PURPOSE OF THIS PRACTICAL GUIDE
One of the most exciting and useful advances in nuclear cardiology is the opportunity to measure myocardial blood flow (MBF) routinely as part of myocardial perfusion imaging (MPI) with positron emission tomography (PET). Incorporating MBF into the MPI evaluation improves diagnosis and assessment of extent and severity of epicardial coronary artery disease (CAD), reclassifies prognosis from assessment based solely on perfusion defects, confirms effectiveness of pharmacologic stress, and can identify coronary microvascular disease with or without epicardial CAD.1–3
PET measurement of MBF assesses the entire coronary circulation, including focal obstruction and diffuse disease of the epicardial coronary arteries, the functioning of the microvasculature, and the ability of the cell membrane to transport the radionuclide into the cell. MBF can be measured with the majority of dedicated PET or hybrid PET/CT scanners in use today. When using modern scanners, quantification of MBF adds no additional radiation or acquisition time. MBF measurement is currently recognized by the Centers of Medicare and Medicaid Services as a Category 1 add-on code for a PET MPI study. As such, quantification of MBF can and should be performed in appropriate patients as an adjunct to spatially relative MPI.3
At present, 82rubidium chloride (82Rb) and 13N- ammonia (13NH3) are the most commonly used radiotracers for PET MPI and are both FDA-approved. 15O- water is more frequently used in Europe and is not FDA- approved. 18F-flurpiridaz is investigational and under evaluation with a second phase III trial. 13NH3 and 15O- water require an on-site cyclotron due to their short halflives. 82Rb also has a short half-life and is generator produced. Measurement of MBF has been validated for 82Rb, 13NH3, and 15O-water.2 For this document, we have focused on PET quantification of MBF with 82Rb due to its wider and increasing use, particularly in centers where there may not be a sophisticated technical infrastructure.
There are two recommended references on PET MPI1 and principles of quantifying MBF.2 Interpreting physicians are encouraged to read and understand these. An important issue is the day-to-day implementation of MBF as a routine part of a PET MPI study. Busy clinical laboratories will need to develop technical infrastructure to set design protocols, optimize quality control, train technologists, educate interpreting physicians, and formulate clinical reports that incorporate clinically meaningful MBF measurements. The purpose of this ‘‘primer’’ is to simplify and demystify MBF quantification, with the objective that practitioners develop practical strategies for incorporating MBF measurement into daily clinical practice for care of their patients.
IMPORTANT POINTS
Measuring MBF with cardiac PET does not increase radiation or imaging time.
MBF can be measured with almost all PET scanners in use today, with proper attention to injected activity and acquisition protocols.
MBF measurement improves diagnostic and prognostic assessment, and thus may impact patient management.
Measurement and reporting of MBF can be performed with existing personnel.
PET MBF assesses the entirety of the coronary delivery system including the epicardial coronaries and the microvasculature.
CLINICAL VALUE OF QUANTIFYING MBF
Traditional MPI relies on regional relative differences in tracer uptake to diagnose flow-limiting CAD. This has proved to be a valuable technique for population-based risk stratification and triaging patients for coronary angiography and interventions. However, traditional MPI is intrinsically spatially relative and a myocardial region with apparently normal radiotracer uptake may be supplied by a coronary artery with a flow-limiting lesion. As such, MPI tends to underestimate the full extent of obstructive CAD4,5 and, in some cases, can appear normal despite prognostically important disease.4 Quantification of MBF offers usefulness over and above traditional spatially relative perfusion defect analysis in six distinctive areas:
Improved diagnosis of epicardial CAD6
Improved assessment of extent and severity of epicardial CAD7–9
Selection of patients for coronary interventions and/ or medical therapy17,18
Diagnosis of coronary microvascular disease, a common cause of cardiac-related symptoms.19 Coronary microvascular disease, with or without epicardial CAD, has prognostic and quality of life significance in both women and men, and is addressable by targeted therapies.
Confirmation of adequate pharmacologic stress in patients who may not respond to pharmacologic stressors and go totally unrecognized with traditional MPI, with the risk of an apparently normal scan in the presence of severe coronary disease.2 The only way to be certain that vasodilation and hence augmentation of blood flow has occurred is by measuring MBF.
The ASNC and SNMMI have prepared and endorsed this document because these benefits can be critically important to the comprehensive assessment of patients being referred for pharmacologic MPI.
IMPORTANT POINTS
Traditional SPECT or PET MPI detects obstructive CAD but potentially under-assesses CAD extent and severity.
MBF measurement improves assessment of epicardial CAD.
MBF improves stratification of risk for major adverse cardiac events.
MBF measurement improves selection of patients for coronary interventionsand/or medical therapy alone.
MBF measurement can identify coronary microvascular disease.
MBF measurement provides assurance that vasodilator stress has been effective.
PRACTICAL TIPS FOR ENSURING CONSISTENT MBF MEASUREMENTS
MBF is dynamic, responding to changes in heart rate and systolic blood pressure (double product), the two main determinants of myocardial work. As such, there will be variability in a patient’s MBF from a physiologic perspective. Another source of measurement variability that can be controlled is adherence to patient preparation and imaging protocols. The following help to minimize procedural sources of variability.
Utilize a free-flowing intravenous line for both stressor and tracer. Optimally, this will be a large (#18 or #20-gauge) needle in a forearm vein. Hand injection should be avoided.
Degree of hyperemia varies in relation to time after stressor injection.20 Time to maximal hyperemia is not well defined for adenosine, dipyridamole, or regadenoson. Regadenoson is the stressor most often used in the United States. Peak hyperemia likely occurs about one or two minutes following its injection,20 so a concomitant time delay between its injection and beginning the tracer infusion would seem prudent.
Be consistent in the time duration between stressor injection/infusion and the tracer injection/infusion. A stopwatch is helpful for this purpose.20
Utilize the same pharmacologic stressor as much as possible for MBF measurements. The degree of hyperemia is known to vary between different stressors.20
IMPORTANT POINTS
Ensure good free-flowing forearm IV.
Timing is critical. Be consistent in the time interval between stressor injection/infusion and the start of the tracer infusion.
Try to use the same pharmacologic stressor.
TECHNICAL REQUISITES FOR ACCURATE MBF QUANTIFICATION
While quantification of MBF is relatively simple with currently available software programs, the programs depend on certain assumptions that are critical to accurate measurements. Practices must ensure that these essential requisites are met before reporting MBF measurements, regardless of the tracer employed, imaging equipment used, or the analysis software. These requirements are enumerated below, and some of the technical terminologies addressed are defined in the Definitions Section above.
Data acquisition needs to begin prior to the tracer arriving at the heart. For 82Rb, the counts in the first frame must be zero. In the case of 13NH3 or 18F- flurpiridaz, counts that remain from an earlier acquisition must be subtracted as part of the quantification.
For modeling the perfusion tracer uptake, a dynamic scan acquisition must start prior to the injection of the tracer and continue throughout the study to observe the kinetic transport of the tracer from the blood pool to the myocardium.
For retention methods, the blood pool tracer concentration integrated over time and a delayed image is used to model MBF. The blood pool tracer concentration for the retention model can be acquired either dynamically or as a single static frame. For those programs that employ a single static frame, acquisition steps (start time, dose timing, decay correction, and generator setting) must be consistent because post acquisition quality control may be limited.
Proper and consistent location of the blood pool region of interest (ROI) must be confirmed (left atrium or left ventricular cavity) and should not be touching adjacent structures. Likewise, the placement of the myocardial ROI must be checked for accuracy.These placements may differ for different software programs.
The dynamic sequence of images should be free of patient body motion so that the blood pool ROI remains centered in the left atrial or left ventricular cavity for the entire scan duration. Any motion should be manually or automatically corrected with software algorithms prior to MBF quantification. This is particularly important for compartment model approaches.
Detector saturation must be avoided during the first pass of the tracer by limiting the injected activity concentration, according to scanner count-rate abilities. These artifacts can range from a loss of quantitative accuracy to complete saturation of the detector, preventing any counts from being recorded. Any under-estimation of the blood input curve will result in over-estimation of MBF.21 Detector saturation is considerably more common in 3D acquisition mode than 2D. Some scanner models will routinely saturate during the dynamic scan acquisition using standard radiotracer dose administration rates and acquiring in 3D. Dose adjustments may be required. In these systems, 2D acquisition of the dynamic scan data may be preferred. Lower injected doses may be adopted for the 3D PET scanners. Failure to follow vendor recommended administration settings can damage the 82Rb generator elution column leading to strontium breakthrough and unintended radiation exposure. Constant activity infusion protocols may help maintain image quality while reducing saturation.
The following are requirements for high-quality images:
The myocardial ROIs need to accurately segment the myocardium and exclude adjacent non-cardiac structures that may be taking up tracer.
Emission and transmission (i.e., radionuclide source or CT) images must be aligned in X-Y-Z dimensions automatically and/or manually as needed for accurate attenuation correction. The CT image needs to be assessed for image artifacts due to metal objects and motion while line source images need to be assessedfor motion.22
Activity in surrounding structures (liver, lung, bowel, stomach) needs to be assessed and confirmed to be absent of spillover into the myocardial ROI.
There needs to be adequate counts to ensure a good signal-to-noise ratio.
IMPORTANT POINTS
Acquisition and processing technologies (if different) must have available the quality control tools to be sure that the above essential requisites have been met. If not met, the interpreting physician should be alerted before the patient has been dismissed, in case that part or the entire study needs to be repeated.
Interpreting physicians must have the quality control tools available to be certain that the essentials above have been met before including MBF results into the study interpretation.
KNOW YOUR SOFTWARE (RETENTION AND COMPARTMENT MODELS)
Compartment Model Approach
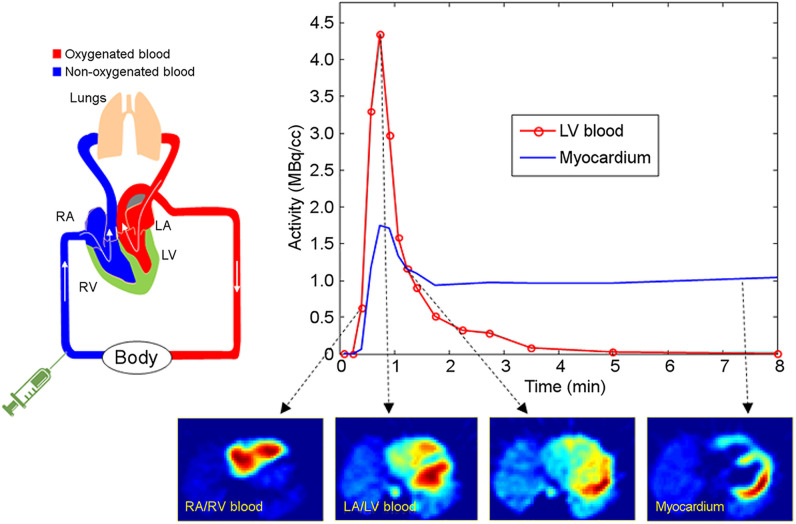
The compartment model approach for calculating MBF requires a series of dynamic images, shown in Figure 1, that record the tracer kinetics from the blood into the myocardium then washout back into the blood. This approach uses a non-linear fit of the model to the acquired data to solve for the regional MBF, tracer washout, and partial volume artifacts. Regional partial- volume correction from variable wall thickness or wall motion can be included but may be sensitive to patient body motion.
Figure 1.
Dynamic PET imaging starts with intravenous injection (Time = 0) and follows the tracer distribution first through the right heart cavities (RA/RV blood pool), then the lungs and into the left heart (LA/LV blood pool), and gradually extracted from the blood pool and retained in the heart tissue (myocardium).
Retention Model Approach
The retention model approach for MBF assumes that tracer retention can be determined by the blood pool concentration of tracer and the irreversible extraction of the tracer from the blood pool into the myocardium. The amount of radiotracer retained in the myocardium is corrected for or normalized to the amount of radiotracer delivered to the myocardium via arterial blood (‘‘the arterial input function’’) and thus the integral of the arterial radiotracer concentration (assumed to be derived from the initial two-minute images). The observed retention of radiotracer in the myocardium is then corrected for the flow-dependent or Kj-specific extraction fraction. By assuming the tracer does not significantly washout from the myocardium, the blood flow can be determined based on the tracer uptake. This model does not consider tracer washout and assumes a partial volume correction factor. This shortcoming can be somewhat mitigated by using a short dynamic scan acquisition time for the acquiring MBF. Patient body motion can be difficult to evaluate and correct depending on the number of blood pool and/or uptake images acquired. If the myocardial ROI matches the uptake frame and the blood pool ROI stays within the visible blood pool region, patient motion should have a minimal effect on quantitative MBF values.
Absolute MBF estimates will differ depending on the kinetic models (especially net retention and dynamic models) and software employed; this variability will be less for MBFR than for peak stress or rest absolute measurements.
IMPORTANT POINTS
Net retention software programs work on a wide range of instrumentation from dedicated PET frame mode to modern list-mode acquisition systems.
Compartment models are suited to newer generation list-mode systems.
Both models are widely used in clinical practice.
A STEP-BY-STEP APPROACH TO PET MBF QUALITY CONTROL FOR INTERPRETING PHYSICIANS
There are many FDA-approved software products for quantifying MBF with PET. Each product is based on a specific tracer kinetic model and strategies for making the necessary measurements to calculate MBF. If the assumptions used by the software are violated, the model will not produce reliable results. Therefore, it is important to understand the software program, and to acquire and process data according to its specifications.
Interpreting physicians should never accept a MBF value without review of simple quality control metrics. The quality control evaluation is an essential first step in the decision whether to report the MBF measurement. This evaluation does require the interpreting physician have appropriate software tools, knows how to use them, and understands them in relation to the software program being used.
Regardless of the model employed or the specific software program being used, the interpreting physician needs to be able to review the co-registration of emission and transmission scans, review the placement of the blood pool and myocardial regions of interest, and review both rest and stress blood pool and myocardial time-activity curves. If the quality review fails, an attempt at correction can be considered; if correction is not feasible, then the values should not be reported. The MBF measurements should generally be consistent with other study results; if they are highly discordant there needs to be even greater rigor in quality assessment before reporting.
Correct Registration of Emission and Attenuation Correction Scans
The alignment of the PET rest and stress emission images and the transmission attenuation correction images need to be assessed in the transaxial, coronal, and sagittal views, preferably using a fused display. Misalignment may be due to patient and/or respiratory motion and result in image artifacts on the relative perfusion images and corresponding regional decreases in MBF. Any required correction of misalignment must be performed on the PET scanner console with repeat reconstruction after proper alignment.
Placement of Blood and Myocardial Regions of Interest (ROIs)
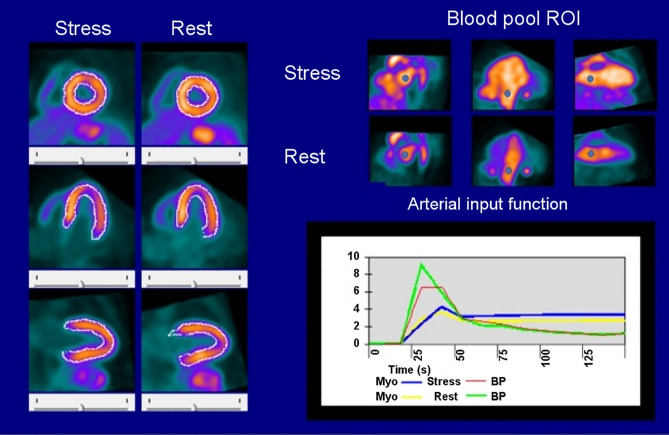
The placement of the ROIs for blood pool sampling (left atrium or left ventricular cavity) needs to be reviewed. The blood pool ROIs should be in the same location for rest and stress and should not touch the walls of either the left atrium or the left ventricle to avoid spillover (Figure 2).
Figure 2.
Quality assurance schematic for PET MBF using a dynamic net retention model.
The myocardial ROIs also must accurately encompass the myocardium during all frames that will be used for determining the tracer uptake. This ROI needs to be inspected to ensure that it is accurately tracing the myocardium and excludes adjacent non-cardiac structures that may contain tracer. Ideally, the software program will support manual adjustment of boundaries if needed (Figure 2).
Time-Activity Curves
The process for inspecting the time-activity curves is different for net retention and compartment models, however, they do share some common quality requirements. Specifically, they are as follows:
The time-activity curve must begin prior to the infusion or injection of the tracer.
The time-activity curve must demonstrate an initial increase in blood pool counts followed by a precipitous drop in counts because of tracer uptake.
The myocardial count time-activity curve must demonstrate a steady uptake of tracer followed by a plateau of counts. For longer acquisitions, this plateau could gently increase or decrease depending on tracer kinetics. However, there should not be any abrupt changes in myocardial counts during the time-activity curve.
Elevated Rest MBF due to High Rate- Pressure Product (RPP) and Effect on MBFR Calculation
PET MBF at rest ranges from 0.4 to 1.2 mL/min/g and in general varies with myocardial workload with higher MBF in patients with increased heart rate or blood pressure.2 Myocardial blood flow reserve (MBFR) is the ratio of stress MBF divided by rest MBF. If heart rate or blood pressure is significantly increased (e.g., due to holding of medications on day of study), the MBFR ratio may appear abnormally low because the resting flow is unusually elevated thus increasing the denominator in the flow ratio. In such cases, one of two approaches can be used. The rest MBF can be ‘‘adjusted’’ using the rate-pressure product (RPP) and a reference value such as 9000. A common way of adjusting is to divide the resting MBF by the RPP and multiplying the result by the reference value. An alternate approach is to explain in the report that MBFR is artifactually low because of high resting MBF, and default to the peak MBF.2 Either approach is reasonable for clinical reporting.
Normal values for peak hyperemia MBF (stress) vary to some degree within the different software programs, but generally should be > 1.7 ml/min/gm. There has been considerable interest in the interplay between MBFR, rest MBF, and peak stress MBF. In the presence of an elevated rest MBF out of proportion to RPP, and with no apparent technical explanation, the peak stress MBF may be an important indicator of normal versus abnormal flows.16 By combining the interpretation of stress MBF with MBFR, i.e., normal stress MBF and low MBFR due to high rest MBF may still be interpreted as normal. Very high resting MBFs out of proportion to the RPP should raise suspicion of detector saturation.
In the absence of significant anatomic abnormalities such as critical aortic stenosis or asymmetric hypertrophy, normal-appearing rest images should not show large variances in absolute regional flow. Misregistration of CT maps is the most likely cause of large regional flow differences in normal-appearing rest images. An attempt at re-registering the PET and CT data is possible provided that the raw image data are still available. Although not typically stored permanently, it is important to retain the raw data until completion of the flow calculations in order to make sure that corrections for PET and CT misalignments are still possible.
Software-Dependent Considerations
Net Retention Model: Placement of ROIs and Time-Activity Curves In the net retention model, the myocardial ROI is generally determined from a single myocardial scan (usually the last frame). This ROI needs to be inspected to ensure that it is accurately tracing the myocardium and excludes adjacent non-cardiac structures that may contain tracer. Ideally, the software program will support manual adjustment of boundaries if needed. It is important to remember that the net retention model does not solve for the partial volume correction. It is an external parameter determined by the scanner resolution and reconstruction parameters. Because of this, it must be adjusted whenever different scanners or reconstruction filters are used.
The net retention model relies on measuring the integrated concentration of the tracer in the blood pool up until the acquisition time of the myocardial uptake frame. The integration can be made by simply acquiring two images: one blood pool and one myocardial uptake. The limitation of this approach is that it is difficult to quality check. Changes in bolus shape, infusion start time, or excessive patient motion, could alter MBF values. Another approach is to measure a dynamic blood pool concentration. This has the advantage that each dynamic frame can be decay corrected to the uptake time. It is also less susceptible to changes in the shape of the input bolus. Blood pool curves for the net retention model should demonstrate the following:
Near zero counts in the first dynamic frame of the 82Rb acquisition
A strong peak of activity between 25 and 75 seconds after start of infusion
Good blood pool clearance prior to the myocardial uptake frame. The myocardium should be clearly defined and brighter than the blood pool during the final uptake frame of the dynamic series.
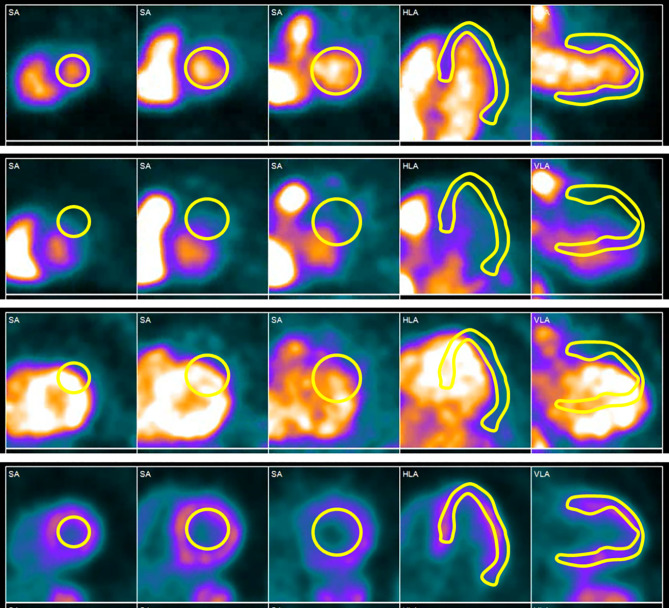
Compartment Model: Placement of ROIs and Time-Activity Curves In the compartment model, the myocardial ROI needs to be reviewed for correct placement and avoidance of adjacent structures in each frame of the acquisition (Figure 3). If there is motion between the frames, this should be corrected. Motion in the blood pool phase is particularly important to correct. Post-reconstruction alignment can improve accuracy, but AC misalignment artifacts may remain and any study with patient motion should be interpreted with caution.
Figure 3.
Patient motion during the uptake phase of a 82Rb stress MPI scan. The temporal sequence is from top to bottom. At the beginning of the acquisition, the blood pool and myocardial ROIs are in correct place, but as the scan progresses, the ROIs are no longer positioned over their respective regions. To obtain accurate blood flow measurements, this motion during the dynamic phase must be corrected.
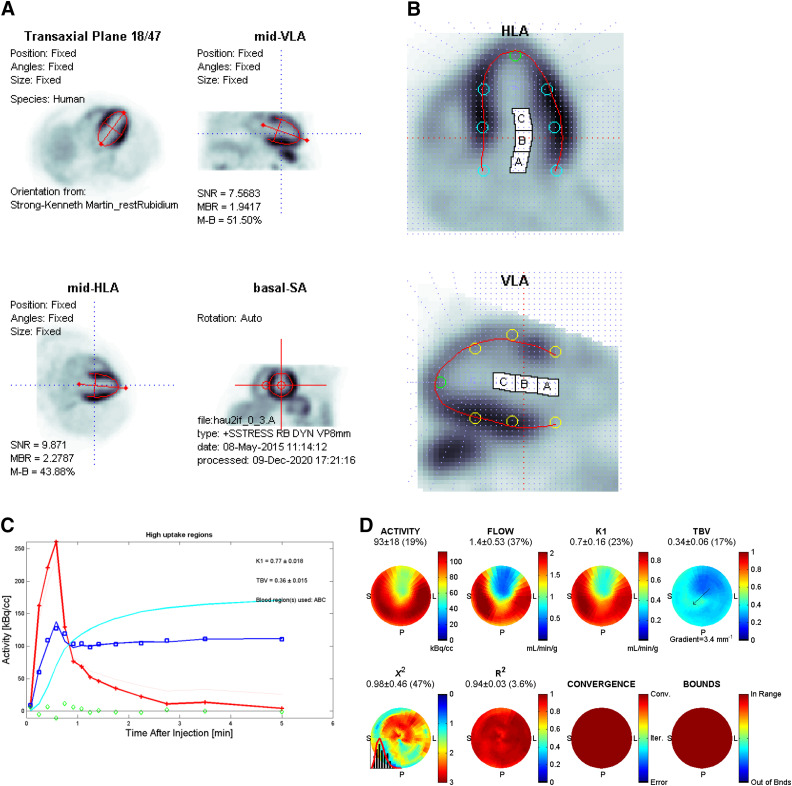
With a compartment model, the dynamic time- activity curves show the blood pool and myocardial activities, the model fit to the measured myocardial activity, as well as blood pool spillover and the partial- volume-corrected myocardial curve (Figure 4).
Figure 4.
Quality assurance data for 82Rb stress PET MBF analysis using a one-tissue compartment model. (A) Orientation of left ventricular long axis to center of short axis, (B) LV tissue contours (red) centered on the myocardium in normal and abnormal regions, and the LV blood pool region (white cavity, base, and atrium) not overlapping the myocardium, (C) Dynamic time-activity curves for arterial blood pool input (red), myocardial tissue (blue squares), spillover and partial-volume-corrected myocardium (cyan line), kinetic model curve (blue solid line) fitting through the myocardial tissue points, with residuals (green diamonds) randomly scattered around zero, and (D) Regional polar maps showing relative uptake (ACTIVITY), MBF (FLOW), Uptake rate constant (K1), total blood volume fraction (TBV), and goodness-of-fit parameters including the normalized v2 (ideally < 3), R2 (ideally close to 1), Convergence (Conv) of the model fitting within the iterations (Iter) limit or other Errors, and whether the fitted parameters are within the expected Bounds (upper and lower limits).
The blood pool and myocardial activity curves should start at zero (at least one background frame prior to radiotracer infusion) and have single peaks (multiple peaks suggest patient motion, radiotracer infusion issues, or detector saturation).
The rest and stress blood pool curves should have similar shapes and peaks, assuming similar injected radiotracer doses, although stress peaks are somewhat lower due to increased heart rate and/or stroke volume resulting in faster clearance of tracer from the blood pool.
The uptake and MBF maps should have similar regional distributions. If available, the goodness-of-fit parameter polar maps should be homogeneous with high values for the R2 map (correlation of the fitted model to the raw data) and low values for the v2 map (random distribution of noise).
Blood pool spillover polar maps used for partial- volume correction should be carefully examined. Asymmetric spillover distribution may suggest patient motion, particularly during the blood pool phase or improper contours for the myocardium.
IMPORTANT POINTS
When interpreting MBF measurements, physicians need to use quality control tools to ensure measurement integrity:
Correct registration of emission and transmission scans.
Correct placement of blood pool and myocardial ROIs. These placements are dependent on the software and kinetic model being used.
Time-activity curves for the software program should be reviewed to ensure they captured the necessary data and to evaluate the placement of the blood pool and myocardial ROIs.
Review of the time-activity curves should establish the entire tracer input was captured during the dynamic acquisition and the placement of the ROIs are correct throughout the entire dynamic acquisition.
INTERPRETING AND REPORTING MYOCARDIAL BLOOD FLOW
The incremental value of PET stress MBF and MBFR for the diagnosis of epicardial CAD and determining prognosis compared to relative MPI has been recently reviewed.2 Diagnostic sensitivity is improved but specificity may be decreased for CAD due to impairment of stress MBF and MBFR with microvascular disease and diffuse atherosclerosis, thereby identifying a previously unrecognized disease process. However, the negative predictive value for high-risk CAD with a normal MBFR > 2 is high. Similarly, a normal MBFR > 2 is associated with an excellent prognosis (Table 1).
Table 1.
General interpretation and classification of risk in relation to global MBFR
| MBFR | Interpretation | Relative risk |
|---|---|---|
| >2 | Normal | Low |
| 1.7-2 | Mildly abnormal | Intermediate |
| 1.2—< 1.7 | Abnormal | High |
| <1.2 with a perfusion defect | Highly abnormal | Very high |
| <1.2 without a perfusion defect | Consider non-diagnostic study | Indeterminate |
Cutoffs are generally arbitrary and may vary slightly between labs, software used, stressors used, and published studies. The principle is that the lower the flow reserve the greater the relative risk
The approach to interpretation of MBF should be additive to the cardiac PET perfusion and functionaldata and should await evaluation after those two components are assessed. As mentioned in the previous sections, before interpreting the MBF data, the reader should be assured that the quality control is adequate. We recommend evaluation of the rest and stress MBF and MBFR data, globally, for each vascular territory, and segmentally within each vascular territory. It should be noted that the prognostic data in the literature are primarily based on global MBFR and small variations in each vascular territory do exist. In certain circumstances in which there is a small reversible defect confined to one vascular territory, the MBFR for the entire vascular region may be in the normal range (for example the LAD). Interrogation of the individual segments in the vascular distribution will provide more specific information about vessel branches (Figure 5). However, precision of MBF measurement decreases with smaller segments due to more statistical noise. Care must be taken to avoid over-interpretation of small reductions in MBF in small regions.
The MBF and MBFR information should be interpreted in a clinical context as the presence or absence of CAD changes the approach to the data. MBFR has value in both patients with known CAD as well as those with suspected CAD (Table 2). The case examples that follow will illustrate many of the key values of MBF assessment in conjunction with myocardial perfusion data.
Table 2.
Clinical value of myocardial blood flow reserve
| No known CAD |
| High negative predictive value in combination with normal perfusion |
| Confirm an abnormality is CAD |
| Predict more severe disease: e.g., 1-vessel abnormal perfusion, 2-3-vessel abnormal MBFR |
| Confirm single vessel disease: 1 vessel abnormal perfusion, 1 vessel abnormal MBFR Normal perfusion, abnormal MBFR: identify balanced CAD, microvascular disease |
| Identify non-responder: all patients |
| Known CAD |
| Often abnormal after CABG, CAD history, myocardial infarction |
| Cardiomyopathy less useful but if normal, helps exclude CAD |
| Renal failure patients generally abnormal |
| Post PCI may be abnormal, but most useful if pre-PCI data available |
| Identify non-responder: all patients |
The reporting of PET studies with MBF must address the question of the referring physician and the clinical context such as possible ischemia in a patient with chest pain or CAC, hemodynamic significance ofknown CAD lesion, possible transplant vasculopathy, or possible microvascular disease. A list of useful sentences for reporting PET MBF studies is found in Table 3.
Table 3.
Examples of useful sentences for reporting PET MBFR
| In the Description |
| There was a rise in MBF between rest and stress |
| There was no rise in MBF between rest and stress |
| Global MBFR was (provide number) |
| In the Conclusion |
| Normal MBFR confirms study normalcy, which indicates lower risk of CAD beyond normal perfusion and predicts a low risk for major coronary-related events |
| Despite normal myocardial perfusion, MBFR is abnormal, placing the patient in a higher risk category for CAD and cardiac- related events in patients with no known CAD |
| There is a perfusion defect in a single coronary territory along with corresponding regional reduction in MBFR. Normal MBFR within the remainder of the myocardium makes more extensive CAD unlikely |
| While the perfusion indicates single vessel disease, MBFR is globally reduced, raising concern for more extensive CAD |
| The absence of a rise in MBF with normal perfusion does not exclude CAD |
| MBFR is not reported in this patient due to technical or patient-specific concerns that can affect accuracy and inappropriate clinical decisions |
A rest/stress PET MPI scan provides traditional relative perfusion images, rest global function, rest regional wall motion and thickening, peak stress global function, peak stress regional wall motion and thickening, transient ischemic dilation (TID) ratio, and CAC (in case of hybrid cameras or when a cardiac-appropriate CT scanner is available). In the current era, rest global and regional MBF, peak stress global and regional MBF, and global and regional MBFR measurements are also routinely available and should be considered for inclusion in reports. Referring physicians will not initially know how to incorporate MBF measurements into management decisions; therefore, the wording needs to assist with how the MBF measurements affect overall study results with respect to diagnosis and risk. It may be advisable early on to limit reporting of MBFs to global and regional MBFR. Table 1 provides someguidance on how to regard different global MBFR measurements in relation to perfusion appearances.
MBF measurements are derived on a pixel-by-pixel basis, averaged into segments, further averaged into presumed coronary distributions, and finally averaged for the entire myocardium (global). Practically, emphasis is placed on the global MBFR because that is the parameter associated most closely with prognosis. However, there is also important information provided by the MBFR measurements in relation to coronary distributions. In addition, it can be useful to look at the segmental scores as shown in Figure 5. Sometimes, side-branch territories can have very low MBFR, ‘‘hidden’’ by normal flows in the major vessel distribution. Measurement of MBF may result in differing results depending on the software, radiotracer, and protocols used for imaging and analysis. Serial patient MBF measurements are best done using the identical approach for imaging and analysis.
Figure 5.
Review segmental measurements of MBF, as sometimes the global and coronary territory scores can be normal, despite abnormal values in side-branch territories. Note reduced flows in the distal territory of the LCx coronary artery, despite the global LV and LCx MBFR 2x resting flows.
IMPORTANT POINTS
Global MBFR >2 has been shown in numerous studies to correlate with an excellent prognosis.
Low MBFR in the setting of no known CAD will usually require further testing such as invasive coronary angiography or CTA to rule out epicardial CAD. However, there will not be a 1:1 correlation of low MBFR and epicardial CAD, as some patients will have microvascular disease alone or in combination with mild-moderate epicardial CAD.
The lower the MBFR the greater the likelihood of multivessel obstructive CAD,9 the worse the prognosis and the greater the likelihood of benefit from revascularization.18
Because MBF measurements may be unfamiliar to referring physicians, the way in which they are reported needs to be sufficiently instructive that the measurement has clinical meaning.
The interpreting physician should recognize that MBFR calculations maybe less helpful in patients with prior CABG, prior large infractions, and end- stage renal disease. In addition, the calculation will be invalid in the presence of severe mitral or aortic regurgitation.
A COMMON DISCORDANCE BETWEEN PERFUSION AND MBF
Perhaps the most challenging result from the standpoint of an interpretative report is the situation where the images appear visually and semi-quantitatively normal but MBFR is low or very low. First, the interpreter needs to be certain that there are no technical or patient-specific explanations that would either erode confidence in the accuracy of MBFR or suggest that reporting of flow data would simply confuse decisionmaking for the referring clinician.
Absent the above considerations, this result can be reflective of any of 4 pathophysiologies: multivessel CAD, coronary microvascular disease, a combination of moderate diffuse epicardial CAD and coronary microvascular disease, or a circulating pharmacologic stress inhibitor (most commonly caffeine-containing substances). The interpreting physician should first consider the presence of an inhibitor. A repeat conversation with the patient will be helpful to reduce likelihood of this possibility. If there remains uncertainty, the test could be repeated the same day with dobutamine or on another day after at least 24 hours of no caffeine or other inhibitors.
A CACS can be pivotal in deciding next best steps. In the presence of high CAC, the next recommendation would be invasive coronary angiography to differentiate epicardial CAD from microvascular disease. When the CAC is low, CTA or no further testing may be recommended.
In some cases, the finding of non-diseased coronary arteries by either invasive coronary angiography or CTA may be sufficient to initiate empiric treatment for microvascular disease. If empiric treatment fails to improve symptoms, invasive studies may be helpful to elucidate the cause of the microvascular disease, so that more disease-focused therapies can be selected.
CASE EXAMPLES
Measurement of MBF will only be helpful clinically if incorporated into the report in a way that is sufficiently directive that the referring physician understands how this extra data affects diagnosis, prognosis, and decision-making. This added information has been shown to be helpful in more specific scenarios than can be fully covered in this ‘‘Practical Guide.’’ However, in daily practice, there are some commonly encountered clinical scenarios where reporting of MBFR will be helpful beyond perfusion defect evaluation. It is not difficult to educate referring physicians about how to use MBFR information in these cases. We suggest that these cases are good examples of how blood flow data should influence study results and clinical decisions.
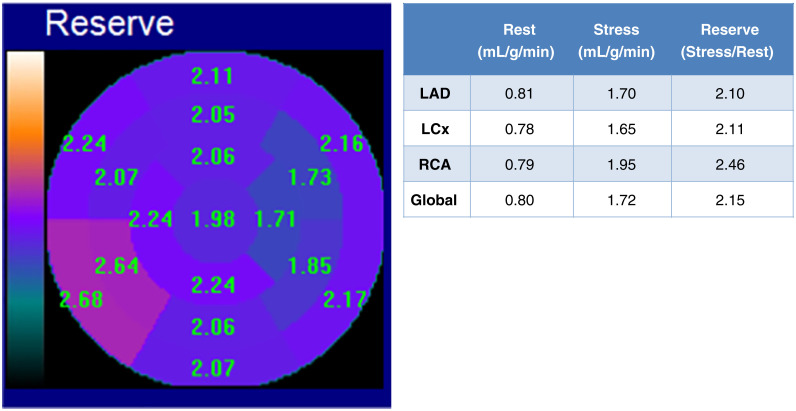
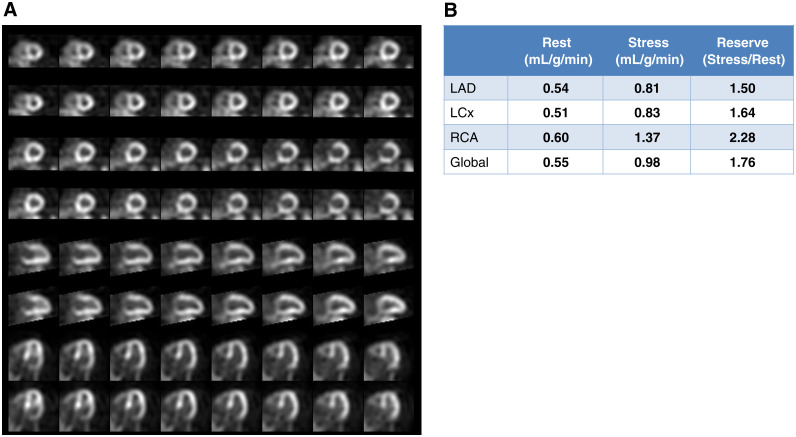
Case 1. Normal Myocardial Perfusion and Normal Myocardial Blood Flow Reserve (Figure 6)
Figure 6.
Case 1 (A) Dipyridamole stress and rest 82Rb MPI PET images, (B) MBF and MBFR, (C) CT for attenuation correction and assessment of coronary calcium.
A 49-year-old woman presented with intermittent exertional chest discomfort. Her only CAD risk factor was dyslipidemia. The referral question was ‘‘Assess for ischemia.’’ She underwent dipyridamole 82Rb PET MPI.
The study was reported as (1) No ischemia or regions of infarction; (2) Normal regional and global LV function at rest; (3) Normal augmentation of LVEF with stress; (4) Normal MBFR globally and in the distributions of all three coronary arteries; (5) The CACS is zero.
Teaching Points Normal relative stress perfusion images and normal MBFR are associated with a very low risk of cardiac death (<0.5% per year).10 Note that the normal-appearing perfusion images alone do not rule out extensive CAD, 50% left main CAD, severe coronary microvascular disease, balanced flow reduction, or non-responsiveness to the vasodilator. The normal MBFR establishes physiologic normality of the epicardial coronaries and the microvasculature. The absence of CAC is important in ruling out subclinical CAD.
Myocardial perfusion alone does not exclude: >50% left main disease, extensive CAD, balanced MBreduction, extensive microvascular disease, non-response to vasodilation.
Normal MBFR in addition to normal perfusion establishes physiologic normality of the epicardial arteries and the microvasculature.
Normal MBFR confirms study normalcy, which indicates lower risk of CAD beyond normal relative perfusion and predicts a low risk for major coronary- related events.
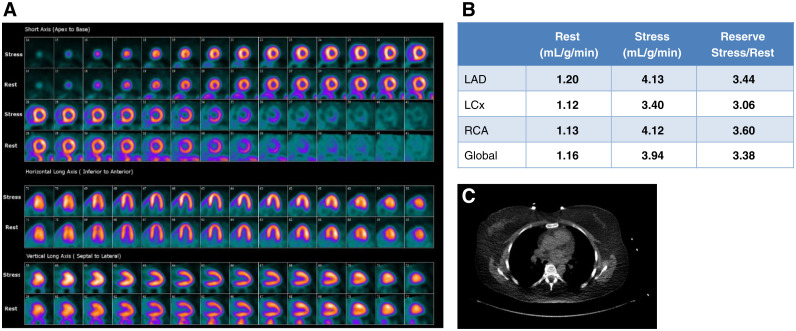
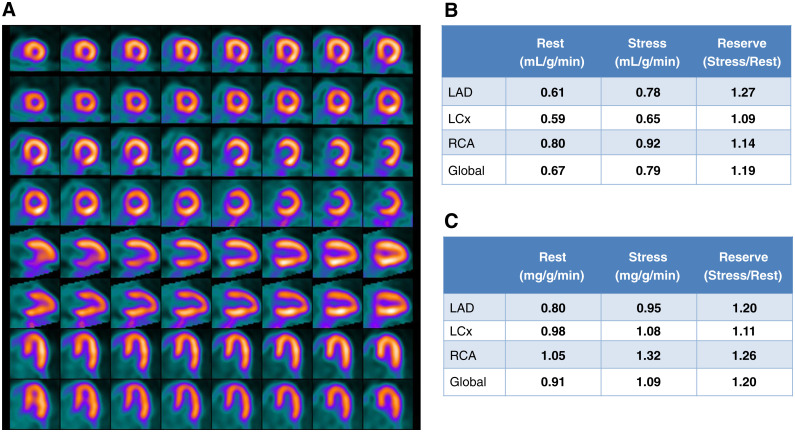
Case 2: Normal Myocardial Perfusion But Abnormal MBFR (Figure 7)
Figure 7.
Case 2. (A) Regadenoson stress and rest 82Rb MPI PET images, (B) MBF and MBFR, (C) CAC scan.
A 92-year-old man with a 6-month history of atypical chest pains, not responsive to an array of therapies was evaluated with regadenoson rest/stress 82Rb PET MPI.
The study was reported as (1) No ischemia or regions of infarction; (2) Normal regional and global LV function at rest; (3) Absence of augmentation of LVEF with stress; (4) Abnormally low MBFR globally and in the distributions of all 3 coronary arteries; (5) CACS is 1345. (6) Despite the normal scan appearance, this study is high risk for major adverse cardiac events based on the high CACS and the low MBFR. MBFR data was used in the decision to send the patient for cardiac cathereterization. This patient had severe 3-vessel disease and underwent coronary artery bypass graft surgery.
Teaching Points An important value of MBFR measurement involves the reclassification of a normal MPI from low risk to high-risk study.10 The low MBFR along with the high CACS is suggestive of multivessel CAD. Coronary angiography would be reasonable in follow-up. Although many of these cases will demonstrate multivessel CAD, some will have moderate epicardial CAD along with microvascular disease. Such patients remain at high risk for adverse outcomes despite the absence of a need for revascularization.
The high CACS establishes the presence of CAD.
Abnormal MBFR despite normal perfusion reclassifies the study from low risk to high risk for cardiac- related events.
The low MBFR in conjunction with a high CACS increases suspicion for significant obstructive multivessel CAD.
The report could include the following conclusion statement: despite normal relative myocardial perfusion, MBFR is abnormal, placing the patient in a high-risk category for multivessel CAD and cardiac- related events.
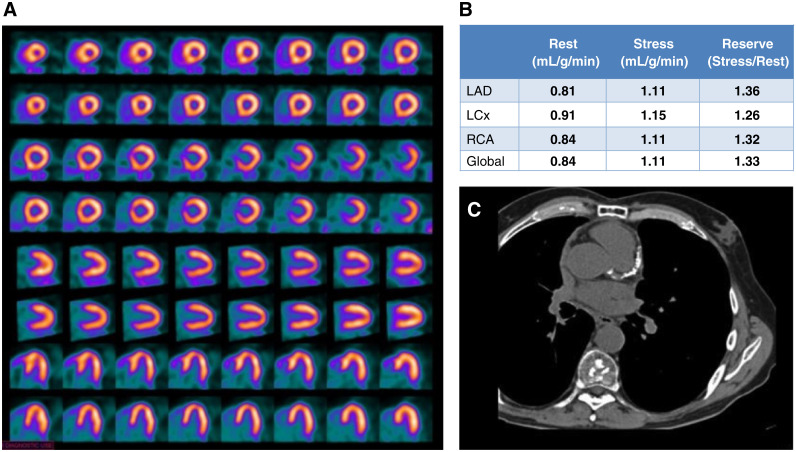
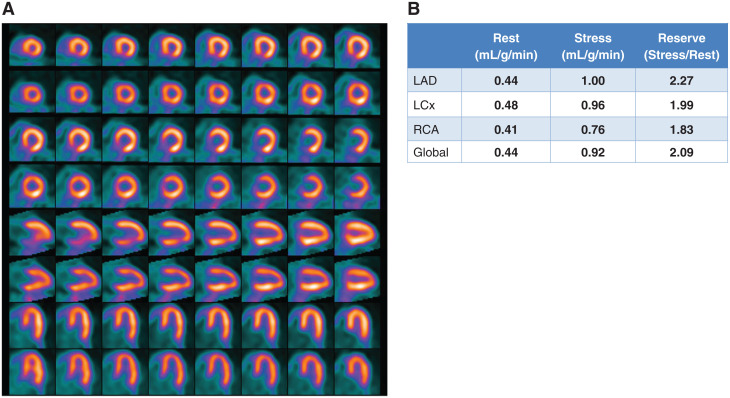
Case 3: A Case of a Non-responder to Vasodilation (Figures 8 and 9)
Figure 8.
Case 3. (A) Regadenoson stress and rest 82Rb MPI PET images, (B) MBF and MBFR.
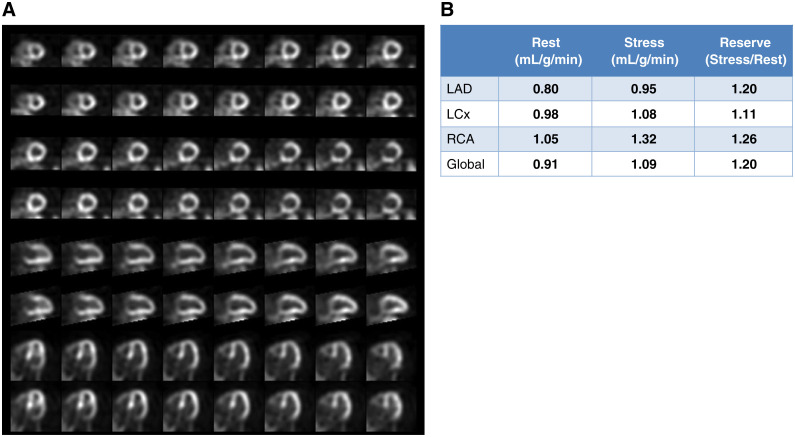
Figure 9.
Follow-up images for Case 3. (A) Regadenoson stress and rest 82Rb MPI PET images, (B) MBF and MBFR.
A 54-year-old morbidly obese man presented to hospital with palpitations, dyspnea, and chest pressure. He was found to have atrial fibrillation and rapid ventricular response. His ECG showed mild ST depression and his serum troponin peaked at 0.07 ng/ml. He spontaneously converted to sinus rhythm two hours after onset of his symptoms. The ED physician started oral diltiazem and apixaban and ordered a 82Rb PET MPI with MBFR.
The study was reported as follows: (1) Poor quality study likely due to the patient’s very large size (BMI 52); (2) No definite ischemia or regions of infarction; (3) Normal regional and global function at rest with LVEF 49%, augmenting normally to 54% with vasodilation. (4) No change in MBF globally and in the distribution of all 3 coronary arteries. (5) A MBFR of <1.2 in setting of no definite perfusion defects raises likelihood of a nondiagnostic test due to vasodilator inhibition. The rest flows are high and contributed to the low MBFR values. However, the global and regional stress flows are low as well and may be due to inadequate vasodilation. Recommend repeating the test off caffeine for at least 24 hours.
After it was determined the patient had consumed caffeine prior to the first test, a repeat study was performed following 24 hours of being caffeine free. Results are shown in Figure 9. The repeat study was reported as follows: (1) There are large and severe reversible perfusion defects anteriorly and laterally; (2) The left ventricle transiently dilates; (3) Left ventricular function is normal at rest regionally and globally, with LVEF 55%; (4) Left ventricular function deteriorates at peak vasodilation, with decreased contraction anteriorly, apically, and laterally, and drop in LVEF to 42%; (5) MBFR is globally abnormal and is especially low in LAD and LCx territories; (6) High-risk study suggestive of left main and/or multivessel CAD.
The patient underwent coronary angiography and was found to have 80% narrowing of the left main coronary artery with diffuse mild-moderate other coronary disease.
Teaching Points Lack of increase in MBF after administration of a vasodilator should always raise suspicion of a non-diagnostic test. Minimal increases can be seen in the setting of severe CAD, but in those cases, there is normally sufficient non-uniformity of tracer uptake to favor a diagnosis of CAD. Options are to wait 24 hours with strict patient preparation, or to test with dobutamine. Inadequate patient preparation (ideally no caffeine for 24 hours; hold for medications known to be vasodilator inhibitors) is commonly encountered in patients recently admitted to hospital, and even in the out-patient setting. There may also be patients with high levels of adrenergic tone in whom the vasodilator effect is not realized.
Normal perfusion with low or very low MBFR is commonly encountered. Explanations can include severe balanced flow reduction due to multivessel disease, severe microvascular disease, a combination of moderate severity epicardial disease and microvascular disease, and non-responsiveness to the vasodilator. Further testing is needed. An important first step is establishing whether inhibitors such as caffeine might have affected test results. If this seems unlikely, a CACS would be very useful in deciding whether invasive coronary angiography or CTA should be next steps.
Homogeneous lack of global increase of MBF and therefore MBFR of 0.8-1.2 in the setting of normal myocardial perfusion raises the possibility of a nondiagnostic test.
Minimal increases in MBF can be seen in the setting of severe CAD, but in those cases, there is normally sufficient non-uniformity of tracer uptake to favor a diagnosis of CAD.
The test should be repeated if a source for an antagonist to vasodilation can be found such as unreported caffeine consumption. If not, alternative explanations should be sought. Knowledge of CAC can help guide decision-making.
The first test conclusion should include the following: The absence of a rise in MBF with stress and normal relative perfusion images does not exclude CAD.
Case 4: A Female Patient with Coronary Microvascular Disease (Figure 10)
Figure 10.
Case 4. (A) Regadenoson stress and rest 82Rb MPI PET images, (B) Day 1 MBF and MBFR data. (C) Day 2 MBF and MBFR data.
A 72-year-old woman complains of exertional dyspnea. She has a long history of poorly controlled essential hypertension. Normal pulmonary function tests were reported. Her LVEF was 68% by echocardiogram. The patient was referred for 82Rb PET MPI to determine if the dyspnea was an anginal equivalent.
The study was interpreted as follows: (1) Normal perfusion at stress and rest; (2) Normal regional and global left ventricular function at rest with LVEF 70%, augmenting normally to 74% with vasodilation stress; (3) Abnormal MBFR averaging 1.2 times baseline flows, balanced within all three coronary territories; (4) CACS is 2; (5) Overall, given the normal perfusion with severe reduction in MBFR with very low calcium score, the study is most consistent with coronary microvascular disease, although it is possible that this patient had inhibitors of vasodilation. A repeat study should be considered with rigid control of all medications known to be competitive antagonists to regadenoson.
Teaching Points Many patients with symptoms suggestive of angina (exertional chest pain and/or dyspnea) and normal-appearing scans have abnormally low MBFR. A CAC screen is a reasonable next test—if the score is high, epicardial CAD remains a consideration. However, when CAC is absent or the score is low, the diagnosis of coronary microvascular disease is more likely. It is important to rule out other potential causes of dyspnea including lung diseases, anemia, endocrine disorders, other forms of heart disease, and deconditioning. Invasively determined coronary hemodynamics can also be useful for confirmation as well as sometimes elucidating a specific etiology. When MBFR isextremely low, as in this case, it may be necessary to obtain a confirmatory repeat measurement, as was done here.
The presence of a low CACS in the setting of abnormal MBFR generally suggests coronary microvascular disease rather than epicardial CAD.
Case 5: A Patient with Apparent Single Vessel Epicardial CAD and Globally Normal MBFR (Figure 11)
Figure 11.
Case 5. (A) Regadenoson rest and stress 82Rb PET MPI, (B) MBF and MBFR data.
An 80-year-old man with known chronic total occlusion of the RCA, presents with worsening exertional dyspnea. He undergoes a rest/regadenoson stress 82Rb PET MPI study.
The study was interpreted as follows: (1) There is a moderate-sized severe reversible perfusion defect of the inferior and inferoseptal walls, in the distribution of the known occluded right coronary artery; (2) At rest, all walls thicken and contract normally and LVEF is 63%; (3) At peak stress, the inferior wall becomes akinetic, but LVEF increases to 68%; (4) MBFR is globally normal at 2.1 times baseline flows and is normal for LAD and LCx territories. However, MBFR is abnormally low for the inferior wall; (5) Overall, the findings are consistent with known occluded RCA and non-flow- limiting disease of either the LAD or LCx.
Teaching Points Cardiac PET MPI is often performed in patients with known CAD. Many have undergone prior coronary angiograms and interventions. When patients become concerned about changes in how they feel, a stress MPI is frequently ordered. Because of a priori knowledge of coronary anatomy, the focus is on unexpected findings. The problem with spatially relative assessments is that a severe abnormality might garner all the attention, when in fact what is of concern is the regions with better perfusion. MBFR becomes of paramount importance in recognizing whether MBF physiology is intact in the other regions. Imagine how the management of this case would have changed, if the MBFR was abnormally low for the LCx and LAD distributions!
The presence of single vessel ischemia is confirmed by abnormal MBFR in that region only, suggesting single vessel CAD at catheterization.
The presence of single vessel ischemia but abnormal MBFR in 2 or 3 vascular territories indicates a likelihood of multivessel CAD at catheterization.
The report might include in the conclusion: Relative myocardial perfusion and MBFR indicate single vessel disease.
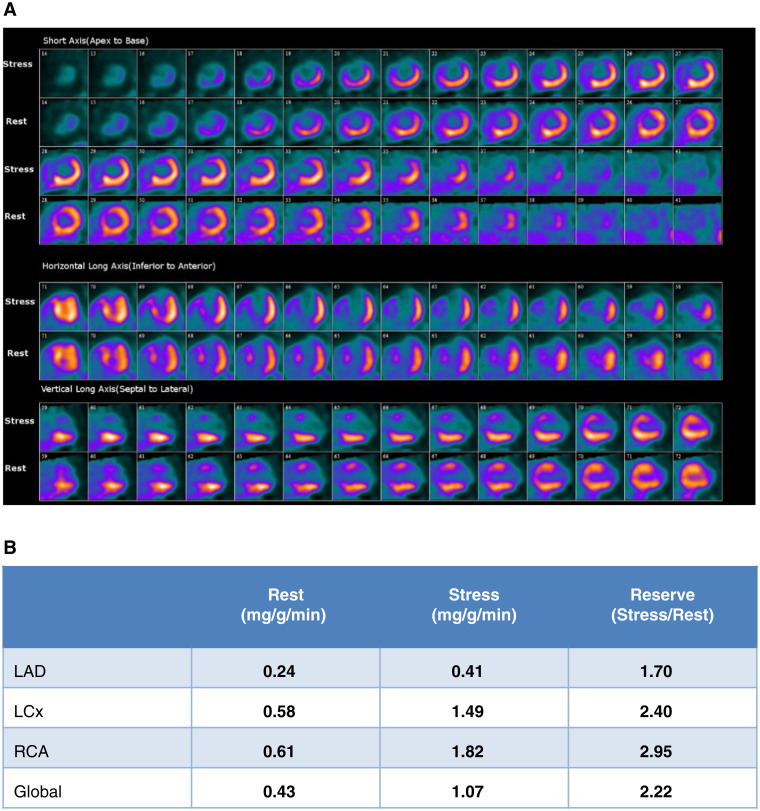
Case 6: Overestimation of MBFR in a Patient with Extensive Transmural Scar (Figure 12)
Figure 12.
Case 6. (A) Dipyridamole stress and rest 82Rb MPI PET images, (B) MBF and MBFR
A 61-year-old man with remote history of large anterior myocardial infarction and totally occluded LAD, subsequent development of severe left mainstenosis treated with left main and LCx stenting 2 years ago returns with recurrent angina. Assess for ischemia.
The dipyridamole 82Rb PET MPI study was reported as (1) Large area of severely reduced tracer uptake in the anterolateral, anterior, anteroseptal, and septal walls and apex with mild improvement on rest imaging consistent with a large area of mild ischemia, non-transmural scar, and transmural scar in the LAD distribution; (2) Moderately dilated LV, severely reduced EF (rest 21%, stress 17%) and global mild hypokinesis with akinesis of the septum, mid, and apical anterior wall and apex during rest and stress; (3) MBFR is reduced in the LAD territory due to extensive scar (0.24). Note also the very low absolute stress MBF. MBFR values for the RCA and LCx territories are within normal limits.
The patient underwent coronary angiography showing proximal occlusions of the LAD and first diagonal branch, patent stents to the LM and LCx and diffuse mild-to-moderate disease of the LCx and RCA. Medical management was recommended.
Teaching Points MBFR may be relatively preserved in the area of infarction in patients with low rest and stress values (and contribute to a higher-than- expected global MBFR). In this patient, the absolute increase in stress MBF compared to rest was severely reduced in the LAD territory and consistent with the occluded LAD. The calculated MBFR values should be reported for the non-infarct regions, but the global MBFR will be contaminated by the misleading values for the infarct region (since both stress and rest flow are proportionally lower in the infarct zone) and should not be reported.
Regions of infarction often have low resting flows.
MBF in regions of predominant infarction may increase proportionally with vasodilation, such that MBFR appears normal. Usually, peak MBF remains low.
MBFR in regions of predominant infarction may be normal or possibly high which can be misleading and affect global MBFR values. In such cases, the focus of MBFR reporting should be more on non-infarct territories.
CONCLUSIONS
This Practical Guide to interpretation and reporting of MBF with cardiac PET MPI was developed to encourage and assist clinicians in the implementation of this relatively new approach to evaluate patients with known or suspected CAD. As has been emphasized, MBF evaluation provides complementary information to MPI that adds considerably to the value of the testing procedure in the diagnosis and risk stratification of CAD and cardiac events. This Practical Guide should provide interpreting physicians with the knowledge to take advantage of this new tool.
Disclosures
Dr. Bateman receives research grant support from Bracco, GE Healthcare, and Jubilant Draxlmage. He serves as a consultant to AIM, AstraZeneca, Curium, and GE Healthcare. He has an equity interest in Cardiovascular Imaging Technologies. He has intellectual property rights for Imagen PET and SPECT software. Dr. Heller is a Medical Advisor for Molecular Imaging Services and consultant to GE Healthcare. Dr. Beanlands is a Career Investigator supported by the Heart and Stroke Foundation of Canada; the University of Ottawa Heart Institute Vered Chair in Cardiology, and Tier 1 Chair in Cardiovascular Research from the University of Ottawa. He is or has been a consultant for and receives grant funding from GE Healthcare, Lantheus Medical Imaging, Jubilant DraxImage. Dr. Calnon has no relevant relationships. Dr. Case receives research grant support from Bracco, GE Healthcare, and Jubilant DraxImage. He has an equity interest in Cardiovascular Imaging Technologies. He has intellectual property rights for Imagen PET and SPECT software. DrdeKemp is a consultant for and has received grant funding from Jubilant DraxImage. He receives revenues from Rubidium-82 generator technology licensed to Jubilant DraxImage and from sales of FlowQuant software. Dr. DePuey has no relevant relationships. Dr. Di Carli has received research grant support from Spectrum Dynamics and consulting honoraria from Sanofi and GE Healthcare. Dr. Guler has no relevant relationships. Dr. Murthy receives research support and funding from INVIA Medical Imaging Solutions, research grants and lecture honoraria from Siemens Medical Imaging, an expert witness testimony payment on behalf of Jubilant Draximage, and advisory board payments from Curium and Ionetix. Dr. Murthy has stock in General Electric and Cardinal Health, and stock options in Ionetix. Dr. Rosenblatt has no relevant relationships. Dr. Sher has no relevant relationships. Dr. Slomka participates in software royalties for QPS software at Cedars-Sinai Medical Center. He has received research grant support from Siemens Healthineers. Dr. Ruddy has received research grant support from GE Healthcare and Advanced Accelerator Applications.
Glossary
Abbreviations
- AC
Attenuation correction
- ASNC
American Society of Nuclear Cardiology
- CAC
Coronary artery calcium
- CACS
Coronary artery calcium score
- CAD
Coronary artery disease
- CT
Computed tomography
- CTA
Computed tomography angiography
- LAD
Left anterior descending
- LCx
Left circumflex
- LVEF
Left ventricular ejection fraction
- MBF
Myocardial blood flow
- MBFR
Myocardial blood flow reserve
- MPI
Myocardial perfusion imaging
- PET
Positron emission tomography
- RCA
Right coronary artery
- RPP
Rate pressure product
- SNMMI
Society of Nuclear Medicine and Molecular Imaging
Glossary
Definitions
- Blood pool input curve
Measures the concentration of radiotracer activity in the blood as a function of time. Typically measured in MBq/ (cm2s) or mCi/(cm2s)
- Blood pool region of interest
Software specific, small area, selected for measuring the blood pool input curve
- Detector saturation
Occurs when the number of singles events being received at the scanner exceeds the rate at which they can be accurately counted
- Dynamic scan
Consecutive series of short tomographic scans typically used to measure radiotracer kinetics
- Fused display
Overlay of the anatomical volume reconstruction (usually from a CT) and the emission reconstruction (PET). Used for quality control to detect misregistration and anatomical localization of tracer uptake
- Partial volume correction
Corrects the radiotracer uptake measurement for the finite resolution of the PET scanner
- Retention methods
Used to determine the myocardial blood flow from the integral of the blood pool input curve and the final myocardial tracer uptake values
- Single tissue compartment model
Uses a dynamic model of the arterial input function, myocardial uptake, and washout to calculate blood flow
REFERENCES
- 1. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187-226. [DOI] [PubMed] [Google Scholar]
- 2. Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges- Neto S, Chareonthaitawee P, et al. Clinical quantification of myocardial blood flow using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med 2018; 59: 273-293; J Nucl Cardiol. 2018;25:269-297. [DOI] [PubMed] [Google Scholar]
- 3. Schindler TC, Bateman TM, Berman DS, Chareonthaitawee P, De Blanche LE, Dilsizian V, et al. Appropriate use criteria for PET myocardial perfusion imaging. J Nucl Med. 2020;61:1222-65. [DOI] [PubMed] [Google Scholar]
- 4. Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14:521-8. [DOI] [PubMed] [Google Scholar]
- 5. Lima RS, Watson DD, Goode AR,, Siadaty MS, Ragosta M, Beller GA, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42:64-70. [DOI] [PubMed] [Google Scholar]
- 6. Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. J Am Coll Cardiol Img. 2009;2:751-8. [DOI] [PubMed] [Google Scholar]
- 7. Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, et al. Preserved coronary flow reserve effectively excludes high risk coronary artery disease on angiography. J Nucl Med. 2014;55:248-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JM, Kim CH, Koo BK, Hwang D, Park J, Zhang J, et al. Integrated myocardial perfusion imaging diagnostics improve detection of functionally significant coronary artery stenosis by 13N-ammonia positron emission tomography. Circ Cardiovasc Imaging. 2016. 10.1161/circimaging.116.004768. [DOI] [PubMed] [Google Scholar]
- 9. Ziadi MC, DeKemp RA, Williams K, Guo A, Renaud JM,, Chow BJ, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19:670- 80. [DOI] [PubMed] [Google Scholar]
- 10. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli M, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease: role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639-53. [DOI] [PubMed] [Google Scholar]
- 12. Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52:726-32. [DOI] [PubMed] [Google Scholar]
- 13. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography: Added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150-6. [DOI] [PubMed] [Google Scholar]
- 14. Ziadi MC, DeKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, et al. Impaired myocardial flow reserve on rubidium- 82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740-8. [DOI] [PubMed] [Google Scholar]
- 15. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and Modifies the effect of early revascularization. Circulation. 2015;131:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel KK, Spertus JA, Chan PS, Sperry BW, Thompson RC, Al Badarin F, et al. Myocardial blood flow reserve assessed by positron emission tomography myocardial perfusion imaging identifies patients with a survival benefit from early revascularization. Eur Heart J. 2020;41:759-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schindler TH, Dilsizian V. Coronary microvascular dysfunction: Clinical considerations and noninvasive diagnosis. J Am Coll Cardiol Img. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):140-155. [DOI] [PubMed] [Google Scholar]
- 20. Johnson NP, Gould KL. Regadenoson versus dipyridamole hyperemia for cardiac PET imaging. J Am Coll Cardiol. 2015;8:438-47. [DOI] [PubMed] [Google Scholar]
- 21. Lassen ML, Manabe O, Otaki Y, Eisenberg E, Huynh PT, Wang F, et al. 3D PET/CT 82 Rb PET myocardial blood flow quantification: Comparison of half-dose and full-dose protocols. Eur J Nucl Med Mol Imaging. 2020;47(13):3084-3093. [DOI] [PubMed] [Google Scholar]
- 22. Slomka PJ, Diaz-Zamudio M, Dey D, Motwani M, Brodov Y, Choi D, et al. Automatic registration of misaligned CT attenuation correction maps in Rb-82 PET/CT improves detection of angio- graphically significant coronary artery disease. J Nucl Cardiol. 2015;22(6):1285-95. [DOI] [PubMed] [Google Scholar]