Supplemental Digital Content is available in the text.
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is actively ongoing around the world to prevent coronavirus disease (COVID-19) infection that may be associated with dramatic outcomes, particularly in kidney transplant recipients (KTRs). We recently published a very low humoral response rate at 3.8% (n=3/78), 28 d after a single dose of the BNT162b2 vaccine (Pfizer-BioNTech).1
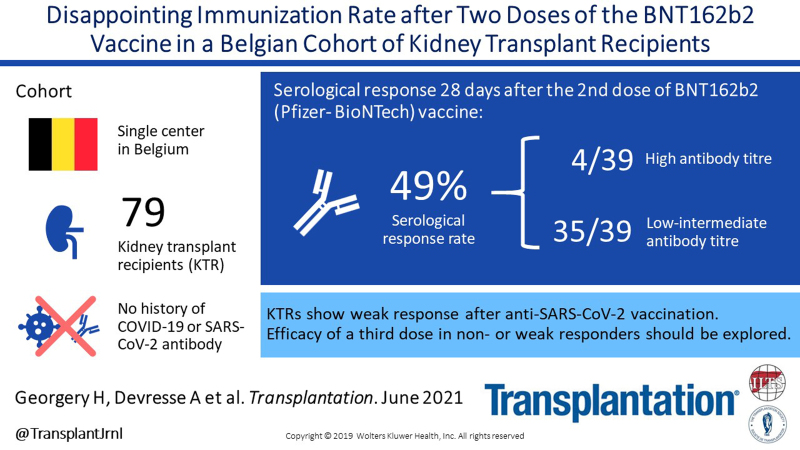
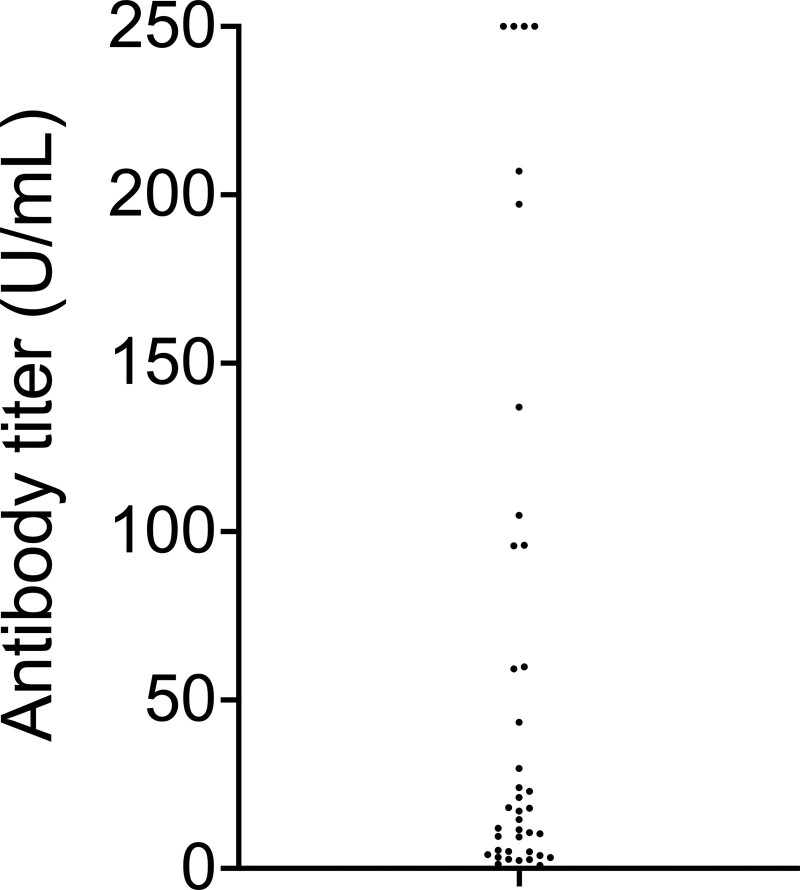
This article reports the serological response rate 28 d after the second dose of vaccine using an immunoassay detecting antibodies against the spike protein receptor-binding domain (Elecsys anti-SARS-CoV-2, Roche Diagnostics GmbH, Mannheim, Germany; positive threshold >0.8 U/mL). All patients had no history of COVID-19 infection and tested negative for anti-SARS-CoV-2 antibody before vaccination. Seventy-nine patients were included. The median age was 61 (range, 18–88) y; 48% were male individuals. The median time since transplantation was 105 (range, 5–609) mo, and 63% were transplanted >6 y ago. Forty-seven percent were treated with an association of tacrolimus, mycophenolate, and steroids, and 29% received an antimetabolite-free immunosuppressive therapy. At day 28, after the second vaccine injection, 39 patients (49%) mounted a serological response. Among responders (Figure 1), only 4 (10%) had high antibody titers (>250 U/mL—the upper detection limit of our assay), whereas 35 (90%) had intermediate response with a median antibody titer of 11.9 U/mL (range, 0.93–207). From the 3 patients who seroconverted after the first dose,1 2 had antibody titer >250 U/mL after the second injection, and 1 had intermediate value (59.8 U/mL). No patient developed COVID-19 infection after the second vaccine injection.
FIGURE 1.
Antibody titer in patients who seroconverted 28 d after 2 doses of the BNT162b2 vaccine. Four patients had antibody titers >250 U/mL (the upper detection limit of our assay).
These results are in line with those previously published.2-4 Cucchiari et al2 showed a 29.9% immunization rate 2 wk after the second dose of the mRNA-1273 (Moderna) vaccine in 133 SARS-CoV-2-naïve kidney or kidney-pancreas transplant recipients. Benotmane et al3 reported an immunization rate of 48% 1 mo after the second dose of the mRNA-1273 vaccine in 205 KTRs. They also found that patients with a first KT, a longer time from transplantation, better kidney function, and less immunosuppression were more likely to seroconvert. Boyarski et al4 showed a seroconversion rate of 54% in 658 solid organ transplanted patients 29 d after the second injection of an mRNA vaccine (47% and 53% of patients were vaccinated with the mRNA-1273 and the BNT162b2 vaccine, respectively). The use of antimetabolites was associated with poor antibody response.
Interestingly, all these reports indicate that even among responders, a large number of patients have intermediate levels of antibody titers after the second dose. Although no threshold has been clearly established for protective immunity, antibody levels in our study and in others2-5 seem lower than in immunocompetent individuals.5 Consequently, it is not excluded that “weak” responders might actually remain unprotected against severe infection.
Overall, there is growing evidence that KTRs show a weak response after anti-SARS-CoV-2 vaccination and consequently remain at risk of severe COVID-19 infection. Thus, investigating alternatives, such as increasing the number or the dose of vaccine in nonresponders or weak responders or modulating immunosuppression during vaccination, is needed.
Supplementary Material
Footnotes
H.G. and A.D. contributed equally to this work.
H.G., A.D., and N.K. participated in research idea, study design, and data analysis. H.G. participated in data acquisition. A.S. and B.K. performed serologic analysis. H.G., A.D., A.S., B.K., T.D., A.B., J.D.G., L.B., J.-C.Y., E.G., and N.K. took care of the patients. All authors discussed and reviewed the article.
This work received IRB approval.
Supplemental Visual Abstract; http://links.lww.com/TP/C256.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Georgery H, Devresse A, Yombi JC, et al. Very low immunization rate in kidney transplant recipients after one dose of the BNT162b2 vaccine: beware not to lower the guard! Transplantation. 2021;105:e148–e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta. 2021;518:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.