Abstract
The Pfizer-BioNTech COVID-19 vaccine has shown excellent clinical effectiveness; however, adverse events of the vaccine remain a concern in Korea. We surveyed adverse events in 2498 healthcare workers vaccinated with the Pfizer-BioNTech COVID-19 vaccine at a university hospital. The survey was conducted using a diary card for 7 days following each injection. The questionnaire response rate was 75.1% (1876/2498) for the first dose and 73.8% (1840/2493) for the second dose. Among local reactions, pain was the most commonly reported (84.9% after the first dose and 90.4% after the second dose). After the second dose, two people visited the emergency room due to severe local pain, but no hospitalization or skin necrosis occurred. Among systemic reactions, fatigue was most frequently reported (52.8% after the first dose and 77.0% after the second dose), followed by myalgia (49.0% and 76.1%), headache (28.7% and 59.2%), chills (16.7% and 54.0%), and arthralgia (11.4% and 39.2%). One or more critical adverse events occurred in 0.2% and 0.7% of the vaccinees. Except for urticaria, more adverse events were reported after the second dose than after the first dose. In the future, adverse events should be investigated in older adults, and a future study with a longer observation period should be conducted.
Keywords: COVID-19, vaccine, adverse event, injection site reaction, mRNA vaccine
First reported in November 2019, COVID-19 has evolved into a pandemic and caused over 3.4 million deaths (as of May 19, 2021) worldwide.1,2 Vaccination has been proposed as a means to end the pandemic. Although there are concerns regarding virus mutations and uncertainties surrounding the duration of the vaccination’s preventative effects, the immunogenicity3 and clinical effectiveness of COVID-19 vaccination have so far been excellent.4,5 Several vaccines are being developed, and after their commercialization in January 2021, vaccination drives have begun in several countries.6 As of May 19, 2021, 1.18 million people in Korea have received two doses of the COVID-19 vaccine.7
The Pfizer-BioNTech COVID-19 vaccine (BNT162b2) was introduced on February 27, 2021, in Korea. The COVID-19 vaccine manufactured by Pfizer is a novel mRNA vaccine. The vaccine injects mRNA encoding the SARS-CoV-2 spike protein: ribosomes translate the mRNA sequence to produce proteins that can stimulate immunity.8 mRNA vaccines have several advantages over conventional vaccines, such as low antigen degradation and mutation potential and rapid mass production, and have shown 95% immunogenicity9 and high effectiveness in clinical trials conducted in Israel and Scotland.10,11 However, information on its adverse events is lacking. Adverse events are particularly concerning because the vaccine was developed in a very short time, and this is the first commercialized mRNA vaccine.12 The Pfizer-BioNTech COVID-19 vaccine’s reports on adverse events have been published through phase 3 trials and the FDA Adverse Event Reporting System of the United States.5 However, most of these data were obtained from Caucasian or American-African individuals, with only limited data obtained from Asian individuals. In Korea, the COVID-19 Vaccination Management System (https://is.kdca.go.kr/) is being used to collect reports of possible adverse events following COVID-19 vaccination. Anyone, including health care workers, patients, and related family members, can make a report via the webpage or phone. While the systemic adverse events of the Pfizer-BioNTech COVID-19 vaccine are known to be more severe after the second dose, no data have been reported on this in Korea.5 Therefore, we conducted a study to investigate the adverse events of two-dose Pfizer-BioNTech COVID-19 vaccination.
Healthcare workers in a tertiary hospital (Incheon, Republic of Korea, 920 beds) received the Pfizer-BioNTech COVID-19 vaccine between March 9 and March 12, 2021 (first dose) and between March 30 and April 2, 2021 (second dose, 3 weeks apart). The vaccine was subcutaneously administered into the deltoid muscle at a volume of 0.3 mL. An obligatory observation period of 15–30 minutes was maintained in the room next to the injection room for monitoring anaphylaxis. A diary card was used for the survey, which was collected within 1 week after the questionnaire. The participants were considered respondents when they reported adverse events on at least 1 day. Ethics approval was obtained from the Institutional Review Board of Inha University Hospital (No.: 2021-04-048). The requirement for informed consent was waived under the approval of the Inha University Institutional Review Board.
The survey items were largely divided into local (redness, edema, and pain) and systemic (fever, fatigue, headache, chills, myalgia, arthralgia, vomiting, and diarrhea) adverse events. The severity of each adverse event was divided into five stages: local swelling and redness, grade 0 for <2 cm, grade 1 for 2–5 cm, grade 2 for 5–10 cm, grade 3 for >10 cm, and grade 4 (critical) for skin necrosis; fever: grade 0 for <38°C, grade 1 for 38°C–38.4°C, grade 2 for 38.4°C–38.9°C, grade 3 for 39.0°C–39.9°C, and grade 4 (critical) for ≥40°C; local tenderness, headache, fatigue, chills, myalgia, arthralgia, and itching, grade 0 for no symptoms, grade 1 for symptoms not interfering with daily activity, grade 2 for symptoms causing some interference with daily activity, grade 3 for symptoms severely interfering with daily activity, and grade 4 (critical) for a visit to the emergency room or hospital admission requirement; nausea, grade 0 for non-specific, grade 1 for 2 times/day, grade 2 for >2 times/day, grade 3 for intravenous hydration requirement, and grade 4 (critical) for a visit to the emergency room or hospital admission requirement; diarrhea, grade 0 for non-specific, grade 1 for 2–5 times/day, grade 2 for 4–5 times/day, grade 3 for ≥6 times/day, and grade 4 (critical) for a visit to the emergency room or hospital admission requirement; urticaria, grade 0 for non-specific, grade 1 for ≤20, grade 2 for 21–50, grade 3 for ≥50, and grade 4 (critical) for a visit to the emergency room or hospital admission requirement. The chi-square test was used to compared adverse events after the first and second doses. The Jonckheere-Terpstra test was used for analysis by age group. A p value of <0.05 was considered to indicate statistical significance. Data analysis was performed using SPSS statistical software, version 21 (IBM Corp., Armonk, NY, USA).
A total of 2498 workers aged 20–65 years were vaccinated. Five of 2498 workers were excluded due to adverse effects or pregnancy at the second dose. Of all vaccinated individuals, 76.6% were women. Among the 2498 healthcare workers, 900 (36.0%) were in their 20s, 619 (24.8%) in their 30s, 460 (18.4%) in their 40s, 402 (16.1%) in their 50s, and 107 (4.3%) in their 60s. The questionnaire response rates were 75.1% (1876/2498) for the first dose and 73.8% (1840/2493) for the second dose. No serious adverse events, such as anaphylaxis, occurred after the first or second dose.
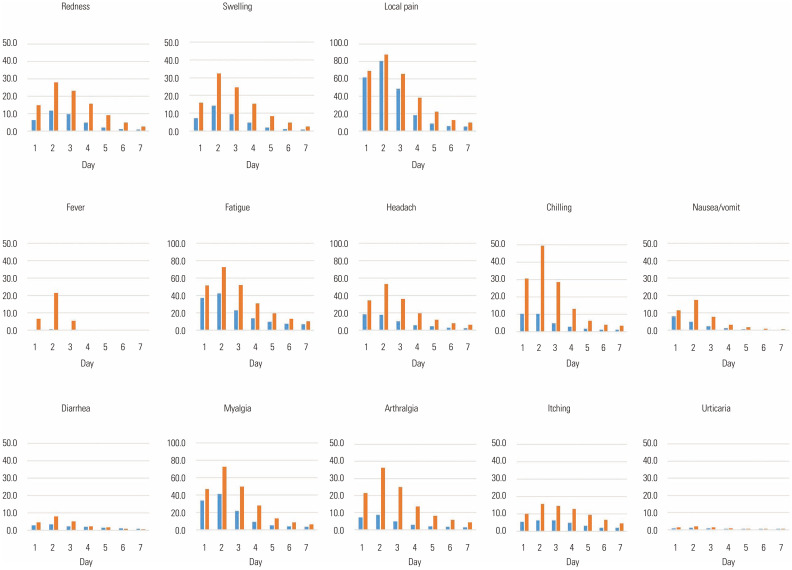
The most common local adverse event was pain, which was reported in 84.9% (1592/1876) of responders after the first dose and 90.4% (1664/1840) of responders after the second dose, followed by swelling [16.5% (309/1876) and 35.5% (653/1840), respectively] and redness [14.1% (264/1876) and 31.9% (587/1840), respectively] (Table 1). No critical adverse events were reported after the first dose. After the second dose, two patients visited the emergency room with severe pain; however, neither of them progressed to skin necrosis. Local adverse events were significantly more frequent after the second dose than after the first dose. The onset of symptoms was most common on the second day after vaccination, both after the first and second doses (Fig. 1); however, four people reported the worsening of swelling after the fifth day.
Table 1. Local Adverse Events among Healthcare Workers.
| Dose 1 (n=1876) | Dose 2 (n=1840) | p value | ||
|---|---|---|---|---|
| Redness* | ||||
| Grade 1 (Mild) | 232 (12.4) | 400 (21.7) | ||
| Grade 2 (Moderate) | 26 (1.4) | 157 (8.5) | ||
| Grade 3 (Severe) | 6 (0.3) | 30 (1.7) | ||
| Grade 4 (Critical) | 0 (0.0) | 0 (0.0) | ||
| Any (Grade 1–4) | 264 (14.1) | 587 (31.9) | <0.001 | |
| Swelling* | ||||
| Grade 1 (Mild) | 268 (14.3) | 455 (24.7) | ||
| Grade 2 (Moderate) | 38 (2.0) | 173 (9.4) | ||
| Grade 3 (Severe) | 3 (0.2) | 25 (1.4) | ||
| Grade 4 (Critical) | 0 (0.0) | 0 (0.0) | ||
| Any (Grade 1–4) | 309 (16.5) | 653 (35.5) | <0.001 | |
| Pain at the injection site† | ||||
| Grade 1 (Mild) | 895 (47.7) | 654 (35.5) | ||
| Grade 2 (Moderate) | 652 (34.8) | 797 (43.3) | ||
| Grade 3 (Severe) | 45 (2.4) | 211 (11.5) | ||
| Grade 4 (Critical) | 0 (0.0) | 2 (0.1) | ||
| Any (Grade 1–4) | 1592 (84.9) | 1664 (90.4) | <0.001 | |
Data are presented as n (%).
*Grade 0=lesser than 2 cm, Grade 1=2–5 cm, Grade 2=5–10 cm, Grade 3=above 10 cm, critical=necrosis; †Grade 0=no symptom, Grade 1=symptom does not interfere with daily activity, Grade 2=symptom causes some interference with daily activity, Grade 3=symptom severely interferes with daily activity, critical=visit to the emergency room or hospital admission required.
Fig. 1. Prevalence of adverse events over time after vaccination. The blue bars represent the prevalence of side effects by date for the first dose, and the red bars represent the prevalence of adverse events by date for the second dose. Local pain, myalgia, headache, and fatigue have a maximum value of 100% on the vertical axis. The maximum value is 50% for other side effects.
Among systemic adverse events, fatigue was the most common [52.8% (991/1876) after the first dose and 77.0% (1417/1840) after the second dose], followed by myalgia [49.0% (920/1876) and 76.1% (1400/1840), respectively], headache [28.7% (538/1876) and 59.2% (1090/1840), respectively], chills [16.7% (313/1876) and 54.0% (994/1840), respectively], itching [12.0% (226/1876) and 22.7% (417/1840), respectively], nausea/vomiting [11.4% (214/1876) and 22.6% (416/1840), respectively], and urticaria [2.3% (43/1876) and 2.7% (50/1840), respectively]. Fever was reported only in 1% (18/1876) of responders after the first dose, which significantly increased to 24.7% (454/1840) after the second dose (Table 2). Systemic adverse events were the most common on the second day after vaccination (Fig. 1). Critical systemic adverse events occurred in three patients after the first dose and in 10 patients after the second dose. These patients were all treated in the emergency room, and hospitalization was not required for any patient. The incidence of adverse events according to age group is described in Table 3. In the second dose, pain, fever, headache, and nausea/vomiting were reported more frequently among younger participants than among older participants, whereas arthralgia and itching were more severe in older adults.
Table 2. Systemic Adverse Events among Healthcare Workers.
| Dose 1 (n=1876) | Dose 2 (n=1840) | p value | ||
|---|---|---|---|---|
| Fever* | ||||
| Grade 1 (Mild) | 12 (0.6) | 332 (18.0) | ||
| Grade 2 (Moderate) | 5 (0.3) | 96 (5.2) | ||
| Grade 3 (Severe) | 1 (0.1) | 25 (1.4) | ||
| Grade 4 (Critical) | 0 (0.0) | 1 (0.1) | ||
| Any (Grade 1–4) | 18 (1.0) | 454 (24.7) | <0.001 | |
| Fatigue† | ||||
| Grade 1 (Mild) | 620 (33.0) | 406 (22.1) | ||
| Grade 2 (Moderate) | 344 (18.3) | 660 (35.9) | ||
| Grade 3 (Severe) | 26 (1.4) | 348 (18.9) | ||
| Grade 4 (Critical) | 1 (0.1) | 3 (0.2) | ||
| Any (Grade 1–4) | 991 (52.8) | 1417 (77.0) | <0.001 | |
| Headache† | ||||
| Grade 1 (Mild) | 349 (18.6) | 370 (20.1) | ||
| Grade 2 (Moderate) | 166 (8.8) | 513 (27.9) | ||
| Grade 3 (Severe) | 22 (1.2) | 202 (11.0) | ||
| Grade 4 (Critical) | 1 (0.1) | 5 (0.3) | ||
| Any (Grade 1–4) | 538 (28.7) | 1090 (59.2) | <0.001 | |
| Chills† | ||||
| Grade 1 (Mild) | 214 (11.4) | 337 (18.3) | ||
| Grade 2 (Moderate) | 90 (4.8) | 411 (22.3) | ||
| Grade 3 (Severe) | 8 (0.4) | 238 (12.9) | ||
| Grade 4 (Critical) | 1 (0.1) | 8 (0.4) | ||
| Any (Grade 1–4) | 313 (16.7) | 994 (54.0) | <0.001 | |
| Nausea/vomiting‡ | ||||
| Grade 1 (Mild) | 182 (9.7) | 320 (17.4) | ||
| Grade 2 (Moderate) | 30 (1.6) | 87 (4.7) | ||
| Grade 3 (Severe) | 2 (0.1) | 7 (0.4) | ||
| Grade 4 (Critical) | 0 (0.1) | 2 (0.1) | ||
| Any (Grade 1–4) | 214 (11.4) | 416 (22.6) | <0.001 | |
| Diarrhea§ | ||||
| Grade 1 (Mild) | 103 (5.5) | 164 (8.9) | ||
| Grade 2 (Moderate) | 27 (1.4) | 45 (2.4) | ||
| Grade 3 (Severe) | 2 (0.1) | 4 (0.2) | ||
| Grade 4 (Critical) | 0 (0.1) | 0 (0.0) | ||
| Any (Grade 1–4) | 132 (7.0) | 213 (11.6) | <0.001 | |
| Myalgia† | ||||
| Grade 1 (Mild) | 559 (29.8) | 404 (22.0) | ||
| Grade 2 (Moderate) | 320 (17.1) | 628 (34.1) | ||
| Grade 3 (Severe) | 40 (2.1) | 363 (19.7) | ||
| Grade 4 (Critical) | 1 (0.1) | 5 (0.3) | ||
| Any (Grade 1–4) | 920 (49.0) | 1400 (76.1) | <0.001 | |
| Arthralgia† | ||||
| Grade 1 (Mild) | 140 (7.5) | 255 (13.9) | ||
| Grade 2 (Moderate) | 57 (3.0) | 305 (16.6) | ||
| Grade 3 (Severe) | 14 (0.7) | 157 (8.5) | ||
| Grade 4 (Critical) | 2 (0.1) | 4 (0.2) | ||
| Any (Grade 1–4) | 213 (11.4) | 721 (39.2) | <0.001 | |
| Itching† | ||||
| Grade 1 (Mild) | 176 (9.4) | 283 (15.4) | ||
| Grade 2 (Moderate) | 46 (2.5) | 131 (7.1) | ||
| Grade 3 (Severe) | 4 (0.2) | 2 (0.1) | ||
| Grade 4 (Critical) | 0 (0.0) | 1 (0.1) | ||
| Any (Grade 1–4) | 226 (12.0) | 417 (22.7) | <0.001 | |
| Urticaria¶ | ||||
| Grade 1 (Mild) | 40 (2.1) | 36 (2.0) | ||
| Grade 2 (Moderate) | 0 (0.0) | 8 (0.4) | ||
| Grade 3 (Severe) | 2 (0.1) | 4 (0.2) | ||
| Grade 4 (Critical) | 1 (0.1) | 2 (0.1) | ||
| Any (Grade 1–4) | 43 (2.3) | 50 (2.7) | 0.407 | |
Data are presented as n (%).
*Grade 0=lower than 38°C; Grade 1=38°C–38.4°C; Grade 2=38.4°C–38.9°C, Grade 3=39.0°C–39.9°C, critical=40°C or above; †Grade 0=no symptom, Grade 1=symptom does not interfere with daily activity, Grade 2=symptom causes some interference with daily activity, Grade 3=symptom severely interferes with daily activity, critical=visit to the emergency room or hospital admission; ‡Grade 0=non-specific, Grade 1=two times/day, Grade 2=more than 2 times/day, Grade 3=requires intravenous hydration, critical=visit to the emergency room or hospital admission; §Grade 0=non-specific, Grade 1=two to three times/day, Grade 2=four to five times/day, Grade 3=six or more times/day, critical=visit to the emergency room or hospital admission required; ¶Grade 0=non-specific, Grade 1=20 or less than 20, Grade 2=21–50, Grade 3=50 or more than 50, critical=visit to the emergency room or hospital admission.
Table 3. Adverse Events by Age Group.
| Dose 1 (n=1876) | Dose 2 (n=1840) | ||||
|---|---|---|---|---|---|
| Events/ participants (%) | p value | Events/ participants (%) | p value | ||
| Redness | 0.006 | 0.365 | |||
| 20–29 | 121/735 (16.5) | 250/741 (33.7) | |||
| 30–39 | 67/488 (13.7) | 140/480 (29.2) | |||
| 40–49 | 43/331 (13.0) | 107/317 (33.7) | |||
| 50–59 | 28/269 (10.4) | 75/257 (29.2) | |||
| 60–69 | 5/53 (9.4) | 15/45 (33.3) | |||
| Swelling | 0.005 | 0.870 | |||
| 20–29 | 144/735 (19.6) | 250/741 (33.7) | |||
| 30–39 | 75/488 (15.4) | 170/480 (35.4) | |||
| 40–49 | 44/331 (13.3) | 111/317 (35.0) | |||
| 50–59 | 38/269 (14.1) | 95/257 (37.0) | |||
| 60–69 | 8/53 (15.1) | 15/45 (33.3) | |||
| Pain at the injection site | <0.001 | 0.024 | |||
| 20–29 | 618/735 (84.1) | 654/741 (88.3) | |||
| 30–39 | 428/488 (87.7) | 451/480 (94.0) | |||
| 40–49 | 296/331 (89.4) | 303/317 (95.6) | |||
| 50–59 | 216/269 (80.3) | 223/257 (86.8) | |||
| 60–69 | 34/53 (64.2) | 33/45 (73.3) | |||
| Fever | 0.252 | <0.001 | |||
| 20–29 | 8/735 (1.1) | 202/741 (27.3) | |||
| 30–39 | 6/488 (1.2) | 139/480 (29.0) | |||
| 40–49 | 4/331 (1.2) | 79/317 (24.9) | |||
| 50–59 | 0/269 (0.0) | 32/257 (12.5) | |||
| 60–69 | 0/53 (0.0) | 2/45 (4.4) | |||
| Fatigue | 0.755 | 0.070 | |||
| 20–29 | 376/736 (51.1) | 562/741 (75.8) | |||
| 30–39 | 271/488 (55.5) | 393/480 (81.9) | |||
| 40–49 | 192/331 (58.0) | 247/317 (77.9) | |||
| 50–59 | 127/269 (47.2) | 184/257 (71.6) | |||
| 60–69 | 25/53 (47.2) | 31/45 (68.9) | |||
| Headache | 0.374 | 0.001 | |||
| 20–29 | 212/736 (28.8) | 450/741 (60.7) | |||
| 30–39 | 146/488 (29.9) | 140/480 (29.2) | |||
| 40–49 | 99/331 (29.9) | 184/317 (58.0) | |||
| 50–59 | 67/269 (24.9) | 138/257 (53.7) | |||
| 60–69 | 14/53 (26.4) | 20/45 (44.4) | |||
| Chills | 0.219 | 0.142 | |||
| 20–29 | 105/736 (14.3) | 377/741 (50.9) | |||
| 30–39 | 91/488 (18.6) | 305/480 (63.5) | |||
| 40–49 | 76/331 (23.0) | 185/317 (58.4) | |||
| 50–59 | 35/269 (13.0) | 116/257 (45.1) | |||
| 60–69 | 6/53 (11.3) | 11/45 (24.4) | |||
| Nausea/vomiting | 0.585 | 0.035 | |||
| 20–29 | 84/736 (11.4) | 180/741 (24.3) | |||
| 30–39 | 59/488 (12.1) | 109/480 (22.7) | |||
| 40–49 | 41/331 (12.4) | 80/317 (25.2) | |||
| 50–59 | 27/269 (10.0) | 43/257 (16.7) | |||
| 60–69 | 3/53 (5.7) | 4/45 (8.9) | |||
| Diarrhea | 0.006 | 0.390 | |||
| 20–29 | 63/736 (8.6) | 75/741 (10.1) | |||
| 30–39 | 37/488 (7.6) | 64/480 (13.3) | |||
| 40–49 | 20/331 (6.0) | 45/317 (14.2) | |||
| 50–59 | 9/269 (3.3) | 23/257 (8.9) | |||
| 60–69 | 3/53 (5.7) | 6/45 (13.3) | |||
| Myalgia | 0.013 | 0.525 | |||
| 20–29 | 378/736 (51.4) | 548/741 (74.0) | |||
| 30–39 | 244/488 (50.0) | 393/480 (81.9) | |||
| 40–49 | 157/331 (47.4) | 246/317 (77.6) | |||
| 50–59 | 125/269 (46.5) | 185/257 (72.0) | |||
| 60–69 | 26/53 (49.1) | 28/45 (62.2) | |||
| Arthralgia | <0.001 | <0.001 | |||
| 20–29 | 65/736 (8.8) | 240/741 (32.4) | |||
| 30–39 | 50/488 (10.2) | 196/480 (40.8) | |||
| 40–49 | 45/331 (13.6) | 157/317 (49.5) | |||
| 50–59 | 44/269 (16.4) | 108/257 (42.0) | |||
| 60–69 | 9/53 (17.0) | 20/45 (44.4) | |||
| Itching | 0.249 | <0.001 | |||
| 20–29 | 83/737 (11.3) | 142/741 (19.2) | |||
| 30–39 | 56/488 (11.5) | 97/480 (20.2) | |||
| 40–49 | 43/331 (13.0) | 78/317 (24.6) | |||
| 50–59 | 37/269 (13.8) | 80/257 (31.1) | |||
| 60–69 | 7/53 (13.2) | 20/45 (44.4) | |||
| Urticaria | 0.603 | 0.715 | |||
| 20–29 | 18/737 (2.4) | 19/741 (2.6) | |||
| 30–39 | 13/488 (2.7) | 13/480 (2.7) | |||
| 40–49 | 5/331 (1.5) | 9/317 (2.8) | |||
| 50–59 | 4/269 (1.5) | 8/257 (3.1) | |||
| 60–69 | 3/53 (5.7) | 1/45 (2.2) | |||
Researchers have reported on the safety of COVID-19 vaccination in Korea.13,14 However, these studies either focused on AstraZeneca’s ChAdOx1 COVID-19 vaccine or were limited to reported adverse events after the first dose. Because the booster dose of the Pfizer-BioNTech COVID-19 vaccine is essential for sustaining the efficacy of the vaccine, studies on adverse events after two doses are important. One previous survey study conducted in Korea reported on adverse reactions after the second dose in Korea, but was limited to a relatively small number of vaccinees (342 healthcare workers).15 The most reliable study on the safety of two doses is the Pfizer-BioNTech COVID-19 vaccine’s Phase 3 trial.16 Our survey was designed based on this Pfizer Phase 3 trial, and the age group in our study was similar to the 18–55 years age group of the Pfizer Phase 3 trial. Therefore, these two studies can be compared indirectly. Fever is an objective indicator and is simple to measure. The incidences of fever in the Pfizer Phase 3 trial were 3.7% after the first dose and 15.8% after the second dose, similar to our study results (0.9% and 16.0%, respectively). However, the incidences of fatigue, myalgia, headache, and arthralgia, which are more subjective than fever, were higher in our study than in the Pfizer-BioNTech COVID-19 vaccine’s Phase 3 trial. Adjustments for factors, such as race and age, however, should be made. Also, because our study was not a case-controlled study, it may cause such differences. The incidence of critical adverse events was 0.2% after the first dose and 0.7% after the second dose in our study, which was higher than 0.01% (after both the first and second doses) of the Pfizer Phase 3 trial. Because all respondents in our study who reported critical adverse events visited the emergency room, their adverse events were classified as Grade 4. However, we should consider that healthcare workers have better access to emergency medical centers than non-medical workers.
In this study, the higher incidence of adverse events after the second dose should be noted. The incidence of all systemic adverse events, except urticaria, was significantly higher after the second dose than after the first dose in this study. Therefore, we suggest that systemic adverse events occur more frequently after the second dose than after the first dose of the Pfizer-BioNTech COVID-19 vaccine in Koreans as well. Depending on the progression of the pandemic or emergence of variants of the SARS-CoV-2 virus, a third vaccination dose or annual vaccination may be considered. In that case, further research on the adverse events after the additional booster dose is needed.
This study has some limitations. First, the number of vaccinees studied was relatively small. Second, because the study population comprised only healthcare workers, it cannot represent the entire population in Korea. Third, no control group was included. Finally, this study did not determine the effects of antipyretics that could affect symptoms after vaccination. A large-scale, case-control study is warranted to overcome these limitations and validate our findings.
In conclusion, pain was the most common local adverse event, and fatigue and myalgia were the most common systemic adverse events of the Pfizer-BioNTech COVID-19 vaccine among healthcare workers in Korea. The incidence of systemic adverse events was higher after the second dose than after the first dose. Further large-scale studies should be undertaken in a population of a broader age group and high-risk groups.
ACKNOWLEDGEMENTS
This work was supported by a research grant from Inha University Hospital.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jin-Soo Lee and Ji Hyeon Baek.
- Data curation: Ji Hyeon Baek, Eunyoung Lee, Yeongju Seo, Yuran Lee, Yoonkyoung Jang, Soyeon Yu, Yeonju Maeng, Soyeon Park, Seohee Park, and Jiah Kim.
- Formal analysis: Jae Hyoung Im and Eunjung Kim.
- Writing—original draft: Jae Hyoung Im.
- Writing—review & editing: Jin-Soo Lee and Ji Hyeon Baek.
- Approval of final manuscript: all authors.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus (COVID-19) dashboard. [accessed on 2021 May 25]. Available at: https://covid19.who.int/
- 3.Tregoning JS, Brown ES, Cheeseman HM, Flight KE, Higham SL, Lemm NM, et al. Vaccines for COVID-19. Clin Exp Immunol. 2020;202:162–192. doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung J. Preparing for the coronavirus disease (COVID-19) vaccination: evidence, plans, and implications. J Korean Med Sci. 2021;36:e59. doi: 10.3346/jkms.2021.36.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korea Disease Control and Prevention Agency (KCDC) Current status of COVID-19 vaccination in Korea. [accessed on 2021 May 25]. Available at: https://ncv.kdca.go.kr/
- 8.Kyriakidis NC, López-Cortés A, Gonzalez EV, Grimáldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chagla Z. The BNT162b2 (BioNTech/Pfizer) vaccine had 95% efficacy against COVID-19 ≥7 days after the 2nd dose. Ann Intern Med. 2021;174:JC15. doi: 10.7326/ACPJ202102160-015. [DOI] [PubMed] [Google Scholar]
- 10.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbasi J. COVID-19 and mRNA vaccines—first large test for a new approach. JAMA. 2020;324:1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- 13.Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36:e107. doi: 10.3346/jkms.2021.36.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021;36:e153. doi: 10.3346/jkms.2021.36.e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. [accessed on 2021 May 25]. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-manufacturer%2Fpfizer%2Freactogenicity.html .