Summary
The mushroom bodies of Drosophila contain circuitry compatible with race models of perceptual choice. When flies discriminate odor intensity differences, opponent pools of αβ core Kenyon cells (on and off αβc KCs) accumulate evidence for increases or decreases in odor concentration. These sensory neurons and “antineurons” connect to a layer of mushroom body output neurons (MBONs) which bias behavioral intent in opposite ways. All-to-all connectivity between the competing integrators and their MBON partners allows for correct and erroneous decisions; dopaminergic reinforcement sets choice probabilities via reciprocal changes to the efficacies of on and off KC synapses; and pooled inhibition between αβc KCs can establish equivalence with the drift-diffusion formalism known to describe behavioral performance. The response competition network gives tangible form to many features envisioned in theoretical models of mammalian decision making, but it differs from these models in one respect: the principal variables—the fill levels of the integrators and the strength of inhibition between them—are represented by graded potentials rather than spikes. In pursuit of similar computational goals, a small brain may thus prioritize the large information capacity of analog signals over the robustness and temporal processing span of pulsatile codes.
Highlights
-
•
Mushroom body output neurons respond with excitation to odor on- and offset
-
•
On and off responses reflect the convergence of oppositely tuned Kenyon cells (KCs)
-
•
Opponent KCs compete by eliciting inhibitory feedback from a common interneuron pool
-
•
KCs and interneurons communicate through graded potentials rather than spikes
Vrontou et al. demonstrate the convergence of oppositely tuned Kenyon cell (KC) populations onto the same mushroom body output neurons (MBONs). The rival KCs vie for control of MBON activity via independently plastic synapses and inhibitory feedback from a common interneuron pool, as proposed in response competition models of perceptual choice.
Introduction
Two-alternative forced-choice tasks, in which a subject must commit to one of two alternatives, sometimes under time pressure and nearly always with uncertain information, are a commonly studied laboratory simplification of real-world decision making. The neural processes that culminate in a binary choice have been compared to the deliberations of a jury before a verdict:1 neurons, like jurors, gather evidence from witnesses over the course of a trial and then reconcile their divergent views in a majority vote.
The problem of how neural circuits implement this form of trial by jury has been approached in a range of species, from primates and rodents to fish and flies. A pioneering and influential body of work is built on a two-alternative forced-choice task in which monkeys distinguish directions of motion in a noisy random dot display.2 Recordings of correlated neuronal activity suggest that motion-sensitive neurons in the middle temporal visual area (MT or V5) provide momentary evidence2,3 that is temporally integrated in lateral intraparietal cortex (LIP) before passing an unspecified thresholding mechanism.4,5 Although the precise role attributed to LIP is a matter of debate,6 the principle that ephemeral sensory signals flow into integrators whose fill levels rise to a response threshold appears general; similar arrangements have been inferred to support visual motion discrimination in zebrafish7,8 and odor intensity discrimination in the fly.9,10
In Drosophila, a rate-limiting integration step takes place in a particular group of third-order olfactory neurons.9,10 When flies decide on the direction of an odor concentration change, the membrane potentials of Kenyon cells (KCs) in the αβ core (αβc) division of the mushroom bodies drift noisily toward action potential threshold,10 just as accumulating evidence would drift toward a response bound.1,11, 12, 13, 14 Consistent with the proposed correspondence of membrane voltage and integrated sensory information, and of action potential and decision thresholds, neurometric functions based on the average timing of the first odor-evoked spikes in the αβc KC population can account for the speed and accuracy of the decision-making animal;10 psychophysical estimates of noise in the decision process match the measured membrane potential noise of αβc KCs;10 and genetically targeted manipulations that alter the latencies of αβc KC spikes have the expected impact on reaction times.9,10
Two functionally separate groups of αβc KCs, termed up and down or on and off cells, respond to increases or decreases in odor concentration10,15 and can therefore represent the strength of evidence for either of the two alternatives in the choice.10 This explicit representation of support for each of the competing hypotheses (as opposed to an aggregate representation of the extent to which one hypothesis is favored over the other) suggests that a decision involves a race between two integrators12,16,17—one built from neurons that accumulate evidence for an increase in odor concentration and another composed of “antineurons”2 that do the opposite. Changes in odorant receptor occupancy at the periphery alter the baseline activity of olfactory receptor neurons and the second-order projection neurons (PNs) with which they form receptor-specific glomerular channels. Large odor concentration changes in a channel’s preferred direction drive high-frequency transmission from PNs to αβc KCs that promotes steep depolarizations to spike threshold and fast, accurate decisions, whereas small concentration changes in the preferred direction, or any change in the null direction, cause only a trickle of synaptic release; shallow, undulating membrane potential rises; and long spike delays that lead to slow, error-prone choices.10,14
In this study, we examine whether the circuitry downstream of αβc KCs is compatible with a model of two competing integrators. We test three predictions of such a model. First, to adjudicate the rival hypotheses advocated by on and off αβc KCs, mushroom body output neurons (MBONs)18,19 sampling the cores of the αβ lobes must listen to both. We therefore expect that each core-innervating MBON is excited by increases as well as decreases in odor concentration. Second, as an animal learns the rules of the two-alternative forced-choice task—that an increase in odor intensity predicts imminent electric shock, whereas a decrease signals protection9—the influence of αβc KCs championing the correct choice should be enhanced while that of proponents of the incorrect choice should be diminished. In other words, we expect antagonistic changes in the strengths of connections of on and off αβc KCs with the same action selection neurons if evidence for the competing alternatives is accumulated separately. Third, race models become equivalent to a drift-diffusion process—the formalism shown accurately to describe the psychophysics of the decision-making animal9,10—only if they include an element of mutual or pooled inhibition12,20,21 to establish response competition between the integrators.22 Inhibition is needed to ensure that the integrators are anti-correlated so that evidence for one choice simultaneously counts as evidence against the other. We therefore predict the existence of inhibitory interactions between αβc KCs.
Results
Core-innervating MBONs respond to odor on- and offset
Seven classes of KC (of which αβc KCs are one) elaborate parallel, tightly packed axon bundles that fill the cross-sections of the mushroom body lobes.18,19 As they run the lengths of each lobe, KC axons cross five or six compartments defined by the non-overlapping dendritic fields of MBONs.19 Each compartment functions as a semi-autonomous memory unit that records the predictive value of the animal’s olfactory experience for a set of motivated actions. Information is stored when local dopaminergic reinforcement adjusts the connection strengths of KCs with the compartment’s inherently valued MBON.14,19,23, 24, 25, 26, 27, 28 As in an electronic memory, matched pairs of data input and output lines—dopaminergic neurons (DANs) and MBONs, respectively—supply each data storage location.19,23
The system is arranged so that MBONs driving approach read KC activity in the vertical (α and α’) lobes, peduncle, and heel (i.e., the γ1 compartment), while MBONs driving aversion sample the horizontal (β, β’, and γ) lobes.25 This arrangement is mirrored in the segregation of positively and negatively reinforcing DANs: the axonal projections of negatively reinforcing neurons in the paired posterior lateral cluster 1 (PPL1) target the vertical lobes and heel,23,29 while those of rewarding neurons in the paired anterior medial cluster (PAM) are confined to the horizontal lobes.30,31 Because coincident dopamine release onto active KC-to-MBON synapses causes synaptic depression,24,26 PPL1 reinforcement diminishes an odor’s appeal by attenuating how strongly its KC representation activates attractive MBONs, while PAM reinforcement does the opposite by weakening KC coupling to aversive outputs.
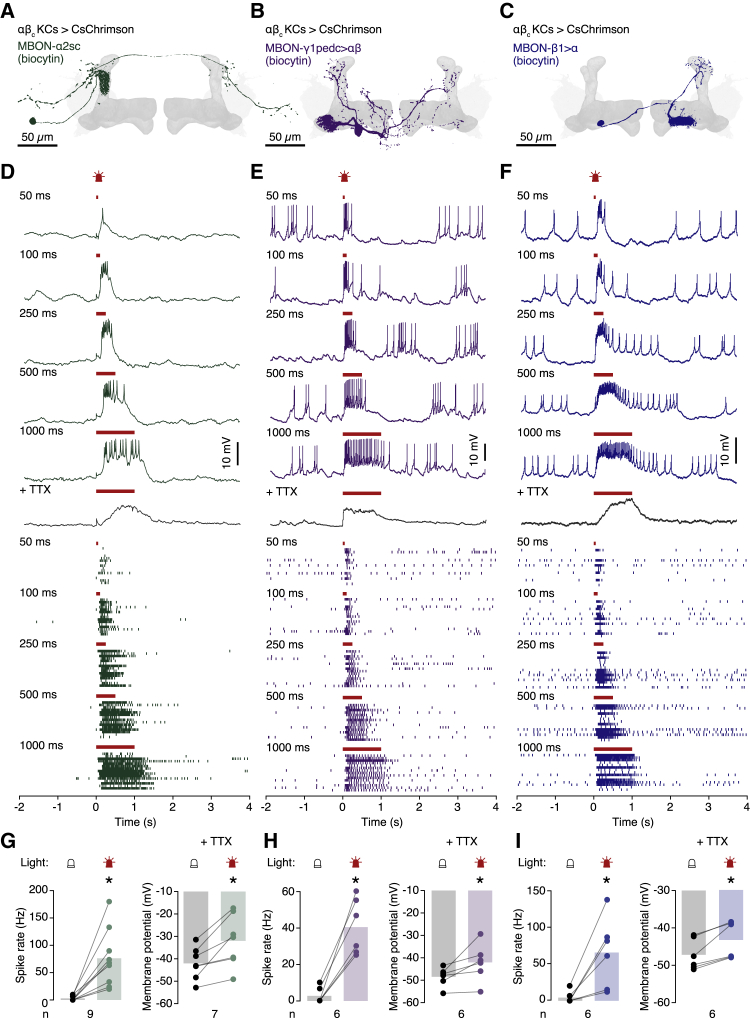
Anatomical analyses19,32 suggest that αβc KCs are a major, but not necessarily the only, source of presynaptic input to three MBON types: MBON-α2sc in the α lobe (Figure 1A); MBON-γ1pedc>αβ in the peduncle (Figure 1B); and MBON-β1>α in the β lobe (Figure 1C). We verified the existence of functional connections in targeted whole-cell recordings from these MBONs in vivo, using selective GAL4 drivers to label individual neurons (Figures 1 and S1). Different output neurons showed characteristic rates and patterns of baseline activity, ranging from quiescence or sparse firing (MBON-α2sc; Figure 1D) to persistent irregular spiking or bursting (MBON-γ1pedc>αβ and MBON-β1>α; Figures 1E and 1F) or alternating UP and DOWN states (MBON-γ4>γ1γ2, which lacks demonstrable core innervation19,32 and served as our specificity control; Figures S1A and S1B). These different MBON personalities have approximate counterparts in the corresponding DANs (see below).
Figure 1.
Three types of core-innervating MBON
(A–C) Biocytin fills of MBON-α2sc (A), MBON-γ1pedc>αβ (B), and MBON-β1>α (C).
(D–F) Example voltage traces (top) and spike rasters (bottom) of MBON-α2sc (D), MBON-γ1pedc>αβ (E), and MBON-β1>α (F) during optogenetic activation of αβc KCs for the indicated durations.
(G–I) Average spike rates (left) and membrane potentials (right) before and during optogenetic activation of αβc KCs, in the absence (left) or presence of TTX (right). Illumination increased the spike rates and membrane potentials of MBON-α2sc (G; p = 0.0312 and p = 0.0084; Wilcoxon test and paired t test, respectively), MBON-γ1pedc>αβ (H; p = 0.0312 and p = 0.0469; Wilcoxon test and paired t test, respectively), and MBON-β1>α (I; p = 0.0312 and p = 0.0312, respectively; Wilcoxon tests). ∗p < 0.05. Data are means ± SEM; n, number of cells.
See also Figure S1.
Optogenetic activation33 of αβc KCs expressing CsChrimson caused depolarizations of up to 20 mV that elevated the firing rates of all three core-innervating MBONs (Figures 1D–1I), but not of MBON-γ4>γ1γ2 (Figures S1B and S1C). Blocking voltage-gated sodium channels with tetrodotoxin (TTX) eliminated action potentials but preserved the voltage deflection on which these action potentials normally ride (Figures 1D–1I), while adding the nicotinic acetylcholine receptor antagonist mecamylamine34 on top of TTX leveled also the subthreshold depolarization and thereby ruled out leaky expression of the optogenetic actuator in the MBON itself (Figures S1D–S1F).
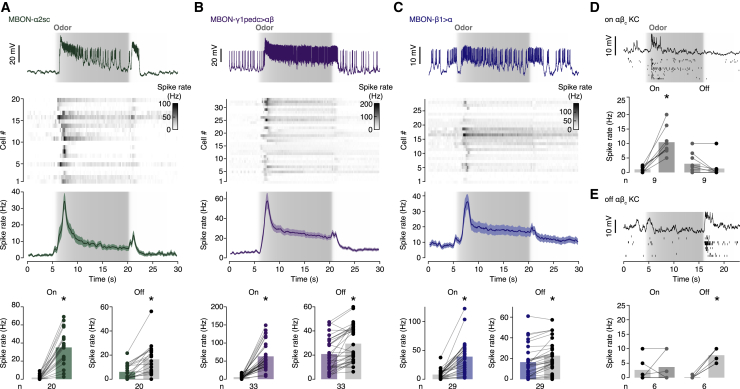
Long odor pulses elicited voltage and spiking responses in core-innervating MBONs whose sharp onset resembled that during optogenetic core KC activation (Figures 2A–2C). A few seconds into the pulse, after the initial depolarization to peak, the membrane potentials and spike rates declined and stabilized at slightly (MBON-α2sc) or moderately elevated plateaux (MBON-β1>α and MBON-γ1pedc>αβ) before rising again to a second peak at the end of the pulse (Figures 2A–2C). We attribute the peaks at odor on- and offset to transmission dominated by on and off αβc KCs (Figures 2D and 2E) whose distinct contributions became visible when a long odor pulse separated them widely in time.15 Under more realistic conditions, such as short odor exposures resembling concentration fluctuations within an odor plume, the on and off responses bled into each other (Figures S2A and S2B).
Figure 2.
Responses of core-innervating MBONs to odor on- and offset
(A–C) Responses of MBON-α2sc (A), MBON-γ1pedc>αβ (B), and MBON-β1>α (C) during 15-s odor pulses (gray shading indicates measured concentration time courses). Top to bottom: example voltage traces, heatmaps of the spike rates of individual cells, spike rate averages (±SEM), and spike rate comparisons before and after odor intensity changes. Odor on- and offset increased the spike rates of MBON-α2sc (A; p < 0.0001 and p < 0.0001, respectively; Wilcoxon tests), MBON-γ1pedc>αβ (B; p < 0. 0001 and p < 0.0001, respectively; Wilcoxon tests), and MBON-β1>α (C; p < 0.0001 and p < 0.0001; Wilcoxon test and paired t test, respectively).
(D and E) Responses of on (D) and off αβc KCs (E) during 10-s odor pulses (gray shading indicates measured concentration time courses). Top to bottom: example voltage traces, spike rasters of individual cells, and spike rate changes caused by odor on- and offset. Odor onset, but not odor offset, increased the spike rates of on αβc KCs (D; p = 0.0039 and p = 0.1250, respectively; Wilcoxon tests), while odor offset, but not odor onset, increased the spike rates of off αβc KCs (E; p = 0.0312 and p = 0.7500, respectively; Wilcoxon tests). ∗p < 0.05. Data are means ± SEM.
See also Figure S2.
Although the numerical excess of on over off αβc KCs is modest at best,10,15 the off responses of all three MBON types were uniformly smaller than their on responses (Figures 2A–2C). The relative strengths of these responses could simply be handed down to αβc KCs from their presynaptic partners, or the KC group that becomes active first—here, on αβc KCs at odor onset—could partially suppress later responders through response competition, as envisioned in our third prediction. Noting this subtlety (but putting it to one side for the moment), we conclude that Figure 2 confirms our first prediction: each core-innervating MBON responds to increases as well as decreases in odor concentration.
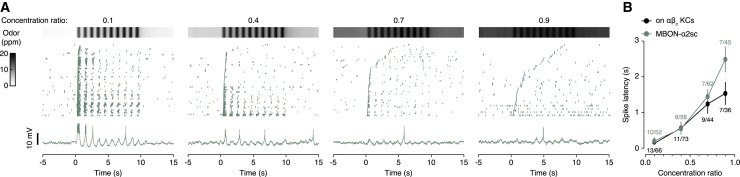
Spike latencies of MBONs versus αβc KCs
If αβc KCs perform a rate-limiting integration step in the decision process,10 they must be rate limiting also for the odor responses of their postsynaptic partners. To establish this point, we concentrated on MBON-α2sc, whose sparse basal activity (Figure 1D) allowed precise measurements of spike latencies. When flies were exposed to a stream of 4-methyl-cyclohexanol (MCH) switching repeatedly between a base concentration that varied between trials (2–18 ppm) and a peak concentration that remained constant (20 ppm), the latency of the first MBON-α2sc spike increased monotonically with diminishing intensity contrast (Figure 3A). The growing MBON spike delays matched those of αβc KCs under the same conditions10 and paralleled increases in decision time when flies discriminate ever smaller intensity differences.9 Averaged across trials, the action potentials of MBON-α2sc closely tracked or slightly trailed—but never led—those of αβc KCs (Figure 3B), which therefore represent the temporal bottleneck in the process.
Figure 3.
Spike latencies of MBON-α2sc versus αβc KCs
(A) Top to bottom: MCH concentrations, spike rasters, and example voltage traces of MBON-α2sc during ten odor intensity cycles between a variable base (2–18 ppm) and a constant peak (20 ppm) MCH concentration. Measured MCH concentration time courses at the different base-to-peak ratios (0.1–0.9, left to right) are displayed on top. The spike rasters are sorted, in ascending order from the bottom, by the latency of the first spike.
(B) Spike latencies of MBON-α2sc and on αβc KCs as functions of MCH concentration ratio. Data are means ± SEM; sample sizes (cells/trials) are indicated near data points. The spike latencies of αβc KCs are replotted from Groschner et al.10
An extensive analysis of odor tuning in the KC ensemble showed that any given KC will respond to the onset of some odors and the offset of others (Jan Kropf, Clifford B. Talbot, and G.M., unpublished data). Put differently, the same neuron will be classified as an on or an off cell (but rarely as both)15 in different olfactory contexts. Because the KCs responsible for the on and off responses are functionally interchangeable (for example, by choosing different odors), a spike latency match similar to the one we have demonstrated for MCH at odor onset (Figure 3B) is expected to hold true also at odor offset.
Reciprocal plasticity of on and off responses
Flies learn to perform odor intensity discrimination by analyzing temporal relations between events. If a neutral odor reliably precedes an unpleasant experience, such as an electric shock, the odor itself acquires negative valence: its presentation generates the anticipation of danger and its fading the anticipation of relief, while the converse is true if the sequence of events is reversed.35 The physical expression of these changed expectations is altered synaptic weights between KCs and MBONs.24,26, 27, 28 A second prediction of our model is, therefore, that positive or negative reinforcement of an odor will change the coupling strengths of its on and off αβc KCs with the same MBONs in opposite directions.
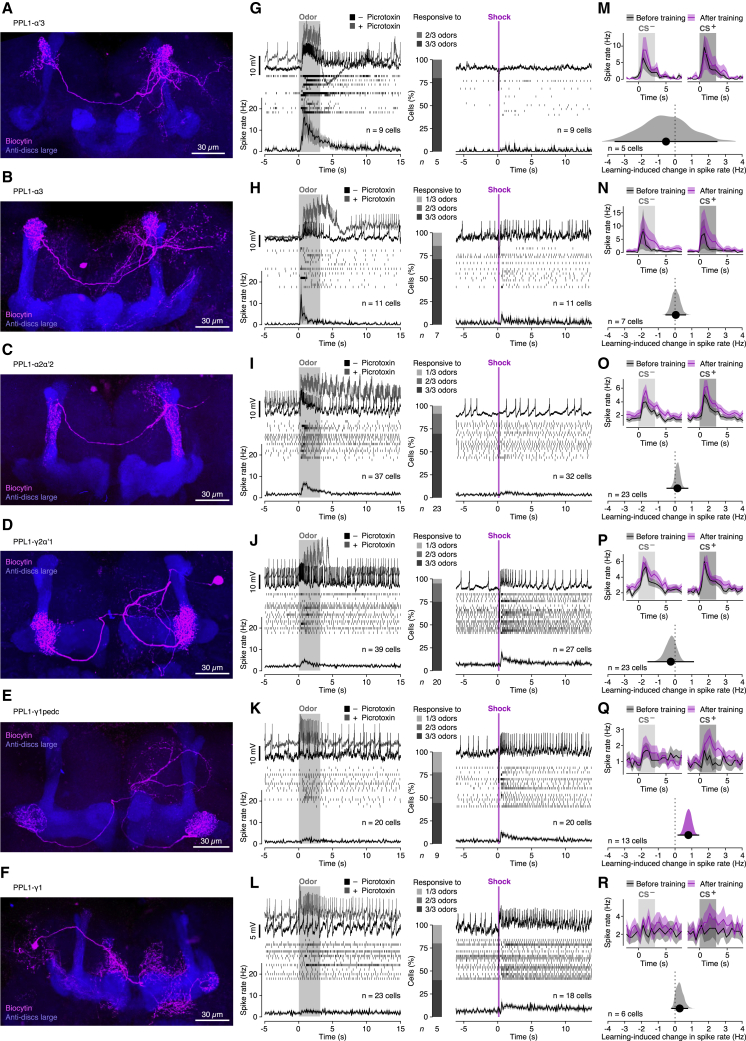
Dopaminergic neurons encode the error signals that guide learning when reality and expectation collide.23, 36, 37 Because flies in our two-alternative forced-choice task are trained by unexpected electric shock,9 members of the 12-strong PPL1 cluster of negatively reinforcing DANs are the relevant carriers of these signals.23 As a prelude to exploring the types of plasticity these neurons instruct, we characterized their activity in whole-cell recordings. On the basis of their axonal projection patterns (Figures 4A–4F), we could distinguish 10 morphological PPL1 types, six of which targeted the mushroom bodies:19,38 the tips of the α’ (PPL1-α’3; Figure 4A) and α lobes (PPL1-α3; Figure 4B); the vertical stalk (PPL1-α’2α2; Figure 4C); the junction between the vertical and horizontal lobes (PPL1-γ2α’1; Figure 4D); the α, β, and γ portions of both heels (PPL1-γ1pedc; Figure 4E); and the γ portion of the contralateral heel (PPL1-γ1; Figure 4F).
Figure 4.
Functional classification of PPL1 neurons
(A–F) Biocytin fills of mushroom-body-innervating PPL1 neurons (magenta). Synaptic structures of the mushroom body lobes were counterstained with an antibody against discs large (blue).
(G–L) Top to bottom: example voltage traces, spike rasters, and spike rate averages (±SEM) of different types of PPL1 neuron during 3-s exposures to isopentyl acetate (left, gray) or 10-ms exposures to electric shock (right, magenta). Stacked column graphs in the center show response percentages to the odors isopentyl acetate, MCH, and OCT. The left-hand panels include example voltage traces after the addition of picrotoxin (gray).
(M–R) Top: spike rate averages (±SEM) of different types of PPL1 neuron during exposures to MCH (CS−, left) or OCT (CS+, right), before (black) and after (magenta) electric shock reinforcement. Bottom: effect size plots of training-induced spike rate changes in different types of PPL1 neuron: (CS+after − CS+before) − (CS−after − CS−before). Shaded areas with solid bars at their bases represent bootstrapped sample distributions and 95% confidence intervals, respectively. Magenta color indicates a statistically significant training effect only for PPL1-γ1pedc (Q; p = 0.0022; one-sample t test). ∗p < 0.05. Data are means ± SEM.
See also Figure S3.
The six mushroom-body-innervating PPL1 neurons fell into two broad classes based on their differential sensitivity to punitive cues (Figures 4G–4L). Applications of electric shock caused prominent firing rate increases in one group (PPL1-γ2α’1, PPL1-γ1pedc, and PPL1-γ1; Figures 4J–4L) but had muted effects on the other (PPL1-α’3, PPL1-α3, and PPL1-α’2α2; Figures 4G–4I). Conversely, presentations of odors caused the largest firing rate modulations in cells with the faintest shock responses (Figures 4G–4I). Where odor responses were detected, they were broadly tuned: at least two of the three odors tested elicited spiking in the majority of responsive neurons (Figures 4G–4I). A tentative interpretation of these observations, which mesh with earlier imaging analyses,38,39 is that the PPL1 cluster contains two anatomically and functionally separable dopaminergic systems: a group of sporadically firing cells (PPL1-α’3, PPL1-α3, and PPL1-α’2α2), which report sudden changes in sensory input (novelty or salience),39 and a group of more persistently active neurons (PPL1-γ1pedc, PPL1-γ1, and PPL1-γ2α’1), which offer a running commentary on the animal’s hedonic state (valence).
Closer scrutiny, however, revealed that the separation between odor- and pain-responsive cells was not absolute. Cases in point are PPL1-γ1pedc and PPL1-γ1, which often showed, in addition to prominent spike rate increases after electric shock, muffled odor responses that could be boosted substantially by blocking GABAA receptors with picrotoxin (Figures 4K, 4L, and S3A). GABA exerted at least some of its inhibitory effect directly on these DANs, as spatially restricted RNA-mediated interference (RNAi) with the expression of the GABAA receptor Rdl also amplified their odor responses (Figure S3B). All PPL1 neurons thus receive broadly tuned excitatory olfactory input, but balanced inhibition normally suppresses the responses of valence-encoding cells to behaviorally neutral odors.
Formal accounts of learning posit that neurons carrying reinforcement signals become responsive to neutral stimuli after these stimuli acquire motivational significance (for example, as predictors of reward or pain).36 The presence of broadly tuned odor responses that are normally concealed by inhibition suggests a simple mechanism for transferring activity from a primary reinforcer to a conditioned stimulus: odor-specific changes in the balance of excitation and inhibition could unmask the suppressed response to the conditioned odor. Because the pain-responsive DANs are latent odor generalists (Figures 4J–4L), such a mechanism could rapidly associate any odor with a motivational dopamine signal.
To examine whether learning would indeed expose or augment a cryptic dopamine response to the conditioned odor,40 we recorded from PPL1 neurons during conditioning trials that paired two blocks of six 3-s presentations of the odor 3-octanol (OCT) with applications of electric shock. Presentations of OCT with shock reinforcement were interlaced with presentations of a second odor, MCH, without reinforcement. Five minutes after the last training cycle, we challenged the animals with the two odors in random sequence. Of the six mushroom-body-innervating PPL1 neurons (Figures 4M–4R), only PPL1-γ1pedc showed enhanced responses to the conditioned odor after training (Figure 4Q). MBON-γ1pedc>αβ, the output neuron twinned with PPL1-γ1pedc, therefore took center stage in our search for reciprocal changes in its coupling strengths to on and off αβc KCs that are expected to underlie short-term changes in behavior (Figures 5A and 5B). We obtained current-clamp recordings from MBON-γ1pedc>αβ and exposed flies to a total of six 15-s odor pulses that each overlapped with two 1.5-s epochs of electric shock. This training protocol altered the on and off responses of MBON-γ1pedc>αβ antagonistically, in keeping with our second prediction: the on response was depressed while the off response was potentiated (Figures 5C and S4A).
Figure 5.
Reciprocal plasticity of on and off responses
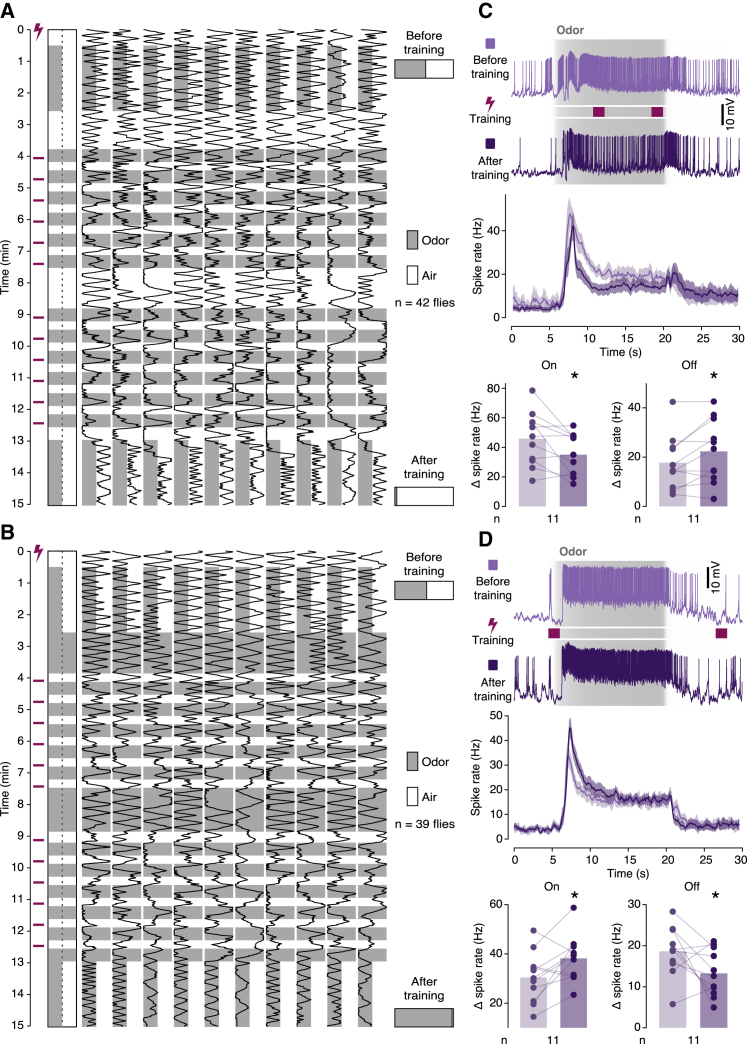
(A and B) Behavioral plasticity induced by electric shock reinforcement after odor onset (A) or odor offset (B). Example movement traces of 10 flies in individual chambers. The flies were allowed to choose between MCH (gray) and air during test periods before and after the electric shock reinforcement of MCH (A) or air (B), as diagrammed on the left. Bar graphs on the right indicate the fractions of choices in favor of MCH (gray) and air (white) before and after training.
(C) Synaptic plasticity induced by electric shock reinforcement after odor onset. Responses of MBON-γ1pedc>αβ to 15-s odor pulses (gray shading indicates measured concentration time courses), before and after electric shock reinforcement (light and dark colors) at times indicated by magenta bars. Top to bottom: example voltage traces, spike rate averages (±SEM), and spike rate changes caused by odor on- and offset. The on and off responses were depressed and potentiated, respectively (p = 0.0262 and p = 0.0325; paired t tests), after training.
(D) Synaptic plasticity induced by electric shock reinforcement after odor offset. Responses of MBON-γ1pedc>αβ to 15-s odor pulses (gray shading indicates measured concentration time courses), before and after electric shock reinforcement (light and dark colors) at times indicated by magenta bars. Top to bottom: example voltage traces, spike rate averages (±SEM), and spike rate changes caused by odor on- and offset. The on and off responses were potentiated and depressed, respectively (p = 0.0050 and p = 0.0471; paired t tests), after training. ∗p < 0.05. Data are means ± SEM.
See also Figures S4 and S5.
Plasticity rules
The differential plasticity of on and off responses is a likely consequence of the order in which on and off αβc KCs become active relative to PPL1-γ1pedc: the activation of on αβc KCs precedes the shock-induced dopamine release, whereas the activation of off αβc KC trails it (Figures 5A and 5C). KC-to-MBON synapses are sensitive to the timing of dopaminergic reinforcement and undergo depression or potentiation after forward or backward pairing.28 Applying these rules to the dual representation of odors by on and off KCs could explain the antagonistic changes in the MBON’s on and off responses: because each odor is represented twice (once by on and once by off αβc KCs; Figure 2), it is also conditioned twice—forward at onset and backward at offset (Figures 5A and 5C). Inverting these timing relationships (by delivering electric shock during the air interval between odor pulses; Figures 5B and 5D) turned learned aversion into attraction (compare Figures 5A and 5B) and produced mirror-symmetric changes in the on and off responses of MBON-γ1pedc>αβ (compare Figures 5C and 5D).
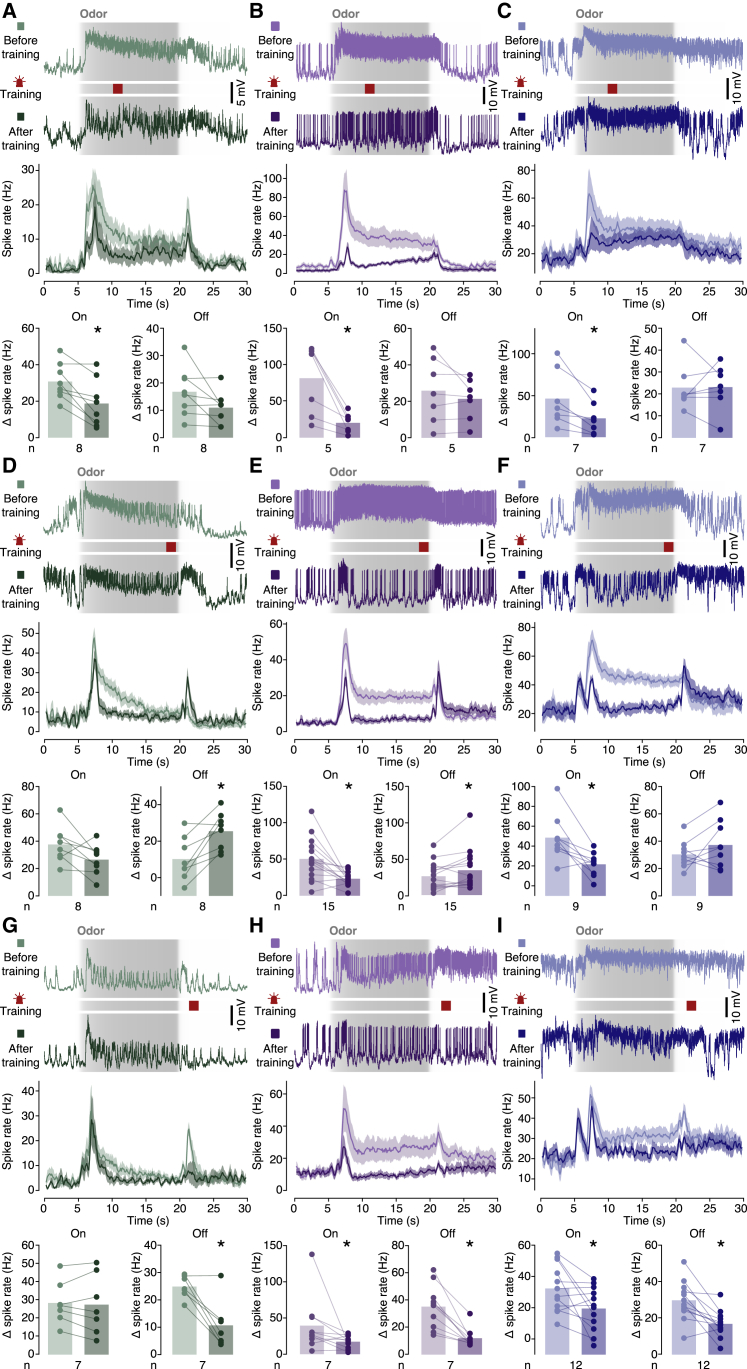
To examine whether similar plasticity rules held for all core-innervating MBONs, we replaced the electric shock reinforcer with direct optogenetic activation of the affine DANs: of PPL1-γ1pedc for MBON-γ1pedc>αβ, of PPL1-α’2α2 for MBON-α2sc, and of PAM neurons for MBON-β1>α. Nesting a block of optogenetic reinforcement within the 15-s odor pulse preserved the sequential activation of on KCs, DANs, and off KCs and recapitulated the pattern of plastic changes seen during standard electric shock training (Figure 5C); it produced depressed on and potentiated off responses in MBON-α2sc (Figure S5A) and MBON-γ1pedc>αβ (Figures S4B and S5B). Confining reinforcement to the first few seconds of the odor pulse selectively depressed the on response but left the off response unchanged (Figures 6A–6C), whereas restricting reinforcement to the period immediately before odor offset potentiated most off responses while continuing to depress some on responses (Figures 6D–6F). The efferent synapses of KCs therefore remained plastic long after the peak in odor-evoked activity, as is evident also behaviorally: flies correctly credited pain to predictive odor intensity changes experienced some 18 s earlier (Figures 5A and 5B). Consistent with a slowly decaying eligibility trace in αβc KC terminals, DAN activation after odor offset depressed not only the off but sometimes also the still pliant on response (Figures 6G–6I).
Figure 6.
Plasticity rules
Responses of MBON-α2sc (left column), MBON-γ1pedc>αβ (center column), or MBON-β1>α (right column) to 15-s odor pulses (gray shading indicates measured concentration time courses), before and after the optogenetic activation of the affine PPL1 or PAM neurons (light and dark colors) at times indicated by crimson bars. Top to bottom: example voltage traces, spike rate averages (±SEM), and spike rate changes caused by odor on- and offset.
(A–C) Dopaminergic reinforcement after odor onset depressed all on responses (A, p = 0.0277; B, p = 0.0156; C, p = 0.0172; paired t [A and C] or Wilcoxon tests) but left the off responses unchanged (A, p = 0.0764; B, p = 0.2537; C, p = 0.9596; paired t tests).
(D–F) Dopaminergic reinforcement before odor offset potentiated most off responses (D, p = 0.0056; E, p = 0.0256; F, p = 0.2100; paired t [D and F] or Wilcoxon tests) and depressed most on responses (D, p = 0.0709; E, p = 0.0023; F, p = 0.0064; paired t tests).
(G–I) Dopaminergic reinforcement after odor offset depressed all off responses (G, p = 0.0312; H, p = 0.0020; I, p = 0.0019; paired t [I] or Wilcoxon tests) as well as some on responses (G, p = 0.6690; H, p = 0.0273; I, p = 0.0248; paired t [G and I] or Wilcoxon tests). ∗p < 0.05. Data are means ± SEM.
Inhibitory competition between αβc KCs
The division between on and off αβc KCs does not respect fixed anatomical or gene expression boundaries but instead changes dynamically with sensory input (Jan Kropf, Clifford B. Talbot, and G.M., unpublished data). This fluid, odor-specific membership of individual neurons in the two functional KC classes, and the consequent lack of genetic markers for these classes, made an experimental test of our third prediction—that on and off αβc KCs inhibit one another—difficult.
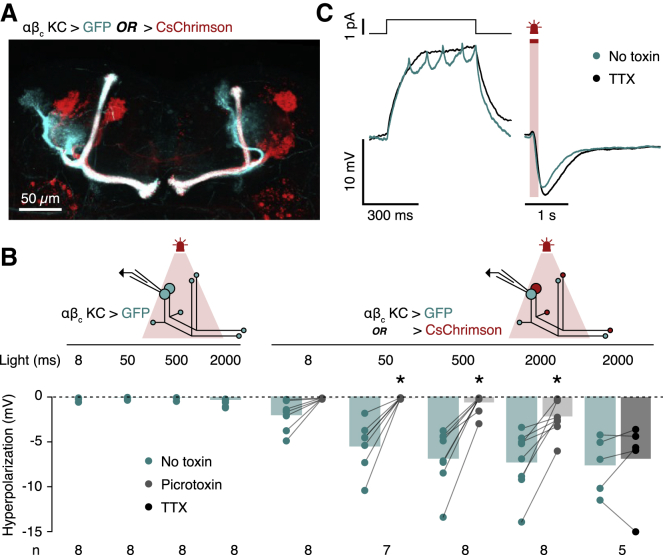
To circumvent this difficulty, we generated a GAL4-responsive FLP-out construct41 for the mutually exclusive expression of CsChrimson or GFP in random subsets of αβc KCs. We then used these non-overlapping populations of differentially responsive neurons as proxies for on and off KCs (in the original context, on and off KCs are differentially responsive to odor concentration changes; here, the two complementary KC sets are differentially responsive to light). The CsChrimson-expressing population was also tagged with tdTomato, which permitted us to visualize the two KC sets and verify the absence of overlap between them (Figure 7A). Recombination, mediated by low-level uninduced expression of an hsFLP transgene,42 typically took place at the neuroblast stage, with all descendants of each of the four lineages that give rise to the mushroom body43 belonging to one or the other αβc KC set (Figure 7A). As predicted, current-clamp recordings from GFP-positive (and, therefore, CsChrimson-negative) αβc KCs showed deep, photon dose-dependent hyperpolarizations of up to 15 mV below the membrane potential baseline when the CsChrimson-positive complement of αβc KCs was stimulated by light (Figures 7B and S6). Picrotoxin blocked the inhibitory response, identifying it as GABAergic (Figure 7B). Because KCs themselves are cholinergic and excitatory,34 within-core inhibition must involve an intermediary that signals through GABA, is activated by KCs, and provides feedback inhibition to KCs. The anterior paired lateral neuron (APL), a GABAergic neuron present in a single copy per hemisphere, fits this bill precisely.18,32,44,45 APL forms reciprocal synapses with KCs throughout the mushroom body18,32 and delivers local, KC division- or compartment-restricted inhibition.44, 45, 46
Figure 7.
Inhibition between αβc KCs
(A) Mutually exclusive FLP-mediated expression of CD8::GFP (cyan) or CsChrimson::tdTomato (crimson) in αβc KCs.
(B) Peak hyperpolarizations of CD8::GFP-positive αβc KCs during light pulses of the indicated durations, in the absence (left) or presence (right) of FLP-mediated recombination to generate a CsChrimson-expressing αβc KC population, before (green columns) or after the addition of picrotoxin (light gray columns) or TTX (dark gray column). Two-way repeated-measures ANOVA detected significant effects of light exposure and picrotoxin on the amplitude of hyperpolarization (p = 0.0034 and p < 0.0001, respectively), while TTX had no effect (p = 0.6250; Wilcoxon test). ∗p < 0.05 in post hoc comparisons. Data are means ± SEM.
(C) Example voltage responses of a CD8::GFP-positive αβc KC during a 500-ms depolarizing current step (left) and a 200-ms light pulse (right), before and after the addition of TTX (green and black traces, respectively).
See also Figure S6.
The subthreshold nature of the integration process,10 however, poses a conundrum: if on and off αβc KCs accumulate sensory evidence by integrating synaptic potentials to spike threshold,10 how can APL exert inhibitory feedback when neither competitor emits an action potential before the race is decided? A likely answer lies in the peculiar structure and biophysics of the feedback loop. αβc KCs and APL are densely interconnected not only in the mushroom body lobes, which contain the axons of KCs, but also in the calyx, where KC dendrites reside.18 Here, both neuronal partners form numerous presynaptic active zones,32,47 indicative of bidirectional communication between them: each αβc KC excites APL through an average of 13.4 synapses—that is, roughly one contact per 20 μm of dendrite—and is inhibited by APL via 10.0 reciprocal synapses.46 Like its locust analog, the giant GABAergic neuron (GGN), APL is a non-spiking cell whose rate of GABA release tracks local voltage changes in a graded fashion;48 in GGN and other non-spiking interneurons, depolarizations of 2–5 mV suffice to trigger secretion.48,49
But how is transmission at the dendritic release sites32,47 of αβc KCs controlled? Membrane potential measurements suggest that these synapses also function in analog, graded-potential mode, whereas transmission at axonal KC-to-MBON terminals is digital and spike dependent. Action potentials backpropagating from the axon initial segment of αβc KCs arrived at the somatic recording site with severely attenuated amplitudes of 1.95 ± 0.09 mV (mean ± SEM; n = 34 cells), barely larger than those of single excitatory postsynaptic potentials (EPSPs) advancing through the dendritic tree (mean ± SEM = 1.25 ± 0.05 mV; n = 36 cells). In the dendrites themselves (which are inaccessible to direct measurements), the relative magnitude of the potentials may flip, with the now unattenuated EPSPs exceeding the size of backpropagating spikes. EPSPs, not action potentials, would then be the dominant form of depolarization in KC dendrites and, as such, the principal drivers of transmitter release. Indeed, the light-induced hyperpolarizations of CsChrimson-negative αβc KCs persisted unabated when action potentials were blocked with TTX (Figures 7B and 7C). All circuit elements are therefore in place for pooled inhibition12,20, 21, 22 between on and off αβc KCs, with APL representing the interneuron “pool” fed by the competing integrators.
Discussion
The idea that decisions are based on the accumulated spikes of oppositely tuned sensors was born in early attempts to unite psychophysical and neurophysiological measurements under the umbrella of signal detection theory.2,3 The recorded spike count distributions of direction-selective units in the monkey’s area MT to motion in the preferred or null directions were taken to represent the responses of two neurons—the recorded neuron and its imagined antineuron conjugate—to movement in the neuron’s preferred direction. The likely direction of motion can then be inferred as the probability that a draw from the neuron’s response distribution yields a larger spike count than a draw from the antineuron’s.2,3 At minimal motion strengths, when the two distributions are congruent, these odds are even and choices are random, but as the neuron responds ever more vigorously to increasingly coherent motion while the antineuron’s response stays flat, the distributions unmix and the probability of a correct choice rises toward one. Comparing the spike counts of two sensors rather than thresholding the output of one removes shared sources of variation and with them the need of adjusting the discrimination threshold to achieve the best separation of the changing response distributions: a neuron-antineuron pair always returns a quantity proportional to likelihood ratio, the optimal hypothesis test.1,11 Although opponent sensory channels in one or another guise feature prominently in many decision-making models,1, 2, 3,11,12,14,20, 21, 22,50 their involvement in the brain is unproven: neurons and antineurons owe their status to each other, as inputs to comparator circuits, but these circuits remain uncharacterized.
Our study draws back the curtain on one such circuit in the fly. Changes in odor intensity are registered by pools of on and off αβc KCs, which represent the strengths of the accumulated evidence for an increase or decrease in odor concentration.10 These pools of sensory neurons and antineurons couple to a second layer of neurons and antineurons, the core-innervating MBONs (Figures 1 and 2), which bias behavioral intent in opposite ways.25 Members of both neuronal pools in the sensory layer connect to both types of MBON in the action selection layer via plastic synapses (Figures 2 and 6). With two sets of neurons and antineurons and all-to-all feedforward connectivity between them (Figure 2), the comparator circuit allows for approach or avoidance following judgments of upward or downward changes in odor intensity—that is, it comprises neural pathways representing the possible contingencies seen behaviorally (Figure 5). The perceptual decision is won—correctly or incorrectly—by the αβc KC pool that reaches spike threshold first (Figure 3), and it is expressed in the behavior instructed by that pool’s favored MBON partners.
Unlike neurons comprising the ON and OFF pathways of motion vision,51,52 on and off αβc KCs cannot be distinguished and manipulated genetically. We have therefore exploited the sensitivity of KC-to-MBON synapses to the timing of reinforcement28 to reveal the convergence of separate on and off channels onto the same MBONs. KC-to-MBON synapses in their ground state exert finely balanced drive on the MBON ensemble, so that votes cast by its members cancel one another as in a hung jury, but experience can shift the synaptic weight distribution and the resulting pattern of MBON activation away from net zero.25,27 We have documented such shifts for the approach-advocating25 MBON-γ1pedc>αβ: pairing odor on- or offset with electric shock weakens transmission from the on αβc KC pool and strengthens transmission from the off αβc KC pool (or vice versa), synergistically changing odor preference (Figure 5). The underlying mechanism is a switch from synaptic depression to synaptic potentiation28 when the order of odor-evoked KC activation and dopaminergic reinforcement is reversed (Figure 5). This mechanism operates at KC connections with all core-innervating MBONs but is likely engaged at different timescales that may reflect sequential memory phases; to demonstrate the mechanism’s ubiquity, we have artificially collapsed this temporal sequence by photostimulating DANs directly (Figure 6). Within the short time frame of our behavioral experiments (Figure 5), only PPL1-γ1pedc, but not PPL1-α’2α2, shows significant pain responses that modulate its sensitivity to a predictive odor (Figure 4), consistent with the view that PPL1-γ1pedc and its cognate MBON-γ1pedc>αβ represent the core circuit for the storage and expression of short-term aversive memories.25,29
A crucial element of many neural network models of decision making is inhibitory feedback from a common interneuron pool driven by the competing integrators,12,20, 21, 22 which helps to amplify small differences in conflicting sensory evidence until, eventually, one integrator prevails. The response competition circuit we have delineated contains such an inhibitory element but with the intriguing twist that the key variables are represented by membrane voltages rather than spikes (Figure 7).10 Analog processing may be a consequence of numerical constraints: if the mushroom bodies lack the neuron numbers needed to approximate continuous quantities with discrete-time action potentials,53 there may be little choice but to swap the advantages regenerative spikes could provide (such as long time windows for adding and retaining sensory evidence)53 for the greater information capacity of graded potentials.54,55 Perhaps more is different.56
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal 4F3 anti-discs large | Developmental Studies Hybridoma Bank, University of Iowa | RRID: AB_528203 |
| AlexaFluor568-conjugated goat anti-mouse IgG | Invitrogen (Thermo Fisher) | A-11031 RRID: AB_144696 |
| Chemicals, peptides, and recombinant proteins | ||
| Biocytin | Sigma-Aldrich | B4261 |
| AlexaFluor633-conjugated streptavidin | Invitrogen (Thermo Fisher) | S21375 |
| Paraformaldehyde | Electron Microscopy Sciences | 15713 |
| Phosphate buffered saline tablets | Oxoid | BR0014G |
| Vectashield antifade mounting medium | Vector Laboratories | H-1000 |
| 4-methyl-cyclohexanol (MCH) | Sigma-Aldrich | 153095 |
| 3-octanol (OCT) | Sigma-Aldrich | 218405 |
| Isopentyl acetate | Sigma-Aldrich | 306967 |
| Ethyl acetate | Sigma-Aldrich | 270989 |
| Eicosane | Sigma-Aldrich | 219274 |
| Thermoplastic wax (52°C melting point) | Agar Scientific | AGG3881 |
| TES | Sigma-Aldrich | T5691 |
| NaCl | Sigma-Aldrich | S7653 |
| KCl | Sigma-Aldrich | P9333 |
| NaHCO3 | Sigma-Aldrich | S6297 |
| NaH2PO4 | Sigma-Aldrich | S8282 |
| CaCl2 | Sigma-Aldrich | 21115 |
| MgCl2 | Sigma-Aldrich | M1028 |
| Trehalose | Sigma-Aldrich | T9531 |
| Glucose | Sigma-Aldrich | G7528 |
| Sucrose | Sigma-Aldrich | S0389 |
| HEPES | Sigma-Aldrich | H4034 |
| MgATP | Sigma-Aldrich | A9187 |
| Na3GTP | Sigma-Aldrich | G6129 |
| EGTA | Sigma-Aldrich | E4378 |
| all-trans retinal | Sigma-Aldrich | R2500 |
| Dimethyl sulfoxide | Sigma-Aldrich | D2650 |
| Tetrodotoxin citrate | Tocris | 1069 |
| Picrotoxin | Tocris | 1128 |
| Mecamylamine hydrochloride | Sigma-Aldrich | M9020 |
| Experimental models: organisms/strains | ||
| Drosophila, Canton-S | Bloomington Drosophila Stock Center | 64349 |
| Drosophila, hsFLP | Bloomington Drosophila Stock Center42 | 26902 |
| Drosophila, NP6024-GAL4 | Kyoto Stock Center18 | 105080 |
| Drosophila, NP7175-GAL4 | Kyoto Stock Center18 | 114120 |
| Drosophila, R58F02-GAL4 | Bloomington Drosophila Stock Center57 | 39186 |
| Drosophila, MB112C-GAL4 | Bloomington Drosophila Stock Center19 | 68263 |
| Drosophila, MB80C-GAL4 | Bloomington Drosophila Stock Center19 | 68285 |
| Drosophila, MB433B-GAL4 | Bloomington Drosophila Stock Center19 | 68324 |
| Drosophila, MB320C-GAL4 | Bloomington Drosophila Stock Center26 | 68253 |
| Drosophila, MB099C-GAL4 | Bloomington Drosophila Stock Center19,26 | 68290 |
| Drosophila, R12G04-LexA | Bloomington Drosophila Stock Center26 | 52448 |
| Drosophila, R58E02-LexA | Bloomington Drosophila Stock Center31 | 52740 |
| Drosophila, R34B02-LexA | Bloomington Drosophila Stock Center26 | 53631 |
| Drosophila, UAS-CD8::GFP | Bloomington Drosophila Stock Center58 | 32186 |
| Drosophila, UAS-Rdl.RNAi.8-10 | Bloomington Drosophila Stock Center59 | 89903 |
| Drosophila, UAS-Dcr-2 | Bloomington Drosophila Stock Center60 | 24650 |
| Drosophila, 10xUAS-IVS-Syn21-Chrimson::tdT-3.1 (su(Hw)attP1) “UAS-CsChrimson::tdTomato” | Gift from D. Anderson61 | N/A |
| Drosophila, 20xUAS-IVS-CsChrimson.mVenus (attp40) “UAS-CsChrimson::mVenus” | Bloomington Drosophila Stock Center62 | 55135 |
| Drosophila, lexAop-rCD2::GFP | Bloomington Drosophila Stock Center63 | 66544 |
| Drosophila, 13xLexAop2-IVS-CsChrimson.mVenus (attp2) “lexAop-CsChrimson” | Bloomington Drosophila Stock Center62 | 55139 |
| Drosophila, 13xLexAop-IVS-GFP-p10 (su(Hw)attP5) “lexAop-GFP” | Gift from G. Rubin64 | N/A |
| Drosophila, 10x-UAS-FRT > -IVS-mCD8::GFP-STOP-FRT > -IVS-CsChrimson::tdTomato (attp40) | This study | N/A |
| Recombinant DNA | ||
| Plasmid, pJFRC177-10XUAS-FRT>-dSTOP-FRT>-myr::GFP | Addgene65 | 32149 |
| Software and algorithms | ||
| MATLAB | Mathworks | https://www.mathworks.com |
| LabVIEW | National Instruments | https://www.ni.com |
| ImageJ | NIH | https://imagej.nih.gov |
| Signal | Cambridge Electronic Design Ltd. | http://ced.co.uk |
| pClamp 10 | Molecular Devices | https://www.moleculardevices.com |
| Igor Pro | Wavemetrics | https://www.wavemetrics.com |
| NeuroMatic | NeuroMatic | http://neuromatic.thinkrandom.com |
| Python 3.7.1 | Python Software Foundation | https://www.python.org |
| pyABF 2.2.3 | Harden Technologies | https://pypi.org/project/pyabf/ |
| dabest 0.3.1 | https://pypi.org/project/dabest/ | |
| Prism | GraphPad | https://www.graphpad.com |
| Other | ||
| Borosilicate glass capillaries | Sutter Instruments | BF150-86-10 |
Resource availability
Lead contact
Requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Gero Miesenböck (gero.miesenboeck@cncb.ox.ac.uk).
Materials availability
All unique reagents generated in this study are available from the lead contact.
Experimental model and subject details
Experimental flies were heterozygous for all transgenes. For patch-clamp recordings, the expression of UAS-CD8::GFP58 was targeted to MBON-α2sc, MBON-γ1pedc>αβ, MBON-β1>α, or MBON-γ4>γ1γ2 using MB080C-GAL4, MB112C-GAL4, or MB433B-GAL4, respectively;19 to PPL1 neurons using TH-GAL4;66 or to αβc KCs using NP6024-GAL4, NP7175-GAL4, or R58F02-GAL4.18,57 To deplete the GABAA receptor Rdl from PPL1 neurons, MB320C-GAL426 controlled the expression of UAS-Rdl.RNAi.8-1059 and UAS-Dcr-2.60
In combined photostimulation and whole-cell recording experiments, the following transgene combinations were used to drive the expression of optogenetic actuator and fluorescent marker:18,19,26,57,58,61, 62, 63, 64 αβc KCs (R58F02-LexA > lexAop-CsChrimson) and MBONs (MB080C-GAL4, MB112C-GAL4, or MB433B-GAL4>UAS-CD8::GFP); PPL1-α’2α2 (MB099C-GAL4>UAS-CsChrimson::tdTomato) and MBON-α2sc (R34B02-LexA>lexAop-GFP); PPL1-γ1pedc (MB320C-GAL4>UAS-CsChrimson::mVenus) and MBON-γ1pedc>αβ (R12G04-LexA>lexAop-rCD2::GFP); and PAM-β1 DANs (R58E02-LexA>lexAop-CsChrimson) and MBON-β1>α (MB433B-GAL4>UAS-CD8::GFP). Not all drivers exhibited absolute specificity for the neurons of interest:19,26,31 MB433B-GAL4 expresses in both MBON-β1>α and MBON-γ4>γ1γ2; MB099C-GAL4 labels PPL1-α’2α2 and PPL1-γ2α’1 and stochastically PPL1-α’3 and/or PPL1-α3; and MB320C-GAL4 captures PPL1-γ1pedc and with low frequency also PPL1-α’2α2 and/or PPL1-α’3.
To achieve the mutually exclusive expression of CD8::GFP or CsChrimson::tdTomato in αβc KCs, a CD8::GFP coding sequence followed by the SV40 transcriptional terminator was flanked by FRT sites and inserted between the UAS promoter and the CsChrimson::tdTomato coding sequence, using pJFRC177-10XUAS-FRT>-dSTOP-FRT>-myr::GFP65 as the backbone. The construct was integrated into the attp40 site and transcribed under the control of R58F02-GAL4.57 The transcript encoded CD8::GFP by default and CsChrimson::tdTomato after the FLP-mediated excision41 of the FRT>–CD8::GFP–stop–FRT>cassette. Low levels of basal expression of the hsFLP transgene, without additional heat shock, were sufficient to produce recombination events42 whose visible signature was a mosaic of green and red fluorescent αβc KCs (Figure 7A). The presence of both KC populations (or the absence of red fluorescence in flies lacking hsFLP) was confirmed by live microscopy before each experiment. Recordings from CsChrimson-positive αβc KCs showed that the membrane potentials of these neurons were correctly modulated by light (Figure S6).
Fly strains were grown on standard cornmeal agar under a 12 h light:12 h dark cycle at 25°C unless they carried the hsFLP transgene; these experimental animals and their controls were raised at 18°C. Flies expressing CsChrimson were transferred to food supplemented with 0.6–2 mM all-trans retinal in dimethyl sulfoxide upon eclosion and raised in darkness thereafter.
Method details
Electrophysiology
For whole-cell patch-clamp recordings in vivo, male or female flies aged 2–11 days were fixed to a custom mount with eicosane or soft thermoplastic wax (Agar Scientific). Cuticle, adipose tissue, trachea, and glial sheath were surgically removed to expose the brain, which was continuously superfused with extracellular solution (pH 7.3) containing 5 mM TES, 103 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 1 mM NaH2PO4, 1.5 mM CaCl2, 4 mM MgCl2, 8 mM trehalose, 10 mM glucose, and 7 mM sucrose (275 mOsM, equilibrated with 5% CO2 and 95% O2). The green-fluorescent somata of target cells (MBONs, PPL1 neurons, αβc KCs) were visually identified using 40 × , 0.8 NA or 60 × , 1.0 NA water immersion objectives (LUMPLFLN40XW or LUMPLFLN60XW, Olympus) and a combination of epifluorescence and differential interference contrast. Patch pipettes (11–22 MΩ, depending on target cell type) were fabricated from borosilicate glass capillaries with outer and inner diameters of 1.5 and 0.86 mm (Sutter Instruments), using a PC-10 micropipette puller (Narishige) or a DMZ Universal Electrode Puller (Zeitz), and filled with solution (pH 7.3) containing 10 mM HEPES, 140 mM potassium aspartate, 1 mM KCl, 4 mM MgATP, 0.5 mM Na3GTP, 1 mM EGTA, and 10 mM biocytin (265 mOsM). Signals were recorded at room temperature (21–23°C) in current-clamp mode with a MultiClamp 700B amplifier (Molecular Devices), lowpass-filtered at 10–20 kHz, and sampled at 20–50 kHz using a Power1401-3A data acquisition interface controlled through Signal (Cambridge Electronic Design Ltd.), an ITC-18 board (InstruTECH) controlled through Igor Pro (WaveMetrics), or a Digidata 1440A digitizer controlled through pCLAMP 10 (Molecular Devices). Data were analyzed with custom procedures, using the NeuroMatic package (http://neuromatic.thinkrandom.com) in Igor Pro (WaveMetrics) and the pyABF module (https://pypi.org/project/pyabf/) in Python 3.7 (Python Software Foundation). Only cells with a measured resting potential below –30 mV and a spiking response to depolarizing current injections were characterized further. Where indicated, mecamylamine (500 μM), picrotoxin (10 μM as standard; up to 250 μM in some cases), or tetrodotoxin (TTX, 1μM) were perfused into the bath to verify cholinergic, GABAergic, or monosynaptic or graded transmission, respectively.
For photostimulation of CsChrimson-expressing cells, a 630-nm LED (Multicomp OSW-4388) controlled by a TTL-triggered dimmable LED driver (Recom RCD-24-0.70/W/X3) was focused on the head of the fly with a mounted 60 mm lens (Thorlabs). The light source delivered 11–80 mW cm-2 of optical power.
Custom solenoid valve systems (The Lee Company) under LabVIEW control (National Instruments) directed mass flow-controlled (CMOSens, Sensirion) streams of clean or odor-infused air (containing 1–20 ppm 4-methyl-cyclohexanol [MCH], 3-octanol [OCT], isopentyl acetate, or ethyl acetate) at constant flow rates of 0.25–0.5 l/min toward the fly’s head.67 With the exception of the experiments shown in Figure 4, the four odors were used randomly and interchangeably. Steady-state odor concentrations were estimated with the help of a ppbRAE 3000 photoionization detector (RAE systems); the kinetics of odor concentration changes at the position of the fly were monitored with a 200B miniPID (Aurora Scientific) at 10 kHz. Pressure changes caused by the opening and closing of valves were balanced by a set of pressure-compensating valves and monitored periodically with a mass flow sensor (FBAL001DU, Sensor Technics).
Electric shocks were applied in constant current or constant voltage regimes. In the former, 10-ms current pulses (20–100 μA, Digitimer DS3) were passed through a pair of 50-μm platinum wires touching the fly’s abdomen; in the latter, a printed circuit board (PCB) supporting the fly’s legs was intermittently connected to a 90-V source (Digitimer DS2A). A CCD camera (Guppy F-033; Allied Vision Technologies) equipped with a 3.3x Macro Zoom Lens (Edmund Optics) was used to observe the fly’s reactions and titrate the intensity of the applied current or verify contact with the PCB. PPL1 odor response plasticity was quantified after two training cycles in the constant current regime. Each cycle consisted of a block of twelve 3 s odor pulses at 20 s intervals: six OCT pulses (CS+) paired with a single 10-ms current activation at 2 s after TTL-triggered odor onset, and six MCH pulses (CS–) without electric shock. Plastic changes in the on and off responses of MBON-γ1pedc>αβ were analyzed after conditioning flies with six 15 s odor pulses, separated by 15 s epochs of clean air, in the constant voltage regime. The PCB was charged twice for 1.5 s, starting at 5.5 and 13.5 s after the TTL-triggered opening of the odor valve (Figure 5C), or at 6.5 and 14.5 s after its TTL-triggered closing (Figure 5D). For optogenetic reinforcement, DAN activity was photostimulated once for 1.5 s, starting at 5.5 s (Figures 6A–6C) or 13.5 s (Figures 6D–6F) after the TTL-triggered odor onset, or at 1.5 s after odor offset (Figures 6G–6I). Measured intensity changes at the antennae lagged by 0.5–2 s behind valve switching commands, with shorter delays at odor offset.
Spikes were detected as maxima in the time derivative of the membrane potential trace; firing rates were calculated as 400-ms moving averages and sampled at 1 kHz. Odor on and off responses were quantified as baseline-subtracted spike rate averages; the mean spike rate in a 1 s window preceding the valve opening command served as the baseline. The measurement windows were anchored to the firing rate peak within 3 s after valve switching. The window for quantifying the on response extended from 250 ms before until 500 ms after the peak; the more transient off response was quantified as the firing rate peak. In spike latency comparisons (Figure 3), occasional trials without spikes at the highest difficulty level (concentration ratio 0.9) were excluded from the analysis.
Behavior
The odor choices of male Canton-S flies aged 7–8 days were analyzed individually in transparent plexiglass chambers (50 mm long, 5 mm wide, 1.3 mm high).9,23 Independently controlled (CMOSens, Sensirion) streams of odorless or MCH-infused air entered the chambers at flow rates of 0.25 l/min through ports at the distal ends, converged at the center, and left through lateral vents. PCBs, connected via solid-state relays (Fairchild HSR312L) to a 70-V power supply, served as floors and ceilings. For electric shock reinforcement, the relays were activated once for 2 s, either at 18 s after odor onset, during twelve consecutive 20 s presentations of 2 ppm MCH, which were interleaved with 20 s exposures to air (reinforcing the odor on response; Figure 5A), or once for 2 s, at 18 s after odor offset, during twelve 20 s presentations of air, which were interleaved with 20 s exposures to 2 ppm MCH (reinforcing the odor off response; Figure 5B). Twenty chambers were operated simultaneously in an incubator (Sanyo MIR-154) at 25°C. The chambers were backlit by 940-nm LEDs (TSAL6100, Vishay) and imaged using a Stingray F080B CCD camera (Allied Vision Technologies) with an 18-mm lens (Edmund Optics). A virtual instrument written in LabVIEW controlled the delivery of odors and electric shock and recorded the positions of the 20 flies as functions of time.23
Data were processed offline in MATLAB (The MathWorks). Preferences were quantified as the percentages of decisions in favor of MCH or air during 2-min test intervals before and after electric shock reinforcement. Flies making fewer than 2 decisions per test interval were excluded from the analysis.
Imaging
Brains of male flies were dissected 2 days after eclosion, fixed in 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS; 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.3) for 20 min at room temperature, washed four times for 15 min in PBS containing 0.2% (v/v) Triton X-100, and mounted in Vectashield (Vector Labs) for imaging native fluoresecence. For immunostaining, fixed brains were permeabilized and blocked in PBS containing 0.2% (v/v) Triton X-100 and 5% (v/v) goat serum for 1 h. To label synaptic structures, the samples were first incubated with mouse monoclonal anti-discs large antibody 4F3 (Developmental Studies Hybridoma Bank, University of Iowa, 1:50) and then with Alexa Fluor 568-conjugated goat anti-mouse IgG (1:200) plus Alexa Fluor 633-conjugated streptavidin (1:150). Each incubation lasted for 48 h at 4°C and was followed by four 20-min washes in PBS. Stained samples were mounted in Vectashield and imaged on a Leica TCS SP5 confocal microscope equipped with an HCX PL APO 40 × /1.3 CS oil immersion objective (Leica). Images were processed in ImageJ.
Quantification and statistical analysis
Data were analyzed in Prism 9 (GraphPad). Group means were compared by paired, unpaired, or one-sample two-sided t tests, as indicated in figure legends, or by two-way repeated-measures ANOVA, followed by post hoc analyses using Holm–Šídák’s multiple comparisons test. Where the assumption of normality was violated (as indicated by Shapiro–Wilk test), group means were compared using two-sided Wilcoxon or two-sided Mann-Whitney test. Bootstrapped sample distributions and 95% confidence intervals were computed in Python 3.7 using the dabest 0.3.1 software package.68
Acknowledgments
We thank Kiara Delos Santos and Manrique Moreno Gasulla for technical support, Andrew Lin for an estimate of linear APL synapse densities, and David Anderson, Serge Birman, Ronald Davis, Barry Dickson, Kent Golic, Kei Ito, Vivek Jayaraman, Tzumin Lee, Liqun Luo, Gerald Rubin, and the Bloomington and Kyoto Stock Centers for reagents. Clifford B. Talbot helped with instrumentation. This work was supported by grants from Wellcome (209235/Z/17/Z, 106988/Z/15/Z, and 090309/Z/09/Z), the Gatsby Charitable Foundation (GAT3237), and the National Institutes of Health (RO1 DA30601).
Author contributions
E.V., L.N.G., and G.M. designed the study, analyzed the data, and prepared the manuscript. E.V., L.N.G., and S.S. performed electrophysiological experiments; R.B. generated fly strains; and A.K. analyzed odor preferences.
Declaration of interests
The authors declare no competing interests.
Published: October 4, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2021.09.008.
Supplemental information
Data and code availability
The published article contains all data generated during this study. Custom instrument control and analysis code used in this study is available from the lead contact.
References
- 1.Gold J.I., Shadlen M.N. The neural basis of decision making. Annu. Rev. Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 2.Newsome W.T., Britten K.H., Movshon J.A. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- 3.Britten K.H., Shadlen M.N., Newsome W.T., Movshon J.A. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shadlen M.N., Newsome W.T. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 5.Roitman J.D., Shadlen M.N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz L.N., Yates J.L., Pillow J.W., Huk A.C. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature. 2016;535:285–288. doi: 10.1038/nature18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragomir E.I., Štih V., Portugues R. Evidence accumulation during a sensorimotor decision task revealed by whole-brain imaging. Nat. Neurosci. 2020;23:85–93. doi: 10.1038/s41593-019-0535-8. [DOI] [PubMed] [Google Scholar]
- 8.Bahl A., Engert F. Neural circuits for evidence accumulation and decision making in larval zebrafish. Nat. Neurosci. 2020;23:94–102. doi: 10.1038/s41593-019-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DasGupta S., Ferreira C.H., Miesenböck G. FoxP influences the speed and accuracy of a perceptual decision in Drosophila. Science. 2014;344:901–904. doi: 10.1126/science.1252114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groschner L.N., Chan Wah Hak L., Bogacz R., DasGupta S., Miesenböck G. Dendritic integration of sensory evidence in perceptual decision-making. Cell. 2018;173:894–905.e13. doi: 10.1016/j.cell.2018.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold J.I., Shadlen M.N. Neural computations that underlie decisions about sensory stimuli. Trends Cogn. Sci. 2001;5:10–16. doi: 10.1016/s1364-6613(00)01567-9. [DOI] [PubMed] [Google Scholar]
- 12.Bogacz R., Brown E., Moehlis J., Holmes P., Cohen J.D. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 2006;113:700–765. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- 13.Ratcliff R., McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groschner L.N., Miesenböck G. Mechanisms of sensory discrimination: insights from Drosophila olfaction. Annu. Rev. Biophys. 2019;48:209–229. doi: 10.1146/annurev-biophys-052118-115655. [DOI] [PubMed] [Google Scholar]
- 15.Ito I., Ong R.C.-Y., Raman B., Stopfer M. Sparse odor representation and olfactory learning. Nat. Neurosci. 2008;11:1177–1184. doi: 10.1038/nn.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaBerge D. A recruitment theory of simple behavior. Psychometrika. 1962;27:375–396. [Google Scholar]
- 17.Vickers D. Evidence for an accumulator model of psychophysical discrimination. Ergonomics. 1970;13:37–58. doi: 10.1080/00140137008931117. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N.K., Tanimoto H., Ito K. Neuronal assemblies of the Drosophila mushroom body. J. Comp. Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 19.Aso Y., Hattori D., Yu Y., Johnston R.M., Iyer N.A., Ngo T.T., Dionne H., Abbott L.F., Axel R., Tanimoto H., Rubin G.M. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usher M., McClelland J.L. The time course of perceptual choice: the leaky, competing accumulator model. Psychol. Rev. 2001;108:550–592. doi: 10.1037/0033-295x.108.3.550. [DOI] [PubMed] [Google Scholar]
- 21.Wang X.-J. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 22.Teodorescu A.R., Usher M. Disentangling decision models: from independence to competition. Psychol. Rev. 2013;120:1–38. doi: 10.1037/a0030776. [DOI] [PubMed] [Google Scholar]
- 23.Claridge-Chang A., Roorda R.D., Vrontou E., Sjulson L., Li H., Hirsh J., Miesenböck G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Séjourné J., Plaçais P.-Y., Aso Y., Siwanowicz I., Trannoy S., Thoma V., Tedjakumala S.R., Rubin G.M., Tchénio P., Ito K., et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat. Neurosci. 2011;14:903–910. doi: 10.1038/nn.2846. [DOI] [PubMed] [Google Scholar]
- 25.Aso Y., Sitaraman D., Ichinose T., Kaun K.R., Vogt K., Belliart-Guérin G., Plaçais P.Y., Robie A.A., Yamagata N., Schnaitmann C., et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hige T., Aso Y., Modi M.N., Rubin G.M., Turner G.C. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron. 2015;88:985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owald D., Felsenberg J., Talbot C.B., Das G., Perisse E., Huetteroth W., Waddell S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86:417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handler A., Graham T.G.W., Cohn R., Morantte I., Siliciano A.F., Zeng J., Li Y., Ruta V. Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell. 2019;178:60–75.e19. doi: 10.1016/j.cell.2019.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aso Y., Siwanowicz I., Bräcker L., Ito K., Kitamoto T., Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke C.J., Huetteroth W., Owald D., Perisse E., Krashes M.J., Das G., Gohl D., Silies M., Certel S., Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C., Plaçais P.-Y., Yamagata N., Pfeiffer B.D., Aso Y., Friedrich A.B., Siwanowicz I., Rubin G.M., Preat T., Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 32.Scheffer L.K., Xu C.S., Januszewski M., Lu Z., Takemura S.Y., Hayworth K.J., Huang G.B., Shinomiya K., Maitlin-Shepard J., Berg S., et al. A connectome and analysis of the adult Drosophila central brain. eLife. 2020;9:e57443. doi: 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zemelman B.V., Lee G.A., Ng M., Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 34.Barnstedt O., Owald D., Felsenberg J., Brain R., Moszynski J.-P., Talbot C.B., Perrat P.N., Waddell S. Memory-relevant mushroom body output synapses are cholinergic. Neuron. 2016;89:1237–1247. doi: 10.1016/j.neuron.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanimoto H., Heisenberg M., Gerber B. Event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- 36.Montague P.R., Dayan P., Sejnowski T.J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao Z., Davis R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front. Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattori D., Aso Y., Swartz K.J., Rubin G.M., Abbott L.F., Axel R. Representations of novelty and familiarity in a mushroom body compartment. Cell. 2017;169:956–969.e17. doi: 10.1016/j.cell.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riemensperger T., Völler T., Stock P., Buchner E., Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 41.Struhl G., Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 42.Golic K.G., Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 43.Ito K., Awano W., Suzuki K., Hiromi Y., Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- 44.Lin A.C., Bygrave A.M., de Calignon A., Lee T., Miesenböck G. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat. Neurosci. 2014;17:559–568. doi: 10.1038/nn.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inada K., Tsuchimoto Y., Kazama H. Origins of cell-type-specific olfactory processing in the Drosophila mushroom body circuit. Neuron. 2017;95:357–367.e4. doi: 10.1016/j.neuron.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 46.Amin H., Apostolopoulou A.A., Suárez-Grimalt R., Vrontou E., Lin A.C. Localized inhibition in the Drosophila mushroom body. eLife. 2020;9:e56954. doi: 10.7554/eLife.56954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christiansen F., Zube C., Andlauer T.F., Wichmann C., Fouquet W., Owald D., Mertel S., Leiss F., Tavosanis G., Luna A.J., et al. Presynapses in Kenyon cell dendrites in the mushroom body calyx of Drosophila. J. Neurosci. 2011;31:9696–9707. doi: 10.1523/JNEUROSCI.6542-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papadopoulou M., Cassenaer S., Nowotny T., Laurent G. Normalization for sparse encoding of odors by a wide-field interneuron. Science. 2011;332:721–725. doi: 10.1126/science.1201835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burrows M., Siegler M.V. Graded synaptic transmission between local interneurones and motor neurones in the metathoracic ganglion of the locust. J. Physiol. 1978;285:231–255. doi: 10.1113/jphysiol.1978.sp012569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machens C.K., Romo R., Brody C.D. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science. 2005;307:1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 51.Joesch M., Schnell B., Raghu S.V., Reiff D.F., Borst A. ON and OFF pathways in Drosophila motion vision. Nature. 2010;468:300–304. doi: 10.1038/nature09545. [DOI] [PubMed] [Google Scholar]
- 52.Clark D.A., Bursztyn L., Horowitz M.A., Schnitzer M.J., Clandinin T.R. Defining the computational structure of the motion detector in Drosophila. Neuron. 2011;70:1165–1177. doi: 10.1016/j.neuron.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbott L.F., DePasquale B., Memmesheimer R.M. Building functional networks of spiking model neurons. Nat. Neurosci. 2016;19:350–355. doi: 10.1038/nn.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Ruyter van Steveninck R.R., Laughlin S.B. The rate of information transfer at graded-potential synapses. Nature. 1996;379:642–645. [Google Scholar]
- 55.Sarpeshkar R. Analog versus digital: extrapolating from electronics to neurobiology. Neural Comput. 1998;10:1601–1638. doi: 10.1162/089976698300017052. [DOI] [PubMed] [Google Scholar]
- 56.Anderson P.W. More is different. Science. 1972;177:393–396. doi: 10.1126/science.177.4047.393. [DOI] [PubMed] [Google Scholar]
- 57.Jenett A., Rubin G.M., Ngo T.-T.B., Shepherd D., Murphy C., Dionne H., Pfeiffer B.D., Cavallaro A., Hall D., Jeter J., et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee T., Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 59.Liu X., Krause W.C., Davis R.L. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dietzl G., Chen D., Schnorrer F., Su K.C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 61.Hoopfer E.D., Jung Y., Inagaki H.K., Rubin G.M., Anderson D.J. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife. 2015;4:e11346. doi: 10.7554/eLife.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klapoetke N.C., Murata Y., Kim S.S., Pulver S.R., Birdsey-Benson A., Cho Y.K., Morimoto T.K., Chuong A.S., Carpenter E.J., Tian Z., et al. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai S.L., Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 64.Pfeiffer B.D., Truman J.W., Rubin G.M. Using translational enhancers to increase transgene expression in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nern A., Pfeiffer B.D., Svoboda K., Rubin G.M. Multiple new site-specific recombinases for use in manipulating animal genomes. Proc. Natl. Acad. Sci. USA. 2011;108:14198–14203. doi: 10.1073/pnas.1111704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friggi-Grelin F., Coulom H., Meller M., Gomez D., Hirsh J., Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 67.Shang Y., Claridge-Chang A., Sjulson L., Pypaert M., Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho J., Tumkaya T., Aryal S., Choi H., Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat. Methods. 2019;16:565–566. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article contains all data generated during this study. Custom instrument control and analysis code used in this study is available from the lead contact.