Abstract
Objective:
To evaluate in vitro the shear bond strength of brackets recycled by sandblasting with aluminum oxide particles of different sizes or reconditioned industrially after successive rebonding.
Materials and Methods:
Eighty brackets were bonded and debonded sequentially three times. After the first debonding, brackets were divided into four groups: (group 1) sandblasting with aluminum oxide particles of 25 μ, (group 2) 50 μ, and (group 3) 110 μ, and (group 4) industrial recycling. Bond strength and adhesive material remaining on debonded bracket bases were evaluated for each successive debond.
Results:
No significant differences were detected between the four groups following the first recycle (P > .05). After the second recycle, bond strength was significantly greater for the industrially recycled group than the other groups (P < .016). When shear bond strength was compared within each recycling method, the bond strength of sandblasted brackets decreased with the increase of particle size and with each recycle; for the industrially recycled group, no significant differences were detected between the three sequences (P > .016). In the evaluation of bond material remnant, the industrially recycled group left significantly less bond material after successive recycling than the other groups did (P < .016). Within each recycling method, the adhesive remnant decreased significantly after successive debond (P < .016).
Conclusions:
Industrial recycling obtained better results than sandblasting after three successive debondings. The brackets' shear bond strength decreased as the size of the aluminum oxide particle used for sandblasting increased and as recycling was repeated.
Keywords: Recycled, Sandblasting, Industrial recycling, Shear bond strength
INTRODUCTION
Bracket debonding, whether accidental or performed deliberately by the orthodontist, is a fairly frequent event as orthodontic treatments proceed. Regardless of the cause of debonding, the orthodontist must decide whether to rebond the same bracket or bond a new one.1,2 Brackets can be recycled indirectly by sending them to external specialized reconditioning services or directly in the orthodontic clinic.
Among the methods used in industrial recycling, the most commonly used apply heat to burn the bond agent followed by electrolytic polishing to eliminate the remaining oxide, or they use chemical agents to dissolve the bond agent in combination with high-frequency vibration and electrochemical polishing.3–5 Various researchers have observed a reduction in shear bond strength (SBS) after industrial bracket recycling of 6%–20%,6 reaching 35% for finer mesh-type brackets.4 However, one in vivo study7 compared the clinical behavior of industrially recycled brackets and new brackets with a 12-month follow-up but found no significant differences in bond failure percentages. Other studies have reported some metal loss in parts of the bracket and a reduction in the diameter of the mesh wires among commercially recycled brackets, whether reconditioned using heat or chemicals.4,6,8 Nevertheless, these changes did not seem to affect bond strength.1,8
The other option—recycling in the clinic—can use various techniques: mechanical (micro sandblasting), thermal (direct burning), or mixed. Nowadays, sandblasting is widely used, and numerous studies9–14 have shown that sandblasting increases the bond strength and survival time of new brackets. Research comparing reconditioning methods used in the clinic has concluded that sandblasting is the most effective method for removing bond material, while no significant differences in bond strength were identified between brackets recycled by this means and new brackets.15–17
Studies of brackets that have undergone successive recycling show contradictory results. Regan et al.18 found no significant differences in SBS among metal brackets that had been recycled up to five times, while Buchwald5 found that the percentage of brackets that could be reused decreased with each successive recycle. Martina et al.19 found no significant dimensional changes in industrially reconditioned ceramic brackets recycled up to 10 times but did find slight reductions in SBS in comparison with new brackets. For Matasa,11 the main advantage of recycling is the economic savings, which can reach 90% if a single bracket is recycled five times.
As far as we are aware, no studies to date have compared brackets recycled by means of sandblasting (with aluminum oxide particles of different sizes) with brackets reconditioned industrially, nor has any research been carried out into the effects of various sequential recycles of a single bracket by means of these procedures.
The aim of this study was to evaluate in vitro the effects of reconditioning metal brackets by sandblasting with aluminum oxide particles of different sizes or by industrial recycling after first and second rebondings/recycles.
MATERIALS AND METHODS
The study used 300 bovine upper central incisors. They were washed in distilled water and submerged in 0.1% thymol solution for 24 hours. Afterward, the teeth were placed in distilled water, which was changed every 24 hours until the moment of use to avoid deterioration.
The SBS test used 240 teeth, while the remaining 60 teeth underwent scanning electron microscope (SEM) observation.
Brackets
The study used 125 upper central incisor brackets (Victory Series, 3M Unitek Dental Products, Monrovia, Calif), of which 80 were used for SBS testing and 45 for SEM observation. The bracket base area was calculated using image analysis equipment and MIP4 software (Microm Image Processing Software, Digital Systems, Barcelona, Spain), obtaining a mean area of 10.25 mm2.
Bonding Procedure
The vestibular surfaces of the teeth were cleaned by brushing with prophylactic cream (Detartrine, Septodont, France), washed, and dried with compressed air.
Then, 80 brackets were bonded to the vestibular surfaces of the teeth with Transbond Plus Self Etching Primer (3M Unitek Dental Products) and Transbond XT Paste (3M Unitek Dental Products) following the manufacturer's instructions for each of the products involved. Excess resin around the bracket base was removed with a dental probe. Brackets were polymerized for 10 seconds on each side of the bracket with a polymerization lamp (Demetron LC, Kerr Corporation, Orange, Calif). Specimens were submerged in distilled water at 37°C for 24 hours.
The SBS was measured using a universal test machine (Autograph AGS-1KND, Shimadzu, Kyoto, Japan) with a 1KN load cell, connected to a metal bar with one end angled at 30°. The machine head speed was 1 mm/min.
The specimens were placed in the machine so that the angled end of the test bar would act at the point between the bracket base and tooth surface in the incisor-apical direction.
The force required to debond each bracket was recorded in Newtons (N) and converted into Megapascales (MPa) by the following formula: MPa = N/mm2.
After debonding brackets, pairs of images were captured using a Leica Z6 APO macroscope and a Leica DC500 camera connected to a PC; the first image was captured normally and the second used a Leica fluorescence illuminator with I3 filter. Images were then generated using Leica Application Suite V 2.5.0 R1 software (Leica Microsystems, Switzerland) and magnified 75×.
The bracket base area covered with bond material was measured using Image Pro Plus image analysis software (IPP 5.1 for Windows; Media Cybernetics, Silver Spring, Md).
The percentage of area occupied by bond material after debonding was calculated as the difference between 100% and the percentage of area covered by bond material.
Intraexaminer Error Assessment
All measurements were made by a single technician. Measurements of two different groups were made twice, with the two separated by an interval of 30 days. Data were analyzed applying Student's t-test for paired samples and Pearson's correlation test. Significance was set at P < .05.
No significant differences were detected between the first and second bond material measurements between the first evaluation time and the second (P = .09); the Pearson correlation test detected a significant correlation between the two measurement times (P = .00 and r = .96).
Bracket Base Cleaning Procedures
Brackets were divided randomly into four groups according to cleaning method:
Group 1: sandblasting with 25 μ Al2O3
Group 2: sandblasting with 50 μ Al2O3
Group 3: sandblasting with 110 μ Al2O3
Group 4: industrial reconditioning
For sandblasting, an instrument was designed that would allow the perpendicular placement of brackets at a distance of 5 mm from the tip of the micro etcher (Danville Engineering, Inc, Danville, Calif). Microparticles were blasted at 5 bar pressure. Each bracket was sandblasted until no adhesive material remained on the base. The time taken to sandblast each bracket was recorded.
The brackets to be reconditioned industrially were sent away to the company Ortho-Service (La Seyne-sur-Mer, France). The brackets were cleaned by the Laserblow method, which uses an Nd-YAG laser to release the bond material particles adhered to the metal structure. The Nd-YAG laser uses an impulse of 10−8 seconds and a high energy of 108 W. Following this method, and according to the manufacturer, the bracket did not heat (only 10° up to 1-µm depth). Afterward, the brackets were polished at high speed using only biocompatible natural components. Ortho-Service offers a certified quality (ISO 9001, ISO 13485) and has enjoyed this CE certification for reconditioning brackets and archwires since 1999.
Bracket Bonding and Rebonding
Bonding/debonding procedures were repeated three times for each bracket. A new tooth was used for each successive rebonding. The brackets were all bonded using the same procedure as described above.
Statistical Analysis
The SBS and percentage of area occupied by remnant bond material for the four cleaning procedures were compared after each debonding sequence. Comparisons were also performed to determine whether significant differences existed in SBS and percentage of area of bond material remaining on teeth between the three debonding sequences within each bracket base cleaning procedure. The Kolmogorov-Smirnov normality test (P < .05) and the Levene homogeneity of variance test (P < .05) were applied to the data for bond strength and percentage of area of bond material remaining on teeth after debonding. As it was seen that data were not distributed normally or failed to fulfill the criteria for variance homogeneity, they were analyzed using the Kruskall-Wallis test (P < .05), identifying those groups that were significantly different with the Mann-Whitney test for two independent samples. To avoid an accumulation of errors due to multiple comparisons, the significance level was modified, dividing this (P < .05) by the number of comparisons made (Bonferroni correction), and P < .016 was considered significant when three comparisons were made and P < .008 for six comparisons.
Data registering the time taken to clean brackets with the three sizes of particle for each debonding sequence were analyzed; when the data fulfilled the criteria for normality and homogeneity of variance by means of variance analysis for one factor and when that data were not distributed normally or failed to fulfill the criteria for variance homogeneity, they were analyzed using the Kruskall-Wallis test (P < .05).
SEM Observation
Forty-five brackets were examined under SEM, divided into five groups:
Control group: new brackets
Group 1: sandblasting with 25 μ Al2O3
Group 2: sandblasting with 50 μ Al2O3
Group 3: sandblasting with 110 μ Al2O3
Group 4: industrial recycling.
To assess the remaining bond material and any possible structural damage done to the bracket bases, brackets were examined when unused and thereafter following each of the four reconditioning/recycling protocols (three sizes of particle or industrial reconditioning) after each debonding sequence. The same bracket bonding and rebonding protocol was followed as for SBS testing, except for the fact that brackets were debonded using debonding pliers (ETM 345RT, Unitek/3M).
Brackets were cleaned in distilled water in an ultrasonic tank for 30 minutes, dried with compressed air, and then examined using an SEM (Jeol 6100, Tokyo, Japan), at an operating voltage of 15 kV and at a 20-mm distance. Images were captured and enlarged 100× and 1500×.
RESULTS
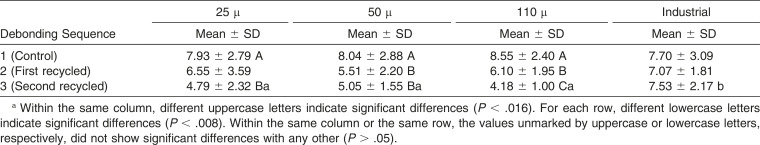
No significant differences were detected between the four groups after the first (control; P = .65) and second (first recycle; P = .05) debonding. At the third sequence (second recycle), the bond strength achieved by the industrially reconditioned brackets was significantly higher than the rest of the groups (25 μ, P = .00; 50 μ, P = .00; 110 μ, P = .00; Table 1).
Table 1. .
Mean Shear Bond Strength (MPa) and Standard Deviation (SD) for Each Group After Different Debonding/Recycling Sequencesa
When the SBS of the three sequences were compared within each cleaning procedure, it was found that bond strength for the 25 μ group was significantly lower at the third sequence (second recycle) than at the first (control; P = .000). The 50 μ group showed significantly greater bond strength at the first sequence (control) than at the second (first recycle) and the third (second recycle; P = .002 and P = .000, respectively). For the 110 μ group, bond strength at the first sequence (control) was significantly greater than at the second (first recycle) and the third (second recycle; P = .001 and P = .000, respectively); likewise, bond strength at the second sequence (first recycle) was significantly higher than at the third (second recycle; P = .000). With the industrial reconditioning, no significant differences were found between the three sequences (P = .86; Table 1).
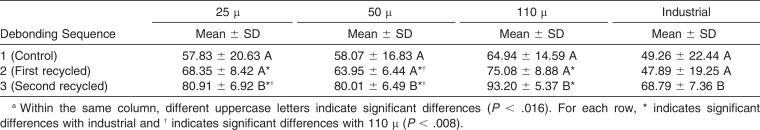
For all four reconditioning procedures, the percentage of tooth occupied by bond material remnant after debonding was higher after the third sequence (second recycle) than the first (control; 25 μ, P = .000; 50 μ, P = .000; 110 μ, P = .000) and the second sequence (first recycle; 25 μ, P = .000; 50 μ, P = .000; 110 μ, P = .000).
The data showed no significant differences between the four groups at the first sequence (control; P = .05). At the second sequence (first recycle), the percentage of tooth area occupied by bond material was significantly less in the industrially reconditioned group than the other groups (25 μ, P = .001; 50 μ, P = .004; 110 μ, P = .000), and when cleaning was carried out by sandblasting with 50 μ particles, significantly less bond material remained on teeth than with 110 μ particles (P = .000; Table 2). In the industrially reconditioned group at the third debonding sequence (second recycle), significantly less bond material was observed than in the other groups (25 μ, P = .000; 50 μ, P = .000; 110 μ, P = .000). Both the 25 μ and the 50 μ groups left significantly less bond material on teeth than the 110 μ group (P = .00 and P = .000, respectively).
Table 2. .
Mean and Standard Deviation (SD) of the Percentage of Tooth Area Occupied by Bond Material for Each Groupa
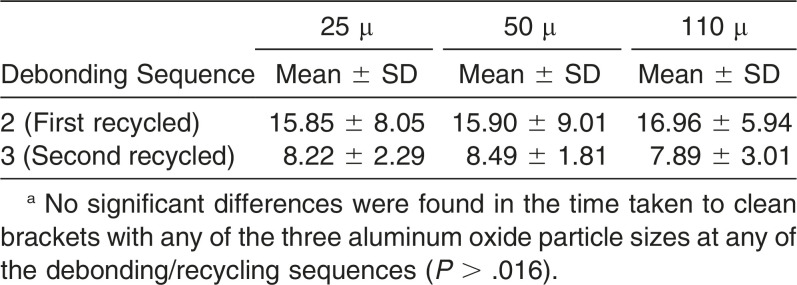
As for the time needed to clean bond material from bracket bases, no significant differences were observed between the three sizes of particle at any of the sequences (sequence 1, P = .25; sequence 2, P = .73; Table 3).
Table 3. .
Mean and Standard Deviation (SD) Time (in Seconds) Taken to Clean Bracket Basesa
Bracket base examination under SEM revealed that the control bracket had a smooth base with a multistranded wire structure and clean retentive areas in between the wires (Figure 1). After reconditioning, brackets showed higher loss of definition in retentive areas as aluminum oxide particle size increased, due to higher quantities of bond material remaining on bracket bases. Furthermore, more obliteration of the wire mesh was observed after the second recycle in all groups. The industrial group underwent greater metal mesh destruction than the other groups, but bond material remaining on bracket bases was observed only after the second recycle (Figure 2).
Figure 1. .
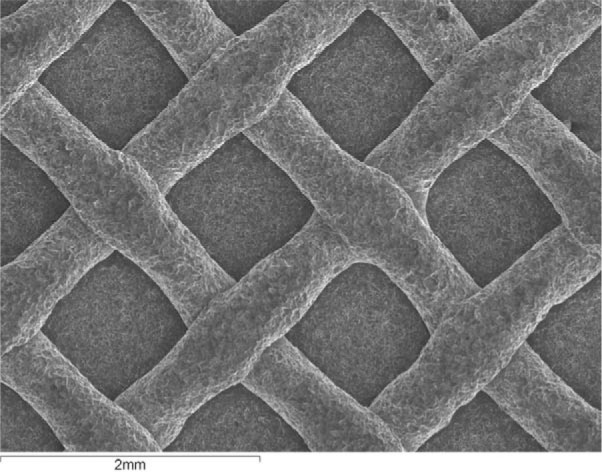
Scanning electron micrograph of bracket base of a new bracket (control group). Original magnification 100×.
Figure 2. .
Scanning electron micrographs of bracket bases after first and second recycled. Group 1: sandblasting with 25 μ Al2O3. Group 2: sandblasting with 50 μ Al2O3. Group 3: sandblasting with 100 μ Al2O3. Group 4: industrial recycling. Original magnification 100×.
DISCUSSION
One of the aims of this study was to evaluate the SBS obtained after reconditioning and rebonding the same bracket. The results found no significant differences in SBS between the four study groups after brackets were recycled for the first time, a finding that agrees with other studies that have evaluated SBS after rebonding new brackets and brackets reconditioned by micro sandblasting15,17,20 or by industrial methods.7 However, Chung et al.21 found that brackets reconditioned by sandblasting needed a bond booster to prevent a loss of SBS compared with new brackets.
After the second recycle, SBS was significantly higher for industrially reconditioned brackets than for sandblasted brackets. These data indicate that clinical sandblasting is a useful practice for a first recycle, while for repeated recycling of brackets, industrial reconditioning is functionally more effective.
For the industrially reconditioned group, evaluation of the different rebonding sequences identified no significant differences in bond strength. This agrees with the study by Regan et al.,18 who found no significant reduction in SBS for metal brackets recycled industrially up to five times. However, Buchwald5 found that the percentage of brackets that could be reused decreased with each recycling procedure.
The study also assessed the influence of particle size in sandblasting after repeated debonding sequences, finding that the larger the particle size, the greater the loss of SBS at successive rebondings. This could be explained by the events observed under SEM, whereby as particle size increased, so did the obliteration of the bracket's mesh by bond material remnants and a loss of definition in the retentive areas, which could contribute to decreases in SBS.
As for the effects of bond material remnants, it was found that as the particle size and the number of debonds/recycles increased, bond failure became more frequent at the bracket-bond material interface than at the tooth-bond material interface, which again could be attributed to greater obliteration of the metal mesh as observed under SEM, leading to loss of retentive capacity. These results agree with Basudan and Al-Emran,22 who studied brackets reconditioned by micro sandblasting with 50 μ aluminum oxide particles at 5 bars (72.5 psi) for 20–40 seconds (until no clinically observable resin remained on the bracket base); these authors also observed bond failure mainly at the bracket-bond material interface, but they did not obtain significant differences in SBS in comparison with the control group or damage to the bracket base's multistrand structure.
The literature lacks studies that compare the effects of particle size for micro sandblasting brackets. It might be thought that using a particular particle size would save time in bracket reconditioning, but no significant differences in the time needed to clean bracket bases were observed between the three sizes assayed at any of the debonding sequences.
The main advantages of reusing brackets are economic, in terms of both the cost of materials used during treatment and the ecological savings.11 The possibility of litigation resulting from using recycled brackets23,24—labeled by the manufacturers as intended for single use—can be eliminated by reconditioning in the clinic, as the same bracket is used again for the same patient. However, as seen from the present results, SBS decreases in relation to the number of rebondings of brackets reconditioned in the clinic by micro sandblasting, which does not occur with industrially reconditioned brackets.
CONCLUSIONS
No significant differences in SBS were found after the first recycle, although after the second recycle. SBS was significantly greater for industrially reconditioned brackets than for sandblasted brackets.
With sandblasting, as the sizes of the aluminum oxide particle increased, SBS decreased with successive rebonding.
Less bond material remnant was observed when brackets were reconditioned industrially than when sandblasted.
With sandblasting, the greater the aluminum oxide particle size and the greater the number of rebondings, the greater the percentage of bond material remained on the teeth; in other words, more bond failures occurred at the bracket-adhesive interface.
No significant differences were found in the time needed to clean bracket bases clinically using sandblasting with different aluminum oxide particle sizes.
Industrial recycling obtained better results than sandblasting after three successive debondings, but for recycling in the dental clinic, the use of sandblasting with an aluminum oxide particle size of 25 μ is recommended.
REFERENCES
- 1.Wright WL, Powers JM. In vitro tensile bond strength of reconditioned brackets. Am J Orthod. 1985;87:247–252. doi: 10.1016/0002-9416(85)90046-6. [DOI] [PubMed] [Google Scholar]
- 2.Regan D, LeMasney B, van Noort R. The tensile bond strength of new and rebonded stainless steel orthodontic brackets. Eur J Orthod. 1993;15:125–135. doi: 10.1093/ejo/15.2.125. [DOI] [PubMed] [Google Scholar]
- 3.Buchman DJL. Effects of recycling on metallic direct-bond orthodontics brackets. Am J Orthod. 1980;77:654–668. doi: 10.1016/0002-9416(80)90157-8. [DOI] [PubMed] [Google Scholar]
- 4.Mascia VE, Chen SR. Shearing strengths of recycled direct bonding brackets. Am J Orthod. 1982;82:211–216. doi: 10.1016/0002-9416(82)90141-5. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald A. A three-cycle in vivo evaluation of reconditioned direct-bonding brackets. Am J Orthod Dentofacial Orthop. 1989;95:352–354. doi: 10.1016/0889-5406(89)90170-4. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler JJ, Ackerman RJ., Jr Bond strength of thermally recycled metal brackets. Am J Orthod. 1983;83:181–186. doi: 10.1016/0002-9416(83)90081-7. [DOI] [PubMed] [Google Scholar]
- 7.Cacciafesta V, Sfondrini MF, Melsen B, Scribante A. A 12 month clinical study of bond failures of recycled versus new stainless steel orthodontic brackets. Eur J Orthod. 2004;26:449–454. doi: 10.1093/ejo/26.4.449. [DOI] [PubMed] [Google Scholar]
- 8.Postlethwaite KM. Recycling bands and brackets. Br J Orthod. 1992;19:157–163. doi: 10.1179/bjo.19.2.157. [DOI] [PubMed] [Google Scholar]
- 9.Egan FR, Alexander SA, Cartwright GE. Bond strength of rebonded orthodontic brackets. Am J Orthod Dentofacial Orthop. 1996;109:64–70. doi: 10.1016/s0889-5406(96)70164-6. [DOI] [PubMed] [Google Scholar]
- 10.Sharma-Sayal SK, Rossouw PE, Kulkarni GV, Titley KC. The influence of orthodontic bracket base design on shear bond strength. Am J Orthod Dentofacial Orthop. 2003;124:74–82. doi: 10.1016/s0889-5406(03)00311-1. [DOI] [PubMed] [Google Scholar]
- 11.Matasa CG. Pros and cons of the reuse of direct bonded appliances. Am J Orthod Dentofacial Orthop. 1989;96:72–76. doi: 10.1016/0889-5406(89)90232-1. [DOI] [PubMed] [Google Scholar]
- 12.Millett D, McCabe JF, Gordon PH. The role of sandblasting on the retention of metallic brackets applied with glass ionomer cement. Br J Orthod. 1993;20:117–122. doi: 10.1179/bjo.20.2.117. [DOI] [PubMed] [Google Scholar]
- 13.MacColl GA, Rossouw PE, Titley KC, Yamin C. The relationship between bond strength and orthodontic bracket base surface area with conventional and microetched foil-mesh bases. Am J Orthod Dentofacial Orthop. 1998;113:276–281. doi: 10.1016/s0889-5406(98)70297-5. [DOI] [PubMed] [Google Scholar]
- 14.Espinar-Escalona E, Barrera-Mora JM, Llamas-Carreras JM, Solano-Reina E, Rodríguez D, Gil FJ. Improvement in adhesion of the brackets to the tooth by sandblasting treatment. J Mater Sci Mater Med. 2012;23:605–611. doi: 10.1007/s10856-011-4509-y. [DOI] [PubMed] [Google Scholar]
- 15.Quick AN, Harris AM, Joseph VP. Office reconditioning of stainless steel orthodontic attachments. Eur J Orthod. 2005;27:231–236. doi: 10.1093/ejo/cjh100. [DOI] [PubMed] [Google Scholar]
- 16.Halwai HK, Kamble RH, Hazarey PV, Gautam V. Evaluation and comparison of the shear bond strength of rebonded orthodontic brackets with air abrasion, flaming, and grinding techniques: an in vitro study. Orthodontics (Chic) 2012;13(1):e1–9. [PubMed] [Google Scholar]
- 17.Sonis AL. Air abrasion of failed bonded metal brackets: a study of shear bond strength and surface characteristics as determined by scanning electron microscopy. Am J Orthod Dentofacial Orthop. 1996;110:96–98. doi: 10.1016/s0889-5406(96)70094-x. [DOI] [PubMed] [Google Scholar]
- 18.Regan D, Van Noort R, O'Keeffe C. The effects of recycling on the tensile bond strength of new and clinically used stainless steel orthodontic brackets: an in vitro study. Br J Orthod. 1990;17:137–145. doi: 10.1179/bjo.17.2.137. [DOI] [PubMed] [Google Scholar]
- 19.Martina R, Laino A, Cacciafesta V, Cantiello P. Recycling effects on ceramic brackets: a dimensional, weight and shear bond strength analysis. Eur J Orthod. 1997;19:629–636. doi: 10.1093/ejo/19.6.629. [DOI] [PubMed] [Google Scholar]
- 20.Grabouski JK, Staley RN, Jakobsen JR. The effect of microetching on the bond strength of metal brackets when bonded to previously bonded teeth: an in vitro study. Am J Orthod Dentofacial Orthop. 1998;114:452–460. doi: 10.1016/s0889-5406(98)70192-1. [DOI] [PubMed] [Google Scholar]
- 21.Chung CH, Fadem BW, Levitt HL, Mante FK. Effects of two adhesion boosters on the shear bond strength of new and rebonded orthodontic brackets. Am J Orthod Dentofacial Orthop. 2000;118:295–299. doi: 10.1067/mod.2000.104810. [DOI] [PubMed] [Google Scholar]
- 22.Basudan AM, Al-Emran SE. The effects of in-office reconditioning on the morphology of slots and bases of stainless steel brackets and on the shear/peel bond strength. J Orthod. 2001;28:231–236. doi: 10.1093/ortho/28.3.231. [DOI] [PubMed] [Google Scholar]
- 23.DiPasquale TJ. Reconditioning and reuse of orthodontic devices. Am J Orthod Dentofacial Orthop. 1992;102:187–189. doi: 10.1016/S0889-5406(05)81229-6. [DOI] [PubMed] [Google Scholar]
- 24.DiPasquale TJ. Reconditioning and reuse of orthodontic devices. Am J Orthod Dentofacial Orthop. 1992;102:285–287. doi: 10.1016/S0889-5406(05)81065-0. [DOI] [PubMed] [Google Scholar]