Abstract
Understanding how neurons of the striatum are formed and integrate into complex synaptic circuits is essential to provide insight into striatal function in health and disease. In this review, we summarize our current understanding of the development of striatal neurons and associated circuits with a focus on their embryonic origin. Specifically, we address the role of distinct types of embryonic progenitors, found in the proliferative zones of the ganglionic eminences in the ventral telencephalon, in the generation of diverse striatal interneurons and projection neurons. Indeed, recent evidence would suggest that embryonic progenitor origin dictates key characteristics of postnatal cells, including their neurochemical content, their location within striatum, and their long-range synaptic inputs. We also integrate recent observations regarding embryonic progenitors in cortical and other regions and discuss how this might inform future research on the ganglionic eminences. Last, we examine how embryonic progenitor dysfunction can alter striatal formation, as exemplified in Huntington's disease and autism spectrum disorder, and how increased understanding of embryonic progenitors can have significant implications for future research directions and the development of improved therapeutic options.
SIGNIFICANCE STATEMENT This review highlights recently defined novel roles for embryonic progenitor cells in shaping the functional properties of both projection neurons and interneurons of the striatum. It outlines the developmental mechanisms that guide neuronal development from progenitors in the embryonic ganglionic eminences to progeny in the striatum. Where questions remain open, we integrate observations from cortex and other regions to present possible avenues for future research. Last, we provide a progenitor-centric perspective onto both Huntington's disease and autism spectrum disorder. We suggest that future investigations and manipulations of embryonic progenitor cells in both research and clinical settings will likely require careful consideration of their great intrinsic diversity and neurogenic potential.
Keywords: embryonic progenitors, lateral ganglionic eminence, medial ganglionic eminence, neuronal diversity, striatum, basal ganglia, spiny projection neurons, interneurons
Introduction
Understanding how neuronal cell identity and precise synaptic circuits in the brain emerge during development remains a fundamental goal in neuroscience. The discovery of radial glial cells (RGCs) as the main progenitor cell in the developing nervous system, and observations that RGCs can give rise not only to neurons, but also to a diverse population of additional progenitors, have made understanding the functional roles and contributions of progenitors to brain development a key focus for neuroscientists. Indeed, it is becoming increasingly clear that the remarkable diversity of embryonic progenitors is directly linked to the neuronal diversity, synaptic connectivity, and circuit function in a variety of regions in the adult brain (Yu et al., 2009; 2012; Tyler et al., 2015; Kelly et al., 2018; Ellender et al., 2019; Guillamon-Vivancos et al., 2019; Matsushima and Graybiel, 2020; van Heusden et al., 2021). This review will focus on the roles for diverse embryonic progenitors in shaping the development and properties of one brain region, the striatum.
The striatum is the main input nucleus of the basal ganglia, a group of interconnected subcortical nuclei that have critical functional roles in motor behavior, learning, and cognition (Graybiel et al., 1994; Grillner et al., 2005; Yin and Knowlton, 2006; Kravitz et al., 2010; Cui et al., 2013; Tecuapetla et al., 2016), and it has been ascribed key computational roles in action selection, decision-making, and reinforcement learning (Redgrave et al., 1999; Reynolds et al., 2001; Samejima et al., 2005; Bogacz and Gurney, 2007; Yartsev et al., 2018). The striatum is a relatively large brain nucleus, consisting of over a million neurons in the mouse. The neurons can be divided into the GABAergic spiny projection neurons (SPNs), which make up ∼95% of all striatal neurons, and a diverse population of interneurons, which make up the remaining ∼5% (Kreitzer and Malenka, 2008; Tepper et al., 2010, 2018). The SPNs are classically divided into the direct pathway dopamine D1 receptor-expressing SPN (dSPN) and the indirect pathway dopamine D2 receptor-expressing SPN (iSPN) types, respectively forming the striatonigral and striatopallidal pathways and sending major projections to the substantia nigra pars reticulata (SNr)/internal globus pallidus (GPi) or the external GP (GPe; Gerfen et al., 1990; Day et al., 2008; Gertler et al., 2008). The resident striatal interneuron population can be subdivided into cholinergic interneurons (CINs) and a diverse group of GABAergic interneurons.
At first glance, the striatum has a less obvious structure than other brain regions. For example, the cortex exhibits a distinct laminar organization with various layers forming sequentially during progressive embryonic periods, and each layer consisting of distinct cell types (Douglas and Martin, 2004). In contrast, the striatum seems to consist of vast numbers of intermingled dSPNs, iSPNs, and interneurons. However, several organizing principles of the striatum exist and are applicable to large populations of diverse striatal cells. These include distinct functional domains related to specific anatomic subregions of striatum (Graybiel and Ragsdale, 1978; Alexander et al., 1986; Graybiel, 1990; Haber, 2008; Pan et al., 2010; Oh et al., 2014; Hintiryan et al., 2016; Hunnicutt et al., 2016; McGregor et al., 2019; Lee et al., 2020). Indeed, one classical distinction divides the striatum into the dorsolateral striatum (DLS) and dorsomedial striatum (DMS), with each anatomic subregion receiving innervation from different cortical and thalamic areas (McGeorge and Faull, 1989; Voorn et al., 2004; Smith et al., 2014). Other distinctions are based on differential expression of a set of neurochemical markers, for example, the μ-opioid receptor, which segregates large populations of dSPNs, iSPNs, and associated interneurons into μ-opioid-rich striosome/patch compartments and μ-opioid-poor matrix compartments (Pert et al., 1976; Graybiel and Ragsdale, 1978; Herkenham and Pert, 1981; Graybiel, 1990; Crittenden and Graybiel, 2011), which are thought to differentially control reward-guided behavior (Gerfen, 1984, 1989; Fujiyama et al., 2011). This review will discuss the role of diverse embryonic progenitors in shaping these and other striatal subregions.
Aberrant development and integration of diverse striatal neurons into circuits can lead to a wide range of disorders with motor and cognitive symptoms (Arber et al., 2015; Peixoto et al., 2019). We further this discussion by addressing how embryonic progenitors generate diverse populations of striatal neurons as well as exploring a growing body of literature suggesting that pathologies such as Huntington's disease (HD) and autism spectrum disorder (ASD) arise from aberrant embryonic progenitor behavior. Furthermore, we discuss throughout the recent advances in technology that allow more sophisticated labeling and manipulation of embryonic progenitors, thus opening possibilities for both novel investigations and potential development of treatment options. Finally, where questions remain regarding the development of the striatum, we provide hypotheses and insights from studies in the cortex and other brain regions.
Embryonic progenitors of the ventral telencephalon
All neural progenitors descend from the neuroepithelial cells that form the neural tube in the developing embryo. After closure of the neural tube, distinct rostral, medial, and caudal regions develop to ultimately give rise to the frontal, middle, and hindbrain regions of the brain (Stiles and Jernigan, 2010). This review mainly focuses on the developing rostral region, the telencephalon, in relation to the striatum; for a focus on basal ganglia development, see the study by Rubenstein and Campbell (2020).
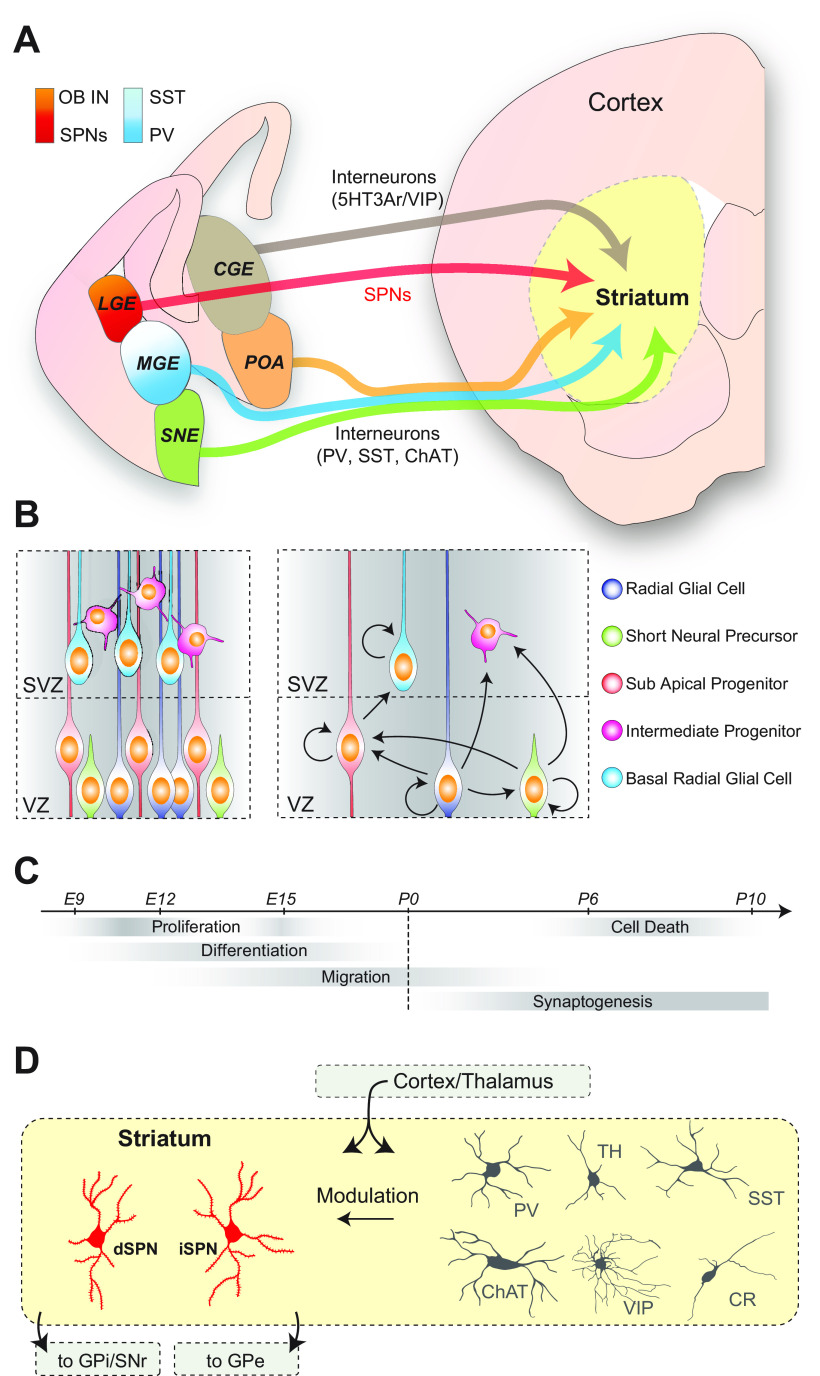
A combination of morphogenetic movements and proliferation between embryonic day 9 (E9) and E11 in mice establishes further discrete proliferative regions in the rostral telencephalon, a dorsal region that gives rise to the cortex, a ventrolateral region that forms the lateral ganglionic eminence (LGE) and mainly gives rise to the SPNs of the striatum (Deacon et al., 1994; Olsson et al., 1998; Wichterle et al., 2001; Nery et al., 2002); and a ventromedial region forming the medial ganglionic eminence (MGE) that gives rise to the interneurons of the striatum, globus pallidus, and cortex, among others (Marin et al., 2000; Anderson et al., 2001; Rallu et al., 2002; Butt et al., 2005; Flandin et al., 2010; Dodson et al., 2015). In addition, adjacent ventral structures such as the caudal ganglionic eminence (CGE; Nery et al., 2002; Ma et al., 2012; Muñoz-Manchado et al., 2016), preoptic area (POA)/anterior entopeduncular area (Marin et al., 2000; Gelman et al., 2011), and septal neuroepithelium (SNE; Magno et al., 2017) also give rise to interneurons (Marin et al., 2000; Fig. 1A). Initial gradients of diffusible factors (Rallu et al., 2002) and distinct transcription factor cascades (Schuurmans and Guillemot, 2002; Silberberg et al., 2016) contribute to this dorsoventral regional patterning.
Figure 1.
Striatal cells arise from diverse progenitor populations in the ganglionic eminences and neighboring structures. A, The embryonic domains that give rise to striatal fated cells include the LGE, MGE, CGE, POA, and SNE. Further gradients can be found within the eminences (e.g., dorsal LGE is a major source of OB interneurons and ventral LGE gives rise to SPNs), and SST interneurons are preferentially derived from dorsal MGE, while PV interneurons are preferentially derived from ventral MGE. B, Left, Embryonic progenitors can be segregated into different groups: the apical progenitors of the VZ, including radial glial cells; subapical progenitors and short neural precursors; and the basal progenitors of the SVZ, which can be separated into bRGCs and other IPs (e.g., basal progenitors). Right, The arrows represent the possible outcomes of progenitor division. C, Cells undergo broadly conserved steps of maturation, including proliferation, differentiation, and migration followed by refinement of circuitry through synaptogenesis and controlled apoptosis. D, The main excitatory inputs to striatum come from cortex and thalamus, which innervate both the SPNs (left) and interneurons (right). SPNs include both the dSPNs (in red) and the iSPNs (in red), which send axonal projections to downstream basal ganglia nuclei including, respectively, the SNr/GPi and GPe. The local populations of diverse interneurons are integrated within the striatum and can modulate the activity of SPNs. VIP, Vasoactive intestinal polypeptide.
The first neuronal progenitor cells (NPCs) in each of these regions consist mainly of RGCs, which divide at the ventricular wall to generate further progenitors that inhabit both the ventricular zone (VZ) and subventricular zone (SVZ) and young neurons that migrate to the primordial striatum and other structures (Marin et al., 2000). The daughter progenitor cells consist of additional RGCs plus a diverse population of intermediate progenitors (IPs; Fig. 1B). These IPs can amplify the number of concurrently actively dividing cells in the developing brain (Noctor et al., 2004) and, as discussed later, convey unique properties to their daughter neurons. As progenitors divide, postmitotic neurons of the ventral telencephalon follow a well defined developmental sequence starting with their migration from their birthplace to their designated brain regions (Villar-Cerviño et al., 2015), progressively differentiating toward their final identity. During later postnatal stages, these immature neurons initially connect widely followed by periods of synaptic refinement and controlled apoptosis in maturing circuits (Fig. 1C). At approximately E18 in mice, the neurogenic divisions within the embryonic brain switch and become gliogenic, generating both astrocytes and oligodendrocytes (Anthony and Heintz, 2008; Minocha et al., 2017; Turrero Garcia and Harwell, 2017). Although this review will mainly focus on progenitor-derived neurons, the extent to which the diversity of embryonic progenitors is related to astrocyte and oligodendrocyte diversity is likely an interesting line of future research.
Embryonic progenitors of the LGE and their progeny
The formation of the LGE as a clearly visible structure in the subpallium occurs around E11 in mice and is followed by the onset of neurogenesis by a diverse population of neurogenic progenitors that inhibit the VZ and SVZ (Halliday and Cepko, 1992; Sheth and Bhide, 1997; Olsson et al., 1998; Stenman et al., 2003; Gotz and Huttner, 2005; Mason et al., 2005; Sousa and Fishell, 2010; Pilz et al., 2013; Kelly et al., 2018; van Heusden, 2021, #6256). The VZ of the LGE is thought to contain several types of embryonic progenitor cells, of which the following two divide at the ventricular surface: classic RGCs with a bipolar morphology that exhibit a basal and apical process during division; and short neural precursors (SNPs), which exhibit a rounded morphology and tend to lack processes during division. Other progenitors (OPs) in the VZ have been shown to divide at subapical positions away from the ventricular surface; these have been named subapical progenitors (SAPs; Pilz et al., 2013). Finally, the SVZ contains progenitor types that lack a process during division and resemble basal progenitors (BPs), as well as progenitors that retain one or more processes and resemble RGCs. The latter progenitors divide in the basal aspects of the LGE and are called basal RGCs (bRGCs; Pilz et al., 2013; Fig. 1B). Detailed analysis of lineage progression among these progenitor types suggests that the majority of LGE RGCs generates daughter progenitor cells, which continue to divide without directly generating postmitotic neurons (Pilz et al., 2013). This is unlike RGCs in the cortex and MGE (Kriegstein and Alvarez-Buylla, 2009) and suggests that most striatal SPNs are generated from IPs. Indeed, lineage analysis suggests that LGE RGCs generate mainly additional RGCs, SNPs, or SAPs. In turn, the SNPs mostly generate further SNPs or SAPs, while SAPs generate further SAPs, BPs, or postmitotic neurons (Pilz et al., 2013; Fig. 1B). Many of these embryonic progenitors are not unique to the LGE and have also been characterized in detail in proliferative zones of the cortex (Noctor et al., 2001, 2004; Gal et al., 2006; Kowalczyk et al., 2009; Stancik et al., 2010; Shitamukai et al., 2011; Wang et al., 2011; Franco and Müller, 2013; Taverna et al., 2014), although their properties can differ between these structures. For example, cortical SNPs tend to have relatively long cell cycle kinetics and often generate neurons directly (Gal et al., 2006; Stancik et al., 2010; Tyler and Haydar, 2013), whereas those in the LGE tend to have relatively short cell cycle kinetics and produce further progenitors (Pilz et al., 2013).
The morphologic diversity of LGE progenitors coexists alongside broader divisions of LGE based on differential transcription factor expression. For example, the transcription factor ETV1/Er81 delineates the dorsal regions of the LGE, which can be further subdivided into a lateral subregion, with high expression of the transcription factors paired-box protein 6 (Pax6) and Genetic-Screened Homeobox 2 (Gsh2) and bordering the cortex (Yun et al., 2001; 2003), and a more medial region with low Pax6 expression but high Gsh2 expression (Flames et al., 2007). Other studies have revealed that the dorsally situated Etv1/Er81+ progenitors tend to generate olfactory bulb (OB) fated interneurons, whereas the more ventrally located Isl1+ progenitors supply SPNs of the striatum, thus providing the first evidence that distinct progenitor domains generate distinct neuron populations (Yun et al., 2001; Stenman et al., 2003; Fig. 1A).
Specific transcription factors, such as GS Homeobox 1/2 (Gsx1/2), achaete-scute homolog 1 (Ascl1), and Distal-Less Homeobox 1/2 (Dlx1/2), as well as Notch signaling, mediate cell-autonomous and nonautonomous regulation of neurogenesis in the LGE and control ordered production of striatal neurons (Yun et al., 2002; Mason et al., 2005). These can further delineate different LGE VZ and SVZ regions (Puelles et al., 2000; Toresson et al., 2000; Yun et al., 2001; Stenman et al., 2003; Flames et al., 2007; Petryniak et al., 2007; Wang et al., 2013). Key in this process are the Gsx (Corbin et al., 2000; Toresson et al., 2000; Yun et al., 2001; 2003; Wang et al., 2013; Roychoudhury et al., 2020; Salomone et al., 2021) and Dlx gene families expressed during the maturation of both progenitors and neurons in the LGE (Porteus et al., 1991; 1994; Anderson et al., 1997; Liu et al., 1997; Eisenstat et al., 1999) and governing further downstream transcriptional networks controlling LGE and striatal development (Long et al., 2009; Lindtner et al., 2019). Indeed, it has been suggested that the early LGE contains Gsx1/2+ neuroepithelial cells that produce multiple progenitor types characterized by Ascl1 and Dlx expression (Yun et al., 2002; Martín-Ibáñez et al., 2012). The Ascl1+/Dlx1/2- and Ascl1+/Dlx1/2+ progenitors are inferred to emerge in sequence (Martín-Ibáñez et al., 2012) and interact through Notch-mediated lateral inhibition to coordinate both proliferation and neurogenesis (Mason et al., 2005). The progenitors within the LGE can be further distinguished through differential transcription factor expression from those found in neighboring eminences. For example, the MGE expresses the transcription factors NK2 Homeobox 1 (Nkx2.1) and LIM/homeobox protein 6 (Lhx6), whereas the LGE does not (Chen et al., 2017; Mayer et al., 2018).
From this transcriptional and morphologically diverse population of embryonic progenitors in the LGE, the vast majority of postmitotic neurons become GABAergic striatal SPNs, with a smaller population maturing into OB interneurons (Wichterle et al., 1999; Corbin et al., 2001; Wichterle et al., 2001; Stenman et al., 2003). The generation of SPNs starts at approximately E10.5 and continues until birth, E19.5, in mice (Deacon et al., 1994; Sheth and Bhide, 1997; Matsushima and Graybiel, 2020; Fig. 2B), although some are also born during early postnatal stages (Das and Altman, 1970; Bayer, 1984; Wright et al., 2013). The orderly production of early- and late-born SPNs within the LGE is regulated in part through various downstream transcription factors (e.g., Ebf1, Isl1, Sp9; Zhang et al., 2016; Merchan-Sala et al., 2017), which can regulate SPN subtype generation and survival, as well as allowing for their selective labeling during early development (Merchan-Sala et al., 2017). Indeed, for the generation of dSPNs it has been shown that the transcription factor Isl1 is important (Ehrman et al., 2013; Lu et al., 2014), with conditional loss leading to early cell death of newly born dSPNs (Ehrman et al., 2013), likely through loss of Foxo1 expression (Waclaw et al., 2017). Additional factors such as ebf1 also play a role in SPN survival (Lobo et al., 2006; 2008), but with loss mainly affecting dSPNs during later stages of neurogenesis. For the generation and survival of iSPNs, it has been shown that the transcription factors Ikaros and Helios are important and also regulate the expression of the iSPN marker enkephalin (Martín-Ibáñez et al., 2010, 2012). In addition, the expression of the transcription factors sp8 and sp9 (Long et al., 2009; Zhang et al., 2016) are further required for iSPN survival as double knockout (KO) results in a nearly complete loss of iSPNs (Xu et al., 2018), similar to KO of their downstream transcription factor six3 (Song et al., 2021).
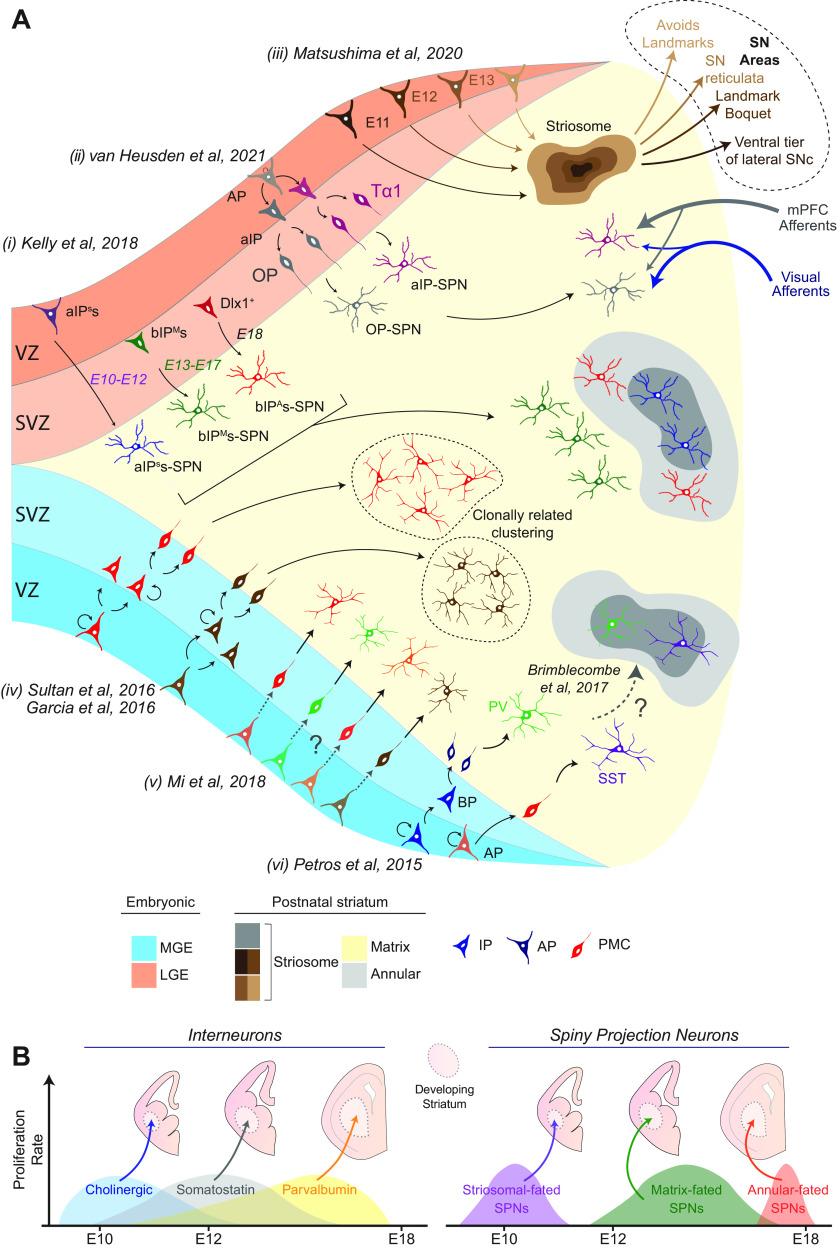
Figure 2.
Embryonic progenitor origin controls many aspects of mature striatal neuron position and connectivity. A, i, LGE aIPs and bIPs generate SPNs fated to the striosomes (aIPSs-SPN) and matrix (bIPMs-SPN) compartments of the adult striatum. SPNs fated for the annular region (bIPAs-SPN) are generated later in development from specific bIPs expressing the transcription factor Dlx1. ii, IPs expressing Tα1 in the LGE generate SPNs that receive stronger innervation from the mPFC, whereas OPs, which do not express Tα1, generate SPNs that receive stronger innervation from visual cortex afferents. iii, The time of birth of SPNs in the LGE determines their spatial arrangement within striosomes and, consequently, facilitates the formation of different long-range synaptic connections with the SN. iv, It is debated whether lineage and/or clonal relationships govern the spatial distribution of MGE-derived neurons. v, The transcriptional identity of mature MGE-derived interneurons is reflected in early postmitotic cells (PMCs); however, whether the underlying progenitor cells predetermine this is not known. vi, Apical neurogenesis in the MGE biases toward the generation of SST interneurons, whereas basal neurogenesis preferentially generates PV interneurons. Unlike the SPNs, it is not known whether this bias extends to the spatial distribution between striosomes and matrix neurochemical compartments in the striatum. B, For both MGE- and LGE-derived neurons, the time of birth appears to be a critical factor that facilitates the generation of the chemical identity and spatial distribution of a neuron.
Embryonic progenitors of the MGE and their progeny
Striatal interneurons, which exert a strong regulatory control over SPN activity and consequently striatal output, are derived primarily from the MGE (Fig. 1A), Therefore, we will first outline what is currently known about MGE progenitors and subsequently introduce the progenitors in other structures that produce the remaining striatal interneurons.
Like the LGE, the MGE arises from the ventral region of the neural tube at approximately E9.5 and is divided into the VZ and SVZ progenitor zones. In the VZ, the primary progenitor is the RGC, which, in addition to self-replication, can give rise to other progenitors and neurons (Turrero Garcia and Harwell, 2017). Other progenitors in the MGE include SAPs and SNPs of the VZ, in addition to other IPs and bRGCs of the SVZ (Turrero Garcia and Harwell, 2017; Fig. 1B). The generation of such progenitors occurs in a lineage-specific manner; for example, SNPs arise from the direct division of RGCs, whereas the generation of bRGs is achieved through SAP intermediates (Pilz et al., 2013; Petros et al., 2015; Fig. 1B).
As in the LGE, the morphologic diversity of embryonic progenitors in the MGE is accompanied by heterogeneity in the combinatorial expression of different transcription factors (Flames et al., 2007; Flandin et al., 2010; Lopes et al., 2012). In situ hybridization studies for multiple target genes such as Dlx2, Pax6, Nkx2.1, Lhx6, and Lhx7 have revealed subdomains formed by groups of transcriptionally similar progenitors that are localized to specific regions of the MGE (Flames et al., 2007). Each of these genes plays a distinct role in the control of cell identity within the subdomains. For example, Dlx genes drive the upregulation of the GABA-synthesizing enzyme glutamic acid decarboxylase and delineate the GABAergic interneurons (Stuhmer et al., 2002; Le et al., 2017). Local populations of APs and BPs can also be further divided into subpopulations based on their transcriptional identity, which controls their metabolism, cell cycle dynamics, or overall neurogenic role. For example, one population of APs displays high expression of the genes required for oxidative phosphorylation (Atp5e and Cox6c), whereas another population of APs highly expresses DNA replication genes (Mcm5, Mcm6, and Mcm7) and translation regulators (Eif4g1, Eif2s1, and Eif3b; Chen et al., 2017). On the other hand, BPs can be divided into two populations based on the expression levels of glutamic acid decarboxylase 2 (Gad2) and aristaless-related homeobox (Arx), which represses the inhibition of cell cycle progression (Lim et al., 2019). Coexpression of Coup-TF1 and Coup-TF2 in distinct progenitor subdomains of the MGE directly controls cell cycle dynamics and neurogenic differentiation (Hu et al., 2017). Upon CoupTF-2 ablation, cell proliferation is perturbed, and the resulting cell fate is shifted. Thus, distinct progenitors in the MGE show different transcriptional and functional properties, which likely contribute to the generation of striatal interneuron diversity.
MGE progenitors give rise to a widely heterogeneous and dispersed population of interneurons that populate brain areas such as the cortex, hippocampus, globus pallidus, and nucleus accumbens (Marin et al., 2000), and can be differentiated based on their chemical, electrical, and morphologic properties. The primary striatal interneuron subtypes are identified by their expression of parvalbumin (PV), somatostatin (SST), or choline acetyltransferase (ChAT) and are diverse regarding their connectivity patterns and intrinsic properties. For example, PV+ fast-spiking interneurons form short-range connections with SPNs and exert strong inhibition that can regulate action potential initiation in both dSPNs and iSPNs, thus mediating feedforward inhibition over striatal output (Mallet et al., 2005; Gittis et al., 2011; O'Hare et al., 2017; Owen et al., 2018). These fast-spiking interneurons receive dense innervation from the cortex, with smaller inputs from both thalamic projections and striatal ChAT+ CINs, and output to multiple SPNs with firing rates of up to 400 Hz through their dense axonal field (Kita, 1993). In contrast, SST+ interneurons coexpress one or both of the neurochemical markers neuropeptide Y (NPY) and nitric oxide synthase, and are commonly referred to as low-threshold spiking interneurons (Kawaguchi et al., 1995; Muñoz-Manchado et al., 2016, 2018). These neurons are also primarily innervated by monosynaptic, excitatory inputs coming from cortex, but they differ from PV+ interneurons in that they form longer-range connections with SPNs and show significantly lower levels of connectivity (Assous et al., 2019). Moreover, these neurons are innervated by CINs and mediate cholinergic-mediated feedforward inhibition (English et al., 2011). Finally, the CINs display a range of transcriptional, morphologic, and physiological properties (Magno et al., 2017; Muñoz-Manchado et al., 2018). For example, it has been shown that the transcription factors Lhx6 and ETV1/Er81 segregate striatal CINs into functional subtypes (Lozovaya et al., 2018; Ahmed et al., 2019, 2021). In particular, the Lhx6-expressing CINs, also called cholinergic-GABAergic interneurons (Lozovaya et al., 2018), display different physiological properties with higher firing rates and larger dendritic fields compared with other CINs. Indeed, coexpression of neurotransmitters such as acetylcholine, glutamate, and GABA in different CINs further highlights their functional diversity (Nelson et al., 2014; Granger et al., 2016). Newly developed approaches [e.g., adeno-associated virus (AAV)-based tools; Vormstein-Schneider et al., 2020; Table 1] will help to selectively label and further study these diverse interneurons.
Table 1.
Recent technological advances in embryonic progenitor research
| Methods | Key findings and references |
|---|---|
| Analysis of gene expression | |
| sc-RNAseq Quantification of RNA transcripts with single-cell resolution |
Uncovered new MGE-derived NPC subtypes and transcriptionally defined cortical interneurons (Tasic et al., 2016; Mayer et al., 2018; Mi et al., 2018; Saunders et al., 2018; Tasic et al., 2018) AP and BP populations divide into two subtypes (Chen et al., 2017) Further subdivisions within dSPN and iSPN and additional striatal SPN types (Gokce et al., 2016; Saunders et al., 2018; Zeisel et al., 2018; Martin et al., 2019) Uncovered gradients of transcriptional heterogeneity correlating with positional information of SPNs (Stanley et al., 2020) Whole-exome sequencing identifying ASD risk genes and the critical importance of interneurons within ASD etiology (Satterstrom et al., 2020) sc-RNAseq of human striatal progenitors and young neurons (Bocchi et al., 2021) |
| Patch-Seq Investigating transcriptional profiles and physiological properties of single cells |
Reveals seven main classes of striatal SPNs and interneurons with gradients of gene expression that vary from cortical and hippocampal interneurons (Muñoz-Manchado et al., 2018) Transcriptional classification of aIP- and OP-derived cortical neurons (Ellender et al., 2019) |
| Spatial transcriptomics Visualizing RNA transcripts in specific areas with spatial resolution (50 μm) |
Spatial transcriptomics enables generation of a whole-brain atlas and uncovers new spatial domains in the striatum (Lein et al., 2017; Ortiz et al., 2020) |
| MERFISH Visualizing RNA transcripts with subcellular compartmentalization |
MERFISH enables spatial RNA profiling of individual cells in different subcellular compartments and in transcriptionally distinct cell cycle phases. This technique is useful to study cell fate and regulation of gene expression (Xia et al., 2019) |
| Lineage tracing and cell fate assays | |
| MADM MADM provides genetic dissection of intrinsic gene function |
This genetic mosaic strategy enables sparse altering of single cells while maintaining a “normal” local microenvironment. This study showed that Lgl1 is a critical regulatory element for embryonic cortical neurogenesis and cell-autonomous control of RGC-mediated glia genesis and postnatal NPCs (Beattie et al., 2017) |
| FlashTag Label, track and isolate isochronic cohorts of newborn cells in the CNS |
This powerful technique, first described in the neocortex, can be used in many brain regions to date the birth of and isolate any type of progenitor in contact with the VZ and to follow cell migration of newly born neurons (Telley et al., 2016; Govindan et al., 2018) |
| In utero electroporation Label embryonic progenitors and track their progeny through prenatal and postnatal periods |
In utero electroporation of constructs driving recombinase systems (e.g., Cre) under the control of promoter sequences specific for certain progenitors in combination with reporter constructs allows for labeling of progenitors and progeny. Used to label apical IPs in striatal and cortical proliferative regions (Gal et al., 2006; Stancik et al., 2010; Tyler and Haydar, 2013; Ellender et al., 2019; van Heusden et al., 2021), and basal IP cells and/or bRGCs in cortical proliferative regions (Tyler et al., 2015; Li et al., 2020) |
| Transplantation assays Human pluripotent stem cell-derived neurons |
In this study, the authors reveal a differentiation protocol to direct hPSCs to mature neurons in 37 d in vitro (Comella-Bolla et al., 2020). Transplantation experiments show that NPCs survive and differentiate (for at least 3 months) in the mouse striatum (Martínez-Cerdeño et al., 2010; Noakes et al., 2019; Comella-Bolla et al., 2020) |
| FUCCI Fluorescence ubiquitination cell cycle indicator analyzes the temporal dynamics of cell cycle progression (live cell imaging) |
Genetically encoded fluorescent probes to visualize cell cycle transition from G1 to S phase (individual G1 phase nuclei in red, S/G2/M phases in green; Sakaue-Sawano et al., 2008) |
| Clonal relationships Dispersion of clonally related interneurons |
Study the clonal or progenitor origin that influences the spatial distribution of mature interneurons (Ciceri et al., 2013; Harwell et al., 2015; Mayer et al., 2015; Sultan et al., 2016; Turrero Garcia et al., 2016) |
| Connectivity and cell activity | |
| Viral transfections RV: retrograde monosynaptic tracing AAV: labeling of distinct neuronal subtypes |
Mapping of synaptic inputs to projection neurons and cholinergic interneurons in the dorsal striatum using modified rabies virus tracing (Guo et al., 2015) Identification of multiple new enhancers to target functionally distinct neuronal subtypes in mice, primates, and humans (Vormstein-Schneider et al., 2020) |
| Studying progenitors in humans | |
| Brain organoids In vitro models that replicate some developmental processes of the human brain |
Study of the transcriptional regulation of progenitor fate that is altered in ASD—for example revealing that the overexpression of FoxG1 leads to the overproduction of interneurons (Mariani et al., 2015) |
| Perturb-Seq Introduction of mutations in specific genes by gene editing (e.g. knock-out candidate genes in mice embryos), followed by single-cell transcriptomic analysis |
Alteration of cortical lineages in the developing mouse brain and analysis of 35 ASD risk genes in five cells classes, including projections neurons, inhibitory neurons, astrocytes, oligodendrocytes and microglia. They revealed that cell type composition remains unaffected, but cell state is affected (Jin et al., 2020). This method can be applied across diseases from diverse tissues, such as human PSCs or brain organoids |
MERFISH, Multiplexed error-robust FISH; MADM, mosaic analysis with double markers; FUCCI, fluorescence ubiquitination cell cycle indicator.
Other embryonic progenitors and their progeny
While most striatal interneurons are derived from the MGE, smaller populations originate from other embryonic structures, including the POA and SNE, which are both situated ventrally to the MGE, as well as the CGE (Marin et al., 2000; Ma et al., 2012; Fig. 1A). The CGE is a chemically distinct proliferation domain originating from the caudal merging of the MGE and LGE, and is classically defined by the expression of the 5HT3a serotonin receptor (Nery et al., 2002; Lee et al., 2010; Fig. 1C). The peak proliferation of CGE-derived NPCs occurs 3 d after that of MGE-derived progenitors (Miyoshi et al., 2010). Approximately 20% of the CGE-derived neurons contribute to a population of striatal PV+ fast-spiking interneurons (Miyoshi et al., 2010) and specifically express 5HT3a (Muñoz-Manchado et al., 2016). The remaining interneurons include a unique population of late-spiking neurogliaform cells and low-threshold spiking cells, both of which lack the expression of known interneuron markers (Muñoz-Manchado et al., 2018). Other striatal interneuron subtypes include a substantial population of tyrosine hydroxylase-positive (TH+) interneurons comprising electrophysiologically distinct cell subtypes (Mao et al., 2019). Striatal TH+ interneurons are not dopaminergic, but rather are a type of GABAergic interneuron that expresses TH without the other requisite enzymes or transporters to operate as dopaminergic neurons. These interneurons play an important role in striatal function through fast GABAergic synaptic transmission. They respond to local or cortical stimulation with glutamatergic EPSPs and exert widespread GABAergic inhibition onto both dSPNs and iSPNs, and between CINs (Xenias et al., 2015; Dorst et al., 2020). Modulation of the properties of TH+ interneurons by dopamine and acetylcholine may play important roles in mediating the striatal effects of these neuromodulators, with potentially important implications in disorders affecting the striatum (Ibáñez-Sandoval et al., 2015). The positional fate, morphology, and neurochemical identity of CGE-derived interneurons in cortex were shown to be dependent on the progenitor domain from which they arise (Torigoe et al., 2016), but no evidence has directly reported whether this extends to the CGE-derived interneurons of the striatum.
Interestingly, progenitors of the POA and SNE express the transcription factor Nkx2.1 and also generate neurons expressing ChAT+, PV+, and SST+ (Marin et al., 2000; Fig. 1A). As smaller contributors to the overall interneuron populations in the cortex and striatum, these regions have been somewhat neglected, so further investigation is needed. This is highlighted by the fact that the morphologic properties of SNE and POA progenitors are not yet clearly defined within the literature. Yet, it is known that POA progenitors are transcriptionally distinct from those in the MGE, expressing transcriptional markers such as brain homeobox protein 1 (Dbx1) and sonic hedgehog (Shh; Gelman et al., 2009).
Finally, a small subpopulation of Empty Spiracles Homeobox 1-lineage (Emx1) cells originating in the cortical proliferative zones seem to migrate into the developing striatum during early prenatal development (Willaime-Morawek et al., 2006) and differentiate primarily into DARPP-32+ SPNs and a small number of calretinin-positive (CR+) striatal interneurons (Cocas et al., 2009). In addition, a small population of SPNs has also been shown to arise from the CGE (Nery et al., 2002).
It is largely unknown how the heterogeneity of embryonic progenitors based on their location of division (e.g., VZ and SVZ), morphology, and cell cycle kinetics maps onto the transcriptional heterogeneity seen in the ganglionic eminences. This is important to understand, not only to further our understanding of progenitor diversity and lineage progression, but also because it might reveal a cohesive framework for labeling and tracking these populations of progenitors during embryogenesis, as well as following their development and neuronal progeny. Endeavors to map the genetic diversity within the ganglionic eminences at the single-cell level (Mayer et al., 2018; Mi et al., 2018) will further these efforts but is complicated because of the highly dynamic nature of their transcriptional profiles (Li et al., 2020).
From ganglionic eminences to postnatal striatum
The LGE and MGE generate the majority of the neurons found in the postnatal striatum. From these embryonic domains, postmitotic cells must first migrate through the mantle zone, the superficial layer beyond the SVZ that contains neurons at various stages of migration and differentiation, before proceeding to the primordial striatum, where they integrate into functional striatal circuits. From the LGE, postmitotic cells migrate predominantly radially over a short distance, following a number of migratory cues toward the striatum (Bayer, 1984; Halliday and Cepko, 1992; Song and Harlan, 1994; de Carlos et al., 1996; Hamasaki et al., 2003; Newman et al., 2015; Kelly et al., 2018; Xu et al., 2018; Chen et al., 2020), where they actively intermix (Tinterri et al., 2018). From the MGE, interneurons migrate longer distances to both the cortex and the striatum, again relying on differential expression of guidance molecules and receptors. For example, it is known that migration to cortical regions is guided by chemoattraction of semaphorin ligands (Sema3A and SemaF), and neuropilin receptors (Nrp1, Nrp2; Marin and Rubenstein, 2001; Andrews et al., 2017) expressed in cortical fated cells, whereas migration to the striatal region is regulated by neuregulin 1 and ErbB4 receptor tyrosine kinase 4 signaling (Villar-Cerviño et al., 2015). Any change in the expression of these transcription factors in postmitotic cells will redirect cells fated to a specific brain region (Villar-Cerviño et al., 2015). The rapid downregulation of Nkx2.1 acts as a postmitotic transcriptional switch (Fig. 3B) in cortical fated cells, as it transcriptionally inhibits cortical migration cues such as Nrp2 (Butt et al., 2008; Nóbrega-Pereira et al., 2008). In contrast, striatal fated cells maintain Nkx2.1 expression into adulthood, preventing cortical migration (Villar-Cerviño et al., 2015).
Figure 3.
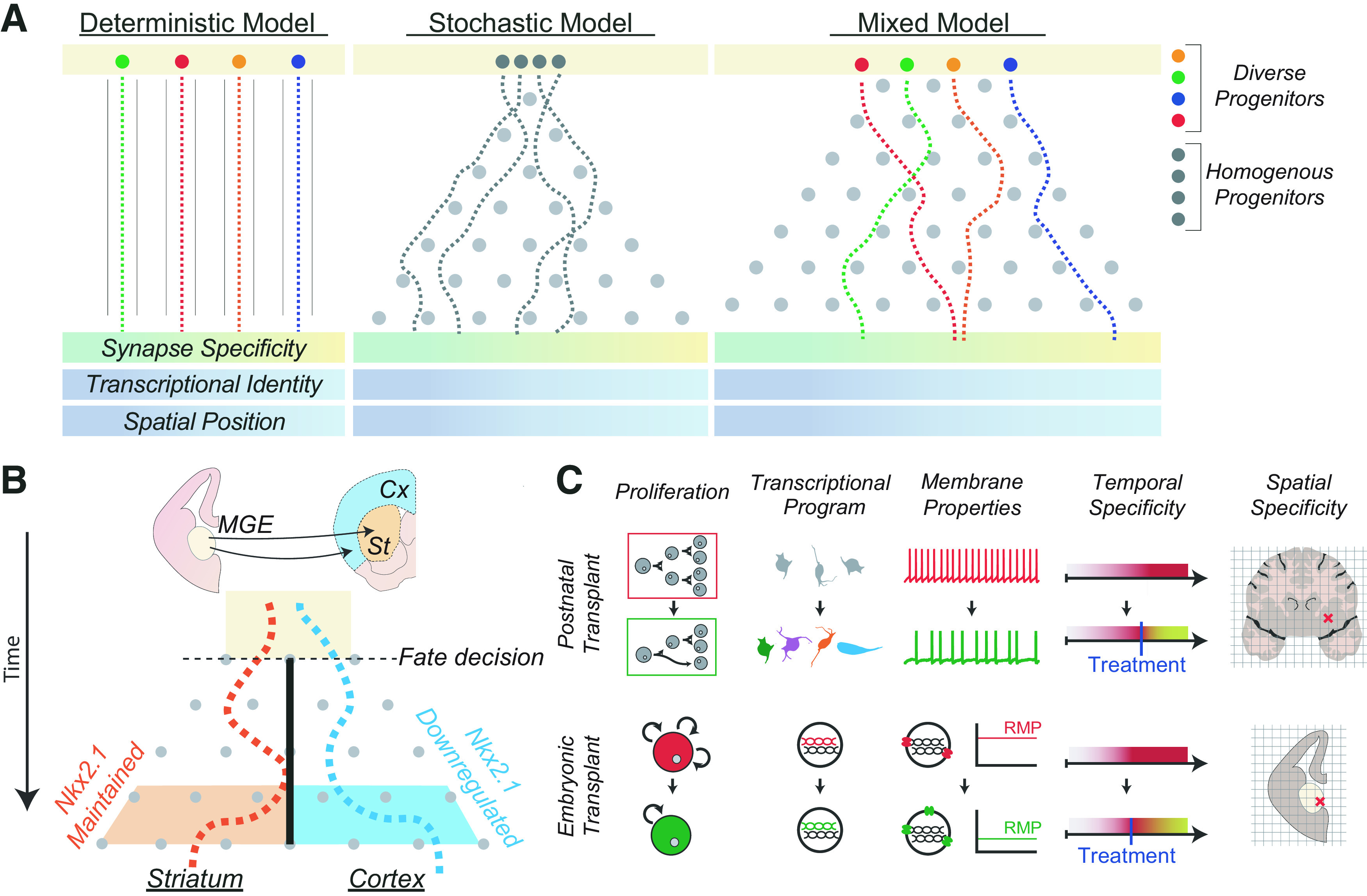
The relationship between diversity in embryonic progenitors and diversity in postnatal neurons. A, Different models have been proposed to explain the neuronal diversity observed in the postnatal brain. In the deterministic model (left), different progenitor pools (different colors; Villar-Cerviño et al., 2015) generate neurons that have specific characteristics (e.g., biased synaptic inputs, transcriptional identity, and/or spatial positioning). In the stochastic model (middle), these characteristics are mainly attained shortly after birth through a seemingly random process (Llorca et al., 2019; Klingler and Jabaudon, 2020). These two models could also coexist for distinct progenitor populations, and they are not mutually exclusive: a mixed model (right) is possible. B, It is possible that some developmental cues are irreversible, permanently shifting the outcome of a stochastic system. In this example, the dark line represents a restriction within the stochastic system: after a cell downregulates the transcription factor Nkx2.1, it becomes fated for the cortex instead of striatum. The result cannot be reversed, regardless of intrinsic or extrinsic cues. C, Top, To effectively restore neural physiology with cell transplants in the postnatal brain, multiple factors must be considered, including transplanting a sufficient number of cells with appropriate transcriptional identities and intrinsic properties (Noakes et al., 2019). Bottom, Because neurodevelopmental pathologies can arise from dysfunctional progenitors, modulation of existing progenitors in situ or transplanting progenitors prenatally might restore a healthy developmental trajectory. This will also necessitate the generation of progenitors with appropriate cell cycle dynamics (Wang et al., 2020), transcriptional states (Satterstrom et al., 2020), and other intrinsic properties, including resting membrane potential (RMP; Vitali et al., 2018). Both adult and embryonic transplants would require transplanting cells at the correct time within a developmental or disease process, as well as in the correct location in the brain.
The smaller populations of cells derived from the CGE, SNE, and POA must also migrate to the striatum; however, they follow different migratory routes, regulated by different genetic cues. Unlike the MGE and LGE, the CGE has two separate caudorostral migratory routes that cells use to invade the striatum, hippocampus, and cortex (Nery et al., 2002; Touzot et al., 2016). These cells regulate migration through specificity protein 8 (Sp8), Prox1, and CoupTF-I/TF-II signaling, which when perturbed, disrupts the ability of cells to successfully integrate into these diverse circuits (Touzot et al., 2016). In the next section, we explore how certain organizational aspects of striatum are governed by embryonic progenitor origin (Fig. 2).
Progenitors shaping striatal neuron positioning.
How is the position of a neuron in the striatum related to the embryonic progenitor it is derived from? It is known that early-born SPNs are located in the caudal parts of the striatum, while later-born SPNs are found in more rostral parts (Newman et al., 2015; Kelly et al., 2018). The differential localization of these SPNs must, to some extent, be related to the populations of progenitors that are actively dividing during early and later stages of neurogenesis. As the proportion of IPs is greater at later stages of neurogenesis, this would suggest a larger contribution of certain IPs (e.g., BPs) to the generation of rostral SPNs (Pilz et al., 2013; Newman et al., 2015; Kelly et al., 2018). Both dSPNs and iSPNs are found intermingled throughout the striatum in a mosaic (Gangarossa et al., 2013), which, at least for the matrix compartment, seems to arise from active intermingling of newly born SPNs (Tinterri et al., 2018). This intermingling suggests that clonal clusters of SPNs might be spread out more extensively throughout the striatum than clones found in the cortex (Yu et al., 2009; Brown et al., 2011; Shi et al., 2017), although this has not been systematically tested. Further positional information of SPNs in the striatum can be related to differential gene expression. For example, clear gradients of expression of the genes Crym and Cnr1 in SPNs can be observed from ventrolateral to dorsomedial striatum (Stanley et al., 2020). However, whether these gradients result from different developmental origins of the SPNs is currently unknown.
Like SPNs, striatal interneurons do not distribute homogeneously within the striatum. Interneuron migration follows a ventral to dorsal gradient and interneurons sequentially populate the lateral and medial regions of the striatum (Chen et al., 2020). In particular, early-born interneurons such as the PV+ interneurons and CINs tend to accumulate in the lateral part of the striatum (Marin et al., 2000; Fig. 2B). Similarly, different types of CR+ interneurons do not distribute homogeneously; for example, those coexpressing the Ca2+ binding protein secretagogin and Sp8 are preferentially located in the rostral parts of the striatum, while other subsets of CR+ interneurons are located more uniformly (Garas et al., 2018). Interestingly, the expression of secretagogin also defines a subpopulation of PV+ interneurons found in more caudal parts of the striatum (Garas et al., 2016). A direct link between progenitor identity and the final location of MGE-derived cells is yet to be elucidated, and the extent of spatial distribution of lineage-related interneurons is a current matter of debate. It has been proposed that lineage relationships do not determine interneuron allocation to particular regions (Mayer et al., 2015) and that clonally related interneurons can be widely dispersed (Harwell et al., 2015). Indeed, studies tracing clonally related neurons (predominantly after retrovirus labeling of progenitors embryonically) reveal that they disperse across the cortex, hippocampus, and striatum, with no apparent clustering (Reid and Walsh, 2002; Ciceri et al., 2013; Turrero Garcia et al., 2016; Fig. 2A, Table 1). However, other studies analyzing the same dataset suggest that lineage (i.e., clonal or progenitor origin) does form clusters of neurons in the postnatal brain (Sultan et al., 2016). Further studies of clonally related striatal interneurons from different embryonic regions and different progenitor cell types are likely necessary to unambiguously answer this question.
Progenitors shaping striatal neurochemical compartments.
As mentioned in the Introduction, striatal dSPNs and iSPNs are intermingled within several functionally and anatomically distinct subregions. SPNs born in the early phases of neurogenesis become preferentially incorporated into striosomes/patches and the later-born SPNs settle in the surrounding matrix (Graybiel and Hickey, 1982; van der Kooy and Fishell, 1987; Song and Harlan, 1994; Mason et al., 2005; Newman et al., 2015). Whether there is further fine-scale organization within these compartments and to what extent this relates to the diversity of embryonic progenitors in the LGE was until recently largely unknown. Several recent studies have started to provide some key insights, however (Kelly et al., 2018; Tinterri et al., 2018; Matsushima and Graybiel, 2020; Fig. 2).
The first study used elegant fate-mapping experiments to provide insight into the roles of distinct types of progenitor cells in the LGE in generating SPNs fated to either the striosome/patch or matrix compartments (Kelly et al., 2018). In this study, the authors used lineage-tracing analysis of embryonic progenitors [using tamoxifen-inducible, NGF-inducible protein (Tis21)- and Ascl1-Cre recombinase mouse driver lines] and demonstrated that the LGE contains two types of IPs, both derived from a RGC lineage. During early stages of neurogenesis (E9.5 to E12.5), apical IPs (aIPs) express the transcription factor Ascl1 and have limited capacity to produce striosomal SPNs. During later phases of neurogenesis (E13.5 to E18.5), basal IPs (bIPs) expressing both Ascl1 and Dlx1 produce matrix SPNs. It is possible that Ascl1, in conjunction with Gsx2, in SAPs inhibits neurogenesis and promotes initial proliferation of these large numbers of basal IPs (Roychoudhury et al., 2020). Both types of embryonic progenitor (apical and basal IPs) were shown to generate both dSPNs and iSPNs (Kelly et al., 2018).
The second study (Tinterri et al., 2018) used a combination of transgenic animals and time-lapse video imaging to provide insight into the seemingly uniform distribution of both dSPNs and iSPNs. Indeed, they were able to show that late-born iSPNs actively intermix with early-born dSPNs and that this, at least for the matrix compartments, depends on the expression of the transcription factor ebf1 in dSPNs (Tinterri et al., 2018).
Last, using a combination of transgenic Dlx1-Cre recombinase mice under the control of a fast-acting version of tamoxifen (4-OHT), Matsushima and Graybiel (2020) revealed that the striosomes/patches are formed through a center-surround rule, in which early-born SPNs are predominantly found in the center of the striosomes/patch compartments and are surrounded by increasingly later-born SPNs. They found that this center-surround rule was universal and was used in both anterior and posterior parts of the striatum, despite absolute differences in the birth date of SPNs in these distinct parts. Moreover, they found that a key anatomic structure, the so-called striosome-dendron bouquet, forms during a very specific period of neurogenesis in the mouse (i.e., approximately E12 to E13; Matsushima and Graybiel, 2020; Fig. 2).
Striatal interneurons also allocate differently between the striosome/patch and matrix compartments, which affects how these local microcircuits of SPNs are modulated (Banghart et al., 2015; Friedman et al., 2015). Often found at higher density in the matrix, CINs, PV, NPY, and CR-expressing interneurons are frequently located along striosomal borders in anatomically and functionally defined areas called “peristriosomal boundaries” (Prager and Plotkin, 2019). CINs and SST+ interneurons located at the interface between striosomes and matrix have dendrites and axons that traverse across compartmental borders (Kubota and Kawaguchi, 1993; Bernacer et al., 2012; Brimblecombe and Cragg, 2015; Matamales et al., 2016). Such interneurons might provide a functional bridge and modulate activity in both compartments (e.g., as demonstrated for the CINs; Crittenden et al., 2017). Yet, the precise roles of many other striatal interneurons in functionally linking striosome/patch and matrix microcircuits and intercompartmental communication remain poorly explored and form an interesting area for future study (Amemori et al., 2011).
Progenitors shaping striatal long-range excitatory synaptic circuits.
How does embryonic progenitor origin shape the specificity of synaptic connections in the striatum? As mentioned in the Introduction, the striatum can be split into distinct functional domains depending on anatomic subregion (e.g., DLS and DMS). These distinct anatomic domains contain a mixture of dSPNs and iSPNs that is thought to process and integrate excitatory inputs from distinct brain regions and also interact with each other via lateral inhibitory connections (Taverna et al., 2008; Planert et al., 2010; Chuhma et al., 2011; Burke et al., 2017; Krajeski et al., 2019). These anatomic domains are thought to be part of larger parallel functional pathways through the basal ganglia (Graybiel and Ragsdale, 1978; Alexander et al., 1986; Graybiel, 1990; Haber, 2008; Pan et al., 2010; Oh et al., 2014; Hintiryan et al., 2016; Hunnicutt et al., 2016; McGregor et al., 2019; Lee et al., 2020). At a more local level, the clear segregation of these functional pathways is less clear. Indeed, individual SPNs exhibit considerable heterogeneity in their afferent connectivity patterns (Pan et al., 2010), and populations of SPNs with diverse excitatory inputs are intermingled in striatum (Oh et al., 2014; Hintiryan et al., 2016; Hunnicutt et al., 2016). Moreover, it has been shown that long-range glutamatergic synapses from different cortical regions can converge onto single SPNs (Reig and Silberberg, 2014) or diverge and form biased synaptic connections on either dSPNs or iSPNs (Wall et al., 2013; Johansson and Silberberg, 2020). Considering that young SPNs exhibit complex migratory pathways and intermix in striatum during development (Tinterri et al., 2018), a question remains regarding how these precise striatal excitatory synaptic circuits develop and whether there is a role for distinct progenitor lineages.
A recent study has provided the first evidence that synapse specificity, of at least corticostriatal afferents, can arise from the embryonic origin of SPNs (van Heusden et al., 2021). In this study, the authors used in utero electroporation of a combination of constructs to label two active pools of embryonic progenitor in the VZ of the LGE at E15.5, based on the differential expression of the tubulin α1 (Tα1) promoter (Table 1). Interestingly, different tubulin isotypes can shape the properties of proliferating cells and might therefore provide a good target for future delineation of further progenitor types (Ramos et al., 2020). The study by van Heusden et al. (2021) combined a Tα1–Cre recombinase construct with a reporter construct incorporating a flexible excision (FLEx) CβA-FLEx cassette, so that Cre recombination permanently switches expression from the fluorescent protein TdTomato to GFP (Franco et al., 2012). Using this methodology, the authors showed that progenitors labeled with GFP (i.e., expressing Tα1) in the VZ had characteristics of both the SNP and SAP populations of LGE progenitors, including a rounded morphology during division, location of division, and fast cell cycle kinetics (Pilz et al., 2013; Kelly et al., 2018; van Heusden et al., 2021). Measures of cell cycle kinetics in this and previous studies (Stancik et al., 2010) were evaluated through labeling with the mitotic marker phosphohistone-3, but new technology [e.g., FUCCI (fluorescence ubiquitination cell cycle indicator); Sakaue-Sawano et al., 2008] will allow for more detailed insights (Table 1). Conversely, the progenitors that expressed TdTomato (i.e., not expressing Tα1 and likely consisting of a more heterogeneous population of progenitors) resembled the population of RGCs in that they had slower cell cycle kinetics and frequently exhibited a radial morphology during division (Pilz et al., 2013; Kelly et al., 2018; van Heusden et al., 2021). As many SAPs derive from SNPs and hence are closely lineally related (Pilz et al., 2013), and as both divide in the apical aspects of the LGE proliferative zone, the GFP+ Tα1-expressing progenitors were collectively referred to as “apical intermediate progenitors,” and the TdTomato+ non-Tα1-expressing progenitors simply as “other progenitors” (Fig. 2A). This also conforms to nomenclature of similar cortical (Tyler and Haydar, 2013; Ellender et al., 2019) and embryonic LGE progenitors (Kelly et al., 2018).
Using this approach, the authors followed the progeny of labeled cells and found that both progenitor pools predominantly generated striatal GABAergic SPNs; they referred to these cells as aIP- and OP-derived SPNs. Notably, both progenitor pools generated both dSPNs and iSPNs, which were intermingled, mostly in the DMS, and had similar properties (van Heusden et al., 2021). The authors explored whether aIP- and OP-derived SPNs differentially sample excitatory input coming from distinct cortical regions using local injections of AAV1-ChR2 in two different regions that send strong projections to DMS [Pan et al., 2010; Oh et al., 2014; Guo et al., 2015; Hunnicutt et al., 2016; i.e., the medial prefrontal cortex (mPFC); Laubach et al., 2018] and visual cortex (VC; Khibnik et al., 2014); this allowed optogenetic activation of afferents and whole-cell patch-clamp recordings of aIP- and OP-derived striatal SPNs. Strikingly, they found that embryonic progenitor origin conveyed significant biases in the strength of the long-range synaptic inputs coming from cortex, in that mPFC strongly innervated the aIP-derived SPNs, whereas the VC strongly innervated the OP-derived SPNs (Fig. 2A). The study by van Heusden et al. (2021), together with recent observations in cortex (Ellender et al., 2019), suggest that the lineage of a neuron may be a key contributor to synapse specificity. In utero electroporation and other techniques such as Flash-Tag (Telley et al., 2016; Govindan et al., 2018) are powerful approaches to label progenitors and follow their progeny (Table 1) to further our understanding of how progenitor identity relates to final function.
Unlike for SPNs, no study has yet investigated the correlation between the identities of progenitor cells (transcriptional, morphologic, or otherwise) and the subsequent excitatory synaptic connectivity pattern of mature striatal interneurons in detail. However, it is possible to infer a link between the two, based on current knowledge of striatal development. Indeed, distinct classes of striatal interneurons receive different glutamatergic inputs along the mediolateral axis. For example, CINs in the DMS receive more inputs from the pedunculopontine nucleus than the CINs in DLS (Assous et al., 2019), and, similarly, PV+ interneurons in the DMS, and not those in the DLS, receive glutamatergic inputs from the cingulate cortex (Monteiro et al., 2018). In the MGE, newly postmitotic, late-born CINs can be defined by the expression of the Gbx2 transcription factor (Chen et al., 2010), which might be related to their preferred pedunculopontine innervation in the DMS CINs. However, the extent to which the identity of the newly formed postmitotic cell is controlled by the transcriptional profile of the underlying progenitor is only beginning to be understood (for review, see Mi et al., 2018; Fig. 2).
Searching for answers in the cortex
Many questions remain regarding the role of embryonic progenitors in shaping postnatal striatal neuronal identity and circuits. For example, how do specific progenitor-derived cells map onto modern transcriptomic classifications of striatal neurons? What is the contribution of progenitor types other than the ones studied so far? We will now discuss some of these outstanding questions in light of the relevant literature, mainly from studies in the cortex, and discuss how these may guide future research in the striatum.
What is the contribution of other embryonic progenitors to the striatum?
As discussed above, the LGE contains a heterogeneous mix of progenitor types including those that divide in the apical aspects of the LGE (e.g., RGC and aIP) and those that divide in the basal aspects of the LGE (e.g., bRGCs and BPs; Olsson et al., 1998; Stenman et al., 2003; Pilz et al., 2013; Kelly et al., 2018; van Heusden et al., 2021). Although a recent study has started to provide insight into the contribution of the apically dividing progenitors to the striatal cellular and circuit organization (van Heusden et al., 2021), much less is known about the contribution of other more basally dividing progenitors. It has been proposed that the number and relative proportion of basal progenitors are responsible for the extensive growth of the neocortex in humans (LaMonica et al., 2013; Florio and Huttner, 2014; Lewitus et al., 2014) and have underpinned an evolutionary step driving our unique cognitive abilities. However, bIPs may not solely exist to increase brain size, but instead offer alternative contributions to the development of cortical as well as striatal circuits.
In the striatum, the striosome and matrix compartments differ substantially in size, but their approximate 1:4 size ratio is conserved across many mammalian species (Brimblecombe and Cragg, 2017). The findings that distinct IP types with different neurogenic capacities are fate restricted to generate SPNs destined for either striosome or matrix compartments provide a plausible explanation for this observation (Kelly et al., 2018). Indeed, Kelly et al. (2018) demonstrated that larger numbers of bIPs are generated from RGCs during a long, late phase in embryogenesis and that individual bIPs undergo more rounds of transitory amplification compared with early aIPs. This results in early aIPs generating many of the striosome SPNs, and later bIPs generating many of the matrix SPNs (Fig. 2A,B). Moreover, these authors demonstrated that the progeny of distinct types of bIPs at various stages of embryonic development inhabit distinct matrix compartments (Kelly et al., 2018), suggesting that bIP diversity can also inform the formation of distinct regions in striatum. What further properties, if any, are conveyed by bIPs is currently largely unknown.
In cortex, in utero electroporation of T-box brain protein 2 (Tbr2) Cre-recombinase constructs and fate mapping of their progeny made it possible to show that the cortical progeny of Tbr2+ bIPs had distinctive electrical and morphologic properties compared with neurons derived from other progenitors (Tyler et al., 2015). It might be possible to label bIPs in the LGE using similar approaches, as Tbr2 is embryonically expressed in the LGE (Kimura et al., 1999). Considering that the MGE gives rise to both striatal and cortical interneurons, it is possible that the mechanisms demonstrated for cortical neurogenesis can be extended to the striatum.
Interestingly, bIPs have been shown to selectively contribute to interneuron diversity (Petros et al., 2015). Indeed, apical progenitors appear to preferentially generate SST+ interneurons, whereas bIPs contribute to PV+ interneurons, confirming a distinct role for bIPs in the MGE (Fig. 2A). Especially during later stages of embryonic development, bIPs become the primary proliferative cells in both ganglionic eminences (Smart, 1976), and it will interesting to explore whether they might convey further characteristics related to cell identity, synaptic connectivity, and/or intrinsic electrical properties.
Do diverse embryonic progenitor types map onto defined postnatal neuron populations?
The advent of single-cell RNA sequencing (sc-RNAseq) technology has provided great insight into the vast diversity of postnatal neurons in the brain, including the striatum (Table 1). Indeed, this has provided evidence for SPN types beyond the classical distinction of dSPNs and iSPNs (Saunders et al., 2018; Martin et al., 2019), additional subdivisions within the dSPNs or iSPNs (Gokce et al., 2016; Zeisel et al., 2018; Stanley et al., 2020), and gradients of transcriptional heterogeneity correlating with SPN position in the striatum (Stanley et al., 2020). Whether and to what extent this great diversity of SPN types maps onto distinct embryonic progenitor pools is largely unknown. It has been shown that different progenitor pools in LGE, such as aIP and OP (van Heusden et al., 2021), and bIP (Kelly et al., 2018), can each generate both dSPNs and iSPNs. It seems that dSPN and iSPN share common progenitors (i.e., both AP and BP) and that lineage commitment is established during the postmitotic transition, as shown in humans as well (Bocchi et al., 2021). This suggests that factors beyond the embryonic pool of origin likely contribute to the generation of SPN transcriptional subtypes (Tepper et al., 1998; Lobo et al., 2006; Franco et al., 2012; Kelly et al., 2018; Anderson et al., 2020; Sharma et al., 2020). Many other factors could act on progenitors and young neurons, including epigenetic modifications (Yoon et al., 2018; Zahr et al., 2018; Telley et al., 2019), factors related to migration (Lim et al., 2018), or further differential transcription factor expression (Lu et al., 2014; Zhang et al., 2016; Bocchi et al., 2021), to prime or post-transcriptionally regulate protein expression (Nowakowski et al., 2013; Zahr et al., 2018; Li et al., 2020).
Despite the above findings, there is some early evidence linking transcriptionally defined cortical neurons to defined populations of embryonic progenitor (Ellender et al., 2019). In this study, the authors used a modified Patch-seq approach (Muñoz-Manchado et al., 2018; Mahfooz and Ellender, 2021; Table 1) to transcriptionally map aIP- and OP-derived cortical neurons to a published large-scale sc-RNAseq neuronal classification of cortex (Tasic et al., 2018). They found that the cortical aIPs, as defined by the selective expression of the Tα1 promoter during early development, were more restricted in the types of cortical neurons they generated than OPs, which consisted of a more heterogeneous population of progenitors (Ellender et al., 2019). This restricted output from aIPs supports the idea that intermediate progenitors emerged to increase the representation of particular postmitotic cell types (Martínez-Cerdeño et al., 2006; Tyler and Haydar, 2013; Taverna et al., 2014; Guillamon-Vivancos et al., 2019) and also supports the idea that VZ neuronal progenitors can exhibit different degrees of lineage restriction (Franco et al., 2012; Gil-Sanz et al., 2015; Llorca et al., 2019). At the same time, as aIPs are derived from RGCs, these findings are compatible with a general model in which a single neuronal progenitor cell type ultimately gives rise to the full complement of excitatory cortical neuronal cell types (Franco and Müller, 2013; Taverna et al., 2014). Last, the data indicate that multiple excitatory progenitor pools, and intermediate progenitor pools in particular, have not simply evolved to expand brain structure volume, but can also contribute to cell diversity.
How progenitor cell diversity in the MGE shapes interneuron transcriptional diversity in the mature brain has been a long-standing question in neural development. Of particular interest is whether a single MGE-derived progenitor can generate both striatal and cortical interneurons (Reid et al., 1995; Reid and Walsh, 2002). As previously described, postmitotic transcriptional switches such as Nkx2.1 can determine cortical versus striatal fate (Villar-Cerviño et al., 2015); but it is not known whether this is predetermined at a progenitor level. Currently, two distinct models have been suggested. In one model, across its proliferative life span, a single progenitor can generate both cortical and striatal fated neurons, which, when mature, can have vastly different functional properties (McConnell and Kaznowski, 1991; Desai and McConnell, 2000; Llorca et al., 2019). Alternatively, a single progenitor may be fate locked to the generation of either striatal or cortical cells. In this latter paradigm, progenitor cell diversity directly drives neuron heterogeneity (Franco et al., 2012; Garcia-Moreno and Molnar, 2015; Fig. 3). Both possible mechanisms raise questions. For example, if cortical and striatal interneurons are derived from different progenitors, are these progenitors spatially segregated within the VZ of the MGE (Flames et al., 2007; Mi et al., 2018)? Or are they randomly distributed, with a stochastic system of probabilistic decisions delineating striatal from cortical MGE-derived interneurons, as has been shown for excitatory neurons in the cortex (Llorca et al., 2019; Klingler and Jabaudon, 2020). Alternatively, specific molecules could separate progenitors giving rise to both striatal and cortical interneurons. For example, the ETV1/Er81 transcription factor is expressed from E10.5 in the MGE and segregates subtypes of progenitor cells in the VZ and SVZ. It has been shown to play a critical role during development, impacting several properties, including neuronal identity (Flames et al., 2007; Doitsidou et al., 2013) and excitability of cortical (Dehorter et al., 2015), as well as striatal interneurons (Ahmed et al., 2021). However, how the ETV1/Er81 transcription factor relates to the distinct progenitor cells discussed so far is largely unknown. It would be interesting to further investigate whether this specific molecule dictates MGE-derived cell fate and participates in the emergence of functional diversity within the striatum.
The question of transcriptional identity is closely related to the more general question: to what extent is embryonic progenitor diversity related to neuronal diversity (Fig. 3)? Because cortical development has been studied to a greater extent than that of other brain regions (including striatum), it may provide some insight into this question. Recently, it was shown that the progenitors that give rise to cortical pyramidal neurons follow a stochastic system of differentiation, wherein their random exposure to different developmental cues differentiates subsequent cellular properties (Llorca et al., 2019; Klingler and Jabaudon, 2020). Indeed, stochastic modeling could predict the clonal size, spatial distribution, and volumetric heterogeneity of cortical pyramidal neurons. This model provides an explanation for how diverse progeny can arise from a relatively homogenous group of progenitors (Klingler and Jabaudon, 2020). However, a completely homogeneous population of progenitors that followed a stochastic mechanism did not fully explain all experimental observations. Indeed, the authors had to trace the progeny from two distinct progenitors, which could then accurately predict the laminar position and their clonal size. This implies that even under a stochastic system, having multiple types of progenitor cells in the embryonic brain is required to generate the required cellular diversity of the postnatal brain (Llorca et al., 2019). Longitudinal sc-RNAseq studies encompassing extended periods of perinatal development, as recently achieved for cortical structures (Di Bella et al., 2021; La Manno et al., 2021), will allow for deeper probing of these questions.
What controls the local connectivity among striatal neurons?
We previously discussed how embryonic progenitor origin generates biases in the long-range excitatory connectivity from different cortical regions onto SPNs and could contribute to the generation of separate functional striatal pathways. Within the striatum, the SPNs (and associated interneurons) form local inhibitory synapses with which they regulate each other's activity; these have also been shown to be selective and biased. For example, iSPNs form more frequent and stronger synaptic connections than dSPNs (Taverna et al., 2008; Planert et al., 2010; Chuhma et al., 2011; Cepeda et al., 2013; Burke et al., 2017; Krajeski et al., 2019), and fast-spiking interneurons make more frequent connections onto dSPNs than onto iSPNs (Gittis et al., 2010; Planert et al., 2010). What rules govern these observed biases in local inhibitory connectivity? Is there evidence for involvement of progenitors?
A recent study investigated whether embryonic progenitor origin affected the strength of local inhibitory connections among SPNs and found no evidence (van Heusden et al., 2021). In this study, an optogenetic circuit-mapping approach was used to study the strength of inhibitory synaptic connections from aIP-derived SPNs to either aIP-derived or OP-derived SPNs and found no difference in their strength. Instead of progenitor origin, the birth dates of SPNs influenced the strength of connections, in that SPNs tended to form strong inhibitory synaptic connections with SPNs born during similar stages of neurogenesis, over and above SPNs born at other developmental stages (van Heusden et al., 2021; Fig. 2B). This is in contrast to recent findings in cortex where embryonic progenitor origin was shown to impact the incidence of local synaptic connectivity among the excitatory neurons in both layer 4 and layer 2/3 of the somatosensory cortex (Ellender et al., 2019). Here, the authors demonstrated that neurons tended to make preferential synaptic connections with other neurons derived from a different embryonic progenitor pool (Ellender et al., 2019).
The results in striatum described above are a first indication of increased interactions among SPNs with similar birth dates, but they do not provide insight into the emergence of preferred connectivity between dSPNs and iSPNs. It is known that the preferred connectivity patterns between SPNs emerge early in postnatal development (Krajeski et al., 2019), suggesting that they could result from synaptic plasticity driven by early neural activity (Cinotti and Humphries, 2021; Lopez-Huerta et al., 2021) and neuromodulation (Goffin et al., 2010). Regarding striatal interneurons, a recent study revealed that in the absence of the Er81 transcription factor, striatal CINs shifted toward less PV–CIN and CIN–CIN synaptic connections (Ahmed et al., 2021). Considering the MGE contains a population of progenitors expressing Er81, it is possible that the cholinergic interneurons derived from these progenitors are fated to a specific connectivity pattern.
Embryonic progenitors and striatal pathology.
Understanding the role of embryonic progenitors in relation to striatal development has the potential to further our understanding of striatal dysfunction in both neurodevelopmental and neurodegenerative disorders. Indeed, recent evidence suggests that defects in the division and differentiation of these progenitors are associated with diseases such as Huntington's disease and autism spectrum disorder.
The earliest symptoms of HD are often subtle, including problems with mood or cognition; these are followed by a general lack of coordination and an unsteady gait. As the disease advances, uncoordinated, involuntary body movements worsen. The cause of HD is typically genetic: a mutation in the huntingtin gene (HTT) is inherited from an affected parent (Barnat et al., 2020) or arises from de novo mutations. The resulting mutant protein (mHtt) leads to the eventual death of striatal cells, particularly affecting the iSPNs (Zheng and Kozloski, 2017). Recent findings have suggested that mHtt can affect progenitor cells during embryonic periods (Wiatr et al., 2018; Barnat et al., 2020). Indeed, using an HD mouse model, it was shown that mHtt affects levels of neurogenesis and can result in increased numbers of embryonic progenitors (Lorincz and Zawistowski, 2009), something that also has been observed in postmortem samples from humans with HD (Curtis et al., 2003). More recently, it was established that these mutations also severely affect the developing cortex, causing mislocalization of both mHtt and junctional complex proteins, defects in embryonic progenitor cell polarity and differentiation, abnormal ciliogenesis, and changes in mitosis and cell cycle progression in both humans and mice (Barnat et al., 2020). In addition, there are suggestions that mature striosomes exhibit increased vulnerability in HD (Hedreen and Folstein, 1995; Friedman et al., 2020); given that striosomal SPNs are generated mainly from aIPs during early stages of neurogenesis (Kelly et al., 2018), the selective impact of mutations in HTT in this population of progenitors could be interesting to study and to test novel treatments (Lin et al., 2015). Together, these recent findings suggest that HD has a substantial neurodevelopmental component and is not solely a neurodegenerative disorder. See also a recent review on altered striatal development in HD (Lebouc et al., 2020).
ASD is a group of neurodevelopmental pathologies that cause significant social and communication challenges and restrictive/repetitive behaviors. Evidence from human postmortem brain studies (Cheffer et al., 2020) and human-derived iPSCs identify early embryonic development as a critical period for this disorder (Cheffer et al., 2020; Griesi-Oliveira et al., 2021; Hohmann et al., 2020). Stem cells derived from people with autism show higher rates of proliferation (Cheffer et al., 2020; Adhya et al., 2021), reduced differentiation potential, and a different genetic profile than those from control donors (Grunwald et al., 2019; Shen et al., 2019; Wang et al., 2020; Adhya et al., 2021). Recent whole-exome sequencing studies of ASD risk genes have shed light on the critical importance of interneurons in ASD etiology (Satterstrom et al., 2020). For example, striatal interneurons show reduced expression of postmitotic neural differentiation factors (Close et al., 2012), including SATB Homeobox 1 (SatB1), which regulates the survival of SST+ and PV+ postmitotic interneurons (Close et al., 2012), and Ephrin type-B receptor 1 (Ephb1), a regulator of striatal and cortical interneuron migration (Villar-Cerviño et al., 2015). Although interneuron numbers might normalize during development, the early alterations can lead to long-lasting changes in neuronal circuit function that affect behavior (Magno et al., 2021). Further work is necessary to directly attribute early alterations in neural progenitor cells and neural circuit formation to the disease mechanisms in ASD.
These studies highlight a clear role for embryonic progenitors in two different disorders and suggest that further research is needed into the impact of the altered behavior of progenitors on the developing brain. One opportunity is the growing use of in vitro models to further dissect disease mechanism and etiology. Despite the limitations (e.g., reproducibility, scalability, and long-term survival; Quesnel-Vallieres et al., 2019; Wang et al., 2020; Pintacuda et al., 2021), the “disease-in-a-dish” approach allows for precisely timed analyses and offers an opportunity to further probe the cellular and molecular alterations in brain development in health and disease (Chan et al., 2020). Stem cell-derived model systems, such as three-dimensional organoids (Di Lullo and Kriegstein, 2017; Pollen et al., 2019), air–liquid interface cerebral organoids from mouse or human iPSCs (Giandomenico et al., 2019), and combining different organoids in “assembloids” (Miura et al., 2020) have opened new experimental avenues for investigating aspects of development and pathology of the human brain (Table 1). Notably, determining how brain cells derived from diverse human genetic backgrounds respond to specific drugs might ultimately allow for personalized medicine approaches for disorders such as HD and ASD (Mariani et al., 2015; Maussion et al., 2019; Wang et al., 2020).
Progenitors supporting neurologic restoration.
As outlined above, remarkable progress has been made in our understanding of progenitors, stem cells, and their progeny, allowing us to shape progenitor cell development to generate many functional mature neural cell types (Arber et al., 2015). A major objective is to reproduce the maturation steps of brain cells and provide new insights into the pathophysiology of various disorders in vitro (Tyson and Anderson, 2014; Mariani et al., 2015; Noakes et al., 2019; Comella-Bolla et al., 2020; Wang et al., 2020). A further objective is to harness this knowledge and develop new cell-based treatment options, including cell transplantation, which would allow for restoration (or modulation) of neural circuit defects in brain disorders. Below, we highlight a few recent articles and would like to refer also this recent review (Bjorklund and Parmar, 2020).
So far, transplantation studies of embryonic progenitor cells in animals and humans have generated some positive results with regard to the ability of delivering cells that become functionally integrated into the postnatal brain. For example, it has been shown that isolated E12.5 to E13.5 or E14.5 MGE progenitor cells can differentiate into interneurons and integrate into early postnatal circuits (Alvarez-Dolado et al., 2006; Martínez-Cerdeño et al., 2010). Moreover, transplantation of embryonic progenitor cells into the postnatal brain has been successfully trialed in preclinical models as potential replacement strategies for the treatment of disorders such as Parkinson's disease and epilepsy (Martínez-Cerdeño et al., 2010; Hunt and Baraban, 2015; Upadhya et al., 2019; Doi et al., 2020; Guo et al., 2021). However, accurate programming of induced cells into specific progenitors, striatal neurons, or mixtures of neurons (Reddington et al., 2014) is likely critical when considering cell transplantation as a possible treatment option for HD or ASD and other disorders.
Directing human stem cells into specific neuronal types is complex and will require accurate differentiation protocols that mimic endogenous neuronal development, integrating aspects of cell maturation (e.g., morphology and electrical properties) and circuit formation (Fig. 3C). Indeed, this will also likely require consideration of the distinct transcriptional programs and developmental sequential events that guide newborn neurons (Telley et al., 2016; Vitali et al., 2018). Recent work has started to examine the properties of human pluripotent stem cells (hPSCs) grafted onto the postnatal mouse (Comella-Bolla et al., 2020) and rat (Noakes et al., 2019) striatum. The cells adopted cellular profiles similar to those found in the human striatum (Table 1). In the latter study, CR+ interneurons were the predominant cells; CINs, while present within the graft, were absent in the in vitro culture; and SST+ and PV+ cells, originally absent in the graft, were detected in the culture. Potential reasons for the differences in cellular composition between the graft and cell culture could be a subtype-dependent survival bias or environment-driven redirection of interneuron fate, creating a shift in the subtype composition to match the region where the cells were grafted (Quattrocolo et al., 2017). Further elucidation of the survival and subtype composition achieved by the grafts is necessary to shed light on the relative influence of intrinsic and extrinsic cues on neuronal fate. The degree of fate commitment present at the progenitor stage could potentially be tested by transplanting hPSC-derived progenitors from a specific ganglionic eminence into the neonatal striatum using neuron type-specific hPSC reporter lines or reprogramming of endogenous cells into neurons (Weinberg et al., 2017). Recent sequencing of mouse (Mayer et al., 2018; Mi et al., 2018; Loo et al., 2019) and human striatal progenitors and young neurons (Bocchi et al., 2021) has provided insight into their lineages and can facilitate the development and the efficacy of cell replacement, showing great potential to improve therapeutic avenues.
Conclusions
The postnatal striatum is a highly complex brain structure with multiple levels of organization, some aspects of which, as outlined in this review, are related to embryonic progenitor cell origin. Here, we highlighted recent studies delineating the crucial importance of progenitor origin in shaping the spatial position, cellular identity, and synaptic connectivity of both striatal spiny projection neurons and interneurons during development. Understanding these novel roles of diverse embryonic progenitors in shaping striatal development provides a useful framework through which to view the vast complexity of neuronal circuits in the postnatal brain, and it can help shape future research directions and the development of cell-based therapies.
Footnotes
This work is supported through an Australian Government Research Training Program scholarship and the Peter Gauge Memorial Supplementary Scholarship from The Australian National University to R.K.; National Health and Medical Research Council Project Grant APP1144145 to N.D.; and Medical Resarch Council Career Development Award MR/M009599/1 to T.E.
The authors declare no competing financial interests.
References
- Adhya D, Swarup V, Nagy R, Dutan L, Shum C, Valencia-Alarcon EP, Jozwik KM, Mendez MA, Horder J, Loth E, Nowosiad P, Lee I, Skuse D, Flinter FA, Murphy D, McAlonan G, Geschwind DH, Price J, Carroll J, Srivastava DP, et al. (2021) Atypical neurogenesis in induced pluripotent stem cells from autistic individuals. Biol Psychiatry 89:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed NY, Knowles R, Dehorter N (2019) New insights into cholinergic neuron diversity. Front Mol Neurosci 12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed NY, Ranjbar-Slamloo Y, Abed ASA, Gao L, Sontani Y, H'Cheo-Gauthier AR, Arabzadeh E, Dehorter N (2021) Er81 transcription factor fine-tunes striatal cholinergic interneuron activity and drives habit formation. J Neurosci 41:4392–4409. 10.1523/JNEUROSCI.0967-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JL, Alvarez-Buylla A, Baraban SC (2006) Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci 26:7380–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemori K, Gibb LG, Graybiel AM (2011) Shifting responsibly: the importance of striatal modularity to reinforcement learning in uncertain environments. Front Hum Neurosci 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AG, Kulkarni A, Harper M, Konopka G (2020) Single-cell analysis of Foxp1-driven mechanisms essential for striatal development. Cell Rep 30:3051–3066.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL (1997) Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 19:27–37. 10.1016/S0896-6273(00)80345-1 [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL (2001) Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128:353–363. [DOI] [PubMed] [Google Scholar]
- Andrews WD, Barber M, Nemitz M, Memi F, Parnavelas JG (2017) Semaphorin3A-neuropilin1 signalling is involved in the generation of cortical interneurons. Brain Struct Funct 222:2217–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TE, Heintz N (2008) Genetic lineage tracing defines distinct neurogenic and gliogenic stages of ventral telencephalic radial glial development. Neural Dev 3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodriguez TA, Rosser AE, Dunnett SB, Li M (2015) Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development 142:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Dautan D, Tepper JM, Mena-Segovia J (2019) Pedunculopontine glutamatergic neurons provide a novel source of feedforward inhibition in the striatum by selectively targeting interneurons. J Neurosci 39:4727–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart MR, Neufeld SQ, Wong NC, Sabatini BL (2015) Enkephalin disinhibits mu opioid receptor-rich striatal patches via delta opioid receptors. Neuron 88:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnat M, Capizzi M, Aparicio E, Boluda S, Wennagel D, Kacher R, Kassem R, Lenoir S, Agasse F, Braz BY, Liu JP, Ighil J, Tessier A, Zeitlin SO, Duyckaerts C, Dommergues M, Durr A, Humbert S (2020) Huntington's disease alters human neurodevelopment. Science 369:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA (1984) Neurogenesis in the rat neostriatum. Int J Dev Neurosci 2:163–175. 10.1016/0736-5748(84)90008-X [DOI] [PubMed] [Google Scholar]
- Beattie R, Postiglione MP, Burnett LE, Laukoter S, Streicher C, Pauler FM, Xiao G, Klezovitch O, Vasioukhin V, Ghashghaei TH, Hippenmeyer S (2017) Mosaic analysis with double markers reveals distinct sequential functions of Lgl1 in neural stem cells. Neuron 94:517–533.e3. [DOI] [PubMed] [Google Scholar]
- Bernacer J, Prensa L, Gimenez-Amaya JM (2012) Distribution of GABAergic interneurons and dopaminergic cells in the functional territories of the human striatum. PLoS One 7:e30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Parmar M (2020) Neuronal replacement as a tool for basal ganglia circuitry repair: 40 years in perspective. Front Cell Neurosci 14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi VD, Conforti P, Vezzoli E, Besusso D, Cappadona C, Lischetti T, Galimberti M, Ranzani V, Bonnal RJP, De Simone M, Rossetti G, He X, Kamimoto K, Espuny-Camacho I, Faedo A, Gervasoni F, Vuono R, Morris SA, Chen J, Felsenfeld D, et al. (2021) The coding and long noncoding single-cell atlas of the developing human fetal striatum. Science 372:eabf5759. 10.1126/science.abf5759 [DOI] [PubMed] [Google Scholar]
- Bogacz R, Gurney K (2007) The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput 19:442–477. 10.1162/neco.2007.19.2.442 [DOI] [PubMed] [Google Scholar]
- Brimblecombe KR, Cragg SJ (2015) Substance P weights striatal dopamine transmission differently within the striosome-matrix axis. J Neurosci 35:9017–9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Cragg SJ (2017) The striosome and matrix compartments of the striatum: a path through the labyrinth from neurochemistry toward function. ACS Chem Neurosci 8:235–242. [DOI] [PubMed] [Google Scholar]
- Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, Ding L, Lopez-Cruz A, Saur D, Anderson SA, Huang K, Shi SH (2011) Clonal production and organization of inhibitory interneurons in the neocortex. Science 334:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DA, Rotstein HG, Alvarez VA (2017) Striatal local circuitry: a new framework for lateral inhibition. Neuron 96:267–284. 10.1016/j.neuron.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G (2005) The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48:591–604. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G (2008) The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron 59:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Galvan L, Holley SM, Rao SP, Andre VM, Botelho EP, Chen JY, Watson JB, Deisseroth K, Levine MS (2013) Multiple sources of striatal inhibition are differentially affected in Huntington's disease mouse models. J Neurosci 33:7393–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Griffiths R, Price DJ, Mason JO (2020) Cerebral organoids as tools to identify the developmental roots of autism. Mol Autism 11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheffer A, Flitsch LJ, Krutenko T, Roderer P, Sokhranyaeva L, Iefremova V, Hajo M, Peitz M, Schwarz MK, Brustle O (2020) Human stem cell-based models for studying autism spectrum disorder-related neuronal dysfunction. Mol Autism 11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chatterjee M, Li JY (2010) The mouse homeobox gene Gbx2 is required for the development of cholinergic interneurons in the striatum. J Neurosci 30:14824–14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Lu KM, Ko HA, Huang TH, Hao JH, Yan YT, Chang SL, Evans SM, Liu FC (2020) Parcellation of the striatal complex into dorsal and ventral districts. Proc Natl Acad Sci U S A 117:7418–7429. 10.1073/pnas.1921007117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Friedman BA, Ha C, Durinck S, Liu J, Rubenstein JL, Seshagiri S, Modrusan Z (2017) Single-cell RNA sequencing identifies distinct mouse medial ganglionic eminence cell types. Sci Rep 7:45656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S (2011) Functional connectome of the striatal medium spiny neuron. J Neurosci 31:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marin O (2013) Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci 16:1199–1210. [DOI] [PubMed] [Google Scholar]
- Cinotti F, Humphries MD (2021) Bayesian mapping of the striatal microcircuit reveals robust asymmetries in the probabilities and distances of connections. bioRxiv. Advance online publication. Retrieved October 4, 2021. 10.1101/2021.06.08.447507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, Rudy B, Fishell G (2012) Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci 32:17690–17705. 10.1523/JNEUROSCI.3583-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocas LA, Miyoshi G, Carney RS, Sousa VH, Hirata T, Jones KR, Fishell G, Huntsman MM, Corbin JG (2009) Emx1-lineage progenitors differentially contribute to neural diversity in the striatum and amygdala. J Neurosci 29:15933–15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comella-Bolla A, Orlandi JG, Miguez A, Straccia M, Garcia-Bravo M, Bombau G, Galofre M, Sanders P, Carrere J, Segovia JC, Blasi J, Allen ND, Alberch J, Soriano J, Canals JM (2020) Human pluripotent stem cell-derived neurons are functionally mature in vitro and integrate into the mouse striatum following transplantation. Mol Neurobiol 57:2766–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G (2000) The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development 127:5007–5020. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G (2001) Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci 4:1177–1182. 10.1038/nn749 [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM (2011) Basal ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat 5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Lacey CJ, Weng FJ, Garrison CE, Gibson DJ, Lin Y, Graybiel AM (2017) Striatal cholinergic interneurons modulate spike-timing in striosomes and matrix by an amphetamine-sensitive mechanism. Front Neuroanat 11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM (2013) Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL (2003) Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A 100:9023–9027. 10.1073/pnas.1532244100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das GD, Altman J (1970) Postnatal neurogenesis in the caudate nucleus and nucleus accumbens septi in the rat. Brain Res 21:122–127. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ (2008) Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci 28:11603–11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carlos JA, Lopez-Mascaraque L, Valverde F (1996) Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci 16:6146–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW, Pakzaban P, Isacson O (1994) The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: neural transplantation and developmental evidence. Brain Res 668:211–219. 10.1016/0006-8993(94)90526-6 [DOI] [PubMed] [Google Scholar]
- Dehorter N, Ciceri G, Bartolini G, Lim L, del Pino I, Marín O (2015) Tuning of fast-spiking interneuron properties by an activity-dependent transcriptional switch. Science 349:1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AR, McConnell SK (2000) Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development 127:2863–2872. [DOI] [PubMed] [Google Scholar]
- Di Bella DJ, Habibi E, Stickels RR, Scalia G, Brown J, Yadollahpour P, Yang SM, Abbate C, Biancalani T, Macosko EZ, Chen F, Regev A, Arlotta P (2021) Molecular logic of cellular diversification in the mouse cerebral cortex. Nature 595:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lullo E, Kriegstein AR (2017) The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Larvin JT, Duffell JM, Garas FN, Doig NM, Kessaris N, Duguid IC, Bogacz R, Butt SJ, Magill PJ (2015) Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, Amano N, Nomura M, Umekage M, Morizane A, Takahashi J (2020) Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun 11:3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsidou M, Flames N, Topalidou I, Abe N, Felton T, Remesal L, Popovitchenko T, Mann R, Chalfie M, Hobert O (2013) A combinatorial regulatory signature controls terminal differentiation of the dopaminergic nervous system in C. elegans. Genes Dev 27:1391–1405. 10.1101/gad.217224.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorst MC, Tokarska A, Zhou M, Lee K, Stagkourakis S, Broberger C, Masmanidis S, Silberberg G (2020) Polysynaptic inhibition between striatal cholinergic interneurons shapes their network activity patterns in a dopamine-dependent manner. Nat Commun 11:5113. 10.1038/s41467-020-18882-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA (2004) Neuronal circuits of the neocortex. Annu Rev Neurosci 27:419–451. [DOI] [PubMed] [Google Scholar]
- Ehrman LA, Mu X, Waclaw RR, Yoshida Y, Vorhees CV, Klein WH, Campbell K (2013) The LIM homeobox gene Isl1 is required for the correct development of the striatonigral pathway in the mouse. Proc Natl Acad Sci U S A 110:E4026–E4035. 10.1073/pnas.1308275110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstat DD, Liu JK, Mione M, Zhong W, Yu G, Anderson SA, Ghattas I, Puelles L, Rubenstein JL (1999) DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol 414:217–237. [DOI] [PubMed] [Google Scholar]
- Ellender TJ, Avery SV, Mahfooz K, Scaber J, von Klemperer A, Nixon SL, Buchan MJ, van Rheede JJ, Gatti A, Waites C, Pavlou HJ, Sims D, Newey SE, Akerman CJ (2019) Embryonic progenitor pools generate diversity in fine-scale excitatory cortical subnetworks. Nat Commun 10:5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koos T (2011) GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci 15:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O (2007) Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci 27:9682–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Kimura S, Rubenstein JL (2010) The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci 30:2812–2823. 10.1523/JNEUROSCI.4228-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio M, Huttner WB (2014) Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141:2182–2194. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Müller U (2013) Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron 77:19–34. 10.1016/j.neuron.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Müller U (2012) Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 337:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, Riad MH, Graybiel AM (2015) A corticostriatal path targeting striosomes controls decision-making under conflict. Cell 161:1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Hueske E, Drammis SM, Toro Arana SE, Nelson ED, Carter CW, Delcasso S, Rodriguez RX, Lutwak H, DiMarco KS, Zhang Q, Rakocevic LI, Hu D, Xiong JK, Zhao J, Gibb LG, Yoshida T, Siciliano CA, Diefenbach TJ, Ramakrishnan C, et al. (2020) Striosomes mediate value-based learning vulnerable in age and a huntington's disease model. Cell 183:918–934.e49. 10.1016/j.cell.2020.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T (2011) Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci 33:668–677. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF (2006) Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci 26:1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, Mailly P, De Bundel D, de Kerchove d'Exaerde A, Hervé D, Girault JA, Valjent E, Krieger P (2013) Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front Neural Circuits 7:124. 10.3389/fncir.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garas FN, Shah RS, Kormann E, Doig NM, Vinciati F, Nakamura KC, Dorst MC, Smith Y, Magill PJ, Sharott A (2016) Secretagogin expression delineates functionally-specialized populations of striatal parvalbumin-containing interneurons. Elife 5:e16088. 10.7554/eLife.16088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garas FN, Kormann E, Shah RS, Vinciati F, Smith Y, Magill PJ, Sharott A (2018) Structural and molecular heterogeneity of calretinin-expressing interneurons in the rodent and primate striatum. J Comp Neurol 526:877–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Moreno F, Molnar Z (2015) Subset of early radial glial progenitors that contribute to the development of callosal neurons is absent from avian brain. Proc Natl Acad Sci U S A 112:E5058–E5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marin O (2011) A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci 31:16570–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nóbrega-Pereira S, Pierani A, Kessaris N, Marín O (2009) The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci 29:9380–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR (1984) The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311:461–464. 10.1038/311461a0 [DOI] [PubMed] [Google Scholar]
- Gerfen CR (1989) The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science 246:385–388. 10.1126/science.2799392 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ (2008) Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci 28:10814–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, Lakatos A, Lancaster MA (2019) Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci 22:669–679. 10.1038/s41593-019-0350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sanz C, Espinosa A, Fregoso SP, Bluske KK, Cunningham CL, Martinez-Garay I, Zeng H, Franco SJ, Müller U (2015) Lineage tracing using Cux2-Cre and Cux2-CreERT2 Mice. Neuron 86:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30:2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Leventhal DK, Fensterheim BA, Pettibone JR, Berke JD, Kreitzer AC (2011) Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci 31:15727–15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin D, Ali AB, Rampersaud N, Harkavyi A, Fuchs C, Whitton PS, Nairn AC, Jovanovic JN (2010) Dopamine-dependent tuning of striatal inhibitory synaptogenesis. J Neurosci 30:2935–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, Rothwell PE, Fuccillo MV, Sudhof TC, Quake SR (2016) Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-Seq. Cell Rep 16:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788. 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- Govindan S, Oberst P, Jabaudon D (2018) In vivo pulse labeling of isochronic cohorts of cells in the central nervous system using FlashTag. Nat Protoc 13:2297–2311. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Mulder N, Saunders A, Sabatini BL (2016) Cotransmission of acetylcholine and GABA. Neuropharmacology 100:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13:244–254. 10.1016/0166-2236(90)90104-I [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Hickey TL (1982) Chemospecificity of ontogenetic units in the striatum: demonstration by combining [3H]thymidine neuronography and histochemical staining. Proc Natl Acad Sci U S A 79:198–202. 10.1073/pnas.79.1.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW Jr (1978) Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A 75:5723–5726. 10.1073/pnas.75.11.5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M (1994) The basal ganglia and adaptive motor control. Science 265:1826–1831. 10.1126/science.8091209 [DOI] [PubMed] [Google Scholar]
- Griesi-Oliveira K, Fogo MS, Pinto BGG, Alves AY, Suzuki AM, Morales AG, Ezquina S, Sosa OJ, Sutton GJ, Sunaga-Franze DY, Bueno AP, Seabra G, Sardinha L, Costa SS, Rosenberg C, Zachi EC, Sertie AL, Martins-de-Souza D, Reis EM, Voineagu I, et al. (2021) Transcriptome of iPSC-derived neuronal cells reveals a module of co-expressed genes consistently associated with autism spectrum disorder. Mol Psychiatry 26:1589–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA (2005) Mechanisms for selection of basic motor programs–roles for the striatum and pallidum. Trends Neurosci 28:364–370. [DOI] [PubMed] [Google Scholar]
- Grunwald L-M, Stock R, Haag K, Buckenmaier S, Eberle M-C, Wildgruber D, Storchak H, Kriebel M, Weißgraeber S, Mathew L, Singh Y, Loos M, Li KW, Kraushaar U, Fallgatter AJ, Volkmer H (2019) Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with schizophrenia and autism. Transl Psychiatry 9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamon-Vivancos T, Tyler WA, Medalla M, Chang WW, Okamoto M, Haydar TF, Luebke JI (2019) Distinct neocortical progenitor lineages fine-tune neuronal diversity in a layer-specific manner. Cereb Cortex 29:1121–1138. 10.1093/cercor/bhy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wang D, He X, Feng Q, Lin R, Xu F, Fu L, Luo M (2015) Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PLoS One 10:e0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Tang L, Tang X (2021) Current developments in cell replacement therapy for Parkinson's disease. Neuroscience 463:370–382. [DOI] [PubMed] [Google Scholar]
- Haber S (2008) Parallel and integrative processing through the basal ganglia reward circuit: lessons from addiction. Biol Psychiatry 64:173–174. 10.1016/j.biopsych.2008.05.033 [DOI] [PubMed] [Google Scholar]
- Halliday AL, Cepko CL (1992) Generation and migration of cells in the developing striatum. Neuron 9:15–26. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Goto S, Nishikawa S, Ushio Y (2003) Neuronal cell migration for the developmental formation of the mammalian striatum. Brain Res Brain Res Rev 41:1–12. [DOI] [PubMed] [Google Scholar]
- Harwell CC, Fuentealba LC, Gonzalez-Cerrillo A, Parker PR, Gertz CC, Mazzola E, Garcia MT, Alvarez-Buylla A, Cepko CL, Kriegstein AR (2015) Wide dispersion and diversity of clonally related inhibitory interneurons. Neuron 87:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen JC, Folstein SE (1995) Early loss of neostriatal striosome neurons in Huntington's disease. J Neuropathol Exp Neurol 54:105–120. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB (1981) Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature 291:415–418. [DOI] [PubMed] [Google Scholar]
- Hintiryan H, Foster NN, Bowman I, Bay M, Song MY, Gou L, Yamashita S, Bienkowski MS, Zingg B, Zhu M, Yang XW, Shih JC, Toga AW, Dong HW (2016) The mouse cortico-striatal projectome. Nat Neurosci 19:1100–1114. 10.1038/nn.4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann SS, Ilieva M, Michel TM (2020) In vitro models for ASD-patient-derived iPSCs and cerebral organoids. Prog Mol Biol Transl Sci 173:355–375. [DOI] [PubMed] [Google Scholar]
- Hu JS, Vogt D, Lindtner S, Sandberg M, Silberberg SN, Rubenstein JLR (2017) Coup-TF1 and Coup-TF2 control subtype and laminar identity of MGE-derived neocortical interneurons. Development 144:2837–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, Mao T (2016) A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5:e19103. 10.7554/eLife.19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Baraban SC (2015) Interneuron transplantation as a treatment for epilepsy. Cold Spring Harb Perspect Med 5:a022376. 10.1101/cshperspect.a022376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Sandoval O, Xenias HS, Tepper JM, Koós T (2015) Dopaminergic and cholinergic modulation of striatal tyrosine hydroxylase interneurons. Neuropharmacology 95:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Simmons SK, Guo A, Shetty AS, Ko M, Nguyen L, Jokhi V, Robinson E, Oyler P, Curry N, Deangeli G, Lodato S, Levin JZ, Regev A, Zhang F, Arlotta P (2020) In vivo Perturb-Seq reveals neuronal and glial abnormalities associated with autism risk genes. Science 370:eaaz6063. 10.1126/science.aaz6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson Y, Silberberg G (2020) The functional organization of cortical and thalamic inputs onto five types of striatal neurons is determined by source and target cell identities. Cell Rep 30:1178–1194.e3. 10.1016/j.celrep.2019.12.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18:527–535. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Raudales R, He M, Lee JH, Kim Y, Gibb LG, Wu P, Matho K, Osten P, Graybiel AM, Huang ZJ (2018) Radial glial lineage progression and differential intermediate progenitor amplification underlie striatal compartments and circuit organization. Neuron 99:345–361.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khibnik LA, Tritsch NX, Sabatini BL (2014) A direct projection from mouse primary visual cortex to dorsomedial striatum. PLoS One 9:e104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Nakashima K, Ueno M, Kiyama H, Taga T (1999) A novel mammalian T-box-containing gene, Tbr2, expressed in mouse developing brain. Brain Res Dev Brain Res 115:183–193. [DOI] [PubMed] [Google Scholar]
- Kita H (1993) GABAergic circuits of the striatum. Prog Brain Res 99:51–72. 10.1016/s0079-6123(08)61338-2 [DOI] [PubMed] [Google Scholar]
- Klingler E, Jabaudon D (2020) Do progenitors play dice? Elife 9:e54042. 10.7554/eLife.54042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF (2009) Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex 19:2439–2450. 10.1093/cercor/bhn260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajeski RN, Macey-Dare A, van Heusden F, Ebrahimjee F, Ellender TJ (2019) Dynamic postnatal development of the cellular and circuit properties of striatal D1 and D2 spiny projection neurons. J Physiol 597:5265–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y (1993) Spatial distributions of chemically identified intrinsic neurons in relation to patch and matrix compartments of rat neostriatum. J Comp Neurol 332:499–513. [DOI] [PubMed] [Google Scholar]
- La Manno G, Siletti K, Furlan A, Gyllborg D, Vinsland E, Mossi Albiach A, Mattsson Langseth C, Khven I, Lederer AR, Dratva LM, Johnsson A, Nilsson M, Lönnerberg P, Linnarsson S (2021) Molecular architecture of the developing mouse brain. Nature 596:92–96. 10.1038/s41586-021-03775-x [DOI] [PubMed] [Google Scholar]
- LaMonica BE, Lui JH, Hansen DV, Kriegstein AR (2013) Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun 4:1665. 10.1038/ncomms2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR (2018) What, if anything, is rodent prefrontal cortex? eNeuro 5:ENEURO.0315-18.2018. 10.1523/ENEURO.0315-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TN, Zhou QP, Cobos I, Zhang S, Zagozewski J, Japoni S, Vriend J, Parkinson T, Du G, Rubenstein JL, Eisenstat DD (2017) GABAergic interneuron differentiation in the basal forebrain is mediated through direct regulation of glutamic acid decarboxylase isoforms by Dlx homeobox transcription factors. J Neurosci 37:8816–8829. 10.1523/JNEUROSCI.2125-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebouc M, Richard Q, Garret M, Baufreton J (2020) Striatal circuit development and its alterations in Huntington's disease. Neurobiol Dis 145:105076. [DOI] [PubMed] [Google Scholar]
- Lee J, Wang W, Sabatini BL (2020) Anatomically segregated basal ganglia pathways allow parallel behavioral modulation. Nat Neurosci 23:1388–1398. 10.1038/s41593-020-00712-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B (2010) The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30:16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein E, Borm LE, Linnarsson S (2017) The promise of spatial transcriptomics for neuroscience in the era of molecular cell typing. Science 358:64–69. [DOI] [PubMed] [Google Scholar]
- Lewitus E, Kelava I, Kalinka AT, Tomancak P, Huttner WB (2014) An adaptive threshold in mammalian neocortical evolution. PLoS Biol 12:e1002000. 10.1371/journal.pbio.1002000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Tyler WA, Zeldich E, Santpere Baro G, Okamoto M, Gao T, Li M, Sestan N, Haydar TF (2020) Transcriptional priming as a conserved mechanism of lineage diversification in the developing mouse and human neocortex. Sci Adv 6:eabd2068. 10.1126/sciadv.abd2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Pakan JMP, Selten MM, Marques-Smith A, Llorca A, Bae SE, Rochefort NL, Marín O (2018) Optimization of interneuron function by direct coupling of cell migration and axonal targeting. Nat Neurosci 21:920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Cho IT, Shi X, Grinspan JB, Cho G, Golden JA (2019) Arx expression suppresses ventralization of the developing dorsal forebrain. Sci Rep 9:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Yuan J, Sander B, Golas MM (2015) In vitro differentiation of human neural progenitor cells into striatal GABAergic neurons. Stem Cells Transl Med 4:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindtner S, Catta-Preta R, Tian H, Su-Feher L, Price JD, Dickel DE, Greiner V, Silberberg SN, McKinsey GL, McManus MT, Pennacchio LA, Visel A, Nord AS, Rubenstein JLR (2019) Genomic resolution of DLX-orchestrated transcriptional circuits driving development of forebrain GABAergic neurons. Cell Rep 28:2048–2063.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Ghattas I, Liu S, Chen S, Rubenstein JL (1997) Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dyn 210:498–512. [DOI] [PubMed] [Google Scholar]
- Llorca A, Ciceri G, Beattie R, Wong FK, Diana G, Serafeimidou-Pouliou E, Fernandez-Otero M, Streicher C, Arnold SJ, Meyer M, Hippenmeyer S, Maravall M, Marin O (2019) A stochastic framework of neurogenesis underlies the assembly of neocortical cytoarchitecture. Elife 8:e51381. 10.7554/eLife.51381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW (2006) FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci 9:443–452. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Yeh C, Yang XW (2008) Pivotal role of early B-cell factor 1 in development of striatonigral medium spiny neurons in the matrix compartment. J Neurosci Res 86:2134–2146. [DOI] [PubMed] [Google Scholar]
- Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JL (2009) Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol 512:556–572. 10.1002/cne.21854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo L, Simon JM, Xing L, McCoy ES, Niehaus JK, Guo J, Anton ES, Zylka MJ (2019) Single-cell transcriptomic analysis of mouse neocortical development. Nat Commun 10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes R, Verhey van Wijk N, Neves G, Pachnis V (2012) Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc Natl Acad Sci U S A 109:3119–3124. 10.1073/pnas.1109251109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Huerta VG, Denton JA, Nakano Y, Jaidar O, Garcia-Munoz M, Arbuthnott GW (2021) Striatal bilateral control of skilled forelimb movement. Cell Rep 34:108651. 10.1016/j.celrep.2020.108651 [DOI] [PubMed] [Google Scholar]
- Lorincz MT, Zawistowski VA (2009) Expanded CAG repeats in the murine Huntington's disease gene increases neuronal differentiation of embryonic and neural stem cells. Mol Cell Neurosci 40:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya N, Ben-Ari Y, Hammond C (2018) Striatal dual cholinergic/GABAergic transmission in Parkinson disease: friends or foes? Cell Stress 2:147–149. 10.15698/cst2018.06.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KM, Evans SM, Hirano S, Liu FC (2014) Dual role for Islet-1 in promoting striatonigral and repressing striatopallidal genetic programs to specify striatonigral cell identity. Proc Natl Acad Sci U S A 111:E168–E177. 10.1073/pnas.1319138111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Zhang Q, Cai Y, You Y, Rubenstein JL, Yang Z (2012) A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb Cortex 22:2120–2130. [DOI] [PubMed] [Google Scholar]
- Magno L, Barry C, Schmidt-Hieber C, Theodotou P, Häusser M, Kessaris N (2017) NKX2-1 is required in the embryonic septum for cholinergic system development, learning, and memory. Cell Rep 20:1572–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magno L, Asgarian Z, Pendolino V, Velona T, Mackintosh A, Lee F, Stryjewska A, Zimmer C, Guillemot F, Farrant M, Clark B, Kessaris N (2021) Transient developmental imbalance of cortical interneuron subtypes presages long-term changes in behavior. Cell Rep 35:109249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfooz K, Ellender TJ (2021) Combining whole-cell patch-clamp recordings with single-cell RNA sequencing. Methods Mol Biol 2188:179–189. [DOI] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F (2005) Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25:3857–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao M, Nair A, Augustine GJ (2019) A novel type of neuron within the dorsal striatum. Front Neural Circuits 13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, Gerstein M, Grigorenko EL, Chawarska K, Pelphrey KA, Howe JR, Vaccarino FM (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL (2001) A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2:780–790. [DOI] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL (2000) Origin and molecular specification of striatal interneurons. J Neurosci 20:6063–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Calvigioni D, Tzortzi O, Fuzik J, Warnberg E, Meletis K (2019) A spatiomolecular map of the striatum. Cell Rep 29:4320–4333. e4325. [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Kriegstein AR (2006) The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex 16:i152–161. 10.1093/cercor/bhk017 [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR (2010) Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell 6:238–250. 10.1016/j.stem.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Ibáñez R, Crespo E, Urbán N, Sergent-Tanguy S, Herranz C, Jaumot M, Valiente M, Long JE, Pineda JR, Andreu C, Rubenstein JLR, Marín O, Georgopoulos K, Mengod G, Fariñas I, Bachs O, Alberch J, Canals JM (2010) Ikaros-1 couples cell cycle arrest of late striatal precursors with neurogenesis of enkephalinergic neurons. J Comp Neurol 518:329–351. [DOI] [PubMed] [Google Scholar]
- Martín-Ibáñez R, Crespo E, Esgleas M, Urban N, Wang B, Waclaw R, Georgopoulos K, Martinez S, Campbell K, Vicario-Abejon C, Alberch J, Chan S, Kastner P, Rubenstein JL, Canals JM (2012) Helios transcription factor expression depends on Gsx2 and Dlx1&2 function in developing striatal matrix neurons. Stem Cells Dev 21:2239–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HA, Rakowiecki SM, Raftopoulou M, Nery S, Huang Y, Gridley T, Fishell G (2005) Notch signaling coordinates the patterning of striatal compartments. Development 132:4247–4258. [DOI] [PubMed] [Google Scholar]
- Matamales M, Gotz J, Bertran-Gonzalez J (2016) Quantitative imaging of cholinergic interneurons reveals a distinctive spatial organization and a functional gradient across the mouse striatum. PLoS One 11:e0157682. 10.1371/journal.pone.0157682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A, Graybiel AM (2020) Combinatorial developmental controls on striatonigral circuits. Cell Rep 31:107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maussion G, Rocha C, Bernard G, Beitel LK, Durcan TM (2019) Patient-derived stem cells, another in vitro model, or the missing link toward novel therapies for autism spectrum disorders? Front Pediatr 7:225. 10.3389/fped.2019.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Jaglin XH, Cobbs LV, Bandler RC, Streicher C, Cepko CL, Hippenmeyer S, Fishell G (2015) Clonally related forebrain interneurons disperse broadly across both functional areas and structural boundaries. Neuron 87:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Hafemeister C, Bandler RC, Machold R, Batista Brito R, Jaglin X, Allaway K, Butler A, Fishell G, Satija R (2018) Developmental diversification of cortical inhibitory interneurons. Nature 555:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE (1991) Cell cycle dependence of laminar determination in developing neocortex. Science 254:282–285. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29:503–537. [DOI] [PubMed] [Google Scholar]
- McGregor MM, McKinsey GL, Girasole AE, Bair-Marshall CJ, Rubenstein JLR, Nelson AB (2019) Functionally distinct connectivity of developmentally targeted striosome neurons. Cell Rep 29:1419–1428.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Sala P, Nardini D, Waclaw RR, Campbell K (2017) Selective neuronal expression of the SoxE factor, Sox8, in direct pathway striatal projection neurons of the developing mouse brain. J Comp Neurol 525:2805–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi D, Li Z, Lim L, Li M, Moissidis M, Yang Y, Gao T, Hu TX, Pratt T, Price DJ, Sestan N, Marín O (2018) Early emergence of cortical interneuron diversity in the mouse embryo. Science 360:81. 10.1126/science.aar6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha S, Valloton D, Arsenijevic Y, Cardinaux JR, Guidi R, Hornung JP, Lebrand C (2017) Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development. Sci Rep 7:43093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Li M-Y, Birey F, Ikeda K, Revah O, Thete MV, Park J-Y, Puno A, Lee SH, Porteus MH, Pașca SP (2020) Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat Biotechnol 38:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G (2010) Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci 30:1582–1594. 10.1523/JNEUROSCI.4515-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Barak B, Zhou Y, McRae R, Rodrigues D, Wickersham IR, Feng G (2018) Dichotomous parvalbumin interneuron populations in dorsolateral and dorsomedial striatum. J Physiol 596:3695–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Manchado AB, Foldi C, Szydlowski S, Sjulson L, Farries M, Wilson C, Silberberg G, Hjerling-Leffler J (2016) Novel striatal GABAergic interneuron populations labeled in the 5HT3a(EGFP) mouse. Cereb Cortex 26:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Manchado AB, Bengtsson Gonzales C, Zeisel A, Munguba H, Bekkouche B, Skene NG, Lonnerberg P, Ryge J, Harris KD, Linnarsson S, Hjerling-Leffler J (2018) Diversity of interneurons in the dorsal striatum revealed by single-cell RNA sequencing and PatchSeq. Cell Rep 24:2179–2190.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Bussert TG, Kreitzer AC, Seal RP (2014) Striatal cholinergic neurotransmission requires VGLUT3. J Neurosci 34:8772–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG (2002) The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci 5:1279–1287. [DOI] [PubMed] [Google Scholar]
- Newman H, Liu FC, Graybiel AM (2015) Dynamic ordering of early generated striatal cells destined to form the striosomal compartment of the striatum. J Comp Neurol 523:943–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes Z, Keefe F, Tamburini C, Kelly CM, Cruz Santos M, Dunnett SB, Errington AC, Li M (2019) Human pluripotent stem cell-derived striatal interneurons: differentiation and maturation in vitro and in the rat brain. Stem Cell Rep 12:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O (2008) Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron 59:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409:714–720. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7:136–144. [DOI] [PubMed] [Google Scholar]
- Nowakowski TJ, Fotaki V, Pollock A, Sun T, Pratt T, Price DJ (2013) MicroRNA-92b regulates the development of intermediate cortical progenitors in embryonic mouse brain. Proc Natl Acad Sci U S A 110:7056–7061. 10.1073/pnas.1219385110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, et al. (2014) A mesoscale connectome of the mouse brain. Nature 508:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare JK, Li H, Kim N, Gaidis E, Ade K, Beck J, Yin H, Calakos N (2017) Striatal fast-spiking interneurons selectively modulate circuit output and are required for habitual behavior. Elife 6:e26231. 10.7554/eLife.26231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Bjorklund A, Campbell K (1998) Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience 84:867–876. [DOI] [PubMed] [Google Scholar]
- Ortiz C, Navarro JF, Jurek A, Martin A, Lundeberg J, Meletis K (2020) Molecular atlas of the adult mouse brain. Sci Adv 6:eabb3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Berke JD, Kreitzer AC (2018) Fast-spiking interneurons supply feedforward control of bursting, calcium, and plasticity for efficient learning. Cell 172:683–695.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Mao T, Dudman JT (2010) Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front Neuroanat 4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RT, Chantranupong L, Hakim R, Levasseur J, Wang W, Merchant T, Gorman K, Budnik B, Sabatini BL (2019) Abnormal striatal development underlies the early onset of behavioral deficits in Shank3B–/– mice. Cell Rep 29:2016–2027.e4. 10.1016/j.celrep.2019.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH (1976) Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A 73:3729–3733. 10.1073/pnas.73.10.3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Bultje RS, Ross ME, Fishell G, Anderson SA (2015) Apical versus basal neurogenesis directs cortical interneuron subclass fate. Cell Rep 13:1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL (2007) Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron 55:417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz GA, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snippert HJ, Clevers H, Godinho L, Guillemot F, Borrell V, Matsuzaki F, Gotz M (2013) Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun 4:2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintacuda G, Martin JM, Eggan KC (2021) Mind the translational gap: using iPS cell models to bridge from genetic discoveries to perturbed pathways and therapeutic targets. Mol Autism 12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJ, Grillner S, Silberberg G (2010) Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci 30:3499–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Bhaduri A, Andrews MG, Nowakowski TJ, Meyerson OS, Mostajo-Radji MA, Di Lullo E, Alvarado B, Bedolli M, Dougherty ML, Fiddes IT, Kronenberg ZN, Shuga J, Leyrat AA, West JA, Bershteyn M, Lowe CB, Pavlovic BJ, Salama SR, Haussler D, et al. (2019) Establishing cerebral organoids as models of human-specific brain evolution. Cell 176:743–756.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Ciaranello RD, Rubenstein JL (1991) Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron 7:221–229. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL (1994) DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci 14:6370–6383. 10.1523/JNEUROSCI.14-11-06370.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Plotkin JL (2019) Compartmental function and modulation of the striatum. J Neurosci Res 97:1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL (2000) Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol 424:409–438. [DOI] [PubMed] [Google Scholar]
- Quattrocolo G, Fishell G, Petros TJ (2017) Heterotopic transplantations reveal environmental influences on interneuron diversity and maturation. Cell Rep 21:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel-Vallieres M, Weatheritt RJ, Cordes SP, Blencowe BJ (2019) Autism spectrum disorder: insights into convergent mechanisms from transcriptomics. Nat Rev Genet 20:51–63. [DOI] [PubMed] [Google Scholar]
- Rallu M, Corbin JG, Fishell G (2002) Parsing the prosencephalon. Nat Rev Neurosci 3:943–951. [DOI] [PubMed] [Google Scholar]
- Ramos SI, Makeyev EV, Salierno M, Kodama T, Kawakami Y, Sahara S (2020) Tuba8 drives differentiation of cortical radial glia into apical intermediate progenitors by tuning modifications of tubulin C termini. Dev Cell 52:477–491.e8. 10.1016/j.devcel.2020.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddington AE, Rosser AE, Dunnett SB (2014) Differentiation of pluripotent stem cells into striatal projection neurons: a pure MSN fate may not be sufficient. Front Cell Neurosci 8:398. 10.3389/fncel.2014.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K (1999) The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89:1009–1023. 10.1016/S0306-4522(98)00319-4 [DOI] [PubMed] [Google Scholar]
- Reid CB, Walsh CA (2002) Evidence of common progenitors and patterns of dispersion in rat striatum and cerebral cortex. J Neurosci 22:4002–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CB, Liang I, Walsh C (1995) Systematic widespread clonal organization in cerebral cortex. Neuron 15:299–310. [DOI] [PubMed] [Google Scholar]
- Reig R, Silberberg G (2014) Multisensory integration in the mouse striatum. Neuron 83:1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR (2001) A cellular mechanism of reward-related learning. Nature 413:67–70. [DOI] [PubMed] [Google Scholar]
- Roychoudhury K, Salomone J, Qin S, Cain B, Adam M, Potter SS, Nakafuku M, Gebelein B, Campbell K (2020) Physical interactions between Gsx2 and Ascl1 balance progenitor expansion versus neurogenesis in the mouse lateral ganglionic eminence. Development 147:dev185348. 10.1242/dev.185348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Campbell K (2020) Neurogenesis in the basal ganglia. In: Patterning and cell type specification in the developing CNS and PNS (Rubinstein J, Rakic P, eds), pp 399–426. London: Oxford Academic. [Google Scholar]
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A (2008) Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132:487–498. [DOI] [PubMed] [Google Scholar]
- Salomone J, Qin S, Fufa TD, Cain B, Farrow E, Guan B, Hufnagel RB, Nakafuku M, Lim HW, Campbell K, Gebelein B (2021) Conserved Gsx2/Ind homeodomain monomer versus homodimer DNA binding defines regulatory outcomes in flies and mice. Genes Dev 35:157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M (2005) Representation of action-specific reward values in the striatum. Science 310:1337–1340. [DOI] [PubMed] [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, Stevens C, Reichert J, Mulhern MS, Artomov M, Gerges S, Sheppard B, Xu X, Bhaduri A, Norman U, Brand H, et al. (2020) Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180:568–584.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA (2018) Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174:1015–1030.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F (2002) Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol 12:26–34. [DOI] [PubMed] [Google Scholar]
- Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, Ginty DD (2020) The emergence of transcriptional identity in somatosensory neurons. Nature 577:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Faouzi S, Bastide A, Martineau S, Malka-Mahieu H, Fu Y, Sun X, Mateus C, Routier E, Roy S, Desaubry L, Andre F, Eggermont A, David A, Scoazec JY, Vagner S, Robert C (2019) An epitranscriptomic mechanism underlies selective mRNA translation remodelling in melanoma persister cells. Nat Commun 10:5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth AN, Bhide PG (1997) Concurrent cellular output from two proliferative populations in the early embryonic mouse corpus striatum. J Comp Neurol 383:220–230. [DOI] [PubMed] [Google Scholar]
- Shi W, Xianyu A, Han Z, Tang X, Li Z, Zhong H, Mao T, Huang K, Shi SH (2017) Ontogenetic establishment of order-specific nuclear organization in the mammalian thalamus. Nat Neurosci 20:516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F (2011) Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci 31:3683–3695. 10.1523/JNEUROSCI.4773-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg SN, Taher L, Lindtner S, Sandberg M, Nord AS, Vogt D, McKinsey GL, Hoch R, Pattabiraman K, Zhang D, Ferran JL, Rajkovic A, Golonzhka O, Kim C, Zeng H, Puelles L, Visel A, Rubenstein JLR (2016) Subpallial enhancer transgenic lines: a data and tool resource to study transcriptional regulation of GABAergic cell fate. Neuron 92:59–74. 10.1016/j.neuron.2016.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH (1976) A pilot study of cell production by the ganglionic eminences of the developing mouse brain. J Anat 121:71–84. [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T, Bolam JP (2014) The thalamostriatal system in normal and diseased states. Front Syst Neurosci 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Chen H, Shang Z, Du H, Li Z, Wen Y, Liu G, Qi D, You Y, Yang Z, Zhang Z, Xu Z (2021) Homeobox gene Six3 is required for the differentiation of D2-type medium spiny neurons. Neurosci Bull 37:985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Harlan RE (1994) Genesis and migration patterns of neurons forming the patch and matrix compartments of the rat striatum. Brain Res Dev Brain Res 83:233–245. [DOI] [PubMed] [Google Scholar]
- Sousa VH, Fishell G (2010) Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain. Curr Opin Genet Dev 20:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF (2010) Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci 30:7028–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley G, Gokce O, Malenka RC, Sudhof TC, Quake SR (2020) Continuous and discrete neuron types of the adult murine striatum. Neuron 105:688–699.e8. 10.1016/j.neuron.2019.11.004 [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K (2003) Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci 23:167–174. 10.1523/JNEUROSCI.23-01-00167.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhmer T, Anderson SA, Ekker M, Rubenstein JL (2002) Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development 129:245–252. [DOI] [PubMed] [Google Scholar]
- Sultan KT, Han Z, Zhang XJ, Xianyu A, Li Z, Huang K, Shi SH (2016) Clonally related GABAergic interneurons do not randomly disperse but frequently form local clusters in the forebrain. Neuron 92:31–44. 10.1016/j.neuron.2016.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, Bertagnolli D, Goldy J, Shapovalova N, Parry S, Lee C, Smith K, Bernard A, Madisen L, Sunkin SM, Hawrylycz M, et al. (2016) Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19:335–346. 10.1038/nn.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, Goldy J, Garren E, Economo MN, Viswanathan S, Penn O, Bakken T, Menon V, Miller J, Fong O, Hirokawa KE, Lathia K, Rimorin C, Tieu M, Larsen R, et al. (2018) Shared and distinct transcriptomic cell types across neocortical areas. Nature 563:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Gotz M, Huttner WB (2014) The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol 30:465–502. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ (2008) Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci 28:5504–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Jin X, Lima SQ, Costa RM (2016) Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166:703–715. [DOI] [PubMed] [Google Scholar]
- Telley L, Govindan S, Prados J, Stevant I, Nef S, Dermitzakis E, Dayer A, Jabaudon D (2016) Sequential transcriptional waves direct the differentiation of newborn neurons in the mouse neocortex. Science 351:1443–1446. [DOI] [PubMed] [Google Scholar]
- Telley L, Agirman G, Prados J, Amberg N, Fievre S, Oberst P, Bartolini G, Vitali I, Cadilhac C, Hippenmeyer S, Nguyen L, Dayer A, Jabaudon D (2019) Temporal patterning of apical progenitors and their daughter neurons in the developing neocortex. Science 364:eaav2522. 10.1126/science.aav2522 [DOI] [PubMed] [Google Scholar]
- Tepper JM, Sharpe NA, Koos TZ, Trent F (1998) Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci 20:125–145. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koós T, Ibanez-Sandoval O, Tecuapetla F, Faust TW, Assous M (2018) Heterogeneity and diversity of striatal GABAergic interneurons: update 2018. Front Neuroanat 12:91. 10.3389/fnana.2018.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinterri A, Menardy F, Diana MA, Lokmane L, Keita M, Coulpier F, Lemoine S, Mailhes C, Mathieu B, Merchan-Sala P, Campbell K, Gyory I, Grosschedl R, Popa D, Garel S (2018) Active intermixing of indirect and direct neurons builds the striatal mosaic. Nat Commun 9:4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K (2000) Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development 127:4361–4371. [DOI] [PubMed] [Google Scholar]
- Torigoe M, Yamauchi K, Kimura T, Uemura Y, Murakami F (2016) Evidence that the laminar fate of LGE/CGE-derived neocortical interneurons is dependent on their progenitor domains. J Neurosci 36:2044–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot A, Ruiz-Reig N, Vitalis T, Studer M (2016) Molecular control of two novel migratory paths for CGE-derived interneurons in the developing mouse brain. Development 143:1753–1765. [DOI] [PubMed] [Google Scholar]
- Turrero Garcia M, Harwell CC (2017) Radial glia in the ventral telencephalon. FEBS Lett 591:3942–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrero Garcia M, Mazzola E, Harwell CC (2016) Lineage relationships do not drive MGE/PoA-derived interneuron clustering in the brain. Neuron 92:52–58. 10.1016/j.neuron.2016.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Haydar TF (2013) Multiplex genetic fate mapping reveals a novel route of neocortical neurogenesis, which is altered in the Ts65Dn mouse model of Down syndrome. J Neurosci 33:5106–5119. 10.1523/JNEUROSCI.5380-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WA, Medalla M, Guillamon-Vivancos T, Luebke JI, Haydar TF (2015) Neural precursor lineages specify distinct neocortical pyramidal neuron types. J Neurosci 35:6142–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JA, Anderson SA (2014) GABAergic interneuron transplants to study development and treat disease. Trends Neurosci 37:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya D, Hattiangady B, Castro OW, Shuai B, Kodali M, Attaluri S, Bates A, Dong Y, Zhang SC, Prockop DJ, Shetty AK (2019) Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci U S A 116:287–296. 10.1073/pnas.1814185115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Fishell G (1987) Neuronal birthdate underlies the development of striatal compartments. Brain Res 401:155–161. [DOI] [PubMed] [Google Scholar]
- van Heusden F, Macey-Dare A, Gordon J, Krajeski R, Sharott A, Ellender T (2021) Diversity in striatal synaptic circuits arises from distinct embryonic progenitor pools in the ventral telencephalon. Cell Rep 35:109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Cerviño V, Kappeler C, Nóbrega-Pereira S, Henkemeyer M, Rago L, Nieto MA, Marín O (2015) Molecular mechanisms controlling the migration of striatal interneurons. J Neurosci 35:8718–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali I, Fievre S, Telley L, Oberst P, Bariselli S, Frangeul L, Baumann N, McMahon JJ, Klingler E, Bocchi R, Kiss JZ, Bellone C, Silver DL, Jabaudon D (2018) Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell 174:1264–1276.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004) Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27:468–474. [DOI] [PubMed] [Google Scholar]
- Vormstein-Schneider D, Lin JD, Pelkey KA, Chittajallu R, Guo B, Arias-Garcia MA, Allaway K, Sakopoulos S, Schneider G, Stevenson O, Vergara J, Sharma J, Zhang Q, Franken TP, Smith J, Ibrahim LA, Astro KJM, Sabri E, Huang S, Favuzzi E, et al. (2020) Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans. Nat Neurosci 23:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Ehrman LA, Merchan-Sala P, Kohli V, Nardini D, Campbell K (2017) Foxo1 is a downstream effector of Isl1 in direct pathway striatal projection neuron development within the embryonic mouse telencephalon. Mol Cell Neurosci 80:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC (2013) Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Long JE, Flandin P, Pla R, Waclaw RR, Campbell K, Rubenstein JL (2013) Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol 521:1561–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wei PC, Lim CK, Gallina IS, Marshall S, Marchetto MC, Alt FW, Gage FH (2020) Increased neural progenitor proliferation in a hiPSC model of autism induces replication stress-associated genome instability. Cell Stem Cell 26:221–233.e6. 10.1016/j.stem.2019.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai JW, LaMonica B, Kriegstein AR (2011) A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci 14:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Criswell HE, Powell SK, Bhatt AP, McCown TJ (2017) Viral vector reprogramming of adult resident striatal oligodendrocytes into functional neurons. Mol Ther 25:928–934. 10.1016/j.ymthe.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiatr K, Szlachcic WJ, Trzeciak M, Figlerowicz M, Figiel M (2018) Huntington disease as a neurodevelopmental disorder and early signs of the disease in stem cells. Mol Neurobiol 55:3351–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A (1999) Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci 2:461–466. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A (2001) In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128:3759–3771. [DOI] [PubMed] [Google Scholar]
- Willaime-Morawek S, Seaberg RM, Batista C, Labbe E, Attisano L, Gorski JA, Jones KR, Kam A, Morshead CM, van der Kooy D (2006) Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol 175:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J, Stanic D, Thompson LH (2013) Generation of striatal projection neurons extends into the neonatal period in the rat brain. J Physiol 591:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenias HS, Ibáñez-Sandoval O, Koós T, Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci 35:6584–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Fan J, Emanuel G, Hao J, Zhuang X (2019) Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc Natl Acad Sci U S A 116:19490–19499. 10.1073/pnas.1912459116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Liang Q, Song X, Zhang Z, Lindtner S, Li Z, Wen Y, Liu G, Guo T, Qi D, Wang M, Wang C, Li H, You Y, Wang X, Chen B, Feng H, Rubenstein JL, Yang Z (2018) SP8 and SP9 coordinately promote D2-type medium spiny neuron production by activating Six3 expression. Development 145:dev165456. 10.1242/dev.165456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartsev MM, Hanks TD, Yoon AM, Brody CD (2018) Causal contribution and dynamical encoding in the striatum during evidence accumulation. Elife 7:e34929. 10.7554/eLife.34929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat Rev Neurosci 7:464–476. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Vissers C, Ming GL, Song H (2018) Epigenetics and epitranscriptomics in temporal patterning of cortical neural progenitor competence. J Cell Biol 217:1901–1914. 10.1083/jcb.201802117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YC, Bultje RS, Wang X, Shi SH (2009) Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YC, He S, Chen S, Fu Y, Brown KN, Yao XH, Ma J, Gao KP, Sosinsky GE, Huang K, Shi SH (2012) Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature 486:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL (2001) Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development 128:193–205. [DOI] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, Hrabe de Angelis M, Weinmaster G, Rubenstein JL (2002) Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development 129:5029–5040. [DOI] [PubMed] [Google Scholar]
- Yun K, Garel S, Fischman S, Rubenstein JL (2003) Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol 461:151–165. [DOI] [PubMed] [Google Scholar]
- Zahr SK, Yang G, Kazan H, Borrett MJ, Yuzwa SA, Voronova A, Kaplan DR, Miller FD (2018) A translational repression complex in developing mammalian neural stem cells that regulates neuronal specification. Neuron 97:520–537.e6. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular architecture of the mouse nervous system. Cell 174:999–1014.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang Y, Wang C, Xu Z, Liang Q, An L, Li J, Liu Z, You Y, He M, Mao Y, Chen B, Xiong ZQ, Rubenstein JL, Yang Z (2016) The zinc finger transcription factor Sp9 is required for the development of striatopallidal projection neurons. Cell Rep 16:1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Kozloski J (2017) Striatal network models of Huntington's disease dysfunction phenotypes. Front Comput Neurosci 11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]