Abstract
Granular, microgel-based materials have garnered interest as promising tissue engineering scaffolds due to their inherent porosity, which can promote cell infiltration. Adapting these materials for 3D bioprinting, while maintaining sufficient void space to enable cell migration, can be challenging, since the rheological properties that determine printability are strongly influenced by microgel packing and void fraction. In this work, we propose a strategy to decouple printability and void fraction by blending UV-crosslinkable gelatin methacryloyl (GelMA) microgels with sacrificial gelatin microgels to form composite inks. We observe that inks with an apparent viscosity greater than ~100 Pa⋅s (corresponding to microgel concentrations ≥ 5 wt%) have rheological properties that enable extrusion-based printing of multilayered structures in air. By altering the ratio of GelMA to sacrificial gelatin microgels, while holding total concentration constant at 6 wt%, we create a family of GelMA:gelatin microgel inks that allows for tuning of void fraction from 0.20 to 0.57. Furthermore, human umbilical vein endothelial cells (HUVEC) seeded onto printed constructs are observed to migrate into granular inks in a void fraction-dependent manner. Thus, our family of microgel inks holds promise for use in 3D printing and tissue engineering applications that rely upon cell infiltration.
Keywords: 3D printing, gelatin methacryloyl, microgels, sacrificial ink, void fraction, endothelial cells, cell infiltration
Graphical Abstract
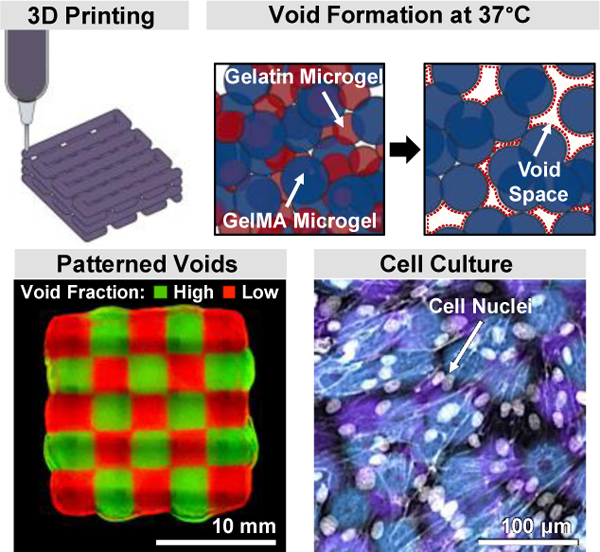
The inherent porosity of microgel-based materials makes them promising for promoting cell infiltration. But adapting these materials to 3D bioprinting requires optimizing rheological properties using methods that strongly influence porosity. A method is reported to decouple void space and printability by blending UV-crosslinkable and sacrificial microgels. The resulting family of inks have void fractions that influence cellular infiltration.
1. Introduction
3D bioprinting promises to allow scalable biofabrication of structurally complex, functional tissue mimics to both study and ameliorate human disease.[1–3] An additive manufacturing approach, 3D bioprinting produces three-dimensional constructs through layer-by-layer deposition of biomaterial inks.[4] These biomaterial inks must serve two primary roles: first, as raw materials that physically enable fabrication of structured constructs, and second, as downstream cell-instructive niches that support cell function. Hydrogels have emerged as ideal biomaterial inks due to their capacity to maintain high cell viability, while also leveraging traditional biomaterials strategies to direct cell phenotypes.[5,6] A challenge with bulk hydrogels, however, is that their homogenous structure can restrict cell mobility and hinder the diffusion of oxygen and nutrients, thus limiting cell viability and infiltration.[7,8]
While microgels have been used as sacrificial porogens to promote cellular mobility and oxygen diffusion,[9,10] the development of fully granular, microgel-based biomaterial inks with independently tunable porosity and printability remains to be demonstrated. Microgel biomaterials are composed of packed solid microgels, which can have variable size and composition to suit the desired application.[11–14] The interstitial spaces between adjacent microgels inherently impart granular inks with interconnected voids (often called micropores) that can promote cell mobility and enhance transport of oxygen and nutrients.
To be used in extrusion 3D bioprinting, microgel-based materials must be formulated to have specific viscoelastic properties. The frictional, non-covalent, and electrostatic forces between neighboring microgels critically affect these mechanical properties and become increasingly influential with greater surface area-to-volume ratio (i.e. microgel size) and with increased particle packing (i.e. microgel concentration). With sufficient microgel interaction forces, the material can exhibit gel-like rheological properties. These solid-like, jammed microgels also exhibit shear-thinning behavior and can flow as a viscous fluid upon the application of force, which is required for extrusion-based biofabrication strategies.[15] Further, because the solid-like state relies solely on non-covalent interactions, jammed microgel inks can rapidly self-heal, making microgel-based granular inks ideal for satisfying the mechanical requirements of 3D printing.
In addition to modifying the mechanical properties of granular materials, altering microgel size and concentration will also result in different types of pores. The size, connectivity, and geometry of pores are all known to influence a range of cell behaviors, including cell proliferation, migration and infiltration, and extracellular matrix (ECM) deposition.[16–19] Thus, increasing the void fraction within porous materials to enhance cell function is often a common goal of biomaterials design for tissue engineering.[20,21] Several approaches have been used to control the void space within granular materials. First, microgel concentration may be used to adjust the void fraction, which is highly related to particle packing density.[12] In general, lowering microgel concentration will increase the overall void space (thereby improving mass transfer and cell motility), but will decrease bulk viscosity (and hence decrease 3D printability). Microgel size is another factor that can be used to control void fraction.[16,22,23] In one example, controlling the particle size from 10 μm to 100 μm resulted in void fractions ranging from 0.12 to 0.29.[22] This closely matches the predicted theoretical limit of void fraction for deformable microgels of uniform size (~0.26).[12] Granular materials containing microgels of heterogeneous sizes will also influence void space, with previous reports showing that using a mixture of small and large microgels causes the small microgels to fill the voids between their larger neighbors, thereby reducing void fraction.[24] Taken together, this background suggests that a microgel-based material that allows tuning of total void fraction while maintaining rheological properties suitable for bioprinting would result in fabricated structures that promote cell migration and infiltration.
Towards this goal, we propose a strategy to control the total void fraction within 3D bioprinted granular inks by using sacrificial gelatin microgels blended with gelatin methacryloyl (GelMA) microgels. After printing and light-induced crosslinking, the sacrificial gelatin microgels can be easily removed by incubation and washing at 37℃. The remaining scaffold consists of a crosslinked network of GelMA microgels. Microgel concentration was found to be an important variable for controlling the rheological properties of these inks, with 6-wt% microgel inks displaying properties ideal for 3D bioprinting. Altering the ratio of smaller gelatin microgels to larger GelMA microgels, while keeping microgel concentration constant at 6 wt%, resulted in a family of inks with tunable control over void fraction, spanning from 0.20 ± 0.02 to 0.57 ± 0.06. To demonstrate the biological relevance of this range of void fraction, human umbilical vein endothelial cells (HUVEC) were seeded on top of 3D printed constructs and shown to migrate inside of the granular material in a void fraction-dependent manner. This is the first demonstration of decoupling granular ink printability from the void fraction of printed scaffolds. To demonstrate the scientific impact of this novel technology, we show how it enables (1) the design of 3D printed scaffolds that have control over the rate of endothelial cell infiltration and (2) demonstration of multi-material printing to form different void fractions patterned within a single printed structure. As cell biology is known to be greatly impacted by scaffold voids, this ink strategy will enable new capabilities in the bioprinting of scaffolds for tissue engineering.
2. Results and Discussion
2.1. Design of GelMA:gelatin Microgel Inks for Tuning of Total Void Fraction
To design an ink capable of being printed into self-supporting structures while possessing a large void fraction, we prepared a jammed slurry of gelatin and GelMA microgels. We hypothesized that 3D printing of this slurry would result in an interpenetrating network of the two microgel populations (Figure 1a, left two panels). Post-printing, the network of GelMA microgels can be stabilized through ultraviolet (UV) light-initiated crosslinking. In contrast, the unmodified, gelatin-only microgels do not participate in this crosslinking process and can be melted and removed at 37°C, leaving behind an interconnected void space (Figure 1a, right two panels).
Figure 1.
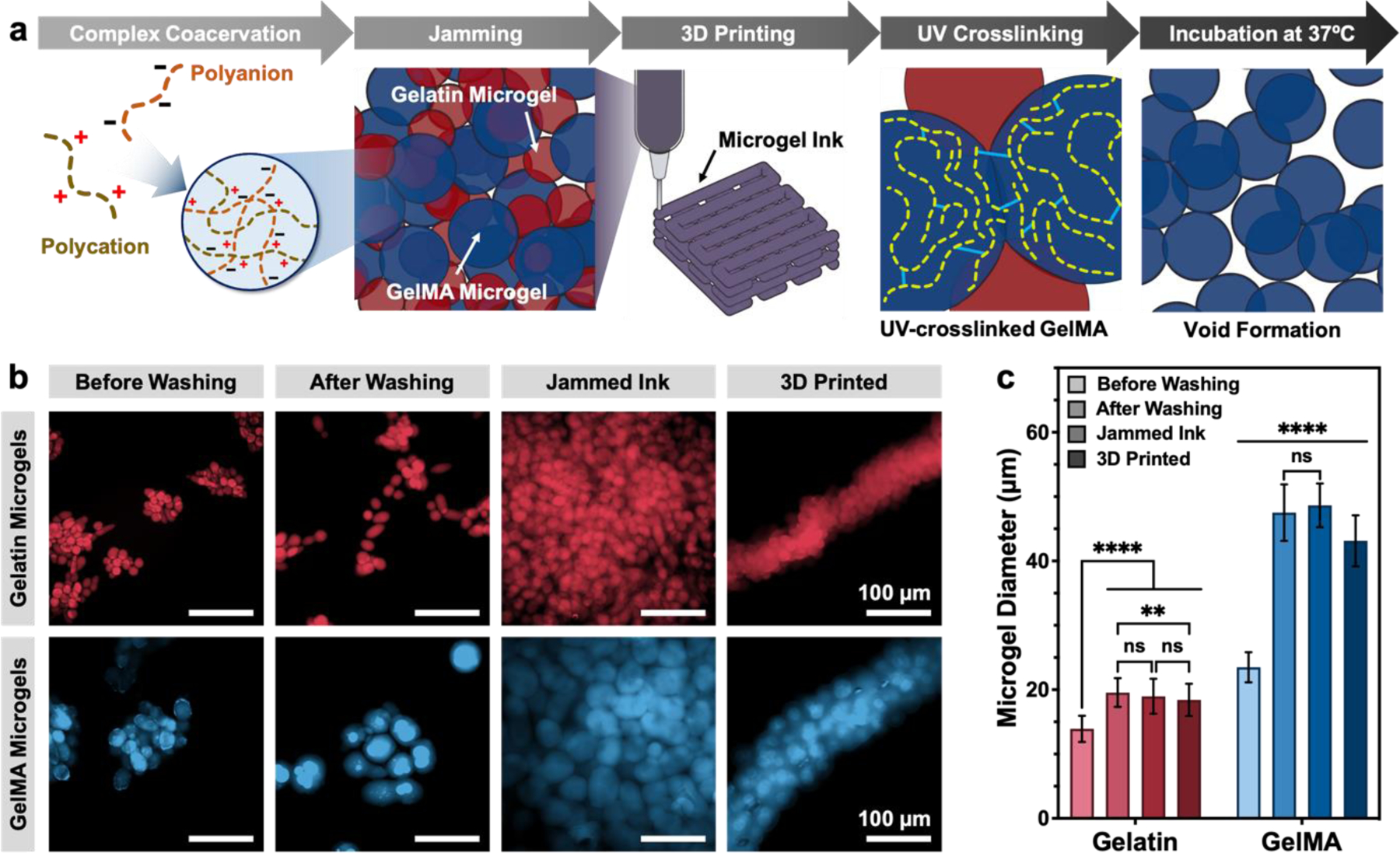
Design of microporous GelMA microgel inks with sacrificial gelatin microgels. (a) Schematic illustration depicting the process for producing 3D printed constructs with tunable void fraction. Gelatin and GelMA microgels are fabricated via complex coacervation method. Jammed composite inks containing a blend of crosslinkable GelMA microgels and sacrificial gelatin microgels are 3D printed, UV crosslinked, and incubated at 37°C to form constructs with tunable void space. (b) False-colored image of a representative sample of rhodamine B-stained gelatin and GelMA microgels before washing, after washing, after jamming, and after 3D printing. (c) Particle diameter of GelMA and gelatin at each stage. Data are plotted as mean ± standard deviation, n = 100 replicates. Scale bars are 100 μm in panel b. Statistical significance tested by one-way ANOVA with Tukey’s post-hoc analysis; n.s. = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Prior to microgel fabrication, a portion of gelatin was modified using methacrylic anhydride (MAA), as first reported by Van Den Bulcke et al., to form GelMA with a degree of substitution of 93.3%.[25] The conjugation of lysine with MAA was observed by proton nuclear magnetic resonance (1H NMR) analysis (Figure S1, Supporting Information). The peaks corresponding to methylene lysine protons (2 H) around 2.8 ppm (peak c) were not present in the spectra of GelMA, indicating the conjugation of free lysines with MAA. Further, the peaks corresponding to the acrylic protons (2 H, around 5.25 ppm) and the methyl protons (3 H, around 1.75 ppm) of grafted methacrylamide groups were observed in GelMA samples, verifying successful gelatin conjugation with MAA.[26]
GelMA and gelatin microgels were prepared separately using a complex coacervation method as reported previously by others.[3,27] Briefly, the positively charged gelatin interacts electrostatically with a polyanion (in our case, acacia gum) to form a liquid-liquid phase that is dispersed as droplets by stirring in a water/ethanol mixture (Figure 1a). Because of the reduced charge and increased hydrophobicity of GelMA relative to gelatin after reaction of the lysine residues with methacrylic anhydride, twice as much ethanol was used to fabricate GelMA microgels compared to gelatin microgels (2 vs. 1 mL ethanol/g of solution, respectively) to induce successful liquid-liquid phase separation of GelMA into droplets. Microfluidic-based microgel fabrication has been used to produce more monodisperse samples than those produced through other approaches; however, this technique requires long production times and sometimes suffers from low throughput.[12,15,16] The complex coacervation method for fabricating granular gels is able to quickly produce large batches of both granular bioinks and support baths. On average, for batch volumes of 50 mL, our protocol had a coacervate yield of 92.57 ± 0.04% (n = 3) and as a proof-of-concept of scalability, when the batch volume increased to 1000 mL, we observed that the yield did not change significantly (91.3%, n = 1).
Both gelatin (red) and GelMA (blue) microgels formed aggregates before washing with saline (Figure 1b). Both types of microgels swelled and remained intact after washing to remove any remaining ethanol, due to the hydration of polymer chains (Figure 1c). After washing, the microgels were jammed by removing excess water through filtration without causing an appreciable change in particle size (Figure 1c). Gelatin microgels had a narrower size distribution than GelMA (polydispersity index, PDI, of 0.20 vs. 0.44, respectively), with mean diameters of 18.0 ± 3.97 μm and 50.55 ± 14.31 μm, for gelatin and GelMA microgels, respectively (Figure S2a). This is within the range of microgel size that has been previously used to study the effect of granular hydrogel porosity on cell behaviors.[16] Additionally, we observed that the microgel shape was slightly different for the two samples. The GelMA microgels had a more uniform, spherical shape (aspect ratio of 1.16 ± 0.12), while the gelatin population had a higher prevalence of elongated microgels (aspect ratio of 1.35 ± 0.26) (Figure S2b).
After washing, the GelMA and gelatin microgel slurries were separately concentrated by vacuum filtration to form jammed precursor inks. After each vacuum filtration step, phosphate-buffered saline (PBS) with 1 mm of lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) photoinitiator was added to achieve a final total microgel mass ranging from 2 wt% to 6 wt%. These microgel inks could be further blended together to form composite GelMA:gelatin inks. All microgel inks were 3D printed via layer-by-layer microextrusion into air, with no additional support material, using a custom-built 3D bioprinter.
2.2. Rheological Properties and Printability of GelMA:Gelatin Microgel Inks
The printability of a biomaterial ink depends upon both its rheological properties and the 3D printing protocol. Here, we use extrusion-based bioprinting without support materials. Employing this approach calls for an ink that can first be extruded through a fine nozzle and is then able to build self-supporting structures, two behaviors that are predominantly influenced by the yield stress (τy) and the shear modulus (G′) of the material, respectively.[28] For jammed microgels, the rheological properties strongly depend on the microgel packing fraction, which controls friction between the surfaces of adjacent microgels.[12,29,30] In our system, the microgel packing fraction was easily controlled by altering microgel concentration, as described above.[31]
Oscillatory shear rheology was used to characterize the mechanical properties of our GelMA microgel inks. The lowest microgel concentration (2 wt%) did not form a gel, as characterized by a loss modulus (G″) greater than the storage modulus (G′) (Figure 2a; see Figure S3, Supporting Information, for individual plots of each ink formulation). While the 3 wt% microgel concentration did form a gel, we predicted that its low plateau storage modulus (G′ ~ 5 Pa) would not allow for the fabrication of self-supporting structures, thus precluding its use as a robust ink (Figure 2a). For microgel concentrations above 4 wt%, each composition exhibited a linear viscoelastic region across a range of applied stresses (Figure 2a). As expected, increasing the weight fraction of GelMA microgels from 4 to 6 wt% resulted in an increase in the plateau storage modulus (G′ ~ 100, 600, and 1000 Pa for 4, 5, and 6 wt%, respectively). Additionally, increasing the weight fraction of GelMA microgels also increased the observed yield stress (τy of ~10, 60, and 140 Pa, respectively).
Figure 2.
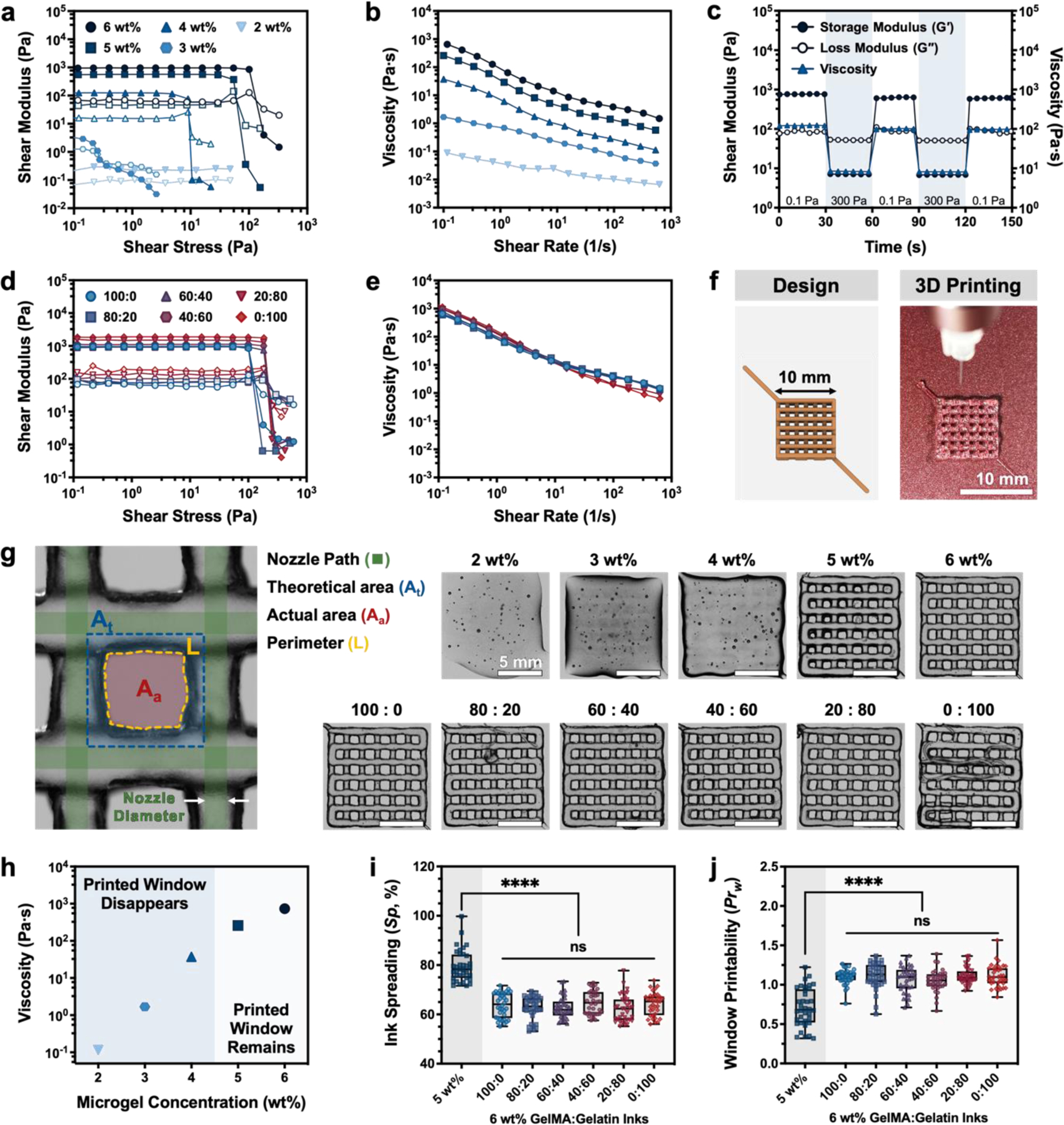
Rheological characteristics and printability of GelMA and gelatin microgel inks without UV crosslinking. (a) Storage moduli (G′, filled symbols) and loss moduli (G″, open symbols) of GelMA microgel inks with a range of total microgel concentration (2–6 wt%) as a function of shear stress (0.1–1000 Pa). (b) Shear viscosity with increasing shear rates (0.1–1000 s−1) demonstrates that GelMA microgel inks are shear-thinning. (c) Evaluation of the self-recovery of a 6-wt% GelMA microgel ink under alternating shear stress (0.1 Pa and 300 Pa). (d) Storage moduli (G′, filled symbols) and loss moduli (G″, open symbols) of composite GelMA:gelatin microgel inks with different ratios (all GelMA: 100:0; all gelatin: 0:100) as a function of shear stress (0.1–1000 Pa). (e) Shear viscosity with increasing shear rates (0.1–1000 s−1) of sacrificial gelatin microgel-laden composite inks. (f) 3D model and representative image of an uncrosslinked lattice structure 3D printed in air using a 6-wt% microgel ink. (g) Optical microscopic images of 3D printed lattice structures made using GelMA with different concentration from 2 to 6 wt% (top row) and 6 wt% GelMA:gelatin microgel composite inks (bottom row) with different ratios from 0:100 to 100:0. (h) Apparent viscosity of GelMA microgel inks at an applied shear rate of 0.1 s−1 as a function of GelMA microgel concentration, with printable (“Printed Window Remains”) and not printable (“Printed Window Disappears”) inks labeled based on printing studies shown in (g,i, and j). (i) The quantified ink spreading (Sp) and (j) window printability (Prw) of 6-wt% inks with different GelMA:gelatin microgel ratios compared to a 5-wt% microgel ink. Data in (i) and (j) plotted as a box and whisker plot, with whiskers showing the minimum and maximum values, and a superimposed scatter plot of all points; n = 36 windows. Scale bars are 10 mm in panel f and 5 mm in panel g. Statistical significance tested by one-way ANOVA with Tukey’s post-hoc analysis; n.s. = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Viscosity is another critical determinant of ink printability that allows inks to resist unwanted material flow after extrusion and results in improved shape fidelity, or the ability to accurately print a desired shape.[32,33] The viscosity of GelMA microgel inks was observed to gradually increase with increasing microgel concentration (Figure 2b). In this system, GelMA microgel inks demonstrate shear-thinning behavior, i.e. a reduction in viscosity in response to increased shear rate. This allows the inks to be extruded through a low-diameter nozzle (inner diameter = 210 μm; see Table S1, Supporting Information, for detailed printing conditions) with reasonable pressure drops. Ideally, the inks will also rapidly self-heal once extruded, i.e. the material will fully regain its original viscoelastic properties. Therefore, an ideal ink for extrusion-based bioprinting would be one that readily flows upon the application of shear stress (i.e. shear-thinning) and rapidly stabilizes after extrusion (i.e. self-healing) to form a robust, solid-like gel.[6] To characterize the ink’s ability to self-heal, the 6-wt% GelMA microgel ink was subjected to alternating low (0.1 Pa) and high (300 Pa) shear stress (Figure 2c). When experiencing low shear stress, as in the printing cartridge prior to extrusion, the microgel ink exhibits solid-like, elastic behavior (G′ ~ 760 Pa, G″ ~ 80 Pa) and maintains high viscosity (120 Pa⋅s). In response to high shear stress, such as that experienced during extrusion through a nozzle, the ink rapidly transits to a liquid-like, viscous state (G′ ~ 7 Pa, G″ ~ 50 Pa). Solid-like behavior is quickly recovered upon return to low shear stress conditions (within 1 s), as is desired after deposition. After two repeated cycles of shear-thinning and self-healing, the storage and loss moduli were similar (within 78.7%) to the original moduli, further indicating self-healing behavior.
Particle packing theory states that the theoretical threshold for void fraction is between 0.26 and 0.476 when identically-sized particles are ideally packed into a structure.[34] Within this range, both microgel concentration and diameter have been used to control the void fraction between annealed microgels in granular biomaterials.[16,22,23,31] Although modulation of microgel concentration is an effective method to tailor the void fraction in cast hydrogels, altering microgel concentration in the context of bioprinting drastically alters ink rheological properties (as shown in Figure 2a, b), and thus is not an ideal strategy. To simultaneously control the void fraction between annealed microgels and maintain the appropriate viscosity, we developed an alternative strategy using sacrificial gelatin microgels (as shown in Figure 1a). The gelatin and GelMA microgels were mixed at different ratios ranging from 100% w/w GelMA (0:100) to 100% w/w gelatin (0:100) to form composite inks, while maintaining a constant total microgel concentration of 6 wt%.
All of the 6-wt% GelMA:gelatin microgel composites tested displayed a linear viscoelastic region, with a slight increase in storage modulus as the gelatin microgel content was increased (Figure 2d; see Figure S4, Supporting Information, for individual plots of each ink formulation). Similarly, composite inks with greater gelatin microgel content had slightly higher viscosity at low shear rates, indicating an overall increase in interparticle friction in these gelatin microgel-dominated composites (Figure 2e).[12,29] Because the total microgel concentration remained fixed, inherent differences between the GelMA and gelatin microgels likely underlie the variation seen in composite ink mechanical properties. In particular, microgel size influences the contact area between adjacent microgels, which in turn modulates their friction and apparent mechanical properties. The difference in size between the smaller gelatin microgels (diameter = 18.0 ± 3.97 μm) and the larger GelMA microgels (diameter = 50.55 ± 14.31 μm) could be sufficient to cause an increase in friction within the jammed ink, even at the same total microgel concentration. The effect of microgel size on ink rheological properties could be investigated further by using other microgel fabrication methods, such as microfluidic device-based fabrication, to create granular inks with tighter control over microgel size and homogeneity. Additionally, modification of gelatin to form GelMA disrupts the triple-helix secondary structure that allows gelatin to solidify at room temperature, resulting in a lower storage modulus for GelMA compared to gelatin (Figure S5, Supporting Information). Therefore, an increase in the amount of smaller, stiffer gelatin microgels in proportion to the larger, softer GelMA microgels resulted in an increase in the overall storage modulus and viscosity of composite microgel inks. We note that all composite ink formulations had an apparent viscosity at an applied shear rate of 0.1 s−1 that was above 100 Pa⋅s.
GelMA microgel ink extrudability and shape fidelity was first qualitatively assessed by visually inspecting the accuracy of a printed lattice, in particular the retention of open windows formed by the lattice structures (Figure 2f).[35] Inks with concentrations ranging from 2 to 6 wt% were used to print a 10-mm by 10-mm lattice composed of four stacked layers (Figure 2g). Low concentration microgel inks (2, 3, and 4 wt%) were not capable of maintaining the open windows within the lattice design and could not be stacked layer-by-layer. Meanwhile, when the ink concentration was increased to 5 and 6 wt%, the square windows of the lattice were somewhat maintained, and individual filaments held their shape. From this, it can be concluded that GelMA microgel inks with concentration 4-wt% or lower do not have sufficiently high shear modulus, yield stress, and viscosity to maintain their printed shape, as could be expected based upon the rheological measurements. Conversely, 5-wt% and 6-wt% inks do have the appropriate rheological properties that allow good shape fidelity. By plotting the ink apparent viscosity (η) at an applied shear rate of 0.1 s−1 as a function of microgel concentration and identifying the ink formulations that were visually able to achieve an “open” printed window structure, an empirical cut-off value of η ~ 100 Pa⋅s was observed for printable ink formulations (Figure 2h). Consistent with our observations for GelMA-only microgel inks, all composite 6-wt% GelMA:gelatin microgel inks allowed for stable fabrication of square, open windows and layer-by-layer stacking in our test lattice prints (Figure 2g), suggesting that printability is dominated by total microgel concentration and can be decoupled from sacrificial gelatin microgel content, and therefore void fraction after gelatin removal.
While rheological properties and extrudability play a crucial role in determining ink printability, quantitative assessment of print accuracy (referred to as “shape fidelity”) is crucial to successfully validating an ink for 3D printing.[33,36] Therefore, to demonstrate the decoupling of printability from the GelMA:gelatin ratio suggested by visual inspection (Figure 2g), two metrics of shape fidelity were quantified from printed lattice structures. This lattice structure is a common print geometry used to test shape fidelity, as the windows (i.e. open spaces) formed within the lattice can be readily measured (Figure 2g).[35,37,38] In a perfect lattice, such as the 3D model used for printing (Figure 2f, “Design”), the area of one of these windows can be described as the area of a square. In practice, the actual area of the window (Aa) often differs from this theoretical area (At) (Figure 2g, diagram at left). For example, at the intersection of two printed filaments, the overlapped hydrogels can spread due to gravity, making Aa smaller than At. Here we define ink spreading (Sp, termed diffusion rate in some manuscripts [37,38]) as the relative difference between actual and theoretical window area (see Equation 1 in Experimental Section). When the total GelMA concentration increased from 5 wt% to 6 wt%, Sp decreased from 78% to 64% indicating that the higher ink concentration had greater shape fidelity. In contrast, there was no statistically significant difference in Sp among the GelMA:gelatin microgel ratios at a fixed total concentration of 6 wt%, indicating that the ratio of GelMA and gelatin microgels did not influence concentration-dominated shape fidelity in our ink system (Figure 2i). We further explored shape fidelity by quantifying window printability (Prw), which used the perimeter (L) and area (Aa) of the printed window to determine its similarity to a perfect square as done in previous studies (Figure 2g, Equation 2).[35,38] Using this parameter, Prw < 1 when an under-gelled ink with low viscosity forms windows that are rounded or circular. Prw = 1 when windows are perfectly square, which indicates well-defined filaments, ideal printability, and often optimal rheological properties Prw > 1 when an over-gelled, high viscosity ink fractures during extrusion to form jagged or uneven filaments. Lattices printed using a 5-wt% microgel ink had windows with a rounded shape (Figure 2g) and a Prw value of 0.7 (Figure 2j). Meanwhile, all GelMA:gelatin microgel inks with a total concentration of 6 wt% had a Prw in the range of 1.0 – 1.1, which indicates high shape fidelity (Figure 2j). These quantitative analyses reveal that the 6-wt% microgel inks exhibit both low ink spreading (Sp) and a reasonable range of window printability (Prw) across all GelMA:gelatin microgel ratio changes, demonstrating that shape fidelity — a crucial component of ink printability — was not impacted by the incorporation of sacrificial gelatin microgels. Interestingly, we noted that there was no statistically significant difference between the 100:0 (DS = 93.3%) and 0:100 (DS = 0%) inks for either shape fidelity measurement, suggesting that the degree of gelatin methacryloylation does not impact printability in our system.
More complex test designs were 3D printed to further explore the versatility and printability of these composite inks across several metrics. Printing soft materials into air often suffers from poor shape retention due to print collapse or viscous flow beyond the intended filament diameter.[39–41] This is especially true in tall prints with narrow cross-sections, where the material must support its own weight. During initial assessments of printability, each microgel ink was capable of printing a 4-layer lattice structure (Figure 2g). We next explored the ability of GelMA:gelatin microgel inks to build tall vertical shapes without the use of support materials. Specifically, the ability to vertically stack inks without collapse, a component of shape fidelity and ink printability, was investigated through the height maintenance (HM) of a tall, printed cylinder compared to theoretical height. For this measurement, a continuous vertical spiral was printed using a 27-gauge needle (inner diameter = 210 µm) to form a 10-mm tall hollow cylinder (Figure 3a). Inks with a total microgel concentration of 4 wt% were only capable of maintaining 62.8% of the theoretical height. Meanwhile, both 5-wt% and 6-wt%, 100:0 microgel inks were capable of forming a stable cylinder, which suggest that inks with at least 5-wt% microgel content are appropriate for constructing tall vertical shapes without collapse (Figure 3b; Figure S6, Supporting Information). Among 6-wt% inks with different GelMA:gelatin microgel ratios, all ink formulations had a HM of around 100% (Figure 3c; Figure S6, Supporting Information). This result demonstrates that the printability of 6-wt% inks, as measured by HM, is primarily independent of the GelMA:gelatin microgel ratio before removal of the sacrificial gelatin component. Next, to investigate the stability of these inks after gelatin removal, printed and UV-crosslinked cylinders were filled with 37°C water, which rapidly solubilized the sacrificial gelatin microgels (Figure 3d). Here, we again used height maintenance, HM, where the theoretical height becomes the pre-melting height. As expected, a crosslinked cylinder printed using only GelMA microgels (i.e. an ink with a GelMA:gelatin ratio of 100:0) was able to stably retain warm water within the structure (Figure 3d; Figure S7, Supporting Information). Conversely, less than 20 seconds after filling with warm water, cylinders printed with either a 20:80 ink or a 0:100 ink underwent a dramatic height loss in response to the melting of sacrificial gelatin microgels that resulted in structural collapse and water leakage. In stark contrast, we found that a cylinder printed with only 60% GelMA content (the 60:40 ink) was capable of retaining its cylindrical shape and holding water while maintaining its pre-melting height. We also investigated the ability of our microgel inks to print branched structures similar to patterns seen in nature (Figure S8, Supporting Information). In all test prints, UV-crosslinked structures could be handled with no apparent loss in structural integrity.
Figure 3.
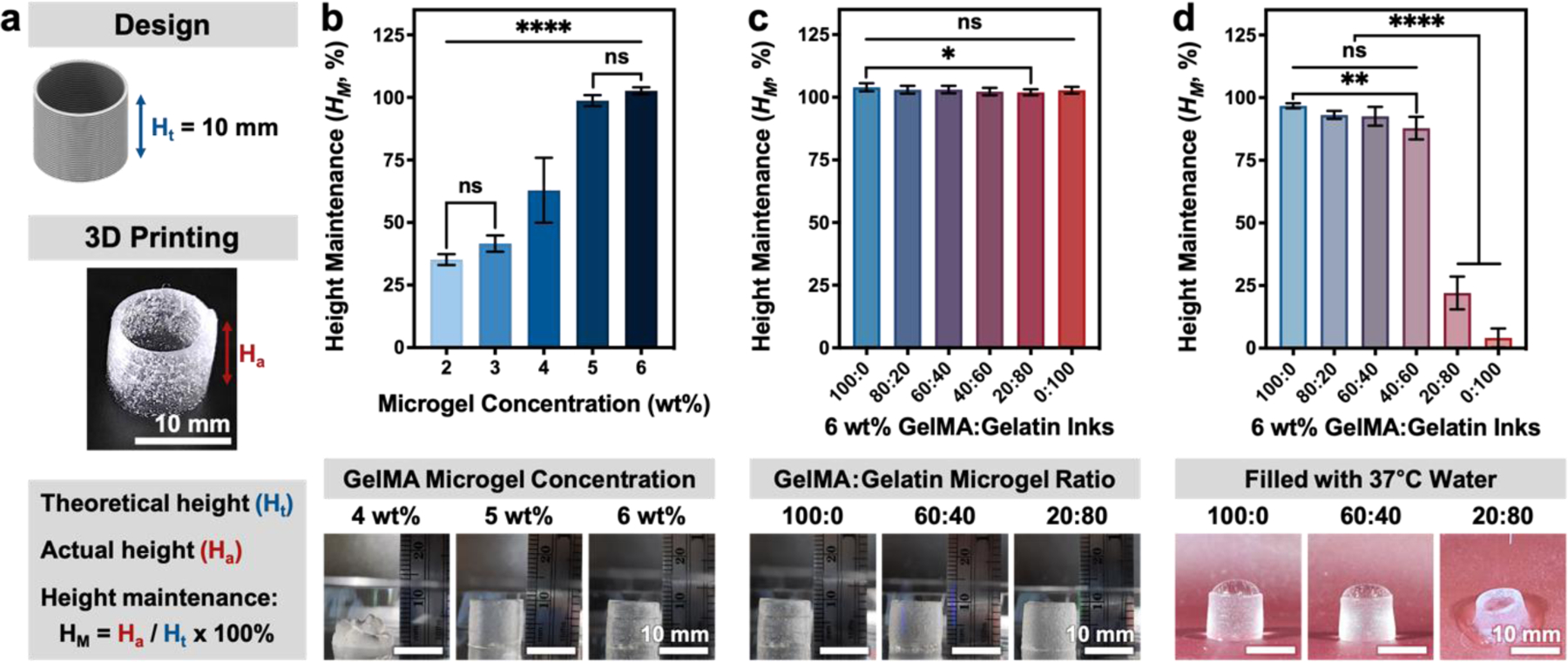
Ability to print self-supporting, stacked structure quantified by height maintenance, HM, of microgel inks. (a) A 1-cm (10 mm) tall cylinder was printed via continuous extrusion through a 27-gauge needle to investigate the ability of various microgel inks to support layer stacking, measured by height maintenance (HM). Height maintenance (HM) of uncrosslinked, 3D printed cylindrical structures made using (b) 100:0 inks with concentrations ranging from 2 to 6 wt% and (c) GelMA:gelatin microgel composite inks with ratios from 0:100 to 100:0 (n = 12 printed cylinders). (d) UV-crosslinked cylinders printed with inks of varying GelMA:gelatin ratio were filled with 37°C water to assess their ability to maintain their height after melting sacrificial gelatin microgels (n = 6 printed cylinders; additional images in Figure S6 and Figure S7, Supporting Information). Data are plotted as mean ± standard deviation. Statistical significance tested by one-way ANOVA with Tukey’s post-hoc analysis; n.s. = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
2.3. Analysis of Void Fraction within GelMA:Gelatin Composite Microgel Inks
Melting of the sacrificial gelatin microgels was characterized by comparing the shear modulus and viscosity of a pure gelatin microgel ink (0:100) at 25°C to that 30 seconds after raising the temperature to 37°C (Figure S9, Supporting Information). The solid-like, elastic gelatin microgel ink transitioned to a liquid-like, viscous state when the temperature was increased to 37°C, verifying that the sacrificial gelatin microgels melt rapidly (Figure S9a, Supporting Information). Additionally, the viscosity (measured at 0.1 s−1) dramatically decreased from 1300 Pa⋅s to 0.16 Pa⋅s at 25°C and 37°C, respectively (Figure S9b, Supporting Information). Taken together, these data validate the use of sacrificial gelatin microgels to control void fraction within UV-crosslinked GelMA:gelatin microgel inks.
We next sought to directly validate the use of sacrificial gelatin microgels as a means to control void fraction by quantifying total void volume within our GelMA:gelatin microgel inks. Prior to UV crosslinking, the GelMA microgels are also temperature sensitive. Increasing the temperature would cause both the gelatin and GelMA microgels to liquefy, which would affect void fraction by causing the microgels to fuse and the inter-microgel voids to collapse. To prevent this unfavorable temperature effect, we held the printing temperature constant at 25°C (Table S1, Supporting Information, lists all printing parameters). The printed structures were then UV-crosslinked immediately, immersed in 2,000-kDa, FITC-labeled dextran, and incubated for 24 h at 37°C. As the sacrificial gelatin microgels melted, the FITC-labeled dextran easily diffused throughout the void space, but did not penetrate the crosslinked GelMA microgels (Figure 4a). Constructs 3D printed using a 100% GelMA microgel ink (100:0) had a total void fraction of about 0.20 ± 0.02, which is formed by the interstitial voids between adjacent microgels (Figure 4b). This value is similar to that reported for other systems of homogenous, deformable microgels.[16] As the relative proportion of gelatin microgels increased within the composite inks, the void space formed between crosslinked GelMA microgels also gradually increased. Specifically, GelMA:gelatin microgel ratios ranging from 80:20 to 60:40 to 40:60 had average void fractions that increased from ~ 0.28 ± 0.04 to 0.41 ± 0.02 to 0.57 ± 0.06, respectively. This approach of employing sacrificial gelatin microgels to incorporate greater void space allowed the 40:60 microgel ink to exceed the maximum theoretical void fraction that can be obtained by packing equally sized microparticles into a cubic lattice (void fraction of 0.476).[34] A percolation network of crosslinked GelMA microgels was observed in all printed samples, which allowed the constructs to maintain their shape and be self-supporting despite possessing a large total void fraction (Figure 4a, GelMA:gelatin microgel ratio of 40:60). In contrast, while scaffolds were successfully printed using an ink with a GelMA:gelatin microgel ratio of 20:80, the constructs could not maintain their shape during gelatin removal and fragmented at 37°C (Figure S10, Supporting Information).
Figure 4.
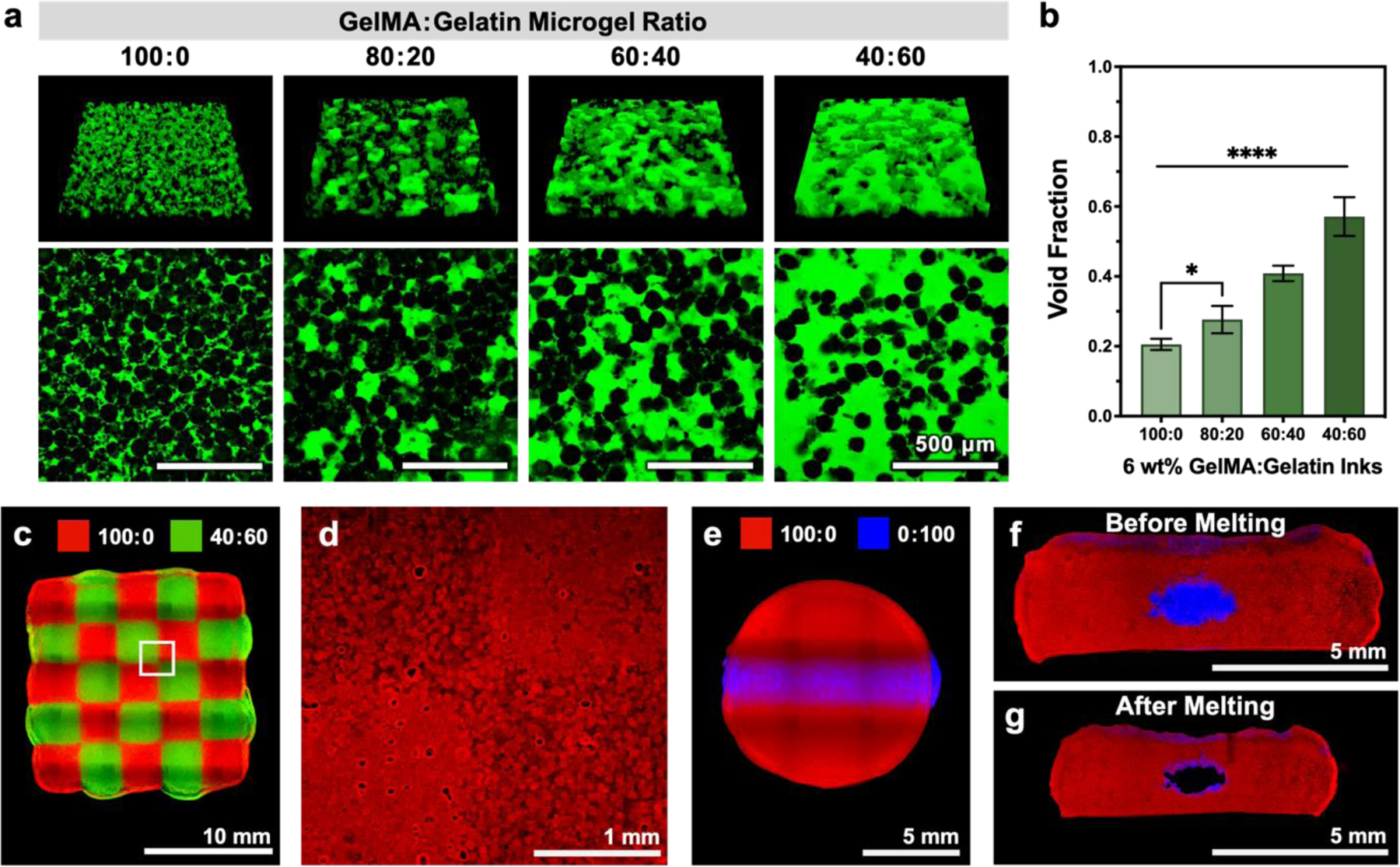
Representative images and quantification of void fraction in samples printed with GelMA:gelatin microgel inks after UV crosslinking and sacrificial gelatin removal. (a) Microgels are shown in black, and voids (which are filled with 2,000-kDa FITC-dextran) are shown in green. (b) Quantified void fraction for inks of varying GelMA:gelatin microgel ratios; mean ± standard deviation, n = 6. 3D printed structures demonstrating the versatility of GelMA:gelatin microgel inks. (c) A chessboard pattern was printed from two different microgel composite inks (100:0 and 40:60, visualized with red and green fluorescent microparticles, respectively). (d) After UV-crosslinking and melting of sacrificial gelatin microgels, regions of patterned void structure were observed by diffusing rhodamine B (red) into the GelMA microgels. (e) An embedded cylindrical channel was printed with two different microgel composite inks (100:0 and 0:100, visualized with red and blue fluorescent microparticles, respectively). Cross-sectional images of the embedded channel (f) pre-melt and (g) post-melt. Scale bars are 500 μm in a, 10 mm in c, 1 mm in d, and 5 mm in e-g. Statistical significance tested by one-way ANOVA with Tukey’s post-hoc analysis; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Patterning of multiple materials into a cohesive scaffold represents an important next step in the evolution of bioprinting toward more biomimetic structures.[39,42] As a matrix cue, void space has been reported to influence several aspects of cell phenotype, including cell proliferation, extracellular matrix secretion, and migration.[16–19] To demonstrate patterning of void fraction within a composite scaffold, we printed a two-material chessboard pattern consisting of 100:0 and 40:60 microgel inks (Figure 4c). After UV crosslinking GelMA microgels and melting sacrificial gelatin microgels, the chessboard remained intact, with distinctly different void structures present within the two different inks (Figure 4d). Sacrificial inks have also been widely used to form hollow channels within larger printed shapes.[43–46] To illustrate the potential use of our 0:100 material as a traditional sacrificial ink, we printed an elliptical cylinder of sacrificial gelatin microgels (0:100) embedded within a larger disc of UV-crosslinkable GelMA microgels (100:0) (Figure 4e,f). After crosslinking and sacrificial gelatin removal at 37°C, a hollow channel was formed (Figure 4g). While the melting process was observed to cause the entire printed scaffold to shrink, the overall shape was maintained (Figure 4g). These proof-of-concept demonstrations suggest that our combination of sacrificial and UV-crosslinkable microgel-based inks may be useful in the future fabrication of a range of tissue-mimetic structures.
2.4. Effect of Sacrificial Gelatin Microgel Removal on Rheological Properties of Crosslinked Inks
The introduction of a large amount of void space into GelMA:gelatin microgel inks was expected to affect the mechanical properties of the final UV-crosslinked constructs. Therefore, the shear moduli of 3D printed scaffolds were measured before and after sacrificial gelatin microgel melting and removal at 37°C (Figure 5). Interestingly, the 100:0, 80:20, and 60:40 UV-crosslinked GelMA:gelatin microgel inks had similar plateau storage moduli at 25°C despite a reduction in UV-crosslinking sites with increased sacrificial gelatin microgel content (Figure 5a). Presumably this is due to the triple-helix secondary structure readily adopted by unmodified gelatin present within the stiffer sacrificial gelatin microgels. As the ratio between UV-crosslinkable GelMA microgels and sacrificial gelatin microgels was altered from 100:0 to 60:40, the increasing proportion of stiffer sacrificial gelatin microgels could partially compensate for the loss of covalent UV crosslinks between GelMA microgels. The mean storage moduli of 40:60 and 20:80 microgel inks were significantly lower than that of the 100:0 formulation (29% and 48% lower, respectively), indicating that the loss of UV crosslinks between GelMA microgels becomes significant in these conditions.
Figure 5.
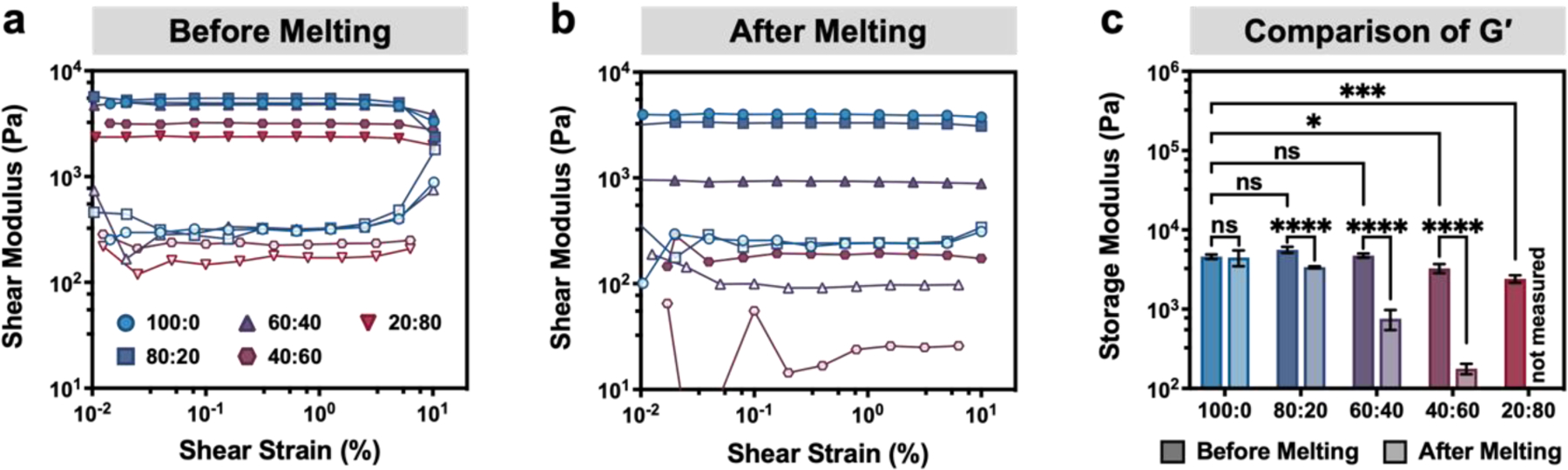
Shear moduli of UV-crosslinked GelMA:gelatin inks before and after melting sacrificial gelatin microgels. (a,b) Representative shear moduli (G′, filled symbols; G″, open symbols) of UV-crosslinked GelMA:gelatin microgel inks (a) before melting (25°C) and (b) after melting (37°C) sacrificial gelatin microgels. (c) Comparison of pre- and post-melting storage moduli (G′) plotted as mean ± standard deviation, n = 3. Statistical significance was evaluated using one-way ANOVA with Tukey’s post-hoc analysis; n.s. = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
UV-crosslinked scaffolds were then incubated at 37°C to remove the gelatin microgels before measuring the rheological properties at 37°C. As expected, these samples demonstrated a decrease in storage modulus with increasing void fraction (Figure 5b). The pure GelMA microgel ink (100:0) did not show any significant change in storage modulus in response to incubation at 37°C (Figure 5c). As the ratio of GelMA and sacrificial gelatin microgels was altered from 100:0 to 40:60, inks with greater sacrificial gelatin microgel content showed a significant reduction in storage modulus compared to their pre-melting values (approximately 40%, 84%, and 95% reduction in storage modulus for 80:20, 60:40, and 40:60, respectively). Constructs 3D printed with a GelMA:gelatin ratio of 20:80 were too weak after melting to withstand washing and rheological measurement. After removal of sacrificial gelatin microgels and introduction of void space, the highest and lowest measured storage moduli were 4.5 ± 1.0 kPa and 177 ± 26 Pa for the 100:0 and 40:60 ink formulations, respectively. Because soft tissues typically have storage moduli ranging from ~100 Pa to ~10 kPa, we concluded that the post-melting mechanical properties of our GelMA:gelatin microgel inks were within a reasonable range for use in bioprinting and cell culture applications.[47,48] Therefore, we chose to use inks with GelMA:gelatin microgel ratios ranging from 100:00 to 40:60 for further 3D printing and in vitro characterization.
2.5. GelMA:gelatin Microgel Inks Support HUVEC Viability and Infiltration
We next sought to demonstrate the use of our inks as a platform material for altering cell infiltration through void fraction. Infiltration of regenerative cell types is required for many applications in vitro and in vivo.[17,49] In particular, vascularization, which relies on the migration of endothelial cells, has been demonstrated to enable the formation of thick tissue mimics in vitro and is required to support long-term survival of newly-developed tissue and implanted constructs in vivo.[43,50–52] Endothelial cells natively possess the capacity for robust infiltration, dynamically switching in the body between a stable, quiescent phenotype and a more migratory phenotype in response to their surrounding environment.[53–55] What is more, granular materials have been shown to promote endothelial cell infiltration in vivo and in vitro.[17] Thus, endothelial cells were selected as a proof-of-concept model to evaluate the potential effect of ink void fraction on cellular infiltration.
Circular discs (8 mm in diameter, 1 mm in height) were printed from each microgel ink (100:0, 80:20, 60:40, and 40:60, all at 6 wt%). Flat, homogenous hydrogels cast from GelMA served as a negative control. After crosslinking, all hydrogels were incubated at 37°C for 24 h and thoroughly washed in DPBS to remove sacrificial gelatin microgels before seeding human umbilical vein endothelial cells (HUVEC). HUVEC were cultured for up to 7 days in growth medium supplemented with vascular endothelial growth factor (VEGF), a potent migratory cue.[56,57] We first confirmed that our microgel inks maintained both acute and long-term cell viability, which is a prerequisite for cell infiltration. All conditions supported high (> 95%) cell viability after one day of culture (Figure 6a; Figure S11a, Supporting Information). Over one week of culture, cell metabolism steadily increased in all conditions, suggesting that the cells were viable and proliferative (Figure 6b; Figure S11b, Supporting Information).
Figure 6.
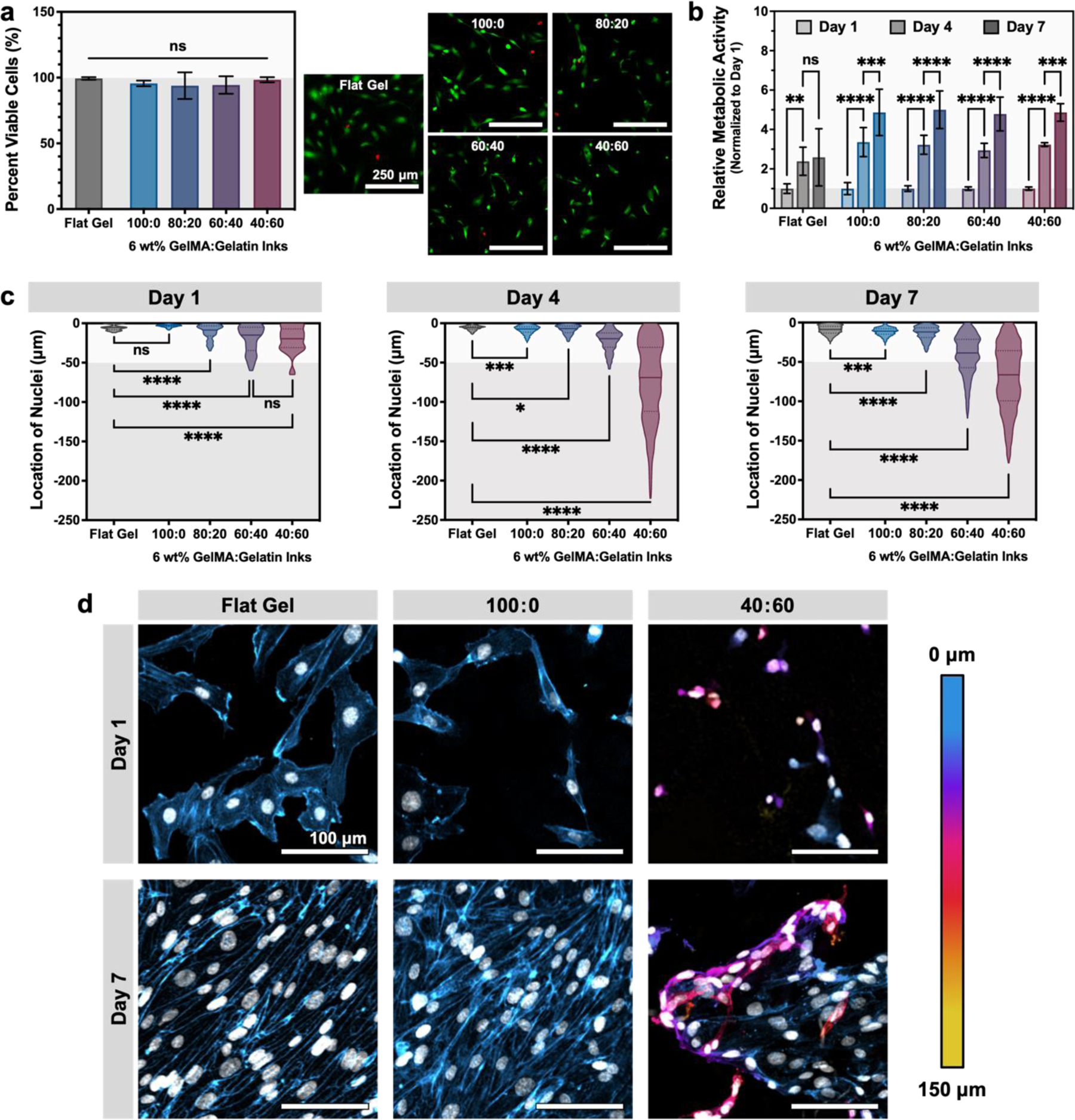
Microgel-based inks with greater void fraction enhance cell infiltration into 3D printed constructs. (a) Live/Dead™ staining after 1 day of culture demonstrates that all material conditions support HUVEC viability (calcein AM = green, live; ethidium homodimer-1 = red, dead; scale bars are 250 μm). For comparison, HUVEC were also cultured on a control substrate of flat GelMA (labeled “Flat Gel”). Data are plotted as mean ± standard deviation, n = 6 images; N = 2–3 replicates. (b) Quantification of cell metabolism shows an increase in overall metabolism per gel over time in all conditions, normalized to day 1 values. Data are plotted as mean ± standard deviation, n = 6 replicates. (c) Quantification of nuclear z-position within GelMA:gelatin microgel inks demonstrating increased number of cells are found deeper within inks with greater total void fraction (n = 35–3,961 cells per condition; N = 3 sample replicates). Data are plotted as violin plots, with median and quartiles denoted by solid and dashed lines, respectively. The top surface of the construct (50 µm) is denoted by a white band. (d) Representative images of HUVEC cultured on a negative control surface of flat GelMA and GelMA:gelatin microgel inks at days 1 and 7. Actin is false-colored to denote z-position within the scaffold; nuclei are false-colored white. Scale bars are 100 μm. Statistical significance tested by one-way ANOVA (a, c) or two-way ANOVA (b) with Tukey’s post-hoc analysis; n.s. = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Endothelial cell migration is a complex biological phenomenon that is impacted not only by scaffold geometry, but also scaffold mechanics, the density and spacing of cell-adhesive ligands, and the presence of cytokines.[58,59] Therefore, to demonstrate that our inks provide the appropriate microenvironment to induce endothelial cell migration, we performed a cell infiltration assay. Quantification of nuclear z-position showed an increase in HUVEC infiltration into inks with greater void fraction (Figure 6c). At day 1, nuclear position remained near the surface of the microgel construct for all conditions, with most nuclei appearing within the top 50 µm, which is the length-scale of surface roughness expected for constructs printed with ~50-µm microgels (GelMA microgel diameter = 50.55 ± 14.31 μm). For constructs with greater porosity (i.e. those printed with inks having a higher fraction of sacrificial gelatin microgels), nuclear z-position at day 1 was slightly more dispersed, presumably due to the increased surface roughness of these more porous materials after gelatin removal. After 4 days of culture, HUVEC showed significant migration into the 40:60 ink (void fraction of 0.57 ± 0.06) that then continued through 7 days of culture. By day 7, the 60:40 ink (void fraction of 0.41 ± 0.02) also began displaying marked cell infiltration. Interestingly, the extent of endothelial cell infiltration into 40:60 constructs decreased slightly between day 4 (median = 69.01, 75th percentile = 111.9, maximum = 222.4) and day 7 (median = 66.39, 75th percentile = 99.45, maximum = 178.2). An increase in cell metabolism was also observed at this time point, suggesting that this reduction in cell infiltration is not likely due to cell death (Figure 6b). Instead, this may be evidence of endothelial cell regression. In this system, we chose to use a single angiogenic growth factor (vascular endothelial growth factor, VEGF) to reduce experimental variables while initiating robust endothelial migration. While VEGF is often used to initiate endothelial cell outgrowth and vascularization, VEGF activation alone is known to produce leaky and unstable vessels that can regress.[60,61] Additional growth factors such as platelet-derived growth factor (PDGF) and angiopoietin-1 (ANG-1) help to stabilize nascent vessels during later stages of vascularization and have been used in concert with VEGF to sequentially induce and stabilize endothelial migration.[58,62] Without this stabilization, endothelial cells have been shown to regress in vivo and in vitro. In the future, the granular inks presented herein could be used to explore the role of endothelial migration and stabilization in microporous materials by using sequential delivery of angiogenic signals.
In addition to infiltration depth, changes were also observed in HUVEC morphology for the different printed constructs. In particular, the 100:0 samples (i.e. those with only interstitial pores between GelMA microgels; void fraction of 0.20 ± 0.02) appeared most similar to the flat negative control samples, with cells forming an apparent monolayer after 7 days in culture (Figure 6d). When looking at depths below the top surface (< −50 µm), no HUVEC nuclei were observable in the 100:0 samples at day 7 (Figure 6b, Figure 7). In contrast, for the 40:60 samples, HUVEC nuclei were observed both near the surface of the construct and at depths below 50 µm, where they appeared to wrap around GelMA microgels (Figure 6b, Figure 7). Together, these data demonstrate that granular inks formulated with sacrificial gelatin microgels can result in constructs with a tunable range of void fraction that provides a permissive migratory environment for endothelial cells.
Figure 7.
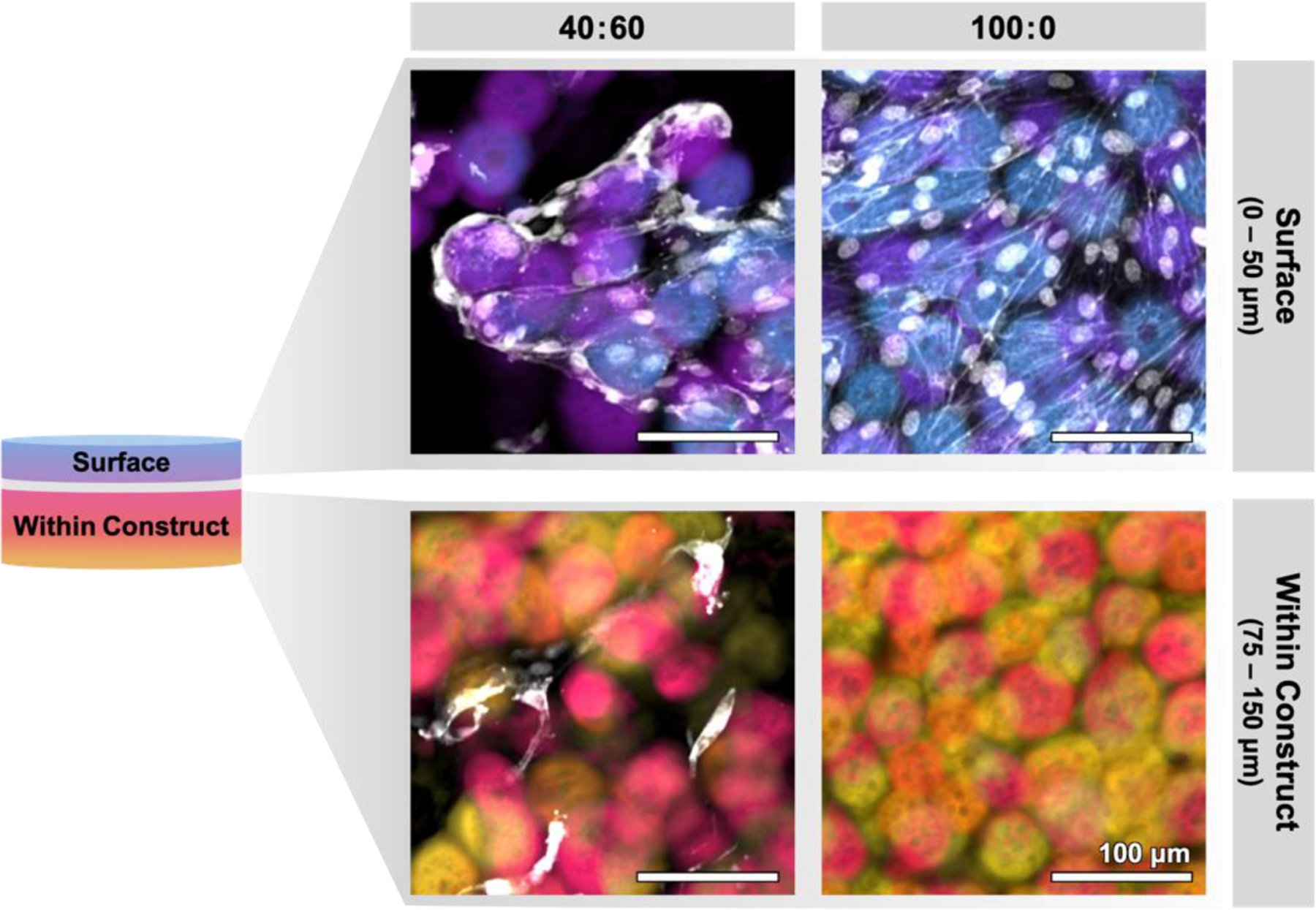
Representative projections of surface (0–50 μm) and internal (75–150 μm) zones within 3D printed microgel constructs after 7 days of culture. In 40:60 inks (left), cells are seen to spread along voids introduced by sacrificial gelatin microgels, allowing them to infiltrate deeper into the construct. In contrast, cells seeded onto constructs printed without sacrificial gelatin microgel (100:0, right) spread along the surface and are not found within the construct. Scale bars represent 100 μm.
In the future, endothelial cells could be cultured for longer times to observe if further infiltration and tubule formation within the microgel constructs may occur.[63] Additionally, an important next step would be in vivo implantation of constructs printed with different GelMA:gelatin microgel blend inks to observe potential effects of void fraction on host vessel infiltration. Taken together, the work herein represents a proof-of-principle for the decoupling of microgel-based ink printability and final void fraction through the blending of crosslinkable and sacrificial microgel components. This strategy of blending multiple microgel populations to decouple ink material properties could readily be extended to other materials, or combinations thereof. While the gelatin and GelMA materials used here readily formed microgels through the simple process of complex coacervation, the extension of this ink strategy to different material systems could employ other microgel fabrication strategies such as the use of microfluidic devices.[13,64]
3. Conclusion
Granular, microgel-based materials have shown promise as tissue engineering scaffolds due to their inherent porosity. Yet the inherent link between total void fraction, ink rheological properties, and cell infiltration often make it challenging to precisely adapt these materials for 3D bioprinting applications. Here, we present a strategy to decouple printability and void fraction by blending UV-crosslinkable GelMA microgels with sacrificial gelatin microgels to form composite inks. By altering the particle packing through concentration, we determined the optimal printing concentration to be 6 wt%. Mixing the GelMA and sacrificial gelatin microgels from 100% GelMA to 100% gelatin, at a constant total concentration of 6 wt%, produced a family of microgel-based inks with high printability. Quantification of void fraction confirmed that these inks possess a range of void fraction from 0.20 ± 0.02 to 0.57 ± 0.06 (for 100:0 and 40:60, respectively) after removal of sacrificial gelatin microgels, exceeding the theoretical limit for microgels without sacrificial components. Various, more complex test structures were 3D printed using our family of blend inks to demonstrate their versatility and the ability to precisely pattern void fraction within printed constructs. Finally, in vitro studies demonstrated the utility of these inks as a cell culture platform to influence endothelial cell infiltration. Specifically, cells maintained viability up to 7 days in culture, and showed migration into 3D printed constructs to an extent that depended upon void fraction. Overall, this work presents a method for building on the promise of granular, microgel-based materials by leveraging traditional 3D printing strategies, such as the use of sacrificial inks, to expand their applicability to an even wider range of biomedical applications.
4. Experimental Section
4.1. GelMA synthesis
To synthesize gelatin methacryloyl (GelMA), type A gelatin (300 bloom, Sigma-Aldrich) was dissolved at 20 wt% in 0.1 m carbonate-bicarbonate (CB) buffer comprising 3.18 g sodium carbonate and 5.86 g sodium bicarbonate in 1 L distilled water. The pH was adjusted to 9 with 3 m sodium hydroxide. Subsequently, methacrylic anhydride (MAA; 94 %, Sigma-Aldrich) was added to the gelatin solution at 0.1 mL per gram of gelatin under magnetic stirring at 500 rpm. The reaction proceeded at 50°C for 2 h, after which the reaction mixture was cooled to room temperature and stored for later use.[26]
4.2. Determination of GelMA Degree of Substitution
A TNBS (2,4,6-trinitrobenzene sulfonic acid, ThermoFisher) assay was performed to quantify the degree of GelMA substitution, according to the manufacturer’s instructions. Briefly, GelMA and gelatin samples were separately dissolved at 1.6 mg/mL in 0.5 mL of 0.1 m sodium bicarbonate buffer. Then, each sample was mixed with 0.5 mL of 0.01% TNBS solution in 0.1 m sodium bicarbonate buffer and incubated at 37°C for 2 h. Next, 0.25 mL of 1 m hydrochloric acid and 0.5 mL of 10 wt% sodium dodecyl sulfate (SDS) were added to stop the reaction. The absorbance of each sample was measured at 335 nm, and the extent of substitution was calculated by comparing the amount of remaining free amine groups in GelMA to that of an unmodified gelatin control. 1H NMR measurement was also performed to verify the degree of substitution. Briefly, GelMA and gelatin samples were separately dissolved at approximately 50 mg/mL in deuterium oxide, and the NMR spectra were recorded on a Varian Unity INOVA 500 NMR spectrometer (1H NMR, 499.75 MHz).
4.3. Preparation of Gelatin and GelMA Microgels
Microgels were formed using a complex coacervation method that employed a blend of positively (gelatin, GelMA) and negatively (gum arabic) charged biopolymers to drive phase separation.[3] For gelatin-only microgels, a solution of 6.4 wt% gelatin type A (300 bloom, Sigma-Aldrich), 0.5 wt% Pluronic® F-127 (Sigma-Aldrich), and 0.2 wt% gum arabic (Alfa Aesar) in DI water was boiled in a microwave. 1 mL of ethanol was added per gram of gelatin solution under magnetic stirring at 500 rpm. For GelMA microgels, a solution of 6.4 wt% GelMA, 1.6 wt% gelatin type A (300 bloom, Sigma-Aldrich), 0.5 wt% Pluronic® F-127 (Sigma-Aldrich), and 0.1 wt% gum arabic (Alfa Aesar) in DI water was similarly boiled, and ethanol was added at 2 mL/g GelMA solution under magnetic stirring at 500 rpm. The beakers were sealed with parafilm to minimize evaporation and allowed to cool to room temperature while stirring overnight. A Büchner funnel was used to collect and wash the resulting microgel slurries. Microgels were washed a total of three times with Dulbecco’s phosphate-buffered saline (DPBS, Corning) to remove residual ethanol and Pluronic® F-127. Prior to 3D printing, the microgel slurries were compacted by vacuum filtration. The concentration of each compacted microgel sample was determined as the percent dry mass of a 50 mg aliquot after thorough drying in a vacuum oven (2 h, 60°C). The final concentration was then adjusted accordingly for 3D printing, as described below.
To visualize microgel size and aspect ratio, compacted gelatin and GelMA microgel slurries were diluted in DPBS and stained with rhodamine B (1 mg/ml, Sigma-Aldrich) for 5 min. The microgels were then imaged using a Leica THUNDER fluorescence microscope, and the microgel diameter and aspect ratio (Figure 1c, n = 100; Figure S2, Supporting Information, n = 3000) were determined using Fiji.[65]
4.4. Gelatin and GelMA Microgel Ink Preparation for 3D Printing
For assessments of printability, GelMA microgels were diluted to appropriate concentrations (from 2 wt% to 6 wt%) with DPBS. To control post-melt void fraction, 6 wt% inks were prepared with different ratios of GelMA and gelatin microgels (GelMA % (w/w) : gelatin % (w/w): 100:0, 80:20, 60:40, 40:60, 20:80, 0:100). For all inks, the photoinitiator lithium phenyl-2,4,6-trimethylbenzo-ylphosphinate (LAP, Sigma-Aldrich) was added to a final concentration of 1 mm. The inks were then thoroughly mixed, briefly centrifuged to remove bubbles, and loaded into a 2.5 mL Hamilton syringe fitted with a blunt-end 27-gauge needle.
4.5. 3D Printing of GelMA and Gelatin Microgel Inks
3D printing was performed with a MakerGear M2 Rev E plastic 3D printer modified to allow two-material bioprinting.[3,47,66] Lattice structures were 10-mm length x 10-mm width and composed of four stacked layers. Single-extrusion cylinders were 10 mm in diameter and 10 mm in height. Discs used for rheological characterization and cell culture were 8 mm in diameter and 1 mm in height. Tool paths for single material lattice and cylinder constructs were generated by hand. Single-material disc and multi-material 3D models were first created using Fusion360 and sliced using Simplify3D. A custom tool-change script was introduced during slicing to calibrate the relative position of each nozzle and allow for simultaneous, layer-by-layer deposition of two materials. To allow for complete removal of sacrificial gelatin microgels, the embedded cylinder structure (dimensions: 10-mm diameter x 2.4-mm height for bulk gel; and 1.5-mm z-axis diameter x 3-mm x-axis diameter x 10-mm length for inner cylinder) was 3D printed with inks composed of all GelMA and all gelatin microgels (GelMA % (w/w) : gelatin % (w/w): 100:0, 0:100, respectively). A two-material chessboard pattern (dimensions: 15-mm width x 15-mm length x 3-mm height for the full structure; 3-mm width x 3-mm length x 3-mm height for each section) with alternating void fractions was fabricated using the 100:0 and 40:60 ink compositions. All of the structures were printed at room temperature to prevent unwanted microgel melting and fusion before UV crosslinking. Table S1, Supporting Information, lists all printing parameters for all printed structures presented within the manuscript. After printing, constructs were crosslinked for 3 min under a UV lamp (365 nm), incubated at 37°C for 24 h to remove gelatin microgels, and washed with DPBS.
4.6. Rheological measurements of gelatin and GelMA microgel inks
To measure the rheological properties of uncrosslinked gelatin and GelMA microgel inks, samples were loaded onto an ARG2 stress-controlled rheometer (TA Instruments) equipped with a 40-mm diameter parallel plate geometry with a 1.0-mm gap height. Samples were subjected to shear stress-sweep experiments over a stress range of 0.1 to 100 Pa at a frequency of 1 Hz at 25°C. The viscosity was measured at 25°C using a range of shear rate from 0.1 to 1000 s−1. The step-stress was measured at both a high magnitude stress (300 Pa) and a low magnitude stress (0.1 Pa). To characterize the temperature dependence of microgel ink mechanical properties, stress sweeps (0.1 to 1000 Pa) and viscosity measurements (shear rates from 0.1 to 1000 s−1) were also performed at 37°C.
3D printed circular discs were used to measure the rheological properties of crosslinked inks before (25°C) and after (37°C) melting gelatin microgels. Briefly, 6-wt% inks containing different ratios of GelMA and gelatin microgels were 3D printed and UV crosslinked as described above. A strain-controlled amplitude sweep (0.01 % to 10 % strain) at a frequency of 1 Hz with a parallel plate geometry (8-mm diameter) was used to determine the linear viscoelastic region at 25°C for microgels prior to melting. Samples were then incubated at 37°C for 24 h to melt gelatin microgels. Afterwards, the post-melt shear modulus at 37°C was measured using a strain-controlled amplitude sweep (0.01 % to 10 % strain) at a frequency of 1 Hz.
4.7. Assessment of lattice shape fidelity
Microgel ink printability was tested by both qualitatively and quantitatively assessing the shape fidelity of a printed lattice, in particular the retention and shape of square, open windows formed by the lattice and unwanted material spreading. After printing, lattice structures were imaged using a Leica THUNDER fluorescence microscope with a tile scan function. The ink spreading (Sp) and window printability (Prw) were determined using Equations (1) and (2), respectively.
| (1) |
| (2) |
where At and Aa are the theoretical and actual areas of the printed window, respectively, and L is the perimeter of the window. For an ink with ideal shape fidelity, which forms perfectly square windows, Sp = 0 (i.e., At = Aa) and Prw = 1. Values plotted represent measurements from the 36 windows of a lattice for each GelMA and gelatin microgel ink.
4.8. Height analysis of stacked cylinders
To assess the ability of GelMA:gelatin microgel inks to build tall vertical shapes without the use of support materials, a continuous vertical spiral was printed with a 27-gauge needle (inner diameter = 210 µm) to form a 10-mm tall, thin-walled hollow cylinder. The height maintenance (HM) of each printed was measured for each GelMA:gelatin microgel ink. The height maintenance (HM) after melting sacrificial gelatin microgels was analyzed by filling UV-crosslinked cylinders with 37°C water and waiting for 1 h. In each case, the height maintenance was determined using Equation (3).
| (3) |
where Ha and Ht are the actual (pre-melting) height and theoretical (or post-melting) height, respectively. The height maintenance of a cylinder structure without any structural collapse is 100% (i.e., Ha = Ht). Three replicates were measured for each condition.
4.9. Void Fraction Analysis
UV-crosslinked discs made from each GelMA and gelatin microgel ratio were incubated in DPBS containing 1 mg/mL 2,000-kDa fluorescein isothiocyanate-dextran (FITC-dextran, Sigma-Aldrich) for 24 h at 37°C to fill the void space between GelMA microgels. The labelled samples were then imaged using a Leica SPE confocal microscope to obtain 100-μm z-stacks. The void space volumes were quantified as a fraction of the total volume represented by the z-stack using Fiji. A minimum of 6 measurements were taken for each microgel ratio.
4.10. HUVEC Expansion and Seeding onto Printed Discs
Circular discs (8-mm diameter x 1-mm height) were printed from each 6 wt% microgel ink (100:0, 80:20, 60:40, and 40:60), as previously described. Flat, homogenous hydrogels cast from 19 wt% GelMA allowed reproducible creation of flat discs, and served as a negative control (G′ ~ 21.6 kPa). Human umbilical vein endothelial cells (HUVEC, PromoCell) were expanded in endothelial growth medium-2 (EGM-2 bullet kit, Lonza), and culture medium was changed every other day. In preparation for seeding onto 3D printed discs, HUVEC were briefly washed two times with DPBS (Corning), incubated for 5 min in 0.05 % Trypsin-EDTA (Gibco), collected with EGM-2 supplemented with 10 % FBS, centrifuged for 4 min at 1000 rpm, and resuspended to count. Meanwhile, sterile printed discs were retrieved from 37°C and washed with warm DPBS to remove melted gelatin. A suspension of 105 cells/mL was then prepared, and 50 μL was seeded onto each prepared disc. Samples were then returned to 37°C and cells were allowed to adhere for 60 min before the addition of 1 mL EGM-2 with 50 ng/mL additional VEGF (R&D Systems, 293-VE). Cell culture medium was refreshed every other day thereafter.
4.11. Assessment of Cell Viability and Metabolism
After one day of culture, cell viability was characterized using Live/Dead™ staining (Life Technologies). Briefly, live (calcein AM) and dead (ethidium homodimer-1) stains were diluted in DPBS according to the manufacturer’s protocol, and 100 μL was pipetted onto a clean coverslip. Samples were then inverted onto the coverslip and imaged using a Leica SPE confocal microscope (n = 3). CellProfiler was used to measure the number of cells with intact or damaged membranes, and results were plotted in GraphPad Prism 9.0.[67]
Cell metabolism was quantified using CellTiter-Blue (Promega) according to the manufacturer’s protocol (n = 5). After 1, 4, and 7 days in culture, the media in each well was replaced with a 1:5 dilution of CellTiter-Blue reagent in EGM-2, and samples were returned to the incubator. After 3 h, 100 μL was transferred from each well into a 96-well plate, and the fluorescence was read using a SpectraMax M2 plate reader (Molecular Devices) at an excitation wavelength of 560 nm and an emission wavelength of 590 nm.
4.12. Characterizing Cell Morphology and Localization
After 1, 4, or 7 days of culture, HUVEC nuclei and actin cytoskeleton were stained to visualize cell morphology and location. Samples were fixed with 4 % paraformaldehyde in DPBS for 45 min at 37°C, and then permeabilized and blocked for 1 hour at room temperature in DPBS containing 0.25 % Triton X-100 (DPBST) and 1 wt% bovine serum albumin (BSA, Roche). The cell nucleus and actin cytoskeleton were then stained through incubation with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI, Cell Signaling Technology, 1:2000) and TRITC-phalloidin (Sigma-Aldrich, 1:200) in DPBST for 1 h at room temperature. Samples were washed with DPBST and imaged using a Leica SPE confocal microscope (n = 3). Nuclear z-position was quantified using CellProfiler, and results were plotted in GraphPad Prism 9.0.[56,58] Representative images were prepared in Fiji.[65]
4.13. Statistical analysis
Statistical analysis and plotting were performed using GraphPad Prism 9.0. In vitro experiments had three independent gel samples in each experiment. Statistical significance was assessed using a one-way ANOVA with Tukey post-hoc test, unless otherwise noted. All errors are reported as the standard deviation of error (SD).
Supplementary Material
Acknowledgements
A.J.S and S.C.S contributed equally to this work. The authors acknowledge Pam Cai for assistance with NMR characterization; Aidan Gilchrist for consultation on image analysis; Julien Roth and Bauer LeSavage for helpful discussion on cell experiments; and Sarah Hull and Lucia Brunel for helpful discussion on bioprinting protocols. We acknowledge financial support from the National Science Foundation (DMR 1808415 to S.C.H) and the National Institutes of Health (R01 HL142718 to S.C.H.).
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Alexis J. Seymour, Department of Bioengineering, Stanford University, Stanford, CA 94305, USA.
Sungchul Shin, Department of Materials Science & Engineering, Stanford University, 476 Lomita Mall, McCullough Room 246, Stanford, CA 94305, USA.
Sarah C. Heilshorn, Department of Materials Science & Engineering, Stanford University, 476 Lomita Mall, McCullough Room 246, Stanford, CA 94305, USA
References
- [1].Daly AC, Davidson MD, Burdick JA, Nat. Commun 2021, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mandrycky C, Wang Z, Kim K, Kim D-H, Biotechnol. Adv 2016, 34, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee A, Hudson A, Shiwarski D, Tashman J, Hinton T, Yerneni S, Bliley J, Campbell P, Feinberg A., Science 2019, 365, 482. [DOI] [PubMed] [Google Scholar]
- [4].Groll J, Burdick JA, Cho D-W, Derby B, Gelinsky M, Heilshorn SC, Juengst T, Malda J, Mironov VA, Nakayama K., Biofabrication 2018, 11, 013001. [DOI] [PubMed] [Google Scholar]
- [5].Freeman FE, Pitacco P, van Dommelen LH, Nulty J, Browe DC, Shin J-Y, Alsberg E, Kelly DJ, Sci. Adv 2020, 6, eabb5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morgan FL, Moroni L, Baker MB, Adv. Healthcare Mater 2020, 9, 1901798. [DOI] [PubMed] [Google Scholar]
- [7].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A., Biomaterials 2010, 31, 5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB, Nature 2020, 584, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shao L, Gao Q, Xie C, Fu J, Xiang M, Liu Z, Xiang L, He Y., Bio-Des. Manuf 2020, 3, 30. [Google Scholar]
- [10].Jeon O, Lee YB, Hinton TJ, Feinberg AW, Alsberg E., Mater. Today Chem 2019, 12, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kessel B, Lee M, Bonato A, Tinguely Y, Tosoratti E, Zenobi-Wong M., Adv. Sci 2020, 7, 2001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Daly AC, Riley L, Segura T, Burdick JA, Nat. Rev. Mater 2020, 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mealy JE, Chung JJ, Jeong HH, Issadore D, Lee D, Atluri P, Burdick JA, Adv. Mater 2018, 30, 1705912. [DOI] [PubMed] [Google Scholar]
- [14].Khan OF, Voice DN, Leung BM, Sefton MV, Adv. Healthcare Mater 2015, 4, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Highley CB, Song KH, Daly AC, Burdick JA, Adv. Sci 2019, 6, 1801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Truong NF, Kurt E, Tahmizyan N, Lesher-Pérez SC, Chen M, Darling NJ, Xi W, Segura T., Acta Biomater 2019, 94, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nih LR, Sideris E, Carmichael ST, Segura T., Adv. Mater 2017, 29, 1606471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X., Nat. Mater 2015, 14, 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Han LH, Yu S, Wang T, Behn AW, Yang F., Adv. Funct. Mater 2013, 23, 346. [Google Scholar]
- [20].Guex AG, Puetzer JL, Armgarth A, Littmann E, Stavrinidou E, Giannelis EP, Malliaras GG, Stevens MM, Acta Biomater 2017, 62, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ratner BD, Regener. Biomater 2016, 3, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Caldwell AS, Campbell GT, Shekiro KM, Anseth KS, Adv. Healthcare Mater 2017, 6, 1700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Darling NJ, Xi W, Sideris E, Anderson AR, Pong C, Carmichael ST, Segura T., Adv. Healthcare Mater 2020, 9, 1901391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Muir VG, Qazi TH, Shan J, Groll J. r., Burdick JA, ACS Biomater. Sci. Eng 2021. [DOI] [PMC free article] [PubMed]
- [25].Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H., Biomacromolecules 2000, 1, 31. [DOI] [PubMed] [Google Scholar]
- [26].Shirahama H, Lee BH, Tan LP, Cho N-J, Sci. Rep 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].De Kruif CG, Weinbreck F, de Vries R., Curr. Opin. Colloid Interface Sci 2004, 9, 340. [Google Scholar]
- [28].Lee J, Oh SJ, An SH, Kim W-D, Kim S-H, Biofabrication 2020, 12, 035018. [DOI] [PubMed] [Google Scholar]
- [29].Menut P, Seiffert S, Sprakel J, Weitz DA, Soft Matter 2012, 8, 156. [Google Scholar]
- [30].Behringer RP, Chakraborty B., Rep. Prog. Phys 2018, 82, 012601. [DOI] [PubMed] [Google Scholar]
- [31].de Aguiar IB, Schroën K, Meireles M, Bouchoux A., Colloids Surf., A 2018, 553, 406. [Google Scholar]
- [32].Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A., Adv. Healthcare Mater 2017, 6, 1601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gillispie G, Prim P, Copus J, Fisher J, Mikos AG, Yoo JJ, Atala A, Lee SJ, Biofabrication 2020, 12, 022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Glover P., University of Aberdeen, UK 2000.
- [35].Ouyang L, Yao R, Zhao Y, Sun W., Biofabrication 2016, 8, 035020. [DOI] [PubMed] [Google Scholar]
- [36].Schwab A, Levato R, D’Este M, Piluso S, Eglin D, Malda J., Chem. Rev 2020, 120, 11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J., Sci. Rep 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Habib A, Sathish V, Mallik S, Khoda B., Materials 2018, 11, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Levato R, Jungst T, Scheuring RG, Blunk T, Groll J, Malda J., Adv. Mater 2020, 32, 1906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McCormack A, Highley CB, Leslie NR, Melchels FP, Trends Biotechnol 2020, 38, 584. [DOI] [PubMed] [Google Scholar]
- [41].Kyle S, Jessop ZM, Al-Sabah A, Whitaker IS, Adv. Healthcare Mater 2017, 6, 1700264. [DOI] [PubMed] [Google Scholar]
- [42].Ashammakhi N, Ahadian S, Xu C, Montazerian H, Ko H, Nasiri R, Barros N, Khademhosseini A., Mater. Today Bio 2019, 1, 100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Skylar-Scott MA, Uzel SG, Nam LL, Ahrens JH, Truby RL, Damaraju S, Lewis JA, Sci. Adv 2019, 5, eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Proc. Natl. Acad. Sci. U.S.A 2016, 113, 3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Highley CB, Rodell CB, Burdick JA, Adv. Mater 2015, 27, 5075. [DOI] [PubMed] [Google Scholar]
- [46].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X., Nat. Mater 2012, 11, 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hull SM, Lindsay CD, Brunel LG, Shiwarski DJ, Tashman JW, Roth JG, Myung D, Feinberg AW, Heilshorn SC, Adv. Funct. Mater 2021, 31, 2007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Guimarães CF, Gasperini L, Marques AP, Reis RL, Nat. Rev. Mater 2020, 5, 351. [Google Scholar]
- [49].Qazi TH, Burdick JA, Biomater. Biosyst 2021, 1, 100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Griffith CK, Miller C, Sainson RC, Calvert JW, Jeon NL, Hughes CC, George SC, Tissue Eng 2005, 11, 257. [DOI] [PubMed] [Google Scholar]
- [51].Kim JJ, Hou L, Huang NF, Acta Biomater 2016, 41, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Massé S, Kim J, Reis L., Nat. Mater 2016, 15, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Du Y, Herath SC, Wang Q.-g., Wang D.-a., Asada HH, Chen PC, Sci. Rep 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shamloo A, Mohammadaliha N, Heilshorn SC, Bauer AL, Ann. Biomed. Eng 2016, 44, 929. [DOI] [PubMed] [Google Scholar]
- [55].Wei Z, Schnellmann R, Pruitt HC, Gerecht S., Cell Stem Cell 2020, 27, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Simons M, Gordon E, Claesson-Welsh L., Nat. Rev. Mol. Cell Biol 2016, 17, 611. [DOI] [PubMed] [Google Scholar]
- [57].Shamloo A, Xu H, Heilshorn S., Tissue Eng. Part A 2012, 18, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Richardson TP, Peters MC, Ennett AB, Mooney DJ, Nat. Biotechnol 2001, 19, 1029. [DOI] [PubMed] [Google Scholar]
- [59].Freiman A, Shandalov Y, Rozenfeld D, Shor E, Segal S, Ben-David D, Meretzki S, Egozi D, Levenberg S., Stem Cell Res. Ther 2016, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N., Nature 2008, 456, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li S, Nih LR, Bachman H, Fei P, Li Y, Nam E, Dimatteo R, Carmichael ST, Barker TH, Segura T., Nat. Mater 2017, 16, 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shin Y, Jeon JS, Han S, Jung G-S, Shin S, Lee S-H, Sudo R, Kamm RD, Chung S., Lab Chip 2011, 11, 2175. [DOI] [PubMed] [Google Scholar]
- [63].Ben-Shaul S, Landau S, Merdler U, Levenberg S., Proc. Natl. Acad. Sci. U.S.A 2019, 116, 2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sideris E, Griffin DR, Ding Y, Li S, Weaver WM, Di Carlo D, Hsiai T, Segura T., ACS Biomater. Sci. Eng 2016, 2, 2034. [DOI] [PubMed] [Google Scholar]
- [65].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B., Nat. Methods 2012, 9, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pusch K, Hinton TJ, Feinberg AW, HardwareX 2018, 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McQuin C, Goodman A, Chernyshev V, Kamentsky L, Cimini BA, Karhohs KW, Doan M, Ding L, Rafelski SM, Thirstrup D., PLoS Biol 2018, 16, e2005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


