Abstract
Background
Indwelling urethral catheters are often used for bladder drainage in hospital. Urinary tract infection is the most common hospital‐acquired infection, and a common complication of urinary catheterisation. Pain, ease of use and quality of life are important to consider, as well as formal economic analysis. Suprapubic catheterisation can also result in bowel perforation and death.
Objectives
To determine the advantages and disadvantages of alternative routes of short‐term bladder catheterisation in adults in terms of infection, adverse events, replacement, duration of use, participant satisfaction and cost effectiveness. For the purpose of this review, we define 'short‐term' as intended duration of catheterisation for 14 days or less.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings (searched 26 February 2015), CINAHL (searched 27 January 2015) and the reference lists of relevant articles.
Selection criteria
We included all randomised and quasi‐randomised trials comparing different routes of catheterisation for short‐term use in hospitalised adults.
Data collection and analysis
At least two review authors extracted data and performed 'Risk of bias' assessment of the included trials. We sought clarification from the trialists if further information was required.
Main results
In this systematic review, we included 42 trials.
Twenty‐five trials compared indwelling urethral and suprapubic catheterisation. There was insufficient evidence for symptomatic urinary tract infection (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.61 to 1.69; 5 trials, 575 participants; very low‐quality evidence). Participants with indwelling catheters had more cases of asymptomatic bacteriuria (RR 2.25, 95% CI 1.63 to 3.10; 19 trials, 1894 participants; very low quality evidence) and more participants reported pain (RR 5.62, 95% CI 3.31 to 9.55; 4 trials, 535 participants; low‐quality evidence). Duration of catheterisation was shorter in the indwelling urethral catheter group (MD ‐1.73, 95% CI ‐2.42 to ‐1.05; 2 trials, 274 participants).
Fourteen trials compared indwelling urethral catheterisation with intermittent catheterisation. Two trials had data for symptomatic UTI which were suitable for meta‐analysis. Due to evidence of significant clinical and statistical heterogeneity, we did not pool the results, which were inconclusive and the quality of evidence was very low. The main source of heterogeneity was the reason for hospitalisation as Hakvoort and colleagues recruited participants undergoing urogenital surgery; whereas in the trial conducted by Tang and colleagues elderly women in geriatric rehabilitation ward were recruited. The evidence was also inconclusive for asymptomatic bacteriuria (RR 1.04; 95% CI 0.85 to 1.28; 13 trials, 1333 participants; very low quality evidence). Almost three times as many people developed acute urinary retention with the intermittent catheter (16% with urethral versus 45% with intermittent); RR 0.45, 95% CI 0.22 to 0.91; 4 trials, 384 participants.
Three trials compared intermittent catheterisation with suprapubic catheterisation, with only female participants. The evidence was inconclusive for symptomatic urinary tract infection, asymptomatic bacteriuria, pain or cost.
None of the trials reported the following critical outcomes: quality of life; ease of use, and cost utility analysis.
Authors' conclusions
Suprapubic catheters reduced the number of participants with asymptomatic bacteriuria, recatheterisation and pain compared with indwelling urethral. The evidence for symptomatic urinary tract infection was inconclusive.
For indwelling versus intermittent urethral catheterisation, the evidence was inconclusive for symptomatic urinary tract infection and asymptomatic bacteriuria. No trials reported pain.
The evidence was inconclusive for suprapubic versus intermittent urethral catheterisation. Trials should use a standardised definition for symptomatic urinary tract infection. Further adequately‐powered trials comparing all catheters are required, particularly suprapubic and intermittent urethral catheterisation.
Plain language summary
Which route of short‐term bladder drainage is best for adults in hospital?
The evidence for this question is up‐to‐date as of 26 February 2015
Number of trials: 42
Number of participants: 4577
Key messages:
This Cochrane review found that there was not enough evidence to determine whether one route of bladder drainage was more likely to reduce urinary tract infection than another. The evidence suggests that participants with suprapubic catheters were less likely to have catheter‐associated pain compared with those with indwelling urethral catheters. The quality of evidence in this review was low, and many of the trials did not report important outcomes such as catheter‐associated quality of life and ease of use. The included trials reported few adverse effects, but it is not clear if this is because the adverse effects did not occur or were simply not reported. Because of the limited evidence, we need more high‐quality trials. It is important that these trials report symptomatic urinary tract infection, pain from using catheters, quality of life, adverse effects and ease of use.
Background: what routes of short‐term bladder drainage are there?
Urinary catheters are tubes that drain urine from the bladder. They are often used in people who are unable to go to the toilet easily during their hospital stay. About one in four hospital patients requires short‐term bladder drainage using a urinary catheter. Catheters can be used in different ways. The main routes of urinary catheterisation are:
1. Urethral : a drainage tube is inserted into the bladder via the urethra, and is either left in place (indwelling catheter), or removed after the bladder is emptied (intermittent catheter).
2. Suprapubic catheterisation: a drainage tube is inserted into the bladder through a small cut in the abdominal wall.
A common complication of short‐term bladder drainage is urinary tract infection. Infections have many serious implications for patients and healthcare providers. Insertion of a suprapubic catheter may also be associated with more risks than urethral routes, such as bleeding or damage to the bowel.
Key results
The Cochrane review looked at studies which made one of three comparisons:
1. Indwelling versus suprapubic catheterisation 2. Indwelling versus intermittent catheterisation 3. Suprapubic versus intermittent catheterisation
1. Twenty‐five trials (2622 participants) compared indwelling urethral and suprapubic catheterisation. There was not enough evidence from five trials to determine whether people had a lower risk of symptomatic urinary tract infection with indwelling urethral or suprapubic catheterisation. There was low quality evidence from four trials that people with indwelling urethral catheters were at greater risk of catheter‐associated pain compared with participants with suprapubic catheters. None of the twenty‐five trials reported ease of use, quality of life or economic outcomes.
2. Fourteen trials (1596 participants) compared indwelling and intermittent urethral catheterisation. There was very low quality evidence from two trials reporting on urinary tract infection, and the review could not determine which route of bladder drainage had a lower risk. None of the fourteen trials reported pain, ease of use, quality of life or economic outcomes.
3. Three trials (359 participants) compared suprapubic and intermittent urethral catheterisation. Only one trial reported on urinary tract infection. The evidence was inconclusive and of low quality. Only one trial had evidence on pain. Again, the evidence was inconclusive and the quality of the evidence was very low. None of the three trials reported ease of use, quality of life or economic outcomes.
Concluding messages
Although many trials have been conducted not enough have looked at important outcomes. Many questions are still unanswered about short‐term bladder drainage. Which route is the least likely to cause urinary tract infection? Is one route associated with more pain than the others? Is there a significant difference in cost or convenience for patients and hospitals between the three routes? Until these questions are answered with higher‐quality evidence, we need more and better trials.
Summary of findings
Background
Description of the condition
Indwelling urinary catheters are commonly used for bladder drainage during hospital care. The most common complication is infection. Urinary tract infections (UTIs) account for about 20% of hospital‐acquired (nosocomial) infections (Smyth 2008), and about 80% of these are associated with urinary catheters. Such infections not only prolong hospital stay and are expensive to treat (Elvy 2009; Nasr 2010), but also cause unpleasant symptoms such as fever and chills in up to 30% of the patients. Patients with infection of the urinary tract can go on to develop bacteraemia (presence of bacteria within circulation). About 17% of hospital bacteraemia is due to catheter‐associated UTI (Gould 2009) . When used in patients who are acutely ill, the risk of a catheter‐associated infection may be higher and hence pose a greater threat to life.
Bacteria get into the catheterised bladder by direct inoculation at the time of catheter insertion and via the following routes: extraluminally by ascending from the urethral meatus along the catheter‐urethral interface, and intraluminally by reflux of the organisms into the catheter lumen (Tambyah 1999; Warren 2001). The normal mechanical wash‐out effect of the urinary stream is interrupted when there is a urinary catheter. The presence of a urinary catheter, plus other factors such as the formation of biofilm, allows bacteria to multiply quickly (Elvy 2009; Newman 2010; Trautner 2004).
There are various micro‐organisms that can cause catheter‐associated urinary tract infection. The most common of these is Escherichia coli, a gram‐negative coliform. Other common infective organisms include Candida spp, Enterococcus spp, Pseudomonas aeruginosa, Klebsiella pneumonia and Enterobacter spp. This list, however, is not exhaustive, with other less common micro‐organisms also causing catheter‐associated urinary tract infection (Elvy 2009).
The Center for Disease Control and Prevention (CDC) defines catheter‐associated UTI as a UTI in the presence of an indwelling catheter which has been in place for more than two calendar days on the day of UTI; or the catheter was in place on the day of the UTI or the previous day and then removed. The UTI criteria must be met on the day of catheter removal or the following day. The UTI CDC criteria must also be met, which include at least one of the following symptoms: fever, suprapubic tenderness, frequency, dysuria, costovertebral pain or tenderness. As well as signs and symptoms, a positive urine culture of 10⁵ colony‐forming units (CFU)/ml with no more than two species of micro‐organisms must be identified. CDC includes intermittent catheters but not suprapubic in its definition (CDC 2015).
The Infectious Diseases Society of America (IDSA) published guidelines for diagnosing catheter‐associated UTIs, which include all three types of catheter: indwelling, intermittent and suprapubic catheters. It recommended that a diagnosis be made when symptoms and signs compatible with UTI were present, plus at least 10³ cfu/ml of one or more bacterial species in a single catheter urine specimen or in a midstream voided urine specimen from a patient whose catheter was removed in the previous 48 hours.
Signs and symptoms that are compatible with UTI include:
new onset or worsening fever
rigors
altered mental status
malaise, or lethargy with no other identified cause
flank pain
costovertebral angle tenderness
acute haematuria
pelvic discomfort
dysuria
urgent or frequent urination
suprapubic pain or tenderness (Hooton 2010).
In this review, we use the IDSA definition of catheter‐associated UTI as it includes indwelling urethral, intermittent urethral and suprapubic catheters in its definition. The CDC definition includes urethral catheters, but does not include suprapubic and we therefore did not use this definition.
The IDSA defines asymptomatic bacteriuria as the presence of at least 10⁵ cfu/ml of one or more bacterial species in a sample of urine in a patient with no symptoms of urinary tract infection (Hooton 2010).
Management of symptomatic UTI varies greatly. Some clinicians will treat patients with asymptomatic bacteriuria, some will use prophylactic antibiotics and others will only treat patients with symptomatic UTI. The European and Asian guidelines on Management and Prevention of Catheter‐Associated Urinary Tract Infections recommend that asymptomatic bacteriuria should not be treated with antibiotics, as the infection will not be eradicated or, if it is, it will return rapidly. They recommend that symptomatic infections in catheterised patients be treated with broad‐spectrum systemic antibiotics, as well as removal and replacement of the catheter (Tenke 2008).
Description of the intervention
In this review, we consider only short‐term urinary catheterisation in hospitalised adults. We define 'short‐term' as 14 days or less.
Indwelling urethral catheterisation
Indwelling urethral catheterisation is most commonly used, in both the short term and the long term. It involves the insertion of a catheter through the urethra into the bladder, although it is associated with various complications. The most common of these is UTI, which can have a heavy cost for both patient and healthcare provider. The first step in reducing UTIs and other complications is to avoid unnecessary catheterisation; the second is to remove the catheter as soon as possible. Indications for indwelling urethral catheterisation include acute urinary retention or bladder outlet obstruction, the need for precise urinary output monitoring, and in patients undergoing urological or gynaecological surgery who might be expected to be unable to micturate immediately after surgery (Gould 2009).
While the most common method of bladder drainage is indwelling urethral catheterisation, intermittent catheterisation and suprapubic catheterisation are alternative approaches. From a theoretical point of view, it is possible that either of these methods may be associated with a lower risk of UTI.
Suprapubic catheterisation
Suprapubic catheterisation involves insertion of a catheter into the bladder through an incision in the abdominal wall. Bacterial colonisation of the urethral tract is less likely because of the lower density of (gram‐negative) micro‐organisms on the abdominal skin than in the periurethral area. However, suprapubic catheterisation involves puncturing the bladder after inserting the catheter through the abdominal wall; the concern is unintended visceral or vascular injury. Contraindications for suprapubic catheterisation include bladder cancer, anticoagulation and antiplatelet treatment, abdominal wall sepsis and presence of subcutaneous vascular graft in the suprapubic region (Harrison 2011).
Intermittent catheterisation
Intermittent catheterisation involves inserting and removing a sterile urethral catheter using an aseptic technique. Micro‐organisms are less likely to gain entry into the bladder by tracking along the catheter wall because the catheter is no longer constantly present.
As with other catheters, intermittent catheterisation can be used diagnostically and therapeutically. Diagnostically, it can be used to obtain a sample of urine, or to assess urodynamics or urinary output. Therapeutically, it is indicated in patients who have problems with bladder voiding due to various reasons, such as spinal cord injury, non‐neurogenic bladder dysfunction or incomplete emptying due to intravesical obstruction. Intermittent catheterisation is contraindicated in patients with priapism, and urethral catheterisation generally is contraindicated in patients with suspected or confirmed urethral damage, or urethral cancer (Geng 2006).
How the intervention might work
Once a catheter is in place, the aim is to minimise the risk of infection. There are two accepted basic principles: keeping the catheter system closed, and removing the catheter when it is no longer needed. Antibiotic prophylaxis is controversial and is addressed in another Cochrane review (Lusardi 2013). Another possible strategy for reducing the risk of infection due to indwelling (i.e. urethral or suprapubic) catheters is to use different materials, such as catheters coated with an antibacterial substance. The effects of using different types of indwelling urethral catheters have been evaluated in another Cochrane review (Lam 2014).
Why it is important to do this review
The aim of this review is to assess the effectiveness of different routes of catheterisation (urethral (indwelling or intermittent) or suprapubic) for short‐term use in hospitalised adults, primarily focused on symptomatic urinary tract infection. Other important outcomes included adverse effects, the need for replacement, duration of use, patient satisfaction and cost effectiveness. For the purposes of this review, we define short term as intended duration of catheterisation for up to 14 days.
Objectives
To determine the advantages and disadvantages of alternative routes of short‐term bladder catheterisation in adults in terms of infection, adverse events, replacement, duration of use, participant satisfaction and cost effectiveness. For the purpose of this review, we define 'short‐term' as intended duration of catheterisation for 14 days or less.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐randomised trials (quasi‐RCTs) comparing alternative routes of short‐term catheterisation in hospitalised adults. We define 'short‐term' as intended duration of catheterisation for 14 days or less. Where the intended duration of catheterisation was not stated, we used the reason for hospitalisation as an indicator of the approximate length of catheterisation.
Types of participants
We included studies of adults requiring short‐term urethral catheterisation in hospital for any reason such as urine monitoring, investigations, acute retention problems, and after surgery. These included those suffering from acute illness, urinary retention, perioperative, postoperative, during labour, and during or following surgery.
Types of interventions
The interventions considered were urethral (indwelling or intermittent) or suprapubic catheterisation.
We used the following definition for this review:
Indwelling catheterisation: The European Association of Urology (EAU) definition for indwelling catheterisation: indwelling catheterisation was defined by the passage of a catheter into the urinary bladder via the urethra using an inflatable balloon or other means to retain it in position (EAU 2014).
Intermittent catheterisation: The EAU definition for intermittent catheterisation: intermittent catheterisation, also known as in‐out catheterisation, was defined as emptying of the bladder via the urethra by a catheter that is removed after the procedure, mostly at regular intervals (EAU 2014).
Suprapubic catheterisation: The European Association of Urology Nurses (EAUN) definition of suprapubic catheterisation: suprapubic catheterisation was defined as the insertion of a catheter into the bladder via the anterior abdominal wall, using sutures or other means to retain it in position (Geng 2012).
We have not considered the following interventions for inclusion in this review:
catheterisation insertion techniques (e.g. clean, sterile, with or without antiseptic or antibiotic cream);
meatal care management techniques (e.g. routine hygiene, antiseptic or antibiotic cream);
types of drainage (e.g. continuous, clamp‐and‐release, pressure valves);
types of drainage container (e.g. flexible, rigid, disposable);
treatment of drainage bag (e.g. washouts, use of antiseptic or clean washing techniques, use of antiseptic solutions in the bag);
antibiotic prophylaxis;
type of catheter material (e.g. latex rubber, silicone latex, silicone);
type of catheter coating (e.g. silver alloy, antibiotic coated, electrified).
We made three specific comparisons: 1. indwelling urethral catheterisation versus suprapubic catheterisation; 2. indwelling urethral catheterisation versus intermittent catheterisation; 3. suprapubic catheterisation versus intermittent catheterisation.
Types of outcome measures
Primary outcomes
Number of participants with symptomatic urinary tract infection (UTI)
We used the IDSA definition. If UTI was reported but symptoms were not described, then we reclassified the outcomes as asymptomatic bacteriuria.
Secondary outcomes
Participant‐reported:
number of participants with pain;
ease of use for participant;
participant discomfort;
participant satisfaction;
need to change catheters;
number of catheters used.
Clinician‐reported:
ease of use for practitioner;
length of time catheters used.
Complications/adverse effects:
asymptomatic bacteriuria, using IDSA definition (Hooton 2010) or as defined by trialists;
urethral stricture;
wound infection (for suprapubic catheters);
urgency/bladder spasms/detrusor overactivity;
other adverse effects of intervention (other than UTI).
Co‐interventions:
use of rescue antibiotics.
Health status/Quality of life:
quality of life (using SF‐36, Ware 1992) or other standard tool;
psychological outcome measures (e.g. HADS, Zigmond 1983).
Economic outcomes:
cost utility analysis (using EQ‐5D) or other standard tool of cost effectiveness or cost utility;
costs of intervention(s);
resource implications of differences in outcomes;
formal economic analysis (cost effectiveness, cost utility).
Other outcomes:
any other non‐prespecified outcomes judged to be important when performing the review.
Quality of evidence:
We assessed the quality of evidence using the GRADE approach. We organised a group discussion through the Urological Cancer Charity (UCAN) with participants who underwent urethral or suprapubic catheterisation, in order to identify outcomes which were important from their perspective. We identified five individuals (four men and one woman) who had undergone urethral catheterisation or suprapubic catheterisation. Most of the participants had both types of catheterisation during different stages of their treatment. The participants suggested that infections, pain and discomfort were certainly the most important outcomes from their point of view. They described the pain sensation of suprapubic catheterisation in a number of different ways, such as " very painful", "extremely painful" and "quite painful". They also stressed the impact of catheterisation on their quality of life and identified it as an important outcome. These results were similar to the focus group conducted by Omar 2013 for another Cochrane review of the types of indwelling urethral catheters for short‐term catheterisation in hospitalised adults (Lam 2014). We finally selected the following outcomes for 'Summary of findings' tables:
Number of participants with symptomatic UTI;
Asymptomatic bacteruria;
Number of participants with pain;
Ease of use for participant;
Quality of life (using SF‐36);
Cost utility analysis (using EQ‐5D).
Search methods for identification of studies
We did not impose language or other restrictions on any of the searches described below.
Electronic searches
This review drew on the search strategy developed for the Cochrane Incontinence Group. We identified relevant trials from the Cochrane Incontinence Group Specialised Register of trials. For more details of the search methods used to build the Specialised Register please see the Group's module in the Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings. Most of the trials in the Cochrane Incontinence Group Specialised Register are also contained in CENTRAL. The date of the last search was: 26 February 2015.
We searched the Incontinence Group Specialised Register using the Group's own keyword system. The search terms used are given in Appendix 1.
For this update we also searched CINAHL (on EBSCO) covering 1 January 1981 to 27 January 2015 (searched on 27 January 2015). The search strategy used is given in Appendix 1.
Searching other resources
We also searched the reference lists of all relevant articles.
Data collection and analysis
Selection of studies
Two review authors independently assessed all titles and abstracts of potentially eligible studies identified by the search. Where there was any possibility that the study might be included, we obtained the full‐text paper. We resolved any disagreements that could not be resolved by discussion by consultation with an independent third person.
Data extraction and management
Two review authors extracted data independently and compared them. If the data in trials had not been fully reported, we sought clarification directly from the trialists. We entered extracted data into Review Manager 5 software (RevMan 5.3). We processed the included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' assessment tool for judging the risk of bias of the included studies (Higgins 2011). We assessed the following areas for each of the studies:
random sequence generation (selection bias)
allocation concealment (selection bias)
blinding of participants (performance bias)
blinding of personnel (performance bias)
blinding of microbiological outcome assessment
blinding of outcome assessment (detection bias)
incomplete outcome data (attrition bias)
selective reporting (reporting bias)
other bias
Two of the review authors independently assessed the studies, and rated each as 'low risk', 'unclear risk' or 'high risk'.
Measures of treatment effect
For categorical outcomes, the numbers reporting an outcome were related to the numbers at risk in each group to derive a risk ratio (RR), and the number needed to treat for an additional beneficial outcome (NNT). For continuous variables, we used means and standard deviations to derive a mean difference (MD). As a general rule, we combined the outcome data using a fixed‐effect model to calculate pooled estimates and their 95% confidence intervals (CIs). However, we considered the use of a random‐effects model where there were concerns that heterogeneity might have been complicating an analysis. When appropriate, we undertook meta‐analysis.
Unit of analysis issues
In single parallel‐group designed trials, the primary analysis was by participant in the trial. In trials with a non‐standard design, such as multiple observations taken, cluster‐randomised trials and cross‐over trials, we conducted the analysis as directed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We used an intention‐to‐treat (ITT) analysis where possible, meaning that participants were analysed based on the intervention group to which they were randomised, regardless of whether they actually received the intervention they were originally assigned. We tried to contact trialists from eight of the trials for which we required further information (Barry 1992 PE; Dixon 2010; Hakvoort 2011; Ichsan 1987; Korkes 2008; Kringel 2010; Naik 2005; Ratnaval 1996).
If we had noted a difference in dropout rates between the randomised groups, we would have performed sensitivity analysis.
Assessment of heterogeneity
We considered the likelihood of important clinical heterogeneity in each meta‐analysis. We assessed heterogeneity visually using forest plots to assess overlap of 95% confidence intervals, the Chi² test for statistical heterogeneity and the I² statistical test (Higgins 2011). If the P value for the Chi² test was low (P < 0.10) or if the I² test was higher than 50%, we considered it statistically significant. Values of the I² test and the corresponding level of heterogeneity are detailed in the Cochrane Handbook for Systematic Reviews of Interventions. If there was significant heterogeneity, we used a random‐effects model.
Assessment of reporting biases
In order to reduce the risk of reporting and publication bias, we searched multiple databases and other sources comprehensively. If the meta‐analysis included more than 10 trials, we used a funnel plot to assess reporting biases (Higgins 2011).
Data synthesis
We combined trials if we considered the interventions to be sufficiently similar, and used a fixed‐effect model to carry out meta‐analysis. If there was significant heterogeneity, we used a random‐effects analysis.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for:
different types of surgery (urogenital versus non‐urogenital surgery or other reason for catheterisation)
women in labour versus caesarean section
gender: men versus women
antibiotic prophylaxis used or not
timing of taking of urine sample
Sensitivity analysis
We conducted sensitivity analyses where there was significant heterogeneity, comparing different types of surgical intervention. We also performed sensitivity analysis on any trials that did not report their definition for symptomatic UTI and asymptomatic bacteriuria.
Results
Description of studies
Results of the search
We screened 958 records found by the literature search for this review: we further assessed the full text of 74 of these articles for eligibility for inclusion. Fifty‐one reports of 42 studies were included in the review, and 16 reports of 16 studies were excluded from the review. There were six reports of six ongoing studies, details of which can be found in the Characteristics of ongoing studies table. We are still seeking one conference abstract (Kringel 2007) that appears to be a further report of the included study Kringel 2010, and has been detailed in the Characteristics of studies awaiting classification table pending its arrival. The flow of literature through the assessment process is shown in the PRISMA flow diagram (Figure 1).
1.
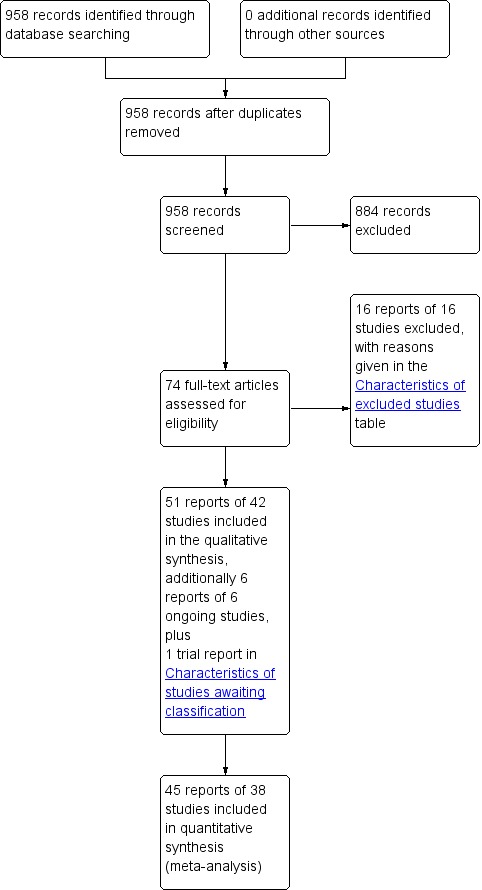
PRISMA study flow diagram
New included trials
In this update, we re‐assessed 18 reports of trials that were excluded in the previous version of the review. Of these, we found 13 reports of 11 trials to be eligible for inclusion. Two were reports of an already‐included trial (Hakvoort 2011), and another two were reports of the same trial (Naik 2005). Overall, we included 11 additional reports of 10 new trials (Barry 1992 PE; Ichsan 1987; Katz 1992; Kerr‐Wilson 1986; Michelson 1988; Naik 2005; Rasmussen 1977; Ratnaval 1996; Skelly 1992; Tangtrakul 1994).
We identified six reports after performing a new search. Of these, a further three new trials were eligible for inclusion (Dixon 2010; Halleberg 2013; Rivard 2012).
Included studies
The trials are described in detail in the Characteristics of included studies table.
We required more information for eight of the included trials. Of these, we were able to contact six: five by email (Dixon 2010; Hakvoort 2011; Korkes 2008; Kringel 2010; Naik 2005) and one by ResearchGate (Ratnaval 1996). We could not find contact details for two trials (Barry 1992 PE; Ichsan 1987). Only three of the six trialists we contacted responded (Dixon 2010; Korkes 2008; Ratnaval 1996).
Design
The review includes 40 randomised trials and two quasi‐randomised trials, as listed below:
RCTs (Ahmed 1993; Andersen 1985; Baan 2003; Barry 1992 PE; Bergman 1987; Botsios 1997; Carpiniello 1988; Dixon 2010; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Harms 1985; Ichsan 1987; Jannelli 2007; Katz 1992; Kerr‐Wilson 1986; Knight 1996; Korkes 2008; Kringel 2010; Millet 2012; Naik 2005; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Rivard 2012; Schiotz 1989; Sethia 1987; Skelly 1992; Stekkinger 2011; Tang 2006; Tangtrakul 1994; Van den Brand 2001; Vandoni 1994; Wiser 1974)
quasi‐RCTs (Barents 1978; Michelson 1988)
Sample sizes
The number randomised in the included trials ranged from 24 participants (Nwabineli 1993) to 344 participants (Hammarsten 1992). In total, 4577 participants were randomised in the 42 trials.
25 trials including 2622 participants compared indwelling urethral and suprapubic catheterisation
14 trials including 1596 participants compared indwelling urethral and intermittent urethral catheterisation
3 trials including 359 participants compared suprapubic and intermittent urethral catheterisation
Participants
We included 42 trials with 4577 participants, who received one route of catheterisation.
Reason for hospitalisation
Thirty‐seven trials had participants hospitalised for a surgical procedure. They were as follows:
Urogenital surgery (Ahmed 1993; Andersen 1985; Barents 1978; Bergman 1987; Dixon 2010; Hakvoort 2011; Hammarsten 1992; Harms 1985; Jannelli 2007; Korkes 2008; Kringel 2010; Prasad 2014; Schiotz 1989; Stekkinger 2011; Wiser 1974)
Abdominal surgery (Baan 2003; Barry 1992 PE; Botsios 1997; Dobbs 1997; Naik 2005; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Rasmussen 1977; Ratnaval 1996; Sethia 1987)
Orthopaedic surgery (Carpiniello 1988; Halleberg 2013; Knight 1996; Michelson 1988; Skelly 1992; Van den Brand 2001)
Caesarean section (Kerr‐Wilson 1986; Tangtrakul 1994)
General surgery (Piergiovanni 1991; Vandoni 1994)
Cardiac surgery (Katz 1992)
The remaining five trials included participants hospitalised for non‐surgical reasons.
Women in labour: three trials (Evron 2008; Millet 2012; Rivard 2012)
Acute urinary retention: one trial (Ichsan 1987)
Elderly women admitted to a geriatric rehabilitation ward for persistent abnormal post‐void residual volumes: one trial (Tang 2006)
We describe reasons for hospitalisation, reason for catheterisation and type of surgery in more detail in Table 4.
1. Types of participants.
| Study ID | Reason for Hospitalisation | Reason for catheterisation | Type of surgery | Gender |
| Ahmed 1993 | Urogenital surgery | Acute urinary retention | TURP for men who present with AUR | Men only |
| Andersen 1985 | Urogenital surgery | Postoperative bladder drainage | Colposuspension or vaginal repair for SUI and/or genital descensus | Women only |
| Baan 2003 | Abdominal surgery | Surgery‐indicated catheterisation | Elective laparotomy | Men and women |
| Barents 1978 | Urogenital surgery | Unclear | Vaginal surgery | Women only |
| Barry 1992 PE | Abdominal surgery | Major abdominal surgery | Elective abdominal surgery | Men and women |
| Bergman 1987 | Urogenital surgery | Surgery‐indicated catheterisation | Vaginal urethropexy (+ hysterectomy) in women with SUI | Women only |
| Botsios 1997 | Abdominal surgery | Surgery‐indicated catheterisation | Elective abdominal surgery of long length | Men and women |
| Carpiniello 1988 | Orthopaedic surgery | Prevent postoperative urinary complications | Total joint replacement | Women only |
| Dixon 2010 | Urogenital surgery | Prevent postoperative urinary retention | Surgery for pelvic organ prolapse and/or SUI | Women only |
| Dobbs 1997 | Abdominal surgery | Prevent postoperative urinary retention | Total hysterectomy for non‐malignant reasons under general anaesthetic | Women only |
| Evron 2008 | Labour | Prevent intrapartum urinary retention | Labour with epidural | Women only |
| Hakvoort 2011 | Urogenital surgery | Abnormal PVR following vaginal prolapse surgery | Vaginal prolapse surgery | Women only |
| Halleberg 2013 | Orthopaedic surgery | Prevent postoperative urinary retention | Hip fracture or hip replacement surgery | Men and women |
| Hammarsten 1992 | Urogenital surgery | Postoperative bladder drainage | TURP | Men only |
| Harms 1985 | Urogenital surgery | Postoperative bladder drainage | Vaginal hysterectomy with front plastic | Women only |
| Ichsan 1987 | AUR | AUR | None | Men and women |
| Jannelli 2007 | Urogenital surgery | Postoperative bladder drainage | Surgery for SUI or anterior vaginal wall prolapse | Women only |
| Katz 1992 | Cardiac surgery | Major cardiac surgery | Coronary artery bypass graft | Men only |
| Kerr‐Wilson 1986 | Caesarean section | Avoid trauma to the bladder during surgery and to ensure unobstructed access to the lower uterine segment | Elective caesarean under epidural anaesthesia | Women only |
| Knight 1996 | Orthopaedic surgery | Prevent postoperative urinary retention | Primary total hip or knee arthroplasty | Men and Women |
| Korkes 2008 | Urogenital surgery | Prevent postoperative urinary retention | Open prostatectomy for BPH | Men only |
| Kringel 2010 | Urogenital surgery | Postoperative bladder drainage | Anterior colporrhaphy plus optional additional procedure (i.e. hysterectomy) | Women only |
| Michelson 1988 | Orthopaedic surgery | Prevent postoperative urinary complications | Total joint replacement | Men and women |
| Millet 2012 | Labour | Prevent intra‐ and postpartum urinary retention | Labour with epidural | Women only |
| Naik 2005 | Abdominal surgery | Postoperative bladder dysfunction | Radical hysterectomy for early stage cervical cancer | Women only |
| Nwabineli 1993 | Abdominal surgery | Prevent postoperative urinary complications (retention and inability to void) | Stage IB or IIA cervical cancer with view for radical hysterectomy | Women only |
| O'Kelly 1995 | Abdominal surgery | Postoperative bladder drainage | Abdominal surgery with full‐length abdominal incision | Men and Women |
| Perrin 1997 | Abdominal surgery | Postoperative bladder drainage | Rectal surgery | Men and women |
| Piergiovanni 1991 | General surgery | Bladder drainage for non‐urological reasons (perioperative, urinary retention, prostatic hypertrophy, incontinence, nursing) | Information not given | Men and women |
| Prasad 2014 | Urogenital surgery | Postoperative bladder drainage | Robot‐assisted laparoscopic radical prostatectomy for newly diagnosed prostate cancer | Men only |
| Rasmussen 1977 | Abdominal surgery | Postoperative bladder drainage | Abdomino‐perineal resection or low anterior resection for rectal cancer | Men and women |
| Ratnaval 1996 | Abdominal surgery | Monitoring of urine during surgery | Pelvic colorectal surgery | Men only |
| Rivard 2012 | Labour | Indicated during birth | Labour with epidural | Women only |
| Schiotz 1989 | Urogenital surgery | Postoperative bladder drainage | Vaginal plastic surgery | Women only |
| Sethia 1987 | General surgery | Monitor urine output postoperatively | Extensive pelvic dissection with or without an anastomosis. | Men and Women |
| Skelly 1992 | Orthopaedic surgery | Postoperative urinary retention | Surgical repair of hip fracture | Men and women |
| Stekkinger 2011 | Urogenital surgery | Prevent postoperative urinary retention | Anterior colporrhaphy ± hysterectomy ± PRS for pelvic prolapse | Women only |
| Tang 2006 | Persistently abnormal PVR | Persistently abnormal PVR in elderly patients | None | Women only |
| Tangtrakul 1994 | Caesarean section | Avoid bladder injury during surgery | Caesarean section | Women only |
| Van den Brand 2001 | Orthopaedic surgery | Prevent postoperative urinary retention | Primary total hip or knee arthroplasty | Men and Women |
| Vandoni 1994 | General surgery | Monitoring or nursing reasons in surgical patients | Information not given | Men and women |
| Wiser 1974 | Urogenital surgery | Postoperative bladder drainage | Vaginal hysterectomy and anterior‐posterior repair | Women only |
AUR: acute urinary retention BPH: benign prostatic hyperplasia PVR: post‐void residual SUI: stress urinary incontinence TURP: trans‐urethral resection of prostate
Gender (Men/Women)
Six trials enrolled only men (Ahmed 1993; Hammarsten 1992; Katz 1992; Korkes 2008; Prasad 2014; Ratnaval 1996).
21 trials enrolled only women (Andersen 1985; Barents 1978; Bergman 1987; Carpiniello 1988; Dixon 2010; Dobbs 1997; Evron 2008; Hakvoort 2011; Harms 1985; Jannelli 2007; Kerr‐Wilson 1986; Kringel 2010; Millet 2012; Naik 2005; Nwabineli 1993; Rivard 2012; Schiotz 1989; Stekkinger 2011; Tang 2006; Tangtrakul 1994; Wiser 1974).
Fifteen trials enrolled both men and women (Baan 2003; Barry 1992 PE; Botsios 1997; Halleberg 2013; Ichsan 1987; Knight 1996; Michelson 1988; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Rasmussen 1977; Sethia 1987; Skelly 1992; Van den Brand 2001; Vandoni 1994).
Age
There was a wide range of ages in the included studies. Five trials did not report the age of participants (Barents 1978; Barry 1992 PE; Harms 1985; Ichsan 1987; Wiser 1974). In trials that did report the age of participants it was reported for each study arm, overall, or both:
< 30 years old: five trials. These trials were all in pregnant women. (Evron 2008; Kerr‐Wilson 1986; Millet 2012; Rivard 2012; Tangtrakul 1994);
40 to 60 years old: seven trials (Bergman 1987; Dobbs 1997; Jannelli 2007; Katz 1992; Naik 2005; Nwabineli 1993; Prasad 2014);
60 to 75 years old: 22 trials (Ahmed 1993; Andersen 1985; Baan 2003; Botsios 1997; Carpiniello 1988; Dixon 2010; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Knight 1996; Korkes 2008; Kringel 2010; Michelson 1988; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Ratnaval 1996; Schiotz 1989; Sethia 1987; Stekkinger 2011; Van den Brand 2001; Vandoni 1994);
≥ 75 years old: two trials (Skelly 1992; Tang 2006).
One trial (Rasmussen 1977) reported the number of participants who were under 70 years old and those aged 70 years or older in each arm. In the indwelling group, eight participants were less than 70 years old and seven were 70 years or older. In the suprapubic group, 25 participants were less than 70 years old and 15 were 70 years or older .
We describe the age of participants in detail in Table 5.
2. Age of participants.
| Study ID | Intervention A | Intervention B | Age (A), years | Age (B), years | Age (overall), years |
| Ahmed 1993 | Indwelling urethral | Suprapubic | 71.6 (mean) | 71.9 (mean) | Not reported |
| Andersen 1985 | Indwelling urethral | Suprapubic | Not reported | Not reported | 61 (34 ‐ 86) (median, range) |
| Baan 2003 | Indwelling urethral | Suprapubic | 59.8 (26 ‐ 81) (mean, range) | 60.4 (37 ‐ 87) (mean, range) | Not reported |
| Barents 1978 | Indwelling urethral | Suprapubic | Not reported | Not reported | Not reported |
| Barry 1992 PE | Indwelling urethral | Suprapubic | Not reported | Not reported | Not reported |
| Bergman 1987 | Indwelling urethral | Suprapubic | Not reported | Not reported | 53 (35 ‐ 68) (mean, range) |
| Botsios 1997 | Indwelling urethral | Suprapubic | 64.3 (1.2) (mean, SD) | 63.8 (1.4) (mean, SD) | Not reported |
| Carpiniello 1988 | Indwelling urethral | Intermittent urethral | 70 (8.6) (mean, SD) | 73 (6.6) (mean, SD) | Not reported |
| Dixon 2010 | Indwelling urethral | Intermittent urethral | 66 (median) | 57 (median) | Not reported |
| Dobbs 1997 | Indwelling urethral | Intermittent urethral | 45 (mean) | 42.6 (mean) | Not reported |
| Evron 2008 | Indwelling urethral | Intermittent urethral | 26 (4) (mean, SD) | 25 (4) (mean, SD) | Not reported |
| Hakvoort 2011 | Indwelling urethral | Intermittent urethral | 61 (10) (mean, SD) | 60 (12) (mean, SD) | Not reported |
| Halleberg 2013 | Indwelling urethral | Intermittent urethral | 72.1 (12.7) (mean, SD) | 71.9 (12.1) (mean, SD) | Not reported |
| Hammarsten 1992 | Indwelling urethral | Suprapubic | 73 (7) (mean, SE) | 71 (7) (mean, SE) | Not reported |
| Harms 1985 | Indwelling urethral | Suprapubic | Not reported | Not reported | Not reported |
| Ichsan 1987 | Indwelling urethral | Suprapubic | Not reported | Not reported | Not reported |
| Jannelli 2007 | Suprapubic | Intermittent urethral | 54.6 (13.7) (mean, SD) | 55.0 (10.5) (mean, SD) | Not reported |
| Katz 1992 | Indwelling urethral | Suprapubic | 60 (9) (mean, SD) | 55 (8) (mean, SD) | Not reported |
| Kerr‐Wilson 1986 | Indwelling urethral | Intermittent urethral | 29.5 (0.97) (mean, SD) | 27.0 (1.03) (mean, SD) | Not reported |
| Knight 1996 | Indwelling urethral | Intermittent urethral | Not reported | Not reported | 66 (35‐86) (mean, SD) |
| Korkes 2008 | Indwelling urethral | Suprapubic | 71.4 (8.0) (52‐84) (mean, SD, range) | 74.1 (6.8) (61 ‐ 91) (mean, SD, range) | Not reported |
| Kringel 2010 | Indwelling urethral | Suprapubic | 63.5 (11.3) (mean, SD) | 61.1 (9.92) (mean, SD) | 64.2 (10.6) (mean, SD) |
| Michelson 1988 | Indwelling urethral | Intermittent urethral | 65.7 (mean) | 61.7 (mean) | 63.5 (mean) |
| Millet 2012 | Indwelling urethral | Intermittent urethral | 27.1 (5.6) (mean, SD) | 28.2 (5.8) (mean, SD) | Not reported |
| Naik 2005 | Suprapubic | Intermittent urethral | Not reported | Not reported | 45 (20‐78) (median, range) |
| Nwabineli 1993 | Indwelling urethral | Suprapubic | 45 (mean) | 42 (mean) | Not reported |
| O'Kelly 1995 | Indwelling urethral | Suprapubic | 65 (42‐81) (median, range) | 68 (35‐79) (median, range) | Not reported |
| Perrin 1997 | Indwelling urethral | Suprapubic | 62 (mean) | 64 (mean) | Not reported |
| Piergiovanni 1991 | Indwelling urethral | Suprapubic | 63 (mean) | 64 (mean) | Not reported |
| Prasad 2014 | Indwelling urethral | Suprapubic | 57.6 (8.6) (mean, SD) | 60.0 (6.4) (mean, SD) | Not reported |
| Rasmussen 1977 | Indwelling urethral | Suprapubic | < 70 years old: 8 participants ≥ 70 years old: 7 participants | < 70 years old: 25 participants ≥ 70 years old: 15 participants | Not reported |
| Ratnaval 1996 | Indwelling urethral | Suprapubic | 63 (42‐80) (median, range) | 64 (32‐81) (median, range) | 66 (32 ‐ 81) (median, range) |
| Rivard 2012 | Indwelling urethral | Intermittent urethral | 27.6 (mean) | 28.7 (mean) | Not reported |
| Schiotz 1989 | Indwelling urethral | Suprapubic | 63.8 (9.1) (mean, SD) | 63.6 (8.5) (mean, SD) | Not reported |
| Sethia 1987 | Indwelling urethral | Suprapubic | 62.3 (mean) | 63.7 (years) | Not reported |
| Skelly 1992 | Indwelling urethral | Intermittent urethral | 78 (8.2) (mean, SD) | 78 (8.6) (mean, SD) | Not reported |
| Stekkinger 2011 | Indwelling urethral | Suprapubic | 61.7 (11.2) (mean, SD) | 62.2 (11.5) (mean, SD) | Not reported |
| Tang 2006 | Indwelling urethral | Intermittent urethral | 81.4 (8.9) (mean, SD) | 80.0 (6.8) (mean, SD) | Not reported |
| Tangtrakul 1994 | Indwelling urethral | Intermittent urethral | 30.4 (4.6) (mean, SD) | 29.1 (4.5) (mean, SD) | Not reported |
| Van den Brand 2001 | Indwelling urethral | Intermittent urethral | 68.6 (8.8) (42 ‐ 85) (mean, SD, range) | 68.2 (9.0) (36 ‐ 84) (mean, SD, range) | Not reported |
| Vandoni 1994 | Indwelling urethral | Suprapubic | 66.4 (mean) | 66 (mean) | Not reported |
| Wiser 1974 | Indwelling urethral | Suprapubic | Not reported | Not reported | Not reported |
SD: standard deviation
Participants who received antibiotics during hospitalisation
There was variation between trials in participants receiving antibiotic therapy, which is likely to be linked to the reason for hospitalisation. Fifteen trials did not report whether antibiotic prophylaxis was used or not (Andersen 1985; Barry 1992 PE; Botsios 1997; Dixon 2010; Evron 2008; Harms 1985; Katz 1992; Korkes 2008; O'Kelly 1995; Prasad 2014; Ratnaval 1996; Rivard 2012; Schiotz 1989; Skelly 1992;Tang 2006). Antibiotic prophylaxis use was as follows in the remaining trials:
Participants received antibiotic therapy: 18 trials (Baan 2003; Bergman 1987; Carpiniello 1988; Dobbs 1997; Hakvoort 2011; Hammarsten 1992; Jannelli 2007; Knight 1996; Kringel 2010; Michelson 1988; Naik 2005; Nwabineli 1993; Perrin 1997; Rasmussen 1977; Sethia 1987; Stekkinger 2011; Van den Brand 2001; Vandoni 1994);
Participants did not receive antibiotics during hospitalisation: five trials (Ahmed 1993; Ichsan 1987; Kerr‐Wilson 1986; Tangtrakul 1994; Wiser 1974);
Some participants received antibiotic therapy and others did not: three trials. It was not reported which participants received antibiotic (Halleberg 2013; Millet 2012; Piergiovanni 1991);
Participants stratified according to antibiotic use: one trial (Barents 1978).
We give more information about antibiotic therapy during the trials in Table 6.
3. Use of antibiotic prophylaxis.
| Study ID | Comparison | With or without antibiotic prophylaxis | Details |
| Ahmed 1993 | 1 | Without | Routine prophylactic antibiotics were not used in either group |
| Andersen 1985 | 1 | Not reported | Not reported |
| Baan 2003 | 1 | With | Prophylactic antibiotics were used in all participants perioperatively for 24 hours |
| Barents 1978 | 1 | Both | Results were stratified according to antibiotic prophylaxis or not. It was not reported whether the prophylactic protocol was the same for all participants |
| Barry 1992 PE | 1 | Not reported | Not reported |
| Bergman 1987 | 1 | With | All participants received the same antibiotic prophylaxis (cefoxitin 2 g intramuscularly 1 hour before and 6 and 12 hours after surgery) |
| Botsios 1997 | 1 | Not reported | Not reported |
| Carpiniello 1988 | 2 | With | Prophylactic cefazolin sodium (Ancef) or clindamycin (Cleocin) until 3rd postoperative day |
| Dixon 2010 | 3 | Not reported | Not reported |
| Dobbs 1997 | 2 | With | Participants received Augmentin® at the induction of general anaesthetic and again 6 hours after surgery (1.2 g); it was not explicitly stated whether other antibiotics except prophylaxis were administered pre‐ or postoperatively |
| Evron 2008 | 2 | Not reported | Not reported if antibiotic prophylaxis used in study but women on antibiotics were excluded |
| Hakvoort 2011 | 2 | With | All participants received prophylactic antibiotics during surgery |
| Halleberg 2013 | 2 | Both | During surgery: cefuroxime, clindamycine, cloxacillin, No antibiotic prophylaxis |
| Hammarsten 1992 | 1 | With | Participants received pivmecillinam and pivampicillin if had no bacteriuria at time of operation as prophylaxis, or if had bacteriuria at time of operation was used as treatment. First dose was given 1 hour preoperatively, and last dose on the day the catheter was removed |
| Harms 1985 | 1 | Not reported | Not reported |
| Ichsan 1987 | 1 | Without | None of the participants who completed the trial received antibiotics |
| Jannelli 2007 | 3 | With | All participants received appropriate preoperative antibiotics |
| Katz 1992 | 1 | Not reported | Not reported |
| Kerr‐Wilson 1986 | 2 | Without | Antibiotic prophylaxis was not used |
| Knight 1996 | 2 | With | All participants received routine antibiotic prophylaxis (cefazolin) every 8 hours for 48 hours; it was not explicitly stated whether other antibiotics except prophylaxis were administered pre‐ or postoperatively |
| Korkes 2008 | 1 | Not reported | Not reported |
| Kringel 2010 | 1 | With | All participants received 2 g cefotiam i.v. before starting surgery as antibiotics prophylaxis |
| Michelson 1988 | 2 | With | Perioperative prophylactic antibiotic therapy (cephalosporin with or without gentamicin) was given to all participants. Participants requiring secondary Foley catheter received antibiotics while device was in place |
| Millet 2012 | 2 | Both | Some women received antibiotics during labour, some received antibiotics in postpartum period and some received no antibiotics |
| Naik 2005 | 3 | With | All women received a single dose of intraoperative antibiotics. Prophylactic antibiotics were not given at any other time in the study. Antibiotics were prescribed when clinically indicated, i.e. positive urine sample or positive SPC site swab |
| Nwabineli 1993 | 1 | With | All participants received the same antibiotic prophylaxis (a single dose of 5 g of methyl penicillin). Antibiotics were not administered routinely in the postoperative period |
| O'Kelly 1995 | 1 | Not reported | Not reported |
| Perrin 1997 | 1 | With | All participants received a single dose of tinidazole and/or ticarcillin |
| Piergiovanni 1991 | 1 | Both | Some participants received antibiotics (65% in each group) |
| Prasad 2014 | 1 | Not reported | Not reported |
| Rasmussen 1977 | 1 | With | Neomycin sulphate + bacitracin were given, 1.5 g every 6 hours 3 days before operation |
| Ratnaval 1996 | 1 | Not reported | Not reported |
| Rivard 2012 | 2 | Not reported | Not reported |
| Schiotz 1989 | 1 | Not reported | Not reported |
| Sethia 1987 | 1 | With | All participants received the same antibiotic prophylaxis (single dose of metronidazole 500 mg and cephadrine 1 g intravenously on induction of anaesthesia). These antibiotics were continued for 48 hours in high‐risk participants and 5 days when sepsis was already present |
| Skelly 1992 | 2 | Not reported | Not reported |
| Stekkinger 2011 | 1 | With | All women received a single dose of prophylactic antibiotics (cefazolin 1 g and metronidazole 500 mg) during surgery |
| Tang 2006 | 2 | Not reported | Not reported |
| Tangtrakul 1994 | 2 | Without | No participants received prophylactic antimicrobial drug |
| Van den Brand 2001 | 2 | With | 1 dose of cefazolin, 1 g, intravenously immediately before surgery; no postoperative antibiotics were used |
| Vandoni 1994 | 1 | With | Identical single‐dose pre‐operative antibiotic prophylaxis was routinely applied (2 g of cefacetrile and 500 mg of metronidazole) |
| Wiser 1974 | 1 | Without | Did not use antibiotic prophylaxis |
SPC: suprapubic catheter
Interventions
The trials compared a number of different routes of catheterisation:
Indwelling: 39 trials (Ahmed 1993; Andersen 1985; Baan 2003; Barents 1978; Barry 1992 PE; Bergman 1987; Botsios 1997; Carpiniello 1988; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Harms 1985; Ichsan 1987; Katz 1992; Kerr‐Wilson 1986; Knight 1996; Korkes 2008; Kringel 2010; Michelson 1988; Millet 2012; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Rivard 2012; Schiotz 1989; Sethia 1987; Skelly 1992; Stekkinger 2011; Tang 2006; Tangtrakul 1994; Van den Brand 2001; Vandoni 1994; Wiser 1974);
Suprapubic: 28 trials (Ahmed 1993; Andersen 1985; Baan 2003; Barents 1978; Barry 1992 PE; Bergman 1987; Botsios 1997; Dixon 2010; Hammarsten 1992; Harms 1985; Ichsan 1987; Jannelli 2007; Katz 1992; Korkes 2008; Kringel 2010; Naik 2005; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Schiotz 1989; Sethia 1987; Stekkinger 2011; Vandoni 1994; Wiser 1974);
Intermittent: 17 trials (Carpiniello 1988; Dixon 2010; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Jannelli 2007; Kerr‐Wilson 1986; Knight 1996; Michelson 1988; Millet 2012; Naik 2005; Rivard 2012; Skelly 1992; Tang 2006; Tangtrakul 1994; Van den Brand 2001).
We describe the interventions in detail in Table 7.
4. Interventions.
| Study ID | Gender of Participants | Intervention A | Intervention B |
| Ahmed 1993 | Men only | Indwelling urethral catheterisation placed preoperatively using 1& Xylocaine gel under aseptic technique | Suprapubic catheter (Stamey‐type, 12 French or 14 French), placed preoperatively under local anaesthetic |
| Andersen 1985 | Women only | Indwelling urethral catheterisation (Charriere16, Foley) inserted preoperatively | Suprapubic catheter (Charriere 12, Ingram) introduced after termination of the operation |
| Baan 2003 | Men and women | Indwelling urethral catheter (Foley) placed before surgery after surgical scrub | Suprapubic catheter (Braun) placed at the time of surgery |
| Barents 1978 | Women only | Indwelling urethral catheter (Silicath Foley) introduced after termination of the operation | Suprapubic catheter (12 Charriere, Silastic Cystocath) introduced perioperatively or after termination of the operation |
| Barry 1992 PE | Men and women | Indwelling urethral catheterisation. Inserted at induction | Suprapubic catheterisation. Inserted at laparotomy |
| Bergman 1987 | Women only | Indwelling urethral catheterisation (14 F Foley) introduced before surgery | Suprapubic catheterisation (5F Bonnano) introduced after termination of the operation |
| Botsios 1997 | Men and women | Indwelling urethral catheterisation (14‐ or 16‐french Foley) introduced after induction of anaesthesia | Suprapubic catheterisation (Cystofix B) introduced intraoperatively |
| Carpiniello 1988 | Women only | Indwelling urethral catheter (Foley) placed preoperatively and maintained for 24 hours | Intermittent catheter performed in recovery room |
| Dixon 2010 | Women only | Indwelling suprapubic catheter inserted in theatre, left on free drainage for 48 hours postoperatively | Intermittent catheterisation postoperatively if unable to pass urine within 6 hours of return from theatre or earlier if uncomfortable or if passing frequent (< 2‐hourly), small volumes of urine (< 200 ml). Continued until can void > 200 ml with post‐void residual volumes < 100 ml |
| Dobbs 1997 | Women only | Indwelling urethral catheter (14 F, Foley) inserted under anaesthetic and removed the night after surgery (about 36 hours after operation). In case of urinary retention thereafter, a urethral catheter was inserted for a further 24 hours | Intermittent catheterisation: 'In‐out' catheterisation with a disposable female catheter. Participants who felt the need to pass urine but were unable to do so, or had not passed urine by 12 hours after surgery, had a further IC. When, thereafter, participants required IC again, a urethral catheter was inserted for 24 hours |
| Evron 2008 | Women only | Indwelling urethral catheterisation (multi‐orifice Foley catheter) placed 90 minutes after epidural induction (average 3 cm cervical dilation) and removed after delivery | Intermittent catheterisation (multi‐orifice Foley catheter) placed 90 minutes after epidural induction (average 6 cm cervical dilation) and removed. Process repeated when clinical indication of urinary retention |
| Hakvoort 2011 | Women only | Indwelling urethral (14 french silicone) catheter was inserted by nursing staff for 3 days on first postoperative day if PVR ≥ 150 ml | Intermittent A SpeediCath® (Coloplast, Humlebaek, Denmark) catheter was inserted with maximum interval 6 hours over 3 days on first postoperative day if PVR ≥ 150 ml |
| Halleberg 2013 | Men and women | Indwelling Foley catheter inserted by registered nurses (RNs) or assistant nurses (ANs). Participants with hip fracture had catheter inserted upon arrival on orthopaedic ward. Participants with osteoarthritis were given the catheter in the morning on the day of the surgery | Intermittent catheterisation introduced if participant was unable to urinate and bladder scan indicated ≥ 400 ml urine in the bladder |
| Hammarsten 1992 | Men only | Indwelling urethral catheter (either teflon‐ or PVC‐coated) | Suprapubic catheter (PVC) |
| Harms 1985 | Women only | Indwelling urethral catheterisation (14 Charriere Foley) introduced after termination of the operation | Suprapubic catheterisation (Cystofix) |
| Ichsan 1987 | Men and women | Indwelling urethral catheter inserted by members of nursing staff. Urine sample obtained every 2 days until catheter removed for bacteriological culture, organism count + repeat specimens | Suprapubic catheter inserted by resident medical officers. Urine sample obtained every 2 days until catheter removed for bacteriological culture, organism count + repeat specimens |
| Jannelli 2007 | Women only | Bonanno suprapubic catheter placed intraoperatively | CISC (14French disposable vinyl catheter) started on 1st postoperative day. (16 FRench silicone Foley catheter placed intraoperatively to monitor urine output in the immediate postoperative period) |
| Katz 1992 | Men only | Indwelling urethral catheterisation (12F silicone‐coated or Teflon‐coated Foley catheter lubricated with paraffin oil) in the operating room after anaesthetic or after surgery completion | Suprapubic catheterisation (8F Cystocath manufactured by Dow Corning Corporation) in the operating room after completion of surgery |
| Kerr‐Wilson 1986 | Women only | Indwelling urethral catheterisation (Foley catheter) inserted immediately before surgery after epidural had been inserted. Removed once the participant was ambulant. | Intermittent catheterisation ‘in‐out’ (Nelaton catheter) inserted immediately before surgery after epidural had been inserted. Removed at the end of operation |
| Knight 1996 | Men and women | Indwelling urethral catheter (Foley) placed just prior to surgery. Remained in place for 48 hours. Thereafter, urinary retention was treated with intermittent catheterisation | Intermittent catheterisation every 6 hours if participants were unable to void or were voiding in volumes of 50 ml or less |
| Korkes 2008 | Men only | Discharged with indwelling urethral catheter following surgery | Discharged with suprapubic catheter following surgery |
| Kringel 2010 | Women only | Indwelling urethral catheter (silicone Foley) placed intraoperatively left indwelling for 24 or 96 hours | Suprapubic catheter (silicone Foley) placed intraoperatively left for 96 hours |
| Michelson 1988 | Men and women | Indwelling urethral catheter inserted just before surgery. Removed the morning after surgery. Urinary retention was treated with intermittent catheterisation following this. If retention continued > 48 hours, indwelling catheter was inserted again | Intermittent catheterisation performed postoperatively by nursing staff only if urinary retention occurred. Performed at least every 6 hours. If retention continued > 48 hours, indwelling catheter was inserted |
| Millet 2012 | Women only | Indwelling Foley catheter (14 French Bard Foley tray, with Bardex Lubricath, anti‐reflux chamber drainage bag, and EZ lock sampling port) inserted after epidural placement. Removed in the 2nd stage of labour at the start of pushing | Intermittent catheter (Bard™ urethral catheterisation tray and 15Fr red, rubber catheter) every 4 hours and as needed after epidural placement. Stopped at delivery |
| Naik 2005 | Women only | Suprapubic catheterisation. Insertion of Bonanno suprapubic catheter (Becton Dickenson, Franklin Lakes, New Jersey, USA) at the time of surgery. On free drainage for 5 days. Woman asked to pass urine normally every 4 hrs, then measure residual volume using catheter. Catheter was removed when residual volume < 100 ml | Intermittent catheterisation. Transurethral indwelling catheter was inserted at the time of surgery. Removed on day 5, women would pass urine every 4 hours then measure residual volume using intermittent catheter. Intermittent catheterisation ceased when residual volume < 100 ml. (hydrophilic coated LoFric – Astra Tech Ltd, Stroudwater Business Park, Stonehouse) |
| Nwabineli 1993 | Women only | Indwelling urethral catheterisation placed before operation | Suprapubic catheterisation introduced after termination of the operation |
| O'Kelly 1995 | Men and Women | Indwelling urethral catheterisation (14‐Fr; Foley) before operation | Suprapubic catheterisation (14‐Fr; Foley) after the abdomen was opened |
| Perrin 1997 | Men and women | Indwelling urethral catheterisation (16‐French; Foley) inserted following induction of anaesthesia | Suprapubic catheterisation (16‐French; Foley) inserted after the opening of the abdomen |
| Piergiovanni 1991 | Men and women | Indwelling urethral catheterisation (Charriere 12 to 20; Foley ) | Suprapubic catheterisation (Charriere 10; Cystofix) |
| Prasad 2014 | Men only | Indwelling urethral catheter placed intraoperatively, removed on postoperative day 7 | Suprapubic catheter placed 24 hours after surgery, removed on postoperative day 7. Had indwelling urethral catheter prior to this |
| Rasmussen 1977 | Men and women | Indwelling urethral catheter (Foley, No. 16 French) was inserted before surgery and kept on during first 24 hours. After this was closed and opened every 6 hours. Removed on 5th day. | Suprapubic catheter (No. 5 French polyethylene tube) was inserted before surgery and drained continuously for 24 hours. After this, was opened and closed for 10 minutes every 6 hours. Removed when post‐voidal volume < 100 ml during each of 2 subsequent measurements |
| Ratnaval 1996 | Men only | Indwelling urethral catheter placed during surgery. Removed based on participant well‐being | Suprapubic Bonanno catheter placed at end of surgery. When suprapubic catheter was going to be removed, it was clamped and the residual volume measured. If it was < 50 ml, the catheter was removed |
| Rivard 2012 | Women only | Indwelling urethral catheter. Removed during 2nd stage of labour when woman started pushing | Intermittent catheter inserted every 2 to 4 hours |
| Schiotz 1989 | Women only | Indwelling urethral catheter (No.14, Foley) introduced at the end of surgery | Suprapubic catheter (No.10, Cystofix) introduced at the end of surgery |
| Sethia 1987 | Men and women | Indwelling urethral catheterisation (14 F Foley) inserted immediately before operation | Suprapubic catheterisation (14 F Foley) placed perioperatively |
| Skelly 1992 | Men and women | Indwelling urethral catheter inserted preoperatively and left in place until 48 hours after surgery. If could not void in following 24 hours, intermittent catheterisation performed every 8 hrs for 24 hrs. If still not able to void indwelling catheter inserted again for 48 hours | Intermittent catheter inserted every 6 ‐ 8 hrs, with 400 ‐ 600 ml of urine removed each time. Catheterisation stopped when residual volume of urine < 150 ml on 2 consecutive occasions |
| Stekkinger 2011 | Women only | Indwelling urethral catheterisation using 14 French (brand not specified) placed intraoperatively, removed postoperative day 3; measurements begun in morning of postoperative day 4 | Suprapubic catheterisation using 15Fr Cystofix™ SPT catheter, sutured to participant's skin (B. Braun Medical, Oss, Netherlands) placed intraoperatively, clamped on the 3rd night after surgery |
| Tang 2006 | Women only | Indwelling Foley catheter, placed after randomisation. Removed at least once weekly, replaced if PVR > 300 ml. | CISC, monitored by bladder scan 3 times a day. CISC performed when PVR > 500 ml or > 300 ml and symptomatic. |
| Tangtrakul 1994 | Women only | Indwelling urethral catheter placed just before operation. Removed following day after operation | Intermittent catheterisation. Catheterised just before operation. Removed immediately after operation |
| Van den Brand 2001 | Men and women | Indwelling urethral catheter (Foley) introduced in the operating room just before the start of surgery. Catheter remained in place for 48 hours | In‐out catheterisation every 6 hours or earlier when clinically needed by a trained staffed nurse until spontaneous voiding occurred |
| Vandoni 1994 | Men and women | Indwelling urethral catheterisation (Charriere 12 latex Foley catheter) | Suprapubic catheterisation (Cystofix(R), Braun‐SSC, Switserland) |
| Wiser 1974 | Women only | Indwelling urethral catheterisation (16 Foley) inserted postoperatively | Suprapubic catheterisation (16 Foley) |
CISC: clean intermittent self catheterisation PVR: post‐void residual
Indication for catheter removal
Only some of the included trials reported an indication or day that the urinary catheter should be removed.
Fourteen trials did not indicate when the catheter should be removed (Ahmed 1993; Baan 2003; Barents 1978; Barry 1992 PE; Evron 2008; Harms 1985; Ichsan 1987; Jannelli 2007; Katz 1992; Ratnaval 1996; Rivard 2012; Sethia 1987; Tang 2006; Vandoni 1994). Among the trials that did report an indication for catheter removal, they were either based on clinical indication, on timing, or on a mixture of the two.
Fourteen trials removed the catheter at a specific time point (Bergman 1987; Botsios 1997; Carpiniello 1988; Dobbs 1997; Hakvoort 2011; Knight 1996; Korkes 2008; Kringel 2010; Perrin 1997; Piergiovanni 1991; Prasad 2014; Schiotz 1989; Tangtrakul 1994; Wiser 1974), ranging from immediately after surgery (Carpiniello 1988; Dobbs 1997; Tangtrakul 1994) to seventh postoperative day (Korkes 2008; Prasad 2014).
Seven trials removed catheters based on clinical indication (Dixon 2010; Halleberg 2013; Hammarsten 1992; Kerr‐Wilson 1986; Millet 2012; O'Kelly 1995; Stekkinger 2011). The clinical indications included post‐void residual volume < 150 ml (Halleberg 2013; Stekkinger 2011), or during the second stage of labour (Millet 2012).
The remaining seven trials combined a specific time point for clamping or trialing removal of the catheter and clinical indication.
Outcomes
The trials used a number of different ways to define microbiological outcomes. Eight trials were classified as reporting symptomatic UTI (Baan 2003; Barry 1992 PE; Dixon 2010; Hakvoort 2011; Korkes 2008; Kringel 2010; Schiotz 1989; Tang 2006). We used the following definitions:
≥ 10⁵ cfu/ml and at least one symptom of UTI (e.g. fever, suprapubic tenderness, dysuria) (Baan 2003; Dixon 2010; Hakvoort 2011; Kringel 2010; Schiotz 1989);
> 10⁴ cfu/ml and leucocituria with at least one sign or symptom (e.g. fever, malaise, haematuria, pain, etc.) (Korkes 2008)
Fever in the absence of other sites of infection (Tang 2006);
No definition (Barry 1992 PE). The only report of this trial that we could find was an abstract. It is unsurprising, therefore, that no definition of symptomatic UTI was supplied. We assumed that the trial used an appropriate definition.
The time that the urine sample was taken for diagnosis of symptomatic UTI also varied between trials. The urine samples were taken at the following times:
Preoperatively (Dixon 2010; Schiotz 1989);
At catheter removal (Hakvoort 2011; Schiotz 1989);
Sample taken based on clinical symptoms (Dixon 2010);
-
Postoperatively:
daily (Barry 1992 PE);
4th postoperative day (Kringel 2010);
1st and 14th day of hospitalisation (Tang 2006)
48 hours after catheter removal (Baan 2003)
Follow‐up: 6 to 8 weeks postoperatively (Schiotz 1989)
We describe the methods of measuring symptomatic UTI in the trials in Table 8.
5. Measurement of symptomatic urinary tract infection.
| Study ID | Outcome as defined by trialists | Definition | When urine sample was taken | Outcome as defined by IDSA criteria |
| Baan 2003 | UTI | ≥ 1 clinical symptoms (fever, increased micturition frequency, burning pain during voidance, pain in lower abdomen); positive sediment (> 10 leukocytes); positive urine culture of > 10⁵ bacterial colonies + < 3 bacterial species | 48 hours after catheter removal | Symptomatic UTI |
| Barry 1992 PE | UTI | No definition | Daily until catheter removal | Unknown, so collect data assuming symptomatic UTI |
| Dixon 2010 | UTI | Catheter specimen of urine or a midstream urine specimen showing a single bacterium growing at a colony count of > 105 cfu/ml. Specimen only taken if UTI suspected on the basis of: pyrexia > 37.5° C, frequent voiding + discomfort when passing urine and positive urinalysis for leukocytes + nitrites | Preoperatively, and postoperatively if UTI suspected | Symptomatic UTI |
| Hakvoort 2011 | UTI | > 10⁵ cfu/ml + ≥ 1 of the following: fever, urinary frequency (> 7 voids/day), dysuria, lower abdominal pain | After PVR had normalised and catheterisation had stopped | Symptomatic UTI |
| Korkes 2008 | UTI | No definition | No information | Unknown, so collect data assuming symptomatic UTI |
| Kringel 2010 | Symptomatic UTI | CDC definition: Indwelling urinary catheter was in place for > 2 calendar days on the date of event, with day of device placement being Day 1, AND an indwelling urinary catheter was in place on the date of event or the day before. If an indwelling urinary catheter was in place for > 2 calendar days and then removed, the UTI criteria must be fully met on the day of discontinuation or the next day. Patient has at least one of the following signs or symptoms: fever (> 38.0° C), suprapubic tenderness, costovertebral angle pain or tenderness, urinary urgency, urinary frequency, dysuria. Patient has a urine culture with no more than 2 species of organisms, at least one of which is a bacteria of ≥ 10⁵ cfu/ml. | 4th postoperative day | Symptomatic UTI |
| Ratnaval 1996 | UTI | No definition | Prior to removal of catheter and if participants developed urinary symptoms after catheter removal | Unknown, so collect data assuming symptomatic UTI |
| Schiotz 1989 | UTI | Bacteriuria > 105 organisms/ml AND ≥ 1 of following: dysuria, pain, fever (rectal temp > 38.5° C measured twice), rigors, sepsis | Preoperatively, at catheter removal and at follow‐up 6 ‐ 8 weeks postoperatively | Symptomatic UTI |
| Tang 2006 | symptomatic UTI | Fever in the absence of other sites of infection with or without symptoms of dysuria or suprapubic discomfort on day 14 | 1st and 14th days | Symptomatic UTI |
PVR: post‐void residual
Twenty trials reported asymptomatic bacteriuria. However, an additional 13 trials that said they were reporting UTI were actually reporting asymptomatic bacteriuria (without clinical symptoms), and we reclassified them. Thirty‐three trials reported asymptomatic bacteriuria, using the following definitions:
≥ 10³ cfu/ml (Bergman 1987; Vandoni 1994);
≥ 10⁴ cfu/ml (Perrin 1997; Piergiovanni 1991; Sethia 1987; Stekkinger 2011; Wiser 1974). One of these trials used a count of > 10⁴ but < 10⁵ cfu/ml as not being diagnostic of bacteriuria (Wiser 1974). Another trial used > 10⁴ cfu/ml to define bacteriuria in participants who still had a catheter present, on the basis that a smaller density of bacteria may be significant (Sethia 1987);
≥ 10⁵ cfu/ml (Ahmed 1993; Andersen 1985; Baan 2003; Barents 1978; Botsios 1997; Carpiniello 1988; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Harms 1985; Jannelli 2007; Kerr‐Wilson 1986; Knight 1996; Kringel 2010; Michelson 1988; Millet 2012; Nwabineli 1993; O'Kelly 1995; Rasmussen 1977; Sethia 1987; Skelly 1992; Tang 2006; Tangtrakul 1994; Van den Brand 2001; Wiser 1974);
No definition (Naik 2005; Ratnaval 1996). No definition for bacteriuria was provided. The outcome was named "positive CSU (catheter specimen of urine)/MSU (midstream of urine)" by Naik 2005. Whereas Ratnaval 1996 reported using culture positive urine samples for diagnosing bacteriuria. We assumed the trialists used an appropriate bacterial culture level.
The time that the urine sample was taken also varied greatly between trials:
At time of catheter insertion (Ahmed 1993; Kerr‐Wilson 1986; Millet 2012; Perrin 1997; Piergiovanni 1991; Rasmussen 1977; Sethia 1987);
At time of catheter removal (Ahmed 1993; Barents 1978; Hakvoort 2011; Kerr‐Wilson 1986; Perrin 1997; Piergiovanni 1991; Stekkinger 2011);
Day of discharge (Halleberg 2013; Millet 2012);
-
Postoperatively:
daily (Nwabineli 1993; O'Kelly 1995; Sethia 1987; Vandoni 1994);
every 2 days (Bergman 1987);
2nd postoperative day (Dobbs 1997; Jannelli 2007; Knight 1996; Michelson 1988; Van den Brand 2001);
3rd postoperative day (Carpiniello 1988; Naik 2005; Tangtrakul 1994);
4th postoperative day (Kringel 2010; Wiser 1974);
5th postoperative day (Andersen 1985; Barents 1978; Knight 1996; Naik 2005; Rasmussen 1977; Skelly 1992);
6th postoperative day (Harms 1985);
7th postoperative day (Barents 1978; Carpiniello 1988; Jannelli 2007; Michelson 1988; Naik 2005);
14th postoperative day (Naik 2005);
21st postoperative day (Naik 2005);
two days after catheter removal (Baan 2003; Botsios 1997; O'Kelly 1995; Sethia 1987)
-
At follow‐up:
four weeks (Halleberg 2013);
six weeks (Ahmed 1993);
three months (Rasmussen 1977).
We describe the methods of measuring asymptomatic bacteriuria in the trials in Table 9.
6. Measurement of asymptomatic bacteriuria.
| Study ID | Outcome as defined by trialists | Definition | When urine sample was taken | Outcome as defined by IDSA criteria |
| Ahmed 1993 | bacteriuria | urine culture with bacterial count > 10⁸ colonies/millilitre | At time of catheterisation and repeated if delay in the operation; at the time of catheter removal, and at 6 weeks follow‐up if symptomatic of UTI. | bacteriuria |
| Andersen 1985 | postoperative urinary infection | “Postoperative urinary infection was defined as significant bacteriuria (>100000 colony‐forming units per ml urine)” | 5th postoperative day (catheter removed by POD 5) | bacteriuria |
| Baan 2003 | postoperative culture | positive urine culture (> 10⁵ bacterial colonies + < 3 bacterial species) within 6 weeks of surgery) | 48 hours after catheter removal | bacteriuria |
| Barents 1978 | significant bacteriuria | ≥ 10⁵ micro‐organism/ml | 5th and 7th postoperative day, and on day of catheter removal | bacteriuria |
| Bergman 1987 | bacteriuria | > 1000 colonies per millimetre by 5th postoperative day | Before surgery, and every 2 days thereafter | bacteriuria |
| Botsios 1997 | bacteriuria | Culture growing >10⁵ organisms/ml was considered positive | 2 days after catheter removal | bacteriuria |
| Carpiniello 1988 | positive postoperative urine culture | Positive urine culture – 105 colonies/millilitre | Preoperatively and in the 1st postoperative week, after the cessation of antibiotics on 3rd postoperative day | bacteriuria |
| Dobbs 1997 | urinary tract bacteriuria/infected MSU | on 2nd postoperative day, positive culture > 10⁵ organisms/µL | 2nd postoperative day | bacteriuria |
| Evron 2008 | postpartum urinary infection | ≥ 105 colonies of same species of bacteria per ml of urine found in 2 consecutive specimens of midstream voided urine at 24 hours and 48 hours | 24 hours and 48 hours | bacteriuria |
| Hakvoort 2011 | significant bacteriuria | > 10⁵ colony‐forming units in a culture | After PVR had normalised and catheterisation had stopped | bacteriuria |
| Halleberg 2013 | nosocomial UTI | negative urine culture results at arrival + positive urine culture results at discharge (≥ 105 cfu/ml) with ≤ 2 species of organisms | Arrival at hospital and before discharge. If positive urine culture at discharge, sample taken 4 weeks after discharge | bacteriuria |
| Harms 1985 | significant bacteriuria | Significant bacteriuria was defined as 105 cfu/ml. Measured on 6th postoperative day | 6th postoperative day | bacteriuria |
| Jannelli 2007 | significant bacteriuria | significant bacteriuria was defined as > 105 cfu/ml on postoperative day 2 or 7 | 2nd and 7th postoperative day | bacteriuria |
| Kerr‐Wilson 1986 | significant bacteriuria | > 10⁵ organisms/ml with or without pus cells | At time of catheter insertion and catheter removal | bacteriuria |
| Knight 1996 | UTI | UTI defined as > 105 colonies of a predominant organism | 2nd and 5th postoperative day | bacteriuria |
| Kringel 2010 | asymptomatic bacteriuria | (CDC definition) a positive urine culture of ≥ 10⁵ cfu/ml with no more than 2 species of uropathogen micro‐organisms AND, a positive blood culture with at least 1 matching uropathogen micro‐organism to the urine culture, or at least 2 matching blood cultures drawn on separate occasions if the matching pathogen is a common skin commensal. Elements of the criterion must occur within a timeframe that does not exceed a gap of 1 calendar day between 2 adjacent elements. | 4th postoperative day | bacteriuria |
| Michelson 1988 | UTI/bacteriuria ‐ve preoperative culture | > 10⁵ cfu/ml | 2nd and 7th postoperative day | bacteriuria |
| Millet 2012 | bacteriuria (CDC definition) | Participant with indwelling catheter within 7 days before culture and 1 urine culture with 105 cfu/ml with ≤ 2 species OR Participant without indwelling catheter within 7 days before culture and 2 urine cultures with 105 cfu/ml of same organism with ≤ 2 species, AND no fever (38° C), dysuria, urgency, frequency, or suprapubic tenderness. Clean‐catch, catheter, aspiration (no catheter tips, not from a bag) | As soon after epidural insertion as possible, and the day of discharge | bacteriuria |
| Millet 2012 | bacteriuria (IDSA definition) | Clean‐catch voided urine in women: 2 consecutive voided specimens with isolation of the same bacterial strain in counts of ≥ 105 cfu/ml. Clean‐catch voided urine in men: single voided specimen with 1 species in counts of ≥ 105 cfu/ml. Catheterised urine in women and men: single catheterised specimen with 1 species in counts of ≥ 100 cfu/ml. | As soon after epidural insertion as possible, and the day of discharge | bacteriuria |
| Naik 2005 | bacteriuria | "positive CSU/MSU rate" ‐ no further definition | 3rd, 5th, 7th, 14th and 21st postoperative days | Unknown, so collect data assuming bacteriuria |
| Nwabineli 1993 | UTI | Bacterial count > 105 ml‐1 | Taken daily until the catheter was removed. Participants who were discharged with a catheter had specimens taken at every readmission for the trial of catheter removal | bacteriuria |
| O'Kelly 1995 | UTI | culture yielded greater than 10⁵ colony‐forming units per ml | Taken daily until the catheter was removed. Final sample taken 2 days after catheter removal | bacteriuria |
| Perrin 1997 | significant bacteriuria | > 10⁴ organisms per ml | At time of catheter insertion, if clinically indicated and at catheter removal | bacteriuria |
| Piergiovanni 1991 | UTI | > 10⁴ bacteria/ml in urine after 24 hours incubation | At time of catheter insertion and catheter removal | bacteriuria |
| Rasmussen 1977 | bacteriuria | > 10⁵/ml | At time of catheter insertion, 5 days later and 3‐month follow‐up | bacteriuria |
| Ratnaval 1996 | postoperative urinary tract infection | "culture positive urine samples" | Not reported | bacteriuria |
| Schiotz 1989 | bacteriuria | more than 105 cfu/ml | Specimens were obtained preoperatively, at catheter removal, at follow‐up 6 ‐ 8 weeks postoperatively and when clinically indicated | bacteriuria |
| Sethia 1987 | UTI | culture of midstream specimen collected within 48 hours of removal of catheter yielded ≥ 105 colony‐forming units/ml OR culture of catheter specimen yielded ≥ 104/ml as a smaller growth could be significant in a participant on continuous catheter drainage | At time of catheter insertion, daily thereafter and 2 days after catheter removal | bacteriuria |
| Skelly 1992 | urinary tract infection | ≥ 10⁵ cfu/ml | 5th postoperative day | bacteriuria |
| Stekkinger 2011 | UTI | > 10⁴ cfu/ml in culture | Before removing or clamping the catheter (3rd postoperative day) | bacteriuria |
| Tang 2006 | bacteriuria | Growth ≥ 10⁵ bacteria/ml on day 14 | Day 1 and day 14 | bacteriuria |
| Tangtrakul 1994 | urinary tract infection | ≥ 105 organisms/ml on 3rd postoperative day | 3rd postoperative day | bacteriuria |
| Van den Brand 2001 | UTI/bacteriuria | Postoperative bacteriuria or urinary tract infection was defined as positive urine sediment for bacteria or white blood cells with a positive urine culture of > 105 colonies | Day before surgery and 2nd postoperative day | bacteriuria |
| Vandoni 1994 | bacteriuria | > 10³ cfu/ml | Taken daily | bacteriuria |
| Wiser 1974 | not significant bacteriuria | > 10⁴ < 10⁵ cfu/ml | 4th postoperative day | bacteriuria |
| Wiser 1974 | significant bacteriuria | > 10⁵ cfu/ml | 4th postoperative day | bacteriuria |
MSU: midstream urine POD: postoperative day PVR: post‐void residual UTI: urinary tract infection
Excluded studies
We excluded 16 studies from the review, for the following reasons:
Design was not appropriate i.e. non‐randomised trials (Abrams 1980; Frymire 1971; Hofmeister 1970; Horgan 1992; Park 2010; Schumm 2008; Shapiro 1982)
Intervention was not relevant (Allardice 1988; Cardenas 2010; Dunn 2003; Ghalayini 2005)
Catheterisation was intended for long‐term use, defined as intended catheterisation more than 14 days (Chartier‐Kastler 2011; Grundy 1983; Sicilia 2013; Suprasert 2002; Turi 2006)
We detail further characteristics in the Characteristics of excluded studies table.
Newly‐excluded trials
We have excluded from this update one trial that was included in the previous version of this review (Shapiro 1982). The trial quasi‐randomised participants with postoperative urinary retention. Those who required catheterisation for any other reason were given indwelling urethral catheterisation. The results were not separated into participants who were quasi‐randomised and those who were not, so the trial is now excluded.
Risk of bias in included studies
We give details of the quality of each individual trial in the table of Characteristics of included studies. The 'Risk of bias' graph and 'Risk of bias' summary figures also give further information (Figure 2; Figure 3).
2.
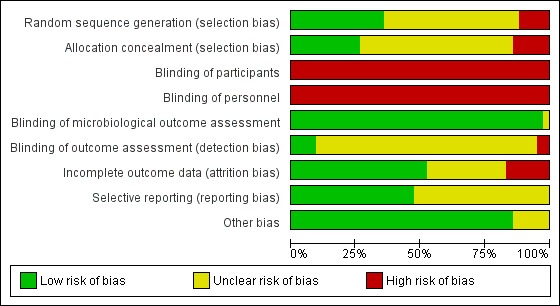
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
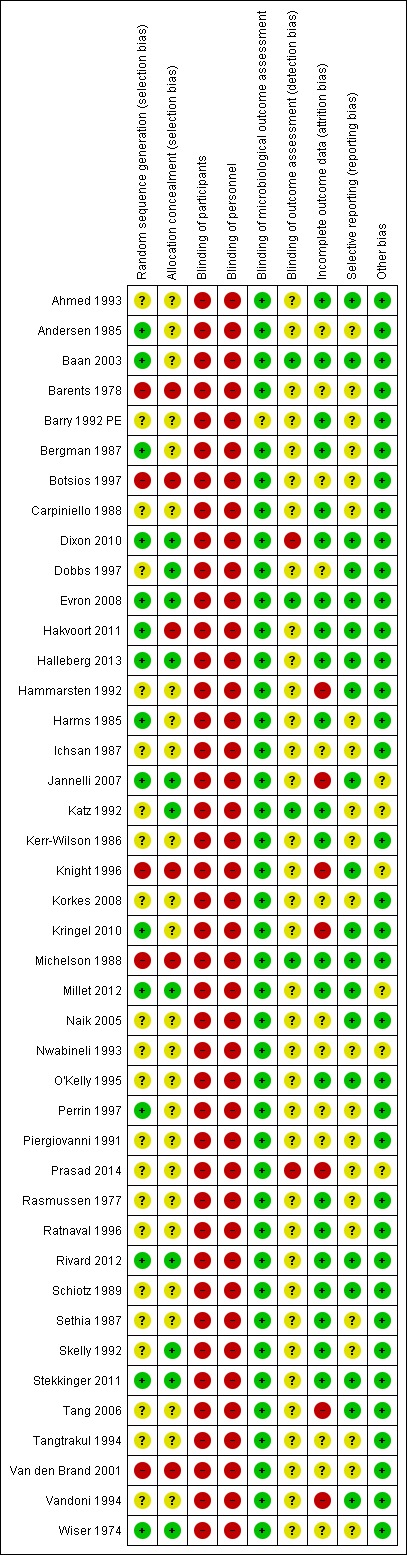
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Random sequence generation was adequate (low risk of bias) in 15 trials (Andersen 1985; Baan 2003; Bergman 1987; Dixon 2010; Evron 2008; Hakvoort 2011; Halleberg 2013; Harms 1985; Jannelli 2007; Kringel 2010; Millet 2012; Perrin 1997; Rivard 2012; Stekkinger 2011; Wiser 1974).
Five trials had an inadequate method of random sequence generation (high risk of bias), as they used quasi‐randomisation (Barents 1978; Botsios 1997; Knight 1996; Michelson 1988; Van den Brand 2001).
The remaining 22 trials had insufficient information to assess the method of random sequence generation (unclear risk of bias) (Ahmed 1993; Barry 1992 PE; Carpiniello 1988; Dobbs 1997; Hammarsten 1992; Ichsan 1987; Katz 1992; Kerr‐Wilson 1986; Korkes 2008; Naik 2005; Nwabineli 1993; O'Kelly 1995; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Schiotz 1989; Sethia 1987; Skelly 1992; Tang 2006; Tangtrakul 1994; Vandoni 1994).
Allocation concealment
We deemed 11 trials to have adequate allocation concealment (low risk of bias) (Dixon 2010; Dobbs 1997; Evron 2008; Halleberg 2013; Jannelli 2007; Katz 1992; Millet 2012; Rivard 2012; Skelly 1992; Stekkinger 2011; Wiser 1974).
Six trials had inadequate allocation concealment (high risk of bias) (Barents 1978; Botsios 1997; Hakvoort 2011; Knight 1996; Michelson 1988; Van den Brand 2001).
The remaining 25 trials had insufficient information to judge allocation concealment (unclear risk of bias) (Ahmed 1993; Andersen 1985; Baan 2003; Barry 1992 PE; Bergman 1987; Carpiniello 1988; Hammarsten 1992; Harms 1985; Ichsan 1987; Kerr‐Wilson 1986; Korkes 2008; Kringel 2010; Naik 2005; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Schiotz 1989; Sethia 1987; Tang 2006; Tangtrakul 1994; Vandoni 1994)
Blinding
Blinding of participants
The review authors decided that blinding of participants to the route of catheterisation they received was not possible. We therefore rated all 42 included trials at high risk of bias, in accordance with the current recommendation of the Cochrane Bias Method Group.
Blinding of personnel
We deemed all 42 trials be high risk of bias, on the assumption that blinding was not possible when it was not reported.
Blinding of microbiological outcomes
We assumed that microbiological outcomes were assessed by a pathologist who was not aware of the route of catheterisation.
We rated 41 of the trials as being at low risk of bias for blinding of microbiological outcomes. (Ahmed 1993; Andersen 1985; Baan 2003; Barents 1978; Bergman 1987; Botsios 1997; Carpiniello 1988; Dixon 2010; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Harms 1985; Ichsan 1987; Jannelli 2007; Katz 1992; Kerr‐Wilson 1986; Korkes 2008; Knight 1996; Kringel 2010; Michelson 1988; Millet 2012; Naik 2005; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Rivard 2012; Schiotz 1989; Sethia 1987; Skelly 1992; Stekkinger 2011; Tang 2006; Tangtrakul 1994; Van den Brand 2001; Vandoni 1994; Wiser 1974).
One trial did not give a definition for UTI, so it was not possible to identify who assessed it. We therefore assigned it an unclear risk of bias (Barry 1992 PE).
Blinding of outcome assessment
Two trials reported no blinding of outcome assessment, so we judged them to be at high risk of bias (Dixon 2010; Prasad 2014).
Four trials reported blinding of the principal investigator or clinician assessing the participant, and we deemed them to be at low risk of bias (Baan 2003; Evron 2008; Katz 1992; Michelson 1988).
The remaining 36 trials did not report blinding of outcome assessment, and we assigned them an unclear risk of bias (Ahmed 1993; Andersen 1985; Barents 1978; Barry 1992 PE; Bergman 1987; Botsios 1997; Carpiniello 1988; Dobbs 1997; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Harms 1985; Ichsan 1987; Jannelli 2007; Kerr‐Wilson 1986; Knight 1996; Korkes 2008; Kringel 2010; Millet 2012; Naik 2005; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Rasmussen 1977; Ratnaval 1996; Rivard 2012; Schiotz 1989; Sethia 1987; Skelly 1992; Stekkinger 2011; Tang 2006; Tangtrakul 1994; Van den Brand 2001; Vandoni 1994; Wiser 1974).
Incomplete outcome data
We judged 22 trials to be at low risk of bias due to incomplete outcome data. In these trials there was either:
no dropouts (Baan 2003; Barry 1992 PE; Bergman 1987; Carpiniello 1988; Harms 1985; Kerr‐Wilson 1986; Ratnaval 1996; Skelly 1992; Stekkinger 2011); or,
no significant differential dropout. (Ahmed 1993; Dixon 2010; Evron 2008; Hakvoort 2011; Halleberg 2013; Katz 1992; Michelson 1988; Millet 2012; O'Kelly 1995; Rasmussen 1977; Rivard 2012; Schiotz 1989; Sethia 1987).
Seven trials had incomplete outcome data. Four of these trials had differential dropout between study arms (Hammarsten 1992; Jannelli 2007; Tang 2006; Vandoni 1994), one trial had a significant dropout rate (Knight 1996), one trial was ended prematurely due to interim analysis (Prasad 2014), and one trial had one study arm stopped prematurely (Kringel 2010).
Thirteen trials had insufficient information to assess incomplete outcome data (Andersen 1985; Barents 1978; Botsios 1997; Dobbs 1997; Ichsan 1987; Korkes 2008; Naik 2005; Nwabineli 1993; Perrin 1997; Piergiovanni 1991; Tangtrakul 1994; Van den Brand 2001; Wiser 1974).
Selective reporting
We assessed selective reporting without being able to access protocols, due to a lack of resources. Our assessments for selective reporting were based on the outcomes stated in the Methods, the results reported, and whether all expected outcomes were reported.
We rated 20 trials at low risk of bias, based on this assessment (Ahmed 1993; Baan 2003; Dixon 2010; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Jannelli 2007; Knight 1996; Kringel 2010; Michelson 1988; Millet 2012; Naik 2005; O'Kelly 1995; Rivard 2012; Schiotz 1989; Stekkinger 2011; Tang 2006; Vandoni 1994).
The remaining 22 trials did not have clear information in their Methods, and we therefore judged them to be at an unclear risk of bias (Andersen 1985; Barents 1978; Barry 1992 PE; Bergman 1987; Botsios 1997; Carpiniello 1988; Harms 1985; Ichsan 1987; Katz 1992; Kerr‐Wilson 1986; Korkes 2008; Nwabineli 1993; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Sethia 1987; Skelly 1992; Tangtrakul 1994; Van den Brand 2001; Wiser 1974).
Other potential sources of bias
Six trials had an unclear risk of bias:
Three trials excluded participants who did not receive the allocated intervention (Jannelli 2007; Knight 1996; Prasad 2014);
One trial did not use an ITT analysis i.e. two participants in group B received suprapubic catheter and one participant in group C had an indwelling urethral catheter introduced following surgery due to previous surgery in the lower abdomen (Katz 1992);
One trial deviated from their study protocol as mentioned in the Characteristics of included studies table (Millet 2012).
One trial was a "pilot study" (Nwabineli 1993)
The remaining 36 trials appeared to be free from other sources of bias, and we judged them to be at low risk (Ahmed 1993; Andersen 1985; Baan 2003; Barents 1978; Barry 1992 PE; Bergman 1987; Botsios 1997; Carpiniello 1988; Dixon 2010; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Hammarsten 1992; Harms 1985; Ichsan 1987; Kerr‐Wilson 1986; Korkes 2008; Kringel 2010; Michelson 1988; Naik 2005; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Rasmussen 1977; Ratnaval 1996; Rivard 2012; Schiotz 1989; Sethia 1987; Skelly 1992; Stekkinger 2011; Tang 2006; Tangtrakul 1994; Van den Brand 2001; Vandoni 1994; Wiser 1974).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Indwelling urethral catheterisation compared to suprapubic catheterisation for short‐term catheterisation in adults.
| Indwelling urethral catheterisation compared to suprapubic catheterisation for short‐term catheterisation in adults | ||||||
| Patient or population: Adults with short‐term catheterisation Settings: Hospital Intervention: indwelling urethral catheterisation Comparison: suprapubic catheterisation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Suprapubic catheterisation | Indwelling urethral catheterisation | |||||
| Number of participants with symptomatic UTI | Study population | RR 1.01 (0.61 to 1.69) | 575 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 121 per 1000 | 122 per 1000 (74 to 204) | |||||
| Asymptomatic bacteruria | Study population | RR 2.25 (1.63 to 3.10) | 2316 (19 studies) | ⊕⊝⊝⊝ very low4,5 | ||
| 125 per 1000 |
282 per 1000 (204 to 288) |
|||||
| Number of participants with pain | Study population | RR 5.62 (3.31 to 9.55) | 535 (4 studies) | ⊕⊕⊝⊝ low3,6 | ||
| 73 per 1000 | 413 per 1000 (243 to 701) | |||||
| Ease of use for participants ‐ not reported | Not estimable | ‐ | not reported | |||
| Quality of life ‐ not reported | Not estimable | ‐ | not reported | |||
| Cost utility analysis ‐ not reported | Not estimable | ‐ | not reported | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for study design (random sequence generation unclear in 3/5 trials; allocation concealment unclear in all 5 trials; participants and personnel not blinded) 2Downgraded two level for imprecision as 95% Confidence Interval is very wide (0.61 to 1.69) and crosses the line of no effect. 3Funnel plot cannot be used as there are fewer than 10 trials and the evidence was not down‐graded for publication bias.
4Downgraded two level for study design (random sequence generation unclear in 9/19 trials and high risk in 2/19 trials; allocation concealment unclear in 15/19 trials and high risk in 2/19 trials; participants and personnel not blinded)
5Downgraded one level for publication bias (There was some evidence of publication bias on interpretation of the funnel plot, as trials with low sample size or negative results were not represented) 6Downgraded two level for study design (Random sequence generation is unclear in 2/4 trials; and high risk in 1/4 trials in the meta‐analysis. Allocation concealment is unclear in 3/4 trials and judged to be high risk in 1/4 trials. Participants were not blinded)
Summary of findings 2. Indwelling urethral catheterisation compared to intermittent urethral catheterisation for short‐term catheterisation in adults.
| Indwelling urethral catheterisation compared to intermittent urethral catheterisation for short‐term catheterisation in adults | ||||||
| Patient or population: Patients with short‐term bladder drainage Settings: Hospital Intervention: indwelling urethral catheterisation Comparison: intermittent urethral catheterisation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intermittent urethral catheterisation | Indwelling urethral catheterisation | |||||
| Number of participants with symptomatic UTI | See comment | see comment | Not estimable | 162 (2 studies; not pooled) | ⊕⊝⊝⊝ very low1,2 | Due to evidence of significant clinical and statistical heterogeneity, we did not pool the results, which were inconclusive (Analysis 2.1). The main source of heterogeneity was the reason for hospitalisation:
|
| Asymptomatic bacteruria | 199 per 1000 |
207 per 1000 (169 to 255) |
RR 1.04 (0.85 to 1.28 | 143 (1610 studies) | ⊕⊝⊝⊝ very low3,4,5 | |
| Number of patients with pain ‐ not reported | Not estimable | ‐ | not reported | |||
| Ease of use for participants ‐ not reported | Not estimable | ‐ | not reported | |||
| Quality of life ‐ not reported | Not estimable | ‐ | not reported | |||
| Cost utility analysis ‐ not reported | Not estimable | ‐ | not reported | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for study design (Random sequence generation is unclear in 1/2 trials. Allocation concealment is unclear in 1 trial and judged to be high risk for the other. Participants and personnel not blinded.) 2Downgraded two level for inconsistency (see comment). 3Downgraded two level for study design (random sequence generation unclear in 6/13 trials and high risk in 3/13 trials; allocation concealment unclear in 4/13 trials and high risk in 4/13 trials; participants and personnel not blinded)
4Downgraded one level for imprecision as 95% Confidence Interval is wide (0.85 to 1.28) and crosses the line of no effect.
5Downgraded one level for publication bias (There was some evidence of publication bias on interpretation of the funnel plot, as trials with low sample size or negative results were not represented)
Summary of findings 3. Suprapubic catheterisation compared to intermittent urethral catheterisation for short‐term catheterisation in adults.
| Suprapubic catheterisation compared to intermittent urethral catheterisation for short‐term catheterisation in adults | ||||||
| Patient or population: patients with short‐term catheterisation Settings: Hospital Intervention: suprapubic catheterisation Comparison: intermittent urethral catheterisation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intermittent urethral catheterisation | Suprapubic catheterisation | |||||
| Number of participants with symptomatic UTI | Study population | RR 1.67 (0.68 to 4.10) | 72 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 167 per 1000 | 0 per 1000 (0 to 0) | |||||
| Asymptomatic bacteruria | Study population | |||||
| 359 per 1000 | 187 per 1000 (72 to 485) | RR 0.52 (0.20 to 1.35) | ⊕⊝⊝⊝ very low2,3,4,5 | |||
| Number of patients with pain | Not estimable | 72 (1 study) | ⊕⊝⊝⊝ very low6,7 | |||
| Ease of use for participants ‐ not reported | Not estimable | ‐ | not reported | |||
| Quality of life ‐ not reported | Not estimable | ‐ | not reported | |||
| Cost utility analysis ‐ not reported | Not estimable | ‐ | not reported | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded two level for imprecision as 95% CI very wide (0.68 to 4.10). 2Funnel plot cannot be used as there are fewer than 10 trials and the evidence was not down‐graded for publication bias.
3Downgraded one level for study design (random sequence generation and allocation concealment unclear in 1/2 trials; participants and personnel not blinded)
4Downgraded two level for imprecision as 95% Confidence Interval is very wide (0.20 to 1.35) and crosses the line of no effect.
5Downgraded one level for inconsistency (There was evidence of statistical heterogeneity as I2 value was 64%
6Downgraded one level for study design (Participants were not blinded and could influence the outcome of interest) 7 Downgraded two level for imprecision as 95% CI very wide (0.68 to 4.10).
Comparison 1: Indwelling urethral catheterisation compared with suprapubic catheterisation
Twenty‐five trials including 2622 participants addressed this comparison (Ahmed 1993; Andersen 1985; Baan 2003; Barents 1978; Barry 1992 PE; Bergman 1987; Botsios 1997; Hammarsten 1992; Harms 1985; Ichsan 1987; Katz 1992; Korkes 2008; Kringel 2010; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Prasad 2014; Rasmussen 1977; Ratnaval 1996; Schiotz 1989; Sethia 1987; Stekkinger 2011; Vandoni 1994; Wiser 1974). The sample size of these trials was small (the largest trial (Hammarsten 1992) included 344 participants). All the trials were RCTs, apart from one quasi‐RCT (Barents 1978). Two trials did not contribute to meta‐analysis (Ichsan 1987; Katz 1992). We summarise the quality of the evidence for the five most important outcomes in Table 1.
Symptomatic urinary tract infection
Five trials (575 participants) reported symptomatic UTI (Baan 2003; Barry 1992 PE; Korkes 2008; Kringel 2010; Schiotz 1989). There was insufficient evidence to determine if indwelling urethral or suprapubic catheterisation reduced symptomatic UTI (32/376, 8.5% versus 24/199, 12.1%: RR 1.01, 95% 0.61 to 1.69; Analysis 1.1). We assessed the quality of evidence to be very low (Table 1).
1.1. Analysis.
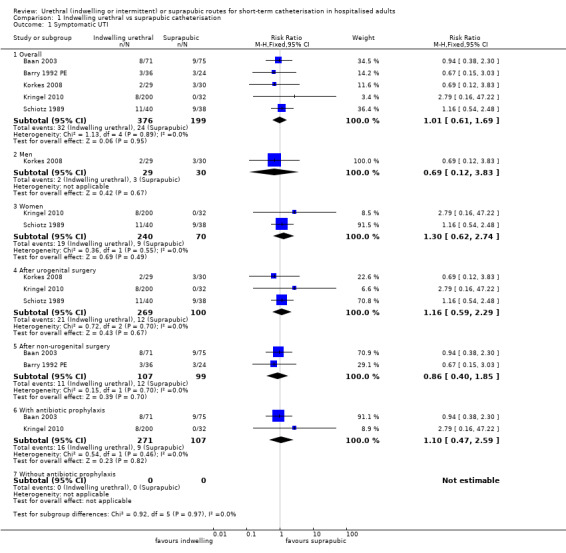
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 1 Symptomatic UTI.
We performed subgroup analyses comparing (a) men versus women, (b) participants undergoing urogenital surgery versus other surgery, and (c) two trials which used antibiotic prophylaxis. In both subgroups there was also no statistical difference in symptomatic UTI (Analysis 1.1.2,3,4,5,6,7). We also performed a sensitivity analysis by excluding Barry 1992 PE, as it did not have a definition for symptomatic UTI. There was no difference in the pooled effect when we excluded this trial , so it remains in the meta‐analysis.
Asymptomatic bacteriuria
In 15 of the 19 trials (1894 participants) asymptomatic bacteriuria was more common after indwelling urethral catheterisation; in nine of these the result was statistically significant. There was evidence of heterogeneity between trials (I² = 55%), so we compared the random‐effects and fixed‐effect models (fixed‐effect model, RR 2.47 (95% CI 2.04 to 3.00) and random‐effects model, RR 2.25 (95% CI 1.63 to 3.10)) (Analysis 1.2). We decided to use the random‐effects model as this would provide a more conservative estimate. We explored heterogeneity in subgroup analyses by gender, type of surgery, antibiotic prophylaxis use and time of collection of the urine sample (while catheterised or afterwards). These all favoured suprapubic catheterisation, although each subgroup analysis had very wide 95% CIs.
1.2. Analysis.
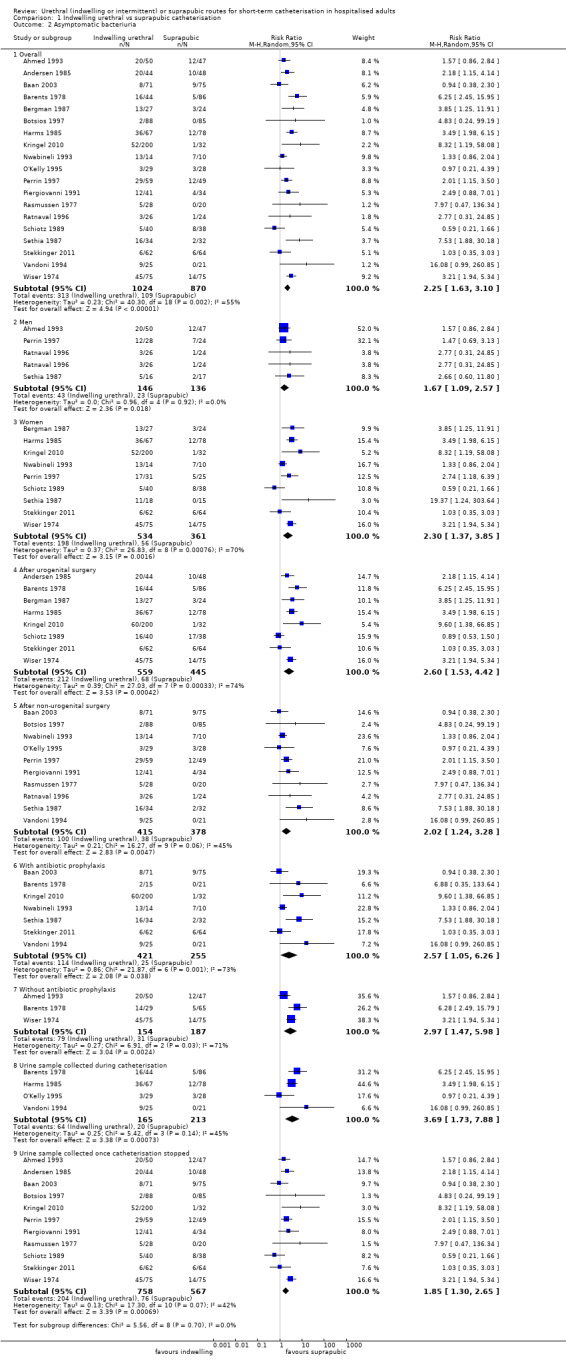
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 2 Asymptomatic bacteriuria.
Fewer participants with suprapubic catheters developed asymptomatic bacteriuria compared with indwelling urethral catheters (RR 2.25, 95% CI 1.63 to 3.10; Analysis 1.2) This is equivalent to about five patients being managed with a suprapubic catheter to avoid one case of bacteriuria (risk difference (RD) 0.19, 95% CI 0.10 to 0.27). As there were more than 10 trials included in this comparison, we used a funnel plot to assess publication bias. There was some evidence of publication bias on interpretation of the funnel plot, as trials with low sample size or negative results were not represented Figure 4.
4.
Funnel plot of comparison: 1 INDWELLING URETHRAL CATHETERISATION VS SUPRAPUBIC CATHETERISATION, outcome: 1.2 Asymptomatic bacteriuria.
Recatheterisation
Signficicantly more people needed to be re‐catheterised in the urethral group than in the suprapubic group (100/590, 17% versus 42/590, 7%). There was evidence of heterogeneity (I² = 58%), so we compared the fixed‐effect and random‐effects models (fixed‐effect model, RR 2.40 (95% CI 1.71 to 3.35) and random‐effects model, RR 2.21 (95% CI 1.19 to 4.09) (Analysis 1.3; 11 trials, 1180 participants)). We decided to use the random‐effects model, as this would provide a more conservative estimate.
1.3. Analysis.
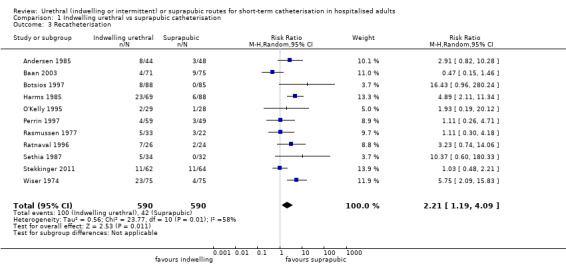
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 3 Recatheterisation.
Participants with a urethral catheter had a higher rate of recatheterisation than those with a suprapubic catheter overall (RR 2.21, 95% CI 1.19 to 4.09; Analysis 1.3). This is equivalent to about 11 patients having to receive a suprapubic catheter to avoid one patient needing to be recatheterised (RD 0.09, 95% CI 0.03 to 0.16).
As there were more than 10 trials included in this comparison, we used a funnel plot to assess publication bias. There was some evidence of publication bias on interpretation of the funnel plot, as trials with low sample size or negative results were not represented (Figure 5).
5.
Funnel plot of comparison: 1 INDWELLING URETHRAL CATHETERISATION VS SUPRAPUBIC CATHETERISATION, outcome: 1.3 Recatheterisation.
Duration of catheterisation
Data related to duration of catheterisation were available for 17 trials.
Two trials (274 participants) reported mean duration of catheterisation with standard deviations (SDs) (Hammarsten 1992; Schiotz 1989). From these two trials, participants managed with indwelling urethral catheterisation had a shorter duration of catheterisation compared with participants managed with suprapubic catheterisation, with a mean difference of ‐1.73 days, 95% CI ‐2.42 to ‐1.05; Analysis 1.4. The remaining 15 trials had insufficient data to include in a meta‐analysis (Analysis 1.5). Overall, the pattern does not consistently favour one route of catheterisation over the other.
1.4. Analysis.
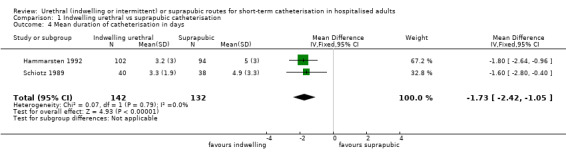
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 4 Mean duration of catheterisation in days.
1.5. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 5 Duration of catheterisation.
| Duration of catheterisation | ||
|---|---|---|
| Study | INDWELLING URETHRAL | SUPRAPUBIC |
| Ahmed 1993 | mean 8.0 days (n = 50) |
mean 8.3 days (n = 47) |
| Baan 2003 | median 5.9 days (n = 71) |
median 6.5 days (n = 75) |
| Barry 1992 PE | mean 5 days (n = 36) |
mean 5 days (n = 24) |
| Bergman 1987 | mean 6.8 days (n = 27) | mean 3.7 days (n = 24) |
| Botsios 1997 | mean 4.4 days (range 3 ‐ 11) (n = 88) | mean 3.3 days (range 3 ‐ 10) (n = 85) |
| Hammarsten 1992 | mean 5.0 (SE ± 3) days (n = 94) |
PVC mean 2.9 (SE ± 2) days Latex mean 3.2 (SE ± 3) days (n = 102) |
| Harms 1985 | mean 8.4 days (n = 69) | mean 6.7 days (n = 88) |
| Nwabineli 1993 | mean
16.5 days median, range 9 (7 ‐ 63) (n = 14) |
mean
13.1 days mean, range 11 (7 ‐ 26) (n = 10) |
| O'Kelly 1995 | median 4 days (range 2 ‐ 11) (n = 29) | median 5 days (range 4 ‐ 10) (n = 28) |
| Ratnaval 1996 | mean 7.5 days (range 2 ‐ 13) (n = 26) |
mean 7.2 days (3 ‐ 14) (n = 24) |
| Stekkinger 2011 | median 4.0 days (range 3 ‐ 18) (n = 62) |
median 4.0 days (range 2 ‐ 69) (n = 64) |
| Vandoni 1994 | mean 4.96 days (n = 25) | mean 4.48 days (n = 25) |
| Wiser 1974 | 5.8 days (n = 75) | 3.9 days (n = 75) |
In two trials (Bergman 1987; Perrin 1997) duration was expressed as the number of participants catheterised for more than five days (Analysis 1.6). These two trials showed significant heterogeneity (I² = 93%), and we therefore did not perform meta‐analysis. The source of the clinical heterogeneity is likely to be because the participants had different reasons for hospitalisation: Bergman 1987 included women undergoing vaginal continence surgery (urethropexy/needle suspension) and vaginal hysterectomy, whereas Perrin 1997 included people undergoing rectal surgery.
1.6. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 6 Number of participants catheterised more than five days.
Urinary retention
Two trials (282 participants) reported the number of participants who developed acute urinary retention following catheterisation (Kringel 2010; Ratnaval 1996). One trial included women undergoing anterior prolapse repair (colporrhaphy, Kringel 2010), and the other included men undergoing pelvic colorectal surgery (Ratnaval 1996). The available evidence was insufficient to conclude whether indwelling urethral or suprapubic catheterisation reduced the risk of acute urinary retention (RR 0.83, 95% CI 0.35 to 1.94; Analysis 1.7).
1.7. Analysis.

Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 7 Number of participants with acute urinary retention.
One trial reported the number of participants who developed chronic urinary retention following catheterisation (Ratnaval 1996). Only one participant out of 26 developed chronic urinary retention in the indwelling group, and none out of 24 in the suprapubic group. The evidence was therefore inconclusive for chronic urinary retention (Analysis 1.8).
1.8. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 8 Number of participants with chronic urinary retention.
Bladder dysfunction
Two trials (276 participants) reported bladder dysfunction. One trial reported participants with post‐void residual volume > 500 ml (Stekkinger 2011) and the other reported voiding difficulty, with no further detail given (Wiser 1974). A small number of participants developed bladder dysfunction (9/137 participants with indwelling urethral catheters versus 6/139 participants with suprapubic catheters (RR 1.53, 95% CI 0.56 to 4.18; Analysis 1.9)).
1.9. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 9 Number of participants with bladder dysfunction.
Pain
Data describing pain were available from eight trials. Five trials reported the number of participants with pain (Baan 2003; Botsios 1997; Kringel 2010; O'Kelly 1995; Piergiovanni 1991). There was evidence of significant heterogeneity (I² = 76%). Only one trial found more participants with suprapubic catheters reporting pain than participants with indwelling urethral catheters (Baan 2003), although this was not statistically significant. This may be due to how pain was assessed. Participants were questioned on "pain in the abdomen". The population in this trial was people undergoing major abdominal surgery, so it is likely that the participants were reporting on pain associated with the surgery rather than pain from the route of catheterisation. Because of this, we excluded Baan 2003 from the meta‐analysis, which eliminated the heterogeneity (I² = 0%).
Sources of heterogeneity
The sources of clinical heterogeneity between trials included:
1. The types of surgery differed between trials:
Abdominal surgery (Baan 2003; Botsios 1997; O'Kelly 1995)
Urogenital surgery (Kringel 2010)
Unspecified surgery (Piergiovanni 1991)
2. The participants differed between trials:
Men and women (Baan 2003; Botsios 1997; O'Kelly 1995; Piergiovanni 1991)
Women only (Kringel 2010)
3. Duration of catheterisation differed between trials:
< 96 hours (Kringel 2010);
< 5 days (Botsios 1997; O'Kelly 1995);
≥ 5 days (Baan 2003; Piergiovanni 1991).
From the remaining four trials (535 participants), more participants with indwelling urethral catheters reported pain compared to participants with suprapubic catheters (RR 5.62, 95% CI 3.31 to 9.55; Analysis 1.10). In these trials, pain was defined using the authors' criteria without validation. We rated the evidence as low quality (Table 1). This is equivalent to three patients being managed with a suprapubic catheter to avoid one patient having (urethral) pain (RD 0.32, 95% CI 0.26 to 0.39).
1.10. Analysis.
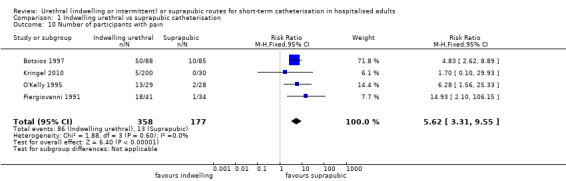
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 10 Number of participants with pain.
One trial (O'Kelly 1995) also reported that there were significantly more catheter days with pain in the indwelling urethral catheter group (37/126 days versus 6/142; RR 6.95, 95% CI 3.03 to 15.92; Analysis 1.11).
1.11. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 11 Number of catheter days with pain.
One trial had different results from the other trials that reported pain. Prasad 2014 reported the mean pain score of participants using a 10‐point visual analogue scale (VAS), but did not report SDs. This trial found no difference in pain scores between the indwelling urethral and suprapubic groups (Analysis 1.12). However, this trial was stopped prematurely following interim analysis. Originally, the trial reported that a minimum of 102 participants were required to find a "statistically significant and clinically meaningful" result, a difference of one point between groups on the VAS on postoperative day one. However, only 58 participants were included in the final analysis. It is possible that this study was inadequately powered to find a difference between the indwelling urethral and suprapubic groups.
1.12. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 12 Mean pain score (VAS 0 ‐ 10).
| Mean pain score (VAS 0 ‐ 10) | |||
|---|---|---|---|
| Study | Indwelling urethral | Suprapubic | p‐value |
| Prasad 2014 | Postoperative day 0: 2.9 Postoperative day 1: 2.5 Postoperative day 7: 1.0 |
Postoperative day 0: 3.5 Postoperative day 1: 3.0 Postoperative day 7: 1.5 |
0.41 0.39 0.26 |
Discomfort
Four trials reported participant discomfort (Botsios 1997; Harms 1985; Perrin 1997; Piergiovanni 1991). As for pain, discomfort was defined using authors' terms without validation. There was evidence of significant heterogeneity (I² = 78%). One trial (Piergiovanni 1991) included a different patient population from the other trials. Piergiovanni 1991 included participants who were hospitalised for various reasons (including surgical and non‐surgical); whereas the remaining trials included participants undergoing abdominal or urogenital surgery. When we excluded Piergiovanni 1991 from meta‐analysis, the heterogeneity fell to I² = 0%, and so we excluded this trial from the meta‐analysis.
Sources of heterogeneity
1. The type of surgery differed between trials:
Abdominal surgery (Botsios 1997; Perrin 1997)
Urogenital surgery (Harms 1985)
Unspecified surgery (Piergiovanni 1991)
2. Antibiotic prophylaxis use differed between trials:
Used antibiotic prophylaxis (Perrin 1997)
Some participants received antibiotic prophylaxis and others did not (Piergiovanni 1991)
Not reported (Botsios 1997; Harms 1985)
From the remaining trials in the meta‐analysis (438 participants), there were significantly more participants with discomfort in the indwelling urethral catheter group compared with the suprapubic catheter group (RR 3.77, 95% CI 2.68 to 5.32, Analysis 1.13). This is equivalent to three patients having a suprapubic catheter to avoid one patient experiencing discomfort (RD 0.39, 95% CI 0.31 to 0.47).
1.13. Analysis.
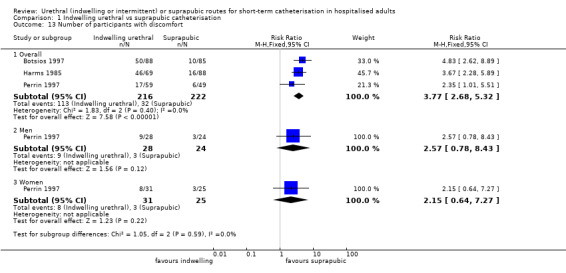
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 13 Number of participants with discomfort.
Catheter obstruction
There was evidence of significant heterogeneity (I² = 58%), so we compared the fixed‐effect and random‐effects models. The fixed‐effect model RR 0.45 (95% CI 0.22 to 0.93), and the random‐effects model RR 0.37 (95% CI 0.08 to 1.78) were similar, so we used the random‐effects model. The five trials that reported catheter obstruction included participants undergoing anterior colporrhaphy (Kringel 2010), rectal surgery (Perrin 1997), vaginal plastic surgery (Schiotz 1989;Wiser 1974) and cystocoele repair (Stekkinger 2011). Catheter obstruction was a rare adverse event: 7/436 participants managed with an indwelling urethral catheter had an obstruction compared with 13/258 managed with a suprapubic catheter. This small difference between groups was not statistically significant (RR 0.37, 95% CI 0.08 to 1.78; Analysis 1.14).
1.14. Analysis.

Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 14 Number of participants with catheter obstruction.
Catheter failure
Two trials (276 participants) reported on those whose catheters fell out (Stekkinger 2011; Wiser 1974). There was evidence of heterogeneity so we did not combine the results of the two trials (Analysis 1.15). The catheters were inserted at different times: Stekkinger 2011 inserted the catheters intraoperatively and Wiser 1974 inserted them postoperatively.
1.15. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 15 Number of participants with catheter that fell out.
One trial reported participants who had urine leak around the catheter (Stekkinger 2011). More participants with a suprapubic catheter had urine leakage compared with indwelling (indwelling 4/62 versus 17/64; RR 0.24, 95% CI 0.09 to 0.68; Analysis 1.16).
1.16. Analysis.
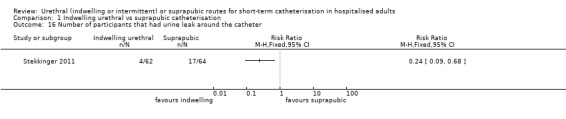
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 16 Number of participants that had urine leak around the catheter.
Haematuria
Four trials (557 participants) (Botsios 1997; Perrin 1997; Stekkinger 2011; Wiser 1974) reported gross haematuria. These trials did not give information about when the haematuria occurred (i.e. while the catheter was in situ or after catheter removal). People who had urethral catheterisation had a statistically significantly reduced risk of gross haematuria (6/284, 2% versus 16/273, 6% with a suprapubic catheter), although the 95% CI approached one (RR 0.39, 95% CI 0.16 to 0.96; Analysis 1.17).
1.17. Analysis.

Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 17 Number of participants with gross haematuria.
The results from the two trials (330 participants) with data on microscopic haematuria were not consistent. One trial (Harms 1985) did not find a statistically significant difference between suprapubic and indwelling urethral catheterisation for microscopic haematuria (RR 0.93, 95% CI 0.72 to 1.20), whereas the other (Botsios 1997) found that participants with a urethral catheter had twice the risk (RR 2.17, 95% CI 1.31 to 3.61, Analysis 1.18) . We did not combine the results because of this heterogeneity. A possible explanation is that participants in each of the trials were undergoing different types of surgery. Additionally, neither gave a definition for microscopic haematuria, so this may be another source of heterogeneity.
1.18. Analysis.
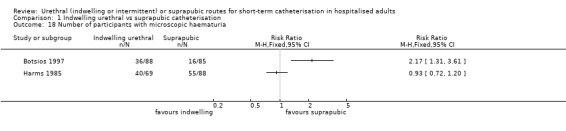
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 18 Number of participants with microscopic haematuria.
Pyuria
There was evidence of heterogeneity (I² = 67%), so we compared the fixed‐effect (RR 2.09 (95% CI 1.63 to 2.68) and random‐effects models (RR 2.35 (95% CI 1.13 to 4.90). We decided to use the random‐effects model as this would provide a more conservative estimate. Participants with indwelling urethral catheters were twice as likely to develop pyuria as those with suprapubic catheters, although the CI was very wide (RR 2.35, 95% CI 1.13 to 4.90, Analysis 1.19; 330 participants).
1.19. Analysis.
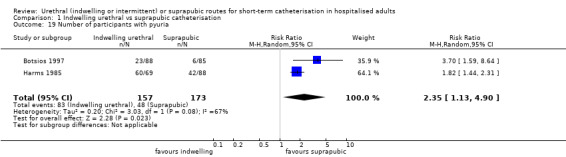
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 19 Number of participants with pyuria.
Urethral stricture
In four trials (516 participants) (Ahmed 1993; Hammarsten 1992; Katz 1992; Korkes 2008), more participants with an indwelling catheter developed urethral stricture than participants using a suprapubic catheter. This was statistically significant but with a very wide CI (RR 2.38, 95% CI 1.02 to 5.56; Analysis 1.20).
1.20. Analysis.
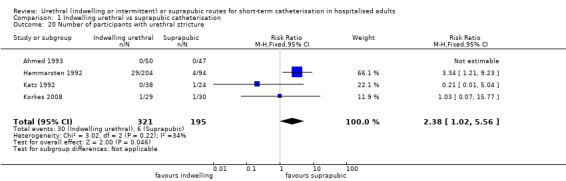
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 20 Number of participants with urethral stricture.
A variety of definitions of urethral stricture were used. Hammarsten 1992 and Katz 1992 reported urethral stricture at six months. Hammarsten 1992 defined urethral stricture as < 19 mm, using urethroscopy, and Katz 1992 used voiding cystourethrogram, with no definition given by the authors. Ahmed 1993 and Korkes 2008 gave no information on when or how the urethral stricture diagnosis was made.
Nursing staff preference
None of the trials reported this outcome.
Urinary symptoms after surgery
One small trial (52 participants) reported on urinary symptoms at six‐month follow‐up (Katz 1992). The participants were asked about symptoms of weakening of stream, urgency, frequency and increased nocturia.Thirteen out of 31 managed with indwelling urethral catheter developed postoperative urinary symptoms compared with 6/21 who were managed with suprapubic catheter. The results of this trial were not statistically significant (RR 1.47, 95% CI 0.66 to 3.24; Analysis 1.21).
1.21. Analysis.
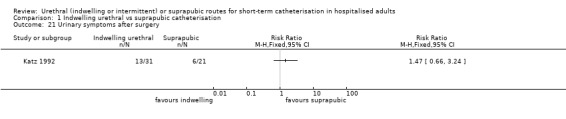
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 21 Urinary symptoms after surgery.
Epididymitis
Only two trials (156 participants) reported epididymitis (Ahmed 1993; Korkes 2008), reporting that five men had this adverse effect. There was evidence of heterogeneity (I² = 54%), so we compared the fixed‐effect and random‐effect models (fixed‐effect model RR 2.45 (95% CI 0.47 to 12.72) and random‐effects model RR 1.82 (95% CI 0.08 to 43.16)). We decided to use the random‐effects model as this would provide a more conservative estimate. In these two trials, the CI was very wide and the evidence was insufficient to determine if there was any difference in the incidence of epididymitis between groups (RR 1.82, 95% CI 0.08 to 43.16; Analysis 1.22).
1.22. Analysis.

Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 22 Number of participants with epididymitis.
Postoperative pyrexia
One trial (97 participants) reported the number of participants with postoperative pyrexia (Ahmed 1993). This was not defined as symptomatic UTI, as it did not include a urine culture, and the reason for pyrexia in this trial was not specified.There was no statistically significant difference between indwelling urethral and suprapubic catheter groups (RR 1.18, 95% CI 0.51 to 2.72; Analysis 1.23). One trial (Bergman 1987) (51 participants) reported the mean fever index (degree times number of hours patient's temperature was above 37.2° C). The results were better in the suprapubic catheter group in terms of fewer participants with febrile morbidity (Analysis 1.24).
1.23. Analysis.
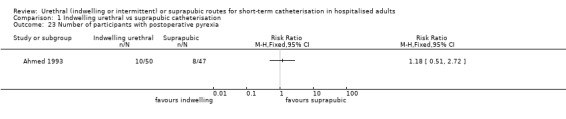
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 23 Number of participants with postoperative pyrexia.
1.24. Analysis.
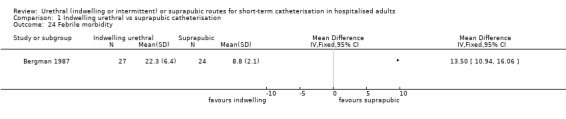
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 24 Febrile morbidity.
Drug therapy
Two trials (254 participants) reported patients who required antibiotic therapy (Ahmed 1993; Harms 1985). Participants managed with indwelling urethral catheter had more than twice the risk of requiring antibiotic therapy than participants with suprapubic catheter (RR 2.10, 95% CI 1.36 to 3.24; Analysis 1.25). This is the equivalent to about five patients being managed with suprapubic catheter to prevent one case of a patient requiring antibiotic therapy (RD 0.19, 95% CI 0.08 to 0.30). One trial (Wiser 1974; 150 participants) reported the number of participants requiring drugs for relief of dysuria.
1.25. Analysis.
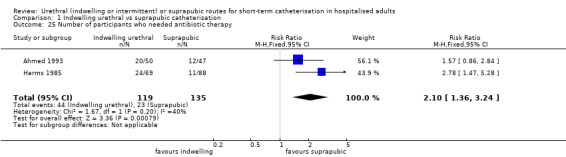
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 25 Number of participants who needed antibiotic therapy.
Fewer participants with a suprapubic catheter required drugs for relief of dysuria (pain, discomfort or burning sensation during urination) compared to an indwelling catheter (RR 1.68, 95% CI 1.23 to 2.28; Analysis 1.26).
1.26. Analysis.
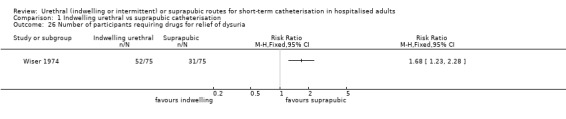
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 26 Number of participants requiring drugs for relief of dysuria.
Duration of hospital stay
One trial (Baan 2003) reported the median duration of hospital stay. Participants with a suprapubic catheter had a median hospital stay of 13.1 days, compared to participants with an indwelling urethral catheter who had a median hospital stay of 15.6 days (Analysis 1.27). However, we could not assess the statistical significance of these results due to insufficient data presentation in the trial report.
1.27. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 27 Duration of hospital stay.
| Duration of hospital stay | |||
|---|---|---|---|
| Study | Indwelling | Suprapubic | Notes |
| Baan 2003 | Median 15.6 days (n = 71) |
Median 13.1 days (n = 75) |
‐ |
Four trials (430 participants) reported mean and SD duration of hospital stay. There was evidence of heterogeneity, so we did not pool the results (Analysis 1.28). The type of surgery differed between trials, which contributed to clinical heterogeneity between trials:
1.28. Analysis.
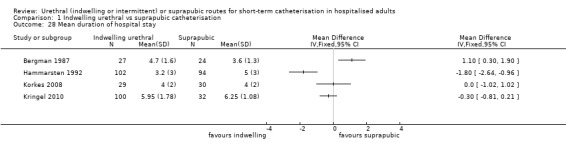
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 28 Mean duration of hospital stay.
Prostatectomy: transurethral resection of prostate (TURP) (Hammarsten 1992), and open prostatectomy (Korkes 2008) which may have affected duration of hospital stay
Urogenital surgery in women: continence surgery (vaginal urethropexy and needle suspension) (Bergman 1987), and prolapse (cystocoele) repair (Kringel 2010).
One trial (150 participants) reported duration of hospital stay as extended hospital stay due to catheter‐associated complications (Wiser 1974). In participants with indwelling catheters, 25/75 had an extended hospital stay compared with 14/75 who received suprapubic catheters. This difference between groups was statistically significant but with a wide CI (RR 1.79, 95% CI 1.01 to 3.16; Analysis 1.29).
1.29. Analysis.
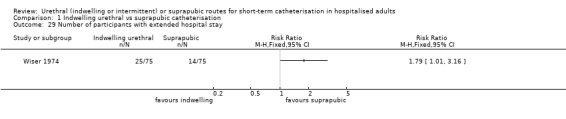
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 29 Number of participants with extended hospital stay.
Economic outcomes
Only one trial (Ichsan 1987) comparing indwelling urethral and suprapubic catheterisation reported economic outcomes (Analysis 1.30). It reported the cost of catheter, other equipment required for catheterisation and labour. Indwelling urethral catheterisation was calculated to be AUD 33.20 (Australian dollars) in total. Suprapubic catheterisation was calculated to be AUD 27.77 in total. The costs of both routes of catheterisation were adjusted according to recatheterisation required. The additional cost incurred in participants with UTI was noted, but was "unable to be quantified because of variability of cost". The trial did not give details of how labour costs were calculated.
1.30. Analysis.
Comparison 1 Indwelling urethral vs suprapubic catheterisation, Outcome 30 Cost.
| Cost | |||
|---|---|---|---|
| Study | Indwelling | Suprapubic | Notes |
| Ichsan 1987 | Catheter: AUD 1.10 Other: AUD 7.67 Labour: AUD 4 Adjustment: x 2.6 Total: AUD 33.20 (n = ?) |
AUD 14 AUD 7.47 AUD 5 x 1.05 AUD 27.77 (n = ?) |
‐ |
Post‐catheter quality of life
None of the trials reported this outcome.
Patient satisfaction
None of the trials reported this outcome.
Comparison 2: Indwelling urethral catheterisation compared with intermittent catheterisation
Fourteen trials in 1596 participants assessed this comparison (Carpiniello 1988; Dobbs 1997; Evron 2008; Hakvoort 2011; Halleberg 2013; Kerr‐Wilson 1986; Knight 1996; Michelson 1988; Millet 2012; Rivard 2012; Skelly 1992; Tang 2006; Tangtrakul 1994; Van den Brand 2001). Only one trial did not contribute to meta‐analysis (Rivard 2012).
Symptomatic urinary tract infection
Two trials had data for symptomatic UTI which were suitable for meta‐analysis (Hakvoort 2011; Tang 2006). Due to evidence of significant clinical and statistical heterogeneity, we did not pool the results, which were inconclusive (Analysis 2.1). The main source of heterogeneity was the reason for hospitalisation:
2.1. Analysis.
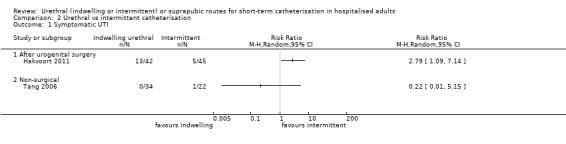
Comparison 2 Urethral vs intermittent catheterisation, Outcome 1 Symptomatic UTI.
Urogenital surgery (Hakvoort 2011);
Elderly women in geriatric rehabilitation ward (Tang 2006).
We rated the quality of evidence as very low (Table 2).
Asymptomatic bacteriuria
The data describing asymptomatic bacteriuria were available from 13 trials (1333 participants). Overall, the rate of bacteriuria was not statistically significantly different between groups (141/650, 22% with indwelling catheterisation versus 136/683, 20% with intermittent catheterisation: RR 1.04, 95% CI 0.85 to 1.28; Analysis 2.2) but the wide confidence interval makes it difficult to say that there would truly be no difference between the groups. As there were more than 10 trials included in this comparison, we used a funnel plot to assess publication bias. There was some evidence of publication bias, as trials with low sample size or negative results were under‐represented on interpretation of the funnel plot (Figure 6)
2.2. Analysis.
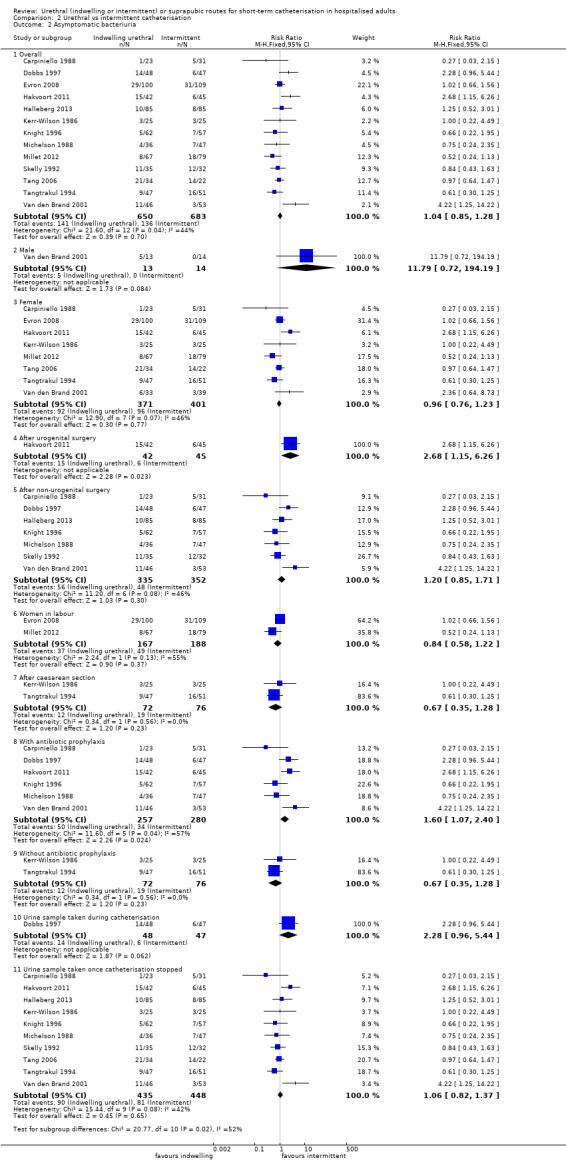
Comparison 2 Urethral vs intermittent catheterisation, Outcome 2 Asymptomatic bacteriuria.
6.
Funnel plot of comparison: 2 URETHRAL CATHETERISATION VS INTERMITTENT CATHETERISATION, outcome: 2.2 Asymptomatic bacteriuria.
Subgroup analyses (Analysis 2.2)
We conducted subgroup analyses by gender, indicator condition for catheterisation (type of surgery or other), antibiotic prophylaxis or not, in pregnant women, and timing of taking of the urine sample (Analysis 2.2). One trial analysed the effect in men only, but the data were too few to assess whether effects differed (Van den Brand 2001; Analysis 2.2.2). One trial in people having urogenital surgery (Hakvoort 2011) showed that twice as many had bacteriuria with indwelling rather than with intermittent catheterisation (Analysis 2.2.4). However, the trial was underpowered to detect such differences. Trials in women in labour or after caesarean section were inconclusive (Analysis 2.2.6,7). If participants received antibiotic prophylaxis, there was more bacteriuria with indwelling than with intermittent catheterisation (RR 1.60, 95% CI 1.07 to 2.40, Analysis 2.2.8), whereas the result was inconclusive in the two small trials where participants did not receive prophylaxis (Kerr‐Wilson 1986; Tangtrakul 1994; Analysis 2.2.9). The majority of the trials of timing of taking a urine sample occurred after the catheter had been removed, and thus reflect the overall analysis (Analysis 2.2.11).
Duration of catheterisation
Three trials had data on duration of catheterisation. Only one trial (66 participants) reported mean and SD (Tang 2006). There was no statistically significant difference in duration of catheterisation between groups (MD 0.60, 95% CI ‐1.17 to 2.37; Analysis 2.3). One trial (50 participants) reported the median and range of duration of catheterisation in hours (Kerr‐Wilson 1986). Participants had indwelling catheterisation for 72 hours (range 72 to 144 hours), whereas intermittent catheterisation was for 18 hours (range 5 to 112 hours). One trial reported only the mean, with less than 15 hours difference in duration of catheterisation on average (indwelling one day versus intermittent 9 hours 37 minutes; Analysis 2.4). One trial (66 participants) reported the number of participants with catheter at 14 days (Tang 2006). There was no significant difference in the number of participants still using a catheter at 14 days (RR 0.76, 95% CI 0.39 to 1.45; Analysis 2.5).
2.3. Analysis.
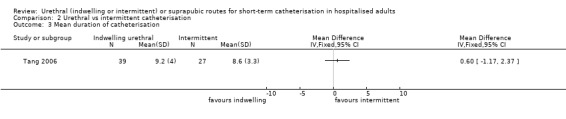
Comparison 2 Urethral vs intermittent catheterisation, Outcome 3 Mean duration of catheterisation.
2.4. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 4 Duration of catheterisation.
| Duration of catheterisation | |||
|---|---|---|---|
| Study | Indwelling | Intermittent | Notes |
| Hakvoort 2011 | median, range 72 (72 ‐ 144) hours (n = 42) |
median, range 18 (5 ‐ 112) hours (n = 45) |
‐ |
| Kerr‐Wilson 1986 | mean 1 day after surgery (n = 25) |
mean 9 hours 37 minutes (n = 25) |
‐ |
2.5. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 5 Number of participants using catheter at 14 days.
Urinary retention
In four trials (Knight 1996; Michelson 1988; Skelly 1992; Tangtrakul 1994) (384 participants), 88 people using an intermittent catheter out of 196 (45%) developed acute urinary retention compared with 30/188 (16%) people with indwelling urethral catheter. There was evidence of heterogeneity (I² = 62%), so we compared the fixed‐effect and random‐effects models (fixed‐effect model RR 0.37 (95% CI 0.26 to 0.53) and random‐effects model RR 0.45 (95% CI 0.22 to 0.91)). We decided to use the random‐effects model as this would provide a more conservative estimate.
More people with intermittent urethral catheters developed acute urinary retention compared to people with indwelling urethral catheters (RR 0.45, 95% CI 0.22 to 0.91; Analysis 2.6). This is equivalent to four patients being managed with indwelling urethral catheter to avoid one case of urinary retention (RD ‐0.26, 95% CI ‐0.46 to ‐0.07).
2.6. Analysis.
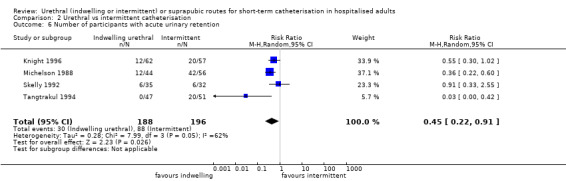
Comparison 2 Urethral vs intermittent catheterisation, Outcome 6 Number of participants with acute urinary retention.
Bladder dysfunction/Inability to void
Three reported bladder dysfunction (Knight 1996; Michelson 1988; Skelly 1992) (286 participants) and one reported inability to void after catheterisation (Kerr‐Wilson 1986; 50 participants). For bladder dysfunction, there was a small difference between groups, which was not statistically significant (indwelling 25/141 versus intermittent 34/145; RR 0.75, 95% CI 0.48 to 1.19; Analysis 2.7). Eleven out of 25 participants managed with intermittent catheters were unable to void, compared with none in the indwelling catheter group (RR 0.02, 95% CI 0.00 to 0.045; Analysis 2.8).
2.7. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 7 Number of participants with bladder dysfunction.
2.8. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 8 Number of participants unable to void after catheterisation.
Urinary symptoms after surgery
One trial (95 participants) reported urinary symptoms after surgery (Dobbs 1997). No information was given on what the urinary symptoms were. Eleven out of 48 participants with indwelling urethral catheters developed postoperative urinary symptoms, compared with 7/47 participants with intermittent urethral catheters. The difference between the groups was not statistically significant (RR 1.54, 95% CI 0.65 to 3.63; Analysis 2.9).
2.9. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 9 Urinary symptoms after surgery.
Postoperative pyrexia
One trial (95 participants) reported the number of participants with postoperative pyrexia (Dobbs 1997). In this trial, there was no statistically significant difference in the number of participants developing postoperative pyrexia (RR 1.11, 95% CI 0.63 to 1.95; Analysis 2.10).
2.10. Analysis.
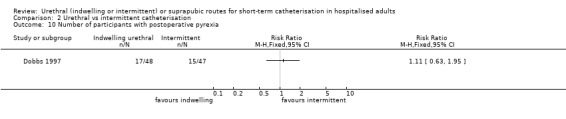
Comparison 2 Urethral vs intermittent catheterisation, Outcome 10 Number of participants with postoperative pyrexia.
Other adverse effects
None of the trials reported the following outcomes: pain, discomfort, catheter obstruction, catheter failure, haematuria, pyuria, urethral stricture, epididymitis or postoperative pyrexia.
Nursing staff preference
One trial (72 participants) reported nursing staff preference for catheter (Rivard 2012). Nurses showed a clear preference for indwelling urethral catheterisation over intermittent. Thirty‐four nurses preferred indwelling urethral catheterisation, two nurses preferred intermittent urethral catheterisation and one nurse had no preference (RR 0.06, 95% CI 0.02 to 0.23; Analysis 2.11). There was no information on how bias was assessed in this trial. The trial stated that the nurses found indwelling urethral catheterisation less time‐consuming. However, it did not report on whether indwelling or intermittent urethral catheterisation was standard care prior to this trial. This may have affected nursing staff preference, if they were more familiar with one method over the other.
2.11. Analysis.
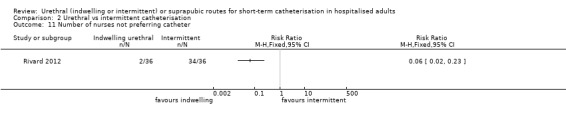
Comparison 2 Urethral vs intermittent catheterisation, Outcome 11 Number of nurses not preferring catheter.
Drug therapy
None of the trials reported this outcome.
Duration of hospital stay
Only one trial (87 participants) provided data on duration of hospital stay (Hakvoort 2011), which reported the median and range. The intermittent urethral group had a median hospital stay of two days compared to indwelling urethral group, which had a median hospital stay of four days (Analysis 2.12).
2.12. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 12 Duration of hospital stay.
| Duration of hospital stay | |||
|---|---|---|---|
| Study | Indwelling | Intermittent | Notes |
| Hakvoort 2011 | median, range 4 (1 ‐ 7) days (n = 42) |
2 (1 ‐ 6) days (n = 45) | ‐ |
Economic outcomes
Two trials (218 participants) reported cost for the first 48 hours after surgery (Knight 1996; Van den Brand 2001). Costs were lower for indwelling urethral catheters than intermittent urethral catheters in both trials, though statistical analysis was not possible. However, there were marked differences between the trials in this respect, with one suggesting a six‐fold difference, and the other a relatively small difference.
One trial (139 participants) calculated the cost of indwelling and intermittent urethral catheterisation based on the average number of catheterisations, and the cost of the catheter kit used (Rivard 2012). Indwelling urethral catheterisation was very slightly more expensive than intermittent (USD 7.37 for indwelling versus USD 6.28 for intermittent). One trial (169 participants) calculated the cost of each route of catheterisation based on total material and labour cost (Halleberg 2013). This trial also compared the cost in participants who developed UTI and those who did not. Intermittent catheterisation was slightly more expensive than indwelling (EUR 16.62 for indwelling versus EUR 17.98 for intermittent). They also found that there was a significantly higher direct cost of catheterisation and cost for length of stay for participants who developed UTI compared to those who did not (Analysis 2.13).
2.13. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 13 Cost.
| Cost | |||
|---|---|---|---|
| Study | INDWELLING URETHRAL | INTERMITTENT URETHRAL | Notes |
| Halleberg 2013 | EUR 16.62 EUR 3.80 EUR 2.45 EUR 16.60 (13.1) n = 85 EUR 13.00 (7.7) n = 85 EUR 45.00 (10.2) n = 85 EUR 3954 (1743) n = 85 EUR 3791 (1736) n = 85 EUR 5173 (1306) n = 85 |
EUR 17.98 EUR 8.90 EUR 3.26 EUR 18.00 (13.6) n = 84 EUR 16.00 (11.8) n = 84 EUR 41.00 (6.5) n = 84 EUR 3642 (1605) n = 84 EUR 3619 (1638) n = 84 EUR 3862 (1329) n = 84 |
Total material + labour cost Unit price for catheter Costs incurred due to UTI (mean) Catheterisation cost (mean, SD, N) Catheterisation cost with no UTIs (mean, SD, N) Catheterisation cost with UTIs (mean, SD, N) Total costs (mean, SD, n) Total costs with no UTIs (mean, SD, n) Total costs with UTIs (mean, SD, n) |
| Kerr‐Wilson 1986 | GBP 0.53 | GBP 0.10 | Cost of catheter |
| Knight 1996 | USD 8.33 (n = 62) USD 17.96 (n = 10) |
USD 53.20 (n = 57) USD 34.58 (n = 20) |
Total cost per patient within 1st 48 hours Total cost per patient after 48 hours |
| Rivard 2012 | USD 6.28 (n = 72) | USD 5.98 (n = 67) | Cost per catheter |
| Van den Brand 2001 | USD 6.15 (n = 46) | USD 7.75 (n = 53) | Total cost per patient within 1st 48 hours |
One trial carried out a cost‐effectiveness analysis (Halleberg 2013). It used health state scores from EQ‐5D, EQ visual analogue scale (EQ VAS) and SF‐6D to calculate quality‐adjusted life years (QALYs) gained at four‐month follow‐up. There was no significant difference between groups in QALYs gained between discharge and four months (Analysis 2.14). As there was no significant difference in these outcomes, the trialists did not calculate the incremental cost‐effectiveness ratio (ICER) as no route of catheterisation was more cost effective than the other.
2.14. Analysis.
Comparison 2 Urethral vs intermittent catheterisation, Outcome 14 Post‐catheter quality of life.
| Post‐catheter quality of life | |||
|---|---|---|---|
| Study | Transurethral Catheter | Clean Intermittent Catheter | Significance |
| Postcatheter Pain Score (VAS 0‐100) | |||
| Hakvoort 2011 | 34 (n = 42) | 29 (n = 45) | P = 0.45 |
| Catheterisation difficulty (VAS 0‐100) | |||
| Hakvoort 2011 | 36 (n = 42) | 28 (n = 45) | P = 0.20 |
| Postcatheter Patient Satisfaction (VAS 0‐100) | |||
| Hakvoort 2011 | 76 (n = 42) | 80 (n = 45) | P = 0.41 |
| EQ‐5D scores; mean score (n of patients) | |||
| Halleberg 2013 | Discharge 0.32 (n = 52) 4 weeks 0.62 (n = 52) 4 months 0.68 (n = 52) Gained QALYs 0.093 (n = 52) |
0.32 (n = 57) 0.56 (n = 57) 0.73 (n = 57) 0.090 (n = 57) |
P = 0.904 |
| EQ VAS scores; mean score (n of patients) | |||
| Halleberg 2013 | Discharge 0.52 (n = 51) 4 weeks 0.65 (n = 51) 4 months 0.68 (n = 51) Gained QALYs 0.044 (n=51) |
0.52 (n = 54) 0.63 (n = 54) 0.69 (n = 54) 0.045 (n = 54) |
P = 0.978 |
| SF‐6D scores; mean scores (n of patients) | |||
| Halleberg 2013 | Discharge 0.50 (n = 45) 4 weeks 0.60 (n = 45) 4 months 0.63 (n = 45) Gained QALYs 0.036 (n = 45) |
0.51 (n = 45) 0.58 (n = 45) 0.65 (n = 45) Gained QALYs 0.032 (n = 45) |
P = 0.616 |
Post‐catheter quality of life
Two trials (198 participants) reported on post‐catheter quality of life (Hakvoort 2011; Halleberg 2013). Pain, catheterisation difficulty and participant satisfaction did not differ between the groups (Hakvoort 2011; Analysis 2.14). Quality of life was quantified by calculating QALYs using health state score from EQ‐5D, EQ VAS and SF‐6D. They found no difference in QALYs gained between the groups at four‐month follow‐up (Halleberg 2013; Analysis 2.14)
Patient satisfaction
None of the trials reported this outcome.
Comparison 3: Suprapubic catheterisation compared with intermittent catheterisation
Three trials in 359 participants assessed this comparison (Dixon 2010; Jannelli 2007; Naik 2005). All three trials only included women. Two of the three trials contributed to meta‐analysis (Jannelli 2007; Naik 2005).
Symptomatic urinary tract infection
Only one trial (72 participants) comparing suprapubic and intermittent urethral catheterisation reported symptomatic UTI (Dixon 2010). In this trial, more participants developed UTI in the suprapubic catheter group than in the intermittent urethral group (10/36 for suprapubic versus 6/36 for intermittent), although this was not statistically significant (RR 1.67, 95% CI 0.68 to 4.10; Analysis 3.1). We rated the quality of evidence as low.
3.1. Analysis.
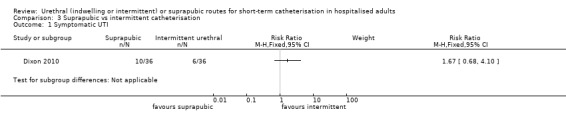
Comparison 3 Suprapubic vs intermittent catheterisation, Outcome 1 Symptomatic UTI.
Asymptomatic bacteriuria
Two trials reported asymptomatic bacteriuria (Jannelli 2007; Naik 2005). There was evidence of heterogeneity (I² = 64%) so we compared the fixed‐effect and random‐effects models (fixed‐effect model RR 0.63 (95% CI 0.42 to 0.95) and random‐effects model RR 0.52 (95% CI 0.20 to 1.35)). We decided to use the random‐effects model as this would provide a more conservative estimate. There was no significant difference in the incidence of asymptomatic bacteriuria between the groups (RR 0.52, 95% CI 0.20 to 1.35; Analysis 3.2).
3.2. Analysis.
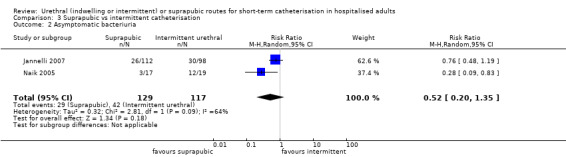
Comparison 3 Suprapubic vs intermittent catheterisation, Outcome 2 Asymptomatic bacteriuria.
Duration of catheterisation
Three trials had data on duration of catheterisation (Dixon 2010; Jannelli 2007; Naik 2005). The results did not consistently show one route of catheterisation to have a shorter duration of catheterisation over the other (Analysis 3.3).
3.3. Analysis.
Comparison 3 Suprapubic vs intermittent catheterisation, Outcome 3 Duration of catheterisation.
| Duration of catheterisation | |||
|---|---|---|---|
| Study | Suprapubic | Intermittent | Notes |
| Dixon 2010 | median, range 5 (4 ‐ 36) days (n = 38) |
median, range 4 (2 ‐ 36) days (n = 37) |
‐ |
| Jannelli 2007 | mean, SD 5.3 (7.0) days (n = 112) |
mean, SD 5.2 (7.4) days (n = 98) |
2 participants were excluded from analysis of duration of catheterisation as had prolonged urinary retention no report of which group they are in. |
| Naik 2005 | median, range 20 (7 ‐ 28) days (n = 19) |
median, range 17 days (7 ‐ 90) (n = 21) |
‐ |
Pain
Only one trial (72 participants) comparing suprapubic and intermittent catheterisation reported the number of participants with pain (Dixon 2010). The trial was too small to be conclusive: 10/36 participants with suprapubic catheter had pain compared to 6/36 participants with intermittent catheter. This was not statistically significant (RR 1.67, 95% CI 0.68 to 4.10; Analysis 3.4). We rated the quality of evidence as very low (Table 3).
3.4. Analysis.
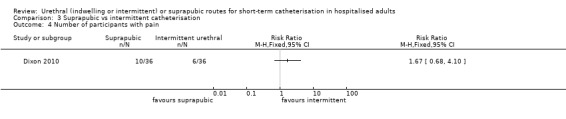
Comparison 3 Suprapubic vs intermittent catheterisation, Outcome 4 Number of participants with pain.
Other adverse effects
None of the trials reported the following outcomes: Urinary retention, bladder dysfunction, discomfort, catheter obstruction, catheter failure, haematuria, pyuria, urethral stricture, nursing staff preference, urinary symptoms after surgery, epididymitis and postoperative pyrexia.
Drug therapy
None of the trials reported this outcome.
Duration of hospital stay
Only one trial (72 participants) had data for duration of hospital stay (Dixon 2010), reported as the median and range. Participants with suprapubic catheter had a median hospital stay of six days, and those with an intermittent urethral catheters had a median hospital stay of five days (Analysis 3.5). However, we were unable to calculate the statistical significance of these results.
3.5. Analysis.
Comparison 3 Suprapubic vs intermittent catheterisation, Outcome 5 Duration of hospital stay.
| Duration of hospital stay | |||
|---|---|---|---|
| Study | Suprapubic | Intermittent | Notes |
| Dixon 2010 | median, range 6 (2 ‐ 15) (n = 38) |
median, range 5 (2 ‐ 19) (n = 37) |
‐ |
Economic outcomes
One trial (72 participants) calculated cost, based on staff time and consumable costs (Dixon 2010). Suprapubic catheter was more expensive (GBP 30.30 for suprapubic versus GBP 26.80 for intermittent urethral catheterisation), although this did not take into account hospitalisation costs (Analysis 3.6).
3.6. Analysis.
Comparison 3 Suprapubic vs intermittent catheterisation, Outcome 6 Cost.
| Cost | |||
|---|---|---|---|
| Study | Suprapubic | Intermittent | Notes |
| Dixon 2010 | GBP 30.30 (n = 38) | GBP 26.80 (n = 37) | consumable + staff costs (based on nursing time) |
Post‐catheter quality of life
None of the trials reported this outcome.
Patient satisfaction
Patient satisfaction was reported in one trial (Jannelli 2007; 210 participants). By postoperative day two, women using intermittent catheterisation reported less interest in using the method again (P < 0.001), more difficulty using the catheter (P < 0.001), more frustration (P < 0.001) and more pain from intermittent catheterisation (P < 0.001) compared to women using suprapubic catheterisation. By postoperative day seven, women using intermittent catheterisation reported more difficulty using the catheter (P = 0.003), more frustration with catheter use (P = 0.01) and less interest in using the method again than women using a suprapubic catheter (P = 0.04), using a visual analogue scale to rate these outcomes. In short, women preferred suprapubic catheterisation. However, there was no difference between groups in overall pain score or limitation in social activities.
Discussion
Summary of main results
This review compared three routes for adults requiring short‐term catheterisation. These three routes were: indwelling urethral, suprapubic and intermittent urethral. The participants included in the trials were hospitalised for many different reasons, such as surgery, acute medical conditions and labour.
Comparison 1: Indwelling urethral vs suprapubic catheterisation
We found 25 trials with 2622 participants comparing indwelling urethral and suprapubic catheterisation. A rare but potentially serious complication of suprapubic catheterisation is bowel perforation. While this was not reported in any of the trials, this would not be expected to occur with either route of urethral catheterisation.
Symptomatic UTI
Only five of the 25 trials reported symptomatic UTI and could be included in the meta‐analysis. The evidence suggesting that there was little difference in symptomatic UTIs after indwelling urethral or suprapubic catheterisation was of very low quality (Table 1) and the confidence interval was wide.
Pain
Fewer participants with suprapubic catheters had pain compared to participants with indwelling urethral catheters. Participants with indwelling urethral catheters were five times more likely to suffer from pain than participants with suprapubic catheters. We rated the evidence as low quality.
Ease of use
None of the trials reported ease of use of catheter.
Quality of life
None of the trials reported quality of life.
Cost utility analysis
None of the trials comparing indwelling urethral and suprapubic catheter included a cost utility analysis. One trial reported that the cost of catheterisation was similar for both routes (Ichsan 1987). As the trial was conducted almost 30 years ago, the relevance of these costs may be limited.
Comparison 2: Indwelling urethral vs intermittent urethral
We found 14 trials with 1596 participants which compared indwelling and intermittent urethral catheters. The sample sizes were relatively small: the largest trial had 209 participants (Evron 2008). All of the trials were RCTs apart from one, which was a quasi‐RCT (Michelson 1988).
Symptomatic UTI
Only two of the 14 trials reported symptomatic UTI and we could not combine them in meta‐analysis due to heterogeneity. The evidence was of very low quality (Table 2).
Pain
None of the included trials reported pain.
Ease of use
None of the included trials reported ease of use of catheter.
Quality of life
Two trials reported on quality of life, although we could not combine them in meta‐analysis as SDs were not reported. One trial reported quality‐of‐life outcomes that were: post‐catheter pain score, catheterisation difficult and post‐catheter participant satisfaction (all using 0 ‐ 100 visual analogue scale) (Hakvoort 2011). We found no difference in mean scores. Another trial reported quality of life using EQ‐5D, EQ‐VAS and SF‐6D, and found no difference at four weeks and four months follow‐up between groups (Halleberg 2013).
Cost utility analysis
None of the included trials reported cost utility analysis.
One trial (Halleberg 2013) included a cost‐effectiveness analysis, which found no significant difference in QALYs gained or cost of catheterisation. We therefore did not calculate an incremental cost‐effectiveness ratio.
Two trials reported the cost of catheters; both found very little difference in cost between groups (Kerr‐Wilson 1986; Rivard 2012). Kerr‐Wilson 1986 reported the unit cost of indwelling urethral catheter to be GBP 0.53 and intermittent urethral catheter GBP 0.10. There were conflicting results in the total cost per participant in the first 48 hours in the two trials reporting it. Knight 1996 reported that intermittent urethral catheterisation was over six times more expensive than indwelling urethral catheterisation. In contrast, Van den Brand 2001 found that the costs were similar.
Comparison 3: Suprapubic vs intermittent urethral
We found three trials with 359 participants comparing suprapubic and intermittent urethral catheterisation. The sample sizes were small, ranging from 40 to 244. All of the trials were RCTs.
Symptomatic UTI
One trial reported symptomatic UTI, and found no significant difference between groups. The quality of evidence was low (Table 3).
Pain
One trial which reported the number of participants with pain found no significant difference between suprapubic and intermittent urethral groups. The evidence was of very low quality.
Ease of use
None of the trials reported ease of use of catheter.
Quality of life
None of the trials reported quality of life.
Cost utility analysis
None of the trials reported cost utility analysis.
The cost of catheterisation, based on consumables and staff costing, was not significantly different between groups in one trial (Dixon 2010).
Overall completeness and applicability of evidence
For this review, we prespecified five important participant‐centred outcomes: symptomatic UTI, pain, ease of use, quality of life and cost utility analysis. Only the primary outcome, symptomatic UTI, was reported by at least one trial in each of the three comparisons. The number of participants with pain was reported in the comparison of indwelling urethral versus suprapubic (comparison 1) and suprapubic versus intermittent urethral catheterisation (comparison 3). Ease of use was not reported in any of the included trials, although it is an important outcome for patients and healthcare providers. One trial comparing indwelling and intermittent urethral catheterisation reported quality of life, but did not report SDs so could not be included in meta‐analysis and assessment of quality. None of the trials reported cost utility analysis, though some reports of costs were available.
Selection of critical outcomes
A previous Cochrane review reported the findings of a focus group aimed at identifying important outcomes for patients undergoing short‐term urinary catheterisation (Omar 2013). The outcomes were identified by clinicians, nurses, a health economist and patients who had experience with urethral catheterisation. The healthcare professionals identified:
symptomatic UTI with/without microbiological evidence;
patient discomfort whilst catheter is in situ;
bacterial resistance to the antimicrobial agent;
urinary sepsis.
The patients in the focus group identified infection and discomfort as critical outcomes for short‐term urinary catheterisation. They also identified length of hospital stay and duration of catheterisation. Quality of life issues such as impact on self esteem and ability to wear clothes comfortably were also deemed important. Patients also had concerns about catheter insertion.
The five critical outcomes identified for this review were informed by and hence very similar to the ones identified by the focus group. Ease of use could impact positively or negatively on a patient’s experience of being catheterised, or the need to perform catheterisation themselves. If a patient or healthcare worker found one route of catheterisation more difficult to use than another, they might be less likely to want to use that route again.
An outcome not identified by the focus group was cost utility analysis; the patients in this focus group were unlikely to have identified cost as a primary concern as they were being treated in the NHS, which is free at the point of access. However, the cost implications of catheterisation and their outcomes need to be considered. In England, one episode of symptomatic UTI in a catheterised patient costs on average GBP 2291, in 2012 (Health Committee 2013).
Time span of included trials
The age of the included trials should also be noted for this review. The oldest trial (Wiser 1974) was over 40 years old. The majority of the trials in this review were published in the 1990s (Ahmed 1993; Barry 1992 PE; Botsios 1997; Dobbs 1997; Hammarsten 1992; Katz 1992; Knight 1996; Nwabineli 1993; O'Kelly 1995; Perrin 1997; Piergiovanni 1991; Ratnaval 1996; Skelly 1992; Tangtrakul 1994; Vandoni 1994). Practice may have significantly changed since then, and the data from older trials may not be as relevant to current practice.
Definiton of UTI and asymptomatic bacteriuria
The available evidence for symptomatic UTI was limited by the diagnostic definitions that were used. Of 24 trials that purported to be reporting symptomatic UTI, only seven trials were actually reporting it. We included an additional trial (Barry 1992 PE) that reported UTI with no definition, and performed a sensitivity analysis with and without it. The definition of 'symptomatic UTI' was taken from the IDSA 2010 guideline on the prevention, treatment and management of catheter‐associated UTI (Hooton 2010). This definition includes positive urine culture and, crucially, signs or symptoms of UTI. We reclassified the 16 trials that did not actually report symptomatic UTI as reporting asymptomatic bacteriuria, since only a positive urine culture was required to diagnose symptomatic UTI, with no mention of signs or symptoms.
Various guideline panels recommend diverse diagnostic criteria for symptomatic UTI. The criteria for a positive urine culture and time the catheter was in place are where some of the differences lie. Those conducting trials and other forms of research are left to select which definition to use, if they follow recommendations from guidelines at all. Two trials included in this review used a recommended definition of symptomatic UTI, taken from the CDC and IDSA guidelines (Kringel 2010; Millet 2012).
The EAU and IDSA both recommend that asymptomatic bacteriuria should not be screened for or treated in any patients during short‐term catheterisation, as complications are very rare when patients are not treated (Grabe 2015; Hooton 2010). Patients with symptomatic UTI should be treated with antimicrobial therapy. Certain groups of patients may be more at risk of infection. For example, following TURP there is a higher rate of bacteraemia and sepsis compared to other surgery. About 60% of patients undergoing TURP with asymptomatic bacteriuria go on to develop bacteraemia. In these patients, between 6% and 10% have clinical signs of sepsis (Nicolle 2005).
It is interesting to note that significantly more trials reported asymptomatic bacteriuria, the less clinically relevant condition in the majority of patients. The question must be asked why the majority of trials in this review reported the less clinically important outcome of asymptomatic bacteriuria, instead of symptomatic UTI.
Treatment for at‐risk patient groups
Certain patient groups are at increased risk of complications of asymptomatic bacteriuria and should be screened and treated for it. These groups are:
pregnant women;
patients undergoing urologic procedures in which visible mucosal bleeding is anticipated (e.g. TURP);
immunocompromised patients (EAU 2014; Hooton 2010).
Core Outcome Measures in Effectiveness Trials
The Core Outcome Measures in Effectiveness Trials initiative (COMET) aims to help co‐ordinate information about core outcome sets. Core outcome sets comprise the minimum outcomes that should be measured and reported in all clinical trials, audits of practice and any other research for a specific condition. The development of a core outcome set for short‐term urinary catheterisation would be beneficial for future trials and systematic reviews. This core outcome set should specify which outcomes should be reported and also how they should be measured. We would suggest that the five outcomes which we selected for the 'Summary of findings' tables, and which are important for decision making from patients' perspective, should be collected and reported in any future trials.
Future research into routes of catheterisation should report on the outcomes with no or limited usable evidence, using standardised definitions for symptomatic UTI and asymptomatic bacteriuria. Trials including men comparing suprapubic and intermittent urethral catheters are required.
Adverse effects
From the trials that we included which compared suprapubic catheterisation to indwelling urethral catheterisation, it would appear that there are many benefits to suprapubic catheterisation. The evidence suggests that fewer patients have pain with suprapubic catheters compared to indwelling, and the incidence of asymptomatic bacteriuria is reduced with suprapubic catheters. However, these trials have very limited, if any, reporting, on the complications associated with insertion of a suprapubic catheter. Suprapubic catheters have to be inserted under anaesthetic, unlike urethral catheterisation. As the majority of the trials included in this review studied surgical patients, this aspect of suprapubic catheterisation was not a primary concern. However, the insertion of a suprapubic catheter is associated with both intraoperative and postoperative complications. An audit of three NHS urology institutions found a suprapubic insertion intraoperative complication rate of 10%, with complications including:
anaesthetic‐related complications;
inability to position patient correctly for catheter insertion;
bowel injury/perforation;
catheter malpositioning/expulsion (Ahluwalia 2006).
It is hard to know whether the trials included in our review comparing suprapubic catheterisation had any incidences of insertion complications, or whether these complications were simply not reported. The CDC recommends future research into the risks and benefits of suprapubic catheters as an alternative to urethral catheters, with a particular focus on complications related to catheter insertion or catheter site (Gould 2009). The EAU recommend that suprapubic and intermittent catheterisation is “preferable to indwelling in the appropriate patient”. This is a recommendation based on evidence from well‐conducted clinical trials, though not RCTs (Grabe 2015). Future trials should measure and report complications associated with catheter insertion.
Quality of the evidence
For this review, we assessed methodological flaws of the included trials using the reports of the trials, which relied on the quality of reporting. Many of the trials had poor reporting, which led to 'Risk of bias' domains being assigned an unclear risk of bias. The domains that were affected by poor reporting in this review were:
random sequence generation;
allocation concealment;
blinding of other outcome assessment.
Many of the included trials failed to report our prespecified outcomes, which left limited evidence for the outcomes. As a result, many of the pooled results were not significant, with wide CIs.
Future trials should follow the CONSORT checklist, to allow trials to be assessed adequately for systematic reviews, but also so that readers can have confidence in the results that are being detailed.
Assessment of the quality of evidence followed the GRADE approach. The quality of evidence for the reported outcomes was low or very low. We downgraded quality of evidence by one or two levels for the following reasons:
Limitations in design and implementation, i.e. there was evidence of methodological flaws;
Indirectness of evidence, i.e. the population, intervention, comparator and outcome were not directly related to the outcome of interest;
Inconsistency of results,where there was unexplained statistical or clinical heterogeneity;
Imprecision of results, where the pooled effect crossed the line of no effect and the 95% CI was wide;
Publication bias. We used a funnel plot to assess publication bias.
Further information on the quality of evidence for the five GRADE outcomes can be found in Table 1; Table 2; Table 3, and the risks of bias of the trials are also presented visually (Figure 2; Figure 3).
Potential biases in the review process
We searched all the relevant databases, with no language restrictions, which allowed as many reports of trials as possible. We also included trial registries in this search, and contacted authors for further information about trials that were reported as completed. It is possible that not all eligible trials were included in the databases that we searched. For some of the older reports of trials there were limited usable data, and contacting their authors was challenging. To reduce the risk of bias, we used the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Another potential source of bias may have been introduced when selecting GRADE outcomes to assess the quality of the evidence. It is possible that, as all the previously‐included data from the original review were included, the review authors were influenced to pick the outcomes with the most available data. We minimised this risk of bias by looking at the results of a focus group that picked outcomes important to patients who had experience of urinary catheterisation. We also researched common problems affecting patients undergoing short‐term urinary catheterisation.
Agreements and disagreements with other studies or reviews
We found the following relevant reviews or guidelines:
An earlier Cochrane review comparing short‐term urinary catheter policies restricted to people after urogenital surgery found insufficient evidence to favour indwelling urethral or suprapubic catheterisation to reduce the incidence of symptomatic UTI; this was similar to our review (Phipps 2006).
The CDC 2009 guideline on catheter‐associated urinary tract infection (CAUTI) prevention also recommended that neither route of catheterisation appeared to be more effective than the other, although this was based on low quality of evidence (Gould 2009).
The EAU 2015 evidence‐based guideline on urological infection found that intermittent urethral catheterisation was associated with a decrease in the incidence of bacteriuria compared with indwelling urethral catheterisation. However, they could not make any recommendations for symptomatic UTI (Grabe 2015).
When comparing suprapubic catheterisation and intermittent urethral catheterisation for the reduction of symptomatic UTI, the IDSA guideline on CAUTI was also unable to make recommendation on which route was more effective (Hooton 2010).
Healy 2012 is a systematic review and meta‐analysis comparing suprapubic catheterisation with urethral (both indwelling and intermittent) catheterisation for short‐term catheterisation following gynaecological surgery. It found that suprapubic catheterisation significantly decreased the incidence of UTI compared with urethral routes of catheterisation. However, this review defined UTI as both symptomatic and asymptomatic bacteriuria. This may explain why they found a significant result, whereas our review found the evidence to be inconclusive.
Our updated review comparing routes of catheterisation for short‐term use found that indwelling urethral catheterisation was associated with more pain than with suprapubic catheterisation. This result is similar to a systematic review conducted by McPhail 2006, which found that patients with indwelling urethral catheters were more likely to have associated pain or discomfort than patients with suprapubic catheters. This is unsurprising, as all their included trials were included in our own review. However, the magnitude of effect in the McPhail review was smaller than ours, which is probably due to the fact our review includes more trials. Additionally, our review had pain and discomfort as separate outcomes, whereas McPhail 2006 combined them as one.
Authors' conclusions
Implications for practice.
Comparison 1: Indwelling urethral catheterisation vs suprapubic catheterisation
There was insufficient evidence to determine which route of catheterisation reduced the risk of symptomatic UTI. Suprapubic catheterisation reduced the risk of asymptomatic bacteriuria compared to indwelling urethral catheterisation, although it is unclear if this is clinically meaningful. Fewer participants with a suprapubic catheter reported pain and discomfort, and were less likely to require recatheterisation. Ease of use, quality of life and cost utility analysis were not reported.
Comparison 2: Indwelling urethral catheterisation vs intermittent urethral catheterisation
There was insufficient evidence for both symptomatic UTI and asymptomatic bacteriuria to draw conclusions for clinical practice. Indwelling urethral catheterisation had a reduced risk of acute urinary retention compared to intermittent urethral catheterisation. We could not assess the remaining outcomes such as pain, ease of use, quality of life and cost utility analysis because of lack of usable data.
Comparison 3: Suprapubic catheterisation vs intermittent urethral catheterisation
From the small number of trials available for this comparison, there was insufficient evidence for any of the outcomes. Symptomatic UTI and pain were reported in one trial, but no significant difference were identified between suprapubic and intermittent urethral group.
The evidence from this review failed to determine which route of catheterisation is most effective for reducing the incidence of catheter‐associated symptomatic UTI. The evidence also failed to address key outcomes such as pain, ease of use, quality of life and cost utility analysis. Additionally, the majority of the trials did not report adverse events associated with suprapubic catheter insertion. There is currently insufficient evidence to support changes based on limited evidence with few statistically or clinically significant results.
Implications for research.
This review found insufficient evidence to select a route of catheterisation to reduce symptomatic UTI.
We need more adequately‐powered RCTs reporting the primary outcome of symptomatic UTI, using a standardised definition from the CDC, IDSA or EAU guidelines. Urine samples for symptomatic UTI should be collected based on clinical indications.
The trials included in this review reported a wide range of outcomes. To guide future trials on what outcomes to report, a core outcome set for short‐term urinary catheterisation should be developed. This core outcome set should include outcomes important to patients, healthcare providers, and other stakeholders. We suggest that the following outcomes should be reported: symptomatic UTI, other adverse effects, pain during use, ease of use, quality of life and cost utility analysis using standardised measures.
As well as future trials reporting the most important outcomes appropriately, consideration should be given to which clinical scenarios actually require short‐term urinary catheterisation. As can be seen from this review, there are many associated complications of urinary catheterisation, such as infection, discomfort, and urethral trauma. By safely reducing the number of patients who have a urinary catheter inserted, the number of patients affected by adverse events will decrease by default, as well as a reduction in the associated costs and resource implications for healthcare providers.
Future trials comparing suprapubic and intermittent urethral catheterisation for short‐term use in hospitalised men should be conducted, as we found none when searching for this review. Trials including men under 40 years of age should also be conducted. The participant‐centred outcomes of symptomatic UTI, ease of use, quality of life and cost utility analysis all require further evidence comparing any two of the three catheters. We need more evidence for pain in trials comparing indwelling urethral versus intermittent urethral, and suprapubic versus intermittent urethral catheterisation.
Future trials should be reported adequately, following the CONSORT statement. Trials presenting continuous data should report standard deviations.
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2015 | New search has been performed | In this update, the review authors have added 25 trials. They performed 'Risk of bias' assessment on all 42 trials in accordance with the current methodology. We held a group discussion with participants who underwent urethral or suprapubic catheterisation in order to identify outcomes which were important from their perspective. We used these outcomes to assess the quality of evidence with the GRADE approach. |
| 1 December 2015 | New citation required but conclusions have not changed | The review was updated however the conclusions did not changed. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 13 October 2008 | Amended | Converted to new review format. |
| 15 August 2007 | New search has been performed | An updated search (performed on 29 May 2006) of the Incontinence Group Specialised Register found no new relevant trials for this review. The existing synopsis was replaced by a plain language summary in accordance with Cochrane guidelines. |
| 25 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors would like to acknowledge the contribution of Peterhans J Van den Broek and Barbara S Niël‐Weise for their contribution to the previous versions of the review.
For the 2015 update, the review authors would like to acknowledge Anna Sierawska, who translated a German paper.
Thanks to the following authors who supplied information about trials:
Liz Dixon (Dixon 2010)
Dr N Harke, MD, FEBU (NCT02108431 2013)
Vicky Arakelian from St. George's Hospital, Kogarah (Ichsan 1987)
Mr Ridzuan Farouk (Ratnaval 1996)
Dr Fernando Korkes (Korkes 2008)
The review authors would like to acknowledge Shaun Treweek for helping us with writing the plain language summary. The structure of the plain language is based on Shaun’s original idea but the review authors take full responsibility for the actual content of the plain language summary.
We would like to thank Thomas Lam who provided expert advice on clinical aspects of the review.
We would like to acknowledge Patrick Woodman for offering some help with abstract screening and data abstraction.
We would also like to thank the following Cochrane Incontinence editorial base staff members for their help and support with this review: Cathryn Glazener, Sheila Wallace, Suzanne Macdonald and Erika Magnago.
We would also like to acknowledge Linda Pennet and Deborah Munro for arranging participants for group discussion and are grateful to all five participants who shared their views. This was extremely helpful for selecting outcomes for the 'Summary of findings' Tables.
Appendices
Appendix 1. Search strategies
Incontinence Group Specialised Register
We searched the Incontinence Group Specialised Register using the Group's own keyword system. The date of the last search was: 26 February 2015. The search terms used were:
(design.rct* or design.cct*) AND ({intvent.mech.cath*} or {intvent.mech.device*} or {intvent.mech.sheaths.} or {intvent.prevent.antibiotics*} or {intvent.prevent.antinfect.*} or {intvent.prevent.cath*} or {intvent.prevent.cleaning fluids*} or {intvent.prevent.surg*} or {intvent.surg.intraoperativemanagement*} or {intvent.surg.postsurgman*} or {intvent.surg.presurgman*.} or {intvent.surg.urethrotomy.})
(All searches were of the keyword field of Reference Manager 2012).
CINAHL
CINAHL (on EBSCO) covering 1 January 1981 to 27 January 2015 (searched on 27 January 2015). The search strategy used is given below:
| # | Query |
| S29 | (S23 AND S28) |
| S28 | S24 OR S25 OR S26 OR S27 |
| S27 | TI urin* N6 catheter* OR AB urin* N6 catheter* |
| S26 | (MH "Catheter Removal") OR (MH "Sheath Removal") OR (MH "Urinary Catheter Care (Saba CCC)") OR (MH "Urinary Catheter Insertion (Saba CCC)") OR (MH "Urinary Catheter Irrigation (Saba CCC)") OR (MH "Urinary Tract Infections, Catheter‐Related") OR (MH "Urinary Catheterization+") OR (MH "Catheters, Urinary+") |
| S25 | (MH "Catheter Occlusion") |
| S24 | (MH "Catheter Care, Urinary+") |
| S23 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 |
| S22 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | (PT Clinical Trial) OR (PT "randomized controlled trial") |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
Data and analyses
Comparison 1. Indwelling urethral vs suprapubic catheterisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic UTI | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall | 5 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.61, 1.69] |
| 1.2 Men | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.83] |
| 1.3 Women | 2 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.62, 2.74] |
| 1.4 After urogenital surgery | 3 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.59, 2.29] |
| 1.5 After non‐urogenital surgery | 2 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.40, 1.85] |
| 1.6 With antibiotic prophylaxis | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.59] |
| 1.7 Without antibiotic prophylaxis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Asymptomatic bacteriuria | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Overall | 19 | 1894 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.63, 3.10] |
| 2.2 Men | 4 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.09, 2.57] |
| 2.3 Women | 9 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.37, 3.85] |
| 2.4 After urogenital surgery | 8 | 1004 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.53, 4.42] |
| 2.5 After non‐urogenital surgery | 10 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.24, 3.28] |
| 2.6 With antibiotic prophylaxis | 7 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.05, 6.26] |
| 2.7 Without antibiotic prophylaxis | 3 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [1.47, 5.98] |
| 2.8 Urine sample collected during catheterisation | 4 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 3.69 [1.73, 7.88] |
| 2.9 Urine sample collected once catheterisation stopped | 11 | 1325 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.30, 2.65] |
| 3 Recatheterisation | 11 | 1180 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.19, 4.09] |
| 4 Mean duration of catheterisation in days | 2 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐1.73 [‐2.42, ‐1.05] |
| 5 Duration of catheterisation | Other data | No numeric data | ||
| 6 Number of participants catheterised more than five days | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Number of participants with acute urinary retention | 2 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.35, 1.94] |
| 8 Number of participants with chronic urinary retention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Number of participants with bladder dysfunction | 2 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.56, 4.18] |
| 10 Number of participants with pain | 4 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.62 [3.31, 9.55] |
| 11 Number of catheter days with pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Mean pain score (VAS 0 ‐ 10) | Other data | No numeric data | ||
| 13 Number of participants with discomfort | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Overall | 3 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.77 [2.68, 5.32] |
| 13.2 Men | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.78, 8.43] |
| 13.3 Women | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.64, 7.27] |
| 14 Number of participants with catheter obstruction | 5 | 694 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.78] |
| 15 Number of participants with catheter that fell out | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 Number of participants that had urine leak around the catheter | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 Number of participants with gross haematuria | 4 | 557 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.16, 0.96] |
| 18 Number of participants with microscopic haematuria | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19 Number of participants with pyuria | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 2.35 [1.13, 4.90] |
| 20 Number of participants with urethral stricture | 4 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.02, 5.56] |
| 21 Urinary symptoms after surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22 Number of participants with epididymitis | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.08, 43.16] |
| 23 Number of participants with postoperative pyrexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24 Febrile morbidity | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25 Number of participants who needed antibiotic therapy | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.36, 3.24] |
| 26 Number of participants requiring drugs for relief of dysuria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 27 Duration of hospital stay | Other data | No numeric data | ||
| 28 Mean duration of hospital stay | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 29 Number of participants with extended hospital stay | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 30 Cost | Other data | No numeric data |
Comparison 2. Urethral vs intermittent catheterisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic UTI | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 After urogenital surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Non‐surgical | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Asymptomatic bacteriuria | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Overall | 13 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.85, 1.28] |
| 2.2 Male | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.79 [0.72, 194.19] |
| 2.3 Female | 8 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.23] |
| 2.4 After urogenital surgery | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.15, 6.26] |
| 2.5 After non‐urogenital surgery | 7 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.85, 1.71] |
| 2.6 Women in labour | 2 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.22] |
| 2.7 After caesarean section | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.28] |
| 2.8 With antibiotic prophylaxis | 6 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.07, 2.40] |
| 2.9 Without antibiotic prophylaxis | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.28] |
| 2.10 Urine sample taken during catheterisation | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.96, 5.44] |
| 2.11 Urine sample taken once catheterisation stopped | 10 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.82, 1.37] |
| 3 Mean duration of catheterisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Duration of catheterisation | Other data | No numeric data | ||
| 5 Number of participants using catheter at 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Number of participants with acute urinary retention | 4 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.22, 0.91] |
| 7 Number of participants with bladder dysfunction | 3 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.48, 1.19] |
| 8 Number of participants unable to void after catheterisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Urinary symptoms after surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Number of participants with postoperative pyrexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Number of nurses not preferring catheter | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Duration of hospital stay | Other data | No numeric data | ||
| 13 Cost | Other data | No numeric data | ||
| 14 Post‐catheter quality of life | Other data | No numeric data | ||
| 14.1 Postcatheter Pain Score (VAS 0‐100) | Other data | No numeric data | ||
| 14.2 Catheterisation difficulty (VAS 0‐100) | Other data | No numeric data | ||
| 14.3 Postcatheter Patient Satisfaction (VAS 0‐100) | Other data | No numeric data | ||
| 14.4 EQ‐5D scores; mean score (n of patients) | Other data | No numeric data | ||
| 14.5 EQ VAS scores; mean score (n of patients) | Other data | No numeric data | ||
| 14.6 SF‐6D scores; mean scores (n of patients) | Other data | No numeric data |
Comparison 3. Suprapubic vs intermittent catheterisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic UTI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Asymptomatic bacteriuria | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.20, 1.35] |
| 3 Duration of catheterisation | Other data | No numeric data | ||
| 4 Number of participants with pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Duration of hospital stay | Other data | No numeric data | ||
| 6 Cost | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 1993.
| Methods |
RCT or quasi‐RCT: 3‐arm RCT Setting: district hospital in UK Period: participants recruited over period of one year |
|
| Participants |
Population: Men who underwent TURP who presented with acute urinary retention Inclusion criteria: not reported Exclusion criteria: not reported Age (mean): A 71.6 year; B 71.9 years; C 72.4 years. Number of participants:
Dropouts (n of participants + reasons): Not described. Follow‐up: outpatient review |
|
| Interventions | A (n = 50): Transurethral catheterisation (type not listed) placed preoperatively using 1& Xylocaine gel under aseptic technique B (n = 47): Suprapubic catheter (Stamey‐type, 12 French or 14 French), placed preoperatively under local anaesthetic C (n = 63): No preoperative bladder drainage Intended duration of intervention: "Short term basis" |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Preoperative bacteriuria: A: 16/50; B: 7/47; C: 5/63 Postoperative bacteriuria: A: 20/50; B: 12/47; C: 4/63, P < 0.001 Definition of bacteriuria: urine culture with bacterial count > 10⁸ colonies/litre. Urine cultures were obtained at the time of catheterisation and repeated if delay in the operation; at the time of catheter removal, and at 6 weeks follow‐up if symptomatic of UTI Number of participants who received antibiotics as indicated by clinical course: A: 20/50; B: 12/47; C: 4/63 Positive cultures from prostatic chippings: A: 26/38; B: 16/38; C: 10/51 Positive culture for pathogenic organisms from prostatic chippings: A: 12/38; B: 5/38; C: 6/51 Number of participants with epididymitis at 6 weeks follow‐up: A: 4/50; B: 0/47; C: 0/63 Urethral stricture: A: 0/50; B: 0/47; C: 0/63 Secondary haemorrhage: A: 0/50; B: 1/47; C: 1/63 Duration of preoperative catheterisation (mean) (days): A: 8.3; B: 8.0 |
|
| Sponsorship/Funding | Not reported | |
| Notes | No power analysis. Routine prophylactic antibiotics were not used in either group Prostatic chippings were obtained in all participants, usually from the superficial part of the middle lobe |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "these patients were divided into 3 groups" "patients presenting with urinary retention were randomly included" ‐ not clear on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given but can assume no blinding as indwelling vs suprapubic vs no catheter |
| Blinding of personnel | High risk | "Both types of catheter were introduced by residents on call" ‐ no information on blinding but can assume no blinding occurred if involved in insertion |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know the catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 160 participants randomised. Incomplete data for prostatic chippings culture but evenly distributed between 3 intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods have data reported in Results. Length of preoperative catheterisation has results but no information given in Methods. Unable to access protocol so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Andersen 1985.
| Methods |
RCT or quasi‐RCT: RCT Setting: Denmark Period: not reported |
|
| Participants |
Population:women undergoing colposuspension or vaginal repair for stress urinary incontinence and/or genital descensus Inclusion criteria: women with stress urinary incontinence and/or genital descensus; colposuspension or vaginal repair operations indicated Exclusion criteria:Recurrent UTI, treatment with steroids, significant bacteriuria at the time of operation, if protocol not followed strictly Age (median, range): overall 61 years (34 ‐ 86 years) Number of participants:
Dropouts (n of participants + reasons): 15 patients were excluded according to the exclusion criteria Follow‐up: 1 year postoperatively |
|
| Interventions | A (n = 44): indwelling urethral catheterisation (Charriere16, Foley) inserted preoperatively B (n = 48): Suprapubic catheter (Charriere 12, Ingram) introduced after termination of the operation Intended duration of intervention: The SPC was clamped on the 3rd postoperative day and removed if the participant could micturate spontaneously and the residual urine was < 80 ml. The UC was left until the 5th postoperative day |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: Overall: A:20/44; B:10/48 In subgroups: a) After colposuspension: A:7/17; B:1/18. b) After vaginal repair: A:13/27; B:9/30 Definition of bacteriuria: 105 cfu/ml on the 5th postoperative day. Specimens were obtained by the catheter in the control group and by midstream in the treatment group Recatheterisation: A:8/44; B:3/48 Mean duration of catheterisation: A: 5.0 days; B: 3.7 days |
|
| Sponsorship/Funding | Simonsen & Weel Company Ltd., Albertslund, Denmark – supplied Ingram® catheters (suprapubic) | |
| Notes | A participant was recatheterised when the volume of residual urine was > 150 ml on the 7th postoperative day despite receiving bladder tonica Not reported whether prophylactic antibiotics were used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomized... using random numbers (Geigy's tables)" ‐ randomisation using random numbers table is an adequate method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given but as suprapubic vs transurethral can assume no blinding |
| Blinding of personnel | High risk | No information given, but transurethral inserted preoperatively and suprapubic intraoperatively can assume clinician not blinded |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 76 participants (83%) met for the follow‐up trial, 16 (17%) lost to follow‐up at 1 year. No details given of number in each intervention group or reason for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | 1‐year data not presented by type of catheter because numbers deemed too small. Unable to access protocol so uncertainty about reporting bias |
| Other bias | Low risk | Catheters supplied by Simonson & Weel. No other funding source reported. Unlikely to have influenced outcomes. |
Baan 2003.
| Methods |
RCT or quasi‐RCT: RCT Setting: The Netherlands Period: not reported |
|
| Participants |
Population: patients undergoing major abdominal procedure who required standard bladder catheterisation Inclusion criteria: patients undergoing major abdominal procedure who required standard bladder catheterisation Exclusion criteria: < 18 years old; UTI or urinary incontinence at the time of catheterisation; history of urological disease or renal insufficiency; immunocompromised; unable to speak and write the Dutch language; undergone a rectum extirpation and an ileoanal pouch; history of surgery for thoracic or abdominal aortic aneurysm Age (mean, range): A 59.8 years (26 ‐ 81); B 60.4 years (37 ‐ 87) Number of participants:
Dropouts (n of patients + reasons): 13 participants for "protocol deviation" (A: 3; B: 10) Follow‐up: Outcomes recorded during hospital stay and 6 weeks postoperatively |
|
| Interventions | A (n = 71): Urethral catheter (Foley) placed before surgery after surgical scrub B (n = 75): Suprapubic catheter (Braun) placed at the time of surgery Intended duration of intervention: Urethral catheters were removed when condition stable, or when the epidural catheter had been removed 24 hours. SPC was removed after spontaneous voiding with a PVR of < 50 ml. |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 8/71; B: 9/75 Definition of symptomatic UTI: At least 1 or more of the clinical symptoms (fever, increased micturition frequency, burning pain during voidance, and pain in the lower abdomen), a positive sediment (> 10 leukocytes), and a positive urine culture (> 10⁵ bacterial colonies and < 3 bacterial species) within 6 weeks after surgery Bacteriuria: A 8/71; B 9/75 Definition of bacteriuria: "positive culture" > 10⁵ bacterial colonies and < 3 bacterial species Postoperative hospital stay (median) (days): A: 15.6; B: 13.1 Number of re‐operations (laparotomies): A: 4/71; B: 7/75 Number of participants requiring recatheterisation: A: 4/71; B: 9/75 Duration of catheterisation (median) (days): A: 5.9; B: 6.5 UTI in participants who received their allocated catheter: A: 8/68; B: 8/65 Incidence of UTI in participants by sex: 4/82 men, 13/64 women Participant opinions about comfort and pain: no differences |
|
| Sponsorship/Funding | Not reported | |
| Notes | Urine cultures sent 48 hours after catheter removal. Recatheterisation done for relaparotomy or sepsis Prophylactic antibiotics were used in all participants perioperatively for 24 hours |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly allocated ‐ stratified for sex... using a computerized randomization program" ‐ adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | "blinding for patients... was not possible" ‐ blinding did not occur |
| Blinding of personnel | High risk | "blinding for nursing staff was not possible" ‐ blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI would be assessed by microbiologist who would not know the type of catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "assessment of primary end point... was performed blinded" "a coded data form of each patient ... was provided to two of the investigators who independently scored for urine tract infections" ‐ primary outcome of UTI was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 146 participants randomised: 75 suprapubic, 71 transurethral No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes in Methods all have data in Results. Unable to access protocol so cannot fully assess risk of reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Barents 1978.
| Methods |
RCT or quasi‐RCT: quasi‐RCT Setting: The Netherlands |
|
| Participants |
Population: patients undergoing vaginal operations Inclusion criteria: women with sterile urine undergoing vaginal operations Exclusion criteria: initial positive urine cultures Age: not reported Number of participants:
Dropouts (n of participants + reasons): 20 patients were excluded because of initial positive urine cultures: (A: 6; B: 14) |
|
| Interventions | A (n = 50): Indwelling urethral catheter (Silicath Foley) introduced after termination of the operation B (n = 100): Suprapubic catheter (12 Charriere, Silastic Cystocath) introduced perioperatively or after termination of the operation |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A:16/44; B:5/86 Subgroups: a) In participants without antibiotic prophylaxis: A: 14/29; B: 5/65. b) In participants with antibiotic prophylaxis: A: 2/15; B: 0/21 Definition of bacteriuria: 105 cfu/ml or more on postoperative day 5. Specimens were aspirated from the drainage tube on the 5th and 7th postoperative day and on the day of catheter removal. Mean duration of catheterisation: At least 7 days in both groups |
|
| Sponsorship/Funding | Not reported | |
| Notes | Results were stratified according to antibiotic prophylaxis or not. It was not reported whether the prophylactic protocol was the same for all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised 1st and 2nd patients to Group I (suprapubic) 3rd patient to group II (urethral) – no randomisation |
| Allocation concealment (selection bias) | High risk | No allocation concealment based on quasi‐randomisation method |
| Blinding of participants | High risk | Can assume no blinding occurred as suprapubic vs urethral |
| Blinding of personnel | High risk | Can assume no blinding occurred as suprapubic vs urethral |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know the catheter participants had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Initially 150 patients allocated – suprapubic:100 + urethral: 50; outcome reporting suprapubic: 86 + urethral: 44 – no information on reason for exclusion/dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information given. Unable to access protocol, some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
Barry 1992 PE.
| Methods |
RCT or quasi‐RCT: RCT Setting: Dublin, Ireland Period: not reported |
|
| Participants |
Population: patients undergoing bladder catheterisation during elective surgery Inclusion criteria: unclear Exclusion criteria: preoperative urinary tract infection Age (mean, SD)/(median, range): not reported Number of participants: · Eligible: 60 · Randomised: 60 · Reported: 60 Dropouts (n of participants + reasons): 0 Follow‐up: not reported |
|
| Interventions |
Time of intervention:
A (n = 36): indwelling urethral catheterisation. Inserted at induction B (n = 24):suprapubic catheterisation. Inserted at laparotomy Intended duration of catheterisation: Not reported |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 3/36; B: 3/24 Definition of symptomatic UTI: not reported Bacteriuria: not reported Definition of bacteriuria: not reported Quality of life: not reported Duration of catheterisation, days (mean, n): A: 5, 36; B: 5, 24 Gross haematuria: A: NR; B: 1/24 Catheter leaks: A: NR; B: 2/24 Technical failures of insertion: A: NR; B: 4/24 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Assume 0 for group B where number of events not reported? Antibiotic prophylaxis use not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given on method of allocation concealment |
| Blinding of participants | High risk | No information given. As suprapubic vs indwelling can assume that blinding did not occur |
| Blinding of personnel | High risk | No information given. As suprapubic vs indwelling can assume that blinding did not occur |
| Blinding of microbiological outcome assessment | Unclear risk | No definition given for symptomatic UTI so do not know if assessed by microbiologist from urine culture, or assessed by clinician based on symptoms |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 participants randomised (suprapubic 24, urethral 36). No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Outcomes that were to be reported not stated. Unable to access protocol so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Bergman 1987.
| Methods |
RCT or quasi‐RCT: RCT Setting: California, USA |
|
| Participants |
Population: patients undergoing vaginal urethropexy (Pereyra procedure) (and hysterectomy) Inclusion criteria: women with stress urinary incontinence and negative urine culture undergoing vaginal urethropexy (Pereyra procedure) (and hysterectomy). Stress urinary incontinence was diagnosed clinically and urodynamically Exclusion criteria: not reported Age (mean, range): 53 years (35 ‐ 68) Dropouts (n of participants + reasons): none Follow‐up: length of hospital stay |
|
| Interventions | A (n = 27): Indwelling urethral catheterisation (14 F Foley) introduced before surgery B (n = 24): Suprapubic catheterisation (5F Bonnano) introduced after termination of the operation. During surgery participants had indwelling urethral catheter. Duration of intervention: The suprapubic and indwelling urethral catheters were clamped on the 3rd postoperative day and the participant was allowed to micturate spontaneously with a symptomatic full bladder. The catheter was removed when the residual urine was < 50 ml on 2 consecutive occasions. In the urethral group, the residual urine was measured by reinserting a fresh Foley catheter. Some participants were discharged with a catheter and were seen every other day in the outpatient clinic. |
|
| Outcomes |
Bacteriuria: A:13/27; B:3/24 Definition of bacteriuria: more than 1000 cfu/ml in the 1st 5 postoperative days. Specimens were aspirated from the drainage tube before surgery and every 2 days thereafter Duration of catheterisation (mean): A: 6.8 days; B: 3.7 days Febrile morbidity (measured by 'Fever index') (mean ± SD): A: 22.3 ± 6.4; B: 8.8 ± 2.1 Number of participants leaving hospital with catheter: A: 15/27; B:4/24 Hospital stay (mean ± SD): A: 4.7 ± 1.6; B: 3.6 ± 1.3 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Comment: All participants had urethral catheters during the operation.
Mean fever index was calculated as (degree) X (hours a participant's temperature exceeded 37.2 C, except for the first 48 hours).
Comment: In 3 participants (1 in the urethral group and 2 in the suprapubic group) UTI was not the source of fever All participants received the same antibiotic prophylaxis (cefoxitin 2 g intramuscularly 1 hour before and 6 and 12 hours after surgery). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were assigned (using a randomisation table)" |
| Allocation concealment (selection bias) | Unclear risk | No information given on method of allocation concealment |
| Blinding of participants | High risk | No information given but as suprapubic or transurethral can assume no blinding |
| Blinding of personnel | High risk | No information given but as surgical procedure can assume clinician not blinded |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know which catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 51 participants randomised; suprapubic 24, Foley 27. No dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | No detailed information given on outcomes in Methods, therefore cannot judge if selective reporting occurred. Unable to access protocol so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Botsios 1997.
| Methods |
RCT or quasi‐RCT: RCT Setting: Greece Period: March 1992 ‐ December 1995 |
|
| Participants |
Population: patients undergoing elective abdominal surgery of long duration Inclusion criteria: patients undergoing elective abdominal surgery of long duration Exclusion criteria: patients with preoperative dysuric complaints, abnormal findings at urine analysis, rectal diseases in which urethral catheterisation is the preferable method Age (mean, SD): A: 64.3 (1.2) years; B 63.8 (1.4) years Dropouts (n of participants + reasons): 7 participants were excluded because of faults in urine sampling or processing |
|
| Interventions | A (n = 88): Indwelling urethral catheterisation (14‐or 16‐french Foley) introduced after induction of anaesthesia B (n = 85): Suprapubic catheterisation (Cystofix B) introduced intraoperatively Duration of intervention: The SPC was clamped on the 2nd postoperative day and removed the next day if the participant could micturate normally and the residual urine was ≤ 100 ml. The UC was removed on the 3rd or 4th postoperative day, depending on the severity of the operation |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 2/88; B: 0/85 Definition of bacteriuria: Significant bacteriuria was defined as > 105 cfu/ml. A midstream specimen was taken on the 2nd day after removal of the catheter Recatheterisation: A: 8/88; B: 0/85 Mean duration (range) of catheterisation: A: 4.4 days (3 ‐ 11); B: 3.3 days (3 ‐ 10) Number of participants with pain: A: 50/88; B: 10/85 Gross haematuria: A: 4/88; B: 4/85 Microscopic haematuria: A: 36/88; B: 16/85 Pyuria: A: 23/88; B: 6/85 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Recatheterisation occurred when normal micturition failed The urine was inspected daily for gross haematuria and specimens were taken daily for biochemical analysis and microscopy. The last was considered abnormal if it showed > 6 white blood cells and/or 2 red blood cells per high‐power field Each participant kept a daily record of catheter‐related pain, scoring discomfort on a standard visual analogue scale (no pain = 0; worst possible pain = 10) Not reported whether prophylactic antibiotics were used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomly assigned (using their departmental record number)" ‐ non‐random sequence generation |
| Allocation concealment (selection bias) | High risk | "randomly assigned (using their departmental record number)" ‐ allocation could be easily broken using departmental record number, high risk of selection bias |
| Blinding of participants | High risk | No information given, but as suprapubic vs indwelling can assume blinding did not occur |
| Blinding of personnel | High risk | No information given, but as suprapubic inserted perioperatively and indwelling pre‐operatively can assume no blinding |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 180 randomised, 7 excluded. Remaining 173 participants: indwelling 88, suprapubic 85 "7 were excluded because their urine samples were either insufficient in number or poorly collected and processed" ‐ no information given on which arm of trial 7 participants were in. |
| Selective reporting (reporting bias) | Unclear risk | Results on recatheterisation and duration of catheterisation not detailed in Methods. Unable to access protocol, so some uncertainty about risk of reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Carpiniello 1988.
| Methods |
RCT or quasi‐RCT: RCT Setting: Pennsylvania, USA Period: November 1985 ‐ March 1986 |
|
| Participants |
Population: Elderly women undergoing total joint replacement Inclusion criteria: Elderly women undergoing total joint replacement Exclusion criteria: Men; non‐primary total joint replacement; positive preoperative urine cultures (> 105 cfu/ml); receiving general anaesthesia; confined to postoperative bed rest Age (mean, SD): A: 70 (8.6) years; B: 73 (6.6) years Number of participants:
Dropouts (n of participants + reasons): none |
|
| Interventions | A (n = 23): Indwelling urethral catheter (Foley) placed preoperatively and maintained for 24 hours B (n = 31): Intermittent catheter performed in recovery room C (n = 23): No catheter used Intended duration of intervention: For approximately 24 hours postoperatively in Group A |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 1/23; B: 5/31; C: 2/23 Definition of bacteriuria: 105 cfu/ml Straight catheter volume obtained after recovery room (mean, SD) (ml): A: N/A; B: 457 (640); C: 454 (494) Recatheterisation after Foley removal: A: 1/23; B: 20/31; C: 13/23 "Deep sepsis": A: 0/23; B: 0/31; C: 0/23. (No definition of deep sepsis) |
|
| Sponsorship/Funding | Not reported | |
| Notes | Treatment Group B (CISC) had urethral catheter replaced once in recovery room, and repeated if clinically in urinary retention after recovery room Pre‐operative positive culture was defined as > 105 cfu/ml. No distinction made between Treatment B participants who needed a repeat in‐and‐out catheter and those in Treatment A or Control who needed a Foley placed because of urinary retention, which was not defined Prophylactic antibiotics were used until postoperative day 3; either prophylactic cefazolin sodium (Ancef) or clindamycin (Cleocin) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly assigned to one of three groups" ‐ no information given on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given on method of allocation concealment |
| Blinding of participants | High risk | No information given but possible that group A and B remained blinded (A: intermittent in recovery room; B: no catheterisation performed in recovery room). Group C (indwelling) unlikely to remain blinded, therefore high risk of bias |
| Blinding of personnel | High risk | No information given but unlikely that clinician was blinded in perioperative or postoperative period |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77 participants randomised; Group A 31, Group B 23, Group C 23 No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Outcomes detailed in Methods covered in Results: urine cultures, number + volume of catheterisations, need for + duration of Foley catheter, results of any urologic tests (including occurrence of UTI) Unable to access protocol so some uncertainty about reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Dixon 2010.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK Period: March 2004 ‐ July 2004 |
|
| Participants |
Population: women undergoing surgery for pelvic organ prolapse and/or stress urinary incontinence Inclusion criteria: women electively admitted for surgery for pelvic organ prolapse and/or stress urinary incontinence Exclusion criteria: • women undergoing surgery where postoperative catheterisation is not routinely used • women requiring continuous postoperative bladder drainage Age (median): A: 66 years; B: 57 years Number of participants: · Eligible: · Randomised: 75 · Reported: 72 Dropouts (n of participants + reasons): 2 from group A (operative bladder injury, latex allergy); 1 from group B (cancelled operation) Follow‐up: duration of hospital stay |
|
| Interventions |
Time of intervention: A (n = 38): indwelling suprapubic catheter inserted in theatre, left on free drainage for 48 hours postoperatively B (n = 37): intermittent catheterisation postoperatively if unable to pass urine within 6 hrs of return from theatre or earlier if uncomfortable or if passing frequent (< 2 hourly), small volumes of urine (< 200ml). Continued until can void > 200 ml with post‐void residual volumes < 100 ml Intended duration of catheterisation: A: 48 hours; B: until able to void > 200 ml and post‐void residual volumes < 100 ml |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 10/36; B: 6/36. (P = 0.44) from text A: 9/36; B: 13/36 (P = 0.44) from table Definition of symptomatic UTI: Catheter specimen of urine or a midstream urine specimen showing a single bacterium growing at a colony count of > 105 cfu/ml. Specimen only taken if UTI suspected on the basis of pyrexia > 37.5° C, frequent voiding + discomfort when passing urine and positive urinalysis for leukocytes + nitrites Bacteriuria: Not reported Definition of bacteriuria: Not reported Length of postoperative stay in days (median, range, N): A: 6 (2 ‐ 15); B: 5 (2 ‐ 19) (P = 0.003) Days of catheterisation (median, range, N): A: 5 (4 ‐ 36); 4 (2 ‐ 36). (P = 0.01) Any pain: A: 10/36; B: 6/36 Hayward pain score* (1 ‐ 5 scale, where 1 is no pain) (total score per group, N): A: 31 (36); B: 15 (36) Consumable costs per participant: A: GBP 24.90; B: GBP 10.60 Nursing time (mins/participant): A: 30; B: 90 Nursing time costs per participant: A: GBP 5.40; B: GBP 16.20 Total costs per participant: A: GBP 30.30; B: GBP 26.80 Quality of life: Not reported |
|
| Sponsorship/Funding | No funding received | |
| Notes | Contacted Liz Dixon (liz.dixon@nuth.nhs.uk) and received information about median age of each group, and confirmed days of catheterisation is median, range (not SD as reported in trial) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization sequence was generated using a random number generator programme” – adequate method of randomisation, low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | “using opaque sealed envelopes” – adequate method of concealment |
| Blinding of participants | High risk | “No blinding of patient, surgeon, nurses, or outcome assessor was feasible.” |
| Blinding of personnel | High risk | “No blinding of patient, surgeon, nurses, or outcome assessor was feasible.” |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “No blinding of patient, surgeon, nurses, or outcome assessor was feasible.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75 randomised, 38 SPC and 37 IC. SPC had 2 withdrawals (1 operative bladder injury, 1 latex allergy), IC had 1 withdrawal (cancelled operation) |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes all had results reported. Unable to access protocol so uncertainty about reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Dobbs 1997.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK Period: not reported |
|
| Participants |
Population: patients undergoing total abdominal hysterectomy for non‐malignant reasons under general anaesthetic Inclusion criteria:100 women undergoing total hysterectomy for non‐malignant reasons under general anaesthetic Exclusion criteria: not reported Age (mean): A: 45 years; B: 42.6 years Dropouts (n of participants + reasons): 5 participants were excluded because of incomplete follow‐up data |
|
| Interventions | A (n = 48): Indwelling urethral catheter (14 French) inserted under anaesthetic and removed the night after surgery (about 36 hours after operation). In case of urinary retention thereafter, an urethral catheter was inserted for a further 24 hours. B (n = 47): Intermittent catheterisation: 'In‐out' catheterisation with a disposable female catheter. Participants who felt the need to pass urine but were unable to do so, or had not passed urine by 12 hours after surgery, had a further intermittent catheter. When patients thereafter required intermittent catheter, a urethral catheter was inserted for 24 hours. Intended duration of catheterisation: 36 hours after surgery |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 14/48; B: 6/47 Definition of bacteriuria: Significant bacteriuria was defined as > 10⁵ cfu/ml. Specimens were collected on the second postoperative day Urinary symptoms immediately after surgery: A: 11/48; B: 7/47 Postoperative pyrexia (> 38° C): A: 17/48; B: 15/47 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Postoperative pyrexia defined as > 38° C Antibiotics: Participants received amoxicillin and clavulanate potassium at the induction of general anaesthetic and again 6 hours after surgery (1.2 g); it was not explicitly stated whether other antibiotics except prophylaxis were administered pre‐ or postoperatively For postoperative pain management, participant‐controlled analgesia was given to participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised by random allocation of a number before the onset of the trial" ‐ no information given on the method of random sequence generation |
| Allocation concealment (selection bias) | Low risk | "allocated numbers were sealed in an envelope, which was opened at the time of surgery" ‐ conceals allocation to participants + investigators |
| Blinding of participants | High risk | No information given but can assume not blinded |
| Blinding of personnel | High risk | No information given but can assume personnel not blinded as intermittent vs indwelling |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100 participants recruited, data only available for 95 participants. Indwelling 48, intermittent 47. No reason for 5 participants being lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes detailed in Methods all had data in Results. Unable to access protocol so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Evron 2008.
| Methods |
RCT or quasi‐RCT: RCT Setting: Israel Period: not reported |
|
| Participants |
Population: Women admitted for labour who had epidural analgesia Inclusion criteria: ASA physical status I and II, primiparous parturients who received patient‐controlled epidural analgesia for labour Exclusion criteria: Patients who had precipitous deliveries, pregnancy complications or history of drug abuse, those taking antibiotic therapy, and those with a history of urinary bladder pathology or UTI Age (mean, SD): A: 26 (4) years; B: 25 (4) years Dropouts (n of participants + reasons) 6 (3 in each group) due to precipitous labour Follow‐up: 48 hours after delivery |
|
| Interventions | A (n = 100): Urethral catheterisation (multi‐orifice Foley catheter) placed 90 minutes after epidural induction (average 3 cm cervical dilation) and removed after delivery B (n = 109): Intermittent catheterisation (multi‐orifice Foley catheter) placed 90 minutes after epidural induction (average 6 cm cervical dilation) and removed. Process repeated when clinical indication of urinary retention Intended duration of intervention: Until spontaneous voiding returned after delivery |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 29/100; B: 31/109. P = 0.9 Definition of Bacteriuria: 105 or more colonies of the same species of bacteria per ml of urine found in 2 consecutive specimens of midstream voided urine, at 24 hours and 48 hours Number of catheterisations necessary: One: A: 100/100; B: 66/109 Two: A: 0/100; B: 23/109 Three: A: 0/100; B: 5/109 Rate of false positive urinary retention: A: 0/100; B: 8/109 Length of 1st stage of labour (mean + SD) (min): A: 373 ± 186; B: 374 ± 178. (P = 0.92) Length of the 2nd stage of labour (mean + SD) (min): A: 105 + 72 ; B: 75 + 52. (P = 0.002) Total fluid input (mean + SD) (ml): A: 2047 + 820 ml; B: 1780 + 620 ml. (P = 0.012, more fluid intake with urethral catheterisation) Total urine output (mean + SD): A: 690 + 470 ml; B: 540 + 380 ml. (P = 0.029, more urine output with urethral catheterisation) Spontaneous delivery: A: 78/100; B: 91/109. (P = 0.2) Instrumental delivery: A: 3/100; B: 4/109 Cesarean section: A: 19/100; B: 14/109 |
|
| Sponsorship/Funding | "There was no financial support of this work" | |
| Notes | Participants' labours were managed under the same passive and active labour protocol for both trial groups. Treatment group was heavier (average 4 kg), had oxytocin augmentation more frequently, and required higher doses of ropivacaine than the control group Not reported if prophylactic antibiotics used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized via computer‐generated code" ‐ adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | "maintained in sequentially numbered opaque envelopes" ‐ adequate method of allocation concealment |
| Blinding of participants | High risk | No information ‐ can be assumed participants could not be blinded as intermittent vs indwelling catheterisation |
| Blinding of personnel | High risk | No information ‐ can be assumed that personnel could not be blinded as required in participant care |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators were blinded as to treatment allocation, as the indwelling bladder catheter was hidden" ‐ adequate method of outcome assessor being blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 215 parturients who were recruited to the trial, 6 were excluded (3 each from the 2 trial groups) because of precipitous labors" ‐ no differential exclusion |
| Selective reporting (reporting bias) | Low risk | Outcomes given in Methods are reported in Results. Unable to access protocol so some uncertainty on selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Hakvoort 2011.
| Methods |
RCT or quasi‐RCT: RCT Setting: The Netherlands Period: not reported |
|
| Participants |
Population: all women with abnormal PVR after vaginal prolapse surgery Inclusion criteria: women, > 18 years old, abnormal PVR (> 150 ml) by bladder scan after vaginal prolapse surgery Exclusion criteria: Any neurologic or anxiety disorder, need for concomitant anti‐incontinence surgery Age (mean, SD): A: 61 (10) years; B: 60 (12) years Number of participants:
Dropouts (n of participants + reasons): none Follow‐up: duration of hospitalisation |
|
| Interventions |
Time of intervention: 1st post‐operative day if PVR ≥ 150 ml A (n = 42): indwelling urethral catheter (14 french silicone) was inserted by nursing staff for 3 days B (n = 45): intermittent A SpeediCath® (Coloplast, Humlebaek, Denmark) catheter was inserted with maximum interval 6 hours over 3 days Intended duration of catheterisation: Minimum of 3 days |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 13/42; B: 5/45 Definition of symptomatic UTI: > 10⁵ cfu plus symptoms Bacteriuria: A: 15/42 (38%); B: 6/45 (14%). (P = 0.02, significant difference) Definition of bacteriuria: > 10⁵ cfu in voided culture obtained upon normalisation of PVR and cessation of catheterisation Duration of catheterization until normalisation of PVR (PVR < 150 ml) (median, range) (hours): A: TIC 72 (72 ‐ 144); B: 18 (5 ‐ 112) (P < 0.001) Number of catheter introductions (median, range): A: TIC 1 (1 ‐ 2); B: 3 (1 ‐ 18) (P < 0.001) Duration of hospitalisation (median, range) (days): A: 4 (1 ‐ 7); B: 2 (1 ‐ 6) (P < 0.001) Pain scores as a result of catheterisation (VAS 0 ‐ 100) (mean, SD): A: 34 (27); B: 29 (24) (P = 0.45) Difficulty with catheter use (VAS 0 ‐ 100) (mean, SD) A: 36 (32); B: 28 (25). (P = 0.20, NS) Participant satisfaction (VAS 0 ‐ 100) (mean, SD): A: 76 (24); B: 80 (22) (P = 0.41) Number of participants that would choose the same treatment again: A: 33/35; B: 37/38 (P = 0.60) |
|
| Sponsorship/Funding | "Funding: none" | |
| Notes | All participants received prophylactic antibiotics, vaginal packing and 14 Fr indwelling catheter immediately after surgery PVRs determined by bladder scan. Urine cultures sent after completion of intervention Appropriate definitions for bacteriuria and UTI used Adequately powered No loss to follow‐up All participants received prophylactic antibiotics during surgery |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerised block randomisation was performed" ‐ adequate method of randomisation |
| Allocation concealment (selection bias) | High risk | "Blinding of the next treatment allocation was not possible" |
| Blinding of participants | High risk | "because of the obvious dissimilarity of the intervention, blinding of the next treatment allocation was not possible" ‐ blinding did not occur |
| Blinding of personnel | High risk | "because of the obvious dissimilarity of the intervention, blinding of the next treatment allocation was not possible" ‐ blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Microbiologist would assess urine culture, and would not know which type of catheter the participant had received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 lost to follow‐up in each group for pain scores. No differential dropout |
| Selective reporting (reporting bias) | Low risk | Outcome measures are all reported in Results. Unable to access protocol, some uncertainty of selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Halleberg 2013.
| Methods |
RCT or quasi‐RCT: RCT Setting: orthopaedic department at a Swedish University Hospital Period: September 2009 – May 2011 |
|
| Participants |
Population: patients undergoing hip surgery Inclusion criteria: patients undergoing hip fracture surgery or hip replacement surgery that was due to osteoarthritis Exclusion criteria: < 50 years, indwelling urinary catheter or cognitive impairment at admission or if, for any reason, the patients could not give their informed consent. Cognitive impairment was defined as disorientation in time, place or room, irrelevant conversation, disorganised thinking or agitation and assessed by the nurse on duty. Age (mean, SD): A: 72.1 (12.7); B: 71.9 (12.1) Number of participants: · Eligible: 459 · Randomised: 182 · Reported: 164 Dropouts (n of participants + reasons): Excluded: 8 excluded from group A (1 withdrawal; 1 deceased; 6 urine culture missing). 4 excluded from group B (1 had hip fracture twice + included twice, but excluded before analysis 2nd time; 1 withdrawal; 2 urine culture missing) Dropout: 1 dropout from group B at 4‐week follow‐up (deceased); 3 dropouts from group A (deceased) and 2 dropouts from group B at 4‐month follow‐up Follow‐up: 4 months after discharge |
|
| Interventions |
Time of intervention: A (n = 93): indwelling Foley catheter inserted by registered nurses (RNs) or assistant nurses (ANs). Participants with hip fracture had catheter inserted upon arrival on orthopaedic ward. Participants with osteoarthritis were given the catheter in the morning on the day of the surgery B (n = 89): intermittent catheterisation introduced if participant was unable to urinate and bladder scan indicated ≥ 400 ml urine in the bladder Intended duration of catheterisation: A: catheter was removed on postoperative day 2. If bladder volume was ≥ 400 ml on bladder scan and participant was unable to urinate, they were recatheterised. B: unclear |
|
| Outcomes |
Primary outcome (symptomatic UTI): NR Definition of symptomatic UTI: NR Bacteriuria: A: 10/85; B: 8/85 Definition of bacteriuria: ≥ 105 cfu/ml with no more than 2 species of organisms Quality of life: EQ‐5D (n): Discharge: A: 0.32 (52); B: 0.32 (57) 4 weeks: A: 0.62 (52); B: 0.56 (57) 4 months: A: 0.68 (52); B: 0.73 (57) EQ VAS (n): Discharge: A: 0.52 (51); B: 0.52 (54) 4 weeks: A: 0.65 (51); B: 0.63 (54) 4 months: A: 0.68 (51); B: 0.69 (54) SF‐6D (n): Discharge: A: 0.50 (45); B: 0.51 (45) 4 weeks: A: 0.60 (45); B: 0.58 (45) 4 months: A: 0.63 (45); B: 0.65 (45) QALYs gained (n): EQ‐5D: A: 0.093 (52); B: 0.090 (57) (P = 0.904) EQ‐VAS: A: 0.044 (51); B: 0.045 (54) (P = 0.978) SF‐6D: A: 0.036 (45); B: 0.032 (45) (P = 0.616) Time to normal bladder function (hours) (median, IQR, n): A: 48 (43 ‐ 55), 85; B: 24 (13 ‐ 48), 85 (P < 0.001) Number of intermittent catheterisations (median, IQR, n): A: 0 (0 ‐ 0), 85; B: 1 (0 ‐ 2), 85 (P < 0.001) Number of bladder scans to normal bladder function (median, IQR, n): A: 2 (1 ‐ 3), 85; B: 6 (4 ‐ 9), 85 (P < 0.001) Length of hospital stay, days (median, IQR, n): A: 8 (7 ‐ 12), 85; B: 8 (6 ‐ 11), 85 Total Costs (n): A: EUR 16.62, 85; B: EUR 17.98, 85 Costs incurred due to UTIs (EUR, n): A: 2.45, 85; B: 3.26, 85 Catheterisation costs, EUR (mean, SD, n): A: 16.6 (13.1), 85; B: 18.0 (13.6), 84 Catheterisation costs with no UTIs, EUR (mean, SD, n): A 13 (7.7), 85; B 16 (11.8), 84 Catheterisation costs, with UTIs EUR (mean, SD, n): A: 45 (10.2), 85; B 41 (6.5), 84 Total costs, EUR (mean, SD, n): A: 3954 (1743), 85; B: 3642 (1605), 84 Total costs, with no UTIs, EUR (mean, SD, n): A: 3791 (1736), 85; B: 3619 (1638), 84 Total costs, with UTIs, EUR (mean, SD, n): A: 5173 (1306), 85; B: 3862 (1329), 84 |
|
| Sponsorship/Funding | “supported by research grants from Örebro County Council Research Committee and the Swedish Association of Health Professionals.” “Astra Tech provided Lofric Primo‐catheters for the patients in the intermittent group” |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “a random computer‐generated sequence was prepared by a statistician” – adequate method of randomisation, low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | “sealed opaque envelopes” – adequate method of allocation concealment, low risk of bias |
| Blinding of participants | High risk | No information given. Can assume as intermittent vs indwelling that blinding was not possible for participants |
| Blinding of personnel | High risk | No information given. Can assume as intermittent vs indwelling that blinding was not possible for personnel |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 182 randomised, 89 intermittent 93 indwelling. Intermittent had 4 withdrawals (1 had 2 hip fractures and included twice but only analysed once; 1 withdrawn; 2 urine culture missing). Indwelling had 8 excluded (1 withdrawn, 1 deceased, 6 urine culture missing). 85 analysed in each group. 1 lost to 4‐week follow‐up in intermittent (deceased). 4‐month follow‐up, intermittent lost 2 (deceased); indwelling lost 3 (deceased). 82 completed trial in each group. Small differential dropout, but low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes are reported in Results. Unable to access protocol so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Catheters provided by Astra Tech for intermittent catheterisation. Unlikely to have had any influence on trial |
Hammarsten 1992.
| Methods |
RCT or quasi‐RCT: RCT Setting: Sweden Period: not reported |
|
| Participants |
Population: men undergoing TURP Inclusion criteria: men undergoing TURP Exclusion criteria: History of urethral stricture; presence of urethral stricture at preliminary urethroscopy Age (mean, standard error): A: 73 (7) years; B: 73 (8) years; C: 71 (7) years Dropouts (n of participants + reasons): ad mortem before follow‐up (A: 3; B: 5; C: 6), additional catheter before follow‐up (A: 2; B: 4; C: 13), lost to follow‐up (A: 3; B: 2; C:4), history of urethral stricture (A: 1; B: 3; C: 0) |
|
| Interventions | A (n = 94): Urethral catheter (22f, teflon‐coated latex) B (n = 102): Urethral catheter (PVC) C (n = 102): Suprapubic catheter (PVC) Intended duration of intervention: A: 5.0 + 3 days; B: 2.9 + 2 days; C: 3.2 + 3 days |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Total post‐operative strictures: A: 14/102; B: 15/102; C: 4/94 Postoperative anterior urethral strictures: A: 11/102; B: 10/102; C: 1/94 Participant dissatisfied with TURP: A: 29/102; B: 23/102; C: 10/94 Dissatisfied participants with bladder neck strictures: A: 3/102; B: 3/102; C: 1/94 Dissatisfied participants with anterior urethral strictures: A: 9/102; B: 8/102; C: 1/94 Postoperative catheter time (mean, SE) (days): A: 5.0 (3); B: 2.9 (2); C: 3.2 (3) Time in hospital after resection (mean, SE) (days) : A: 5.7 (3); B: 5.1 (3); C: 7.1 (4) |
|
| Sponsorship/Funding | Not reported | |
| Notes | Strictures assessed at 6 ‐ 24 months postoperatively Strictures were defined as urethra with diameters < 19 mm in size Treatment groups had higher rates of residual adenoma and recurrent cancer than controls (NS) Anterior urethral strictures not counted towards the total number of strictures among the 3 groups No power analysis Participants received Pivmecillinam and Pivampicillin if they had no bacteriuria at time of operation as prophylaxis, or if they had bacteriuria at time of operation it was used as a treatment. 1st dose was given 1 hour preoperatively, and last dose on the day the catheter was removed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly divided into 3 groups" ‐ no information given on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given. Can be assumed 2 groups receiving transurethral catheter remained blinded but suprapubic did not |
| Blinding of personnel | High risk | No information given. Can be assumed that surgeons inserting catheter were not blinded. Again, clinicians not involved in surgery likely to be blinded to material of transurethral catheter but not blinded to suprapubic group |
| Blinding of microbiological outcome assessment | Low risk | No microbiological outcomes reported, therefore no risk of bias from reporting of microbiological outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "suprapubic drainage group 13 patients were excluded because they needed a transurethral catheter before followup" ‐ none excluded from transurethral, differential dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes detailed in Methods matched in outcomes reported in Results. Unable to access protocol so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Harms 1985.
| Methods |
RCT or quasi‐RCT: RCT Setting: Germany Period: November 1979 ‐ September 1980 |
|
| Participants |
Population: women undergoing vaginal hysterectomy Inclusion criteria: women undergoing vaginal hysterectomy with front (resp. front and back) plastic Exclusion criteria: not reported Age: not reported Dropouts (n of participants + reasons): 12 Participants for the outcome bacteriuria: 10 participants in suprapubic group because of positive culture at the moment of trial inclusion, 2 in the indwelling urethral group for the same reason. |
|
| Interventions | A (n = 69): Indwelling urethral catheterisation (14 Charriere Foley) introduced after termination of the operation B (n = 88): Suprapubic catheterisation (Cystofix) Duration of intervention: In both groups the catheter was intermittently clamped beginning the 5th postoperative day. In general, the catheter was removed on the 7th postoperative day. For participants in the SPC group, the catheter was removed if the residual urine was < 100 ml on 3 consecutive occasions. For participants in the UC group, if a participant could not micturate or if the residual urine was > 300 ml despite receiving bladder tonica, a catheter was reinserted |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 36/67; B: 12/78 Definition of bacteriuria: Significant bacteriuria was defined as 105 cfu/ml on 6th postoperative day Recatheterisation: A: 23/69; B: 6/88 Duration of catheterisation: A: 8.4 days; B: 6.7 days Number of participants with discomfort: A: 46/69; B: 16/88 Microscopic haematuria on the 6th postoperative day: A: 40/69; B: 55/88 Pyuria on the 6th postoperative day: A: 60/69; B: 42/88 Need for antibiotic therapy: A: 24/69; B: 11/88 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Catheter specimens were taken preoperatively and on the 6th and 12th postoperative days for microscopy and microbiological culture. In case of catheter removal, specimens were obtained by a once‐only catheterisation. Recatheterisation occurred when normal micturition failed and for participants in the treatment group also if the residual urine was > 300 ml despite receiving bladder tonica. 'Microscopic haematuria' was defined as > 1 red blood cell im Sedimentbefund (Normalfeld) i.e. more than 5 red blood cell per field (magnification not reported. Pyuria was defined as > 5 white blood cells im Sedimentbefund (Normalfeld) i.e. more than 5 white blood cells per field (magnification not reported. No definition was given for participants' discomfort. Not reported whether prophylactic antibiotics were used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Die Zuteilung der Probandinnen zur suprapubischen bzw. tranurethralen Vergleichsgruppe erfolgte durch Wüflen; damit war eine zufällige Verteilung sicher gewährlestet" ‐ randomisation done using die. Adequate method of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information but as urethral vs suprapubic can assume that blinding did not occur |
| Blinding of personnel | High risk | No information but as urethral vs suprapubic can assume that blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 157 participants, none lost to follow‐up. 12 excluded due to positive preoperative urine cultures |
| Selective reporting (reporting bias) | Unclear risk | No information given about outcomes to be studied |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ichsan 1987.
| Methods |
RCT or quasi‐RCT: RCT Setting: Australia Period: February 1984 – May 1984 |
|
| Participants |
Population: patients who presented with acute urinary retention via emergency room or developing retention whilst in hospital Inclusion criteria: not reported Exclusion criteria: not reported Age (mean, SD)/(median, range): not reported Number of participants: · Eligible: · Randomised: 60 · Reported: 52 Dropouts (n of participants + reasons): 2 excluded due to history of urethral stricture. 3 had infected initial urine culture. 3 required for antibiotics for other reasons. Not reported which group. Follow‐up: 12 days |
|
| Interventions |
Time of intervention: A: indwelling urethral catheters inserted by members of nursing staff. Urine sample obtained every 2 days until catheter removed for bacteriological culture, organism count + repeat specimens B: suprapubic catheters inserted by resident medical officers. Urine sample obtained every 2 days until catheter removed for bacteriological culture, organism count + repeat specimens Intended duration of catheterisation: not reported |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: Proportion of men with infection at 12 days follow‐up: A: 80%; B: 40% Proportion of men with infection at 12 days follow‐up, analysed with ‘life‐table procedure’: A: 90%; B: 25% Definition of bacteriuria: ≥ 10⁸ organisms Quality of life: Catheter‐associated discomfort: A: 17/?; B: 0/? Dysuria: A: 19/?; B: NR Erythema around catheter: A: NR; B: 4/? Catheter ceased draining: A: NR; B: 1/? Macroscopic haematuria: A: 0/?; B: 4/20 Cost (AUD): Catheter: A: 1.10; B: 14.00 Other equipment: A: 7.67; B: 7.47 Labour: A: 4.00; B: 5.00 Adjustment for recatheterisations: A: x 2.6; B: x 1.05 Total: A: 33.20; B: 27.77 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Number in each group not reported “Suprapubic catheters were inserted by resident medical officers whereas urethral catheters were inserted by members of the nursing staff” – bias as groups not treated equally Contacted St. George Hospital for contact details for Dr. David R Hunt. Retired and no contact details available, unable to contact |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given on method of allocation concealment |
| Blinding of participants | High risk | No information given on blinding, but as suprapubic vs indwelling can assume blinding did not occur. |
| Blinding of personnel | High risk | “Suprapubic catheters were inserted by resident medical officers whereas urethral catheters were inserted by members of the nursing staff” |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on blinding. No information given on primary outcome so unclear risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given on number of participants randomised to each intervention. 60 participants randomised, 52 completed trial |
| Selective reporting (reporting bias) | Unclear risk | No information given on outcomes being reported. Unable to access protocol so uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Jannelli 2007.
| Methods |
RCT or quasi‐RCT: RCT Setting: USA Period: November 2000 ‐ April 2005 |
|
| Participants |
Population: women undergoing surgery for stress urinary incontinence or anterior vaginal wall prolapse Inclusion criteria: women scheduled for surgery for stress urinary incontinence or anterior vaginal wall prolapse Exclusion criteria: Preoperative bacteriuria, urinary retention, voiding dysfunction, h/o urethral trauma, unable to perform CISC Number of participants:
Age (mean, SD): A: 54.6 (13.7) years; B: 55.0 (10.5) years Dropouts (n of participants + reasons): 34 women were excluded. 3 did not have the expected surgery, 6 refused their allocated assignment, 5 requested a switch to the other allocation group, 4 were unable to learn CISC, 1 was assigned to 1 group but received the wrong treatment, and 8 were lost for "other" reasons |
|
| Interventions | A (n = 112): Bonanno suprapubic catheter placed intra‐operatively B (n = 98): CISC (14 French disposable vinyl catheter) started on 1st postoperative day. (16 French silicone Foley catheter placed intraoperatively to monitor urine output in the immediate postoperative period) Intended duration of catheterisation: until participants returned to adequate voiding (PVR < 100 ml or < 30% of voided volume on 2 consecutive voids) |
|
| Outcomes |
Bacteriuria: A: 26/112; B: 30/98 Definition of bacteriuria: > 105 cfu/ml on postoperative day 2 or 7 Patient satisfaction by VAS on 7th postoperative day (mean, SD ): Overall pain: A: 3.4 (2.4); B: 3.4 (2.7) Pain from catheter: A: 1.9 (2.3); B: 1.4 (1.8) Ease of catheter use: A: 1.4 (2.2) ; B: 2.5 (2.9) Catheter frustration: A: 1.6 (2.5); B: 2.7 (3.3) (P = 0.01; CISC caused more frustration) Catheter limit social activities: A: 1.2 (2.2); B: 1.3 (2.5) Use this method again: A: 8.4 (2.5); B: 7.1 (3.3) Length of catheter use (mean, SD)(days) (2 participants with prolonged urinary retention were excluded, negating ITT) (n = 208): A: 5.3 (7.0); B: 5.2 (7.4) Catheter‐related complications: Unable to perform allocated catheter procedure: A: not reported; B: 5/98 Cellulitis around catheter (definition of cellulitis: presence of erythema and/or tenderness at the suprapubic site and treated with appropriate antibiotics in the out‐patient setting) A: 9/11; B: not reported Leakage around catheter: A: 3/112; B: not reported Catheter obstruction: A: 1/112; B: not reported |
|
| Sponsorship/Funding | Not reported | |
| Notes | Urinanalysis was performed on postoperative days 2 and 7, with the urine being sent to look for leukocyte esterase and nitrates Unclear whether statistical differences in VAS scores were clinically significant According to power analysis, needed 113 per group to detect no difference in bacteriuria rates, and did not achieve this number Negated ITT analysis with post hoc exclusion of 2 participants with extended urinary retention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random number table in blocks of 4" ‐ adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | "Assignments were kept in sealed, sequentially numbered, opaque envelopes" ‐ low chance of allocation being revealed |
| Blinding of participants | High risk | "masking of the physician or patient to the assignment was not feasible given the nature of the intervention" |
| Blinding of personnel | High risk | "masking of the physician or patient to the assignment was not feasible given the nature of the intervention" |
| Blinding of microbiological outcome assessment | Low risk | Primary outcome of bacteriuria is low risk of bias. Microbiologist would assess this outcome, and they would not know what type of catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In SPC group, 123 participants underwent expected surgery but 117 received suprapubic catheter ‐ no reason given. At same point in trial, 19 participants who underwent expected surgery did not do CISC Differential dropout ‐ at randomisation 120 CISC and 124 SPC; completed follow‐up and analysed 98 CISC and 112 SPC. Almost double number of participants lost in CISC compared to SPC |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes detailed in Background and Methods have results. Unable to access protocol, so some uncertainty about selective reporting. |
| Other bias | Unclear risk | 2 excluded due to prolonged urinary retention. Did not appear to perform ITT analysis. |
Katz 1992.
| Methods |
RCT or quasi‐RCT: RCT Setting: Israel Period: not reported |
|
| Participants |
Population: men undergoing coronary artery bypass graft (CABG) surgery Inclusion criteria: men undergoing CABG Exclusion criteria: patients who already had a catheter, had CABG in the past, had a history of lower urinary tract surgery. Age (mean, SD): A: 60 (9); B: 58 (8); C: 55 (8) Number of participants: · Eligible: 75 · Randomised: 62 · Reported: 62 (52 for 6‐month follow‐up, questionnaire) Dropouts (n of participants + reasons): Excluded (13): 5 already had Foley catheter, 3 had prostatectomy, 5 had CABG in past Follow‐up: 6 months after operation |
|
| Interventions |
Time of intervention: A (n = 21): indwelling urethral catheterisation (12F silicone‐coated or Teflon‐coated Foley catheter lubricated with paraffin oil) in the operating room after anaesthetic before surgery. (FB) B (n = 17): indwelling urethral catheterisation (12F silicone‐coated or Teflon‐coated Foley catheter lubricated with paraffin oil) in the operating room after completion of surgery. (FA) C (n = 24): suprapubic catheterisation (8F Cystocath manufactured by Dow Corning Corporation) in the operating room after completion of surgery. (FY) Intended duration of catheterisation: 48 hours after surgery |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: not reported Definition of bacteriuria: not reported Quality of life: not reported Complication rate (%, n): A: 10, 21; B: 16, 17; C: 26, 24. no significant difference Response rate for questionnaire at 6 months: A: 16/21; B: 15/17; C: 21/24 Patients with score > 0 for difficulty in voiding symptoms (weakening of stream, urgency, frequency, increased nocturia. 1 point for each symptom, 0 = no symptoms present, 4 = all symptoms present): A: 10/16; B 3/15; C: 6/21 Complaint score (mean, SD, n): A: 1.7 (1.5), 16; B: 0.4 (1.1), 15; C: 0.7 (1.2), 21 Urethral Stricture (n, %): A: 0/21; B: 0/17; C: 1/24 (4.2) |
|
| Sponsorship/Funding | Not reported | |
| Notes | 2 participants in group B received suprapubic catheter. 1 participant in group C had indwelling urethral catheter introduced following surgery due to previous surgery in lower abdomen Use of prophylactic antibiotics not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “nurse who picked an envelope from one of 75 sealed envelopes” – no mention if envelopes are shuffled |
| Allocation concealment (selection bias) | Low risk | “sealed envelopes” – adequate method of allocation concealment, low risk of selection bias |
| Blinding of participants | High risk | No information on blinding of participants. Could have been blinded to time of insertion, but not to type of catheter |
| Blinding of personnel | High risk | No information on blinding of personnel. As catheters placed during surgery, unlikely surgeons blinded. |
| Blinding of microbiological outcome assessment | Low risk | No microbiological outcomes reported, therefore no risk of bias from reporting of microbiological outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “A urologic evaluation was done by a urologist who was not involved in the treatment of the patients during their CABG.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 75 patients entered trial, 13 excluded (5 already had catheter, 3 had prostatectomy, 5 had CABG in past). 62 randomised – 21 to indwelling inserted before procedure, 19 to indwelling inserted after procedure, 22 to suprapubic inserted after procedure. 2 participants in indwelling group inserted after procedure had SPC inserted during procedure so were analysed in SPC group Small differential dropout, not large enough to increase risk of bias |
| Selective reporting (reporting bias) | Unclear risk | No information in Methods about outcomes; primary outcome is urethral stricture which is reported. Unable to access protocol so uncertainty surrounding reporting bias risk |
| Other bias | Unclear risk | Unsure if ITT took place |
Kerr‐Wilson 1986.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK Period: Not reported |
|
| Participants |
Population: patients undergoing elective caesarean section under epidural analgesia Inclusion criteria: patients undergoing elective caesarean section under epidural analgesia Exclusion criteria: not reported Age (mean, SEM): A: 29.5 (0.97); B: 27.0 (1.03) Number of participants: · Eligible: NR · Randomised: 50 · Reported: 50 Dropouts (n of participants + reasons): 0 Follow‐up: duration of hospital stay |
|
| Interventions |
Time of intervention: after epidural had been inserted, immediately before surgery A (n = 25): indwelling catheterisation (Foley catheter) inserted immediately before surgery after epidural had been inserted. Removed once the participant was ambulant B (n = 25): intermittent catheterisation ‘in‐out’ (Nelaton catheter) inserted immediately before surgery after epidural had been inserted. Removed at the end of operation Participants who became distressed by inability to pass urine were recatheterised Intended duration of catheterisation: A: beginning of surgery until participant was ambulant B: duration of surgery |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: A: 3/25; B: 3/25 Definition of bacteriuria: > 10⁵ organisms/ml with or without pus cells Participants requiring recatheterisation: A: 0/25; B: 11/25 Duration of catheterisation: A: 1 day after surgery; B: 9 hours 37 mins after surgery (mean) Participants unable to spontaneously void after catheterisation: A: 0/25; B: 11/25 Cost per catheter: A: 53p (Foley); B: 10p (Nelaton) Quality of life: not reported |
|
| Sponsorship/Funding | Not reported | |
| Notes | Antibiotic prophylaxis was not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants | High risk | No information given but as intermittent vs indwelling unlikely blinding of participants occurred |
| Blinding of personnel | High risk | No information given but as intermittent vs indwelling unlikely blinding of participants occurred |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on blinding of outcome assessment. Urinary retention appears to be primary outcome, but no method given on how it was diagnosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 50 participants in trial, 25 allocated to each group. No loss to follow‐up or dropouts |
| Selective reporting (reporting bias) | Unclear risk | No information given in Methods on outcomes being studied. Unable to access protocol so uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Knight 1996.
| Methods |
RCT or quasi‐RCT: RCT Setting: USA |
|
| Participants |
Population: patients undergoing total hip or knee arthroplasty Inclusion criteria:patients undergoing a primary total hip arthroplasty or total knee arthroplasty with sterile urine cultures Exclusion criteria:History of chronic UTIs or a structural urinary abnormality, revision arthroplasty, long‐term suppressive antibiotic therapy Age (mean, range): 66 (35 ‐ 86) years. Number of participants:
Dropouts (n of participants + reasons): 55 participants were excluded: 35 did not have cultures obtained on postoperative day 5; 5 allocated to treatment group received a urethral catheter; 7 allocated to control group received an IC; 8 required prolonged use of a urethral catheter for medical reasons Follow‐up: until participants were voiding without problems |
|
| Interventions | A (n = 62): Indwelling urethral catheter (Foley) placed just prior to surgery. Remained in place for 48 hours.
Thereafter, urinary retention was treated with intermittent catheterisation. B (n = 57): Intermittent catheterisation every 6 hours if participants were unable to void or were voiding in volumes of ≤ 50 ml. |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 5/62; B: 7/57 (P = 0.45) Definition of bacteriuria: > 105 colonies of a predominant organism Number of participants with urinary retention 48 hours after surgery: A:12/62; B:20/57 Costs per participant for the first 48 hours after surgery: A: USD 8.33; B: USD 53.20 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Urinary retention was defined as the inability to pass urine or if participants were voiding in volumes of ≤ 50 ml
Analysis of cost was conducted for the 2 methods of management based on both materials and nursing time components of costs. Antibiotics: All participants received routine antibiotic prophylaxis (cefazolin) every 8 hours for 48 hours; it was not explicitly stated whether other antibiotics except prophylaxis were administered pre‐ or postoperatively. Participant‐controlled analgesia was given to those who had a general anaesthetic. Participants who had an epidural anaesthetic were maintained on the epidural for analgesia for 48 hours. The average number of intermittent catheterisations was 8 per participant (participants were catheterised more frequently than the planned 6‐hour trial interval) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Eligible patients were randomized into one of two groups according to the last digit of their medical record number without regard to age, sex or procedure” – sequence generated by rule based on medical record number; not truly random |
| Allocation concealment (selection bias) | High risk | “Eligible patients were randomized into one of two groups according to the last digit of their medical record number without regard to age, sex or procedure” – allocation not concealed as participant could be identified by medical record number |
| Blinding of participants | High risk | No information provided but can assume participants were not blinded as indwelling vs intermittent |
| Blinding of personnel | High risk | “Patients... received an indwelling Foley catheter in the operating room just prior to start of surgery” “Patients… were intermittently straight catheterized every six hours” – can assume that clinicians were not blinded during insertion of catheter and in postoperative care |
| Blinding of microbiological outcome assessment | Low risk | Urine culture for symptomatic UTI would be assessed by microbiologist, who would not know the allocation of participant to either type of catheter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Fifty‐five patients who were enrolled in the trial were eliminated for various reasons: 35 patients did not have cultures obtained on postoperative day 5; five patients to be in group 2 received a Foley; 7 patients to be in group 1 did not receive a Foley catheter; eight patients required prolonged use of a Foley catheter (to accurately monitor intake and output) for medical reasons.” – dropout rate of 55 participants is high. |
| Selective reporting (reporting bias) | Low risk | Reported all expected outcomes. Unable to access protocol, some uncertainty about selective reporting |
| Other bias | Unclear risk | "five patients to be in group 2 received a Foley, 7 patients to be in group 1 did not receive a Foley" ‐ these participants were excluded from analysis |
Korkes 2008.
| Methods |
RCT or quasi‐RCT: RCT Setting: Brazil Period: 2000 ‐ 2004 |
|
| Participants |
Population: men undergoing open prostatectomy for BPH Inclusion criteria: All patients undergoing open prostatectomy for BPH (prostate > 80 g) Exclusion criteria: Not defined Age (mean, SD): A 71.4 (8.0) years; B 74.1 (6.8) years. Number of participants:
Dropouts (n of participants + reasons): not reported Follow‐up: mean follow‐up A: 19.7 months, B: 21.4 months |
|
| Interventions | All participants received both a urethral catheter (22f 3‐way) and a suprapubic catheter (20f) placed intraoperatively. One of these catheters was removed during the inpatient stay "once the urine had cleared." A: Discharged with urethral catheter B: Discharged with suprapubic catheter Duration of Intervention: Catheter removed on postoperative day 7 if the "patient voided well with a minimal PVR." |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 2/29; B: 3/30 Definition of symptomatic UTI: UTI for this subset of men was defined as leucocituria AND positive culture (> 10⁴ cfu/ml) AND symptoms (e.g. fever, malaise, haematuria, pain, etc.) Overall complications: A: 10/29; B: 9/30. Early complications: A: 6/29; B: 5/30 Late complications: A: 4/29; B: 4/30 Number of participants with wound infection: A: 4/29; B: 1/30 Number of participants with urethral stricture: A: 1/29 (3.4%); B: 1/30 (3.3%) Number of participants with epididymitis: A: 0/29; B: 1/30 Number of participants with stress incontinence (any participant who had urinary leakage as a complaint or when questioned): A: 1/29; B: 2/30 Number of participants with bladder neck contracture: A: 1/29; B: 1/30 Number of participants with meatal stenosis: A: 1/29; B: 0/30 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Contacted Dr. Korkes (fkorkes@terra.com.br) about the definition used for UTI in the study. Response received, with the following definition: "UTI for these subset of men was defined as leucocituria AND positive culture (>104 cfu/ml) AND symptoms (e.g..: fever, malaise, hematuria, pain, etc.)" No power analysis performed to determine whether it can support "no difference between groups for any outcomes" Mean follow‐up: A: 21.4 months; B: 19.7 months Complication outcomes not well defined Antibiotic prophylaxis use not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospectively randomized in two groups" ‐ no further information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given but as urethral vs suprapubic can assume no blinding occurred |
| Blinding of personnel | High risk | No information given but as urethral vs suprapubic can assume no blinding occurred. |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI would be assessed by a microbiologist for positive urine culture, who would not know the type of catheter a participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given on number randomised, dropout rate and numbers lost to follow‐up. Most of the results reported as percentage instead of raw numbers |
| Selective reporting (reporting bias) | Unclear risk | Method stated "data regarding complication rates and postoperative outcomes were then assessed" ‐ Results cover expected complications. Unable to access protocol, some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Kringel 2010.
| Methods |
RCT or quasi‐RCT: 3‐arm RCT Setting: Germany |
|
| Participants |
Population: women undergoing anterior colporrhaphy plus an optional further procedure Inclusion criteria: women undergoing anterior colporrhaphy plus an optional further procedure (i.e. hysterectomy). Exclusion criteria: Preoperative asymptomatic bacteriuria, symptomatic UTI, previous vaginal prolapse surgery, unable to give consent Number of participants:
Age (mean, SD): A 63.5 (11.3) years; B 61.1 (9.92) years Dropouts (n of participants + reasons): None |
|
| Interventions | A (n = 100): Indwelling urethral catheter (silicone Foley) placed intraoperatively, left indwelling for 24 hours B (n = 100): Indwelling urethral catheter (silicone Foley) placed intraoperatively, left indwelling for 96 hours C (n = 32): Suprapubic catheter (silicone Foley) placed intraoperatively, left for 96 hours Intended duration of Intervention: 24 or 96 hours |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 2/100; B: 6/100; C: 0/32. (P = 0.155) Definition of symptomatic UTI: urine sample on postoperative day 4. Defined using CDC definition of catheter‐associated UTI Bacteriuria: A: 27/100; B: 25/100; C: 1/32 (P = 0.016; higher rates of Asymptomatic bacteriuria (ASB) in treatment groups) Definition of bacteriuria: urine sample on postoperative day 4. ASB defined using CDC definition Overall catheter‐related complications: A: 5/100; B: 5/100; C: 7/32 (P = 0.003; higher complication rate in group C) Urinary retention: A: 3/100; B: 2/100; C: 2/32 Pyelectasia: A: 0/100; B: 0/100; C: 1/32 Catheter blockage: A: 0/100; B: 0/100; C: 4/32 Dysuria without UTI: A: 2/100; B: 3/100; C: 0/32 Subjective well‐being score (1 (optimal) to 6 (bad)) (mean, SD): A: 2.22 ± 0.91; B: 2.27 ± 1.06; C: 2.96 ± 1.33 (P = 0.003; group C less tolerable) Mean hospital stay (mean, SD) (days): A: 5.62 ± 1.10; B: 5.95 ± 1.78; C: 6.25 ± 1.08 (P = 0.043) |
|
| Sponsorship/Funding | University of Rostock (Germany) | |
| Notes | UTI and bacteruria were defined using CDC criteria UA and urine culture were performed before and 96 hours after surgery Subjective well‐being score: 1 was optimal and 6 was poor tolerability According to the power analysis, the investigators needed 100 participants per arm to show no significant difference between groups. However, they stopped the suprapubic arm with only 32 participants, due to "complications" and tolerability index, but the complication rate at which they would stop the trial was NOT defined prior to the trial Clinical characteristics of participant subgroups ‐ significant difference in preoperative bladder control problems (P = 0.029) and preoperative recurrent UTIs (P = 0.047). 96‐hour SUC had greater incidence of bladder control problems and recurrent UTIs All participants received 2 g Cefotiam i.v. before starting surgery as antibiotics prophylaxis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "as randomization procedure, the permuted block randomization with variable block length was used" ‐ adequate method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | "using sealed envelopes" ‐ not specified if envelopes were transparent or opaque |
| Blinding of participants | High risk | No information given but as suprapubic vs urethral can assume blinding did not occur |
| Blinding of personnel | High risk | No information given but as suprapubic vs urethral can assume blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI and bacteriuria would be assessed by microbiologist who would not know which catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At randomisation, 100 randomised to IUC 24 hours and IUC 96 hours each, and 32 to SPC 96 hours due to "preterm discontinuation of the SUC arm after pre‐planned interim data monitoring" |
| Selective reporting (reporting bias) | Low risk | Reported all primary and secondary outcomes in Methods. Unable to access protocol, some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
Michelson 1988.
| Methods |
RCT or quasi‐RCT: quasi‐RCT Setting: Hospital of the University of Pennsylvania, USA Period: July 1985 – June 1986 |
|
| Participants |
Population: patients undergoing total joint replacement surgery (knee or hip) Inclusion criteria: patients undergoing total joint replacement surgery (knee or hip) Exclusion criteria: NR Age (mean): Overall: 63.5; A: 65.7; B: 61.7 Number of participants: · Eligible: not reported · Randomised: 96 · Reported: 89 (infection outcome), 96 or 100 (n of procedures) Dropouts (n of participants + reasons): 4 excluded from group A and 3 from group B for UTI outcome due to preoperative or postoperative urinary cultures not being available Follow‐up: 7 days postoperatively |
|
| Interventions |
Time of intervention: A (n = 41): indwelling catheter inserted just before surgery. Removed the morning after surgery. Urinary retention was treated with intermittent catheterisation following this. If retention continued > 48 hours, indwelling catheter was inserted again B (n = 55): intermittent catheterisation performed postoperatively by nursing staff only if urinary retention occurred. Performed at least every 6 hours. If retention continued > 48 hours, indwelling catheter was inserted Intended duration of catheterisation: A: < 24 hours; B: until resolution of urinary retention |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: A: 4/36; B: 7/47 Definition of bacteriuria: > 10⁵ cfu/ml no distinction made between bacteriuria and symptomatic UTI “because in patients with large implant (such as total hip or total knee device) both are potential sources of bacterial seeding to the implant.” On day 2 or 7, not detailed which Participants with retention requiring recatheterisation: Postoperative day 1: A 9/41; B 29/55 Postoperative day 2: A 7/41; B 5/55 Postoperative day 3: A 3/41; B 1/55 Postoperative day 4: A 1/41; B 1/55 Postoperative day 5: A 1/41; B 0/55 Total number of participants requiring straight catheterisation for urinary retention: A: 12/44; B: 42/56. (P < 0.002) Participants with bladder dysfunction requiring secondary indwelling catheter: A 8/44; B 12/56 Overall urinary retention: A 12/44; B 42/56 Quality of life: not reported |
|
| Sponsorship/Funding | Not reported | |
| Notes | Table 2: group A = 41; group B = 55 Text in results: group A = 41; group B = 51. “Although the patients were informed of the study and given the option to decline participation, formal consent was not obtained, since the treatments under study are both practiced widely.” Perioperative prophylactic antibiotic therapy (cephalosporin with or without gentamicin) was given to all participants. Participants requiring secondary Foley catheter received antibiotics while device was in place |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “patients were randomly assigned according to chart number” |
| Allocation concealment (selection bias) | High risk | As patient chart number used for random sequence generation, it could be easily broken and allocation concealment would not have occurred |
| Blinding of participants | High risk | Comparing indwelling vs intermittent can assume participant was not blinded |
| Blinding of personnel | High risk | No information given. As indwelling vs intermittent catheterisation, unlikely blinding could take place |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “the principal investigator was blinded to each patient’s name, attending physician, disease and surgery until the final compilation of data” – adequate blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential withdrawal, adequate explanation for excluding participants from some analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes in the Methods are reported in the Results. Unable to access protocol, so some uncertainty about risk of reporting bias |
| Other bias | Low risk | Appears to be free from other sources of bias |
Millet 2012.
| Methods |
RCT or quasi‐RCT: RCT Setting: Hawaii |
|
| Participants |
Population: women in labour requesting epidural analgesia Inclusion criteria: Women, > age 18, singleton gestation, > 37 + 0 weeks GA, spontaneous or induced labour, desires epidural analgesia Exclusion criteria: < 18 yrs age, GA < 37 + 0 weeks, multiple gestation, declined epidural analgesia, clinical chorioamnionitis at admission, symptoms of UTI or pyelonephritis, antibiotics usage within 2 weeks of admission, congenital urinary tract abnormalities, HIV/AIDS, lupus, pregestational diabetes, pre‐eclampsia, or chronic corticosteroid use Number of participants:
Age (mean, SD): A 27.1 (5.6) years; B 28.2 (5.8) years. Dropouts (n. of patients + reasons): 5 patients excluded for bacteriuria at first sample + 9 excluded for missing postpartum urine samples |
|
| Interventions | All participants had 2 urine cultures for evaluation. 1st culture was taken from 1st catheterisation after epidural; 2nd culture was taken the day of hospital discharge by catheterised or clean‐catch voided sample (if participant declined catheterisation) Time of intervention: after epidural placement A (n = 76): indwelling Foley catheter (14 French Bard Foley tray, with Bardex Lubricath, anti‐reflux chamber drainage bag, and EZ lock sampling port) inserted after epidural placement. Removed in the 2nd stage of labour at the start of pushing. B (n = 84): intermittent catheter (Bard™ urethral catheterisation tray and 15Fr red, rubber catheter) every 4 hours and as needed after epidural placement. Stopped at delivery |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: Number of postpartum urine cultures (sample 2) that met the criteria for bacteriuria (using either CDC or IDSA definition) in each catheter group CDC Definition A: 1/67; B: 7/79 (P < 0.05) IDSA Definition A: 8/67; B: 18/79 (P < 0.05) Significantly higher difference in bacteriuria rates in those receiving intermittent vs indwelling Definition of bacteriuria: Using both CDC and IDSA definitions of asymptomatic bacteriuria from sample 2 of urine samples CDC: • Participant with indwelling catheter within 7 days before culture and 1 urine culture with 105 cfu/ml with ≤ 2 species OR • Participant without indwelling catheter within 7 days before culture and 2 urine cultures with 105 cfu/ml of same organism with ≤ 2 species, AND • No fever (38° C), dysuria, urgency, frequency, or suprapubic tenderness • Clean‐catch, catheter, aspiration (no catheter tips, not from a bag) IDSA: • Clean‐catch voided urine in women: 2 consecutive voided specimens with isolation of the same bacterial strain in counts of ≥ 105 cfu/ml • Clean‐catch voided urine in men: single voided specimen with 1 species in counts of ≥ 105 cfu/ml • Catheterised urine in women and men: single catheterised specimen with 1 species in counts of ≥ 100 cfu/ml Number of catheter introductions 1 ‐ 2 catheterisations 53/81 (65%) 3 ‐ 4 catheterisations 21/81 (26%) 5 ‐ 6 catheterisations 7/81 (8.6%) Mean number of CICs in labour was 2.3 ± 1.5 (range 0 ‐ 8) Number of catheter introductions and IDSA bacteriuria 1 ‐ 2 catheterisations 15/53 (28%) 3 ‐ 4 catheterisations 2/21 (9.5%) 5 ‐ 6 catheterisations 1/7 (14.3%) Number of catheter introductions and CDC bacteriuria 1 ‐ 2 catheterisations 6/53 (11.3%) 3 ‐ 4 catheterisations 2/21 (9.5%) 5 ‐ 6 catheterisations 0/7 No significant correlation between an increasing number of CICs and the rates of bacteriuria Duration of catheterisation (hours) in CIF group and meeting the IDSA bacteriuria criteria Removed within 1 ‐ 5 hours 4/9 (44%) 11 ‐ 15 hours 3/9 (33%) 16 ‐ 20 hours 1/9 (11%) 26 ‐ 30 hours 1/9 (11%) Duration of catheterisation (hours) in CIF and meeting the CDC bacteriuria criteria Removed within 1 ‐ 5 hours 1/9 (11%) No significant correlation between increasing hours of CIF exposure and the rates of bacteriuria No significant difference in the mean number of catheterisations or mean hours of catheterisation among the negative, mixed, and bacteriuric cultures Number of symptomatic bacteriuria: 24/26 (92.3%) were asymptomatic vs 2/26 (7.7%) symptomatic |
|
| Sponsorship/Funding | The trial was sponsored by and conducted at the Kapi‘olani Medical Center for Women and Children in Honolulu, HI, USA | |
| Notes | 11 participants (15%) in the CIC group received CIF for clinical reasons 2 participants in the CIF group (2.9%) received CIC. When the participants who received both catheter types were removed from the analysis, there was still a significant difference in bacteriuria rates among those receiving CIC vs CIF 157 of 180 nurses (87%) completed an online educational tool that reviewed proper sterile technique of bladder catheterisation Most nurses in the hospital were used to CIF during labour (paradigm shift) 2 definitions of asymptomatic bacteriuria were used (CDC and IDSA) No specific data were collected regarding type and dosing of epidural anaesthesia Antibiotics: some women received antibiotics during labour and in the postpartum period. Other women did not receive any antibiotics Trial was adequately powered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is reported that “Computer‐generated randomization cards were created, placed in opaque envelopes that were labelled solely with the trial number, and opened only after consent completion.” We therefore judged it to be low risk |
| Allocation concealment (selection bias) | Low risk | It is reported that “Computer‐generated randomization cards were created, placed in opaque envelopes that were labelled solely with the study number, and opened only after consent completion.” We therefore judged it to be low risk |
| Blinding of participants | High risk | Participants were not blinded and it is reported “We conducted a randomized, nonblinded, prospective trial.” Judged to be high risk |
| Blinding of personnel | High risk | Participants were not blinded and it is reported “We conducted a randomized, nonblinded, prospective trial.” Judged to be high risk |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assess by microbiologist, who would not know what type of catheter the participants received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The primary outcome measure was the number of postpartum urine cultures that met the criteria for bacteriuria in each catheter group. This would be checked by a microbiologist and therefore would be blinded. However, it is not clear if the other outcome assessors were blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trialists have provided reason for dropout. In the IC group there was 5 dropouts and in the CIF group there were 9 dropouts. Judged to be low risk |
| Selective reporting (reporting bias) | Low risk | The trialists have reported all the outcomes which they mentioned in the Methods section. Unable to access protocol so some uncertainty about selective reporting |
| Other bias | Unclear risk | There was a learning curve for CISC, since the team was "not used" to those techniques. Protocol specifically states that a catheterised specimen would be obtained pre‐op, but 25% refused, and a voided specimen was obtained (protocol deviation, possible higher levels of bacteriuria in the those who gave a voided specimen) |
Naik 2005.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK Period: 1 July 1999 – 31 June 2002 |
|
| Participants |
Population: women treated by radical hysterectomy for early‐stage cervical cancer Inclusion criteria: women undergoing radical hysterectomy for early‐stage cervical cancer Exclusion criteria: not reported Age (median, range): 45 (20 ‐ 78) Number of participants: · Eligible: Not reported · Randomised: 40 · Reported: 36 Dropouts (n of participants + reasons): 4 removed following randomisation – 2 in each group. 1 developed ureteric fistula, 1 developed postoperative confusion, 1 had a stroke, 1 died. Not detailed which groups they were in. Follow‐up: 12 weeks |
|
| Interventions |
Time of intervention: A (n = 19): suprapubic catheterisation. Insertion of Bonanno suprapubic catheter (Becton Dickenson, Franklin Lakes, New Jersey, USA) at the time of surgery. On free drainage for 5 days. Woman asked to pass urine normally every 4 hours, then measure residual volume using catheter. Catheter was removed when residual volume < 100 ml B (n = 21): intermittent catheterisation. Transurethral indwelling catheter (hydrophilic coated LoFric – Astra Tech Ltd, Stroudwater Business Park, Stonehouse) was inserted at the time of surgery. Removed on day 5, women would pass urine every 4 hours then measure residual volume using intermittent catheter. Intermittent catheterisation ceased when residual volume < 100 ml. Intended duration of catheterisation: Until residual volume < 100 ml |
|
| Outcomes |
Primary outcome (symptomatic UTI): NR Definition of symptomatic UTI: NR Bacteriuria (“positive CSU/MSU rate”): Day 3: A: 1/17 (6%); B: 8/19 (42%) (P = 0.05) Day 5: A: 3/17 (18%); B: 12/19 (63%) (P = 0.004) Day 7: A: 6/17 (36%); B: 7/19 (37%) (P = 0.4) Day 14: A: 9/17 (53%); B: 4/19 (21%) (P = 0.16) Day 21: A: 2/17 (12%); B: 2/19 (11%) (P = 0.21) Definition of bacteriuria: NR Duration of catheterisation, days(median, range, N): A: 20 (7 ‐ 28), 17; B: 17 (7 ‐ 90), 19 (P = 0.83) Adverse effects: A: 8/17 (symptoms/problems with suprapubic catheter site, of which 4 had positive wound swab requiring antibiotics); B: NR Quality of life: Various questionnaires reported only in terms of between‐group differences expressed in P values, for postoperative weeks 3, 6 and 12 |
|
| Sponsorship/Funding | Not reported | |
| Notes | All women received a single dose of intraoperative antibiotics. Prophylactic antibiotics were not given at any other time in the trial. Antibiotics were prescribed when clinically indicated, i.e. positive urine sample or positive SPC site swab 2 participants randomised to group A received transurethral indwelling catheter instead because of suprapubic site problems. 1 randomised to group A requested removal of suprapubic catheter and further bladder care by intermittent self catheterisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomisation was performed via sealed envelopes and an independent administrator” – not enough information of method of randomisation to be able to assess risk of selection bias |
| Allocation concealment (selection bias) | Unclear risk | “Sealed envelopes” no further information given, not enough information to be able to asses risk of selection bias |
| Blinding of participants | High risk | No information given. As intermittent self catheterisation vs suprapubic, unlikely blinding could take place |
| Blinding of personnel | High risk | No information given. As intermittent self catheterisation vs suprapubic, unlikely blinding could take place |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what type of catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 women randomised – intermittent 21, suprapubic 19 4 participants withdrawn following surgery: intermittent 2, suprapubic 2. (1 developed ureteric fistula, 1 developed postoperative confusion, 1 had perioperative cerebrovascular accident, 1 died). No information on which groups the 4 participants were in. |
| Selective reporting (reporting bias) | Low risk | All data that were planned to be collected in the Methods have results reported. Unable to access protocol, so some uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Nwabineli 1993.
| Methods |
RCT or quasi‐RCT: RCT Setting: Glasgow, UK |
|
| Participants |
Population: women undergoing radical hysterectomy for cervical cancer Inclusion criteria: women with stage IB or IIA carcinoma of the cervix with a view of radical hysterectomy and ≤ 50 years of age Exclusion criteria:Voiding problems preoperatively, patients who underwent radiotherapy, patients taking drugs likely to affect bladder function Comment: It is not clear whether patients with initial positive urine cultures were excluded Age (mean): A: 45 years; B: 42 years Number of participants:
Dropouts (n of participants + reasons): none |
|
| Interventions | A (n = 14): Indwelling urethral catheterisation placed before operation B (n = 10): Suprapubic catheterisation introduced after termination of the operation Comment: Participants had urethral catheters during operation Duration of intervention: Trial of voiding started on the 5th postoperative day. Catheters were removed when residual urine was ≤ 100 ml |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 13/14; B: 7/10 Definition of bacteriuria: Significant bacteriuria was defined as more than 105 cfu/ml. Daily catheter specimens were examined bacteriologically until the catheter was removed. In case of participants who went home specimens were taken each time readmission occurred for trial of removal of catheter Duration of catheterisation: A: mean 16.5 days (median 9 days, range 7 ‐ 63 days); B: mean 13.1 days (median 11 days, range 7 ‐ 26 days) |
|
| Sponsorship/Funding | Not reported | |
| Notes | All participants received the same antibiotic prophylaxis (a single dose of 5 g of methyl penicillin). Antibiotics were not administered routinely in the postoperative period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Random numbers were obtained from random sampling numbers” – not enough information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information but as urethral vs suprapubic can assume no blinding occurred |
| Blinding of personnel | High risk | No information but as urethral vs suprapubic can assume no blinding occurred |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria assessed by microbiologists, who would not know allocation of patient to suprapubic or urethral catheter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on blinding of other outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting of number of excluded patients and why ‐ potential source of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Unclear, unable to access protocol, so uncertainty about selective reporting |
| Other bias | Unclear risk | "pilot study" |
O'Kelly 1995.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK |
|
| Participants |
Population: patients undergoing abdominal surgery Inclusion criteria: patients undergoing abdominal surgery and a full‐length abdominal incision Comment: It is not clear whether patients with initial positive urine cultures were excluded Exclusion criteria: not reported Age (median, range): A: 65 (42 ‐ 81) years; B: 68 (35 ‐ 79) years Number of participants:
Dropouts (n of participants + reasons): 5 participants:2 died shortly after operation and in 3 participants no catheter was inserted. Authors did not report from which group participants were lost |
|
| Interventions | A (n = 29): Indwelling urethral catheterisation (14 French) before operation B (n = 28): Suprapubic catheterisation (14 French) after the abdomen was opened Duration of intervention: Catheters were removed when this was appropriate on clinical grounds, but it was recommended that suprapubic catheters should remain in place until the 5th postoperative day. Before removal the SPC was clamped and was withdrawn when the participant could micturate spontaneously and the residual urine was < 100 ml |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 3/29; B: 3/28 Definition of bacteriuria: Significant bacteriuria was defined as > 105 cfu/ml. Specimens were daily aspirated from the catheter and a final midstream was obtained 2 days after the catheter was removed Recatheterisation: A: 2/29; B: 1/28 Duration of catheterisation (median): A: 4 days (range 2 ‐ 11); B: 5 days (range 4 ‐ 10) Number of participants with pain: A: 13/29; B: 2/28 Number of catheter days with pain: A: 37/126; B: 6/142 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Each participant kept a daily record of catheter‐related pain, scoring discomfort on a standard VAS (no pain = 0; worst possible pain = 10) Not reported whether prophylactic antibiotics were used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "62 patients were randomized into two groups" ‐ no information given on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No method of allocation concealment given |
| Blinding of participants | High risk | No information given but as suprapubic vs urethral can assume blinding of participants did not occur |
| Blinding of personnel | High risk | "All catheters were inserted by the surgeon in the operating theatre" ‐ based on this statement, can assume surgeons not blinded "patients were reviewed each day by one of the authors (T.J.O.K. or A. Mathew)" ‐ based on this statement, can assume authors not blinded |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI assessed by microbiologist who would not know the route of catheter the participant had inserted |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients died shortly after operation and in three cases a catheter was not inserted" ‐ reason unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Methods gives some details of what data will be measured, for which there are results. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
Perrin 1997.
| Methods |
RCT or quasi‐RCT: RCT Setting: Australia |
|
| Participants |
Population: patients undergoing rectal surgery Inclusion criteria:patients undergoing rectal surgery Exclusion criteria: If the rectum was not mobilised; if a urethral catheter was required as part of the operation technique; already having an indwelling catheter; infected urine at time of operation Age ("average age"): A: 62 years; B: 64 years Number of participants:
Dropouts (n of participants + reason): 28 patients were excluded: 17 according to exclusion criteria, 6 because of bacteriuria at baseline, 4 because of no initial sample had been taken and 1 because the SPC could not been inserted. Comment: 1) In the SPC group there were 49 participants, in the UC group 59 participants; 2) 137 participants reported randomised, but 28 participants were excluded and 108 patients were analysed (= 136 participants) |
|
| Interventions | A (n = 59): Indwelling urethral catheterisation (16‐French; Foley) inserted following induction of anaesthesia B (n = 49): Suprapubic catheterisation (16‐French; Foley) inserted after the opening of the abdomen Duration of intervention: Until the 5th postoperative day or when the medical condition did not necessitate continuing the monitoring of urine output. The suprapubic catheters were clamped prior to removal to ensure satisfactory voiding (residual volume of < 100 ml). For the UC the residual volume of urine was only measured when voiding did not occur after 6 hours or the participant became uncomfortable |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: Overall A:29/59; B:12/49 In subgroups: a)In males A:12/28; B:7/24 b)In females A:17/31; B:5/25 Definition of bacteriuria: Significant bacteriuria was defined as greater than 10 000 cfu/ml. Specimens were aspirated from the drainage tube at the time of catheterisation, if clinically indicated and immediately prior to removal. Recatheterisation: A: 4/59; B: 3/49 Number of participants catheterised > 5 days: Overall: A: 33/59; B: 44/49 In subgroups: a) men A: 15/28; B: 23/24 b) women A: 18/31; B: 21/25 Discomfort: Overall: A: 17/59; B: 6/49 In subgroups: a) men A: 9/28; B: 3/24 b) women A: 8/31; B: 3/25 Haemorrhage A: 0/59; B: 0/49 Blockage A: 0/59; B: 2/49 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Duration of catheterisation:
Prolonged in the suprapubic group (see outcomes) No definition was given for participants' discomfort All participants received a single dose of tinidazole and/or ticarcillin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated (by computer generated random numbers" ‐ random component in sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given but as suprapubic vs urethral can assume participants not blinded |
| Blinding of personnel | High risk | No information given but as both catheters inserted during surgery can assume clinicians were not blinded |
| Blinding of microbiological outcome assessment | Low risk | Low risk of bacteriuria being affected by bias as based on culture |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All patients entered into the study were questioned specifically by the treating doctor in regard to catheter discomfort." ‐ no information given on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "An additional 11 patients were excluded (6 had infected urine at time of operation, 4 had no initial sample taken, and in 1 patient a suprapubic catheter was unable to be inserted)" ‐ does not give reason for other 17 participants excluded "28 patients of total 137 who were initially entered in this trial were excluded. Of the remaining 108 patients..." 137 minus 28 equals 109, not 108 |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not detailed in Methods section but mentions data that will be collected. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Piergiovanni 1991.
| Methods |
RCT or quasi‐RCT: RCT Setting: Switzerland |
|
| Participants |
Population: patients requiring bladder drainage while hospitalised Inclusion criteria: patients with sterile urine needing a bladder drainage for non‐urological indications (54% perioperative, 29 % urinary retention, 11% prostatic hypertrophy, 6% incontinence and nursing) Age (mean): A: 63 years; B: 64 years Number of participants:
Dropouts (n of participants + reasons) 25 patients were excluded: 10 patients had bacteriuria at admission, 8 died, 5 already had a catheter and 2 were transferred to another hospital |
|
| Interventions | A (n = 41): Indwelling urethral catheterisation (Charriere 12 to 20; Foley) B (n = 34): Suprapubic catheterisation (Charriere 10; Cystofix) Duration of intervention: At least 5 days |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 12/41; B: 4/34 Definition of bacteriuria: Significant bacteriuria was defined 104 cfu/ml. The urine was monitored at catheter insertion and 3 days after removal of the catheter Discomfort: A: 38/41; B: 18/34 Participants with pain: A: 18/41; B: 1/34 |
|
| Sponsorship/Funding | Not reported | |
| Notes | No definition was given for the outcome measure 'discomfort' No definition was given for the outcome measure 'pain' Some participants received antibiotics (65% in each study arm) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomization into two groups” “les deux groups ont été tires au sort avant le début de l’étude, de manière aléatoire” [the two groups were chosen by lot before the start of the study, randomly] – not enough information about method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants | High risk | No information given but as transurethral and suprapubic can assume that participants were not blinded |
| Blinding of personnel | High risk | “Elles ont été mises en place soit par le personnel infirmier, soit par les chirurgiens ou les anesthésistes” [they (catheters) were put in place by nurse, surgeon or anaesthetists] – no mention of blinding of personnel, can assume those involved in insertion were not blinded |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria assessed by microbiologists, who would not know allocation of participant to suprapubic or transurethral catheter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “25 malades ont été retires de l’étude selon les critères suivants: infection des urines au moment du sondage 10; décès 8; sonde à demeure 5; transferts dans d’autres hôpitaux 2.” [25 patients were withdrawn from the study according to the following criteria: UTI at time of catheter insertion 10; 8 deaths ; catheter remained indwelling 5; transfers to other hospitals 2] unclear which intervention group in, could be significant |
| Selective reporting (reporting bias) | Unclear risk | Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Prasad 2014.
| Methods |
RCT or quasi‐RCT: RCT Setting: USA Period: August 2011 |
|
| Participants |
Population: Men with newly diagnosed prostate cancer and undergoing robot‐assisted laparoscopic radical prostatectomy Inclusion criteria: men with biopsy‐proven prostate cancer with a BMI < 40 (kg/m²) Exclusion criteria: Age (mean, SD): A: 57.7 (8.6) years; B: 60.0 (6.4) years Number of participants: · Eligible: 95 · Randomised: 66 · Reported: 58 Dropouts (n of participants + reasons): 4 participants (2 in each group) dropped out before day 1 of 7. Another 4 participants (1 indwelling urethral and 3 suprapubic) did not receive allocated intervention, excluded from analysis Follow‐up: minimum 1 year |
|
| Interventions |
Time of intervention: A (n = 32): Indwelling urethral catheter placed intraoperatively, removed on postoperative day 7 B (n = 34): Suprapubic catheter placed 24 hours after surgery, removed on postoperative day 7. Had indwelling urethral catheter prior to this Intended duration of catheterisation: 7 days |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: not reported Definition of bacteriuria: not reported Quality of life: not reported Postoperative pain (mean, n): POD 0: A: 2.9 (29); B: 3.5 (29) (P = 0.41) POD 1: A: 2.5 (29); B: 3.0 (29) (P = 0.39) POD 7: A: 1.0 (29); B: 1.5 (29) (P = 0.26) |
|
| Sponsorship/Funding | Cook Medical provided suprapubic catheters | |
| Notes |
Contact details: Dr Sandip Prasad, e‐mail: prasads@musc.edu Antibiotic prophylaxis use not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used block randomisation, unclear how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | If block randomisation was performed by surgeon, high risk of sequence generation being broken |
| Blinding of participants | High risk | “Non‐blinded patient” – was not possible for participants to be blinded |
| Blinding of personnel | High risk | Surgeons and other staff involved in surgery and care could not be blinded to which catheter participant had |
| Blinding of microbiological outcome assessment | Low risk | Microbiological outcomes not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “the impact of emotional factors regarding treatment allocation to the nonblinded patient may have influenced the pain score. Since most patients expressed a desire to be allocated to the SPT group” – highly likely that lack of blinding affected participant pain scores |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Based on this analysis the trial was terminated for futility and all results reported are based on interim data.” – aimed to recruit 102 but stopped early with only 58 participants Similar drop out between groups, but reasons for dropout not reported. |
| Selective reporting (reporting bias) | Unclear risk | Only primary outcome reported in Methods |
| Other bias | Unclear risk | “All subjects were analyzed on an intent to treat basis. However, 4 patients who did not receive the assigned intervention and another 4 who dropped out before day 1 of 7 were excluded from analysis.” – should have included participants who received wrong intervention |
Rasmussen 1977.
| Methods |
RCT or quasi‐RCT: RCT Setting: Denmark Period: October 1974 – June 1976 |
|
| Participants |
Population: patients undergoing surgery by abdomino‐perineal resection (APR) or low anterior resection (LAR) for rectal cancer Inclusion criteria: patients undergoing surgery by abdomino‐perineal resection (APR) or low anterior resection (LAR) for rectal cancer Exclusion criteria: patients with preoperative bacteriuria, patients who required urethral catheterisation due to tumour invasion Age (mean, SD)/(median, range): < 70: A: 8; B: 7 ≥ 70: A: 25; B: 15 Number of participants: · Eligible: · Randomised: 55 · Reported: 55 Dropouts (n of participants + reasons): B: 1 man as Intracath needle too short to penetrate bladder wall; 2 women allocated SPC swapped to urethral (1 ‐ tube slipped out of bladder, 1 – obstruction); 1 woman SPC catheter became obstructed and then died from pulmonary embolism (1 month after surgery) Follow‐up: 3 months |
|
| Interventions |
Time of intervention: A (n = 33): indwelling urethral catheter (Foley, No. 16 French) was inserted before surgery and kept on during first 24 hours. After this was closed and opened every 6 hours. Removed on 5th day. B (n = 22): suprapubic catheter (No. 5 French polyethylene tube) was inserted before surgery and drained continuously for 24 hours. After this was opened and closed for 10 minutes every 6 hours. Removed when post‐voidal volume < 100 ml during each of 2 subsequent measurements Intended duration of catheterisation: A: 5 days; B: post‐voidal volume < 100 ml |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: 5 days: A: 5/28; B: 0/20 (P < 0.05) 3 months: A: 5/33; B: 5/22 Definition of bacteriuria: ≥ 10⁵/ml Recatheterisation: A: 5/33; B: 3/22 MEN ONLY daily number of micturitions (median, range, n): A: 6 (3 ‐ 10), 15; B: 4 (3 ‐ 6), 14 Reasons for antibiotic therapy (postoperative hospital stay): Wound Infection: A: 2/33; B: 2/22 Peritoneal contamination: A: 5/33; B: 1/22 Bacteriuria: A: 5/33; B: 5/22 Other Reasons: A: 5/33; B: 1/22 No antibiotics: A: 16/33; B: 13/22 Quality of life: Not reported |
|
| Sponsorship/Funding | Not reported | |
| Notes | 2 participants did not receive intervention as allocated (received urethral instead of suprapubic after 1 day because the tube had slipped out from the bladder in 1 case and had become obstructed in another). Neomycin sulphate + bacitracin were given, 1.5 g every 6 hours 3 days before operation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information on method of allocation concealment |
| Blinding of participants | High risk | No information on blinding. Can assume as suprapubic vs indwelling that blinding did not occur |
| Blinding of personnel | High risk | No information on blinding. Can assume as suprapubic vs indwelling that blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding. No information on primary outcome – some of the outcomes would be at risk of bias if blinding did not occur |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential dropout. Adequate explanation for withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Outcomes being studied are not stated clearly in the Methods. Unable to access protocol, so uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free of other sources of bias |
Ratnaval 1996.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK Period: not reported |
|
| Participants |
Population: men undergoing pelvic colorectal surgery Inclusion criteria: men undergoing pelvic colorectal surgery Exclusion criteria: not reported Age (median, range): Overall: 66 (32 ‐ 81) A: 63 (42 ‐ 80); B: 64 (32 ‐ 81) Number of participants: · Eligible: not reported · Randomised: 50 · Reported: 50 Dropouts (n of participants + reasons): 0 Follow‐up: duration of catheterisation |
|
| Interventions |
Time of intervention: A (n = 26): indwelling urethral catheter placed during surgery. Removed based on participant well‐being B (n = 24): suprapubic Bonano catheter placed at end of surgery. When suprapubic catheter was going to be removed, it was clamped and the residual volume measured. If it was < 50 ml, the catheter was removed Intended duration of catheterisation: Not reported |
|
| Outcomes |
Primary outcome (symptomatic UTI): no definition Definition of symptomatic UTI: no definition Bacteriuria: A: 3/26; B: 1/24 (P > 0.05) Definition of bacteriuria: based on culture positive urine samples Days of catheterisation (mean, range, n): A: 7.5 (2 ‐ 13), 26; B: 7.2 (3 ‐ 14), 24 Acute urinary retention: A: 6/26; B: 5/24 (P > 0.05) Chronic urinary retention: A: 1/26; B: 0/24 (P > 0.05) Recatheterisation: A: 7/26; B: 2/24 (P > 0.05) Frequent voiding: A: 11/26; B: 2/24 (P < 0.05) Catheter pulled out: A: 0/26; B: 1/24 (P > 0.05) Quality of life: Not reported |
|
| Sponsorship/Funding | Not reported | |
| Notes | Not reported if used prophylactic antibiotics Contacted Mr Ridzuan Farouk via Researchgate.net. Reported that postoperative urinary tract infection was based on "culture positive urine samples" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were then randomised alternately to each form of catheterisation” |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given. As suprapubic vs indwelling, can assume that it was not possible for blinding to occur |
| Blinding of personnel | High risk | No information given. As suprapubic vs indwelling, can assume that it was not possible for blinding to occur as catheters inserted during surgery |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by a microbiologist who would not know what type of catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Information on outcomes not reported in Methods. Unable to access protocol, so some uncertainty about reporting bias |
| Other bias | Low risk | Appears to be free from other sources of bias |
Rivard 2012.
| Methods |
RCT or quasi‐RCT: RCT Setting: USA Period: July 2009 – November 2009 |
|
| Participants |
Population: singleton pregnancies in active labour or undergoing induction of labour who were anticipated to have vaginal delivery Inclusion criteria: adult women (age > 18) with a singleton pregnancy who presented to the labour and delivery unit in active labour or for labour induction, were anticipated to have a vaginal delivery, and chose epidural for pain control Exclusion criteria: scheduled for caesarean delivery, had multi‐fetal gestations, required tocolysis, and/or were on magnesium prophylaxis Age (mean): A: 27.6; B: 28.7 Number of participants: · Eligible: 139 · Randomised: 139 · Reported: 138 Dropouts (n of participants + reasons): 1 in intermittent catheter group due to physician withdrawal Follow‐up: duration of labour |
|
| Interventions |
Time of intervention: Enrolled participants randomised after physician determined clinical need for catheterisation A (n = 72): indwelling catheter. Removed during 2nd stage of labour when woman started pushing B (n = 67): intermittent catheter inserted every 2 to 4 hours Intended duration of catheterisation: A: until 2nd stage of labour B: unclear |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: not reported Definition of bacteriuria: not reported Cost per catheter: A: USD 6.28; B: USD 5.98 Number of catheterisations (mean, SD not reported, n): A: NR; B: 2.5 Nurses’ perceptions: Preference: A: 30/37; B: 7/37 *Better care: A: 28/37; B: 9/37 **Time requirement: A: 31/37; B: 6/37 Preference for method to be standard care: A: 34/37; B: 2/37 Quality of life: Not reported |
|
| Sponsorship/Funding | “No financial disclosures” | |
| Notes | *Nurses’ perception of care they provide to patients **i.e. nurses perceived A (indwelling) to be less time‐consuming Not reported if used prophylactic antibiotics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer‐generated list of randomization using permuted block” – adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | “Sequentially numbered opaque envelopes displaying only the randomization number on the outside” – low risk of allocation of intervention being revealed |
| Blinding of participants | High risk | No information given on blinding of participants. Can assume that blinding did not occur as indwelling vs intermittent |
| Blinding of personnel | High risk | No information given on blinding of personnel. As indwelling vs intermittent can assume blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Microbiological outcomes not reported, therefore no risk of bias from reporting of microbiological outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 146 enrolled, 7 excluded (2 magnesium prophylaxis, 2 no epidural, 3 delivered prior to randomisation). 139 randomised, 72 indwelling, 67 intermittent 1 withdrawn from intermittent due to physician recommendation. 72 indwelling, 66 intermittent None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the Results. Unable to access protocol, so uncertainty surrounding selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Schiotz 1989.
| Methods |
RCT or quasi‐RCT: RCT Setting: Norway |
|
| Participants |
Population: women undergoing vaginal plastic surgery Inclusion criteria: women undergoing vaginal plastic surgery Age (mean, SD): A: 63.8 (9.1) years; B: 63.6 (8.5) years Number of participants:
Dropouts (n of participants + reasons): 12 patients were excluded because of positive preoperative urine cultures |
|
| Interventions | A (n = 40): Indwelling urethral catheter (No.14,Foley) introduced at the end of surgery B (n = 38): Suprapubic catheter (No.10, Cystofix) introduced at the end of surgery Intended duration of catheterisation: The suprapubic catheter was clamped on the 3rd postoperative day and removed if the participant could micturate spontaneously. The indwelling urethral catheter was removed on the 3rd postoperative day and the participants were catheterised intermittently if required |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 11/40; B: 9/38 Definition of symptomatic UTI: > 105 cfu/ml, associated with dysuria and/or pain and/or fever and/or rigors and/or sepsis. During hospital stay. Bacteriuria: A: 5/40; B: 8/38 Definition of bacteriuria: > 105 cfu/ml. Specimens were obtained preoperatively, at catheter removal, at follow‐up 6 ‐ 8 weeks postoperatively, and when clinically indicated Bacteriuria (asymptomatic and symptomatic) at 6 ‐ 8 week follow‐up: A: 5/40; B: 4/38 Symptomatic UTI at 6 ‐ 8 week follow‐up: A: 4/40; B: 4/38 Bacteriuria at 6 ‐ 8 week follow‐up: A: 1/40; B: 0/38 Duration of catheterisation: A: 3.3 days (SD 1.9); B: 4.9 days (SD 3.3) Catheter obstruction: A: 0/40; B: 2/38 |
|
| Sponsorship/Funding | Not reported | |
| Notes | A participant was recatheterised when intermittent catheterisation was necessary more than twice Not reported whether prophylactic antibiotics were used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospective randomized study" ‐ no further information given |
| Allocation concealment (selection bias) | Unclear risk | "Allocation to either method was done 'blind' on admission, by a nurse not involved in the construction of the study protocol" ‐ no information on method of allocation concealment |
| Blinding of participants | High risk | No information given but as suprapubic vs transurethral can assume no blinding |
| Blinding of personnel | High risk | No information given but as suprapubic vs transurethral can assume clinicians were not blinded |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI and bacteriuria were assessed by a microbiologist who was not aware of which catheter the patient had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "excluded from the study were 12 patients with positive preoperative cultures" ‐ reasons unlikely to be related to true outcomes |
| Selective reporting (reporting bias) | Low risk | Have reported all the outcomes which were expected. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sethia 1987.
| Methods |
RCT or quasi‐RCT: RCT Setting: UK |
|
| Participants |
Population: patients undergoing general surgery Inclusion criteria: Patients requiring general surgical operations. Indications for catheterisation were the need to monitor postoperative urine output and after an extensive pelvic dissection with or without an anastomosis Exclusion criteria: history of urological disease Comment: It is not clear whether patients with initial positive urine cultures were excluded Age (mean): A: 62.3 years; B: 63.7 years Number of participants:
Dropouts (n of participants + reasons): 5 participants were excluded. Reasons were inadequacies either in the number of specimens obtained or in their processing |
|
| Interventions | A (n = 34): Indwelling urethral catheterisation (14 F Foley) inserted immediately before operation B (n = 32): Suprapubic catheterisation (14 F Foley) placed perioperatively Duration of catheterisation: No protocol described; Median period was 5 days for each group. |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: Overall: A: 16/34; B: 2/32 In subgroups: a) men A: 5/16; B: 2/17 b) women A: 11/18; B: 0/15 Definition of bacteriuria: Significant bacteriuria was defined as 104 cfu/ml of a catheter yielded specimen and 105 cfu/ml of a midstream specimen. Specimens were obtained immediately following catheterisation and daily thereafter aspirated from the drainage tube. A further midstream specimen was obtained 2 days after removal of the catheter. Recatheterisation (after catheter removal): A: 5/34; B: 0/32 |
|
| Sponsorship/Funding | Not reported | |
| Notes | All participants received the same antibiotic prophylaxis (single dose of metronidazole 500 mg and cephadrine 1 g intravenously on induction of anaesthesia). These antibiotics were continued for 48 hours in high‐risk participants and 5 days when sepsis was already present | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All patients undergoing general surgical operations who were thought to require catheterization were randomly allocated to one of two groups" ‐ no information on method |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given but as suprapubic vs transurethral can assume no blinding |
| Blinding of personnel | High risk | No information given but as suprapubic vs transurethral can assume clinicians were not blinded |
| Blinding of microbiological outcome assessment | Low risk | The primary outcome UTI was assessed by a microbiologist; who would not know the type of catheter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "71 patients were entered into the trial but five were excluded because of inadequacies either in the number of specimens obtained or in their processing" ‐ missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | No information given in Methods on outcomes being measured. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Skelly 1992.
| Methods |
RCT or quasi‐RCT: RCT Setting: orthopaedic unit in a general hospital (St. Joseph’s Hospital, Hamilton, Ontario, Canada) Period: November 1986 – December 1987 |
|
| Participants |
Population: patients ≥ 60 years old admitted for surgical repair of hip fracture Inclusion criteria: patients ≥ 60 years old admitted for surgical repair of hip fracture Exclusion criteria: patients with intractable incontinence, patients admitted with indwelling catheter in place, patients who were unable to provide informed consent Age (mean, SD): A: 78 (8.2); B: 78 (8.6) Number of participants: · Eligible: 76 · Randomised: 67 · Reported: 67 Dropouts (n of participants + reasons): 5 medically unstable, 2 died before surgery, 2 did not have urinary retention after surgery Post‐randomisation: none reported Follow‐up: 5 days |
|
| Interventions |
Time of intervention: preoperative A (n = 35): indwelling catheter inserted preoperatively and left in place until 48 hours after surgery. If could not void in following 24 hours, intermittent catheterisation performed every 8 hours for 24 hours. If still not able to void indwelling catheter inserted again for 48 hours B (n = 32): intermittent catheter inserted every 6 ‐ 8 hours, with 400 ‐ 600 ml of urine removed each time Catheterisation stopped when residual volume of urine < 150 ml on 2 consecutive occasions Intended duration of catheterisation: A: 48 hours B: 5 postoperative days if normal voiding returned. If not, catheter remained in until voiding returned Voiding defined as > 100 ml and residual volume < 150 ml on 2 consecutive occasions |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: A: 11/35; B: 12/32 Definition of bacteriuria: ≥ 10⁵ cfu/ml on postoperative day 5 Postoperative urinary retention : A: 6/35; B: 6/32 Participants voiding on postoperative day 5: A: 13/35; B: 21/32 Days until return of voiding (mean, n): A: 9.4, 35; B: 5.1, 32 Incontinent after postoperative day 5: A: 5/35; B: 2/32 Quality of life: not reported |
|
| Sponsorship/Funding | “This work was supported by a research grant from Ontario Ministry of Health” | |
| Notes | Not reported if used prophylactic antibiotics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “use of a randomization technique involving block sizes of four” – method of randomisation not given, not clear if selection bias risk is high or low |
| Allocation concealment (selection bias) | Low risk | “sequentially numbered sealed envelope” – adequate method of allocation concealment, low risk of selection bias |
| Blinding of participants | High risk | No information given. As indwelling vs intermittent can assume blinding did not occur for participants, therefore high risk of bias |
| Blinding of personnel | High risk | No information given. As indwelling vs intermittent can assume blinding did not occur for personnel, therefore high risk of bias |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given. Primary outcome is urinary retention, but no method reported on how urinary retention was diagnosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76 agreed to participate in trial – 9 excluded (5 at physician’s request as medically unstable, 2 died before surgery, 2 did not have urinary retention). 67 randomised. All randomised participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No clear information given in Methods about the outcomes being studied. Unable to access protocol, so uncertainty surrounding reporting bias |
| Other bias | Low risk | Appears to be free from other sources of bias |
Stekkinger 2011.
| Methods |
RCT or quasi‐RCT: RCT Setting: Deventer Ziekenhuis, Deventer, Netherlands Period: July 2005 ‐ August 2007 |
|
| Participants |
Population: patients undergoing cystocoele repair Inclusion criteria: All women scheduled for anterior colporrhaphy (AC) surgery ± hysterectomy, ± PRS for pelvic prolapse Exclusion criteria: Those without AC, those with incontinence surgery; h/o urinary retention, urologic disease, renal insufficiency; those with preoperative UTI by dipstick; those unable to read and write Dutch Number of participants:
Age (mean, SD): A: 61.7 (11.2) years; B: 62.2 (11.5) years Dropouts (n of participants + reasons): suprapubic group (2 never placed, 3 clogged, 5 fell out), but analysed with ITT. |
|
| Interventions | A (n = 62): indwelling urethral catheterisation using 14 French (brand not specified) placed intra‐operatively, removed in the afternoon of postoperative day 3; measurements begun in morning of postoperative day 4 B (n = 64): suprapubic catheterisation using 15Fr Cystofix™ SPT catheter, sutured to participant's skin (B. Braun Medical, Oss, Netherlands) placed intra‐operatively, clamped on the 3rd night after surgery; measurements begun in the morning of postoperative day 4 All participants received pre‐operative antibiotics (1 g Cefazolin and 500 mg of Metronidazole) Duration of Intervention: Until participant returned to adequate voiding (PVR < 150 ml) |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 6/62; B: 6/64 (P = 0.93) (NS) Definition of bacteriuria: Presence of > 10⁴ cfu/ml in culture Proportion of participants with inadequate post‐operative voiding (PVR > 150 ml) on postoperative day 4: A: 14; B): SPC 13, (P = 0.76) (NS) Proportions able to void spontaneously: A: 60/62; B: 62/64 (NS) Number of days until residual volume < 150 ml (days)(median, minimum‐maximum): A: 4 (3 ‐ 18); B: SPC 4 2 ‐ 69); (P = 0.024) (NS) PVR volume (ml)(median, minimum‐maximum): A: 56 (0 ‐ 700); B: 85 (0 ‐ 650) (P = 0.76) (NS) Length of hospital stay (days)(median, minimum‐maximum): A: 4 (3 ‐ 7); B: 4 (3 ‐ 13) (P = 0.71) (NS) PVR volume > 500 ml: A: 5/62; B: 5/64; (P = 0.96) (NS) Need for recatheterisation: A: 11/62; B: 11/64 (P = 0.94) (NS) Catheter‐related complications: A: 7/62; B: 30/64 ‐Catheter fell out: A: 0/62; B: 5/64 (P = 0.058) (NS) ‐Serious haematuria: A: 0/62; B: 5/64 (P = 0.058) (NS) ‐Urine leakage: A: 4/62; B: 17/64 (P = 0.003) (Significant Difference) ‐Catheter blockage: A: 3/62; B: 3/64 (P = 1.0) (NS) |
|
| Sponsorship/Funding | Not reported | |
| Notes | Although there was no statistically significant difference for the primary outcome, the sample size of 114 was calculated for a difference of 15% between groups; therefore the trial did NOT have the power to detect a lesser difference (only 2%) in this sample. Complication rate was reported as total number of complications, although participants may have developed more than one complication, thereby lowering the reported rate. All women received a single dose of prophylactic antibiotics (cefazolin 1 g and metronidazole 500 mg) during surgery |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "women were randomly assigned to two groups... sequentially numbered sealed, opaque envelopes that were opened before surgery" ‐ adequate method of randomisation |
| Allocation concealment (selection bias) | Low risk | " sequentially numbered sealed,opaque envelopes" ‐ adequate method of allocation concealment |
| Blinding of participants | High risk | No information given but as suprapubic vs transurethral can assume blinding did not occur |
| Blinding of personnel | High risk | No information given but as suprapubic vs transurethral can assume blinding did not occur |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI would be assessed by microbiologist who would not know which catheter participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 126 randomised. None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes mentioned in Methods are reported in Results. Unable to access protocol, so some uncertainty around selective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
Tang 2006.
| Methods |
RCT or quasi‐RCT: RCT Setting: women's geriatric rehabilitation ward of a convalescent hospital, China Period: June 1999 ‐ December 2000 |
|
| Participants |
Population: elderly women admitted to a convalescent hospital Inclusion criteria: women, > age 65, with PVR persistently > 300 ml by bladder scan ultrasound, admitted to a geriatric rehab ward Exclusion criteria: Terminally‐ill patients; indwelling catheter required for continuous monitoring of urine output Age (mean, SD): A: 81.4 (8.9); B: 80.0 (6.8) Number of participants:
Dropouts (n of participants + reasons): 15 participants were lost (A: 6/45; B: 9/36), with the main reason "for dropout was transfer to an acute hospital and clinical deterioration". 1 in group B refused further intermittent catheterisation from day 2. 1 in group B switched to indwelling catheterisation due to bilateral hydronephrosis. 2 in group A died. |
|
| Interventions | A (n = 45): Indwelling Foley catheter, placed after randomisation. Removed at least once weekly, replaced if PVR > 300 ml B (n = 36): CISC, monitored by bladder scan 3 times a day. CISC performed when PVR > 500 ml or > 300 ml and symptomatic |
|
| Outcomes |
Primary outcome (symptomatic UTI): A: 0/34; B: 1/22 (P = 0.400) Definition of symptomatic UTI: Symptomatic UTI on day 14: either having a fever in the absence of other sites of infection with or without symptoms of dysuria or suprapubic discomfort Bacteriuria: A: 21/34; B: 14/22 (P = 0.888) Definition of bacteriuria: Growth of ≥ 10⁵ bacteria/ml on 14th day Proportion of participants catheter‐free with PVR < 150 ml on day 14: A: 27/39; B: 16/27 (P = 0.403) PVRU on day 14 (ml) (mean, SD): A: 54.4 (49.1); B: 77.6 (48.2) Time to become catheter‐free (mean, SD)(days): A: 9.2 (4.0); B: 8.6 (3.3) |
|
| Sponsorship/Funding | Not reported | |
| Notes | CISC group catheterised a mean of 3 times a day Urine cultures sent on day 1 and day 14. Bacteriuria defined as 105 cfu/ml on culture. Symptomatic UTI defined as fever with or without dysuria or suprapubic pain. According to power analysis, required 80 participants to determine no significant difference between groups; after losses, there were not enough participants. Incomplete outcome data for 15 lost participants after randomisation, and missing data for bacteriuria. Not reported if used prophylactic antibiotics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by "random number table" ‐ not enough information |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given but as indwelling vs intermittent can assume no blinding |
| Blinding of personnel | High risk | No information given but as indwelling vs intermittent can assume no blinding |
| Blinding of microbiological outcome assessment | Low risk | Symptomatic UTI and bacteriuria would be assessed by microbiologist who would not know the type of catheter the participant had received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 dropouts which are differential when you exclude women who died. Also incomplete data for secondary outcome measures |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes in Methods are all reported in Results. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free of other sources of bias |
Tangtrakul 1994.
| Methods |
RCT or quasi‐RCT: RCT Setting: Department of Obstetrics + Gynaecology, Ramathibodi Hospital, Thailand Period: August 1991 – December 1991. |
|
| Participants |
Population: women undergoing caesarean section Inclusion criteria: women undergoing caesarean section, non‐private patient, never been catheterised before operation, no history of UTI or anomaly of urinary tract, no medical complication, did not receive antimicrobial drugs 1 week before operation Exclusion criteria: NR Age (mean, SD): A: 30.4 (4.6); B: 29.1 (4.5) Number of participants: · Eligible: 107 · Randomised: unclear · Reported: 98 (table 2 data only reported for 55 participants) Dropouts (n of participants + reasons): 9 women due to urine cultures from initial catheterisation that were positive Follow‐up: 3rd postoperative day |
|
| Interventions |
Time of intervention: preoperatively A (n = 47): indwelling catheter placed just before operation. Removed following day after operation B (n = 51): intermittent catheterisation. Catheterised just before operation. Removed immediately after operation If participant in either group had postoperative urinary retention they were treated with intermittent catheterisation. Any participant who required intermittent catheterisation more than twice had indwelling Foley catheter inserted for 24 hours. Urinary retention was defined as unable to void in presence of clinically apparent bladder distention, or at least every 6 hours while awake Intended duration of catheterisation: A day after operation B ≥ every 6 hours while awake |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Definition of symptomatic UTI: not reported Bacteriuria: A: 9/47 (19.1); B: 16/51 (31.4) (P > 0.05) Definition of bacteriuria: ≥ 105 organisms/ml on 3rd postoperative day Urinary retention (n): A: 0/47; B: 20/51 Number of intermittent catheterisation (n): 1 time: A: 0/47; B: 11/51 2 times: A: 0/47; B: 7/51 3 times (Foley): A: 0/47; B: 2/51 Quality of life: not reported |
|
| Sponsorship/Funding | “This study was supported by Ramathibodi Research Grant No. 9/1992” | |
| Notes | Used non‐private patients in Thailand, overall generalisability of the trial affected No participants received prophylactic antimicrobial drug |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “They were randomly allocated into 2 groups according to their initial urinary management in the operating room” – no information given on method of randomisation. Reporting not very clear, participants may have been stratified |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants | High risk | No information given. As comparing intermittent vs indwelling unlikely blinding occurred |
| Blinding of personnel | High risk | No information given. As comparing intermittent vs indwelling unlikely blinding occurred |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria would be assessed by microbiologist who would not know what catheter the participant had |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information given unclear. Intermittent had 51 participants, Indwelling had 47. 9 women excluded due to positive cultures initially. Clinical data of participants with labour pain only reports on total of 55 participants (intermittent 32, indwelling 23). No reason given for missing data. |
| Selective reporting (reporting bias) | Unclear risk | No information given on outcomes to be studied. Unable to access protocol, so some uncertainty about reporting bias |
| Other bias | Low risk | Appears to be free from other sources of bias |
Van den Brand 2001.
| Methods |
RCT or quasi‐RCT: RCT Setting: The Netherlands |
|
| Participants |
Population: patients undergoing total hip or knee replacement Inclusion criteria: primary total hip and primary total knee arthroplasty patients Exclusion criteria: Chronic or recurrent UTI; steroid medication; long‐term antibiotic therapy; endocarditis antibiotic prophylaxis; preoperative bacteriuria; UTI. Age (mean, SD): A: 68.6 (8.8) years; B: 68.2 (9.0) years Number of participants:
Dropouts (n of participants + reasons): 14 participants were excluded: 3 because of Foley catheter after operation inserted, 2 because of Foley catheter removed after day 2, 7 because of no urine culture or sediment postoperative, and 2 because of complications |
|
| Interventions | A (n = 46): Indwelling urethral catheter (Foley) introduced in the operating room just before the start of surgery. Catheter remained in place for 48 hours B (n = 53): Intermittent catheterisation every 6 hours or earlier when clinically needed by a trained staff nurse until spontaneous voiding occurred. No prophylactic catheterisation was performed immediately postoperatively because of a greater risk of bacteruria |
|
| Outcomes |
Bacteriuria: A: 11/46; B: 3/53 In subgroups: a) men: A: 5/13; B: 0/14 b) women: A: 6/33; B: 3/39 Definition of bacteriuria: Significant bacteriuria was defined as a positive urine sediment for bacteria and white blood cells with a positive urine culture of 10⁵ cfu/ml. Midstream clean‐catch urine specimens for sediment were taken on the day before surgery and the 2nd postoperative day (after removal of the indwelling catheter), for sediment and culture on the 5th postoperative day Costs per patient for the first 48 hours after surgery: A: USD 6.15 per patient; B: USD 7.75 per patient |
|
| Sponsorship/Funding | "No benefits or funds were received in support of this study" | |
| Notes | OPM: When sediment was positive on the 2nd postoperative day, urine was cultured and antibiotics were started. Analysis of cost per participant was conducted for the2 methods of management based on both catheter materials and nursing time components of costs Antibiotics: 1 dose of cefazolin, 1g, intravenously immediately before surgery; no postoperative antibiotics were used Postoperative pain management did not occur by epidural anaesthesia The average number of intermittent catheterisations was 1.6 per participant Participant characteristics, 5 in group A and 0 in group B had diabetes (confounder for infection) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "All enrolled patients were randomized into 2 groups according to their patient number" ‐ not a method of randomisation |
| Allocation concealment (selection bias) | High risk | Patients were randomised according to patient number, therefore the sequence could be easily broken |
| Blinding of participants | High risk | No information but can assume participants could not be blinded as intermittent vs indwelling |
| Blinding of personnel | High risk | No information but can assume personnel could not be blinded, especially when inserting catheter |
| Blinding of microbiological outcome assessment | Low risk | "All results of the urinalysis preoperatively and postoperatively were reviewed independently by urologist blinded for the patient data" ‐ blinding of primary outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 113 enrolled into trial, 14 withdrew throughout trial ‐ does not say their intervention assignment |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes are reported. UTI and postoperative bacteriuria defined as the same thing, uncertain data for one of these outcomes was not reported. Unable to access protocol, so some uncertainty about selective reporting. |
| Other bias | Low risk | Appears to be free from other sources of bias |
Vandoni 1994.
| Methods |
RCT or quasi‐RCT: RCT Setting: Switzerland |
|
| Participants |
Population: surgical patients who required urinary catheterisation Inclusion criteria: surgical patients needing urinary catheterisation either for monitoring purposes or nursing reasons Exclusion criteria: Patients receiving antibiotics < 2 weeks prior to admission; already having a urinary catheter; patients with known or suspected bladder tumour; patients admitted for urologic surgery; bacteriuria at admission (not clear whether hospital admission or trial admission) Age (mean): A: 66.4 years; B: 66 years Number of participants:
Dropouts (n of participants + reasons): 4 participants in the SPC group. Reasons were failure to catheterise because of obesity |
|
| Interventions | A (n = 25): Indwelling urethral catheterisation (Charriere 12 latex Foley catheter) B (n = 25): Suprapubic catheterisation (Cystofix(R), Braun‐SSC, Switzerland) Duration of intervention: Authors did not report the protocol |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 9/25; B: 0/21 Definition of bacteriuria: Significant bacteriuria was defined as 1000 cfu/ml or more. Specimens were daily aspirated from the drainage tube Duration of catheterisation: A: mean 4.96 days; B: mean 4.48 days |
|
| Sponsorship/Funding | The trial was funded by Bayer (Switzerland) AG and Braun‐SSC (Switzerland) | |
| Notes | Identical single dose preoperative antibiotic prophylaxis was routinely applied (2 g of cefacetrile and 500 mg of metronidazole) Comment: 2 participants spontaneously related their preferences for suprapubic catheterisation: It is not clear whether these 2 participants were included in the treatment group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly allocated" ‐ no further information on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information given on concealment method |
| Blinding of participants | High risk | No information given. "Two of them [participants] had already experienced an urethral catheter and spontaneously related their preference for suprapubic catheterisation." ‐ can assume participants not blinded |
| Blinding of personnel | High risk | "All catheters were placed by the authors" ‐ does not state for rest of personnel but can assume were not blinded |
| Blinding of microbiological outcome assessment | Low risk | "Our laboratory staff, unaware of the type of catheter used, examined every sample" ‐ outcome of bacteriuria at low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear risk of detection bias for other outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "This is also why we had to exclude four obese patients whose bladder was impossible to find via the percutaneous suprapubic route" ‐ difference between intervention arms of trial |
| Selective reporting (reporting bias) | Low risk | Have reported primary outcome of interest and other relevant outcomes. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
Wiser 1974.
| Methods |
RCT or quasi‐RCT: RCT Setting: Tennessee, USA |
|
| Participants |
Population: women undergoing vaginal hysterectomy and anterior‐posterior repair Inclusion criteria: women undergoing vaginal hysterectomy and anterior‐posterior repair Exclusion criteria: Endometriosis, chronic pelvic inflammatory disease, pelvic masses, women who were emotionally unstable or uncooperative, asymptomatic bacteriuria, medical disorders, urological disorders, neurologic disorders Age: not reported Number of participants:
Dropouts (n of participants + reasons): Not reported; However, in table 3 there were 2 results missing in the treatment group |
|
| Interventions | A (n = 75): Indwelling urethral catheterisation (16 Foley) inserted postoperatively B (n = 75): Suprapubic catheterisation (16 Foley) Duration of intervention: In both groups the catheter was clamped for 4‐hour intervals beginning the3rd postoperative day. The catheter was removed the 4th postoperative day |
|
| Outcomes |
Primary outcome (symptomatic UTI): not reported Bacteriuria: A: 45/75; B: 14/75 Definition of bacteriuria: Significant bacteriuria was defined as > 104 cfu/ml on 4th postoperative day Recatheterisation: A: 23/75; B: 4/75 Duration of catheterisation: A: mean 5.8 days; B: mean 3.9 days Extended hospital stay: A: 25/75; B: 14/75 Number of participants requiring drugs for relief of dysuria: A: 52/75; B: 31/75 Haematuria: A: 2/75; B: 7/75 Catheter obstruction: A: 4/75; B: 2/75 Catheter inadvertently removed: A: 5/75; B: 4/75 Voiding difficulty: A 4/75; B 1/75 |
|
| Sponsorship/Funding | Not reported | |
| Notes | Specimens were taken from the catheter preoperatively, immediately postoperatively, on discontinuing the drainage system (on the 4th postoperative day) and 6 weeks postoperatively Extended hospital stay was defined as hospitalised > 7 days No use of prophylactic antibiotics. Antibiotics were not administered routinely in the postoperative period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were divided into two groups by the selection of a closed slip of paper denoting either "suprapubic" or "Foley" by an unbiased third party" ‐ method of randomisation appropriate |
| Allocation concealment (selection bias) | Low risk | "closed slide of paper... by an unbiased third party" ‐ low risk of allocation being broken |
| Blinding of participants | High risk | No information given but as suprapubic vs transurethral can assume no blinding |
| Blinding of personnel | High risk | No information given but as suprapubic vs transurethral can assume clinicians were not blinded |
| Blinding of microbiological outcome assessment | Low risk | Bacteriuria at low risk of detection bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information given on other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Table 3 ‐ suprapubic drainage group have 2 patient missing from discharge day postoperatively, no reason given |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not clearly stated. Unable to access protocol, so some uncertainty about selective reporting |
| Other bias | Low risk | Appears to be free from other sources of bias |
ASB: Asymptomatic Bacteriuria BMI: body mass index BPH: benign prostatic hyperplasia CISC: clean intermittent self catheterisation GA: gestational age ITT: intention to treat i.v.: intravenous NR: not reported POD: postoperative day PVR: post‐void residual RCT: randomised controlled trial SPC: suprapubic catheter SUC: suprapubic urethral catheter TURP: trans‐urethral resection of prostate UC: urethral catheter UTI: urinary tract infection VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 1980 | Not RCT |
| Allardice 1988 | Comparing 2 different materials for indwelling urethral catheterisation |
| Cardenas 2010 | CISC only |
| Chartier‐Kastler 2011 | Population is men with neurogenic bladder, long‐term catheterisation rather than short‐term |
| Dunn 2003 | Comparing different durations of indwelling urethral catheterisation |
| Frymire 1971 | Not RCT |
| Ghalayini 2005 | Compares CISC to TURP, not another type of catheterisation |
| Grundy 1983 | Catheter follow‐up 32 and 33 days; inclusion criteria only up to 14 days |
| Hofmeister 1970 | Not RCT |
| Horgan 1992 | Not RCT |
| Park 2010 | Not RCT |
| Schumm 2008 | Discusses the trial design of CATHETER trial, which does not meet inclusion criteria |
| Shapiro 1982 | Not RCT |
| Sicilia 2013 | Patient population: stroke, long‐term catheterisation rather than short‐term |
| Suprasert 2002 | Appears to be long‐term catheterisation rather than short‐term |
| Turi 2006 | Trial population is patients with neurogenic bladder and patients with inoperable BPH. Long‐term catheterisation rather than short term |
BPH: benign prostatic hyperplasia CISC: clean intermittent self catheterisation TURP: trans‐urethral resection of prostate
Characteristics of studies awaiting assessment [ordered by study ID]
Kringel 2007.
| Methods | RCT |
| Participants | Patients undergoing anterior colporrhaphy |
| Interventions | A: indwelling urethral catheters B: suprapubic catheters |
| Outcomes | "rate of complications and infections" |
| Notes | Poster presentation of already included study (Kringel 2010) |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000618651.
| Trial name or title | Indwelling urinary catheter during epidural anaesthesia in labour for preventing postpartum urinary tract infection Setting: Ho Chi Minh City, Vietnam |
| Methods | RCT |
| Participants | Inclusion criteria: Women with a singleton pregnancy, head presentation, admit to Hung Vuong hospital, and who are anticipated to undergo vaginal delivery during current condition and require epidural anaesthesia during labour. Exclusion criteria: Women pregnant who have planned caesarean section, previous caesarean section, women who have indication for indwelling urinary catheter postpartum such as: severe pre‐eclampsia, eclampsia, severe postpartum haemorrhage, severe internal medical diseases, and women who have urinary tract infection within 2 weeks of admission |
| Interventions | A: indwelling catheter inserted after epidural anaesthesia until time of delivery B: intermittent catheter used after insertion of epidural anaesthesia as required during course of labour |
| Outcomes | Primary outcome: Midwives will measure bladder residual volume by bladder scanner. Severe postpartum urinary retention will be diagnosed if bladder residual volume ≥ 400 ml.Timepoint: within 6 hours |
| Starting date | 16th June 2014 |
| Contact information | Dr. Phan Thi Hang Hung Vuong Hospital, 218 Hong Bang street, district 5th, Ho Chi Minh City, Vietnam phanhangbvhv@yahoo.com |
| Notes |
NCT01465594 2011.
| Trial name or title | Randomized study comparing urinary diversion by suprapubic catheter with transurethral catheter in patients after radical prostatectomy |
| Methods | RCT |
| Participants | Inclusion criteria: Adenocarcinoma of the prostate, participants treated by conventional or robotic‐assisted laparoscopic prostatectomy, be willing/able to adhere to follow‐up visits Exclusion criteria: people treated by retropubic or perineal prostatectomy; people with known bladder cancer, contraindications for anticholinergic drugs, waist measurement > 100 cm, no written informed consent, age < 18 years, people with known narrow‐angle glaucoma |
| Interventions | A: indwelling urethral catheter B: suprapubic catheter |
| Outcomes | Primary outcome: superiority of suprapubic catheter after EERPE regarding quality of life/participant comfort. Assessed on 2nd postoperative day. Secondary outcomes: QoL measured by visual analogue (pain) scale, EORTC QlQ ‐C 30 and QLQ ‐ PR 25 questionnaires, incontinence rate, complication rate regarding insufficiency and strictures of vesicourethral anastomoses and urinary tract infection; demand for re‐catheterisation due to urinary retention and demand for antispasmodics. Assessed on 1st and 3rd until 5th postoperative day. |
| Starting date | November 2011 |
| Contact information | Dr. Christian Arsov University Hospital, Urological department Düsseldorf, Germany, 40225 christian.arsov@med.uni‐duesseldorf.de |
| Notes |
NCT02108431 2013.
| Trial name or title | Superior postoperative patient comfort in suprapubic drainage versus transurethral catheterization following robot‐assisted radical prostatectomy: a prospective randomized clinical trial |
| Methods | RCT |
| Participants | Men with prostate cancer (18 years old and over) undergoing robot‐assisted radical prostatectomy Exclusion Criteria: BMI > 40, history of catheterisation, history of radiation, history of chemotherapy, participating in any other research, unable to provide informed consent |
| Interventions | A: suprapubic catheterisation B: transurethral catheterisation |
| Outcomes | Primary outcome: Change of postoperative pain related to urinary drainage objectified by numeric rating scale (NRS) questionnaire within 5 days. Time frame: postoperatively on day 1, 2, 3, 4 and 5 in the morning, at noon and in the evening Secondary outcomes: Number of participants with bacteriuria. Time frame: 5th day after the surgery. Second void urine sample after catheter removal |
| Starting date | February 2013 |
| Contact information | Dr. J.H. Witt, MD., St. Antonius Hospital Gronau urologie@st‐antonius‐gronau.de Dr. N. Harke, MD., FEBU. nina.harke@st‐antonius‐gronau.de Director of Scientific Research |
| Notes | Correspondence with Dr. Harke 12th February 2015 regarding trial. On 12th February 2015, trial was in publication process and planning to submit paper for publication in 2 weeks. Asked for further information about what journal the trial was being submitted to, in order to look for to include in future updates of review. |
NCT02198157 2014.
| Trial name or title | Effect of intermittent versus continuous bladder catheterization during labor on second stage duration |
| Methods | RCT |
| Participants | Inclusion criteria: age 18 ‐ 45 years, nulliparous women, gestational age 24 ‐ 42 weeks, epidural anaesthesia, vertex presentation, singleton |
| Interventions | A: indwelling urethral catheterisation B: intermittent urethral catheterisation |
| Outcomes | Primary outcome: duration of second stage of labour Secondary outcomes: chorioamnionitis, postpartum haemorrhage |
| Starting date | July 2014 (not recruiting 13th February 2015) |
| Contact information | Abeer Suleiman, MD Dep. OB/GYN, HaEmek Medical Center, Afula, Israel abeersulim@gmail.com |
| Notes |
NTR2806.
| Trial name or title | Catheter management and complications for symptomatic postpartum urinary retention. |
| Methods | RCT |
| Participants | Inclusion criteria: 1. Women who deliver in the participating hospitals; 2. Vaginally and by caesarean section; 3. 18 years and older; 4. Are unable to void within 6 hours postpartum. Exclusion criteria: 1. Age < 18 years; 2. Insufficient knowledge or understanding of the Dutch language; 3. Congenital urinary tract abnormalities; 4. Pre‐existent and treated urinary tract infection < 1 week before the delivery; 5. An indwelling catheter before delivery for parturition‐related reasons; 6. History of chronic neurological disease, including diabetic neuropathy. |
| Interventions | A: indwelling catheter B: intermittent catheter |
| Outcomes | Primary outcome: bladder‐related quality of life 3 months after randomisation for symptomatic PUR (UDI‐6 questionnaire) Secondary outcomes: 1. Development of a risk profile for overt PUR; 2. Prevalence of overt PUR (bladder scan); 3. The prevalence of clinical urinary tract infections due to different catheterisation methods; 4. Cost effectiveness analysis for different treatments of overt PUR; 5. Patient preference for different methods of catheterisation. |
| Starting date | 1st February 2011 |
| Contact information | Dr. Femke Mulder Academisch Medisch Centrum Meibergdreef 9 ‐ H4 205 1105 AZ Amsterdam The Netherlands f.e.mulder@amc.uva.nl |
| Notes | Contacted for further information as planned closing date was 1st June 2012. Trial website: www.studies‐obsgyn.nl/campur/page.asp?page_id=944 |
Wilson 2013.
| Trial name or title | Identifying and applying a targeted evidence‐based practice change in the maternal/ child health inpatient setting |
| Methods | RCT (randomisation envelopes opened just before epidural placement) |
| Participants | Term‐gestation singleton pregnancy primiparous women on the labour and delivery unit who requested an epidural |
| Interventions | A: indwelling urethral catheterisation B: intermittent urethral catheterisation |
| Outcomes | Length of second stage of labour Number of catheters during birth Incidence of UTI after discharge |
| Starting date | Not reported |
| Contact information | Barbara L. Wilson barbara.wilson@ nurs.utah.edu |
| Notes | Contacted on 30th January 2015. In results: "At present, the data have been collected and are currently being analyzed". Contacted to find out if any publication of results. |
BMI: body mass index EERPE: Endoscopic Extraperitoneal Radical Prostatectomy PUR: postpartum urinary retention QoL: quality of life UTI: urinary tract infection
Differences between protocol and review
For this update, the review authors have adopted the GRADE approach for assessing the quality of evidence of five outcomes which were included in the 'Summary of findings' tables. In this update we added the subgroup 'Timing of taking of urine sample', as this is clinically important.
Contributions of authors
For the 2015 update, Emily Kidd (EK) and Muhammad Imran Omar (MO) screened abstracts and full‐text reports of potentially eligible trials. Fiona Stewart (FS), EK and MO performed data extraction and 'Risk of bias' assessment independently. Quality of evidence was assessed by MO, EK and FS. Data analysis was done by EK, FS and MO. EK took the lead in writing the manuscript of the review. FS, PW, NK, EH and MO made comments and suggestions on the manuscript, which were incorporated in the review. EK and MO addressed referees' and editor's comments. For group discussion, MO developed the interview guide, moderated the group and analysed the qualitative data.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Incontinence Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Emily Kidd: none known Fiona Stewart: none known Nadine Kassis: none known Emily Hom: none known Muhammad Imran Omar: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahmed 1993 {published data only}
- Ahmed MS. Urinary tract infection in relation to pre‐operative catheterization: suprapubic versus urethral catheters. Pakistan Journal of Surgery 1993;9(2):43‐8. [sr‐incont35911] [Google Scholar]
Andersen 1985 {published data only}
- Andersen JT, Heisterberg L, Hebjorn S, Petersen K, Stampe Sorensen S, Fischer‐Rasmussen W, et al. Suprapubic versus transurethral bladder drainage after colposuspension/vaginal repair. Acta Obstetricia et Gynecologica Scandinavica 1985;64(2):139‐43. [sr‐incont616] [DOI] [PubMed] [Google Scholar]
Baan 2003 {published data only}
- Baan AH, Vermeulen H, Meulen J, Bossuyt P, Olszyna D, Gouma DJ. The effect of suprapubic catheterization versus transurethral catheterization after abdominal surgery on urinary tract infection: a randomized controlled trial. Digestive Surgery 2003;20(4):290‐5. [sr‐incont23330] [DOI] [PubMed] [Google Scholar]
Barents 1978 {published data only}
- Barents JW, Dankert J, Ilic P, Laanbroek HJ, Vries H. The indwelling catheter in gynecology and the development of bacteriuria; a comparative study of patients with the transurethral and the suprapubic catheter. Nederlands Tijdschrift voor Geneeskunde 1978;122(36):1321‐7. [sr‐incont7620] [PubMed] [Google Scholar]
Barry 1992 PE {published data only}
- Barry M, Gillen P, McNicholas M, Travnor O, Hyland J. A prospective, randomized controlled trial of urethral vs. suprapubic bladder catheterisation in abdominal surgery (Abstract number 69). Irish Journal of Medical Science 1992;161(8):540. [18538] [Google Scholar]
Bergman 1987 {published data only}
- Bergman A, Matthews L, Ballard CA, Roy S. Suprapubic versus transurethral bladder drainage after surgery for stress urinary incontinence. Obstetrics and Gynecology 1987;69(4):546‐9. [sr‐incont551] [PubMed] [Google Scholar]
Botsios 1997 {published data only}
- Botsios D, Demetriades C, Goulimaris I, Kanellos I, Dadoukis I. Suprapubic percutaneous cystostomy versus urethral catheterisation in abdominal surgery. A prospective randomised controlled study. Digestive Surgery 1997;14:404‐8. [sr‐incont6768] [Google Scholar]
Carpiniello 1988 {published data only}
- Carpiniello VL, Cendron M, Altman HG, Malloy TR, Booth R. Treatment of urinary complications after total joint replacement in elderly females. Urology 1988;32(3):186‐8. [sr‐incont1179] [DOI] [PubMed] [Google Scholar]
Dixon 2010 {published data only}
- Dixon L, Dolan M, Brown K, Hilton P. RCT of urethral versus suprapubic catheterization. British Journal of Nursing 2010;19(18):s7‐s13. [ISRCTN66645527; sr‐incont40340] [DOI] [PubMed] [Google Scholar]
- ISRCTN66645527. Intermittent urethral versus indwelling suprapubic catheterisation in the management of voiding after urogynaecological surgery: a randomised single centre controlled trial. isrctn.org/ISRCTN66645527 (accessed 12th November 2015). [ISRCTN66645527; sr‐incont64580]
Dobbs 1997 {published data only}
- Dobbs SP, Jackson SR, Wilson AM, Maplethorpe RP, Hammond RH. A prospective, randomized trial comparing continuous bladder drainage with catheterization at abdominal hysterectomy. British Journal of Urology 1997;80(4):554‐6. [sr‐incont6720] [DOI] [PubMed] [Google Scholar]
Evron 2008 {published data only}
- Evron S, Dimitrochenko V, Khazin V, Sherman A, Sadan O, Boaz M, et al. The effect of intermittent versus continuous bladder catheterization on labor duration and postpartum urinary retention and infection: a randomized trial. Journal of Clinical Anesthesia 2008;20(8):567‐72. [sr‐incont31242] [DOI] [PubMed] [Google Scholar]
- Rigini N, Evron S, Sadan O, Szmuk P, Ezri T. Intermittent versus continuous bladder catheterization and labor epidural (Abstract). Anesthesiology 2006;105:A907. [23732] [Google Scholar]
Hakvoort 2011 {published data only}
- Hakvoort RA, Thijs S, Bouwmeester F, Ruhe I, Vernooil M, Broekman A, et al. Optimal management of bladder retention following vaginal prolapse surgery: a randomized controlled trial comparing clean intermittent catheterization (CIC) and transurethral indwelling catheterisation (TIC) (Abstract number 58). Neurourology and Urodynamics 2010;29(6):892‐3. [40128] [Google Scholar]
- Hakvoort RA, Thijs SSD, Bouwmeester FW, Broekman AM, Ruhe IM, Vernooij MM, et al. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG 2011;118(9):1055‐60. [sr‐incont42075] [DOI] [PubMed] [Google Scholar]
- NTR1152. A randomised trial determining whether intermittent catheterisation is a better option for treating urinary retention than a transurethral indwelling bladder catheter for the outcomes duration of catheterisation, urinary tract infection and patient satisfaction ‐ CATH trial. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1152 (accessed 12th November 2015). [NTR1152; sr‐incont65505]
Halleberg 2013 {published data only}
- Halleberg Nyman M, Gustafsson M, Langius‐Eklof A, Johansson JE, Norlin R, Hagberg L. Intermittent versus indwelling urinary catheterisation in hip surgery patients: a randomised controlled trial with cost‐effectiveness analysis. International Journal of Nursing Studies 2013;50(12):1589‐98. [sr‐incont61995] [DOI] [PubMed] [Google Scholar]
Hammarsten 1992 {published data only}
- Hammarsten J, Lindqvist K. Suprapubic catheter following transurethral resection of the prostate: a way to decrease the number of urethral strictures and improve the outcome of operations. Journal of Urology 1992;147(3):651‐2. [sr‐incont1271] [DOI] [PubMed] [Google Scholar]
Harms 1985 {published data only}
- Harms E, Christmann U, Klock FK. Suprapubic urinary diversion following gynecologic operations. [German]. Geburtshilfe und Frauenheilkunde 1985;45(4):254‐60. [sr‐incont1140] [DOI] [PubMed] [Google Scholar]
Ichsan 1987 {published data only}
- Ichsan J, Hunt DR. Suprapubic catheters: a comparison of suprapubic versus urethral catheters in the treatment of acute urinary retention. Australian and New Zealand Journal of Surgery 1987;57(1):33‐6. [DOI] [PubMed] [Google Scholar]
Jannelli 2007 {published data only}
- Jannelli ML, Wu JM, Plunkett LW, Williams KS, Visco AG. A randomized controlled trial of clean intermittent self‐catheterization versus suprapubic catheterization after urogynecologic surgery. American Journal of Obstetrics and Gynecology 2007;197(1):72‐4. [sr‐incont23807] [DOI] [PubMed] [Google Scholar]
Katz 1992 {published data only}
- Katz G, Milgalter E, Landau Y, Borman JB. Prevention of urethral strictures following coronary artery bypass graft surgery. Urology 1992;39(5):433‐5. [DOI] [PubMed] [Google Scholar]
Kerr‐Wilson 1986 {published data only}
- Kerr‐Wilson RH, McNally S. Bladder drainage for caesarean section under epidural analgesia. BJOG 1986;93(1):28‐30. [DOI] [PubMed] [Google Scholar]
Knight 1996 {published data only}
- Knight RM, Pellegrini VD Jr. Bladder management after total joint arthroplasty. Journal of Arthroplasty 1996;11(8):882‐8. [sr‐incont4765] [DOI] [PubMed] [Google Scholar]
Korkes 2008 {published data only}
- Korkes F, Moniz RR, Silva FA, Guidoni LRM, Silva DB, Fernandes RC, et al. Urethral vs suprapubic catheter following open prostatectomy: surgeon's choice. Revista Chilena de Urologia 2008;73(4):272‐6. [sr‐incont39873] [Google Scholar]
Kringel 2010 {published data only}
- ISRCTN46098701. Comparison of the postoperative rate of complications and infections in indwelling urinary catheters and suprapubic catheters after anterior colporrhaphia: a three‐arm prospective randomised trial. isrctn.org/ISRCTN46098701 (accessed 12th November 2015). [ISRCTN46098701; sr‐incont62940 ]
- Kringel U, Reuimer T, Tomczak S, Green S, Kundt G, Gerber B. Postoperative infections due to bladder catheters after anterior colporrhaphy: a prospective, randomized three‐arm study. International Urogynecology Journal and Pelvic Floor Dysfunction 2010;21(12):1499‐504. [ISRCTN46098701; sr‐incont41072] [DOI] [PubMed] [Google Scholar]
Michelson 1988 {published data only}
- Michelson JD, Lotke PA, Steinberg ME. Urinary‐bladder management after total joint‐replacement surgery. New England Journal of Medicine 1988;319(6):321‐6. [DOI] [PubMed] [Google Scholar]
Millet 2012 {published data only}
- Millet L, Shah S, Bartholomew ML. Rates of bacteriuria in laboring women with epidural analgesia: continuous vs. intermittent bladder catheterization. American Journal of Obstetrics and Gynecology 2012;206(4):316.e1‐7. [sr‐incont44529] [DOI] [PubMed] [Google Scholar]
- Millet L, Shaha S, Bartholomew ML. Bacteriuria in laboring women with epidural analgesia: Continuous vs intermittent bladder catheterization (Abstract number 52). American Journal of Obstetrics and Gynecology 2012;206(1 Suppl 1):S33. [sr‐incont65102] [DOI] [PubMed] [Google Scholar]
Naik 2005 {published data only}
- Naik R, Maughan K, Nordin A, Lopes A, Godfrey KA, Hatem MH. A prospective randomised controlled trial of intermittent self‐catheterisation vs. supra‐pubic catheterisation for post‐operative bladder care following radical hysterectomy. Gynecologic Oncology 2005;99(2):437‐42. [sr‐incont21564] [DOI] [PubMed] [Google Scholar]
- Roberts K, Naik R. Catheterization options following radical surgery for cervical cancer. British Journal of Nursing 1006;15(19):1038‐44. [sr‐incont22222] [DOI] [PubMed] [Google Scholar]
Nwabineli 1993 {published data only}
- Nwabineli NJ, Walsh DJ, Davis JA. Urinary drainage following radical hysterectomy for cervical carcinoma ‐ A pilot comparison of urethral and suprapubic routes. International Journal of Gynecological Cancer 1993;3(4):208‐10. [sr‐incont7143] [DOI] [PubMed] [Google Scholar]
O'Kelly 1995 {published data only}
- O'Kelly TJ, Mathew A, Ross S, Munro A. Optimum method for urinary drainage in major abdominal surgery: a prospective randomized trial of suprapubic versus urethral catheterization. British Journal of Surgery 1995;82(10):1367‐8. [sr‐incont2866] [DOI] [PubMed] [Google Scholar]
Perrin 1997 {published data only}
- Perrin LC, Penfold C, McLeish A. A prospective randomized controlled trial comparing suprapubic with urethral catheterization in rectal surgery. Australian and New Zealand Journal of Surgery 1997;67(8):554‐6. [sr‐incont5587] [DOI] [PubMed] [Google Scholar]
Piergiovanni 1991 {published data only}
- Piergiovanni M, Tschantz P. [Urinary catheterization: transurethral or suprapubic approach?]. [French]. Helvetica Chirurgica Acta 1991;58(1‐2):201‐5. [sr‐incont1280] [PubMed] [Google Scholar]
Prasad 2014 {published data only}
- Prasad SM, Large MC, Patel AR, Famakinwa O, Galocy RM, Karrison T, et al. Early removal of urethral catheter with suprapubic tube drainage versus urethral catheter drainage alone after robot‐assisted laparoscopic radical prostatectomy. Journal of Urology 2014;192(1):89‐95. [sr‐incont66322] [DOI] [PubMed] [Google Scholar]
Rasmussen 1977 {published data only}
- Rasmussen OV, Korner B, Møller‐Sørensen P, Kronborg O. Suprapubic versus urethral bladder drainage following surgery for rectal cancer. Acta Chirurgica Scandinavica 1977;143(6):371‐4. [PubMed] [Google Scholar]
Ratnaval 1996 {published data only}
- Ratnaval CD, Renwick P, Farouk R, Monson JR, Lee PW. Suprapubic versus transurethral catheterisation of males undergoing pelvic colorectal surgery. International Journal of Colorectal Disease 1996;11(4):177‐9. [DOI] [PubMed] [Google Scholar]
Rivard 2012 {published data only}
- NCT00959920. Study of obstetric Foley therapy (SOFT trial). clinicaltrials.gov/show/NCT00959920 (accessed 12th November 2015). [NCT00959920; TrialID.SOFT; sr‐incont64492]
- Rivard C, Awad M, Liebermann M, Dejong M, Massey SM, Sinacore J, et al. Bladder drainage during labor: a randomized controlled trial. The Journal of Obstetrics and Gynaecology Research 2012;38(8):1046‐51. [NCT00959920; sr‐incont49710] [DOI] [PubMed] [Google Scholar]
Schiotz 1989 {published data only}
- Schiotz HA, Malme PA, Tanbo TG. Urinary tract infections and asymptomatic bacteriuria after vaginal plastic surgery. A comparison of suprapubic and transurethral catheters. Acta Obstetricia Gynecologica Scandinavica 1989;68(5):453‐5. [sr‐incont1165] [DOI] [PubMed] [Google Scholar]
Sethia 1987 {published data only}
- Sethia KK, Selkon JB, Berry AR, Turner CM, Kettlewell MG, Gough MH. Prospective randomized controlled trial of urethral versus suprapubic catheterization. British Journal of Surgery 1987;74(7):624‐5. [sr‐incont1114] [DOI] [PubMed] [Google Scholar]
Skelly 1992 {published data only}
- Skelly JM, Guyatt GH, Kalbfleisch R, Singer J, Winter L. Management of urinary retention after surgical repair of hip fracture. Canadian Medical Association Journal 1992;146(7):1185‐9. [PMC free article] [PubMed] [Google Scholar]
Stekkinger 2011 {published data only}
- NTR1528. A comparison of suprapubic and transurethral catheterization on postoperative urinary retention after vaginal prolapse repair ‐ CAD TRIAL. www.trialregister.nl/trialreg/admin/rctview.asp?TC=1528 (accessed 12th November 2015). [NTR1528; sr‐incont65499] [DOI] [PubMed]
- Stekkinger E, Linden PJQ. A comparison of suprapubic and transurethral catheterization after vaginal prolapse repair: a randomized controlled trial. Gynecologic and Obstetric Investigation 2011;72(2):109‐16. [sr‐incont42069] [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling catheterization in older female patients. Maturitas 2006;53(3):274‐81. [sr‐incont22495] [DOI] [PubMed] [Google Scholar]
Tangtrakul 1994 {published data only}
- Tangtrakul S, Taechaiya S, Suthutvoravut S, Linasmita V. Post‐cesarean section urinary tract infection: a comparison between intermittent and indwelling catheterization. Journal of the Medical Association of Thailand 1994;77(5):244‐8. [PubMed] [Google Scholar]
Van den Brand 2001 {published data only}
- Brand I, Castelein RM. Total joint arthroplasty and incidence of postoperative bacteriuria with an indwelling catheter or intermittent catheterization with one‐dose antibiotic prophylaxis: a prospective randomized trial. Journal of Arthroplasty 2001;16(7):850‐5. [sr‐incont13007] [DOI] [PubMed] [Google Scholar]
Vandoni 1994 {published data only}
- Vandoni RE, Lironi A, Tschantz P. Bacteriuria during urinary tract catheterization: suprapubic versus urethral route: a prospective randomized trial. Acta Chirurgica Belgica 1994;94(1):12‐6. [sr‐incont30] [PubMed] [Google Scholar]
Wiser 1974 {published data only}
- Wiser WL, Morrison JC, Loveday GL, McIntosh RE, Kennedy BS, Shaw BH, et al. Management of bladder drainage following vaginal plastic repairs. Obstetrics and Gynecology 1974;44(1):65‐71. [sr‐incont874] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 1980 {published data only}
- Abrams PH, Shah PJ, Gaches CG, Ashken MH, Green NA. Role of suprapubic catheterization in retention of urine. Journal of the Royal Society of Medicine 1980;73(12):845‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allardice 1988 {published data only}
- Allardice JT, Standfield NJ, Wyatt AP. Acute urinary retention: which catheter?. Annals of the Royal College of Surgeons of England 1988;70(6):366‐8. [PMC free article] [PubMed] [Google Scholar]
Cardenas 2010 {published data only}
- Cardenas D, Moore KN, Dannels‐McClure A, Scelza W, Granves D, Brooks M. Intermittent catheterisation with hydrophilic‐coated catheters delays the onset of urinary tract infection in patients with acute spinal cord injury: an international, multicenter, randomised controlled trial (Abstract number 126). Neurourology and Urodynamics 2010;29(6):990‐1. [sr‐incont40143] [Google Scholar]
Chartier‐Kastler 2011 {published data only}
- Chartier‐Kastler E, Lauge I, Ruffion A, Goossens D, Charvier K, Biering‐Sorensen F. Safety of a new compact catheter for men with neurogenic bladder dysfunction: a randomised, crossover and open‐labelled study. Spinal Cord 2011;49(7):844‐50. [NCT00990093; sr‐incont42729] [DOI] [PubMed] [Google Scholar]
Dunn 2003 {published data only}
- Dunn TS, Shlay J, Forshner D. Are in‐dwelling catheters necessary for 24 hours after hysterectomy?. American Journal of Obstetrics and Gynecology 2003;189(2):435‐7. [DOI] [PubMed] [Google Scholar]
Frymire 1971 {published data only}
- Frymire LJ. Comparison of suprapubic versus Foley drains. Obstetrics and Gynecology 1971;38(2):239‐44. [PubMed] [Google Scholar]
Ghalayini 2005 {published data only}
- Ghalayini IF, Al Ghazo MA, Pickard RS. A prospective randomized trial comparing tranurethral prostatic resection and clean intermittent self‐catheterization in men with chronic urinary retention. BJU International 2005;96(1):93‐7. [sr‐incont21452] [DOI] [PubMed] [Google Scholar]
Grundy 1983 {published data only}
- Grundy DJ, Fellows GJ, Gillett AP, Nuseibeh I, Silver JR. A comparison of fine‐bore suprapubic and an intermittent urethral catheterisation regime after spinal cord injury. Paraplegia 1983;21(4):227‐32. [sr‐incont7525] [DOI] [PubMed] [Google Scholar]
Hofmeister 1970 {published data only}
- Hofmeister FJ, Martens WE, Strebel RL. Foley catheter or suprapubic tube?. American Journal of Obstetrics and Gynecology 1970;107(5):767‐79. [DOI] [PubMed] [Google Scholar]
Horgan 1992 {published data only}
- Horgan AF, Prasad B, Waldron DJ, O'Sullivan DC. Acute urinary retention. Comparison of suprapubic and urethral catheterisation. British Journal of Urology 1992;70(2):149‐51. [sr‐incont18523] [DOI] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park HC, Son JH, Jang SH. Rethinking suprapubic cystostomy in voiding dysfunction: new trial with timed drainage. Korean Journal of Urology 2010;51(12):847‐52. [sr‐incont41042] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumm 2008 {published data only}
- Schumm K, N'Dow J, Norrie J, and the CATHETER Study Group. Recruitment challenges in a large randomsed controlled trial with complex processes (Abstract number P06). Proceedings of the 29th Society for Clinical Trials Annual Meetibng, 18‐21 May 2008, St. Louis (MO). 2008. [sr‐incont27304]
Shapiro 1982 {published data only}
- Shapiro J, Hoffmann J, Jersky J. A comparison of suprapubic and transurethral drainage for postoperative urinary retention in general surgical patients. Acta Chirurgica Scandinavica 1982;148(4):323‐7. [sr‐incont46935] [PubMed] [Google Scholar]
Sicilia 2013 {published data only}
- Sicilia I, Mattioni A, Cenciarelli S, Mazzoli T, Gallinella E, Greco LM, et al. Intermittent versus permanent bladder catheterization in acute stroke patients (Abstract number 600). Cerebrovascular Diseases 2013;35(Suppl 3):663. [sr‐incont59886] [Google Scholar]
Suprasert 2002 {published data only}
- Suprasert P, Srisomboon J, Phongnarisorn C. A prospective randomized study comparing voiding time between suprapubic catheterization and intermittent self‐catheterization following radical hysterectomy and pelvic lymphadenectomy for cervical cancer. Thai Journal of Obstetrics & Gynecology 2002;14(1):73‐9. [sr‐incont26731] [Google Scholar]
Turi 2006 {published data only}
- Turi MH, Hanif S, Fasih Q, Shaikh MA. Proportion of complications in patients practicing clean intermittent self‐catheterization (CISC) vs indwelling catheter. Journal of the Pakistan Medical Association 2006;56(9):401‐9. [sr‐incont22267] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kringel 2007 {published data only}
- Kringel U, Gerber B. Comparision of the postoperative rate of complications and infections in indwelling urinary catheters and suprapubic catheters after anterior kolporrhaphia: a 3‐arm prospective randomised trial. Interim analysis after 30 patients (Abstract: Poster presentation). Proceedings of the North German Society of Obstetrics and Gynaecology (NGGG) meeting, 2007 Sept 7‐9, Göttingen, Germany. 2007. [ISRCTN46098701; sr‐incont64686]
References to ongoing studies
ACTRN12614000618651 {published data only}
- ACTRN12614000618651. Randomized control trial of continuous indwelling urinary catheter versus intermittent urinary catheter in pregnant women during epidural anesthesia in labour to prevent severe post partum urinary retention. www.anzctr.org.au/ACTRN12614000618651.aspx (accessed 12th November 2015). [ACTRN12614000618651; sr‐incont62933]
NCT01465594 2011 {published data only}
- NCT01465594. Randomized study comparing urinary diversion by suprapubic catheter with transurethral catheter in patients after radical prostatectomy. clinicaltrials.gov/show/NCT01465594 (accessed 12th November 2015). [NCT01465594; sr‐incont50317]
NCT02108431 2013 {published data only}
- NCT02108431, Habibzada J, Godes M, Witt JH. Superior postoperative patient comfort in suprapubic drainage versus transurethral catheterization following robot‐assisted radical prostatectomy: a prospective randomized clinical trial. clinicaltrials.gov/show/NCT02108431 (accessed 12th November 2015). [NCT02108431; sr‐incont60954] [DOI] [PubMed]
NCT02198157 2014 {published data only}
- NCT02198157. Effect of intermittent versus continuous bladder catheterization during labor on second stage duration. clinicaltrials.gov/show/NCT02198157 (accessed 12th November 2015). [NCT02198157; sr‐incont62945]
NTR2806 {published data only}
- NTR2806. Catheter Management and complications for Symptomatic Postpartum Urinary Retention ‐ CAMPUR. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2806 (accessed 12th November 2015). [NTR2806; TrialID.CAMPUR.; sr‐incont66314]
Wilson 2013 {published data only}
- Wilson B, Phelps C. Identifying and applying a targeted evidence‐based practice change in the maternal/child health inpatient setting. Nursing for Women's Health 2013;17(6):490‐7. [sr‐incont65833] [DOI] [PubMed] [Google Scholar]
Additional references
Ahluwalia 2006
- Ahluwalia RS, Johal N, Kouriefs C, Kooiman G, Montgomery BSI, Plail RO. The surgical risk of suprapubic catheter insertion and long‐term sequelae. Annals of the Royal College of Surgeons of England 2006;88(2):210‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2015
- National Healthcare Safety Network (NHSN), Centers for Disease Control and Prevention. Urinary tract infection (Catheter‐Associated Urinary Tract Infection [CAUTI] and Non‐Catheter‐Associated Urinary Tract Infection [UTI]) and other urinary system infection [USI]) events, Chapter 7 ‐ 13 device‐associated module. CDC NHSN Patient Safety Component (PSC) Manual. Atlanta, GA. (available at: cdc.gov/nhsn/pdfs/pscManual/7pscCAUTIcurrent.pdf) (accessed 4 February 2015): Centers for Disease Control and Prevention, 5 January 2015. [Google Scholar]
COMET
- Core Outcome Measures in Effectiveness Trials (COMET) Initiative. COMET Initiative. www.comet‐initiative.org/ (accessed 27 May 2015).
EAU 2014
- European Assocation of Urology. EAU Guidelines. 25th Edition. Arnhem, The Netherlands: EAU Guidelines Office, 2014. [Google Scholar]
Elvy 2009
- Elvy J, Colville A. Catheter associated urinary tract infection: what is it, what causes it and how can we prevent it?. Journal of Infection Prevention 2009;10(2):36‐41. [Google Scholar]
Geng 2006
- Geng V, Emblem EL, Gratzl S, Incesu O, Jensen K. EAUN Good Practice in Healthcare: Urethral Catheterisation. Section 2: Male, female and paediatric intermittent catheterization. Arnhem, The Netherlands: European Assocation of Urology, 2006. [Google Scholar]
Geng 2012
- Geng V, Cobussen‐Boekhorst H, Farrell J, Gea‐Sanchez M, Pearce I, Schwennesen T, et al. Catheterisation ‐ Indwelling catheters in adults: urethral and suprapubic. 1st Edition. Arnhem, The Netherlands: European Association of Urology Nurses, 2012. [Google Scholar]
Gould 2009
- Gould CV, Umscheid CA, Agarwal RK, Kunst G, Pegues DA, and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Prevention of Catheter‐Associated Urinary Tract Infections 2009. cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Atlanta, GA: Healthcare Infection Control Practices Advisory Committee (HICPAC), Centers for Disease Control and Prevention (CDC), (accessed 4th February 2015).
Grabe 2015
- Grabe M, Bartoletti R, Bjerklund Johansen TE, Cai T, Cek M, Koves B, et al. Urological infections. In: European Assocation of Urology, editor(s). European Association of Urology Guidelines. 2015 Edition. Arnhem, The Netherlands: EAU Guidelines Office, 2015:7‐13. [Google Scholar]
Harrison 2011
- Harrison SCW, Lawrence WT, Morley R, Pearce I, Taylor J. British Association of Urological Surgeons' suprapubic catheter practice guidelines. BJU International 2011;107(1):77‐85. [DOI] [PubMed] [Google Scholar]
Health Committee 2013
- Health Committee, House of Commons. Written evidence from Bard Ltd (PEX 17) (2012 Oct); Eleventh report of session 2012‐13 ‐ Public expenditure on health and care services – Volume II ‐ Additional written evidence (HC 2012‐13, 651). www.publications.parliament.uk/pa/cm201213/cmselect/cmhealth/651/651vw.pdf. London: The Stationery Office Limited, (accessed 28 May 2015).
Healy 2012
- Healy EF, Walsh CA, Cotter AM, Walsh SR. Suprapubic compared with transurethral bladder catheterization for gynecologic surgery: a systematic review and meta‐analysis. Obstetrics and Gynecology 2012;120(3):678‐87. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from cochrane‐handbook.org. The Cochrane Collaboration.
Hooton 2010
- Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention and treatment of catheter‐associated urinary tract infections in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical Infectious Diseases 2010;50(5):625‐63. [DOI] [PubMed] [Google Scholar]
Lam 2014
- Lam TBL, Omar MI, Fisher E, Gillies K, MacLennan S. Types of indwelling urethral catheters for short‐term catheterisation in hospitalised adults. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD004013.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lusardi 2013
- Lusardi G, Lipp A, Shaw C. Antibiotic prophylaxis for short‐term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD005428.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McPhail 2006
- McPhail M J, Abu‐Hilal M, Johnson CD. A meta‐analysis comparing suprapubic and transurethral catheterization for bladder drainage after abdominal surgery. British Journal of Surgery 2006;93(9):1038‐44. [DOI] [PubMed] [Google Scholar]
Nasr 2010
- Nasr A. State of the globe: catheterizations continue to cultivate urinary infections. Journal of Global Infectious Diseases 2010;2(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 2010
- Newman DK. Prevention and management of catheter‐associated UTIs. Infectious Disease 2010;Special Edition:13‐20. [Google Scholar]
Nicolle 2005
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clinical Infectious Diseases 2005;40(5):643‐54. [DOI] [PubMed] [Google Scholar]
Omar 2013
- Omar MI, Lam TBL, MacLennan S. Critical outcomes in a Cochrane systematic review: patients' perspective. 21st Cochrane Colloquium 2013;Suppl:2. [Google Scholar]
Phipps 2006
- Phipps S, Lim YN, McClinton S, Barry C, Rane A, N'Dow JMO. Short term urinary catheter policies following urogenital surgery in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004374.pub2] [DOI] [PubMed] [Google Scholar]
Reference Manager 2012 [Computer program]
- Thomson Reuters. Reference Manager Professional Edition. Version Version 12. New York: Thomson Reuters, 2012.
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smyth 2008
- Smyth E, McIlveny G, Enstone J, Emmerson A, Humphreys H, Fitzpatrick F, et al. Hospital Infection Society Prevalence Survey Steering Group. Four country healthcare associated infection prevalence survey 2006: overview of the results. Journal of Hospital Infection 2008;69(3):230‐48. [DOI] [PubMed] [Google Scholar]
Tambyah 1999
- Tambyah PA, Halvorson KT, Maki DG. A prospective study of pathogenesis of catheter‐associated urinary tract infections. Mayo Clinic Proceedings 1999;74(2):131‐6. [15410] [DOI] [PubMed] [Google Scholar]
Tenke 2008
- Tenke P, Kovacs B, Bjerklund Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management andprevention of catheter‐associated urinary tract infections. International Journal of Antimicrobial Agents 2008;31(1):68‐78. [DOI] [PubMed] [Google Scholar]
Trautner 2004
- Trautner BW, Darouiche RO. Catheter associated infections: pathogenesis affects prevention. Archives of Internal Medicine 2004;164(8):842‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Warren 2001
- Warren JW. Catheter‐associated urinary tract infections. International Journal of Antimicrobial Agents 2001;17(4):299‐303. [15414] [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]


