Abstract
Introduction: Overdose fatalities associated with the opioid epidemic are predictably attributable to drug-induced respiratory depression. In terms of illicit opioid abuse, fentanyl is the synthetic opioid responsible for the largest number of overdose deaths. There is, therefore, an urgent need to identify safe and effective therapeutics that can attenuate fentanyl-induced respiratory depression. Identification of effective alternate analgesic strategies that lessen the respiratory depression associated with narcotics would also help improve current strategies for pain management. Our laboratory recently reported that the G protein-biased CB2 cannabinoid receptor agonist LY2828360 suppressed chemotherapy-induced neuropathic nociception and attenuated both morphine tolerance and physical dependence in paclitaxel-treated mice. However, the impact of LY2828360 on other undesirable side effects of opioids, such as opioid-induced respiratory depression, remains unknown.
Materials and Methods: We used whole-body plethysmography to assess the impact of the CB2 cannabinoid agonist LY2828360 on fentanyl-induced respiratory depression using wild-type (WT) and CB2 knockout (CB2KO) mice.
Results: Fentanyl reduced minute ventilation and respiratory frequency without altering tidal volume in both WT and CB2KO mice. In WT mice, the high dose of fentanyl (0.2 mg/kg intraperitoneal [i.p.]) produced a greater suppression of respiratory parameters compared with the low dose of fentanyl (0.1 mg/kg i.p.). Coadministration of a behaviorally active dose of LY2828360 (3 mg/kg i.p.) with fentanyl (0.2 mg/kg i.p.) attenuated fentanyl-induced respiratory depression in WT mice. Notably, LY2828360 (3 mg/kg i.p.) did not attenuate fentanyl-induced respiratory depression in CB2KO mice, consistent with mediation by CB2 receptors. Moreover, LY2828360 (3 mg/kg i.p.) alone lacked intrinsic effects on respiratory parameters in either WT or CB2KO mice.
Conclusion: The combination of a CB2 agonist with fentanyl may represent a safer adjunctive therapeutic strategy compared with a narcotic analgesic alone by attenuating the development of opioid-induced respiratory depression. Moreover, the CB2 agonist, administered alone, did not alter respiration. Our findings suggest that the CB2 cannabinoid agonist LY2828360 may provide CB2-mediated protection against fentanyl-induced respiratory depression, a detrimental and unwanted side effect of opioid use and abuse.
Keywords: CB2 receptor, endocannabinoid, fentanyl, opioid overdose, respiratory depression
Introduction
Opioid-related overdose deaths have been driven primarily by the synthetic opioid fentanyl and its analogs.1,2 Fentanyl, originally developed as a rapidly acting analgesic, is much more potent than natural opioids such as morphine.3 Accidental contamination or intentional mixing of street drugs with fentanyl also creates an opioid mix of unpredictable and variable potency and increases fatal overdose risk.4,5 Fentanyl readily enters the central nervous system where it decreases respiratory drive and increases mechanical resistance to breathing (by decreasing thoracic compliance), potentially inducing fatality within minutes of administration.6–8 Naloxone, a nonselective and competitive opioid receptor antagonist, is the only pharmacological treatment available to reverse such overdoses. However, due to the high potency of fentanyl and its longer duration of action, multiple naloxone doses are often required to provide sustained overdose reversal, increasing the risk of unsuccessful treatment and death.9,10 Reports of unsuccessful attempts to revive patients with naloxone persist despite administration of multiple or escalating doses due to the short window between drug intake and overdose.9,11–14 Thus, identification of safe and effective alternative/supplementary therapeutic strategies to attenuate opioid-induced respiratory depression remains an unmet clinical need.
The endocannabinoid system consists of CB1 and CB2 cannabinoid receptors, their endogenous lipid signaling molecules (endocannabinoids), and enzymes that control their synthesis and degradation.15,16 This neuromodulatory system can mitigate unwanted side effects of opioids.17 Endocannabinoids depress respiration in a CB1-mediated manner.18,19 Furthermore, selective CB1 agonists induce respiratory depression in a manner blocked by CB1 antagonists.20–23 By contrast, the CB2 receptor has been postulated as a potential therapeutic target to overcome the side effects of direct acting CB1 agonists or opioids. CB2 receptors are predominantly expressed in cells within the immune system24–27 and are upregulated in response to injury or inflammation.28–30 Functional CB2 receptors are also present in neuroanatomical regions controlling respiration, including the brainstem and are activated by elevated levels of endocannabinoids.27,31,32 Furthermore, low levels of CB2 receptors are also reported in respiratory tissues such as the lungs.24,33,34 Although CB2 mechanisms help maintain opioid-induced analgesic efficacy through synergistic interactions with μ-opioid receptors,35–38 their effects on opioid-induced respiratory depression, remain unexplored.
Our laboratories recently reported that the G protein-biased cannabinoid CB2 receptor agonist LY2828360 attenuated morphine tolerance and physical dependence while also attenuating neuropathic nociception in a paclitaxel model of chemotherapy-induced neuropathy.35 This LY2828360-induced attenuation of morphine tolerance was absent in paclitaxel-treated CB2 knockout (CB2KO) mice, consistent with CB2-receptor mediation.35 Moreover, morphine-dependent CB2KO mice treated with paclitaxel showed higher levels of naloxone-precipitated opioid withdrawal compared with their wild-type (WT) counterparts.35 LY2828360 was previously evaluated in a phase-2 clinical trial for osteoarthritic pain where it failed for lack of efficacy but was otherwise safe in humans (www.clinicaltrials.gov identifier: NCT01319929).39 These observations raise the possibility that CB2 activation by LY2828360 may safely restore homeostasis and counter the impact of aberrant challenges such as opioid-induced side effects.
In this study, we used whole-body plethysmography to examine the impact of the CB2 agonist LY2828360 on respiratory depression induced by the synthetic opioid fentanyl. We characterized the impact of acute fentanyl administration on distinct respiratory parameters (i.e., minute ventilation, respiratory frequency, and tidal volume) in otherwise naive mice. We also assessed the impact of LY2828360 treatment on fentanyl-induced changes in respiratory parameters using both WT and CB2KO mice, to confirm CB2-receptor mediation.
Materials and Methods
Animals
Sixty adult male mice weighing 30–40 g and ∼3–4 months old were used in these studies. Thirty adult male C57BL/6J WT mice were purchased from Jackson Laboratories (Bar Harbor, ME). Thirty adult male CB2KO mice (B6.129P2-CNR2 [tm1Dgen/J]) were bred at Indiana University for >20 generations onto a C57BL/6J background. Mice were group housed in a 12:12 h light/dark cycle, temperature and humidity-controlled facility with ad libitum food and water. All experimental procedures were approved by the Bloomington Institutional Animal Care and Use Committee of Indiana University (IACUC Protocol 19-037).
Drugs and chemicals
LY2828360 [8-(2-chlorophenyl)-2-methyl-6-(4-methylpiperazin-1-yl)-9-(tetrahydro-2H-pyran-4-yl)-9Hpurine] was synthesized and obtained from Sai Biotech (Mumbai, India). Fentanyl citrate was purchased from Sigma Aldrich (St. Louis, MO). All compounds were dissolved in a vehicle containing 3% dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO) with the remaining 97% consisting of emulphor (Alkamuls EL-620; Solvay, Princeton, NJ), 95% ethanol, and 0.9% saline in a 1:1:18 ratio, respectively, and administered through intraperitoneal (i.p.) injection in a final volume of 10 mL/kg.
Assessment of respiratory parameters
A whole-body plethysmography apparatus (DSI, St. Paul, MN) was used to assess respiratory parameters in freely moving mice. Mice first received room air for 2 days before testing to allow for chamber habituation (30 min each day). On the subsequent day, mice were additionally handled to reduce potential experimental stress. Finally, on the test day, mice received air mixed with 10% CO2 to induce an increase in minute ventilation without inducing stress, similar to previous studies.40 Mice were recorded for 50 min to allow time for acclimation to CO2 and the establishment of a stable baseline before pharmacological manipulations. Mice were briefly removed from the chambers, given a single i.p. injection, and returned to the chamber for 30 min of postinjection recording. Then, mice were immediately removed from the plethysmography chambers to terminate the experiment. Three distinct respiratory parameters were recorded for the entire 80 min observation interval: minute ventilation (i.e., volume of ventilation, mL/min), respiratory frequency (i.e., breaths per minute), and tidal volume (i.e., volume of each breath, mL/kg). Parameters were averaged into 5 min bins. In experiment 1, the impact of high- (0.2 mg/kg) and low-dose (0.1 mg/kg) fentanyl on respiratory parameters was assessed in WT mice. The dose of fentanyl and duration of postinjection monitoring was similar to previous studies of fentanyl-induced respiratory depression.6,41 In experiment 2, the impact of LY2828360 (3 mg/kg) alone, fentanyl (0.2 mg/kg) alone, fentanyl (0.2 mg/kg) + LY2828360 (3 mg/kg) or vehicle were assessed in WT mice. In experiment 3, the impact of LY2828360 alone (3 mg/kg), fentanyl alone (0.2 mg/kg), fentanyl (0.2 mg/kg) + LY2828360 (3 mg/kg) or vehicle were assessed in CB2KO mice to ascertain whether LY2828360-induced effects on fentanyl-induced respiratory depression were CB2 mediated.
Statistical analysis
Respiratory parameter data were analyzed using two-way repeated measures analysis of variance (ANOVA, i.e., performed across the entire pre- and postinjection observation interval associated with CO2 exposure), followed by Bonferroni's post hoc and multiple comparison tests. Bonferroni's multiple comparison tests, which provide more statistical power by making a more limited set of comparisons, were used to compare effects of fentanyl to all other groups, thereby reducing chances of a type II error.42 Effects of LY2828360 alone (i.e., in the absence of fentanyl) were additionally compared with vehicle using two-way repeated measures ANOVA followed by Bonferroni's post hoc tests. All data were analyzed using GraphPad Prism version 7.05 (GraphPad Software, Inc., La Jolla, CA). p<0.05 was considered statistically significant.
Results
Respiratory parameters did not differ between groups during the baseline (i.e., preinjection) recordings at any timepoint (−50 to −5 min) in any study (Figs. 1–3). All respiratory parameters changed across time (i.e., −50 min preinjection to 30 min postinjection) irrespective of drug treatment (p<0.0001; Figs. 1–3).
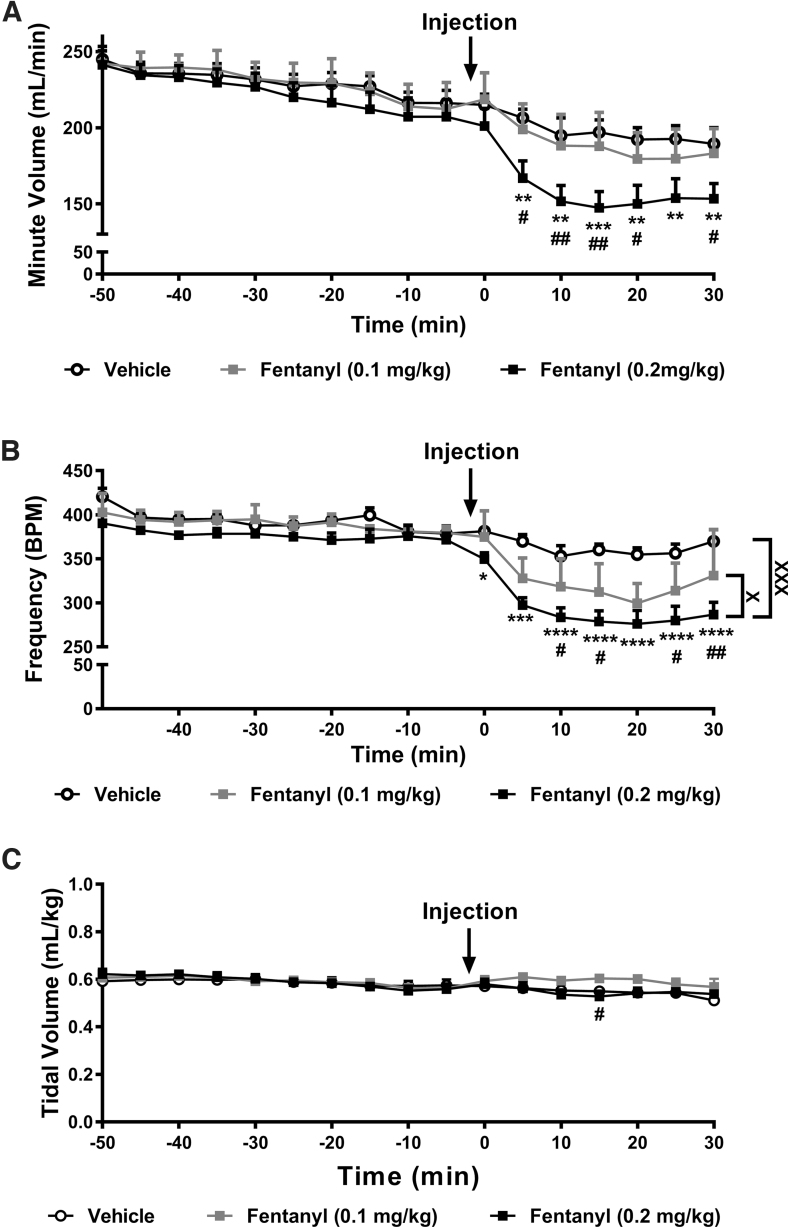
FIG. 1.
Fentanyl suppresses minute ventilation and respiratory frequency without altering tidal volume. (A) Fentanyl suppressed minute ventilation in a time-dependent manner. Fentanyl (0.2 mg/kg) suppressed minute ventilation compared with both vehicle and fentanyl (0.1 mg/kg) (p<0.05 at each timepoint). Fentanyl (0.1 mg/kg) did not reliably alter minute ventilation compared with vehicle. No differences in minute ventilation were noted before the drug injection. (B) Fentanyl suppressed respiratory frequency in a time and dose-dependent manner. Fentanyl (0.2 mg/kg) decreased respiratory frequency overall when compared with both vehicle and fentanyl (0.1 mg/kg) groups (p<0.05). Fentanyl (0.1 mg/kg) decreased respiratory frequency compared with the vehicle-treated group (p<0.05; significance marks not shown). No differences in respiratory frequency were observed between groups before the drug injection. (C) Fentanyl altered respiratory tidal volume in a time-dependent manner, but no overall effect of drug across the entire observation interval was observed. All respiratory parameters changed across time irrespective of drug treatment. Post hoc comparisons failed to reveal differences between groups at any timepoint. Data are expressed as mean±SEM (n=6 per group). “*” indicates high-dose fentanyl group versus vehicle group where ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05, “#” indicates high-dose fentanyl group versus low-dose fentanyl group with the same symbol indications, “X” indicates main drug effect of the selected groups with the same symbol indications, two-way repeated measures ANOVA followed by Bonferroni's post hoc or multiple comparisons test. ANOVA, analysis of variance; SEM, standard error of the mean.
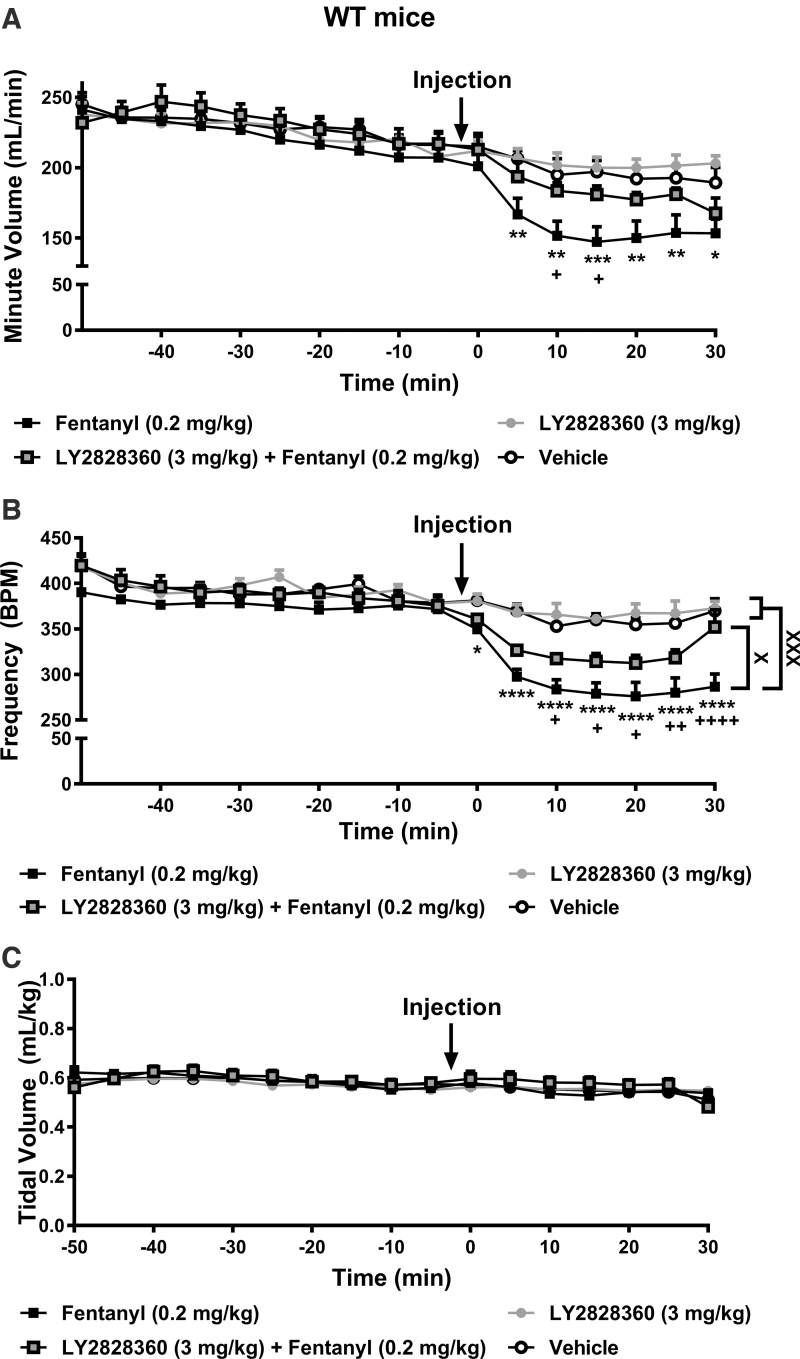
FIG. 2.
The CB2 agonist LY2828360 attenuates fentanyl-induced depression of minute ventilation and respiratory frequency in WT mice. (A) In WT mice, fentanyl (0.2 mg/kg) decreased respiratory minute ventilation compared with vehicle 5–30 min postinjection (p<0.01 at each timepoint). Minute ventilation was higher in the LY2828360 (3 mg/kg) + fentanyl (0.2 mg/kg) group compared with fentanyl (0.2 mg/kg) alone group from 10 to 15 min postinjection (p<0.05 at each timepoint). (B) Fentanyl decreased respiratory frequency compared with vehicle from 0 to 30 min postinjection in WT mice (p<0.05 at each timepoint). Respiratory frequency was higher overall in the LY2828360 + fentanyl group compared with the fentanyl alone group (p<0.05). Respiratory frequency was higher in the LY2828360 + fentanyl compared with the fentanyl alone group from 10 to 30 min postinjection (p<0.05 at each timepoint). (C) Tidal volume differed between groups in a time-dependent manner but no differences were observed between the LY2828360 + fentanyl group compared with the fentanyl alone group at any timepoint. (A–C) LY2828360 administered alone did not alter minute ventilation, respiratory frequency, or tidal volume compared with vehicle at any timepoint (p>0.05). All respiratory parameters changed across time irrespective of drug treatment. Data are expressed as mean±SEM (n=6 per group). “*” indicates fentanyl alone group versus vehicle group where ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05, “+” indicates fentanyl alone group versus LY2828360 + fentanyl group with the same symbol indications, “X” indicates main drug effect of the selected groups with the same symbol indications, two-way repeated measures ANOVA followed by Bonferroni's post hoc or multiple comparisons test. WT, wild-type.
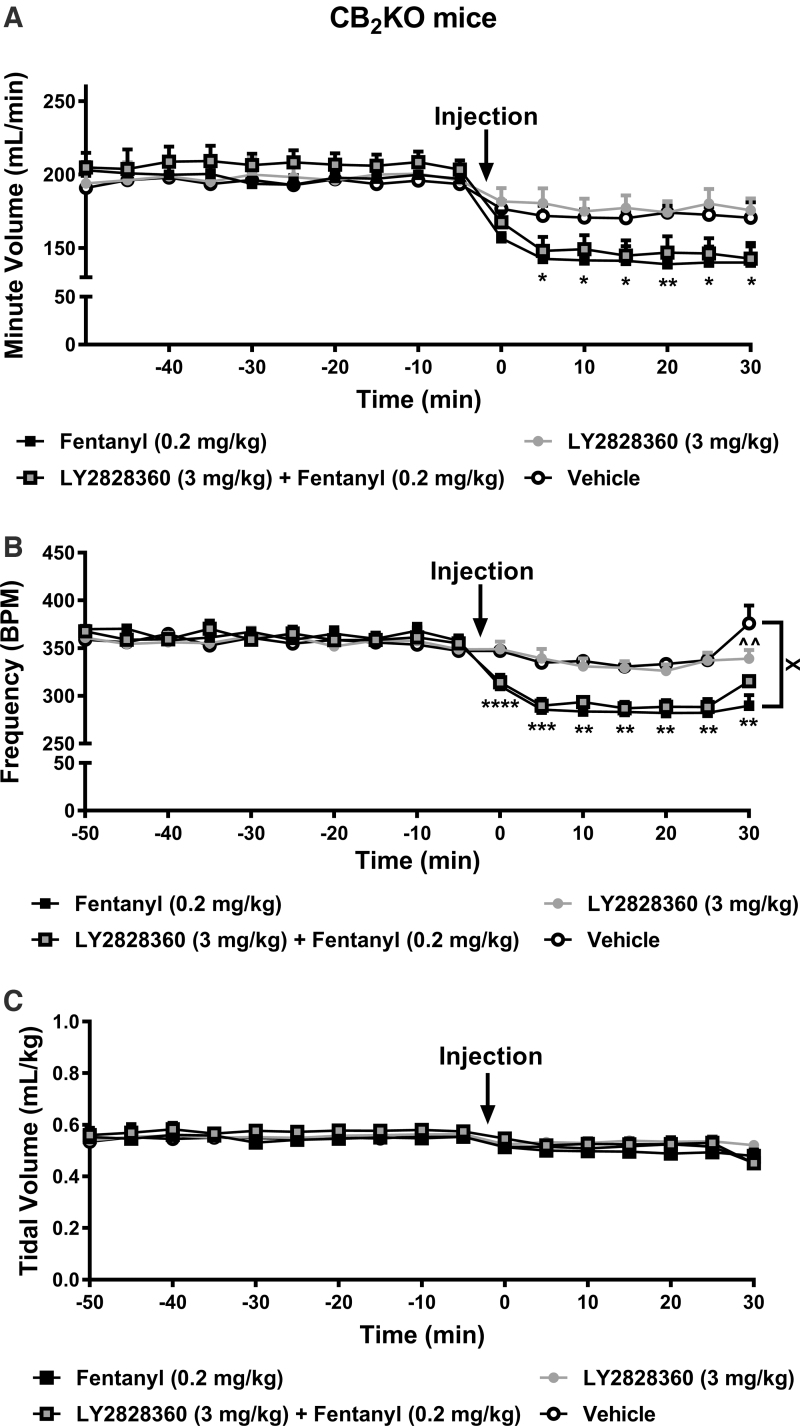
FIG. 3.
LY2828360 does not attenuate fentanyl-induced depression of minute ventilation and respiratory frequency in CB2KO mice. (A) In CB2KO mice, fentanyl (0.2 mg/kg) suppressed minute ventilation compared with vehicle from 5 to 30 min postinjection (p<0.05 at each timepoint). Minute ventilation did not differ between groups receiving ether fentanyl (0.2 mg/kg) alone or LY2828360 (3 mg/kg) + fentanyl (0.2 mg/kg) at any timepoint (p>0.05). (B) In CB2KO mice, fentanyl alone suppressed respiratory frequency compared with vehicle from 0 to 30 min postinjection (p<0.05 at each timepoint). Respiratory frequency was reduced overall in the fentanyl (0.2 mg/kg) alone group compared with the vehicle-treated group (p<0.05). In CB2KO mice, LY2828360 + fentanyl produced similar depression of respiratory frequency compared with fentanyl alone (p>0.05). (C) Drug treatments altered respiratory tidal volume in a time-dependent manner, but no overall effect of drug was detected in the testing interval. Post hoc comparisons failed to reveal differences between groups at any timepoint. (A–C) LY2828360 administered alone did not alter minute ventilation, respiratory frequency, or tidal volume compared with vehicle at any timepoint except where noted at the terminal timepoint for respiratory frequency (p>0.05). All respiratory parameters changed across time irrespective of drug treatment. Data are expressed as mean±SEM (n=8 per group except the LY2828360 + fentanyl group where n=6). “*” indicates fentanyl alone group versus vehicle group where ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05, “^” indicates LY2828360 alone group versus vehicle group with the same symbol indications, “X” indicates main drug effect of the selected groups with the same symbol indications, two-way repeated measures ANOVA followed by Bonferroni's post hoc or multiple comparisons test. CB2KO, CB2 knockout.
Fentanyl produces respiratory depression
Fentanyl depressed minute ventilation in a time-dependent manner (interaction: F32,240=3.578, p<0.0001; Fig. 1A). Minute ventilation did not differ between groups overall and decreased across the observation interval irrespective of drug treatment (drug: F2,15=2.263, p=0.1383; time: F16,240=82.37, p<0.0001; Fig. 1A). Post hoc analyses revealed that high-dose fentanyl (0.2 mg/kg) depressed minute ventilation compared with vehicle and low-dose (0.1 mg/kg) fentanyl (p<0.05 from 5 to 30 min postinjection with one exception; fentanyl doses did not differ at 25 min postinjection). Minute ventilation did not differ between low-dose fentanyl and the vehicle-treated group.
Fentanyl depressed respiratory frequency in a time-dependent manner (interaction: F32,240=4.345, p<0.0001; Fig. 1B). Respiratory frequency differed between groups overall and decreased across the observation interval irrespective of drug treatment (drug: F2,15=13.4, p=0.0005; time: F16,240=58, p<0.0001; Fig. 1B). Post hoc analyses revealed that, across the observation interval, high-dose fentanyl reduced respiratory frequency compared with vehicle (p=0.0003) and low-dose fentanyl (p=0.0420). Both high- and low-dose fentanyl depressed respiratory frequency compared with vehicle from 0 to 30 and 5 to 30 min postinjection, respectively (p<0.05 at each timepoint). In general, high-dose fentanyl produced a greater suppression of respiratory frequency compared with low-dose fentanyl (p<0.05 from 10 to 30 min postinjection).
Finally, fentanyl produced a modest time-dependent change in tidal volume (interaction: F32,240=2.543, p<0.0001; Fig. 1C). Tidal volume did not differ between groups overall but changed in a time-dependent manner irrespective of drug treatment across the observation interval (drug: F2,15=0.4209, p=0.6640; time: F16,240=13.95, p<0.0001; Fig. 1C). Post hoc analyses revealed that high-dose fentanyl depressed tidal volume compared with low-dose fentanyl only at the 15 min postinjection timepoint (p=0.0262). Tidal volume in groups receiving either dose of fentanyl did not differ from vehicle at any timepoint.
LY2828360 does not alter respiration in the absence of fentanyl
In the absence of fentanyl, LY2828360 (3 mg/kg) did not alter respiratory parameters compared with vehicle in either WT or CB2KO mice, with the exception of a single timepoint in CB2KO mice (detailed as follows; Figs. 2 and 3).
LY2828360 attenuates fentanyl-induced respiratory depression in WT mice
In WT mice, fentanyl (0.2 mg/kg), administered in the presence or absence of LY2828360 (3 mg/kg), depressed minute ventilation in a time-dependent manner (interaction: F48,320=3.953, p<0.0001; Fig. 2A). Drug condition alone did not impact minute ventilation overall and minute ventilation decreased across time irrespective of drug treatment (drug: F3,20=1.664, p=0.2067; time: F16,320=78.85, p<0.0001; Fig. 2A). Post hoc analyses revealed that fentanyl depressed minute ventilation compared with vehicle and LY2828360 alone (p<0.05 from 5 to 30 min postinjection). Minute ventilation was higher in the LY2828360 + fentanyl compared with the fentanyl alone group from 10 to 15 min postinjection (p<0.05 at each timepoint). Thus, LY2828360 attenuated fentanyl-induced decreases in respiratory minute ventilation.
In WT mice, fentanyl, administered in the presence or absence of LY2828360, altered respiratory frequency in a time-dependent manner (interaction: F48,320=5.396, p<0.0001). Respiratory frequency differed between groups overall and decreased across the observation interval (drug: F3,20=10.61, p=0.0002; time: F16,320=65.45, p<0.0001; Fig. 2B). Post hoc analyses revealed that, across the observation interval, fentanyl decreased respiratory frequency compared with vehicle (p=0.0004), LY2828360 alone (p=0.0002) or LY2828360 + fentanyl (p=0.0303) groups. Strikingly, respiratory frequency was lower in the fentanyl alone group compared with the LY2828360 + fentanyl group from 10 to 30 min postinjection (p<0.05 at each timepoint). Thus, LY2828360 attenuated fentanyl-induced depression of respiratory frequency.
In WT mice, fentanyl, administered in the presence or absence of LY2828360, altered respiratory tidal volume in a time-dependent manner (interaction: F48,320=2.179, p<0.0001; Fig. 2C). Tidal volume did not differ between groups overall but decreased across the observation interval irrespective of drug treatment (drug: F3,20=0.1384, p=0.9359; time: F16,320=22, p<0.0001; Fig. 2C). Post hoc analyses failed to reveal any significant differences between the groups at any timepoint.
LY2828360 attenuates fentanyl-induced respiratory depression through a CB2-mediated mechanism
In CB2KO mice, fentanyl (0.2 mg/kg), administered in the presence or absence of LY2828360 (3 mg/kg) depressed minute ventilation in a time-dependent manner (interaction: F48,416=6.375, p<0.0001; Fig. 3A). Minute ventilation did not differ between groups overall but decreased across time irrespective of drug treatment (drug: F3,26=0.7048, p=0.5578; time: F16,416=86.81, p<0.0001; Fig. 3A). Post hoc analyses revealed that fentanyl depressed minute ventilation in CB2KO groups compared with vehicle and LY2828360 alone (p<0.05 from 5 to 30 min postinjection). Strikingly, minute ventilation did not differ in CB2KO groups receiving fentanyl in either the presence or absence of LY2828360 at any postinjection timepoint (p>0.05 at each timepoint). Thus, depressive effects of fentanyl on minute ventilation were intact in CB2KO mice and coadministration of LY2828360 with fentanyl failed to prevent these depressive effects.
In CB2KO mice, fentanyl, administered in the presence or absence of LY2828360 depressed respiratory frequency in a time-dependent manner (interaction: F48,416=9.351, p<0.0001; Fig. 3B). Respiratory frequency differed between groups overall and decreased across time irrespective of drug treatment (drug: F3,26=3.248, p=0.0380; time: F16,416=70.22, p<0.0001; Fig. 3B). Post hoc analyses revealed that, across the observation interval, fentanyl decreased respiratory frequency compared with vehicle (p=0.0488) but not LY2828360 alone (p>0.05) or LY2828360 + fentanyl (p>0.05). Respiratory frequency was lower in the fentanyl alone group compared with vehicle from 0 to 30 min postinjection (p<0.05 at each timepoint). Strikingly, respiratory frequency did not differ in groups receiving fentanyl in either the presence or absence of LY2828360 at any postinjection timepoint (p>0.05 at each timepoint). Thus, the depressive effects of fentanyl on respiratory frequency were intact in CB2KO mice and coadministration of LY2828360 with fentanyl failed to prevent these depressive effects. Respiratory frequency was higher in the vehicle group compared with the LY2828360 alone group only at the 30 min postinjection timepoint (p<0.01).
In CB2KO mice, fentanyl, administered in the presence or absence of LY2828360, altered respiratory tidal volume in a time-dependent manner (interaction: F48,416=1.582, p=0.0104; Fig. 3C). Tidal volume did not differ between groups overall but decreased across time irrespective of drug treatment (drug: F3,26=0.3635, p=0.7799; time: F16,416=25.56, p<0.0001; Fig. 3C). Post hoc analyses failed to reveal any significant differences between the groups at any timepoint.
Discussion
The opioid epidemic has been marked by a dramatic increase in synthetic opioid overdose fatalities.1,43 These fatalities are primarily driven by the propensity of illicit fentanyl and its analogs to produce heightened respiratory depression, resulting in enhanced lethality.2,3,9 In our study, fentanyl (0.2 mg/kg) decreased minute ventilation and respiratory frequency without altering tidal volume. Strikingly, the CB2 agonist LY2828360 attenuated fentanyl-induced respiratory depression in WT but not CB2KO mice, consistent with CB2 mediation. Importantly, LY2828360 also lacked intrinsic effects on all respiratory parameters assessed in both WT and CB2KO mice.
In rhesus monkeys, fentanyl dose-dependently decreased minute ventilation, but cannabinoid agonists Δ9-tetrahydrocannabinol or CP55,940, at doses that enhance opioid-induced antinociception, did not alter fentanyl-induced respiratory depression.44 The CB1 antagonist SR141716A did not alter heroin-induced respiratory depression in monkeys.23 These prior studies tested effects of nonselective pan cannabinoid agonists or CB1 antagonists. Here we show, for the first time, that a selective CB2 agonist attenuates opioid-induced respiratory depression without intrinsically altering respiratory parameters. Thus, CB2-receptor-mediated mechanisms may reverse opioid-induced respiratory depression.
WIN55,212-2, a nonselective cannabinoid receptor agonist and JWH133, a selective CB2 receptor agonist attenuated HCl- and capsaicin-induced bronchoconstriction in guinea pigs.45,46 These beneficial effects were blocked by a CB2 (SR144528) but not a CB1 (SR141716) receptor antagonist.45,46 Furthermore, bronchoconstriction in these models is shown to be caused by several receptor types, including μ-opioid receptors localized to the sensory nerve endings in the airway.47,48 CB2 receptors may also convey their protective effects by suppressing sensory nerve activation45,46,49 (but see Calignano et al.50). Opioid-induced microglial activation opposes opioid analgesia due to release of proinflammatory mediators and exacerbates opioid-induced side effects such as respiratory depression through nonstereoselective activation of toll-like receptor 451 (but see Zwicker et al.52). Systemic coadministration of the microglial inhibitor minocycline attenuates morphine-induced reductions in tidal volume, minute volume, inspiratory force and expiratory force, and reductions in blood oxygen saturation while potentiating morphine analgesia.53 Opioid-induced hypoxia is a potent stimulus for such microglial activation and this microglial activation is inhibited by minocycline.54 CB2 receptor activation may help reduce this opioid-induced microglial activation and proinflammatory factor release, thereby attenuating opioid-induced respiratory depression.55–57 Finally, JWH133 alleviates lung ischemia-reperfusion injury58,59 and paraquat-induced lung edema and lung histopathological changes60; thus, CB2-receptor-mediated reduction of inflammation may help improve lung functioning.
Our studies suggest that adjunctive therapeutic strategies combining LY2828360 with a narcotic analgesic to attenuate development of respiratory depression may provide a safer therapeutic strategy. However, almost 39% of people who died from unintentional opioid overdoses did not have a previous diagnosis of chronic pain.61 It is important to determine whether treatments aimed at ameliorating adverse side effects of opioids reverse opioid-induced respiratory depression and are efficacious in the absence of a pathological pain state. Our findings suggest that CB2 receptors are functional in the absence of a pathological pain state in fentanyl-treated mice and are engaged by LY2828360 to attenuate opioid-induced respiratory depression even in the absence of a neuropathic pain state (i.e., where CB2 expression would not necessarily be induced or upregulated30). We previously reported that CB2KO mice have greater levels of naloxone-precipitated opioid withdrawal compared with WT mice.35 Thus, CB2 receptor deletion in the CB2KO mice may, in fact, unmask a heightened opioid response. The WT and CB2KO mice used here were not littermates and, consequently we did not evaluate whether fentanyl-induced respiratory depression is greater in CB2KO mice compared with WT mice. Comparisons using littermate CB2KO and WT mice is warranted in future studies to address this question.
Our findings are potentially clinically relevant because coadministration of LY2828360 with fentanyl may be able to prevent opioid-induced respiratory depression before the harmful effects of an overdose ensue. An adjunctive pharmacological approach could be particularly advantageous for postsurgical pain and holds several advantages over naloxone, which is only a rescue mechanism and must be administered through injection or nasal spray within a narrow timeframe after opioid overdose to restore respiratory function.11 Importantly, naloxone administration causes a reversal of opioid-induced analgesia,62–64 in contrast to the opioid analgesic synergism displayed by CB2 agonists.35–38 Moreover, the short half-life of naloxone compared with fentanyl can cause “re-narcotization” leading to a reoccurrence of respiratory depression after naloxone tissue levels decline.12,65 Naloxone overantagonism can also cause iatrogenic harm and induce highly aversive withdrawal symptoms, pulmonary edema, and cardiac arrhythmias.66,67 Therefore, identification of a pharmacological treatment that lacks toxicity but safely blocks opioid-induced side effects such as respiratory depression before they manifest while sparing analgesia is an urgent clinical need.
LY2828360 is a potent G protein-biased CB2 agonist that does not recruit β-arrestin.35 Although reports have highlighted the importance of “biased opioid agonism” in the development of opioid-induced respiratory depression,68 a more recent study has refuted this claim.69 More research is necessary to extend our findings to other CB2 agonists and opioids and to test reversal dosing regimens using mice of both sexes. No serious adverse effects were reported in patients receiving LY2828360 (80 mg/day for 4 weeks) in a phase-2 clinical trial (www.clinicaltrials.gov identifier: NCT01319929).39 Although shown ineffective for treatment of osteoarthritic pain, this trial did not include opioid users; therefore, the impact of LY2828360 on opioid-induced side effects were not evaluated and remain unknown. Our studies suggest that LY2828360 could be repurposed for a new adjunctive therapeutic indication that attenuates fentanyl-induced respiratory depression.
Abbreviations Used
- ANOVA
analysis of variance
- CB2KO
CB2 knockout
- i.p.
intraperitoneal
- SEM
standard error of the mean
- WT
wild-type
Author Disclosure Statement
A.G.H. and K.M. are coinventors on a patent related to CB2-opioid analgesic mechanisms. None of the other authors report any conflicts of interest.
Funding Information
This study is supported by the National Institutes of Health National Institute on Drug Abuse (Grants DA047858, DA041229, DA009158, and DA042584 [A.G.H. and K.M.)], the National Cancer Institute (Grant CA200417 [A.G.H.]), an Indiana Addiction Grand Challenge Grant (A.G.H.), purchase of the plethysmograph was supported in part, with support from the Indiana Clinical and Translational Sciences Institute, funded in part by UL1 TR002529, an Advanced Summer Research Scholarship Program (C.A.Z.), and the Harlan Scholars Research Program (V.I.).
Cite this article as: Zavala CA, Thomaz AC, Iyer V, Mackie K, Hohmann AG (2020) Cannabinoid CB2 receptor activation attenuates fentanyl-induced respiratory depression, Cannabis and Cannabinoid Research 6:5, 389–400, DOI: 10.1089/can.2020.0059.
References
- 1. Wilson N, Kariisa M, Seth P, et al. Drug and opioid-involved overdose deaths-United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Donnell J, Gladden RM, Goldberger BA, et al. Notes from the field: opioid-involved overdose deaths with fentanyl or fentanyl analogs detected—28 states and the district of Columbia, July 2016–December 2018. MMWR Morb Mortal Wkly Rep. 2020;69:271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region-United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuczynska K, Grzonkowski P, Kacprzak L, et al. Abuse of fentanyl: an emerging problem to face. Forensic Sci Int. 2018;289:207–214. [DOI] [PubMed] [Google Scholar]
- 5. Marinetti LJ, Ehlers BJ. A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. J Anal Toxicol. 2014;38:592–598. [DOI] [PubMed] [Google Scholar]
- 6. Hill R, Santhakumar R, Dewey W, et al. Fentanyl depression of respiration: comparison with heroin and morphine. Br J Pharmacol. 2020;177:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100:747–758. [DOI] [PubMed] [Google Scholar]
- 8. Burns G, DeRienz RT, Baker DD, et al. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin Toxicol (Phila). 2016;54:420–423. [DOI] [PubMed] [Google Scholar]
- 9. Somerville NJ, O'Donnell J, Gladden RM, et al. Characteristics of fentanyl overdose-Massachusetts, 2014–2016. MMWR Morb Mortal Wkly Rep. 2017;66:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutter ME, Gerona RR, Davis MT, et al. Fatal fentanyl: one pill can kill. Acad Emerg Med. 2017;24:106–113. [DOI] [PubMed] [Google Scholar]
- 11. Fairbairn N, Coffin PO, Walley AY. Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: challenges and innovations responding to a dynamic epidemic. Int J Drug Policy. 2017;46:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson AB, Gladden RM, Delcher C, et al. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65:844–849. [DOI] [PubMed] [Google Scholar]
- 14. Schumann H, Erickson T, Thompson TM, et al. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46:501–506. [DOI] [PubMed] [Google Scholar]
- 15. Gregg LC, Jung KM, Spradley JM, et al. Activation of type 5 metabotropic glutamate receptors and diacylglycerol lipase-alpha initiates 2-arachidonoylglycerol formation and endocannabinoid-mediated analgesia. J Neurosci. 2012;32:9457–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piomelli D, Hohmann AG, Seybold V, et al. A lipid gate for the peripheral control of pain. J Neurosci. 2014;34:15184–15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiese B, Wilson-Poe AR. Emerging evidence for cannabis' role in opioid use disorder. Cannabis Cannabinoid Res. 2018;3:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benyo Z, Ruisanchez E, Leszl-Ishiguro M, et al. Endocannabinoids in cerebrovascular regulation. Am J Physiol Heart Circ Physiol. 2016;310:H785–H801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iring A, Hricisak L, Benyo Z. CB1 receptor-mediated respiratory depression by endocannabinoids. Respir Physiol Neurobiol. 2017;240:48–52. [DOI] [PubMed] [Google Scholar]
- 20. Padley JR, Li Q, Pilowsky PM, et al. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br J Pharmacol. 2003;140:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfitzer T, Niederhoffer N, Szabo B. Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. Br J Pharmacol. 2004;142:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmid K, Niederhoffer N, Szabo B. Analysis of the respiratory effects of cannabinoids in rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:301–308. [DOI] [PubMed] [Google Scholar]
- 23. Vivian JA, Kishioka S, Butelman ER, et al. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther. 1998;286:697–703. [PubMed] [Google Scholar]
- 24. Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. [DOI] [PubMed] [Google Scholar]
- 25. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. [DOI] [PubMed] [Google Scholar]
- 26. Schatz AR, Lee M, Condie RB, et al. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol. 1997;142:278–287. [DOI] [PubMed] [Google Scholar]
- 27. Onaivi ES, Ishiguro H, Gong JP, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. [DOI] [PubMed] [Google Scholar]
- 28. Maresz K, Carrier EJ, Ponomarev ED, et al. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Hoffert C, Vu HK, et al. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. [DOI] [PubMed] [Google Scholar]
- 30. Wotherspoon G, Fox A, McIntyre P, et al. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. [DOI] [PubMed] [Google Scholar]
- 31. Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. [DOI] [PubMed] [Google Scholar]
- 32. Gong JP, Onaivi ES, Ishiguro H, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. [DOI] [PubMed] [Google Scholar]
- 33. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. [DOI] [PubMed] [Google Scholar]
- 34. Yu Y, Fuscoe JC, Zhao C, et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat Commun. 2014;5:3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin X, Dhopeshwarkar AS, Huibregtse M, et al. Slowly signaling G protein-biased CB2 cannabinoid receptor agonist LY2828360 suppresses neuropathic pain with sustained efficacy and attenuates morphine tolerance and dependence. Mol Pharmacol. 2018;93:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li AL, Lin X, Dhopeshwarkar AS, et al. Cannabinoid CB2 agonist AM1710 differentially suppresses distinct pathological pain states and attenuates morphine tolerance and withdrawal. Mol Pharmacol. 2019;95:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuill MB, Hale DE, Guindon J, et al. Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol Pain. 2017;13:1744806917728227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grenald SA, Young MA, Wang Y, et al. Synergistic attenuation of chronic pain using mu opioid and cannabinoid receptor 2 agonists. Neuropharmacology. 2017;116:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pereira A, Chappell A, Dethy J, et al. A proof-of-concept (POC) study including experimental pain models (EPMs) to assess the effects of a CB2 agonist (LY2828360) in the treatment of patients with osteoarthritic (OA) knee pain. Clin Pharmacol Ther. 2013;93:S56–S57. [Google Scholar]
- 40. Hill R, Lyndon A, Withey S, et al. Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology. 2016;41:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith WD. A comparison in mice of naloxone and nalorphine as antagonists to neuroleptanalgesic drugs. Br J Anaesth. 1976;48:1039–1044. [DOI] [PubMed] [Google Scholar]
- 42. Motulsky H. Intuitive biostatistics: a nonmathematical guide to statistical thinking. Oxford University Press: New York, 2010. [Google Scholar]
- 43. Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths-United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weed PF, Gerak LR, France CP. Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur J Pharmacol. 2018;833:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cui YY, D'Agostino B, Risse PA, et al. Cannabinoid CB(2) receptor activation prevents bronchoconstriction and airway oedema in a model of gastro-oesophageal reflux. Eur J Pharmacol. 2007;573:206–213. [DOI] [PubMed] [Google Scholar]
- 46. Yoshihara S, Morimoto H, Ohori M, et al. The cannabinoid receptor agonist WIN 55212-2 inhibits neurogenic inflammations in airway tissues. J Pharmacol Sci. 2005;98:77–82. [DOI] [PubMed] [Google Scholar]
- 47. Belvisi MG, Chung KF, Jackson DM, et al. Opioid modulation of non-cholinergic neural bronchoconstriction in guinea-pig in vivo. Br J Pharmacol. 1988;95:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karlsson JA, Lanner AS, Persson CG. Airway opioid receptors mediate inhibition of cough and reflex bronchoconstriction in guinea pigs. J Pharmacol Exp Ther. 1990;252:863–868. [PubMed] [Google Scholar]
- 49. Patel HJ, Birrell MA, Crispino N, et al. Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol. 2003;140:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Calignano A, Katona I, Desarnaud F, et al. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. [DOI] [PubMed] [Google Scholar]
- 51. Watkins LR, Hutchinson MR, Rice KC, et al. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zwicker JD, Zhang Y, Ren J, et al. Glial TLR4 signaling does not contribute to opioid-induced depression of respiration. J Appl Physiol (1985). 2014;117:857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hutchinson MR, Northcutt AL, Chao LW, et al. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008;22:1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167–171. [DOI] [PubMed] [Google Scholar]
- 55. Fernandez-Lopez D, Martinez-Orgado J, Nunez E, et al. Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr Res. 2006;60:169–173. [DOI] [PubMed] [Google Scholar]
- 56. Ashton JC, Rahman RM, Nair SM, et al. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. [DOI] [PubMed] [Google Scholar]
- 57. Merighi S, Gessi S, Varani K, et al. Cannabinoid CB(2) receptor attenuates morphine-induced inflammatory responses in activated microglial cells. Br J Pharmacol. 2012;166:2371–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang W, Xiong Y, Chen Y, et al. NOX2 is involved in CB2-mediated protection against lung ischemia-reperfusion injury in mice. Int J Clin Exp Pathol. 2020;13:277–285. [PMC free article] [PubMed] [Google Scholar]
- 59. Zeng J, Li X, Cheng Y, et al. Activation of cannabinoid receptor type 2 reduces lung ischemia reperfusion injury through PI3K/Akt pathway. Int J Clin Exp Pathol. 2019;12:4096–4105. [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Z, Wang Y, Zhao H, et al. CB2 receptor activation ameliorates the proinflammatory activity in acute lung injury induced by paraquat. Biomed Res Int. 2014;2014:971750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Olfson M, Wall M, Wang S, et al. Service use preceding opioid-related fatality. Am J Psychiatry. 2018;175:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manage. 2002;23:48–53. [DOI] [PubMed] [Google Scholar]
- 63. Latasch L, Zimmermann M, Eberhardt B, et al. [Treatment of morphine-induced constipation with oral naloxone]. Anaesthesist. 1997;46:191–194 (Article in German). [DOI] [PubMed] [Google Scholar]
- 64. Sykes NP. An investigation of the ability of oral naloxone to correct opioid-related constipation in patients with advanced cancer. Palliat Med. 1996;10:135–144. [DOI] [PubMed] [Google Scholar]
- 65. Lotsch J. Pharmacokinetic-pharmacodynamic modeling of opioids. J Pain Symptom Manage. 2005;29(5 Suppl):S90–S103. [DOI] [PubMed] [Google Scholar]
- 66. Neale J, Strang J. Naloxone—does over-antagonism matter? Evidence of iatrogenic harm after emergency treatment of heroin/opioid overdose. Addiction. 2015;110:1644–1652. [DOI] [PubMed] [Google Scholar]
- 67. van Dorp EL, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf. 2007;6:125–132. [DOI] [PubMed] [Google Scholar]
- 68. Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. [DOI] [PubMed] [Google Scholar]
- 69. Kliewer A, Gillis A, Hill R, et al. Morphine-induced respiratory depression is independent of beta-arrestin2 signalling. Br J Pharmacol. 2020. [Epub ahead of print]; DOI: 10.1111/bph.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]