Abstract
Introduction
Blood donor studies offer a unique opportunity to screen healthy populations for the presence of antibodies to emerging infections. We describe the use of blood donor specimens to track the ‘first-wave’ of the COVID-19 pandemic in Ireland.
Methodology
A random selection of donor samples received by the Irish Blood Transfusion Service (IBTS) between February and September 2020 (n = 8,509) were screened by multiple commercial SARs-CoV-2 antibody assays. The antibody detection rate was adjusted to the population to determine the SARS-CoV-2 seroprevalence in Ireland.
Results
SARS-CoV-2 antibody detection rose significantly during the first peak of COVID-19 infection, increasing from 0.3% in March, to 2.9% in April (p < 0.0001, The first SARS-CoV-2 antibody positive donor samples were collected on the 17th February 2020, 2 weeks prior to the first official notification. This is the earliest serological evidence of SARS-CoV-2 circulating in the Irish population. Our results also show a significantly higher antibody prevalence in the Capital city and in donors less than 40 years of age.
Conclusions
The present study demonstrates evidence of SARS-CoV-2 antibody reactivity across all age groups and counties. The critical value of blood donor seroprevalence studies is apparent in this report which identified the earliest serological evidence of SARS-CoV-2 infection in Ireland, as well as documenting the evolution of COVID-19 pandemic in Ireland over time.
Keywords: Ireland, SARS-CoV-2, Epidemiology, Blood, Donor, Seroprevalence
1. Introduction
The first case of SARS-CoV-2 in the Republic of Ireland was reported on February 29th 2020 in a young male tourist who had returned from Northern Italy; however it was later reported that a case of community transmission had already presented to hospital in the southern part of the country [1], [2], [3], [4]. A rapidly evolving response was demanded of Irish health services, to identify, test and quarantine cases of infection before health services became overwhelmed. Molecular-based testing was promptly established but testing capacity was limited by reagent supply. By March 12th 2020 high level public health restrictions were imposed. Travel was permitted for essential work only and persons greater than seventy years of age were advised to ‘cocoon’ indoors. An easing of restrictions over the summer of 2020 heralded a resurgence of cases leading to the “2nd wave” of cases, peaking in October 2020 [1], [2], [3], [4], [5].
The Irish Blood Transfusion Service (IBTS) responded to the emerging threat of SARS CoV-2 by introducing a 28-day deferral for those with a travel–related risk, symptoms suggestive of, or contact with known cases of COVID-19. In accordance with the evolving national guidance on a novel emerging infectious disease with an uncharacterized transfusion-transmissibility risk, restrictions remained in place at the IBTS even after it was confirmed that SARS-CoV-2 did not pose a transfusion-transmission risk [6], [7], [8]. These measures protected staff, blood donors, the blood supply and the recovering donors themselves.
It is now understood that asymptomatic COVID-19 infection occurs at a rate of 33–75% [9], [10], [11]. This is reflected in our national data which estimates that approximately 60% of those with detectable viral RNA were documented as ‘symptomatic’ in Irish surveillance reports. As a result, and consistent with many infectious disease outbreaks, the full extent of the SARS-CoV-2 pandemic in Ireland is likely under-recorded [10, 12]. Blood donor studies offer a unique opportunity to screen healthy populations for the presence of antibodies to new and emerging infections [13]. This is particularly relevant for COVID-19 as it is expected that blood donor behaviours, which may be associated with a lower incidence of some infections, are unlikely to be protective against a respiratory infection [14], [15], [16], [17], [18]. Furthermore, detailed seroprevalence data is essential to develop appropriate national vaccination strategies, and for the evaluation of the effectiveness of the various infection control measures.
In the present study, we describe the use of blood donor specimens to track the ‘first-wave’ of the COVID-19 pandemic in Ireland. Specifically, we provide evidence of the SARS-CoV-2 antibodies circulating in the Irish blood donor population prior to the first official notification of the disease. In addition, the significance of donor age, blood group and geographical location were analysed.
2. Materials & methods
2.1. Study design and ethical approval
A random selection of blood donor plasma samples from donations received by the Irish Blood Transfusion Service (IBTS) between February and September 2020 (n = 8509) were chosen for inclusion in the study. This study was approved by the National Office for Research Ethics Committee. Irish blood donors were asymptomatic and provided consent at donation for the use of their blood samples in anonymised research. Limited demographic information was retrieved from the blood management system, eProgesa version 5.0.3, prior to anonymisation, and included gender, age, donation clinic, ABO blood group and Anti-D (RhD) status.
2.2. Donor SARS-CoV-2 antibody testing
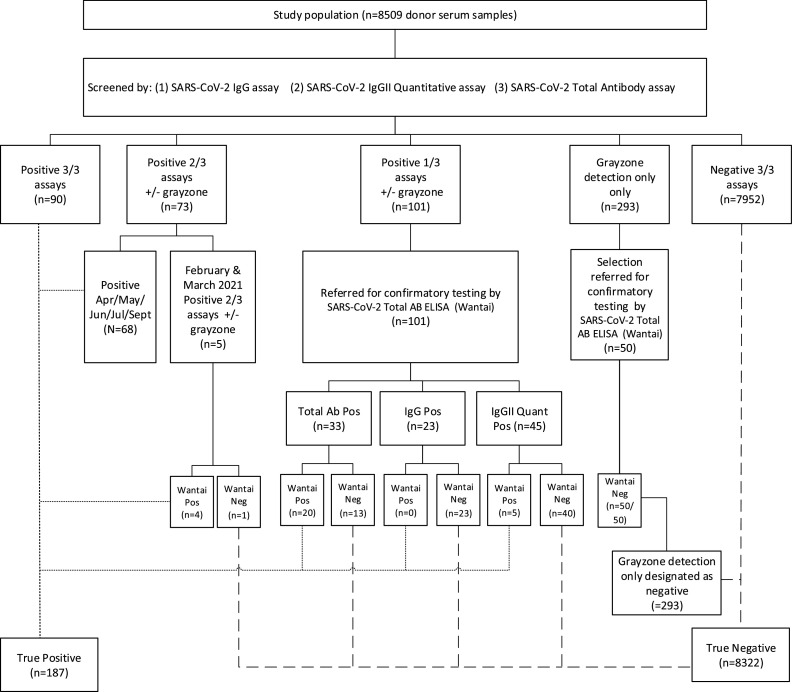
All samples were tested according to the study algorithm outlined in Fig. 1 , and in accordance with the manufacturer's instructions, as follows:
-
(i)
SARS-CoV-2 IgG assay (Abbott Diagnostics): This assay was carried out using the Abbott ARCHITECT™ serological testing platform and qualitatively detected IgG antibodies to the SARS-CoV-2 nucleocapsid protein. An index value of ≥1.40 S/CO was considered positive, and a value of 0.49–1.39 S/CO was equivocal or in the ‘grey zone’ detection range.
-
(ii)
SARS-CoV-2 IgG II Quantitative assay (Abbott Diagnostics): This assay was carried out using the Abbott ARCHITECT™ serological testing platform and quantitatively detected IgG antibodies to SARS-CoV-2 spike protein. Samples with a result ≥50.0 AU/mL were considered positive.
-
(iii)
SARS-CoV-2 Total Antibody assay (Abbott Diagnostics): This assay was carried out using the Abbott ARCHITECT™ serological testing platform and qualitatively detected IgA, IgM and IgG antibodies to SARS-CoV-2 nucleocapsid and spike proteins. Samples with an index value ≥1.0 S/CO were considered positive.
-
(iv)
SARS-CoV-2 Total AB ELISA (Wantai, Fortress Diagnostics: Confirmatory testing of inconclusive samples was carried out following referral of samples to the National Virus Reference Laboratory (NVRL), using the Fortress Diagnostics Wantai assay . As this assay was carried out at an independent reference Laboratory and has been previously shown to have optimum sensitivity, we utilized this assay as the confirmatory assay for borderline results [19], [20], [21]. Testing was performed per manufacturer's instructions. This assay qualitatively detected IgM and IgG antibodies to SARS-CoV-2 spike protein. Samples with an index value ≥1.1 A/CO were considered positive.
Fig. 1.
Irish blood donors SARS-CoV-2 antibody testing algorithm.
2.3. Seroprevalence and statistical analysis
Statistical analyses were performed using the statistical software package IBM SPSS (Version 27) and MedCalc (www.medcalc.org). Crude seroprevalence rates were calculated as the number of reactive samples divided by the total number of samples tested, stratified by donor demographics and time. Seroprevalence rates were adjusted, where appropriate, to reflect the Irish population demographics. The 2016 CENSUS data provided information on age and sex distributions, as well as the population levels in each of the 4 provinces in the Republic of Ireland (Leinster, Munster, Connaught and Ulster (part of)). Population age distributions included ages of eligible donors only (18 – 70). The adjustment was calculated as per Lewin et al. [22] by multiplying the crude seroprevalence by the population adjustment factors calculated according to the following formula:
= pPopulation AGE* SEX* PROVINCE / pDonor Sample AGE* SEX *PROVINCE
Descriptive statistics are presented as percentages and numbers. The Chi-Square test and confidence intervals were used to assess associations between donor demographic variables. A value of p < 0.05 was considered statistically significant. Multivariable regression analyses were carried out, where appropriate, to control for possible gender and age confounding.
3. Results
The donor sample population comprised of eligible blood donors, aged between 18 and 70 years, all of whom would have been excluded from donation if they had symptoms compatible with, and/or known exposure to COVID-19 in the previous 28 days. Approximately 250 to 500 donor samples per week were analysed and the increase in weekly sample testing corresponded with the first peak of COVID-19 infection in Ireland.
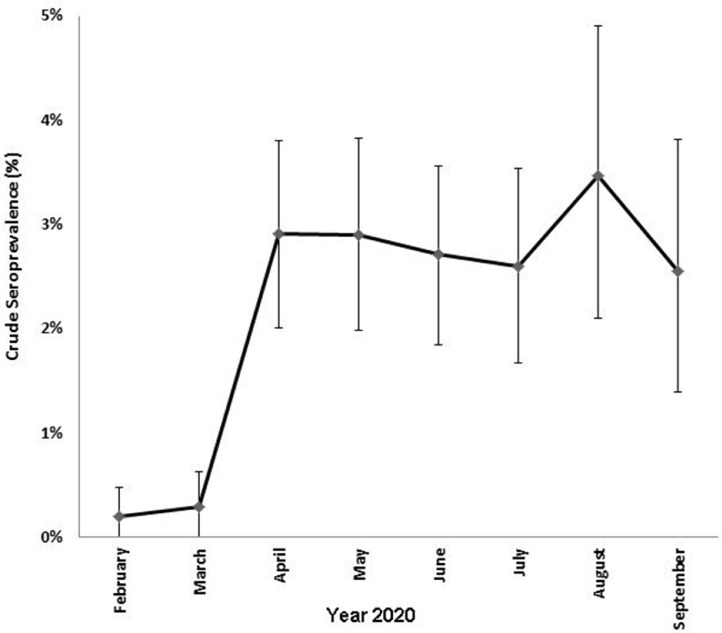
A number of different commercial assays were used to screen the anonymised donor specimens and a specific testing algorithm outlined in Fig. 1, was designed to assign donors as confirmed positive or confirmed negative. Briefly, donor specimens negative by all three screening assays were considered truly negative. The majority of samples positive for SARS-CoV-2 antibodies on at least two out of three screening assays were considered as confirmed positive. Inconclusive results were referred for additional testing at the National Virus Reference Laboratory. Five samples from donors bled in February and March 2020 reactive on two out of 3 screening assays, were subject to additional testing at the NVRL to enhance the specificity of the testing algorithm at a time of low prevalence of SARS-CoV-2 antibodies in the population. The remaining sixty-eight samples in this category were not referred for further testing as they pertained to a period when SARS-Cov-2 was well established and circulating at high levels in Ireland (April-September 2020). Overall, 187/8509 (2.2%) samples were confirmed positive for the presence of SARS-CoV-2 antibodies. SARS-CoV-2 antibody detection rates for all donor demographics are listed in Table 1 . SARS-CoV-2 antibody detection rose significantly during the first peak of COVID-19 infection, increasing from 0.2% and 0.3% in February and March, to 2.9% in April (p < 0.0001, Fig. 2 ). The seroprevalence rate stabilised at 2.5–3.5% for the remainder of the study period.
Table 1.
Donor sample population demographics and SARS-CoV-2 antibody detection.
| Study Variable | Total | Donors with SARS-CoV-2 antibody detected | Crude seroprevalence(% [95%CI]) | Adjusted seroprevalence*(% [95%CI]) | p-value | ||
|---|---|---|---|---|---|---|---|
| Donor population | 8509 | 187 | 2.20% [1.89 – 2.54] | 2.41% [2.09 – 2.76] | |||
| Month | February | 1047 | 2 | 0.19% [0.02 – 0.69] | |||
| March | 1033 | 3 | 0.29% [0.06 – 0.85] | ||||
| April | 1342 | 39 | 2.91% [2.07 – 3.97] | <0.0001 | |||
| May | 1277 | 37 | 2.90% [2.04 – 3.99] | ||||
| June | 1365 | 37 | 2.71% [1.91 – 3.74] | ||||
| July | 1116 | 29 | 2.60% [1.74 – 3.73] | ||||
| August | 663 | 23 | 3.47% [2.20 – 5.21] | ||||
| September | 666 | 17 | 2.55% [1.49 – 4.09] | ||||
| Gender | Male | 4842 | 104 | 2.15% [1.76 – 2.60] | 1.86% [1.50 – 2.29] | ||
| Female | 3667 | 83 | 2.26% [1.80 – 2.81] | 2.43% [1.97 – 2.96] | |||
| Age (yrs) | 18–29 | 1133 | 46 | 4.06% [2.97 – 5.42] | 5.30% [4.04 – 6.82] | <0.0001 | |
| 30–39 | 1664 | 46 | 2.76% [2.02 – 3.69] | 3.55% [2.70 – 4.57] | <0.0001 | ||
| 40–49 | 2271 | 39 | 1.72% [1.22 – 2.35] | 1.50% [1.04 – 2.09] | |||
| 50–59 | 2241 | 37 | 1.65% [1.16 – 2.28] | 1.21% [0.79 – 1.75] | |||
| ≥60 | 1200 | 19 | 1.58% [0.95 – 2.47] | 1.67% [1.02 – 2.57] | |||
| Province | Leinster | 4955 | 136 | 2.74% [2.30 – 3.25] | 2.95% [2.48 – 3.47] | <0.0001 | |
| Munster | 2257 | 37 | 1.64% [1.15 – 2.26] | 1.86% [1.34 – 2.52] | |||
| Ulster | 648 | 9 | 1.39% [0.64 – 2.64] | 1.39% [0.63 – 2.63] | |||
| Connacht | 649 | 5 | 0.77% [0.25 – 1.80] | 1.23% [0.53 – 2.43] | |||
| Blood Grouping | ABO | A | 2367 | 67 | 2.83% [2.19 – 3.58] | ||
| B | 964 | 17 | 1.76% [1.03 – 2.82] | ||||
| AB | 203 | 6 | 2.96% [1.09 – 6.43] | ||||
| O | 4975 | 97 | 1.95% [1.58 – 2.38] | ||||
| A antigen | + | 2570 | 73 | 2.84% [2.22 – 3.57] | <0.0001 | ||
| − | 5939 | 114 | 1.92% [1.58 – 2.31] | ||||
| RhD | + | 6248 | 139 | 2.22% [1.87 – 2.63] | |||
| − | 2261 | 48 | 2.12% [1.57 – 2.82] | ||||
Adjusted to National demographic distributions recorded in the 2016 CENSUS www.CSO.ie.
Fig. 2.
SARS-CoV-2 antibody detection in donations received between February 2020 and September 2020. Error bars represent 95% confidence intervals.
Two SARS-CoV-2 seroreactive donors were identified in February 2020. Both of these samples were collected on February 17th 2020, which is 12 days before the first official COVID-19 case was reported in the Republic of Ireland [1], [2], [3], [4]. This significant finding, in our belief, likely reflects the earliest evidence of SARS-CoV-2 infection in Ireland, and indicates that the virus was circulating prior to the first notified case on the 29th of February. The total antibody assay was reactive in these two asymptomatic donors from February 17th, but SARS-CoV-2 IgG was not detected by the Abbott qualitative IgG assay or the Abbott quantitative spike IgG assay. Antibody detection was confirmed by total antibody testing using the Wantai SARS-CoV-2 Total AB ELISA (Fortress Diagnostics). The two seropositive donors identified donated in geographically distinct parts of the country; one donor attended a clinic in Munster, and the other in Ulster.
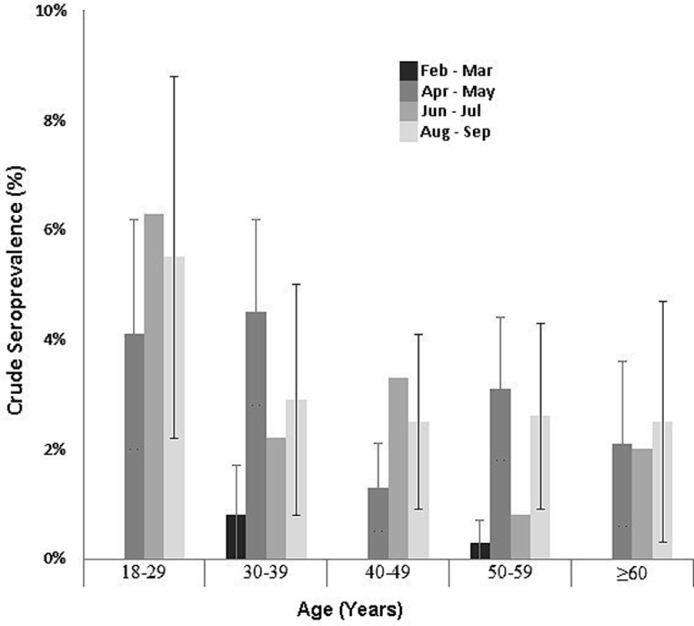
As expected, antibody detection varied greatly by age group, with the highest rate of 5.3% (crude 4.1%) observed in the youngest age category of 18–29 years of age (Fig. 3 ). The 18–29 year donor age group had a consistently elevated rate of SARS-CoV-2 antibody detection from April 2020 onwards, ranging from 4.1% to 6.3%. The SARS-CoV-2 antibody detection rate in donors aged 60 years and older was the lowest at 1.7%. Seroprevalence rates in the 30–39 and 50–59 years age groups peaked in the April and May, and the 40–49 years age group peaked in June and July. Overall, SARS-CoV-2 antibody detection was significantly greater in donors less than 40 years of age (p < 0.0001).
Fig. 3.
SARS-CoV-2 antibody detection in donors age groups during the first-wave of the COVID-19 pandemic. Error bars represent 95% confidence intervals.
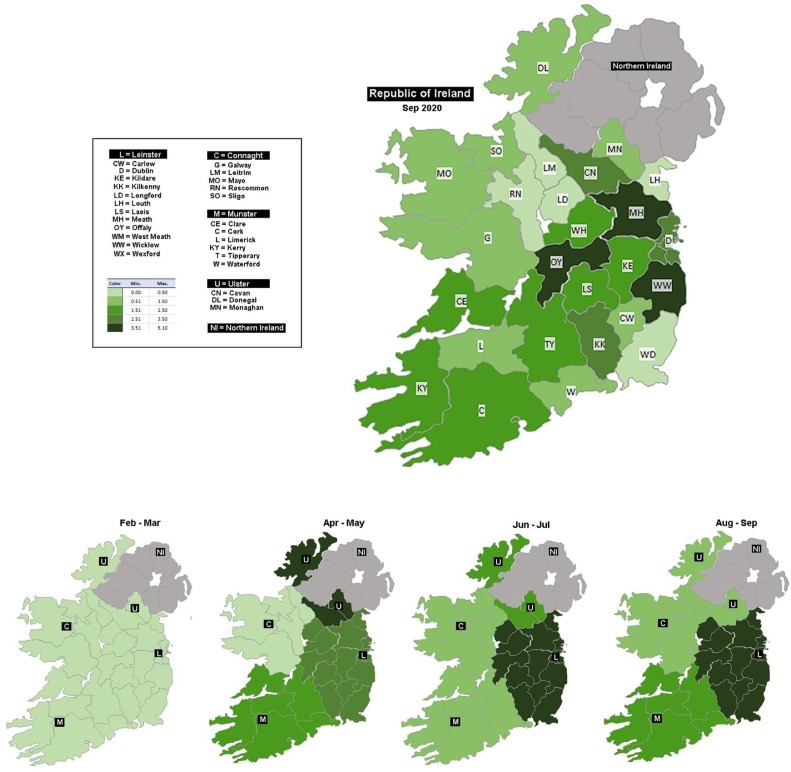
Donations were collected from all 26 counties in the Republic of Ireland. Overall, 58.2% of donors attended a blood donation clinic in the East (Leinster), 26.5% attended in the South (Munster), 7.6% donated in the West (Connacht) and the remaining 7.6% attended at clinic in the North (Ulster, part of). The highest antibody detection rate was observed in the samples received from Leinster-based donation clinics at 2.95% (n = 136/4955, p < 0.0001). This is compared to a detection rate of 1.9% (n = 37/2257) in Munster, 1.4% (n = 9/648) in Ulster and 1.2% (n = 5/649) in Connacht. There was a notable increase in detection rates in Munster and Ulster April and May. However, the antibody detection rate in Connacht remained low throughout the study period (Table 1, Fig. 4 ).
Fig. 4.
Comparison of SARS-CoV-2 antibody detection in donations received from different geographical locations throughout Ireland.
SARS-CoV-2 antibody detection was compared with donor demographics, including blood groups. The presence of the blood group A antigen was significantly higher in donors samples with detectable SARS-CoV-2 antibody. This difference remained significant following adjustment for possible confounding by donor age and gender (p < 0.001).
4. Conclusions
Blood services can provide valuable epidemiological data on emerging infections informing policy and national surveillance programmes [16], and can assess the dynamics of viral circulation, and model the evolution of infectious disease outbreaks, such as the COVID-19 pandemic. The first SARS-CoV-2 antibody positive donor samples were collected on the 17th February 2020. This is the earliest serological evidence of SARS-CoV-2 circulating in the Irish population. These donations were received at sites which were in geographic proximity to the first documented case of community transmission in Ireland, and the first case diagnosed by PCR on the island of Ireland, respectively [1], [2], [3], [4]. Current evidence indicates that the IgG response to SARS-CoV-2 peaks 3 to 7 weeks post-infection. This acute antibody response is followed by a plateau phase, and subsequently slowly declines [16]. IgG was not detected in these early reactive donors. We propose these results reflect the presence of SARS-CoV-2 IgM antibody in donor plasma, suggesting that SARS-CoV-2 infection occurred within the previous two weeks in early February 2020. It is now apparent that COVID-19 was circulating in Europe earlier than first official notifications to the European Centre for Disease Prevention and Control (ECDC) [1,4]. Retrospective PCR testing of a stored respiratory sample from a patient hospitalised in France in December 2019 confirmed SARS-CoV-2 infection [23]. The relatively high rate of asymptomatic infection has played a large part in the widespread global transmission of SARS-CoV-2.
Our study is consistent with national surveillance data which indicates that widespread community transmission did not occur before March 2020 [1]. The potential for SARS-CoV-2 IgG antibody decline was mitigated by incorporating multiple screening assays, into the donor testing algorithm [16, [24], [25], [26]]. Seroprevalence rates increased significantly in April but remained stable at this level until the end of the study, possibly reflecting compliance with public health measures implemented during this time. The donor seroprevalence rate of 2.4% was higher than that reported for in the first wave of COVID-19 infections in Ireland by direct methods, confirming a higher rate of infection in the community than was diagnosed using PCR testing [1,4]. This finding is comparable to what was observed in similar blood donor studies across Europe, such as Denmark and the Netherlands, which reported a SARS-CoV-2 donor seroprevalence of 1.9% and 3.1% between April and May 2020, respectively [17, 27]. Donor studies are extremely suitable for such international comparison as all donors are carefully pre-selected using similar guidelines [16].
Dublin, located in the Eastern Irish province, Leinster, is the most populated city and has consistently reported the largest cumulative number of cases. However, incidence rates have been variable across the country [28]. The Study to investigate COVID-19 Infection in People Living in Ireland (SCOPI) showed significantly higher antibody prevalence in Dublin compared to the more rural Irish western province of Connaught in the summer of 2020. The results from these two locations were extrapolated to estimate an overall national seroprevalence of 1.7% (95% CI; 1.1–2.4%) [29]. Although, our estimate of overall seroprevalence falls within the confidence intervals of that calculated by the SCOPI study; the rate is higher than previously estimated [29]. Donor samples were obtained from all 26 counties and may be more representative of the true seroprevalence. Notably, a different antibody detection temporal trend was observed for Ulster compared to the other provinces which all maintained a steady rate after the initial increase. The counties of Ulster border Northern Ireland, where public health restrictions were implemented and lifted at different times [30]. In addition, cross border movement of people may have contributed to differing levels of infection in that area.
The Irish case fatality ratio is reported as 1.86%, with the heaviest burden of COVID-19 disease and mortality in the older age groups [28]. In direct contrast to those severely impacted by COVID-19 disease, the highest rate of circulating antibodies was detected in donors less than 40 years of age, and lowest in the older age categories. Several factors may have influenced this, such as different age-related responses to public health restrictions, social behaviours, the likelihood of asymptomatic infection in younger individuals, recovery time, pre-selection of younger donors during public health restrictions and the individual risk perception surrounding blood donation during the pandemic [31].
The impact of ABO blood group on SARS-CoV-2 susceptibility remains unclear [[32], [33], [34], [35], [36], [37]]. Blood groups are known to influence individual susceptibility to other viruses such as SARS-CoV-1 and norovirus [34]. A significantly higher rate of seropositivity was observed in Irish donors with the blood group A antigen. Possible mechanisms for the observed difference include anti-A antibodies binding to viral antigens, resulting in a protective effect by blocking the Spike and ACE-2 protein interaction required for viral entry [36]. However, the clinical significance of this finding remains unclear and merits further study.
In conclusion, the present study of over 8000 blood donors, sampled during the first wave of the COVID-19 pandemic, demonstrates evidence of SARS-CoV-2 antibody reactivity across all age groups and counties. The critical value of a blood donor seroprevalence study is apparent in this report which identified the earliest serological evidence of SARS-CoV-2 infection in Ireland, as well as documenting the evolution of COVID-19 pandemic in Ireland over time. Studies of this kind emphasise the role of blood services in providing ‘real-time’ seroprevalence data to policy-makers to inform decision-making in relation to national pandemic management, infection prevention and control strategies and vaccination policy and in assessing the impact of these measures on rates of infection and immunity for established and emerging infectious diseases.
5. Financial support
This work was supported by the Irish Blood Transfusion Service internal research and development funding, and by Abbott Diagnostics. Abbott diagnostics provided reagents for testing; however, has had no involvement in study design; sample collection, analysis and interpretation of data; writing of the report; and in the decision to submit the article for publication.
CRediT authorship contribution statement
Dearbhla Butler: Methodology, Data curation, Formal analysis, Writing – original draft. Dermot Coyne: Methodology, Data curation, Supervision, Funding acquisition. Louise Pomeroy: Conceptualization, Methodology, Writing – review & editing. Pádraig Williams: Resources, Project administration. Paul Holder: Methodology, Funding acquisition. Alex Carterson: Methodology, Data curation. Stephen Field: Conceptualization, Supervision, Funding acquisition. Allison Waters: Data curation, Writing – original draft, Project administration, Formal analysis. Niamh O'Flaherty: Conceptualization, Methodology, Writing – review & editing, Project administration, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: This work was supported by the Irish Blood Transfusion Service internal research and development funding, and by Abbott Diagnostics. Abbott diagnostics provided reagents for testing; however, has had no involvement in study design; sample collection, analysis and interpretation of data; writing of the report; and in the decision to submit the article for publication.
Acknowledgements
The IBTS is indebted to our dedicated donors who have continued to attend blood donor clinics during these challenging times. We also kindly acknowledge the contribution of our colleagues at the National Virus Reference Laboratory for their time and help with confirmatory SARS-CoV-2 antibody testing. We also acknowledge the contribution of Abbott Laboratories to the design and funding of the testing algorithm.
References
- 1.Conway R., Kelly D.M., Mullane P., Ni Bhuachalla C., O'Connor L., Buckley C., Kearney P.M., Doyle S. Corrigendum to: epidemiology of COVID-19 and public health restrictions during the first wave of the pandemic in Ireland in 2020. J. Public Health (Oxf) 2021 doi: 10.1093/pubmed/fdab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Public Health Emergency Team. Public health framework approach in providing advice to government in relation to reducing social distancing measures introduced in response to COVID-19. 2020 May 1. https://www.gov.ie/en/publication.
- 3.Faller E., Lapthorne S., Barry R., Shamile F., Salleh F., Doyle D., O'Halloran D., Prentice M., Sadlier C. The presentation and diagnosis of the first known community-transmitted case of SARS-CoV-2 in the Republic of Ireland. Ir. Med. J. 2020;113(5):78. May 7. [PubMed] [Google Scholar]
- 4.Kennelly B., O'Callaghan M., Coughlan D., Cullinan J., Doherty E., Glynn L., Moloney E., Queally M. The COVID-19 pandemic in Ireland: an overview of the health service and economic policy response. Health Policy Technol. 2020;9(4):419–429. doi: 10.1016/j.hlpt.2020.08.021. Dec10.1016/j.hlpt.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perumal V., Curran T., Hunter M. First case of covid-19 in Ireland. Ulster Med. J. 2020;89(2):128. [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 measures taken by IBTS to protect donors and staff: Irish blood transfusion service. (www.giveblood.ie) Accessed 02-06- 2021.
- 7.Corman V.M., Rabenau H.F., Adams O., Oberle D., Funk M.B., Keller-Stanislawski B., Timm J., Drosten C., Ciesek S. SARS-CoV-2 asymptomatic and symptomatic patients and risk for transfusion transmission. Transfusion. 2020;60(6):1119–1122. doi: 10.1111/trf.15841. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiely P., Hoad V.C., Seed C.R., Gosbell I.B. Severe acute respiratory syndrome coronavirus-2: implications for blood safety and sufficiency. Vox Sang. 2021;116(2):155–166. doi: 10.1111/vox.13009. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. Aug. [DOI] [PubMed] [Google Scholar]
- 10.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann. Intern. Med. 2021;174(5):655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S.L., Mertens A.N., Crider Y.S., Nguyen A., Pokpongkiat N.N., Djajadi S., Seth A., Hsiang M.S., Colford J.M., Jr, Reingold A., Arnold B.F., Hubbard A., Benjamin-Chung J. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat. Commun. 2020;11(1):4507. doi: 10.1038/s41467-020-18272-4. Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy D., McAloon C., Collins A., Hunt K., Butler F., Byrne A., Casey-Bryars M., Barber A., Griffin J., Lane E.A., Wall P., More S.J. Relative infectiousness of asymptomatic SARS-CoV-2 infected persons compared with symptomatic individuals: a rapid scoping review. BMJ Open. 2021;11(5) doi: 10.1136/bmjopen-2020-042354. May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien S.F., Lieshout-Krikke R.W., Lewin A., Erikstrup C., Steele W.R., Uzicanin S., Custer B. Surveillance, risk assessment, policy sub-group of the ISBT transfusion transmitted infectious diseases working party. Research initiatives of blood services worldwide in response to the covid-19 pandemic. Vox Sang. 2021;116(3):296–304. doi: 10.1111/vox.12995. Mar. [DOI] [PubMed] [Google Scholar]
- 14.Castro Dopico X., Muschiol S., Christian M., Hanke L., Sheward D.J., Grinberg N.F., Rorbach J., Bogdanovic G., Mcinerney G.M., Allander T., Wallace C., Murrell B., Albert J., Karlsson Hedestam G.B. Seropositivity in blood donors and pregnant women during the first year of SARS-CoV-2 transmission in Stockholm, Sweden. J. Intern. Med. 2021 doi: 10.1111/joim.13304. May. doi 18:10.1111/joim.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer B., Knabbe C., Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Euro Surveill. 2020;25(28) doi: 10.2807/1560-7917.ES.2020.25.28.2001285. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewin A., Drews S.J., Lieshout-Krikke R., Erikstrup C., Saeed S., Fady H., Uzicanin S., Custer B., O'Brien S.F.; Surveillance, risk assessment, policy, the virology sub-groups of the ISBT transfusion transmitted infectious diseases working party. An international comparison of anti-SARS-COV-2 assays used for seroprevalence surveys from blood component providers. Vox Sang. 2021 Apr 29:10.1111/vox.13100. doi: 10.1111/vox.13100. [DOI] [PMC free article] [PubMed]
- 17.Slot E., Hogema B.M., Reusken C.B.E.M., Reimerink J.H., Molier M., Karregat J.H.M., IJlst J., Novotný V.M.J., van Lier R.A.W., Zaaijer H.L. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat. Commun. 2020;11(1):5744. doi: 10.1038/s41467-020-19481-7. Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sughayer M.A., Mansour A., Al Nuirat A., Souan L., Ghanem M., Siag M. Dramatic rise in seroprevalence rates of SARS-CoV-2 antibodies among healthy blood donors: the evolution of a pandemic. Int. J. Infect. Dis. 2021;107:116–120. doi: 10.1016/j.ijid.2021.04.059. Jundoi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harritshøj L.H., Gybel-Brask M., Afzal S., Kamstrup P.R., Jørgensen C.S., Thomsen M.K., Hilsted L., Friis-Hansen L., Szecsi P.B., Pedersen L., Nielsen L., Hansen C.B., Garred P., Korsholm T.L., Mikkelsen S., Nielsen K.O., Møller B.K., Hansen A.T., Iversen K.K., Nielsen P.B., Hasselbalch R.B., Fogh K., Norsk J.B., Kristensen J.H., Schønning K., Kirkby N.S., Nielsen A.C.Y., Landsy L.H., Loftager M., Holm D.K., Nilsson A.C., Sækmose S.G., Grum-Schwensen B., Aagaard B., Jensen T.G., Nielsen D.M., Ullum H., Dessau R.B. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J. Clin. Microbiol. 2021;59(5) doi: 10.1128/JCM.02596-20. Apr 20e02596-20. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marlet J., Petillon C., Ragot E., Abou El Fattah Y., Guillon A., Marchand Adam S., Lemaignen A., Bernard L., Desoubeaux G., Blasco H., Barin F., Stefic K., Gaudy-Graffin C. Clinical performance of four immunoassays for antibodies to SARS-CoV-2, including a prospective analysis for the diagnosis of COVID-19 in a real-life routine care setting. J. Clin. Virol. 2020;132 doi: 10.1016/j.jcv.2020.104633. Novdoi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bal A., Pozzetto B., Trabaud M.A., Escuret V., Rabilloud M., Langlois-Jacques C., Paul A., Guibert N., D'Aubarède-Frieh C., Massardier-Pilonchery A., Fabien N., Goncalves D., Boibieux A., Morfin-Sherpa F., Pitiot V., Gueyffier F., Lina B., Fassier J.B., Trouillet-Assant S., COVID SER Study Group Evaluation of high-throughput SARS-CoV-2 serological assays in a longitudinal cohort of patients with mild COVID-19: clinical sensitivity, specificity, and association with virus neutralization test. Clin. Chem. 2021;67(5):742–752. doi: 10.1093/clinchem/hvaa336. Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewin A., Therrien R., De Serres G., et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can. J. Public Health. 2021:1–11. doi: 10.17269/s41997-021-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deslandes A., Berti V., Tandjaoui-Lambotte Y., Alloui C., Carbonnelle E., Zahar J.R., Brichler S., Cohen Y. SARS-CoV-2 was already spreading in France in late December 2019. Int. J. Antimicrob. Agents. 2020;55(6) doi: 10.1016/j.ijantimicag.2020.106006. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muecksch F., Wise H., Batchelor B., Squires M., Semple E., Richardson C., McGuire J., Clearly S., Furrie E., Neil G., Hay G., Templeton K., Lorenzi J.C.C., Hatziioannou T., Jenks S., Bieniasz P.D. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. medRxiv [Preprint] 2020 doi: 10.1101/2020.08.05.20169128. Aug 6:2020.08.05.20169128. doi: [DOI] [Google Scholar]
- 25.Perreault J., Tremblay T., Fournier M.J., Drouin M., Beaudoin-Bussières G., Prévost J., Lewin A., Bégin P., Finzi A., Bazin R. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136(22):2588–2591. doi: 10.1182/blood.2020008367. Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O'Byrne A., Kouphou N., Galao R.P., Betancor G., Wilson H.D., Signell A.W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J.M., Lista M.J., Temperton N., Snell L.B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M.K.I., O'Connell L., O'Hara G., MacMahon E., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J.D., Neil S.J.D., Malim M.H., Doores K.J. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erikstrup C., Hother C.E., Pedersen O.B.V., Mølbak K., Skov R.L., Holm D.K., Sækmose S.G., Nilsson A.C., Brooks P.T., Boldsen J.K., Mikkelsen C., Gybel-Brask M., Sørensen E., Dinh K.M., Mikkelsen S., Møller B.K., Haunstrup T., Harritshøj L., Jensen B.A., Hjalgrim H., Lillevang S.T., Ullum H. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin. Infect. Dis. 2021;72(2):249–253. doi: 10.1093/cid/ciaa849. Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epidemiology of COVID-19 in Ireland- daily infographic https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/casesinireland/epidemiologyofcovid-19inireland/Health Protection Surveillance Centre. Updated daily. Accessed 02-06- 2021.
- 29.Preliminary report of the results of the Study To investigate COVID-19 infection in people living in Ireland (SCOPI): a national seroprevalence study, June-July 2020. Health Service Executive. 2020 [Google Scholar]
- 30.Coughlin S.S., Yiǧiter A., Xu H., Berman A.E., Chen J. Early detection of change patterns in COVID-19 incidence and the implementation of public health policies: a multi-national study. Public Health Pract. (Oxf) 2021;2 doi: 10.1016/j.puhip.2020.100064. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haw J., Holloway K., Masser B.M., Merz E.M., Thorpe R. Blood donation and the global COVID-19 pandemic: areas for social science research. Vox Sang. 2021;116(4):363–365. doi: 10.1111/vox.12974. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goel R., Bloch E.M., Pirenne F., Al-Riyami A.Z., Crowe E., Dau L., Land K., Townsend M., Jecko T., Rahimi-Levene N., Patidar G., Josephson C.D., Arora S., Vermeulen M., Vrielink H., Montemayor C., Oreh A., Hindawi S., van den Berg K., Serrano K., So-Osman C., Wood E., Devine D.V. Spitalnik SL; ISBT COVID-19 working group. ABO blood group and COVID-19: a review on behalf of the ISBT COVID-19 working group. Vox Sang. 2021 doi: 10.1111/vox.13076. Feb 12:10.1111/vox.13076. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Wang X., Chen J., Cai Y., Deng A., Yang M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br. J. Haematol. 2020;190(1):24–27. doi: 10.1111/bjh.16797. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miotto M., Di Rienzo L., Gosti G., Milanetti E., Ruocco G. Does blood type affect the COVID-19 infection pattern? PLoS ONE. 2021;16(5) doi: 10.1371/journal.pone.0251535. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto F., Yamamoto M., Muniz-Diaz E. Blood group ABO polymorphism inhibits SARS-CoV-2 infection and affects COVID-19 progression. Vox Sang. 2021;116(1):15–17. doi: 10.1111/vox.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J., Yang Y., Huang H., Li D., Gu D., Lu X., Zhang Z., Liu L., Liu T., Liu Y., He Y., Sun B., Wei M., Yang G., Wang X., Zhang L., Zhou X., Xing M., Wang P.G. Relationship between the abo blood group and the coronavirus disease 2019 (COVID-19) susceptibility. Clin. Infect. Dis. 2021;73(2):328–331. doi: 10.1093/cid/ciaa1150. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zietz M., Zucker J., Tatonetti N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020;11(1):5761. doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]