Abstract
Objectives
The goal of this study was to describe outcomes and associated characteristics of patients who were intubated during the initial (3/2020‐4/2020) New York City surge of the severe acute respiratory syndrome coronavirus 2 (COVID‐19) pandemic, during which time we were confronted by an unknown and unprecedented respiratory distress syndrome with extremely high degrees of morbidity and mortality. Our secondary aim was to analyze our physician's rapidly evolving approaches to COVID‐19 airway management.
Methods
A retrospective cohort analysis of all patients intubated at two emergency departments (EDs) for COVID‐19 suspected respiratory failure. In addition, a survey was done to analyze clinician airway management trends and attitudes as they evolved during that period.
Results
Ninety‐five patients met inclusion criteria for the study. Primary outcomes looked at the spectrum of mortality outcomes ranging from died on arrival (DOA) to the ED, died in the ED (DED), died an inpatient (DIH), and survival to discharge. Overall mortalitywas 71.6% with an average age of 62.7 years. Female sex, as a demographic, was associated with higher rates of survival to discharge at 42.3% when compared to males at 23.2% (P < 0.001). Mean age was 70.8 years DOA, 65.6 years DED, 62.9 years DIH, and 60.0 years for survivors (P = 0.0037). Initial lactate levels were 8.15 mmol/L DED, 3.56 mmol/L DIH, and 2.61 mmol/L survivors (P < 0.0001). Initial creatinine levels were 3.38 mg/dL DED, 1.94 mg/dL DIH, and 1.77 mg/dL survivors (P = 0.0073). D‐dimer levels were 7520.5 ng/mL DED, 5932.4 ng/mL DIH, and 1133.9 ng/mL survivors (P = 0.0045). Physician survey respondents reported high levels (69%) of laryngeal edema and prolonged post intubation hypoxia (>50% of time) and >80% remained concerned for their safety. There was a dramatic shift from early (73% of time) to late intubation strategies (67% of time) or non‐invasive approaches (28% of time) as the first surge of the pandemic evolved.
Conclusion
Our findings demonstrate that several demographic, clinical and laboratory parameters correlated with mortality in our cohort of patients intubated during the initial phase of the COVID‐19 pandemic. These included male sex, advanced age, high levels of initial lactic acidosis, elevated D‐dimer, and chronic kidney disease/acute kidney injury. In contrast, presenting respiratory characteristics were not correlated with mortality. In addition, our findings demonstrate that physician attitudes and strategies related to COVID‐19 airway management evolved significantly and rapidly over the initial phase of the pandemic.
Keywords: airway management, COVID‐19, difficult airway, respiratory failure
1. INTRODUCTION
1.1. Background
New York City (NYC) was the epicenter of the severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐19) pandemic crises in March and April 2020. Patients presenting with coronavirus disease (COVID‐19) infections described a myriad of symptoms including intermittent fever, chills, dry cough, dyspnea, myalgia, as well as gastrointestinal complaints of nausea, vomiting, and diarrhea.The most critically ill patients; however, presented with acute and rapidly progressing respiratory failure. 1 , 2 Airway management, including both invasive and non‐invasive modalities, was recognized to be the most important aspect of COVID‐19 management for an unprecedented number of patients initially presenting to the emergency department (ED). 3 Early in the NYC COVID‐19 surge, similar to established practice throughout the world, endotracheal intubation via rapid sequence induction (RSI) or a modified RSI was considered to be the treatment of choice for patients presenting with hypoxic respiratory failure. 4 , 5 Data has shown that over the course of this initial COVID‐19 surge, the borough of the Bronx was disproportionately impacted by both patient volumes and high levels of presenting acuity. 6
1.2. Importance
Our department serves as one of the primary centers for providing emergency care to the Bronx, an area with the highest mortality and hospitalization rates related to the COVID‐19 infection, as of April 2020.7 Social determinants of health and underlying health disparities leading to endemic chronic illnesses (asthma, diabetes, hypertension, obesity, chronic obstructive pulmonary disease, and psychiatric disease) affecting our patient population led to extreme levels of COVID‐19 morbidity and mortality, despite the Bronx having some of the highest number of hospital beds available in NYC per 100,000 patients and the smallest number of older adults (aged ≥65 years).7,8
1.3. Goals of this investigation
This study aims to capture a unique moment in emergency medicine history where we found ourselves confronting an unknown and unprecedented respiratory illness with extremely high degrees of morbidity and mortality as well as a clear infectious danger to the staff with incomplete knowledge as to contagion or virulence. We strove to further describe clinical trends and patient outcomes in a cohort of COVID‐19 ED patients undergoing early airway management for respiratory failure in two urban academic emergency departments (ED) during the height of the first COVID‐19 surge in NYC.
2. METHODS
2.1. Study design and setting
Our department consists of 2 clinical sites, both located in the Bronx, New York. One site is an urban, level 1 trauma center with an average census of 100,000 ED visits per year serving as the primary academic site for one of the largest emergency medicine residency programs in the country. The second ED is a community hospital with an average census of 50,000 visits per year and is staffed by attending physicians and non‐physician advanced practice clinicians. Both EDs are staffed by attendings from the same Department of Emergency Medicine.
Department‐wide intubation policies and procedures were implemented at the beginning of the COVID‐19 surge in‐line with World Health Organization recommendations at the time. 9 Non‐cardiac arrest intubations were performed using standard RSI with either ketamine or etomidate and rocuronium. Video laryngoscopy was established as the primary strategy with either an angulated LoPro or DirectView MAC (Spectrum single use GLIDESCOPE) blade, based on physician preference. Ventilators were set up a priori and connected immediately after endotracheal tube placement (direct to circuit) to avoid any use of bag valve mask ventilation and subsequent aerosolization of virus.
Pre‐oxygenation was maximized before endotracheal intubation. Intubations took place in a closed‐door or negative pressure room. All staff wore personal protective equipment (PPE) consisting of, at minimum, N95 mask, eye goggles, face shield, and fluid resistant gown/suit over work clothes. The use of an intubating box or intubating sheet was employed in some cases. Staff in the room during the procedure was restricted to the intubator (typically an emergency medicine resident), an emergency medicine attending (and as needed, a senior emergency medicine resident), a nurse, and a respiratory therapist.
2.2. Study population
A retrospective cohort analysis of 95 patients intubated in two EDs for COVID‐19 suspected of respiratory failure from March 2, 2020 to April 18, 2020. Patients who met study criteria and were analyzed for outcomes, demographics, presenting vital signs, initial imaging and laboratory studies, and interventions recorded.
Our primary study aims and design sought to describe the association between pre‐selected demographic, clinical, and laboratory findings as they related to mortality for this unique patient cohort.
2.3. Data collection
The study's patient population was determined by a predetermined series of inclusion and exclusion criteria.
Inclusion criteria included all patients over the age of 18 that were intubated within the ED with “high suspicion of COVID‐19 respiratory failure” were included in the study. Criteria to be considered “high suspicion for COVID‐19 respiratory failure” was defined as any patient intubated for respiratory distress, respiratory failure, or cardiac arrest presentation with at least 1 of the following 8 clinical features: (1) known COVID‐19‐positive or known antecedent exposure to a patient with COVID‐19, (2) cough, (3) fever, (4) shortness of breath/dyspnea, (5) hypoxia, (6) altered mental status, (7) syncope, (8) chest pain, (9) weakness/malaise, or (10) influenza‐like illness. Of note, early on in the initial surge, there were no accurate and consistent modalities for testing available at the hospital level.
Patients were excluded by criteria if they were intubated in the ED for a clear non‐COVID‐19 etiology. These included patients who were intubated secondary to traumatic injury, burns, intracerebral hemorrhage, and intoxication leading to airway compromise.
Eligible patients were screened by generating a list from the electronic health record (EHR) of all patients who had documentation of endotracheal intubation. The report included patient demographics of age, gender, height, weight, initial vital signs, pertinent medical history, imaging and laboratory data. The data report was exported to a password protected standard data collection form. Each patient's chart was then reviewed for eligibility and complete data collection by 1 of a team's 5 abstractors (SM, EP, SS, MT, and JM). After an initial training, a set of standardized data field definitions and abstraction procedures was provided to all abstractors. The data collection form was piloted for reliability before finalization.
After data collection began formally, investigators met on a regular basis to ensure continued uniformity of data collection procedures. The chart review process detailed the past medical history of the patients, relevant clinical details about their ED visit, subsequent hospitalization, and final disposition/outcome. All data were entered in either ordinal, categorical, or continuous numerical formats, in accordance with the data dictionary. All unclear questions related to inclusion or exclusion criteria and/or questions about clinical course or outcome were adjudicated by committee.
The Bottom Line.
Physicians' decision to intubate, this study of 95 patients intubated during the initial NYC COVID‐19 pandemic surge demonstrated that demographic factors, such as age, gender, as well as biomarker derangement including initial lactic acidosis, elevated D‐dimer, and acute kidney injury/chronic kidney disease (AKI/CKD) correlated more with mortality than initial respiratory characteristics. The study showed how physicians’ attitudes and airway management strategies changed rapidly during the initial NYC COVID‐19 surge period.
2.4. Measures
Final patient disposition served as the study's primary outcome. The four clinical outcomes considered were as follows:
Dead on arrival (DOA):if the patient arrived in cardiac arrest with high suspicion of COVID‐19 and was unable to be resuscitated.
Died in ED (DED): the patient did not survive ED resuscitation attempts.
Died as an inpatient/died in hospital (DIH).
Survived to hospital discharge.
In addition to the aforementioned primary outcomes a series of patient characteristics were also considered in describing the patient cohort. Patient demographics included age, sex, height, and weight. Presenting initial vital signs and selected laboratory findings were likewise analyzed. Furthermore, underlying medical conditions including reactive airway disease (asthma/chronic obstructive pulmonary disease), hypertension, coronary artery disease, congestive heart failure, diabetes mellitus, and HIV were also considered.
2.5. Data analysis
Our data analysis was conducted to specifically consider the impact of pre‐identified demographic, clinical, and laboratory characteristics on primary patient outcomes of those included in the study. This was done via the following statistical analysis: Categorical variables are presented as means with SD. Continuous variables are presented as frequencies (%). An ANOVA analysis was used to compare outcomes across the primary outcome groups and data are presented with P values. A χ2 was used for categorical values and the P‐values are reported. The delta SpO2 was defined as the difference between the first recorded hospital SpO2 and the first post intubation Sp02. It was initially hypothesized to potentially correlate with success of resuscitation and favorable outcomes.
Based on our initial analysis, 3 respiratory factors considering initial hypoxemia (initial SpO2, delta SpO2, and initial respiratory rate) and 3 systemic indicators (initial creatinine, initial lactate, and initial D‐dimer) were selected for comparison between patients who died in the ED versus those who survived the ED but died later in their hospital course. To compare the groups, we performed a one‐way ANOVA with a Sidak post‐hoc comparison. Data that violated the homoscedastic error assumption were natural logarithm (Ln)‐transformed. To offset availability bias from complete‐case analysis, we used mean imputation for missing data among the 6 variables of interest.
To explore secular changes in hypoxemia severity at the time of intubation over the study period, we fitted a Loess regression on pre‐intubation SpO2 as a function of date. All analysis was performed in SAS University Edition (SAS Institute, Cary, NC).
2.6. Physician survey analysis
To assess physicians’ evolving attitudes and perceptions concerning intubation for respiratory distress during the initial COVID‐19 surge, a 10‐question SurveyMonkey was administered to faculty and residents. The survey design included multiple choice and Likert responses that assessed the intubating experience, perceptions of staff safety, approach to non‐invasive strategies, as well as the dynamic practice environment during the study period. The survey was sent to staff on May 13 and closed on June 1 and consisted of 10 discrete questions. Data are presented as simple response percentages and the survey instrument is available for review in Appendix 1.
3. RESULTS
One‐hundred‐sixty‐five (165) consecutive patients were identified as having been intubated during the designated study period, and 95 patients ultimately met study inclusion criteria. All 95 patients were successfully intubated in the ED with no need for surgical airway intervention or the use of adjunct rescue devices. All patients were followed through 1 of the 4 primary outcomes including 4 DOA (4%), 12 DED (12.6%), 52 DIH (55%), and 27 patients survived to discharge (28.5%). All patients had reached a final disposition by 120 days.
Primary study aims looked to assess patient outcomes from this unique patient cohort related to underlying demographic characteristics including age and sex. Overall mortality among the cohort was found to be 71.6%. A total of 72.6% of included patients were male. Of the 26 women who met criteria for inclusion, 11 (42.3%) survived to discharge in contrast to only 16 of 69 males (23.2%) who survived to discharge. Male versus female mortality was 76.8% versus 57.7% (P < 0.001). In addition to sex, patient age was also identified as having a significant association with patient outcome. The average age of the included cohort was 62.7 years. The average ages of patients based on patient outcome were 70.8 years DOA, 65.6 years DED, 62.9 DIH, and 60.0 years for those discharged (P = 0.0037).
In addition to underlying demographic characteristics, initial presenting clinical parameters were assessed for their impact on patient outcomes. Mean initial room air SpO2 was 80.9% and initial recorded SpO2, following intubation, was 90.2%. These findings were thought to be of particular importance considering the disease's prevalence for respiratory involvement. Sub‐cohort analysis based on primary patient disposition was also considered. Average initial room air SpO2 were: N/A DOA, 78.5% DED, 79.2% DIH, and 84.7% for those discharged. Following intubations, patient initial SpO2 and delta SpO2 were also considered. Initial post intubation SpO2 were: N/A DOA, 90.3% DED, 88.1% DIH, and 94.0% for those discharged.
In addition to respiratory findings, hemodynamic parameters were also initially considered for their possible association with patient outcomes. This included the prevalence of mild tachycardia, with an average heart rate of 108 beats per minute, without hypotension. The median blood pressure for patients on arrival was normotensive at 132/75 mm HgB. Of note, despite the infectious nature of the disease process, and its use as a major screening criteria throughout the pandemic, most of the cohorts patients were not febrile on presentation with an mean and median temperature of 37.2°C and 36.9°C, respectively.
Several laboratory studies were found to correlate with primary patient outcomes. These included values for initial lactate, creatinine, and D‐dimer. Average initial lactate levels were N/A DOA, 8.15 mmol/L DED, 3.56 mmol/L DIH, and 2.61 mmol/L for those discharged (P < 0.0001). Average initial creatinine levels were N/A DOA, 3.38 mg/dL DED, 1.94 mg/dL DIH, and 1.77 mg/dL for those discharged (P = 0.0073). Average initial D‐dimer levels were N/A DOA, 7520.5 ng/mL DED, 5932.4 ng/mL DIH, and 1133.9 ng/mL for those discharged (P = 0.0045). The data on these initial patient demographics and characteristics related to primary outcomes can be found in Tables 1 and 2.
TABLE 1.
Patient demographics
| Variable | No. | Mean | SD |
|---|---|---|---|
| Demographics/exam findings | |||
| Age (y) | 95 | 62.71579 | 13.55812 |
| BMI (body mass index) | 86 | 31.60591 | 20.84101 |
| Room air SpO2 | 87 | 80.86207 | 15.0458 |
| Initial SpO2 | 87 | 90.18391 | 9.746232 |
| Delta SpO2 | 87 | 9.321839 | 13.00223 |
| Initial respiratory rate | 85 | 30.95294 | 27.23876 |
| Initial systolic blood pressure | 87 | 132.4253 | 31.50734 |
| Initial diastolic blood pressure | 87 | 75.71264 | 18.93128 |
| Initial mean arterial pressure | 87 | 94.61686 | 21.18734 |
| Initial heart rate | 89 | 108.3303 | 25.93134 |
| Initial temperature (°C) | 74 | 37.24324 | 1.181951 |
| Laboratory findings | |||
| WBC (white blood cell) | 84 | 12.02155 | 5.575656 |
| Platelet | 84 | 248.7857 | 110.5428 |
| Absolute lymphocyte count | 84 | 3.349286 | 19.20136 |
| Initial creatinine | 86 | 2.026744 | 1.703521 |
| Initial lactate | 65 | 3.396923 | 3.071134 |
| Initial troponin | 67 | 0.064627 | 0.155897 |
| Initial D‐dimer | 48 | 4698.96 | 9673.95 |
| Initial CRP | 53 | 209.534 | 140.024 |
| Initial LDH | 68 | 757.9235 | 551.8407 |
| Initial ferritin | 50 | 1610.22 | 1412.34 |
Abbreviations: CRP, C Reactive Protein; LDH, Lactate Dehydrogenase serum level.
TABLE 2.
Primary outcomes
| DOA (n = 4) | DED (n = 12) | DIP (n = 52) | LIVED (n = 27) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age mean, y | 70.8 | 65.6 | 62.9 | 60 | P = 0.0037 |
| BMI mean | 24.9 | 29 | 30 | 35.7 | P = 0.068 |
| Gender | |||||
| Female | 2 | 6 | 7 | 11 | M v F |
| Male | 2 | 6 | 45 | 16 | P < 0.001 |
| Exam findings | |||||
| Initial SPO2 RA | – | 90.3 | 88.1 | 94 | P = 0.675 |
| Delta SPO2 | – | 11.8 | 8.82 | 9.33 | P = 0.389 |
| RR | – | 24.9 | 35.1 | 25.7 | P = 0.244 |
| Labs (initial) | |||||
| Lactate | – | 8.15 | 3.56 | 2.62 | P < 0.0001 |
| Creatinine | – | 3.39 | 1.95 | 1.77 | P = 0.0073 |
| D‐dimer | – | 7520 | 5930 | 1130 | P = 0.0045 |
| WBC | – | 10.4 | 11.8 | 12.9 | P = 0.0027 |
| Risk factors | |||||
| Hypertension | 4 | 6 | 34 | 16 | P = 0.67 |
| Diabetes mellitus | 1 | 6 | 26 | 14 | P = 0.319 |
| Congestive heart failure (CHF) | 0 | 2 | 4 | 6 | P = .475 |
| Coronary artery disease (CAD) | 0 | 1 | 10 | 1 | P = 0.0067 |
| Reactive airway disease (asthma/COPD) | 1 | 1 | 11 | 17 | P = 0.597 |
| HIV | 0 | 0 | 2 | 2 | |
| Medications | |||||
| Oral steroids | 0 | 0 | 1 | 0 | |
| ACE inhibitor | 0 | 2 | 8 | 4 | |
| RAAs | 0 | 2 | 8 | 4 | |
| Beta blocker | 1 | 3 | 10 | 8 | |
Abbreviations: RR, respiratory rate; RAAS, A class of blood pressure medication Renin‐Angtiotensin‐Aldosterone System.
Table 3 and Figure 1 represent an additional analysis of a selection of both respiratory indicators (Sp02, Delta Sp02, and respiratory rate) and systemic indicators (creatinine, lactate, and D‐dimer) and their association with the primary outcomes.
TABLE 3.
Initial respiratory and systemic indicators in COVID‐19 patients intubated in the ED who died in the ED versus in hospital
| Markers | Died in ED | Died in hospital | Difference (95% CI) | P | P ANOVA |
|---|---|---|---|---|---|
| Respiratory indicators | |||||
| SpO2 | 78.6% | 79.3% | −0.7% (−10% to 8.6%) | P = 0.88 | P = 0.26 |
| Delta SpO2 a | 11.8% | 8.8% | 3.0% (−5.2% to 11.2%) | P = 0.47 | P = 0.77 |
| Respirations (breaths/min) | 24.9 | 29.9 | −5.0 (−12.1 to 2.2) | P = 0.17 | P = 0.10 |
| Systemic indicators | |||||
| Creatinine (mg/dL) | 3.4 | 1.9 | 1.4 (0.4 to 2.5) | P = 0.0064 | P = 0.0123 |
| Lactate (mmol/L) | 8.2 | 3.6 | 4.6 (3.0 to 6.2) | P < 0.0001 | P < 0.0001 |
| Ln (D‐dimer) | 8.90 | 7.91 | 0.99 (0.34 to 1.64) | P = 0.0030 | P < 0.0001 |
| D‐dimer (ng/mL) b | 7310 | 2716 | 4594 (1,116 to 11,217) | ||
Abbreviations: CI, confidence interval; ED, emergency department; Ln, natural logarithm; SpO2, oxygen saturation measured by pulse oximetry.
Displays the mean initial value of each indicator in patients who died in the ED versus who died in hospital, as well as the mean difference between groups with 95% CI from the Sidak post‐hoc comparison. The P‐value column displays to the P‐value corresponding the post‐hoc comparison whereas the P ANOVA column displays the P‐value corresponding to the F statistic for the overall ANOVA.
aDelta SpO2 defined as the first SpO2 after introduction of supplemental oxygen minus the initial room air SpO2.
bDisplays the back‐transformed values from the Ln‐transformed D‐dimer analysis for purposes of interpretation.
FIGURE 1.
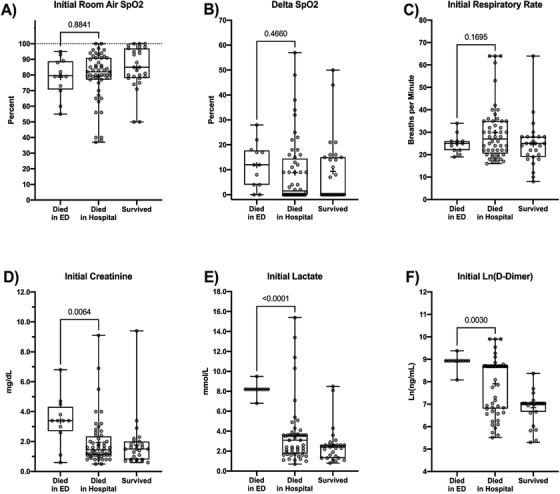
Box‐and‐whisker plots for initial pulmonary and systemic indicators at ED arrival for patients who died in the ED versus died in hospital versus survived to discharge. Boxes indicate 25th to 75th percentile, whiskers represent minimum and maximum values. Horizontal bar indicates group median. “+” indicates group mean. The P‐value from the Sidak post‐hoc comparison of patients who died in the ED versus those who died in hospital is displayed over the bracket. Because D‐dimer displayed a beta distribution that violated the homoscedastic error assumption, the natural logarithm transformed values were used for analysis
Although underlying demographic and exam characteristics were found to contribute to patient outcome following ED intubation, another variable that also seemed to show significant correlation was temporally related to date of patient presentation. Before March 20, none of the intubated patients presented to the ED with an initial SpO2 of < 90%. Between March 20 and April 5, roughly half the intubated patients presented with SpO2 levels below 90% with patients presenting with SpO2 levels between 60% and 90%. After April 8, hypoxic presentations requiring intubation significantly reduced in frequency (Figures 2 and 3).
FIGURE 2.
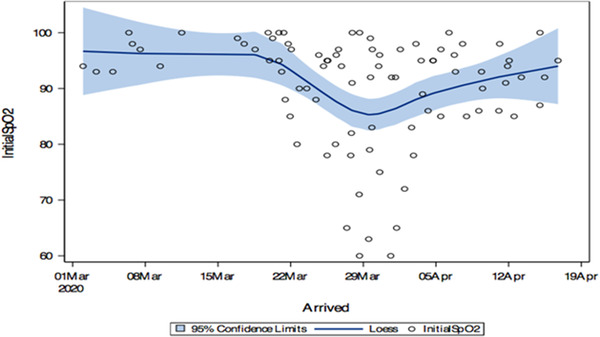
Initial patient presentation SpO2 by date
FIGURE 3.
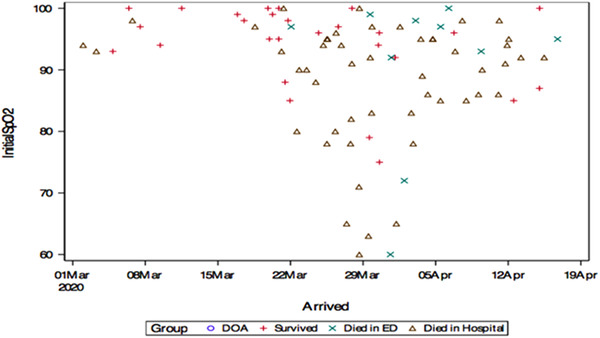
Initial patient presentation SpO2 by date and patient outcome
To better understand the evolution of physician attitudes toward COVID‐19 intubation practices, a qualitative survey was conducted. A total of 121 surveys were sent (81 residents and 40 faculty) and 98 were returned (81% response rate). Findings demonstrated that 98% of all intubations were performed with video (GLIDESCOPE) laryngoscopy. A total of 69% of respondents encountered significant laryngeal edema either always (28%) or very frequently (41%). Pre‐intubation hypoxia was encountered either always or very frequently (70%), with post‐intubation hypoxia encountered roughly half the time (33%), very frequently (40%), or always (10%). A total of 82% of staff were either extremely or very concerned about infectious exposure during the initial COVID‐19 surge. In terms of evolving approaches to airway management during the initial COVID surge, in the first 3 weeks of the surge (March 7‐March 21), 73% of respondents intubated earlier than usual, and only 2.6% respondents focused on non‐invasive strategies first. Although not defined in relation to a specific point in time, the question of early intubation sought to subjectively assess if clinicians chose to intubate earlier in a patient's clinical course due to the unique nature of the pandemic. By the second 3 weeks of the surge (April 1‐April 21), 67% of respondents were intubating later than usual, and 28% of respondents were focused on non‐invasive strategies first. By the end of the initial surge (third week in April), 44% of respondents reported being very successful with non‐invasive approaches to reduce intubations, and 15% of respondents reported being extremely or always successful with non‐invasive approaches. Success was defined as limiting intubations in the ED via other ventilatory strategies. The long‐term impact of those strategies, however, including possible need for subsequent inpatient intubation, exceeded the scope of this study.
4. LIMITATIONS
This is a single department, single geographic region, retrospective chart review. There was no plan a priori to review ED intubations in this COVID‐19 surge. During this early COVID‐19 surge, nearly every patient with respiratory symptoms we were evaluating had COVID‐19. Unfortunately, testing was not universally available for all patients, and there was a definite false–negative rate to COVID testing at the time. Therefore, we included all patients during this time frame who were either COVID‐19‐positive or where there was high suspicion for COVID‐19 based on clinical presentation or symptoms. It is possible that a small number of patients included in this study had an alternative diagnosis for their respiratory symptoms.
Because of the complex nature and continuously evolving understanding of this unprecedented disease process, long‐term morbidity was thought to exceed the parameters of the study, and primary outcomes focused on mortality to assess for primary patient outcomes. Furthermore, decisions as to out‐of‐hospital clinical protocols and assessment of mortality exceeded the parameters of this study, and as such, all patients presenting to the ED were included regardless of the outcomes of initial resuscitation efforts.
The physician survey was conducted ≈6 weeks after the COVID‐19 surge. Although timely, factors such as recall biases and the over or under emphasized what the physicians remember experiencing during this highly stressful pandemic period. Furthermore, the survey tool itself was not previously validated, and as such, subjectivity and aforementioned biasing factors could have impacted findings.
5. DISCUSSION
This study describes a unique moment in emergency medicine history when NYC experienced an unprecedented COVID‐19 patient surge at the beginning of the pandemic in the United States. During this 6‐week time frame, the ventilatory management approach to COVID‐19 patients evolved drastically. In the first few weeks, the department approach was to intubate patients early in their ED stay and avoid high flow nasal cannula and noninvasive ventilation out of fear of viral aerosolization and infecting staff. Whether this fear was justified is an area of debate. 10 Patients were also intubated early given the levels of hypoxia noted in these COVID‐19 patients despite some of these patients having clinical pictures that looked better than their pulse oximetry readings. This was the approach our colleagues in other NYC EDs were taking early in the NYC surge as well.
Several weeks into this COVID‐19 surge, it became clear that patients after intubation were spending weeks on mechanical ventilation with high mortality rates. 11 In early April, our department started applying alternative treatment methods to avoid intubation, such as placing patients in a prone position to improve oxygenation and the use of high flow nasal cannula and/or noninvasive ventilations. 12 , 13 , 14 This helped avoid a number of intubations. As a department, we accepted a larger degree of hypoxia while these other strategies were implemented, thus setting a stricter threshold for proceeding to intubation. We know of no other clinical entity that has resulted in such a drastic change to basic ventilatory support strategies in such a compressed time frame.
Rapid sequence induction (RSI) is the standard of care in ED practice for the routine establishment of definitive airway access. During the initial phases of the COVID‐19 pandemic, unresolved questions related to the unique challenges of this unknown pathogen compelled ED leadership to modify well‐established airway practices in consideration of both patient and practitioner safety. Despite the unique challenges of the COVID‐19 presentations, our rapid sequence endotracheal intubations were 100% successful without the requirement for any surgical airways or the use of adjunct devices. This is even more significant because 69% of our physicians reported finding significant laryngeal edema that has been described elsewhere. 15 COVID‐19 intubations are also more challenging because of the severity of respiratory failure, the significant hypoxia typically present before intubation that is only partially responsive to standard pre‐oxygenation, the layers of PPE that can restrict normal movement and visibility on the part of the intubator, and less physicians in the room to serve as back‐up or extra hands in difficult airway scenarios. At the time, there was heightened anxiety for many of our physicians because of the highly infectious nature of intubation for COVID‐19 patients.
A total of 83% of the EM physicians in our group reported that COVID‐19 patients suffered from an extended period of profound hypoxia after intubation. Collectively, our group felt this phenomenon was unique and had not been previously seen in other disease processes. It is worth noting that this was the most hypoxic group of patients that most of us have ever encountered, and subjectively, many of our physicians likened the initial surge of patients as feeling more like a chemical attack or an anti‐metabolic toxin than an infectious agent. This post‐intubation hypoxia was out of proportion to hypoxia that is normally predicted to develop because of severe acute hypoxic respiratory failure with minimal respiratory reserve. These hypoxic episodes would often last 10 to 30 minutes with pulse oximetry readings dropping into the 60s/70s or even lower despite aggressive ventilatory management. This would occur despite quick and successful intubations, confirmatory capnography tracings, and FIO2 of 100%. Further studies should look at how often this occurs, if there are any mitigating factors that can be used, and what the optimal initial ventilator settings would be.
Our study cohort presented with advanced COVID‐19 respiratory failure, uniformly in extremis and with an overall mortality of 71.6%. A total of 16.6% of our patient population did not even survive their resuscitation attempts in the ED, with 4% DOA and an additional 12.6% dying after intubation and during ED resuscitation. We found that within this cohort, male sex, advanced age, a history of coronary artery disease, the non‐respiratory findings of lactic acidosis, elevated D‐dimer, and CKD/AKI were correlated with death either in the ED or during the patient's hospital course. Fever, hypotension, and even initial respiratory status (including our analysis of the pre and post Sp02 [the Sp02 delta]) were not strongly correlated with outcomes (likely because of the fact that all patients were severely hypoxic on arrival). Increased BMI did not correlate with mortality. Although the explanation for this finding exceeded the scope of our study, it was hypothesized to be to the unique nature of the study cohort including patients of advanced age from nursing homes who are often cachectic at baseline because of underlying chronic medical conditions.
We thought, based on changes in patient selection for intubation, that there would be a change in mortality for patients that were intubated over the study time period. One hypothesis was that the mortality might go up because patients who were more likely to survive would not be intubated at all. Unfortunately, the number of cases is too small to show a definite trend. Data on patients treated with non‐invasive ventilatory strategies was out of the scope of this study.
This study describes all COVID‐19 intubations in 2 urban‐academic ED during the NYC March/April surge that was early in the US pandemic. In our cohort of intubated COVID‐19 patients, male sex, advanced age, and certain laboratory tests (lactic acidosis, elevated D‐dimer, and CKD/AKI) were correlated with death either in the ED or during the patient's hospital course.
Physician airway management strategies evolved rapidly during the course of this surge; however, the possible impact of those changes on patient outcomes exceeds the scope of our study. Additional research should evaluate the best strategies for RSI intubation of COVID‐19 patients to avoid periods of post‐intubation hypoxia as well as evaluate the best management of acute respiratory failure and hypoxia including timing of noninvasive strategies versus intubation.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
JDA, SM, EP, SS, MT, JO, DL, MJ, JM, and JS conceived the study and designed the study design. JDA, SM, EP, SS, MT, JM, and JS conducted and supervised data collection. JDA, DL, MJ, JM, and JS undertook managing the data, including quality control and data analysis. JDA, SM, EP, SS, MT, JO, and JS drafted the manuscript. All authors contributed substantially to the manuscript revision. JDA takes responsibility for the paper as a whole.
Supporting information
Supporting Information
Biography
Jason Z. D'Amore, MD, MBA, is the Vice Chair of Emergency Medicine at Jacobi Medical Center/North Central Bronx Hosptial in Bronx, New York.

D'Amore J, Meigher S, Patterson E, et al. Intubation outcomes and practice trends during the initial New York SARS‐COV‐19 surge at an academic, level 1 trauma, urban emergency department. JACEP Open. 2021;2:e12563. 10.1002/emp2.12563
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships.
Supervising Editor: Nicholas Caputo, MD, MSc.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duca A, Memaj I, Zanardi F, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the emergency department during Italian novel coronavirus SARS‐CoV2 outbreak: preliminary data on the role of Helmet CPAP and non‐invasive positive pressure ventilation. EClinicalMedicine. 2020;24:100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID‐19 Disease is Suspected: Interim Guidance. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/331446. License: CC BY‐NC‐SA 3.0 IGO [Google Scholar]
- 5. Yao W, Wang T, Jiang B, et al, collaborators . Emergency tracheal intubation in 202 patients with COVID‐19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125(1):e28‐e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx New York. Metabolism. 2020;108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ross J, Diaz CM, Starrels JL. The Disproportionate burden of COVID‐19 for immigrants in the Bronx, New York. JAMA Intern Med. 2020;180(8):1043‐1044. [DOI] [PubMed] [Google Scholar]
- 8. Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID‐19 hospitalizations and deaths across New York city boroughs. JAMA. 2020;323(21):2192‐2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID‐19 Disease is Suspected: Interim Guidance. World Health Organization; 2020. [Google Scholar]
- 10. Leonard S, Atwood CW Jr, Walsh BK, et al. Preliminary findings of control of dispersion of aerosols and droplets during high velocity nasal insufflation therapy using a simple surgical mask: implications for high flow nasal cannula. Chest. 2020;S0012‐3692(20)30579‐1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi‐center prospective cohort study. Crit Care. 2020;24:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang LG, LeBaron J, Bodnar D, et al. Conscious proning: an introduction of a proning protocol for nonintubated, awake, hypoxic emergency department COVID‐19 patients. Acad Emerg Med. 2020;27(7):566‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caputo ND, Strayer RJ, Levitan R. Early self‐proning in awake, non‐intubated patients in the emergency department: a single ED's experience during the COVID‐19 pandemic. Acad Emerg Med. 2020;27(5):375‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGrath BA BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID‐19 complicating airway management. Anaesthesia. 2020;75(7):972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


