Abstract
A wide variety of wires are available for use in interventional radiology, with wires demonstrating differences in construction, diameter, length, coating, shape, and taper. It is crucial to understand the difference in characteristics between these wires to select the most effective and safe wire for the intended purpose when undertaking a procedure. This article reviews the qualities and functions of different types of wires to aid in this decision-making process.
Keywords: interventional radiology, technology, guidewires, design
The interventional radiologist's (IR) armamentarium consists of an array of wires, each with a unique set of design properties and functional characteristics that make the instrument suited for a particular procedure. Operators select wires based on personal preferences generated through experience; however, an understanding of general principles is paramount for both trainees and seasoned IRs as device properties can guide procedural planning and intraprocedural troubleshooting.
Wires: General Characteristics
Broadly, wires are subcategorized into access wires, navigational wires, and working wires ( Fig. 1 ). Wire selection is contingent on properties that determine whether a given wire is suitable for an intended procedural step. Important considerations include diameter, length, shape, taper, composition (hydrophilicity, hydrophobicity), and stiffness.
Fig. 1.
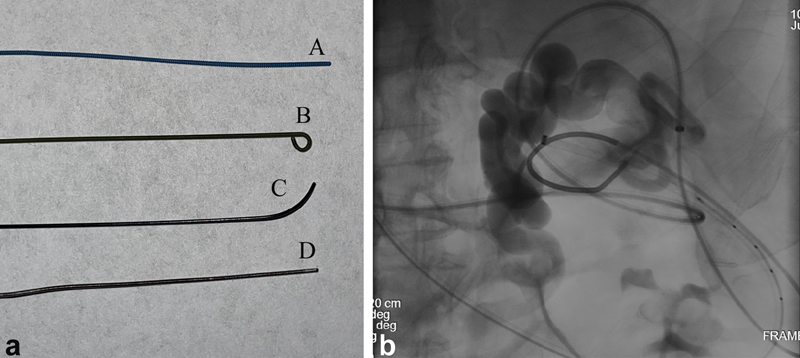
( a ) Commonly used wires. A—The Amplatz Super Stiff Guidewire (Boston Scientific) is a stiff working wire that allows for stable exchanges and excellent catheter and device trackability. It has a stainless-steel core with flat-wire coil and polytetrafluoroethylene (PTFE) coating, and comes in several lengths and tip configurations. B—Rosen Guidewire (Cook Medical) is a working wire that has a tightened 1.5-mm “J” configuration to allow for atraumatic vessel or stent navigation. C—Angled Glidewire (Terumo) is a soft, hydrophilic wire that may be used for navigation across a tight stenosis or other fluid-filled structure. The angled tip provides steerability; multiple different angle configurations are available. D—Magic Torque Guidewire (Boston Scientific) has a stainless steel core and spring coil tip. The distal 10 cm has a hydrophilic coating, while the proximal end does not, allowing the wire to be used as both a navigational and working wire in certain situations. The distal end also has radiopaque markers (two shown with black arrows) at 2- and 1-cm intervals to allow for calibration for device size during a case. ( b ) A 63-year-old man with cirrhosis, portal hypertension, status post transjugular intrahepatic portosystemic shunt (TIPS) creation now with hepatic encephalopathy and large splenorenal shunt, for which he presents for closure. After obtaining antegrade trans-TIPS access and retrograde access through the left renal vein to the splenorenal shunt, a Magic Torque wire is positioned in the field of view to allow for measurement calibration for vascular plug sizing. Black arrows—1-cm markings on the wire.
Wire diameter reflects the outer measurement (inches) with sizes typically ranging from 0.010 to 0.038.” The most commonly utilized diameter wires are 0.018″ and 0.035″, as they accommodate the most commonly stocked catheters. Wires are typically paired with a needle or end-hole catheter; 0.018″ wires can be accommodated by 3-Fr catheters and 22-gauge or larger needles, while 0.035″ wires are accommodated by 5-Fr catheters or 18-gauge or larger needles. Oversizing the catheter to a wire (i.e., using a 0.018″ wire with a 5-Fr catheter) may result in a step-off between the wire and catheter tip, which can prevent advancement of the catheter across a branching vessel or through soft tissue. Typically, 0.018″ wires are utilized to gain initial access into a blood vessel or nonvascular space (i.e., renal collecting system or biliary system) prior to upsizing to a 0.035″ system. In general, a 0.035″ wire is stronger than a 0.018″ wire, as strength is directly related to wire diameter. This allows for more wire pushability and support for tracking devices such as catheters, balloons, or stents. On the other hand, smaller wires provide more flexibility which helps in navigating small vessels or other body cavities. Moreover, 0.014″ to 0.018″ microwires can be utilized in smaller diameter vessels with microcatheters where a larger system may result in injury or lack of maneuverability.
Wire length is typically determined by the distance from the skin-entry site to beyond the target area of intervention, as well as the length of the expected catheter and sheath combination. Common wire lengths range from 80 to 260 cm, with 145 to 180 cm being the most commonly used. Wire length should be double the length of the intended catheter; this ensures adequate wire length for any necessary catheter exchanges that may arise during a procedure. Exchange-length wires ranging from 260 to 300 cm are commonly used in cases where stable endovascular or intraluminal access must be maintained (e.g., during stenting). Handling exchange-length wires requires care as the unwieldy length of these wires poses potential for contamination.
Wire stiffness is dictated by its construction. Commonly used spring wires are generally constructed with a stiff mandrel core around which an outer wire is tightly coiled 1 2 ( Fig. 2 ). The mandril is connected to both the proximal and distal ends of the outer wire coil. The existence of this safety wire (or metal ribbon) prevents the outer wire coil from unwinding from around the mandril. The thickness of this mandrel and coiled outer wire determine wire stiffness. Common core materials include stainless steel and nitinol (nickel–titanium alloy). A small, thin safety wire is also present in a longitudinal orientation extending the length of the wire.
Fig. 2.
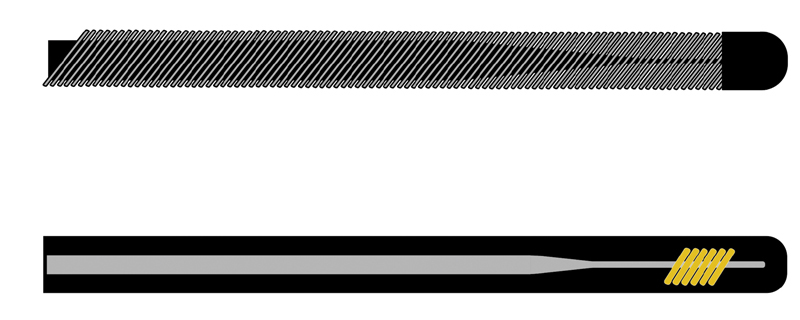
Top: Spring wire construction demonstrating inner mandrel core with surrounding tightly wound wire. Below: Hydrophilic wire construction with inner mandrel core with plastic coating.
Wire shape , defined by the distal end of the wire, serves two functions: the ability to determine how traumatic the wire may be to surrounding structures and the ability to steer the wire to select a vessel or other luminal structure. Spring wires risk destruction when manual shaping is attempted, as the mandrel is attached only to the tip of the wire by the thin safety wire. However, there exist spring wires with inherently curved safety wires allowing for elastic J-tips (e.g., 1.5-mm radius). These tips allow for atraumatic navigation of vascular structures (especially in the setting of atheromatous plaque) as well as nonvascular structures. J-tip wires may also be used when crossing bare metal stents to ensure the wire remains in the stent lumen and does not cross into stent interstices.
Many other preformed wire shapes are available, and describe the curve of the distal tip of the wire (the last few centimeters). Curves typically are of 30 to 90 degrees; however, complex wire curves with more than one shape can also be used. In general, the wire curve should approximate the angle of the vessel or structure to be traversed. Tip shape is useful in the selection of a structure; other wire characteristics (stiffness, taper, hydrophilicity) facilitate catheter tracking.
Wire taper refers to the diameter of the inner core or mandril of the wire. If the mandril extends to the very end of the outer wire coil, there would be no tapering and the wire would feel equally stiff along its entire length. If the inner mandril is tapered, the wire feels stiffest at the back end of the wire and softest at the lead end. This taper allows for easy catheter trackability along the stiff portion of the wire and little likelihood for vessel trauma at the lead end. If the wire tip is too floppy (soft), however, it may be difficult to have a catheter track over the floppiest portion. The trade off, therefore, is to choose a wire with enough of a taper to allow for atraumatic placement, while still facilitating catheter advancement. Wire tapers generally are from 2 to 18 cm in length. Moreover, variations in the taper between a stiff wire shaft and soft tip allow wires to have unique “tip load” characteristics, trackability, and flexibility without kinking. 3
Wire coating is also an important characteristic to consider when choosing a wire. The most important distinction is between hydrophobic and hydrophilic wires. Hydrophobic wires typically have Teflon or polytetrafluoroethylene (PTFE) coating to decrease the coefficient of friction and increase lubricity. 3 Some wires also contain a heparin coating to transiently reduce thrombogenicity; this heparin effect typically lasts 5 to 10 minutes. 4 5 6 7 Common hydrophobic wires include the Amplatz (Boston Scientific, Marlborough, MA), Bentson (Cook Medical), and Rosen wires (Cook Medical).
In comparison to Teflon-coated wires, hydrophilic wires are made of a similar core, but with a plastic sheathing coated with hydrophilic material to maximize lubricity. The most commonly used hydrophilic wire is the Glidewire (Terumo, Tokyo, Japan), which has a nitinol core. They can be straight or angled at their tip. Because hydrophilic wires are slippery, they are often steered more easily than Teflon-coated wires via placement of a torque device at the back end of the wire. Hydrophilic wires are also considered to be less thrombogenic than their nonhydrophilic counterparts. 4 8
Hydrophilic guidewires require activation and continual lubrication when outside of the body. They are often stored in saline-flushed housings, flushed catheters, and saline bowls, or regularly wiped with saline during procedures. Their hydrophilic constitution makes them especially slick and movable when wet, but very sticky when allowed to dry. The utmost care must be taken to maintain wire position during catheter exchanges over a hydrophilic wire, usually with the use of hemostats or using one's fingernails to secure the wire. Care should also be taken to avoid shearing forces, such as pulling a hydrophilic wire across a needle bevel during a procedure, as the hydrophilic material has been known to become striped and may embolize 9 10 ( Fig. 3 ). Nitinol core hydrophilic wires are generally resistant to shaping, but like unwrapped steel wires, can obtain a curvature when drawn between the thumb and a shaping device or stiff cannula. 3 A nitinol wire can also be purposefully acutely kinked outside the body to navigate sharp corners. 3
Fig. 3.
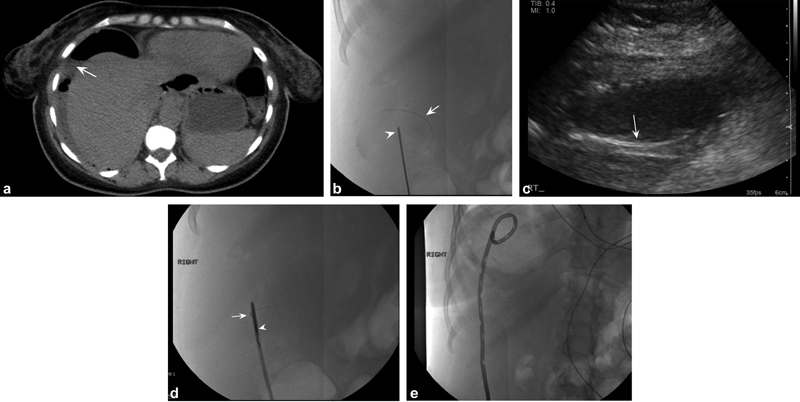
A 43-year-old woman with abdominal pain and computed tomographic (CT) findings consistent with perforated viscus. ( a ) Axial noncontrast CT images demonstrate large air- and fluid-filled collection in the right upper quadrant (white arrow) for which drainage was requested. ( b ) After ultrasound-guided access to the collection with an 18-gauge needle (white arrowhead), a Glidewire (Terumo) was advanced into the collection. During manipulation of the wire, the hydrophilic coating sheared off the wire into the abscess cavity (white arrow). ( c ) Ultrasound imaging demonstrates linear echogenic structure in the cavity corresponding to the sheared wire coating (white arrow). ( d ) The system was upsized to a 5-Fr sheath, through which a loop snare was advanced (white arrowhead) to capture and remove the coating (white arrow). ( e ) Completion image demonstrates complete removal of the Glidewire coating with a pigtail drain in place.
Access Wires
Access wires are utilized during the initial stages of a procedure to secure stable access during both endovascular and non-endovascular procedures (e.g., renal collecting system, biliary tree, and abscess cavity). Access wires are typically made of a metal core and do not have an outer braided layer. Commonly used access wires are 0.018″ short wires with floppy, atraumatic straight-tips such as the Cope Mandril wire (Cook Medical), which is easily accepted into a standard 21-gauge micropuncture needle for endovascular procedures, or a short nitinol wire included in nonvascular access systems such as the AccuStick Needle (Boston Scientific). The Nitrex wire (Medtronic, Minneapolis, MN), with a nitinol core and relatively atraumatic gold tungsten tip, can also be considered in instances when maneuverability is desired during initial access.
Navigational Guidewires and Working Wires
In general, other than access wires guidewires can be further defined as navigational wires and working wires. Navigational wires are less stiff than working wires and often have soft, shaped, and/or angled tips to facilitate selecting vessels. They are typically used to cross tortuous structures—especially where the operator may expect to experience resistance—such as a vascular stenosis, clogged catheter, 7 or fluid-filled nonvascular structures (e.g., urinary tract, biliary tree, and alimentary tract). While hydrophilic and non-hydrophilic wires can both be used as navigational wires, hydrophilic wires are more commonly used since this hydrophilicity allows the wire to easily overcome resistance in fluid-filled vascular and non-vascular structures.
Once a navigational wire is used to reach the target destination, it is often exchanged via the catheter for the working wire, which is typically a stiffer, non-hydrophilic wire. As discussed earlier, the working wire must be approximately twice the length of the catheter used during a catheter exchange to allow for subsequent removal of the catheter without losing wire position. The most important characteristic of a working wire is its relative stiffness. The stiffness of these wires allows for tracking of catheters and endovascular devices (e.g., angioplasty balloons, stents) without wire kinking or herniation. Moreover, non-hydrophilic wire composition makes these wires easier to manually grip, resulting in less susceptibility to movement during wire exchanges. Notably, care should be taken when removing non-hydrophilic wires through vascular systems, as friction between the wire and vessel wall can result in endothelial injury and patient discomfort during wire exchanges.
It is critical to consider the diameter, shape, and taper of the guidewire in relation to the catheter being used. Wire shape and taper will largely influence catheter direction and steerability, while hydrophilicity and stiffness largely facilitate catheter exchanges. Moreover, mismatch between the outer diameter of the guidewire and the inner diameter of the catheter will result in difficulty moving the catheter over the wire due to this size mismatch and “step off” or “shouldering of the catheter on the wire. This can result in endothelial or soft-tissue injury, catching of bifurcations with subsequent inability of the catheter tip to track, and potential wire kinking if there is a large step-off between the stiff catheter tip and floppy wire.
Microwires
Commonly used microwires range in diameter from 0.012″ to 0.016″, with common lengths between 200 and 300 cm. Microwires are used in combination with microcatheters, typically in a coaxial fashion through a base catheter, to navigate small vessels that are difficult or dangerous to catheterize with a larger 4–to 5-Fr system. Similar to larger wires, microwires come with numerous tip shapes to catheterize difficult-to-reach vessels. Other wire characteristics (e.g., stiffness, taper) are also variable from wire to wire. Many of the currently available microwires are hydrophilic and have relatively atraumatic (floppy) tips to minimize the risk of vasospasm and dissection when navigating a small vessel. Shapeable tip microwires such as the Fathom (Boston Scientific) and Transcend (Stryker, Kalamazoo, MI) wires have unique physical characteristics that allow the operator to shape and reshape the wire tip as needed, depending on the takeoff angle of the target vessel or endoluminal structure.
Tip-Deflecting wires
Tip-deflecting wires are steerable guidewires with a straight or J-shaped deflectable tip that can be manipulated through a trailing handle. The external handle allows the operator to select the curvature of the wire tip depending on the degree of tension used on the handle 2 ( Fig. 4 ). Catheter tips will conform to the shape of the wire. Importantly, due to the relatively stiff (traumatic) tip, a deflecting wire is typically not advanced beyond the end of the catheter. 1 Tip-deflecting wires are useful for navigating tortuous structures and can be used for selective angiography. 11 Other uses include inferior vena cava (IVC) filter retrieval, 12 IVC filter repositioning, 13 or foreign body retrieval or manipulation, including repositioning of malpositioned central venous catheter tips 14 15 ( Fig. 4 ).
Fig. 4.
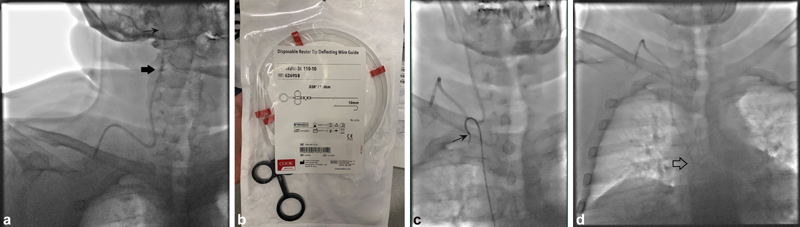
A 49-year-old man with metastatic renal cell carcinoma undergoing chemotherapy who presented due to sensation of audible noise next to his right ear when his port was being flushed. ( a ) Scout image prior to the procedure demonstrates a malpositioned catheter tip, which had migrated cephalad into the right internal jugular vein (black arrows). Right common femoral vein access was obtained, but initial attempts to reposition the port catheter with a Sos Omni 2 catheter (AngioDynamics, Latham, NY) were unsuccessful due to lack of support provided by the catheter. ( b ) A Reuter Tip Deflecting Wire (Cook Medical) was used to assist with repositioning. The handle at the back of the wire can be used to shape the wire to deflect the catheter tip to varying degrees. ( c ) Sos Omni 2 catheter hooked around the catheter, with radiopaque tip deflecting wire (black arrow) within the catheter to provide additional support. This system was used to pull the port catheter caudally. ( d ) Final image demonstrating the port catheter tip in appropriate position at the superior cavoatrial junction (arrow).
Conclusion
Wire choice is an important decision to optimize the chances for success during a case. It is crucial to understand the differences between different types of access, navigational, and working wires. The different wires available to IRs have unique benefits and disadvantages based on their construction, stiffness, coating, and tip. Careful planning and continued experience help both trainees and experienced IRs decide the optimal wire type for particular situation.
Acknowledgments
None.
Funding Statement
Funding This study was not supported by any funding.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Kaufman J A, Lee M J. 2nd ed. Saunders; 2013. Vascular and Interventional Radiology: The Requisites; pp. 25–55. [Google Scholar]
- 2.Mauro M A. 2nd ed. Philadelphia, PA: Saunders/Elsevier; 2014. Image-guided interventions. Expert Radiology Series; pp. e15–e23. [Google Scholar]
- 3.Schröder Jr. New York: Thieme; 2013. Peripheral Vascular Interventions: An Illustrated Manual; pp. 7–41. [Google Scholar]
- 4.Kido D K, King P D, Manzione J V, Simon J H.The role of catheters and guidewires in the production of angiographic thromboembolic complications Invest Radiol 198823(Suppl 2):S359–S365. [DOI] [PubMed] [Google Scholar]
- 5.Lee K H, Han J K, Byun Y. Heparin-coated angiographic catheters: an in vivo comparison of three coating methods with different heparin release profiles. Cardiovasc Intervent Radiol. 2004;27(05):507–511. doi: 10.1007/s00270-003-4035-5. [DOI] [PubMed] [Google Scholar]
- 6.Raininko R, Söder H. Clot formation in angiographic catheters–an in vitro comparative study. Effects of heparin and protein coating of the catheter. Acta Radiol. 1993;34(01):78–82. [PubMed] [Google Scholar]
- 7.Huang S Y, Engstrom B I, Lungren M P, Kim C Y. Management of dysfunctional catheters and tubes inserted by interventional radiology. Semin Intervent Radiol. 2015;32(02):67–77. doi: 10.1055/s-0035-1549371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leach K R, Kurisu Y, Carlson J E. Thrombogenicity of hydrophilically coated guide wires and catheters. Radiology. 1990;175(03):675–677. doi: 10.1148/radiology.175.3.2343111. [DOI] [PubMed] [Google Scholar]
- 9.Rosen L E, Singh R I, Mahon B. Myocardial hydrophilic polymer emboli following cardiac catheterization: a case report and literature review. Cardiovasc Pathol. 2014;23(03):175–177. doi: 10.1016/j.carpath.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Grundeken M J, Li X, Kurpershoek C E. Distal embolization of hydrophilic-coating material from coronary guidewires after percutaneous coronary interventions. Circ Cardiovasc Interv. 2015;8(02):e001816. doi: 10.1161/CIRCINTERVENTIONS.114.001816. [DOI] [PubMed] [Google Scholar]
- 11.Nakao N, Miura K, Takayasu Y, Uchida H. Tip-deflecting wire for percutaneous transhepatic portography. Radiology. 1982;143(01):258. doi: 10.1148/radiology.143.1.7199751. [DOI] [PubMed] [Google Scholar]
- 12.Sista A K, Vedantham S, Kaufman J A, Madoff D C. Endovascular interventions for acute and chronic lower extremity deep venous disease: state of the art. Radiology. 2015;276(01):31–53. doi: 10.1148/radiol.2015132603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson R B, Fowl R J, Lubbers D J, Vu D N, Kempczinski R F. Repositioning of partially dislodged Greenfield filters from the right atrium by use of a tip deflection wire. J Vasc Surg. 1990;12(01):70–72. doi: 10.1067/mva.1990.20088. [DOI] [PubMed] [Google Scholar]
- 14.Nemcek A A, Jr, Vogelzang R L. Modified use of the tip-deflecting wire in manipulation of foreign bodies. AJR Am J Roentgenol. 1987;149(04):777–779. doi: 10.2214/ajr.149.4.777. [DOI] [PubMed] [Google Scholar]
- 15.Morris D C, Scott I R, Jamieson W R. Pacemaker electrode repositioning using the loop-snare technique. Pacing Clin Electrophysiol. 1989;12(06):996–999. doi: 10.1111/j.1540-8159.1989.tb05037.x. [DOI] [PubMed] [Google Scholar]


