Abstract
Background
Infants born preterm are at increased risk of developing cognitive and motor impairment compared with infants born at term. Early developmental interventions have been provided in the clinical setting with the aim of improving overall functional outcomes for these infants. Long‐term benefits of these programmes remain unclear.
Objectives
Primary objective To compare the effectiveness of early developmental intervention programmes provided post hospital discharge to prevent motor or cognitive impairment in preterm (< 37 weeks) infants versus standard medical follow‐up of preterm infants at infancy (zero to < three years), preschool age (three to < five years), school age (five to < 18 years) and adulthood (≥ 18 years).
Secondary objectives
To perform subgroup analyses to determine the following.
• Effects of gestational age, birth weight and brain injury (periventricular leukomalacia (PVL)/intraventricular haemorrhage (IVH)) on cognitive and motor outcomes when early intervention is compared with standard follow‐up.
∘ Gestational age: < 28 weeks, 28 to < 32 weeks, 32 to < 37 weeks.
∘ Birth weight: < 1000 grams, 1000 to < 1500 grams, 1500 to < 2500 grams.
∘ Brain injury: absence or presence of grade III or grade IV IVH or cystic PVL (or both) or an abnormal ultrasound/magnetic resonance image (MRI) before initiation of the intervention.
• Effects of interventions started during inpatient stay with a post‐discharge component versus standard follow‐up care.
• Effects of interventions focused on the parent‐infant relationship, infant development or both compared with standard follow‐up care.
To perform sensitivity analysis to identify the following.
• Effects on motor and cognitive impairment when early developmental interventions are provided within high‐quality randomised trials with low risk of bias for sequence generation, allocation concealment, blinding of outcome measures and selective reporting bias.
Search methods
The search strategy of the Cochrane Neonatal Review Group was used to identify randomised and quasi‐randomised controlled trials of early developmental interventions provided post hospital discharge. Two review authors independently searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Advanced, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO and EMBASE (1966 to August 2015).
Selection criteria
Studies included had to be randomised or quasi‐randomised controlled trials of early developmental intervention programmes that began within the first 12 months of life for infants born before 37 weeks' gestational age. Interventions could commence on an inpatient basis but had to include a post‐discharge component for inclusion in this review. Outcome measures were not prespecified, other than that they had to assess cognitive outcomes, motor outcomes or both. Rates of cerebral palsy were documented.
Data collection and analysis
Two independent review authors extracted and entered data. Cognitive and motor outcomes were pooled by four age groups: infancy (zero to < three years), preschool age (three to < five years), school age (five to < 18 years) and adulthood (≥ 18 years). Meta‐analysis using RevMan 5.1 was carried out to determine the effects of early developmental interventions at each age range. Subgroup analyses focused on gestational age, birth weight, brain injury, commencement of the intervention, focus of the intervention and study quality.
Main results
Twenty‐five studies met the inclusion criteria (3615 randomly assigned participants). Only 12 of these studies were randomised controlled trials with appropriate allocation concealment. Variability was evident with regard to focus and intensity of the intervention, participant characteristics and length of follow‐up. Meta‐analysis led to the conclusion that intervention improved cognitive outcomes at infancy (developmental quotient (DQ): standardised mean difference (SMD) 0.32 standard deviations (SDs), 95% confidence interval (CI) 0.16 to 0.47; P value < 0.001; 16 studies; 2372 participants) and at preschool age (intelligence quotient (IQ); SMD 0.43 SDs, 95% CI 0.32 to 0.54; P value < 0.001; eight studies; 1436 participants). However, this effect was not sustained at school age (IQ: SMD 0.18 SDs, 95% CI ‐0.08 to 0.43; P value = 0.17; five studies; 1372 participants). Heterogeneity between studies for cognitive outcomes at infancy and at school age was significant. With regards to motor outcomes, meta‐analysis of 12 studies showed a significant effect in favour of early developmental interventions at infancy only; however, this effect was small (motor scale DQ: SMD 0.10 SDs, 95% CI 0.01 to 0.19; P value = 0.03; 12 studies; 1895 participants). No effect was noted on the rate of cerebral palsy among survivors (risk ratio (RR) 0.82, 95% CI 0.52 to 1.27; seven studies; 985 participants). Little evidence showed a positive effect on motor outcomes in the long term, but only five included studies reported outcomes at preschool age (n = 3) or at school age (n = 2).
Authors' conclusions
Early intervention programmes for preterm infants have a positive influence on cognitive and motor outcomes during infancy, with cognitive benefits persisting into preschool age. A great deal of heterogeneity between studies was due to the variety of early developmental intervention programmes tested and to gestational ages of included preterm infants; thus, comparisons of intervention programmes were limited. Further research is needed to determine which early developmental interventions are most effective in improving cognitive and motor outcomes, and to discern the longer‐term effects of these programmes.
Plain language summary
Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants
Review question: In preterm infants, do early developmental intervention programmes provided post hospital discharge compared with standard medical follow‐up better improve cognitive and motor development at infancy (zero to < three years), preschool age (three to < five years), school age (five to < 18 years) and adulthood (≥ 18 years)?
Background: Preterm infants (babies born before 37 weeks) are at risk for developmental problems, including cognitive and motor delays. Cognitive development refers to thinking and learning abilities, and motor development refers to the ways children move, such as by sitting, crawling and walking. Early developmental interventions aim to reduce cognitive and motor problems; however, the benefits of these programmes are not clear.
Study characteristics: Twenty‐five studies met the inclusion criteria (3615 randomly assigned participants). Only 12 of these studies were randomised controlled trials with appropriate allocation concealment. Variability was noted with regard to focus and intensity of the intervention, participant characteristics and length of follow‐up.
Key findings: Evidence suggests that early developmental interventions improve cognitive outcomes up to preschool age. Evidence also indicates that early developmental interventions improve motor outcomes during infancy; however, these effects are small. Little evidence was found of an effect on long‐term cognitive or motor outcomes (up to school age). The early developmental intervention programmes described in this review had to begin within the first 12 months of life, had to focus on the parent‐infant relationship and/or infant development and, although they could begin while the baby was still in hospital, had to include a component that was delivered post discharge from hospital. The early developmental intervention programmes included in this review vary by content and by frequency and focus of the intervention.
Conclusions: This review of 25 trials supports early developmental intervention programmes provided to preterm infants post hospital discharge with the goal of improving cognitive development over the short to medium term (up to preschool age). Variability among these early developmental intervention programmes limits the conclusions that can be drawn about their effectiveness.
Background
Description of the condition
Infants born preterm or at low birth weight (LBW) are at increased risk of developing motor, cognitive and behavioural impairment compared with infants born at term (Pedersen 2000; Bhutta 2002; Doyle 2004; Spittle 2013). Despite improving rates of survival for extremely low birth weight (ELBW) infants since the 1990s, the rate of disability has remained relatively constant, with up to 50% of these infants later exhibiting developmental disabilities such as motor, cognitive or behavioural impairment (Bhutta 2002; Doyle 2004). Five to fifteen per cent of children will have cerebral palsy (CP) (Tin 1997; Vohr 2005; Spittle 2007).
These neurosensory impairments are complex and are often subtle, and may affect various aspects of the child's development. At school age, children born preterm experience problems across most educational domains. They tend to have difficulty learning, particularly in applying mathematical concepts (Anderson 2003). Attentional problems and hyperactivity are commonly reported in children born prematurely (Horwood 1998). These can substantially affect academic achievement and social integration (Hoy 1992; Sommerfelt 1996; Botting 1998; Spittle 2009b). Minor motor impairments, which are similar to those seen in children with developmental co‐ordination disorder (Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM‐IV)), have been found to be more prevalent in very preterm infants (Williams 2010). These motor problems persist into adolescence and can affect school performance and self esteem (Powls 1995). In adulthood, very low birth weight (VLBW) infants continue to exhibit higher rates of neurosensory impairment, with lower academic scores and a lower high school graduation rate compared with adults born at normal birth weight (Hack 2002).
Learning, behaviour and motor impairment in preterm children can be associated with medical risk factors (e.g. birth weight, gestational age, periventricular leukomalacia (PVL), intraventricular haemorrhage (IVH), respiratory distress syndrome (RDS), necrotising enterocolitis (NEC)); however, such problems account for only a portion of the variance associated with these long‐term outcomes (Vohr 2000). Non‐medical factors such as social class, parental education, parenting style, parental mental health, family structure, family functioning and the home environment are also associated with developmental outcomes of children born preterm (Laucht 1997; Hogan 2000; Treyvaud 2010).
Description of the intervention
Early developmental interventions have been used in the clinical setting with the aim of improving overall functional outcomes for these infants. As a result of the complex biological, medical and environmental elements that contribute to development, early intervention may encompass many different components, and services may be provided through a variety of disciplines (Berger 1998). Early intervention for preterm infants may focus on different aspects of early development, depending on targeted outcomes.
Developmental care, an intervention that focuses on the environment and the infant, is designed to minimise stress for the infant in the neonatal intensive care unit (NICU) (Als 1997; Symington 2003). Several systematic reviews have described variable short‐term benefits of developmental care such as reduced oxygen dependency and improved neurodevelopmental outcomes up to 12 months; however, benefits were not sustained at two years (Jacobs 2002; Symington 2003).
How the intervention might work
Early intervention programmes that focus on development post hospital discharge and into the community setting may have a greater impact on long‐term morbidity, as they focus more on family factors and the home environment. Interventions aimed at enhancing the parent‐infant relationship focus on sensitising parents to infant cues and on teaching appropriate and timely responses to infant needs. Evidence suggests that early high‐quality parent‐infant interactions positively influence cognitive and social development in children (Melnyk 2001). Recent evidence also shows that effects of early intervention on cognitive outcomes for preterm children do not appear to be specific to the type of therapy received, and receipt of any early intervention for preterm infants is associated with improved cognitive function at between one and two years (McManus 2012). Several types of interventions such as physiotherapy and infant stimulation programmes focus on infant development. Physiotherapy trials aim to optimise motor development but vary in the theoretical rationale underlying the intervention programme. Some physiotherapy interventions are based on principles of neuro‐developmental therapy (NDT), which aims to modify sensory input and/or abnormal movement patterns with the goal of improving motor outcomes through active and/or passive techniques (Brown 2001; Blauw‐Hospers 2005). Systematic reviews of the effects of NDT in children with neurological dysfunction have been inconclusive. A review by Brown 2001 showed that NDT was beneficial in six out of 15 studies. A review by Ottenbacher 1986 showed a small treatment effect on motor outcomes compared with the comparison group. Infant stimulation programmes may involve multi‐sensory stimulation such as auditory, visual, vestibular and tactile stimulation. Environmental and social factors are well recognised as influencing the development of children, especially those at increased biological risk (Shonkoff 2003). Increasing evidence supports implementation of environmental enrichment programmes in which the intervention aims to improve at least one aspect of cognitive or motor outcomes by providing an optimal environment for learning. Early intervention programmes that include enhancement of parent‐infant interactions; adaptation of the environment to promote motor, social or cognitive skills; and parent education about supporting skill development comprise strategies that provide an infant with an enriched environment. This approach has been shown to be of benefit for infants with CP, but less is known about these interventions for children born preterm (Morgan 2013).
Why it is important to do this review
For the purposes of this review, an early developmental intervention is considered to be a programme beginning within the first year of life, with or without an inpatient hospital component, for which the aim is to enhance infant development. Interventions have been grouped to focus on the parent‐infant relationship, development of the infant or both. Although some interventions may specifically target motor or cognitive development, a strong relationship between these areas has been noted. For example, by influencing motor function, such interventions may improve cognitive outcomes, as they allow infants greater opportunity to interact with their environment (Thelen 1996; Becker 1999). Different models of intervention programmes may have different goals such as prevention, remediation or treatment of a specific delay or disability (Majnemer 1998). When an intervention is begun at an early age for infants at high risk of neurodevelopmental problems, the intervention has a preventative focus, with strategies aimed at minimising the effects of prematurity and promoting optimal development. However, during the course of an intervention, if a specific dysfunction becomes apparent or a diagnosis is made, strategies are then focused on preventing further delay and compensating for deficits to promote best function and independence for the child. It is important for the care provider to understand the effectiveness of these intervention programmes in the high‐risk preterm infant population.
Objectives
Primary objective
To compare the effectiveness of early developmental intervention programmes provided post hospital discharge to prevent motor or cognitive impairment in preterm (< 37 weeks) infants versus standard medical follow‐up of preterm infants at infancy (zero to < three years), preschool age (three to < five years), school age (five to < 18 years) and adulthood (≥ 18 years).
Secondary objectives
To perform subgroup analyses to determine the following:
-
Effects of gestational age, birth weight and brain injury (periventricular leukomalacia (PVL)/intraventricular haemorrhage (IVH)) on cognitive and motor outcomes when early intervention is compared with standard follow‐up care.
Gestational age: < 28 weeks, 28 to < 32 weeks, 32 to < 37 weeks.
Birth weight: < 1000 grams, 1000 to < 1500 grams, 1500 to < 2500 grams.
Brain injury: absence or presence of grade III or IV IVH or cystic PVL (or both) or an abnormal ultrasound/magnetic resonance image (MRI) before initiation of the intervention.
Effects of interventions started during inpatient stay with a post‐discharge component versus standard follow‐up care.
Effects of interventions focused on the parent‐infant relationship, infant development or both compared with standard follow‐up care.
To perform sensitivity analysis to identify the following:
Effects on motor and cognitive impairment when early developmental interventions are provided within high‐quality randomised trials with low risk of bias for sequence generation, allocation concealment, blinding of outcome measures and selective reporting bias.
Methods
Criteria for considering studies for this review
Types of studies
We included all trials using random or quasi‐random allocation that met the inclusion criteria for types of participants, interventions and outcomes.
Types of participants
Preterm infants born at less than 37 weeks' gestational age (according to best obstetrical estimate at the time of delivery). We excluded studies that did not report outcomes for preterm infants separately from those for infants born at term.
Types of interventions
We included early developmental intervention programmes that aimed to improve cognitive or motor outcomes. Enrolment in early intervention programmes could occur while the infant was an inpatient during the primary hospitalisation or post hospital discharge. Intervention had to begin within the first 12 months of post‐term age and could be provided at home, in hospital or at the community centre. The intervention must have been carried out by a health professional such as a physiotherapist, a doctor, a psychologist, an occupational therapist or a nurse. Types of interventions could include physiotherapy, occupational therapy, psychological therapy, neurodevelopmental therapy, parent‐infant relationship enhancement, infant stimulation, infant development, developmental care and early intervention (education). Interventions could focus on the parent‐infant relationship, development of the infant or both.
Types of outcome measures
Primary outcomes
Following are some of the outcome measures that may have been used to assess cognitive and motor development. We included only standardised objective measures of cognitive and motor outcomes.
Cognitive outcomes
Continuous
Infant age (zero to < three years): Bayley Scales of Infant Development ‐ Mental Development Index Edition I (BSID‐MDI‐I; Bayley 1969), Bayley Scales of Infant Development ‐ Mental Development Index Edition II (BSID‐MDI‐II; Bayley 1993), Bayley Scales of Infant and Toddler Development ‐ Edition III Cognitive Scale (BSITD‐III) (Bayley 2005) and the Griffiths Mental Development Scale ‐ General Cognitive Index (GCI) (Griffiths 1954; Griffiths 1970)
Preschool age (three to < five years): Stanford‐Binet Intelligence Scale (3rd Edition, 1972) (Terman 1973), McCarthy Scales of Children's Abilities (McCarthy 1972), Wechsler Preschool and Primary Scale of Intelligence ‐ Revised (WPPSI‐R) (Wechsler 1989) and Differential Abilities Scale Edition II (DAS‐II; Elliot 2007)
School age (five to 17 years): WPPSI, Wechsler Intelligence Scale for Children ‐ Full Scale IQ (WISC‐III) (Wechsler 1991), Kaufman Assessment Battery for Children ‐ Mental Processing Composite (Kaufman 1983), Griffiths Mental Development Scale (Griffiths 1970) and British Abilities Scale (BAS) (Elliot 1996)
Adulthood (≥ 18 years): Wechsler Abbreviated Scale of Intelligence (WASI)
Motor outcomes
Continuous
Infant age (zero to < three years): Bayley Scales of Infant Development ‐ Psychomotor Development Index Edition I (BSID‐PDI‐I; Bayley 1969), Bayley Scales of Infant Development ‐ Psychomotor Development Index Edition II (BSID‐PDI‐II; Bayley 1993), Bayley Scales of Infant and Toddler Development ‐ Total Motor Quotient Edition III (BSITD‐III; Bayley 1993) and the Griffiths Locomotor Subscale (Griffiths 1954; Griffiths 1970), Test of Infant Motor Performance (TIMP) (Campbell 1995), Alberta Infant Motor Scale (AIMS) (Piper 1994) and Peabody Developmental Motor Scales Editions I and II (Folio 2000)
Preschool and school age: Movement Assessment Battery for Children (MABC) Editions 1 and 2 (Henderson 1992; Henderson 2007), Bruininks‐Oseretsky Test of Motor Proficiency (BOTMP) (Bruininks 1978), Griffiths Locomotor Subscale (Griffiths 1970) and McCarthy Scales of Children's Abilities (McCarthy 1972) Motor Scales
Adulthood (≥ 18 years): Bruininks‐Oseretsky Test of Motor Proficiency (Bruininks 1978)
Secondary outcomes
Rates of Cerebral Palsy (CP)
Rates of non‐CP motor impairment: MABC scores < 5th centile
Search methods for identification of studies
Electronic searches
We used the search strategy for the Cochrane Neonatal Review Group (CNRG). See Cochrane Neonatal Group, search strategy for Specialised Register, in The Cochrane Library. Review authors undertook a comprehensive search of databases such as the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 2), MEDLINE Advanced (1966 to August 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to August 2015), PsycINFO (1966 to August 2015) and EMBASE (1988 to August 2015). This is the third update of this review.
The following search strategy was used.
Infant‐premature OR infant‐low birth weight.
AND early intervention (education) OR developmental care OR physical therapy OR occupational therapy OR psychology OR parent‐infant relationship OR rehabilitation OR exercise OR neurodevelopmental therapy OR infant stimulation.
AND child development OR infant development OR cognition OR intellectual disability OR developmental disabilities OR psychomotor performance OR psychomotor disorders OR cerebral palsy OR developmental co‐ordination disorder OR movement disorders OR motor skill disorders.
NOT drug therapy OR genetics OR chest physiotherapy OR cardiac.
We included studies that were reported in English or in a language for which a translator was available.
Searching other resources
Review authors cross‐referenced relevant literature including identified trials and existing review articles.
Data collection and analysis
Selection of studies
We used the standard methods of the CNRG; however, we also included studies in which allocation concealment was not used. Two of the review authors who work in the fields of early intervention (AS, JO) independently assessed the eligibility of studies for inclusion. We reviewed studies yielded by the initial search on the basis of title and abstract, and we excluded studies that did not meet the inclusion criteria. Review authors then evaluated the full text of remaining articles that appeared to meet the inclusion criteria.
Data extraction and management
Two review authors independently extracted and entered study data.
Assessment of risk of bias in included studies
We evaluated the methodological quality of included trials by using the CNRG methodological scheme, whereby each article was assessed for selection (blinding of randomisation), performance (blinding of intervention), attrition (completeness of follow‐up) and detection (blinding of outcome measures). We classified allocation concealment as adequate (A), unclear (B), inadequate (C) or not used (D) as another criterion for assessment of validity. We requested additional information from the authors of trials to clarify methods used and to obtain missing data (to perform analyses on an intention‐to‐treat basis), when necessary. Two review authors (AS, JO) independently rated methodological quality.
For the current update, we assessed risk of bias for each study by using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed risk of bias of included studies by using the following criteria.
-
Sequence generation (checking for possible selection bias): For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
-
Allocation concealment (checking for possible selection bias): For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
-
Blinding (checking for possible performance bias): For each included study, we categorised methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We categorised methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel; and
low risk, high risk or unclear risk for outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations): For each included study and for each outcome, we described data completeness including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomly assigned participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
-
Selective reporting bias: For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed methods as:
low risk (when it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk (when not all of the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
-
Other sources of bias: For each included study, we described important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early because of some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
Measures of treatment effect
We used Review Manager 5.1 (RevMan 2011) software to conduct data management and analysis. We used the standard methods of CNRG to synthesise the data. For data analysis, 'intervention group' refers to infants who were involved in early developmental intervention programmes, and 'follow‐up group' refers to infants who had received standard medical follow‐up. Standard follow‐up varied between studies, as different hospitals/institutions used different standard follow‐up procedures. For individual trials, when possible, we reported mean values for treatment and control groups (and 95% confidence intervals (CIs)) for continuous variables. For the meta‐analysis of continuous outcomes, we calculated standardised mean differences (SMDs), as a variety of outcome measures (with different standard deviations (SDs)) measured the same outcome. For example, cognitive outcomes at infancy can be measured by Bayley MDI (Edition I, II or III). For dichotomous outcomes, we reported risk ratio (RR) and risk difference (RD) (and 95% CIs) for treatment and follow‐up groups. We pooled cognitive and motor outcome data into four age groups ‐ infancy (zero to < three years), preschool age (three to < five years), school age (five to < 18 years) and adulthood (≥ 18 years). When studies reported data at more than one time point within an age group, we used data from the latest assessment. For example, if a study reported cognitive outcomes at 12 months and 24 months, we used only 24‐month data.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting forest plots and quantifying the impact of heterogeneity using the I2 statistic. We explored possible causes of statistical heterogeneity by using prespecified subgroup analysis (e.g. differences in study quality, participants, intervention regimens, outcome assessments).
Data synthesis
We calculated pooled treatment effects across trials by using a fixed‐effect model when more than one trial assessed treatment effects for the same outcome in similar populations and used similar outcome measures. However, when we observed substantial heterogeneity between studies, we used the I2 statistic and a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We explored possible reasons for heterogeneity by scrutinising included studies and, when appropriate, performing subgroup analyses.
Sensitivity analysis
We performed a sensitivity analysis based on the methodological quality of trials: randomised controlled trials with allocation concealment versus quasi‐randomised trials with unclear allocation concealment.
Results
Description of studies
Results of the search
The original review (Spittle 2007) identified 16 randomised or quasi‐randomised controlled trials of early developmental interventions provided post hospital discharge. Authors of the updated review excluded the study of Piper 1986 because it included infants born at > 37 weeks' gestation.
The second updated review identified an additional six studies, resulting in inclusion of a total of 21 studies in the second updated review. Of these six studies, five were new studies (Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009) that were identified through the search of databases; an additional study (Sajaniemi 2001) was identified by a review of the reference list of another systematic review on this topic (Vanderveen 2009).
The third and current update of this review identified three new trials (Kyno 2012; Wu 2014; Dusing 2015) by searching databases, and another trial by reviewing another systematic review on the topic (Teti 2009). In addition to new trials, longer‐term outcomes were available for the original studies of Koldewijn 2009 at five years, Spittle 2009 at four years and Kaaresen 2006 at five years and seven years.
Included studies
The 25 trials that met the inclusion criteria yielded a total of 58 publications, as most studies had published several papers related to cognitive and motor outcomes at different ages. Following is a description of each of these studies.
The 'Avon Premature Infant Program' (APIP) (APIP 1998) conducted a multi‐centre randomised controlled trial comparing two interventions versus standard follow‐up. All eligible infants (N = 309) born over a two and one‐half year period were randomly assigned to one of three groups at seven to 10 days after birth (Portage = 111, Parent Advisor = 99, standard follow‐up = 99). Consent to participate in the study was obtained post randomisation to evaluate the acceptability and impact of the intervention in population terms. This resulted in 284 families of infants consenting to participate in the study, among the 309 infants randomly assigned to the three groups (Portage = 97, Parent Advisor = 90, standard follow‐up = 97). Families of infants who did not consent to the intervention were invited to participate in outcome assessments, and outcome data at two years (but not at five years) and results were reported on an intention‐to‐treat basis. Both intervention groups were enrolled in a programme directed by research nurses at home upon discharge from hospital until two years. Visits were weekly for the first two months, were reduced to one or two per month for the next 12 months, then to one per month until two years of corrected age. The frequency of visits was tailored to suit the family. The Portage programme is a home visiting educational service for children with additional support needs and their families. It takes place in the child's own home and aims to equip parents with the skills and confidence that they need to help their child. Portage offers practical help and ideas to encourage a child's interests while making learning fun for the entire family. The primary focus of the Portage group was the developmental progress of the child, although parental support was provided as part of the delivery. A second intervention group was used to control for the parent support given through the Portage group. The parent support group received supportive counselling for parents but no advice on infant development. Details of care for the standard follow‐up group were not given. At two years, cognitive development was assessed using the Griffiths GCI, and at five years with the BAS. Motor outcome was assessed at five years with the Movement ABC. A cutoff equivalent to the upper quartile of term reference group scores was used to define motor impairment. Rates of CP were also reported at five years. All outcomes were measured by a blinded assessor. Data from the two intervention groups were combined and compared with those from the standard follow‐up group for all analyses, except for the subgroup analysis of 'Focus of intervention'.
Bao 1999 conducted a multi‐centre quasi‐randomised controlled trial of an intervention package that focused on infant development versus standard follow‐up. Parents of infants in the intervention group (n = 52) were taught to carry out the programme, as implemented by a doctor, from term equivalent age to two years of age. The programme aimed to enhance motor, cognitive and speech development, and to improve social behaviour. This programme involved checking development of the infant, then instructing parents on how to carry out a home programme until the next examination. The home programme included exercise and suggestions for toys, books and pictorials appropriate to the child's age. One visit occurred per month for the first year, and one every two months for the second year. Parent education classes were reported to occur "sometimes". Details of care for the standard follow‐up group (N = 51) were not given. Cognitive and motor outcomes were measured by a blinded assessor at 18 months and at 24 months using the BSID‐II MDI and PDI.
Barrera 1986 conducted a multi‐centre randomised controlled trial comparing two types of intervention programmes versus standard follow‐up. Eighty preterm infants were randomly assigned to one of three groups: parent‐infant intervention (N = 22), developmental intervention (N = 16) or standard follow‐up (N = 21). Twenty‐one infants did not complete the study for a variety of reasons (e.g. death of infant, family moved). The number of infants in each group was reported only for infants who completed the programme. The parent‐infant intervention aimed to improve the quality of interaction between parents and child by enhancing parents' observational skills and teaching them to be mutually responsive to their infant. The developmental programme aimed to improve infants' cognition, communication, gross and fine motor development, socio‐emotional skills and self help skills. Parents worked with therapists to plan and implement developmental activities. Both interventions were implemented by one of four therapists with training in speech pathology, occupational therapy or early childhood education. Sessions were provided weekly for three to four months, bi‐weekly for the next six months, then monthly for three months. The mean number of home visits was 23 (range 12 to 28). The standard follow‐up group received home visits for assessment purposes only. During these visits, the examiner answered parents' questions about their child's development and about reading material or community resources. Cognitive and motor outcomes were obtained at four, eight, 12 and 16 months through the BSID‐I MDI and PDI. At 4.5 to 5 years, the child's cognitive development was assessed with McCarthy Scales of Children's Abilities ‐ GCI. All outcome assessors were blinded to children's group allocation. Data from the two intervention groups were combined and were compared with data from the standard follow‐up group for all relevant analyses.
Cameron 2005 conducted a single‐centre randomised controlled trial to investigate the effects of a physiotherapy early intervention programme versus standard follow‐up. The intervention group (N = 34) received a physiotherapy programme that aimed to improve motor outcomes by promoting symmetry, muscle balance and movement using postural support and facilitation techniques. The intervention began while the infant was an inpatient, with daily (weekdays) sessions provided from birth to discharge. It was then provided on a needs‐oriented basis post discharge up to four months. This included advice on play activities to encourage the infant's development based on infant progress. The standard follow‐up group (N = 38) received no physiotherapy and no placebo interventions. Investigators assessed motor development at four months by using the AIMS, and assessors blinded to the child's group allocation reported rates of CP at 18 months.
Dusing 2015 performed a small pilot randomised trial to assess the feasibility of completing a trial of the Supporting Play Exploration and Early Development Intervention (SPEEDI). A group of 10 infants born at < 34 weeks' gestational age (GA) were recruited from a single‐centre NICU and were randomly assigned. Intervention (SPEEDI) and usual care groups received standard care in the NICU and in the community. The SPEEDI group received the intervention in two phases. Phase 1 commenced in the NICU from 35 weeks' GA to term or hospital discharge, and Phase 2 from the latter point to three months of age. Phase 1 provided infants with daily movement experiences designed to vary positioning and to support interaction and early development. These were supported by a physiotherapist at a frequency of five 20‐minute sessions per week. Parents received 10 study visits and two meetings with the therapist during phase 1 to develop goals for the intervention and to demonstrate activities, and parents received a booklet that provided details of the interventions. Phase 2 aimed to assist parents in developing a routine for developmentally appropriate play, and to teach parents about infants' cues and readiness for interaction. Parents were encouraged to complete the intervention for 20 minutes a day, five times a week. The therapist met with each parent and infant every two weeks until three months post discharge. The frequency of therapist intervention and of parent intervention was monitored in a log book for phase 1 and phase 2. Information about other community services received was gathered through a questionnaire. The Test of Infant Motor Performance (TIMP) was performed at baseline (recruitment age), then at zero, three and four months' corrected age (CA). The Bayley III was performed at six months' CA by a blinded assessor for the purpose of assessing motor and cognitive outcomes.
Field 1980 carried out a randomised controlled study to assess the effects of combined risks of being born preterm to a teenage mother and to evaluate the effects of an intervention programme. The study comprised 60 preterm infants with teenage mothers who were randomly assigned to intervention (N = 30) or to standard follow‐up (N = 30). Teenage mothers were younger than 19 years of age, had an average of 10 years of education and were unmarried but living with a parent. The intervention consisted of home visits made by a two‐person team: a trained interventionist and a female student. Aims were to educate the mother regarding developmental milestones and child‐rearing practices, to teach the mother age‐appropriate stimulation to facilitate cognitive and social interaction and communication skills and to facilitate mother‐infant relationships. Some tasks were based on infant assessments. Intervention was targeted to the at‐risk mother, even when the infant was cared for by grandparents during the day. Home activities were prescribed, and adherence to the programme monitored. The intervention began post discharge and was provided biweekly for four months, then once a month for the next four months (up to eight months). Details of care for the standard follow‐up group were not given. An assessor blinded to group allocation measured cognitive and motor outcomes at eight months using the BSID‐I MDI and PDI.
Gianni 2006 performed a pilot randomised controlled trial on the effects of an early post‐discharge developmental intervention on neurodevelopmental outcomes at 12, 24 and 26 months of age versus standard follow‐up. Of the 61 infants initially assessed for eligibility, 25 were excluded from and 36 were included in the analysis at 36 months. The intervention group (N = 18) was seen by a psychologist and by a psychometrician twice a month in the outpatient department for 1.5 hours. The psychologist's intervention involved supporting mental health issues associated with preterm birth, and the psychometrician's intervention targeted the infant and the mother‐child interaction. It is reported that mother‐child pairs (four to six pairs) attended group meetings from three to 12 months' corrected age. The control group (N = 18) and the intervention group received periodic paediatrician follow‐up but no other interventions. The Griffiths Mental Developmental Scale (no reference to the version is given) assessed developmental outcomes at 12 and 24 months' corrected age and at 36 months' chronological age.
Goodman 1985 conducted a quasi‐randomised controlled trial to investigate the effects of early NDT versus standard follow‐up. A total of 107 infants were assessed as being 'normal' or 'at risk' on the basis of a neurodevelopmental score and were then alternatively assigned to intervention or control groups. Study authors stated that before beginning the study, their intention was to study 40 infants in the intervention and follow‐up groups. To allow for attrition, they enrolled 107 infants into the study at three months. However, the formal study ceased when 40 infants in each category had been followed for 12 months. Therefore, investigators presented data for only 80 of the 107 infants enrolled in the study. The intervention group (N = 40) received monthly outpatient NDT at the hospital as provided by a physiotherapist for 12 months. Duration of treatment was at least 45 minutes, during which time parents were shown exercises for use at home, where they were expected to carry out the programme on a daily basis. Infants in both treatment and standard follow‐up groups (N = 40) were seen at the hospital's follow‐up clinic, which was staffed by neonatologists, physiotherapists, speech and hearing therapists, ophthalmologists, public health nurses and social workers, at six weeks' and at three, six, nine and 12 months' corrected age. In addition to scheduled visits, infants in either group could attend when clinically indicated. At 12 months and at six years of age, a blinded assessor measured motor and cognitive development by using the Griffiths GCI and Locomotor Subscales.
The 'Infant Health and Development Program' (IHDP) (I.H.D.P. 1990) is the largest multi‐centre trial conducted to investigate the effects of early intervention versus standard follow‐up. To minimise the cost of the study, investigators randomly assigned one‐third of participants to intervention (N = 377) and two‐thirds to standard follow‐up (N = 608). The intervention programme began post discharge from the neonatal nursery and continued until 36 months' CA. Education professionals provided the intervention. The intervention group received home visits, visited a child development centre and attended parent group meetings. Home visits were provided weekly for the first year and biweekly for the second and third years. These visits emphasised cognitive, linguistic and social development via a programme of games to be used by the parent with the child and aimed at helping parents manage self identified problems. Children in the intervention group attended child development centres five days per week from 12 to 36 months' corrected age. Teachers at the centre continued with the above curriculum, while taking into account the child's needs and developmental levels. Parent group meetings held bi‐monthly from 12 months provided information on child rearing, health and safety and other parental concerns. The standard follow‐up group underwent medical, developmental and social assessments, with referral to other services as indicated. Compliance with the programme was variable. The mean number of home visits in the first year was 34.0 (SD 10.2, range 0 to 51); second year 17.4 (SD 7.2, range 0 to 29) and third year 15.4 (SD 7.4, range 0 to 26). Mean number of visits to child centres for the second year was 132.5 (SD 76.2, range 0 to 235) and for the third year 134.9 (SD 78.5, range 0 to 241). The mean number of attendances at parent meetings in the second year was 2.1 (SD 1.9, range 0 to 7) and in the third year 1.6 (SD 1.7, range 0 to 6). Cognitive outcome was measured at 12 and 24 months with the BSID‐I MDI, at three years with the Stanford‐Binet Intelligence Scale, at five years with the WPPSI, at eight years with the WISC‐III and at 18 years with the WASI and Peabody Picture Vocabulary Test Edition III (PPVT‐III). Motor outcome was assessed at 12 and 24 months with the BSID‐I PDI. Not all data were published, and we requested missing data from study authors. All outcome assessors were blinded to children's group allocation.
Johnson 2009 carried out a cluster‐randomised controlled trial with a cross‐over design that included six neonatal units across the UK and commenced the intervention from the first weeks after birth. The intervention programme consisted of weekly one‐hour sessions, beginning in hospital and continuing up to a maximum of six sessions post discharge. The intervention programme (N = 112), which was called the 'Parent Baby Interaction Program', included strategies to enhance parent‐infant interaction and facilitate attachment, while sensitising parents to their baby's cues and providing education about developmental care. The intervention was targeted at the mother and was delivered by a research nurse who was trained in the intervention. Of 112 infants recruited to the treatment group, 108 attended at least one intervention session, for a median of eight sessions (interquartile range (IQR) five to 11). Most sessions occurred in hospital, and a median of two sessions were provided post discharge (IQR two to seven). Families in the control group received standard care (N = 121); however, details of this care were not provided. Assessors blinded to group allocation assessed development at two years using the BSID‐II.
Kaaresen 2006 modified the Mother‐Infant Transaction Program as originally described by Nurcombe 1984 in a randomised controlled trial of 146 infants born at < 2000 grams. The intervention group (N = 72) received an initial briefing session, which was followed by daily one‐hour sessions with both parents and infants on seven consecutive days, starting one week before discharge, and four home visits at three, 14, 30 and 90 days after discharge. A team of nurses implemented the programme, which included education on behavioural cues, parent‐infant interactions and appropriate stimulation of the infant. The control group received standard care provided by a physiotherapist and doctor consultation at discharge. Investigators assessed cognitive and motor outcomes at two and three years by using the Norwegian version of the BSID‐II, and at five years with the WPPSI and McCarthy Scales of Children's Abilities. Assessors blinded to children's group allocation assessed cognitive outcomes at seven and nine years of age using the WISC‐III.
Koldewijn 2009 carried out a multi‐centre (seven sites) randomised controlled trial of the effects of the 'Infant Behavioural Assessment and Intervention Program' (IBAIP) in infants born at < 32 weeks' gestational age, at < 1500 grams or both. The intervention group (N = 86) received one‐hour sessions, with the first session provided just before discharge, followed by six to eight home visits up to six months' CA. The intervention was available as part of a commercially available training package intended to enhance parents' abilities to read and respond to their infants' cues throughout everyday life. The intervention is described in detail in the referenced publications. The control group (N = 90) and the intervention group received standard care, which consisted of regular paediatrician outpatient visits. The paediatrician could refer the child for physiotherapy if necessary, but for infants in the control group, referral could not be made to an IBAIP‐trained physiotherapist, and those in the intervention group could receive only an additional three home visits with their IBAIP‐trained physiotherapist. At six and 24 months, investigators blinded to group assignment assessed infants using the BSID‐II, and at 44 months using the Pediatric Evaluation of Disability Inventory (PEDI). At 44 months, they assessed motor co‐ordination using the Developmental Test of Visual Motor Integration (VMI) and the Pediatric Evaluation of Disability Inventory (PEDI). At 5.5 years, 76% of children returned for follow‐up and were assessed with the WPPSI‐III, the MABC‐2 and the Developmental Test of VMI.
Kyno 2012 conducted a randomised controlled trial for preterm infants (30.0 to 35.6 weeks' GA) using the Mother‐Infant Transaction Program (MITP), originally described by Nurcombe 1984. Researchers randomly assigned infants in the NICU to intervention (n = 61) and control (n = 57) groups. The MITP consisted of 11 one‐hour sessions of semi structured pre‐discharge and post‐discharge interventions. The first seven sessions, which were provided during the last week of hospitalisation and were followed by four home visits at 3, 14, 30 and 90 days after discharge, focused on infant development and the parent‐infant relationship. Investigators reported no details about the control group with regards to the intervention. At 36 months, researchers assessed infants by using the Mullen Scale of Early Learning (MSEL), which includes gross motor, fine motor, visual reception and receptive and expressive language. The early learning composite score is a measure of global cognitive function. The assessor was not blinded to group allocation.
Lekskulchai 2001 conducted a randomised controlled trial to evaluate the effects of a physiotherapy motor development programme in improving motor performance among preterm infants. Investigators used the TIMP assessment to classify the 84 infants in terms of risk of developmental delay. The motor developmental programme (N = 43) began at 40 weeks' postmenstrual age, and three additional visits were provided at one, two and three months' corrected age. A physiotherapist instructed primary caregivers on how to perform three activities with the infant during each session that were to be carried out at home. Before the next visit, the principal researcher evaluated the previous month's programme with caregivers through an interview and demonstration of activities by the caregiver. A research assistant assessed the standard follow‐up group (N = 41) (using the TIMP) at one, two, three and four months, and parents were able to discuss any concerns with the principal researcher. A physiotherapist blinded to group allocation at one, two, three and four months' corrected age used the TIMP to assess motor outcomes.
Melnyk 2001 carried out a quasi‐randomised pilot project to compare the 'creating opportunities for parent empowerment' (COPE) programme versus placebo intervention. The intervention programme was carried out in blocks (related to date of admission) to avoid contamination of the comparison group by staff and parents in the treatment group. The COPE programme (N = 26) was a four‐phase programme that consisted of audiotaped and written information and workbooks on infant behaviour and parental roles. The first three sessions occurred two to four days after admission to hospital, and the last session occurred approximately one week after discharge. The comparison programme (N = 29) was delivered at the same four time points and involved audiotaped and written information on hospital services, routine discharge and immunisations. An assessor blinded to infant group allocation measured cognitive outcomes by using the BSID‐II MDI at three and six months.
Nelson 2001 conducted a randomised controlled study to investigate the effects of an infant stimulation programme versus standard follow‐up. Infants were randomly assigned to an intervention group (N = 21) or to standard follow‐up (N = 16) at 33 weeks of age and were eligible to commence the intervention programme after this point. The intervention group received a multi‐sensory stimulation programme that included auditory, tactile, visual and vestibular stimuli in response to infant behavioural and physiological cues. A research assistant provided the intervention in the hospital twice daily five days per week until discharge. Mothers were taught the intervention, which they continued to administer at home until infants reached two months' corrected age. Standard follow‐up and intervention groups received a baseline of care in the nursery that was designed to optimise development, reduce stress and facilitate sleep cycles and motor development. All infants also received a home programme of physiotherapy intervention. Investigators used the BSID‐II MDI and PDI to assess cognitive and motor outcomes at 12 months.
Nurcombe 1984 was the first randomised controlled trial of the 'the Mother‐Infant Transaction Program' (MITP), also known as the Vermont Intervention Program. The intervention group (N = 38) received a programme designed to enhance mother‐infant interaction and infant development by teaching mothers to be more sensitive and responsive to babies' physiological, behavioural and social cues. Intervention consisted of a total of 11 sessions delivered by a trained neonatal intensive care nurse. Seven sessions were conducted in hospital before discharge, and four were provided at home during the first three months following discharge. The first seven inpatient sessions focused on educating the mother (and the father if available) with regard to the infant's motor system, state regulations, social interaction, daily care and preparation for home. Information given at these sessions was then consolidated into the first session provided post discharge. The remaining three sessions at home involved discussion regarding mutual enjoyment through play and understanding of temperamental patterns. Researchers did not report details of care for the standard follow‐up group (N = 40). They observed a significant difference in SES between intervention and standard follow‐up groups despite randomisation. Study authors provided data that had been adjusted to account for differences in SES. They measured cognitive and motor outcomes at six, 12 and 24 months by using the BSID‐I MDI and PDI. At three and four years, they assessed cognitive development by using the McCarthy Scale of Children's Abilities. At seven and nine years, they assessed cognitive development by using the Kaufman Assessment Battery for Children. All outcome assessors were blinded to children's group allocation.
Ohgi 2004 conducted a randomised controlled trial to determine the effects of an early intervention programme for preterm infants at high risk for CP versus standard follow‐up. The intervention group (N = 12) received a behaviour‐based intervention combined with developmental support designed to enhance infants' development and parent‐infant relationships. The intervention began in the NICU and lasted until six months' corrected age. The programme had two components. The first was designed to facilitate mother‐infant interactions and involved three or four 30‐minute sessions provided at 36 to 40 weeks' postmenstrual age, before the time of discharge. The second component was presented to parents during visits to the hospital and focused on advising mothers on how to handle their infants according to infant abilities and developmental needs. After discharge, the intervention group received weekly or biweekly outpatient sessions, each for 40 to 60 minutes. The standard follow‐up group (N = 12) received the same care as the treatment group with respect to attendance at clinics and referral to developmental services, if infants showed signs of neurological dysfunction or developmental delay. An assessor blinded to infants' group allocation assessed motor and cognitive outcomes at six months using the BSID‐I MDI and PDI.
Resnick 1988 conducted a quasi‐randomised controlled trial designed to evaluate a programme of hospital‐ and home‐based intervention versus standard follow‐up care. An early childhood development specialist delivered two developmental interventions per day to infants in the treatment group (N = 21) while infants were in the NICU. These interventions involved a stimulation programme (auditory, visual, vestibular and tactile) and passive movements. After discharge, a nurse visited the home weekly until infants reached term‐corrected age. From term age until 12 months' corrected age, an early childhood developmental specialist visited the infant and caregiver twice monthly for 60 to 90 minutes. The post‐discharge programme focused on language enrichment, social skills, cognitive development, parenting activities and muscular development. The standard follow‐up group (N = 20) received a full range of services, including social services, physiotherapy and occupational therapy based on the baby's condition. For outcome assessments at six and 12 months of age, investigators used the BSID‐I MDI and PDI.
Rice 1979 conducted the first randomised controlled trial of infant stimulation for preterm infants versus standard follow‐up. The intervention group (N = 15) received a tactile‐kinaesthetic stimulation programme administered by their mothers, which was designed to enhance parent‐infant relationships while giving infants appropriate levels of stimulation. The programme consisted of a stroking treatment for 15 minutes, followed by infant rocking and cuddling for another five minutes. Nurses taught mothers to deliver the intervention four times a day for a period of 30 days, beginning the day the infant was discharged from the hospital. The standard follow‐up group (N = 15) received normal discharge information and was visited regularly (number of visits was not reported by study authors) by the researcher and by other public health nurses, who provided social reinforcement for appropriate mothering behaviour. An assessor blinded to group allocation assessed cognitive and motor development at four months by using the BSID‐I MDI and PDI.
Sajaniemi 2001 aimed to assess the effects of an early occupational therapy intervention in a randomised controlled trial of infants born at < 1000 grams. Investigators matched infants in pairs in accordance with their perinatal risk scores and allocated them successively to intervention or non‐intervention groups. The intervention group (N = 63) received a one‐hour weekly home‐based intervention from six to 12 months aimed at supporting parent‐infant interactions and enhancing motor control. The average number of sessions was 20. The non‐intervention group (N = 63) and the intervention group could access additional occupational therapy or physiotherapy. Children with CP and mental retardation were excluded from the study as diagnoses were made. Infants were followed up at 24 months' corrected age with the BSID‐I MDI, and at four years' CA with the WPPSI.
Spittle 2009 performed a randomised controlled trial of a preventive care programme for infants born at < 30 weeks' gestational age, called 'VIBeS Plus' (Victorian Infant Brain Studies Plus). The intervention group (N = 61) received nine visits at home post hospital discharge by a team consisting of a physiotherapist and a psychologist; each session lasted 90 to 120 minutes from one week post hospital discharge until 11 months' corrected age. The preventive care programme aimed to improve infant development while supporting parents' mental health. The standard follow‐up group (N = 59) and the intervention group were seen by their maternal child health nurses and could be referred for early intervention services if their paediatrician or nurse believed it was needed. At 12 months' corrected age, researchers used the AlMS and the Neuro Sensory Motor Developmental Assessment (NSMDA) to assess motor development. At 24 months' corrected age, they used the Bayley III to assess cognitive, language and motor performance. At four years' corrected age, investigators assessed cognitive development by using the Differential Abilities Scale (DAS‐II) and used the General Cognitive Ability score as the primary outcome. Motor outcome was assessed with the MABC‐2. An assessor blinded to group allocation performed all follow‐up assessments.
Teti 2009 conducted a randomised controlled trial to assess the effects of an intervention on low birth weight infants of African American mothers. The study recruited 173 infants and mothers, 171 of whom were premature. Mothers were excluded if they had a positive toxicology screen or were younger than 18 years of age, and infants were excluded if they had a chromosomal abnormality. The intervention began in the NICU at 32 weeks' GA for infants born at < 32 weeks and at between 32 and 36 weeks' GA for infants born at ≥ 32 weeks. Investigators used the NBAS and video to provide eight sessions over a 20‐week period to the intervention group (N = 99), which included an infant tactile stimulation component and two psychoeducational components. Care details for the control group (N = 95) were not described. The intervention continued until approximately four months' corrected age, and infants were assessed with the BSID‐II MDI and PDI at between three and four months. Whether assessors were blinded to group allocation is not reported.
Wu 2014 conducted a randomised controlled trial comparing three interventions ‐ a clinic‐based intervention programme (CBIP; n = 57), a home‐based intervention programme (HBIP; n = 63) and a standard care programme (n = 58) ‐ in very low birth weight infants from the first week after birth until 12 months of age. Infants were low‐risk preterm infants born at < 1500 grams, at < 37 weeks' GA, singleton or first born of multiple births, with no congenital or brain abnormalities. The CBIP and the HBIP provided similar child‐, parent‐ and dyad‐focused services and interventions but differed in terms of the location at which the intervention was provided. All three groups received health surveillance for five sessions during the hospitalisation period and attended a neonatal clinic visit for eight sessions post discharge. These began at one week and were spaced out until 12 months post discharge. In addition, CBIP and HBIP groups had eight sessions with a physiotherapist at the time of the neonatal visits, which focused on environment modulation, developmental skills, feeding, massage, parental support and education. Investigators assessed cognitive, motor and language outcomes at 24 months by using the Bayley‐III.
Yigit 2002 carried out a randomised controlled trial investigating the effects of early physiotherapy intervention versus standard follow‐up for low‐risk preterm infants. Study authors did not report how many infants were initially randomly assigned to each group; however, they did report that 39 infants were dropped from the study within the first 12 months for lack of participation. This resulted in 80 infants in the physiotherapy intervention group and 80 infants in the standard follow‐up group at 12 months. Infants were registered for the study before the time of hospital discharge; however, it is unclear when the study began. The physiotherapy intervention was based on the principles of infant stimulation and NDT. It is reported that infants attended an early intervention programme and were also given a home programme; however, researchers provide no details on either programme. It is reported that all study infants were seen by the same physiotherapist once a month for the first nine months, then once every three months until 18 to 24 months of age. However, it is unclear whether the physiotherapist provided intervention or assessments at these sessions. No further details of care provided for the standard follow‐up group were reported. Motor outcomes were assessed throughout the intervention on the basis of reflexes and motor milestones, and rates of CP were reported. It is not clear whether assessors were blinded to infants' group allocation.
Types of studies
Nineteen of the 25 included studies were randomised controlled trials (Rice 1979; Field 1980; Nurcombe 1984; Barrera 1986; I.H.D.P. 1990; APIP 1998; Lekskulchai 2001; Nelson 2001; Yigit 2002; Ohgi 2004; Cameron 2005; Gianni 2006; Kaaresen 2006; Koldewijn 2009; Spittle 2009; Teti 2009; Kyno 2012; Wu 2014; Dusing 2015), five were quasi‐randomised controlled trials of early developmental programmes (Goodman 1985; Resnick 1988; Bao 1999; Melnyk 2001; Sajaniemi 2001) and one was a cluster‐randomised controlled trial (Johnson 2009). However, randomisation methods for six of the studies were not clear (Rice 1979; Field 1980; Barrera 1986; Nelson 2001; Yigit 2002; Gianni 2006). For a summary of included studies, see the Characteristics of included studies table.
Types of participants
All studies included infants who were born preterm, with a range of gestational age from < 37 weeks or birth weight from < 2500 grams (Rice 1979; Field 1980; Nurcombe 1984; Barrera 1986; I.H.D.P. 1990; Bao 1999; Lekskulchai 2001; Melnyk 2001; Nelson 2001). Inclusion criteria for remaining studies varied; three studies included infants born at < 34 weeks' gestational age or at < 1800 grams (Goodman 1985; Resnick 1988; Yigit 2002); two studies infants born at < 33 weeks' gestational age (APIP 1998; Cameron 2005); one study infants born at < 34 weeks' gestational age (Dusing 2015); one study infants born at < 2000 grams (Kaaresen 2006); one study infants born at < 32 weeks' gestational age, at < 1500 grams or both (Koldewijn 2009); one study infants born at < 1500 grams (Wu 2014); one study infants born at < 30 weeks' gestational age (Spittle 2009); and one study infants born at < 1000 grams (Sajaniemi 2001). Kyno 2012 included infants with gestational age of 30.0 to 35.6 weeks. Two studies included only infants born preterm with cerebral injuries (Nelson 2001; Ohgi 2004). The study by Teti 2009 included infants of African American mothers born at low birth weight or preterm, with 171 of 173 born at < 37 weeks' gestational age.
Types of interventions
Aims of interventions
Aims of intervention programmes varied between studies, with most programmes aiming to improve both cognitive and motor outcomes (Rice 1979; Field 1980; Nurcombe 1984; Barrera 1986; Resnick 1988; I.H.D.P. 1990; APIP 1998; Bao 1999; Nelson 2001; Sajaniemi 2001; Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009 ; Teti 2009 ; Kyno 2012; Wu 2014 ; Dusing 2015). The main aim of the four studies that involved physiotherapy was to improve motor outcomes in the intervention group (Goodman 1985; Lekskulchai 2001; Yigit 2002; Cameron 2005). Melnyk 2001 aimed to improve only cognitive outcomes in the intervention group.
Focus of interventions
Each study was classified according to the main focus of the intervention programme, with possible classifications of 'parent‐infant relationship', 'infant development' and 'infant development and parent‐infant relationship'. Enhancing the parent‐infant relationship and infant development was the focus of most studies (Nurcombe 1984; Resnick 1988; I.H.D.P. 1990; Nelson 2001; Sajaniemi 2001; Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009 ; Teti 2009; Kyno 2012; Wu 2014; Dusing 2015). Infant development alone was the focus of six studies (Rice 1979; Goodman 1985; Bao 1999; Lekskulchai 2001; Yigit 2002; Cameron 2005). One study focused on enhancing the parent‐infant relationship alone (Melnyk 2001). Two studies included two intervention groups and a control group; Barrera 1986 included one group that received a parent/infant‐focused intervention and one that received an infant development‐focused intervention, and APIP 1998 had one group that received an infant development intervention and one that was given 'parent support'. An additional classification of 'parent support' was added for this study.
Types of interventions
Although intervention programmes were focused on improving cognitive or motor outcomes, or both, theoretical constructs and components of these programmes varied greatly. Programmes were implemented by doctors (Bao 1999), physiotherapists (Goodman 1985; Lekskulchai 2001; Yigit 2002; Cameron 2005; Koldewijn 2009; Spittle 2009 ; Wu 2014; Dusing 2015), nurses (Rice 1979; Nurcombe 1984; Resnick 1988; APIP 1998; Kaaresen 2006; Johnson 2009 ; Kyno 2012), intervention therapists (Nurcombe 1984), education professionals (Resnick 1988; I.H.D.P. 1990), psychologists (Gianni 2006; Spittle 2009 ; Teti 2009), occupational therapists (Barrera 1986; Sajaniemi 2001) and/or speech pathologists (Barrera 1986). Theoretical constructs of intervention programmes included teaching parents about infant development and milestones (Barrera 1986; Resnick 1988; I.H.D.P. 1990; Bao 1999; Ohgi 2004; Cameron 2005; Kaaresen 2006; Koldewijn 2009; Spittle 2009), understanding behavioural cues (Nurcombe 1984; Barrera 1986; Bao 1999; Melnyk 2001; Ohgi 2004; Cameron 2005; Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009) and providing infant stimulation (Rice 1979; Field 1980; Nurcombe 1984; Nelson 2001), physiotherapy (Goodman 1985; Lekskulchai 2001; Nelson 2001; Yigit 2002; Cameron 2005; Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009), occupational therapy (Sajaniemi 2001), early educational intervention (I.H.D.P. 1990; Bao 1999) and enhancement of the parent‐infant relationship (Field 1980; Nurcombe 1984; Resnick 1988; I.H.D.P. 1990; Melnyk 2001; Sajaniemi 2001; Ohgi 2004; Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009).
Frequency of interventions
The frequency and duration of intervention programmes ranged from four sessions over approximately one month (Melnyk 2001) to weekly sessions over 12 months, followed by bi‐weekly sessions for another two years (I.H.D.P. 1990). Most interventions began post discharge from the hospital (Rice 1979; Field 1980; Goodman 1985; Barrera 1986; I.H.D.P. 1990; APIP 1998; Bao 1999; Lekskulchai 2001; Yigit 2002; Gianni 2006; Spittle 2009), and in ten studies, interventions began when the infant was still an inpatient (Nurcombe 1984; Resnick 1988; Ohgi 2004; Cameron 2005; Johnson 2009; Koldewijn 2009 ; Teti 2009; Kyno 2012; Wu 2014 ; Dusing 2015).
Types of outcome measures
Cognitive outcomes
At infancy (zero to < three years), 21 studies reported cognitive outcomes: Eight studies reported cognitive outcomes using the BSID‐I MDI (Rice 1979; Field 1980; Nurcombe 1984; Barrera 1986; Resnick 1988; I.H.D.P. 1990; Bao 1999; Sajaniemi 2001), seven with the BSID‐II MDI (Melnyk 2001; Nelson 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Teti 2009), three with the Bayley‐III (Spittle 2009; Wu 2014; Dusing 2015) and three with the Griffiths (Goodman 1985; APIP 1998; Gianni 2006).
At preschool age (three to < five years), eight studies reported cognitive outcomes using the Stanford‐Binet Intelligence Scale (I.H.D.P. 1990), the McCarthy Scales of Children's Abilities (Nurcombe 1984; Barrera 1986), Griffiths Mental Development Scale (Gianni 2006), WPPSI (Sajaniemi 2001) or BSID‐II (Kaaresen 2006) or the Differential Abilities Scale II (Spittle 2009). Kyno 2012 used the Mullen Scales of Early Learning.
At school age (five to 17 years), five studies reported cognitive outcomes using WPPSI and WISC‐III (I.H.D.P. 1990; Kaaresen 2006; Koldewijn 2009), the Kaufman Assessment Battery for Children ‐ Mental Processing Composite (Nurcombe 1984) or the British Abilities Scale (APIP 1998).
At 18 years of age, I.H.D.P. 1990 reported cognitive outcomes with WASI and PPVT‐III.
Motor outcomes
At infancy (zero to < three years), 21 studies reported motor outcomes using standardised measurement tools including BSID‐I PDI (Rice 1979; Field 1980; Nurcombe 1984; Barrera 1986; Resnick 1988; I.H.D.P. 1990; Bao 1999), BSID‐II PDI (Nelson 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Teti 2009), Bayley‐III (Spittle 2009; Wu 2014; Dusing 2015), Griffiths Locomotor Subscale (Goodman 1985; Gianni 2006), Test of Infant Motor Performance (Lekskulchai 2001; Dusing 2015) and Alberta Infant Motor Scale (Cameron 2005). An additional study (Yigit 2002) reported on the age of acquisition of motor skills such as sitting and crawling.
At preschool age (three to < five years), Gianni 2006 reported on motor development using the Griffiths Locomotor Subscale at 36 months, Koldewijn 2009 reported motor outcomes using the PEDI at 44 months and Spittle 2009 used the Movement ABC at four years.
At school age (five to 17 years), four studies reported motor outcomes using a variety of measures including the Movement ABC (APIP 1998; Koldewijn 2009), the McCarthy Scales of Children's Abilities (Kaaresen 2006) or the Griffiths Locomotor Subscale (Goodman 1985).
Seven studies reported rates of CP (Goodman 1985; APIP 1998; Yigit 2002; Cameron 2005; Koldewijn 2009; Spittle 2009; Wu 2014).
No studies reported on rates of developmental co‐ordination disorder; however, three studies used different cutoffs and reported on the number of children classified as having a motor impairment (APIP 1998; Koldewijn 2009; Spittle 2009).
Excluded studies
The original search strategy yielded 1092 references; we excluded 1035 publications upon review of titles and abstracts. The remaining 57 publications required more detailed examination by two independent review authors.
The second updated search strategy revealed an additional 421 publications; we excluded 414 of these upon review of titles and abstracts. Review authors excluded 17 publications from the original review and seven more from the updated review, as they did not fit all of the inclusion criteria (see Characteristics of excluded studies table).
Publications excluded from the third update are provided in the reference list.
Risk of bias in included studies
Allocation
Of the 25 included studies, 12 described adequate concealment of allocation (Nurcombe 1984; I.H.D.P. 1990; APIP 1998; Lekskulchai 2001; Ohgi 2004; Cameron 2005; Kaaresen 2006; Koldewijn 2009; Spittle 2009; Teti 2009; Kyno 2012; Wu 2014), eight did not clearly state randomisation methods (Rice 1979; Field 1980; Barrera 1986; Bao 1999; Nelson 2001; Sajaniemi 2001; Yigit 2002; Gianni 2006) and remaining studies did not use allocation concealment (Goodman 1985; Resnick 1988; Melnyk 2001; Johnson 2009).
Blinding
Melnyk 2001 was the only study that included a comparison treatment group; therefore, it is the only study that may have blinded participants to the intervention. APIP 1998 included two intervention groups ‐ one that received a developmental intervention, and one that received parent support only ‐ to control for the parent support component of an intervention that occurs with any family contact. Barrera 1986 also had two intervention groups; however, this study compared two types of interventions. All other studies involved comparison of the intervention programme versus standard follow‐up; therefore, families were not blinded to the intervention. No studies reported masking of therapists who delivered the interventions. Masking of therapists delivering the interventions often is not feasible unless the programme is similar to the one described in the study by Melnyk 2001, in that the intervention is described on audiotape and in written material.
All studies had at least one blinded outcome measure, except for Yigit 2002, in which it is unclear whether assessors were blinded to participants' intervention status, and Teti 2009, which did not include blinded assessors.
Incomplete outcome data
Completeness of follow‐up varied greatly both within and between studies. Twelve studies had greater than 80% follow‐up at one point (Field 1980; Nurcombe 1984; I.H.D.P. 1990; APIP 1998; Bao 1999; Lekskulchai 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009; Wu 2014). Assessing the completeness of follow‐up for some studies was difficult because the number of participants included at the start of these trials was not clearly stated (Rice 1979; Barrera 1986; Resnick 1988; Gianni 2006). Studies that began in the NICU had greater potential for lower follow‐up rates, as survival of infants was not always as apparent as when infants were recruited post hospital discharge. For example, Cameron 2005 began the intervention programme in hospital and reported only 83% follow‐up at four months of age, as 7% (five infants) of infants in the study died before the first outcome assessment was performed at four months. Goodman 1985 stopped this study after 20 infants in four subgroups had completed the study, despite enrolling 107 infants. Sajaniemi 2001 excluded infants with CP and mental retardation post randomisation; this resulted in low follow‐up rates for children who were initially randomly assigned.
Effects of interventions
This review found 21 quasi‐randomised or randomised controlled trials involving 3133 infants. The primary objective was to determine the effects of early developmental intervention programmes post hospital discharge for preterm infants on cognitive and motor development compared with standard medical follow‐up during infancy (zero to two years of age), preschool age (three to < five years of age), school age (five to 17 years of age) and adulthood. The following section describes cognitive and motor outcomes for each group and for the whole group and subsequent subgroup analyses.
Early developmental intervention versus standard follow‐up (all studies) (Comparison 1)
Cognitive outcome at infancy (outcome 1.1)
Sixteen studies reported sufficient data on cognitive outcomes to be pooled for meta‐analysis. Infants who received early developmental intervention (N = 1152) scored a standardised mean DQ 0.32 SD (95% CI 0.16 to 0.47; P value < 0.001) higher than infants who received standard follow‐up (N = 1220). Of these 16 studies, only three (I.H.D.P. 1990; Bao 1999; Melnyk 2001) reported differences that were statistically significant. Heterogeneity between studies was significant (I2 = 63%) and reflected the diversity of the early intervention programmes but limited conclusions that could be drawn from the results (Analysis 1.1).
1.1. Analysis.
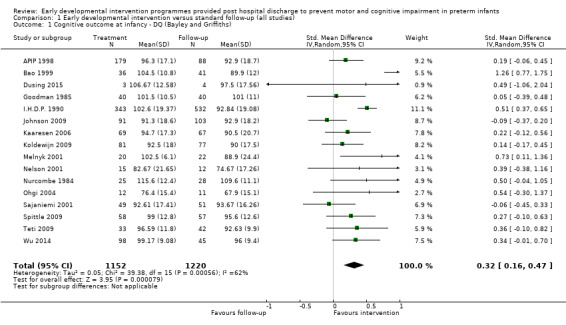
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 1 Cognitive outcome at infancy ‐ DQ (Bayley and Griffiths).
An additional five studies did not provide adequate data for meta‐analysis. Three of these reported significant differences in favour of the intervention group (Rice 1979; Field 1980; Resnick 1988), and two found no differences (Barrera 1986; Gianni 2006). Rice 1979 reported a significant difference (P value < 0.05) in favour of the intervention group (N = 15) at four months of age compared with the control group (N = 15) on the BSID‐I; however, this study did not report means and SDs. Field 1980 reported that the intervention group (N = 27) scored a mean of nine DQ points higher than the control group (N = 25) on the BSID‐I at eight months; however, these study authors did not report SDs (P value < 0.001). Resnick 1988 reported a significant difference in favour of the treatment group at 12 months (P value = 0.04) but not at six months. Gianni 2006 reported no differences between intervention and control groups at 12 and 24 months; however, study authors presented no data.
Cognitive outcome at preschool age (outcome 1.2)
Eight studies (Nurcombe 1984; Barrera 1986; I.H.D.P. 1990; Sajaniemi 2001; Gianni 2006; Kaaresen 2006; Spittle 2009; Kyno 2012) reported cognitive outcomes; these data were pooled for meta‐analysis. At preschool age, children who had received early developmental intervention (N = 619) had a standardised mean IQ 0.42 SD (95% CI 0.32 to 0.53; P value < 0.001) higher than children who received standard follow‐up (N = 817). Some heterogeneity between studies was noted (I2 = 10%) (Analysis 1.2).
1.2. Analysis.
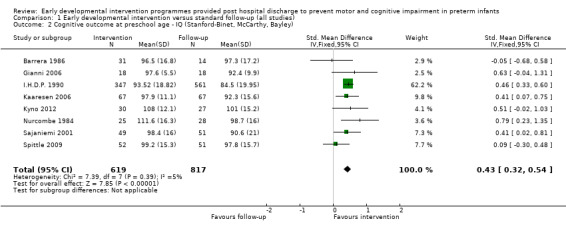
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy, Bayley).
Cognitive outcome at school age (outcome 1.3)
Five studies reported sufficient data for meta‐analysis (Nurcombe 1984; I.H.D.P. 1990; APIP 1998; Kaaresen 2006; Koldewijn 2009). At school age, children who received early developmental intervention (N = 619) did not score significantly higher on IQ measures than those who received standard follow‐up (N = 790) (IQ; SMD 0.18 SD, 95% CI ‐0.08 to 0.43; P value = 0.16). Significant heterogeneity (I2 = 72%) between studies limited the conclusions that could be drawn from these results (Analysis 1.3).
1.3. Analysis.
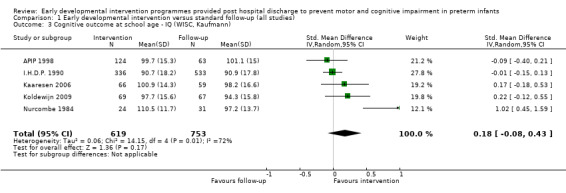
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 3 Cognitive outcome at school age ‐ IQ (WISC, Kaufmann).
Cognitive outcome at adulthood
I.H.D.P. 1990 reported outcomes at 18 years of age with a follow‐up rate of 65%. Investigators found no overall group differences between intervention and standard follow‐up groups on WASI and PPVT‐III.
Motor outcome at infancy (outcome 1.4)
Twelve studies provided sufficient data for meta‐analysis with the Bayley PDI (Edition I, II or III) and the Griffiths Locomotor Subscale (Nurcombe 1984; Goodman 1985; I.H.D.P. 1990; Bao 1999; Nelson 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009; Wu 2014; Dusing 2015). A small significant difference in motor outcome was reported for infants who received early developmental intervention (N = 872) compared with those given standard follow‐up (N = 1023) (DQ: SMD 0.10 SD, 95% CI 0.01 to 0.19; P value = 0.03). Only one of these 10 studies reported a significant difference between intervention and standard follow‐up (Koldewijn 2009). No heterogeneity between studies was noted (I2 = 0%) (Analysis 1.4).
1.4. Analysis.
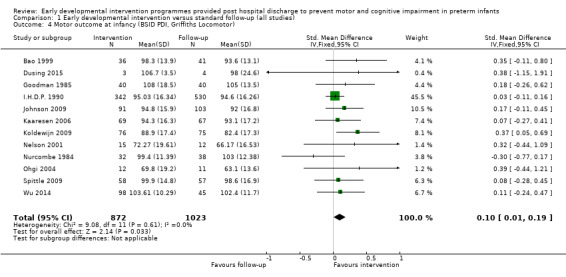
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 4 Motor outcome at infancy (BSID PDI, Griffiths Locomotor).
An additional 10 studies (Rice 1979; Field 1980; Barrera 1986; Resnick 1988; Lekskulchai 2001; Yigit 2002; Cameron 2005; Gianni 2006; Teti 2009; Kyno 2012) reported motor outcomes; however, these studies were not appropriate for use in meta‐analysis (because of the type of assessment tool used or because of missing data). Lekskulchai 2001 was the only one of these studies that reported a significant difference in favour of the intervention group (P value < 0.001) when the Test of Infant Motor Performance was used at four months of age.
Kyno 2012 found no differences between groups on gross and fine motor subscales of the Mullen Scales of Early Learning at 36 months.
Motor outcome at preschool age (outcome 1.5)
Three studies (Gianni 2006; Kaaresen 2006; Spittle 2009) reported motor outcomes and showed no differences between intervention (N = 133) and control groups (N = 131) (DQ: SMD 0.08 SD, 95% CI ‐0.16 to 0.32; P value = 0.53). In contrast, Koldewijn 2009 reported that the intervention group scored significantly higher on mobility skills and mobility assistance subscales of the PEDI at 44 months (Analysis 1.5).
1.5. Analysis.
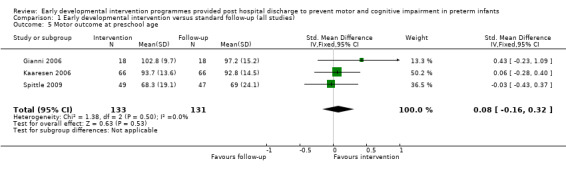
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 5 Motor outcome at preschool age.
Motor outcome at school age (continuous variables) (outcome 1.6)
Goodman 1985 and Koldewijn 2009 were the only studies that reported motor outcomes upon using the Griffith Locomotor Subscale or Movement ABC at six years of age; they described no differences in motor outcomes for children who received early intervention (SMD ‐0.18, 95% CI ‐0.47 to 0.11; P value = 0.22). Kaaresen 2006 reported no differences in total motor score upon using the McCarthy Scales of Children's Ability at five years; however, summary data were not reported. Children in the intervention group were significantly more skilled in standing on one foot (P value = 0.03) and in placing the right keys in a box when using the non‐dominant hand (P value = 0.03) (Analysis 1.6).
1.6. Analysis.
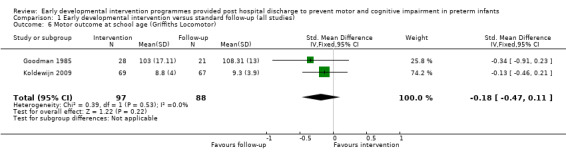
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 6 Motor outcome at school age (Griffiths Locomotor).
Motor outcome at school age (dichotomous variables) (outcome 1.7)
APIP 1998 and Koldewijn 2009 reported numbers of children with motor impairment at five years when the Movement ABC was used. They reported no differences in motor outcomes for children who received early intervention (typical RR 1.12, 95% CI 0.87 to 1.44; P value = 0.40) (Analysis 1.7).
1.7. Analysis.
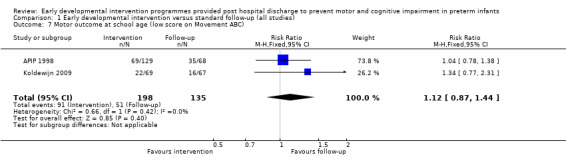
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 7 Motor outcome at school age (low score on Movement ABC).
Rate of cerebral palsy (outcome 1.8)
Six studies reported rates of CP (Goodman 1985; APIP 1998; Yigit 2002; Cameron 2005; Koldewijn 2009; Wu 2014). Investigators reported no difference in the RR of CP between intervention and standard follow‐up groups (typical RR 0.82, 95% CI 0.52 to 1.27; P value = 0.37) (Analysis 1.8).
1.8. Analysis.
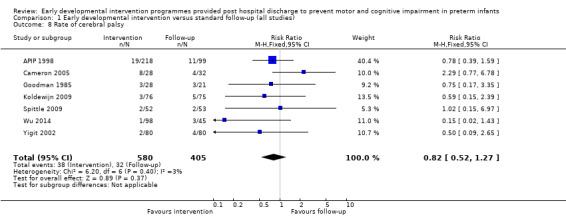
Comparison 1 Early developmental intervention versus standard follow‐up (all studies), Outcome 8 Rate of cerebral palsy.
Subgroup analysis: gestational age (Comparison 2)
Most studies included infants born at a wide range of gestational ages and reported no outcomes related to subgroups of gestational age.
Cognitive outcome at infancy (outcome 2.1)
The only study that reported data that could be used in a meta‐analysis was APIP 1998. Researchers reported that infants born at a gestational age < 28 weeks (DQ; SMD 0.39, 95% CI ‐0.06 to 0.83; P value = 0.09) benefited more from the intervention programme than infants born at gestational age ≥ 28 weeks (DQ; SMD 0.09, 95% CI ‐0.25 to 0.43; P value = 0.60); however, effects for both subgroups were not statistically significant, nor were findings on the overall test for subgroup differences (P value = 0.31). Koldewijn 2009 reported no interaction between gestational age < 28 weeks and ≥ 28 weeks and intervention, and Johnson 2009 reported no differences in outcomes between infants born at gestational age < 28 weeks and ≥ 28 weeks.
Cognitive outcome at preschool age
No studies reported outcomes in relation to gestational age.
Cognitive outcome at school age
No studies reported outcomes in relation to gestational age.
Motor outcome at infancy
Koldewijn 2009 found no interaction between gestational age < 28 and ≥ 28 weeks and intervention, and Johnson 2009 reported no differences in outcomes between infants born at gestational age < 28 weeks and ≥ 28 weeks.
Motor outcome at preschool age
No studies reported outcomes in relation to gestational age.
Motor outcome at school age (continuous variables)
No studies reported outcomes in relation to gestational age.
Motor outcome at school age (dichotomous variables)
No studies reported outcomes in relation to gestational age.
Rate of cerebral palsy
No studies reported CP outcomes in relation to gestational age.
Subgroup analysis: birth weight (Comparison 3)
Cognitive outcome at infancy (outcome 3.1)
Four studies investigated the impact of birth weight on effects of early developmental interventions. However, only three of these studies (Barrera 1986; Sajaniemi 2001; Teti 2009) used the birth weight subgroups of LBW (1500 to 2499 grams), VLBW (1000 to 1499 grams) and ELBW (< 1000 grams). Barrera 1986 carried out subgroup analyses of heavier infants (1500 to 1999 grams) and lighter infants (< 1500 grams) and reported that infants born at lower birth weight in both intervention groups made significant therapeutic gains compared with infants born at higher birth weight. However, investigators did not report means and SDs. I.H.D.P. 1990 analysed the results of a higher weight subgroup (2001 to 2499 grams) and a lower weight subgroup (< 2000 grams) and reported that infants born at higher birth weight benefited more from the intervention programme. Heavier infants who received intervention (N = 125) scored 0.75 SD (95% CI 0.52 to 0.98; P value < 0.001) higher than heavier infants who received standard follow‐up (N = 197), and investigators reported no differences in outcomes between infants born at lighter birth weight (N = 218) who received intervention and those who received standard follow‐up (N = 355). These data could not be added to the meta‐analysis because reported weight categories were different. APIP 1998 reported data for infants born at higher birth weight (≥ 1250 grams) and at lighter birth weight (< 1250 grams). They found that lighter birth weight infants in the 'Portage group' (N = 22) scored 5.3 DQ points higher (95% CI 0.2 to 10.2; P value < 0.05) than infants in the control group (N = 29), whereas they observed no difference at 12 months between infants in the heavier subgroup who had received intervention versus those given standard follow‐up. Conflicting evidence was found regarding differences in benefit derived from early developmental interventions according to birth weight. Sajaniemi 2001 included only infants who were ELBW, and Teti 2009 found no differences in cognitive outcomes at infancy when comparing infants born ELBW and VLBW (Analysis 3.1).
3.1. Analysis.
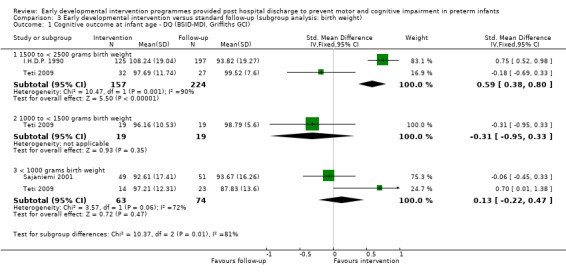
Comparison 3 Early developmental intervention versus standard follow‐up (subgroup analysis: birth weight), Outcome 1 Cognitive outcome at infant age ‐ DQ (BSID‐MDI, Griffiths GCI).
Cognitive outcome at preschool age (outcome 3.2)
I.H.D.P. 1990, the only study that reported outcomes according to birth weight at preschool age, indicated that higher birth weight infants who received intervention scored 0.70 SD (95% CI 0.47 to 0.93; P value < 0.001) higher than infants given standard follow‐up. Lighter weight infants who received intervention scored 0.41 (95% CI 0.02 to 0.81; P value = 0.04) higher than those given standard follow‐up. Sajaniemi 2001 included only infants who were ELBW and reported a significant difference in favour of the intervention group at four years (Analysis 3.2).
3.2. Analysis.
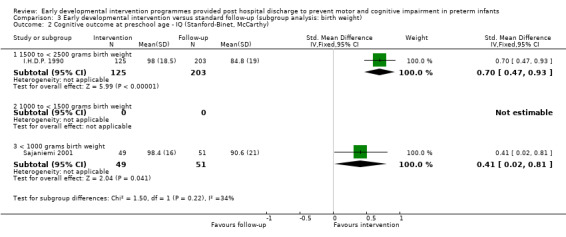
Comparison 3 Early developmental intervention versus standard follow‐up (subgroup analysis: birth weight), Outcome 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy).
Cognitive outcome at school age
I.H.D.P. 1990 at school age reported that heavier birth weight infants who received intervention scored 4.4 IQ points higher than heavier infants who received standard follow‐up; however, they found no differences in the lighter weight group (SDs were not reported). At five years, APIP 1998 found no differences related to birth weight between intervention and control groups. Conflicting evidence was found regarding the benefits of early developmental interventions according to birth weight.
Cognitive outcome at adulthood
I.H.D.P. 1990 reported that the higher weight subgroup at 18 years performed better on PPVT‐III after adjustments were made for perinatal risk factors; however, investigators reported no differences in the lower weight subgroup and no differences on the WASI for infants of any birth weight.
Motor outcome at infancy
I.H.D.P. 1990 reported no differences in motor outcomes between infants in intervention and control groups born at heavier (≥ 2000 grams) or lighter (< 2000 grams) weight. Barrera 1986 also reported no differences in motor outcomes between infants in intervention and control groups born at heavier (1500 to 1999 grams) or lighter (< 1500 grams) weight.
Motor outcome at preschool age
No studies reported motor outcomes in relation to birth weight.
Motor outcome at school age (continuous variables)
No studies reported motor outcomes in relation to birth weight.
Motor outcome at school age (dichotomous variables)
No studies reported motor outcomes in relation to birth weight.
Rate of cerebral palsy
No studies reported CP outcomes in relation to birth weight.
Subgroup analysis: brain injury (Comparison 4)
Cognitive outcome at infancy (outcome 4.1)
Most of the included studies did not report separate results for infants who had PVL or IVH. Two studies included only infants who were at risk for adverse neurological outcomes due to PVL or IVH, or both (Nelson 2001; Ohgi 2004). These two studies showed no significant differences in cognitive outcomes between intervention and control groups at infancy (DQ; SMD 0.50, 95% CI ‐0.12 to 1.13; P value = 0.11). APIP 1998 was the only study that reported cognitive outcomes for infants in intervention and control groups who had abnormal ultrasound results compared with those who had normal ultrasound results. Researchers reported that infants at risk for adverse neurological outcomes derived significant benefit from early developmental intervention, whereas infants who were not at risk showed no cognitive benefit associated with the intervention programme.
Cognitive outcome at preschool age
No studies reported cognitive outcomes in relation to brain injury.
Cognitive outcome at school age
APIP 1998, the only study that reported outcomes in relation to normal and abnormal ultrasound findings, reported no differences between groups at school age.
Motor outcome at infancy (outcome 4.2)
Ohgi 2004 and Nelson 2001 included infants at risk for adverse neurological outcomes due to PVL or IVH, or both, and showed no significant differences between intervention and follow‐up groups (DQ; SMD 0.47, 95% CI ‐0.15 to 1.10; P value = 0.14). Standard follow‐up groups in both studies were given access to physiotherapy services as required.
Motor outcome at preschool age
No studies reported motor outcomes in relation to brain injury.
Motor outcome at school age (continuous variables)
No studies reported motor outcomes in relation to brain injury.
Motor outcome at school age (dichotomous variables)
No studies reported motor outcomes in relation to brain injury.
Rate of cerebral palsy
No studies reported CP outcomes in relation to brain injury.
Subgroup analysis: commencement of intervention programme (inpatient vs post hospital discharge) (Comparison 5)
Cognitive outcome at infancy (outcome 5.1)
Among the 16 studies that reported sufficient data for meta‐analysis, ten began when infants were in the NICU (Nurcombe 1984; Melnyk 2001; Nelson 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Teti 2009; Wu 2014; Dusing 2015) and six began post hospital discharge (Goodman 1985; I.H.D.P. 1990; APIP 1998; Bao 1999; Sajaniemi 2001; Spittle 2009). Programmes that began while infants were inpatients had a significant impact on cognitive outcome at infancy (DQ; SMD 0.24, 95% CI 0.08 to 0.40; P value = 0.003), as did programmes that commenced post hospital discharge (DQ; SMD 0.35, 95% CI 0.07 to 0.63; P value = 0.01) compared with standard follow‐up. Effects of interventions that commenced in the NICU were less heterogeneous (I2 = 20%) than those of interventions that commenced post hospital discharge (I2 = 80%) (Analysis 5.1).
5.1. Analysis.
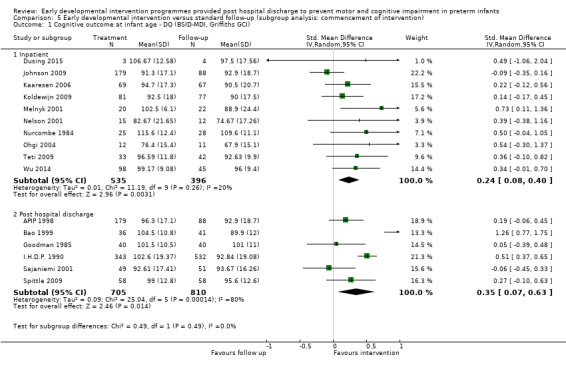
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 1 Cognitive outcome at infant age ‐ DQ (BSID‐MDI, Griffiths GCI).
Cognitive outcome at preschool age (outcome 5.2)
Three studies began in the NICU (Nurcombe 1984; Kaaresen 2006; Kyno 2012) and reported a significant effect in favour of the intervention group (IQ; SMD 0.51, 95% CI 0.26 to 0.77; P value < 0.001). The five studies that began post discharge showed a significant effect in favour of the intervention group (IQ; SMD 0.41, 95% CI 0.29 to 0.53; P value < 0.0001) (Analysis 5.2).
5.2. Analysis.
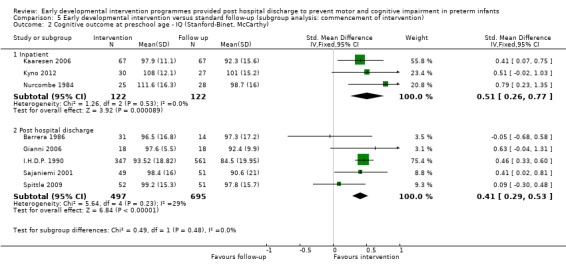
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy).
Cognitive outcome at school age (outcome 5.3)
Nurcombe 1984, Kaaresen 2006 and Koldewijn 2009 are the only studies that began in the NICU and reported outcomes at school age and effects at preschool age showing a significant effect in favour of the intervention group (IQ; SMD 0.49, 95% CI 0.09 to 0.88; P value = 0.02) with significant heterogeneity among groups (65%). The two studies that began post hospital discharge showed no differences between outcomes (IQ; SMD ‐0.02, 95% CI ‐0.15 to 0.10; P value = 0.70). Sample sizes of studies in which interventions began in the NICU (N = 322) were much smaller than sample sizes of studies in which interventions began post hospital discharge (N = 1056) (Analysis 5.3).
5.3. Analysis.
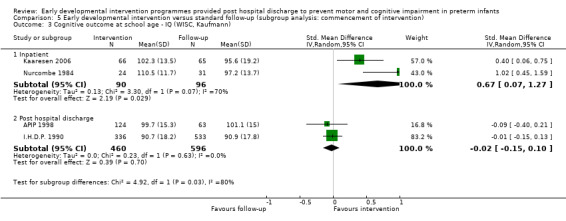
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 3 Cognitive outcome at school age ‐ IQ (WISC, Kaufmann).
Motor outcome at infancy (outcome 5.4)
Among studies that provided sufficient data for meta‐analysis, six began when infants were in the NICU (Nurcombe 1984; Nelson 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009) and four began post hospital discharge (Goodman 1985; I.H.D.P. 1990; Bao 1999; Spittle 2009). Programmes that began in hospital had a slightly greater impact on motor outcomes at infancy (DQ; SMD 0.19, 0.05 to 0.34; P value = 0.01) than did programmes that began post hospital discharge (DQ; SMD 0.07, 95% CI ‐0.05 to 0.18; P value = 0.28); however, findings on the overall test for subgroup differences were not significant (P value = 0.19) (Analysis 5.4).
5.4. Analysis.
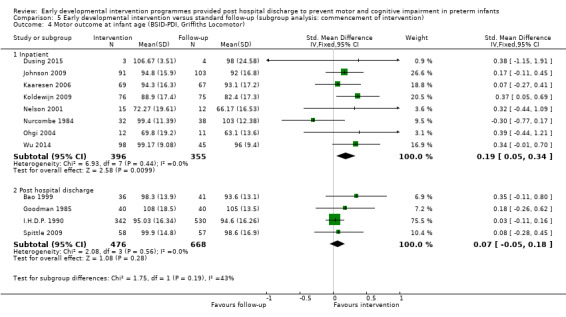
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 4 Motor outcome at infant age (BSID‐PDI, Griffiths Locomotor).
Motor outcome at preschool age (outcome 5.5)
One study commenced in the NICU and reported data at preschool age showing no differences between groups (Kaaresen 2006). Koldewijn 2009, which also commenced in the NICU, reported that the intervention group scored significantly higher on mobility skills and mobility assistance subscales of the PEDI at 44 months. Gianni 2006 commenced post discharge and reported no differences in motor outcomes (Analysis 5.5).
5.5. Analysis.
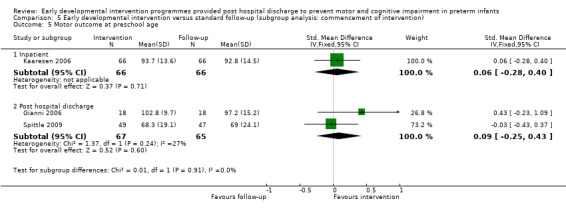
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 5 Motor outcome at preschool age.
Motor outcome at school age (continuous variables) (outcome 5.6)
Goodman 1985 began post hospital discharge and showed no differences between groups (Analysis 5.6).
5.6. Analysis.
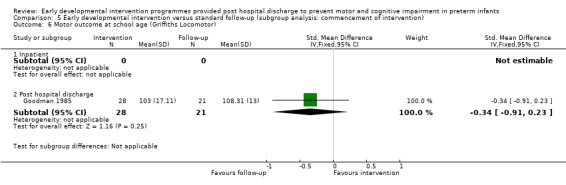
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 6 Motor outcome at school age (Griffiths Locomotor).
Motor outcome at school age (dichotomous variables) (outcome 5.7)
APIP 1998, the only study to report rates of motor impairment, found no differences between groups (Analysis 5.7).
5.7. Analysis.
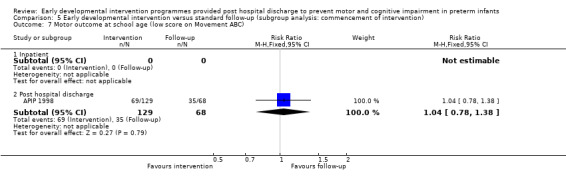
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 7 Motor outcome at school age (low score on Movement ABC).
Rate of cerebral palsy (outcome 5.8)
Cameron 2005 and Koldewijn 2009, the only studies that began in the NICU and reported rates of CP, described no differences between groups. Goodman 1985,APIP 1998,Yigit 2002 and Spittle 2009 began post hospital discharge and found no differences between groups in rates of CP (Analysis 5.8).
5.8. Analysis.
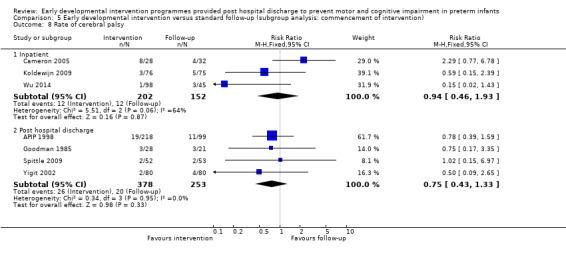
Comparison 5 Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention), Outcome 8 Rate of cerebral palsy.
Subgroup analysis: focus of intervention (parent‐infant relationship vs infant development vs combination) (Comparison 6)
Cognitive outcome at infancy (outcome 6.1)
One study focused primarily on the parent‐infant relationship (Melnyk 2001). Infants who received early intervention that focused on the parent‐infant relationship alone scored a standardised mean DQ 0.73 SD (95% CI 0.11 to 1.36; P value = 0.02) higher than infants who received standard follow‐up. Three studies that focused primarily on infant development (Goodman 1985; APIP 1998; Bao 1999) reported no significant differences between groups when the intervention focused on infant development alone. However, significant heterogeneity (I2 = 87) between these studies reflects the diversity of early intervention programmes that focus on infant development. Remaining studies focused on both the parent‐infant relationship and infant development (Nurcombe 1984; I.H.D.P. 1990; Nelson 2001; Sajaniemi 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009; Teti 2009; Wu 2014; Dusing 2015). I.H.D.P. 1990 was the only study that reported a significant difference in favour of the intervention group, whereas the other studies reported a trend in favour of the treatment group. Overall, infants who received intervention that focused on both the parent‐infant relationship and infant development scored a standardised mean DQ 0.26 SD (95% CI 0.11 to 0.41; P value < 0.001) higher than that of infants who received standard follow‐up. APIP 1998 investigated the effects of parent support or infant development (with parent support) versus standard follow‐up and reported no differences between groups (Analysis 6.1).
6.1. Analysis.
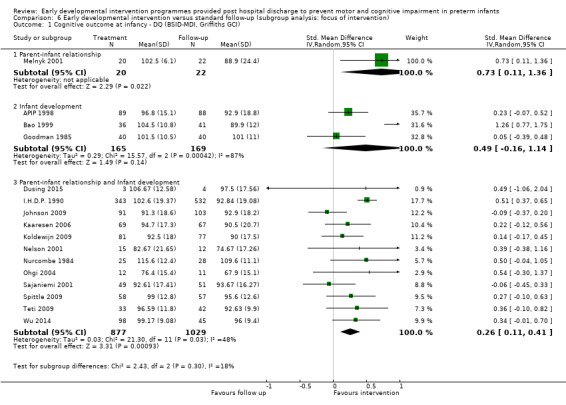
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 1 Cognitive outcome at infancy ‐ DQ (BSID‐MDI, Griffiths GCI).
Cognitive outcome at preschool age (outcome 6.2)
In Barrera 1986, one treatment group focused on infant development and the other on the parent‐infant relationship. Only pooled results of the two different intervention groups were reported; therefore, these data were not included in the analysis. Infants who received intervention that focused on both the parent‐infant relationship and infant development scored a standardised mean IQ 0.48 SD (95% CI 0.35 to 0.61; P value < 0.001) higher than infants who received standard follow‐up at preschool age. All seven remaining studies (Nurcombe 1984; I.H.D.P. 1990; Sajaniemi 2001; Gianni 2006; Kaaresen 2006; Spittle 2009; Kyno 2012) focused on both infant development and the parent‐infant relationship and reported a large difference of 0.44 in standardised mean IQ in favour of the intervention (95% CI 0.33 to 0.55; P value < 0.0001) (Analysis 6.2).
6.2. Analysis.
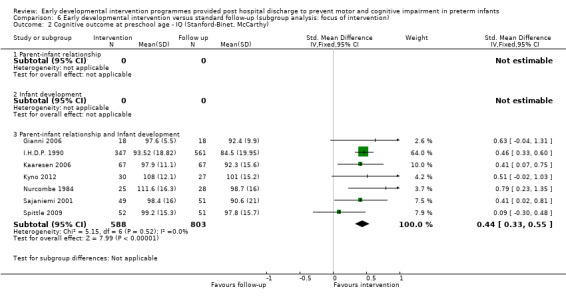
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy).
Cognitive outcome at school age (outcome 6.3)
At school age, four studies focused on the parent‐infant relationship and infant development (Nurcombe 1984; I.H.D.P. 1990; Kaaresen 2006; Koldewijn 2009), and one study focused on infant development (APIP 1998). The four studies that focused on both the parent‐infant relationship and infant development reported different results, with I.H.D.P. 1990 and Kaaresen 2006 showing no effect and Nurcombe 1984 and Koldewijn 2009 showing significant differences in favour of the intervention group. Heterogeneity (I2 = 80%) was significant because of differences in sample size and in study outcomes. Overall comparison of cognitive development at school age revealed no significant differences between children who received any of the three types of early intervention programmes and those who received standard follow‐up as infants (Analysis 6.3).
6.3. Analysis.
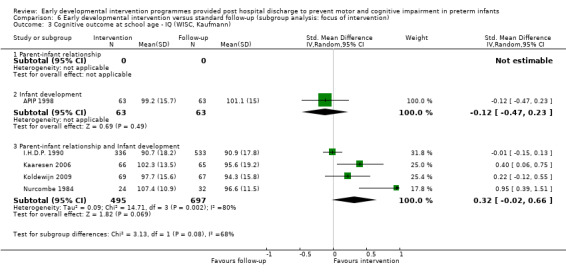
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 3 Cognitive outcome at school age ‐ IQ (WISC, Kaufmann).
Motor outcome at infancy (outcome 6.4)
Studies that reported motor outcome at infancy focused on both the parent‐infant relationship and infant development (Nurcombe 1984; I.H.D.P. 1990; Nelson 2001; Ohgi 2004; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009) or on infant development alone (Goodman 1985; Bao 1999). Programmes that focused on infant development had a slightly greater impact on motor outcomes at infancy (DQ: SMD 0.26 SD, 95% CI ‐0.05 to 0.58; P value = 0.10) than those that focused on the parent‐infant relationship and infant development together (DQ: SMD 0.09 SD, 95% CI ‐0.01 to 0.19; P value = 0.07); however, the effect on neither group was significant (Analysis 6.4).
6.4. Analysis.
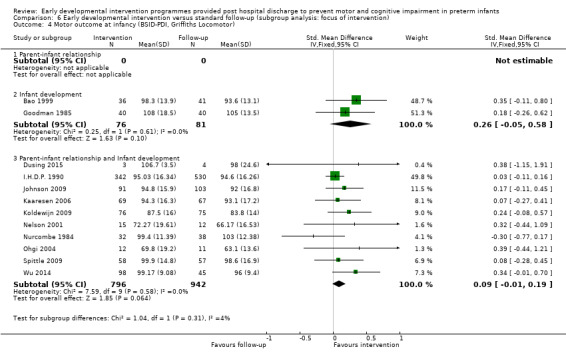
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 4 Motor outcome at infancy (BSID‐PDI, Griffiths Locomotor).
Motor outcome at preschool age (outcome 6.5)
Gianni 2006, Kaaresen 2006 and Spittle 2009 focused on the parent‐infant relationship and infant development and showed no differences between groups.
Motor outcome at school age (continuous variables) (outcome 6.6)
Goodman 1985 focused on infant development and reported no significant differences between groups.
Motor outcome at school age (dichotomous variables)
APIP 1998 focused on infant development and reported no differences between groups.
Rate of cerebral palsy (outcome 6.7)
The seven studies that reported rates of CP focused on infant development and reported no differences between groups (Goodman 1985; APIP 1998; Yigit 2002; Cameron 2005; Koldewijn 2009; Spittle 2009; Wu 2014) (Analysis 6.7).
6.7. Analysis.
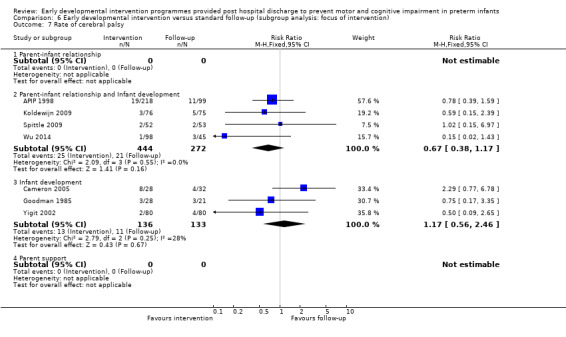
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 7 Rate of cerebral palsy.
Subgroup analysis: quality of studies (higher vs lower) (Comparison 7)
Eight trials were considered to have high quality, as they were randomised controlled trials with adequate allocation concealment, and they provided sufficient data for meta‐analysis (Nurcombe 1984; I.H.D.P. 1990; APIP 1998; Ohgi 2004; Kaaresen 2006; Koldewijn 2009; Spittle 2009; Wu 2014).
Cognitive outcome at infancy (outcome 7.1)
Eight of the studies included in this meta‐analysis were of high quality and reported a significant treatment effect supporting the intervention group (DQ; SMD 0.34 SD, 95% CI 0.21 to 0.46; P value < 0.001). Less heterogeneity (24%) was noted between higher‐quality studies than between low‐quality studies (78%) (Analysis 7.1).
7.1. Analysis.
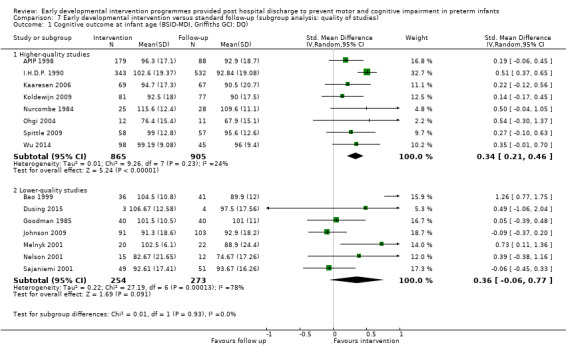
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 1 Cognitive outcome at infant age (BSID‐MDI, Griffiths GCI: DQ).
Cognitive outcome at preschool age (outcome 7.2)
At preschool age, high‐quality studies were homogenous and demonstrated a significant treatment effect supporting the intervention group (IQ; SMD 0.44 SD, 95% CI 0.32 to 0.55; P value < 0.001) (Analysis 7.2).
7.2. Analysis.
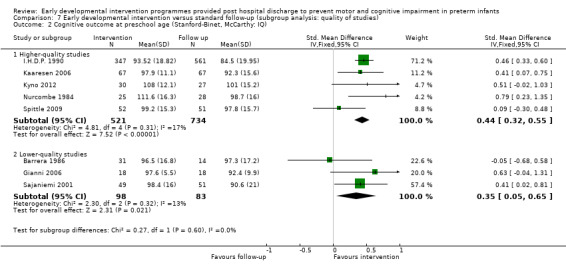
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 2 Cognitive outcome at preschool age (Stanford‐Binet, McCarthy: IQ).
Cognitive outcome at school age (outcome 7.3)
At school age, all five studies were of high quality but showed little evidence of a treatment effect (IQ; SMD 0.18 SD, 95% CI ‐0.08 to 0.43; P value = 0.17) (Analysis 7.3).
7.3. Analysis.
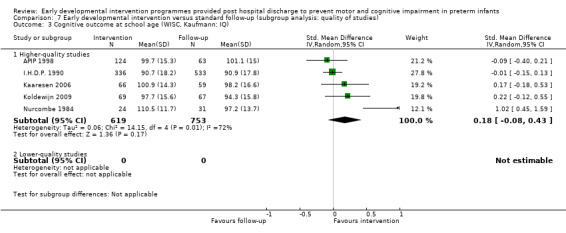
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 3 Cognitive outcome at school age (WISC, Kaufmann: IQ).
Motor outcome at infancy (outcome 7.4)
When only the six high‐quality studies were considered, no significant difference between groups was observed (IQ; SMD 0.06 SD, 95% CI ‐0.04 to 0.17; P value = 0.25) (Analysis 7.4).
7.4. Analysis.
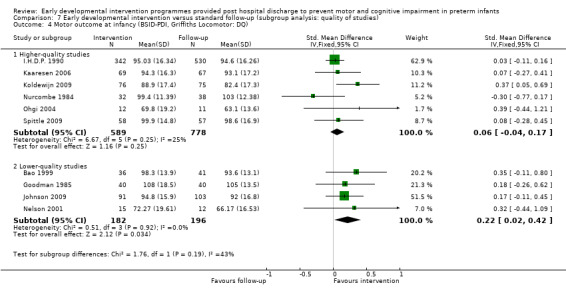
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 4 Motor outcome at infancy (BSID‐PDI, Griffiths Locomotor: DQ).
Motor outcome at preschool age (outcome 7.5)
Kaaresen 2006 and Spittle 2009, the only high‐quality studies to report on this outcome, revealed no differences between groups (Analysis 7.5).
7.5. Analysis.
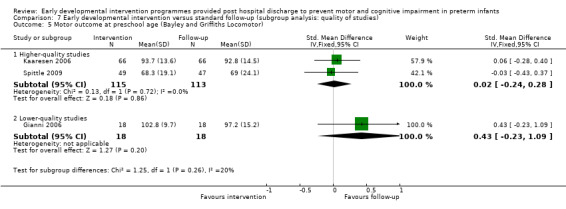
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 5 Motor outcome at preschool age (Bayley and Griffiths Locomotor).
Motor outcome at school age (continuous variables)
No high‐quality studies reported motor outcomes.
Motor outcome at school age (dichotomous variables) (outcome 7.6)
APIP 1998, the only high‐quality study to report rates of motor impairment, described no differences between groups (Analysis 7.6).
7.6. Analysis.
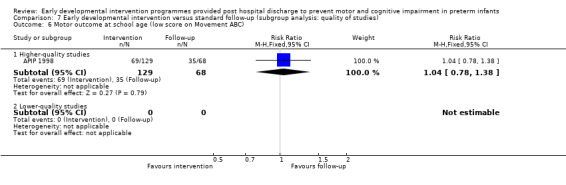
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 6 Motor outcome at school age (low score on Movement ABC).
Rate of cerebral palsy (outcome 7.7)
APIP 1998, Cameron 2005, Spittle 2009 and Wu 2014, the only high‐quality studies to report rates of CP, found no differences between groups (Analysis 7.7).
7.7. Analysis.
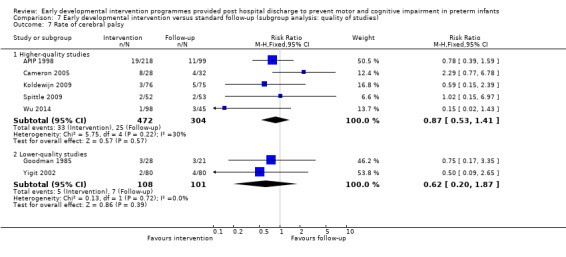
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 7 Rate of cerebral palsy.
Discussion
The primary goal of this updated review was to compare effects of early developmental intervention programmes provided post hospital discharge for preterm infants on cognitive and motor development versus standard medical follow‐up at infancy (zero to < three years), preschool age (three to < five years), school age (five to < 18 years) and adulthood (≥ 18 years). We included in this review 25 randomised or quasi‐randomised controlled trials of early developmental interventions for preterm infants; however, not all studies provided sufficient data for inclusion in the meta‐analysis. Meta‐analysis demonstrated a treatment effect of early intervention on cognitive outcomes at infancy of approximately one‐third of SD on standardised cognitive tests, and at preschool age of approximately one‐half of SD on standardised IQ tests; however, no difference at school age or into adulthood was noted. With regards to motor outcomes, this updated review demonstrated a small but significant difference in favour of intervention at infancy; however, only a few studies reported longer‐term outcomes and described conflicting results. The size of the reported effect on cognitive outcomes is considered to be of clinical importance; however, the effect on motor development is small.
This updated review includes four new trials and longer‐term outcomes of three trials that were included in previous versions of the review. Although data from an additional seven studies have been included in this review update, overall results have not changed.
The previous updated review included six new studies ‐ one study that was not previously identified (Sajaniemi 2001) and five that were completed more recently (Gianni 2006; Kaaresen 2006; Johnson 2009; Koldewijn 2009; Spittle 2009) and had not reported outcomes when the original review was published (Spittle 2007). The addition of these studies to the meta‐analysis for the second update at infancy led to a change in effect size for both cognitive and motor outcomes when compared with the previous review but resulted in no further change with the third update.
The study by Kaaresen 2006 of the Mother‐Infant Transaction Program (MITP) showed diminished differences between intervention and control in time as related to cognitive outcome. The intervention group showed significantly higher cognitive outcomes at five years, and these data were included in the meta‐analysis of school‐age outcomes for the second update of this Cochrane review. However, when the cohort was assessed at seven and nine years of age, no differences in cognitive outcomes were noted between groups. The current review has demonstrated diminishing effects of early developmental interventions over time. These diminishing effects support the need for further intervention around school age possibly targeted towards school readiness.
Considerable debate has examined how best to reduce or prevent long‐term impairments while improving cognitive and motor outcomes for children born preterm. This high level of interest is reflected in the large numbers of randomised controlled trials and systematic reviews on the topic. To our knowledge, our first review provided the first reported meta‐analysis of early developmental interventions provided post discharge from hospital for preterm infants. However, Vanderveen 2009 more recently published a systematic review on early intervention programmes for preterm infants involving parents. The Vanderveen review also included interventions that were based only in the hospital, such as the Newborn Individualized Developmental Care and Assessment Program (NIDCAP), along with interventions post hospital discharge, and their findings were similar to those reported in our review. The Vanderveen review identified 25 studies that used a variety of interventions including parent education, infant stimulation, home visits and individualised developmental care. Meta‐analysis showed early improvement in cognitive and motor performance that was not sustained at school age. Blauw‐Hospers 2005 published a systematic review investigating the effects of early intervention on motor outcomes for all infants at risk for, or diagnosed with, developmental motor disorders. Review authors defined early intervention as "multidisciplinary services provided to children from birth to five years of age to promote child health and well‐being, enhance emerging competencies, minimise developmental delays, existing or emerging disabilities, prevent functional deterioration, and promote adaptive parenting and overall family functioning". They reported that NIDCAP, an inpatient programme comprising interventions that focus on specific motor training such as treadmill training, and general development programmes that aim to increase the child's exploration have a positive effect on motor outcomes for high‐risk infants. The Blauw‐Hospers 2005 systematic review was broader than the current review in that it included all early intervention programmes that began from birth to 18 months for all infants at risk for, or diagnosed with, developmental motor disorders. Review authors did not include preterm infants at 'low risk' of developmental motor disorders; therefore, many of the studies included in the current review were not included by Blauw‐Hospers 2005. Inclusion criteria for early developmental interventions programmes applied in the current review were very specific in an attempt to limit variability among intervention programmes. As Blauw‐Hospers 2005 reported, the term 'early intervention' can be understood in one of two ways: intervention that occurs early in life, or intervention that occurs early in expression of the condition. We have included only intervention programmes that began early in life ‐ within the first 12 months, when the brain is highly plastic (Hadders‐Algra 2001). These intervention programmes are more likely to be targeted at prevention because longer‐term problems may not be apparent and specific diagnoses are less likely.
The current review has a number of limitations. Interventions received by 'treatment groups' in this review varied in theoretical content, environmental context, intensity and duration of follow‐up. This contributed to significant levels of heterogeneity when cognitive outcomes were pooled and, therefore, limits the conclusions that can be drawn from these results. Subgroup analysis was useful for investigating areas of variation between trials.
In our first review, most of the included studies recruited infants in the 1980s, when the mean gestational age (GA) at entry was older than in studies conducted in the 1990s. Advances in perinatal care meant that pooling results across eras may have resulted in comparisons of different groups of infants with respect to outcomes. With the addition of nine studies in the second and third updates of this review, effects on cognitive development have decreased, although they are still significant, and effects on motor development have increased. However, this may reflect differences in the types of early intervention programmes provided, for example, the fact that two studies included physiotherapy, rather than differing characteristics among participants. To account for differences in GA and birth weight of infants across studies, subgroup analysis was planned for both of these factors. Most studies did not report outcomes according to GA or birth weight, thus limiting subgroup analysis. I.H.D.P. 1990 reported that infants who were born at between 2000 grams and 2500 grams had greater response to intervention. Barrera 1986 and APIP 1998 found the opposite response, with infants born at very low birth weight (VLBW) having greater cognitive response at infancy. Sajaniemi 2001 included only extremely low birth weight (ELBW) infants and reported significant effects on cognitive development. No studies reported differences in motor outcomes between intervention and control groups related to GA or birth weight. The presence of brain injury is important to consider when effects of interventions on preterm infants are assessed. Only two studies included infants with periventricular leukomalacia (PVL) or intraventricular haemorrhage (IVH), and these studies reported no differences in cognitive and motor outcomes between intervention and control groups (Nelson 2001; Ohgi 2004). The standard follow‐up group in both of these studies received physiotherapy as required, which may have affected the motor outcomes of these infants. APIP 1998 reported that infants with abnormal cranial ultrasound findings had a positive cognitive response to intervention, but infants who received intervention or standard follow‐up with normal cranial ultrasound showed no differences in outcomes. The other studies did not report whether they excluded infants with abnormal imaging, PVL or IVH; therefore, they could not be included in the subgroup analysis.
Other variables such as socioeconomic status (SES) are also important to consider when different studies are compared. Rice 1979 and Field 1980 included only infants who were born to mothers of low SES. APIP 1998 and Nurcombe 1984 reported significant differences in SES between groups despite randomisation. The control group in the study by APIP 1998 included a higher percentage of mothers who were educated beyond 16 years of age, were in non‐manual occupations and had use of a car compared with the intervention groups. All three of these variables were independently associated with Griffiths quotients and may explain some of the variance in outcomes. Using a regression model to account for SES differences between groups, study authors reported improvement in IQ scores for both Portage and Parent Advisor groups compared with the standard follow‐up group. Nurcombe 1984 reported that results were adjusted to account for differences in SES between groups. Koldewijn 2009 reported both unadjusted and adjusted results for several variables, including SES. When results were adjusted, a significant effect on motor development was noted at infancy, but not when results were unadjusted. Teti 2009 targeted infants of African American mothers because of associated high rates of premature birth and noted that at least half of families were living below the poverty threshold. When adjusted for poverty status, results revealed a greater intervention effect on cognitive outcome (Mental Development Index (MDI)) for infants of mothers living above the poverty threshold. Field 1980 and Rice 1979 included only families of low SES, which limits generalisability of these results to the general population.
Two types of subgroup analyses were performed in relation to types of intervention programmes provided. The first subgroup analysis compared interventions that were begun while the infant was still in hospital versus those that were begun post hospital discharge. Both types of programmes had a significant effect on cognitive outcomes at infancy and preschool age. At school age, the only study that demonstrated a difference in favour of the intervention group began with participants belonging to an inpatient group. Interventions that began when infants were inpatients were more homogenous, as all focused on improving the parent‐infant relationship and on enhancing parents' abilities to read and respond appropriately to infants' behavioural cues. Significant heterogeneity between interventions that occurred post hospital discharge can be explained by the variety of intervention programmes provided. Interventions that began post hospital discharge had a greater impact on motor outcomes in infants; however, this effect was not significant. The second subgroup analysis was related to the focus of the intervention programme as parent‐infant relationship, infant development or both. Interventions with a component that focused on the parent‐infant relationship had a greater impact on cognitive outcomes at infancy and preschool age when compared with interventions that focused on infant development or parent support alone.
This review has compared early developmental interventions versus standard follow‐up; however, details of standard follow‐up were not always reported. Many of the studies that reported details of follow‐up indicated that infants and families still had access to developmental services such as physiotherapy and social services. Although evidence for some of these services is limited (Wang 2006), it could be considered unethical to prevent access to them. Services received by the standard follow‐up group may improve outcomes, making a treatment effect more difficult to detect. For example, the standard follow‐up group in four studies (Goodman 1985; Resnick 1988; Nelson 2001; Ohgi 2004) received physiotherapy in accordance with institutional policies, which may have influenced motor outcomes of the standard follow‐up group. Contamination of control and intervention groups with additional treatments or other therapies is also problematic, as families of preterm infants may seek additional treatment for a child who is perceived to be 'at risk' for developmental difficulties. None of the included studies reported the 'dosage' of other interventions received by the standard follow‐up group, and none performed analyses in relation to services accessed by the follow‐up group.
The extent of intervention received by infants and families in treatment groups varied greatly between studies (from four to 336 sessions) and within studies. The relationship between intervention dosage and compliance with the intervention is important. Compliance may be estimated by attendance at designated visits or by therapists' recordings of impressions of compliance. I.H.D.P. 1990 reported that higher levels of participation were related to better outcomes on the MDI and higher IQ scores at 24 and 36 months. In the study by Cameron 2005, a better motor outcome was reported at four months for families with good compliance. However, subjective measurement of compliance by study investigators may be biased, and more objective measurements would be optimal. Dusing 2015 used parent recording and questionnaires to record dosage and to monitor compliance, and decided a priori that infants who received less than 70% of the prescribed intervention would be dropped from the study. Study authors reported that one infant was excluded from analysis on the basis of this premise. Although this approach may provide a more objective analysis of the effects of an intervention, it may create bias by increasing attrition to unacceptable levels.
A wide variety of measurement tools were used in these studies, restricting the ability of review authors to pool data. Fewer measurement tools were utilised to assess cognitive development than were used to assess motor development; this made it possible for review authors to pool cognitive data for meta‐analysis at different ages. Effects of early intervention on motor development could be subjected to meta‐analysis at infancy only when the Bayley (Edition I, II or III) or Griffiths Locomotor Subscale was used. Bayley and Griffiths provide broad measures of motor development and do not specifically evaluate minor motor problems, which are common among preterm infants. These studies might have no effect on motor outcomes, or measures used may not have been sensitive enough to allow detection of effects of interventions on motor problems. Other motor measurement tools that assess movement quality and motor performance in greater detail, such as the Alberta Infant Motor Scale (AIMS), the Test of Infant Motor Performance (TIMP) and Movement ABC, were used by individual studies; however, pooling these data for meta‐analysis would not have been appropriate. The diversity of motor assessment tools and lack of data at older ages limit the ability of review authors to compare results between studies. The meta‐analysis of long‐term effects of early developmental interventions on motor and cognitive outcomes was limited not only by the small number of studies, but by the low rates of follow‐up reported.
The meta‐analysis in this review sought to examine motor and cognitive outcomes using standardised assessments. Since the 2000s, a shift has occurred in how disability is measured. Instead of measurement via a medical framework, disability is measured on the basis of functional outcomes, activity limitations and participation restrictions as part of a social and environmental framework (Simeonsson 2003). The World Health Organization now uses the International Classification of Functioning, Disability and Health, rather than the old model of International Classification of Impairments, Disabilities and Handicaps (WHO 2001). Early intervention may not be able to change the physical outcomes of a motor disorder such as cerebral palsy (CP); however, it may change how affected individuals function and participate in society. Intervention may affect motor outcomes in a functional way. For example, the infant may be able to play outside on uneven surfaces, whereas without intervention the child may be restricted to indoor activities. Outcome measures such as the Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID‐PDI), Bayley‐III and the Griffiths Locomotor Subscale measure global motor development in a controlled environment. The controlled environment in which these assessments occur is often a quiet room set up to allow the infant or the child to perform in the best way possible. The skill level achieved in this setting may not be achievable in another setting, so it is important that skills are assessed away from the testing situation and in children's own specific environments. This review has included only traditional outcome measures of motor and cognitive outcomes; however, as functional measures become more widely used, review authors must consider their inclusion. Furthermore, early developmental interventions may affect other areas of development, seen in improvements in behaviour, or parents themselves, seen in decreased levels of anxiety and depression; this has been demonstrated by several studies (Kaaresen 2006; Koldewijn 2009; Spittle 2009).
The methodological quality of included studies was variable, and only eight randomised controlled trials reported adequate concealment allocation and greater than 85% follow‐up. Sensitivity analysis was performed to assess effects of study quality on cognitive and motor outcomes. When only higher‐quality studies were included in the meta‐analysis, an intervention effect on cognitive outcomes was evident at infancy and at preschool age. However, no significant effects on motor outcomes were noted. Trials of developmental interventions have had some limitations. It is not feasible to mask the person implementing the intervention nor the recipient of the intervention (in this case, mother and baby) unless a comparison group providing an alternative intervention is used instead of a control group. Only one study provided a comparison treatment instead of a non‐treatment control (Melnyk 2001); however, this was not a randomised trial. When interventions are delivered, whether they focus on infant development or on the parent‐infant relationship, the component of parental support may affect outcomes. APIP 1998 was the only study to control for parent support by including three groups: one that received an infant development programme, one that received parent support only and a control group. Methodological quality assessments used in this review did not take sample size into account. Sample size is important the outcomes of individual programmes are assessed, as some studies may not have had enough power to demonstrate differences between groups. I.H.D.P. 1990 was by far the largest, including 985 infants, followed by APIP 1998, with 308 infants. Significant results reported by I.H.D.P. 1990 and a large sample size influenced overall results of the meta‐analysis. I.H.D.P. 1990 is very different from other early developmental programmes in terms of frequency and duration of interventions; this should be considered when results of this review are interpreted. Spittle 2009 involved all of the authors of this systematic review, and although we have adopted Cochrane review methods in conducting this review, readers must consider that we may be biased.
This systematic review has not investigated which aspect of early developmental interventions has a greater effect on outcome ‐ optimal duration, timing, frequency or focus of the intervention. Further research is needed to identify components of intervention that are most effective on the basis of cost and benefit. I.H.D.P. 1990 was estimated to cost USD15,146 per year per child. Investigators suggest that this value could be reduced to USD8806 if centres were located in the community, and if teacher‐child ratios were decreased. However, this remains a costly intervention as compared with interventions provided by Nurcombe 1984, which reported better long‐term outcomes and was less costly to implement, as only 11 sessions were provided over four months versus an intensive programme provided over three years for infants in the intervention group in I.H.D.P. 1990.
Authors' conclusions
Implications for practice.
Meta‐analysis demonstrated that early developmental interventions post hospital discharge for preterm infants have a significant impact on cognitive and motor development at infancy and preschool age, and have a small effect on motor development at infancy. At school age, only five studies have investigated the long‐term effects of intervention on cognitive outcomes, and three have investigated these effects on motor outcomes, with none demonstrating substantial differences in long‐term outcomes. Interventions that focus on both the parent‐infant relationship and infant development have the greatest impact on cognitive development over the short to medium term. Heterogeneity between early developmental intervention programmes with regards to content, focus and intensity limits the conclusions that can be drawn in this review.
Implications for research.
Additional high‐quality randomised controlled trials are needed to identify effective components of successful early developmental interventions for preterm infants. Greater selectivity of high‐risk populations may reveal those infants who may benefit the most from an intervention. Targeting an intervention to address the needs of the infant and family more specifically may reduce costs and increase effectiveness. Further research is required to determine effects of intervention programmes on long‐term motor outcomes, as only limited conclusions could be made from this review. Long‐term follow‐up high‐quality randomised controlled trials of interventions focusing on both motor and cognitive outcomes for preterm infants are needed. Measurement tools must be sensitive enough to detect changes in motor performance and to identify minor neurological problems. This review has not investigated effects on behaviour, parental outcomes (such as depression and anxiety), function, activity levels or participation, all of which may be influenced by early developmental intervention programmes.
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2015 | New citation required but conclusions have not changed | This review identified an additional 4 trials, along with 3 additional long‐term outcomes studies for trials that were previously included. A total of 25 trials are included in this review |
| 21 August 2015 | New search has been performed | This updates the review, "Early developmental intervention programs post‐hospital discharge to prevent motor and cognitive impairments in preterm infants", published in the Cochrane Database of Systematic Reviews (Spittle 2007) |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 1 October 2012 | New citation required and conclusions have changed | This updated review identified an additional 6 studies, resulting in inclusion of a total of 21 studies in this review |
| 17 September 2008 | Amended | Converted to new review format |
| 1 December 2006 | New citation required and conclusions have changed | Substantive amendments made |
Acknowledgements
Professor Jenny Keating, School of Primary Health Care, Monash University, for providing support and guidance at the commencement of this review. Dr. Klebenov for providing 12‐ and 24‐month motor data from I.H.D.P. 1990. Dr. Ohgi for clarifying follow‐up rates of participants.
Data and analyses
Comparison 1. Early developmental intervention versus standard follow‐up (all studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infancy ‐ DQ (Bayley and Griffiths) | 16 | 2372 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.16, 0.47] |
| 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy, Bayley) | 8 | 1436 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.32, 0.54] |
| 3 Cognitive outcome at school age ‐ IQ (WISC, Kaufmann) | 5 | 1372 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.08, 0.43] |
| 4 Motor outcome at infancy (BSID PDI, Griffiths Locomotor) | 12 | 1895 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.01, 0.19] |
| 5 Motor outcome at preschool age | 3 | 264 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.16, 0.32] |
| 6 Motor outcome at school age (Griffiths Locomotor) | 2 | 185 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.47, 0.11] |
| 7 Motor outcome at school age (low score on Movement ABC) | 2 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 8 Rate of cerebral palsy | 7 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.52, 1.27] |
Comparison 2. Early developmental intervention versus standard follow‐up (subgroup analysis: gestational age).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infant age DQ (BSID‐MDI, Griffiths GCI) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 32 to < 37 weeks | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 28 to < 32 weeks | 1 | 153 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.25, 0.43] |
| 1.3 < 28 weeks | 1 | 87 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.06, 0.83] |
2.1. Analysis.
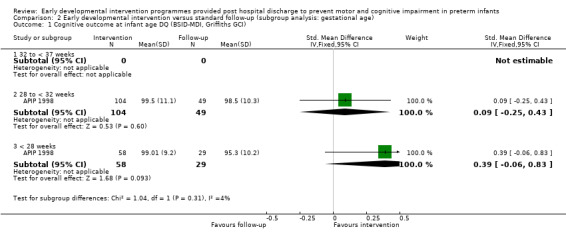
Comparison 2 Early developmental intervention versus standard follow‐up (subgroup analysis: gestational age), Outcome 1 Cognitive outcome at infant age DQ (BSID‐MDI, Griffiths GCI).
Comparison 3. Early developmental intervention versus standard follow‐up (subgroup analysis: birth weight).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infant age ‐ DQ (BSID‐MDI, Griffiths GCI) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1500 to < 2500 grams birth weight | 2 | 381 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.38, 0.80] |
| 1.2 1000 to < 1500 grams birth weight | 1 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.95, 0.33] |
| 1.3 < 1000 grams birth weight | 2 | 137 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.22, 0.47] |
| 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 1500 to < 2500 grams birth weight | 1 | 328 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.47, 0.93] |
| 2.2 1000 to < 1500 grams birth weight | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 < 1000 grams birth weight | 1 | 100 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.02, 0.81] |
Comparison 4. Early developmental intervention versus standard follow‐up (subgroup analysis: brain injury).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infant age ‐ DQ (BSID‐MDI, Griffiths GCI) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Absence of PVL/IVH | 1 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.01, 0.70] |
| 1.2 Presence of PVL/IVH | 2 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.12, 1.13] |
| 2 Motor outcome at infant age (BSID‐PDI, Griffiths Locomotor) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Absence of PVL/IVH | 1 | 143 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.24, 0.47] |
| 2.2 Presence of PVL/IVH | 2 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐0.15, 1.10] |
4.1. Analysis.
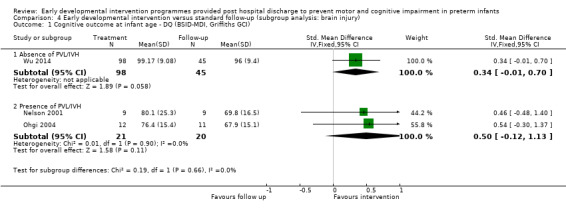
Comparison 4 Early developmental intervention versus standard follow‐up (subgroup analysis: brain injury), Outcome 1 Cognitive outcome at infant age ‐ DQ (BSID‐MDI, Griffiths GCI).
4.2. Analysis.
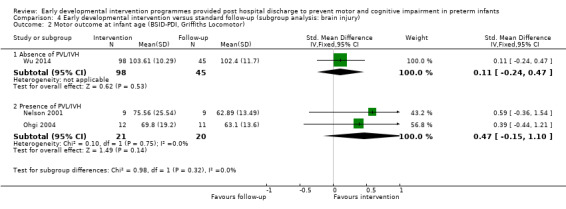
Comparison 4 Early developmental intervention versus standard follow‐up (subgroup analysis: brain injury), Outcome 2 Motor outcome at infant age (BSID‐PDI, Griffiths Locomotor).
Comparison 5. Early developmental intervention versus standard follow‐up (subgroup analysis: commencement of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infant age ‐ DQ (BSID‐MDI, Griffiths GCI) | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Inpatient | 10 | 931 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 1.2 Post hospital discharge | 6 | 1515 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.07, 0.63] |
| 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy) | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Inpatient | 3 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.26, 0.77] |
| 2.2 Post hospital discharge | 5 | 1192 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.29, 0.53] |
| 3 Cognitive outcome at school age ‐ IQ (WISC, Kaufmann) | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Inpatient | 2 | 186 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.07, 1.27] |
| 3.2 Post hospital discharge | 2 | 1056 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.15, 0.10] |
| 4 Motor outcome at infant age (BSID‐PDI, Griffiths Locomotor) | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Inpatient | 8 | 751 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.05, 0.34] |
| 4.2 Post hospital discharge | 4 | 1144 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.05, 0.18] |
| 5 Motor outcome at preschool age | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Inpatient | 1 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.28, 0.40] |
| 5.2 Post hospital discharge | 2 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.25, 0.43] |
| 6 Motor outcome at school age (Griffiths Locomotor) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Inpatient | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Post hospital discharge | 1 | 49 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.91, 0.23] |
| 7 Motor outcome at school age (low score on Movement ABC) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Inpatient | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Post hospital discharge | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 8 Rate of cerebral palsy | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Inpatient | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.93] |
| 8.2 Post hospital discharge | 4 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.43, 1.33] |
Comparison 6. Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infancy ‐ DQ (BSID‐MDI, Griffiths GCI) | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Parent‐infant relationship | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [0.11, 1.36] |
| 1.2 Infant development | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.16, 1.14] |
| 1.3 Parent‐infant relationship and Infant development | 12 | 1906 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.41] |
| 2 Cognitive outcome at preschool age ‐ IQ (Stanford‐Binet, McCarthy) | 7 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Parent‐infant relationship | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Infant development | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Parent‐infant relationship and Infant development | 7 | 1391 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.33, 0.55] |
| 3 Cognitive outcome at school age ‐ IQ (WISC, Kaufmann) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Parent‐infant relationship | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Infant development | 1 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.47, 0.23] |
| 3.3 Parent‐infant relationship and Infant development | 4 | 1192 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.02, 0.66] |
| 4 Motor outcome at infancy (BSID‐PDI, Griffiths Locomotor) | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Parent‐infant relationship | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Infant development | 2 | 157 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.05, 0.58] |
| 4.3 Parent‐infant relationship and Infant development | 10 | 1738 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.01, 0.19] |
| 5 Motor outcome at preschool age (Bayley and Griffiths Locomotor) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Parent‐infant relationship | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Infant development | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Parent‐infant relationship and Infant development | 3 | 264 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.16, 0.32] |
| 6 Motor outcome at school age (Griffiths Locomotor) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐5.31 [‐13.74, 3.12] |
| 6.1 Parent‐infant relationship | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Infant development | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐5.31 [‐13.74, 3.12] |
| 6.3 Parent‐infant relationship and Infant development | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Rate of cerebral palsy | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Parent‐infant relationship | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Parent‐infant relationship and Infant development | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.17] |
| 7.3 Infant development | 3 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.56, 2.46] |
| 7.4 Parent support | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.5. Analysis.
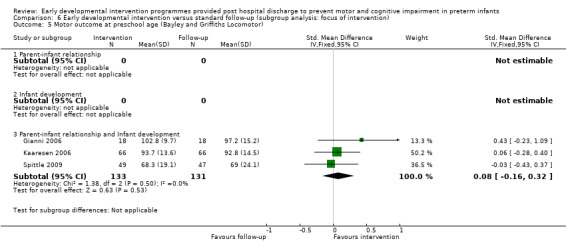
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 5 Motor outcome at preschool age (Bayley and Griffiths Locomotor).
6.6. Analysis.
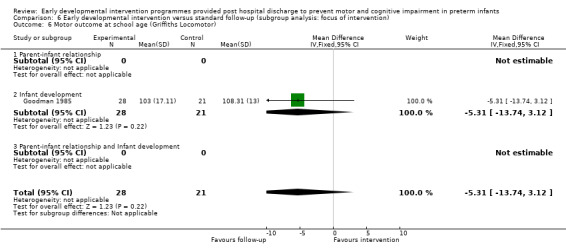
Comparison 6 Early developmental intervention versus standard follow‐up (subgroup analysis: focus of intervention), Outcome 6 Motor outcome at school age (Griffiths Locomotor).
Comparison 7. Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive outcome at infant age (BSID‐MDI, Griffiths GCI: DQ) | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Higher‐quality studies | 8 | 1770 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.21, 0.46] |
| 1.2 Lower‐quality studies | 7 | 527 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.06, 0.77] |
| 2 Cognitive outcome at preschool age (Stanford‐Binet, McCarthy: IQ) | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Higher‐quality studies | 5 | 1255 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.44 [0.32, 0.55] |
| 2.2 Lower‐quality studies | 3 | 181 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.05, 0.65] |
| 3 Cognitive outcome at school age (WISC, Kaufmann: IQ) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Higher‐quality studies | 5 | 1372 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.08, 0.43] |
| 3.2 Lower‐quality studies | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Motor outcome at infancy (BSID‐PDI, Griffiths Locomotor: DQ) | 10 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Higher‐quality studies | 6 | 1367 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.04, 0.17] |
| 4.2 Lower‐quality studies | 4 | 378 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [0.02, 0.42] |
| 5 Motor outcome at preschool age (Bayley and Griffiths Locomotor) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Higher‐quality studies | 2 | 228 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.24, 0.28] |
| 5.2 Lower‐quality studies | 1 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.23, 1.09] |
| 6 Motor outcome at school age (low score on Movement ABC) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Higher‐quality studies | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.78, 1.38] |
| 6.2 Lower‐quality studies | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Rate of cerebral palsy | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Higher‐quality studies | 5 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.41] |
| 7.2 Lower‐quality studies | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.20, 1.87] |
| 8 Motor outcome at school age (Griffiths Locomotor) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Higher‐quality studies | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Lower‐quality studies | 1 | 49 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.91, 0.23] |
7.8. Analysis.
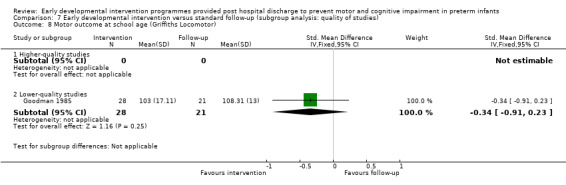
Comparison 7 Early developmental intervention versus standard follow‐up (subgroup analysis: quality of studies), Outcome 8 Motor outcome at school age (Griffiths Locomotor).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
APIP 1998.
| Methods | Multi‐centre randomised controlled trial comparing 2 interventions vs standard follow‐up. All eligible infants born over a 2.5‐year period were randomly assigned to 1 of the 3 groups at 7 to 10 days after birth (Portage, Parent Advisor, Follow‐up). The Portage programme, a home visiting educational service for children with additional support needs and their families, offers practical help and ideas to encourage a child's interests and make learning fun for the entire family. A second intervention group was used to control for the parent support given with the Portage group. The parent support group received supportive counselling for parents but no advice on infant development | |
| Participants | 309 infants
Inclusion criteria: GA < 33 weeks Exclusion criteria: English was not the first language, patient did not live within the study area Characteristics: mean GAs for the 2 treatment groups (Portage and Parent Advisor) and the standard follow‐up group: 31, 30 and 31 weeks, respectively |
|
| Interventions | 2 intervention programmes used ‐ 'Portage' and 'Parent Advisor' ‐ to control for the support aspect of Portage Portage group (N = 111): infant development and parent support Parent advisor group (N = 99): parent support For data analysis, Portage and Parent Advisor groups were included together as the intervention group Standard follow‐up group (N = 99): Details of standard follow‐up for the control group were not reported | |
| Outcomes | Cognitive
Motor
|
|
| Notes | Control group had a higher percentage of mothers who were educated beyond 16 years of age, were in non‐manual occupations and had the use of a car compared with both intervention groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation arranged in blocks of 6 with random number table for each stratum |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Consent to participate in the study was obtained post randomisation to evaluate acceptability and impact of interventions in population terms. To account for potential bias, families of infants who did not consent to the intervention were invited to participate in outcome assessments, and outcome data at 2 years (but not at 5 years) and results are reported on an intention‐to‐treat basis |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Performance bias due to knowledge of allocated interventions by personnel delivering interventions during the study. Unclear whether participants were aware of group allocation, and whether personnel or participants were biased towards 1 intervention as superior to the other intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments performed by assessors masked to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk at 24 months (94%) but high risk at 5 years (66%) for cognitive outcomes |
| Selective reporting (reporting bias) | Low risk | |
Bao 1999.
| Methods | Multi‐centre quasi‐randomised controlled trial of an intervention package that focused on infant development vs standard follow‐up. The programme aimed to enhance motor, cognitive and speech development, and to improve social behaviours by assessing development of the infant, then instructing parents on how to carry out a home programme until the next examination. Home programme included exercise and suggestion of toys, books and pictorials appropriate to the child's age and was delivered by a paediatrician | |
| Participants | 103 infants
Inclusion criteria: GA 28 to 37 weeks Exclusion criteria: not reported Characteristics: mean GA for intervention and standard follow‐up groups: 33.9 (SD 1.8) weeks and 34.2 (SD 2.1) weeks, respectively |
|
| Interventions | Intervention group (N = 52): infant development Standard follow‐up group (N = 51): details not described | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomly assigned according to order of admission to hospital ‐ odd numbers to intervention and even numbers to conventional care |
| Allocation concealment (selection bias) | High risk | Personnel may have been aware of group allocation as a result of the randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither personnel nor participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated whether any infants were withdrawn from the study; 100% follow‐up apparent |
| Selective reporting (reporting bias) | High risk | Not clear whether participants were withdrawn from the study; few details of inclusion criteria and few population characteristics reported |
Barrera 1986.
| Methods | Multi‐centre randomised controlled trial comparing 2 types of intervention programmes vs standard follow‐up. Parent‐infant intervention aimed to improve the quality of the interaction between parent and child by enhancing parents' observational skills and teaching them to be mutually responsive to their infant. Developmental programme aimed to improve infants' cognition, communication, gross and fine motor development, socioemotional skills and self help skills. Parents worked with therapists to plan and implement developmental activities | |
| Participants | 80 infants
Inclusion criteria: BW < 2000 grams or GA ≤ 37 weeks and discharged home from hospital with good prognosis for survival Exclusion criteria: life‐threatening illnesses, did not live within the study area Characteristics: mean GA for all groups: 33 weeks |
|
| Interventions | 2 intervention programmes were compared with standard follow‐up Parent‐infant intervention (N = 22*): parent‐infant relationship Developmental programme (N = 16*): infant development For data analysis, the 2 parent‐infant and developmental groups were included together as the intervention group Standard follow‐up (N = 21*): home visits for assessment purposes only. During these visits, the examiner answered questions from parents related to their child's development, reading material or community resources | |
| Outcomes | Cognitive
Motor
|
|
| Notes | *21 infants did not complete the study. The number of infants in each group listed includes infants who completed the 1‐year programme, as the number of infants randomly assigned to each group at the beginning of the study was not stated. It is reported that no differences between groups in reasons for withdrawal from the study were noted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were block randomly assigned according to sex, BW, SES and antenatal/postnatal complications |
| Allocation concealment (selection bias) | Unclear risk | Unclear what measures were taken to ensure concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 2 intervention groups were included; therefore, participants may have been blinded to group allocation. However, it is not clear whether personnel or participants would have had a bias towards the different intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: high risk at 4, 8, 12 and 16 months (73%) and at 5 years (56%) |
| Selective reporting (reporting bias) | High risk | Number of infants in each group reported only for infants who completed the programme |
Cameron 2005.
| Methods | Single‐centre randomised controlled trial to investigate the effects of a physiotherapy early intervention programme vs standard follow‐up | |
| Participants | 72 infants
Inclusion criteria: BW < 1500 grams and GA < 32 weeks Exclusion criteria: requirement for oxygen at 4 months' corrected age, severe hydrocephalus requiring a shunt, demonstrated signs of drug withdrawal, history of social problems Characteristics: mean GAs for treatment and standard follow‐up groups: 28.7 (SD 2.4) and 29.6 (SD 2.0) weeks, respectively |
|
| Interventions | Intervention group (N = 34): infant development Standard follow‐up group (N = 38): no physiotherapy intervention nor other placebo intervention given | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Toss of a coin |
| Allocation concealment (selection bias) | Low risk | Toss of a coin |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 4 months (83%) |
Dusing 2015.
| Methods | Single‐centre randomised controlled trial to determine the feasibility of completing a clinical trial of Supporting Play Exploration and Early Development Intervention (SPEEDI) for preterm infants. This early and intense intervention is blended with family support to assist in the transition from hospital to home. Intervention has 2 phases: phase 1 in the NICU from 35 weeks' GA to term age; phase 2 in the community from discharge until 3 months of age. Purpose of phase 1 was to provide opportunities for the infant to experience variable movements and social interactions. Phase 2 aimed to assist parents in establishing a routine for developmentally appropriate play after discharge and to teach them to implement these interventions in the home | |
| Participants | 10 infants Inclusion criteria: GA < 34 weeks, medically stable, off ventilator support, thermoregulation by 35 weeks' GA, living within 30 minutes of the hospital. For multiple births, 1 infant was randomly selected to participate Exclusion criteria: genetic syndrome, musculoskeletal deformity Characteristics: mean GA (weeks) and BW (grams) for SPEEDI group: 31 (95% CI 28 to 33) and 1375 (95% CI 920 to 1835); usual care group: 27 (95% CI 25 to 30) and 785 (95% CI 690 to 1600) |
|
| Interventions | Intervention group
Usual care group: This group received standard care in the NICU and in the community. This is not described further. Parents reported on other EI services received during the period of the project |
|
| Outcomes | Cognitive
Motor
|
|
| Notes | Numbers are small but 6‐month data are used in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants randomly assigned to intervention or control, but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear what measures were taken to ensure concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Some infants also received other EI during the programme |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor and physical therapist blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 4 months (70%), 6 months (70%) |
| Selective reporting (reporting bias) | Low risk | Study authors report on numbers of infants not available for assessment at various time points |
Field 1980.
| Methods | Randomised controlled study to assess effects of combined risks of being born preterm to a teenage mother and to evaluate effects of an intervention programme. Aim of the intervention was to educate the mother regarding developmental milestones and child‐rearing practices, to teach the mother age‐appropriate stimulation to facilitate cognitive and social interaction and communication skills and to facilitate mother‐infant relationships | |
| Participants | 60 infants
Inclusion criteria: BW < 2500 grams and GA < 37 weeks. All infants were born to African American teenage mothers with low sociodemographic status Exclusion criteria: serious neonatal complications that would require long periods of intensive care and early separation Characteristics: mean GAs of intervention and standard follow‐up groups: 35.5 and 35.3 weeks, respectively |
|
| Interventions | Intervention group (N = 30): infant development and parent‐infant relationship Standard follow‐up (N = 30): no details on standard follow‐up given | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear (reported that mothers volunteered for intervention, then were randomly assigned to control or intervention) |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 8 months (85.5%) |
Gianni 2006.
| Methods | Pilot randomised controlled trial of an early post‐discharge developmental intervention on neurodevelopmental outcomes. Intervention group was seen by a psychologist and a psychometrician, twice a month. Psychologist's intervention was related to supporting mental health issues associated with preterm birth; psychometrician's intervention involved the infant and mother‐child interaction | |
| Participants | 38 infants
Inclusion criteria: BW < 1250 grams, singleton, infant fed formula Exclusion criteria: congenital heart disease, chromosomal abnormality, brain abnormality or combination seen on MRI; death during inpatient stay Characteristics: mean GAs of intervention and standard follow‐up groups: 28.3 (SD 2.8) and 27.5 (SD 1.8) weeks, respectively |
|
| Interventions | Intervention group (N = 18): infant development and parent‐infant relationship Standard follow‐up (N = 18): no intervention. Standard follow‐up included periodic paediatric visits at 40 weeks and 3, 6, 12, 24 and 36 months of age | |
| Outcomes | Griffiths Mental Developmental Scale (DS) and related subscales at 12, 24 and 36 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear: infants randomly assigned into 2 groups matched for GA; no other information given |
| Allocation concealment (selection bias) | Unclear risk | Unclear as randomisation methods not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 100% at 36 months, no information for 12 and 24 months |
| Selective reporting (reporting bias) | High risk | Unclear how many infants were assessed for eligibility vs consented to the study and whether any withdrawals occurred |
Goodman 1985.
| Methods | Quasi‐randomised controlled trial to investigate effects of early neurodevelopmental therapy vs standard follow‐up. Intervention was delivered by a physiotherapist, and parents were shown exercises for use at home, where parents were expected to carry out the programme on a daily basis | |
| Participants | 107 infants*
Inclusion criteria: BW < 1700 grams or GA < 34 weeks Exclusion criteria: Infants considered neurologically impaired were excluded from the study, as all were given intervention Characteristics: mean GAs of intervention and standard follow‐up groups: 30.9 (SD 1.9) and 31 (SD 1.8) weeks, respectively |
|
| Interventions | Intervention group (N = 40): infant development
Standard follow‐up group (N = 40): Infants in both groups (intervention and standard follow‐up) attended a follow‐up clinic at 6 weeks and at 3, 6, 9 and 12 months' corrected age. This clinic was staffed by a neonatologist, physiotherapists, a speech and hearing therapist, ophthalmologists, public health nurses and a social worker It is not clear how much 'intervention' was provided to infants in the control group during these visits |
|
| Outcomes | Cognitive
Motor
|
|
| Notes | *Prior to commencing study, study authors stated that intention was to study 40 infants in each group. To allow for attrition, 107 infants were enrolled into the study at 3 months However, the formal study ceased when 80 infants were followed up to 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants assessed as 'normal' or 'at‐risk' on the basis of a neurodevelopmental score and then alternatively assigned to intervention or control groups |
| Allocation concealment (selection bias) | High risk | Unclear whether study personnel would be aware of group allocation given that children were then alternatively assigned to intervention or control groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Before beginning the study, study authors stated that their intention was to study 40 infants in the intervention and follow‐up groups. However, they recruited 107 children to allow for withdrawal of infants from the study. They ceased follow‐up when 80 children were assessed and did not report data for remaining 27 children |
I.H.D.P. 1990.
| Methods | Large multi‐centre randomised trial that investigated the effects of early intervention vs standard follow‐up. To minimise the cost of the study, one‐third of participants were randomly assigned to intervention (N = 377) and two‐thirds were randomly assigned to standard follow‐up (N = 608). Intervention group had home visits, attended a child development centre and attended parent group meetings. Home visits were made weekly for the first year and biweekly for the second and third years. Home visits emphasised cognitive, linguistic and social development via a programme of games for the parent to use with the child, and aimed to help parents manage self identified problems. Children in the intervention group attended child development centres 5 days per week, from 12 to 36 months' corrected age | |
| Participants | 985 infants
Inclusion criteria: BW ≤ 2500 grams or GA < 37 weeks Exclusion criteria: congenital abnormalities, genetic disorders, still hospitalised, too sick to participate in the programme at term Characteristics: mean GA for intervention and standard follow‐up groups: 33 weeks |
|
| Interventions | Intervention group (N = 377): infant development and parent‐infant relationship Standard follow‐up group (N = 608): Both groups received medical, developmental and social assessments, with referral to other services as indicated | |
| Outcomes | Cognitive
Motor
|
|
| Notes | Additional data (means and SDs for 12 and 24 months for MDI and PDI) were obtained from study authors for meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised adaptive randomisation method to ensure 2:1 ratio of standard follow‐up to intervention; stratified by study site and BW |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: low risk at 12 months = 90%, 24 months = 89%, 36 months = 93%, 5 years = 82% and 8 years = 89%; high risk at 18 years = 65% |
| Selective reporting (reporting bias) | Low risk | |
Johnson 2009.
| Methods | Cluster‐randomised controlled trial with a cross‐over design including 6 neonatal units across the UK with intervention commenced from the first weeks after birth. Intervention programme consisted of weekly 1‐hour sessions, beginning in hospital, up to a maximum of 6 sessions post discharge. Intervention programme included strategies to enhance parent‐infant interaction, to facilitate attachment, to sensitise parents to their baby's cues and to provide education about developmental care | |
| Participants | 233 infants from 6 neonatal units
Inclusion criteria: GA < 32 weeks Exclusion criteria: illness incompatible with life, residing outside the study catchment area Characteristics: mean GAs for intervention and standard follow‐up groups: 28.5 and 29.0 weeks, respectively |
|
| Interventions | Intervention group (N = 112) received the 'Parent Baby Interaction Program' aimed at parent‐infant relationship and infant development Standard care group (N = 121) received standard care |
|
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster randomisation with cross‐over design |
| Allocation concealment (selection bias) | High risk | Research nurse and parents not blinded to group allocation before recruitment because of cross‐over study design |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 84% follow‐up (195/233 infants assessed at 24 months) |
Kaaresen 2006.
| Methods | Randomised controlled trial of a modified version of the Mother Infant Transaction Program originally described by Nurcombe 1984 vs standard care that included a physiotherapist and doctor consultation at discharge. Intervention included an initial briefing session, followed by daily 1‐hour sessions with both parents and their infants on 7 consecutive days, starting 1 week before discharge, and 4 home visits at 3, 14, 30 and 90 days after discharge. Programme was implemented by a team of nurses and included education on behavioural cues, parent‐infant interaction and appropriate stimulation of the infant | |
| Participants | 146 infants from 6 neonatal units
Inclusion criteria: BW < 2000 grams Exclusion criteria: congenital abnormalities, non‐Norwegian speakers, triplets Characteristics: mean GAs for intervention and standard follow‐up groups: 30.3 (SD 3.) and 30.1 (SD 3.5) weeks, respectively |
|
| Interventions | Intervention group (N = 72): infant development and parent‐infant relationship Standard follow‐up (N = 74): included examination and offer of training in infant massage by physiotherapist, clinical examination of hearing and vision and discharge consultation with doctor | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment arranged in blocks using computer‐generated random numbers and stratified for gestation (< 28 weeks and ≥ 28 weeks) |
| Allocation concealment (selection bias) | Low risk | Allocation performed using opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 93% follow‐up at 2 years, 92% follow‐up at 3 years, 90% follow‐up at 5 years |
Koldewijn 2009.
| Methods | Multi‐centre randomised controlled trial of effects of the 'Infant Behavioural Assessment and Intervention Program' (IBAIP) vs standard follow‐up. Intervention group received 1‐hour sessions, with the first session just before discharge, followed by 6 to 8 home visits up to 6 months' corrected age. Intervention was part of a commercially available training package, which aims to enhance parents' ability to read and respond to their infants' cues throughout everyday life | |
| Participants | 176 infants Inclusion criteria: BW < 1500 grams or GA < 32 weeks, or both (across 7 cites in Amsterdam, the Netherlands) Exclusion criteria: congenital abnormality, non‐Dutch speakers, mothers with history of drug use or severe physical or mental illness or participating in another post‐discharge trial Characteristics: mean GAs for intervention and standard follow‐up groups: 29.6 (SD 2.2) and 30.0 (SD 2.2) weeks, respectively |
|
| Interventions | Intervention group (N = 86): infant development and parent‐infant relationship Standard follow‐up (N = 90): included regular outpatient visits with a paediatrician and neurobehavioural and developmental assessment at term, 3 months and 6 months | |
| Outcomes | Cognitive
Motor
|
|
| Notes | *Data used in meta‐analysis have bend adjusted for gestation, sex, ultrasound, oxygen > 28 days and maternal education | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment computer generated, stratified for GA (< 30 weeks and ≥ 30 weeks) and recruitment site |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Children with congenital abnormalities, non‐Dutch speakers and mothers with history of drug use or severe physical or mental illness or participating in another post‐discharge trial were excluded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 44 months = 86% (low risk), 5 years = 77% (high risk) |
Kyno 2012.
| Methods | Single‐centre randomised controlled trial of the "Mother‐Infant Transaction Program" vs standard care for infants born at 30 to 36 weeks' GA. Intervention commenced 1 week before discharge and involved 7 sessions during this week. Infants were seen 4 times at home at 7, 14, 30 and 90 days after discharge by a nurse trained in the programme | |
| Participants | 118 infants Inclusion criteria: GA ≥ 30 to < 36 weeks, admitted to NICU at Oslo Unviersity Hospital. Mothers had to speak, write and read in Norwegian and had to have no history of psychiatric disorders nor of drug abuse Exclusion criteria: congenital abnormality, hearing loss, chromosomal disorder with expected hospitalisation > 8 days Characteristics: mean GAs for intervention and standard follow‐up groups: 33.6 (SD 1.3) and 33.1 (SD 1.4) weeks, respectively |
|
| Interventions | Intervention group (N = 61): infant development and parent‐infant relationship Standard follow‐up (N =57 ): not described | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers used to allocate groups. Twins randomly assigned to the same group |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Inclusion criteria: mothers of Norwegian speakers, without history of psychiatric illness or drug abuse |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible to blind intervention personnel and parents |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researchers non‐blinded. However, reliability between 10 test situations from each group randomly selected to be filmed, then scored by a blinded tester with low disagreement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 52% at 36 months |
Lekskulchai 2001.
| Methods | Randomised controlled trial to evaluate the effects of a physiotherapy motor developmental programme in improving motor performance for preterm infants vs standard follow‐up. Motor developmental programme began at 40 weeks' postmenstrual age, with a further 3 visits at 1, 2 and 3 months' corrected age. Physiotherapist instructed primary carer on how to perform 3 activities with the infant during each session, which were to be carried out at home. Before the next visit, principal researcher evaluated previous month's programme with the carer through an interview and demonstration of activities by the carer | |
| Participants | 84 infants
Inclusion criteria: BW and GA < 37 weeks, considered to be 'at‐risk' of adverse neurological sequelae, assessed with TIMP at 40 weeks' post‐conceptional age Exclusion criteria: congenital abnormality, genetic disorder, surgery or serious illness including hydrocephalus and PVH (grade III) excluded before randomisation Characteristics: mean GA for intervention and standard follow‐up groups: 31.9 (SD 2.4) and 32.3 (SD 2.2) weeks, respectively |
|
| Interventions | Intervention group (N = 43): infant development Standard follow‐up (N = 41): all families (intervention and standard follow‐up) assessed (using the TIMP) by a research assistant at 1, 2, 3 and 4 months and were able to discuss concerns with principal researcher | |
| Outcomes | Cognitive
Motor
|
|
| Notes | Unable to use data in meta‐analysis as outcome measure (TIMP) was not appropriate for pooling with other outcome measures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Intervention or control slip taken blindly from a container |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Infants were classified as at risk for developmental delay by the Test of Infant Motor Performance provided at the beginning of the study. Congenital abnormalities, genetic disorders, surgery or serious illness including hydrocephalus and PVH (grade III) were excluded before random assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 1, 2, 3 and 4 months = 86% |
Melnyk 2001.
| Methods | Quasi‐randomised pilot project comparing the 'creating opportunities for parent empowerment' (COPE) programme vs placebo intervention. The COPE programme was a 4‐phase programme that consisted of audiotaped and written information and workbooks on infant behaviour and parental roles. First 3 sessions occurred 2 to 4 days after admission to hospital, and last session occurred approximately 1 week after discharge. Comparison programme was delivered at the same 4 time points and involved audiotaped and written information about hospital services, routine discharge information and education about immunisations | |
| Participants | 55 infants
Inclusion criteria: BW < 2000 grams and GA < 34 weeks Exclusion criteria: perinatal hypoxia or abnormal ultrasound with no congenital or chromosomal abnormalities nor metabolic disease Characteristics: mean GAs for intervention and standard follow‐up groups: 31.3 (SD 2.2) and 32.0 (SD 1.6) weeks, respectively |
|
| Interventions | Intervention group (N = 26): parent‐infant relationship Standard follow‐up group (N = 29): received placebo intervention that also consisted of audiotaped and written information in relation to hospital services, routine discharge information and education about immunisations | |
| Outcomes | Cognitive
Motor
|
|
| Notes | This was the only study that included a comparison group receiving a placebo intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation with intervention provided in blocks according to the date admitted to hospital |
| Allocation concealment (selection bias) | High risk | Infants randomly assigned according to date admitted to hospital |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Infants with perinatal hypoxia or abnormal ultrasound, congenital or chromosomal abnormalities or metabolic disease excluded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo intervention used; both participants and staff blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 3 and 6 months = 76% |
Nelson 2001.
| Methods | Randomised controlled study to investigate effects of an infant stimulation programme vs standard follow‐up. Intervention group received a multi‐sensory stimulation programme including auditory, tactile, visual and vestibular stimuli in response to infant behavioural and physiological cues. Intervention was provided by a research assistant at the hospital twice daily, 5 days per week until discharge. Mothers were taught the intervention, which they continued to administer at home until infants reached 2 months' corrected age | |
| Participants | 37 infants
Inclusion criteria: BW < 1500 grams and GA 23 to 26 weeks (group 1), born between 23 and 32 weeks, diagnosed with PVL or grade III IVH (group 2) Exclusion criteria: not medically stable, required mechanical ventilation or was not feeding at commencement of the study (intervention commenced while infants were in the NICU), intrauterine growth restriction, chromosome disorders and NEC Characteristics: mean GAs for intervention and control groups, group 1: 25.6 (SD 1.1) and 25.6 (SD 1.5) weeks; group 2: 27.2 (SD 2.9) and 27.3 (SD 2.4) weeks, respectively |
|
| Interventions | Intervention group (N = 21): infant development and parent‐infant relationship Standard follow‐up (N = 16): All infants (intervention and standard follow‐up groups) received developmental care as inpatients, along with a physiotherapy programme post hospital discharge | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Children who were not medically stable, required mechanical ventilation or were not feeding at the beginning of the study (intervention began while infants were in the NICU) or with intrauterine growth restriction, chromosome disorders and NEC were excluded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 12 months = 70% |
Nurcombe 1984.
| Methods | Randomised controlled trial of the 'the Mother‐Infant Transaction Program' (MITP), also known as the Vermont Intervention Program, vs standard follow‐up. Intervention was designed to enhance mother‐infant interaction and infant development by teaching mothers to be more sensitive and responsive to baby's physiological, behavioural and social cues. Intervention consisted of a total of 11 sessions delivered by a trained neonatal intensive care nurse: 7 sessions were conducted in hospital before discharge and 4 at home during the first 3 months following discharge; focused on infant's motor system, state regulation, social interaction, daily care, preparations for home, mutual enjoyment through play and understanding of temperamental patterns | |
| Participants | 78 infants
Inclusion criteria: BW < 2250 grams and GA < 37 weeks Exclusion criteria: congenital abnormality, severe neurological defect, multiple birth, single mother Characteristics: mean GAs for treatment and standard follow‐up groups: 32.3 (SD 2.4) and 31.9 (SD 2.4) weeks, respectively. Significant difference in SES of intervention and standard follow‐up groups despite randomisation |
|
| Interventions | Intervention group (N = 38): infant development and parent‐infant relationship Standard follow‐up (N = 40): no details reported | |
| Outcomes | Cognitive
Motor
|
|
| Notes | Reported data have been adjusted to control for SES of families | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Toss of a coin |
| Allocation concealment (selection bias) | Low risk | Toss of a coin |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Children with congenital abnormalities, severe neurological defects, multiple births and single mothers were excluded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: low risk at 12 months = 95%; however, longer‐term follow‐up not adequate at 24, 36 and 48 months (68%) and at 7 and 9 years (71%) |
Ohgi 2004.
| Methods | Randomised controlled trial to determine effects of an early intervention programme on preterm infants with high risk of cerebral palsy vs standard follow‐up. Intervention group received a behavioural‐based intervention combined with developmental support designed to enhance infant development and parent‐infant relationship. Intervention began in the NICU and lasted until 6 months' corrected age | |
| Participants | 24 infants Inclusion criteria: BW < 2500 grams and at high risk for neurological problems due to PVL, IVH (as shown by ultrasound) or both Exclusion criteria: multiple births, born in another town and returned there Characteristics: mean GAs for treatment and follow‐up groups: 30.3 (SD 3.3) and 30.3 (SD 2.7) weeks, respectively. No significant differences between groups for infant and maternal factors, social factors, distribution of diagnoses and severity of injury | |
| Interventions | Intervention group (N = 12): infant development and parent‐infant relationship Standard follow‐up (N = 12): All infants in control and intervention groups attended follow‐up clinics and were referred to developmental services if they presented with signs of neurological dysfunction or developmental delay | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using method of minimisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Included only children at high risk of cerebral palsy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 12 months = 96% |
Resnick 1988.
| Methods | Blinding to intervention: no Blinding to outcome assessments: yes Concealment of allocation: inadequate | |
| Participants | 41 infants Inclusion criteria: BW < 1800 grams, GA < 37 weeks Exclusion criteria: not specified Characteristics: mean GAs for intervention and control groups: 31.7 (SD 2.9) and 31.0 (SD 2.0) weeks, respectively | |
| Interventions | Intervention group (N = 21): infant development and parent‐infant relationship Standard follow‐up group (N = 20): given access to a full range of social services, physiotherapy and occupational therapy | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants randomly assigned according to the last digit of hospital number |
| Allocation concealment (selection bias) | High risk | infants randomly assigned according to the last digit of hospital number |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Exclusion criteria not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of follow‐up: unclear, as it is not stated whether any participants withdrew from the study before the 12‐month assessment |
Rice 1979.
| Methods | Randomised controlled trial of infant stimulation for preterm infants vs standard follow‐up. Intervention group received a tactile‐kinaesthetic stimulation programme administered by their mothers that aimed to enhance parent‐infant relationship and to give infants appropriate levels of stimulation | |
| Participants | 30 infants Inclusion criteria: born at GA < 37 weeks between 1974 and 1975; born to mothers of low SES Exclusion criteria: not specified Characteristics: mean GAs for intervention and control groups: not stated but reported to be similar between control and intervention groups | |
| Interventions | Intervention group (N = 15): infant development Standard follow‐up (N = 15): Mothers were given standard discharge information related to caring for their infant. It is reported that mothers were visited regularly by the experimenter and by other public health nurses, who provided social reinforcement for appropriate mothering behaviour | |
| Outcomes | Cognitive
Motor
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given on generation of a randomised sequence |
| Allocation concealment (selection bias) | Unclear risk | No information given on how allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Included only children born to mothers of low SES |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors masked to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated whether any infants withdrew from the study |
Sajaniemi 2001.
| Methods | Randomised controlled trial to assess effects of early occupational therapy. Intervention group received a 1‐hour weekly home‐based intervention from 6 to 12 months aimed at supporting parent‐infant interactions and enhancing motor control | |
| Participants | 126 infants Inclusion criteria: BW < 1000 grams, born between January 1991 and December 1994 and admitted to Helsinki University Central Hospital Exclusion criteria: cerebral palsy, mental retardation Characteristics: mean GAs for intervention and control group: 27.1 (SD 0.3) and 26.4 (SD 0.3) weeks, respectively |
|
| Interventions | Intervention group (N = 63): infant development Standard follow‐up (N = 63): not described; however, children in both groups had access to extra occupational therapy and physiotherapy when required | |
| Outcomes | Cognitive
Motor
|
|
| Notes | Infants were excluded at 2 and 4 years for having cerebral palsy or mental retardation, changing the number of eligible infants at each time point. Follow‐up rates reported in the table are based upon the 126 infants originally allocated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Every second patient was randomly allocated to the control group on a case‐control basis |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether participants or personnel were unaware of random assignment during the study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Children later diagnosed with cerebral palsy or mental retardation excluded from the analysis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 2 years = 79%; 4 years = 76% |
Spittle 2009.
| Methods | Single‐centre randomised controlled trial of a preventive care programme vs standard follow‐up. Intervention group received 9 visits post hospital discharge from a team of a physiotherapist and a psychologist at home, with each session lasting 1.5 to 2 hours from 1 week post hospital discharge to 11 months' corrected age. Preventive care programme aimed to improve infant development and to support parents' mental health | |
| Participants | 120 infants Inclusion criteria: born at GA < 30 weeks Exclusion criteria: non‐English speaking, living > 100 km from the hospital, with or without congenital abnormalities Characteristics: mean GAs for intervention and control groups: 27.3 (SD 1.6) and 27.4 (SD 1.4) weeks, respectively | |
| Interventions | Intervention group (N = 61): infant development and parent‐infant relationship Standard follow‐up (N = 59): not systematic; however, each family had access to maternal child health nurses and could access early intervention if referred by paediatrician or healthcare team | |
| Outcomes | Cognitive
Motor
|
|
| Notes | *This study includes authors of this Cochrane review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation concealment, randomisation via computer‐generated programme and assigned with opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Children from non‐English speaking families and those living > 100 km from the hospital with or without congenital abnormalities excluded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 2 years = 96%, 4 years = 89% |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | All authors of this Cochrane review were involved in this randomised controlled trial |
Teti 2009.
| Methods | Randomised controlled trial of an 8 sessions over 20 weeks intervention programme targeted to very low birth weight children of African American women who were recruited from the NICUs of 4 centres Intervention consisted of 2 psychoeducational components and a parent‐administered infant tactile stimulation component that commenced before discharge from hospital and continued until 4 months' corrected age. Intervention targeted mothers and infants and involved educating mothers about infant behaviour and individual infant abilities to enhance attachment and facilitate social and motor behaviours |
|
| Participants | 173 infants Inclusion criteria: low birth weight infants of African American women Exclusion criteria: mothers with positive toxicology screen or younger than 18 years of age. Infants were excluded if they had a chromosomal abnormality Characteristics: mean GAs for intervention and control groups: 30.6 (SD 3.2) and 29.9 (SD 3.6) weeks, respectively |
|
| Interventions | Intervention group (N = 99): parent education and parent infant attachment strategies Standard follow‐up (N = 95): not described | |
| Outcomes | Cognitive
Motor
|
|
| Notes | MDI data used for meta‐analysis included VLBW and ELBW groups only, as LBW group included 2 term‐age children. PDI not reported separately for preterm infants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information given on how allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Population of infants selected on the basis of having African American mothers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completeness of follow‐up: 4 months = 78% |
| Selective reporting (reporting bias) | Low risk | Study authors report using an intention‐to‐treat approach for the investigation and analyses |
Wu 2014.
| Methods | Randomised controlled trial with 3 arms, comparing a clinic‐based intervention programme (CBIP), a home‐based intervention programme (HBIP) and a usual care program from shortly after birth until 12 months' corrected age. Clinic‐ and home‐based interventions similar in content but different in location (hospital vs home visits). Interventions aimed to improve cognitive, motor and language outcomes by targeting child, parent and dyad, whereas standard care focused only on the child | |
| Participants | 211 low‐risk preterm infants Inclusion criteria: birth weight < 1500 grams, GA < 37 weeks, admission to study hospital within 7 days of birth, singleton birth or first child of multiples, absence of congenital abnormalities and neurological abnormalities (e.g. grade III/IV IVH, seizures, hydrocephalus) Characteristics: mean GA (weeks) and BW (grams) for CBIP = 30.0 (SD 2.6) and 1179 (SD 228), for HBIP = 29.9 (SD 3.2) and 1149 (SD 283) and for standard care = 29.3 (SD 2.7) and 1091 (SD 268), respectively |
|
| Interventions | CBIP (n = 57), HBIP (n = 63): clinic based or home based, focusing on infant, parent and parent‐infant relationship. Intervention involved environment enrichment, including feeding support, massage, interaction activities and parental support Usual care (n = 58): same for all groups, including 5 inpatient sessions and 8 neonatal clinic visits focused on the child's health |
|
| Outcomes | Cognitive
Motor
|
|
| Notes | For purposes of meta‐analysis, outcomes for HBIC and CBIC groups were grouped together for comparison vs standard care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using computer‐generated random numbers and stratified by gestational age and hospital |
| Allocation concealment (selection bias) | Low risk | Reported to be concealed from parents, clinical staff and research assistants; details of how concealment occurred not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Low‐risk preterm population, no congenital or brain abnormalities and recruited only singleton and first‐born infants of multiples. Early dropouts (n = 29) and pilot study (n = 4) excluded from main study, but not clear which group to which they were randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel delivering the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up at 24 months: 80% (not including early dropouts and pilot infants) |
| Selective reporting (reporting bias) | Low risk | Dosage of intervention documented, with intention‐to‐treat analysis |
Yigit 2002.
| Methods | Randomised controlled trial investigating effects of early physiotherapy intervention vs standard follow‐up for low‐risk preterm infants. Study authors did not report how many infants were initially randomly assigned to each group; however, they did report that 39 infants were dropped from the study within the first 12 months for lack of participation. This resulted in 80 infants in the physiotherapy intervention group and 80 in the standard follow‐up group at 12 months. Infants were registered for the study before hospital discharge; however, it is unclear when the study began | |
| Participants | 199 infants*
Inclusion criteria: BW < 2000 grams and GA < 34 weeks
Mean GAs for intervention and standard follow‐up groups: 31.3 (SD 2.2) and 32.0 (SD 1.6) weeks, respectively Exclusion criteria: perinatal hypoxia or abnormal neurosonography |
|
| Interventions | Intervention group (N = 80): infant development Standard follow‐up group (N = 80): both groups seen monthly by the same physiotherapist for first 9 months, then every 3 months until 18 to 24 months of age. Unclear whether this was done for assessment or for intervention | |
| Outcomes | Cognitive
Motor
|
|
| Notes | *It is stated that 39 infants dropped out of the study because of lack of participation at 12 months; however, numbers of infants initially randomly assigned to intervention and standard follow‐up not reported Data could not be used in meta‐analysis, as they were not standardised measures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation techniques used |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether any methods were used to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completeness of follow‐up: 83% follow‐up at 12 months. Study authors did not report how many infants were initially randomly assigned to each group; however, they do report that 39 infants were dropped from the study within the first 12 months for lack of participation |
BSID‐I, Bayley Scales of Infant Development Edition I; BSID‐II, Bayley Scales of Infant Development Edition II; BSID MDI: Bayley Scales of Infant Development ‐ Mental Development Index; BSID PDI: Bayley Scales of Infant Development ‐ Psychomotor Development Index; BW: birth weight; CA: corrected age; CBIP: clinic‐based intervention programme; CI: confidence interval; DAS: Differential Abilities Scale; DQ: developmental quotient; DS: developmental scale; EI: early intervention; ELBW: extremely low birth weight; GA: gestational age; GCI: General Cognitive Index; HBIP: home‐based intervention programme; IVH: intraventricular haemorrhage; LBW: low birth weight; MABC‐2: Movement Assessment Battery for Children Edition 2; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit; PEDI‐NL: Pediatric Evaluation of Disability Inventory; PPVT: Peabody Picture Vocabulary Test; PVH: periventricular haemorrhage; PVL: periventricular leukomalacia; SD: standard deviation; SES: socioeconomic status; SPEEDI: Supporting Play Exploration and Early Development Intervention; TIMP: Test of Infant Motor Performance; VLBW: very low birth weight; WASI: Wechsler Abbreviated Scale of Intelligence; WISC‐III: Wechsler Intelligence Scale for Children ‐ Full Scale IQ test; WPPSI: Wechsler Preschool and Primary Scale of Intelligence.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Badr 2006 | Population: included infants > 37 weeks' gestation |
| Beckwith 1988 | Intervention: no post‐hospital discharge component |
| Beeghly 1995 | Population: infants not preterm |
| Britain 1995 | Method: case studies |
| Chen 2001 | Language: published in Chinese |
| Culp 1989 | Outcome measures: parent focused only |
| Fucile 2012 | Intervention: no post‐hospital discharge component Outcomes: not motor and not cognitive |
| Girolami 1994 | Intervention: no post‐hospital discharge component |
| Guzzetta 2011 | Intervention: no post‐hospital discharge component |
| Hielkema 2010 | Population: included infants > 37 weeks' gestation |
| Hielkema 2011 | Population: included infants > 37 weeks' gestation |
| Israel 2003 | Outcome measures: no results reported Method: unclear |
| Kanda 2004 | Method: case‐control study |
| Kang 1995 | Outcome measures: parent‐infant interaction only |
| Kendrick 2000 | Method: literature review only |
| Kleberg 2000 | Intervention: no post‐hospital discharge component |
| Kleberg 2002 | Intervention: no post‐hospital discharge component |
| Landsem 2014 | Parenting outcomes only |
| Matsuishi 1998 | Method: case‐control study |
| Nair 2009 | Population: included infants > 37 weeks' gestation |
| Newnham 2009 | Outcome: not motor and not cognitive |
| Oberg 2012 | Intervention: inpatient intervention |
| Olafsen 2012 | Outcome: not motor and not cognitive |
| Piper 1986 | Population: included infants > 37 weeks' gestation |
| Ross 1984 | Method: case‐control study |
| Scott 1989 | Method: literature review only |
| Slater 1987 | Method: cohort study |
| Walker 2010 | Population: included infants > 37 weeks' gestation |
| Wasik 1990 | Population: infants not preterm |
| Widmayer 1981 | No intervention post discharge by health or educational professional |
Characteristics of ongoing studies [ordered by study ID]
Sgandurra 2014.
| Trial name or title | Sgandurra 2014 |
| Methods | Multi‐centre RCT |
| Participants | Preterm infants without major complications, 3 to 9 months' corrected age at commencement of intervention with specific gross motor abilities defined by Ages & Stages Questionnaire |
| Interventions | Intervention (n = 20): CareToy group performed 4 weeks of personalised activities with the CareToy system Control (n = 20): received standard care |
| Outcomes | Infant Motor Profile Scale Bayley‐III Alberta Infant Motor Scale |
| Starting date | 2014 ‐ expected completion April 2015 |
| Contact information | gcioni@fsm.unipt.it |
| Notes |
Contributions of authors
Alicia Spittle and Jane Orton wrote the protocol, with contributions from Lex Doyle and Ros Boyd. Alicia Spittle and Jane Orton searched the literature, reviewed all possible trials for inclusion, extracted details of study methods and results, entered data into RevMan, wrote the initial synthesis of results and contributed to all versions of the review. Ros Boyd extracted details of the results and contributed to all versions of the review. Lex Doyle and Peter Anderson contributed to synthesis of the results and to all versions of the review.
Sources of support
Internal sources
Murdoch Childrens Research Institute, Australia.
NHMRC Career Development Fellowship (RB), Other.
External sources
NHMRC (National Health and Medical Research Council) Australia ‐ Grant 284512, Australia.
NHMRC Public Health Scholarship (AJS), Australia.
Allens Arthur Robinsons Grant, Australia.
Cerebral Palsy Alliance, Other.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded by Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
NHMRC Early Career Fellowship (AJS), Other.
NHMRC Centre of Research Excellence in Newborn Medicine, Other.
NHMRC Career Development Fellowship (RNB), Other.
NHMRC Research Fellowship (PJA), Other.
Declarations of interest
All authors involved in this review were involved in a randomised controlled trial of intervention with preterm infants (Spittle 2009).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
APIP 1998 {published data only}
- Avon Premature Infant Project. Randomised trial of parental support for families with very preterm children. Archives of Disease in Childhood. Fetal Neonatal Edition 1998;79(1):F4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Ring W, Anderson P, Marlow N. Randomised trial of parental support for families with very preterm children: outcome at 5 years. Archives of Disease in Childhood 2005;90(9):909‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bao 1999 {published data only}
- Bao X, Sun S, Wei S. Early intervention promotes intellectual development of premature infants: a preliminary report. Chinese Medical Journal 1999;112(6):520‐3. [PubMed] [Google Scholar]
Barrera 1986 {published data only}
- Barrera ME, Cunningham CE, Rosenbaum PL. Low birth weight and home intervention strategies: preterm infants. Journal of Developmental and Behavioural Pediatrics 1986;7(6):361‐6. [PubMed] [Google Scholar]
- Barrera ME, Doucet DA, Kitching KJ. Early home intervention and socio‐emotional development of preterm infants. Infant Mental Health Journal 1990;11(2):142‐57. [Google Scholar]
- Barrera ME, Kitching KJ. A 3 year early home intervention follow‐up study with low birthweight infants and their parents. Topics in Early Childhood Special Education 1991;10(4):14‐28. [Google Scholar]
- Barrera ME, Rosenbaum PL, Cunningham CE. Early home intervention with low‐birth‐weight infants and their parents. Child Development 1986;57(1):20‐33. [DOI] [PubMed] [Google Scholar]
Cameron 2005 {published data only}
- Cameron EC, Maehle V, Reid J. The effects of an early physical therapy intervention for very preterm, very low birth weight infants: a randomized controlled clinical trial. Pediatric Physical Therapy 2005;17(2):107‐19. [DOI] [PubMed] [Google Scholar]
Dusing 2015 {published data only}
- Dusing SC, Brown SE, Drew CM, Thacker LR, Hendricks‐Munoz KD. Supporting play exploration and early development intervention from NICU to home: a feasibility study. Pediatric Physical Therapy 2015;27(3):267‐74. [PUBMED: 26102168] [DOI] [PubMed] [Google Scholar]
Field 1980 {published data only}
- Field TM, Widmayer SM, Stringer S, Ignatoff E. Teenage, lower‐class, black mothers and their preterm infants: an intervention and developmental follow‐up. Child Development 1980;51(2):426‐36. [PubMed] [Google Scholar]
Gianni 2006 {published data only}
- Gianní ML, Picciolini O, Ravasi M, Gardon L, Vegni C, Fumagalli M, et al. The effects of an early developmental mother‐child intervention program on neurodevelopment outcome in very low birth weight infants: a pilot study. Early Human Development 2006;82(10):691‐5. [DOI] [PubMed] [Google Scholar]
Goodman 1985 {published data only}
- Goodman M, Rothberg AD, Houston‐McMillan JE, Cooper PA, Cartwright JD, Velde MA. Effect of early neurodevelopmental therapy in normal and at‐risk survivors of neonatal intensive care. Lancet 1985;2(8468):1327‐30. [DOI] [PubMed] [Google Scholar]
- Rothberg AD, Goodman M, Jacklin LA, Cooper PA. Six‐year follow‐up of early physiotherapy intervention in very low birth weight infants. Pediatrics 1991;88(3):547‐52. [PubMed] [Google Scholar]
I.H.D.P. 1990 {published and unpublished data}
- Berlin LJ, Brooks‐Gunn J, McCarton C, McCormick MC. The effectiveness of early intervention: examining risk factors and pathways to enhanced development. Preventive Medicine 1998;27(2):238‐45. [DOI] [PubMed] [Google Scholar]
- Blair C, Ramey CT, Hardin JM. Early intervention for low birth weight, premature infants: participation and intellectual development. American Journal of Mental Retardation 1995;99(5):542‐54. [PubMed] [Google Scholar]
- Brooks‐Gunn J, Klebanov PK, Liaw F, Spiker D. Enhancing the development of low‐birthweight, premature infants: changes in cognition and behavior over the first three years. Child Development 1993;64(3):736‐53. [DOI] [PubMed] [Google Scholar]
- Brooks‐Gunn J, Liaw F, Klebanov PK. Effects of early intervention on cognitive function of low birth weight preterm infants. Journal of Pediatrics 1992;120(3):350‐9. [DOI] [PubMed] [Google Scholar]
- Brooks‐Gunn J, McCarton CM, Casey PH, McCormick MC, Bauer CR, Bernbaum JC, et al. Early intervention in low‐birth‐weight premature infants. Results through age 5 years from the Infant Health and Development Program. JAMA 1994;272(16):1257‐62. [PubMed] [Google Scholar]
- Hollomon HA, Scott KG. Influence of birth weight on educational outcomes at age 9: the Miami site of the Infant Health and Development Program. Journal of Developmental and Behavioral Pediatrics 1998;19(6):404‐10. [DOI] [PubMed] [Google Scholar]
- McCarton C. Behavioral outcomes in low birth weight infants. Pediatrics 1998;102(5 Suppl E):1293‐7. [PubMed] [Google Scholar]
- McCarton CM, Brooks‐Gunn J, Wallace IF, Bauer CR, Bennett FC, Bernbaum JC, et al. Results at age 8 years of early intervention for low‐birth‐weight premature infants: the Infant Health and Developmental Program. JAMA 1997;277:126‐32. [PubMed] [Google Scholar]
- McCormick MC, Brooks‐Gunn J, Buka SL, Golman J, Yu J, Salganik DT, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics 2006;117:771‐80. [DOI] [PubMed] [Google Scholar]
- McCormick MC, McCarton C, Brooks‐Gunn J, Belt P, Gross RT. The Infant Health and Development Program: interim summary. Journal of Developmental and Behavioral Pediatrics 1998;19:359‐70. [DOI] [PubMed] [Google Scholar]
- McCormick MC, McCarton C, Tonascia J, Brooks‐Gunn J. Early educational intervention for very low birth weight infants: results from the Infant Health and Development Program. Journal of Pediatrics 1993;123:527‐33. [DOI] [PubMed] [Google Scholar]
- The Infant Health and Development Program. Enhancing the outcomes of low‐birth‐weight, premature infants. A multisite randomized trial. JAMA 1990;263(22):3035‐42. [DOI] [PubMed] [Google Scholar]
Johnson 2009 {published data only}
- Johnson S, Whitelaw A, Glazebrook C, Israel C, Turner R, White IR, et al. Randomized trial of a parenting intervention for very preterm infants: outcome at 2 years. Journal of Pediatrics 2009;155(4):488‐94. [DOI] [PubMed] [Google Scholar]
Kaaresen 2006 {published data only}
- Hauglann L, Handegaard BH, Ulvund SE, Nordhov M, Ronning JA, Kaaresen PI. Cognitive outcome of early intervention in preterms at 7 and 9 years of age: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;100(1):F11‐6. [PUBMED: 25249191] [DOI] [PubMed] [Google Scholar]
- Kaaresen PI, Rønning JA, Tunby J, Nordhov SM, Ulvund SE, Dahl LB. A randomized controlled trial of an early intervention program in low birth weight children: outcome at 2 years. Early Human Development 2008;84(3):201‐9. [DOI] [PubMed] [Google Scholar]
- Kaaresen PI, Rønning JA, Ulvund SE, Dahl LB. A randomized, controlled trial of the effectiveness of an early‐intervention program in reducing parenting stress after preterm birth. Pediatrics 2006;118(1):e9‐19. [DOI] [PubMed] [Google Scholar]
- Nordhov SM, Rønning JA, Dahl LB, Ulvund SE, Tunby J, Kaaresen PI. Early intervention improves cognitive outcomes for preterm infants: randomized controlled trial. Pediatrics 2010;126(5):e1088‐94. [DOI] [PubMed] [Google Scholar]
Koldewijn 2009 {published data only (unpublished sought but not used)}
- Koldewijn K, Wolf MJ, Wassenaer A, Meijssen D, Sonderen L, Baar A, et al. The Infant Behavioral Assessment and Intervention Program for very low birth weight infants at 6 months corrected age. Journal of Pediatrics 2009;154(1):33‐8.e2. [PUBMED: 18783797] [DOI] [PubMed] [Google Scholar]
- Koldewijn K, Wassenaer A, Wolf MJ, Meijssen D, Houtzager B, Beelen A, et al. A neurobehavioral intervention and assessment program in very low birth weight infants: outcome at 24 months. Journal of Pediatrics 2010;156(3):359‐65. [PUBMED: 19880139] [DOI] [PubMed] [Google Scholar]
- Meijssen D, Wolf MJ, Koldewijn K, Houtzager BA, Wassenaer A, Tronick E, et al. The effect of the Infant Behavioral Assessment and Intervention Program on mother‐infant interaction after very preterm birth. Journal of Child Psychology and Psychiatry and Allied Disciplines 2010;51(11):1287‐95. [PUBMED: 20345840] [DOI] [PubMed] [Google Scholar]
- Meijssen D, Wolf MJ, Bakel H, Koldewijn K, Kok J, Baar A. Maternal attachment representations after very preterm birth and the effect of early intervention. Infant Behavior & Development 2011;34(1):72‐80. [PUBMED: 21067812] [DOI] [PubMed] [Google Scholar]
- Meijssen DE, Wolf MJ, Koldewijn K, Wassenaer AG, Kok JH, Baar AL. Parenting stress in mothers after very preterm birth and the effect of the Infant Behavioural Assessment and Intervention Program. Child: Care, Health and Development 2011;37(2):195‐202. [PUBMED: 20645992] [DOI] [PubMed] [Google Scholar]
- Hus JWP, Jeukens‐Visser M, Koldewijn, Geldof CJA, Kok JH, Nollet F, et al. Sustained developmental effects of the infant behavioral assessment and intervention program in very low birth weight infants at 5.5 years corrected age. Journal of Pediatrics 2013;162:1112‐9. [PUBMED: 23312690] [DOI] [PubMed] [Google Scholar]
- Verkerk G, Jeukens‐Visser M, Koldewijn K, Wassenaer A, Houtzager B, Kok J, et al. Infant behavioral assessment and intervention program in very low birth weight infants improves independency in mobility at preschool age. Journal of Pediatrics 2011;159(6):933‐8.e1. [PUBMED: 21784445 ] [DOI] [PubMed] [Google Scholar]
Kyno 2012 {published data only}
- Kyno NM, Ravan IH, Lindermann R, Fargeland MW, Smeby N, Torgersen AM. Effect of an early intervention programme on development of moderate and late preterm infants at 36 months: a randomized controlled study. Infant Behaviour and Development 2012;35(4):916‐26. [PUBMED: 23063851] [DOI] [PubMed] [Google Scholar]
Lekskulchai 2001 {published data only}
- Lekskulchai R, Cole J. Effect of a developmental program on motor performance in infants born preterm. Australian Journal of Physiotherapy 2001;47(3):169‐76. [DOI] [PubMed] [Google Scholar]
Melnyk 2001 {published data only}
- Melnyk BM, Alpert‐Gillis L, Feinstein NF, Fairbanks E, Czarniak‐Schultz N, Hust D, et al. Improving cognitive development of low‐birth‐weight premature infants with the COPE program: a pilot study of the benefit of early NICU intervention with mothers. Research in Nursing and Health 2001;24(5):373‐89. [DOI] [PubMed] [Google Scholar]
Nelson 2001 {published data only}
- Nelson NM, White‐Traut RC, Vasan U, Silvestri J, Comiskey E, Meleedy‐Rey P, et al. One‐year outcome of auditory‐tactile‐visual‐vestibular intervention in the neonatal intensive care unit: effects of severe prematurity and central nervous system injury. Journal of Child Neurology 2001;16(7):493‐8. [DOI] [PubMed] [Google Scholar]
Nurcombe 1984 {published data only}
- Achenbach TM, Howell CT, Aoki MF, Rauh VA. Nine‐year outcome of the Vermont Intervention Program for low birth weight infants. Pediatrics 1993;91(1):45‐55. [PubMed] [Google Scholar]
- Achenbach TM, Phares V, Howell CT, Rauh VA, Nurcombe B. Seven‐year outcome of the Vermont Intervention Program for Low‐Birthweight Infants. Child Development 1990;61(6):1672‐81. [PubMed] [Google Scholar]
- Nurcombe B, Howell DC, Rauh V, Teti DM, Ruoff P, Brennan J. An intervention program for mothers of low‐birthweight babies: preliminary results. Journal of the American Academy of Child Psychiatry 1984;23(3):319‐25. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Achenbach TM, Nurcombe BH, Howell CT, Teti DM. Minimizing adverse effects of low birthweight: four‐year results of an early intervention program. Child Development 1988;59(3):544‐53. [PubMed] [Google Scholar]
- Rauh VA, Nurcombe B, Achenbach T, Howell C. The Mother‐Infant Transaction Program: the content and implications of an intervention for the mothers of low‐birthweight infants. Clinics in Perinatology 1990;17(1):31‐45. [PubMed] [Google Scholar]
Ohgi 2004 {published and unpublished data}
- Ohgi S, Fukuda M, Akiyama T, Gima H. Effect of an early intervention programme on low birthweight infants with cerebral injuries. Journal of Paediatric and Child Health 2004;40(12):689‐95. [DOI] [PubMed] [Google Scholar]
Resnick 1988 {published data only}
- Resnick MB, Armstrong S, Carter RL. Developmental intervention program for high‐risk premature infants: effects on development and parent infant interactions. Journal of Developmental and Behavioral Pediatrics 1988;9(2):73‐8. [PubMed] [Google Scholar]
Rice 1979 {published data only}
- Rice RD. The effects of the Rice infants sensorimotor stimulation treatment on the development of high‐risk infants. Birth Defects Original Article Series 1979;15(7):7‐26. [PubMed] [Google Scholar]
Sajaniemi 2001 {published data only}
- Sajaniemi N, Makela J, Salokorpi T, Wendt L, Hamalainen T, Hakamies‐Blomqvist L. Cognitive performance and attachment patterns at four years of age in extremely low birth weight infants after early intervention. European Child & Adolescent Psychiatry 2001;10(2):122‐9. [PUBMED: 11469284] [DOI] [PubMed] [Google Scholar]
- Salokorpi T, Rauito T, Kajantie E, Wednt L. Is early occupational therapy in extremely preterm infants of benefit in the long run?. Pediatric Rehabilitation 2002;5(2):91‐8. [PUBMED: 12490052] [DOI] [PubMed] [Google Scholar]
- Salokorpi T, Sajaniemi N, Rajantie I, Hallback H, Hamalainen T, Rita H, et al. Neurodevelopment until the adjusted age of 2 years in extremely low birth weight infants after early intervention ‐ a case‐control study. Pediatric Rehabilitation 1998;2(4):157‐63. [PUBMED: 10048099] [DOI] [PubMed] [Google Scholar]
Spittle 2009 {published data only}
- Spencer‐Smith MM, Spittle AJ, Doyle LW, Lee KJ, Lorefice L, Suetin A, et al. Long‐term benefits of home‐based preventive care for preterm infants: a randomized trial. Pediatrics 2012;130(6):1094‐101. [PUBMED: 23129084] [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Anderson PJ, Lee KJ, Ferretti C, Eeles A, Orton J, et al. Preventive care at home for very preterm infants improves infant and caregiver outcomes at 2 years. Pediatrics 2010;126(1):e171‐8. [PUBMED: 20547650] [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Ferretti C, Anderson PJ, Orton J, Eeles A, Bates L, et al. Improving the outcome of infants born at <30 weeks gestation ‐ a randomized controlled trial of preventative care at home. BMC Pediatrics 2009;9:73. [PUBMED: 19954550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teti 2009 {published data only}
- Teti DM, Black M, Viscardi R, Glass P, O'Connell, Baker L, Cusson R, Reiner Hess C. Intervention with African American premature infants: four‐month results of an early intervention program. Journal of Early Intervention 2009;31(2):146‐66. [DOI: 10.1177/1053815109331864] [DOI] [Google Scholar]
Wu 2014 {published data only}
- Wu YC, Leng CH, Hsieh WS, Hsu CH, Chen WJ, Gau SS, et al. A randomized controlled trial of clinic‐based and home‐based interventions in comparison with usual care for preterm infants: effects and mediators. Research in Developmental Disabilities 2014;35(10):2384‐93. [PUBMED: 24973546] [DOI] [PubMed] [Google Scholar]
Yigit 2002 {published data only}
- Yigit S, Kerem M, Livanelioglu A, Oran O, Erdem G, Mutlu A, et al. Early physiotherapy intervention in premature infants. The Turkish Journal of Pediatrics 2002;44(3):224‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Badr 2006 {published data only}
- Badr LK, Garg M, Kamath MK. Intervention for infants with brain injury: results of a randomized controlled study. Infant Behaviour & Development 2006;29(1):80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beckwith 1988 {published data only}
- Beckwith L. Intervention with disadvantaged parents of sick preterm infants. Psychiatry 1988;51(3):242‐7. [DOI] [PubMed] [Google Scholar]
Beeghly 1995 {published data only}
- Beeghly M, Brazelton TB, Flannery KA, Nugent JK, Barrett DE, Tronick EZ. Specificity of preventative pediatric intervention effects in early infancy. Journal Developmental and Behavioural Pediatrics 1995;16(3):158‐66. [PubMed] [Google Scholar]
Britain 1995 {published data only}
- Britain LA, Holmes GE, Hassanein RS. High‐risk children referred to an early‐intervention developmental program. Clinical Pediatrics 1995;34(12):635‐41. [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen D, Zhang J, Chen Y. Early intervention on intelligent development of premature infant. Chinese Mental Health Journal 2001;15:55‐7. [Google Scholar]
Culp 1989 {published data only}
- Culp RE, Culp AM, Harmon RJ. A tool for educating parents about their premature infants. Birth 1989;16(1):23‐6. [DOI] [PubMed] [Google Scholar]
Fucile 2012 {published data only}
- Fucile S, McFarland DH, Gisel EG, Lau C. Oral and nonoral sensorimotor interventions facilitate suck‐swallow‐respiration functions and their coordination in preterm infants. Early Human Development 2012;88(6):345‐50. [PUBMED: 21962771] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girolami 1994 {published data only}
- Girolami JL, Campbell SK. Efficacy of a neuro‐developmental treatment program to improve motor control in infants born prematurely. Pediatric Physical Therapy 1994;6:175‐84. [Google Scholar]
Guzzetta 2011 {published data only}
- Guzzetta A, D'Acunto MG, Carotenuto M, Berardi N, Bancale A, Biagioni E, et al. The effects of preterm infant massage on brain electrical activity. Developmental Medicine and Child Neurology 2011;53(Suppl 4):46‐51. [PUBMED: 21950394] [DOI] [PubMed] [Google Scholar]
Hielkema 2010 {published data only}
- Hielkema T, Hamer EG, Reinders‐Messelink HA, Maathuis CG, Bos AF, Dirks T, et al. LEARN 2 MOVE 0‐2 years: effects of a new intervention program in infants at very high risk for cerebral palsy; a randomized controlled trial. BMC Pediatrics 2010;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hielkema 2011 {published data only}
- Hielkema T, Blauw‐Hospers CH, Dirks T, Drijver‐Messelink M, Bos AF, Hadders‐Algra M. Does physiotherapeutic intervention affect motor outcome in high‐risk infants? An approach combining a randomized controlled trial and process evaluation. Developmental Medicine and Child Neurology 2011;53(3):e8‐15. [DOI] [PubMed] [Google Scholar]
Israel 2003 {published data only}
- Israel C. The preterm infant parenting study. Midirs Midwifery Digest 2003;13:239‐41. [Google Scholar]
Kanda 2004 {published data only}
- Kanda T, Pidcock FS, Hayakawa K, Yamori Y, Shikata Y. Motor outcome differences between two groups of children with spastic diplegia who received different intensities of early onset physiotherapy followed for 5 years. Brain and Development 2004;26(2):118‐26. [DOI] [PubMed] [Google Scholar]
Kang 1995 {published data only}
- Kang R, Barnard K, Hammond M, Oshio S, Spencer C, Thibodeaux B, et al. Preterm infant follow‐up project: a multi‐site field experiment of hospital and home intervention programs for mothers and preterm infants. Public Health Nursing 1995;12(3):171‐80. [DOI] [PubMed] [Google Scholar]
Kendrick 2000 {published data only}
- Kendrick D, Elkan R, Hewitt M, Dewey M, Blair M, Robinson J, et al. Does home visiting improve parenting and the quality of the home environment? A systematic review and meta analysis. Archives of Disease in Childhood 2000;82(6):443‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleberg 2000 {published data only}
- Kleberg A, Westrup B, Stjernqvist K. Developmental outcome, child behavior and mother‐child interaction at 3 years of age following Newborn Individualized Developmental Care and Intervention Program (NIDCAP) intervention. Early Human Development 2000;60(2):123‐35. [DOI] [PubMed] [Google Scholar]
Kleberg 2002 {published data only}
- Kleberg A, Westrup B, Stjernqvist K, Lagercrantz H. Indications of improved cognitive development at one year of age among infants born very prematurely who received care based on the Newborn Individualized Developmental Care and Assessment Program (NIDCAP). Early Human Development 2002;68(2):83‐91. [DOI] [PubMed] [Google Scholar]
Landsem 2014 {published data only}
- Landsem IP, Handegard BH, Tunby J, Ulvund SE, Ronning JA. Early intervention program reduces stress in parents of preterms during childhood, a randomized controlled trial. Trials 2014;15:387. [PUBMED: 25282345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuishi 1998 {published data only}
- Matsuishi T, Ishibashi S, Kamiya Y, Shoju J, Yamashita Y, Fukuda S, et al. Early intervention for very‐low‐birth‐weight infants. Brain and Development 1998;20(1):18‐21. [DOI] [PubMed] [Google Scholar]
Nair 2009 {published data only}
- Nair MKC, Philip E, Jeyaseelan L, George B, Mathews S, Padma K. Effect of Child Development Centre model early stimulation among at risk babies ‐ a randomized controlled trial. Indian Pediatrics 2009;46(Suppl):S20‐6. [PubMed] [Google Scholar]
Newnham 2009 {published data only}
- Newnham CA, Milgrom J, Skouteris H. Effectiveness of a modified Mother‐Infant Transaction Program on outcomes for preterm infants from 3 to 24 months of age. Infant Behaviour & Development 2009;32(1):17‐26. [DOI] [PubMed] [Google Scholar]
Oberg 2012 {published data only}
- Oberg GK, Campbell SK, Girolami GL, Ustad T, Jørgensen L, Kaaresen PI. Study protocol: an early intervention program to improve motor outcome in preterm infants: a randomized controlled trial and a qualitative study of physiotherapy performance and parental experiences. BMC Pediatrics 2012;12:15. [PUBMED: 22336194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olafsen 2012 {published data only}
- Olafsen KS, Rønning JA, Handegård BH, Ulvund SE, Dahl LB, Kaaresen PI. Regulatory competence and social communication in term and preterm infants at 12 months corrected age. Results from a randomized controlled trial. Infant Behavior & Development 2012;35(1):140‐9. [PUBMED: 21908049] [DOI] [PubMed] [Google Scholar]
Piper 1986 {published data only}
- Piper MC, Kunos VI, Willis DM, Mazer BL, Ramsay M, Silver KM. Early physical therapy effects on the high‐risk infant: a randomized controlled trial. Pediatrics 1986;78(2):216‐24. [PubMed] [Google Scholar]
Ross 1984 {published data only}
- Ross GS. Home intervention for premature infants of low‐income families. American Journal of Orthopsychiatry 1984;54(2):263‐70. [DOI] [PubMed] [Google Scholar]
Scott 1989 {published data only}
- Scott DT, Spiker D. Research on the sequelae of prematurity: early learning, early interventions and later outcomes. Seminars in Perinatology 1989;13(6):495‐505. [PubMed] [Google Scholar]
Slater 1987 {published data only}
- Slater MA, Naqvi M, Andrew L, Haynes K. Neurodevelopment of monitored versus non‐monitored very low birth weight infants: the importance of family influences. Journal of Developmental and Behavioral Pediatrics 1987;8(5):278‐85. [PubMed] [Google Scholar]
Walker 2010 {published data only}
- Walker SP, Chang SM, Younger N, Grantham‐McGregor SM. The effect of psychosocial stimulation on cognition and behaviour at 6 years in a cohort of term, low‐birthweight Jamaican children. Developmental Medicine and Child Neurology 2010;52(7):e148‐54. [DOI] [PubMed] [Google Scholar]
Wasik 1990 {published data only}
- Wasik BH, Ramey CT, Bryant DM, Sparling JM. A longitudinal study of two early intervention strategies: Project CARE. Child Development 1990;61(6):1682‐96. [PubMed] [Google Scholar]
Widmayer 1981 {published data only}
- Widmayer SM, Field TM. Effects of Brazelton demonstrations for mothers on the development of preterm infants. Pediatrics 1981;67(5):711‐4. [PubMed] [Google Scholar]
References to ongoing studies
Sgandurra 2014 {published data only}
- Sgandurra G, Bartalena L, Giovanni C, Greisen G, Herskind A, Inguaggiato E, et al. Home‐based, early intervention with mechatronic toys for preterm infants at risk of neurodevelopmental disorders (CARETOY): a RCT protocol. BMC Pediatrics 2014;14:268. [PUBMED: 25319764] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Als 1997
- Als H, Gilkerson L. The role of relationship‐based developmentally supportive newborn intensive care in strengthening outcome of preterm infants. Seminars in Perinatology 1997;21(3):178‐89. [DOI] [PubMed] [Google Scholar]
Anderson 2003
- Anderson P, Doyle L, The Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003;289(24):3264‐72. [DOI] [PubMed] [Google Scholar]
Bayley 1969
- Bayley N. Bayley Scales of Infant Development. New York: The Psychological Corporation, 1969. [Google Scholar]
Bayley 1993
- Bayley N. The Bayley Scales of Infant Development. 2nd Edition. New York: The Psychological Corporation, 1993. [Google Scholar]
Bayley 2005
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edition. San Antonio, TX: Harcourt Assessment, 2005. [Google Scholar]
Becker 1999
- Becker P, Grunwald PC, Brazy JE. Motor organization in very low birth weight infants during caregiving: effects of a developmental intervention. Developmental and Behavioral Pediatrics 1999;20(5):344‐54. [DOI] [PubMed] [Google Scholar]
Berger 1998
- Berger S, Holt‐Turner I, Cupoli JM, Mass M, Hageman JR. Caring for the graduate from the neonatal intensive care unit. At home, in the office, and in the community. Pediatric Clinics of North America 1998;45(3):701‐12. [DOI] [PubMed] [Google Scholar]
Bhutta 2002
- Bhutta AT, Cleves MA, Casey PH, Craddock MM, Anand KJ. Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. JAMA 2002;288(6):728‐37. [DOI] [PubMed] [Google Scholar]
Blauw‐Hospers 2005
- Blauw‐Hospers C, Hadders‐Algra M. A systematic review of the effects of early intervention on motor development. Developmental Medicine and Child Neurology 2005;47(6):421‐32. [DOI] [PubMed] [Google Scholar]
Botting 1998
- Botting N, Powls A, Cooke RW, Marlow N. Cognitive and educational outcome of very‐low‐birthweight children in early adolescence. Developmental Medicine and Child Neurology 1998;40(10):652‐60. [DOI] [PubMed] [Google Scholar]
Brown 2001
- Brown GT, Burns SA. The efficacy of neurodevelopmental treatment in paediatrics: a systematic review. British Journal of Occupational Therapy 2001;64(5):235‐44. [Google Scholar]
Bruininks 1978
- Bruininks RH. Bruininks‐Oseretsky Test of Motor Proficiency Examiner's Manual. Circle Pines: American Guidance Service, 1978. [Google Scholar]
Campbell 1995
- Campbell SK, Kolobe TH, Osten ET, Lenke M, Girolami GL. Construct validity of the test of infant motor performance. Physical Therapy 1995;75(7):585‐96. [DOI] [PubMed] [Google Scholar]
Doyle 2004
- Doyle LW, The Victorian Infant Collaborative Study Group. Evaluation of neonatal intensive care for extremely low birthweight infants in Victoria over two decades: I. Effectiveness. Pediatrics 2004;113(3 Pt 1):505‐9. [DOI] [PubMed] [Google Scholar]
Elliot 1996
- Elliot C, Smith P, McCullock K. British Abilities Scales. London: Nelson Publishing Co Ltd, 1996. [Google Scholar]
Elliot 2007
- Elliot C. Differential Ability Scale‐II. San Antonio, TX: Harcourt Assessment, 2007. [Google Scholar]
Folio 2000
- Folio MR, Fewell RR. Peabody Developmental Motor Scales. 2nd Edition. Austin: Pro‐Education Incorporated, 2000. [Google Scholar]
Griffiths 1954
- Griffiths R. The abilities of babies: a study of mental measurement. London: University of London Press, 1954. [Google Scholar]
Griffiths 1970
- Griffiths R. The abilities of young children: a comprehensive system of mental measurement for the first eight years. London: Child Development Research Center, 1970. [Google Scholar]
Hack 2002
Hadders‐Algra 2001
- Hadders‐Algra M. Early brain damage and the development of motor behavior in children: clues for therapeutic intervention?. Neural Plasticity 2001;8(1‐2):31‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 1992
- Henderson SE, Sugden DA. Movement Assessment Battery for Children Checklist. San Antonio, TX: The Psychological Corporation, 1992. [Google Scholar]
Henderson 2007
- Henderson SE, Sugden DA, Barnett AL. Movement assessment battery for children. Second Edition. London: The Psychological Corporation, 2007. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hogan 2000
- Hogan DP, Park JM. Family factors and social support in the developmental outcomes of very‐low birth weight children. Clinics in Perinatology 2000;27(2):433‐59. [DOI] [PubMed] [Google Scholar]
Horwood 1998
- Horwood LJ, Mogridge N, Darlow BA. Cognitive educational and behavioural outcomes at 7 to 8 years in a national very low birthweight cohort. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoy 1992
- Hoy EA, Sykes DH, Bill JM, Halliday HL, McClure BG, Reid MM. The social competence of very‐low‐birth weight children: teacher, peer and self‐perceptions. Journal of Abnormal Child Psychology 1992;20(2):123‐50. [DOI] [PubMed] [Google Scholar]
Jacobs 2002
- Jacobs SE, Sokol J, Ohlsson A. The Newborn Individualized Developmental Care and Assessment Program is not supported by meta‐analyses of the data. Journal of Pediatrics 2002;140(6):699‐706. [DOI] [PubMed] [Google Scholar]
Kaufman 1983
- Kaufman A, Kaufman NL. Kaufman Assessment Battery for Children. Circle Pines: American Guidance Service, 1983. [Google Scholar]
Laucht 1997
- Laucht M, Esser G, Schmidt MH. Developmental outcomes of infants born with biological and psychosocial risks. Journal Child Psychology and Psychiatry 1997;38(7):843‐53. [DOI] [PubMed] [Google Scholar]
Majnemer 1998
- Majnemer A. Benefits of early intervention for children with developmental disabilities. Seminars in Pediatric Neurology 1998;5(1):62‐9. [PUBMED: 9548643] [DOI] [PubMed] [Google Scholar]
McCarthy 1972
- McCarthy D. Manual for McCarthy's Scales of Children's Abilities. New York: Psychological Corporation, 1972. [Google Scholar]
McManus 2012
- McManus BM, Rosenberge SA. Does the persistence of developmental delay predict receipt of early intervention services. Developmental Pediatrics and Early Intervention 2012;12(6):546‐50. [PUBMED: 23088816] [DOI] [PubMed] [Google Scholar]
Morgan 2013
- Morgan C, Novak I, Badawi N. Enriched environments and motor outcomes in cerebral palsy: systematic review and meta‐analysis. Pediatrics 2013;132(2):e735‐46. [PUBMED: 23958771] [DOI] [PubMed] [Google Scholar]
Ottenbacher 1986
- Ottenbacher KJ, Biocca Z, DeCremer G, Gevelinger M, Jedlovec KB, Johnson MB. Quantitative analysis of the effectiveness of pediatric therapy. Emphasis on the neurodevelopmental treatment approach. Physical Therapy 1986;66(7):1095‐101. [DOI] [PubMed] [Google Scholar]
Pedersen 2000
- Pedersen SJ, Sommerfelt K, Markestad T. Early motor development of premature infants with birthweight less than 2000 grams. Acta Paediatrica 2000;89(12):1456‐61. [DOI] [PubMed] [Google Scholar]
Piper 1994
- Piper MC, Darrah J. Motor Assessment of the Developing Infant. Philadelphia: WB Saunders, 1994. [Google Scholar]
Powls 1995
- Powls A, Botting N, Cooke RW, Marlow N. Motor impairment in children 12 to 13 years old with a birthweight less than 1250 g. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;73(2):F62‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Shonkoff 2003
- Shonkoff JP. From neurons to neighbourhoods: old and new challenges for developmental and behavioural pediatrics. Journal of Developmental and Behavioural Pediatrics 2003;24(1):70‐6. [PUBMED: 12584488] [DOI] [PubMed] [Google Scholar]
Simeonsson 2003
- Simeonsson RJ, Leonard M, Lollar D, Bjorck‐Akesson E, Hollenweger J, Martinuzzi A. Applying the International Classification of Functioning, Disability and Health (ICF) to measure childhood disability. Disability and Rehabilitation 2003;25(11‐12):602‐10. [DOI] [PubMed] [Google Scholar]
Sommerfelt 1996
- Sommerfelt K, Troland K, Ellertsen B, Markestad T. Behavioral problems in low‐birthweight preschoolers. Developmental Medicine and Child Neurology 1996;38(10):927‐40. [DOI] [PubMed] [Google Scholar]
Spittle 2009b
- Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, et al. Early emergence of behavior and social‐emotional problems in very preterm infants. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(9):909‐18. [DOI] [PubMed] [Google Scholar]
Spittle 2013
- Spittle AJ, Orton J. Cerebral palsy and developmental coordination disorder in children born preterm. Seminars in Fetal and Neonatal Medicine 2014;19(12):84‐9. [PUBMED: 24290908] [DOI] [PubMed] [Google Scholar]
Symington 2003
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001814] [DOI] [PubMed] [Google Scholar]
Terman 1973
- Terman LM, Merrill MA. Stanford‐Binet Intelligence Scale: Manual for the Third Revision, Form L‐M. Boston: Houghton Miffin Company, 1973. [Google Scholar]
Thelen 1996
- Thelen E, Smith L. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge: MIT Press, 1996. [Google Scholar]
Tin 1997
- Tin W, Wariyar U, Hey E. Changing prognosis for babies of less than 28 weeks' gestation in the north of England between 1983 and 1994. Northern Neonatal Network. British Medical Journal 1997;314(7074):107‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treyvaud 2010
- Treyvaud K, Anderson VA, Lee KJ, Woodward LJ, Newnham C, Inder TE, et al. Parental mental health and early social‐emotional development of children born very preterm. Journal of Pediatric Psychology 2010;35(7):768‐77. [DOI] [PubMed] [Google Scholar]
Vanderveen 2009
- Vanderveen JA, Bassler D, Robertson CM, Kirpalani H. Early interventions involving parents to improve neurodevelopmental outcomes of premature infants: a meta‐analysis. Journal of Perinatology 2009;29(5):343‐51. [DOI] [PubMed] [Google Scholar]
Vohr 2000
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993‐1994. Pediatrics 2000;105(6):1216‐26. [DOI] [PubMed] [Google Scholar]
Vohr 2005
- Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics 2005;116(3):635‐43. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang CJ, McGlynn EA, Brook RH, Leonard CH, Piechuch RE, Hsueh SI, et al. Quality‐of‐care indicators for the neurodevelopmental follow‐up of very low birth weight children: results of an expert panel process. Pediatrics 2006;117(6):2080‐92. [DOI] [PubMed] [Google Scholar]
Wechsler 1989
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence ‐ Revised. San Antonio, TX: The Psychological Corporation, 1989. [Google Scholar]
Wechsler 1991
- Wechsler D. Manual for Wechsler Intelligence Scale for Children. 3rd Edition. San Antonio, TX: The Psychological Corporation, 1991. [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health, 2001. www.who.int/classifications/icf/en/. (accessed 11 November 2012).
Williams 2010
- Williams J, Lee KJ, Anderson PJ. Prevalence of motor‐skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Developmental Medicine and Child Neurology 2010;52(3):232‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Orton 2009
- Orton J, Spittle A, Doyle L, Anderson P, Boyd R. Do early intervention programmes improve cognitive and motor outcomes for preterm infants after discharge? A systematic review. Developmental Medicine and Child Neurology 2009;51(1):851‐9. [DOI] [PubMed] [Google Scholar]
Spittle 2007
- Spittle AJ, Orton J, Doyle LW, Boyd RN. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005495.pub2] [DOI] [PubMed] [Google Scholar]


