London: British Nutrition Foundation, 1999. [Google Scholar]
Browne 2006
- Browne JC, Scott KM, Silvers KM. Fish consumption in pregnancy and omega-3 status after birth are not associated with postnatal depression. Journal of Affective Disorders 2006;90(2-3):131-9. [DOI] [PubMed] [Google Scholar]
Calder 2003
- Calder PC. n-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 2003;38(4):342-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caughey 1996
- Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1B production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. American Journal of Clinical Nutrition 1996;63:116-22. [DOI] [PubMed] [Google Scholar]
Chalon 2006
- Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2006;75(4-5):259-69. [DOI] [PubMed] [Google Scholar]
Chambergo‐Michilot 2021
- Chambergo-Michilot D, Branez-Condorena A, Falvy-Bockos I, Pacheco-Mendoza J, Benites-Zapata VA. Efficacy of omega-3 supplementation on sertraline continuous therapy to reduce depression or anxiety symptoms: a systematic review and meta-analysis. Psychiatry Research 2021;296:113652. [DOI] [PubMed] [Google Scholar]
Cho 1998
- Cho MJ, Kim MH. Use of the Center for Epidemiological Studies Depression (CES-D) scale in Korea. Journal of Nervous and Mental Disease 1998;186:304-10. [DOI] [PubMed] [Google Scholar]
Cipriani 2018
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391(10128):1357-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2021 [Computer program]
- Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org, 2021.
Deeks 2001
- Deeks JJ, Altman DG, Bradbrun MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Publishing Group, 2001:285-312. [Google Scholar]
De la Presa Owens 1999
- De la Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula fed piglets. Journal of Nutrition 1999;129(11):2088-93. [DOI] [PubMed] [Google Scholar]
Delion 1994
- Delion S, Chalon S, Hérault J, Guilloteau D, Besnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotonergic neurotransmission in rats. Journal of Nutrition 1994;124(12):2466-76. [DOI] [PubMed] [Google Scholar]
Delion 1996
- Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. Alpha-linolenic acid dietary deficiency alters age related changes in dopaminergic and serotonergic neurotransmission in the rat frontal cortex. Journal of Neurochemistry 1996;66(4):1582-91. [DOI] [PubMed] [Google Scholar]
Diguer 1993
- Diguer L, Barber J, Luborsky L. Three concommitants: personality disorders, psychiatric severity, and outcome of dynamic psychotherapy of major depression. American Journal of Psychiatry 1993;150(8):1246-8. [DOI] [PubMed] [Google Scholar]
Dupuy 1984
- Dupuy H. The psychological general well-being (PGWB) index. In: Wenger NK, Mattson ME, Furber CD, et al, editors(s). Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York, US: Le Jaq Publishing Inc, 1984:170-83. [Google Scholar]
Edwards 1998
- Edwards R, Peet M, Shay J, Horrobin D. Depletion of docosahexaenoic acid in red blood cell membranes of depressive patients. Biochemical Society Transactions 1998;26:s142. [DOI] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Davey Smith G. Principles of and procedures for systematic reviews. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Publishing Group, 2001:23-42. [Google Scholar]
Ehringer 1990
- Ehringer W, Belcher D, Wassall SR, Stillwell W. A comparison of the effects of linolenic acid (18:3 omega 3) and docosahexaenoic (22:6 omega 3) acids on phospholipid bilayers. Chemistry and Physics of Lipids 1990;54(2):79-88. [DOI] [PubMed] [Google Scholar]
Endicott 1993
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin 1993;29(2):321-6. [PubMed] [Google Scholar]
Firth 2019
- Firth J, Teasdale SB, Allott K, Siskind D, Marx W, Cotter J, et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019;18(3):308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Min-mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189-98. [DOI] [PubMed] [Google Scholar]
Fountoulakis 2013
- Fountoulakis KN, Veroniki AA, Siamouli M, Möller H-J. No role for initial severity on the efficacy of antidepressants: results of a multi-meta-analysis. Annals of General Psychiatry 2013;12(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frangou 2006
- Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. British Journal of Psychiatry 2006;188:46-50. [DOI] [PubMed] [Google Scholar]
Freeman 2008
- Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. Journal of Affective Disorders 2008;110(1-2):142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Gabbay 2018
- Gabbay V, Freed RD, Alonso CM, Senger S, Stadterman J, Davison BA, et al. A double-blind placebo-controlled trial of omega-3 fatty acids as a monotherapy for adolescent depression. Journal of Clinical Psychiatry 2018;79(4):17m11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 2007
- Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, et al. Lipids and essential fatty acids in patients presenting with self-harm. British Journal of Psychiatry 2007;190:112-7. [DOI] [PubMed] [Google Scholar]
GBD 2018
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1789-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giltay 2011
- Giltay EJ, Geleijnse JM, Kromhout D. Effects of n-3 fatty acids on depressivesymptoms and dispositional optimism after myocardial infarction. American Journal of Clinical Nutrition 2011;94(6):1442-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginty 2015
- Ginty AT, Conklin SM. Short-term supplementation of long-chain omega-3 polyunsaturated fatty acids reduces depression symptomology among young adults with depression: A preliminary randomized, double blind, and placebo controlled trial. Psychiatry Research 2015;229(1-2):485-9. [DOI] [PubMed] [Google Scholar]
Goldsmith 2016
- Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry 2016;21(12):1696-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregory 2000
- Gregory J, Foster K, Tyler H, Wiseman M. National Diet and Nutritional Survey of British Adults. London: Her Majesty's Stationary Office, 2000. [Google Scholar]
Grosso 2014
- Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PloS ONE 2014;9(5):e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology, revised. Rockville, MD: National Institute of Mental Health, 1976. [Google Scholar]
Haag 2003
- Haag M. Essential fatty acids and the brain. Canadian Journal of Psychiatry 2003;48(3):195-203. [DOI] [PubMed] [Google Scholar]
Hakkarainen 2004
- Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Is dietary intake of omega-3 fatty acids associated with depression? American Journal of Psychiatry 2004;161(3):567-9. [DOI] [PubMed] [Google Scholar]
Hallahan 2007
- Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double blind randomised controlled trial. British Journal of Psychiatry 2007;190:118-22. [DOI] [PubMed] [Google Scholar]
Hallahan 2016
- Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE, et al. Efficacy of omega-3 highly unsaturatedfatty acids in the treatment of depression. British Journal of Psychiatry 2016;209(3):192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haller 2019
- Haller H, Anheyer D, Cramer H, Dobos G. Complementary therapies for clinical depression: an overview of systematic reviews. BMJ Open 2019;9(8):e028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamazaki 2005
- Hamazaki K, Itomura M, Huan M, Nishizawa H. Effects of w-3 fatty acid-containing phospholipids on blood catecholamine concentrations in healthy volunteers: a randomized, placebo controlled, double blind trial. Nutrition 2005;21(6):705-10. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hibbeln 1998
- Hibbeln JR. Fish consumption and major depression. Lancet 1998;351(9110):1213. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hirashima 2004
- Hirashima F, Parow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, et al. Omega-3 fatty acid treatment and T2 whole brain relaxation times in bipolar disorder. American Journal of Psychiatry 2004;161(10):1922-4. [DOI] [PubMed] [Google Scholar]
Hoehn 1967
- Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology 1967;17(5):427-42. [DOI] [PubMed] [Google Scholar]
Husted 2016
- Husted KS, Bouzinova EV. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina 2016;52(3):139-47. [DOI] [PubMed] [Google Scholar]
Jacka 2017
- Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Medicine 2017;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2000
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. American Journal of Clinical Nutrition 2000;71(1 Suppl):343s-8s. [DOI] [PubMed] [Google Scholar]
Kalkman 2021
- Kalkman HO, Hersberger M, Walitza S, Berger GE. Disentangling the molecular mechanisms of the antidepressant activity of omega-3 polyunsaturated fatty acid: a comprehensive review of the literature. International Journal of Molecular Sciences 2021;22(9):4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keck 2006
- Keck PE Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biological Psychiatry 2006;60(9):1020-2. [DOI] [PubMed] [Google Scholar]
Keshavarz 2018
- Keshavarz SA, Mostafavi SA, Akhondzadeh S, Mohammadi MR, Hosseini S, Eshraghian MR, et al. Omega-3 supplementation effects on body weight and depression among dieter women with co-morbidity of depression and obesity compared with the placebo: a randomized clinical trial. Clinical Nutrition ESPEN 2018;25:37-43. [DOI] [PubMed] [Google Scholar]
Khajehnasiri 2015
- Khajehnasiri F, Akhondzadeh S, Mortazavi SB, Allameh A, Sotoudeh G, Khavanin A, et al. Are supplementation of omega-3 and ascorbic acid effective in reducing oxidative stress and depression among depressed shift workers? International Journal for Vitamin and Nutrition Research 2015;85(5-6):299-310. [DOI] [PubMed] [Google Scholar]
Kirsch 2008
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Medicine 2008;5(2):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirsch 2019
- Kirsch I, Ness AR, Appleton KM. Treatments for depression: Side effects, adverse events and health risks. Journal of Affective Disorders 2019;259:38-9. [DOI] [PubMed] [Google Scholar]
Köhler 2017
- Köhler CA, Freitas TH, Maes M, Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatrica Scandinavica 2017;135(5):373-87. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2019
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Translational Psychiatry 2019;9(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2007
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of anti-depressant efficacy of omega-3 fatty acids. Journal of Clinical Psychiatry 2007;68(7):1056-61. [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, et al. Are omega-3 fatty acids anti-depressants or just mood-improving agents? Molecular Psychiatry 2012;17(12):1161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 1995
- Ma J, Folsom AR, Eckfeldt JH, Lewis L, Chambless LE. Short- and long-term repeatability of fatty acid composition of human plasma phospholipids and cholesterol esters: The Atherosclerosis Risk in Communities (ARIC) Study Investigators. American Journal of Clinical Nutrition 1995;62(3):572-8. [DOI] [PubMed] [Google Scholar]
Mamalakis 2002
- Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids 2002;67(5):311-8. [DOI] [PubMed] [Google Scholar]
Mamalakis 2004
- Mamalakis G, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose polyunsaturated fatty acids in an adolescent group. Prostaglandins, Leukotriens and Essential Fatty Acids 2004;71(5):289-94. [DOI] [PubMed] [Google Scholar]
Mamalakis 2006
- Mamalakis G, Kiriakakis M, Tsibinos G, Hatzis C, Flouri S, Mantzoros C, et al. Depression and serum adiponectin and adipose omega-3 and omega-6 fatty acids in adolescents. Pharmacology, Biochemistry and Behavior 2006;85(2):474-9. [DOI] [PubMed] [Google Scholar]
Marangoni 1993
- Marangoni F, Angeli MT, Colli S, Eligini S, Tremoli E, Sirtori CR, et al. Changes of n-3 and n-6 fatty acids in plasma and circulating cells of normal subjects, after prolonged administration of 20:5 (EPA) and 22:6 (DHA) ethyl esters and prolonged washout. Biochimica et Biophysica Acta 1993;1210(1):55-62. [DOI] [PubMed] [Google Scholar]
Martins 2011
- Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 LC-PUFA supplementation in depression: evidence from an updated meta-analysis of randomized controlled trials. OCL - Oleagineux Corps Gras Lipides 2011;18:188-98. [DOI] [PubMed] [Google Scholar]
Mazaherioun 2018
- Mazaherioun M, Saedisomeolia A, Javanbakht MH, Koohdani F, Zarei M, Ansari S, et al. Long chain n-3 fatty acids improve depression syndrome in type 2 diabetes mellitus. Iranian Journal of Public Health 2018;47(4):575-83. [PMC free article] [PubMed] [Google Scholar]
McNamara 2006
- McNamara RK, Richtand NM, Levant B. Omega-3 fatty acid deficiency decreases dopamine D2 receptor binding and increases serotonin 5-HT2A receptor binding in the adult rat prefrontal cortex. Biological Psychiatry 2006;59:146S. [Google Scholar]
Miller 2016
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology 2016;16(1):22-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miyake 2006
- Miyake Y, Sasaki A, Yokoyama T, Tanaka K, Ohya Y, Fukushima W. Risk of postpartum depression in relation to dietary fish and fat intake in Japan: the Osaka Maternal and Child Health Study. Psychological Medicine 2006;36(12):1727-37. [DOI] [PubMed] [Google Scholar]
Mocking 2016
- Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhé HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Translational Psychiatry 2016;6(3):e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI] [PubMed] [Google Scholar]
Mozaffari‐Khosravi 2013
- Mozaffari-Khosravi H, Yassini-Ardakani M, Karamati M, Shariati-Bafghi SE. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. European Neuropsychopharmacology 2013;23(7):636-44. [DOI] [PubMed] [Google Scholar]
Nasir 2019
- Nasir M, Bloch MH. Trim the fat: the role of omega-3 fatty acids in psychopharmacology. Therapeutic Advances in Psychopharmacology 2019;9:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nemets 2006
- Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. American Journal of Psychiatry 2006;163(6):1098-100. [DOI] [PubMed] [Google Scholar]
NICE 2004
- National Institute for Clinical Excellence. Depression: Management of Depression in Primary and Secondary Care. London, England: National Institute for Clinical Excellence, 2004. [Google Scholar]
Noaghiul 2003
- Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. American Journal of Psychiatry 2003;160(12):2222-7. [DOI] [PubMed] [Google Scholar]
Opiyo 2019
- Opiyo RO, Nyasulu PS, Koigi RK, Obondo A, Ogoyi D, Kogi-Makau W. Effect of fish oil omega‑3 fatty acids on reduction of depressive symptoms among HIV‑seropositive pregnant women: a randomized, double‑blind controlled trial. Annals of General Psychiatry 2018;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2006b
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. American Journal of Psychiatry 2006;163(6):969-78. [DOI] [PubMed] [Google Scholar]
Parletta 2019
- Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutritional Neuroscience 2019;22(7):474-87. [DOI] [PubMed] [Google Scholar]
Peet 1998
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biological Psychiatry 1998;43(5):315-9. [DOI] [PubMed] [Google Scholar]
Peet 2004
- Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. British Journal of Psychiatry 2004;184:404-8. [DOI] [PubMed] [Google Scholar]
Raison 2013
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013;70(1):31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rallidis 2003
- Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis 2003;167(2):237-42. [DOI] [PubMed] [Google Scholar]
Rapaport 2016
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Molecular Psychiatry 2016;21(1):71-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravi 2016
- Ravi S, Khalili H, Abbasian L, Arbabi M, Ghaeli P. Effect of omega-3 fatty acids on depressive symptoms in HIV-positive individuals: a randomized, placebo-controlled clinical trial. Annals of Pharmacotherapy 2016;50(10):797-807. [DOI] [PubMed] [Google Scholar]
Rees 2008
- Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Australian & New Zealand Journal of Psychiatry 2008;42(3):199-205. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan). Version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Reynolds 1995
- Reynolds WM, Kobak KA. Hamilton Depression Inventory. In: Psychological Assessment Resources. Odessa, FL: Psychological Assessment Resources, 1995. [Google Scholar]
Rogers 2008
- Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomized controlled trial. British Journal of Nutrition 2008;99(2):421-31. [DOI] [PubMed] [Google Scholar]
Ross 2007
- Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids in Health and Disease 2007;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruxton 2005
- Ruxton CH, Calder PC, Reed SC, Simpson MJ. The impact of long chain n-3 polyunsaturated fatty acids on human health. Nutrition Research Reviews 2005;18(1):113-29. [DOI] [PubMed] [Google Scholar]
Ryff 1995
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. Journal of Personality and Social Psychology 1995;69(4):719-27. [DOI] [PubMed] [Google Scholar]
Sarris 2019
- Sarris J, Byrne GJ, Stough C, Bousman C, Mischoulon D, Murphy J, et al. Nutraceuticals for major depressive disorder - more is not merrier: an 8-week double-blind, randomised, controlled trial. Journal of Affective Disorders 2019;245:1007-15. [DOI] [PubMed] [Google Scholar]
Sawazaki 1999
- Sawazaki S, Hamazaki T, Yazawa K, Kobayashi M. The effect of docosahexaenoic acid on plasma catecholamine concentrations and glucose tolerance during long-lasting psychological stress: a double blind placebo controlled study. Journal of Nutritional Science and Vitaminology 1999;45(5):655-65. [DOI] [PubMed] [Google Scholar]
Schefft 2017
- Schefft C, Kilarski LL, Bschor T, Kohler S. Efficacy of adding nutritional supplements in unipolar depression: a systematic review and meta-analysis. European Neuropsychopharmacology 2017;27(11):1090-109. [DOI] [PubMed] [Google Scholar]
Serralde‐Zuñiga 2019
- Serralde-Zúñiga AE, Gonzalez Garay AG, Rodríguez-Carmona Y, Melendez G. Fluoxetine for adults who are overweight or obese. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD011688. [DOI: 10.1002/14651858.CD011688.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharifan 2017
- Sharifan P, Hosseini MS, Sharifan A. The interventional relationship between frequent fish consumption and depression symptoms in aging adults: a randomized controlled trial. International Journal of Geriatric Psychiatry 2017;32(12):e116-22. [DOI] [PubMed] [Google Scholar]
Silvers 2002
- Silvers KM, Scott KM. Fish consumption and self-reported physical and mental health status. Public Health Nutrition 2002;5(3):427-31. [DOI] [PubMed] [Google Scholar]
Simopolous 1999
- Simopoulos AP. Evoluntionary aspects of omega-3 fatty acids in the food supply. Prostaglandins, Leukotrienes and Essential Fatty Acids 1999;60(5-6):421-9. [DOI] [PubMed] [Google Scholar]
Sinn 2012
- Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. British Journal of Nutrition 2012;107(11):1682-93. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith MA, Beilin LJ, Mori TA, Oddy WH. Essential fatty acids and mood: a systematic review of observational studies. American Journal of Food and Nutrition 2011;1:14-27. [Google Scholar]
Stahl 2008
- Stahl LA, Begg DP, Weisinger RS, Sinclair AJ. The role of omega-3 fatty acids in mood disorders. Current Opinion in Investigational Drugs 2008;9(1):57-64. [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG, eds, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Publishing Group, 2001:189-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 1999
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega-3 fatty acids in bipolar disorder – a preliminary double-blind, placebo-controlled trial. Archives of General Psychiatry 1999;56(5):407-12. [DOI] [PubMed] [Google Scholar]
Su 2008
- Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2008;69(4):644-51. [DOI] [PubMed] [Google Scholar]
Su 2014
- Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biological Psychiatry 2014;76(7):559-66. [DOI] [PubMed] [Google Scholar]
Su 2016
- Su KP. Omega-3 fatty acids for inflammation-related depression. Biological Psychiatry 2016;Suppl 1:285S. [Google Scholar]
Su 2018
- Su K-P, Yang H-T, Chang JP-C, Shih Y-H, Guu T-W, Satyanarayanan SK, et al. Eicosapentaenoic and docosahexaenoic acids have different effects on peripheral phospholipase A2 gene expressions in acute depressed patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2018;80(Part C):227-33. [DOI] [PubMed] [Google Scholar]
Sublette 2011
- Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. Journal of Clinical Psychiatry Diseases of the Nervous System 2011;72(12):1577-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tajalizadekhoob 2011
- Tajalizadekhoob Y, Sharifi F, Fakhrzadeh H, Mirarefin M, Ghaderpanahi M, Badamchizade Z, et al. The effect of low-dose omega 3 fatty acids on the treatment of mild to moderate depression in the elderly: a double-blind, randomized, placebo-controlled study. European Archives of Psychiatry and Clinical Neuroscience 2011;261(8):539-49. [DOI] [PubMed] [Google Scholar]
Takeuchi 2002
- Takeuchi T, Fukumoto Y, Harada E. Influence of a dietary n-3 fatty acid deficiency on the cerebral catecholamine contents, EEG and learning ability in rat. Behavioral Brain Research 2002;131(1-2):193-203. [DOI] [PubMed] [Google Scholar]
Tanskanen 2001
- Tanskanen A, Hibblen JR, Hintikka J, Haatainen K, Honkalampi K, Viinamäki K. Fish consumption, depression, and suicidality in a general population. Archives of General Psychiatry 2001;58(5):512-3. [DOI] [PubMed] [Google Scholar]
Tappia 1997
- Tappia PS, Ladha S, Clark DC, Grimble RF. The influence of membrane fluidity, TNF receptor binding, cAMP production and GTPase activity on macrophage cytokine production in rats fed a variety of fat diets. Molecular and Cellular Biochemistry 1997;166(1-2):135-43. [DOI] [PubMed] [Google Scholar]
Tayama 2019
- Tayama J, Ogawa S, Nakaya N, Sone T, Hamaguchi T, Takeoka A, et al. Omega-3 polyunsaturated fatty acids and psychological intervention for workers with mild to moderate depression: a double-blind randomized controlled trial. Journal of Affective Disorders 2019;245:364-70. [DOI] [PubMed] [Google Scholar]
Taylor 2005
- Taylor T, Jefferis GS, Koop M, Hill BC, Hastie T, Heit G, et al. Quantitative measurements of alternating finger tapping in Parkinson's disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Movement Disorders 2005;20(10):1286-98. [DOI] [PubMed] [Google Scholar]
Trivedi 2004
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomology, clinician rating (IDS-C) and self report (IDS-SR), and the Quick Inventory of Depressive Symptomology, clinician rating (QIDS-C) and self-report (QIDS-SR) in public sector patients with mood disorders; a psychometric evaluation. Psychological Medical 2004;34(1):73-82. [DOI] [PubMed] [Google Scholar]
Tu 2020
- Tu CH, Chen CM, Yang CC, Galecki P, Su KP. Brain responses to emotional stimuli after eicosapentaenoic acid and docosahexaenoic acid treatments in major depressive disorder: toward personalized medicine with anti-inflammatory nutraceuticals. Journal of Personalized Medicine 2020;10(4):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2008
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008;358(3):252-60. [DOI] [PubMed] [Google Scholar]
Van de Rest 2008
- Van de Rest O, Geleijnse JM, Kok FJ, Van Staveren WA, Hoefnagels WH, Beekman AT, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. American Journal of Clinical Nutrition 2008;88(3):706-13. [DOI] [PubMed] [Google Scholar]
Volkmann 2020
- Volkmann C, Volkmann A, Muller CA. On the treatment effect heterogeneity of antidepressants in major depression: a Bayesian meta-analysis and simulation study. PLoS ONE 2020;15(11):e0241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston MA: The Health Institute, 1993. [Google Scholar]
Williams 2004
- Williams JW Jr, Stellato CP, Cornell J, Barrett JE. The 13- and 20-item Hopkins Symptom Checklist Depression Scale: psychometric properties in primary care patients with minor depression or dysthymia. International Journal of Psychiatry in Medicine 2004;34(1):37-50. [DOI] [PubMed] [Google Scholar]
Yang 2019
- Yang B, Lin L, Bazinet RP, Chien YC, Chang JP, Satyanarayanan SK, et al. Clinical efficacy and biological regulations of omega-3 PUFA-derived endocannabinoids in Major Depressive Disorder. Psychotherapy and Psychosomatics 2019;88(4):215-24. [DOI] [PubMed] [Google Scholar]
Yao 2004
- Yao JK, Magan S, Sonel AF, Gurklis JA, Sanders R, Reddy RD. Effects of omega-3 fatty acid on platelet serotonin responsivity in patients with schizophrenia. Prostaglandins, Leukotrienes and Essential Fatty Acids 2004;71(3):171-6. [DOI] [PubMed] [Google Scholar]
Yesvage 1983
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 1983;17(1):37-49. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Appleton 2014
- Appleton KM, Perry R, Sallis HM, Ness AR, Churchill R. Omega‐3 fatty acids for depression in adults. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD004692. [DOI: 10.1002/14651858.CD004692.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Appleton 2015
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega‐3 fatty acids for depression in adults. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD004692. [DOI: 10.1002/14651858.CD004692.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silvers 2009 (withdrawn)
- Silvers KM, Hackett ML, Scott KM. Omega 3 fatty acids for depression. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD004692. [DOI: 10.1002/14651858.CD004692.pub2] [DOI] [Google Scholar]


