Abstract
Objectives: High levels of morbidity and mortality associated with injection drug use continue to represent a significant public health challenge in many settings worldwide. Previous studies have shown an association between cannabis use and decreased risk of some drug-related harms. We sought to evaluate the association between high-intensity cannabis use and the frequency of injection drug use among people who inject drugs (PWID).
Methods: The data for this analysis were collected from three prospective cohorts of PWID in Vancouver, Canada, between September 2005 and May 2018. Generalized linear mixed-effects models were used to analyze the association between daily cannabis use and the frequency of injecting illegal drugs (i.e., self-reported average number of injections per month).
Results: Among the 2,619 active PWID, the frequency of injection drug use was significantly lower among people who use cannabis daily compared with people who use it less than daily (adjusted odds ratio [AOR]=0.84, 95% confidence interval [CI]: 0.73–0.95). Sub-analyses indicated that this effect was restricted to the frequency of illegal opioid injection (AOR=0.78, 95% CI: 0.68–0.90); the association between daily cannabis use and the frequency of illegal stimulant injection was not significant (AOR=1.08, 95% CI 0.93–1.25).
Discussion: The findings from these prospective cohorts suggest that people who use cannabis daily were less likely to report daily injection of illegal drugs compared with people who use it less than daily. These results suggest the potential value of conducting experimental research to test whether controlled administration of cannabinoids impacts the frequency of illegal opioid injection among PWID.
Keywords: cannabis, injection drug use, people who inject drugs, prospective cohort study
Introduction
An estimated 15.6 million people inject illegal drugs worldwide, and the morbidity and mortality associated with injection drug use continues to be a major public health concern.1 The health and social consequences of injection drug use include higher risks of acquiring blood-borne pathogens, including HIV and hepatitis C virus (HCV), accidental overdose, stigmatization, and criminalization.2–4 Clinical engagement in treatment for HIV infection and substance use disorders can also be compromised by ongoing injection drug use: Patients who inject drugs are more likely to have poor drug treatment outcomes, have higher rates of sub-optimal adherence to antiretroviral therapy (ART), and exhibit a detectable HIV viral load.5–7
The recent legalization of medical and nonmedical cannabis in many settings in the United States and Canada has sparked concerns about the impact of these regulatory reforms on vulnerable populations, for example, through promoting the development of high-risk drug use behaviours.8,9 The so-called gateway hypothesis suggests that cannabis facilitates the transition from legal to illegal substance use and that the impact of cannabis on this transition is causal.9,10 However, many authors have argued that this transition may be attributed to genetic, environmental, and psychosocial risk factors for drug use rather than the effect of gateway substances.9,11 Further, cannabis use may have different effects on other high-risk drug behaviors among different populations, such as marginalized people who use drugs. Recent studies have suggested that cannabis use may have the potential to mitigate the risks of drug-related harms among people who inject drugs (PWID). For example, preliminary studies have reported that cannabis use is associated with reductions in illegal drug use, drug-seeking behavior, injection initiation, and exposure to fentanyl.12–18 In a recent longitudinal study of people who use illegal drugs starting opioid agonist therapies (OAT), high-intensity cannabis use was associated with better levels of retention in treatment at 6 months.19 Given these preliminary findings, and the ongoing crisis borne of unprecedented numbers of drug-related overdose deaths throughout the United States and Canada, thorough examination of the relationships between cannabis use and high-risk drug use is urgently needed.
In October, 2018, Canada became the second country to legalize nonmedical cannabis use for adults at the federal level.20 Cannabis is one of the most commonly used substances in Canada, with approximately half of all Canadians older than the age of 15 reporting lifetime cannabis use.21 Cannabis use is most common among young people and males. Residents of British Columbia report significantly higher rates of past year cannabis use (23%) than other Canadian provinces.22 Vancouver, British Columbia, is also home to high levels of injection drug use among people living in the Downtown Eastside area23,24 and it has followed a de facto policy of decriminalized cannabis use since 2005.25 The characteristics of this setting and the implementation of this cannabis policy provide an opportunity to examine the impact of cannabis use on injection drug use patterns among PWID.
Several studies have evaluated the determinants of injection drug use patterns among PWID, although the majority has focused on injection initiation and injection cessation. Hazardous alcohol use, cocaine use, and poly-substance use have been associated with increased or active injection drug use.26–28 For some PWID, cocaine use significantly increases the risk of injecting binges. With a half-life between 40 and 60 min, people who use cocaine often inject up to 30 times per day.28,29 Specific types of substance use associated with increased rates of injection initiation and decreased rates of injection cessation include crystal methamphetamine, cocaine, crack-cocaine, heroin, alcohol, and benzodiazepines.30–33 Investigations of cannabis use on injection patterns have produced mixed results, with an initial study indicating that cannabis use increased the risk of injection initiation and two more recent analyses found that cannabis use was associated with a decreased likelihood of injection initiation.16,32,34
Given that the legalization and regulation of nonmedical cannabis use is expanding in many jurisdictions throughout North America, and the existing studies suggesting that cannabis may reduce drug-related harms among PWID, further investigation of how cannabis influences injection drug use behaviors is warranted.35 The objective of this study was to evaluate the association between frequent cannabis use and the frequency of injection drug use among three prospective cohorts of PWID in Vancouver, Canada between 2005 and 2018. Since a few studies have investigated the independent effects of cannabis use on opioid and stimulant use, we also performed sub-analyses to evaluate the association between cannabis use and the frequency of stimulant and opioid injection.16,35 Based on the early evidence linking cannabis use to lower rates of other forms of substance use, specifically among marginalized people who use drugs,15,16,18 we hypothesize that cannabis use will be associated with decreased injection drug use frequency among this sample of PWID.
Materials and Methods
The data for this analysis were collected from three open and ongoing community-recruited prospective cohorts of people who use illegal drugs with harmonized procedures for recruitment, follow-up, and data collection in Vancouver, Canada. These cohorts include the Vancouver Injection Drug Users Study (VIDUS), the AIDS Care Cohort to Evaluate exposure to Survival Services (ACCESS), and the At-Risk Youth Study (ARYS). In each study, participants are recruited through extensive community outreach in the Downtown Eastside and Downtown South neighborhoods of Vancouver, Canada. To be eligible for these studies, participants must reside in the Greater Vancouver Regional District, use illegal drugs other than or in addition to cannabis (which was illegal during the study period) in the previous month, and provide informed consent at study enrolment. The VIDUS includes adults who are HIV-negative and injected drugs in the month preceding enrolment; ACCESS includes HIV-positive adults, and ARYS includes street-involved youth aged 14–26 years (Supplementary Table S1). These cohorts have been described in detail in previous studies.36–38
At baseline and every 6-month follow-up visit thereafter, participants completed an interviewer-administered questionnaire, provided blood samples for HIV/HCV serological analysis, and were examined by a study nurse who provides basic medical care and referrals to health and social services if requested. The study questionnaire collects data, including sociodemographic information, substance use patterns, HIV risk behavior, and engagement with health and social services. The items used to assess injection frequency were asked separately for each substance, and these items were identical across questionnaires for each of the study cohorts. At each study visit, participants are remunerated $40 CAD for their time. The VIDUS, ACCESS, and ARYS studies have been approved by the University of British Columbia/Providence Health Care Research Ethics Board.
The analytical sample for this study included all VIDUS, ACCESS, and ARYS participants aged at least 18 years old, reported injection drug use at any point over follow-up, and completed at least one study visit between September 2005 and May 2018. We included all observations from all VIDUS participants, as injection drug use was an inclusion criterion for this cohort. For ACCESS and ARYS participants who reported injecting drugs at their earliest interviews, we included all observations. Among ACCESS and ARYS participants who were injection-naive at baseline, we included all observations after their first report of injection drug use, if any. This included initiates of injection drug use, and this sample was selected to analyze the impact of cannabis use among all participants who reported injection drug use over their history of substance use. The specific impact of cannabis use on injection initiation has been evaluated in previous studies.16,32,34,39
The primary outcome of interest was injection drug use frequency, which was derived from the item: “In the last six months, when you were using, which of the following drugs did you inject and how often?” Participants were provided with a list of commonly injected drugs (e.g., heroin, cocaine, crystal methamphetamine) and were asked to estimate their average frequency of injection in the past 6 months according to the following response options: (1) no use (reference category); (2) less than once per month; (3) one to three times per month; (4) about once per week; (5) two or more times per week; and (6) at least once per day. Pharmaceutical opioid injection was measured through the item: “In the past 6 months, have you injected any of the following prescription opioids? If so, how often did you inject them?” The participants were provided with a list of pharmaceutical opioids with corresponding pictures to facilitate identification. The response options were identical to those listed earlier. Based on these frequency categories, participants who reported daily injection of these substances were coded as “1” for the outcome (i.e., daily injection drug use).18
The primary explanatory variable of interest was cannabis use in the past 6 months (≥daily vs. <daily). Cannabis use was measured based on the item: “In the last six months, how often have you used marijuana?” The response options include, “0=less than once a month, 1=1–3 times a month, 2=once a week, 3=2 or more times a week, 4=at least daily.” These categories were collapsed to ≥ daily vs. <daily due to the low prevalence of people who use occasionally (e.g., “less than once a month” and “1–3 times per month”), and analyzing these as individual categories can produce unstable estimates of effect size.
We also included sociodemographic and substance use variables hypothesized to confound the association between cannabis use and injection drug use frequency based on previous studies. These variables included: age (per year older); gender (male vs. non-male); ethnicity (white vs. non-white); living in a relationship (i.e., legally married, common law or regular partner vs. others); recent incarceration (yes vs. no); licit employment (i.e., having regular, temporary, or self-employed work vs. none); enrollment in OAT, including methadone and buprenorphine/naloxone, (yes vs. no); engagement with alcohol or drug treatment, excluding OAT (yes vs. no); homelessness (yes vs. no); binge drug use, defined as a period of using drugs more often than usual (yes vs. no); non-injection heroin use (≥daily vs. <daily); non-injection cocaine use (≥daily vs. <daily); non-injection crack-cocaine use (≥daily vs. <daily); non-injection crystal methamphetamine use (≥daily vs. <daily); involvement in drug dealing (yes vs. no); time since baseline visit (per year longer); previous injection drug use, based on injection drug use during the study visit preceding the most recent follow-up visit (≥daily vs. <daily); and cohort (ACCESS vs. VIDUS and ARYS vs. VIDUS). All behavioral variables refer to the 6-month period preceding the most recent follow-up, and variable definitions were consistent with previous studies.40
First, we analyzed the baseline characteristics of the analytical sample, stratified by cannabis use, using the Chi-square test for binary measures and the Wilcoxon's rank-sum test for continuous measures. The association between cannabis use and injection drug use frequency was analyzed by using generalized linear mixed-effects models (GLMM) with a logit link function to account for repeated measurements within individuals over time. Random intercepts were included in each of the models to account for the clustering of observations within participants. Specifically, we performed a crude GLMM analysis to evaluate the association between each explanatory variable of interest and the frequency of injection drug use. For the multivariable analysis, we fit a full multivariable model that included cannabis use and all other explanatory variables. The final multivariable model was created through an iterative process, removing the variable that produced the smallest relative change in the cannabis use coefficient. This manual step-wise procedure was repeated until the minimum change in the cannabis use coefficient exceeded 5%. The objective of this method is to retain covariates with a larger relative effect on the association between the primary explanatory variable (i.e., cannabis use) and the outcome (i.e., injection frequency) or, in other words, fit a parsimonious model with the most important confounding variables, consistent with previous studies.41
Cannabis use measures were lagged by one study visit in the analysis to temporally precede the outcome. Time since baseline and study cohort were forced into all models. We also conducted sub-analyses to investigate the impact of cannabis use on the frequency of injection opioid use, and the frequency of injection stimulant use. Injection opioid use included heroin, fentanyl, and prescription opioids; injection stimulant use included cocaine, crack-cocaine, and crystal methamphetamine. Each of these analyses was restricted to participants who use each substance, and the type of substance use was time-updated over follow-up (e.g., the analysis of injection opioid frequency was restricted to people who use injection opioids). The GLMM was also applied to the sub-analyses and followed the same model-building procedure as the whole-sample analysis. The statistical analyses were performed by using SAS version 9.4 (SAS, Cary, NC). All p-values were two-sided with a significance threshold of 0.05.
Results
Between September 2005 and May 2018, 2,619 participants provided at least one report of injection drug use, completed at least one interview during the study period, and were included in these analyses. The median age of participants at baseline was 36.5 (interquartile range [IQR]=25.7–45.7) years, 1,710 (65.3%) were male, and 1,569 (59.9%) self-reported white ancestry. The median number of follow-up visits completed by the study sample was 5 (IQR=2–10).
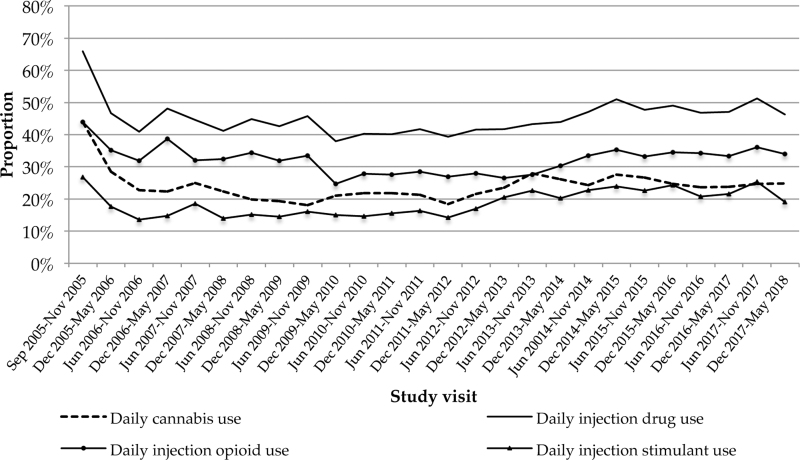
The baseline characteristics of the study sample stratified by daily cannabis use are presented in Table 1. At baseline, daily cannabis use was reported by 729 (27.8%) participants and 1,211 (46.2%) participants reported daily injection drug use. Baseline was defined as the first study visit for each participant when they reported injection drug use. The trends in cannabis use and injection drug use over the study period are displayed in Figure 1 (please see Supplementary Fig. S1 for the trends in injection drug use stratified by daily cannabis use and Supplementary Table S2 for cannabis use trends over follow-up). For participants who reported injection drug use at the outset of the cohort study, this would include their first study visit, and this would include subsequent study visits for participants who did not report injection drug use during their initial study visit.
Table 1.
Baseline Characteristics Stratified by Daily Cannabis Use Among People Who Inject Drugs (n=2,619)
| Characteristic | Total, n (%) | Daily cannabis use |
p | |
|---|---|---|---|---|
| Yes, 729 (27.8%), n (%) | No, 1,890 (72.2%), n (%) | |||
| Age | ||||
| Median | 36.5 | 31.7 | 37.7 | <0.001 |
| IQR | (25.7–45.7) | (23.7–42.7) | (27.3–46.5) | |
| Sex | ||||
| Female | 908 (34.7) | 192 (26.3) | 716 (37.9) | <0.001 |
| Male | 1,710 (65.3) | 537 (73.7) | 1,173 (62.1) | |
| White ancestry | ||||
| Yes | 1,569 (59.9) | 440 (60.4) | 1,129 (59.7) | 0.721 |
| No | 1,040 (39.7) | 285 (39.1) | 755 (39.9) | |
| Relationship | ||||
| Legally married/common law/regular partner | 753 (28.8) | 209 (28.7) | 544 (28.8) | 0.993 |
| Other | 1,858 (70.9) | 516 (70.8) | 1,342 (71.0) | |
| Incarcerationa | ||||
| Yes | 491 (18.7) | 134 (18.4) | 357 (18.9) | 0.827 |
| No | 2,113 (80.7) | 587 (80.5) | 1,526 (80.7) | |
| Employmenta | ||||
| Yes | 683 (26.1) | 242 (33.2) | 441 (23.3) | <0.001 |
| No | 1,935 (73.9) | 487 (66.8) | 1,448 (76.6) | |
| Opioid agonist therapya | ||||
| Yes | 1,065 (40.7) | 248 (34.0) | 817 (43.2) | <0.001 |
| No | 1,541 (58.8) | 474 (65.0) | 1,067 (56.5) | |
| Participation in drug or alcohol treatmenta | ||||
| Yes | 356 (13.6) | 118 (16.2) | 238 (12.6) | 0.012 |
| No | 2,246 (85.8) | 601 (82.4) | 1,645 (87.0) | |
| Homelessnessa | ||||
| Yes | 1,172 (44.7) | 363 (49.8) | 809 (42.8) | 0.002 |
| No | 1,432 (54.7) | 365 (50.1) | 1,067 (56.5) | |
| Binge drug usea | ||||
| Yes | 1,452 (55.4) | 413 (56.7) | 1,039 (55.0) | 0.329 |
| No | 1,149 (43.9) | 307 (42.1) | 842 (44.6) | |
| Non-injection heroin usea | ||||
| ≥Daily | 127 (4.8) | 37 (5.1) | 90 (4.8) | 0.728 |
| <Daily | 2,486 (94.9) | 689 (94.5) | 1,797 (95.1) | |
| Non-injection cocaine usea | ||||
| ≥Daily | 28 (1.1) | 14 (1.9) | 14 (0.7) | 0.008 |
| <Daily | 2,588 (98.8) | 714 (97.9) | 1,874 (99.2) | |
| Non-injection crack cocaine usea | ||||
| ≥Daily | 753 (28.8) | 170 (23.3) | 583 (30.8) | <0.001 |
| <Daily | 1,860 (71.0) | 556 (76.3) | 1,304 (69.0) | |
| Non-injection methamphetamine usea | ||||
| ≥Daily | 200 (7.6) | 70 (9.6) | 130 (6.9) | 0.018 |
| <Daily | 2,417 (92.3) | 658 (90.3) | 1,759 (93.1) | |
| Participation in selling illicit drugsa | ||||
| Yes | 966 (36.9) | 277 (38.0) | 689 (36.5) | 0.463 |
| No | 1,653 (63.1) | 452 (62.0) | 1,201 (63.5) | |
| Cohort | ||||
| VIDUS | 1,214 (46.4) | 277 (38.0) | 937 (49.6) | <0.001 |
| ACCESS | 742 (28.3) | 175 (24.0) | 567 (30.0) | |
| ARYS | 663 (25.3) | 277 (38.0) | 386 (20.4) | |
| Year of study visit | ||||
| Median | 2008 | 2010 | 2008 | 0.006 |
| IQR | (2006–2013) | (2006–2014) | (2006–2013) | |
| Frequency of injection drug usea | ||||
| No use | 41 (1.6) | 15 (2.1) | 26 (1.4) | <0.001 |
| Less than once per month | 455 (17.4) | 151 (20.7) | 304 (16.1) | |
| One to three times per month | 262 (10.0) | 93 (12.8) | 169 (8.9) | |
| Once per week | 215 (8.2) | 54 (7.4) | 161 (8.5) | |
| Two to three times per week | 434 (16.6) | 128 (17.6) | 306 (16.2) | |
| At least daily | 1,211 (46.2) | 288 (39.5) | 923 (48.8) | |
Bold text refers to p-values <0.05.
Refers to activities in the 6 months before the follow-up interview.
ACCESS, AIDS Care Cohort to Evaluate exposure to Survival Services; ARYS, At-Risk Youth Study; IQR, interquartile range; VIDUS, Vancouver Injection Drug Users Study.
FIG. 1.
Trends in cannabis use and injection drug over follow-up (2005–2018).
The specific substances that the participants reported injecting daily at baseline included heroin (n=805, 30.7%), cocaine (n=194, 7.4%), crack-cocaine (n=16, 0.6%), methamphetamine (n=313, 12.0%), speedball (heroin and cocaine; n=70, 2.7%), goofball (heroin and crystal methamphetamine; n=43, 1.6%), and prescription opioids (n=152, 5.8%). There were no participants who reported at least daily injection benzodiazepine use at baseline. At baseline, people who use cannabis daily were more likely to be younger, male, unemployed, homeless, engaged in drug or alcohol treatment (other than OAT), people who use cocaine or crystal methamphetamine daily. At baseline, the distribution of participants from each cohort was significantly different between people who use cannabis daily and those who do not. People who used cannabis were less likely to be engaged in OAT and use crack-cocaine daily.
The bivariable and multivariable GLMM analyses of the frequency of injection drug use are presented in Table 2. The observed frequency of injection drug use in the whole-sample analysis was significantly lower among people who use cannabis daily compared with people who use it less than daily (adjusted odds ratio [AOR]=0.84, 95% confidence interval [CI]: 0.73–0.95; p=0.010). Additional factors retained in the final multivariable model included age (AOR=0.93, 95% CI: 0.92–0.94; p<0.001), non-injection crack-cocaine use (AOR=1.70, 95% CI: 1.50–1.94; p<0.001), time since baseline visit (AOR=1.09, 95% CI: 1.06–1.11; p<0.001), and cohort (ACCESS vs. VIDUS AOR=0.68, 95% CI: 0.55–0.83; p<0.001; ARYS vs. VIDUS AOR=0.60, 95% CI: 0.43–0.84; p<0.001). The interaction between cannabis use and time since baseline was not statistically significant (p=0.099). The analyses of factors associated with injection frequency stratified by study cohort are included in Supplementary Tables S3–S5. Factors associated with injection frequency among exclusive opioid and stimulant users are shown in Supplementary Tables S6 and S7.
Table 2.
Bivariable and Multivariable Generalized Linear Mixed-Effects Analysis of Factors Associated with Injection Drug Use Frequency Among 2,619 Participants
| Characteristic | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Daily cannabis use | ||||
| Yes vs. no | 0.89 (0.78–1.02) | 0.098 | 0.84 (0.73–0.95) | 0.010 |
| Age | ||||
| OR per year older | 0.96 (0.95–0.96) | <0.001 | 0.93 (0.92–0.94) | <0.001 |
| Gender | ||||
| Male vs. female | 0.59 (0.49–0.71) | <0.001 | ||
| Caucasian ethnicity | ||||
| Yes vs. no | 0.88 (0.74–1.05) | 0.168 | ||
| Stable relationshipa | ||||
| Yes vs. no | 0.96 (0.87–1.07) | 0.511 | ||
| Incarcerationa | ||||
| Yes vs. no | 1.94 (1.69–2.22) | <0.001 | ||
| Employmenta | ||||
| Yes vs. no | 0.73 (0.65–0.81) | <0.001 | ||
| Opioid agonist therapya | ||||
| Yes vs. no | 0.46 (0.42–0.51) | <0.001 | ||
| Participation in alcohol or drug treatmenta | ||||
| Yes vs. no | 0.94 (0.81–1.10) | 0.456 | ||
| Homelessnessa | ||||
| Yes vs. no | 1.94 (1.76–2.15) | <0.001 | ||
| Binge drug usea | ||||
| Yes vs. no | 1.32 (1.21–1.43) | <0.001 | ||
| Non-injection heroin usea | ||||
| ≥Daily vs. <daily | 2.19 (1.65–2.91) | <0.001 | ||
| Non-injection cocaine usea | ||||
| ≥Daily vs. <daily | 2.28 (1.36–3.84) | 0.002 | ||
| Non-injection crack cocaine usea | ||||
| ≥Daily vs. <daily | 1.59 (1.42–1.77) | <0.001 | 1.70 (1.50–1.94) | <0.001 |
| Non-injection methamphetamine usea | ||||
| Yes vs. no | 2.17 (1.75–2.68) | <0.001 | ||
| Participation in selling illicit drugsa | ||||
| Yes vs. no | 2.52 (2.28–2.79) | <0.001 | ||
| Time since baseline visit | ||||
| OR per year increase | 0.98 (0.97–1.00) | 0.016 | 1.09 (1.06–1.11) | <0.001 |
| Previous injection drug use | ||||
| ≥Daily vs. <daily | 3.80 (3.45–4.19) | <0.001 | ||
| Cohort | ||||
| ACCESS vs. VIDUS | 0.50 (0.41–0.61) | <0.001 | 0.68 (0.55–0.83) | <0.001 |
| ARYS vs. VIDUS | 1.45 (1.16–1.82) | 0.001 | 0.60 (0.43–0.84) | <0.001 |
Refers to activities in the 6 months before the follow-up interview; cannabis use was lagged by one study visit in the analysis; bold text refers to p-values <0.05.
CI, confidence interval; OR, odds ratio.
The sub-analyses indicated that the association between daily cannabis use and the frequency of illegal opioid injection was statistically significant (AOR=0.78, 95% CI: 0.68–0.90; p<0.001) (Table 3), but it was not significant for the frequency of illegal stimulant injection (AOR=1.08, 95% CI: 0.93–1.25; p=0.320) (Table 4). We defined loss to follow-up as people who stopped contributing follow-up visits at least 3 years before the end of the study period. Using the last included study visit for each participant, 31.6% of daily cannabis users qualified as lost to follow-up whereas 33.0% of non-daily cannabis users qualified as lost to follow-up. There was no significant difference in the proportion of participants lost to follow-up by cannabis use status (Chi square test p-value=0.520). We observed a low proportion of missing data (<1% of observations) for the predictor and outcome variables, as well as the covariates.
Table 3.
Bivariable and Multivariable Mixed-Effects Model Analysis of Factors Associated with Opioid Injection Frequency Among 2,619 Participants
| Characteristic | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Daily cannabis use | ||||
| Yes vs. no | 0.78 (0.67–0.92) | 0.002 | 0.78 (0.68–0.90) | <0.001 |
| Age | ||||
| OR per year older | 0.95 (0.95–0.96) | <0.001 | 0.94 (0.93–0.96) | <0.001 |
| Gender | ||||
| Male vs. female | 0.52 (0.42–0.66) | <0.001 | 0.87 (0.72–1.04) | 0.122 |
| Caucasian ethnicity | ||||
| Yes vs. no | 1.00 (0.80–1.26) | 0.982 | ||
| Stable relationshipa | ||||
| Yes vs. no | 1.07 (0.95–1.21) | 0.268 | ||
| Incarcerationa | ||||
| Yes vs. no | 1.71 (1.48–1.98) | <0.001 | ||
| Employmenta | ||||
| Yes vs. no | 0.72 (0.64–0.82) | <0.001 | ||
| Opioid agonist therapya | ||||
| Yes vs. no | 0.40 (0.35–0.44) | <0.001 | ||
| Participation in alcohol or drug treatmenta | ||||
| Yes vs. no | 0.80 (0.67–0.94) | 0.008 | ||
| Homelessnessa | ||||
| Yes vs. no | 1.99 (1.78–2.22) | <0.001 | ||
| Binge drug usea | ||||
| Yes vs. no | 1.20 (1.09–1.32) | <0.001 | ||
| Non-injection heroin usea | ||||
| ≥Daily vs. <daily | 2.89 (2.14–3.92) | <0.001 | ||
| Non-injection cocaine usea | ||||
| ≥Daily vs. <daily | 1.12 (0.63–1.99) | 0.707 | ||
| Non-injection crack cocaine usea | ||||
| ≥Daily vs. <daily | 1.84 (1.63–2.08) | <0.001 | ||
| Non-injection methamphetamine usea | ||||
| Yes vs. no | 0.92 (0.71–1.17) | 0.487 | ||
| Participation in selling illicit drugsa | ||||
| Yes vs. no | 2.48 (2.22–2.78) | <0.001 | ||
| Time since baseline visit | ||||
| OR per year increase | 0.97 (0.96–0.98) | <0.001 | 1.04 (1.02–1.06) | <0.001 |
| Previous injection drug use | ||||
| ≥Daily vs. <daily | 3.02 (2.72–3.36) | <0.001 | 4.79 (4.27–5.37) | <0.001 |
| Cohort | ||||
| ACCESS vs. VIDUS | 0.33 (0.26–0.43) | <0.001 | 0.59 (0.49–0.72) | <0.001 |
| ARYS vs. VIDUS | 0.93 (0.70–1.23) | 0.591 | 0.46 (0.33–0.62) | <0.001 |
Refers to activities in the 6 months before the follow-up interview; cannabis use was lagged by one study visit in the analysis; bold text refers to p-values <0.05.
Table 4.
Bivariable and Multivariable Mixed-Effects Model Analysis of Factors Associated with Stimulant Injection Frequency Among 2,619 Participants
| Characteristic | Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Daily cannabis use | ||||
| Yes vs. no | 1.16 (0.99–1.36) | 0.066 | 1.08 (0.93–1.25) | 0.320 |
| Age | ||||
| OR per year older | 0.97 (0.96–0.98) | <0.001 | 0.98 (0.97–0.99) | <0.001 |
| Gender | ||||
| Male vs. female | 0.96 (0.77–1.19) | 0.701 | ||
| Caucasian ethnicity | ||||
| Yes vs. no | 0.82 (0.66–1.01) | 0.063 | ||
| Stable relationshipa | ||||
| Yes vs. no | 0.92 (0.81–1.05) | 0.228 | ||
| Incarcerationa | ||||
| Yes vs. no | 1.82 (1.56–2.12) | <0.001 | ||
| Employmenta | ||||
| Yes vs. no | 0.76 (0.66–0.87) | <0.001 | 0.78 (0.67–0.91) | 0.001 |
| Opioid agonist therapya | ||||
| Yes vs. no | 0.97 (0.85–1.10) | 0.596 | ||
| Participation in alcohol or drug treatmenta | ||||
| Yes vs. no | 1.10 (0.92–1.32) | 0.294 | ||
| Homelessnessa | ||||
| Yes vs. no | 1.64 (1.46–1.85) | <0.001 | 1.45 (1.26–1.66) | <0.001 |
| Binge drug usea | ||||
| Yes vs. no | 1.40 (1.27–1.55) | <0.001 | 1.42 (1.27–1.58) | <0.001 |
| Non-injection heroin usea | ||||
| ≥Daily vs. <daily | 1.86 (1.36–2.52) | <0.001 | ||
| Non-injection cocaine usea | ||||
| ≥Daily vs. <daily | 3.74 (2.19–6.37) | <0.001 | ||
| Non-injection crack cocaine usea | ||||
| ≥Daily vs. <daily | 0.80 (0.70–0.92) | 0.001 | 0.94 (0.80–1.09) | 0.395 |
| Non-injection methamphetamine usea | ||||
| Yes vs. no | 4.01 (3.22–4.99) | <0.001 | 2.75 (2.17–3.49) | <0.001 |
| Participation in selling illicit drugsa | ||||
| Yes vs. no | 1.74 (1.54–1.97) | <0.001 | ||
| Time since baseline visit | ||||
| OR per year increase | 1.03 (1.01–1.05) | <0.001 | 1.05 (1.03–1.08) | <0.001 |
| Previous injection drug use | ||||
| ≥Daily vs. <daily | 3.95 (3.47–4.49) | <0.001 | 3.85 (3.38–4.39) | <0.001 |
| Cohort | ||||
| ACCESS vs. VIDUS | 1.15 (0.92–1.45) | 0.222 | 1.38 (1.14–1.67) | <0.001 |
| ARYS vs. VIDUS | 2.40 (1.86–3.10) | <0.001 | 1.44 (1.06–1.94) | 0.019 |
Refers to activities in the 6 months before the follow-up interview; cannabis use was lagged by one study visit in the analysis; bold text refers to p-values <0.05.
Discussion
In this study, we observed that daily cannabis use was common among study participants and reported by 729 (27.8%) participants at baseline. Over the 12-year study period, the odds of being a daily injector were 17% lower among people who use cannabis daily compared with people who use it less than daily. The sub-analyses demonstrated that cannabis use was associated with a decreased frequency of illegal opioid injection; however, it was not significantly associated with the frequency of illegal stimulant injection. The impact of cannabis use on injection frequency did not vary significantly over time, and increased time since baseline was associated with increased odds of daily injection drug use. To our knowledge, this is the first study to analyze the association between cannabis use and the frequency of injection drug use. Previous studies have focused on specific forms of substance use influenced by cannabis use (e.g., crack-cocaine), combined injection and non-injection drug use as an outcome, or analyzed injection drug use patterns such as initiation and cessation.15,16,42,43 These findings contribute to preliminary preclinical, clinical, and ecological evidence describing beneficial associations with cannabis use for people at risk of drug-related harms, including PWID.44–46
Preliminary observational studies have found beneficial associations between cannabis and patterns of other injection and non-injection opioid use. Although a 2001 study found that cannabis use was associated with an increased risk of injection drug use initiation, more recent evidence, including a 2018 study, found that frequent cannabis use was associated with lower rates of initiation of injection drug use among people who use illegal drugs.16,32,34,39 Similarly, a recent urine toxicology analysis reported a negative association between recent exposure to fentanyl and recent exposure to Δ-9-THC, the primary psychoactive constituent of cannabis.15,17 Analysis of population-level data from the United States has also found that medical and recreational cannabis laws are associated with state-level decreases in opioid-related mortality, although a recent study has challenged these findings.47–49 Nevertheless, the association between medical cannabis laws and decreased opioid-related mortality appears to be stronger in settings with more liberal allowances for cannabis dispensaries.50 Also, U.S. states with medical cannabis laws experienced a significant decrease in Medicare prescriptions for medications to treat pain, depression, and anxiety, all highly prevalent among PWID.51,52 Patients living with chronic pain have reported comparable pain relief, reduced medication side effects, improved quality of life, and reduced opiate use associated with medical cannabis use.53,54 Given that up to two thirds of PWID report being denied prescription analgesia and 30–40% of these patients resort to illegal drugs such as heroin to treat pain, increased availability and use of cannabis may provide an alternative to using illegal opiates that are commonly injected.55,56 This is supported by a recent study showing that cannabis use was associated with decreased illegal opioid use among people who use drugs with chronic pain.18
The preliminary observational studies describing beneficial associations between cannabis and opioids have raised the possibility that cannabis might be a useful intervention in the ongoing crisis arising from opioid overdose deaths in many settings in the United States and Canada.57,58 In British Columbia, the number of overdose deaths has reached unprecedented levels in each of the past 4 years, with ∼1,000 deaths per year.59 In the United States, deaths involving synthetic opioids doubled from 2015 to 2016.60 These increases in illegal drug overdose deaths appear to be driven primarily by fentanyl in some jurisdictions, and nearly 80% of drugs analyzed in an illegal drug checking program tested positive for fentanyl.59,61 In our setting, belief in the positive potential of cannabis to address the overdose crisis has led to the establishment of two community-led interventions to distribute no-cost cannabis to people at risk of opioid overdose.62 There is a need to evaluate these harm reduction-based interventions and to conduct clinical trials to identify causal relationships, if any, between controlled administration of cannabinoids and overdose risk.
Evidence is also urgently needed to document the possible risks of this approach, including development of cannabis use disorder or exacerbation of other mental health co-morbidities. Identifying the specific cannabinoids, routes of administration and treatment regimens that are best suited to decrease overdose risk are also important questions to be addressed through these clinical trials. In our study setting (the western Canadian province of British Columbia), the total illegal drug toxicity deaths in May 2020 (n=119) represent the highest ever recorded monthly rate in the province.63 The high prevalence of cannabis use, particularly therapeutic use among PWID and are at risk of drug-related harm,18 supports further evaluation of cannabis harm-reduction services to address high-risk drug use behaviors and overdose risk. Our findings that cannabis use was associated with a decreased frequency of injection opioid use provide evidence to inform policy surrounding the harm reduction application of cannabis for PWID.
Although previous studies have identified an association between cannabis use and decreased use of stimulants, we did not observe a significant association between cannabis use and injection stimulant use.15,16 Given that stimulants such as cocaine are often injected up to 30 times per day and injection cocaine use is an independent predictor of HIV acquisition, further research is needed to evaluate illegal stimulant use among PWID.28 The interaction between cannabis use and time was also not statistically significant, indicating that the effect of cannabis use on injection frequency did not vary significantly during the study period. This indicates that the decreased odds of daily injection drug use associated with cannabis use were consistent regardless of how long participants had been enrolled in the study cohorts. As a result, the potential for cannabis use to decrease injection frequency may apply to study participants at various stages of their substance use history who are at risk of drug-related harm associated with injection drug use. It is also worth noting that the odds of daily injection drug use increased over time. This increase in risk highlights the need for continued and expanded access to substance use treatment interventions and harm-reduction services throughout the substance use history of PWID to decrease the intensity of substance use and associated harm, particularly during the opioid overdose crisis.
This study has limitations. As the study participants were not randomly recruited, our findings may not be generalizable to other populations of PWID. We also recognize that the self-reported drug use behaviors may have been influenced by socially desirable reporting and recall bias, although the reliability and validity of self-report methods among PWID has been previously demonstrated.64 This study did not quantify the THC and CBD of the cannabis consumed and this may have influenced the findings we observed. Since we measured cannabis use frequency and not amount (i.e., number of grams consumed), we were not able to evaluate potential dose-response effects of cannabis on injection drug use frequency. We did not measure exposure to cannabis before study enrolment and during missed follow-up visits, and it is possible that the length of exposure to cannabis use may have influenced our results. Although this sample included a high prevalence of daily cannabis use, this frequency of use may be less common in other settings and the effect of cannabis use on injection drug use frequency may be weaker among people who use cannabis less frequently. People who use cannabis daily are also more likely to report therapeutic uses of cannabis to address pain, nausea, mental health, and HIV, which may be related to injection drug use behaviors.18 As previously mentioned, patients living with HIV often use cannabis to address HIV-associated comorbidities and common side-effects of ART.65–68 Thus, the nature of the relationship between cannabis use and injection drug use may be moderated by engagement with ART and therapeutic cannabis use among people living with HIV. In addition, future research would benefit from examining subgroup effects by sex, gender, and ethnicity and implementing validated measures of cannabis-related harms, including cannabis use disorder, among PWID using cannabis. Lastly, it is possible that residual confounding influenced the association between cannabis use and injection drug use frequency since this was an observational study design.
Conclusions
In conclusion, we observed that the odds of daily injection drug use were significantly lower among participants who used cannabis daily among three prospective cohorts of PWID over a 12-year follow-up period. This association was restricted to injection opioid use, and the association between cannabis use and injection stimulant use was not statistically significant. These findings contribute data to the preliminary preclinical evidence, indicating that cannabis use may have the potential to reduce some drug-related harm among people living with substance use disorders such as opioid use disorder, especially during the ongoing overdose crisis in Canada and the United States.
Supplementary Material
Acknowledgments
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff. They specifically like to thank Carly Hoy, Jennifer Matthews, Peter Vann, Steve Kain, Dr. Lorena Mota, and Ana Prado for their research and administrative support.
Abbreviations Used
- ACCESS
AIDS Care Cohort to Evaluate exposure to Survival Services
- AOR
adjusted odds ratio
- ART
antiretroviral therapy
- ARYS
At-Risk Youth Study
- CBD
cannabidiol
- CI
confidence interval
- CIHR
Canadian Institutes of Health Research
- CTN
Canadian HIV Trials Network
- GLMM
generalized linear mixed-effects models
- HCV
hepatitis C virus
- IQR
interquartile range
- MSFHR
Michael Smith Foundation of Health Research
- OAT
opioid agonist therapy
- OR
adjusted odds ratio
- PWID
people who inject drugs
- THC
tetrahydrocannabinol
- VIDUS
Vancouver Injection Drug Users Study
Author Disclosure Statement
No competing interests to declare.
Funding Information
This study was supported by the U.S. National Institutes of Health (U01-DA038886, U01-DA0251525) and the Canadian Institutes of Health Research (CIHR; MOP–286532). This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine that supports Dr. Evan Wood. This study was supported by the CIHR Canadian HIV Trials Network (CTN 222). H.R. is supported by a Sponsor/CTN Postdoctoral Fellowship Award. K.D.B. is supported by a Michael Smith Foundation of Health Research (MSFHR)/St. Paul's Hospital Foundation–Providence Health Care Career Scholar Award and a CIHR New Investigator Award. M.-J.M. is supported in part by the United States National Institutes of Health (U01-DA021525), a New Investigator Award from CIHR, and a Scholar Award from MSFHR. His institution has received an unstructured gift to support him from NG Biomed, Ltd., a private firm applying for a government license to produce cannabis. He is the Canopy Growth professor of cannabis science, a position established through unstructured gifts to the University of British Columbia from Canopy Growth, a licensed producer of cannabis, and the Ministry of Mental Health and Addictions of the Government of British Columbia. K.H. is supported by a CIHR New Investigator Award (MSH-141971), an MSFHR Scholar Award, and the St. Paul's Foundation. M.-E.S. is supported by an MSFHR/St. Paul's Foundation Scholar Award.
Supplementary Material
Cite this article as: Reddon H, DeBeck K, Socias M-E, Lake S, Dong H, Hayashi K, Milloy M-J (2020) Frequent cannabis use is negatively associated with frequency of injection drug use among people who inject drugs in a Canadian setting, Cannabis and Cannabinoid Research 6:5, 435–445, DOI: 10.1089/can.2019.0104.
References
- 1. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeBeck K, Shannon K, Wood E, et al. Income generating activities of people who inject drugs. Drug Alcohol Depend. 2007;91:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshall BD, Kerr T, Shoveller JA, et al. Homelessness and unstable housing associated with an increased risk of HIV and STI transmission among street-involved youth. Health Place. 2009;15:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller CL, Kerr T, Fischer B, et al. Methamphetamine injection independently predicts hepatitis C infection among street-involved youth in a Canadian setting. J Adolesc Health. 2009;44:302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azar P, Wood E, Nguyen P, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infect Dis. 2015;15:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladak F, Socias E, Nolan S, et al. Substance use patterns and HIV-1 RNA viral load rebound among HIV-positive illicit drug users in a Canadian setting. Antivir Ther. 2019;24:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altice FL, Kamarulzaman A, Soriano VV, et al. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Windle SB, Wade K, Filion KB, et al. Potential harms from legalization of recreational cannabis use in Canada. Can J Public Health. 2019;110:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayet A, Legleye S, Beck F, et al. The gateway hypothesis, common liability to addictions or the route of administration model A modelling process linking the three theories. Eur Addict Res. 2016;22:107–117. [DOI] [PubMed] [Google Scholar]
- 10. Kandel DB, Yamaguchi K, Klein LC. Testing the gateway hypothesis. Addiction. 2006;101:470–472; discussion 4–6. [DOI] [PubMed] [Google Scholar]
- 11. Kleinig J. Ready for retirement: the gateway drug hypothesis. Subst Use Misuse. 2015;50:971–975. [DOI] [PubMed] [Google Scholar]
- 12. Labigalini E Jr., Rodrigues LR, Da Silveira DX.. Therapeutic use of cannabis by crack addicts in Brazil. J Psychoactive Drugs. 1999;31:451–455. [DOI] [PubMed] [Google Scholar]
- 13. Dreher M. Crack heads and roots daughters: the therapeutic use of cannabis in Jamaica. J Cannabis Ther. 2002;2:121–133. [Google Scholar]
- 14. Goncalves JR, Nappo SA. Factors that lead to the use of crack cocaine in combination with marijuana in Brazil: a qualitative study. BMC Public Health. 2015;15:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Socias ME, Kerr T, Wood E, et al. Intentional cannabis use to reduce crack cocaine use in a Canadian setting: a longitudinal analysis. Addict Behav. 2017;72:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reddon H, DeBeck K, Socias ME, et al. Cannabis use is associated with lower rates of initiation of injection drug use among street-involved youth: a longitudinal analysis. Drug Alcohol Rev. 2018;37:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi K, Milloy MJ, Lysyshyn M, et al. Substance use patterns associated with recent exposure to fentanyl among people who inject drugs in Vancouver, Canada: a cross-sectional urine toxicology screening study. Drug Alcohol Depend. 2018;183:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lake S, Walsh Z, Kerr T, et al. Frequency of cannabis and illicit opioid use among people who use drugs and report chronic pain: a longitudinal analysis. PLoS Med. 2019;16:e1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Socias ME, Wood E, Lake S, et al. High-intensity cannabis use is associated with retention in opioid agonist treatment: a longitudinal analysis. Addiction. 2018;113:2250–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cannabis Legalization and Regulation. Department of Justice, Government of Canada, 2018. [Google Scholar]
- 21. Canadian Tobacco, Alcohol and Drugs (CTADS) Survey: detailed tables. Health Canada, 2017. [Google Scholar]
- 22. Rotermann M. Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Health Rep. 2019;30:3–13. [DOI] [PubMed] [Google Scholar]
- 23. Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11:F59–F65. [DOI] [PubMed] [Google Scholar]
- 24. Wood E, Kerr T. What do you do when you hit rock bottom? Responding to drugs in the City of Vancouver. Int J Drug Policy. 2006;17:55–60. [Google Scholar]
- 25. Reddon H, Fast D, DeBeck K, et al. Prevalence and correlates of selling illicit cannabis among people who use drugs in Vancouver, Canada: a ten-year prospective cohort study. Int J Drug Policy. 2019;69:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reed C, Bliss C, Stuver SO, et al. Predictors of active injection drug use in a cohort of patients infected with hepatitis C virus. Am J Public Health. 2013;103:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trenz RC, Scherer M, Harrell P, et al. Early onset of drug and polysubstance use as predictors of injection drug use among adult drug users. Addict Behav. 2012;37:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–893. [DOI] [PubMed] [Google Scholar]
- 29. McCoy CB, Lai S, Metsch LR, et al. Injection drug use and crack cocaine smoking: independent and dual risk behaviors for HIV infection. Ann Epidemiol. 2004;14:535–542. [DOI] [PubMed] [Google Scholar]
- 30. Hadland S, Wood E, Nosova E, et al. Cessation of injecting and preceding drug use patterns among a prospective cohort of street-involved youth. J Adolesc Health. 2017;61:612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evans J, Hahn J, Lum P, et al. Predictors of injection drug use cessation and relapse in a prospective cohort of young injection drug users in San Francisco, CA (UFO Study). Drug Alcohol Depend. 2009;101:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fuller CM, Vlahov D, Arria AM, et al. Factors associated with adolescent initiation of injection drug use. Public Health Rep. 2001;116(Suppl 1):136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Werb D, Kerr T, Buxton J, et al. Crystal methamphetamine and initiation of injection drug use among street-involved youth in a Canadian setting. CMAJ. 2013;185:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hadland SE, Kerr T, Marshall BD, et al. Non-injection drug use patterns and history of injection among street youth. Eur Addict Res. 2010;16:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurd YL. Cannabidiol: swinging the marijuana pendulum from ‘Weed’ to medication to treat the opioid epidemic. Trends Neurosci. 2017;40:124–127. [DOI] [PubMed] [Google Scholar]
- 36. Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. [DOI] [PubMed] [Google Scholar]
- 37. Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. [DOI] [PubMed] [Google Scholar]
- 38. Wood E, Stoltz JA, Montaner JS, et al. Evaluating methamphetamine use and risks of injection initiation among street youth: the ARYS study. Harm Reduct J. 2006;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valdez A, Cepeda A, Neaigus A, et al. Heroin transition risk among daily and non-daily cannabis users who are non-injectors of heroin. Int J Drug Policy. 2008;19:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wood E, Tyndall MW, Spittal PM, et al. Unsafe injection practices in a cohort of injection drug users in Vancouver: could safer injecting rooms help? CMAJ. 2001;165:405–410. [PMC free article] [PubMed] [Google Scholar]
- 41. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. [DOI] [PubMed] [Google Scholar]
- 42. Kral AH, Wenger L, Novak SP, et al. Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend. 2015;153:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyd J, Fast D, Hobbins M, et al. Social-structural factors influencing periods of injection cessation among marginalized youth who inject drugs in Vancouver, Canada: an ethno-epidemiological study. Harm Reduct J. 2017;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lofwall MR, Babalonis S, Nuzzo PA, et al. Opioid withdrawal suppression efficacy of oral dronabinol in opioid dependent humans. Drug Alcohol Depend. 2016;164:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hurd YL, Yoon M, Manini AF, et al. Early phase in the development of cannabidiol as a treatment for addiction: opioid relapse takes initial center stage. Neurotherapeutics. 2015;12:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bachhuber MA, Saloner B, Cunningham CO, et al. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174:1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Livingston MD, Barnett TE, Delcher C, et al. Recreational cannabis legalization and opioid-related deaths in Colorado, 2000–2015. Am J Public Health. 2017;107:1827–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shover CL, Davis CS, Gordon SC, et al. Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proc Natl Acad Sci U S A. 2019;116:12624–12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Powell D, Pacula RL, Jacobson M. Do medical marijuana laws reduce addiction and deaths related to pain killers?. J Health Econ. 2018;58:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bradford AC, Bradford WD. Medical marijuana laws reduce prescription medication use in Medicare part D. Health Aff (Millwood). 2016;35:1230–1236. [DOI] [PubMed] [Google Scholar]
- 52. Reddon H, Pettes T, Wood E, et al. Incidence and predictors of mental health disorder diagnoses among people who inject drugs in a Canadian setting. Drug Alcohol Rev. 2018;37(Suppl 1):S285–S293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17:739–744. [DOI] [PubMed] [Google Scholar]
- 54. Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Voon P, Callon C, Nguyen P, et al. Denial of prescription analgesia among people who inject drugs in a Canadian setting. Drug Alcohol Rev. 2015;34:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lake S, Milloy MJ, Dong H, et al. Initiation into prescription opioid injection and associated trends in heroin use among people who use illicit drugs. Drug Alcohol Depend. 2016;169:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lake S, Milloy MJ. Access to medical cannabis is expanding across North America regardless of the opioid crisis-why not study if it could help? Addiction. 2018;113:1550–1551. [DOI] [PubMed] [Google Scholar]
- 58. Lucas P. Rationale for cannabis-based interventions in the opioid overdose crisis. Harm Reduct J. 2017;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Illicit Drug Overdose Deaths in BC January 1, 2009–March 31, 2019. British Columbia Coroners Service, Ministry of Public Safety & Solicitor General, 2019. [Google Scholar]
- 60. Drug Overdose Deaths in the United States, 1999–2017. National Centre for Health Statistics, Centre for Disease Control and Prevention, 2018. [Google Scholar]
- 61. Karamouzian M, Dohoo C, Forsting S, et al. Evaluation of a fentanyl drug checking service for clients of a supervised injection facility, Vancouver, Canada. Harm Reduct J. 2018;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. High Hopes Foundation hopes to get Vancouverites off opiates—and on cannabis instead. CBC News, 2017. [Google Scholar]
- 63. Illicit Drug Toxicity Deaths in BC January 1, 2010–May 31, 2020. British Columbia Service, Ministry of Public Safety & Solicitor General, 2020. [Google Scholar]
- 64. Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51:253–263; discussion 67–68. [DOI] [PubMed] [Google Scholar]
- 65. Harris GE, Dupuis L, Mugford GJ, et al. Patterns and correlates of cannabis use among individuals with HIV/AIDS in Maritime Canada. Can J Infect Dis Med Microbiol. 2014;25:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mimiaga MJ, Reisner SL, Grasso C, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the centers for AIDS research network of integrated clinical systems cohort. Am J Public Health. 2013;103:1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Okafor CN, Zhou Z, Burrell LE, et al. Marijuana use and viral suppression in persons receiving medical care for HIV-infection. Am J Drug Alcohol Abuse. 2017;43:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Costiniuk CT, Jenabian MA. Cannabinoids and inflammation: implications for people living with HIV. AIDS. 2019;33:2273–2288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.