Supplemental Digital Content is available in the text.
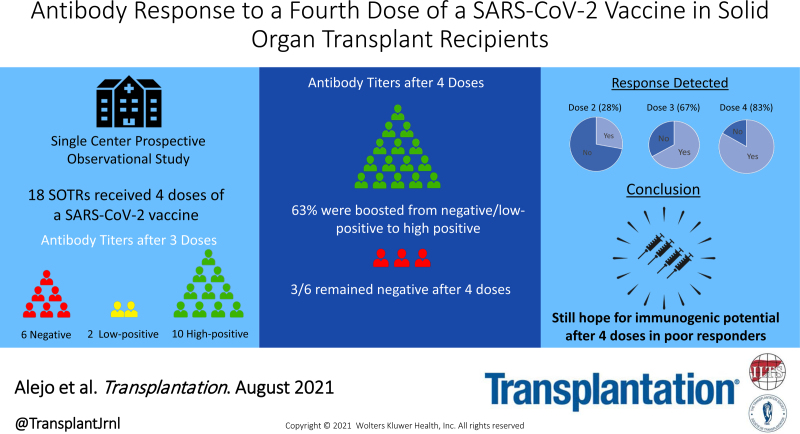
The antibody response after 2 doses of an mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine is excellent in the general population but less robust in transplant patients.1 Severe breakthrough infections in solid organ transplant recipients (SOTRs) have prompted debate on how to protect these individuals.2,3 We previously reported improved antibody responses in ~50% of SOTRs after a third dose (D3) of vaccine.4 In this series, we studied antibody responses to a fourth dose (D4) of SARS-CoV-2 vaccine in 18 SOTRs from April 24, 2021, through June 16, 2021.
Participants were enrolled in an observational study of SARS-CoV-2 vaccination outcomes in SOTRs.1 Eighteen received a D4 of a coronavirus disease 2019 (COVID-19) vaccine and had no known history of COVID-19 infection. Semiquantitative antispike antibody testing was performed using the Roche Elecsys anti–SARS-CoV-2 S or the EUROIMMUN immunoglobulin G enzyme immunoassays 2–6 wk post-D4. We categorized titers as negative, low-positive, and high-positive; low-positive titers were >0.8 U/mL but <50 U/mL (Roche), or >1.1 but <4 AU (EUROIMMUN). High-positive titers were ≥50 U/mL (Roche) or ≥4 AU (EUROIMMUN). This study was approved by the Johns Hopkins Institutional Review Board and participants provided informed consent electronically.
The median age was 58 y (interquartile range [IQR], 50–65). The median time from transplant was 7.1 y (IQR, 2.3–16.2). The median time from D3 to D4 was 28 d (IQR, 21–30). Eleven (61.1%) participants received kidney transplants. Sixteen (88.9%) were on mycophenolate mofetil at the time of vaccination. Pre-D4, there were 6 participants with negative titers, 2 with low-positive, and 10 with high-positive. Post-D4, 5 of 8 (63%) participants with negative or low-positive titers showed boosting to high-positive titers (Table 1). Additionally, among 11 SOTRs serially tested on similar assays, post-D4 titers rose in 7 (63%). Most participants with high-positive pre-D4 titers showed further boosting. The 3 participants with persistently negative titers post-D4 were kidney transplant recipients <5 y posttransplant taking tacrolimus and mycophenolate mofetil, and 2 of 3 were additionally taking corticosteroids. Eleven of 16 participants (69%) receiving antiproliferative agents showed antibody boosting.
TABLE 1.
Antibody titers after each vaccine
| Age, y | Sex | Organ(s) | Time since transplant, y | Antimetabolite | Initial vaccine series | Post-D2 titer | D3 | Post-D3 titer | Post-D3 titer | D4 | Post-D4 titer | Post-D4 titer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 44 | F | Kidney | 4 | Yes | Moderna | Negative | Pfizer | Negative | 0.22 E | Pfizer | Negative | 0.92 E |
| 65 | F | Kidney | 0.5 | Yes | Moderna | Negative | Moderna | Negative | 0.06 E | Moderna | Negative | 0.06 E |
| 44 | M | Kidney | 3 | Yes | Pfizer | Negative | Pfizer | Negative | 0.09 E | J&J | Negative | 0.4 R |
| 63 | M | Liver | 11 | Yes | Pfizer | Negative | J&J | Negative | 0.46 R | Pfizer | High | 54.9 R |
| 57 | M | Kidney | 15 | Yes | J&J | Negative | Moderna | Negative | 0.97 E | Moderna | High | 286.9 R |
| 53 | M | Kidney | 21 | Yes | Pfizer | Negative | Pfizer | Negative | (self-report) | J&J | High | 343 R |
| 61 | F | Kidney | 8 | Yes | Pfizer | Negative | Moderna | Low | 2.75 R | Moderna | High | >2500 R |
| 49 | F | Kidney | 1 | Yes | Moderna | Negative | Pfizer | Low | 7.3 R | Pfizer | High | 82.9 R |
| 52 | F | Kidney-Pancreas | 20 | Yes | Moderna | Negative | Pfizer | High | 504.4 R | Pfizer | High | 845 R |
| 54 | M | Liver | 1 | Yes | Pfizer | Low | Moderna | High | 125.7 R | Moderna | High | >2500 R |
| 69 | M | Heart | 16 | Yes | Pfizer | Negative | Moderna | High | 8.37 E | Moderna | High | >2500 R |
| 68 | M | Heart | 2 | Yes | Pfizer | Negative | Moderna | High | >250 R | Moderna | High | 402.9 R |
| 43 | F | Pancreas | 1 | Yes | Pfizer | Negative | Moderna | High | 4.72 E | Moderna | High | 5.27 E |
| 58 | M | Kidney | 3 | Yes | Moderna | Low | Moderna | High | 6.93 E | Moderna | High | 4.16 E |
| 42 | F | Liver | 5 | No | Moderna | Negative | Pfizer | High | 11.39 E | Pfizer | High | 8.75 E |
| 73 | F | Kidney-Liver | 18 | Yes | Pfizer | Low | Moderna | High | 4.45 E | Moderna | High | 1691 R |
| 67 | F | Kidney | 11 | Yes | Moderna | Low | Pfizer | High | 9.19 E | Pfizer | High | >2500 R |
| 64 | M | Liver | 21 | No | Moderna | Low | Pfizer | High | 7.21 E | Pfizer | High | >2500 R |
D, dose; E, EUROIMMUN assay (parameters: low-positive, ≥1.1 and <4; high-positive, ≥4 AU); F, female; J&J, Johnson & Johnson; M, male; R, Roche assay (parameters: low-positive, ≥0.8 and <50; high-positive, ≥50 U/mL).
To our knowledge, this is the first series describing the antibody response among SOTRs after 4 doses of vaccine against COVID-19. Given neutralizing antibody level may be the best correlate of vaccine-associated immunoprotection to date, it is encouraging that 50% of participants with negative and all with low-positive titers pre-D4 showed boosting to high-positive titers post-D4.5 This echoes previous findings that one-third of negative and all low-positive patients after 2 doses were boosted to high-positive titers after receiving a D3 of vaccine.4 These findings suggest that immunogenic potential exists for these poor responders.
Limitations include small sample size, lack of formal neutralizing antibody, B-cell or T-cell assays, durability of antibody levels, or safety information regarding the D4 given limited time to follow-up. We also lacked CD4 counts or hypogammaglobulinemia information in persistent suboptimal responders.
Though some patients may require additional measures such as immunosuppression modulation to achieve immunity, these data support continued exploration of subsequent vaccine doses in SOTRs.
ACKNOWLEDGEMENTS
The authors thank the participants of the study, without whom this work would be impossible, as well as the Johns Hopkins Transplant Vaccine study team, including Michael T. Ou, BS; Ross S. Greenberg, BA; Jake A. Ruddy, BS; Muhammad Asad Munir, MBBS; Michelle R. Krach, MS; Iulia Barbur, BSE. They also thank Andrew H. Karaba, MD, PhD; and Ms. Yolanda Eby for project support and guidance.
Supplementary Material
Footnotes
This research was made possible with the generous support of the Ben-Dov family. This work was supported by grants 5T32DK007713 (J.L.A.), The ASTS Fryer Resident Scientist Award (J.M.), F32DK124941 (B.J.B.), K01DK114388-03 (M.L.L.), K01DK101677 (A.B.M.), and K23DK115908 (J.M.G.-W.) from the National Institute of Diabetes and Digestive and Kidney Diseases; and grant K24AI144954 (D.L.S.) from the National Institute of Allergy and Infectious Disease.
D.L.S. has received consulting and speaking honoraria from Sanofi, Novartis, CLS Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. M.L.L. is the Social Media Editor for Transplantation. The other authors declare no conflicts of interest. J.L.A., J.M., T.P.-Y.C., A.T.A., B.J.B., R.K.A., A.A.R.T., M.L.L., A.B.M., J.M.G.-W., D.L.S., and W.A.W. participated in conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work of revising it critically for important intellectual content; gave the final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Visual Abstract; http://links.lww.com/TP/C276.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tau N, Yahav D, Schneider S, et al. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am J Transplant. 2021;21:2910–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105:e265–e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.