Abstract
We used an allelogenic Cre/loxP gene targeting strategy in mice to determine the role of cytosolic phosphoenolpyruvate carboxykinase (PEPCK) in hepatic energy metabolism. Mice that lack this enzyme die within 3 days of birth, while mice with at least a 90% global reduction of PEPCK, or a liver-specific knockout of PEPCK, are viable. Surprisingly, in both cases these animals remain euglycemic after a 24-h fast. However, mice without hepatic PEPCK develop hepatic steatosis after fasting despite up-regulation of a variety of genes encoding free fatty acid-oxidizing enzymes. Also, marked alterations in the expression of hepatic genes involved in energy metabolism occur in the absence of any changes in plasma hormone concentrations. Given that a ninefold elevation of the hepatic malate concentration occurs in the liver-specific PEPCK knockout mice, we suggest that one or more intermediary metabolites may directly regulate expression of the affected genes. Thus, hepatic PEPCK may function more as an integrator of hepatic energy metabolism than as a determinant of gluconeogenesis.
Gluconeogenesis is the process whereby glucose is formed from noncarbohydrate metabolic substrates such as lactate and alanine. This metabolic pathway occurs predominately in the liver and kidney and is essential for the production of glucose during extended fasting when glycogen stores have been depleted (18, 23). A key step in gluconeogenesis is the formation of phosphoenolpyruvate from oxaloacetate, which is catalyzed by phosphoenolpyruvate carboxykinase (PEPCK). This reaction has long been thought to be essential for gluconeogenesis since it bypasses the thermodynamically unfavorable conversion of pyruvate to phosphoenolpyruvate by pyruvate kinase (20). Although both a cytosolic and a mitochondrial isoform of PEPCK are expressed in rodents, the cytosolic isoform accounts for over 95% of the activity in the liver and kidney (30, 45).
Several lines of evidence have led to the widely held notion that PEPCK is the rate-determining step in hepatic and renal gluconeogenesis. First, the cytosolic isoform of PEPCK is adaptively regulated at the transcriptional level in a manner that correlates with alterations in gluconeogenic flux (5, 12, 25, 47). Second, treatment of fasted animals with 3-mercaptopicolinic acid, a PEPCK inhibitor, causes hypoglycemia (8). Third, overexpression of PEPCK in both cell lines and transgenic mice causes either increased gluconeogenesis or hyperglycemia (37, 44). However, other data suggest that gluconeogenic flux is determined by alterations in activities of multiple enzymes, not just PEPCK. Indeed, in one study both pyruvate carboxylase and pyruvate kinase were suggested to play a greater role in determining gluconeogenic flux in isolated rat hepatocytes than PEPCK (13). Also, results of a perfused rat liver study indicated that pyruvate carboxylase rather than PEPCK is a primary rate-determining step for gluconeogenesis (10).
Energy metabolism in the liver involves the interconversion of carbohydrates, lipids, and amino acids. The pathways involved are regulated by multiple mechanisms. First, allosteric effectors or covalent modifications affect the activity of certain enzymes. Second, substrate availability itself may play an essential role. Third, hormones and other extrahepatic effectors regulate the expression of genes encoding various enzymes. However, the mechanisms that regulate flux between different pathways have not been thoroughly elucidated. Since PEPCK catalyzes a reaction near the intersection of several fundamentally important pathways, the absence of this enzyme might have unpredictable effects on the accumulation of specific metabolites. Moreover, excess hepatic glucose production is a major factor contributing to fasting hyperglycemia in both type 1 and type 2 diabetes mellitus (6, 7). Thus, knowledge of how gluconeogenesis is regulated is of both fundamental and clinical importance.
To gain greater insights into the role of PEPCK in both gluconeogenesis and hepatic energy metabolism, we used an allelogenic Cre/loxP gene targeting strategy to generate mice that had variable degrees of impaired PEPCK gene expression. In addition, by intercrossing mice with a conditional PEPCK gene locus with a line of albumin (Alb)-cre transgenic animals, we were also able to produce mice with a liver-specific PEPCK gene knockout. Studies of these mice indicate that while PEPCK has a much lower than expected effect on gluconeogenesis, the enzyme plays an unexpected role in integrating metabolic pathways in the liver.
MATERIALS AND METHODS
Targeting vector.
The targeting vector shown in Fig. 1A contains a phosphoglycerol kinase (pgk)-neomycin resistance (neo) gene cassette, a pgk-herpes simplex virus type 1 thymidine kinase (tk) gene cassette, and three loxP sites. The vector was assembled in pNTK(A) using loxP sites from pBS246 (39) and mouse PEPCK gene fragments isolated from a 129/SvJ Bac genomic DNA clone (46). The long arm of the targeting vector, a 6-kb KpnI fragment, was ligated into the ClaI site of pBS.LP, thereby forming BSLP.PEPCK.LA. A 1-kb excisable KpnI DNA fragment containing exons 4 and 5 was ligated into the ClaI site of pNTK(A), thereby forming mPEP.B3. The short arm of the targeting vector was a 2.1-kb KpnI/XbaI fragment that was ligated into the BamHI site of mPEP.B3, forming mPEP.K2. After destroying a SalI site in mPEP.K2, the 6-kb SalI fragment of BSLP.PEPCK.LA was cloned into the remaining SalI site of mPEP.K2, creating the final construct, mPEPCK.KO2. The correct assembly of mPEPCK.KO2 was confirmed by DNA sequencing.
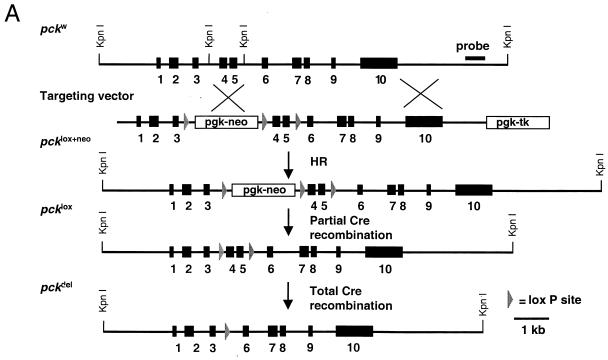
FIG. 1.
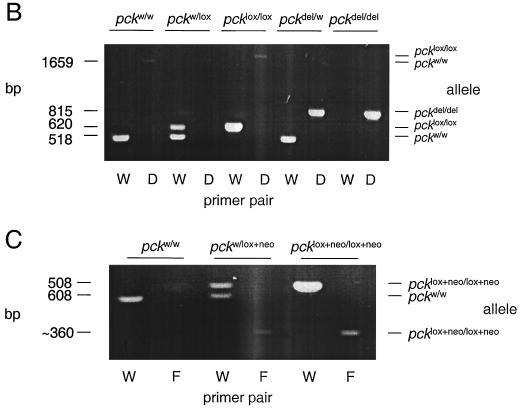
pck alleles generated by gene targeting and Cre-mediated recombination. (A) Top, partial map of the pckw allele. Exons are indicated as solid rectangles. The location of the DNA fragment used as the Southern hybridization probe is shown. Middle, map of the PEPCK gene targeting vector. The vector contains a pgk-neo cassette, a pgk-tk cassette, and three loxP sites (triangles). Two of the loxP sites flank neo, and the third is located between exons 4 and 5 in the PEPCK gene. The pcklox+neo allele was generated by homologous recombination (HR) in ES cells. Bottom, the pcklox and pckdel alleles, derived from pcklox+neo by Cre-mediated recombination. Exons 4 and 5 and neo were excised by Cre microinjection of single-cell pcklox+neo/w embryos. (B and C) PCR genotype analysis. Tail DNA was amplified by both primer pair W (5′-TCTGTCAGTTCAATACCAATCT-3′), 5′-AATGTTCTCTGCAAGTCCTGGTG-3′) and primer pair D (5′-ATCAGCTTTAGTCGTCTCTGGT-3′, 5′-AATGTTCTCTGCAAGTCCTGGTG-3′) or primer pair F (5′-TCTGTCAGTTCAATACCAATCT-3′, 5′-AGCCTCTGTTCCACATACACTTCA-3′. The amplified alleles and their sizes are shown on the right and left, respectively. (D) Western blot analysis of liver homogenates from pckw/w (lane 1), pckdel/w (lane 2), and pckdel/del (lane 3) mice.
Gene targeting and production of chimeras.
Fifty micrograms of the targeting vector was linearized with NotI and then electroporated into 5 × 107 TL-1 embryonic cells (ES) cells, which were derived from 129/SvEvTac mice (22). Southern blot analysis of several clones resistant to both G418 and ganciclovir revealed one clone, 2D7, that had undergone the desired recombination event (data not shown). The ES cell clone was microinjected into C57BL/6 blastocysts, which were then implanted into pseudopregnant female recipients to produce chimeras. Germ line transmission of the targeted pcklox+neo allele was confirmed by Southern blotting and PCR analysis (data not shown).
Conversion of the pcklox+neo allele to a pcklox or pckdel allele.
The pcklox+neo allele was converted to both a pcklox and a pckdel allele by pronuclear microinjection of supercoiled pBS185 (39), a cytomegalovirus-Cre expression plasmid, into single-cell mouse embryos derived from mating pcklox+neo males with superovulated B6D2 F1 hybrid female (1, 35).
Genotype analysis.
Four different pck alleles (pckw, pcklox+neo, pcklox, and pckdel [w and del denote wild type and deletion, respectively]) were routinely distinguished by PCR analysis (Fig. 1B and C). pckw and pcklox were detected using primers 5′-TCTGTCAGTTCAATACCAATCT-3′ and 5′-AATGTTCTCTGCAAGTCCTGGTG-3′. A 518-bp fragment was generated from pckw, and a 620-bp fragment was generated from pcklox. For the pcklox+neo allele, a 360-bp fragment was amplified using 5′-TCTGTCAGTTCAATACCAATCT-3′ and 5′-AGCCTCTGTTCCACATACACTTCA-3′. The pckdel allele was detected as a 815-bp fragment using primers 5′-ATCAGCTTTAGTCGTCTCTGGT-3′ and 5′-AATGTTCTCTGCAAGTCCTGGTG-3′. The Alb-cre transgene (35) was detected by PCR with primers 5′-ACCTGAAGATGTTCGCGATTATCT-3′ and 5′-ACCGTCAGTACGTGAGATATCTT-3′, which yielded a 370-bp fragment.
Animals.
All mice were specific pathogen free, maintained on a 12-h light-dark cycle, and fed a standard rodent chow (Purina Mills, Inc., St. Louis, Mo.). Treatment and housing of animals met guidelines of the American Association for the Accreditation of Laboratory Animal Care, and the protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Northern blot analysis.
Total RNA was isolated using TRIzol (Life Technologies, Inc., Grand Island, N.Y.). Northern blot analysis was performed as previously described (28). The PEPCK probe was a 569-bp HindIII-EcoRI fragment spanning exons 1 to 4 of the mouse cDNA. cDNA clones for rat CYP4A1, rat CYP4A1, and mouse medium-chain fatty acyl coenzyme A (acyl-CoA) dehydrogenase were provided D. Kelly (Department of Internal Medicine, Washington University). Probes for pyruvate carboxylase, fructose-1,6-bisphosphatase, and glucose-6-phosphatase were prepared from human, pig, and rat cDNA clones, respectively. Probes (numbers in parentheses are those of the IMAGE consortium clones from which clones were prepared) for malonyl-CoA decarboxylase (692290), very long-chain fatty acyl-CoA dehydrogenase (2236538), long-chain fatty acyl-CoA dehydrogenase (2225775), acyl-CoA oxidase (1972335), carnitine octanoyltransferase (1451465), carnitine acetyltransferase (1889414), and enoyl- CoA hydratase–l-3-hydroxyacyl-CoA dehydrogenase bifunctional protein (2076790), cytosolic (2064729) and mitochondrial (1972463) aspartate aminotransferase, cytosolic (329958) and mitochondrial (2192286) malate dehydrogenase, citrate synthase (335882), isocitrate dehydrogenase (2136389), succinyl-CoA synthetase (2099765), lactate dehydrogenase (1451692), alanine aminotransferase (1886916), and glyceraldehyde-3-phosphate dehydrogenase (716832) were obtained from Research Genetics, Inc., Huntsville, Ala., and verified by DNA sequencing prior to use. The relative abundance of each mRNA was corrected for loading differences, using cyclophilin cDNA as a control probe.
PEPCK activity measurements and Western blot analysis.
PEPCK activity was measured using an NADH-coupled system to quantitate the conversion of phosphoenolpyruvate into oxaloacetate and subsequent conversion to malate (45). All assays were performed within 3 h of removal of tissue from the animal. Activity was expressed as milliunits per milligram of protein in liver supernatant (1 mU = 1 nmol of oxaloacetate produced/min). The protein content was determined using a Bradford assay kit (Bio-Rad, Hercules, Calif.), with bovine serum albumin as a standard. Western blot analysis was performed as previously described (16). A sheep anti-PEPCK antiserum (a gift from D. K. Granner, Vanderbilt University) was used at a 1:5,000 dilution.
In vivo glucose kinetics.
Glucose turnover rates and gluconeogenic index in the form of plasma [14C]glucose specific activity derived from [14C]lactate were measured as previously described (29), using mice in which both jugular and carotid catheters had been surgically implanted 5 days prior to study. The experimental protocol involved depriving animals of food for 26 h, followed by an experimental period of 220 min, which consisted both of a 100-min equilibration period (−100 to 0 min) and a 120-min experiment period. Basal glucose turnover and gluconeogenic index were determined by 2-μCi bolus injection and constant infusion at 0.5 μCi/min of high-pressure liquid chromatography-purified [3-3H]glucose (NEN Life Science Products, Boston, Mass.) for 220 min and constant infusion at 0.1 μCi/min of [U-14C]lactate (NEN Life Science Products) for 120 min beginning at 0 min. Blood samples were withdrawn from a carotid artery catheter at −30, 0, 30, 60, 90, and 120 min. Liquid chromatography was used to purify radioactive glucose in plasma. The samples were deproteinized by passage through a 9- by 190-mm column filled with 14-μm sulfonate polystyrene cation-exchange resin (Benson BH-4, Reno, Nev.) at a flow rate 2.2 ml/min.
Endurance exercise study.
Male mice that had been fasted for 24 h were acclimated to a treadmill for 2 min each at 13.4 and 16.1 meters/min and then run to exhaustion at 18.8 meters/min. Blood glucose concentration was measured with a Hemocue (Mission Viejo, Calif.) glucose meter immediately before, at 30 min, and at the end of the exercise.
Analytical procedures.
Plasma insulin, glucagon, and corticosterone concentrations were determined by radioimmunoassay using a rat insulin kit (Linco Research, St. Louis, Mo.), a rat glucagon kit (Linco Research), and a Coat-A-Count rat corticosterone kit (DPC, Los Angeles, Calif.), respectively. Plasma free fatty acid (FFA) concentrations were measured with a NEFA C kit (Wako Pure Chemical Industries, Osaka, Japan). Plasma triglyceride concentrations were measured using a colorimetric kit (Sigma, St. Louis, Mo.). Plasma glucose, lactate, glycerol, β-hydroxybutyrate (BHBA), and hepatic malate concentrations were determined by enzymatic assays using microassay procedures (3). Hepatic glycogen and triglyceride contents were analyzed as described elsewhere (3, 40). All results are presented as the mean ± standard error of the mean. Statistical significance was determined by one-way analysis of variance. P values of less than 0.05 were considered statistically significant.
Nucleotide sequence accession number.
The sequences altered in the pcklox allele were deposited in GenBank (accession number AF220498).
RESULTS
Targeting of the mouse pck gene.
We designed a Cre/loxP gene targeting strategy that allowed the creation of three different alleles of the pck gene from a single gene targeting event in ES cells (Fig. 1A). The parental pcklox+neo allele contains three loxP sites and a neo cassette. Two of the loxP sites flank a pgk-neo cassette which is located between exons 3 and 4, whereas a third loxP site lies downstream of exon 5. After its introduction into mice, the pcklox+neo allele was further modified by Cre-mediated recombination in single-cell pcklox+neo embryos. Partial recombination yielded a conditional allele (pcklox) that lacks neo but retains two loxP sites flanking exons 4 and 5. Complete recombination produced a null pck allele (pckdel) that contains a single loxP site and lacks exons 4 and 5. The generation of each allele was confirmed by both PCR (Fig. 1B and C) and Southern blot analysis (data not shown).
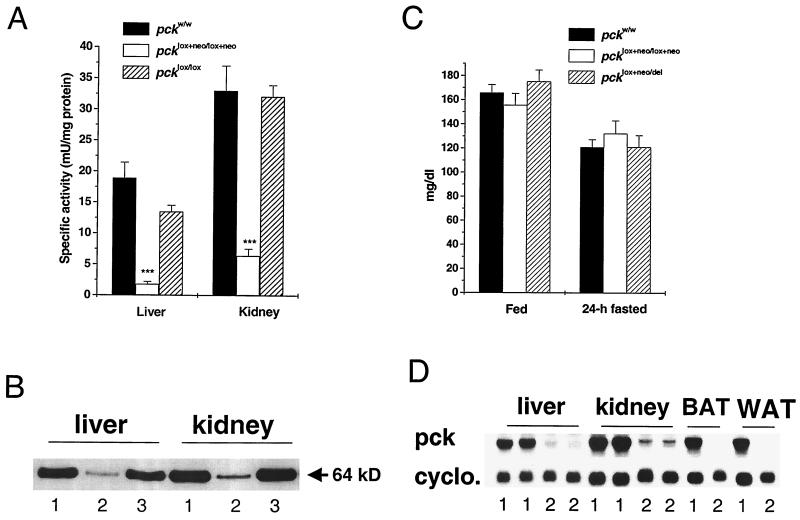
Interbreeding of animals that were heterozygous for either the pcklox+neo or pcklox allele yielded mice that were homozygous for each of these two alleles. In both cases the mice were viable, thereby enabling PEPCK activity and protein content to be determined from both the liver and kidney after a 24-h fast. PEPCK activity and protein amount in the pcklox/lox mice did not differ significantly from those of wild-type mice (Fig. 2A and B). In contrast, PEPCK activity and protein mass in pcklox+neo/lox+neo mice, which contains a neo cassette between exons 3 and 4, was reduced to about ∼10 and 20% of normal in the liver and kidney, respectively (Fig. 2A and B). Thus, the pcklox+neo allele is functionally attenuated, presumably due to interference with normal RNA processing as been observed in other genes that retain a neo cassette within an intron (27). Plasma glucose concentrations in fed and 24-h-fasted mice that were homozygous for the pcklox+neo allele did not differ from those in mice with two pckw alleles (Fig. 2C). However, the relative liver mass in pcklox+neo/lox+neo mice after a 24-h fast was increased by 30% (5.6% ± 0.4% versus 4.3% ± 0.1% of body weight for wild-type mice, P < 0.004, n = 8 to 10). Content of malate, a tricarboxylic acid (TCA) cycle intermediate, in fasting mice was 5.4-fold greater in the liver (1.57 ± 0.21 versus 0.29 ± 0.01 μM/g, P < 0.001, n = 6 to 7) and 2.4-fold greater in the kidney (0.36 ± 0.04 versus 0.15 ± 0.01 μM/g, P < 0.001, n = 6 to 7) of pcklox+neo/lox+neo mice than in wild-type mice. Plasma FFA concentrations in 24-h-fasted pcklox+neo/lox+neo mice were not different from that in control mice (1,345 ± 123 versus 1161 ± 74 μEq/liter n = 7 to 9).
FIG. 2.
Analysis of PEPCK expression in mice that are homozygous for the pcklox+neo and pcklox alleles and that are compound heterozygotes of pcklox+neo and pckdel alleles. (A) Hepatic and renal PEPCK activity in pcklox+neo/lox+neo and pcklox/lox mice fasted for 24 h. ∗∗∗, P < 0.001, n = 4. (B) Western blot analysis of liver and kidney tissues from 24-h-fasted mice. Lanes 1, 2, and 3 represent pckw/w, pcklox+neo/lox+neo, and pcklox/lox mice, respectively. (C) Plasma glucose concentration in pcklox+neo/lox+neo and pcklox+neo/del mice at fed and 24-fasted states (n = 7 to 9). (D) Northern blot analysis of liver, kidney, and brown and white adipose tissues (BAT and WAT) from 24-h-fasted mice. Lanes 1 and 2 represent pckw/w and pcklox+neo/del mice, respectively. cyclo., cyclophilin.
Characterization of pck null pups.
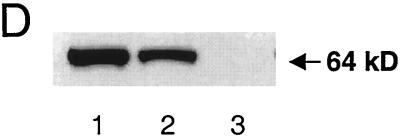
Mice that were homozygous null for PEPCK (i.e., pckdel/del) were obtained by interbreeding of pckdel/w mice. Genotype analysis of 97 1-day-old pups revealed 22 pckw/w (22.7%), 49 pckdel/w (50.5%), and 26 pckdel/del mice (26.8%), consistent with Mendelian inheritance. Western blot analysis of liver extracts confirmed the absence of PEPCK in pckdel/del mice. PEPCK protein content was decreased by approximately one-half in pckdel/w pups, indicating the lack of any significant compensatory changes in gene transcription (Fig. 1D). All pckdel/del pups died within 3 days of birth, mostly at days 2 and 3. Prior to becoming moribund, pckdel/del pups became lethargic and pale and were smaller than heterozygous null or normal littermates. Necropsy of the dead pckdel/del pups revealed empty stomachs and pale livers.
One-day-old pckdel/del mice were markedly hypoglycemic, with plasma glucose concentrations of 32 ± 7 mg/dl compared to control values of 90.3 ± 7.9 mg/dl. FFA concentrations were ∼2-fold greater in pckdel/del pups, but plasma lactate concentrations were unchanged (Table 1). Hepatic glycogen content in pckdel/del pups was only 38% of that in wild-type pups, although hepatic glycogen content of fetuses removed at embryonic day 20 did not differ among the three genotypes (553 ± 11 μM/g in pckw/w pups, 594 ± 26 in pckdel/w pups, and 551 ± 24 in pckdel/del pups, n = 3 to 6) and, in all cases, was greater than in postnatal day 1 (P1) pups (P < 0.001). Hepatic malate concentration was 10-fold greater in livers of the pckdel/del pups than in those of wild-type pups (Table 1). While the malate concentration was increased in the pckdel/w pups, although to a lesser degree, there were no differences in the plasma glucose, FFA, lactate, and hepatic glycogen concentrations of these animals compared to pckw/w pups (Table 1).
TABLE 1.
Plasma and hepatic metabolites in 1-day-old pckdel/del and pckdel/w pupsa
| Pup genotype | Concn (mean ± SE)
|
||||
|---|---|---|---|---|---|
| Plasma
|
Hepatic
|
||||
| Glucose (mg/dl) | FFA (μeq/liter) | Lactate (mg/dl) | Glycogen (μM/g) | Malate (μM/g) | |
| pckw/w | 90.3 ± 7.8 (9) | 830 ± 89 (9) | 37.5 ± 1.9 (13) | 210.0 ± 34.4 (8) | 0.68 ± 0.07 (6) |
| pckdel/w | 93.1 ± 6.9 (20) | 925 ± 92 (14) | 37.9 ± 2.0 (16) | 189.3 ± 14.0 (16) | 1.27 ± 0.14 (7)* |
| pckdel/del | 31.8 ± 7.2 (8)*** | 1,610 ± 210 (10)** | 41.6 ± 1.6 (13) | 79.1 ± 15.6 (7)** | 6.59 ± 0.22 (6)*** |
*, P < 0.05, **, P < 0.01, and ***, P < 0.001 versus pckw/w; n is indicated in parentheses.
PEPCK mRNA abundance was decreased by 42 and 33% in liver and brown adipose tissue of pckdel/w pups (P < 0.01, n = 4), respectively, compared to wild-type pups. Consistent with prior studies of the developmental expression of PEPCK (41), PEPCK mRNA in kidneys of 1-day-old pups was not detected by Northern blot analysis for either wild-type or knockout pups (data not shown). The body weight of pckdel/del pups was 6.8% less than that of pckw/w pups at P1 (P < 0.05).
Adult heterozygous pck null mice.
To assess the impact of the loss of one functional pck allele, we performed further studies on 8- to 10-week-old animals that were heterozygous null for PEPCK (pckdel/w). Hepatic PEPCK activities of fed and 24-h-fasted pckdel/w mice were 60 and 52%, respectively, of that of control animals (Table 2), indicating that a single functional pck allele in liver was only minimally capable of compensating for the loss of the second allele. Similarly, renal PEPCK activity in pckdel/w mice was approximately two-thirds of control level at both fed (7.4 ± 1.0 versus 10.6 ± 0.8 mU/mg of protein, P < 0.04, n = 4) and 24-h-fasted (23.2 ± 0.9 versus 39.4 ± 4.1 mU/mg of protein, P < 0.01, n = 4) states. Mitochondrial PEPCK activity in the liver was less than 2% of the total PEPCK activity and did not differ between pckdel/w and pckw/w mice (data not shown). PEPCK mRNA abundance was decreased by 35% in liver and by 33% in kidney in 24-h-fasted mice (data not shown). However, plasma glucose concentrations did not differ at either fed or fasted states (Table 2). Both hepatic (Table 2) and muscle (data not shown) glycogen contents were also similar. No significant differences were found in plasma FFA, BHBA, lactate, insulin, and hepatic malate concentrations between the two genotypes at both fed and fasted states (Table 2).
TABLE 2.
Plasma insulin concentration, plasma and hepatic metabolite concentrations, and hepatic PEPCK activity in fed and 24-h-fasted pckdel/w and pckw/w micea
| Pup genotype | Concn (mean ± SE)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plasma
|
Hepatic
|
||||||||
| Insulin (pg/ml) | Glucose (mg/dl) | FFA (μeq/liter) | Glycerol (mg/dl) | BHBA (mg/dl) | Lactate (mg/dl) | Glycogen (μM/g) | Malate (μM/g) | PEPCK activity (mU/mg of protein) | |
| Fed | |||||||||
| pckw/w | 908 ± 183 | 192.2 ± 4.7 | 649 ± 76 | 5.7 ± 0.6 | 3.5 ± 0.3 | 34.0 ± 3.2 | 402.4 ± 32.9 | 0.47 ± 0.03 | 6.5 ± 0.6 |
| pckdel/w | 874 ± 104 | 179.5 ± 6.9 | 648 ± 62 | 6.4 ± 0.8 | 3.3 ± 0.3 | 32.2 ± 1.8 | 369.8 ± 18.3 | 0.56 ± 0.07 | 3.9 ± 0.3** |
| 24 h fasted | |||||||||
| pckw/w | 178 ± 56 | 137.0 ± 7.1 | 1,199 ± 78 | 6.2 ± 0.8 | 16.6 ± 1.6 | 35.4 ± 23 | 51.3 ± 17.0 | 0.35 ± 0.03 | 16.2 ± 2.3 |
| pckdel/w | 235 ± 50 | 150.0 ± 6.9 | 1,052 ± 61 | 7.0 ± 0.8 | 13.5 ± 1.2 | 36 ± 2.3 | 58.5 ± 13.6 | 0.35 ± 0.03 | 8.5 ± 1.0* |
Age- and sex-matched mice 8 to 10 weeks old were sacrificed by cervical dislocation at 8 to 10 a.m., and blood was collected from neck vessels. *, P < 0.05, and **, P < 0.01 versus pckw/w; n = 8 except for PEPCK activity (n = 4).
Characterization of compound heterozygotes.
Given the lack of any effect of fasting on the plasma glucose concentration in either the pcklox+neo/lox+neo or pckdel/w mice, we proceeded to intercross pcklox+neo/lox+neo mice with pckdel/w mice. By doing so, we produced compound heterozygotes (pcklox+neo/del) that had 95, 90, and almost 100% reductions of PEPCK gene expression in liver, kidney, and adipose tissues, respectively, as judged Northern blot analysis (Fig. 2D). Plasma glucose concentrations in both fed and 24-h-fasted mice were no different from those in wild-type mice (Fig. 2C). However, in this case the relative liver mass in pcklox+neo/del mice after a 24-h fast was increased by 65% (7.1% ± 0.1% of body weight) and their livers were pale at necropsy.
Liver-specific knockout of PEPCK.
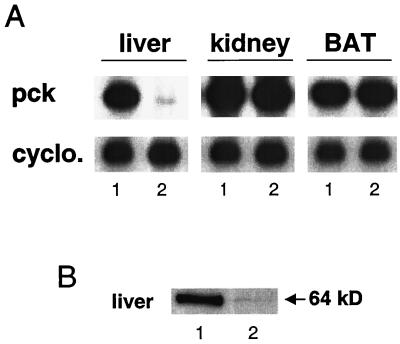
Since liver is the major site of gluconeogenesis, we proceeded to generate liver-specific PEPCK knockout (i.e., pcklox/lox+Alb-cre) mice by mating pcklox/lox with animals that express cre in the liver under control of the albumin promoter. Genotype analysis of 126 mice at weaning revealed frequencies of 72 pcklox/lox mice and 54 pcklox/lox+Alb-cre mice (P < 0.11 according to chi-square test). Southern blot analysis of tissue DNA from 6-week-old pcklox/lox+Alb-cre mice showed over 80% conversion of the pcklox to pckdel allele in the liver, with no sign of recombination in other tissues (data not shown), consistent with previous studies (35). To further assess the efficiency of recombination within hepatocytes, both Western and Northern blot analyses were performed. PEPCK mRNA and protein amounts in 6-week-old pcklox/lox+Alb-cre mice were markedly reduced (Fig. 3), cytosolic PEPCK activity was undetectable, and mitochondrial PEPCK activity was not altered (data not shown). PEPCK mRNA abundance in kidney and brown adipose tissues of pcklox/lox+Alb-cre mice was unaffected (Fig. 3A). The small amount of residual PEPCK protein and mRNA detected in the livers of adult animals by both of these assays is likely due to a nonhepatocyte source, since a previous study of the Alb-cre mice showed them to confer complete recombination by 6 to 8 weeks of age (35). However, recombination using this transgene was age dependent since PEPCK protein levels decreased only by ∼70 and ∼80% at 1 and 7 days after birth, respectively, in pcklox/lox+Alb-cre mice (data not shown).
FIG. 3.
Liver-specific recombination in pcklox/lox+Alb-cre (lanes 1) and pcklox/lox (lanes 2) mice. (A) Northern blot analysis of overnight-fasted 5- to 6-week-old mice; BAT, brown adipose tissue; cyclo., cyclophilin. (B) Western blot analysis of liver tissue from overnight-fasted mice.
Euglycemia in liver-specific PEPCK knockout mice at rest.
Plasma glucose concentrations in pcklox/lox+Alb-cre mice at both fed and fasted states were not different from those in pcklox/lox control mice (Table 3). However, fed (postabsorptive) hepatic glycogen content in the livers of PEPCK knockout mice was only 56% of the level in control mice, and glycogen was depleted completely in 24-h-fasted animals (Table 3). Plasma concentrations of the gluconeogenic substrates, lactate and glycerol, did not differ between the two genotypes, while fasting plasma alanine concentration was increased by 25% in pcklox/lox+Alb-cre mice.
TABLE 3.
Body and liver weight, plasma hormone concentrations, and plasma and hepatic metabolite concentrations in fed and 24-h-fasted pcklox/lox and pcklox/lox+Alb-cre micea
| Pup genotype | Body wt (g) | Relative liver wt (% of body wt) | Concn (mean ± SE)
|
||||
|---|---|---|---|---|---|---|---|
| Plasma
| |||||||
| Insulin (pg/ml) | Glucagon (pg/ml) | Corticosterone (ng/ml) | Glucose (mg/dl) | Glycerol (mg/dl) | |||
| Fed | |||||||
| pcklox/lox | 22.2 ± 0.6 | 5.7 ± 0.1 | 412 ± 61 | NDb | ND | 195.9 ± 8.3 | 5.4 ± 0.9 |
| pcklox/lox+Alb-cre | 21.9 ± 0.9 | 6.7 ± 0.2*** | 357 ± 86 | ND | ND | 185.4 ± 45.2 | 5.3 ± 0.7 |
| 24 h fasted | |||||||
| pcklox/lox | 17.2 ± 0.6 | 4.5 ± 0.1 | 253 ± 76 | 50.0 ± 8.0 | 367.5 ± 45.2 | 131.7 ± 10.7 | 6.5 ± 1.1 |
| pcklox/lox+Alb-cre | 17.8 ± 1.5 | 7.7 ± 0.1*** | 206 ± 34 | 52.1 ± 4.6 | 426 ± 38.9 | 127.9 ± 6.1 | 6.1 ± 0.5 |
| Concn (mean ± SE)
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Plasma
|
Hepatic
|
|||||||
| BHBA (mg/dl) | Lactate (mg/dl) | Alanine (μM) | FFA (μeq/liter) | Triglycerides (mg/dl) | Glycogen (μM/g) | Triglycerides (mg/g) | Malate (μM/g) | Malonyl-CoA (nM/g) |
| 3.6 ± 0.3 | 32.3 ± 2.8 | ND | 558 ± 38 | 116.2 ± 4.6 | 356.7 ± 19.4 | 17.5 ± 3.0 | 0.56 ± 0.07 | ND |
| 3.2 ± 0.3 | 33.4 ± 3.4 | ND | 649 ± 63 | 109.8 ± 8.7 | 198 ± 26.1*** | 19.2 ± 3.7 | 3.64 ± 0.40*** | ND |
| 17.6 ± 1.0 | 31.5 ± 5.5 | 350 ± 21 | 1,050 ± 113 | 61.3 ± 5.3 | 36.7 ± 9.3 | 66.1 ± 7.9 | 0.35 ± 0.02 | 7.2 ± 0.6 |
| 12.5 ± 1.7* | 37.9 ± 3.5 | 439 ± 14** | 1,613 ± 140** | 82 ± 8* | 1.0 ± 0.2*** | 127.8 ± 9.3*** | 3.48 ± 0.24*** | 7.4 ± 0.4 |
Age- and sex-matched mice 8 to 9 weeks old were sacrificed by cervical dislocation at 8 to 10 a.m., and blood was collected from neck vessels. *, P < 0.05, **, P < 0.01, and ***, P < 0.001 versus pcklox/lox; n = 6 to 11.
ND, not determined.
Given the normal plasma glucose concentrations in pcklox/lox+Alb-cre mice after fasting, we measured concentrations of plasma hormones involving regulation of energy metabolism (Table 3). Both fed and fasting insulin concentrations were not different between the two genotypes, nor were the fasting glucagon and corticosterone concentrations, despite hepatic malate concentrations that were increased 5.5- and 9-fold at the fed and fasted states, respectively, in pcklox/lox+Alb-cre mice (Table 3).
The plasma glucose concentration in pcklox/lox+Alb-cre pups was less than that in pcklox/lox pups at P1 (31.7 ± 4.6 versus 62.3 ± 3.1 mg/dl, n = 8 to 14, P < 0.0001). However, plasma glucose concentrations did not differ between the two genotypes at P3 and P7 (data not shown). Hepatic glycogen content in pcklox/lox+Alb-cre pups was decreased by 50% at P1 and by 85% at P3 (data not shown).
To further determine the effect of the lack of hepatic PEPCK on whole body glucose metabolism, we performed a study of basal glucose kinetics in 26-h-fasted pcklox/lox+Alb-cre mice. During the entire 220-min experimental period, plasma glucose concentrations in the liver-specific PEPCK knockout mice did not differ from the control values. The [3-3H]glucose-measured glucose turnover rate and specific activity of plasma [14C]glucose were also not different (Table 4).
TABLE 4.
Basal glucose kinetics in pcklox/lox pcklox/lox+Alb-cre mice after a 26-h fasta
| Pup genotype | Mean ± SE
|
||
|---|---|---|---|
| Plasma glucose (mg/dl) | Glucose turnover rate (mg/min/kg) | Plasma [14C]glucose sp act (cpm/mg) | |
| pcklox/lox | 119.3 ± 7.0 | 13.6 ± 0.7 | 42,820 ± 3,492 |
| pcklox/lox+Alb-cre | 139.6 ± 4.8 | 12.4 ± 0.6 | 35,049 ± 2,457 |
Male mice approximately 7 months old were used; n = 4.
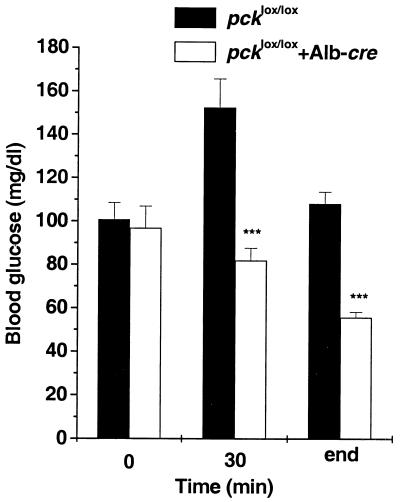
Since pcklox/lox+Alb-cre mice are able to maintain normal glucose metabolism at rest, we tested their response to exercise, a maneuver that increases peripheral glucose utilization. At 30 min and at the end of the exercise, blood glucose concentrations in the knockout mice fell to approximately half of the level in controls (Fig. 4). While the exercise endurance time between the two genotypes did not reach the defined statistically significant level (50.6 ± 7.3 versus 71.4 ± 6.8, P < 0.07, n = 5), the animals lacking hepatic PEPCK appeared to be less tolerant of exercise than the pcklox/lox controls.
FIG. 4.
Changes of blood glucose concentrations during exercise in pcklox/lox+Alb-cre mice. ∗∗∗, P < 0.001 versus pcklox/lox at each time point; n = 5.
Impaired lipid metabolism and up-regulation of genes encoding enzymes of energy metabolism in mice lacking hepatic PEPCK.
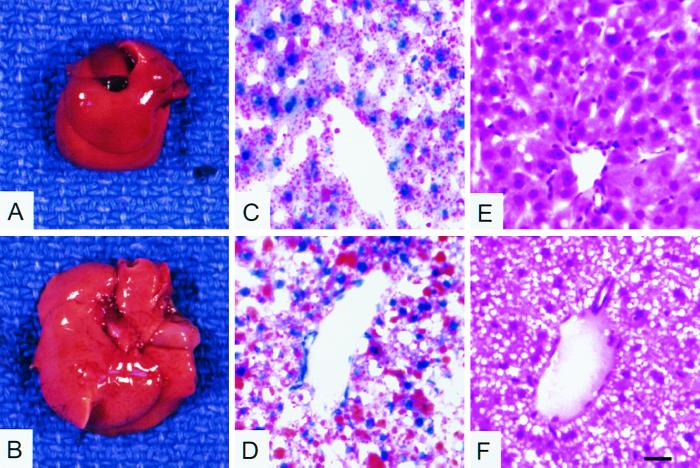
The liver weights in pcklox/lox+Alb-cre mice were increased by 18 and 71% in the fed and 24-fasted states, respectively, compared to pcklox/lox mice (Table 3 and Fig. 5B). Oil red O and hematoxylin-eosin staining of liver sections from pcklox/lox+Alb-cre mice showed larger lipid droplets and vacuoles, respectively (Fig. 5D and F). Although not different in fed mice, hepatic triglyceride content in the 24-h-fasted pcklox/lox+Alb-cre mice was increased by 94% compared with controls. Fasting plasma FFA and triglyceride concentrations were also greater in the knockout mice than in the controls. In contrast, the plasma BHBA concentration was less in the knockout mice (Table 3), suggesting a possible decrease in FFA β oxidation in livers of the knockout mice. Hepatic malonyl-CoA concentrations were not different between the two genotypes at the fasted state (Table 3).
FIG. 5.
Hepatic steatosis in 24-h-fasted pcklox/lox+Alb-cre mice. (A and B) Necropsy indicates increased liver size with pale color in ∼9-week-old pcklox/lox+Alb-cre mice compared to pcklox/lox mice. (C and D) Oil red O histochemistry for neutral lipids in liver sections. Larger lipid droplets (red) are shown in livers of pcklox/lox+Alb-cre mice than in those of pcklox/lox mice. (E and F) Hematoxylin-eosin histological staining for liver sections. Open circles in liver sections of pcklox/lox+Alb-cre mice suggest lipid vacuoles. Scale bars in panels C to F represent 50 μm.
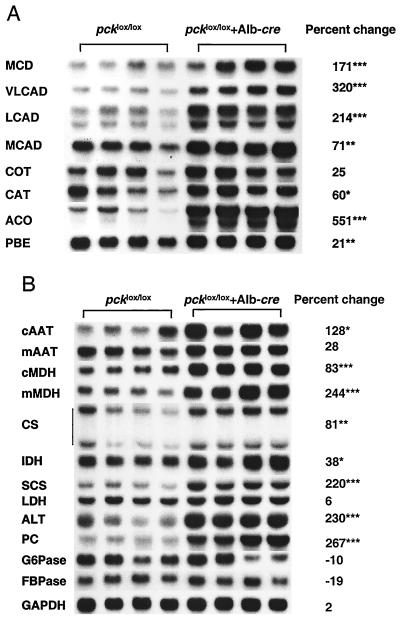
To begin to determine the basis for abnormalities in the regulation of lipid metabolism in these mice, malonyl-CoA decarboxylase mRNA abundance was assessed and found to be increased by 71% in livers of the knockout mice (Fig. 6A). The mRNA levels for the mitochondrial and peroxisomal FFA β-oxidation enzymes, acyl-CoA oxidase, very long-chain fatty acyl-CoA dehydrogenase, and long-chain fatty acyl-CoA dehydrogenase were increased by 551, 320, and 214%, respectively. The mRNA levels for medium-chain fatty acyl-CoA dehydrogenase, carnitine acetyltransferase, and enoyl-CoA hydratase–l-3-hydroxyacyl-CoA dehydrogenase bifunctional protein were also increased, but to smaller extents (Fig. 6A). There was no change in the mRNA abundance for microsomal FFA ω-oxidation enzyme, CYP4A1, and CYP4A4 (data not shown). Palmitate β-oxidation activity in liver homogenates was also increased by 16% (P < 0.01 [data not shown]), consistent with elevated mRNA abundance for FFA oxidation enzymes.
FIG. 6.
Altered gene expression for energy metabolism enzymes in 24-h-fasted pcklox/lox+Alb-cre mice. Northern blots were first probed with the specific cDNA; then the membranes were stripped and reprobed with cyclophilin cDNA. The relative abundance for each mRNA was normalized to the cyclophilin mRNA level. (A) Lipid-metabolizing enzymes. MCD, malonyl-CoA decarboxylase; VLCAD, very long-chain fatty acyl-CoA dehydrogenase; LCAD, long-chain fatty acyl-CoA dehydrogenase; MCAD, medium-chain fatty acyl-CoA dehydrogenase; COT, carnitine octanoyltransferase; CAT, carnitine acetyltransferase; ACO, acyl-CoA oxidase; PBE, enoyl-CoA hydratase–l-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. (B) Gluconeogenic, TCA cycle, and other enzymes. cAAT and mAAT, cytosolic and mitochondrial aspartate aminotransferase, respectively; cMDH and mMDH, cytosolic and mitochondrial malate dehydrogenase, respectively; CS, citrate synthase; IDH, isocitrate dehydrogenase; SCS, succinyl-CoA synthetase; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; PC, pyruvate carboxylase; G6Pase, glucose-6-phosphatase; FBPase, fructose-1,6-bisphosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ∗, P < 0.05, ∗∗, P < 0.01, and ∗∗∗, P < 0.001; n = 4.
DISCUSSION
We used an allelogenic Cre/loxP gene targeting strategy to determine role of cytosolic PEPCK in hepatic gluconeogenesis and energy metabolism. This strategy enabled the creation of a series of three functionally distinct pck alleles from a single ES cell line. In addition, since one of the alleles created was conditional, by intercrossing these animals with a line of Alb-cre transgenic mice that we have previously described (35), we were also able to generate mice with a liver-specific knockout of PEPCK.
PEPCK is essential for life.
The initiation of PEPCK gene expression and gluconeogenesis occur at birth since suckling rodent pups are thought to depend on gluconeogenesis for glucose production (2, 11). Thus, the facts that pckdel/del mice die within 3 days of birth and that 1-day-old pck-null pups are markedly hypoglycemic suggest impaired gluconeogenesis. Moreover, the lack of differences in fetal liver glycogen content between the pckdel/del and pckw/w fetuses, but decreased glycogen content and plasma glucose concentrations in 1-day-old pckdel/del pups, is also consistent with impaired gluconeogenesis in these knockout pups. However, it is not entirely clear whether the cause of death in these pups is actually hypoglycemia. First, glucose-6-phosphatase knockout mice, which have impaired hepatic and renal gluconeogenesis as well as severe hypoglycemia, do not die until 5 weeks of age (24). Second, the liver-specific PEPCK knockout (pcklox/lox+Alb-cre) mice, which at day 1 exhibited hypoglycemia as severe as that in the pckdel/del animals, survived and were euglycemic by 3 days of age. Thus, it remains possible that other factors besides hypoglycemia contribute to the earlier death of PEPCK knockout pups. Indeed, since PEPCK immunoreactivity has been detected in a variety of cells and tissues (48), it is possible that the animals die as a result of loss of PEPCK in one or more of these cell types.
Fasted mice with diminished PEPCK gene expression are euglycemic at rest.
The Cre/loxP strategy used in this study involved placement of a neo cassette between exons 3 and 4 of the PEPCK gene and its subsequent removal by cre plasmid microinjections after germ line transmission. However, the mice that retained the neo cassette have a functionally impaired allele that proved to be valuable in assessing the control strength of PEPCK on gluconeogenesis. Interestingly, both pcklox+neo/lox+neo and pcklox+neo/del mice were viable and did not exhibit any abnormality of blood glucose concentrations after a 24-h fast. Indeed, the fact that mice with a 90% reduction of PEPCK expression globally, as well as animals with a total knockout of hepatic PEPCK, are able to maintain euglycemia after fasting clearly indicates that this enzyme exerts a much weaker control of gluconeogenesis than previously thought. Considering that the isotopic studies in pcklox/lox+Alb-cre mice showed that these animals had similar glucose turnover rates and plasma [14C]glucose specific activities (derived from [14C]lactate) as wild-type mice, this conclusion is virtually inescapable. However, while the isotopic studies indicate that resting mice without hepatic PEPCK remain capable of synthesizing almost the same amount of glucose as a normal animal, how this is achieved remains unclear.
The liver and kidney are the only two gluconeogenic tissues. In humans, the contribution of renal glucose production during the postabsorptive state to systemic glucose appearance has been reported to vary from 5 to 28% (9, 42). The kidney can contribute more than 50% of total glucose production during either a 5- to 6-week starvation in humans (33) or insulin-induced hypoglycemia in dogs (4). Thus, we cannot rule out the possibility that net glucose production in the kidney increases to compensate for diminished hepatic glucose production in pcklox/lox+Alb-cre mice although PEPCK gene expression in the kidney of pcklox/lox+Alb-cre mice is unchanged.
It is also possible that the liver in pcklox/lox+Alb-cre mice is still capable of converting lactate to glucose, although there is no known pathway other than via PEPCK for the conversion of oxaloacetate to phosphoenolpyruvate. Mitochondrial PEPCK, even though it accounts for only ∼2% of total PEPCK activity, may be sufficient to maintain sufficient gluconeogenesis during fasting, provided there is no additional stress such as exercise. There is little reason to think that the thermodynamically unfavorable conversion of pyruvate to phosphoenolpyruvate by pyruvate kinase occurs in these mice, although this possibility may need to be directly tested.
Hepatic gluconeogenesis may also be activated by enhanced pyruvate carboxylation, malate-aspartate shuttle, TCA cycle, and oxidative phosphorylation and ATP production (14, 19, 32). The increased hepatic mRNA levels for pyruvate carboxylase, alanine aminotransferase, mitochondrial malate dehydrogenase, cytosolic malate dehydrogenase, cytosolic aspartate aminotransferase, citrate synthase, and succinyl-CoA synthetase in pcklox/lox+Alb-cre mice (Fig. 6B) after a 24-h fast are all consistent with this possibility.
Glycerol is normally considered a minor gluconeogenic substrate since it accounts for only ∼3% of overall glucose present in the postabsorptive state (31). Plasma glycerol is derived from adipose tissue as a result of lipolysis, which probably is enhanced in fasted pcklox/lox+Alb-cre mice as suggested by increased plasma FFA concentration, thereby increasing glycerol availability. On the other hand, because of the increased hepatic triglyceride content in the knockout mice, more plasma glycerol would be phosphorylated by hepatic glycerol kinase for esterification of fatty acid in the liver of the knockout mice during fasting. Moreover, plasma glycerol concentrations are not changed in pcklox/lox+Alb-cre mice. Thus, it is unlikely that a compensatory increase in gluconeogenesis from glycerol occurs in these mice.
It should also be pointed out that futile cycling of substrates (e.g., glucose and fructose-6-phosphate cycling) might lead to an overestimation of the measurement of glucose turnover as determined by [3-3H]glucose, with glucose utilization in peripheral tissues actually being decreased. Since the plasma glucose concentration reflects the balance of glucose production and utilization, a decrease in glucose utilization would help maintain glucose homeostasis even if glucose production were diminished in pcklox/lox+Alb-cre mice. It is also possible that increased plasma FFA in pcklox/lox+Alb-cre mice compete with glucose for metabolism in tissues like skeleton muscle, a major organ of glucose consumption, thereby impairing glucose utilization in this tissue (36).
Despite a normal fasting glucose concentration at rest, blood glucose concentrations of pcklox/lox+Alb-cre mice decreased with exercise. The exercise response pattern of blood glucose concentrations in the control and knockout mice is similar to that observed in a study of fed rats treated with 3-mercaptopicolinic acid (17). Since the mice were fasted for 24 h before exercise, glycogen stores would not be expected to contribute substantially to glucose production. Thus, the diminished blood glucose concentration in pcklox/lox+Alb-cre mice after 30 min of exercise probably reflects inadequate gluconeogenic flux in the face of increased peripheral glucose utilization. It has previously been shown that the glucose appearance rate in control rats is increased ∼2-fold during submaximal exercise but decreased in 3-mercaptopicolinic acid-treated rats (43). Therefore, the exercise study demonstrates that the liver-specific PEPCK knockout mice have limited gluconeogenic capacity.
While additional studies may be required to confirm our experimental interpretations, the results we have obtained are consistent with the conclusion that PEPCK is much less effective in determining gluconeogenic flux than previously thought. Instead of a single dominant control point, these results support the concept of multienzyme regulation of the gluconeogenic pathway (13, 34). This difference may have significant implications for the development of strategies to inhibit the excess hepatic glucose production that occurs in type 2 diabetes mellitus.
Impaired lipid metabolism in liver-specific PEPCK knockout mice.
While these studies strongly suggest that PEPCK exerts much less control of gluconeogenesis than previously thought, the finding of marked steatosis pcklox/lox+Alb-cre mice during fasting indicates that the enzyme has a previously unsuspected role in regulating hepatic lipid metabolism. The increased hepatic triglyceride accumulation in pcklox/lox+Alb-cre mice may result from the 60% increased plasma FFA concentration in these mice. The hepatic steatosis in pcklox/lox+Alb-cre mice may also be caused by a decrease in hepatic FFA oxidation, as suggested by lower plasma BHBA concentration despite increased plasma FFA concentration in these animals. Malonyl-CoA is a potent inhibitor of carnitine palmitoyltransferase I, the key enzyme for transport of FFA into mitochondria for oxidation (26). Moreover, the concentration of malonyl-CoA in muscle is correlated positively with the sum of citrate and malate concentrations (38). However, the fasting hepatic malonyl-CoA concentration in pcklox/lox+Alb-cre mice was not increased, presumably due to increased malonyl-CoA decarboxylase, as indicated by RNA analysis.
It is also surprising that the expression of genes encoding FFA-oxidizing enzymes both in mitochondria and in peroxisomes is actually elevated in pcklox/lox+Alb-cre mice during fasting. mRNA abundance for both acyl-CoA oxidase and fatty acyl-CoA dehydrogenases was elevated severalfold in the liver-specific knockout mice. Acyl-CoA oxidase is an important enzyme for peroxisomal FFA oxidation, and acyl-CoA oxidase knockout mice also have severely fatty livers (15). Fatty acyl-CoA dehydrogenases are also important enzymes for mitochondrial FFA oxidation, as demonstrated by the mortality rates of long-chain fatty acyl-CoA dehydrogenase knockout mice (21). The mechanism(s) responsible for the increased expression of the genes encoding these enzymes is not known. The activation of peroxisome proliferation-activated receptor alpha (PPARα) signaling by long-chain acyl-CoA, as suggested by studies of the acyl-CoA oxidase knockout mice (15), does not provide an explanation because the expression of other genes known to be regulated by PPARα, such as the CYP4A1, CYP4A3, and carnitine octanoyltransferase genes, is not altered in pcklox/lox+Alb-cre mice.
This finding, as well as the lack of significant alterations in plasma hormone concentrations, suggests that alterations in the concentrations of metabolic intermediates may affect expression of numerous genes encoding various enzymes involved in energy metabolism. Indeed, the markedly elevated expression of genes encoding hepatic FFA oxidation enzymes stands in direct contradiction to the fatty liver phenotype in pcklox/lox+Alb-cre mice.
Concluding statements.
This study provides new and unexpected insights into the role of PEPCK in regulating hepatic energy metabolism. While fasting plasma glucose concentrations are normal in mice with greatly diminished PEPCK gene expression, the absence of PEPCK causes impaired lipid metabolism and marked alterations in the expression of a variety of hepatic genes involved in energy metabolism. These changes occur in the absence of alterations in plasma concentrations of major hormones. Thus, PEPCK appears to play a vital role in the integration of multiple pathways of energy metabolism, a function that has not heretofore been attributed to this enzyme.
ACKNOWLEDGMENTS
We thank D. Wasserman, P. Flakoll, Y. Fujimoto, E. P. Donahue, M.-Y. Zhu, and J. Lindner for help and advice in performing these studies, and we thank A. D. Cherrington and D. K. Granner for reading the manuscript and providing comments. We are also indebted to A. Saha (Boston University) for measuring malonyl-CoA and to D. Kelly (Washington University) for providing cDNA for CYP4A3, CYP4A1, and MCAD.
This study was supported by funding from the National Institutes of Health (grant DK42502). P. She is a recipient of a JDFI postdoctoral fellowship.
REFERENCES
- 1.Araki K, Araki M, Miyazaki J, Vassalli P. Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase. Proc Natl Acad Sci USA. 1995;92:160–164. doi: 10.1073/pnas.92.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard F J, Hanson R W. Phosphoenolpyruvate carboxykinase and pyruvate carboxylase in developing rat liver. Biochem J. 1967;104:866–871. doi: 10.1042/bj1040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmeyer H U. and 1985. Methods of enzymatic analysis. 3rd ed. 4 to 6. Weinheim, Germany: VCH Verlagsgesllschaft; 1984. [Google Scholar]
- 4.Cersosimo E, Molina P E, Abumrad N N. Renal glucose production during insulin-induced hypoglycemia. Diabetes. 1997;46:643–646. doi: 10.2337/diab.46.4.643. . (Erratum, 46:1532.) [DOI] [PubMed] [Google Scholar]
- 5.Cimbala M A, Lamers W H, Nelson K, Monahan J E, Yoo-Warren H, Hanson R W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J Biol Chem. 1982;257:7629–7636. [PubMed] [Google Scholar]
- 6.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38:550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo R A, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 8.DiTullio N W, Berkoff C E, Blank B, Kostos V, Stack E J, Saunders H L. 3-Mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974;138:387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekberg K, Landau B R, Wajngot A, Chandramouli V, Efendic S, Brunengraber H, Wahren J. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes. 1999;48:292–298. doi: 10.2337/diabetes.48.2.292. [DOI] [PubMed] [Google Scholar]
- 10.Exton J H, Park C R. Control of gluconeogenesis in liver. 3. Effects of l-lactate, pyruvate, fructose, glucagon, epinephrine, and adenosine 3′,5′-monophosphate on gluconeogenic intermediates in the perfused rat liver. J Biol Chem. 1969;244:1424–1433. [PubMed] [Google Scholar]
- 11.Girard J, Ferre P, Pegorier J P, Duee P H. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev. 1992;72:507–562. doi: 10.1152/physrev.1992.72.2.507. [DOI] [PubMed] [Google Scholar]
- 12.Granner D, Andreone T, Sasaki K, Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983;305:549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- 13.Groen A K, van Roermund C W, Vervoorn R C, Tager J M. Control of gluconeogenesis in rat liver cells. Flux control coefficients of the enzymes in the gluconeogenic pathway in the absence and presence of glucagon. Biochem J. 1986;237:379–389. doi: 10.1042/bj2370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halestrap A P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989;973:355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters J M, Gonzalez F J, Yeldandi A V, Rao M S, Reddy J K. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J Biol Chem. 1999;274:19228–19236. doi: 10.1074/jbc.274.27.19228. [DOI] [PubMed] [Google Scholar]
- 16.Jetton T L, Magnuson M A. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci USA. 1992;89:2619–2623. doi: 10.1073/pnas.89.7.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John-Alder H B, McAllister R M, Terjung R L. Reduced running endurance in gluconeogenesis-inhibited rats. Am J Physiol. 1986;251:R137–R142. doi: 10.1152/ajpregu.1986.251.1.R137. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Tayek J A. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am J Physiol. 1998;275:E537–E542. doi: 10.1152/ajpendo.1998.275.3.E537. [DOI] [PubMed] [Google Scholar]
- 19.Kraus-Freidmann N, Feng L. The role of intracellular Ca2+ in the regulation of gluconeogenesis. Metabolism. 1996;45:389–403. doi: 10.1016/s0026-0495(96)90296-6. [DOI] [PubMed] [Google Scholar]
- 20.Krebs H A. Considerations concerning the pathways of synthesis in living matter. Synthesis of glycogen from non-carbohydrate precursors. Bull Johns Hopkins Hosp. 1954;95:19. [PubMed] [Google Scholar]
- 21.Kurtz D M, Rinaldo P, Rhead W J, Tian L, Millington D S, Vockley J, Hamm D A, Brix A E, Lindsey J R, Pinkert C A, O'Brien W E, Wood P A. Targeted disruption of mouse long-chain acyl-CoA dehydrogenase gene reveals crucial roles for fatty acid oxidation. Proc Natl Acad Sci USA. 1998;95:15592–15597. doi: 10.1073/pnas.95.26.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labosky P A, Winnier G E, Jetton T L, Hargett L, Ryan A K, Rosenfeld M G, Parlow A F, Hogan B L. The winged helix gene, Mf3, is required for normal development of the diencephalon and midbrain, postnatal growth and the milk-ejection reflex. Development. 1997;124:1263–1274. doi: 10.1242/dev.124.7.1263. [DOI] [PubMed] [Google Scholar]
- 23.Landau B R, Wahren J, Chandramouli V, Schumann W C, Ekberg K, Kalhan S C. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Investig. 1996;98:378–385. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei K J, Chen H, Pan C J, Ward J M, Mosinger B, Jr, Lee E J, Westphal H, Mansfield B C, Chou J Y. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson M A, Quinn P G, Granner D K. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin's effects oppose those of cAMP and dexamethasone. J Biol Chem. 1987;262:14917–14920. [PubMed] [Google Scholar]
- 26.McGarry J D, Leatherman G F, Foster D W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978;253:4128–4136. [PubMed] [Google Scholar]
- 27.Meyers E N, Lewandoski M, Martin G R. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 28.Niswender K D, Postic C, Jetton T L, Bennett B D, Piston D W, Efrat S, Magnuson M A. Cell-specific expression and regulation of a glucokinase gene locus transgene. J Biol Chem. 1997;272:22564–22569. doi: 10.1074/jbc.272.36.22564. [DOI] [PubMed] [Google Scholar]
- 29.Niswender K D, Shiota M, Postic C, Cherrington A D, Magnuson M A. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. J Biol Chem. 1997;272:22570–22575. doi: 10.1074/jbc.272.36.22570. [DOI] [PubMed] [Google Scholar]
- 30.Nordlie R C, Lardy H A. Mammalian liver P-enolpyruvate carboxykinase. J Biol Chem. 1963;238:2259–2263. [PubMed] [Google Scholar]
- 31.Nurjhan N, Consoli A, Gerich J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J Clin Investig. 1992;89:169–175. doi: 10.1172/JCI115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen M R, Halestrap A P. The mechanisms by which mild respiratory chain inhibitors inhibit hepatic gluconeogenesis. Biochim Biophys Acta. 1993;1142:11–22. doi: 10.1016/0005-2728(93)90079-u. [DOI] [PubMed] [Google Scholar]
- 33.Owen O E, Felig P, Morgan A P, Wahren J, Cahill G F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Investig. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilkis S J, Granner D K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 35.Postic C, Shiota M, Niswender K D, Jetton T L, Chen Y, Moates J M, Shelton K D, Lindner J, Cherrington A D, Magnuson M A. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 36.Randle P J, Garland P B, Halers C N, Newsholme E A. The glucose-fatty acid cycle: its role in insulin sensitivity and the metabolic disturbance of diabetes mellitus. Lancet. 1963;i:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 37.Rosella G, Zajac J D, Kaczmarczyk S J, Andrikopoulos S, Proietto J. Impaired suppression of gluconeogenesis induced by overexpression of a noninsulin-responsive phosphoenolpyruvate carboxykinase gene. Mol Endocrinol. 1993;7:1456–1462. doi: 10.1210/mend.7.11.8114759. [DOI] [PubMed] [Google Scholar]
- 38.Saha A K, Laybutt D R, Dean D, Vavvas D, Sebokova E, Ellis B, Klimes I, Kraegen E W, Shafrir E, Ruderman N B. Cytosolic citrate and malonyl-CoA regulation in rat muscle in vivo. Am J Physiol. 1999;276:E1030–E1037. doi: 10.1152/ajpendo.1999.276.6.E1030. [DOI] [PubMed] [Google Scholar]
- 39.Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombination. Methods Enzymol. 1993;225:890–901. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- 40.She P, Hippen A R, Young J W, Lindberg G L, Beitz D C, Richardson L F, Tucker R W. Metabolic responses of lactating dairy cows to 14-day intravenous infusions of glucagon. J Dairy Sci. 1999;82:1118–1127. doi: 10.3168/jds.S0022-0302(99)75335-X. [DOI] [PubMed] [Google Scholar]
- 41.Short M K, Clouthier D E, Schaefer I M, Hammer R E, Magnuson M A, Beale E G. Tissue-specific, developmental, hormonal, and dietary regulation of rat phosphoenolpyruvate carboxykinase-human growth hormone fusion genes in transgenic mice. Mol Cell Biol. 1992;12:1007–1020. doi: 10.1128/mcb.12.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Investig. 1995;96:2528–2533. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turcotte L P, Rovner A S, Roark R R, Brooks G A. Glucose kinetics in gluconeogenesis-inhibited rats during rest and exercise. Am J Physiol. 1990;258:E203–E211. doi: 10.1152/ajpendo.1990.258.1.E203. [DOI] [PubMed] [Google Scholar]
- 44.Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 1994;91:9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiese T J, Lambeth D O, Ray P D. The intracellular distribution and activities of phosphoenolpyruvate carboxykinase isozymes in various tissues of several mammals and birds. Comp Biochem Physiol B. 1991;100:297–302. doi: 10.1016/0305-0491(91)90378-q. [DOI] [PubMed] [Google Scholar]
- 46.Williams C P, Postic C, Robin D, Robin P, Parrinello J, Shelton K, Printz R L, Magnuson M A, Granner D K, Forest C, Chalkley R. Isolation and characterization of the mouse cytosolic phosphoenolpyruvate carboxykinase (GTP) gene: evidence for tissue-specific hypersensitive sites. Mol Cell Endocrinol. 1999;148:67–77. doi: 10.1016/s0303-7207(98)00234-2. [DOI] [PubMed] [Google Scholar]
- 47.Wynshaw-Boris A, Short J M, Loose D S, Hanson R W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. I. Multiple hormone regulatory elements and the effects of enhancers. J Biol Chem. 1986;261:9714–9720. [PubMed] [Google Scholar]
- 48.Zimmer D B, Magnuson M A. Immunohistochemical localization of phosphoenolpyruvate carboxykinase in adult and developing mouse tissues. J Histochem Cytochem. 1990;38:171–178. doi: 10.1177/38.2.1688895. [DOI] [PubMed] [Google Scholar]