Abstract
Symptomatic knee pain is one of the most common joint diseases that affects millions of people worldwide. The treatment for knee pain secondary to osteoarthritis (OA) begins with conservative therapy and progresses to surgical intervention when conservative therapy fails. Genicular artery embolization (GAE) offers an alternative option for patients who are poor surgical candidates. Multiple studies have been conducted worldwide demonstrating the safety and efficacy of GAE in patients with mild to moderate OA. The purpose of this article is to describe the current literature on GAE and highlight the latest findings from a randomized controlled trial comparing GAE versus sham embolization.
Keywords: musculoskeletal intervention, osteoarthritis, genicular artery embolization, interventional radiology
Genicular artery embolization (GAE) was initially described to treat patients with spontaneous hemarthrosis after knee arthroplasty. 1 2 Angiography in these patients demonstrates synovial hypervascularity that can be targeted with embolization to control hemarthrosis. 3 4 Okuno et al were the first to apply this same technique to patients with knee pain secondary to osteoarthritis (OA) who have not undergone arthroplasty. 5 It was successfully demonstrated in consecutive trials that transarterial embolization of genicular arteries in patients with osteoarthritic knee pain refractory to conservative management could significantly improve knee pain and function. 6 7 The presumed mechanism behind the pain reduction involves ischemia of the inflamed synovial tissue, including the irritated neurovascular bundles that course within it. 8 9 10 Since the initial trial by Okuno et al in 2013, multiple studies have been conducted worldwide culminating in a randomized control study in the United States that highlighted the effectiveness of GAE compared to a sham procedure. 11 12 13 14 15 The purpose of this article is to describe the literature on GAE and highlight the latest findings from the randomized sham-controlled trial.
Anatomy
An in-depth understanding of the knee joint arterial anatomy is necessary to successfully navigate and embolize the appropriate vessels. The following arteries may be targeted during GAE: the descending genicular artery, the medial superior genicular artery, the medial inferior genicular artery, the lateral superior genicular artery, the lateral inferior genicular artery, and the anterior tibial recurrent artery. An angiographic classification for the genicular vascular anatomy was proposed recently: medially—M1 (3/3 arteries present) versus M2 (2/3 arteries present), laterally—L1 (3/3 arteries present) versus L2 (2/3 arteries present). 16 Of note, the superior patellar artery and the median genicular artery were studied by Okuno et al but has not been included in any other studies given its small vessel size and limited involvement in the knee joint. When performing GAE, it is important to understand that the knee joint is a highly vascular structure with numerous arterial anastomoses that can potentially lead to nontarget embolization. 17
Procedure
Most commonly, contralateral femoral artery access is used for GAE. After advancing a diagnostic catheter “up and over” the aortic bifurcation, lower extremity digital subtraction angiography is performed to identify the genicular vessels. A microcatheter is then advanced into the individual vessels and selective angiography is performed to assess for hypervascularity appearing as a “tumor blush” ( Fig. 1 ). The operator may choose to interrogate only the genicular arteries that correlate with the region of pain in the knee. The targeted arteries are then embolized very slowly with dilute particles ranging from 75 to 300 μm until the “tumor blush” is no longer evident on subsequent angiography ( Fig. 2 ). Both permanent and absorbable particles have been described for GAE. Patients are generally discharged several hours after the procedure. Most commonly, patients report increased pain during the first few days after the procedure with pain relief and improved function starting 1 to 2 weeks after the procedure.
Fig. 1.
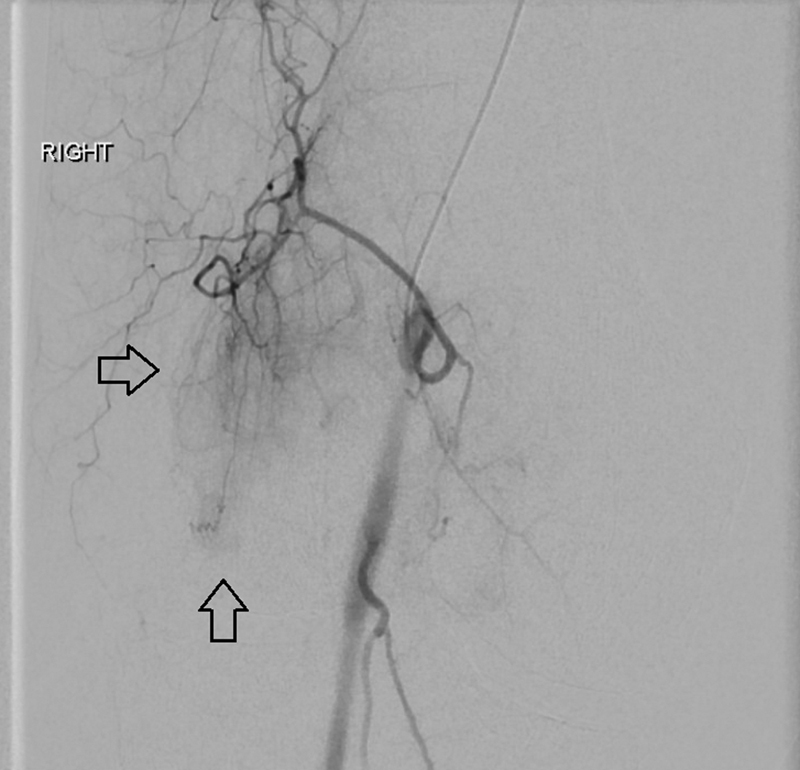
Preembolization angiography from the superior medial genicular artery demonstrating the hypervascularity (arrows) noted in osteoarthritis patients.
Fig. 2.
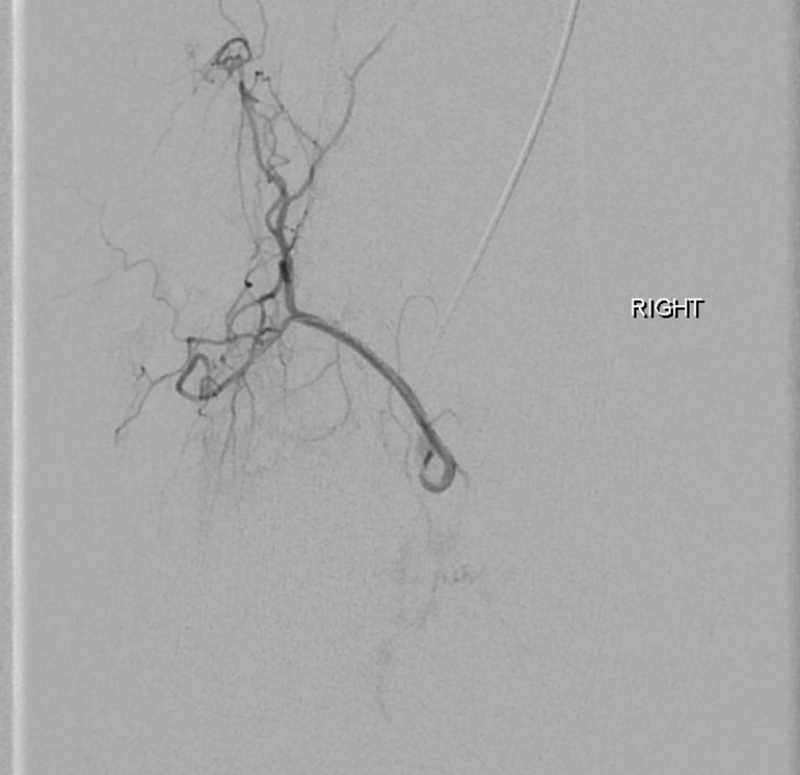
Postembolization angiography with absence of abnormal vessels and return to normal anatomy.
Outcomes
Table 1 summarizes the basic demographic data from the major pilot studies reported in the literature. One study by Okuno et al (2015) is not included in the list since the patients from the first study were also included in the 2017 study. 5 The randomized sham control study by Bagla et al is also not included since it is not comparable to the others but will be discussed later. 12 Okuno et al 6 had the highest sample size of 72 patients, while Lee et al 7 had the highest average age of 67.1. The reported patient body mass index (BMI) was highest with Bagla et al (BMI: 35.0), while gender distribution was skewed toward females consistently in almost all studies. 11 Major differences in study designs included variation in patient scoring tools. Visual analog scale (VAS) was not used by Landers et al, 14 while the Western Ontario and McMaster Universities Arthritis Index (WOMAC) was not used by Lee et al, 7 Landers et al, 14 and Little et al. 15 Instead, both Landers et al 14 and Little et al 15 used the Knee injury and Osteoarthritis Outcome Score (KOOS). Additionally, Embozene was the most used embolic material, but imipenem/cilastatin sodium (IPM/CS) and polyvinyl alcohol have also been used.
Table 1. Summary of demographic data from initial pilot studies.
| Study | n | Age | BMI | M/F | Avg KL grade | VAS | WOMAC | KOOS a | Embolic agent |
|---|---|---|---|---|---|---|---|---|---|
| Okuno et al 6 | 72 | 64.4 | 25.1 | 23/49 | ∼ 2.0 | 72.0 | 42.5 | NA | IPM/CS, Embozene |
| Bagla et al 11 | 20 | 59.4 | 35.0 | 9/11 | 2.4 | 76.0 | 61.0 | NA | Embozene |
| Lee et al 7 | 45 | 67.1 | 24.9 | 17/54 | 2.7 | 5.6 a | NA | NA | IPM/CS |
| Landers et al 14 | 10 | 62.2 | 31.0 | 4/4 | ∼ 1.5 | NA | NA | 45.0 | PVM, Embozene |
| Little et al 15 | 38 | 60.0 | 30.0 | 18/20 | 2.4 | 60.0 | NA | 54.0 | Embosphere |
Abbreviations: BMI, body mass index; IPM/CS, imipenem/cilastatin sodium; KOOS, Knee injury and Osteoarthritis Outcome Score; M/F, male/female; PVM; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Knee injury and Osteoarthritis Outcome Score.
The study by Okuno et al was the earliest and probably the most influential study on GAE. 6 The study demonstrated that GAE for mild to moderate OA led to significant improvements in VAS and WOMAC compared to baseline at the 1-, 4-, 6-, 12-, and 24-month follow-up periods. A temporary embolic composed of IPM/CS, an antibiotic that crystallizes in solution, was used for the majority of patients in this study. If patients were allergic to IPM/CS, a permanent spherical embolic was used (Embozene). No differences in outcomes were noted when comparing patients who received IPM/CS versus Embozene. Of the 72 patients who received GAE, 6 patients underwent repeat GAE within the study period. While Okuno et al focused on patients with mild to moderate OA, Lee et al separated patients into mild to moderate and severe OA subcohorts. 7 Although GAE was effective in the mild to moderate group, 3 of the 12 patients in the severe group required total knee replacement by the end of the trial.
Given the success of GAE in these initial Asian studies, Bagla et al performed a pilot study evaluating the efficacy of GAE in a more obese U.S. population. 11 Significant improvement in pain and disability was again observed in the 6-month follow-up period. Landers et al completed a similar pilot study in Australia with a small sample size of 10 patients. 14 Although the patients had 2-year follow-up, two patients withdrew from the study after 12 months, decreasing the sample size to eight. Additionally, both Embozene and polyvinyl alcohol particles were used as embolic agents instead of a single agent. Investigators from the United Kingdom have published the latest pilot study on GAE with follow-up of 12 months. Although the study supported prior results in the literature, it was the first to use a patient satisfaction survey which demonstrated that 75% of patients considered the procedure a positive experience. Of the 38 patients in the study, 6 patients were not embolized because of the presence of extensive anastomosis ( n = 3), lack of hyperemic target on initial angiography ( n = 2), and the presence of extensive cutaneous supply ( n = 1). The 32 patients who underwent embolization demonstrated significant improvement in pain and function at 6 weeks which maintained to 12 months. Additionally, unlike earlier studies, cone-beam CT and an ice pack to the knee joint were both used to prevent nontarget embolization.
Up to this point, GAE has proven to be a safe procedure with only mild complications reported. 18 Table 2 summarizes the reported adverse events (AEs) from GAE in the literature. Minor AEs include skin discoloration without ulcers and puncture site hematoma which both self-resolved without further intervention. The observed skin discoloration is thought to be secondary to nontarget embolization of small cutaneous arteries. In the Japanese study in which both permanent and absorbable particles were used, none of the patients who received the temporary embolic developed the skin changes. Of the studies that used only permanent embolics, Little et al had one of the lowest rates of discoloration which may be due to placing ice packs on the skin overlying the targeted genicular arteries, resulting in vasoconstriction of cutaneous vessels. 15 Other AEs that have been reported include self-resolving fever in one patient and foot numbness in two patients which resolved with gabapentin.
Table 2. Summary of adverse events.
| Study | Skin discoloration | Puncture-site hematoma | Miscellaneous |
|---|---|---|---|
| Okuno et al 6 | 4/72, 0.1% | 12/72, 0.2% | 0 |
| Bagla et al 11 | 13/20, 65.0% | 1/20, 0.1% | a 2/20, 0.1% |
| Lee et al 7 | 3/45, 0.1% | 5/45, 0.1% | b 1/45, 0.02% |
| Landers et al 14 | 0 | 1/10, 0.1% | 0 |
| Little et al 15 | 4/38, 0.1% | 1/38, 0.02% | 0 |
Toe numbness.
Fever.
Randomized Control Study
The success of the early pilot studies set the stage for the first randomized controlled trial comparing GAE to a sham procedure. 12 Twenty-one patients were recruited and randomly assigned to treatment ( n = 14) or control ( n = 7) arms. Patients in the treatment group received GAE, while patients in the sham group received lower extremity angiograms without embolization. Sham group patients were offered the option to crossover to the treatment group at the 1-month follow-up and all seven patients crossed over to receive GAE. Statistically significant pain reduction was noted in the treatment group versus sham at 1 month (50.1 mm VAS; SE = 10.6, 95% confidence interval: [29.0, 72.3], p < 0.01). Long-term analysis to 12 months confirmed improvements in VAS and WOMAC compared to baseline. Although patients were removed from the study if they required increasing analgesic, sensitivity analyses were conducted to confirm the validity of the results. In summary, this study provides evidence that the effect of GAE in reducing pain and disability in patients with knee OA is significantly greater than the effect of placebo.
Conclusion
Symptomatic knee OA is one of the most common joint problems worldwide, affecting millions of people and causing substantial disability. Although there are many options for conservative therapy prior to surgery, there is yet to be a treatment modality that provides reliable, sustained relief without the risks of chronic medication. The available data for GAE suggest that it may fill this void.
Disclosures
• S.B. is a consultant for Boston Scientific, Varian Medical Systems, Medtronic, Embolx, IMBiotechnologies, and Phillips Medical System.
• A.I. is a consultant for Terumo, ABK Biomedical, and CrannMed.
• No other disclosures or conflicts of interest.
References
- 1.Bagla S, Rholl K S, van Breda A, Sterling K M, van Breda A. Geniculate artery embolization in the management of spontaneous recurrent hemarthrosis of the knee: case series. J Vasc Interv Radiol. 2013;24(03):439–442. doi: 10.1016/j.jvir.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Yoo J H, Oh H C, Park S H, Lee S, Lee Y, Kim S H. Treatment of recurrent hemarthrosis after total knee arthroplasty. Knee Surg Relat Res. 2018;30(02):147–152. doi: 10.5792/ksrr.17.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luyckx E GR, Mondelaers A MP, van der Zijden T, Voormolen M HJ, Van den Bergh F RA, d'Archambeau O C. Geniculate artery embolization in patients with recurrent hemarthrosis after knee arthroplasty: a retrospective study. J Arthroplasty. 2020;35(02):550–556. doi: 10.1016/j.arth.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 4.van Baardewijk L J, Hoogeveen Y L, van der Geest I CM, Schultze Kool L J. Embolization of the geniculate arteries is an effective treatment of recurrent hemarthrosis following total knee arthroplasty that can be safely repeated. J Arthroplasty. 2018;33(04):1177–11800. doi: 10.1016/j.arth.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Okuno Y, Korchi A M, Shinjo T, Kato S. Transcatheter arterial embolization as a treatment for medial knee pain in patients with mild to moderate osteoarthritis. Cardiovasc Intervent Radiol. 2015;38(02):336–343. doi: 10.1007/s00270-014-0944-8. [DOI] [PubMed] [Google Scholar]
- 6.Okuno Y, Korchi A M, Shinjo T, Kato S, Kaneko T. Midterm clinical outcomes and MR imaging changes after transcatheter arterial embolization as a treatment for mild to moderate radiographic knee osteoarthritis resistant to conservative treatment. J Vasc Interv Radiol. 2017;28(07):995–1002. doi: 10.1016/j.jvir.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Lee S H, Hwang J H, Kim D H. Clinical outcomes of transcatheter arterial embolisation for chronic knee pain: mild-to-moderate versus severe knee osteoarthritis. Cardiovasc Intervent Radiol. 2019;42(11):1530–1536. doi: 10.1007/s00270-019-02289-4. [DOI] [PubMed] [Google Scholar]
- 8.Ashraf S, Mapp P I, Walsh D A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63(09):2700–2710. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet C S, Walsh D A. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44(01):7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 10.Mapp P I, Walsh D A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(07):390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 11.Bagla S, Piechowiak R, Hartman T, Orlando J, Del Gaizo D, Isaacson A. Genicular artery embolization for the treatment of knee pain secondary to osteoarthritis. J Vasc Interv Radiol. 2020;31(07):1096–1102. doi: 10.1016/j.jvir.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Bagla S, Piechowiak R, Hartman T S. Multicenter prospective, randomized, sham-controlled study of genicular artery embolization. J Vasc Interv Radiol. 2020;31(03):S6. doi: 10.1016/j.jvir.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Chandrashekhara S. Feasibility, safety and efficacy of genicular artery embolization for relief of knee pain related to osteoarthritis. J Vasc Interv Radiol. 2020;31(03):S7. [Google Scholar]
- 14.Landers S, Hely R, Page R. Genicular artery embolization to improve pain and function in early-stage knee osteoarthritis-24-month pilot study results. J Vasc Interv Radiol. 2020;31(09):1453–1458. doi: 10.1016/j.jvir.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Little M W, Gibson M, Briggs J. Genicular artEry embolizatioN in patiEnts with oSteoarthrItiS of the Knee (GENESIS) using permanent microspheres: interim analysis. Cardiovasc Intervent Radiol. 2021;44(06):931–940. doi: 10.1007/s00270-020-02764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagla S, Piechowiak R, Sajan A, Orlando J, Canario A DH, Isaacson A. Angiographic analysis of the anatomical variants in genicular artery embolization. J Clin Intervent Radiol ISVIR. 2021 doi: 10.1055/s-0041-1729464. [DOI] [Google Scholar]
- 17.Sajan A, Bagla S, Isaacson A. Non-neoplastic disease outside the spine-genicular artery embolization and adhesive capsulitis embolization. Tech Vasc Interv Radiol. 2020;23(04):100702. doi: 10.1016/j.tvir.2020.100702. [DOI] [PubMed] [Google Scholar]
- 18.Casadaban L C, Mandell J C, Epelboym Y. Genicular artery embolization for osteoarthritis related knee pain: a systematic review and qualitative analysis of clinical outcomes. Cardiovasc Intervent Radiol. 2021;44(01):1–9. doi: 10.1007/s00270-021-02819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


