Abstract
High incidences of morbidity and mortality associated with age-related diseases among the elderly population are a socio-economic challenge. Aging is an irreversible and inevitable process that is a risk factor for pathological progression of diverse age-related diseases. Spermidine, a natural polyamine, plays a critical role in molecular and cellular interactions involved in various physiological and functional processes. Spermidine has been shown to modulate aging, suppress the occurrence and severity of age-related diseases, and prolong lifespan. However, the precise mechanisms through which spermidine exerts its anti-aging effects have not been established. In this review, we elucidate on the mechanisms and roles underlying the beneficial effects of spermidine in aging from a molecular and cellular perspective. Moreover, we provide new insights into the promising potential diagnostic and therapeutic applications of spermidine in aging and age-related diseases.
Keywords: spermidine, aging, age-related diseases, longevity, autophagy
1. Introduction
Due to improvements in the quality of life, social security, and medical conditions, life expectancy has been significantly prolonged. However, as the global population ages, socioeconomic challenges are becoming increasingly common, which has attracted global attention. Aging is an inevitable and irreversible biological process that is characterized by a gradual and progressive loss of physiological integrity and functions. It is a predominant risk factor for higher incidences of chronic disorders such as cardiovascular diseases (CVDs), neurodegenerative diseases, metabolic diseases, musculoskeletal diseases and immune-senescence diseases [1, 2]. The elderly population often present with other morbidities that may eventually lead to death [3]. Studies on aging, including those focused on major age-related diseases, are still in their early stages [4]. Due to the rising aging population and the prevalence of age-related diseases, it is important to develop novel preventive and therapeutic interventions to suppress aging and decrease the burden of age-related diseases.
Polyamines are ubiquitous polycations that are found in all cells, tissues, and organs. They can interact with negatively charged molecules such as DNA, RNA, adenosine triphosphate, and proteins. These molecules exert multiple functions in many physiological and pathophysiological processes, including cell proliferation, differentiation, growth, tissue regeneration and gene regulation [5]. Due to its antioxidant functions, anti-inflammatory properties, enhanced proteostasis and improved mitochondrial metabolic functions, spermidine, a naturally occurring polyamine, is involved in a series of biological events, including autophagy induction, apoptosis, transcription, and DNA stability [4, 6]. The concentration of spermidine declines with age, and exogenous spermidine supplementation reverses age-associated adverse changes and prolongs the lifespan [7]. Spermidine is associated with longevity [8, 9]. Given that it interacts with various molecules, spermidine influences aging through diverse mechanisms. However, the roles and mechanisms through which spermidine modulates the process of aging and alters the course of age-related diseases have not been elucidated. Therefore, in this review, we compile and update the latest knowledge regarding how spermidine modulates aging and reveal its potential diagnostic and therapeutic applications in age-related diseases.
2. Mechanisms of spermidine in aging
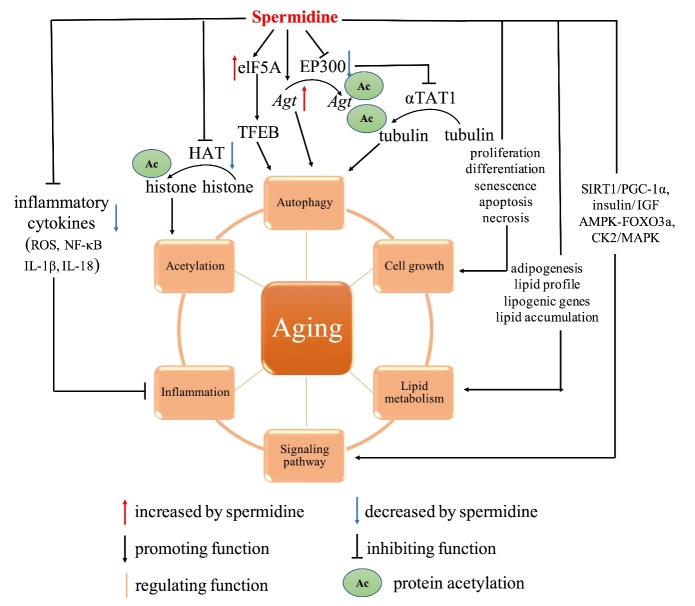
Even though aging is inevitable, it can be modified by biological and genetic interventions, pharmaceuticals, lifestyle, and the systemic environment [10-12]. Spermidine has been shown to be important for prolonging survival outcomes, and abnormal changes in spermidine levels are associated with aging as well as disease development [13]. Intracellular concentrations of spermidine are suppressed during aging. Exogenous spermidine supplementation has been shown to extend the lifespans of flies, nematodes and yeast [14]. Moreover, a diet enriched in spermidine was shown to prolong the lifespans of mice [15]. However, studies on the mechanisms of action of spermidine are rare. Autophagy is the main mechanism of spermidine in delaying aging and prolonging the lifespan. In addition, spermidine exerts its effects through other mechanisms, including anti-inflammation, histone acetylation reduction, lipid metabolism and regulation of cell growth and signaling pathways [16]. In this section, we update on the field of spermidine research and discuss the potential mechanisms of spermidine in aging (Fig. 1).
Figure 1.
Molecular and cellular mechanisms of spermidine in age-related diseases. Spermidine is an inducer of autophagy, which is the main mechanism of anti-aging. First, spermidine triggers autophagy by modulating the expressions of Atg genes. Second, it regulates transcription factor elF5A to promote the synthesis of transcription factor TFEB. Third, spermidine inhibits EP300, which directly promotes the acetylation of Atg genes and indirectly stimulates deacetylation of tubulin due to inhibition of aTAT1. Besides, spermidine exerts potent anti-inflammatory roles by suppressing of multiple inflammatory cytokines, such as ROS, NF-κB, IL-1β and IL-18. Moreover, it is involved in regulation of cell proliferation, differentiation, senescence, apoptosis and necrosis, ultimately promoting cell growth and inhibiting cell death. As an anti-aging agent, spermidine suppresses histone acetylation. Moreover, spermidine regulates lipid metabolism. On the one hand, it promotes the differentiation of preadipocytes into mature adipocytes. On the other hand, it alters lipid profile, modulates lipogenic gene expressions, and represses lipid accumulation. Furthermore, spermidine can delay aging through specific signaling pathways, such as SIRT1/PGC-1α, insulin/ IGF, AMPK-FOXO3a, and CK2/MAPK signaling pathways. Abbreviations: Atg: autophagy-related genes; aTAT1: a-tubulin acetyltransferase 1; EP300: E1A-associated protein p300; ROS: reactive oxygen species; NF-κB: nuclear factor kappa-B; IL: interleukin; SIRT1: Sirtuin-1; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator alpha; IGF: insulin-like growth factor; AMPK: AMP-activated protein kinase; MAPK: mitogen-activated protein kinase
2.1 Autophagy
Autophagy, an intracellular degradation system, is a complex process that delivers damaged or unnecessary cytoplasmic components into lysosomes [17, 18]. It can be divided into microautophagy, macroautophagy, and chaperone-mediated autophagy. The term autophagy is often used in reference to macroautophagy, the most common process through which cytoplasmic contents can be sequestered within the autophagosome, subsequently fusing with a lysosome or vacuole [19]. At basal levels, it is a process that is necessary to mediate proper cellular function, but can be adjusted by certain stimuli, such as aging, oxidative stress, or inflammation [20]. Aging enhances the formation of damaged cellular constituents, including proteins and organelles [21, 22], and suppresses cellular ability to degrade these components [23, 24]. Therefore, autophagy plays an important role in anti-aging and in improving longevity. Autophagy is primarily a cytoprotective mechanism [25, 26]. Induction of autophagy prolongs the lifespan, while its deficiency shortens the lifespan [27].
Spermidine has been shown to induce autophagy in multiple organs, including liver, heart, and muscle in mice [28], as well as in aging yeast, worms, flies, and cultured mammalian cells [14, 29]. Spermidine induces autophagy by adjusting the expression levels of autophagy-related genes (Atg). The Atg genes, such as Atg 7, Atg 15, and Atg 11 were up-regulated upon spermidine supplementation [8], while Atg gene knockout abolished spermidine-induced lifespan extension [14, 30]. Second, spermidine regulates autophagy by inducing the expression of transcription factor, elF5A, to increase the synthesis of transcription factor, TFEB [31]. Third, spermidine initiates autophagy by inhibiting protein acetylation [32]. The E1A-associated protein p300 (EP300) is an acetyltransferase that directly promotes acetylation of multiple autophagy-essential proteins, and indirectly stimulates tubulin deacetylation by inhibiting a-tubulin acetyltransferase 1 (aTAT1) [33, 34]. Spermidine enhances the deacetylation by reducing the expression of EP300. Besides, spermidine decreases acetylation by reducing the availability of acetyl-CoA [29]. Moreover, spermidine can also induce autophagy through other pathways, including regulation of inflammation and lipid metabolism among others [35, 36]. Altogether, autophagy is the most important mechanism through which spermidine exerts its anti-aging effects.
2.2 Anti-inflammation
Inflammation is a double-edged sword. It plays a crucial role in immunity by resisting pathogenic invasion. However, it may disrupt the balance of organisms, which may eventually lead to disease. Excessive inflammatory responses, also referred to as “inflammatory aging”, is a major risk factor for aging [37-39]. In addition, elevated expression levels of pro-inflammatory biomarkers, C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) have been associated with a risk of developing various age-related diseases including cardiovascular diseases, cerebrovascular diseases, chronic kidney diseases, and metabolic syndrome [40-42].
Systemic effects of spermidine play a crucial role in delaying aging, possibly through its involvement in anti-inflammatory processes. In this section, we elucidate on the potential mechanisms through which spermidine suppresses inflammation. Eisenberg et al. reported that spermidine supplementation reduces chronic inflammation by decreasing TNF-α expression levels, thereby suppressing the occurrence and progression of cardiovascular dysfunctions [15]. Besides, Jeong et al. proved that spermidine exerts potent anti-inflammatory effects through various mechanisms [43]. First, it inhibits the accumulation of reactive oxygen species (ROS) and translocation of nuclear factor-kappa B (NF-κB). Second, spermidine is associated with the inhibition of inflammation related migration of immune cells. Spermidine supplementation has been shown to suppress protein expression levels of IL-1β and IL-18 [44]. These findings indicate that anti-inflammation is essential for spermidine-mediated delay of aging.
2.3 Cellular lifecycle
Cellular lifecycle involves proliferation, differentiation, senescence and apoptosis, which are structural and functional bases for organism growth, development, aging and death, respectively. Most cells exhibit a normal lifecycle, while a few cells deviate from a normal lifecycle due to interference of certain factors, including damage, necrosis or cancer. Dysregulated cellular lifecycle has been implicated in the pathogenesis of aging and age-related diseases. Spermidine is involved in regulation the lifecycle of cells [45]. With the relevant cumulative findings, herein we discuss the correlation between spermidine and cellular lifecycle.
2.3.1 Spermidine and cell proliferation
Cell proliferation, which is attributed to cell division, is an important characteristic of living organisms. Cell cycle is responsible for cell growth, survival and death [46]. Spermidine plays a causative role in modulating the cell cycle [47], with small amounts of spermidine shown to sustain normal cell cycles [48]. Landau et al. reported that the absence of spermidine can cause growth cessation at the G1 phase by affecting the expression of cell cycle regulators [49]. Spermidine was also shown to enhance the proportion of S phase cells and maintain mitochondrial membrane potential, thereby improving the senescence of mouse neuroblastoma cells [50].
2.3.2 Spermidine and cell differentiation
Cell differentiation refers to process through which cells from the same source gradually produce cell groups with different morphological structures and functional characteristics. Recent studies have revealed that spermidine is involved in cell differentiation [51, 52]. Emerging evidence indicates a role for spermidine in enhancing differentiation in differentiated chondrocytes and in adult stem cells [53]. Cervelli et al. proved that exogenous supplementation of spermidine impacts on D-gal-induced aging-related skeletal muscle atrophy during skeletal muscle differentiation [54].
2.3.3 Spermidine and cell senescence
Cell senescence is characterized by cessation of replication, loss of proliferation potential, resistance to apoptosis, and increased protein production [55, 56]. Spermidine prevents cell senescence [57]. Elevated spermidine levels were associated with improved functions of “old” B cells, which might reverse immune aging [58]. Zhu et al. demonstrated that spermidine inhibits high glucose and neurotoxicity-induced senescence induced by upregulating the expression of cannabinoid receptor type 1 [59]. Suppressed p21 and p16 expression levels and senescence-associated β-gal staining indicated that spermidine improved bleomycin-stimulated premature cell senescence [60].
2.3.4 Spermidine and cell death
Physiological cell apoptosis and pathological cell necrosis are collectively referred to as cell death. Cell apoptosis is a basic biological phenomenon of cells, which plays an important role in the removal of unwanted or abnormal cells from multicellular organisms. It is involved in the evolution of organisms, maintaining the stability of the internal environment and in the development of multiple systems. Spermidine can modulate cell apoptosis. The interaction between spermidine and mitochondrial membrane induces the release of cytochrome C, which is the prolusion to apoptosis [61]. In addition to interfering with the cell cycle, spermidine has also been found to slow down the aging process by preventing apoptosis [49, 50]. Cell necrosis refers to cell death under the induction of extreme physical, chemical or other serious pathological factors. The role of spermidine in cell necrosis has been reported [62]. Elevated spermidine levels were shown to suppress cell necrosis, prolong the lifespan and improve health in aging yeast [14].
2.4 Histone acetylation
Post-translational modification of histone has been shown to play a significant role in epigenetic changes during aging, with histone acetylation being the most important. Spermidine inhibits histone acetylation, thereby, exerting anti-aging effects [63, 64]. However, the mechanisms through which spermidine affects histone acetylation have not been fully elucidated. Alterations in spermidine concentrations impact on the activities of histone acetylases and deacetylases. Spermidine leads to hypoacetylation by decreasing histone acetylases rather than increasing histone deacetylases [8]. Eisenberg et al. reported that spermidine administration induces histone H3 deacetylation by inhibiting acetyltransferases (HAT) in yeast [14]. Burgio et al. documented that spermidine modulates histone acetylation levels by activating P/CAF, a highly conserved HAT, in vivo and in vitro [65]. These findings show that spermidine plays a crucial role in anti-aging by inhibiting histone acetylation.
2.5 Lipid metabolism
Lipid metabolism is a significant biochemical process that is involved in the synthesis and degradation of lipids, such as steroids, triglycerides, and phospholipids, to produce energy and maintain normal biological functions [66]. Aging is closely associated with lipid metabolism. Disruption of lipid metabolism has detrimental outcomes on health and longevity. A correlation between spermidine and lipid metabolism in aging has been reported [67].
Minois et al. found that increased triglycerides levels as well as altered phospholipid profiles and fatty acids were associated with extended lifespans in spermidine-fed flies [68]. Further research revealed that most of these spermidine-induced changes are largely regulated through autophagy. Gao et al. proved that spermidine regulates lipid metabolism through suppressing the expression of lipogenic genes via an AMP-activated protein kinase (AMPK) signaling pathway [69]. Moreover, spermidine has been shown to suppress necrotic core formation and lipid accumulation by stimulating cholesterol outflow [36]. Ma et al. noted that spermidine feeding reduces plasma lipid profiles and fat mass without affecting body weight, thereby exerting an potential effect in treating obesity [70]. Spermidine/spermine N1-acetyltransferase (SAT1) acetylates spermidine and spermine to generate N1-acetylspermine, N1,12-diacetylspermine, and N1-acetylspermidine. SAT1 activation is closely associated with beige adipocyte biogenesis and low-grade inflammation [71].
In addition to the complex interactions between spermidine and lipid metabolism, spermidine promotes the differentiation of pre-adipocytes into mature adipocytes at the cellular level. α-difluoromethylornithine (DFMO) is a catalytic suicide inhibitor of polyamine synthesis that decreases the expression of transcription factors critical to late adipocyte markers and pre-adipocyte differentiation [72]. Exogenous supplementation of natural spermidine was shown to initiate the differentiation of preadipocytes into mature adipocytes in the presence of DFMO, thereby regulating adipogenesis [73]. Collectively, the role of spermidine in lipid metabolism is one of the important mechanisms of its anti-aging effects.
2.6 Signaling pathways
Multiple signaling pathways are involved in modulation of aging and age-related diseases. Spermidine interacts with various signaling pathways to regulate the aging process [8]. However, the specific mechanisms have not been established. In this section, we elucidate on the relationship between spermidine and specific signaling pathways in aging.
Sirtuin-1/peroxisome proliferator-activated receptor gamma coactivator alpha (SIRT1/PGC-1α) signaling pathway is a major modulator of mitochondrial function and a vital contributor to aging and cardiovascular diseases. Wang et al. confirmed that spermidine stimulates mitochondrial biogenesis through the SIRT1/PGC-1α pathway and could, therefore, be used to prevent cardiac function degradation during aging [74]. In drosophila, dietary spermidine supplementation was associated with extended lifespan by suppressing insulin/ insulin-like growth factor (IGF) signaling [75]. FOXO3a, a downstream effector of AMP-activated protein kinase (AMPK), which is involved in the aging process, has been associated with longevity [76, 77]. Fan et al. showed that spermidine protects against aging-related skeletal muscle atrophy by suppressing apoptosis and enhancing autophagy through the mediation of the AMPK-FOXO3a signaling pathway [78]. The ubiquitous kinase, CK2, has been reported to translate information in the mitogen-activated protein kinase (MAPK) pathway by detecting spermidine levels [79]. Moreover, spermidine upregulates the expression of MAPK family genes and to regulate MAPK phosphorylation [79, 80]. In conclusion, spermidine exerts its anti-aging properties by activating or suppressing signaling pathways.
2.7 Others
In addition to the above mechanisms through which spermidine modulates aging, biological functions of spermidine in protecting replicating DNA from oxidative damage have also been proposed. Oxidative damage by singlet oxygen, 1O2, leads to harmful effects on cells. Spermidine, as a positively charged molecule, can bind and precipitate DNA [81]. Khan et al. documented that spermidine protects DNA against oxidative attack, ensuring the integrity of DNA and RNA, thereby guaranteeing protein synthesis [82].
3. The role of spermidine in age-related diseases
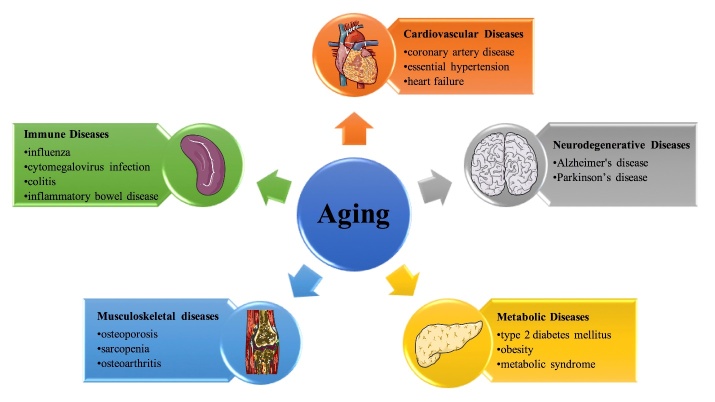
Aging refers to a gradual deterioration of functionality and physiological integrity processes, which enhances susceptibility to age-related diseases (Fig. 2). Since the aging population is rapidly increasing, there is a need to thoroughly understand aging and age-related diseases. Sudies have reported on the mechanisms involved in aging, especially vascular aging [1, 2, 83-90]. Spermidine is a critical factor in aging and age-related diseases, including CVDs, neurodegenerative diseases, metabolic diseases, musculoskeletal diseases, and immune diseases. In this section, we discuss on the role of spermidine in age-related diseases (Table 1).
Figure 2.
The role of aging in age-related diseases. This figure shows examples of age-related diseases where aging is one of the main risk factors.
Table 1.
Roles of spermidine in aged-related diseases.
| Disease | Functions | Potential mechanisms | |
|---|---|---|---|
| Cardiovascular Diseases | CAD | regulate myocardial ischemic reperfusion | modulate arterial blood perfusion [92] |
| CAD | inhibit AS | decrease inflammatory cytokines and improving mitochondrial function [94] | |
| CAD | reduce AS | induce autophagy [95, 96] | |
| CAD | attenuate AS | antagonize platelet aggregation [96] | |
| CAD | protect from AS | promote conversion of PHBP [97] | |
| CAD | inhibit AS | activate eNOS [98] | |
| EH | inhibit EH | regulate NMDA and its receptors [100] | |
| EH | reduce blood pressure | improve age-related diastolic [101] | |
| EH | inhibit EH | regulate angiotensin II [103] | |
| HF | delay HF | prevent cardiac hypertrophy and protect cardiomyocytes [15, 101] | |
| HF | attenuate HF | attenuate mitochondrial dysfunction [105] | |
| HF | protect cardiac | reduce telomere attrition [106] | |
| HF | protect cardiac | regulate apoptosis of myocardial ischemic cells [107] | |
| Neurodegenerative Diseases | AD | reduce memory decline | induce autophagy [110, 111] |
| AD | ameliorate dementia | prevent inflammation and apoptosis of nerve cells [44] | |
| AD | influence memory | stimulate neural actions [112] | |
| PD | protect from PD | maintain the mitochondria in dopaminergic neurons function [113] | |
| PD | protect against PD | induce autophagy [114] | |
| PD | protect against PD | trigger PINK1-PDR1-dependent mitophagy [115] | |
| PD | alleviate PD | inhibit α-synuclein and promote climbing activity [116, 117] | |
| PD | protect against PD | exert anti-inflammatory and antioxidant properties [118] | |
| PD | attenuate PD | regulate SAT1 activity [119] | |
| Metabolic Diseases | T2DM | prevent T2DM | improve insulin sensitivity and maintain glucose homeostasis [120] |
| T2DM | prevent T2DM | promote facultative cell proliferation and maintain glucose homeostasis [121] | |
| T2DM | prevent diabetic complications | inhibit lipid peroxidation, hemoglobin glycation [122] | |
| T2DM | reduce hyperglycemic | enhance glucose utilization [123] | |
| T2DM | reduce nephropathy complications | reduce renal collagen [125] | |
| Obesity | ameliorate obesity | reduce adiposity and hepatic fat accumulation [120] | |
| Obesity | loss of weight | regulate lipid metabolism, inflammatory response, and thermogenesis [70] | |
| Obesity | attenuate obesity | induce autophagy in white adipose tissue [126] | |
| Obesity | alleviate obesity | enhance intestinal barrier function and alternate microbiota composition [127] | |
| Obesity | reduce adiposity | inhibit lipogenic genes expression [69] | |
| Obesity | attenuate obesity | increase energy expenditure [128] | |
| Metabolic syndrome |
correct metabolic syndrome | activate TETA [129] | |
| Metabolic syndrome |
inhibit metabolic syndrome | ameliorate hepatic steatosis and adipose tissue inflammation [70] | |
| Musculoskeletal Diseases | Osteoporosis | enhance bone strength | promote warmth regeneration [130] |
| Osteoporosis | prevent bone loss | disturb osteoclastic activation [131, 132] | |
| Osteoporosis | reduce migration and osteoclastogensis | inhibit RANKLE-mediated signaling pathway, prevent transcription factors [133] | |
| Sarcopenia | ameliorate skeletal muscle atrophy | regulate skeletal muscle differentiation [54] | |
| Sarcopenia | ameliorate muscle defects | induce autophagy [135] | |
| Osteoarthritis | improve osteoarthritis | activate autophagy [136] | |
| Osteoarthritis | alleviate synovitis, osteophyte formation and cartilage degeneration | inhibit TNF-α induced NF-κB/p65 signaling pathway [137] | |
| Osteoarthritis | protect chondrocytes | reduce oxidant and inflammatory responses [138] | |
| Immune Diseases | influenza | improve CD8+ T cell responses | induce autophagy [140] |
| cytomegalovirus infection | improve CD8+ T cell responses | induce autophagy [140] | |
| colitis | attenuate pathology | promote homeostasis differentiation of regulatory T cells [138] | |
| IBD | attenuate inflammation | induce autophagy [141] |
CAD: coronary artery disease; AS: atherosclerosis; EH: essential hypertension; HF: heart failure; AD: Alzheimer's disease; PD: Parkinson’s disease; T2DM: type 2 diabetes mellitus; PHBP: plasma hyaluronan-binding protein; eNOS: endothelial nitric oxide synthase; NMDA: N-methyl-D-aspartate; SAT1: Spermidine/spermine N1-acetyltransferase; TETA: Triethylenetetramine dihydrochloride; TNF-α: tumor necrosis factor-α; NF-κB: nuclear factor kappa-B
3.1 Spermidine and age-related CVDs
Aging is a major risk factor for the development of CVDs, which is a major cause of disability and death in the elderly population. Spermidine prevents against cardiac aging by improving left ventricular elasticity, diastolic function, and mitochondrial function [15]. It has been reported that CVDs such as coronary artery disease (CAD), essential hypertension (EH), and heart failure (HF) are highly influenced by spermidine levels [91].
3.1.1 Spermidine and CAD.
CAD, one of the primary CVDs, is caused by the narrowing or blocking of vascular lumen due to atherosclerotic lesions in coronary arteries. Atherosclerosis (AS) is the major cause of CAD, which is a leading cause of mortality, especially in the elderly population. Studies have reported on the role of spermidine in CAD. Han et al. found an association between spermidine and myocardial ischemic reperfusion [92]. A prospective study found an inverse parallel relationship between spermidine and AS [93]. Tyrrell et al. documented that spermidine supplementation in aged mice inhibits AS via decreasing inflammatory cytokines and improving mitochondrial functions [94]. The risk of AS can also be reduced by the role of spermidine in autophagy [95, 96]. Besides, spermidine attenuates AS due to its antagonistic action on platelet aggregation, which is regarded as a causative factor for AS [96]. Plasma hyaluronan-binding protein (PHBP) is a factor VII activating protease involved in the modulation of vascular function, inflammation, and AS. Spermidine promotes the conversion of PHBP from a single-chain to a two chain form, thereby protecting against AS development [97]. In type 2 diabetes mellitus (T2DM), restoration of endothelial nitric oxide synthase (eNOS) activation by spermidine was found to be blocked by autophagy inhibitors, resulting in AS [98].
3.1.2 Spermidine and EH
EH is characterized by increased vascular resistance, due to endothelial dysfunction and vascular remodeling, representing age-related functional and structural alterations, respectively [99]. Spermidine attenuates the development of EH during aging. Maione et al. reported on the beneficial effects of spermidine on N-methyl-D-aspartate (NMDA) induced EH [100]. Eisenberg et al. proved that dietary spermidine reduces high blood pressure by improving age-related diastolic [101]. Ornithine decarboxylase (ODC) is a crucial enzyme in the polyamine biosynthesis. In hypertensive tissues, spermidine concentrations have been shown to increase in tandem with alterations in ODC activity [102]. Ibrahim et al. proved that spermidine regulates blood pressure because it is an essential component of the blood pressure effect of angiotensin II [103].
3.1.3 Spermidine and HF
HF is a clinical syndrome of aging-related phenotypes. An association between spermidine and HF has been reported. Appropriate induction of autophagy by spermidine might be involved in resistance to HF [104]. Moreover, spermidine supplementation was shown to prevent cardiac hypertrophy and protect cardiomyocytes, thereby delaying HF progression [15, 101]. Mitochondria are crucial in myocardial maintenance and development. Spermidine attenuates mitochondrial dysfunction during aging, which is the primary cause of HF development [105]. Wirth et al. found that the cardioprotective effect of spermidine at the histological level was associated with reduced telomere attrition in cardiac tissues [106]. Moreover, Tantini et al. indicated the effect of spermidine on the apoptosis of myocardial ischemic cells, which inhibited HF development [107].
3.2 Spermidine and age-related neurodegenerative diseases
Neurodegenerative diseases are featured by a progressive loss of selective populations of vulnerable neurons, and they can be classified as Alzheimer's disease (AD), Parkinson’s disease (PD), or motor neuron disease according to clinical characteristics. Spermidine protects against neuronal cell damage by inducing autophagy [108]. Therefore, supplementation with spermidine inhibits multiple neurological pathologies including neurodegeneration, memory loss, cognitive decline, and motor impairment in aging [109].
3.2.1 Spermidine and AD
AD, also referred to as senile dementia, is characterized by progressive cognitive dysfunction and behavioral impairments. Clinically, it manifests as memory impairment, aphasia, agnosia, personality and behavioral alterations, among others. Age-associated memory decline can be attenuated by the autophagic effect of spermidine [110, 111]. Besides, spermidine has been reported to ameliorate age-related dementia [44]. It relieves mitochondrial dysfunction to maintain neuronal energy, prevent nerve cell apoptosis and inflammation as well as improve the expression of neurotrophic factors. Wirth et al. revealed that spermidine exerts a positive influence on memory performance among the elderly, which might be regulated by stimulating the neuromodulators in the memory system [112].
3.2.2 Spermidine and PD
PD, namely paralysis agitans, is a common neurodegenerative disease that is manifested by tremors, myotonia and decreased movement abilities. Degeneration and death of dopaminergic neurons in the substantia nigra is the main pathological basis of PD. McCarty et al. proved that spermidine protects against PD by maintaining dopaminergic neurons functions in the mitochondria [113]. Jadiya et al. reported that spermidine protected against PD by inducing the Atg 7 dependent autophagy pathway in C. elegans [114]. Besides, it also protected cells in a PD model of C. elegans against the toxic effects through the PINK1-PDR1-dependent mitophagy pathway [115]. α-synuclein is considered to be the primary toxic trigger of PD. Previous studies found that higher spermidine concentration alleviates the process of PD through inhibition of α-synuclein and promotion of climbing activity [116, 117]. Guerra et al. suggested that spermidine exhibits neuroprotective effects against PD, which are mediated through its anti-inflammatory and antioxidant properties [118]. DENSPM, a polyamine analogue, and Berenil, a pharmacological agent, increases or decreases SAT1 activities, respectively. It has been confirmed that DENSPM attenuates PD histopathology while Berenil aggravates it [119].
3.3 Spermidine and age-related metabolic diseases
Metabolic diseases are caused by disorders in substance anabolism and catabolism, which are closely correlated with aging. Spermidine is involved in the development of metabolic diseases, such as T2DM, obesity, and metabolic syndrome [120].
3.3.1 Spermidine and T2DM
T2DM is characterized by hyperglycemia due to insulin resistance. Its risk factors are complex, including aging, obesity, a strong family history of diabetes, and physical inactivity. The mechanisms and roles of spermidine in T2DM have been reported. Exogenous spermidine supplementation improves insulin sensitivity and maintains glucose homeostasis [120]. Levasseur et al. clarified that spermidine binds deoxyhypusine synthase (DHPS) in β cells to mRNA translation, which promotes facultative cell proliferation and glucose homeostasis maintenance [121]. Méndez et al. revealed that L-arginine and spermidine plays a inhibitory role in lipid peroxidation and hemoglobin glycation, which may prevent diabetic complications [122]. Besides, Wang et al. proved that spermidine enhanced glucose utilization through AMPK activation in myotubes, possessing a potential hypoglycemic activity in vitro [123]. Serum spermidine oxidase activity has been shown to regulate T2DM and its microvascular complications in patients [124]. Furthermore, Marx et al. elaborated that spermidine and agmatine were involved in renal collagen reduction in diabetic mice, thereby reducing the complications associated with diabetic nephropathy [125].
3.3.2 Spermidine and obesity
Obesity is associated with multiple alterations at hormonal, inflammatory and endothelial levels, thereby enhancing morbidity rates from CVDs. In addition to its role in T2DM, spermidine was found to reduce adiposity and hepatic fat accumulation in diet-induced obese mice [120]. Besides, spermidine dietary can cause a significant weight loss and has the potential for treating obesity due to its beneficial effects in regulating in lipid metabolism, inflammatory responses, and thermogenesis [70]. Spermidine intake was negatively correlated with obesity caused by high-calorie diets and was accompanied by the induction of autophagy in white adipose tissues [126]. Notably, Ma et al. demonstrated that spermidine supplementation alleviated obesity in both mice and humans because its effects in enhancement of intestinal barrier functions and alteration of microbiota composition as well as functions [127]. Moreover, spermidine suppresses adiposity by inhibiting lipogenic genes expression through an AMPK-mediated mechanism [69]. Up-regulation of spermidine is accompanied by down-regulation of nicotinamide N-methyltransferase (Nnmt), which results in nicotinamide salvage regeneration of NAD+, increased energy expenditure, and resistance against obesity [128].
3.3.3 Spermidine and metabolic syndrome
Metabolic syndrome is characterized by insulin resistance, abdominal obesity, hypertension, and hyperlipidemia. Studies have suggested an association between spermidine and metabolic syndrome. Triethylenetetramine dihydrochloride (TETA), a copper-chelator agent, is a safe pharmaceutical that can reduce obesity associated with excessive sucrose intake, high-fat diet, or leptin deficiency, since it can reduce hepatic steatosis and glucose intolerance. It has been shown that the TETA effects depended on the SAT1 activation, which can correct metabolic syndrome [129]. Moreover, Ma et al. confirmed that spermidine inhibited metabolic syndrome in obese mice by ameliorating hepatic steatosis and adipose tissue inflammation [70].
3.4 Spermidine and age-related musculoskeletal diseases
Musculoskeletal diseases are a range of degenerative and inflammatory disorders, which are a vital cause of disability. Spermidine exhibits protective roles against various musculoskeletal diseases, such as osteoporosis, sarcopenia, and osteoarthritis.
3.4.1 Spermidine and osteoporosis
Osteoporosis, the most common metabolic bone disease, is characterized by microarchitectural deterioration and low bone mass. Spermidine concentration is inversely proportional to osteoporosis [130]. Spermidine dietary supplementation enhances bone strength. Besides, increased spermidine biosynthesis in vivo promoted warmth regeneration, which prevented bone loss through gut microbiota. Spermidine was shown to prevent against bone loss by preferentially disturbing osteoclastic activation in ovariectomized mice [131, 132]. Yeon et al. documented that spermidine exerts anti-osteoclastogensis and anti-migration effects by inhibiting RANKLE-mediated signaling pathway and by preventing the expression of transcription factors such as NF-κB [133].
3.4.2 Spermidine and sarcopenia
Skeletal muscles are essential in inhibiting the development of multiple chronic diseases, including CVDs, T2DM, and cancer. Sarcopenia refers to age-associated progressive loss of skeletal muscle mass and function. Spermidine concentrations are associated with sarcopenia [134]. Cervelli et al. hypothesized that spermidine protects against aging-related skeletal muscle atrophy by regulating skeletal muscle differentiation [54]. Chrisam et al. revealed that systemic administration of spermidine induced autophagy in mice, leading to a concurrent amelioration of both ultrastructural and histological muscle defects [135].
3.4.3 Spermidine and osteoarthritis
Osteoarthritis is one of the most prevalent and debilitating chronic joint diseases, which has been associated with a decline and loss in life quality. Sacitharan et al. hypothesized that spermidine is a potential therapy for osteoarthritis because it activates autophagy in osteoarthritic cartilage and reverses the reduction in polyamine synthesis [136]. Chen et al. showed that spermidine alleviates synovitis, osteophyte formation, and cartilage degeneration by inhibiting TNF-α induced NF-κB/p65 signaling pathway in osteoarthritis [137]. In addition, spermidine was shown to exhibit antioxidant, anti-inflammatory, and chondroprotective roles in osteoarthritic chondrocytes [138].
3.5 Spermidine and age-related immune diseases
Immune diseases are caused by imbalances in regulation, which affects immune responses, thereby leading to pathological changes and functional impairments. Spermidine can boost immunity. Autophagy is now recognized as an indispensable cog in the formation of long-lasting immunity, which can help host defense against viruses, bacteria, and parasites. Spermidine treatment ameliorates the decline in autophagy and reverses the senescence of old immune cell functions [31]. Puleston et al. found decreased T cell autophagy in elderly mice, which associated autophagy with immune-senescence [139]. They also reported that spermidine improves memory CD8+ T cell responses to influenza and cytomegalovirus infection by inducing autophagy [140]. In T cell-transfer-induced colitis models, spermidine attenuated tissue pathology by promoting homeostasis differentiation of regulatory T cells within the gut [138]. Liu et al. proved that dietary spermidine supplementation attenuated inflammatory bowel disease (IBD) in mice by inducing autophagy and anti-inflammatory actions associated with mitochondrial ROS-dependent AMPK activation [141].
4. Diagnostic and therapeutic potential of spermidine for age-related diseases
A large proportion of age-related diseases inevitably develop into functional failure or even death, because there are no drug therapies that can reverser the aging process. Therefore, early diagnosis and prompt management are particularly important. In terms of clinical applications, spermidine is a promising prognostic biomarker and a potential therapeutic agent for delaying age-related diseases.
Various pathologies are associated with elevated spermidine concentrations, promoting the possibility of spermidine being a potential disease biomarker. Soda et al. found a positive correlation between spermidine concentrations and CVDs-associated mortality, implying that it could be a prognostic biomarker for CVDs severity [91]. Besides, spermidine is a potential biomarker for the diagnosis of neurocognitive diseases that occur with age, such as AD or PD [31, 142]. Additionally, spermidine can be used in the diagnosis of other age-related diseases, such as cancers [143], T2DM [98], immune diseases (systemic lupus erythematosus) [144], and frailty [145] among others. However, it is worth noting that the association between elevated spermidine levels with various pathologies does not necessarily support its causal involvement.
Moving away from promising biomarker functions, spermidine is also a potential therapeutic agent for treatment of age-related diseases. Madeo et al. postulated that spermidine is a promising pharmaceutical intervention for aging and age-related neurodegenerative, cardiovascular, and malignant diseases [146]. Spermidine plays a protective role in aging heart, and could therefore, be used to protect against CVDs [7, 15]. A growing number of studies are reporting that spermidine is a candidate drug for neurodegenerative diseases such as AD and PD, since it can protect neurons, exert anti-inflammatory and antioxidant activities, and induce autophagy [113, 147]. In addition, there is increasing evidence that spermidine is a therapeutic agent for metabolic diseases by improving insulin sensitivity, maintaining glucose homeostasis, inhibiting lipid metabolism, and promoting thermogenesis [57, 70]. Moreover, due to its anti-inflammatory and antioxidant properties, it might be a potential therapeutic intervention for age-associated acquired immune diseases [43]. Nevertheless, the exact mechanisms have not been established and should, therefore, be evaluated further.
5. Conclusions and perspectives
Aging and age-related diseases share a number of basic mechanistic pillars. Aging is a catalyst in the development of age-related diseases, whereas age-related diseases exacerbate the aging process. Thus, age-induced alterations should be characterized, and aging mechanisms evaluated in order to achieve novel strategies for life extension. Spermidine regulates a wide range of biochemical and physiological aging processes and prolongs a healthy lifespan. Besides, it is a potential prognostic biomarker and therapeutic agent for evaluating and managing age-related diseases. Despite our knowledge on how spermidine brings about its anti-aging effects, there is a need for more studies. Dietary spermidine has a beneficial role in organisms. However, there exists many challenges regarding its administration to humans, including modulatory hurdles, safety and bioavailability, and clinical design issues. Remarkably, elucidation of the precise mechanisms and roles of spermidine in human aging will result in unprecedented health benefits. Therefore, studies should aim at establishing the potential causal relationship between altered spermidine metabolism and associated pathways for disease development and progression. It should also be determined whether exogenous supplementation of spermidine can delay aging, settle age-related diseases, improve life quality and eventually prolong a healthy lifespan.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82071593 and 81770833; the Fundamental Research Funds for the Central Universities of Central South University (NO. 2019zzts354).
Footnotes
Competing Interest
The authors declare that they have no competing interests.
References
- [1].Ni YQ, Zhan JK, Liu YS (2020). Roles and mechanisms of MFG-E8 in vascular aging-related diseases. Ageing Res Rev, 64:101176. [DOI] [PubMed] [Google Scholar]
- [2].Ni YQ, Lin X, Zhan JK, Liu YS (2020). Roles and Functions of Exosomal Non-coding RNAs in Vascular Aging. Aging Dis, 11:164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barbé-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME (2020). The interplay between immunosenescence and age-related diseases. Semin Immunopathol:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Partridge L, Fuentealba M, Kennedy BK (2020). The quest to slow ageing through drug discovery. Nature Reviews Drug Discovery, 19:513-532. [DOI] [PubMed] [Google Scholar]
- [5].Bae DH, Lane DJR, Jansson PJ, Richardson DR (2018). The old and new biochemistry of polyamines. Biochim Biophys Acta Gen Subj, 1862:2053-2068. [DOI] [PubMed] [Google Scholar]
- [6].Ren J, Zhang Y (2018). Targeting Autophagy in Aging and Aging-Related Cardiovascular Diseases. Trends Pharmacol Sci, 39:1064-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang H, Wang J, Li L, Chai N, Chen Y, Wu F, et al. (2017). Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget, 8:64793-64808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Minois N, Carmona-Gutierrez D, Madeo F (2011). Polyamines in aging and disease. Aging (Albany NY), 3:716-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dever TE, Ivanov IP (2018). Roles of polyamines in translation. J Biol Chem, 293:18719-18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Haan G, Lazare SS (2018). Aging of hematopoietic stem cells. Blood, 131:479-487. [DOI] [PubMed] [Google Scholar]
- [11].Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. (2006). Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes, 55:2256-2264. [DOI] [PubMed] [Google Scholar]
- [12].Qian M, Liu B. 2018. Pharmaceutical Intervention of Aging. In Aging and Aging-Related Diseases. 235-254. [DOI] [PubMed] [Google Scholar]
- [13].Hussain SS, Ali M, Ahmad M, Siddique KH (2011). Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv, 29:300-311. [DOI] [PubMed] [Google Scholar]
- [14].Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. (2009). Induction of autophagy by spermidine promotes longevity. Nat Cell Biol, 11:1305-1314. [DOI] [PubMed] [Google Scholar]
- [15].Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med, 22:1428-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Minois N (2014). Molecular basis of the 'anti-aging' effect of spermidine and other natural polyamines - a mini-review. Gerontology, 60:319-326. [DOI] [PubMed] [Google Scholar]
- [17].Feng Y, He D, Yao Z, Klionsky DJ (2014). The machinery of macroautophagy. Cell Res, 24:24-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mizushima N, Komatsu M (2011). Autophagy: renovation of cells and tissues. Cell, 147:728-741. [DOI] [PubMed] [Google Scholar]
- [19].Wen X, Klionsky DJ (2016). An overview of macroautophagy in yeast. J Mol Biol, 428:1681-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fîlfan M, Sandu RE, Zăvăleanu AD, GreşiŢă A, Glăvan DG, Olaru DG, et al. (2017). Autophagy in aging and disease. Rom J Morphol Embryol, 58:27-31. [PubMed] [Google Scholar]
- [21].Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A (2005). Autophagy and aging: the importance of maintaining "clean" cells. Autophagy, 1:131-140. [DOI] [PubMed] [Google Scholar]
- [22].Stadtman ER (2001). Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci, 928:22-38. [DOI] [PubMed] [Google Scholar]
- [23].Madeo F, Tavernarakis N, Kroemer G (2010). Can autophagy promote longevity? Nat Cell Biol, 12:842-846. [DOI] [PubMed] [Google Scholar]
- [24].Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z, Masini M, et al. (2003). Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol, 38:519-527. [DOI] [PubMed] [Google Scholar]
- [25].Jiang M, Liu K, Luo J, Dong Z (2010). Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol, 176:1181-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gottlieb RA, Mentzer RM (2010). Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol, 72:45-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamaguchi O, Otsu K (2012). Role of autophagy in aging. J Cardiovasc Pharmacol, 60:242-247. [DOI] [PubMed] [Google Scholar]
- [28].Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, et al. (2011). Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol, 192:615-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pietrocola F, Lachkar S, Enot DP, Niso-Santano M, Bravo-San Pedro JM, Sica V, et al. (2015). Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ, 22:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yue F, Li W, Zou J, Jiang X, Xu G, Huang H, et al. (2017). Spermidine Prolongs Lifespan and Prevents Liver Fibrosis and Hepatocellular Carcinoma by Activating MAP1S-Mediated Autophagy. Cancer Res, 77:2938-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang H, Alsaleh G, Feltham J, Sun Y, Napolitano G, Riffelmacher T, et al. (2019). Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol Cell, 76:110-125.e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Madeo F, Pietrocola F, Eisenberg T, Kroemer G (2014). Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov, 13:727-740. [DOI] [PubMed] [Google Scholar]
- [33].Mackeh R, Lorin S, Ratier A, Mejdoubi-Charef N, Baillet A, Bruneel A, et al. (2014). Reactive oxygen species, AMP-activated protein kinase, and the transcription cofactor p300 regulate α-tubulin acetyltransferase-1 (αTAT-1/MEC-17)-dependent microtubule hyperacetylation during cell stress. J Biol Chem, 289:11816-11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee IH, Finkel T (2009). Regulation of autophagy by the p300 acetyltransferase. J Biol Chem, 284:6322-6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabandé-Rodríguez E, Gómez de Las Heras MM, Mittelbrunn M (2019). Control of Inflammation by Calorie Restriction Mimetics: On the Crossroad of Autophagy and Mitochondria. Cells, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Michiels CF, Kurdi A, Timmermans JP, De Meyer GRY, Martinet W (2016). Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis, 251:319-327. [DOI] [PubMed] [Google Scholar]
- [37].Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, et al. (2013). The role of inflammation in age-related disease. Aging (Albany NY), 5:84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Morrisette-Thomas V, Cohen AA, Fülöp T, Riesco É, Legault V, Li Q, et al. (2014). Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev, 139:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bektas A, Schurman SH, Sen R, Ferrucci L (2018). Aging, inflammation and the environment. Exp Gerontol, 105:10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bektas A, Schurman SH, Sen R, Ferrucci L (2017). Human T cell immunosenescence and inflammation in aging. J Leukoc Biol, 102:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stepanova M, Rodriguez E, Birerdinc A, Baranova A (2015). Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget, 6:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta, 1813:878-888. [DOI] [PubMed] [Google Scholar]
- [43].Jeong JW, Cha HJ, Han MH, Hwang SJ, Lee DS, Yoo JS, et al. (2018). Spermidine Protects against Oxidative Stress in Inflammation Models Using Macrophages and Zebrafish. Biomol Ther (Seoul), 26:146-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xu TT, Li H, Dai Z, Lau GK, Li BY, Zhu WL, et al. (2020). Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany NY), 12:6401-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yan J, Yan JY, Wang YX, Ling YN, Song XD, Wang SY, et al. (2019). Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br J Pharmacol, 176:3126-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Matsumura I, Tanaka H, Kanakura Y (2003). E2F1 and c-Myc in cell growth and death. Cell Cycle, 2:333-338. [PubMed] [Google Scholar]
- [47].Ou Y, Wang SJ, Li D, Chu B, Gu W (2016). Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A, 113:E6806-e6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chattopadhyay MK, Tabor CW, Tabor H (2002). Absolute requirement of spermidine for growth and cell cycle progression of fission yeast (Schizosaccharomyces pombe). Proc Natl Acad Sci U S A, 99:10330-10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Landau G, Ran A, Bercovich Z, Feldmesser E, Horn-Saban S, Korkotian E, et al. (2012). Expression profiling and biochemical analysis suggest stress response as a potential mechanism inhibiting proliferation of polyamine-depleted cells. J Biol Chem, 287:35825-35837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jing YH, Yan JL, Wang QJ, Chen HC, Ma XZ, Yin J, et al. (2018). Spermidine ameliorates the neuronal aging by improving the mitochondrial function in vitro. Exp Gerontol, 108:77-86. [DOI] [PubMed] [Google Scholar]
- [51].Muñoz-Esparza NC, Latorre-Moratalla ML, Comas-Basté O, Toro-Funes N, Veciana-Nogués MT, Vidal-Carou MC (2019). Polyamines in Food. Front Nutr, 6:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schwarz C, Horn N, Benson G, Wrachtrup Calzado I, Wurdack K, Pechlaner R, et al. (2020). Spermidine intake is associated with cortical thickness and hippocampal volume in older adults. Neuroimage, 221:117132. [DOI] [PubMed] [Google Scholar]
- [53].Borzì RM, Guidotti S, Minguzzi M, Facchini A, Platano D, Trisolino G, et al. (2014). Polyamine delivery as a tool to modulate stem cell differentiation in skeletal tissue engineering. Amino Acids, 46:717-728. [DOI] [PubMed] [Google Scholar]
- [54].Cervelli M, Leonetti A, Duranti G, Sabatini S, Ceci R, Mariottini P (2018). Skeletal Muscle Pathophysiology: The Emerging Role of Spermine Oxidase and Spermidine. Med Sci (Basel), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL (2013). Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest, 123:966-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Khosla S, Farr JN, Tchkonia T, Kirkland JL (2020). The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol, 16:263-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Madeo F, Eisenberg T, Pietrocola F, Kroemer G (2018). Spermidine in health and disease. Science, 359. [DOI] [PubMed] [Google Scholar]
- [58].Metur SP, Klionsky DJ (2020). The curious case of polyamines: spermidine drives reversal of B cell senescence. Autophagy, 16:389-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhu WW, Xiao F, Tang YY, Zou W, Li X, Zhang P, et al. (2018). Spermidine prevents high glucose-induced senescence in HT-22 cells by upregulation of CB1 receptor. Clin Exp Pharmacol Physiol, 45:832-840. [DOI] [PubMed] [Google Scholar]
- [60].Baek AR, Hong J, Song KS, Jang AS, Kim DJ, Chin SS, et al. (2020). Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp Mol Med, 52:2034-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stefanelli C, Stanic I, Zini M, Bonavita F, Flamigni F, Zambonin L, et al. (2000). Polyamines directly induce release of cytochrome c from heart mitochondria. Biochem J, 347 Pt 3:875-880. [PMC free article] [PubMed] [Google Scholar]
- [62].Eisenberg T, Carmona-Gutierrez D, Büttner S, Tavernarakis N, Madeo F (2010). Necrosis in yeast. Apoptosis, 15:257-268. [DOI] [PubMed] [Google Scholar]
- [63].Wang Y, Yuan Q, Xie L (2018). Histone Modifications in Aging: The Underlying Mechanisms and Implications. Curr Stem Cell Res Ther, 13:125-135. [DOI] [PubMed] [Google Scholar]
- [64].Bose R, Kanungo MS (1982). Polyamines modulate phosphorylation and acetylation of non-histone chromosomal proteins of the cerebral cortex of rats of various ages. Arch Gerontol Geriatr, 1:339-348. [DOI] [PubMed] [Google Scholar]
- [65].Burgio G, Corona DF, Nicotra CM, Carruba G, Taibi G (2016). P/CAF-mediated spermidine acetylation regulates histone acetyltransferase activity. J Enzyme Inhib Med Chem, 31:75-82. [DOI] [PubMed] [Google Scholar]
- [66].Xie Y, Li J, Kang R, Tang D (2020). Interplay Between Lipid Metabolism and Autophagy. Front Cell Dev Biol, 8:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Slack C, Werz C, Wieser D, Alic N, Foley A, Stocker H, et al. (2010). Regulation of lifespan, metabolism, and stress responses by the Drosophila SH2B protein, Lnk. PLoS Genet, 6:e1000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Minois N, Rockenfeller P, Smith TK, Carmona-Gutierrez D (2014). Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition. PLoS One, 9:e102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gao M, Zhao W, Li C, Xie X, Li M, Bi Y, et al. (2018). Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem Biophys Res Commun, 505:93-98. [DOI] [PubMed] [Google Scholar]
- [70].Ma L, Ni Y, Hu L, Zhao Y, Zheng L, Yang S, et al. (2021). Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in preexisting obese mice. Life Sci, 265:118739. [DOI] [PubMed] [Google Scholar]
- [71].Yuan F, Zhang L, Cao Y, Gao W, Zhao C, Fang Y, et al. (2018). Spermidine/spermine N1-acetyltransferase-mediated polyamine catabolism regulates beige adipocyte biogenesis. Metabolism, 85:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bachmann AS, Geerts D (2018). Polyamine synthesis as a target of MYC oncogenes. J Biol Chem, 293:18757-18769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hyvönen MT, Koponen T, Weisell J, Pietilä M, Khomutov AR, Vepsäläinen J, et al. (2013). Spermidine promotes adipogenesis of 3T3-L1 cells by preventing interaction of ANP32 with HuR and PP2A. Biochem J, 453:467-474. [DOI] [PubMed] [Google Scholar]
- [74].Wang J, Li S, Wang J, Wu F, Chen Y, Zhang H, et al. (2020). Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging (Albany NY), 12:650-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tain LS, Jain C, Nespital T, Froehlich J, Hinze Y, Grönke S, et al. (2020). Longevity in response to lowered insulin signaling requires glycine N-methyltransferase-dependent spermidine production. Aging Cell, 19:e13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Soerensen M, Dato S, Christensen K, McGue M, Stevnsner T, Bohr VA, et al. (2010). Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell, 9:1010-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fritzen AM, Frøsig C, Jeppesen J, Jensen TE, Lundsgaard AM, Serup AK, et al. (2016). Role of AMPK in regulation of LC3 lipidation as a marker of autophagy in skeletal muscle. Cell Signal, 28:663-674. [DOI] [PubMed] [Google Scholar]
- [78].Fan J, Yang X, Li J, Shu Z, Dai J, Liu X, et al. (2017). Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget, 8:17475-17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Stark F, Pfannstiel J, Klaiber I, Raabe T (2011). Protein kinase CK2 links polyamine metabolism to MAPK signalling in Drosophila. Cell Signal, 23:876-882. [DOI] [PubMed] [Google Scholar]
- [80].Choi YH, Park HY (2012). Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J Biomed Sci, 19:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang D, Liu Q, Wu D, He B, Li J, Mao C, et al. (2018). Isothermal Self-Assembly of Spermidine-DNA Nanostructure Complex as a Functional Platform for Cancer Therapy. ACS Appl Mater Interfaces, 10:15504-15516. [DOI] [PubMed] [Google Scholar]
- [82].Khan AU, Mei YH, Wilson T (1992). A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci U S A, 89:11426-11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li S, Zhan JK, Wang YJ, Lin X, Zhong JY, Wang Y, et al. (2019). Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci, 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lin X, Li S, Wang YJ, Wang Y, Zhong JY, He JY, et al. (2019). Exosomal Notch3 from high glucose-stimulated endothelial cells regulates vascular smooth muscle cell calcification/aging. Life Sci:116582. [DOI] [PubMed] [Google Scholar]
- [85].Lin X, Zhan JK, Wang YJ, Tan P, Chen YY, Deng HQ, et al. (2016). Function, Role, and Clinical Application of MicroRNAs in Vascular Aging. Biomed Res Int, 2016:6021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lin X, Zhan JK, Zhong JY, Wang YJ, Wang Y, Li S, et al. (2019). lncRNA-ES3/miR-34c-5p/BMF axis is involved in regulating high-glucose-induced calcification/senescence of VSMCs. Aging (Albany NY), 11:523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhan JK, Wang YJ, Li S, Wang Y, Tan P, He JY, et al. (2018). AMPK/TSC2/mTOR pathway regulates replicative senescence of human vascular smooth muscle cells. Exp Ther Med, 16:4853-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tan P, Wang YJ, Li S, Wang Y, He JY, Chen YY, et al. (2016). The PI3K/Akt/mTOR pathway regulates the replicative senescence of human VSMCs. Mol Cell Biochem, 422:1-10. [DOI] [PubMed] [Google Scholar]
- [89].Xu F, Zhong JY, Lin X, Shan SK, Guo B, Zheng MH, et al. (2020). Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J Pineal Res, 68:e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].He J, Tu C, Liu Y (2018). Role of lncRNAs in aging and age-related diseases. Aging Med (Milton), 1:158-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Soda K, Kano Y, Chiba F (2012). Food polyamine and cardiovascular disease--an epidemiological study. Glob J Health Sci, 4:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Han LP, Xu CQ, Jiang CM, Li HZ, Zhao YJ, Gong YS, et al. (2008). [Myocardial polyamine metabolism and the ischemia-reperfusion injury in the rat heart]. Zhonghua Xin Xue Guan Bing Za Zhi, 36:346-349. [PubMed] [Google Scholar]
- [93].Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, et al. (2018). Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr, 108:371-380. [DOI] [PubMed] [Google Scholar]
- [94].Tyrrell DJ, Blin MG, Song J, Wood SC, Zhang M, Beard DA, et al. (2020). Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ Res, 126:298-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Matsumoto M (2020). Prevention of Atherosclerosis by the Induction of Microbial Polyamine Production in the Intestinal Lumen. Biol Pharm Bull, 43:221-229. [DOI] [PubMed] [Google Scholar]
- [96].Matsumoto M, Kitada Y, Naito Y (2019). Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yamamoto E, Nishimura N, Okada K, Sekido C, Yamamichi S, Hasumi K (2011). Inhibitors of autoactivation of plasma hyaluronan-binding protein (factor VII activating protease). Biol Pharm Bull, 34:462-470. [DOI] [PubMed] [Google Scholar]
- [98].Fetterman JL, Holbrook M, Flint N, Feng B, Bretón-Romero R, Linder EA, et al. (2016). Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis, 247:207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, et al. (2016). Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res, 110:298-308. [DOI] [PubMed] [Google Scholar]
- [100].Maione S, Berrino L, Pizzirusso A, Leyva J, Filippelli A, Vitagliano S, et al. (1994). Effects of the polyamine spermidine on NMDA-induced arterial hypertension in freely moving rats. Neuropharmacology, 33:789-793. [DOI] [PubMed] [Google Scholar]
- [101].Eisenberg T, Abdellatif M, Zimmermann A, Schroeder S, Pendl T, Harger A, et al. (2017). Dietary spermidine for lowering high blood pressure. Autophagy, 13:767-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mathy C, Carlier P, Yerna N, Rorive G (1987). [Polyamines and cardiovascular hypertrophy in experimental hypertension]. Arch Mal Coeur Vaiss, 80:777-782. [PubMed] [Google Scholar]
- [103].Ibrahim J, Hughes AD, Schachter M, Sever PS (1996). Depletion of resistance vessel polyamines attenuates angiotensin II induced blood pressure rise in rats. Clin Exp Hypertens, 18:811-830. [DOI] [PubMed] [Google Scholar]
- [104].Yamaguchi O (2019). Autophagy in the Heart. Circ J, 83:697-704. [DOI] [PubMed] [Google Scholar]
- [105].Oh CM, Ryu D, Cho S, Jang Y (2020). Mitochondrial Quality Control in the Heart: New Drug Targets for Cardiovascular Disease. Korean Circ J, 50:395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wirth A, Wolf B, Huang CK, Glage S, Hofer SJ, Bankstahl M, et al. (2021). Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tantini B, Fiumana E, Cetrullo S, Pignatti C, Bonavita F, Shantz LM, et al. (2006). Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J Mol Cell Cardiol, 40:775-782. [DOI] [PubMed] [Google Scholar]
- [108].Yang Y, Chen S, Zhang Y, Lin X, Song Y, Xue Z, et al. (2017). Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis, 8:e2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ricke KM, Cruz SA, Qin Z, Farrokhi K, Sharmin F, Zhang L, et al. (2020). Neuronal Protein Tyrosine Phosphatase 1B Hastens Amyloid β-Associated Alzheimer's Disease in Mice. J Neurosci, 40:1581-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, et al. (2014). Spermidine-triggered autophagy ameliorates memory during aging. Autophagy, 10:178-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, et al. (2013). Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci, 16:1453-1460. [DOI] [PubMed] [Google Scholar]
- [112].Wirth M, Benson G, Schwarz C, Köbe T, Grittner U, Schmitz D, et al. (2018). The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex, 109:181-188. [DOI] [PubMed] [Google Scholar]
- [113].McCarty MF, Lerner A (2020). Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson's Disease. Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Jadiya P, Mir SS, Nazir A (2018). Osmotic stress induced toxicity exacerbates Parkinson's associated effects via dysregulation of autophagy in transgenic C. elegans model. Cell Signal, 45:71-80. [DOI] [PubMed] [Google Scholar]
- [115].Yang X, Zhang M, Dai Y, Sun Y, Aman Y, Xu Y, et al. (2020). Spermidine inhibits neurodegeneration and delays aging via the PINK1-PDR1-dependent mitophagy pathway in C. elegans. Aging (Albany NY), 12:16852-16866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Büttner S, Broeskamp F, Sommer C, Markaki M, Habernig L, Alavian-Ghavanini A, et al. (2014). Spermidine protects against α-synuclein neurotoxicity. Cell Cycle, 13:3903-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Antony T, Hoyer W, Cherny D, Heim G, Jovin TM, Subramaniam V (2003). Cellular polyamines promote the aggregation of alpha-synuclein. J Biol Chem, 278:3235-3240. [DOI] [PubMed] [Google Scholar]
- [118].Guerra GP, Rubin MA, Mello CF (2016). Modulation of learning and memory by natural polyamines. Pharmacol Res, 112:99-118. [DOI] [PubMed] [Google Scholar]
- [119].Lewandowski NM, Ju S, Verbitsky M, Ross B, Geddie ML, Rockenstein E, et al. (2010). Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc Natl Acad Sci U S A, 107:16970-16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ramos-Molina B, Queipo-Ortuño MI, Lambertos A, Tinahones FJ, Peñafiel R (2019). Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Front Nutr, 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Levasseur EM, Yamada K, Piñeros AR, Wu W, Syed F, Orr KS, et al. (2019). Hypusine biosynthesis in β cells links polyamine metabolism to facultative cellular proliferation to maintain glucose homeostasis. Sci Signal, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Méndez JD, Balderas FL (2006). Inhibition by L-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed Pharmacother, 60:26-31. [DOI] [PubMed] [Google Scholar]
- [123].Wang LN, Jiang HW, Li JL, Li JY (2018). Enhancement of Glucose Utilization by Loesenerine through AMPK Activation in Myotubes. Chem Pharm Bull (Tokyo), 66:885-886. [DOI] [PubMed] [Google Scholar]
- [124].Seghieri G, Gironi A, Niccolai M, Mammini P, Alviggi L, De Giorgio LA, et al. (1990). Serum spermidine oxidase activity in patients with insulin-dependent diabetes mellitus and microvascular complications. Acta Diabetol Lat, 27:303-308. [DOI] [PubMed] [Google Scholar]
- [125].Marx M, Trittenwein G, Aufricht C, Hoeger H, Lubec B (1995). Agmatine and spermidine reduce collagen accumulation in kidneys of diabetic db/db mice. Nephron, 69:155-158. [DOI] [PubMed] [Google Scholar]
- [126].Fernández Á F, Bárcena C, Martínez-García GG, Tamargo-Gómez I, Suárez MF, Pietrocola F, et al. (2017). Autophagy couteracts weight gain, lipotoxicity and pancreatic β-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis, 8:e2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F, et al. (2020). Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes, 12:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bonhoure N, Byrnes A, Moir RD, Hodroj W, Preitner F, Praz V, et al. (2015). Loss of the RNA polymerase III repressor MAF1 confers obesity resistance. Genes Dev, 29:934-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Castoldi F, Hyvönen MT, Durand S, Aprahamian F, Sauvat A, Malik SA, et al. (2020). Chemical activation of SAT1 corrects diet-induced metabolic syndrome. Cell Death Differ, 27:2904-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chevalier C, Kieser S, Çolakoğlu M, Hadadi N, Brun J, Rigo D, et al. (2020). Warmth Prevents Bone Loss Through the Gut Microbiota. Cell Metab, 32:575-590.e577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yamamoto T, Hinoi E, Fujita H, Iezaki T, Takahata Y, Takamori M, et al. (2012). The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br J Pharmacol, 166:1084-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Yamada T, Park G, Node J, Ozaki K, Hiraiwa M, Kitaguchi Y, et al. (2019). Daily intake of polyamine-rich Saccharomyces cerevisiae S631 prevents osteoclastic activation and bone loss in ovariectomized mice. Food Sci Biotechnol, 28:1241-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yeon JT, Ryu BJ, Choi SW, Heo JC, Kim KJ, Son YJ, et al. (2014). Natural polyamines inhibit the migration of preosteoclasts by attenuating Ca2+-PYK2-Src-NFATc1 signaling pathways. Amino Acids, 46:2605-2614. [DOI] [PubMed] [Google Scholar]
- [134].Uchitomi R, Hatazawa Y, Senoo N, Yoshioka K, Fujita M, Shimizu T, et al. (2019). Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci Rep, 9:10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chrisam M, Pirozzi M, Castagnaro S, Blaauw B, Polishchuck R, Cecconi F, et al. (2015). Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice. Autophagy, 11:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Sacitharan PK, Gharios GB, Edwards JR (2019). Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via EP300: response to correspondence by Borzì et al. Exp Mol Med, 51:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chen Z, Lin CX, Song B, Li CC, Qiu JX, Li SX, et al. (2020). Spermidine activates RIP1 deubiquitination to inhibit TNF-α-induced NF-κB/p65 signaling pathway in osteoarthritis. Cell Death Dis, 11:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].D'Adamo S, Cetrullo S, Guidotti S, Silvestri Y, Minguzzi M, Santi S, et al. (2020). Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free Radic Biol Med, 153:159-172. [DOI] [PubMed] [Google Scholar]
- [139].Puleston DJ, Zhang H, Powell TJ, Lipina E, Sims S, Panse I, et al. (2014). Autophagy is a critical regulator of memory CD8(+) T cell formation. Elife, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Puleston DJ, Simon AK (2015). New roles for autophagy and spermidine in T cells. Microb Cell, 2:91-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Liu R, Li X, Ma H, Yang Q, Shang Q, Song L, et al. (2020). Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic Biol Med, 161:339-350. [DOI] [PubMed] [Google Scholar]
- [142].Saiki S, Sasazawa Y, Fujimaki M, Kamagata K, Kaga N, Taka H, et al. (2019). A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann Neurol, 86:251-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Maksymiuk AW, Sitar DS, Ahmed R, Cheng B, Bach H, Bagchi RA, et al. (2018). Spermidine/spermine N1-acetyltransferase-1 as a diagnostic biomarker in human cancer. Future Sci OA, 4:Fso345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kim HA, Lee HS, Shin TH, Jung JY, Baek WY, Park HJ, et al. (2018). Polyamine patterns in plasma of patients with systemic lupus erythematosus and fever. Lupus, 27:930-938. [DOI] [PubMed] [Google Scholar]
- [145].Dominguez LJ, Barbagallo M (2017). The relevance of nutrition for the concept of cognitive frailty. Curr Opin Clin Nutr Metab Care, 20:61-68. [DOI] [PubMed] [Google Scholar]
- [146].Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G (2019). Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab, 29:592-610. [DOI] [PubMed] [Google Scholar]
- [147].Gruendler R, Hippe B, Sendula Jengic V, Peterlin B, Haslberger AG (2020). Nutraceutical Approaches of Autophagy and Neuroinflammation in Alzheimer's Disease: A Systematic Review. Molecules, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]