Abstract
Post-encoding coordinated reactivation of memory traces distributed throughout interconnected brain regions is thought to be critical for consolidation of memories. However, little is known about the role of neural circuit pathways during post-learning periods for consolidation of memories. To investigate this question, we optogenetically silenced the inputs from both auditory cortex and thalamus in the lateral amygdala (LA) for 15 min immediately following auditory fear conditioning (FC) and examined its effect on fear memory formation in mice of both sexes. Optogenetic inhibition of both inputs disrupted long-term fear memory formation tested 24 h after FC. This effect was specific such that the same inhibition did not affect short-term memory and context-dependent memory. Moreover, long-term memory was intact if the inputs were inhibited at much later time points after FC (3 h or 1 d after FC), indicating that optical inhibition for 15 min itself does not produce any nonspecific deleterious effect on fear memory retrieval. Selective inhibition of thalamic input was sufficient to impair consolidation of auditory fear memory. In contrast, selective inhibition of cortical input disrupted remote fear memory without affecting recent memory. These results reveal a dissociated role of thalamic and cortical input to the LA during early post-learning periods for consolidation of long-term fear memory.
SIGNIFICANCE STATEMENT Coordinated communications between brain regions are thought to be essential during post-learning periods for consolidation of memories. However, the role of specific neural circuit pathways in this process has been scarcely explored. Using a precise optogenetic inhibition of auditory input pathways, either thalamic or cortical or both, to the LA during post-training periods, we here show that thalamic input is required for consolidation of both recent and remote fear memory, whereas cortical input is crucial for consolidation of remote fear memory. These results reveal a dissociated role of auditory input pathways to the LA for consolidation of long-term fear memory.
Keywords: auditory fear conditioning, amygdala, auditory thalamus, auditory cortex, post-training, memory consolidation
Introduction
The coordinated interactions in a distributed network of structures during post-learning periods is thought to be critically involved in consolidation of memories. Evidence from in vivo electrophysiological recordings suggests that coherent activities in the amygdala-hippocampus and amygdala-mPFC circuit during sleep following fear learning participate in the consolidation of fear memory (Popa et al., 2010; Girardeau et al., 2017). The optogenetic manipulations of basolateral amygdala (BLA) inputs to the medial entorhinal cortex after learning revealed that this pathway selectively influences the consolidation of spatial/contextual memory (Wahlstrom et al., 2018).
Consistent with this idea, accumulating evidence suggests that activity in the brain structures involved in learning are required after learning for consolidation of memories. Earlier studies using pharmacological methods have shown that the post-training amygdala activity is involved in consolidation of aversive training, such as inhibitory avoidance (IA) learning (Brioni et al., 1989; Castellano and McGaugh, 1990; Liang et al., 1994; Parent and McGaugh, 1994; Izquierdo et al., 1997; Zanatta et al., 1997). Immediate post-training inactivation of BLA with lidocaine impairs contextual fear conditioning (FC) (Vazdarjanova and McGaugh, 1999). Similar post-training BLA inactivation by TTX disrupts consolidation of both auditory and contextual FC (Sacchetti et al., 1999). More recently, using precise optogenetic manipulations of BLA activity after IA training in rats, it has been shown that specific frequency patterns of activity in the BLA after learning are critical for consolidation of IA memory (Huff et al., 2013). In contrast to these findings, however, there is also evidence against this view (Wilensky et al., 1999). Post-training inactivation of the BLA by muscimol, a GABAA agonist, does not affect memory for Pavlovian FC (Wilensky et al., 1999, 2000), proposing that, for Pavlovian fear conditoning, synaptic activity in the BLA is required only during learning but not after, and consolidation in the amygdala afterward is dependent on intracellular signaling cascades (e.g., cAMP-PKA signaling pathway).
Converging evidence points that activity in the association cortex is required after learning for formation of remote cued fear memory. Post-training lesion or reversible inactivation of the temporal association cortex (Te2) in the rat causes impairment of the retrieval of the remote auditory fear memory (Sacco and Sacchetti, 2010; Grosso et al., 2015; Cambiaghi et al., 2016b). Different from the cortex, reversible pharmacological inactivation of auditory thalamus at a few different time points, as early as 15 min, after auditory FC does not affect memory formation (Sacchetti et al., 1999). Despite these findings, the role of circuit pathways for consolidation has been scarcely explored. Because lesion or pharmacological methods, although useful, often involve a confounding effect because of a functional compensation (Goshen et al., 2011; Otchy et al., 2015) and are inevitably limited by the lack of spatial specificity, especially for the manipulation of particular axonal projections, a more precise method, such as optogenetics, is needed to investigate this issue.
In auditory FC, a tone signal is delivered to the amygdala through both thalamic and cortical inputs. Potentiation of these inputs is believed to be a synaptic mechanism for encoding FC (LeDoux, 2000). FC induces associative plasticity not only in the amygdala but also in regions outside the amygdala, including the various stages of the auditory pathways (Ryugo and Weinberger, 1978; Edeline et al., 1993; Hennevin et al., 1993; Bordi and LeDoux, 1994; Quirk et al., 1997; Maren et al., 2001; Weinberger, 2004; Apergis-Schoute et al., 2005; Moczulska et al., 2013; Yang et al., 2016; Dalmay et al., 2019; Barsy et al., 2020; Taylor et al., 2021). Despite these findings, it is unclear whether communications between the amygdala and auditory system are necessary after learning for consolidation of fear memory. To address this question, we optogenetically silenced the inputs from auditory thalamus (medial geniculate nucleus and adjacent posterior intralaminar nucleus [MGm/PIN]) and cortex (ventral part of the secondary auditory cortex [AuV/TeA]) in the LA for 15 min immediately following auditory FC and examined its effect on long-term fear memory formation in mice.
Materials and Methods
Mice
Adult 129 × C57BL/6 hybrid background mice (2-3 months old, 23-35 g) were used for all experiments. Mice were group-housed (3-5 mice per cage) under a 12 h light/dark cycle at a constant temperature (22 ± 1°C) with 40%-60% humidity. Food and water were available ad libitum throughout the experiments. Behavioral experiments were performed under the light phase. For the behavior experiments, both male and female (total 208 mice) were used in balance. Notably, we did not observe any noticeable differences between male and female in the results. All procedures were approved by the KAIST Institutional Animal Care and Use Committee.
Adeno-associated virus (AAV) viral vector and packaging
AAV was packaged as previously described (Kwon et al., 2014). DNA plasmid coding AAV-CaMKIIα-eNpHR3.0-EYFP, AAV-CaMKIIα-ChR2-Venus, or AAV-CaMKIIα-EGFP was amplified and purified using a Maxiprep kit (QIAGEN). The purified DNA plasmid was co-transfected with the DNA plasmid coding AAV2/1 and pAΔF6 using calcium phosphate precipitation into HEK293T cell. Seventy-two hours after transfection, cells were harvested and virus was purified by iodixanol-gradient ultracentrifugation. Purified viral solution was dissolved in PBS. Viral titers were determined by qPCR (Rotor-Gene Q, QIAGEN) using SYBR Green (204074, QIAGEN). Titer of AAV solution ranged between 9 × 1011 and 2 × 1012 vg ml−1.
Surgery
Mice were anesthetized with pentobarbital (83 mg kg−1 of body weight) by intraperitoneal injections and fixed in a stereotaxic frame. Small holes were drilled with an electrical driller at target sites on both hemispheres. Virus solution was loaded in a glass pipette filled with water and 1.5 µl of mineral oil at the tip. Appropriate volume of virus (0.3 µl per side) was bilaterally injected into the AuV/TeA (AP −2.9 mm, ML ±4.55 mm, DV −3.1 mm) and MGm/PIN (AP −3.1 mm, ML ±1.9 mm, DV −3.5 mm) at a rate of 0.1 µl ml−1 for 3 min. The injection pipette was left at the injection site for an additional 10 min for diffusion of the virus. After the injection electrode was slowly withdrawn, mice were placed on heating pad for recovery and returned to their home cages. Three weeks after viral injection surgery, mice underwent surgery again for implanting optic ferrules (Doric Lenses, 200 µm core diameter, 0.37 NA) above the LA (AP −1.8 mm, ML ±3.55 mm, DV −3.8 mm). Optic ferrules were fixed with dental cement for chronic implantation. Mice were single housed for a week before behavioral experiments.
Behavior
All behavior experiments were conducted at least 4 weeks after virus injection surgery to allow sufficient time for expression of opsins in the presynaptic terminals. After 7 d recovery from implantation of optic ferrules, mice were tethered to fiber-optic patch cords for 5 min per day in 3 consecutive days for habituation to light stimulation procedure. For auditory FC, mice were placed in a conditioning chamber (Coulbourn Instruments) equipped with a grid floor (Coulbourn Instruments) with 70% ethanol as a background odor. After 2 min of free exploration, tone (2.8 kHz, 85 dB) was presented for 30 s, which was co-terminated with a foot shock (0.5 mA, 2 s). Mice then were allowed to remain in the chamber for an additional 30 s. For photoinhibition of eNpHR3.0-expressing terminals, yellow light was generated using diode laser (CrystaLaser) and delivered through surgically implanted optic ferrules. Yellow light (561 nm, 5 mW at fiber tip, each hemisphere) was continuously delivered for 15 min in a neutral context at a few different time points (2 min, 3 h, 1 d) after FC. At the end of 15 min photoinhibition, mice were detached from fiber-optic patch cords and returned to their home cage. For the retention tests, CS-induced freezing was measured in a context-shifted chamber with a white acrylic floor and semi-circular wall. After 2 min of free exploration, freezing responses to the tone stimulus (2.8 kHz, 3 min, 85 dB) were monitored. Freezing level during the first 1 min of tone presentation was used for data analysis. Recent or remote fear memory was tested 1 or 20 d, respectively, after conditioning.
For contextual FC, mice were placed into the conditioning chamber (Coulbourn Instruments) equipped with a grid floor (Coulbourn Instruments) with 70% ethanol as a background odor. Two minutes later, mice received a foot shock (0.5 mA, 2 s). Contextual fear memory test was performed in the same chamber 1 d after conditioning. Freezing level during the first 2 min after mice entered into the chamber was used for data analysis. Mouse behavior was recorded by a camera from above at 4 frames per second. Freezing behavior was automatically scored using FreezeFrame software (version 3.32, Actimetrics).
To mimic rebound-like synaptic responses, 473 nm blue light generated using diode laser (10 Hz, 20 ms pulse width; CrystaLaser) was delivered to ChR2-expressing auditory axon terminals through the surgically implanted optic ferrule. Mice underwent auditory FC after habituation to the light stimulation procedure as above. Seventeen minutes after auditory FC, blue light (10 mW at fiber tip, each hemisphere) was delivered for 0.2 s in a neutral context. One day after the ChR2 stimulation, the freezing response to tone was measured.
Histology
At the end of all behavioral experiments, mice were perfused transcardially with 100 ml PBS followed by same volume of 4% PFA. Brains were extracted and then postfixed in 4% PFA overnight. The brains were sliced in coronal sections at 40 µm thickness using vibratome (VT-1200S, Leica Microsystems). For histologic analysis, sections were mounted on gelatin-coated slides and coverslipped with Vectashield mounting solution (h-1200, Vector Laboratories). Histologic verification of virus expression and ferrule placement was performed with a fluorescence microscope (ECLPSE 80i, Nikon) or confocal microscope (LSM880, Zeiss at the KAIST Bio-Core Center). By reference to the Mouse Brain Atlas (Paxinos and Franklin, 2019), only the animals that showed restricted virus expression mainly in the AuV/TeA and MGm/PIN regions were included for data analysis. Mice that showed unilateral expression, physical damage by the ferrule, or off-target expression in the surrounding areas, such as ventral hippocampus and perirhinal cortex, were excluded. Histologic verification data for all mice included in the data analysis are presented in Figure 6.
Figure 6.
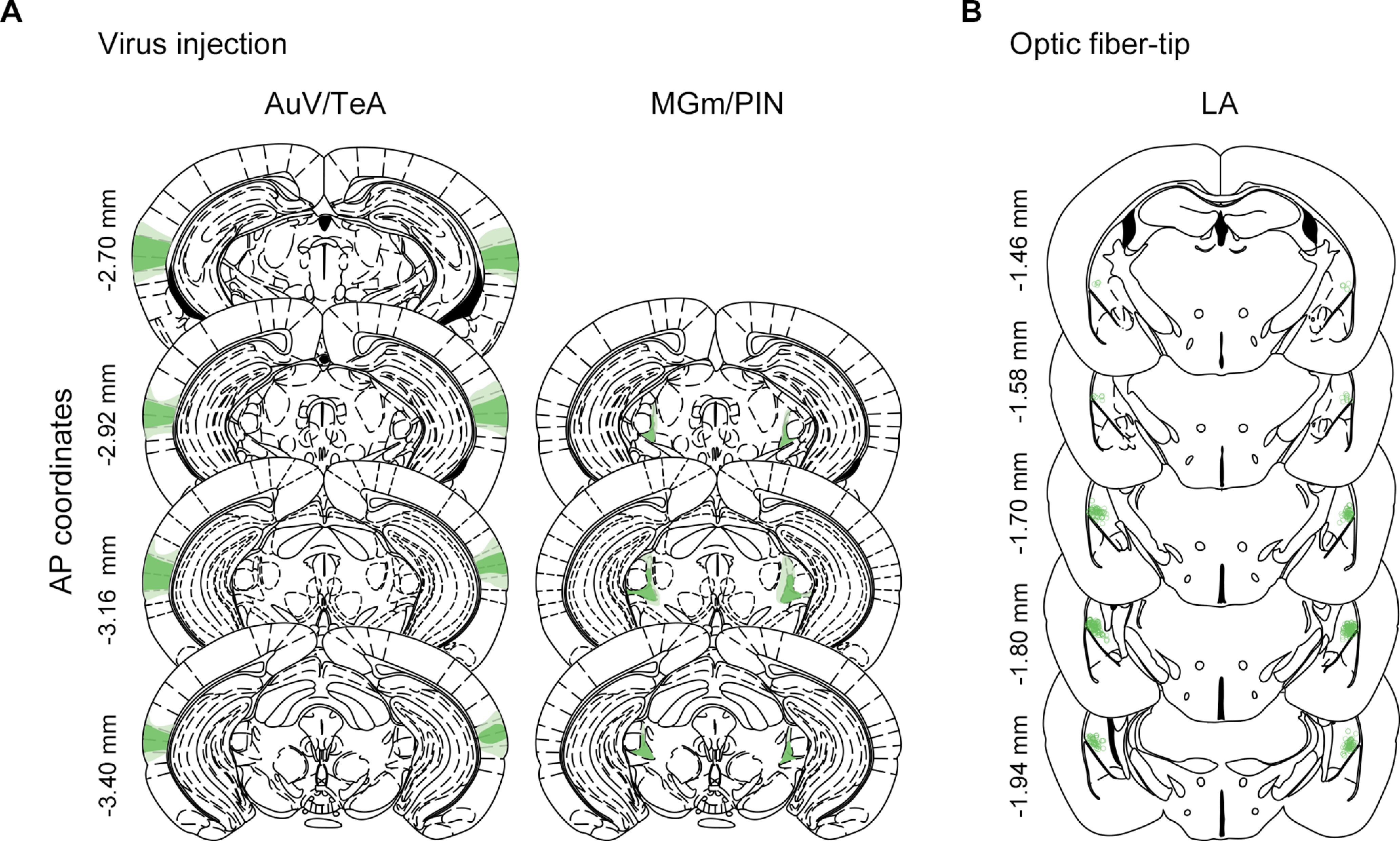
Histologic verification of virus expression and placement of optic fiber tip. A, The largest (light color) and the smallest (deep color) virus expression areas in the AuV/TeA and MGm/PIN for all animals included in data analysis. B, Locations of optic fiber tip for all animals included in data analysis.
Experimental design and statistical analyses
The experiments used a between-subject design to compare experimental group and the control group. Data are presented as mean ± SEM. Statistical significance of data was determined using two-tailed Student's t test or repeated-measures two-way ANOVA followed by Sidak's post hoc test for multiple comparisons. Sidak's post hoc confirmed statistical significance between groups. Prism (version 9.0.0, GraphPad Software) was used for all statistical analyses.
Results
Optogenetic inhibition of both thalamic and cortical auditory inputs to the LA for 15 min immediately following FC impairs long-term fear memory formation
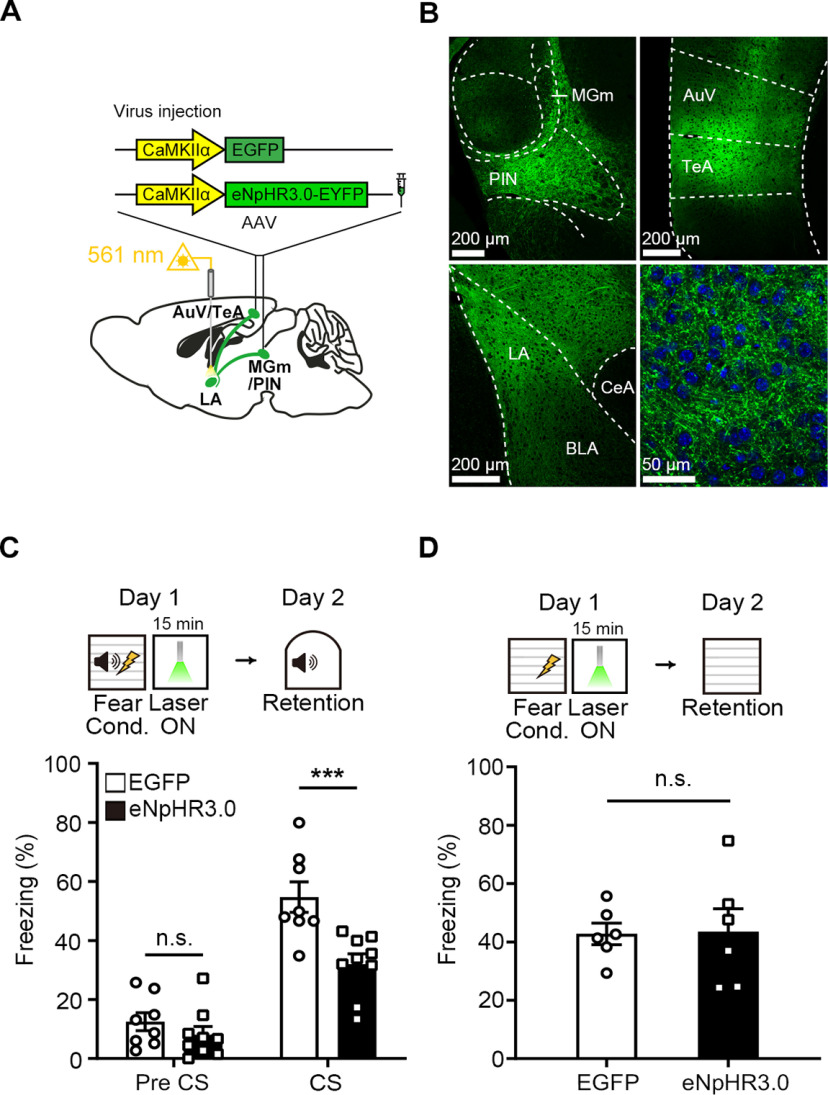
We examined the effect of optogenetic inhibition of auditory inputs to the LA during post-learning periods on fear memory formation. To this end, we used AAV vector containing genes encoding eNpHR3.0 fused to an enhanced yellow fluorescent protein (EYFP) or enhanced green fluorescent protein (EGFP; control) under the Ca2+/calmodulin-dependent protein kinase II-α (CaMKIIα) promoter (Fig. 1A). We bilaterally injected AAV-eNpHR3.0-EYFP or AAV-EGFP virus into the two major auditory brain regions that send direct projections to the LA: the auditory thalamus, including medial division of the MGm/PIN and the AuV/TeA (Fig. 1B). Notably, we did not find any eNpHR3.0-EYFP-positive cells in the LA throughout all brain sections we examined, indicating no detectable transneuronal transports by the AAV 2/1 virus in our virus injection condition (Fig. 1B). Yellow light (561 nm, 5 mW at fiber tip) was delivered to the eNpHR3.0-expressing auditory axons in the bilateral LA through the optic fiber implanted above the LA (Fig. 1A). Four weeks after virus injection to ensure sufficient presynaptic terminal expression, mice were trained for FC in which a neutral tone (CS) was paired with an aversive foot shock (0.5 mA; US). Animals from both eNpHR3.0 and EGFP control groups received a continuous 561 nm light stimulation (activating eNpHR3.0) for 15 min immediately after FC. The next day, animals were tested for fear memory (Fig. 1C). Animals injected with AAV-eNpHR3.0-EYFP displayed a significantly reduced freezing compared with control mice (Fig. 1C; repeated-measures two-way ANOVA, group × time interaction, F(1,15) = 12.84, p = 0.0027; Sidak's post hoc test, p = 0.0003). It is possible that inhibition of auditory inputs for 15 min may nonspecifically cause a decrease in freezing behavior. To test this possibility, we performed the same optogenetic inhibition in animals trained with contextual FC. The next day, animals were re-exposed to the conditioned context to determine contextual fear memory. In this case, we found no significant difference in freezing between groups (Fig. 1D; two-tailed unpaired t test, t(10) = 0.08,971, p = 0.9303), highlighting the specific effect of optical silencing on auditory fear memory. Virus expression and placement of optic fiber tip were histologically verified (see Fig. 6). These results suggest that auditory inputs to the LA are required during post-learning periods for fear memory formation.
Figure 1.
Optogenetic inhibition of auditory inputs to the LA immediately after FC impairs fear memory. A, Illustration of AAV-CaMKIIα-EGFP or AAV-CaMKIIα-eNpHR3.0-EYFP virus vector injection in the bilateral MGm/PIN and AuV/TeA and optic fiber implantation in the bilateral LA for optogenetic inhibition of auditory inputs in the LA. B, Representative confocal microscopic images of eNpHR3.0-EYFP expression in the MGm/PIN and AuV/TeA neurons and their terminals in the LA. High-magnification images show MGm/PIN and AuV/TeA axons in the LA. DAPI-stained cell bodies (blue) within the LA. C, Top, Experimental procedure of auditory FC followed by optogenetic inhibition of auditory inputs in the LA. Bottom, Time spent freezing during the retention test was decreased by photoinhibition in the eNpHR3.0 (n = 9 mice) compared with the EGFP group (n = 8 mice). D, Top, Experimental procedure for optogenetic inhibition of auditory inputs in the LA right after contextual FC. Bottom, Time spent freezing during the contextual fear memory retention was not affected by photoinhibition in the eNpHR3.0 compared with EGFP (n = 6 mice per group). Data are mean ± SEM. ***p < 0.001.
By using in vivo multiunit recording in our recently published study (Jeong et al., 2021), we confirmed an efficient NpHR-mediated silencing of auditory inputs to the LA in our optogenetic experimental setup. Despite this result, we cannot completely rule out that there might have been rebound-like increases of activity in auditory inputs because of the sustained inhibition of axons, and this nonspecifically affected consolidation process. To test this possibility, we performed an additional experiment in which we activated the auditory inputs in the LA by delivering two brief pulses of 473 nm light (activating ChR2) ∼17 min after FC, a time point corresponding to when the 561 nm light was turned off in NpHR experiments, and examined its effect on long-term memory formation. We bilaterally injected AAV-ChR2-Venus or AAV-EGFP virus into the MGm/PIN and AuV/TeA (Fig. 2A,B). Mice underwent exactly the same experimental procedures as above, except that two pulses of 473 nm blue light (10 Hz, 20 ms pulse width), instead of 15 min continuous yellow light, were delivered (Fig. 2C). The next day, we tested these mice and found no significant difference in freezing to tone between groups (Fig. 2C; repeated-measures two-way ANOVA, group × time interaction, F(1,12) = 0.08,972, p = 0.7697; Sidak's post hoc test, p = 0.9637). Thus, it is unlikely that disruption of consolidation by post-training optogenetic silencing of auditory inputs was because of rebound effects.
Figure 2.
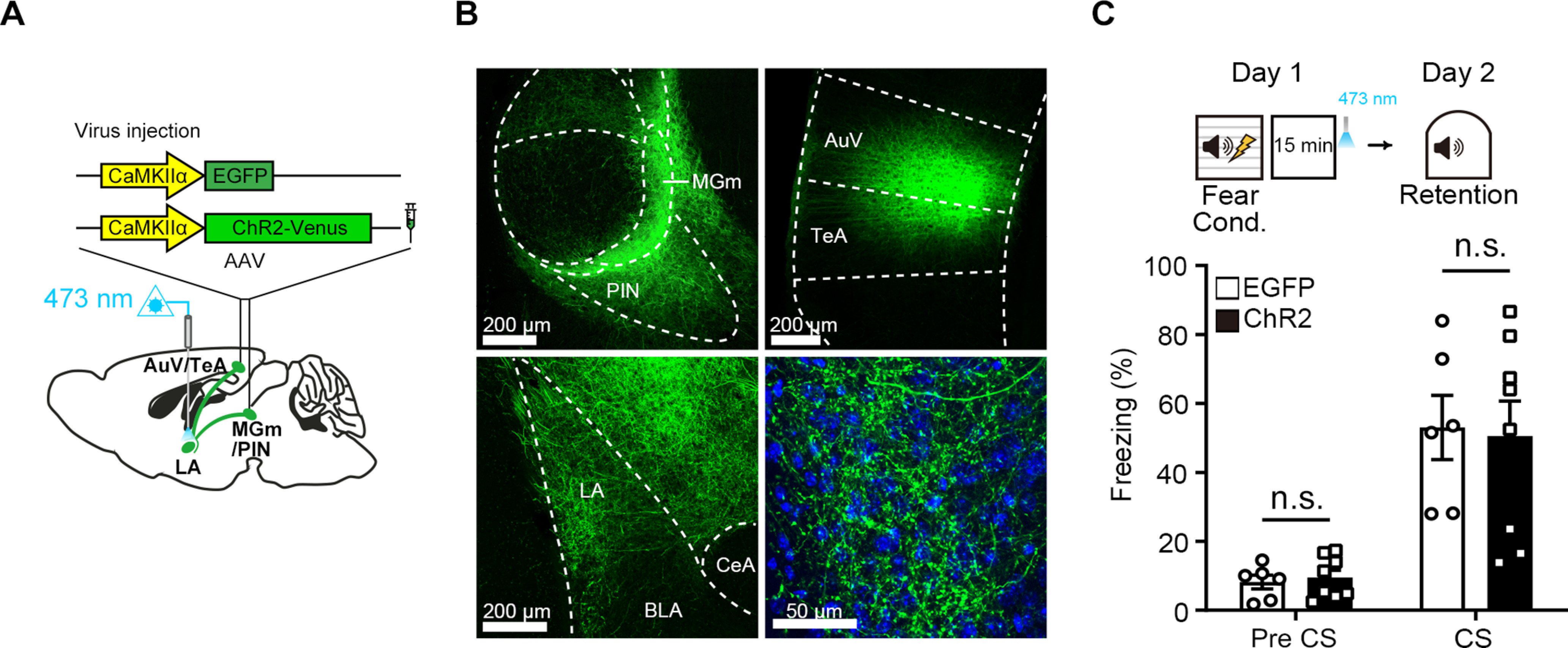
A brief optogenetic stimulation of auditory inputs after FC does not affect consolidation of auditory fear memory. A, Schematic depiction of AAV-CaMKIIα-EGFP or AAV-CaMKIIα-ChR2-Venus virus injection in the bilateral MGm/PIN and AuV/TeA for optogenetic activation of auditory inputs in the LA 17 min after FC. B, Representative confocal microscopic images of ChR2-Venus expression in the MGm/PIN and AuV/TeA neurons and their terminals in the LA. High-magnification images show MGm/PIN and AuV/TeA axons in the LA. DAPI-stained cell bodies (blue) within the LA. C, Top, Experimental procedure for brief optogenetic activation of auditory inputs in the LA ∼17 min after FC. Bottom, Time spent freezing during the retention test. There was no significant difference in freezing level between groups (EGFP, n = 6 mice; eNpHR3.0, n = 8 mice). Data are mean ± SEM.
Auditory inputs to the LA are involved in consolidation of fear memory
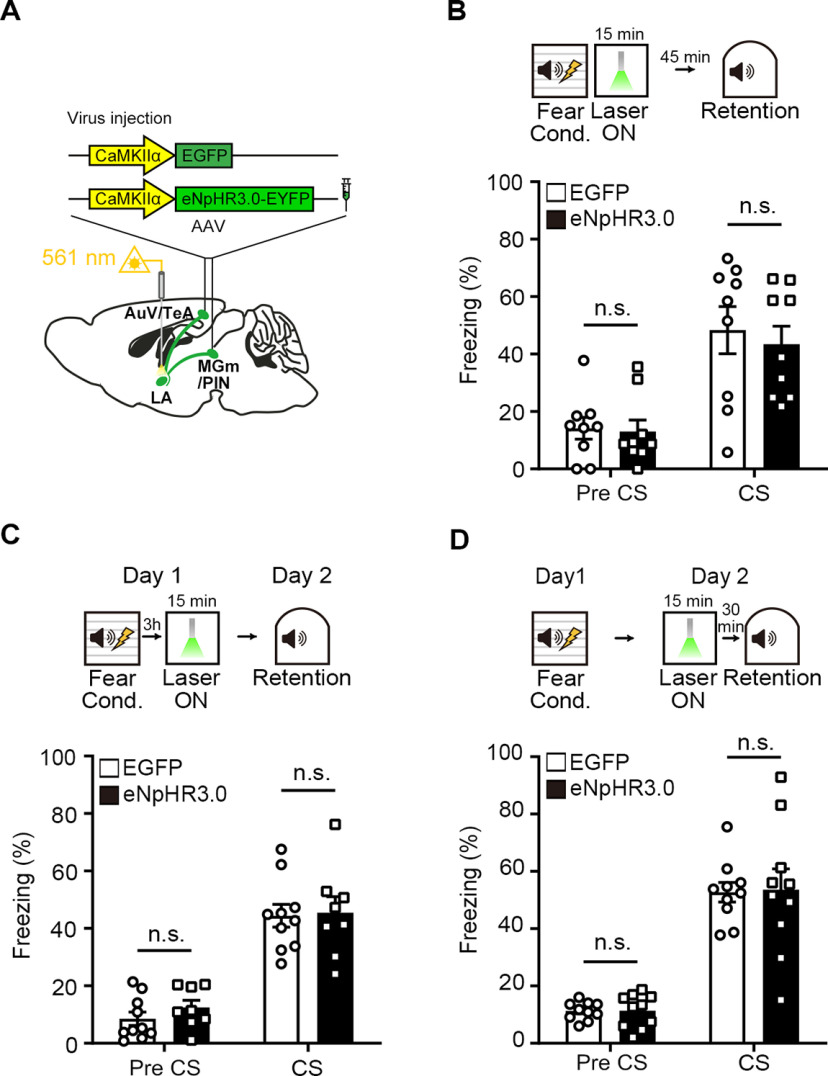
Next, we asked whether the amnesic effect of post-training optogenetic inhibition of auditory inputs to the LA are attributable to the impairment of memory consolidation processes. Because we inhibited the inputs immediately after training, acquisition of memory could be affected. To test this possibility, we examined whether short-term memory formation was normal. AAV-eNpHR3.0-EYFP or AAV-EGFP virus was bilaterally injected into the MGm/PIN and AuV/TeA as above (Fig. 3A). We performed the same experimental procedures as above, except that memory was tested 1 h after training. During the test, we found no significant difference in conditioned freezing to tone between groups (Fig. 3B; repeated-measures two-way ANOVA, group × time interaction, F(1,16) = 0.2685, p = 0.6114; Sidak's post hoc test, p = 0.8075), indicating that acquisition of memory was normal. The inhibition of auditory inputs to the LA any time after FC may nonspecifically cause a deleterious effect on memory retrieval. To test this issue, we trained animals with FC and tested them for fear memory formation 24 h later as before, but light was delivered for 15 min at 3 h or 1 d after training (Fig. 3C,D). When light was delivered 3 h after FC, long-term fear memory was intact. When tested 24 h after training, animals injected with AAV-eNpHR3.0-EYFP virus displayed a similar level of freezing compared with control animals injected with AAV-EGFP (Fig. 3C; repeated-measures two-way ANOVA, group × time interaction, F(1,16) = 0.2727, p = 0.6087; Sidak's post hoc test, p = 0.9777). In another set of experiments, light was delivered 30 min before retrieval test, and we again observed no significant difference in freezing between groups (Fig. 3D; repeated-measures two-way ANOVA, group × time interaction, F(1,18) = 0.01337, p = 0.9092; Sidak's post hoc test, p = 0.9851). Together, these results suggest that auditory inputs to the LA are specifically involved in consolidation of long-term fear memory, likely during an early post-learning period.
Figure 3.
Auditory inputs to the LA are required for consolidation of fear memory. A, Schematic depiction of virus injection site for optogenetic inhibition of auditory inputs in the LA during post-training periods. B, Top, Experimental procedure for optogenetic inhibition of auditory inputs to the LA immediately following auditory FC to investigate the effect on short-term memory. Percentage of time spent freezing during the short-term memory test was not affected by photoinhibition in the eNpHR3.0 compared with the EGFP group (n = 9 mice per group). C, Top, Experimental procedure for 3 h delayed inhibition of auditory inputs in the LA. There was no significant difference in freezing level between groups (EGFP, n = 10 mice; eNpHR3.0, n = 8 mice). D, Experimental procedural for inhibition of auditory inputs in the LA 30 min before CS recall test 24 h after FC. In the subsequent auditory fear memory test, both groups showed similar freezing levels (n = 10 mice per group). Data are mean ± SEM.
Selective inhibition of thalamic but not cortical input to the LA immediately after FC impairs consolidation of 24 h recent fear memory
In order to examine the contribution of each input pathway, either thalamic or cortical, to consolidation of fear memory, we then selectively inhibited one input in the LA immediately after FC. For selective inhibition of thalamic input, we injected AAV-eNpHR3.0-EYFP or AAV-EGFP virus into the bilateral MGm/PIN (Fig. 4A,B). Animals received 561 nm light for 15 min immediately after FC. Fear memory was tested 24 h later. Similar to the inhibition of both inputs, animals injected with AAV-eNpHR3.0-EYFP displayed a significantly less freezing compared with control animals (Fig. 4C; repeated-measures two-way ANOVA, group × time interaction, F(1,17) = 11.13, p = 0.0039; Sidak's post hoc test, p < 0.0001). The 15 min inhibition itself of MGm/PIN input to the LA may produce a nonspecific harmful effect on fear memory 24 h later. To exclude this possibility, we performed the same input inhibition but at 24 h after FC and then tested mice next day. Animals injected with AAV-eNpHR3.0-EYFP displayed no significant difference in freezing level compared with control animals (Fig. 4D; repeated-measures two-way ANOVA, group × time interaction, F(1,18) = 0.3961, p = 0.5370; Sidak's post hoc test, p = 0.6812). For selective inhibition of cortical input, we injected AAV-eNpHR3.0-EYFP or AAV-EGFP virus into the bilateral AuV/TeA (Fig. 4E,F). Different from the inhibition of thalamic input, in this condition we found no significant difference in freezing between groups (Fig. 4G; repeated-measures two-way ANOVA, group × time interaction, F(1,16) = 0.2723, p = 0.6089; Sidak's post hoc test, p = 0.9392). These results suggest that the thalamic input but not cortical input to the LA is involved in consolidation of recent fear memory.
Figure 4.
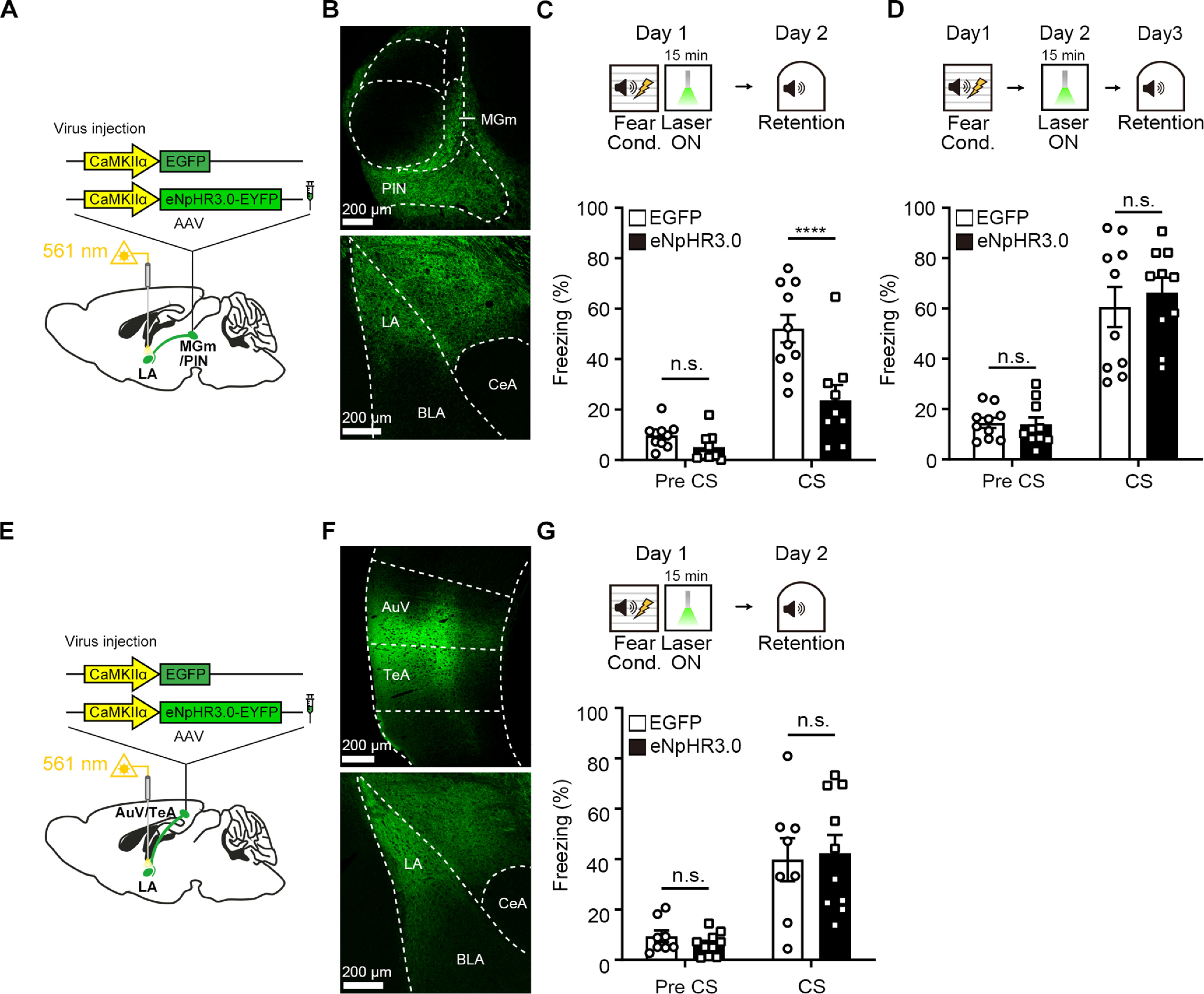
Post-training inhibition of thalamic input alone sufficiently impairs recent (24 h) fear memory. A, Schematic depiction of virus injection and optic fiber implantation to selectively inhibit MGm/PIN-LA projections. B, Representative confocal microscopic images of eNpHR3.0-EYFP expression in the MGm/PIN neurons and their presynaptic axon terminals in the LA. C, Top, Experimental procedure of photoinhibition of thalamic input in the LA after auditory FC. Bottom, Percentage of time spent freezing during the auditory fear memory test was reduced by photoinhibition in the eNpHR3.0 (n = 9 mice) compared with the EGFP group (n = 10 mice). D, Experimental procedure for photoinhibition of thalamic input in the LA 24 h after auditory FC. Bottom, There was no significant difference in tone-induced freezing level between groups at 48 h memory test (n = 10 mice per group). E, Schematic depiction of virus injection and optic fiber implantation to selectively inhibit AuV/TeA-LA projections. F, Representative confocal microscopic images of eNpHR3.0-EYFP expression in the AuV/TeA neurons and their presynaptic axon terminals in the LA. G, Top, Experimental procedure for photoinhibition of cortical input right after auditory FC. Bottom, There was no significant difference in tone-induced freezing level between groups in the recent fear memory test (EGFP, n = 8 mice; eNpHR3.0, n = 10 mice). Data are mean ± SEM. ****p < 0.0001.
Selective inhibition of cortical input to the LA immediately after FC impairs consolidation of remote fear memory
The inhibition of cortical input to the LA did not affect fear memory formation at recent time. One interpretation of this result is that cortical input is not involved in consolidation of fear memory. However, there is also an alternative possibility that it may have a role for remote memory formation. Previous studies suggest that association cortex is engaged after learning for consolidation of remote fear memory. It has been shown that lesions of the auditory cortex after training cause impairment of retention of remote auditory fear memory (Sacco and Sacchetti, 2010; Grosso et al., 2015; Cambiaghi et al., 2016b). Inactivation of the secondary auditory cortex (Te2) in the rat during memory consolidation causes a deficit in the remote fear retention. Moreover, inactivation of the secondary auditory cortex (Te2) in the rat during memory consolidation disrupts the activity between Te2 and BLA, which is detected during fear memory retrieval (Cambiaghi et al., 2016a). Therefore, we examined whether the optical inhibition of cortical input to the LA immediately after FC affects fear memory at remote time. Animals were injected with AAV-eNpHR3.0-EYFP or AAV-EGFP into the bilateral AuV/TeA (Fig. 5A,B) and trained for FC immediately followed by light delivery for 15 min as before. For remote retention of memory, animals were tested 20 d after FC training (Fig. 5C). Animals injected with AAV-eNpHR3.0-EYFP displayed a significantly less freezing to tone compared with a control group (Fig. 5C; repeated-measures two-way ANOVA, group × time interaction, F(1,18) = 9.349, p = 0.0068; Sidak's post hoc test, p = 0.0012). The same light stimulation, however, did not affect remote memory if it was delivered 30 min before retrieval test (Fig. 5D; repeated-measures two-way ANOVA, group × time interaction, F(1,12) = 0.2199, p = 0.6475; Sidak's post hoc test, p > 0.9999), indicating that the 15 min silencing of auditory cortical input itself does not produce a nonspecific deleterious effect on retrieval of remote fear memory. Therefore, our results suggest that the auditory cortical input to the LA selectively contributes to consolidation of remote fear memory.
Figure 5.
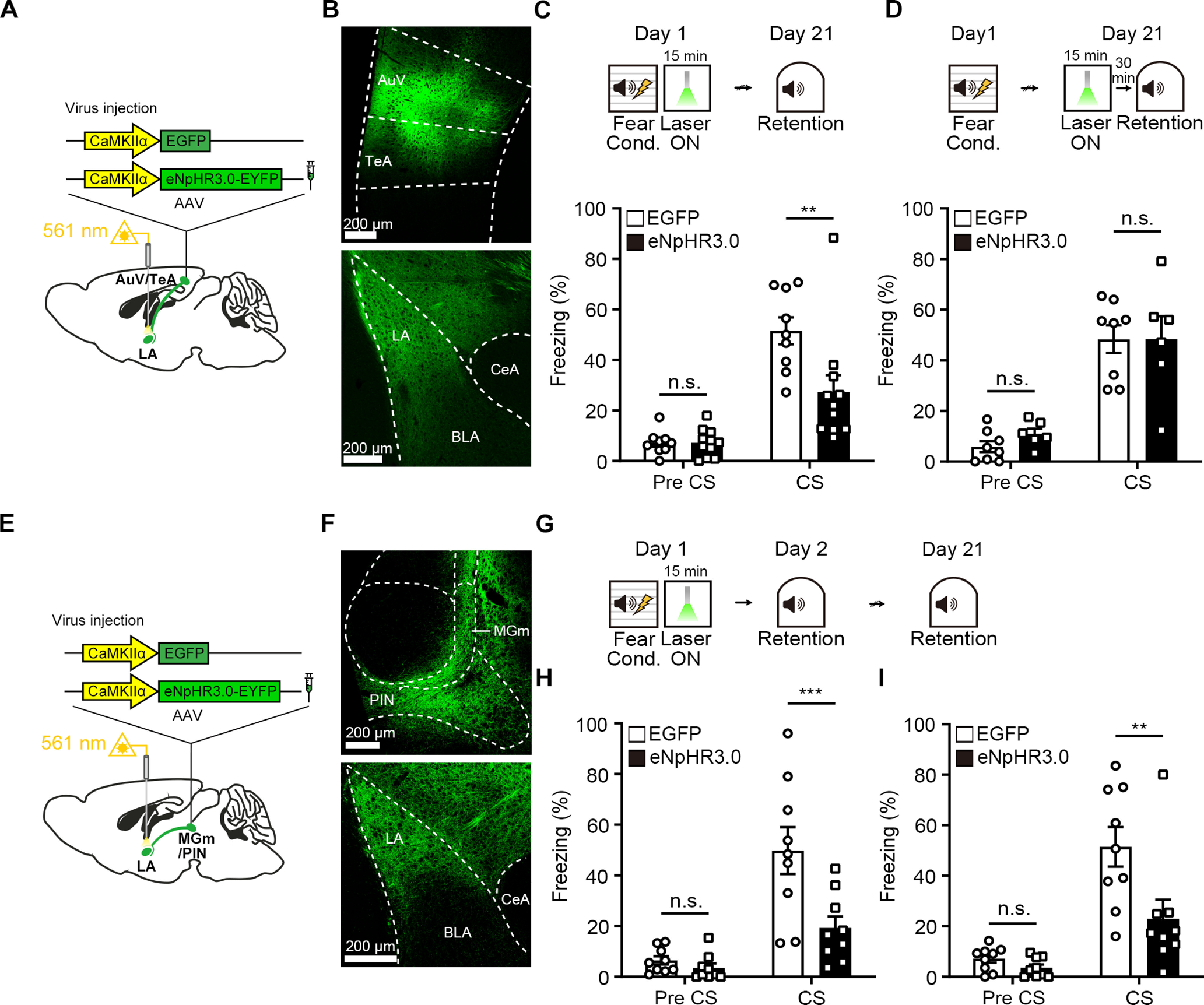
Post-training selective inhibition of cortical input to the LA impairs remote fear memory. A, Schematic depiction of AAV virus injection for eNpHR3.0-EYFP or EGFP expression in the AuV/TeA. B, Representative confocal microscopic images showing expression of eNpHR3.0-EYFP in the AuV/TeA neurons and their terminals in the LA. C, Top, Experimental procedure for photoinhibition of cortical input to the LA immediately after FC. Bottom, Percentage of time spent freezing during remote memory retention was reduced by photoinhibition in the eNpHR3.0 (n = 11 mice) compared with the EGFP group (n = 9 mice). D, Top, Experimental procedure for inhibiting cortical input in the LA 30 min before remote auditory fear memory test. Bottom, There was no significant difference in tone-induced freezing level between groups at remote memory test (EGFP, n = 8 mice; eNpHR3.0, n = 6 mice). E, Schematic depiction of AAV virus injection for eNpHR3.0-EYFP or EGFP expression in the MGm/PIN. F, Representative confocal microscopic images showing expression of eNpHR3.0-EYFP in the MGm/PIN neurons and their terminals in the LA. G, Experimental procedure for photoinhibition of thalamic input to the LA immediately after FC. H, Percentage of time spent freezing during recent memory retention was reduced by photoinhibition in the eNpHR3.0 compared with the EGFP group (n = 9 mice per group). I, In the subsequent remote memory test, mice in the eNpHR3.0 group displayed less freezing than mice in the control EGFP group (n = 9 mice per group). Data are mean ± SEM. ***p < 0.001. **p < 0.01.
Although we found that post-training inhibition of thalamic input disrupted consolidation of recent memory, it is unknown whether this effect is specific to recent memory without affecting remote memory. To test this possibility, we performed an additional optogenetic behavior experiment. As before, mice were injected with either AAV-eNpHR3.0-EYFP or AAV-EGFP virus into the bilateral MGm/PIN and received 561 nm light for 15 min immediately after FC (Fig. 5E–G). This time the same mice were tested twice for fear memory recall 1 and 20 d later (Fig. 5G). Consistent with our previous observation, we found that mice injected with AAV-eNpHR3.0-EYFP displayed significantly less freezing to tone than a control group during recent memory test (Fig. 5H; repeated-measures two-way ANOVA, group × time interaction, F(1,16) = 10.22, p = 0.0056; Sidak's post hoc test, p = 0.0006). When these mice were tested again 20 d after FC, eNpHR3.0-mice still displayed significantly less freezing to tone than the control group (Fig. 5I; repeated-measures two-way ANOVA, group × time interaction, F(1,16) = 6.289, p = 0.0233; Sidak's post hoc test, p = 0.0019). These results suggest that post-training activity in thalamic inputs is not specifically involved in consolidation of recent memory but rather required for permanent memory storage.
Discussion
Our results suggest that inputs from the auditory thalamus and cortex to the LA are required during early post-training periods for consolidation of long-term fear memory. This conclusion is supported by the data showing that an optogenetic inhibition of thalamic input for 15 min immediately after FC impaired fear memory at both recent and remote time point, whereas an optogenetic inhibition of cortical input impaired fear memory at remote time point without affecting recent memory. Because short-term memory was intact with an optogenetic inhibition of both auditory inputs, it is not that memory impairment was caused by disruption of memory acquisition. The disconnection of auditory inputs to the LA for 15 min may nonspecifically affect a general amygdala function required for normal freezing behavior. However, given the same post-training optical inhibition did not affect freezing to conditioned context, this is unlikely. Importantly, the effect of optical inhibition on consolidation of fear memory was contingent on the time point after training such that when light was delivered later time points than immediately after training, fear memory was not affected, indicating that the inhibition of auditory inputs to the LA for 15 min itself does not perturb retrieval process.
Previous works using pharmacological inactivation of the BLA suggested that synaptic activity in the BLA is not required during post-training periods for consolidation of Pavlovian FC (Wilensky et al., 1999, 2000). Based on these findings, it has been generally thought that sensory information (e.g., tone and shock) enters the LA during learning, and afterward consolidation is accomplished by intracellular signaling processes in the amygdala. According to this model, the only post-training manipulations that would affect consolidation must be those that alter intracellular signaling cascades. Our finding that post-training optogenetic inhibition of LA inputs disrupts consolidation challenges this view. Instead, our results propose an updated model that the ongoing activity in auditory inputs to the LA is required even after learning for consolidation of fear memory. One big difference between the prior works and ours is the methodology used to inhibit synaptic activity. Optogenetic tools provide better temporal and spatial precision than drugs. Moreover, pharmacological methods, although useful, often involve a confounding effect because of a functional compensation (Goshen et al., 2011). These differences could explain the discrepancy between the prior works and ours.
We hypothesize that the post-training activity of auditory CS inputs to the LA may contribute to stabilization of an interregional network of cell ensembles that is crucial for later retrieval of memory. As a possible mechanism, we consider that the ongoing activity in these inputs triggers a synaptic reinforcement process that presumably enables formation of a long-lasting form of synaptic changes in an input-specific manner. Consistent with this idea, a previous study has shown that post-training reactivation of NMDAR in the hippocampal CA1 region is crucial for memory consolidation (Shimizu et al., 2000). It is unclear how the auditory inputs to the LA could continue to be active even after training. One intriguing idea is that hormonal or neuromodulatory systems activated by emotionally arousing learning experience somehow keep these inputs active during post-learning periods. Extensive evidence indeed suggests that post-training noradrenergic activation in the BLA is critical for consolidation of emotionally arousing experiences (Liang et al., 1986; LaLumiere et al., 2003; McGaugh and Roozendaal, 2009). We speculate that noradrenergic or possibly other neuromodulatory effects on the auditory axons in the LA or on the neurons in the auditory areas could mediate the prolonged activation of these inputs. These ideas remain to be determined.
The old model of fear learning generally suggested that thalamic inputs, but not cortical inputs, to the LA are important for auditory FC (LeDoux, 2000; Herry and Johansen, 2014). Although either auditory thalamic and cortical pathways can fully support auditory FC (Romanski and LeDoux, 1992), several lines of evidence suggest that thalamic projection is the primary CS pathway in auditory FC. In vitro electrophysiological study found that fear-conditioned rats exhibit enhanced synaptic transmission in the thalamic-amygdala pathway (Mckernan and Shinnick-Gallagher, 1997). LTP can occur in the thalamic pathway in response to tetanic stimulation and FC (Rogan and LeDoux, 1995; Rogan et al., 1997). Plasticity to the CS in the thalamus and the amygdala develops faster than plasticity in the auditory cortex (McEchron et al., 1995, 1996; Quirk et al., 1997). A recent optogenetic study has shown that specific neurons in the lateral thalamus convey the association of tone and shock signals to the LA, and optogenetic silencing of their inputs to the LA prevents auditory FC. Different from this old view, however, by testing memory at remote time, our study reveals a crucial role of auditory cortical inputs to the LA for auditory FC. It is interesting that the inhibition of cortical input pathway during such a short time period (15 min) right after learning selectively affected remote, not recent, fear memory. Memories are thought to undergo a systems-level reorganization over time, known as systems consolidation (Marr, 1971; Frankland and Bontempi, 2005; Bergstrom, 2016). Thus, one possible explanation of our results is that the cortical input to the LA may be critically involved during a short period of time immediately after FC in systems consolidation of auditory cued fear memory. A previous study in rats indicates that a functional connectivity is established between higher-order sensory cortex and basolateral amygdala during consolidation of remote auditory fear memory likely through interactions between these brain structures, and this connectivity is selectively necessary for the retrieval of remote but not recent fear memory (Sacco and Sacchetti, 2010; Cambiaghi et al., 2016a; Manassero et al., 2018). On the basis of these findings, we consider that the cortical input to the LA during early post-training periods may be required for the development of such interregional functional connectivity necessary for remote retrieval of fear memory. Our results suggest that post-training activity in thalamic inputs is required for consolidation of both recent and remote fear memory. This may reflect that plasticity at auditory thalamic inputs to the LA is involved in permanent storage of auditory fear memory. Supporting the involvement of auditory thalamus in remote fear memory retrieval, expression of zif268 is elevated in MGm/PIN at remote time points (28 d) (Kwon et al., 2012; Bergstrom, 2016). In this scenario, the post-training activity in thalamic inputs could contribute to stabilize the functional connectivity established by FC between the auditory thalamus and the LA. Alternatively, the auditory thalamic pathway to the LA may be specifically involved in recent memory, but, perhaps because some processes in recent memory consolidation are sequentially linked to remote memory consolidation, the inhibition of post-training thalamic inputs also affected remote memory. Interestingly, a recent study has demonstrated a specific engagement of thalamic nucleus reuniens inputs to the BLA for extinction of remote, but not recent, contextual fear memory (Silva et al., 2021). It has also been shown that retrieval circuits for auditory fear memory are temporally shifted (Do-Monte et al., 2015). While it has been shown that the auditory thalamic pathway to the LA is crucial for retrieval of recent auditory fear memory (Barsy et al., 2020), whether the same thalamic pathway is also engaged in remote memory is unclear. This question remains to be determined.
Consolidation of memory is thought to depend on post-encoding coordinated reactivation of ensemble of neurons active during an event that are distributed broadly throughout multiple brain structures (Marr, 1971; Buzsáki, 1989; Wilson and McNaughton, 1994; Hoffman and McNaughton, 2002; Girardeau et al., 2009; Carr et al., 2011). Consistent with this idea, it has been shown that post-training suppression of a specific subset of neurons in the LA or dentate gyrus participating in encoding fear memory impairs consolidation of memory (Hsiang et al., 2014; Park et al., 2016). Moreover, optogenetic inhibition of the engram population in the primary visual cortex during post-conditioning sleep disrupts consolidation of fear memory, suggesting that reactivation of learning-activated sensory population during post-learning sleep plays an instructive role for memory consolidation (Clawson et al., 2021). A recently published report found that LA-projecting neurons in the auditory thalamus can control the activity patterns of the amygdala in response to multimodal and associated signals, suggesting that thalamic pathways could contribute to the amygdala oscillatory activity (Paré et al., 1995; Pape and Driesang, 1998; Barsy et al., 2020). In addition, during post-learning offline periods, the synchronous activity of neuronal ensembles, regulated by top-down cortical input, promotes memory consolidation and reactivation of postsynaptic neurons (Miyamoto et al., 2016). Therefore, the auditory inputs may provide necessary inputs for the coordinated reactivation of LA cells within an engram.
In conclusion, our findings suggest that inputs from both the auditory thalamus and cortex to the LA are required during early post-training periods for consolidation of long-term fear memory, with dissociated role for recent (thalamic input) and remote (cortical input) memory formation. These results support the idea that coordinate reactivation of memory traces distributed between the sensory brain areas and the LA is crucial for consolidation of associative fear memory.
Footnotes
This work was supported by the National Research Foundation of Korea Grant 2018R1A2B3004486 (Korea government).
The authors declare no competing financial interests.
References
- Apergis-Schoute AM, Debiec J, Doyère V, LeDoux JE, Schafe GE (2005) Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci 25:5730–5739. 10.1523/JNEUROSCI.0096-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsy B, Kocsis K, Magyar A, Babiczky Á, Szabó M, Veres JM, Hillier D, Ulbert I, Yizhar O, Mátyás F (2020) Associative and plastic thalamic signaling to the lateral amygdala controls fear behavior. Nat Neurosci 23:625–637. 10.1038/s41593-020-0620-z [DOI] [PubMed] [Google Scholar]
- Bergstrom HC (2016) The neurocircuitry of remote cued fear memory. Neurosci Biobehav Rev 71:409–417. 10.1016/j.neubiorev.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE (1994) Response properties of single units in areas of rat auditory thalamus that project to the amygdala: II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res 98:275–286. 10.1007/BF00228415 [DOI] [PubMed] [Google Scholar]
- Brioni JD, Nagahara AH, McGaugh JL (1989) Involvement of the amygdala GABAergic system in the modulation of memory storage. Brain Res 487:105–112. 10.1016/0006-8993(89)90945-1 [DOI] [PubMed] [Google Scholar]
- Buzsáki G (1989) Two-stage model of memory trace formation: a role for 'noisy' brain states. Neuroscience 31:551–570. 10.1016/0306-4522(89)90423-5 [DOI] [PubMed] [Google Scholar]
- Cambiaghi M, Grosso A, Likhtik E, Mazziotti R, Concina G, Renna A, Sacco T, Gordon JA, Sacchetti B (2016a) Higher-order sensory cortex drives basolateral amygdala activity during the recall of remote, but not recently learned fearful memories. J Neurosci 36:1647–1659. 10.1523/JNEUROSCI.2351-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambiaghi M, Grosso A, Renna A, Sacchetti B (2016b) Differential recruitment of auditory cortices in the consolidation of recent auditory fearful memories. J Neurosci 36:8586–8597. 10.1523/JNEUROSCI.0561-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM (2011) Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 14:147–153. 10.1038/nn.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, McGaugh JL (1990) Effects of post-training bicuculline and muscimol on retention: lack of state dependency. Behav Neural Biol 54:156–164. 10.1016/0163-1047(90)91352-c [DOI] [PubMed] [Google Scholar]
- Clawson BC, Pickup EJ, Ensing A, Geneseo L, Shaver J, Gonzalez-Amoretti J, Zhao M, York AK, Kuhn FR, Swift K, Martinez JD, Wang L, Jiang S, Aton SJ (2021) Causal role for sleep-dependent reactivation of learning-activated sensory ensembles for fear memory consolidation. Nat Commun 12:1200. 10.1038/s41467-021-21471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay T, Abs E, Poorthuis RB, Hartung J, Pu DL, Onasch S, Lozano YR, Signoret-Genest J, Tovote P, Gjorgjieva J, Letzkus JJ (2019) A critical role for neocortical processing of threat memory. Neuron 104:1180–1194. 10.1016/j.neuron.2019.09.025 [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Quiñones-Laracuente K, Quirk GJ (2015) A temporal shift in the circuits mediating retrieval of fear memory. Nature 519:460–463. 10.1038/nature14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM (1993) Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci 107:539–551. 10.1037//0735-7044.107.4.539 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B (2005) The organization of recent and remote memories. Nat Rev Neurosci 6:119–130. 10.1038/nrn1607 [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB (2009) Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12:1222–1223. 10.1038/nn.2384 [DOI] [PubMed] [Google Scholar]
- Girardeau G, Inema I, Buzsáki G (2017) Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat Neurosci 20:1634–1642. 10.1038/nn.4637 [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Dynamics of retrieval strategies for remote memories. Cell 147:678–689. 10.1016/j.cell.2011.09.033 [DOI] [PubMed] [Google Scholar]
- Grosso A, Cambiaghi M, Renna A, Milano L, Roberto Merlo G, Sacco T, Sacchetti B (2015) The higher order auditory cortex is involved in the assignment of affective value to sensory stimuli. Nat Commun 6:8886. 10.1038/ncomms9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennevin E, Maho C, Hars B, Dutrieux G (1993) Learning-induced plasticity in the medial geniculate nucleus is expressed during paradoxical sleep. Behav Neurosci 107:1018–1030. 10.1037//0735-7044.107.6.1018 [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP (2014) Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 17:1644–1654. 10.1038/nn.3869 [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL (2002) Coordinated reactivation of distributed memory traces in primate neocortex. Science 297:2070–2073. 10.1126/science.1073538 [DOI] [PubMed] [Google Scholar]
- Hsiang HL, Epp JR, van den Oever MC, Yan C, Rashid AJ, Insel N, Ye L, Niibori Y, Deisseroth K, Frankland PW, Josselyn SA (2014) Manipulating a 'cocaine engram' in mice. J Neurosci 34:14115–14127. 10.1523/JNEUROSCI.3327-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT (2013) Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc Natl Acad Sci USA 110:3597–3602. 10.1073/pnas.1219593110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Schmitz PK, Medina JH (1997) Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur J Neurosci 9:786–793. 10.1111/j.1460-9568.1997.tb01427.x [DOI] [PubMed] [Google Scholar]
- Jeong Y, Cho HY, Kim M, Oh JP, Kang MS, Yoo M, Lee HS, Han JH (2021) Synaptic plasticity-dependent competition rule influences memory formation. Nat Commun 12:1–13. 10.1038/s41467-021-24269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JT, Jhang J, Kim HS, Lee S, Han JH (2012) Brain region-specific activity patterns after recent or remote memory retrieval of auditory conditioned fear. Learn Mem 19:487–494. 10.1101/lm.025502.112 [DOI] [PubMed] [Google Scholar]
- Kwon JT, Nakajima R, Kim HS, Jeong Y, Augustine GJ, Han JH (2014) Optogenetic activation of presynaptic inputs in lateral amygdala forms associative fear memory. Learn Mem 21:627–633. 10.1101/lm.035816.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL (2003) Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci 23:6754–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Liang K, Juler RG, McGaugh J (1986) Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res 368:125–133. 10.1016/0006-8993(86)91049-8 [DOI] [PubMed] [Google Scholar]
- Liang KC, Hon W, Davis M (1994) Pre- and posttraining infusion of N-methyl-D-aspartate receptor antagonists into the amygdala impair memory in an inhibitory avoidance task. Behav Neurosci 108:241–253. 10.1037/0735-7044.108.2.241 [DOI] [PubMed] [Google Scholar]
- Manassero E, Renna A, Milano L, Sacchetti B (2018) Lateral and basal amygdala account for opposite behavioral responses during the long-term expression of fearful memories. Sci Rep 8:518. 10.1038/s41598-017-19074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA (2001) The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci 21:RC135. 10.1523/JNEUROSCI.21-06-j0001.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1971) Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci 262:23–81. 10.1098/rstb.1971.0078 [DOI] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N (1995) Simultaneous single unit recording in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit's classically conditioned heart rate. Brain Res 682:157–166. 10.1016/0006-8993(95)00331-J [DOI] [PubMed] [Google Scholar]
- McEchron MD, Green EJ, Winters RW, Nolen TG, Schneiderman N, McCabe PM (1996) Changes of synaptic efficacy in the medial geniculate nucleus as a result of auditory classical conditioning. J Neurosci 16:1273–1283. 10.1523/JNEUROSCI.16-03-01273.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B (2009) Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 202:3–14. 10.1007/s00213-008-1285-6 [DOI] [PubMed] [Google Scholar]
- McKernan M, Shinnick-Gallagher P (1997) Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390:607–611. 10.1038/37605 [DOI] [PubMed] [Google Scholar]
- Miyamoto D, Hirai D, Fung CC, Inutsuka A, Odagawa M, Suzuki T, Boehringer R, Adaikkan C, Matsubara C, Matsuki N, Fukai T, McHugh TJ, Yamanaka A, Murayama M (2016) Top-down cortical input during NREM sleep consolidates perceptual memory. Science 352:1315–1318. 10.1126/science.aaf0902 [DOI] [PubMed] [Google Scholar]
- Moczulska KE, Tinter-Thiede J, Peter M, Ushakova L, Wernle T, Bathellier B, Rumpel S (2013) Dynamics of dendritic spines in the mouse auditory cortex during memory formation and memory recall. Proc Natl Acad Sci USA 110:18315–18320. 10.1073/pnas.1312508110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, Ölveczky BP (2015) Acute off-target effects of neural circuit manipulations. Nature 528:358–363. 10.1038/nature16442 [DOI] [PubMed] [Google Scholar]
- Pape HC, Driesang RB (1998) Ionic mechanisms of intrinsic oscillations in neurons of the basolateral amygdaloid complex. J Neurophysiol 79:217–226. 10.1152/jn.1998.79.1.217 [DOI] [PubMed] [Google Scholar]
- Paré D, Pape HC, Dong J (1995) Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol 74:1179–1191. 10.1152/jn.1995.74.3.1179 [DOI] [PubMed] [Google Scholar]
- Parent MB, McGaugh JL (1994) Posttraining infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance training. Brain Res 661:97–103. 10.1016/0006-8993(94)91186-x [DOI] [PubMed] [Google Scholar]
- Park S, Kramer EE, Mercaldo V, Rashid AJ, Insel N, Frankland PW, Josselyn SA (2016) Neuronal allocation to a hippocampal engram. Neuropsychopharmacology 41:2987–2993. 10.1038/npp.2016.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB (2019) Paxinos and Franklin's the mouse brain in stereotaxic coordinates. San Diego: Academic. [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Léna C, Paré D (2010) Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci USA 107:6516–6519. 10.1073/pnas.0913016107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE (1997) Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19:613–624. 10.1016/S0896-6273(00)80375-X [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE (1995) LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron 15:127–136. 10.1016/0896-6273(95)90070-5 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE (1997) Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390:604–607. 10.1038/37601 [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE (1992) Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J Neurosci 12:4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Weinberger NM (1978) Differential plasticity of morphologically distinct neuron populations in the medical geniculate body of the cat during classical conditioning. Behav Biol 22:275–301. 10.1016/s0091-6773(78)92351-9 [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C (1999) Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci 19:9570–9578. 10.1523/JNEUROSCI.19-21-09570.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B (2010) Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329:649–656. 10.1126/science.1183165 [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ (2000) NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science 290:1170–1174. 10.1126/science.290.5494.1170 [DOI] [PubMed] [Google Scholar]
- Silva BA, Astori S, Burns AM, Heiser H, van den Heuvel L, Santoni G, Martinez-Reza MF, Sandi C, Gräff J (2021) A thalamo-amygdalar circuit underlying the extinction of remote fear memories. Nat Neurosci 24:964–974. 10.1038/s41593-021-00856-y [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hasegawa M, Benoit CM, Freire JA, Theodore M, Ganea DA, Innocenti SM, Lu T, Gründemann J (2021) Single cell plasticity and population coding stability in auditory thalamus upon associative learning. Nat Commun 12:2438. 10.1038/s41467-021-22421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL (1999) Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci 19:6615–6622. 10.1523/JNEUROSCI.19-15-06615.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom KL, Huff ML, Emmons EB, Freeman JH, Narayanan NS, McIntyre CK, LaLumiere RT (2018) Basolateral amygdala inputs to the medial entorhinal cortex selectively modulate the consolidation of spatial and contextual learning. J Neurosci 38:2698–2712. 10.1523/JNEUROSCI.2848-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM (2004) Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5:279–290. 10.1038/nrn1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE (1999) Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci 19:RC48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE (2000) The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci 20:7059–7066. 10.1523/JNEUROSCI.20-18-07059.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 265:676–679. 10.1126/science.8036517 [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu DQ, Huang W, Deng J, Sun Y, Zuo Y, Poo MM (2016) Selective synaptic remodeling of amygdalocortical connections associated with fear memory. Nat Neurosci 19:1348–1355. 10.1038/nn.4370 [DOI] [PubMed] [Google Scholar]
- Zanatta M, Quillfeldt J, Schaeffer E, Schmitz P, Quevedo J, Medina J, Izquierdo I (1997) Involvement of the hippocampus, amygdala, entorhinal cortex and posterior parietal cortex in memory consolidation. Braz J Med Biol Res 30:235–240. 10.1590/s0100-879x1997000200012 [DOI] [PubMed] [Google Scholar]