Abstract
Animals perform a series of actions in a fixed order during ritualistic innate behaviors. Although command neurons and sensory pathways responding to external stimuli that trigger these behaviors have been identified, how each action is induced in a fixed order in response to multimodal sensory stimuli remains unclear. Here, the sexually dimorphic lateral antennal lobe tract projection neuron 4 (lPN4) in male Drosophila melanogaster mediates the expression of a fixed behavioral action pattern at the beginning of the courtship ritual, in which a male taps a female body and then extends a wing unilaterally to produce a courtship song. We found that blocking the synaptic output of lPN4 caused an increase in the ratio of male flies that extended a wing unilaterally without tapping the female body, whereas excitation of lPN4 suppressed the transition from the tapping phase to the unilateral wing extension phase. Real-time calcium imaging showed that lPN4 is activated by a volatile pheromone, palmitoleic acid, whose responses were inhibited by simultaneous gustatory stimulation with female cuticular hydrocarbons, showing the existence of an “AND-gate” for multimodal sensory inputs during male courtship behaviors. These results suggest that the function of lPN4 is to suppress unilateral wing extension while responding to a female smell, which is released by appropriate contact chemosensory inputs received when tapping a female. As the female smell also promotes male courtship behaviors, the olfactory system is ready to simultaneously promote and suppress the progress of courtship actions while responding to a female smell.
SIGNIFICANCE STATEMENT Although it has been 80 years since Konrad Lorenz and Niko Tinbergen introduced how multiple acts comprising separate innate behaviors are released in a fixed order according to external stimuli, the neural circuits responsible for such fixed action patterns remain largely unknown. The male courtship behavior of Drosophila melanogaster is a good model to investigate how such a fixed behavioral sequence is determined in the brain. Here, we show that lateral antennal lobe tract projection neuron 4 (lPN4) in D. melanogaster functions as an “AND-gate” for volatile and contact chemosensory inputs, mediating the expression of tapping behaviors before unilateral wing extension during male courtship rituals.
Keywords: courtship, fixed action pattern, insect, olfaction, pheromone, sexual behavior
Introduction
During innate behaviors, animals perform a series of actions in a fixed order. For example, Drosophila melanogaster (fruit fly) males, on encountering a potential mate, orient toward the mate, tap the mate with a foreleg, extend and vibrate one of the wings unilaterally, lick the genitalia of the mate with its proboscis, and finally attempt copulation (Greenspan and Ferveur, 2000; Ejima and Griffith, 2007). The male carries out each courtship action in response to multimodal sensory cues. Visual and olfactory cues released by a moving female stimulate the male to initiate courtship by orienting himself toward her and progressing to the next step of tapping her. Gustatory information received by the foreleg triggers unilateral wing extension and vibration to produce the courtship song, which drives the female to slow down, allowing the male to lick her genitalia. Licking provides gustatory information that progresses the courtship to a copulation attempt (Auer and Benton, 2016). External environments such as the existence of food odors affect the male courtship activities (Grosjean et al., 2011), however, do not change the courtship sequence. Recently, increased activity of aSP22 neurons was found to elicit multiple late-stage courtship actions, while ablation of these neurons did not change the behavioral order (McKellar et al., 2019). P1 neurons have also been reported to trigger an entire series of courtship actions (Kohatsu et al., 2011). Activation levels of aSP22 and P1 neurons correlate with which courtship actions are triggered or their frequencies; however, it remains largely unknown how the courtship sequence is determined in the brain.
Among the sensory pathways correlated with courtship behavior, the olfactory and gustatory pathways, which process chemical substances (pheromones) of the mate, have been well investigated. The odorant receptors (Ors) 47b and 88a expressing olfactory sensory neurons (OSNs) respond to fly odors extracted from both males and females (van der Goes van Naters and Carlson, 2007). Or47b OSNs respond to fatty acids, such as palmitoleic acid, that are present on the cuticular surface of males and females, and promote courtship in males (Lin et al., 2016). Conversely, Or65a and Or67d OSNs respond to the male pheromone cis-vaccenyl acetate (cVA; van der Goes van Naters and Carlson, 2007), which works as a courtship-inhibitory pheromone for males (Ejima and Griffith, 2007; Kurtovic et al., 2007). The fly odor information received by these four receptors is transferred to the dorsolateral glomeruli in the primary olfactory center, the antennal lobe. For example, glomerulus VA1d receives input from Or88a OSNs, VA1v from Or47b, DA1 from Or67d, and DL3 from Or65a (Vosshall and Stocker, 2007). Meanwhile, gustatory signals from female cuticular hydrocarbons, such as 7,11-heptacosadiene (7,11-HD) and 7,11-nonacosadiene (7,11-ND), are detected by gustatory neurons in the legs and proboscis, thus promoting male courtship (Liu et al., 2012; Thistle et al., 2012). The gustatory neurons responding to the female cuticular hydrocarbons activate P1 neurons via ascending neurons, such as PPN1 and vAB3 (Clowney et al., 2015; Kallman et al., 2015). These ascending neurons innervate the ventrolateral protocerebrum, in the vicinity of which inhibitory mAL neurons postsynaptic to the vAB3 neurons terminate, forming parallel feedforward excitatory and inhibitory pathways to induce courtship behavior (Clowney et al., 2015; Kallman et al., 2015).
To determine how external multimodal sensory information is integrated and induces male courtship actions that follow a fixed sequence, we aimed to investigate the anatomy downstream of Or47b and Or88a OSNs: medial antennal lobe tract projection neurons (mPNs), mediolateral antennal lobe tract projection neurons (mlPNs), and lateral antennal lobe tract PNs (lPNs; Tanaka et al., 2012; Horne et al., 2018). Among these, we analyzed the morphology and function of lPN4, which innervates both VA1d and VA1v glomeruli and uniquely links these glomeruli with multiple sensory centers such as the subesophageal ganglion/antennal mechanosensory and motor center, the gustatory and auditory centers, and the ventrolateral protocerebrum, the visual center, suggesting that lPN4 integrates multimodal sensory information during courtship behavior (Tanaka et al., 2012).
Materials and Methods
Fly strains.
Flies were reared on a standard corn meal, yeast, and glucose agar medium at 25°C and >50% humidity under a 12 h light/dark cycle. For behavioral experiments, each virgin adult male was collected and maintained separately in a vial containing 1% agar in grape juice, whereas virgin adult females were maintained in groups on the standard medium. The experiments included flies between 4 and 8 d after eclosion. We used w1118 (a gift from Kazuhiro Kume, Nagoya City University, Nagoya City, Japan) as the wild type and the following transgenic strains: NP21- and NP6107-GAL4 strains (Tanaka et al., 2012) obtained from KYOTO Stock Center (Department of Drosophila Genomics and Genetic Resources, Kyoto Institute of Technology, Kyoto, Japan); UAS-mCD8::GFP (Lee and Luo, 1999; a gift from Demian Park, Brandeis University, Waltham; RRID:BDSC_5139); 10XUAS-mCD8::GFP (RRID:BDSC_32186) and 20XUAS-mCD8::GFP (RRID:BDSC_32194) obtained from Tadao Usui, Kyoto University, Kyoto, Japan; 20XUAS-GCaMP6s (Chen et al., 2013) strains (stock #42746 and #42749, Bloomington Drosophila Stock Center; RRID:BDSC_42746 and RRID:BDSC_42749); UAS-TNT and UAS-TNTIN strains (Ejima and Griffith, 2008; provided by Hubert Amrein, Texas A&M College of Medicine, College Station, TX); and UAS-dTrpA1 (Hamada et al., 2008; obtained from Paul Garrity, Brandeis University, Waltham, MA; RRID:BDSC_26263). We outcrossed NP6107-GAL4 into w1118 background over seven consecutive generations. Heterozygous controls were obtained by crossing w1118 with UAS-effector strains.
Immunohistochemistry.
Fly brains were dissected in PBS, pH 7.4, and fixed with 4% paraformaldehyde for 1 h at room temperature. They were then washed three times with PBS for 5 min and blocked for 1 h in blocking solution [10% goat serum in PBS containing 0.2% Triton X-100 (PBST)]. Incubation of brains in the blocking solution containing primary antiserum was performed for 24–48 h with shaking. They were rinsed thoroughly with PBST, incubated in the blocking solution containing secondary antiserum overnight, washed in PBS, and mounted in 80% glycerol in PBS. The primary antibodies used included the following: rabbit anti-GFP (1:500; catalog #A-11122, Thermo Fisher Scientific; RRID:AB_221569); rabbit anti-GABA (1:200; catalog #GTX125988, GeneTex; provided by Kazuhiro Wada, Hokkaido University, Sapporo, Japan); mouse anti-choline acetyltransferase (Takagawa and Salvaterra, 1996; 1:200; catalog #4B1, Developmental Studies Hybridoma Bank at University of Iowa; RRID:AB_528122;); mouse anti-bruchpilot (Wagh et al., 2006; 1:10; catalog #nc82, Developmental Studies Hybridoma Bank at University of Iowa; RRID:AB_2314866), and mouse anti-SYNAPSIN (1:20; catalog #3C11, Developmental Studies Hybridoma Bank at University of Iowa; RRID:AB_528479) antibodies. For detection of primary antibodies, goat anti-rabbit antibodies conjugated with Alexa Fluor 488 (1:200; catalog #A11034, Life Technologies Japan Ltd.; RRID:AB_2576217) or anti-mouse antibodies with Alexa Fluor 568 (1:200; catalog #A11004, Life Technologies Japan Ltd.; RRID:AB_2534072) were used as secondary antibodies.
Reconstruction and analyses of confocal images.
Confocal serial optical images were taken at 0.5–3.8 µm z intervals with a laser scanning confocal microscope (model LSM 700, Zeiss) equipped with Plan-Neofluar 20×/0.50, C-Apochromat 40×/1.2W, and Plan-Apochromat 63×/1.4 lenses. The images were carefully scanned not to saturate the fluorescent signals, which widens the signal areas artificially. To compare the morphologies between males and females, signal intensities detected were arranged at the same level. Three-dimensional reconstruction of confocal images was performed with ZEN 2012 (Zeiss). The brightness, color, and contrast of images were adjusted with Photoshop CS version 5.1 (Adobe). The volumes and lengths of the lPN4 axons, and the numbers of branches were measured by NeuronStudio software (Wearne et al., 2005). The mean sectional area of each lPN4 axon was calculated by dividing its volume by its length.
Courtship behavior recording.
Behavioral experiments were performed at a zeitgeber time of 0–4 in dim red light at 25°C or 31°C and >40% humidity. To count wing extension and copulation attempts, a virgin male was placed into a cell (φ = 8 mm, 3 mm depth; Ejima and Griffith, 2008). After allowing the male to acclimate to the cell for 5 min, a decapitated wild-type virgin female was introduced to the same cell or to a lower cell separated by mesh from the upper cell where the male was present. We also observed decapitated males and males lacking third antennal segments. Males were decapitated under CO2 anesthesia and kept in the cell for >10 min before recording. The third antennal segments were removed under CO2 anesthesia 15–19 h before recording. Wing extension was counted if the wing was opened unilaterally >45°. Copulation attempts were counted when the body length of the male became <70% by bending its abdomen.
To measure the frequency of tapping in males, we placed a decapitated virgin female with both wings removed in the lower cell and a virgin male in the upper cell. After allowing acclimation for 5 min, the mesh between the two cells was removed. The recording began 2 min before the mesh was removed and continued until the male started to extend a wing unilaterally, or for 10 min if the male did not show unilateral wing extension. We considered it to be tapping if a male foreleg contacted a female thorax, abdomen, or the proximal side of a leg. If a male tapped the ventral side of a female body with both forelegs, we did not include it in the analysis, as we could not obtain the correct frequency of tapping. Walking on the female body and touching the female body by accidentally falling from a cell wall or ceiling were not counted as tapping. For the last minute during acclimation, the male behavior was recorded to evaluate movement activity. Experiments were video recorded at 30 or 60 Hz with an MCM-4304 (MicroVision) or GZ4304M (Gazo) camera. The frequency of courtship acts was measured by Behavioral Observation Research Interactive Software (Friard and Gamba, 2016). The movement activities were measured by tracking the males with the MTrack2 plugin in ImageJ Fiji (https://valelab4.ucsf.edu/∼nstuurman/IJplugins/MTrack2.html).
Pheromones.
The cis-vaccenyl acetate (catalog #10421, Pherobank) and palmitoleic acid (catalog #10009871, Cayman Chemical) were diluted at 1% v/v in paraffin oil (catalog #122–04775, Wako). Previously, 7,11-HD and 7,11-ND (catalog #10012567 and #9000314, Cayman Chemical) had been dissolved in ethanol and hexane, respectively, and evaporated for at least 30 min before diluting at 100 ng/ml in paraffin oil.
Calcium imaging.
Four to six days after eclosion, flies were anesthetized in a vial on ice for <1 min and restrained in a plastic dish by wax and epoxy (Tanaka et al., 2009). The tops of antennae were covered by aluminum foil to avoid antennae moistening. The saline solution was composed of the following (in mM): 103 NaCl, 3 KCl, 5 TES, 10 trehalose, 10 glucose, 7 sucrose, 26 NaHCO3, 1 NaH2PO4, 1.5 CaCl2, and 4 MgCl2, adjusted to 280 mOsm with sucrose and pH 7.25 with HCl. It was then applied over the head and a window was opened on the top of the head. After removing fat, air sacs, and digestive system in the head, muscles causing brain movement were cut and the fly was set under a AxoExaminer A1 (Zeiss) equipped with W Plan-Apochromat 40×/1.0 lens and a mercury lamp. In case forelegs were stimulated with pheromones, the middle parts of forelegs were fixed with Parafilm. Immediately after 100 ng/ml 7,11-HD and 7,11-ND were applied to the forelegs, the fly was set under the microscope.
An odor puff (2 s, 0.1 L/min) passing through a tube inserted with filter paper (3 × 25 mm) containing 6.25 µl of either paraffin oil or 1% palmitoleic acid (50 µg included in solvent) or 7.5 µl of either paraffin oil or 1% cVA (70 ng) was applied to the fly every 30 s. The total amount of palmitoleic acid was adjusted to 50 µg, since Or47b-expressing OSNs in both young males and females show robust responses to the pure palmitoleic acid (Lin et al., 2016). The total amount of cVA was adjusted to 70 ng, less than the amount of cVA contained in a male body (between 200 ng and 2 µg; Tachibana et al., 2015).
GCaMP fluorescence was recorded three times for each animal and odor pair with a camera (model EM-CCD, Hamamatsu) at 4 Hz. Mean fluorescence intensity within the most dorsal section of the antennal lobe of the primary axon or the branches in the ventrolateral protocerebrum (lPN4), or arborizations at the horizontal level of the mushroom body pedunculus (P1 neurons) was measured by ImageJ. Mean fluorescence ratio (F/F0; percentage) was then calculated by dividing the mean fluorescence intensity of every frame by that of a 1 s recording before the odor stimulation, and then by taking the average of three replicates. The odor responses (ΔR values) were then calculated by subtracting the mean fluorescent ratio observed in the trials using paraffin oil (solvent) from the value observed using cVA or palmitoleic acid.
Experimental design and statistical analysis.
Data are presented as the mean ± SEM. Using Prism 5 (GraphPad), we applied the D'Agostino–Pearson test and Bartlett's test to determine the normal distribution of data and equal variances, respectively, and performed two-tailed unpaired t test, two-tailed Mann–Whitney test, and two-sided Fisher's exact test. To analyze the frequencies of tapping (see Figs. 3D,I, 4A,F), we performed a generalized linear mixed model with a Poisson distribution using the glmmML package (https://cran.r-project.org/web/packages/glmmML/index.html) running on R version 3.3.3 (https://www.R-project.org/). To calculate r correlation coefficients and ϕ coefficients as effect sizes, we used compute.es (https://cran.r-project.org/web/packages/compute.es/index.html) and psych (https://cran.r-project.org/web/packages/psych/index.html) packages running on R version 3.3.3. Statistical significance was set at p < 0.05. The sample size is shown in the figures.
Figure 3.
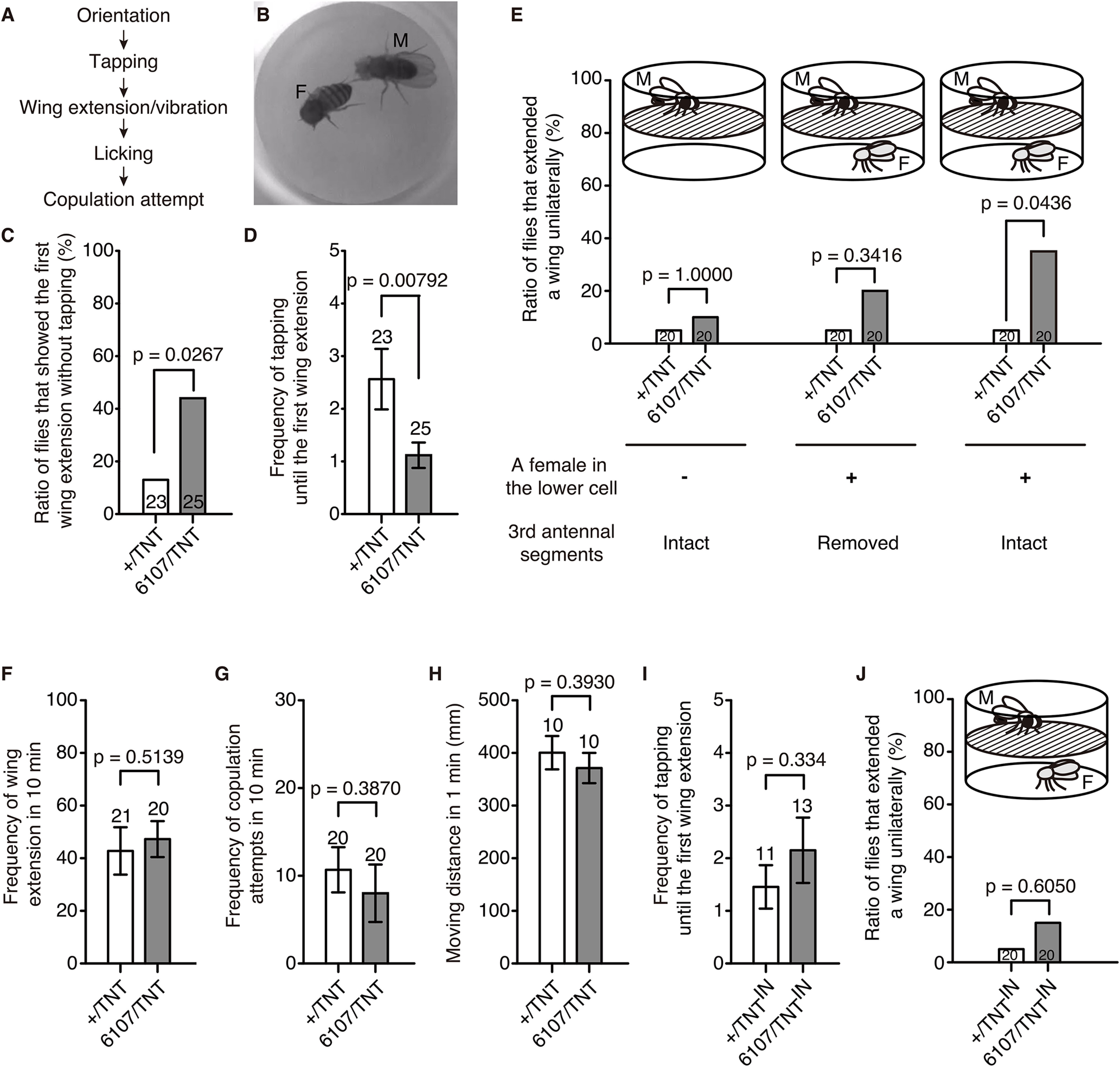
Blocking the synaptic output of lPN4 caused wing extension before tapping the female. A, Courtship sequence of D. melanogaster. B, Tapping of a control male (M) to a decapitated female (F). C–G, Courtship behaviors of males expressing TNT in lPN4 (6107/TNT) and controls (+/TNT). C, Ratio of flies that showed the first unilateral wing extension without tapping the female. D, Frequency of tapping until the first unilateral wing extension in males. E, Ratio of male flies that showed unilateral wing extension in the absence of a female in 10 min (left) and in the presence of a decapitated female in the lower cell in 10 min (middle, right). Middle, The third antennal segments of the male fly were removed. F, Frequency of wing extension observed in 10 min. G, Frequency of copulation attempts in 10 min. H, Movement activities in 1 min. I, J, Courtship behaviors of males expressing TNTIN in the lPN4 (6107/TNTIN) and controls (+/TNTIN). I, Frequency of tapping until the first unilateral wing extension. J, Ratio of male flies with intact third antennal segments that showed unilateral wing extension in the presence of a decapitated female in the lower cell in 10 min. Statistical analyses were performed using two-sided Fisher's exact test (C, E, J), generalized linear mixed model (D, I), and two-tailed Mann–Whitney test (F–H). Numbers within bars and above error bars represent the numbers of animals observed. Error bars indicate the SEM.
Figure 4.
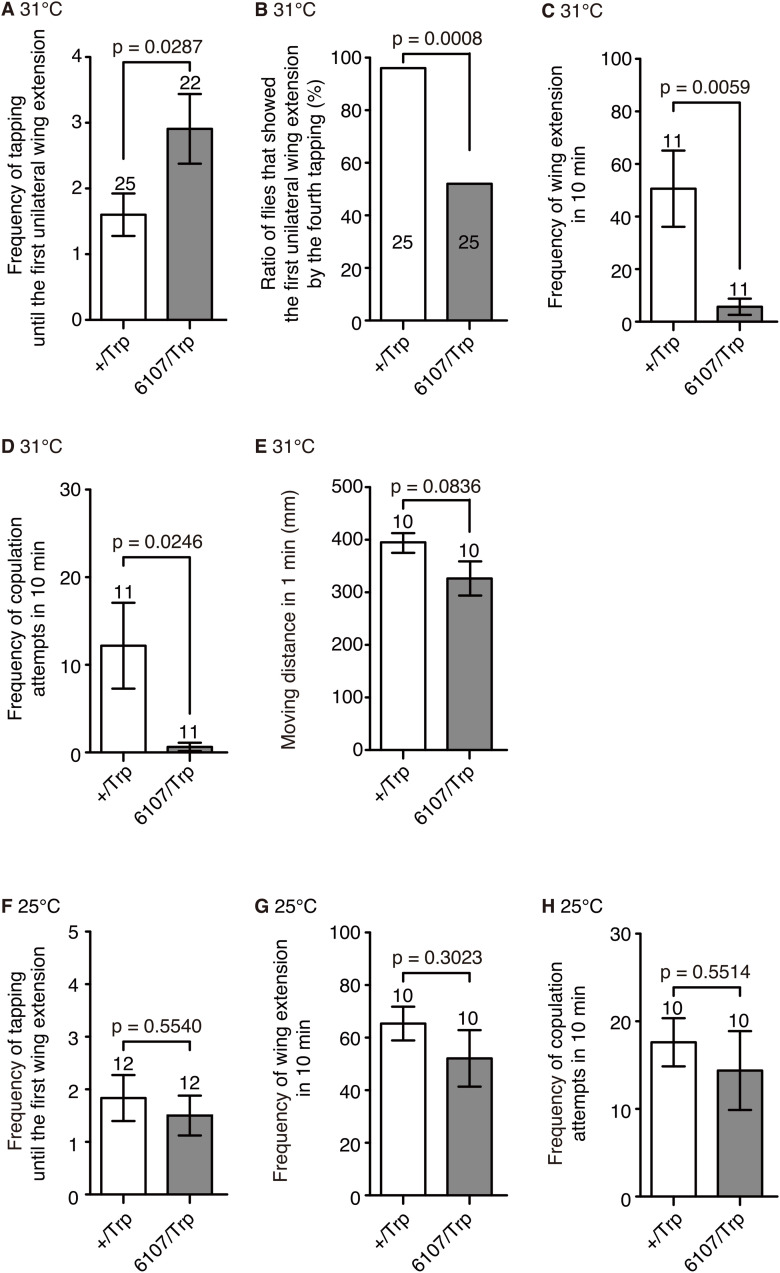
dTrpA1 expression in lPN4 suppressed the expression of post-tapping behaviors in males. A, Frequency of tapping until the first unilateral wing extension at 31°C in the dTrpA1-expressing males (6107/Trp) and controls (+/Trp). B, Ratio of male flies that showed the first unilateral wing extension by the fourth tapping of a female at 31°C. C, Frequency of unilateral wing extension observed in 10 min at 31°C. D, Frequency of copulation attempts in 10 min at 31°C. E, Movement activities in 1 min at 31°C. F–H, The frequencies of tapping until the first unilateral wing extension (F), unilateral wing extension (G), and copulation attempts (H) were not statistically different between controls and those flies expressing dTrpA1 in lPN4 at 25°C. Statistical analyses were performed using a generalized linear mixed model (A, F), two-sided Fisher's exact test (B), two-tailed Mann–Whitney test (C, D), and unpaired two-tailed t test (E, G, H). Numbers within bars and above error bars represent the numbers of animals observed. Error bars indicate the SEM.
Results
Projection patterns and sexual dimorphism of lPN4
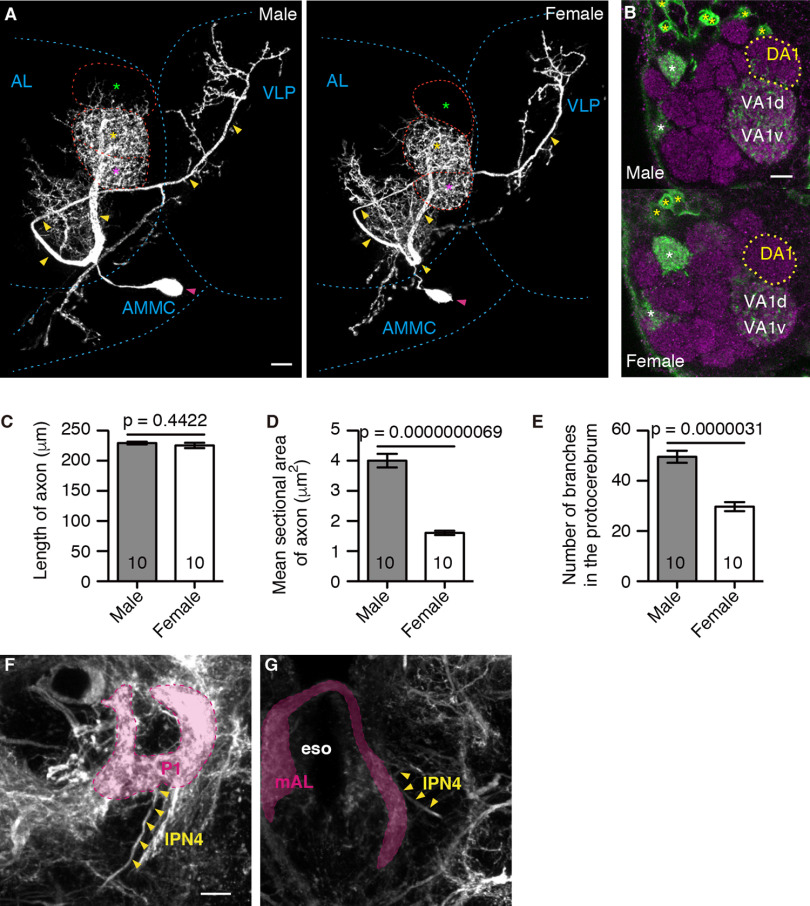
We first compared the lPN4 morphology between males and females using NP6107, a GAL4 enhancer trap strain that specifically labels a single lPN4 (Tanaka et al., 2012), and found that lPN4s are sexually dimorphic in the projection areas, section areas of the axons, and number of branches in the protocerebrum (Fig. 1). lPN4 innervated the DA1 glomerulus in males but not in females (n = 10 neurons in five brains for both sexes; Fig. 1B). Although the lengths of the primary axonal parts running from the root of VA1v to the ventrolateral protocerebrum were not significantly different between males and females (males, 229.3 ± 2.4 µm; females, 225.4 ± 4.3 µm; t(18) = 0.7859, p = 0.4422, two-tailed unpaired t test; effect size, r = 0.18; Fig. 1C), the mean sectional areas were larger in males than in females (males, 4.0 ± 0.2 µm2; females, 1.6 ± 0.1 µm2; t(18) = 10.17, p = 0.0000000069, two-tailed unpaired t test; effect size, r = 0.92; Fig. 1D). In addition, the number of branches in the protocerebrum was also significantly higher in males than in females (males, 49.6 ± 2.4; females, 29.7 ± 1.8; t(18) = 6.648, p = 0.0000031, two-tailed unpaired t test; effect size, r = 0.83; Fig. 1E).
Figure 1.
Sexual dimorphism in the morphology of lPN4 neurons. A, Male (left) and female (right) lPN4s. Primary axons and cell bodies are indicated by yellow and magenta arrowheads, respectively. The DA1 (green asterisks), VA1d (yellow asterisks), and VA1v (magenta asterisks) glomeruli are outlined by red dashed lines. AL, Antennal lobe; AMMC, antennal mechanosensory and motor center; VLP, ventrolateral protocerebrum. B, The innervation of lPN4 (green) into the DA1 glomerulus (yellow dotted lines) in the antennal lobe was observed in males (top), but not in females (bottom). Glomeruli are labeled with the anti-bruchpilot antibodies (magenta). It should be noted that NP6107 also labeled the projection neurons (yellow asterisks) innervating the ordinary glomeruli, DM6, VM7d, and VM7v (white asterisks), which do not respond to fly smells (Seki et al., 2017) and thus do not contribute to the courtship sequence. C–E, Mean length (C) and sectional area (D) of the primary axon, and the number of branches in the protocerebrum (E) of the male and female lPN4s. Comparison between males and females was conducted using two-tailed unpaired t test. Numbers within bars are the numbers of neurons analyzed. Error bars indicate SEM. F, G, The lPN4 axon, indicated by arrowheads, projects to the arborization areas of the P1 neurons (magenta in F) in the VLP and mAL neurons (magenta in G) in the subesophageal zone in the male brain. To label the P1 and mAL neurons in addition to lPN4, we used the fruNP21 GAL4 line, which also labels other neurons. eso, Esophagus. Genotype: NP6107-GAL4 (A, B) or NP21-GAL4 (F, G)/Y or +; 10xUAS-mCD8::GFP/+; 20xUAS-mCD8::GFP/+. Lateral is to the right; dorsal is to the top. Scale bars: A, B, F, 10 μm.
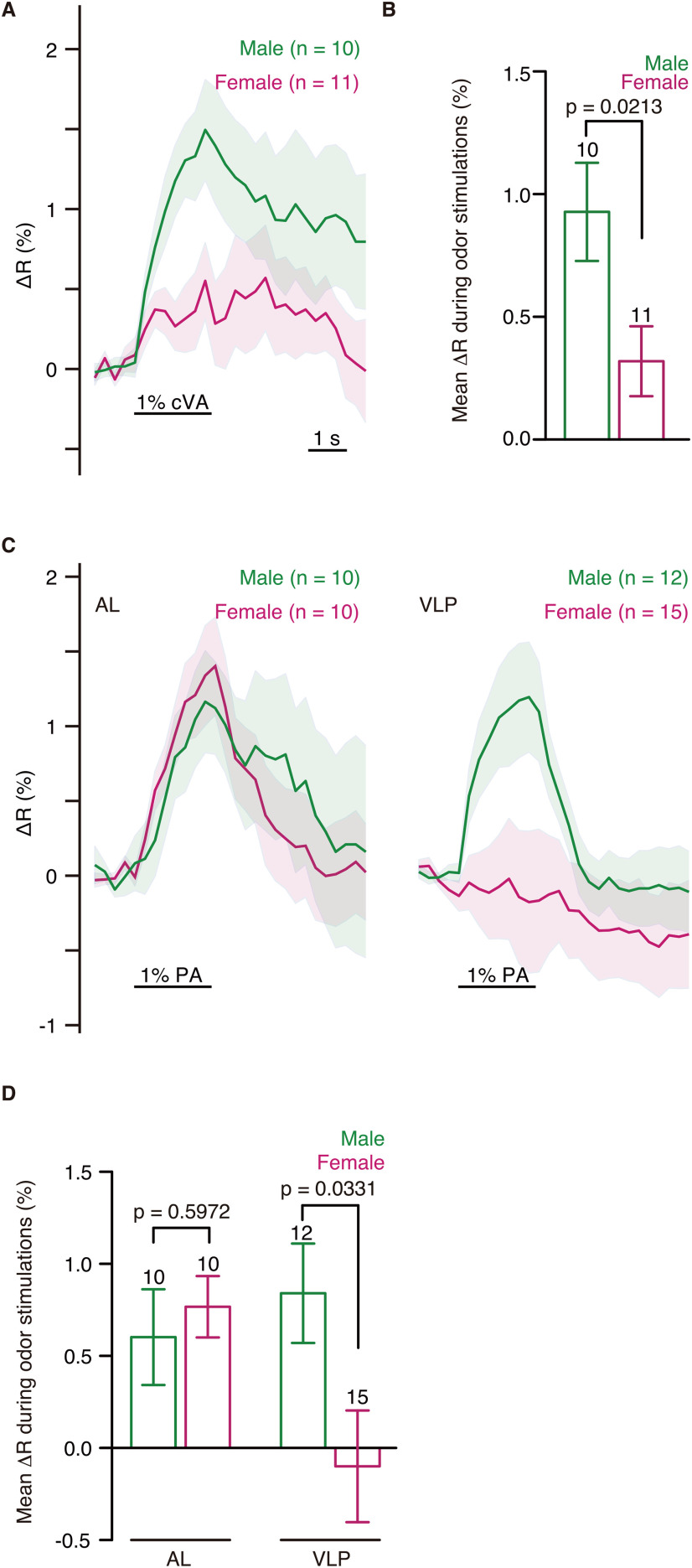
We also found sexual dimorphisms in the odor response patterns. When GCaMP6s (Chen et al., 2013) was expressed in lPN4, the excitatory calcium responses to cVA in the male antennal lobe were significantly larger than those in females (t(19) = 2.509, p = 0.0213, two-tailed unpaired t test; effect size, r = 0.48; Fig. 2A,B), which is consistent with the fact that only the male lPN4 innervated the DA1 glomerulus where OSNs responding to cVA terminate. Calcium responses of lPN4s to palmitoleic acid were recorded in both the antennal lobe and ventrolateral protocerebrum (Fig. 2C). In the antennal lobe, where lPN4 receives olfactory input from the Or47b OSNs, the responses were not significantly different between males and females (t(18) = 0.5380, p = 0.5972, two-tailed unpaired t test; effect size, r = 0.12; Fig. 2D), whereas the excitatory responses in the ventrolateral protocerebrum observed in males were significantly larger than those in females (t(25) = 2.255, p = 0.0331, two-tailed unpaired t test; effect size, r = 0.40; Fig. 2D). This result may reflect that the lPN4 axon is thicker in males than in females, as thicker neurites generally spread depolarization with a greater distance than thinner neurites (Johnston and Wu, 1995), or that the female lPN4 is inhibited at the presynaptic sites in the ventrolateral protocerebrum.
Figure 2.
Sexual dimorphism in the odor response patterns of lPN4. A, Calcium responses of lPN4s to 1% cVA in the antennal lobe. Horizontal bar represents the odor stimulations. Shading indicates the SEM. B, Mean ΔR during cVA stimulations. C, Calcium responses of lPN4s to 1% palmitoleic acid (PA) in the antennal lobe (AL, left) and ventrolateral protocerebrum (VLP, right). D, Mean ΔR during the 1% PA stimulations in the AL and VLP. Mean ΔR during the odor stimulations was compared using unpaired two-tailed t test. Error bars indicate the SEM. Numbers above error bars represent the numbers of animals observed.
We also analyzed the projection patterns of lPN4 using the fruNP21 GAL4 line, which labeled the P1 and mAL neurons as well as lPN4 (Kimura et al., 2005, 2008; Tanaka et al., 2012), and found that the male lPN4 also shared projection areas with the P1 and mAL neurons in the ventrolateral protocerebrum and subesophageal zone, respectively (Fig. 1F,G). These results suggest that the fly odor information of lPN4 is relayed to these neurons in males, which may correlate with male-specific sexual behavior.
Genetic manipulation of lPN4 induced aberrant courtship behavior in males
We next investigated the function of lPN4 in male courtship behavior (Fig. 3A) using NP6107 to express tetanus toxin (TNT), a protease that blocks vesicle fusion to induce chronic inhibition of lPN4 synaptic transmission (DiAntonio et al., 1993). When we paired the TNT-expressing male with a female in a cell in red dim light to exclude visual input during mating, we found that tapping behaviors (Fig. 3B) preceding the first unilateral wing extension were skipped more so in TNT-expressing males than that in controls (11 of 25 TNT-expressing males; 3 of 23 controls; p = 0.0267, two-sided Fisher's exact test; effect size, ϕ = 0.34; Fig. 3C). The mean frequency of tapping until the first unilateral wing extension was lower in the TNT-expressing males than that in controls (1.1 ± 0.2 in TNT-expressing males; 2.6 ± 0.60 in controls; z = −2.655, p = 0.00792, generalized linear mixed model; effect size, r = 0.33; Fig. 3D).
Unilateral wing extension was observed in the TNT-expressing males even when the males were exposed to the odor of a motionless decapitated female in the lower layer of the double-layered chamber separated from the male layer by a mesh barrier (Fig. 3E; Ejima and Griffith, 2007). In this situation, the ratio of flies that extended a wing unilaterally was significantly higher in TNT-expressing males than in controls (7 of 20 TNT-expressing males; 1 of 20 controls; p = 0.0436, two-sided Fisher's exact test; effect size, ϕ = 0.38; Fig. 3E, right). TNT-expressing males that had their third antennal segments, the olfactory organ, surgically removed did not significantly extend their wings (4 of 20 TNT-expressing males; 1 of 20 controls; p = 0.3416, two-sided Fisher's exact test; effect size, ϕ = 0.23; Fig. 3E, middle). In the absence of a female, we observed few unilateral wing extensions in both strains (2 of 20 TNT-expressing males; 1 of 20 controls; p = 1.0000, two-sided Fisher's exact test; effect size, ϕ = 0.09; Fig. 3E, left). We also confirmed that none of the 20 TNT-expressing males showed unilateral wing extension in the presence of a female when they were decapitated. These results indicate that olfactory input drove this abnormal behavior in the TNT-expressing males, as the mesh barrier of the chamber prohibited the male from reaching and tapping the female.
Wing extension without tapping behaviors in TNT-expressing animals was not evoked because of hyperactive courtship motivation, as the overall frequency of wing extension and copulation attempts in TNT-expressing animals was comparable to that of the control males (frequency of wing extension: TNT-expressing males, 47.3 ± 6.9; controls, 42.8 ± 9.0; U = 184.5, p = 0.5139, two-tailed Mann–Whitney test; effect size, r = 0.06; Fig. 3F; copulation attempts: TNT-expressing males, 8.0 ± 3.3; controls, 10.7 ± 2.6; U = 168, p = 0.3870, two-tailed Mann–Whitney test; effect size, r = 0.21; Fig. 3G). We did not observe significant differences in moving activities between the TNT-expressing animals and controls (TNT-expressing males, 371.5 ± 28.6 mm; controls, 400.7 ± 31.5 mm; U = 38.0, p = 0.3930, two-tailed Mann–Whitney test; effect size, r = 0.15; Fig. 3H), suggesting that the abnormal behaviors were not caused by motor defects in the TNT-expressing males. In addition, the males expressing the inactive form of TNT (TNTIN) did not show a significant decrease in the frequency of tapping until the first unilateral wing extension (TNTIN-expressing males, 2.2 ± 0.6; controls, 1.5 ± 0.4; z = 0.9666, p = 0.334, generalized linear mixed model; effect size, r = 0.18; Fig. 3I) or an increase in the ratio of males that showed unilateral wing extension when a male was separated from a female by a mesh barrier (3 of 20 TNTIN-expressing males; 1 of 20 controls; p = 0.6050, two-sided Fisher's exact test; effect size, ϕ = 0.17; Fig. 3J), indicating that the phenotypes in the TNT-expressing animals were not because of a mutation caused by GAL4 insertion. These results indicate that lPN4 suppresses wing extension when males smell female odors; therefore, blocking the synaptic output of lPN4 caused wing extension before tapping the female.
We confirmed that lPN4 plays an inhibitory role in wing extension by expressing Drosophila transient receptor potential channel A1 (dTrpA1; Hamada et al., 2008). When lPN4 was excited at 31°C, we observed that the frequency of tapping until the first unilateral wing extension was significantly increased in the dTrpA1-expressing animals (controls, 1.6 ± 0.3; dTrpA1-expressing males, 2.9 ± 0.5; z = 2.188, p = 0.0287, generalized linear mixed model; effect size, r = 0.30; Fig. 4A). Moreover, excitation of lPN4 suppressed the transition from the tapping phase to the unilateral wing extension phase. In contrast to controls, in which 96% of males showed unilateral wing extension at least once by the fourth tap, a significantly lower rate (only 52%) of the dTrpA1-expressing males showed wing extension by the fourth tap (24 of 25 controls; 13 of 25 dTrpA1-expressing males; p = 0.0008, two-sided Fisher's exact test; effect size, ϕ = 0.50; Fig. 4B). The frequencies of wing extension (controls, 50.6 ± 14.4; dTrpA1-expressing males, 5.7 ± 3.1; U = 18.5, p = 0.0059, two-tailed Mann–Whitney test; effect size, r = 0.54; Fig. 4C) and copulation attempts (controls, 12.2 ± 4.9; dTrpA1-expressing males, 0.6 ± 0.5; U = 29.5, p = 0.0246, two-tailed Mann–Whitney test; effect size, r = 0.45; Fig. 4D) were significantly decreased when lPN4 was excited. The dTrpA1-expressing males showed movement activities comparable to controls at 31°C (dTrpA1-expressing males, 326.5 ± 32.5 mm; controls, 395.2 ± 18.9 mm; t(18) = 1.832, p = 0.0836, two-tailed unpaired t test; effect size, r = 0.38; Fig. 4E), suggesting that the phenotype of the dTrpA1-expressing males at 31°C was not caused by motor defects. We did not observe significant differences between dTrpA1-expressing animals and controls at 25°C in the frequency of tapping until the first unilateral wing extension (idTrpA1-expressing males, 1.5 ± 0.4; controls, 1.8 ± 0.4; z = −0.5915, p = 0.5540, generalized linear mixed model; effect size, r = 0.12; Fig. 4F), frequency of unilateral wing extension (dTrpA1-expressing males, 52.1 ± 10.8; controls, 65.4 ± 6.4 in controls; t(18) = 1.062, p = 0.3023, two-tailed unpaired t test; effect size, r = 0.23; Fig. 4G), and frequency of copulation attempts (dTrpA1-expressing males, 14.4 ± 4.5; controls, 17.6 ± 2.7; t(18) = 0.6071, p = 0.5514, two-tailed unpaired t test; effect size, r = 0.13; Fig. 4H), indicating that the phenotypes in the dTrpA1-expressing animals were not because of a mutation caused by GAL4 insertion. These results indicate that excitation of lPN4 suppresses the expression of unilateral wing extension, and thus decreases the frequency of later actions of the courtship ritual.
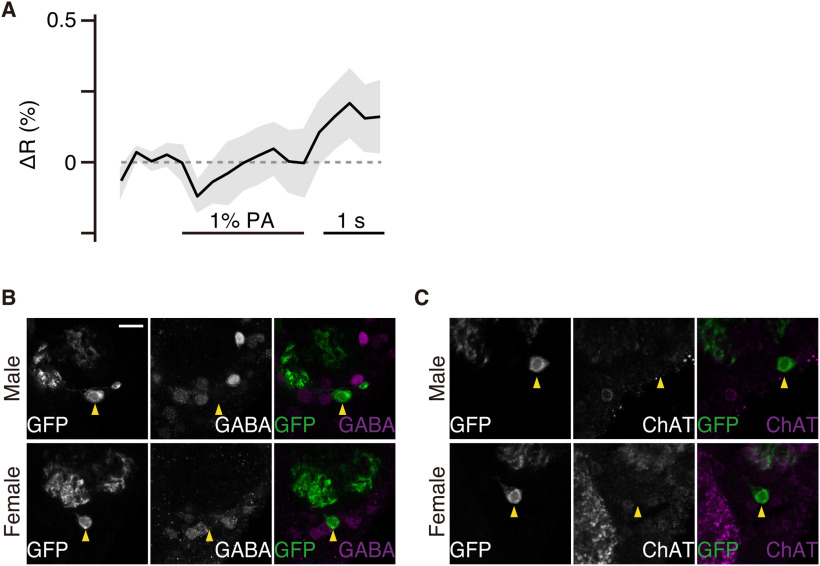
As excitation of lPN4 suppressed post-tapping behaviors, we investigated whether the P1 neurons were inhibited when lPN4 was excited. We performed calcium imaging of P1 neurons to record responses to palmitoleic acid and found that the arborization of P1 neurons around the lPN4 terminals was slightly inhibited during palmitoleic acid stimulation (Fig. 5A). We then tried to identify neurotransmitters in lPN4 that mediate the suppression of post-tapping actions using anti-GABA and anti-choline acetyltransferase antibodies; however, neither anti-GABA nor anti-choline acetyltransferase antibodies labeled lPN4 (Fig. 5B,C).
Figure 5.
Calcium responses of P1 neurons to 1% palmitoleic acid (PA) stimulations. A, Calcium responses of P1 neurons to 1% PA stimulations recorded at the horizontal level of mushroom body pedunculus (n = 11 brains). Horizontal bar represents the odor stimulation. Shading indicates the SEM. Genotype: UAS-GCaMP6s/+; NP21-GAL4/UAS-GCaMP6s. B, C, The signals of anti-GABA (B) and choline acetyltransferase (ChAT; C) antibodies (white signals in middle images; magenta in right images) in male (top) and female (bottom) brains. The positions of the cell bodies of lPN4 (white signals in left images; green in right images) are indicated by yellow arrowheads. Genotype: Y or +/GAL4; 10xUAS-mCD8::GFP/+; 20xUAS-mCD8::GFP/+. Medial is to the left; dorsal is to the top. Scale bar, 10 μm.
lPN4 was inhibited by the application of female cuticular hydrocarbons to forelegs
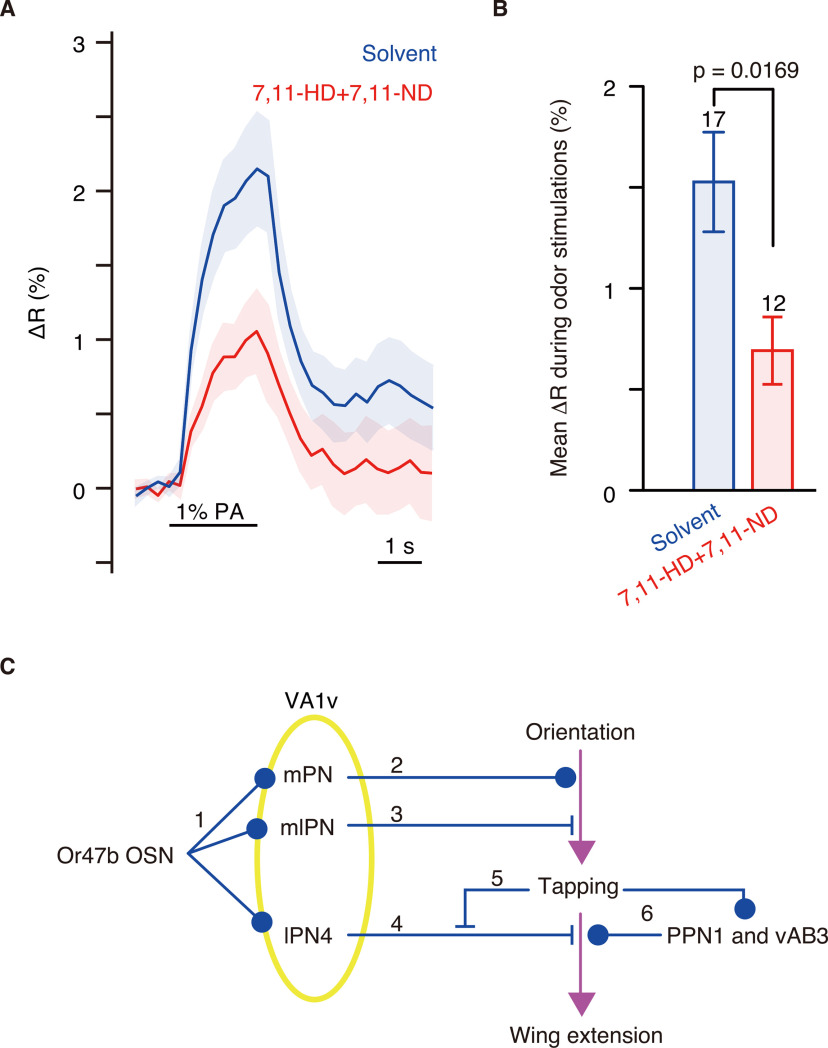
lPN4 was excited by palmitoleic acid, an aphrodisiac odor, but its excitation suppressed post-tapping behavior. These results led us to hypothesize that unilateral wing extension is released through inhibition of lPN4 while tapping a female, as gustatory input received during tapping triggers wing extension. Thus, we investigated whether applying female cuticular pheromones to male forelegs inhibits the odor responses of lPN4 by expressing GCaMP6s (Fig. 6A). We found that the application of 7,11-HD and 7,11-ND to the forelegs significantly reduced calcium responses of lPN4 to palmitoleic acid in the ventrolateral protocerebrum (t(27) = 2.547, p = 0.0169, two-tailed unpaired t test; effect size, r = 0.43; Fig. 6B).
Figure 6.
Application of female cuticular pheromones to male forelegs reduced the calcium responses of lPN4 to 1% palmitoleic acid (PA). A, Calcium responses to 1% PA recorded from lPN4s in the ventrolateral protocerebrum of the males with paraffin oil (solvent, blue) or 1% 7,11-HD and 7,11-ND (red) applied to forelegs. Shade shows the SEM. B, Mean ΔR during the 1% PA stimulations. Numbers above error bars represent the numbers of animals recorded. Error bars indicate the SEM. Two-tailed unpaired t test was performed for statistics. C, Schema of the function of Or47b OSN pathways. Or47b-expressing OSNs excite the projection neurons of multiple tracts in the VA1v glomerulus (1). The output of mPNs promotes courtship behavior to induce tapping (2), whereas the mlPNs control the level of male courting through feedforward inhibition (Wang et al., 2014; 3). The output of lPN4 suppresses wing extension (4). Inhibition of lPN4s mediated by gustatory input during tapping (5) and excitation of PPN1 and vAB3 through tapping (6) release wing extension in males.
Discussion
Horne et al. (2018) reported that there are three lPN4s innervating VA1v, among which we analyzed the projection patterns and functions of one single, sexually dimorphic lPN4 in this study. In Drosophila, fruitless (fru)-expressing neurons, such as mAL neurons and projection neurons innervating DA1, show sexually dimorphic projection patterns (Kimura et al., 2005; Datta et al., 2008). lPN4 is labeled with the fruNP21 GAL4 line (Tanaka et al., 2012), although the lPN4 cell body is not localized where the Fru protein is (Kimura et al., 2005). This implies that Fru expression below the detectable level of the anti-Fru antibodies would cause the sexually dimorphic morphology and thus the odor response patterns.
Our finding that the excitation of lPN4 postsynaptic to the Or47b-expressing OSNs suppressed the post-tapping actions might be contradictory to previous reports in which the Or47b OSNs were required to promote male courtship behavior (Dweck et al., 2015; Lin et al., 2016). Among the three projection neurons (mPNs, mlPNs, and lPN4) that innervate VA1v, where Or47b OSNs terminate (Wong et al., 2002; Tanaka et al., 2012), the excitatory cholinergic mPNs projecting to the mushroom body and lateral horn are the sole candidates that mediate the Or47b-dependent promotion of male courtship behavior. The GABAergic mlPNs project only to the lateral horn, which is regarded as an olfactory center for experience-independent olfactory processing (Heimbeck et al., 2001; Okada et al., 2009). Downregulation of the GABA level in the mlPNs evoked unilateral wing extension and copulation attempts more frequently in males, indicating that the mlPN pathway is involved in controlling the level of male courting through feedforward inhibition of the postsynaptic cells of the mPNs in the lateral horn (Wang et al., 2014). Meanwhile, lPN4 does not project to the lateral horn, but projects to the ventrolateral protocerebrum, where multiple neurons correlated with sexual behavior, such as P1, vAB3, and PPN1, converge (Clowney et al., 2015; Kallman et al., 2015). The vAB3 and PPN1 neurons transmit female cuticular pheromone information detected by gustatory neurons in the forelegs and can excite P1 neurons (Clowney et al., 2015; Kallman et al., 2015). Unlike the mlPNs, blocking the synaptic output from lPN4 did not increase the level of male courting, and excitation of lPN4 suppressed post-tapping actions in males. As the female smell promotes male courtship behaviors, the olfactory system is ready to simultaneously promote and suppress the progress of courtship actions while responding to a female smell.
The lPN4 projection area in the ventrolateral protocerebrum overlaps with that of P1 neurons. Considering that excitation of the P1 neurons triggered an entire series of courtship actions from tapping to copulation (Kohatsu et al., 2011); that P1 neurons were inhibited slightly during palmitoleic acid stimulations, and that lPN4 is inhibited by female cuticular hydrocarbons, we suggest that the early phase of the male courtship ritual is driven by the following processes (Fig. 6C). First, the mPNs, mlPNs, and lPN4 innervating the VA1v glomerulus are simultaneously activated by fly odors during orientation toward the mate. The lPN4 pathway weakly inhibits the P1 neurons, whereas the mPN and mlPN pathways cooperatively excite the P1 neurons, which makes males tap the mate to evaluate its species and sex (Kohatsu et al., 2011) when unilateral wing extension is suppressed. Tapping a female with a foreleg inhibits the odor responses of lPN4 and excites vAB3 and PPN1 neurons (Clowney et al., 2015; Kallman et al., 2015), which, in combination, strongly activate the P1 neurons, resulting in unilateral wing extension to produce the courtship song. Neither anti-GABA nor anti-choline acetyltransferase antibodies labeled lPN4. The inhibitory mAL neurons that are activated by female pheromone stimuli and inhibit the P1 neurons (Clowney et al., 2015) terminate in the vicinity of the lPN4 axon. Therefore, it would be intriguing to examine whether lPN4 inhibits P1 neurons directly or indirectly and whether mAL neurons inhibit lPN4 according to pheromone signals.
Excitation of lPN4 by an aphrodisiac odor is inhibited by gustatory, appropriate female pheromone information, indicating that lPN4 functions as an “AND-gate” for multimodal sensory inputs. In this model, our findings suggest that an aphrodisiac olfactory stimulus activates both the promoter (mPNs in this mating system) and inhibitor (lPN4) pathways. The promoter pathway drives the first action (tapping), whereas the inhibitor pathway suppresses the expression of the second action (wing extension). Sensory inputs (gustatory signals with female cuticular hydrocarbons) received during the first action release the inhibitor pathway, which leads to the second action. This multistep trigger system mediated by lPN4 allows the male to refine the information regarding the potential mate and accordingly progress its courtship behavior. Fixed behavioral sequences are also observed when animals show fixed action patterns that are triggered by just one sign stimulus or releaser (Camhi, 1984), in contrast to the Drosophila courtship sequence that is elicited by the multistep trigger system. The neural circuits responsible for the fixed action patterns remain largely unknown. It would be fascinating to examine whether a similar “AND-gate” system also contributes to a behavioral sequence of a fixed action pattern.
Footnotes
This work was supported by Precursory Research for Embryonic Science and Technology (PRESTO) program of the Japan Science and Technology Agency to N.K.T.; and by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to N.K.T. (Grants 24120509, 22770068, 26830026, 17K07480, and 20K06733) and to A.E. (Grants 22680026 and 18K06329). We thank Kazuhiko Kume, Taro Ueno, Tadao Usui, Paul Garrity, Hubert Amrein, Demian Park, the Kyoto Drosophila Genetic Resource Center, and the Bloomington Drosophila Stock Center for providing fly strains; and Kazuhiro Wada and the Developmental Studies Hybridoma Bank at the University of Iowa for antibodies. We also thank Kazuhide Kiuchi for constructing the fly chambers; and Ken-ichi Kimura, Masayuki Koganezawa, Kazumichi Shimizu, and Kazuaki Ikeda for helpful discussions. In addition, we thank Tadashi Uemura and Tetsuya Tabata for support.
The authors declare no competing financial interests.
References
- Auer TO, Benton R (2016) Sexual circuitry in Drosophila. Curr Opin Neurobiol 38:18–26. 10.1016/j.conb.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Camhi JM (1984) Neuroethology. Sunderland, MA: Sinauer. [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499:295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V (2015) Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87:1036–1049. 10.1016/j.neuron.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R (2008) The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452:473–477. 10.1038/nature06808 [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Parfitt KD, Schwarz TL (1993) Synaptic transmission persists in synaptotagmin mutants in Drosophila. Cell 73:1281–1290. 10.1016/0092-8674(93)90356-u [DOI] [PubMed] [Google Scholar]
- Dweck HK, Ebrahim SA, Thoma M, Mohamed AA, Keesey IW, Trona F, Lavista-Llanos S, Svatoš A, Sachse S, Knaden M, Hansson BS (2015) Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci U S A 112:E2829–E2835. 10.1073/pnas.1504527112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A, Griffith LC (2007) Measurement of courtship behavior in Drosophila melanogaster. CSH Protoc 2007:pdb.prot4847. 10.1101/pdb.prot4847 [DOI] [PubMed] [Google Scholar]
- Ejima A, Griffith LC (2008) Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS One 3:e3246. 10.1371/journal.pone.0003246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Greenspan RJ, Ferveur JF (2000) Courtship in Drosophila. Annu Rev Genet 34:205–232. 10.1146/annurev.genet.34.1.205 [DOI] [PubMed] [Google Scholar]
- Grosjean Y, Rytz R, Farine J-P, Abuin L, Cortot J, Jefferis GSXE, Benton R (2011) An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478:236–240. 10.1038/nature10428 [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA (2008) An internal thermal sensor controlling temperature preference in Drosophila. Nature 454:217–220. 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF (2001) A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A 98:15336–15341. 10.1073/pnas.011314898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Langille C, McLin S, Wiederman M, Lu Z, Xu CS, Plaza SM, Scheffer LK, Hess HF, Meinertzhagen IA (2018) A resource for the Drosophila antennal lobe provided by the connectome of glomerulus VA1v. Elife 7:e37550. 10.7554/eLife.37550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S (1995) Foundations of cellular neurophysiology. Cambridge, MA: MIT. [Google Scholar]
- Kallman BR, Kim H, Scott K (2015) Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. Elife 4:e11188. 10.7554/eLife.11188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K-I, Ote M, Tazawa T, Yamamoto D (2005) Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438:229–233. 10.1038/nature04229 [DOI] [PubMed] [Google Scholar]
- Kimura K-I, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D (2008) Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59:759–769. 10.1016/j.neuron.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D (2011) Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69:498–508. 10.1016/j.neuron.2010.12.017 [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ (2007) A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446:542–546. 10.1038/nature05672 [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22:451–461. 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Lin H-H, Cao D-S, Sethi S, Zeng Z, Chin JSR, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su C-Y, Wang JW (2016) Hormonal modulation of pheromone detection enhances male courtship success. Neuron 90:1272–1285. 10.1016/j.neuron.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Starostina E, Vijayan V, Pikielny CW (2012) Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J Neurosci 32:11879–11889. 10.1523/JNEUROSCI.1376-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar CE, Lillvis JL, Bath DE, Fitzgerald JE, Cannon JG, Simpson JH, Dickson BJ (2019) Threshold-based ordering of sequential actions during Drosophila courtship. Curr Biol 29:426–434. 10.1016/j.cub.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Okada R, Awasaki T, Ito K (2009) Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol 514:74–91. 10.1002/cne.21971 [DOI] [PubMed] [Google Scholar]
- Seki Y, Dweck HKM, Rybak J, Wicher D, Sachse S, Hansson BS (2017) Olfactory coding from the periphery to higher brain centers in the Drosophila brain. BMC Biol 15:56. 10.1186/s12915-017-0389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana S, Touhara K, Ejima A (2015) Modification of male courtship motivation by olfactory habituation via the GABAA receptor in Drosophila melanogaster. PLoS One 10:e0135186. 10.1371/journal.pone.0135186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagawa K, Salvaterra P (1996) Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res 24:237–243. 10.1016/0168-0102(95)00999-x [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Ito K, Stopfer M (2009) Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci 29:8595–8603. 10.1523/JNEUROSCI.1455-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Endo K, Ito K (2012) The organization of antennal lobe-associated neurons in the adult Drosophila melanogaster brain. J Comp Neurol 520:4067–4130. 10.1002/cne.23142 [DOI] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K (2012) Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149:1140–1151. 10.1016/j.cell.2012.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR (2007) Receptors and neurons for fly odors in Drosophila. Curr Biol 17:606–612. 10.1016/j.cub.2007.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF (2007) Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30:505–533. 10.1146/annurev.neuro.30.051606.094306 [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E (2006) Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49:833–844. 10.1016/j.neuron.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Wang K, Gong J, Wang Q, Li H, Cheng Q, Liu Y, Zeng S, Wang Z (2014) Parallel pathways convey olfactory information with opposite polarities in Drosophila. Proc Natl Acad Sci U S A 111:3164–3169. 10.1073/pnas.1317911111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearne SL, Rodriguez A, Ehlenberger DB, Rocher AB, Henderson SC, Hof PR (2005) New Techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neurosci 136:661–680. 10.1016/j.neuroscience.2005.05.053 [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R (2002) Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109:229–241. 10.1016/s0092-8674(02)00707-9 [DOI] [PubMed] [Google Scholar]