Abstract
Pathologic angiogenesis causes blindness in many eye diseases. Crespo-Garcia, Tsuruda, and Dejda et al. employed bioinformatics to characterize cell senescence as a primary factor in the common pathogenesis of retinopathies. They validated their findings using human and mouse retina with proliferative retinopathy. Clearance of senescent cells suppressed neovessel growth.
Pathological angiogenesis is a primary cause of vision loss in proliferative retinopathies. Uncontrolled retinal vessel growth is driven by tissue hypoxia and nutrient deprivation, as a compensatory tissue response to restore oxygen and energy balance. However, these neovessels are fragile and leaky and may cause retinal detachment. Current anti-vascular endothelial growth factor (VEGF; key for vascular and neuronal development) therapy is not always effective in patients and has raised safety concerns as it also inhibits normal vascularization and neuron survival. Therefore, the need to develop selective therapeutic targets to suppress pathologic vessels while promoting normal vessel growth is urgent. Retinal neurons and blood vessels undergo cell senescence induced by stress/damage and aging [1]. However, the impact of cellular senescence on retinopathies is understudied. A recent report by Crespo-Garcia, Tsuruda, and Dejda et al illustrated the importance of the accumulation of senescent cells in the neovascular area in proliferative retinopathy (Figure 1) [2]. Genetic or pharmacologic inhibition of senescent cells promoted the regression of neovessels and regrowth of normal vessels (Figure 1) [2]. B cell lymphoma-extra large (BCL-xL) inhibition with UBX1967, with elimination of senescent vascular cells, could be a potential therapeutic target in treating proliferative retinopathy.
Figure 1. Inhibition of cellular senescence in neovascular tufts suppresses proliferative retinopathy.
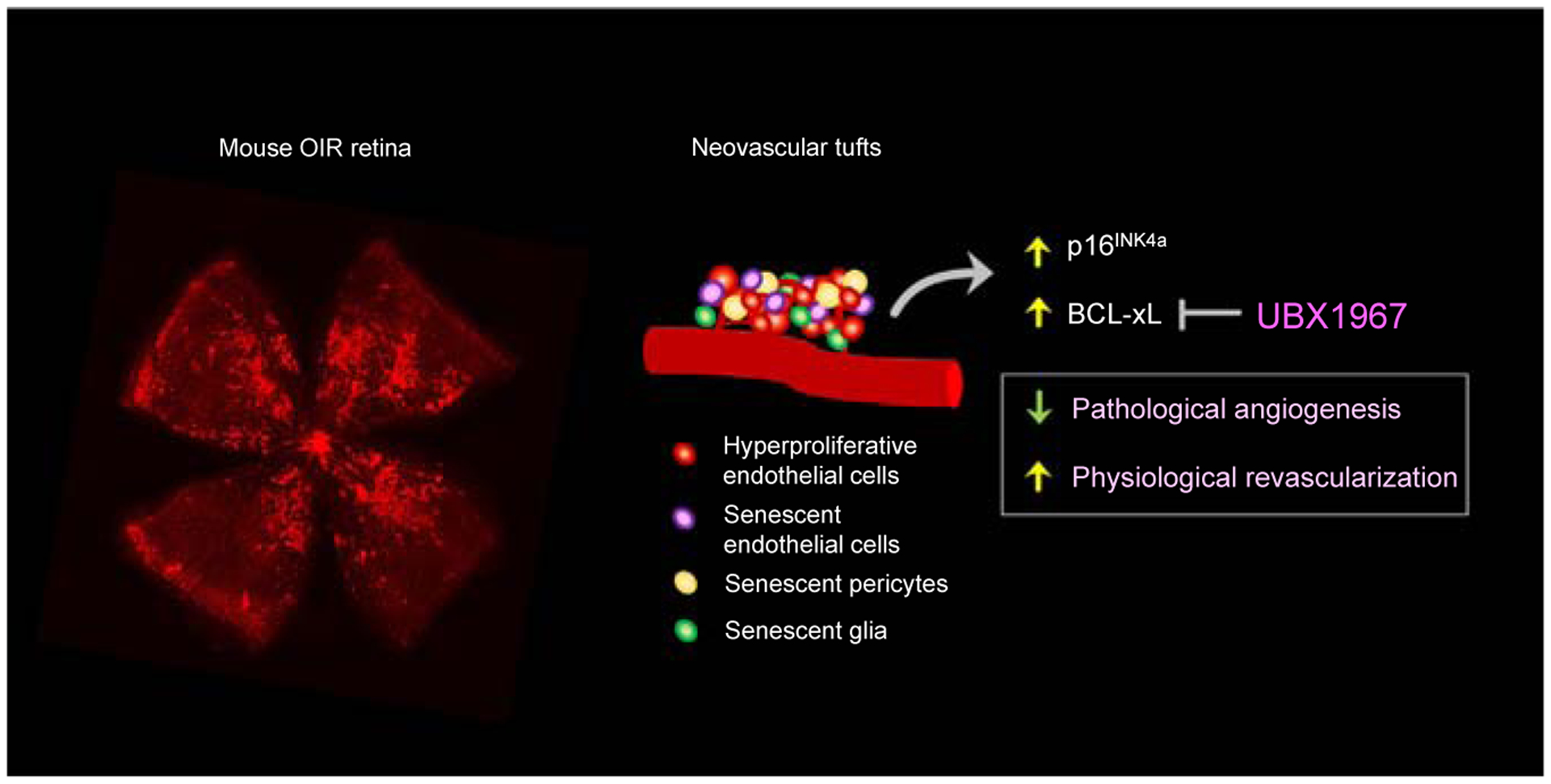
Single-cell RNA sequencing revealed that in mouse oxygen-induced retinopathy (OIR) retinas with pathological angiogenesis, senescence was observed in cells associated with the vascular unit (endothelial cells, pericytes and retinal glia (Müller glia and astrocytes). Increased senescent marker p16INK4a in human retina with proliferative diabetic retinopathy and BCL-xL mouse OIR retina were observed. Inhibition of BCL-xL with UBX1967 to suppress senescence reduced pathological angiogenesis and promoted normal retinal revascularization in mouse OIR retinas.
The authors showed that there was a peak cellular senescence burden at the peak of retinal neovascularization in a mouse model of oxygen-induced retinopathy (OIR), mimicking human proliferative retinopathies. With the use of bulk RNA-seq of retinas at three distinct time points when neovessel formation begins, when it peaks, and when normal vessels regenerate, the greatest gene variation falls when peak neovascularization is seen [2]. Cellular senescence-associated genes were identified and confirmed with traditional senescence markers. The same group has previously reported that retinal ischemia triggers cellular senescence [3] and senescent vascular endothelial cells in OIR attract neutrophils and triggers the production of neutrophil extracellular traps (NETs) to eliminate senescent vasculature, pre-paring the ischemic retina for reparative vascular regeneration [4]. Interruption of neutrophil associated clearance of senescent cells leads to compromised regression of pathologic retinal neovessels [4]. In this study, the authors further characterized the process, showing that the vascular unit (astrocytes, pericytes, endothelial cells, and Müller glia) versus other retinal cells was enriched in cell senescence markers with single-cell RNA sequencing (Drop-seq) [2], allowing selective targeting of the vascular unit.
The authors evaluated the retinal cell senescence status in human proliferative diabetic retinopathy to extend these findings to potential clinical application. They identified induction of the antiproliferative molecule p16TNK4a (a marker of cell senescence) in the ganglion cell layer, in the superficial retinal vascular plexus from which proliferative neovessels arise [2], suggesting the translational value of targeting vascular senescence. The authors found that induction of intraocular apoptosis in p16TNK4a-expressing cells ablated retinal senescent cells, decreased retinal neovessel formation, and promoted normal retinal vessel regrowth in OIR mice [2], providing a rationale for therapeutic targeting of the senescent vascular unit in retinopathies.
As senescence provides both beneficial and detrimental effects [1], the authors tested if local delivery of a senolytic drug, which selectively targets senescent cells, prevented pathological retinal angiogenesis. The authors found that UBX1967, an inhibitor of BCL-xL, initiated retinal apoptosis in senescent cells but not in normal retinas [2]. More intriguingly, UBX1967 promoted normal retinal vessel regrowth and inhibited retinal neovessel formation by selectively eliminating a subpopulation of endothelial cells that expressed type I collagen (Col1a1) [2], further confirming the feasibility of treatment with UBX1967 to selectively target senescent vascular cells to inhibit proliferative retinopathy.
In summary, the study provides a novel approach to treat proliferative retinopathy with the advantages of targeting the proliferative retinal vascular unit selectively, while also promoting normal retinal revascularization. Retinal neuronal function and morphology assessment would provide additional evidence that inhibition of cellular senescence does not affect retinal neurons in proliferative retinopathy. Further studies are needed to understand the initiation of cellular senescence, the factors involved in the balance between beneficial and harmful senescence, as well as the interaction between retinal cellular senescence and metabolic homeostasis. In addition to the lack of oxygen (hypoxia), a well-recognized factor driving neovessel and normal vessel growth is photoreceptor metabolic need [5,6]. The authors also showed retinal pigment epithelium (RPE; photoreceptors support cells) senescence in human proliferative diabetic retinopathy [2]. Senescent cells are highly metabolically active and cellular senescence is accompanied by increased mitochondrial biogenesis and mitochondrial oxidative metabolism [1]. RPE preferably transports glucose to photoreceptors [7], and increasing glucose use in RPE causes photoreceptor glucose shortage and cell death [8]. Therefore, senescent RPE with disturbed metabolism might cause unmet metabolic needs in photoreceptors and induce neovessel growth in proliferative retinopathies. This process might also be implicated in aging retinas. In age-related macular degeneration, a leading cause of blindness in older adults, RPE senescence is a key contributor to disease progression [1]. There is a significant decrease in number and area of mitochondria in human RPE at early stages of the disease [9]. It would be of great interest to further explore the relationship between metabolic imbalance and cellular senescence in RPE and the impact on pathological retinal angiogenesis. Furthermore, endothelial cell metabolism also controls physiological and pathological retinal angiogenesis [10]. It is important to investigate if restoration of endothelial cell metabolic homeostasis at an early stage might prevent cellular senescence and pathologic vessel growth.
Footnotes
Declaration of interests
The authors have no interests to declare.
References
- 1.Sreekumar PG et al. (2020) The emerging role of senescence in ocular disease. Oxidative Med. Cell. Longev 2020, 2583601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crespo-Garcia S et al. (2021) Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 33, 818–832.e7 [DOI] [PubMed] [Google Scholar]
- 3.Oubaha M et al. (2016) Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci. Transl. Med 8, 362ra144. [DOI] [PubMed] [Google Scholar]
- 4.Binet F et al. (2020) Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 369, eaay5356. [DOI] [PubMed] [Google Scholar]
- 5.Fu Z et al. (2017) Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO Mol. Med 10, 76–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyal JS et al. (2016) Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med 22, 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanow MA et al. (2017) Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. eLife 6, 28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao C et al. (2011) mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J. Clin. Invest 121, 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feher J et al. (2006) Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol. Aging 27, 983–993 [DOI] [PubMed] [Google Scholar]
- 10.Eelen G et al. (2018) Endothelial cell metabolism. Physiol. Rev 98, 3–58 [DOI] [PMC free article] [PubMed] [Google Scholar]


