Abstract
This case report describes the treatment of a skeletal Class III malocclusion with autotransplantation of a cryopreserved tooth. To gain an esthetic facial profile and good occlusion, extraction of bimaxillary premolars and surgical therapy were chosen. The patient had chronic apical periodontitis on the lower left first molar. Although she did not feel any pain in that region, the tooth was considered to have a poor prognosis. Therefore, we cryopreserved the extracted premolars to prepare for autotransplantation in the lower first molar area because the tooth would probably need to be removed in the future. The teeth were frozen by a programmed freezer with a magnetic field (CAS freezer) that was developed for tissue cryopreservation and were cryopreserved in −150°C deep freezer. After 1.5 years of presurgical orthodontic treatment, bilateral sagittal split ramus osteotomy was performed for mandible setback. Improvement of the facial profile and the occlusion were achieved in the retention phase. Six years after the initial visit, the patient had pain on the lower left first molar, and discharge of pus was observed, so we extracted the lower left first molar and autotransplanted the cryopreserved premolar. Three years later, healthy periodontium was observed at the autotransplanted tooth. This case report suggests that long-term cryopreservation of teeth by a CAS freezer is useful for later autotransplantation, and this can be a viable technique to replace missing teeth.
Keywords: Autotransplantation, Cryopreservation, Programmed freezer, Magnetic field
INTRODUCTION
Autotransplantation of teeth is useful procedure for recovering occlusal function by replacing missing teeth. Unnecessary wisdom teeth and teeth extracted for orthodontic treatment are mainly used as donor teeth. Autotransplanted teeth can regenerate the alveolar bone of the socket through the osteoinduction ability of periodontal ligament (PDL) cells.1,2 This treatment is reliable and associated with good outcomes.3 However, sometimes patients may not have an available donor tooth because it was previously extracted. To solve this problem, teeth cryopreservation systems have been developed. Many clinical reports and animal experiments show the efficacy of teeth cryopreservation.4–9 However, in some cases, transplantation of a cryopreserved tooth may result in tooth-bone ankylosis or root resorption because of PDL cell damage induced by ice crystal formation inside the cells. Recently, we developed a programmed freezer with a magnetic field called the Cells Alive System (CAS) (ABI Corporation Ltd, Abiko, Japan). A CAS freezer can prevent ice crystal formation, and our previous findings showed that a 0.1-mT magnetic field, a 15-minute hold time, and a plunging temperature of −30°C led to the greatest survival rate for PDL cells.10 However, autotransplantation of human cryopreserved teeth by a CAS freezer has not yet been performed. This case report describes the treatment of a skeletal Class III malocclusion with a tooth autotransplanted after cryopreservation for 6 years.
CASE REPORT
Pretreatment Evaluation
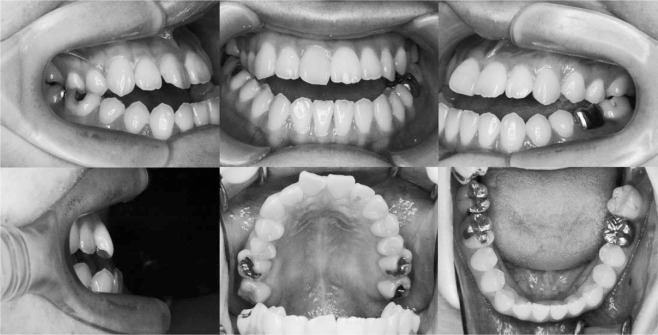
The patient, a woman aged 24 years and 1 month, complained of a mandibular protrusion. Pretreatment facial photographs showed a long face and a concave profile (Figure 1). Intraoral photographs and dental casts revealed a −5.0 mm overjet and a −2.5 mm overbite. The right and left molar relationships were both Class III. The upper dental midline coincided with the facial midline, but the lower midline was shifted to the left by 1 mm (Figure 2). Panoramic radiograph showed a periapical cyst on the lower left first molar, although there was no clinical symptom (Figure 3).
Figure 1.
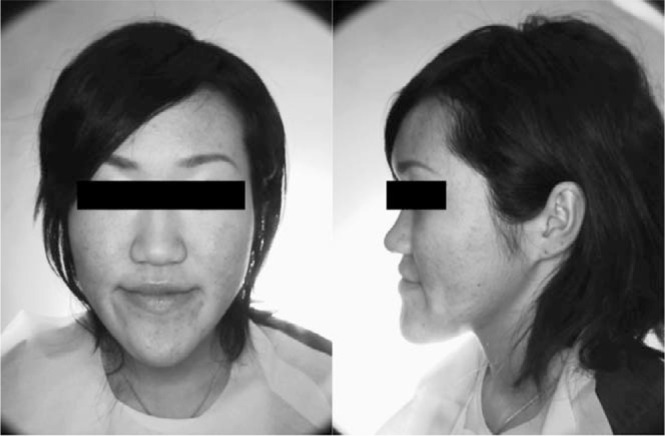
Pretreatment facial photographs.
Figure 2.
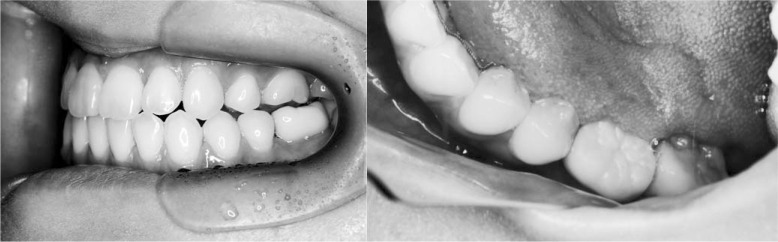
Pretreatment intraoral photographs.
Figure 3.

Pretreatment panoramic radiograph.
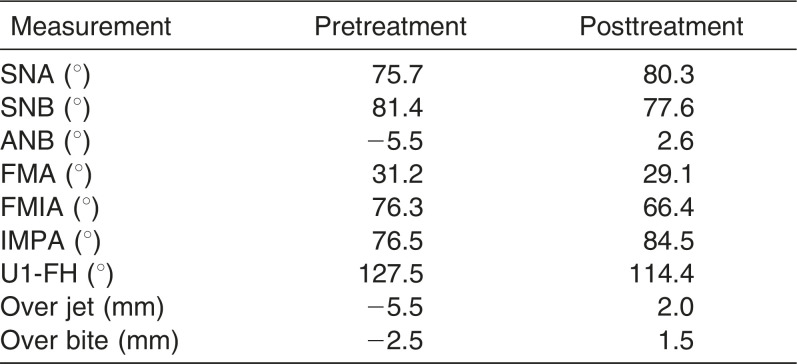
We referred the patient to endodontists. They removed the metal crown for endodontic treatment but suggested that we should observe the progress without treating the radicular cyst because the dentin was very thin and likely to be broken during root canal treatment. Therefore, the temporary crown was set on the lower left first molar and application of orthodontic force was avoided. As shown in the cephalometric measurements, a skeletal Class III relationship (ANB angle, −5.5°) with posterior shift of the maxilla and anterior shift of the mandible were observed. Labial inclination of the upper incisors (U1-FH, 127.5°) and lingual inclination of lower incisors (IMPA, 76.5°) were also evident (Table 1). Based on the information, the patient was diagnosed as having skeletal Class III mandibular protrusion with an open bite. Therefore, we recommended extraction of the upper first premolar for orthodontic treatment with double jaw surgery. We also suggested cryopreservation of the extracted upper first premolars. The study was approved by the Ethics Committee of Hiroshima University (D65-1), and informed consent was obtained before tooth extraction.
Table 1. .
Summary of Cephalometric Measurements
Treatment Plan
The treatment objectives essentially consisted of repositioning of two jaws and extracting the upper first premolars. The treatment plan was as follows.
Extraction of the upper first premolars (cryopreservation)
Lingual inclination of the upper incisors and labial inclination of the lower incisors by presurgical orthodontic treatment
Two jaw surgery (upper: 5 mm advance by Le Fort I osteotomy; lower: 5 mm setback by sagittal split ramus osteotomy)
Postsurgical orthodontic treatment
Treatment Progress
Under local anesthesia, the upper premolars were extracted. The teeth were immediately put into the Teeth Keeper NEO (Neo Dental International Inc, Tokyo, Japan). Then, the teeth were placed in tempered hard-glass vials (SV-10, Nichiden-Rika Glass Co, Ltd, Kobe, Japan) with 5 mL preservative media (Bambanker 2, Lymphotec, Tokyo, Japan) that contained 10% dimethyl sulfoxide. The teeth were frozen by a CAS programmed freezer containing a magnetic field (ABI Corporation Ltd). The CAS freezer was programmed to freeze cells at −0.5°C/min with a 0.1-mT magnetic field, a 15-minutte hold time at −5°C, and a plunging temperature of −30°C according to our previous study.10–12 Then, the teeth were cryopreserved at −150°C.
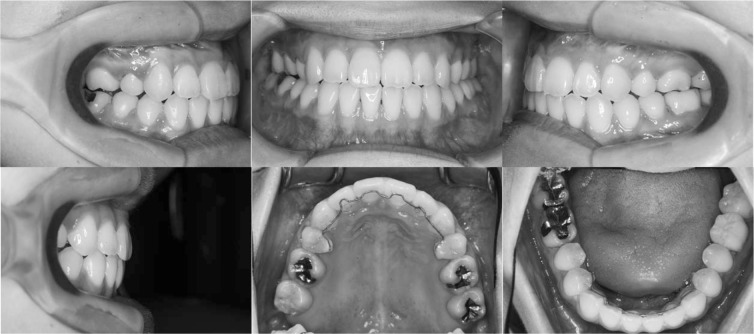
The upper canines and incisors were distalized, and both arches were aligned so that the two jaws fit together properly. After 18 months, the tooth alignment was finished and orthognathic surgery was carried out. The maxilla was moved forward 5 mm by Le Fort I osteotomy, and the mandible was moved backward 5 mm by sagittal split ramus osteotomy. Postsurgical orthodontic treatment took 10 months, and the teeth were well aligned with a good intercuspal relation (Figure 4). After removal of the edgewise brackets, lingual bonded retainers were set on both the upper and lower arches between the premolars.
Figure 4.
Posttreatment intraoral photographs.
Treatment Results
A normal overjet and overbite were achieved and the upper and lower midlines coincided. The upper and lower dental arches were well aligned and a Class II molar relationship was achieved. The facial profile was improved but facial asymmetry and long facial height remained (Figure 5). The posttreatment panoramic radiograph showed that all the roots were almost parallel, and the size of the periapical cyst on the lower left first molar did not change (Figure 6). Cephalometric analysis showed that IMPA changed from 76.5° to 84.5° and the ANB angle changed from −5.5° to 2.6° (Table 1).
Figure 5.
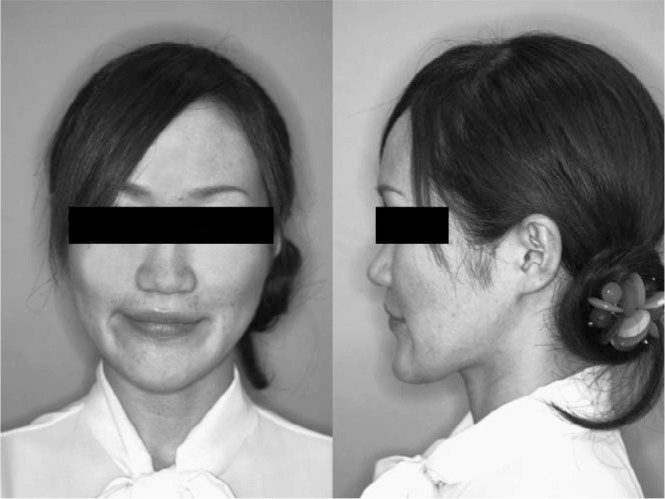
Posttreatment facial photographs.
Figure 6.
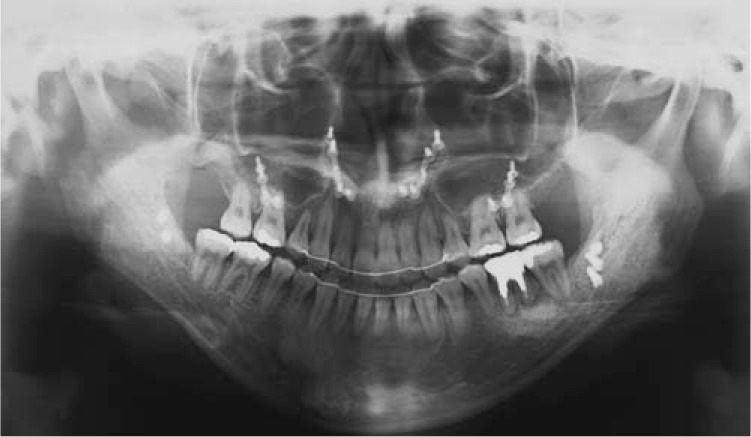
Posttreatment panoramic radiograph.
Autotransplantation of the Cryopreserved Tooth
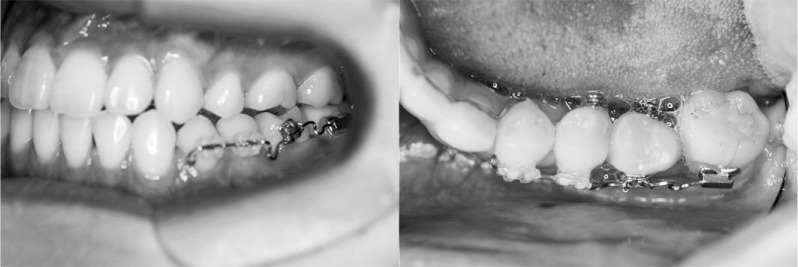
Six years after the premolars were cryopreserved, the patient had percussion pain on the lower left first molar and discharge of pus was observed. The dental x-ray showed that the periapical cyst had grown (Figure 7), and endodontists tried to treat the root canal disease. However, the mesial root was cracked and the tooth was designated for extraction. Therefore, we decided to autotransplant the previously cryopreserved premolar. After extraction of the lower left first molar, pus discharge, softened bone, and granulation tissue were observed, so we removed them carefully by a sharp spoon before autotransplantation. Then, the cryopreserved tooth was applied to a fitness test into the socket after thawing in a 37°C water bath. The tooth was kept in the Teeth Keeper NEO during socket preparation and fixed with a 0.7-mm Co-Cr wire after transplantation (Figure 7). The dental radiograph of the transplanted tooth right after autotransplantation showed mesial inclination, and there was no bone on the mesial side (Figure 8). Two weeks after autotransplantation, endodontic treatment was started and the root canal was filled with gutta-percha point and sealer. One month after autotransplantation, we started to upright the transplanted tooth by a sectional arch (Figure 9). Three years after the autotransplantation, the final dental prosthesis was set on the transplanted tooth, although a little mesial angulation remained (Figure 10). Bone regeneration, physiologic tooth mobility, healthy periodontal ligament, and lamina dura were observed. Inflammatory root resorption and replacement resorption were not observed (Figure 11).
Figure 7.
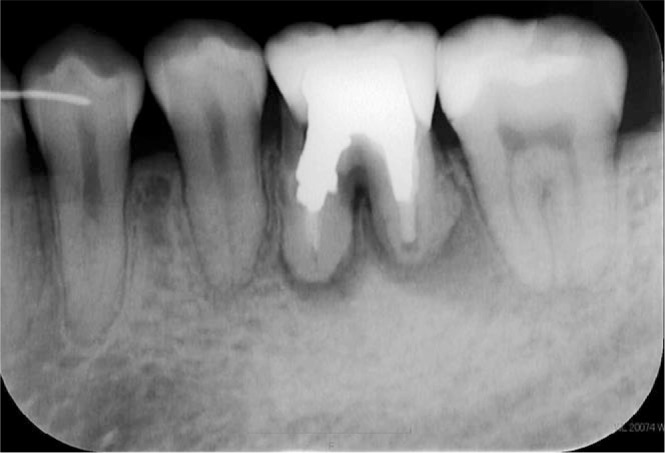
Dental radiograph of the lower left first molar 6 years after the initial visit.
Figure 8.

Dental radiograph of the transplanted tooth right after autotransplantation.
Figure 9.
Intraoral photographs of the transplanted tooth, which was started to upright by a sectional arch 1 month after autotransplantation.
Figure 10.
Intraoral photographs of the transplanted tooth 3 years after autotransplantation.
Figure 11.
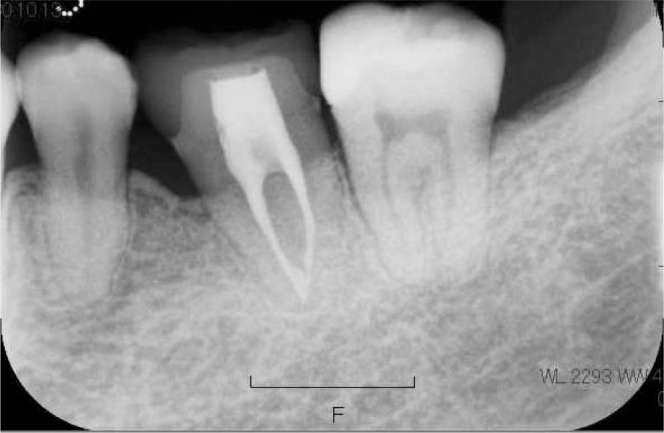
Dental radiograph of the transplanted tooth 3 years after autotransplantation.
DISCUSSION
In the present case, we needed to correct the overjet, molar relationship, and facial profile. Thus, we planned for repositioning of the jaws with extraction of upper first premolars. As a result, the patient's profile, overjet, and molar relationship were improved. However, slight facial asymmetry and long facial height still remained. As for the proportion of the face and frontal view, genioplasty should have been applied.
Andreasen et al.13 reported that autotransplanted teeth with incomplete and complete roots had more than a 95% survival rate after a long observation period. Czochrowska et al14 also reported long-term outcomes of tooth transplantation 17 to 41 years posttreatment and showed that the tooth survival rate was 90%. Therefore, autotransplantation can be a more appropriate alternative treatment for replacing missing teeth. However, opportunities for immediate tooth transplantation are limited. For example, the patient must have a healthy donor tooth at the same time transplantation is needed. When autotransplantation is necessary but the recipient site is too small and orthodontic treatment is needed to gain space, immediate tooth transplantation is also impossible.
Therefore, the study of teeth cryopreservation has been examined in basic studies15–17 and clinical studies.4–9 Periodontal healing after cryopreservation is a critical factor for success after cryopreservation. However, cells are easily damaged by ice crystal formation, which leads to tooth-bone ankylosis and root resorption. Recently, a cryopreservation technology was developed in the food industry. When frozen flesh is thawed, the ruptured cells release water and many of the compounds that provide certain flavors, resulting in a dry, tasteless product. We collaborated with ABI Corporation Ltd, a company that produces freezers for food preservation, to develop a special freezer using the CAS magnetic field. Because cells contain a cluster of water molecules, when the water freezes this cluster grows and injures the cell membrane. However, alternating magnetic fields with an induced electric field generated from the CAS freezer vibrates cells and water molecules by a nonthermal mechanism. These vibrations were amplified in sympathy with mechanical and thermal vibration (CAS vibration). This CAS vibration prevents intracellular ice crystal formation by causing it to vibrate.10 Iwasaka et al.18 clarified the effect and mechanism of magnetic fields on freeze processes in aqueous solutions. They showed that fine ice crystals, which strongly scattered the irradiated light, increased in number in the early phase of the supercooled state when the pulsed train magnetic field was applied. For tissue cryopreservation, a suitable hold time is important to allow the cryoprotectant to pass into the cell membrane, and a suitable plunging temperature is crucial for preventing damage from intracellular ice crystal formation. We found that a 0.1-mT magnetic field, a 15-minute hold time, and a plunging temperature of −30°C led to the greatest survival rate (90% of cells survived immediately after thawing) and viability rate of PDL cells.10 A follow-up study showed no differences in PDL cell appearance between those cryopreserved by CAS for 5 years and controls.11 Moreover, we investigated periodontal healing after replantation of a rat incisor that was cryopreserved by CAS.
No progressive root resorption was found in the teeth that were replanted immediately (control group) or cryopreserved.12 Also in this case report, bone regeneration, healthy periodontal ligament, and lamina dura were observed 3 years after the autotransplantation of cryopreserved tooth. Inflammatory root resorption and replacement resorption were not observed, and the transplanted tooth continued to stay stable. These findings strongly suggested that a CAS freezer can be used for long-term tooth cryopreservation and later for autotransplantation. Mechanical properties such as cracks and fractures of cryopreserved tooth are also critical after autotransplantation. Intracellular ice crystal formation increases the width of the cells in the pulp and the size of the odontoblastic dendrites localized in the dentinal tubules. Then the expansion of the pulp tissue might directly cause cracks in dentin and enamel. However, Kühl et al.19 showed that cracks in dentin after cryopreservation are absent or narrower than 1.84-µm. Therefore, we suppose that cryopreservation may lead to only a very few cracks in the enamel.19 However, periodic checkups and more evidence regarding masticatory function and periodontal healing of many transplanted cryopreserved teeth are needed through randomized clinical studies in the future.
CONCLUSIONS
We cryopreserved premolars extracted for orthodontic treatment for 6 years.
Three years after autotransplantation of cryopreserved tooth, bone regeneration, healthy PDL, and lamina dura were observed.
Inflammatory root resorption and replacement resorption were not observed. Therefore, a CAS freezer is useful for long-term teeth cryopreservation.
ACKNOWLEDGMENT
We would like to express gratitude to Mr N. Ohwada (ABI Corporation Ltd, Abiko, Japan) for valuable information and suggestions.
REFERENCES
- 1.Kristerson L, Johansson LA, Kisch J, Stadler LE. Autotransplantation of third molars as treatment in advanced periodontal disease. J Clin Periodontol. 1991;18:521–528. doi: 10.1111/j.1600-051x.1991.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 2.Shimono M, Ishikawa T, Ishikawa H, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491–502. doi: 10.1002/jemt.10290. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg T, Isaksson S. A clinical follow-up study of 278 autotransplanted teeth. Br J Oral Maxillofac Surg. 1996;34:181–185. doi: 10.1016/s0266-4356(96)90374-5. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz O. Cryopreservation as long-term storage of teeth for transplantation or replantation. Int J Oral Maxillofac Surg. 1986;15:30–32. doi: 10.1016/s0300-9785(86)80008-4. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz O, Rank CP. Autotransplantation of cryopreserved tooth in connection with orthodontic treatment. Am J Orthod Dentofac Orthop. 1986;90:67–72. doi: 10.1016/0889-5406(86)90029-6. [DOI] [PubMed] [Google Scholar]
- 6.Laureys W, Beele H, Cornelissen R, Dermaut L. Revascularization after cryopreservation and autotransplantation of immature and mature apicoectomized teeth. Am J Orthod Dentofac Orthop. 2001;19:346–352. doi: 10.1067/mod.2001.113259. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki N, Hamamoto Y, Nakajima T, Irie K, Ozawa H. Periodontal regeneration of transplanted rat molars after cryopreservation. Arch Oral Biol. 2004;49:59–69. doi: 10.1016/j.archoralbio.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Paulsen HU, Andreasen JO, Schwartz O. Tooth loss treatment in the anterior region: autotransplantation of premolars and cryopreservation. WorldJ Orthod. 2006;7:27–34. [PubMed] [Google Scholar]
- 9.Temmerman L, De Pauw GA, Beele H, Dermaut LR. Tooth transplantation and cryopreservation. Am J Orthod Dentofac Orthop. 2006;129:691–695. doi: 10.1016/j.ajodo.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Kaku M, Kamada H, Kawata T, et al. Cryopreservation of periodontal ligament cells with magnetic field for teeth banking. Cryobiology. 2010;61:73–78. doi: 10.1016/j.cryobiol.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Abedini S, Kaku M, Kawata T, et al. Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues. Cryobiology. 2011;62:181–187. doi: 10.1016/j.cryobiol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Kamada H, Kaku M, Kawata T. In-vitro and in-vivo study of periodontal ligament cryopreserved with a magnetic field. Am J Orthod Dentofac Orthop. 2011;140:799–805. doi: 10.1016/j.ajodo.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen JO, Paulsen HU, Yu Z, Bayer T, Schwartz O. A long-term study of 370 autotransplanted premolars. Part 2. Tooth survival and pulp healing subsequent to transplantation. Eur J Orthod. 1990;12:14–24. doi: 10.1093/ejo/12.1.14. [DOI] [PubMed] [Google Scholar]
- 14.Czochrowska EM, Stenvik A, Bjercke B, Zachrisson BU. Outcome of tooth transplantation: survival and success rates 17–41 years posttreatment. Am J Orthod Dentofac Orthop. 2002;121:110–119. doi: 10.1067/mod.2002.119979. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki N, Hamamoto Y, Nakajima T, Irie K, Ozawa H. Periodontal regeneration of transplanted rat molars after cryopreservation. Arch Oral Biol. 2004;49:59–69. doi: 10.1016/j.archoralbio.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J. Cryopreservation of human teeth for future organization of a tooth bank—a preliminary study. Cryobiology. 2005;51:322–329. doi: 10.1016/j.cryobiol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Izumi N, Yoshizawa M, Ono Y, Kobayashi T, Hamamoto Y, Saito C. Periodontal regeneration of transplanted rat teeth subcutaneously after cryopreservation. Int J Oral Maxillofac Surg. 2007;36:838–844. doi: 10.1016/j.ijom.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaka M, Onishi M, Kurita S, Owada N. Effects of pulsed magnetic fields on the light scattering property of the freezing process of aqueous solutions. J Appl Phys. 2011;109: 07E320:1–3. [Google Scholar]
- 19.Kühl S, Deyhle H, Zimmerli M, et al. Cracks in dentin and enamel after cryopreservation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e5–e10. doi: 10.1016/j.tripleo.2011.06.020. [DOI] [PubMed] [Google Scholar]