Abstract
Introduction: Cannabis smoke contains carcinogens similar to tobacco, in addition to compounds with antitumor activity. Cannabis use reduces the risk of obesity and cannabinoids inhibit chronic inflammation, known causes of cancer. The net effect of Cannabis use on cancer risk is not known.
Objective: To examine the association between Cannabis use and cancer risk in the United States.
Methods: Identify and analyze published data on cancer risk in Cannabis users.
Results: A total of 55 data points, consisting of risk ratios of cancer in Cannabis users and nonusers, were identified from 34 studies. Of these, 5 did not contain data essential for inclusion in the meta-analysis. The remaining data showed a nonsignificant trend to an association with reduced risk (relative risk [RR]=0.90, p>0.06, N=50) although heterogeneity is high (I2=72.4%). Removal of data with high risk of selection bias (defined as those from North Africa and those that failed to adjust for tobacco) and data with high risk of performance bias (defined as those with fewer than 20 cases or controls among Cannabis users) resulted in an RR <1.0 (RR=0.86, p<0.017, N=24) and large effect size (Hedges g=0.66), but did not decrease heterogeneity (I2=74.9). Of all cancer sites, only testicular cancer showed an RR value >1, although this was not significant and had a negligible effect size (RR=1.12, p=0.3, Hedges g=0.02). Following removal of testicular cancers the remaining data showed a decrease in risk (RR=0.87, p<0.025, N=41). Cancers of the head and neck showed a negative association with cancer risk (RR=0.83, p<0.05), with a large effect size (Hedges g=0.55), but high heterogeneity (I2=79.2%). RR did not reach statistical significance in the remaining cancer site categories (lung, testicular, obesity-associated, other). The data are consistent with a negative association between Cannabis use and nontesticular cancer, but there is low confidence in this result due to high heterogeneity and a paucity of data for many cancer types.
Keywords: cancer risk, Cannabis, marijuana, meta-analysis, scoping review
Introduction
The impact of Cannabis use on cancer risk is of considerable interest. Cancer is a leading cause of death in the United States and around the world. In the United States alone, >1.7 million diagnoses and 607,000 deaths are projected from cancer in 2019 (Ref.1) and cancer deaths were responsible for $94.4 billion in lost earnings in 2015 (Ref.2). The real costs are much higher than this, however, because even cancer patients who will make a full recovery face lengthy, expensive, and stressful treatment regimens.
The pharmacological activity of Cannabis is primarily due to the presence of phytocannabinoids, a group of lipid-soluble chemicals. These act by mimicking the cannabinoids produced by the endocannabinoid system (ECs), an important physiological signaling system. The cellular and physiological actions of cannabinoids arise from interaction with a variety of widely distributed receptor types, of which cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 have received the greatest attention.3
The ECs appears to be dysregulated by the modern American diet. In particular, emerging evidence suggests that an elevated dietary ratio of omega-6/omega-3 fatty acids may lead to excess production of the endocannabinoid signals N-arachidonoylethanolamide and 2-arachidonoylglycerol, leading to overstimulation of CB1 and contributing in turn to the emerging epidemic of obesity, metabolic syndrome, and associated diseases.4–6 CB1 is also stimulated by the exogenous cannabinoid Δ9-tetrahydrocannabinol (THC), the main psychoactive cannabinoid in Cannabis.
Paradoxically, however, acute stimulation of CB1 is associated with weight gain and antagonists of CB1 increase metabolic rates,4–6 yet Cannabis users show decreased body mass index (BMI) relative to nonusers.4 This may be due to downregulation of CB1 in Cannabis users, which would desensitize the ECs and counteract the impact of the modern American diet on weight gain.4
Cannabis has both protumor and antitumor actions. Which effect predominates in Cannabis users? Cannabis is emerging as a palliative option for cancer patients.7–14 Medical Cannabis use reduces opioid use,8–11 counteracts multiple side effects of chemotherapy, improves mood and outlook, and provides relief from insomnia.12–14 If Cannabis causes cancer, these therapeutic benefits might be offset in cancer patients by stimulation of existing tumors or creation of new neoplasms. However, if Cannabis reduces cancer risk, the case for inclusion of Cannabis in therapeutic cancer care strategies is augmented. In addition, if moderate, adult Cannabis use is found to reduce risk of cancer, a leading cause of death, this information must be included in estimates of its impact on public health.
Tumorigenic activity of Cannabis
Cannabis has several tumorigenic properties, manifested when smoked. Cannabis smoke contains carcinogens similar to those in tobacco smoke, including tar and polycyclic aromatic hydrocarbons, and these may be at higher concentration than in tobacco smoke.15,16 Users who smoke Cannabis heavily have histological changes, including inflammation, in the lungs and airways resembling those prestaging the initiation of tobacco-related lung cancer.17 Sharing of joints or pipes is hypothesized to increase risk of human papilloma virus exposure, a significant cause of oral and other cancers.18 Furthermore, although cannabinoids usually inhibit cancer cells in vitro and in mouse models,3 they stimulate growth of some cancer cell lines in laboratory studies.19
Antitumor activity of Cannabis
Actions that reduce cancer risk include reduced risk of obesity and diabetes mellitus (DM), and inhibition of inflammation by cannabinoids. In addition, in laboratory studies, cannabinoids and other compounds in Cannabis directly inhibit cancer initiation, growth, and spread at the cellular level.
Decreased obesity rates in Cannabis users
Obesity increases the risk of cancer.20–26 Cancers of the breast, colon, and rectum, prostate, esophagus, stomach (cardia), pancreas, uterine corpus, gallbladder, kidney, liver, ovary, and thyroid, as well as multiple myeloma, are positively associated with obesity.20–26 In the United States, between 1982 and 2000, obesity caused about 20% and 14% of cancer deaths in women and men, respectively.20
Note that obesity rates are substantially higher today than in 2000, and rates of obesity-related cancers are increasing even in young people as the obesity rate continues to rise.23
The percentage of cancer deaths attributable to obesity are therefore likely to be much higher today than they were in 2003, when the study by Calle et al. was published,20 even as tobacco use and lung cancer are declining. However, using the numbers from Calle et al.,20 a minimum of 85,000 to 120,000 of the 607,000 cancer deaths projected for 2019 (Ref.1) are caused by obesity. Similarly, an estimated 3.6% of all new cancer cases worldwide are caused by excess body fat.26
A recent review determined that Cannabis use is associated with reduced obesity risk (mean risk ratio=0.68) and BMI (average decrease in BMI relative to nonusers=6%).4 Like weight loss following bariatric surgery,27 reduced obesity rates in response to Cannabis use may decrease cancer risk. Cannabis use protects against nonalcoholic fatty liver disease,28 a disorder that is strongly associated with obesity and is an independent predictor of gastric and liver cancer, cancer types strongly associated with obesity.24,25 Cannabis users may thus have lower risk of obesity-associated cancer types simply due to decreased risk of obesity.
Decreased inflammation
Chronic inflammation is associated strongly both with obesity and with the initiation and progression of cancer.27–30 A decrease in BMI of 7%, similar in magnitude to the observed 6% decrease in BMI associated with Cannabis use,4 decreases proinflammatory and increases anti-inflammatory gene expression in visceral adipose tissue.31 Cytokines involved in inflammation play a significant role in promoting cancer, and therefore, drugs such as nonsteroidal anti-inflammatory drugs decrease cancer risk.32
A number of cannabinoids have anti-inflammatory effects in laboratory studies, and cannabinoids are therefore of interest for development as a therapy option for chronic inflammation, currently an unmet medical need.33 Thus, Cannabis use may directly inhibit inflammation at the cellular level, and in addition, indirectly reduces inflammation by decreasing BMI and risk of obesity. Both actions should decrease cancer risk. This effect would be offset if inflammatory responses are desensitized with chronic use.4
Improved insulin sensitivity and decreased risk of DM
Cannabis use is associated with reduced risk of DM, reduced fasting insulin levels, and reduced insulin resistance.34–38 In contrast, DM and elevated insulin or C-peptide levels are associated with greater cancer risk, and faster growth and aggressiveness of colorectal, pancreatic, liver, postmenopausal breast, and endometrial cancers, and thus increased mortality from these cancers.39
Probiotic actions of cannabinoids on the gut microbiome
The gut microbial flora interacts closely with the ECs and plays an important role in metabolism.40 Changes in the intestinal microbial flora, including a shift in the ratio of Firmicutes:Bacteroidetes, are correlated with obesity. These changes are also associated with the initiation and progression of systemic inflammation, and increased risk of colorectal cancer.41 Cannabinoids oppose this gut dysbiosis.41 In mice fed a high-fat diet, chronic THC administration not only prevents obesity, but also prevents changes in the gut microbial flora from one characteristic of a lean phenotype to one associated with obesity and increased risk of colorectal cancer.41
Direct antitumor properties of Cannabis
Multiple chemicals in Cannabis, including psychoactive and nonpsychoactive cannabinoids as well as flavonoids, possess antitumor activity.42–45 Cannabinoids inhibit tumor initiation, metastasis, vascular adhesiveness, tissue invasiveness, and angiogenesis, while selectively stimulating apoptosis of cancer cells.3,42–44,46–56 They thus, by multiple mechanisms, inhibit all stages of cancer initiation, development, growth, and spread. As a result, in laboratory studies and animal models, cannabinoids destroy tumors while leaving surrounding cells unharmed.42–44 In laboratory studies, cannabinoids inhibit gliomas, thyroid epithelioma, lymphoma, neuroblastoma, and carcinomas of the oral region, lung, skin, uterus, breast, prostate, pancreas, and colon.42–44,46–56
Hypotheses
The impact of Cannabis use on cancer risk will depend on the relative magnitudes of the carcinogenic and antitumor effects, leading to three hypotheses:
-
1.
the carcinogenic effects predominate, leading to greater cancer risk in users;
-
2.
the carcinogenic and antitumor effects cancel each other leading to no net impact (null hypothesis);
-
3.
the antitumor effects predominate, leading to reduced cancer risk in users.
Adding complexity, the balance between the opposing effects of carcinogens and antitumor compounds may differ among cancer types, and different tissues may be exposed to different concentration ratios of carcinogens and cannabinoids. Tissues of the mouth, pharynx, larynx, and lungs are directly exposed to carcinogens from smoke while other tissues are not. Furthermore, some cell types may be more sensitive to the carcinogenic or antitumor actions than others, and effects of Cannabis use on risk factors, such as obesity or inflammation, also influence the balance between these opposing actions.
Methods
Review of the literature on the effects of Cannabis use on cancer rates
Information sources
PubMed, MEDLINE, Web of Science, and Google scholar were screened for articles presenting data on risk ratios of cancer in Cannabis users and nonusers.
Search strategy
PubMed, MEDLINE, and Web of Science were searched using “(Cannabis or marijuana) AND (cancer) NOT (therapeutic or palliative), most recently in August 2019. Google scholar was also searched for references using the terms “Cannabis and cancer” and “Marijuana and cancer.” Titles were evaluated for relevance, and case studies and those addressing palliative or therapeutic care were rejected. Those potentially reporting risk ratios were selected for further analysis and data extraction. Review and data source articles were also examined for references.
Summary measures
The principal summary measure is the risk ratio of cancer in Cannabis users, relative to nonusers, in the form of adjusted odds ratio (AOR), relative risk (RR), and adjusted hazard ratio (AHR).
Selection of data to use in further analyses
Studies considered for this review included case–control and cohort studies that present risk ratio data (AORs, RR, or AHR), comparing rates of cancer in Cannabis users and nonusers.
Many studies presented multiple values for risk ratios for a single cancer type or site, including values for different user groups (duration of use, frequency of use, quantity of use, etc.) and adjustment for different confounders. The data selected for use in the analysis consisted of ever versus never use, current versus never use, or ever versus nonhabitual use categories, as available (Table 1).
Table 1.
Studies Identified in Literature Searches
| Reference | Study population | Study type | Cancer type | Adjustments | Data used |
|---|---|---|---|---|---|
| Aldington et al.58 | New Zealand | Case–control | Head and neck | Age, sex, ethnicity alcohol consumption, income, and pack-years of cigarette smoking | Ever vs. never user |
| Aldington et al.59 | New Zealand | Case–control | Lung | Sociodemographic factors, tobacco smoking status, and pack-years | Nonsmoker vs. smoker |
| Berthiller et al.60 | North Africa (Magreb) | Lung | Age, occupational exposure, country (in pooled analysis), and lifetime pack-years of tobacco smoking | ||
| Berthiller et al.61 | North and South America, Havana | Case–control | Oral, pharyngeal, laryngeal | Age, sex, race, education, family history of cancer, HPV-16, smoking, and average drinks of alcohol/week | Ever vs. never user |
| Callaghan et al.62 | Sweden | Cohort | Lung | Age, race, education, alcohol use, and tobacco cigarette | Ever vs. never user |
| Callaghan et al.63 | Sweden | Cohort | Testicular | Age, cryptorchidism, paternal history of testicular cancer, frequency of tobacco smoking, alcohol consumption | Ever vs. never user |
| Chacko et al.64 | United States; VA hospitals | Case–control | Transitional cell of bladder | No adjustments | Ever vs. never user |
| Chao et al.65 | United States | Cohort | Karposi's sarcoma | Age, education, study center, tobacco smoking, alcohol use, number of male sexual partners since last visit, lifetime sexual partners at time of enrollment, receptive anal intercourse and condom use, history of STIs, antiviral therapy, and CD4 counts | HIV, HHV-8 coinfected, weekly or more frequent use, recent use |
| Daling et al.66 | Washington State, United States, B.C. Canada | Case–control | Anal | Age, residence, and cigarette smoking (never, former, or current) | Ever vs. never user |
| Daling et al.67 | Washington State, United States | Case–control | Testicular | Age at reference, reference year, alcohol use, current smoking, and history of cryptorchidism | Ever vs. never user |
| Efird et al.68 | California, United States | Cohort | Glioma | Cigars, pipes, sex, race, alcohol, education, and coffee | Ever vs. never user |
| Feng et al.69 | North Africa | Case–control | Nasopharyngeal | Age, SES measures, associated dietary factors, and cigarettes smoked per day | Ever vs. never user |
| Gillison et al.70 | United States Johns Hopkins | Case–control | Head and neck squamous cell | Race; tobacco and alcohol use; number of teeth lost; frequency of tooth brushing; and number of oral sex partners | HPV-16 negative; current users vs. nonusers |
| Han et al.71 | United States | Case–control | Lung | Age, gender, race/ethnicity, education, health insurance, family income, tobacco use, duration of alcohol use, durations of nonmedical use of pain relievers, tranquilizers, stimulants, and sedatives | Never vs. <1 year, 2–10 years, 11+ years. No ever vs. never provided. No case or control numbers provided. |
| Hashibe et al.57 | United States California (Los Angeles) | Case–control | Oral, pharyngeal, laryngeal, esophageal | Age, sex, education, alcohol, cigarette | Weighted average across user groups, >0–60+ joint-years vs. nonuser |
| Holly et al.72 | United States California | Case–control | Non-Hodgkins lymphoma | Age | Used Cannabis >1000×(highest usage rate) |
| Hsairi et al.73 | North Africa | Case–control | Bronchial | Age, sex, and cigarettes/day | User vs. nonuser |
| Lacson et al.74 | United States California | Case–control | Testicular | Cocaine use, amyl nitrite, cryptorchidism, religiosity, education | Ever vs. never user |
| Liang et al.75 | United States Massachusetts | Case–control | Oral, pharyngeal, laryngeal | Adjusted for age, sex, race, education, tobacco use, pack-years of tobacco, alcohol-year | Current user vs. never user |
| Llewellyn et al.76 | United Kingdom | Descriptive | Oral SCC | No adjustments | NA |
| Llewellyn et al.77 | United Kingdom | Case–control | Oral | Alcohol and tobacco | Ever vs. never user |
| Llewellyn et al.78 | United Kingdom | Case–control | Oral | Age, sex, race, education, tobacco use, pack-years of tobacco, alcohol-year | Ever vs. never user |
| Maden et al.79 | Washington State, United States, BC Canada | Case–control | Penile | Age, race, BMI; no tobacco use | Used Cannabis >50 times |
| Marks et al.80 | United States, Latin America | Case–control | Oral, oropharyngeal | Adjusted for tobacco | Ever vs. never user |
| Nelson et al.81 | United States, California | Case–control | Non-Hodgkin's lymphoma | No adjustments | No use vs. any use |
| Rosenblatt et al.82 | United States, Washington State | Case–control | Oral | Age, study, race, sex, education level, pack-year, alcohol duration, pipe smoking duration, cigar smoking duration | Ever vs. never user |
| Sasco et al.83 | North Africa; Morocco | Case–control | Lung | Smoking status, history of chronic bronchitis, passive smoking, occupational exposure, cooking and heat source, lighting source, ventilation of kitchen | Hashish/Kiff users vs. nonusers |
| Sidney et al.84 | California, United States | Retrospective cohort | Breast, cervical, colorectal, lung, melanoma, prostate | Age, race, education, alcohol use, and tobacco cigarette smoking | Ever users vs. nonusers/experimenters |
| Thomas et al.85 | United States, California | Cohort | Bladder | Age, race, education, alcohol use, and tobacco cigarette | User vs. nonuser |
| Trabert et al.86 | United States | Case–control | Testicular | Age, reference year, alcohol use, tobacco use, history of cryptorchidism | Ever vs. never use |
| Voirin et al.87 | North Africa | Case–control | Lung | Age, tobacco, and occupational exposures | Never vs. past use |
| Zhang et al.88 | United States, New York | Case–control | Head and neck squamous cell | Adjusted for age, sex, race, education, drink-years, tobacco use, pack years | Past use vs. never |
| Zhang et al.89 | United States, Canada, United Kingdom, New Zealand | Case–control | Lung | Tobacco, level of alcohol use, respiratory conditions at conscription | Habitual vs. nonhabitual or never users |
| Zhu et al.90 | United States | Case–control | Sinonasal and nasopharyngeal | Cigarettes, age, ethnicity, education level, marital status, having received blood products, exposure to pesticides containing 2,4,5-T, use of barbiturates without a prescription, etc. | User vs. nonuser |
BMI, body mass index; HHV, human herpes virus; HPV, human papilloma virus; NA, not applicable; SCC, squamous cell carcinoma; SES, socioeconomic status; STIs, sexually transmitted infections.
The data from the most fully adjusted model were selected from each study. One study failed to provide a single value for overall user risk,57 but rather assigned data to distinct groups based on cumulative exposure (i.e., 0 to 1, 1 to <10, 10 to <30, 30 to <60, and ≥60 joint years). For these data, to determine the effects of average use, the values for each usage group were weighted by the number of cases in that group, then the weighted average across user groups was determined and used to provide a summary AOR. The references identified, study population, study type (case–control or cohort), cancer type, adjustments, and comparison type (i.e., ever use vs. never use, etc.) from each reference are summarized in Table 1.57–90
Conversion of AOR data to RR data
Risk ratios from the various studies were reported as AOR, RR, or AHR. Odds ratios and RR are similar in value at risk ratios near one, but diverge as risk ratios diverge from one and are therefore not directly comparable.91 To provide a single measurement unit for analysis, AOR data were converted to RR following Zhang and Kai.91 HR data are considered equivalent to RR and were not converted.92
Statistical analysis
Statistical analysis of the data was performed with the software program JASP using a Dersimonian–Laird random effects meta-analysis model.
Effect size was calculated as:
Standard error was calculated as:
where userpos and userneg are number of cancer cases and cancer free in Cannabis users, and nonuserpos and nonuserneg are numbers of cancer cases and cancer free in nonusers.
Examination of heterogeneity in the data
Heterogeneity was examined by selectively removing specific blocks of data, including data with high risk of selection bias, high risk of performance bias, and data for specific cancer sites, and by examining data for each category of cancer type or site independently.
Examination of risk of publication bias
Publication bias was investigated by analyzing funnel plot asymmetry using rank correlation in JASP.
Examination of impact of risk of selection and performance bias on heterogeneity
The results of the literature search consist of a series of articles that present risk ratios for cancer in Cannabis users.57–90 These were screened to identify and remove data with high risk of selection and performance bias, as defined below.
Removal of data with high risk of selection bias
The goal of the study was to examine the data on the risk of cancer due to Cannabis use in the United States. Therefore, data that did not fit this population was determined to be at high risk of selection bias. In the context of this study, high risk of selection bias was defined as failure to adequately adjust for tobacco use. Elimination of studies with high risk of selection bias thus involved eliminating studies that failed to adjust for tobacco use, or those that compared user and nonuser populations that universally or predominantly use tobacco,62,64,72,73,81,87 due to the inability to adequately adjust for tobacco use in this scenario.
Data collected in North Africa were determined to be at high risk of selection bias, and were also removed,60,69,73,83,87 because North African Cannabis users consume Cannabis in the form of kiff or hashish, and mix Cannabis with tobacco.57 These delivery methods are rare in the United States and interfere with adjustment for tobacco as a confounding factor.
Removal of data with high risk of performance bias
Many of the measures used to evaluate risk of performance bias in meta-analyses are not applicable in the current data set, as these data arose from comparison of cancer rates among study populations rather than documentation of changes in response to treatment interventions. Sample size was therefore selected as the most obvious measure of the potential for performance bias. The impact of removal of small sample size data, reporting measures of risk based on 20 or fewer Cannabis-using cases or controls,63,66,68,70,73,77–79,83,88,90 was examined. For studies reporting risk ratios for multiple cancer sites, data from each individual cancer site were assessed for number of cases and controls among Cannabis users, and those with fewer than 20 cases or controls were eliminated, while data for sites with more than 20 cases and controls were retained.
The Grubbs test93 was used to test, for outlier status, the highest and lowest values remaining in the data after removal of data with high risk of selection bias. No outliers were detected.
Examination of data from specific cancer sites
The data were separated into the following five cancer sites: (1) cancers of the head and neck (head and neck squamous cell carcinoma, laryngeal, nasal, nasopharyngeal, oral, pharyngeal, and sinonasal), (2) lung, (3) testicular, (4) obesity related (esophageal, colorectal, breast, prostate, cervical), and (5) other types (melanoma, anal, penile, bladder, glioma, and non-Hodgkin's lymphoma, types that did not fit into the other categories). Each of these subsets was analyzed independently.
Analysis of data
Some studies reported risk ratios for multiple cancer sites (i.e., oral, pharyngeal, laryngeal, etc.). These were considered as independent data points in the analysis. AOR data were converted to RR before statistical analysis.91 Data were imported into the JASP meta-analysis software in the form of log RR. Means and confidence intervals (CIs) were obtained as log RR values from the meta-analysis software, then converted back to RR. Statistically significant differences in risk were accepted when 95% CIs excluded 1.0, or p<0.05 in the output of the meta-analysis software.
Heterogeneity was quantified as I2 (Ref.94). Effect size was calculated as Hedges g, with correction of bias for small samples for cancer sites with low N following Durlak.95
Effect size was calculated as Hedges g, defined as:
Mean 1 is the mean log of RR data (logRR) of users, and mean 2 is 0 (logRR of nonusers, with RR defined as 1.0). Correction for small sample size was performed as:
Exposure dependence
Eight articles provided data amenable to analysis of exposure-dependent effects.57,58,61,75,80,82,84,85 These data were expressed in the original articles in a number of distinct units (joint-years, times/week, ounces/week×years, times, quartiles). To analyze exposure dependence, these units were assigned to low, intermediate, intermediate plus, and high-exposure categories, as shown in Table 4. The mean and 95% CIs of log(RR) were determined for each exposure category, then the data were converted back to RR.
Table 4.
Effects of Removing Data from Individual Cancer Categories on Results of Meta-analysis
| Data set removed | RR | 95% CI | p | Q (p) | I 2 | τ 2 | df | p Funnel plot assym |
|---|---|---|---|---|---|---|---|---|
| Head and neck removed | 0.95 | 0.835–1.08 | 0.44 | 89.6 (<0.001) | 65.41 | 0.075 | 31 | 0.615 |
| Lung cancer removed | 0.90 | 0.79–1.01 | 0.075 | 167.0 (<0.001) | 76.6 | 0.099 | 42 | 0.77 |
| Testicular cancer removed | 0.87 | 0.78–0.98 | 0.021 | 165.2 (<0.001) | 74.9 | 0.092 | 41 | 0.56 |
| Obesity-associated cancer removed | 0.91 | 0.80–1.03 | 0.129 | 168.3 (<0.001) | 77.43 | 0.109 | 42 | 0.66 |
| Other cancer types removed | 0.91 | 0.81–1.02 | 0.115 | 116.4 (<0.001) | 71.55 | 0.081 | 39 | 0.825 |
Data are analyzed with a Dersimonian–Laird model in JASP. Data in italics show RR significantly different from 1.0.
Results
The results of the PubMed, MEDLINE, Web of Science, and Google Scholar searches are presented in Figure 1. The search yielded a total of 34 studies reporting risk ratios,57–90 and these studies provided 55 risk ratio data points from a variety of cancer sites (Table 1). Five data points did not provide numbers of user and nonuser cancer-positive and cancer-negative patients and could not be used in the JASP meta-analysis.
FIG. 1.
PRISMA flow diagram of search strategy and results.
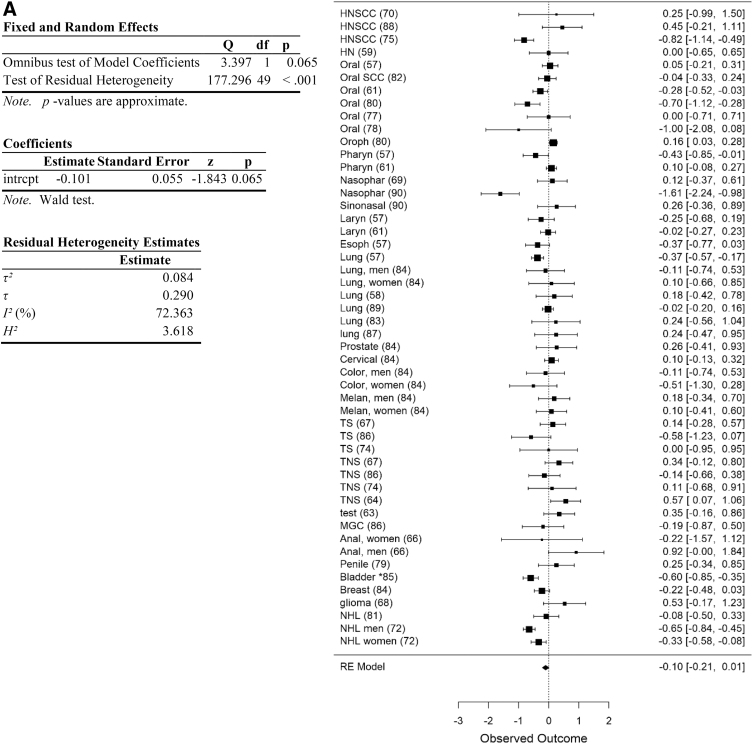
The remaining 50 data points could be analyzed in the meta-analysis (Fig. 2A). These included data from the head and neck (N=18), esophageal cancer (N=1), lung (N=7), melanoma (a single study reported risk separately for men and women; these were treated as independent data points; N=2), prostate (N=1), colorectal cancer (N=2), testicular cancer (N=8), anal cancer (a single study reported risk separately for men and women; these were treated as independent data points, N=2), penis (N=1), bladder cancer (N=2), glioma (N=1), breast cancer (N=1), non-Hodgkin's lymphoma (N=3), and cervical cancer (N=1).
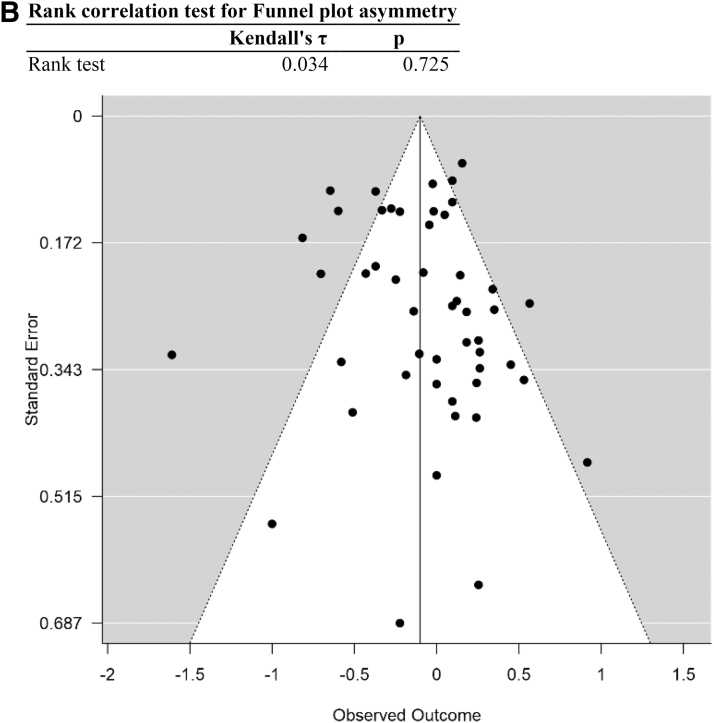
FIG. 2.
Summary of all cancer data obtained in search. (A) Forest plot of cancer data. (B) Funnel plot of data. Data are plotted and analyzed as logRR. Corresponding meta-analytic summary RR value=0.90 (95% CI=0.81–1.01; Table 2). Analyzed with Dersimonian–Laird model. CI, confidence interval; HN, head and neck; HNSCC, head and neck squamous cell carcinoma; MGC, mixed germ cell; NHL, non-Hodgkin's lymphoma; RR, relative risk; TNS, testicular non-seminoma; TS, testicular seminoma.
Relative risk
Analysis of the entire data set indicated a trend toward an association with reduced cancer risk in Cannabis users, but this trend was not significant (meta-analytic RR=0.90, 95% CI=0.81–1.01, p=0.065, df=49; Table 2). No significant evidence of publication bias was apparent (funnel plot asymmetry p=0.725). Following removal of data for high risk of selection and performance bias (as defined), the remaining data support an association with reduced risk of cancer in Cannabis users (meta-analytic RR=0.86, 95% CI=0.76–0.97, p=0.017, df=23; Table 2), with no significant evidence of publication bias (Funnel plot asymmetry p=0.66; Fig. 2B). Heterogeneity remained high, however (I2=74.9) after removing these data (Table 2).
Table 2.
Meta-analysis of the Entire Data Set, and Effects of Removing Data with High Risk of Selection and/or Performance Bias
| Data set | RR | 95% CI | p | Q (p) | I 2 | τ 2 | df | p Funnel plot assym | Hedges g |
|---|---|---|---|---|---|---|---|---|---|
| All data | 0.90 | 0.81–1.01 | 0.065 | 177.3 (<0.001) | 72.4 | 0.084 | 49 | =0.725 | 0.23 |
| Removed selection | 0.97 | 0.71–1.31 | 0.82 | 32.77 (<0.001) | 75.6 | 0.141 | 8 | =0.61 | |
| Remaining selection | 0.90 | 0.81–1.01 | 0.066 | 135.4 (<0.001) | 69.7 | 0.073 | 41 | =0.88 | |
| Removed Performance | 1.0 | 0.76–1.34 | 0.99 | 41.72 (<0.001) | 61.65 | 0.210 | 16 | >0.48 | |
| Remaining performance | 0.91 | 0.81–1.01 | 0.067 | 142.4 (<0.001) | 77.5 | 0.079 | 32 | >0.28 | |
| Elim. selection, performance | 0.86 | 0.76–0.97 | 0.017 | 91.8 (<0.001) | 74.9 | 0.060 | 23 | >0.60 | 0.66 |
Data are analyzed with a Dersimonian–Laird model in the statistical software program JASP. Data in italics show RR significantly different from 1.0.
CI, confidence interval; JASP; RR, relative risk.
The entire data set was divided into the following categories of cancer types: (1) head and neck cancers (airways of the head and neck), (2) lung, (3) testes, (4) obesity-related, and (5) other types; these categories were analyzed separately.
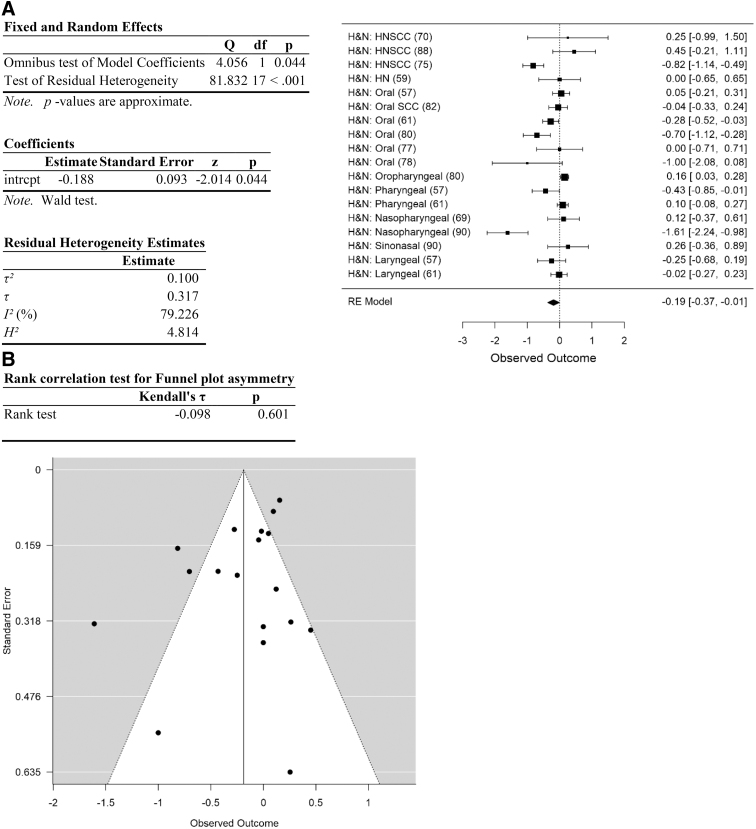
Head and neck cancers showed a significant negative association between Cannabis use and cancer risk (meta-analytic RR=0.83, 95% CI=0.72–0.97, N=18, p<0.05; Table 2; Fig. 3). Analyses of lung cancer data are shown in Figure 4, testicular cancer data in Figure 5, cancer types associated with obesity in Figure 6, and cancer types other than head and neck, lung, testicular, and obesity-related in Figure 7. The remaining categories showed RR values that were not statistically different from 1. Of the categories, only testicular cancer showed RR >1.0, although this was not significant (meta-analytic RR=1.12, 95% CI=0.9–1.4, N=9, p=0.3; Table 3). Elimination of testicular cancers from the data set resulted in a significant decrease in RR in the remaining data (meta-analytic RR=0.87, 95% CI=0.78–0.98, N=41, p<0.025; Table 4). None of the other cancer site categories resulted in RR statistically different from one when removed (Table 4).
FIG. 3.
Analysis of head and neck cancer data. (A) Forest plot. (B) Funnel plot. Data are plotted and analyzed as logRR. Corresponding meta-analytic summary RR value=0.83 (95% CI=0.72–0.99; Table 4). Analyzed with Dersimonian–Laird model.
FIG. 4.
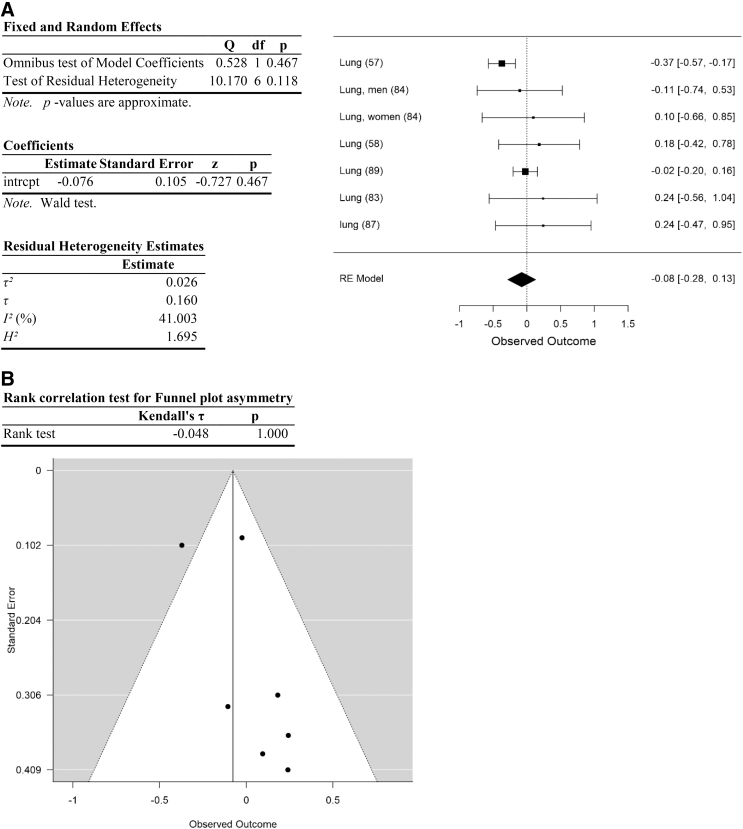
Analysis of lung cancer data. (A) Forest plot. (B) Funnel plot. Data are plotted and analyzed as logRR. Corresponding meta-analytic summary RR value=0.93 (95% CI=0.76–1.14; Table 4). Analyzed with Dersimonian–Laird model.
FIG. 5.
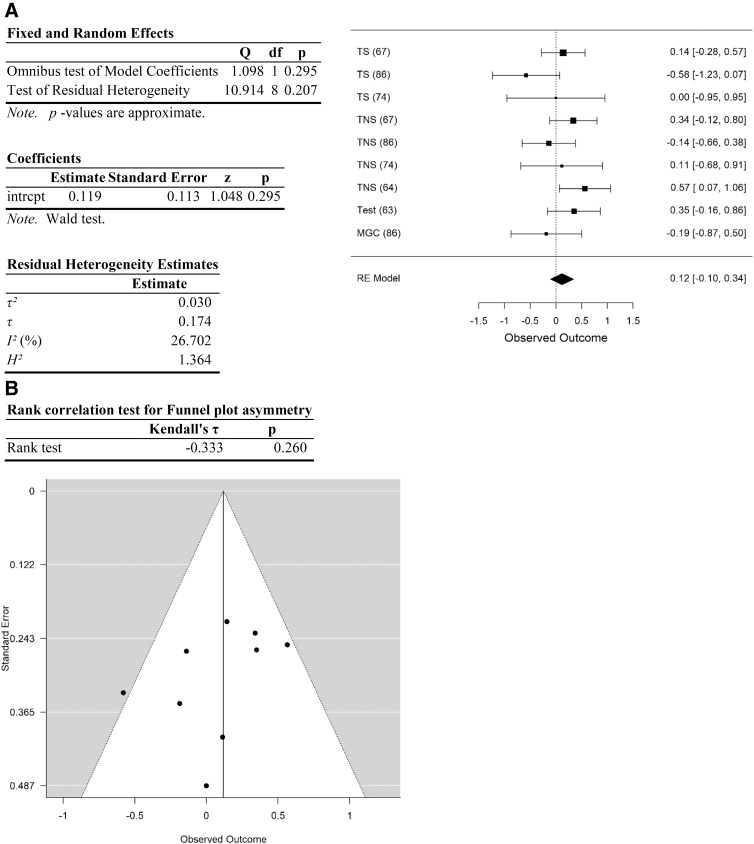
Analysis of testicular cancer data. (A) Forest plot. (B) Funnel plot. Data are plotted and analyzed as logRR. Corresponding meta-analytic summary RR value=1.12 (95% CI=0.9–1.40; Table 4). Analyzed with Dersimonian–Laird model.
FIG. 6.
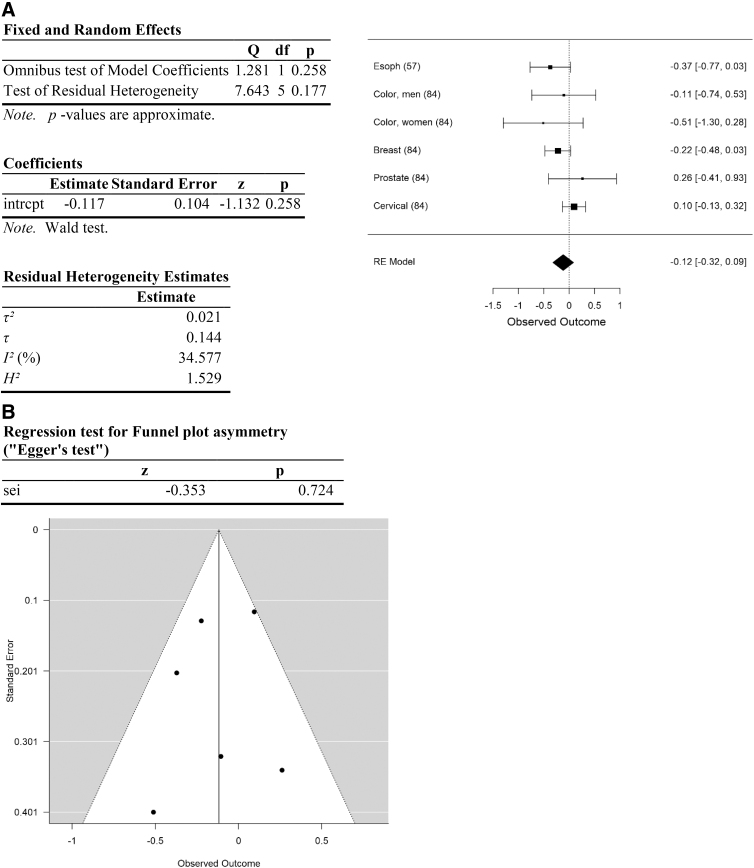
Analysis of cancer types associated with obesity. (A) Forest plot. (B) Funnel plot. Data are plotted and analyzed as logRR. Corresponding meta-analytic summary RR value=0.89 (95% CI=0.73–1.09; Table 4). Analyzed with Dersimonian–Laird model.
FIG. 7.
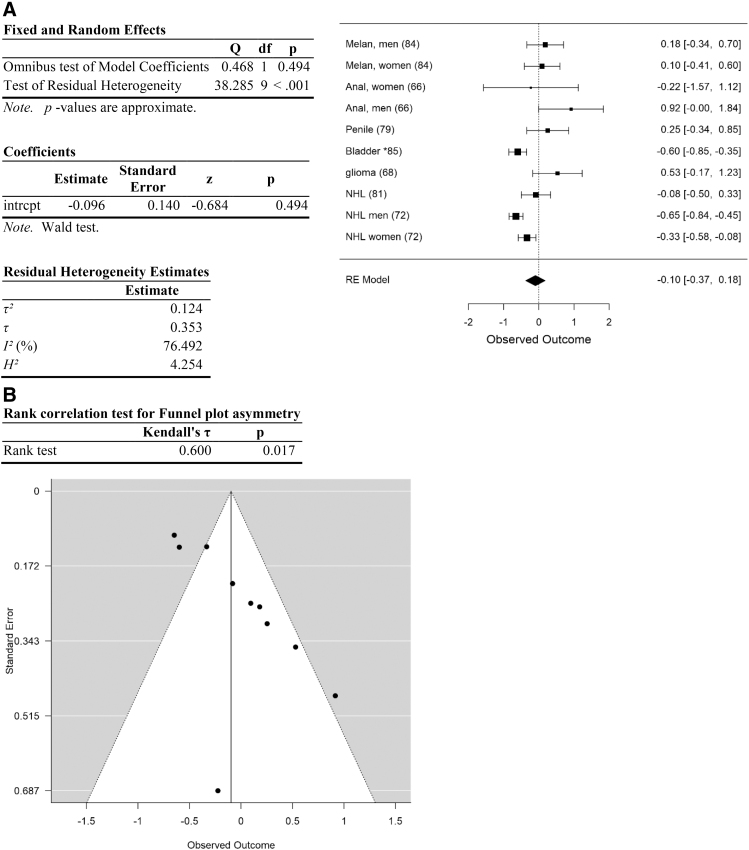
Analysis of cancer types other than head and neck, lung, testicular, and obesity-associated cancers. (A) Forest plot. (B) Funnel plot. Data are plotted and analyzed as logRR. Corresponding meta-analytic summary RR value=0.91 (95% CI=0.69–1.20; Table 4). Analyzed with Dersimonian–Laird model.
Table 3.
Examination of Individual Categories of Cancer Types
| Cancer type category | RR | 95% CI | p | Q (p) | I2 | τ 2 | df | p Funnel plot assym | Hedges g |
|---|---|---|---|---|---|---|---|---|---|
| Head and neck | 0.83 | 0.72–0.99 | 0.044 | 81.8 (<0.001) | 79.2 | 0.10 | 17 | 0.60 | 0.55 |
| Lung cancer | 0.93 | 0.76–1.14 | 0.47 | 10.17 (>0.11) | 41.0 | 0.026 | 6 | 1.0 | 0.23 |
| Testicular | 1.12 | 0.90–1.40 | 0.30 | 10.9 (>0.13) | 26.7 | 0.03 | 8 | 0.26 | 0.02 |
| Obesity | 0.89 | 0.73–1.09 | 0.26 | 7.6 (>0.17) | 34.6 | 0.021 | 5 | >0.72 | 0.69 |
| Other | 0.91 | 0.69–1.20 | 0.49 | 38.3 (<0.001) | 76.5 | 0.124 | 9 | <0.02 | 0.10 |
Data are analyzed with a Dersimonian–Laird model in JASP. Data in italics show RR significantly different from 1.0.
Table 5.
Cancer Types and Exposure Units used to Evaluate Potential Exposure Dependence of the Association between Cannabis Use and Cancer Risk
| Cancer type | Exposure units | Lowest | Intermed | Intermed + | Highest | Ref. |
|---|---|---|---|---|---|---|
| Oral | Joint-years | >0 to <1 | 1–10 | >10 | >30 | 57 |
| Pharyngeal | Joint-years | > 0 to <1 | 1–10 | >10 | >30 | 57 |
| Laryngeal | Joint-years | > 0 to <1 | 1–10 | >10 | >30 | 57 |
| Lung | Joint-years | > 0 to <1 | 1–10 | >10 | >30 | 57 |
| Esophageal | Joint-years | > 0 to <1 | 1–10 | >10 | >30 | 57 |
| HNSCC | Tertile | 1 | 2 | 3 | 59 | |
| Oral | Joint-years | >0–2 | >2–5 | >5 | 61 | |
| Pharyngeal | Joint-years | >0–2 | >2–5 | >5 | 61 | |
| Laryngeal | Joint-years | >0–2 | >2–5 | >5 | 61 | |
| HNSCC | Ounces/week×years | 0–1/16 | 1/16 to <3 | 3 to <7.5 | 7.5+ | 75 |
| Oral | Joint-years | 0–2 | 2–10 | >10 | 80 | |
| Oropharyngeal | Joint-years | 0–2 | 2–10 | >10 | 80 | |
| Oral | Times/week | 1 | 1–7 | 7+ | 82 | |
| Bladder | Times | 11–99 | 100–499 | >500 | 85 | |
| N | 14 | 14 | 14 | 6 |
N-values for each exposure group are shown in bold.
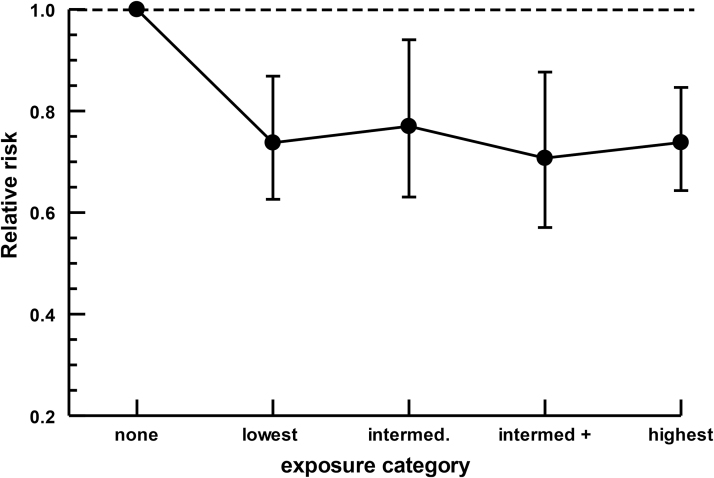
Relative risk data associated with these exposure units are presented in Figure 8.
HNSCC, head and neck squamous cell carcinoma.
Analysis of heterogeneity
There was high heterogeneity among the data.94 The initial data set (including all data) yielded a residual heterogeneity of Q=177.3 (p<0.001), I2=72.4%, df=49 (Table 2; Fig. 2). Heterogeneity remained high after removal of studies with high risk of selection and performance bias: Q=91.8 (p<0.001), I2=74.9%, df=23 (Table 2). Heterogeneity was explored further by analyzing each category of cancer types separately, and by determining the impact of removing each category of cancer types on measures of heterogeneity. Among the five categories of cancer types, heterogeneity was high in head and neck cancers and “other” cancer types, and much lower within lung, testicular, and obesity-related cancers (Table 3). Removal of any one category of cancer types failed to substantially reduce heterogeneity (Table 4).
Effect size
Effect size for the entire data set was determined to be small (Hedges g corrected for bias=0.23 (Ref.95). Removal of data with high risk of selection and performance bias resulted in an increase in Hedges g to 0.66, an effect size between medium and large. Following separation of the data into categories, effect size of cancers of the head and neck was moderate (head and neck: Hedges g corrected for bias=0.55) and of obesity-related cancers was between medium and large (Hedges g=0.69). In contrast, effect size was small or negligible for lung, testicular, and other cancer types (0.23, 0.02, and 0.1, respectively; Table 3).
Exposure level dependence
Preliminary analysis of exposure-dependent data suggests that all exposure categories are associated with reduced cancer risk (Fig. 8). Column effects (exposure) and row effects (study) were both statistically significant (2—way analysis of variance, column effects: F-value=3.19, p<0.025; row effects: F-value=3.096, p<0.006) suggesting differences in response to exposure as well as to study/cancer type. All exposure categories show significantly reduced risk relative to the no-use category (95% CIs exclude 1.0; Fig. 8). There are no significant differences among exposure categories once the no-use category was removed (p>0.9) suggesting that low and high exposures have similar impact on cancer risk. Most of these data are from cancers of the head and neck, and the risk of selection bias in these data is therefore very high.
FIG. 8.
Analysis of potential exposure-dependence. Data from a subset of studies could be assigned to arbitrary exposure categories. Results are expressed as mean RR ±95% CI. Cancer types, and exposure units placed into each category, are presented in Table 5. All exposure categories are significantly different to nonusers but not different to each other.
Conclusions
The hypothesis that Cannabis use increases cancer risk is not supported by the available data. The data instead show a trend toward a negative association between Cannabis use and cancer risk (all data: meta-analytic RR=0.90, p=0.065, N=50). This trend reaches statistical significance upon removal of data determined to be at high risk of selection and performance bias (meta-analytic RR of remaining data=0.86, p<0.02), or removal of data from testicular cancers (meta-analytic RR of remaining data=0.87, p<0.025).
Of the five categories of cancer sites, only one, testicular cancer, showed an RR value >1, (meta-analytic RR=1.12), although the data did not reach statistical significance (p>0.3) and the effect size was negligible (Hedges g=0.02). The remaining cancer sites showed nominal RR values <1, but only cancers of the head and neck showed a statistically significant decrease in risk (meta-analytic RR=0.83, p<0.05, N=18) with medium effect size (Hedges g=0.55). This was also the cancer site with the greatest amount of data.
Heterogeneity was high in the data set. Exploration of this heterogeneity suggests that it arises at least in part from differences among cancer sites rather than from selection or performance bias. When the data were separated into sites, heterogeneity remained high in cancers of the head and neck, and “other” sites, but was substantially reduced in cancers of the lungs, testes, and obesity-related cancers.
The current analysis suggests that Cannabis use in the United States may decrease risk of cancer by 10% (meta-analytic RR=0.90). Only testicular cancers show an RR >1.0, although this was not statistically significant. Upon removal of testicular cancers from the data, the remaining data show an RR significantly below 1.0 (p<0.05; Table 3).
If cancer types do respond differently to Cannabis use, the overall impact of Cannabis use on cancer will depend on the impact of Cannabis use on each cancer type, and the number of cases of and deaths from each cancer type in the population. For example, there are ∼1.7 million cancer cases, and 600,000 deaths, in the United States in 2019 (Ref.1). Of these, ∼7900 cases and 370 deaths are from testicular cancer (2013 data from Centers for Disease Control and Prevention [CDC]: https://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm).
The current analysis suggests an association of Cannabis use with a substantial decrease in risk of nontesticular cancers, with moderate effect size, and a nonsignificant increase in risk of testicular cancer, with negligible effect size. This suggests that Cannabis use may substantially decrease the death rate from cancer in the United States. However, the available data provide little confidence in this conclusion. There appear to be different responses among cancer types, and there are no data, or few/poor data, available for many cancer types.
There is high heterogeneity among studies that is not explained by chance, and no studies estimate the RR of all cancer types in Cannabis users relative to nonusers. Cancers of the head and neck, with the lowest meta-analytic RR of any cancer type, are over-represented in the data, and cancers associated with obesity are under-represented. Furthermore, the data used are epidemiological, rather than experimental, and thus can show association but not causation. It is therefore necessary to exercise caution in interpreting these data. Nevertheless, data suggest that Cannabis use may decrease the risk of cancer in the United States.
Decreased cancer risk in Cannabis users should not be surprising, as Cannabis and cannabinoids decrease obesity, inhibit chronic inflammation, reduce fasting insulin levels and insulin sensitivity, and have direct antitumor actions. Furthermore, the airways and bladder would be exposed to the highest levels of carcinogens from Cannabis smoke, yet risk of cancers of the oral, pharyngeal and laryngeal regions, and the bladder are significantly decreased in Cannabis users (current analysis and Ref.85). This demonstrates that the anticancer effects of Cannabis outweigh the carcinogenic effects even in the airways and bladder, where carcinogen exposure is high.
It is possible that the actual decrease in cancer risk due to Cannabis use is even greater than the estimated 10% decrease in risk emerging from the current analysis, as few data are available for the impact of Cannabis use on the RR of cancers that are not exposed to the carcinogens of smoke, for example, those associated with obesity. Cannabis users show decreased BMI and obesity rates.4,97 This decrease is known to be associated with decreased risk of obesity-related diseases, including DM and nonalcoholic fatty liver disease.34–38,104
Many cancer types are positively associated with obesity, including cancers of the liver, breast, colon and rectum, prostate, stomach (cardia), pancreas, uterine corpus, gallbladder, esophagus, kidney, ovary, and thyroid, as well as multiple myeloma.20–26 Cannabis use4,97 may decrease the risk of these obesity-related cancer types simply due to the reduction in BMI associated with Cannabis use. In addition, however, most of these cancer types are known to be inhibited or destroyed by cannabinoids in laboratory studies.47–56 Cannabis use is therefore very likely to be associated with a substantial reduction in the risk of, and mortality from, obesity-related cancers.
Unfortunately, no recent data, and few data overall, are available for the RR of obesity-related cancers in Cannabis users. Data providing risk ratios for obesity-related cancers are limited to six data points from two studies. The first study, using data from the early 1990s in California, provided data for esophageal (RR=0.67), colorectal (RR men=0.9, RR women=0.6), prostate (RR=1.3), and breast (RR=1.0) cancers.84 Another study, from 2006, also included data on esophageal cancer (AOR=0.67, RRconv=0.69).57 These data provide a mean meta-analytic RR of 0.89, 95% CI=0.72–1.09. While not statistically significant, this is consistent with decreased risk. Note that these are likely to underestimate the impact today, as the obesity rate in the United States was about 25% lower at the time these data were collected than it is today (data from CDC).
Data can be used to provide a rough, preliminary estimate of the potential magnitude of the impact of Cannabis use on cancer diagnoses and deaths. This can be obtained as the product of the annual number of cancer deaths or diagnoses, the fraction of the population using Cannabis, and the decrease in risk of cancer in Cannabis users (1 minus the RR). There are projected to be 1.7 million diagnoses and 607,000 deaths from cancer in the United States in 2019 (Ref.1). According to current census data, the U.S. population age 18 years and above is ∼255 million. A recent poll suggests that 55 million, or about 21%, of these people use Cannabis, and 35 million, or ∼14%, are regular users.96 If the 10% decrease in cancer risk indicated by the current analysis is accurate, and applies across all cancer types, then Cannabis use would prevent as many as 23,800 to 35,700 cancer diagnoses (1,700,000×0.14 or 0.21×0.10), and 8498 to 12,747 cancer deaths (607,000×0.14 or 0.21×0.10) each year.
Some cancer types are relatively common while others are rare, and some types have much greater mortality rates than others. In the current data set, only testicular cancers show a trend to increasing risk in Cannabis users, and testicular cancer is a relatively uncommon cancer with a high survival rate. Furthermore, there is high heterogeneity in the data and no data are available for many cancers. The actual impact of Cannabis use on cancer cases and deaths therefore cannot be estimated with any degree of confidence with the data available at this time. However, given the large number of cancer diagnoses, and the large numbers of people using Cannabis, even a moderate impact on cancer risk will have significant public health implications.
There is increasing awareness that Cannabis provides therapeutic medical benefits. By decreasing risk of cancer, obesity, and DM,4,34–38,97 leading causes of premature death and disability, it is becoming clear that Cannabis use may also meet the definition of preventive medicine. Like all medicines, Cannabis has harmful effects, especially if misused. However, Cannabis use rarely results in fatalities, and is thus safer than many pharmaceuticals.98–103 Therefore, moderate, adult Cannabis use may be associated with a net improvement in public health.
Exposure level dependence
Even the lowest category of use appears to be associated with a significant decrease in cancer risk (Fig. 4). This resembles data in which individuals using Cannabis only one to four times per month show decreased BMI and obesity risk relative to nonusers,34 due at least in part by long-lasting downregulation of CB1 in response to Cannabis use.4 Thus, use of Cannabis only one to four times per month may be sufficient to significantly reduce risk of cancer, DM, and fatty liver disease while avoiding the harmful effects of heavy or frequent use.
How does the current analysis compare with previous studies?
Several prior reviews and meta-analyses have addressed the relationship between Cannabis use and cancer risk.105–110 Three of these reviews detected no association between Cannabis use and cancer risk,105,108,109 one hypothesized a decrease in risk106 and two concluded that Cannabis use is associated with an increased risk of cancer,107,110 of the testes and lungs, respectively.
The current review differed by being the only study to convert AOR data to RR before analysis, then use log RR data in a meta-analyses. The current review also differs from those studies by including all available cancer data, rather than focusing on a specific cancer type. The current study is also the only one to determine the impact of removing studies with high risk of selection bias or those with low sample sizes among Cannabis users or controls. The differences in data selection criteria and treatment of the data resulted in differences in summary measures of risk among these studies.
Implications for use of Cannabis as a cancer cure or treatment
There is an emerging trend in which patients turn away from more mainstream chemotherapy methods and instead attempt to use Cannabis as a stand-alone cancer cure.111 The current analysis does not support average recreational levels of Cannabis use as an effective stand-alone cure for cancer. However, the results do suggest that, in addition to providing significant improvements in the quality of life of cancer patients, adding Cannabis therapy to established cancer treatment regimens may well improve treatment efficacy without stimulating tumor growth.
Limitations
At present, reliable estimates of RR are not available for most cancer types, and no risk data at all are available for many cancer types while certain cancer types (head and neck, lung, testicular) are over-represented in the data. Few data are available for cancer types correlated with obesity, and Cannabis use is associated with reduced risk of obesity.4 No single study addresses the relationship between Cannabis use and the overall risk of cancer. Instead, available studies focus on one or several specific cancer types. Furthermore, the data are associative, rather than a result of controlled clinical trials or interventions. Therefore, the overall impact of Cannabis use on cancer risk cannot be determined with confidence using the available data.
A statistically significant decrease in risk (at p<0.05) was not apparent in the original data set, but emerges after elimination of data with high risk of selection and performance bias, as defined. The elimination of these data was to accomplish two explicit goals. The first goal is to specifically determine the likely impact of Cannabis use on cancer risk in the United States. This was accomplished by rejecting data from North Africa, where Cannabis is consumed with tobacco,57 and data that failed to adjust for tobacco use, as these data conflate the effects of tobacco use with the impact of Cannabis itself. The second goal was to increase the accuracy and reduce the variance of the results by reducing the risk of performance bias introduced by small sample sizes. This may have eliminated data from rare cancers. Elimination of data for testicular cancers in the examination of heterogeneity also resulted in a significant decrease in risk in the remaining data.
A limitation to using the results to estimate the overall impact on cancer rates and deaths arises because the overall impact of Cannabis use on risk of cancer depends on its impact on risk of each individual cancer type, and the frequency of that cancer type in the population. For example, if Cannabis increases risk of a rare cancer, but decreases the risk of a common cancer by an equal amount, the overall effect would be an overall decrease in cancer diagnoses in the population, and vice versa. Similarly, effects of Cannabis use on risk of cancer types with high mortality rates are more significant than effects on risk of less-aggressive cancers or cancers for which established treatment methods are more successful.
The risk ratios used in the analysis arise from self-report data. These are not optimal as patients may be reluctant to divulge Cannabis use. Furthermore, much of the data used in the analysis are from comparisons of ever users versus never users, and so by implication includes people with little or no recent exposure to Cannabis. However, Ngueta and Ndjaboue38 observed lingering impacts of Cannabis use on obesity rates that remained after long-term abstinence, and it is possible that the same is true of cancer, especially considering the strong links between obesity and cancer.20–27 Furthermore, a subset of the data was amenable to exposure dependence analysis, and includes respondents who used Cannabis at high levels, or for long periods of time (30 years or more). These data did not show appreciably different risk in low and high exposure categories of users (Fig. 8).
Acknowledgments
The authors thank Rhonda Culbertson, IUSB librarian, for help designing literature search strategies. Jordan Zaderej, IUSB student, for discussions of inflammation as a contributor to comorbidities of obesity.
Abbreviations Used
- AHR
adjusted hazard ratio
- AOR
adjusted odds ratio
- BMI
body mass index
- CB1
cannabinoid receptor type 1
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DM
diabetes mellitus
- EC
endocannabinoid system
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papilloma virus
- logRR
log of RR data
- RR
relative risk
- RRconv
relative risk converted from AOR data
- SCC
squamous cell carcinoma
- THC
Δ9-tetrahydrocannabinol
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was supported by a sabbatical leave from Indiana University South Bend. No other funding support was utilized in this project.
Cite this article as: Clark TM (2020) Scoping review and meta-analysis suggests that Cannabis use may reduce cancer risk in the United States, Cannabis and Cannabinoid Research 6:5, 413–434, DOI: 10.1089/can.2019.0095.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Islami F, Miller KD, Siegel RL, et al. . National and state estimates of lost earnings from cancer deaths in the United States. JAMA Oncol. 2019;5:e191460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Velasco G, Sánchez C, Guzmán M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12:436–444. [DOI] [PubMed] [Google Scholar]
- 4. Clark TM, Jones JM, Hall AG, et al. . Theoretical explanation for reduced body mass index and obesity rates in Cannabis users. Cannabis Cannabinoid Res. 2018;3:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bisogno T, Maccarrone M. Endocannabinoid signaling and its regulation by nutrients. BioFactors. 2014;40:373–380. [DOI] [PubMed] [Google Scholar]
- 6. Mazier W, Saucisse N, Gatta-Cherifi B, et al. . The endocannabinoid system: pivotal orchestrator of obesity and metabolic disease. Trends Endocrinol Metab. 2015;26:524–537. [DOI] [PubMed] [Google Scholar]
- 7. Martell K, Fairchild A, LeGerrier B, et al. . Rates of Cannabis use in patients with cancer. Curr Oncol. 2018;25:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boehnke KF, Litinas E, Clauw DJ. Medical Cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17:739–744. [DOI] [PubMed] [Google Scholar]
- 9. Haroutounian S, Ratz Y, Ginosar Y, et al. . The effect of medicinal Cannabis on pain and quality-of-life outcomes in chronic pain. Clin J Pain. 2016;32:1036–1043. [DOI] [PubMed] [Google Scholar]
- 10. Bradford AC, Bradford WD, Abraham A, et al. . Association between US state medical Cannabis laws and opioid prescribing in the Medicare Part D population. JAMA Intern Med. 2018;178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen H, Hockenberry JM. Association of medical and adult-use marijuana laws with opioid prescribing for Medicaid enrollees. JAMA Intern Med. 2018;178:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carter GT, Flanagan AM, Earleywine M, et al. . Cannabis in palliative medicine: improving care and reducing opioid-related morbidity. Am J Hosp Palliat Care. 2011;28:297–303. [DOI] [PubMed] [Google Scholar]
- 13. Chakravarti B, Ravi J, Ganju RK. Cannabinoids as therapeutic agents in cancer: current status and future implications. Oncotarget. 2014;5:5852–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ostadhadi S, Rahmatollahi M, Dehpour AR, et al. . Therapeutic potential of cannabinoids in counteracting chemotherapy-induced adverse effects: an exploratory review. Phytother Res. 2015;29:332–338. [DOI] [PubMed] [Google Scholar]
- 15. Wu TC, Tashkin DP, Djahed B, et al. . Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318:347–351. [DOI] [PubMed] [Google Scholar]
- 16. Melamede R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduct J. 2005;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fligiel SE, Roth MD, Kleerup EC, et al. . Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112:319–326. [DOI] [PubMed] [Google Scholar]
- 18. Zwenger SR. Bogarting that joint might decrease oral HPV among Cannabis users. Curr Oncol. 2009;16:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeda S, Yamamoto I, Watanabe K. Modulation of Δ9-tetrahydrocannabinol-induced MCF-7 breast cancer cell growth by cyclooxygenase and aromatase. Toxicology. 2009;259:25–32. [DOI] [PubMed] [Google Scholar]
- 20. Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 21. Taghizadeh N, Boezen HM, Schouten JP, et al. . BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10:e0125261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renehan AG, Tyson M, Egger M, et al. . Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. [DOI] [PubMed] [Google Scholar]
- 23. Sung H, Siegel RL, Rosenberg PS, et al. . Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4:e137–e147. [DOI] [PubMed] [Google Scholar]
- 24. Hamaguchi M, Hashimoto Y, Obora A, et al. . Non-alcoholic fatty liver disease with obesity as an independent predictor for incident gastric and colorectal cancer: a population-based longitudinal study. BMJ Open Gastroenterol. 2019;6:e000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guh DP, Zhang W, Bansback N, et al. . The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arnold M, Pandeya N, Byrnes G, et al. . Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng T, Lyon CJ, Bergin S, et al. . Obesity, inflammation, and cancer. Annu Rev Pathol-Mech. 2016;11:421–449. [DOI] [PubMed] [Google Scholar]
- 28. Vázquez-Bourgon J, de la Foz VOG, Suarez-Pereira I, et al. . Cannabis consumption and non-alcoholic fatty liver disease. A three years longitudinal study in first episode non-affective psychosis patients. Prog Neuro-Psychoph. 2019;95:109677. [DOI] [PubMed] [Google Scholar]
- 29. Munn LL. Cancer and inflammation. WIREs Syst Biol Med. 2017;9:e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. [DOI] [PubMed] [Google Scholar]
- 31. Clement K, Viguerie N, Poitou C, et al. . Weight loss regulates inflammation related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. [DOI] [PubMed] [Google Scholar]
- 32. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. [DOI] [PubMed] [Google Scholar]
- 33. Zurier RB, Burstein SH. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016;30:3682–3689. [DOI] [PubMed] [Google Scholar]
- 34. Rajavashisth TB, Shaheen M, Norris KC, et al. . Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ngueta G, Bélanger RE, Laouan-Sidi EA, et al. . Cannabis use in relation to obesity and insulin resistance in the Inuit population. Obesity. 2015;23:290–295. [DOI] [PubMed] [Google Scholar]
- 36. Alshaarawy O, Anthony JC. Cannabis smoking and serum C-reactive protein: a quantile regressions approach based on NHANES 2005–2010. Drug Alcohol Depen. 2015;147:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med. 2013;126:583–589. [DOI] [PubMed] [Google Scholar]
- 38. Ngueta G, Ndjaboue R. Lifetime marijuana use in relation to insulin resistance in lean, overweight, and obese US adults. J Diabetes. 2019;12:38–47. [DOI] [PubMed] [Google Scholar]
- 39. Tsilidis KK, Kasimis JC, Lopez DS, et al. . Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 40. Cani PD, Plovier H, Van Hul M, et al. . Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol. 2016;12:133. [DOI] [PubMed] [Google Scholar]
- 41. Cluny NL, Keenan CM, Reimer RA, et al. . Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS One. 2015;10:e0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3:745. [DOI] [PubMed] [Google Scholar]
- 43. McAllister SD, Soroceanu L, Desprez PY. The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharm. 2015;10:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galve-Roperh I, Sánchez C, Cortéz ML, et al. . Anti- tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–319. [DOI] [PubMed] [Google Scholar]
- 45. Moreau M, Ibeh U, Decosmo K, et al. . Flavonoid derivative of Cannabis demonstrates therapeutic potential in preclinical models of metastatic pancreatic cancer. Front Oncol. 2019;9:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fraguas-Sánchez AI, Martín-Sabroso C, Torres-Suárez AI. Insights into the effects of the endocannabinoid system in cancer: a review. Br J Pharmacol. 2018;175:2566–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carracedo A, Gironella M, Lorente M, et al. . Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress–related genes. Cancer Res. 2006;66:6748–6755. [DOI] [PubMed] [Google Scholar]
- 48. Cianchi F, Papucci L, Schiavone N, et al. . Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α-mediated ceramide de novo synthesis in colon cancer cells. Clin Cancer Res. 2008;14:7691–7700. [DOI] [PubMed] [Google Scholar]
- 49. Dando I, Donadelli M, Costanzo C, et al. . Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 2013;4:e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Donadelli M, Dando I, Zaniboni T, et al. . Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011;2:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qamri Z, Preet A, Nasser MW, et al. . Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther. 2009;8:3117–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olea-Herrero N, Vara D, Malagarie-Cazenave S, et al. . Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R (+)-Methanandamide and JWH-015: involvement of CB 2. British J Cancer. 2009;101:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vara D, Salazar M, Olea-Herrero N, et al. . Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Diff. 2011;18:1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patsos HA, Hicks DJ, Dobson RR, et al. . The endogenous cannabinoid, anandamide, induces cell death in colorectal carcinoma cells: a possible role for cyclooxygenase 2. Gut. 2005;54:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xian XS, Park H, Choi MG, et al. . Cannabinoid receptor agonist as an alternative drug in 5-fluorouracil-resistant gastric cancer cells. Anticancer Res. 2013;33:2541–2547. [PubMed] [Google Scholar]
- 56. Fonseca BM, Correia-da-Silva G, Teixeira NA. Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. J Physiol Biochem. 2018;74:261–272. [DOI] [PubMed] [Google Scholar]
- 57. Hashibe M, Morgenstern H, Cui Y, et al. . Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–1834. [DOI] [PubMed] [Google Scholar]
- 58. Aldington S, Harwood M, Cox B, et al. . Cannabis use and risk of lung cancer: a case-control study. Eur Respir J. 2008;31:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aldington S, Harwood M, Cox B, et al. . Cannabis use and cancer of the head and neck: case-control study. Otolaryngol Head Neck Surg. 2008;138:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berthiller J, Straif K, Boniol M, et al. . Cannabis smoking and risk of lung cancer in men. A pooled analysis of three studies in Magreb. J Thor Onc. 2008;3:1398–1403. [DOI] [PubMed] [Google Scholar]
- 61. Berthiller J, Yuan-chin AL, Boffetta P, et al. . Marijuana smoking and the risk of head and neck cancer: pooled analysis in the INHANCE Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Cause Control. 2013;24:1811–1820. [DOI] [PubMed] [Google Scholar]
- 63. Callaghan RC, Allebeck P, Akre O, et al. . Cannabis use and incidence of testicular cancer: a 42-year follow-up of Swedish men between 1970 and 2011. Cancer Epidemiol Biomarkers Prev. 2017;26:1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chacko JA, Heiner JG, Siu W, et al. . Association between marijuana use and transitional cell carcinoma. Urology. 2006;67:100–104. [DOI] [PubMed] [Google Scholar]
- 65. Chao C, Jacobson LP, Jenkins FJ, et al. . Recreational drug use and risk of Kaposi's sarcoma in HIV-and HHV-8- coinfected homosexual men. AIDS Res Hum Retrov. 2009;25:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Daling JR, Weiss NS, Hislop TG, et al. . Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987;317:973–977. [DOI] [PubMed] [Google Scholar]
- 67. Daling JR, Doody DR, Xiaofei Sun BS, et al. . Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Efird JT, Friedman GD, Sidney S, et al. . The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neuro-Oncol. 2004;68:57–69. [DOI] [PubMed] [Google Scholar]
- 69. Feng BJ, Khyatti M, Ben-Ayoub W, et al. . Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Brit J Cancer. 2009;101:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gillison ML, D'Souza G, Westra W, et al. . Distinct risk factor profiles for human papillomavirus type 16–positive and human papillomavirus type 16–negative head and neck cancers. J Nat Cancer Inst. 2008;100:407–420. [DOI] [PubMed] [Google Scholar]
- 71. Han B, Gfroerer JC, Colliver JD. Associations between duration of illicit drug use and health conditions: results from the 2005–2007 national surveys on drug use and health. Ann Epidemiol. 2010;20:289–297. [DOI] [PubMed] [Google Scholar]
- 72. Holly EA, Lele C, Bracci PM, et al. . Case-control study of non-Hodgkin's lymphoma among women and heterosexual men in the San Francisco Bay Area, California. Am J Epidemiol. 1999;150:375–389. [DOI] [PubMed] [Google Scholar]
- 73. Hsairi M, Achour N, Zouari B, et al. . Etiologic factors in primary bronchial carcinoma in Tunisia. La Tunise Medicale. 1993;71:265–268. [PubMed] [Google Scholar]
- 74. Lacson JCA, Carroll JD, Tauzon E, et al. . Population- based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012;118:5374–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liang C, McClean MD, Marsit C, et al. . A population- based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res. 2009;2:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Llewellyn CD, Linklater K, Bell J, et al. . Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 2003;39:106–114. [DOI] [PubMed] [Google Scholar]
- 77. Llewellyn CD, Linklater K, Bell J, et al. . An analysis of risk factors for oral cancer in young people: a case control study. Oral Oncol. 2004;40:304–313. [DOI] [PubMed] [Google Scholar]
- 78. Llewellyn CD, Johnson NW, Sarnakulasuriya S. Risk factors for oral cancer in newly diagnosed patients aged 45 years and younger: a case-control study in Southern England. Oral Path Med. 2004;33:525–532. [DOI] [PubMed] [Google Scholar]
- 79. Maden C, Sherman KJ, Beckmann AM, et al. . History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19–24. [DOI] [PubMed] [Google Scholar]
- 80. Marks MA, Chaturvedi AK, Kelsey K, et al. . Association of marijuana smoking with oropharyngeal and oral tongue cancers: pooled analysis from the INHANCE consortium. Cancer Epidemiol Biomarkers Prev. 2014;23:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nelson RA, Levine AM, Marks G, et al. . Alcohol, tobacco and recreational drug use and the risk of non-Hodgkin's lymphoma. Br J Cancer. 1997;76:1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rosenblatt KA, Daling JR, Chen C, et al. . Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64:4049–4054. [DOI] [PubMed] [Google Scholar]
- 83. Sasco AJ, Merrill RM, Dari I, et al. . A case-control study of lung cancer in Casablanca, Morocco. Cancer Causes Control. 2002;13:609–616. [DOI] [PubMed] [Google Scholar]
- 84. Sidney S, Quesenberry CP, Friedman GD. Marijuana use and cancer incidence (California, United States). Cancer Causes Control. 1997;8:722–728. [DOI] [PubMed] [Google Scholar]
- 85. Thomas AA, Wallner LP, Quinn VP, et al. . Association between Cannabis use and the risk of bladder cancer: results from the California Men's Health Study. Urology. 2015;85:388–393 [DOI] [PubMed] [Google Scholar]
- 86. Trabert B, Sigurdson AJ, Sweeney AM, et al. . Marijuana use and testicular germ cell tumors. Cancer. 2011;117:848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Voirin N, Berthiller J, Benhaïm-Luzon V, et al. . Risk of lung cancer and past use of Cannabis in Tunisia. J Thorac Oncol. 2006;1:577–579. [PubMed] [Google Scholar]
- 88. Zhang Z-F, Morgenstern H, Spitz MR, et al. . Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999;8:1071–1078. [PubMed] [Google Scholar]
- 89. Zhang LR, Morgenstern H, Greenland S, et al. . Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhu K, Levine RS, Brann EA, et al. . Case-control study evaluating the homogeneity and heterogeneity of risk factors between sinonasal and nasopharyngeal cancers. Internet J Cancer. 2002;99:119–123. [DOI] [PubMed] [Google Scholar]
- 91. Zhang J, Kai FY. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 92. Stare J, Maucort-Boulch D. Odds ratio, hazard ratio, and relative risk. Metodološki Zvezki. 2016;13:59–67. [Google Scholar]
- 93. Grubbs F. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 94. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 95. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34:917–928. [DOI] [PubMed] [Google Scholar]
- 96. Yahoo News/Marist Poll. Available at: http://maristpoll.marist.edu/yahoo-newsmarist-poll/#sthash.oD494aaA.dpbs. Accessed August 10, 2020.
- 97. Le Strat Y, Le Foll B. Obesity and Cannabis use: results from 2 representative national surveys. Am J Epidemiol. 2011;174:929–933. [DOI] [PubMed] [Google Scholar]
- 98. Meier MH, Caspi A, Cerda M, et al. . Associations between Cannabis use and physical health problems in early midlife a longitudinal comparison of persistent Cannabis vs tobacco users. JAMA Psychiat. 2016;73:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bechtold J, Simpson T, White HR, et al. . Chronic adolescent marijuana use as a risk factor for physical and mental health problems in young adult men. Psychol Addict Behav. 2015;29:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fuster D, Cheng DM, Allensworth-Davies D, et al. . No detectable association between frequency of marijuana use and health or healthcare utilization among primary care patients who screen positive for drug use. J Gen Intern Med. 2014;29:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Calabria B, Degenhardt L, Hall W, et al. . Does Cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of Cannabis use. Drug Alcohol Rev. 2010;29:318–330. [DOI] [PubMed] [Google Scholar]
- 102. Gmel G, Kuendig H, Rehm J, et al. . Alcohol and Cannabis use as risk factors for injury–a case-crossover analysis in a Swiss hospital emergency department. BMC Public Health. 2009;9:40..101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–659. [DOI] [PubMed] [Google Scholar]
- 104. Adejumo AC, Alliu S, Ajayi TO, et al. . Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: a cross-sectional study. PLoS One. 2017;12:e0176416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huang Z-F, Zhang Z-F, Tashkin DP, et al. . An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen AL, Chen TJ, Braverman ER, et al. . Hypothesizing that marijuana smokers are at a significantly lower risk of carcinogenicity relative to tobacco-non-marijuana smokers: evidenced based on statistical reevaluation of current literature. J Psychoactive Drugs. 2008;40:263–272. [DOI] [PubMed] [Google Scholar]
- 107. Gurney J, Shaw C, Stanley J, et al. . Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC Cancer. 2015;15:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. De Carvalho MFF, Dourado MR, Fernandes IB, et al. . Head and neck cancer among marijuana users: a meta-analysis of matched case–control studies. Archives Oral Biol. 2015;60:1750–1755. [DOI] [PubMed] [Google Scholar]
- 109. Mehra R, Moore BA, Crothers K, et al. . The association between marijuana smoking and cancer. A systematic review. Arch Intern Med. 2006;166:1359–1367. [DOI] [PubMed] [Google Scholar]
- 110. Borki R, Fenane H, Harrak L. Cannabis smoking and risk of lung cancer-A systematic review and meta-analysis. Int J Med Surgery. 2014;1:31–37. [Google Scholar]
- 111. Shi S, Brant AR, Sabolch A, et al. . False news of a Cannabis cancer cure. Cureus. 2019;11:e3918. [DOI] [PMC free article] [PubMed] [Google Scholar]