Abstract
Human pluripotent stem cells have emerged as a promising in vitro model system for studying the brain. Two-dimensional and three-dimensional cell culture paradigms have provided valuable insights into the pathogenesis of neuropsychiatric disorders, but they remain limited in their capacity to model certain features of human neural development. Specifically, current models do not efficiently incorporate extracellular matrix-derived biochemical and biophysical cues, facilitate multicellular spatio-temporal patterning, or achieve advanced functional maturation. Engineered biomaterials have the capacity to create increasingly biomimetic neural microenvironments, yet further refinement is needed before these approaches are widely implemented. This Review therefore highlights how continued progression and increased integration of engineered biomaterials may be well poised to address intractable challenges in recapitulating human neural development.
Human cortical development is characterized by a series of highly regulated, dynamic processes that begin with the emergence of neuroepithelial stem cells and culminate in the maturation of neural circuits. Given the limitations inherent in obtaining samples of the human brain, researchers have turned to model systems to provide insight into conserved developmental processes. Crucially, these processes may also be implicated in the aetiology of neurodevelopmental disorders (NDDs). Unfortunately, investigations into the molecular and physiological causes of NDDs have proven challenging, and the translation of research findings into clinical therapeutics has largely underdelivered1. These short-comings can be attributed, in part, to the limitations of conventional preclinical models2–4. Mouse models of brain development are limited by anatomical and physiological differences that have emerged over at least 70 million years of evolutionary time separating mice and humans5–7. For example, the rodent cerebral cortex is lissencephalic, represents a disproportionally smaller percentage of total brain mass and contains approximately 1,000-old fewer neurons than the human cerebral cortex8,9. Furthermore, mouse and human cortical neurons are distinguished by changes in morphology, relative abundance, laminar distribution and gene expression patterns7. Importantly, the gene families with the greatest divergence in expression between humans and rodents include those encoding neurotransmitter receptors and ion channels that are current targets for drugs designed to clinically manage NDDs. Moreover, the prefrontal cortex, a brain region implicated in NDDs10, is significantly larger and more structurally and functionally complex in humans than in rodents11–13. Unlike mice, non-human primates competently emulate the cognitive, behavioural and social traits of humans, but they require extensive resources, and studying such primates raises important ethical discussions14. Although the non-human primate cerebral cortex is more similar to the human cerebral cortex, its use as a model of the human brain is undermined by decreased expansion, a lower diversity of cortical progenitor subtypes and decreased progenitor cell proliferation rates15–17.
While animal models permit direct probing of neurodevelopmental mechanisms in functional brain tissues over time, human studies are limited to the use of post-mortem samples, biopsy samples from surgical resection, neuroimaging and neuropharmacological treatments. Although recent efforts have demonstrated the restoration of some cellular functions after death in brain tissue from pigs18, this approach is considerably limited in its throughput. Healthy human brain biopsy samples, often obtained from individuals with epilepsy for whom pharmacotherapy was not effective, provide functional tissue that can be maintained for weeks19 and modulated with optogenetics20, but they are limited in their ability to accurately model a wide range of disorders and are restricted by their low throughput and high heterogeneity with respect to cell type, morphology and proliferation rate21,22. Functional neuroimaging offers a window into human neural physiology, and pharmacological therapeutics provide information about facets of disease pathology, yet these techniques are limited in their ability to provide mechanistic insights as they lack cellular and molecular resolution23–25. Fundamentally, all these approaches are unable to model NDDs as they are restricted to timescales outside those relevant for brain development and therefore offer a single end point for disorders with dynamic developmental trajectories26,27.
The disparity in certain species-specific developmental milestones and our limited access to functional human brain tissue at specific developmental time points has led to an emphasis on ex vivo investigations into neural development (FIG. 1). While studies that use non-human cell lines have driven significant discoveries and precipitated the generation of compelling cell culture platforms, translating these findings to human brain development will require the use of human pluripotent stem cells (human PS cells). Early efforts to recapitulate neural development in vitro relied on regulating key signalling cascades previously identified in model organisms. The neural induction of human PS cells is often mediated by the exogenous application of small molecules that modulate signalling pathways relevant to neural development28–30. Differentiating human PS cells into neural cells in a two-dimensional (2D) monolayer format has facilitated the generation of various neuronal and glial subtypes31–38, as well as the modelling of cellular processes in neural development39–43 and, by extension, the aetiology of NDDs44–49. The demonstration that somatic cells can be directly reprogrammed into neuronal cells50, and that these neuronal cells maintain hallmarks of ageing such as epigenetic DNA methylation and gene expression51,52, while instrumental to studies of neurodegenerative disorders, is less relevant to investigations into neural development, and therefore is not a focus of this Review. Monolayer cell culture systems are often characterized by less diverse cell type populations and a high degree of control over microenvironmental cues (both biochemical and biophysical). However, although 2D cell culture platforms facilitate high-throughput manipulation of individual cells, they remain limited by technical challenges in maintaining long-term cultures and their inherent inability to emulate the more complex cell–cell interactions occurring in the developing mammalian brain.
Fig. 1 |. The developmental trajectory of in vivo and in vitro model systems.
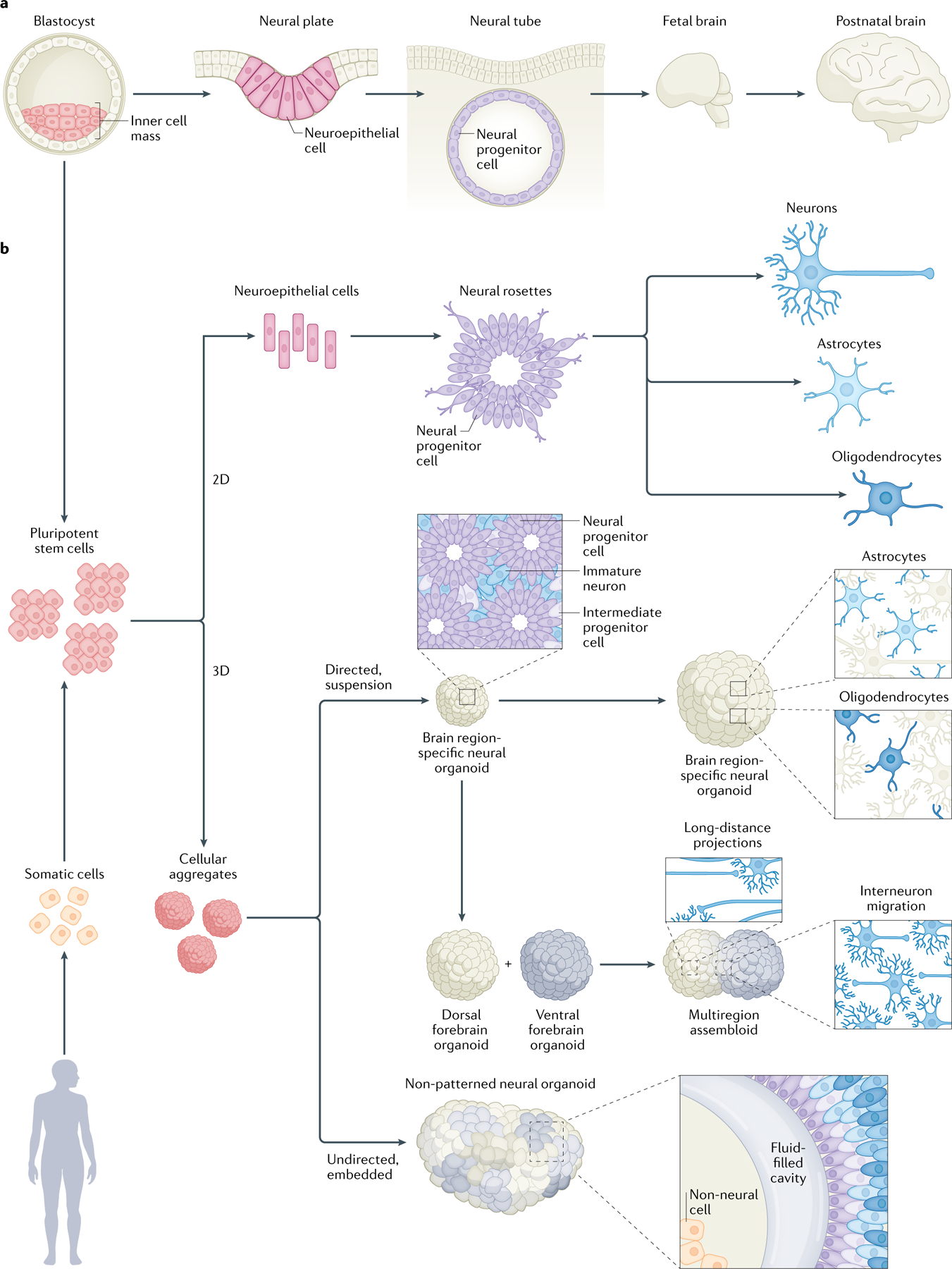
a | During postconception weeks 3 and 4, a layer of neuroepithelial cells expands, elongates and folds to form the neural tube. Following neurulation, the neural progenitor cells within the neural tube are patterned across both rostrocaudal and dorsoventral axes by morphogen gradients. The first neurons emerge at postconception week 6. Once radially migrating neurons reach their target cortical layer, their axons are guided by chemoattractive and chemorepulsive cues, including secreted molecules, cell surface molecules and the extracellular matrix. Neurogenesis and neuronal migration are followed by the generation and maturation of astrocytes and oligodendrocytes. Last, synaptogenesis, myelination and circuit refinement by synapse elimination continue late into the postnatal years. Several excellent reviews present comprehensive discussions of these processes247,335–338. b | Pluripotent stem cells can be derived from the inner cell mass of human blastocysts or by reprogramming somatic cells. The process of guiding pluripotent stem cells into neural cell fates is inspired by in vivo neurogenesis, wherein neuroepithelial cells differentiate into neural progenitors and, eventually, functional neurons and glia. To achieve this progression in vitro, two-dimensional (2D) differentiation relies on exposing a monolayer of cells to a defined concentration of small molecules that modulate signalling pathways implicated in neural cell fate acquisition. Three-dimensional (3D) approaches can generally be subdivided into directed and undirected differentiation. In directed differentiation, a series of patterning molecules are used to drive specific brain regionalization and cell fate. These brain region-specific neural organoids can be fused into assembloids, which elicit cellular migration and circuit formation. In undirected differentiation, cellular aggregates are embedded in an exogenous biomaterial and allowed to stochastically pattern. While both 2D and 3D differentiation paradigms broadly recapitulate the emergence of spatio-temporally appropriate cell types, multiple differences between in vivo and in vitro neurodevelopment remain. Of note, the emergence of radially arranged progenitor cells in neural rosettes reflects, but does not completely emulate, the neural tube.
Human pluripotent stem cells (Human PS cells).
A broad category of human stem cells that includes both embryonic stem cells and induced pluripotent stem cells. Stem cells are defined by their capacity to continuously divide into identical, undifferentiated daughter cells and to differentiate into cells from any of the three germ layers.
Three-dimensional (3D) neural cultures recapitulate many of the cytoarchitectural features of the developing brain, and therefore may be better suited to study the spatio-temporal dynamics of neural development53–55. Although 3D neural tissue constructs can trace their derivation back to dissociation–reaggregation studies pioneered in the 1960s56,57 and progenitor cell-derived neurospheres first developed in the 1990s58–61, the first human PS cell-derived neural organoids were developed in the early 2010s62. In recent years, two distinct approaches for generating human PS cell-derived neural organoids have emerged: undirected (non-patterned) and directed (patterned) differentiation. Undirected differentiation strategies, in which human PS cells are embedded in an extracellular matrix (ECM) and cultured in the absence of inductive signals, are characterized by the emergence of neuroepithelial structures that exhibit various neural fates and non-neural cell types63–67. Alternatively, directed differentiation strategies rely on small molecules and growth factors to reliably guide aggregated human PS cells towards distinct brain region-specific identities68–74 and biologically relevant physiologies, including myelination75,76. Joining brain region-specific organoids together as assembloids, or fusing these organoids with other cell types, permits the study of cell migration, such as interneuron migration from the ventral forebrain to the dorsal forebrain77–81, of interactions between neural cells and microglia82, and of circuit formation with long-range connections, as in cortico-striatal81 or cortico-motor83 assembloids. While undirected neural organoids have been used to study disease phenotypes63,84,85, the higher degree of control imparted in the derivation of directed neuronal organoids67,86 may better facilitate reproducible mechanistic studies69,71,77,87,88. Advances in both undirected and directed neural cell culture approaches have yielded organoids that emulate the cellular diversity of the human fetal cortex86, produce temporally dynamic cerebrospinal fluid-like products89, functionally integrate and vascularize within mouse brains90 and, when cultured over long periods, model forebrain chromatin dynamics91 and the transition into postnatal states92.
Organoids.
Three-dimensional clusters of organ-specific cells of multiple subtypes that self-organize and exhibit some organ-appropriate functions.
Assembloids.
Three-dimensional, self-organizing cultures derived by fusion of organoids with other organoids or cell lineages.
Although innovations in the culture of neural organoids have created in vitro models that bear increasing similarity to their in vivo counterparts, a number of limitations remain. For example, while human PS cell-derived neural organoids can exhibit neuroepithelial wrinkling93,94 and are instructive in studies of Miller–Dieker syndrome87,95, they fail to recapitulate gyrification96,97. This restriction may be due to limits in the diffusion of nutrients within cell culture media, the absence of functional vasculature or a deficiency in extracellular mechanical cues. Owing to a lack of spatio-temporal signalling cues, neural organoids do not recapitulate some cytoarchitectural features of the central nervous system, such as the formation of clearly distinguishable cortical layers97 or an ECM-rich subplate98–103. Recently, cell stress pathways have been proposed to be upregulated in brain organoids104. It remains to be seen whether these features depend on culture conditions (that is, the presence of serum or undefined ECM materials), and subsequent long-term studies with brain region-specific organoids described endoplasmic reticulum and glycolysis trajectories consistent with a homeostatic state91,92. Finally, while the electrophysiological properties of neurons mature within organoids over time68,71,77,81, the lack of sensory input precludes activity-dependent programmes of maturation in vitro. Taken together, although important aspects of in vivo biology have been recapitulated with neural organoids, these systems remain limited by their incomplete and insufficiently controlled incorporation of cellular and acellular microenvironmental cues.
Biomaterials have the potential to address current limitations in cellular models of neural development (FIG. 2). The definition of biomaterials has evolved as a function of their increasingly widespread application105; herein, biomaterials are broadly defined as any material that has been designed to purposefully interact with individual cells or cell constructs. These materials are derived by a range of synthesis strategies and have markedly differing chemical compositions and structural features. The deliberate modulation of these properties results in a breadth of biomaterials that induce morphological and physiological changes in cells. Throughout this Review, we present recent applications of biomaterials for enhancing our understanding of human neural development (TABLE 1). Specifically, we explore biomaterial-based approaches for advancing models of neural development, focusing on matrix-derived biochemical and biophysical cues, spatio-temporally controlled cell patterning and mature neuronal circuit development. Additionally, for each strategy, we provide a forward-looking perspective on innovations in biomaterials science that are poised to create in vitro models that more closely recapitulate key processes of human brain development.
Fig. 2 |. Biochemical and biophysical signalling cues within the neural microenvironment.
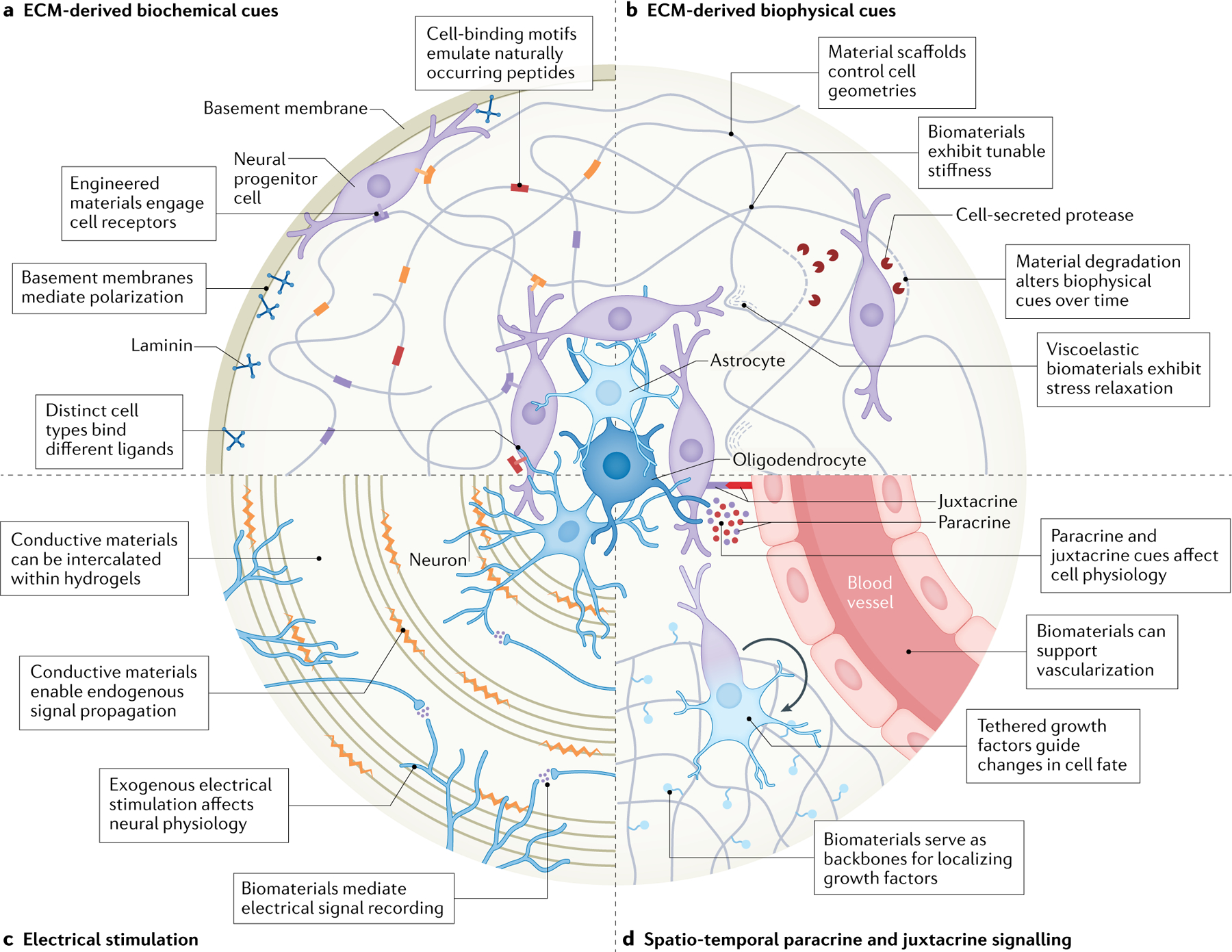
The neural microenvironment is multifaceted, spatio-temporally dynamic and, thus far, insufficiently emulated in vitro. Biomaterials have the potential to recapitulate extracellular matrix (ECM)-derived biochemical and biophysical cues, spatio-temporally conserved paracrine and juxtacrine signalling, and electrical stimulation. a | Biomaterials can be engineered to incorporate cell-interactive domains within their backbones to recapitulate the signalling cues presented by the neural ECM and basement membrane. b | Material scaffolds can be engineered to restrict cell geometry. Encapsulated cells can remodel their local niche by secreting proteases to degrade surrounding materials or by exerting strain on surrounding materials. c | Conductive polymers enable electrical signal propagation, which both promotes neurite outgrowth and enhances functional maturation. d | Various cell type-specific growth factors can be tethered to biomaterials to provide spatio-temporal control over cell fate and morphology. The capacity to pattern the local environment with biomaterials, and techniques that use biomaterials such as three-dimensional bioprinting, may facilitate co-cultures with neural and non-neural cells that induce both paracrine and juxtacrine signalling.
Table 1 |.
Biomaterials used in neural cell culture
| Biomaterial | Material modifications and manipulations | Cell type | Outcome | Refs |
|---|---|---|---|---|
| Decellularized tissues | ||||
| Decellularized brain tissue | Addition of collagen I | Human iNSCs | Enhances NPC differentiation and maturation | 166 |
| Matrigel | Microfabricated compartment | Human ES cells | Drives neural organoid folding through cytoskeletal contraction | 94 |
| – | Human ES cells | Supports the growth of cerebral organoids | 63,152,153 | |
| Microfluidic gradient generation | Human ES cells | Directs rostrocaudal organization during neural differentiation | 43 | |
| – | Human iPS cells | Supports the growth of forebrain organoids | 71 | |
| – | Mouse ES cells | Affects neural differentiation | 159 | |
| 3D bioprinting into alginate scaffold | Human iPS cell-derived NPCs, mouse iPS cell-derived OPCs | Supports a 3D spinal cord tissue-like platform | 266 | |
| Natural biopolymers | ||||
| Agarose | Photopatterned with SHH and CNTF | Mouse NPCs | Directs migration of NPCs towards SHH | 264 |
| Alginate | 3D bioprinting; addition of chitosan and agarose | Human iPS cells | Supports differentiation into neurons and astrocytes | 270 |
| RGD peptide; reduced alginate MW | Human iPS cell-derived NPCs | Reveals NPC sensitivity to differences in stress relaxation | 209 | |
| IKVAV and FGF2 peptide–DNA | Mouse NSCs | Induces transient, controllable NSC migration | 171 | |
| – | Mouse NSCs | Enhances expression of neuronal markers in soft hydrogels | 199 | |
| Addition of hyaluronic acid | Mouse ES cells | Enhances neuronal differentiation | 164 | |
| Photopatterned with NGF | DRGs | Guides neurite outgrowth by patterning NGF | 265 | |
| Collagen | Microfluidic device; co-culture with MSC-derived cells | Human NSCs | Increases neuronal expression compared with glial expression | 255 |
| Microfluidic device; co-culture with human iPS cell-derived ECs | Human ES cell-derived NSCs | Supports the formation of vascular networks within neural spheroids | 289 | |
| Fibrinogen | 3D bioprinting; addition of alginate, genipin and guggulsterone PCL microspheres | Human iPS cell-derived NPCs | Increases expression of neural, astrocytic and oligodendrocytic markers | 271 |
| Hyaluronic acid | Addition of chitosan | Human iPS cells | Promotes neural differentiation in the absence of neural induction medium | 165 |
| RGD, YIGSR and IKVAV peptides | Human iPS cell-derived NPCs | Enhances neuronal differentiation through combination of peptides | 169 | |
| Density gradient multilayer polymerization | Human iPS cell-derived NPCs | Accelerates differentiation and permits neuronal migration | 167 | |
| – | Mouse NPCs | Influences differentiation in a stiffness-dependent manner | 186 | |
| Laminin | – | Human ES cells | Increases NPC expansion, differentiation and neurite outgrowth | 158 |
| Micropatterned substrates | Human ES cells | Mediates ectodermal border formation | 221 | |
| Addition of entactin | Mouse ES cells | Stabilizes the formation of polarized telencephalic neuroepithelium | 163 | |
| – | Mouse NSCs | Reduces tensile strain-mediated oligodendrocyte differentiation | 236 | |
| Vitronectin | Micropatterned substrates | Human ES cells | Guides neuroepithelial and neural plate patterning | 220 |
| Protein-engineered materials | ||||
| Elastin-like protein | RGD and MMP-sensitive peptides | Mouse NPCs | Maintains NPC stemness and enhances differentiation capacity | 211,212 |
| RGD peptide; microfluidic gradient generation | DRGs | Enhances neurite extension or neurite guidance | 256 | |
| Synthetic polymers | ||||
| Carbon nanotubes | Single walled; electrical stimulation; PLGA scaffold | Human iPS cell-derived NSCs | Enhances NSC differentiation via a depolarizing direct current | 311 |
| Multiwalled, multilayered | Mouse NSCs | Facilitates neuronal differentiation and synapse formation | 312 | |
| Graphene | Rolled graphene oxide foam; electrical stimulation | Human NSCs | Directs growth of neural fibres | 318 |
| Electrical stimulation | Mouse NSCs | Yields higher neuron density and more spontaneous synaptic activity | 319 | |
| PCL | Microspheres containing guggulsterone | Human iPS cells | Increases expression of TUJ1 and OLIG2 in human iPS cell aggregates | 252 |
| PEDOT | PEDOT–PSS; granular | Human NPCs | Maintains viability | 328 |
| PEDOT–PSS; electrical stimulation | Human NPCs | Induces more neurons with longer neurites | 322 | |
| Chitosan/gelatin scaffold | Mouse NSCs | Improves adhesion and increases proliferation | 326,327 | |
| Polyethylene glycol | Micropatterned substrates; Matrigel adsorption | Human ES cell-derived NECs | Controls neural organoid polarization | 222 |
| Integrin-binding and MMP-degradable peptides | Human astrocytes | Maintains astrocyte quiescence | 172 | |
| MMP-sensitive peptide; addition of ECM components | Mouse ES cells | Affects cytoskeleton-mediated symmetry breaking and DV patterning | 149 | |
| PLGA | Fibres; Matrigel embedding | Human ES cells, human iPS cells | Guides 3D differentiation of neural organoids | 225 |
| Microspheres containing FGF2 | Human ES cells, mouse NSCs | Maintains cells in less differentiated state through controlled release | 251 | |
| Polyacrylamide | GAG-binding peptide | Human ES cells | Enhances neuronal differentiation on compliant substrates | 195 |
| Oligonucleotide-based crosslinking | Mouse NSCs | Reveals temporal sensitivity in directing neuronal differentiation | 196 | |
| Functionalized with laminin | Mouse NSCs | Promotes neuronal differentiation on soft surfaces | 201 | |
| Polypyrrole | Doped with DBS; electrical stimulation | Human NSCs | Promotes induction of neurons over glial cells | 323 |
| Doped with DBS; electrical stimulation; encapsulation in alginate | Human iPS cell-derived NPCs | Upregulates expression of neurotrophic growth factors | 325 | |
3D, three-dimensional; CNTF, ciliary neurotrophic factor; DBS, dodecylbenzenesulfonate; DRG, dorsal root ganglion; DV, dorsoventral; EC, endothelial cell; ECM, extracellular matrix; ES cell, embryonic stem cell; FGF2, fibroblast growth factor 2; GAG, glycosaminoglycan; IKVAV, isoleucine–lysine–valine–alanine–valine; iNSC, induced neural stem cell; iPS cells, induced pluripotent stem cells; MMP, matrix metallopeptidase; MSC, mesenchymal stem cell; MW, molecular weight; NEC, neuroepithelial cell; NGF, nerve growth factor; NPC, neural progenitor cell; NSC, neural stem cell; OPC, oligodendrocyte progenitor cell; PCL, polycaprolactone; PEDOT, poly(3,4-ethylenedioxythiophene); PLGA, poly(lactide-co-glycolide); PSS, polystyrene sulfonate; RGD, arginine–glycine–aspartate; SHH, sonic hedgehog; YIGSR, tyrosine–isoleucine–glycine–serine–arginine.
ECM-derived biochemical signalling
The neural ECM influences cellular proliferation, differentiation, migration and maturation throughout brain development106–108. Unlike the ECMs of other tissues, the neural ECM consists primarily of hyaluronic acid (HA), chondroitin sulfate proteoglycans (CSPGs) and heparan sulfate proteoglycans, link proteins, tenascins, laminins and reelin106. As it contains a relatively low percentage of fibrous proteins, including collagen and fibronectin, the neural ECM resembles a lattice of amorphous aggregates109 (FIG. 3a). Importantly, the neural ECM exhibits spatio-temporally dynamic prevalence and composition. For example, CSPGs and tenascin R are differentially distributed across spatially distinct brain regions110, and, as a function of time, CSPGs transition from larger to smaller variants, overall HA content decreases and tenascin C is replaced with tenascin R106,111,112. These transitions in ECM composition affect the structure of the matrix itself and coincide with the shift from a rapidly expanding tissue to one wherein growth has ceased and synapses are reinforced109.
Fig. 3 |. Engineered matrices to recreate ecM-derived signalling cues.
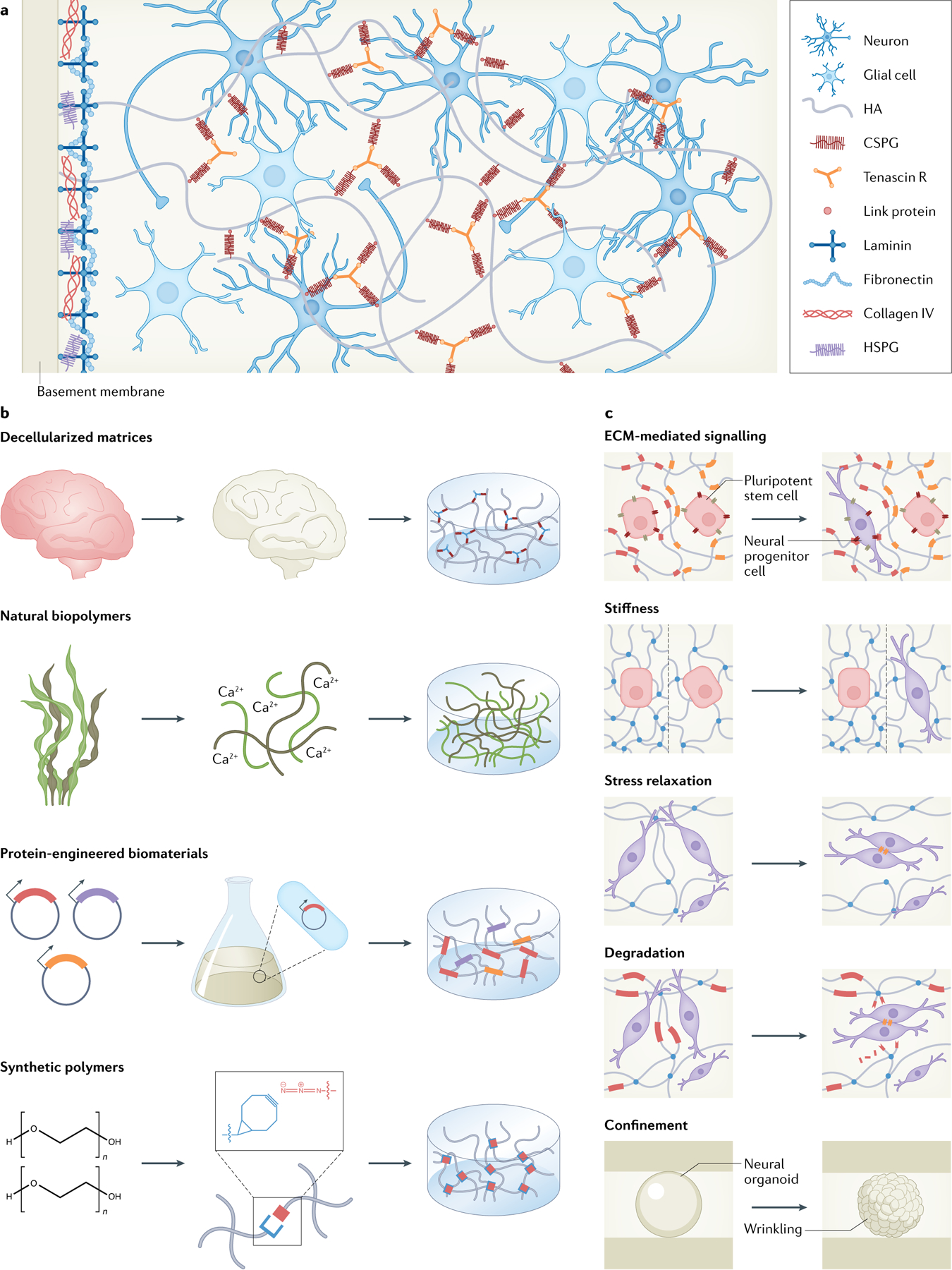
a | The native extracellular matrix (ECM) in the brain presents a complex microenvironment composed primarily of hyaluronic acid (HA), proteoglycans, tenascins, link proteins, laminins, fibronectins and collagens106. Neural ECM is unique insofar as it contains relatively low levels of fibrous proteins (collagen and fibronectin) and high levels of glycosaminoglycans, including chondroitin sulfate proteoglycans (CSPGs). The pericellular neural ECM is described as a supramolecular assembly of HA–link protein–CSPG–tenascin. b | Various materials have been used for modelling the native brain microenvironment, including decellularized brain matrices, natural biopolymers, protein-engineered biomaterials and synthetic polymers. c | Biomaterial strategies designed to manipulate the local cellular environment include controlling either biochemical or biophysical signalling cues presented to cells. The presentation of certain cell-adhesive ligands can increase neural marker expression164. Modulating material stiffness can drive neural differentiation195. Both stress relaxation and degradability have been shown to have a role in maintaining neural progenitor cell stemness maintenance through increased cell–cell contact211. Matrix confinement can drive wrinkling in neural organoids94. HSPG, heparan sulfate proteoglycan.
With regard to the effect of the ECM on neural cell proliferation and differentiation, the higher expression of HA has been associated with decreased neural progenitor cell (NPC)113 and astrocyte proliferation114,115. Moreover, enzymatic disruption of CSPGs, and a concomitant decrease in the abundance of cell-surface CSPGs, reduces NPC proliferation and leads to more NPCs adopting a glial cell fate116, while the addition of exogenous CSPGs stimulates the proliferation of NPCs117. Tenascin C and laminin increase the proliferation rates of NPCs through upregulation of the epidermal growth factor and fibroblast growth factor (FGF) signalling pathways, respectively118–122, while tenascin R decreases proliferation and biases NPCs towards glial cell fates123. Tenascin C also inhibits oligodendrocyte differentiation124–126, and laminin promotes neural differentiation by upregulating the integrin–WNT7A–decorin pathway127.
Neural progenitor cell (NPC).
A neural stem cell with limited capacity for self-renewal.
The effect of the extracellular environment on the migration and outgrowth of brain cells was documented as early as 1928, when Santiago Ramón y Cajal observed a “strange propensity of the nerve sprouts to adhere to supports or pre-established pathways”128. More recent studies have demonstrated that a reduction in the level of either HA or laminin results in a decrease in neuronal projection length129,130, whereas reductions in CSPG levels promote axonal growth131–135. Higher levels of HA mediate increased neural migration112,136 through a receptor for a hyaluronan-mediated motility-mediated signalling cascade137, and tenascin R initiates the tangential migration of forebrain neuroblasts in an activity-dependent manner138. Laminin disruption has resulted in both the disruption of interkinetic nuclear migration139 and the migration of neurons to more superficial cortical layers130.
Finally, the neural ECM has been implicated in regulating the later stages of neural development, namely axon myelination, synaptogenesis and neural circuit formation. Disruptive mutations in genes coding for laminin or laminin receptors result in hypomyelination140–142, whereas both tenascin C and tenascin R inhibit the extension of myelin sheets by oligodendrocytes126,143. Late in prenatal development, synapses are surrounded by an ECM mesh composed of HA, link proteins, CSPGs and tenascin R that is referred to as the ‘perineuronal net’ and has been implicated in stabilizing neural circuits, while limiting the formation of new synapses144–147. This loss of neural plasticity has been reversed via the administration of chondroitinase, implying that the enzymatic degradation of circuit-stabilizing molecules can promote synaptogenesis and functional maturation132,133,148.
Although the neural ECM shapes key developmental processes, 2D and 3D culture approaches rely mostly on heterogenous, insufficiently manipulatable ECM-inspired materials (such as Matrigel) or are cultured as free-floating cell aggregates devoid of exogenous ECM149–151. Therefore, the introduction of biomaterials that better emulate the composition or downstream signalling effects of the endogenous neural ECM may yield cell culture models with more biomimetic neural proliferation, differentiation, migration and maturation (FIG. 3b).
Natural materials.
To date, only two naturally derived biomaterials, Matrigel and purified laminin, have routinely been integrated into human PS cell models of neural development. Matrigel has provided a support scaffold for neural cell growth in several seminal studies of 3D human stem cell-derived models of neural development63,71,152,153. As a murine sarcoma-derived reconstituted basement membrane, Matrigel lacks many neural ECM components that influence brain development, including several glycoproteins and proteoglycans154–156. Moreover, Matrigel contains growth factors that are known to affect neural cell physiology, including transforming growth factor-β, epidermal growth factor, FGF2, platelet-derived growth factor and insulin-like growth factor 1 (REF.157). Importantly, both Matrigel and growth factor-reduced Matrigel have been shown to affect neural differentiation by increasing both neurite length158,159 and the number of dopaminergic neurons159 compared with conventional ECM molecules. Matrigel is commonly used because of its simplicity (that is, its propensity to crosslink at temperatures above 10 °C) and commercial availability, but given its heterogeneity, poor emulation of native neural ECM and capacity to bias differentiation, other biomaterials should be considered for in vitro models of neural development. Unlike sarcoma-derived matrices, protein-based or polysaccharide-based biomaterials have the potential to introduce cell-interactive domains and cell-mediated degradation in a chemically defined, modifiable matrix.
A collection of studies in the early 1980s demonstrated a role for laminin in driving neurite outgrowth160–162, and it has since remained a standard component of 2D neural culture paradigms. More recently, a study comparing various coating materials demonstrated that purified laminin drove the greatest increase in the number of nestin-expressing and TUJ1-expressing (TUJ+) human embryonic stem cell (ES cell)-derived NPCs and immature neurons, respectively158. In 3D serum-free floating cultures of mouse ES cell-derived cortical neuroepithelium, the addition of laminin with entactin induced the formation of a polarized neuroepithelium with a continuous peripheral basement membrane163. Moreover, exogenous laminin prevented the formation of neural rosettes, and the continuous layer of FOXG1+PAX6+EMX1+ telencephalic NPCs gave rise, over time, to mature cell types, including TBR2+ intermediate progenitors, early TBR1+CTIP2+ cortical neurons and late BRN2+CUX1+ cortical neurons. Subsequent 3D neural organoid differentiation protocols, which omit the addition of exogenous ECM components, have documented the emergence of similar cell diversity and spatial arrangement68. However, given that the cortical basement membrane persists at the pial surface throughout neural development, it follows that the mechanism underlying laminin-induced neural polarization and basement membrane integrity is worthy of further exploration.
In an effort to emulate the composition of the neural ECM in vitro, recent studies have applied natural biomaterials to influence neural development164–166. For example, HA hydrogel beads were created by adding HA to an alginate solution that was mixed with mouse ES cells and dropped into calcium chloride164. Compared with fibronectin, the addition of HA drove an increase in both neuronal and glial marker expression, while also biasing neurons towards a glutamatergic subtype. A subsequent study used an HA-based hydrogel, created by resuspending HA and chitosan in a cell-containing dextran–sodium chloride solution, to demonstrate that human induced PS cells (iPS cells) can be differentiated into structures expressing NPC genes after 14 days in the absence of neural induction medium165. Finally, the differentiation of human PS cell-derived NPCs was accelerated within a methacrylate-modified HA167. The density gradient created within this material, achieved by mixing small-molecule density modifiers with the prepolymer solution, mediated the identification of an NDD-associated gene mutation on neuronal migration. By relying primarily on resuspending and mixing commercially available materials, these studies demonstrate that the incorporation of natural biomaterials into culture systems is generally quite feasible. Although decellularized neural ECM may present a higher degree of experimental complexity, the recellularization of decellularized ECM offers several compelling advantages over single-component biomaterials168. Inspired by the prospect of creating a natural biomaterial that recapitulates the temporal dynamics of the neural ECM, laminin-coated silk scaffolds were supplemented with a mixture of type I collagen and decellularized ECM from either a fetal pig or an adult pig166. Culturing human neural stem cells (NSCs) induced from neonatal foreskin fibroblasts within these materials for up to 7 months revealed differences in the abundance of TUJ1+ neuronal projections relative to GFAP+ astrocytes. Taken together, the results of these preliminary studies indicate the potential of influencing neural development with readily available mimics of the endogenous neural ECM.
Natural biomaterials.
Biomaterials derived from natural sources, including proteins, polysaccharides and decellularized tissue matrices.
Neural stem cells (NSCs).
Multipotent cells that maintain the capacity to undergo limitless self-renewing cell divisions and create progeny of restricted lineages that differentiate into mature neural and glial cells.
Designer materials.
Unlike naturally derived materials, synthetic materials are capable of being tailored to accommodate a range of biochemical and biomechanical properties. The high degree of experimenter-driven customization in these materials has enabled tightly controlled experiments that characterize the role of distinct ECM-derived developmental cues. By leveraging statistical modelling, a recent study characterized the effect of ECM-inspired peptide sequences on the survival and differentiation of human iPS cell-derived NPCs within HA hydrogels169. Initial screens of 16 distinct hydrogel formulations with differing concentrations of known adhesive ligands (RGD, YIGSR and IKVAV peptide ligands) revealed that YIGSR promoted a high level of cell survival. Following two additional iterations, to first optimize the RGD concentration and then the IKVAV concentration, the study authors obtained a gel with distinct adhesive ligand concentrations, which increased cell spreading, neurite extension and the expression of SOX2 and DCX, markers of NPCs and immature neurons, respectively. Although this HA hydrogel could be tuned to different stiffnesses by varying the polymer weight percentage, the study authors limited their gels to a single HA concentration. To address a wider array of developmental cues, an automated liquid-dispensing robot was developed to create polyethylene glycol (PEG) hydrogels with unique combinations of stiffness (0.5–8 kPa), degradability and ECM matrix components (laminin, entactin, collagen, fibronectin and perlecan)149,170. This high-throughput system facilitated the observations that intermediate stiffnesses and laminin promote mouse ES cell neural differentiation (as determined by increased SOX1 expression) and polarity (as determined by a higher percentage of actomyosin contractile rings). Additionally, when compared with encapsulating cells within Matrigel, a differentiation-optimized PEG hydrogel resulted in neuroepithelial colonies with more homogeneous spherical shapes, increased polarity and a constrained colony area over time. These studies demonstrate the potential for designer matrices to serve as platforms for high-throughput investigations into the contributions of distinct components of the neural ECM in neural development.
Synthetic matrices have also been used to probe the effects of the temporally dynamic presentation of ECM-derived cues to NSCs. For example, a strategy based on the reversible interaction between complementary DNA tethers was used to evaluate neonatal murine spinal cord-derived NSC migration and proliferation in response to IKVAV and FGF2 (REF.171). Interestingly, binding of IKVAV-linked ‘bioactive’ DNA to the ‘surface’ DNA strands, which were themselves bound to an alginate-coated glass surface, induced migration of NSCs away from a neurosphere, while the addition of a fully complementary ‘displacement’ DNA strand not linked to IKVAV resulted in a retraction of NSCs. This retraction was associated with a reduction in the β1 integrin signal and increased laminin production. Importantly, the addition of an FGF2 ‘bioactive’ strand increased proliferation, while the return of the IKVAV ‘bioactive’ strand prompted repeated neural migration. Overall, these studies illustrate the unique potential for tunable synthetic biomaterials to support high-throughput, temporally dynamic investigations into the influence of the ECM on neural development.
Synthetic biomaterials.
Biomaterials derived from synthetic sources, including metals, ceramics, synthetic polymers and composites.
Outlook.
Natural and synthetic biomaterials have the capacity to emulate neural ECM-derived biochemical cues and drive neural proliferation, migration, differentiation and maturation. Each material class harbours compelling advantages and, as is the case for most systems, important shortcomings. While natural materials such as collagen or Matrigel contain a multitude of cell-interactive domains and are inherently biodegradable, they are limited by batch-to-batch variability and a low degree of tunability. Conversely, synthetic materials such as PEG or poly(lactide-co-glycolide) are highly tunable and well defined yet lack tissue-specific structure and biochemical cues barring further modification. Without these modifications (such as the inclusion of cell-adhesive peptide ligands and sites for protease-mediated degradation172), synthetic biomaterials are typically incapable of supporting essential cellular physiologies149,169. Concerted research efforts in biomaterials science have focused on the identification and manipulation of a minimal set of ECM-mimetic biochemical and biophysical signalling cues. In addition to probing cell–ECM interaction pathways, these studies contribute to an overarching goal of enabling reproducible synthetic materials to emulate the microenvironment of natural biomaterials173,174.
Looking forward, while several studies have characterized ECM deposition by neural cells in vitro149,163,171, the contributions of cell-secreted ECM in an ECM-mimetic biomaterial are poorly understood. To expand on this characterization, future studies could characterize the spatio-temporal dynamics of nascent ECM protein secretion via incorporated methionine analogues and bio-orthogonal fluorophore labelling175. Importantly, this technique is applicable to non-genetically modified cells as the methionine analogue (azidohomoalanine) is simply added to the culture medium. Coupling such an assay with hydrogel–tissue chemistry-based tissue decellularization and immunofluorescent labelling of neural and glial cells could reveal the contributions of distinct cells to the emergence of neural ECM (including neural basement membrane) in vitro (box 1). To further characterize the contributions of individual cells to the neural ECM and expand on work that evaluated the effects of naturally derived biomaterials on the recapitulation of human brain gene expression patterns176, single-cell RNA sequencing could be synergized with high-throughput matrix composition screens. Additionally, following recent evidence that the controlled presentation of ECM to intestinal spheroids directed tissue-wide polarity177, future studies with neural organoids could explore the consequences of adding and removing ECM-mimetic biomaterials for the formation of polarized organizing centres and cortical layers. In keeping with the desire to achieve high-throughput, reproducible characterization, a microengineered multiwell plate and automated cell culture strategy could be used to investigate ECM-derived signalling in neural development178. Finally, protein engineering is a thus far underexplored third class of biomaterial synthesis strategy. Recombinant protein-based biomaterials can be designed to contain a variety of ECM-inspired peptides, and, as such, allow modular control over the biochemical and biomechanical properties of the material microenvironment179,180. Incorporating these chemically defined, tunable protein-engineered biomaterials into studies of neurodevelopment would facilitate mechanistic studies of cell–matrix interactions. While several of these suggestions require high technical proficiency, simply transitioning to a defined culture material will both improve reproducibility and mediate more bottom-up investigations into ECM-derived biochemical differentiation cues.
Box 1 |. Biomaterial-based platforms for characterizing neural development.
In addition to their utility as a medium for modulating the processes that underlie neural development, biomaterials can serve as platforms for characterizing neural development. this potential is reinforced by two recent demonstrations of hydrogel–tissue chemistry. First, by crosslinking acrylamide and bisacrylamide to molecules with available amines within tissue, the CLARITY (clear lipid-exchanged acrylamide-hybridized rigid imaging/immunostaining/in situ hybridization-compatible tissue–hydrogel) tissue clearing technique significantly limits the degree of protein loss339. the thermally initiated hydrogel mesh effectively replaces lipid bilayers with a synthetic biomaterial and serves as a support structure for molecular and cellular information. a collection of hydrogel–tissue hybridization strategies has emerged in the years since CLARITY was introduced, including PACT340, PARS340, ePACT341, ACT-PRESTO342, MAP343, ExM344 and SWITCH345. Second, hydrogel–tissue chemistry was leveraged to achieve in situ three-dimensional (3D) single-cell RNA sequencing through STARmap (spatially resolved transcript amplicon readout mapping)346. By functionalizing an in situ constructed cDNA amplicon with an acrylic acid N-hydroxysuccinimide moiety and copolymerizing it with acrylamide monomers, a hydrogel–amplicon network is created. this network stabilizes the amplicons in 3D space throughout subsequent protein and lipid removal, ensuring that the 3D spatial information of each amplicon is retained. a similar approach for achieving spatially resolved single-cell RNA sequencing was later reported which incorporates the ExM hybridization strategy347. Looking forward, we anticipate there being great value in synergizing techniques that characterize the 3D proteomic and transcriptomic landscapes with techniques to manipulate or model neurodevelopmental processes.
Decellularization.
The process of isolating tissue-specific extracellular matrix by removing cell content.
Protein engineering.
The process of modifying existing protein sequences through substitution, insertion or deletion of nucleotides in an encoding gene.
ECM-derived biophysical signalling
Cells are exquisitely sensitive to mechanical inputs from their surrounding microenvironment, changing shape and cytoskeletal organization in response to matrix tension and relaxation. Stiffness, stress relaxation, degradability and confinement affect neural development in 2D and 3D cell culture microenvironments181 (FIG. 3c). Cells detect and respond to changes in their microenvironment through transmembrane receptors, such as cadherins and integrins, which transduce mechanical stimuli to biochemical responses182,183. Biomaterials with tunable biophysical matrix properties are capable of presenting these mechanical stimuli to cells and have been implicated in a number of pertinent developmental processes, including neural compartmentalization184, differentiation185,186, migration187, proliferation188, axon growth189 and synaptic plasticity190.
Stiffness.
Brain tissue is substantially softer than several other tissues of the human body. Depending on the developmental stage, brain region and method of measurement, an elastic modulus (E) ranging from several hundred pascals to a few thousand pascals has been reported184,191,192. It follows that culturing NSCs on tissue culture plastic (2–4 GPa), approximately seven orders of magnitude stiffer than native brain tissue, could affect cell behaviour in vitro193,194. To address this inconsistency, biomaterials can be designed with a range of physiologically relevant stiffnesses. For example, the stiffness of polyacrylamide hydrogels can be easily tuned by changing their chemical composition and crosslinking density. Tuning matrix stiffness alone has been shown to drive differentiation of monolayer human ES cells into physiologically active neurons in the absence of soluble inductive factors195. Notably, the hydrogels that were most effective in inducing neural differentiation had stiffnesses similar to the stiffness of native brain tissue (E ~ 700 Pa). While static stiffness can influence neural differentiation, a recent study demonstrated that adult rat hippocampal NSCs possess a mechanical memory wherein transient exposure to specific substrate stiffnesses during a defined time window determines their lineage commitment196. While this study also used a polyacrylamide gel, the authors incorporated an additional oligonucleotide-based mechanism to allow dynamic and reversible stiffness modulation without gel swelling and contraction. Interestingly, a pulse of soft substrate (E ~ 300 Pa) delivered from 12 to 36 h after initial differentiation is both necessary and sufficient to direct neuronal differentiation on stiff matrices (E ~ 3 kPa). Conversely, a pulse of stiffness on otherwise soft matrices in the same time window suppressed neuronal differentiation. Mechanistically, stiffness-sensitive neural differentiation is associated with the transcriptional co-activator Yes-associated protein (YAP), either through the inhibition of YAP nuclear localization195 or through YAP–β-catenin interactions196. YAP signalling has been identified to play a role in translating changes in cytoskeletal tension into the expression of ECM-remodelling genes via mechanical activation by Rho GTPases197,198. Although the full repertoire of signalling regulators and pathways remains under investigation, these studies, along with a collection of studies using mouse NSCs199–201, highlight the importance of matrix stiffness in promoting neurogenesis.
Stress relaxation.
Native brain tissue is viscoelastic, exhibiting a time-dependent response to applied forces202,203. Specifically, viscoelastic materials such as brain tissue are often stress relaxing, such that when a force is applied, the material responds to dissipate that force192,204. Throughout neural development, these mechanical forces influence key processes, including neurogenesis, cell migration, compartmentalization and synapse formation182,183. Natural biomaterials, including collagen and fibrin, exhibit stress relaxation; however, the ability to independently tune their stress relaxation rates without altering other properties (that is, stiffness, degradability and adhesive ligand density) poses a significant challenge205. Recently, elegant methods have been developed to overcome this challenge. In alginate hydrogels, for example, the rate of stress relaxation can be independently tuned by altering the chemical composition of the gel and the molecular weight of alginate206. This platform has been used to study the effect of stress relaxation on cells encapsulated in 3D materials, predominantly using cells described as human mesenchymal stem cells (MSCs). For MSCs, fast-relaxing hydrogels promote cell spreading and drive osteogenic differentiation206. While stress relaxation is an important mechanical property of the developing brain with implications in ageing207 and disease pathophysiology208, minimal research has been done to understand its impact in neural development. A study comparing the effects of stiffness, stress relaxation and adhesive ligand density in ionically crosslinked alginate hydrogels showed that differences in stress relaxation drive the greatest differential gene expression in human PS cell-derived NPCs209. Notably, stress relaxation induces the differential expression of genes implicated in cytoskeletal remodelling and oligodendrocyte differentiation, suggesting that the shape and fate of NPCs may be affected by the viscoelastic properties of the matrix. Given the impact of stress relaxation on MSC behaviour, continued mechanistic study of matrix stress relaxation within in vitro models of neural development could similarly provide insight into the effects of brain viscoelasticity on NPC morphology and differentiation.
Matrix degradation.
In addition to cell-induced strain, matrix remodelling can also occur through cell-mediated degradation. This degradation is triggered by the secretion of proteases that degrade the surrounding ECM and, in doing so, provide space for cell proliferation and ECM deposition. While cells can readily expand in sub-confluent 2D cultures, matrix remodelling is required for cells in 3D cultures to spread or migrate210. To facilitate matrix degradation in 3D cultures, protein-engineered biomaterials, such as elastin-like protein hydrogels, have been designed with proteolytically cleavable sequences. In such a system, the matrix preserved the self-renewing potential of adult mouse hippocampal NPCs by increasing cadherin-mediated cell–cell contact211. Furthermore, matrix degradation was shown to enhance the differentiation capacity of mouse NPCs encapsulated in protein-engineered 3D hydrogels as a function of remodelling time212. Even in highly degradable matrices, sufficient remodelling must occur via matrix degradation for mouse NPCs to differentiate into neurons and astrocytes. These results suggest that matrix remodelling may serve to prime the NPCs for differentiation, whereas other biochemical and mechanical cues, such as adhesive ligand density149,169 and stiffness193,196, respectively, may bias the fate of NPC differentiation.
Matrix confinement.
Investigations into the effects of micropatterned cell culture substrates and scaffolds are inspired, in part, by studies which demonstrated that ES cell renewal capacity and differentiation trajectory are influenced by colony geometry213–215. Several subsequent studies revealed that geometric confinement is sufficient to induce reproducible self-organization of human ES cells into all three germ layers following differentiation with BMP4 (REFS216,217). During embryonic development, the process of blastula folding and germ layer differentiation, known as gastrulation, is followed by neurulation in which the neural plate invaginates to form the neural tube. In 1874, Wilhelm His Sr proposed that neurulation is driven by mechanical forces218. In the years that followed, the role of biophysical signalling cues in neural induction, as described previously, became widely accepted219. These signalling cues emerge within a spatially confined microenvironment; thus, recapitulating that confinement in a controlled, reproducible manner may provide unique insights into neurodevelopmental mechanisms. For example, a recent study in which human ES cells were cultured on top of vitronectin-coated circular polydimethylsiloxane islands revealed that cell shape and cytoskeletal contractile force guide neuroepithelial border patterning220. A subsequent study explored the spatio-temporal dynamics of early ectodermal patterning in human ES cells, and observed that neural, neural crest, placodal and epidermal progenitors can reliably be derived on micropatterned surfaces in a geometry that emulates the medial–lateral axis in vivo221.
While the two previously described studies used micropatterned surfaces to study the effects of geometric confinement on neural induction in two dimensons, a similar micropatterned surface was recently used to influence the development of 3D neural constructs222. Seeding human PS cell-derived neuroepithelial cells (NECs) on micropatterned PEG substrates223,224 demonstrated that the initial size and shape of the 2D NECs influence the emergence of singular neural rosettes. Once released from their geometric confinement, NECs exhibited radial outgrowth, while maintaining a singular neuroepithelium within a 3D hemispherical structure, implying that neural organoids with reproducible, singular polarized neuroepithelial regions can be fabricated with micropatterned substrates. A subsequent study cultured human PS cells with poly(lactide-co-glycolide) fibre microfilaments in an effort to physically guide the 3D differentiation of neural organoids in suspension culture from the inside225. The microfilament-engineered cerebral organoids were elongated, contained lower proportions of non-ectodermal cell types and upregulated their expression of forebrain markers such as FOXG1 and EMX1. Controlling human PS cell colony geometry, in both two dimensions and three dimensions, with biomaterials therefore permits reproducible investigations into the effects of colony size, shape and surface area on cell fate acquisition, border formation and polarity.
Neuroepithelial cells (NeCs).
early neural stem cells emerging from neuroectoderm that ultimately give rise to radial glia and other cells in the early developing central nervous system.
Due to their biomimetic cytoarchitecture and cell density, 3D organoid models may be an ideal platform for characterizing biomechanically driven neurodevelopmental processes. Neural organoids have been cultured within a Matrigel scaffold on a microfabricated organ-on-a-chip (OoC) system to physically confine cellular expansion94. Wrinkling of the neural organoid was observed at a critical nuclear density that was achieved by physical confinement. However, treatment with an inhibitor of myosin contractility resulted in loss of surface wrinkling, revealing that the folding may be the result of a combination of core cytoskeletal contractions and cellular expansion at the periphery. While the study authors reported similar results upon reducing the hydrogel density, their OoC model could be leveraged further to understand how manipulating other mechanical cues of the gel (that is, stiffness, stress relaxation or degradability) could alter cytoskeletal contraction and cortical folding.
Organ-on-a-chip (OoC).
A class of microphysiological systems wherein specialized cells are cultured within microfluidic chips.
Outlook.
The previously described studies highlight the importance of mechanical cues in directing neural cell behaviour. In most of these studies, the mechanical properties of the matrix remain constant over time. However, native brain tissue has spatio-temporally dynamic mechanical properties. Atomic force microscopy reveals that the ventricular zone and subventricular zone gradually stiffen over the course of development, and stiffness has been shown to vary across cortical layers226. It follows that biomaterials with dynamic mechanical properties could be leveraged to recapitulate the gradual stiffening of the neural microenvironment. For example, methacrylated HA hydrogels can be gradually stiffened from 150 to 3,000 Pa via photo-activated crosslinking, which activates inert chemical groups upon exposure to UV light227. Enzyme-mediated stiffening has also been demonstrated using PEG–norbornene hydrogels with tyrosinase-mediated stiffening228. These approaches may facilitate characterization of the effects of temporally dependent changes in matrix stiffness on neural development. Spatial control over biophysical properties of biomaterials may be mediated by advanced manufacturing processes, including 3D printing229, gradient photomasks230,231 or temperature fluctuations232. Although creating biomaterials with spatio-temporally tunable mechanical properties may be achievable, consideration must be given to the distinct responses of different neural and glial cell subtypes to the said properties.
Current neuronal cell culture paradigms rarely characterize the effects of strain and shear forces on neural development. Tensile strain, for example, affects radial glia as they retain their apical–basal contacts in the thickening cortex233. Mechanical tension on individual neural cells in two dimensions has been shown to stimulate neurite elongation234,235 and decrease oligodendrocyte differentiation in an integrin α6-dependent manner236; however, the influence of tensile strain on in vitro models of the developing human cortex has not yet been investigated. The flow of cerebrospinal fluid across the ventricles during brain development guides ependymal cell orientation and directs neuroblast migration237,238. Additionally, shear forces induced by blood flow in the neurovascular niche affect metabolic coupling between neural and endothelial cells239 and barrier permeability42. OoC systems permit the exposure of cells to tunable flow as well as ECM-derived signalling cues, and several recent studies have explored their use in relation to neurovascular development240,241; however, there has been less characterization of the effects of such flow on neuronal migration. These approaches to study mechanical strain and shear have yet to be thoroughly explored in a 3D neuronal context, but they have the potential to inform how external forces may influence polarity, cell-cycle dynamics and cell fate in the developing brain.
Finally, to date, neither single-cell encapsulated neural cells nor cortical organoids have achieved folding resembling the gyrification in the cerebral cortex. While recent studies have induced surface wrinkling through spatial confinement94 or PTEN-knockout mediated NPC expansion93, neither demonstrated folding restricted to the cortical plate as occurs in vivo (outer folding and inner smoothness)242. Neural organoids mature beyond the developmental stage wherein gyrification starts92, yet their limited surface area and lack of ECM or associated mechanical forces may preclude the emergence of physiological folding243. If gyrification is achievable for human PS cell-derived in vitro models of the brain, ECM-mediated biophysical signalling cues may prove to play a crucial role242,244. Such cues derived from the neural ECM, including components of the HLC complex (HAPLN1, lumican and collagen), were shown to increase tissue stiffness, upregulate HA deposition and induce folding in explants of human fetal cerebral cortex245. Importantly, leveraging biomaterials to emulate ECM-derived mechanical forces has the potential to facilitate studies characterizing the mechanisms underlying congenital lissencephalies.
Spatio-temporal patterning
Throughout neural development, critical cellular processes such as fate specification, migration and axonal projection are spatio-temporally choreographed by morphogen gradients, cytoarchitecture, cell–cell contacts and paracrine signalling246,247. However, standard in vitro cell culture paradigms routinely present a spatially isotropic environment, which results in uniform cell types or stochastic self-assembly. Moreover, as a consequence of discrete medium change schedules and supplement degradation, the culture environment fluctuates over time in a manner that may or may not reflect the temporal dynamics observed in vivo. Finally, as a consequence of the challenges inherent in localizing specific medium requirements to distinct cell types, multicellular co-cultures, especially those with cells from different germ layers, have proven challenging to maintain. Engineered systems, and biomaterials in particular, have the capacity to mediate the production of cellular models that consistently recapitulate the spatio-temporal signalling dynamics and cellular composition within the brain and, therefore, are a reliable platform for investigating the unique contributions of distinct signalling cues to neural development (FIG. 4a,b).
Fig. 4 |. Biofabrication strategies to modulate multicellular neural maturation.
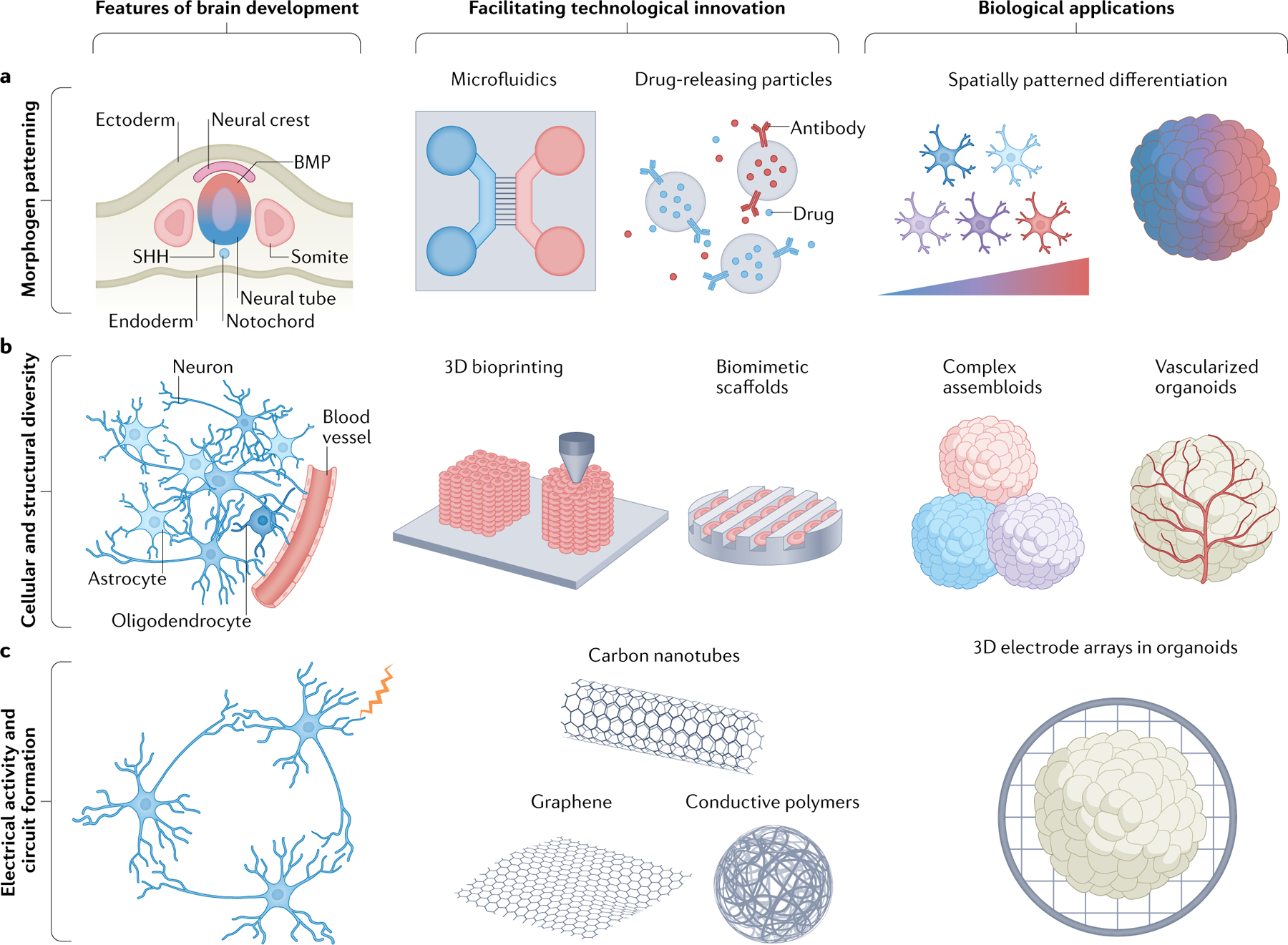
a | Cellular identity is spatially patterned by morphogen gradients in vivo. Similar morphogen or growth factor gradients can be generated by microfluidics, and growth factor release can be stabilized using drug-releasing microparticles or nanoparticles. Such approaches can enable spatially patterned in vitro cultures in two dimensions and three dimensions. b | The developing brain comprises different cell types and structures, including blood vessels. Bioprinting and biomaterial-based scaffolds may enable the generation of more complex structures and human neurovascularization in three dimensions. c | Electrical activity during development can be emulated by electrical stimulation using engineered materials, such as carbon nanotubes, graphene and conductive polymers. Ultimately, such techniques may enable a multi-electrode array-like structure embedded in three-dimensional (3D) cultures. BMP, bone morphogenetic protein; SHH, sonic hedgehog.
Controlled release.
The ECM sequesters soluble growth factors and, in this way, controls the bioavailability of key signalling molecules in the neural microenvironment248. The controlled release of soluble molecules over time has been a focus of materials science for years249,250. In these approaches, a supplement is embedded into particles or tethered to material backbones and slowly released over time, thereby stabilizing fluctuations in concentration. For example, soluble FGF2 rapidly degrades in cell culture, dropping to only 50% of its original concentration within 4 h, and almost completely disappearing over 72 h251. However, embedding FGF2 in polymer microspheres prevents much of this degradation, both allowing the concentration of FGF2 in the medium to remain stable over several days and mediating the stemness maintenance of human ES cell-derived NPCs. Such microsphere approaches can also accommodate various molecules in different matrices. For example, the incorporation of guggulsterone in poly(ε-caprolactone) microspheres induced an increase in the expression of TUJ1 and OLIG2 in human iPS cell aggregates252.
Including biomaterials within OoC systems has emerged as a promising method for controlling the spatial distribution of signalling molecules. The generation of concentration gradients using a polydimethylsiloxane-based microfluidic mixer has been widely reported253,254. A recent study demonstrated the utility of such gradient generators in directing the rostrocaudal organization of stem cells throughout neural differentiation43. Here, a layer of human ES cells on Matrigel was exposed to a gradient of the WNT signalling activator GSK3 inhibitor during conventional dual SMAD neuronal differentiation. A variety of assays, including single-cell RNA sequencing, revealed that the WNT activation gradient causes cells to adopt different identities along the rostrocaudal axis, corresponding to the applied gradient, in advance of expressing neural-specific cell fate markers. Additional studies leveraging natural and protein-engineered biomaterials and OoC systems have demonstrated a role for more defined biomaterials in spatial growth factor presentation. Specifically, encapsulating human fetal telencephalic NSCs within a collagen hydrogel, itself situated in a microfluidic array that facilitated the exchange of MSC-derived glial cell-derived neurotrophic factor enriched medium, resulted in increased neuronal marker (TUJ1 and MAP2) expression compared with glial marker (GFAP and OLIG2) expression255. In another study, chick dorsal root ganglion-derived spheroids encapsulated in elastin-like protein demonstrated enhanced neurite extension towards a nerve growth factor (NGF) gradient as a function of the RGD ligand concentration256. While these studies used single-cell suspensions within their OoC systems, the potential for these systems to recapitulate a range of biochemical and biophysical signalling cues has precipitated the emergence of studies exploring organoids-on-a-chip257. Most of these studies examine non-neural organoids within OoC systems and have been excellently reviewed elsewhere257–259.
The spatial patterning of molecules in a hydrogel substrate is also well established260,261. In these systems, growth factors are bound to a biomaterial, and cells are cultured on top of, or within, it. While this approach can be used to achieve a controlled release of growth factors similar to particle encapsulation, its greater potential lies in its ability to be photopatterned262,263. By conjugating growth factors to light-sensitive carriers, the factors can be selectively bound to specific locations within the hydrogel. For example, an agarose hydrogel with spatially patterned sonic hedgehog (SHH) and ciliary neurotrophic factor (CNTF) was created following sequential irradiation with a two-photon laser in the presence of maleimide–barnase and maleimide–streptavidin and, subsequently, barstar–SHH and biotin–CNTF fusion proteins264. Mouse retinal precursor cells cultured on top of the gels exhibited activation of both the SHH pathway and the CNTF pathway, and subventricular adult mouse NPCs preferentially migrated into gels with SHH. A more recent study developed a one-step binding process using a synthetic photocaging system and biotinylated growth factors, which was able to guide the neurite outgrowth of dorsal root ganglion axons by patterning NGF265. In summary, the biomaterial-mediated controlled release of patterning molecules and growth factors may ameliorate concerns related to signalling cue degradation and facilitates spatial control over signal patterning. Taken together, these advances have the potential to improve the reliability of neural differentiation, while also creating dynamic multicellular microenvironments.
3D bioprinting.
3D bioprinting, a process in which cells are suspended in a hydrogel or hydrogel precursor and the resulting suspension is patterned using 3D printing techniques, has been extensively used to achieve cellular heterogeneity within in vitro tissue constructs. In one such study, human iPS cell-derived spinal NPCs and mouse oligodendrocyte progenitor cells were printed with various hydrogels (gelatin methacrylate, gelatin mixed with fibrin, and diluted Matrigel) into pre-made silicone structures using a custom microextrusion system in a temporally efficient manner266. After several days in culture, both cell types remain viable, extend projections and, in the case of the spinal NPCs, show calcium activity. This work highlights two important considerations for bioprinted constructs: print-time constraints due to desiccation and cell type-specific material optimization. Recently a bio-orthogonal biomaterial crosslinking strategy demonstrated the ability to address both of these requirements267. In this study, human PS cell-derived neural organoids were printed into a reversible gel support bath to limit desiccation within a PEG bioink with mechanical properties previously shown to support mouse NPC expansion268. While promising, the chemical modifications required to mediate such bio-orthogonal crosslinking may be too technically complex to achieve widespread adoption. Alternatively, alginate has emerged as an ideal bioink for neural cells due to its neural biocompatibility269, relative ease of use (that is, crosslinking occurs in the presence of divalent cations) and widespread availability. Human iPS cells printed within an alginate, carboxymethyl chitosan and agarose bioink were able to differentiate into neurons and astrocytes270. As the cells were cultured, they migrated to form embryoid bodies, thereby demonstrating a degree of self-assembly. In another study, human iPS cell-derived NPCs printed within a fibrin, alginate and genipin bioink containing guggulsterone-eluting microspheres exhibited increased expression of neuronal (TUJ1 and tyrosine hydroxylase), astrocytic (GFAP) and oligodendrocytic (O4) markers271. Given the ability to direct not only cell arrangements but also spatial signalling cues with biomaterials, 3D bioprinting has the potential to create biomimetic, cellularly diverse neural tissue constructs.
Vascularization.
The development of human brain vasculature begins with the infiltration of the neural tube by endothelial cell-derived blood vessels, which sprout in tandem with a gradient of neurogenesis in the forebrain272–274. Importantly, both juxtacrine and reciprocal paracrine relationships have been documented between endothelial cells and NPCs275–281. Traditional in vitro cerebral vascularization studies have relied on co-culture systems with mature endothelial cells obtained from both human tissue (for example, human umbilical vein endothelial cells) and adult mouse brains (for example, immortalized mouse brain endothelial cells (bEnd.3 cells))282. These cells exhibit low proliferation rates and high degrees of heterogeneity in genotype and phenotype283. Moreover, the cell culture platforms themselves fail to recapitulate the spatial arrangement of NPCs and endothelial cells, resulting in non-physiological gradients of diffusible signalling cues284. Although microfluidic devices have been used to expose bEnd.3 cells to neural-conditioned medium with defined gradients285, these systems lack the cell–cell contact inherent in native tissue. Partly to capture this cell–cell contact, an embryoid-body cell culture protocol was leveraged to generate 3D vascularized neural spheroids from postnatal rodent cortex286. Additionally, human iPS cell-derived neural organoids were xenografted into an adult mouse and vascularized by endogenous endothelium90. These approaches recapitulated some of the spatial dynamics of neurovascularization, but they are undermined by their dependence on mouse cells. Several recent studies have aimed to circumvent the introduction of non-human cells by culturing human PS cell-derived neural organoids at an air–liquid interface287,288; however, these organoids fail to incorporate juxtacrine and paracrine signalling from endothelial cells.
The use of biomaterials to address challenges associated with modelling human neurovascularization in vitro remains underexplored. Of note, a recent study co-cultured human iPS cell-derived endothelial cells and motor neuron neurospheres within a collagen hydrogel, which was itself injected into a microfluidic device and subjected to intraluminal flow289. Interestingly, while introducing perfusion did not affect vascular network morphology or permeability, the frequency of neuron firing, peak calcium concentration and rate of depolarization all increased. Importantly, in perfusion-lacking paradigms, the 3D co-culture of endothelial cells and motor neuron neurospheres resulted in both paracrine (BDNF) and juxtacrine (DLL4–NOTCH1 and JAG1–NOTCH1) signalling, which increased the expression of mature motor neuron markers, the extent of neurite outgrowth, synapse formation and neural activity. While this study did not use the distinct microfluidic channels within the chip to provide different media to the encapsulated endothelial cells and motor neuron neurospheres, OoC systems have the potential to alleviate concerns related to the challenges of optimizing a single culture medium for different cell types239,290. Taken further, by integrating tunable biomaterials into OoC systems, both soluble and tethered biochemical cues may be tailored to specific populations of cells.
Outlook.
Although a wide breadth of technical approaches to achieve spatial patterning within neural tissue constructs have been described, many opportunities remain to further enhance morphogen release, cytoarchitecture, cell–cell contact and paracrine signalling. While 2D cell culture may provide more control over the administration of these signalling cues to individual cells, adapting these biomaterial-based strategies for 3D cell culture will allow researchers to capitalize on the system’s unique advantages. A recent attempt to engineer morphogen release in 3D neural organoids highlighted the potential utility of biomaterials in controlling spatio-temporal signal presentation. In this study, a cellular organizer consisting of SHH-releasing human PS cells was embedded within a forebrain organoid and, following doxycycline induction, drove the expression of ventral forebrain markers291. By leveraging genetic engineering to create an inducible signalling centre within a 3D tissue construct, the study authors simultaneously derived telencephalic and diencephalic domains of the forebrain and, in doing so, better recapitulated the spatio-temporal dynamics of early cortical development. However, in approximately 25% of cases, the organizer itself split into multiple centres. A material-based artificial signalling centre with acellular control over signalling gradients may ameliorate concerns related to signal integrity and, in doing so, provide improved reproducibility.
Biomaterials can serve as the matrix within which cells are patterned with an exogenously supplied gradient or the medium through which said gradient is created. Although most prior work incorporated single, linear exogenous signalling gradients, a recent study demonstrated that a four-channel microfluidic device generating two spatially orthogonal gradients can pattern collagen-encapsulated mouse ES cells into motor neurons292. Additionally, photoreversible protein patterning and the integration of Boolean logic gates into biomaterials facilitate spatio-temporal control of growth factors and cells293–295. This degree of multifaceted, microenvironment-specific control (enzymatic oligopeptide degradation, reduction-mediated disulfide bond breaking and UV-induced photoscission) could be applied towards recapitulating complex patterns of growth factor presentation throughout neural development. In keeping with leveraging the local niche towards driving spatial patterning, a recent study demonstrated that oligonucleotide aptamer biomaterials with bound growth factors release their cargo upon the application of traction forces from encapsulated cells296. The prospect of a cell-responsive growth factor release mechanism holds great potential as a means of creating cell type-specific signalling niches within engineered 3D microenvironments.
Although the integration of biomaterial-based strategies for engineering vascularized neural constructs in vitro is in its infancy, evidence from tangential studies suggests that biomaterials may eventually play a critical role in modelling neurovascularization. For example, applying flow with millifluidic chips may improve the generation of vascular networks with perfusable lumens297. Given the propensity for endothelial cells to sprout through degradable bioinks towards a gradient of angiogenic factors, intercalating neural constructs within degradable bioinks may facilitate neurovascular interactions298. Additionally, controlling integrin activation with functionalized hydrogels may drive the formation of space-filling, mature vasculature in neural cell–endothelial cell co-cultures299. Finally, printing sacrificial gelatin inks within neural tissue constructs consisting of collagen, Matrigel and thousands of cerebral organoids300, and perfusing the tubular channels with human iPS cell-derived endothelial cells, would allow investigations into the effects of vascularization on a much larger scale than previously considered possible.
Material-driven maturation
The endogenous bioelectric field is instrumental in regulating embryogenesis. Recent evidence has suggested that these bioelectric signalling cues serve instructive roles as patterning agents both within the brain301,302 and as a function of the brain303. To ensure precise and efficacious delivery of electrical stimuli to neural cells in vitro, a range of biomaterial scaffolds have been developed that provide both structural support and electrical stimulation (FIG. 4c).
Carbon-based materials.
Carbon-based materials are a unique subset of conductive materials owing to their high degree of biocompatibility, conductivity, stability, scalability, capacity to be functionalized to promote specific cellular responses (that is, cell adhesion and growth) and potential as nanofillers within hybrid hydrogels304,305. Two distinct forms of carbon biomaterials, carbon nanotubes (CNTs) and graphene, have emerged as promising platforms for controlling neurodevelopmental processes.
CNTs are cylindrical materials that readily form robust 3D microporous scaffolds which remain electrically conductive under mechanical deformation due to the high number of junction points in the percolated network306,307. This stability may prove to be particularly important when synergizing biomechanical stimulation with bioelectrical stimulation in multifaceted models of the neurodevelopmental microenvironment. Several studies from the early 2000s demonstrated the utility of CNTs as substrates that could induce increased neurite branching and action potential frequency through the formation of tight cell–material contacts308–310. Recently, single-walled CNT–polymer composites were shown to affect human iPS cell-derived NSC differentiation following the delivery of a depolarizing direct current311. Subsequent experiments revealed that the fibrous structure of multilayered CNTs could drive synapse formation through integrin-mediated interactions between mouse NSCs and the CNT312. Specifically, stimulated embryonic whole-brain NSCs acquired maturer phenotypes, including an increased prevalence of synaptophysin, longer and more complex neuritic branching, and increased sodium current peaks. The cytotoxicity of CNTs has recently re-emerged as a controversial issue following their inclusion on the ‘Substitute It Now’ list of hazardous chemical and nanomaterials in 2019313. In response, some in the scientific community were quick to call attention to numerous studies demonstrating the relative safety of these materials314,315. While the safety concerns of including CNTs within in vitro models of neural development are minimal, the heterogeneity of CNTs in length, diameter, rigidity and chemical functionalization requires scientists to evaluate cytotoxicity on a case-by-case basis. Finally, while CNTs emulate fibrillar structures found in collagen-rich ECM, the neural ECM more closely resembles amorphous aggregates of proteoglycans within a larger lattice structure. Given the influence of surface geometry on neural differentiation220–222,225, studies comparing CNTs with conductive polymers (CPs) with more biomimetic conformations may yield compelling insights.
Unlike CNTs, which have a tubular structure, graphene is a 2D carbon material in which a single layer of carbon atoms is arranged in a hexagonal lattice. By means of synthetic approaches, such as chemical vapor deposition, graphene can be patterned on substrates with various geometries. In addition to their geometric diversity, graphene sheets are also highly conductive, mechanically robust and well suited to bioimaging316. While the cytotoxicity of graphene and graphene derivatives is dependent on various factors, including size, degree of functionalization and synthesis measures317, graphene is generally considered to be biocompatible. Therefore, graphene biomaterials have been used to modulate neural development in vitro. For example, rolled graphene oxide foams were shown to direct the growth of neural fibres as human ES cell-derived NSCs differentiated into neurons following electrical stimulation318. In response to delivery of 10-s voltage pulses in the early stage of differentiation, NSCs increased their proliferation rate and preferentially differentiated into TUJ1+ neurons compared with GFAP+ glia. The high conductivity and favourable surface chemistry of graphene have also been utilized to promote neuronal network signalling. For example, differentiating rat hippocampal NPCs on graphene sheets increased the number of MAP2+ neurons, spontaneous calcium spikes, and spontaneous and miniature synaptic activity319. Taken together, these results point to a powerful avenue of research in which carbon-based materials are leveraged to promote neural maturation.
Conductive polymers.
Although less extensively explored than carbon-based scaffolds, CPs are promising platforms for electrically stimulating neural cells with several potential advantages, including improved flexibility, dual electronic and ionic conductivity, chemical versatility and tunability, and straightforward processability from solution320. When doped into an oxidized state, CPs can transport electrical charges across the alternating double bonds in their conjugated backbones, which allows them to couple electric fields to nearby cells via sustained electrical currents. Commonly used CPs include polyaniline (PANI), polypyrrole (PPy) and poly(3,4-ethylenedioxythiophene) (PEDOT), as these conjugated polymers are stable in their doped state321. The biocompatibility of this doping process, in addition to the CP itself, needs to be considered when designing cellular studies. For instance, PANI is less often used with cells since it has low conductivity above pH 4 and is usually doped with a strong acid such as hydrochloric acid322. On the other hand, PPy and PEDOT are commonly polymerized in the presence of biocompatible anionic small molecules or polymeric dopants such as dodecylbenzenesulfonate and polystyrene sulfonate (PSS), respectively.
While demonstrations of CPs in studies probing neural development are limited, initial results suggest that CPs can enhance neuronal differentiation and neuritic extension. PPy was shown to be compatible with clinically relevant human brain tissue-derived RenCell CX NSCs323. PPy films were electropolymerized onto gold-coated polymers, then coated with laminin to promote NSC adhesion. Biphasic electrical stimulation on dodecylbenzenesulfonate-doped PPy films induced seeded NSCs to primarily form TUJ1+ neurons over GFAP+ glia and increased the total neurite length per cell. Interestingly, cell clustering on electrically stimulated PPy was observed, with clusters localized to the more conductive regions of the film. Similar results were obtained using spin coating to generate PEDOT–PSS films from a solution containing a polar co-solvent that increases film conductivity by promoting favourable phase separation of PEDOT from PSS322. In this environment, pulsed DC electrical stimulation significantly increased human fetal brain tissue-derived RenCell VM NSC elongation, the percentage of TUJ1+ neurons compared with GFAP+ glia and neurite length. Finally, a recent study demonstrated that genetically modifying neurons to secrete enzymes that catalyse PANI polymerization results in the deposition of CPs at the cell surface324. These locally synthesized materials were shown to induce decreased action potential firing and increased capacitance in acute brain slices. In freely moving Caenorhabditis elegans, the deposition of PANI near cholinergic motor neurons resulted in impaired locomotion, while its deposition near GABAergic motor neurons resulted in increased reversal frequencies and sharp turns.
In addition to electrical conductivity, recent evidence suggests that the geometry of the conductive support may impact cell fate. A study explored the impact of geometry on the behaviour of human NPCs under electrical stimulation by electroplating dodecylbenzenesulfonate-doped PPy onto either a 2D substrate or a plating wire from which the electroplated PPy was subsequently detached to create a freestanding 3D PPy tube325. Human iPS cell-derived NPCs immobilized in alginate hydrogels were seeded either on top of the 2D films or within the 3D tubes. While cells in both cases experienced similar electric field strengths, the geometry impacted the electric field distribution such that cells in 3D tubes experienced more unidirectional stimulation than those seeded on 2D films. Electrical stimulation upregulated neurotrophic growth factors in both two dimensions and three dimensions, although it was more successful at upregulating glial cell-derived and brain-derived neurotrophic factors in the 3D tubes. However, these results are difficult to decouple from the markedly decreased viability of cells within the 3D tubes (19% dead compared with 1.6% dead in two dimensions), which may de due to the impermeability of smooth PPy coatings limiting nutrient and waste diffusion.
Cell viability in 3D geometries can potentially be improved by incorporating CPs into 3D hydrogel scaffolds, combining electrical conductivity with porosity to enable nutrient and waste transport. Compared with the smooth CP structures used as conductive supports in the previously discussed studies, CP-based hydrogels maintain an ECM-like 3D environment for cells while allowing greater contact with electrically conductive components. Although there is some preliminary evidence that CP-based hydrogels can promote NSC proliferation and differentiation into neurons, existing studies either do not involve human PS cell-derived NPCs326,327 or do not include electrical stimulation328. For example, the presence of PEDOT was shown to improve the adhesion and increase the proliferation of rat hippocampal progenitors within biodegradable chitosan–gelatin hydrogel scaffolds326. Moving forward, incorporating electrical stimulation with these hydrogel scaffolds could potentially further shift the balance of differentiation towards neuronal lineages and provide a novel way to clarify the role of scaffold geometry in neural development.
Outlook.
The unique combination of electrical conductivity and structural tunability of conducting scaffolds could, in principle, provide unique physical conduits for electrical coupling between neurons. Realizing the potential of these biomaterials will require advances in both material development and biological characterization.
With respect to developing new biomaterials, one key advance would come from the spatial patterning of stimulation. While clearly influencing the development of cells within their systems, most of the experimental paradigms discussed above deliver stimulation over the entire conductive area. Future systems could use novel materials to deliver chronic, spatially defined stimulations that more closely resemble neuronal inputs in vivo to drive the formation of specific circuits. Individually addressable locations could also enable the recording of extracellular potentials, allowing a multi-electrode array and bidirectional input and output. These materials could be integrated into larger 3D structures, such as mesh electrodes, to allow integration within a neural organoid. Additionally, to support longitudinal studies of development, the systems need to be robust enough to survive hundreds of days of culture and repeated handling. Finally, while efforts have been made to create conductive biomaterials with tunable mechanical properties329,330, further exploration into the synergistic effects of material-provided bioelectric and biophysical signalling cues is required.
There is also a need for comprehensive investigation of the biological effects of these engineered materials, especially over the long time frames that may be required to allow the electrical activity within in vitro neural constructs to resemble those of preterm infants331. Although the materials themselves are often well characterized, previous work has primarily focused on a handful of metrics, such as gene expression or neuritic outgrowth, to draw conclusions about the effects of the materials on cell development. More nuanced biological questions remain. For example, how do changes in electrical activity affect the emergence of neuronal circuits in vitro? Is it possible for conductive substrates to mimic input from other brain regions? Answering these questions would lead to a more complete understanding of neural development in vitro and open avenues for disease modelling.
While not a focus of this Review, biomaterials have also emerged as a compelling means by which to measure neural activity both in vitro and in vivo. Given the potential for biomaterial-based neural interfaces to be implemented in diagnostic and therapeutic settings, studies probing their long-term biocompatibility, mechanical mismatch and signal-to-noise ratio are garnering great interest. Several recent reviews have thoroughly characterized this space332–334.
Finally, as 2D and 3D in vitro neural cell culture platforms continue to acquire increased functional maturation and, by extension, enhanced developmental fidelity, questions related to the ethics of these models will become ever more important (box 2).
Box 2 |. Ethical considerations pertinent to deriving models of the human brain.
Ethical limitations on in vivo neurodevelopmental research have driven researchers to create increasingly biomimetic in vitro models of functioning human brains. Eventually these efforts may yield constructs with such high biological fidelity that ethical issues reappear348,349. anticipated questions of morality can be broadly described as falling within three categories: a neural tissue’s capacity to sense its environment, the consent of a cell donor and the precision of language used by both scientists and the media. First, society generally accepts that many important preclinical studies inflict pain on model organisms (ranging in behavioural complexity from mice to primates), but it demands that the potential benefits justify the pain. Neural organoids with nociceptive sensory neurons have already been developed350. assessing the perception of pain in such organoids is highly important; one group has already called for a moratorium on research with neural organoids until such studies are performed351. second, donors of the somatic cells that are reprogrammed into the stem cells used to make human pluripotent stem cell-derived neural tissue may object to scientists creating in vitro models of functional brain tissue that share their genetic background; therefore, specific informed consent is prudent. Last, organoids (more so than two-dimensional neurons) raise issues associated with communication, as scientific publications balance the desire to broadcast innovative research with the public’s proclivity to jump to visions of scientists probing mature human brains in tanks. the ubiquitous use of the misleading term ‘mini-brain’ suggests that popular science media, as well as a number of highly regarded academic journals, still have room for improvement in this regard. Moreover, researchers should be careful to portray these constructs and their limitations realistically, while concurrently emphasizing the potential value of such models for studying disease aetiology and, eventually, for relieving human suffering. Looking forward, transplanting human organoids into model organisms adds a substantial degree of complexity to all these issues. in an effort to address these concerns, the US National Academies convened a committee to report on ethical, legal and regulatory issues associated with neural chimeras and organoids, and released its findings in a consensus study report352.
Conclusions
The immense potential of human PS cells lies in their ability to recapitulate developmental trajectories. While current strategies to guide human PS cell differentiation have significantly progressed since their inception, they remain limited in their capacity to recreate important facets of the neural microenvironment. Within these niches, cells are exposed to ECM-derived biochemical and biophysical cues, spatio-temporally conserved paracrine and juxtacrine signalling, and electrical stimulation. The direct incorporation of biomaterials, and the application of techniques that rely on biomaterials, has the potential to emulate neurodevelopmental signals and, by extension, to create more biomimetic models of the human brain. These advanced in vitro models will facilitate investigations into previously inaccessible developmental processes and disease aetiologies, and, in turn, mediate the discovery and preclinical validation of therapeutics aimed at ameliorating neurodevelopmental diseases.
Acknowledgements
The authors thank members of the Heilshorn, Paşca, Bao and Cui laboratories for helpful discussions. Specifically, the authors acknowledge B. L. LeSavage and M. J. Kratochvil for their constructive feedback. The authors acknowledge financial support from the US National Institutes of Health (R21NS114549, R01EB027666 and R01EB027171 (S.C.H.)), the US National Science Foundation (DMR1808415 and CBET2033302 (S.C.H.)), the National Science Foundation Future Manufacturing Program under award no. 2037164 (V.R.F., Y.J. and Z.B.), the National Science Foundation Graduate Research Fellowship Program under grant no. DGE-1656518 (J.G.R. and M.S.H.), the Stanford Smith Family Graduate Fellowship (J.G.R.), the Stanford ChEM-H O’Leary-Thiry Graduate Fellowship (M.S.H.), a Stanford Bio-X seed grant (V.R.F., Y.J. and Z.B.) and the Stanford Brain Organogenesis Program in the Wu Tsai Neurosciences Institute (B.C., H.T.G., Z.B., S.P.P. and S.C.H.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Dolmetsch R & Geschwind Daniel, H. The human brain in a dish: the promise of iPSC-derived neurons. Cell 145, 831–834 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews K, Christmas D, Swan J & Sorrell E Animal models of depression: navigating through the clinical fog. Neurosci. Biobehav. Rev 29, 503–513 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Dragunow M The adult human brain in preclinical drug development. Nat. Rev. Drug Discov 7, 659 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Michalon A, Lindemann L, Fontoura P & Santarelli L Drug discovery for autism spectrum disorder: challenges and opportunities. Nat. Rev. Drug Discov 12, 777 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Waterston RH et al. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Rakic P Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci 10, 724–735 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge RD et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herculano-Houzel S The human brain in numbers: a linearly scaled-up primate brain. Front. Hum. Neurosci 3, 31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herculano-Houzel S, Mota B & Lent R Cellular scaling rules for rodent brains. Proc. Natl Acad. Sci. USA 103, 12138–12143 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schubert D, Martens GJM & Kolk SM Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Mol. Psychiatry 20, 795 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakic P A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18, 383–388 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Molnár Z et al. New insights into the development of the human cerebral cortex. J. Anat 235, 432–451 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts AC & Clarke HF Why we need nonhuman primates to study the role of ventromedial prefrontal cortex in the regulation of threat- and reward-elicited responses. Proc. Natl Acad. Sci. USA 116, 26297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izpisua Belmonte JC et al. Brains, genes, and primates. Neuron 86, 617–631 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silbereis JohnC., Pochareddy S, Zhu Y, Li M & Sestan N The cellular and molecular landscapes of the developing human central nervous system. Neuron 89, 248–268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otani T, Marchetto Maria, C., Gage Fred, H., Simons Benjamin, D. & Livesey Frederick, J. 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467–480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa AMM, Meyer KA, Santpere G, Gulden FO & Sestan N Evolution of the human nervous system function, structure, and development. Cell 170, 226–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrselja Z et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature 568, 336–343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz N et al. Human cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures. Sci. Rep 7, 12249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson M et al. Optogenetic control of human neurons in organotypic brain cultures. Sci. Rep 6, 24818 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons HM et al. Cellular composition of human glial cultures from adult biopsy brain tissue. J. Neurosci. Methods 166, 89–98 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Park TI-H et al. Adult human brain neural progenitor cells (NPCs) and fibroblast-like cells have similar properties in vitro but only NPCs differentiate into neurons. PLoS ONE 7, e37742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden DEJ The challenges and promise of neuroimaging in psychiatry. Neuron 73, 8–22 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Alda M Lithium in the treatment of bipolar disorder: pharmacology and pharmacogenetics. Mol. Psychiatry 20, 661–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun U et al. From maps to multi-dimensional network mechanisms of mental disorders. Neuron 97, 14–31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ardhanareeswaran K, Mariani J, Coppola G, Abyzov A & Vaccarino FM Human induced pluripotent stem cells for modelling neurodevelopmental disorders. Nat. Rev. Neurol 13, 265–278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Zhang L & Gage FH Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell 11, 45–59 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers SM et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol 27, 275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc. Natl Acad. Sci. USA 108, 8299–8304 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Kirwan P, Smith J, Robinson HPC & Livesey FJ Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci 15, 477–486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X-J et al. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol 23, 215–221 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Kirwan P & Livesey FJ Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc 7, 1836–1846 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Yan Y et al. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem Cells Transl. Med 2, 862–870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roybon L et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 4, 1035–1048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arber C et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development 142, 1375 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piao J et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 16, 198–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J et al. Generation of serotonin neurons from human pluripotent stem cells. Nat. Biotechnol 34, 89–94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an early demonstration that human PS cells can be differentiated into serotonergic neurons in two dimensions and probed with drug candidates.
- 38.Nolbrant S, Heuer A, Parmar M & Kirkeby A Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat. Protoc 12, 1962–1979 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Edri R et al. Analysing human neural stem cell ontogeny by consecutive isolation of Notch active neural progenitors. Nat. Commun 6, 6500 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Close JL et al. Single-cell profiling of an in vitro model of human interneuron development reveals temporal dynamics of cell type production and maturation. Neuron 93, 1035–1048.e1035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuijlaars J et al. Sustained synchronized neuronal network activity in a human astrocyte co-culture system. Sci. Rep 6, 36529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vatine GD et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24, 995–1005. e1006 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Rifes P et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol 38, 1265–1273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study leverages a gradient-producing microfluidic device to create human PS cell-derived neural tissue that exhibits progressive caudalization from forebrain to midbrain to hindbrain.
- 44.Marchetto MCN et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paşca SP et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med 17, 1657–1662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brennand KJ et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheridan SD et al. Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of fragile X syndrome. PLoS ONE 6, e26203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mertens J et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527, 95–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X et al. Idiopathic autism: cellular and molecular phenotypes in pluripotent stem cell-derived neurons. Mol. Neurobiol 54, 4507–4523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vierbuchen T et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mertens J et al. Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell 17, 705–718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huh CJ et al. Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. eLife 5, e18648 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paşca SP, Panagiotakos G & Dolmetsch RE Generating human neurons in vitro and using them to understand neuropsychiatric disease. Annu. Rev. Neurosci 37, 479–501 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Amin ND & Paşca SP Building models of brain disorders with three-dimensional organoids. Neuron 100, 389–405 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Chiaradia I & Lancaster MA Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat. Neurosci 23, 1496–1508 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Ishii K Reconstruction of dissociated chick brain cells in rotation-mediated culture. Cytologia 31, 89–98 (1966). [DOI] [PubMed] [Google Scholar]
- 57.Garber BB Brain histogenesis in vitro: reconstruction of brain tissue from dissociated cells. Vitro 8, 167–177 (1972). [DOI] [PubMed] [Google Scholar]
- 58.Reynolds BA, Tetzlaff W & Weiss S A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci 12, 4565 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S-C, Wernig M, Duncan ID, Brüstle O & Thomson JA In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol 19, 1129–1133 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Watanabe K et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci 8, 288–296 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Eiraku M et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Eiraku M et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011). [DOI] [PubMed] [Google Scholar]; This is early demonstration that suspended human PS cell-derived neural retina exhibits patterns of self-patterning and self-formation.
- 63.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camp JG et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci. USA 112, 15672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quadrato G et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Renner M et al. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 36, 1316–1329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka Y, Cakir B, Xiang Y, Sullivan GJ & Park I-H Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 30, 1682–1689.e1683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pasca AM et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mariani J et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakaguchi H et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun 6, 8896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian X et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jo J et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sloan SA et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790. e776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon S-J et al. Reliability of human cortical organoid generation. Nat. Methods 16, 75–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madhavan M et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 15, 700–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marton RM et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci 22, 484–491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Birey F et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiang Y et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398.e387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bagley JA, Reumann D, Bian S, Lévi-Strauss J & Knoblich JA Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiang Y et al. hESC-Derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24, 487–497.e487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miura Y et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol 38, 1421–1430 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Y-T et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98, 1141–1154.e1147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andersen J et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929. e1926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study creates the first multisynaptic human circuit consisting of cortical, spinal and skeletal muscle spheroids.
- 84.Li R et al. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell 8, 823–833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ogawa J, Pao GM, Shokhirev MN & Verma IM Glioblastoma model using human cerebral organoids. Cell Rep. 23, 1220–1229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Velasco S et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bershteyn M et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449.e434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan TA et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat. Med 26, 1888–1898 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pellegrini L et al. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369, eaaz5626 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors create a choroid plexus organoid that produces cerebrospinal-like fluid and facilitates screening of drug permeation.
- 90.Mansour AA et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol 36, 432–441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study transplants human neural organoids into the mouse cerebral cortex and observes progressive neural maturation, functional vascularization and graft-to-host synaptic connectivity.
- 91.Trevino AE et al. Chromatin accessibility dynamics in a model of human forebrain development. Science 367, eaay1645 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gordon A et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci 24, 331–342 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes the postnatal transition of cortical organoids at 250–300 days in culture.
- 93.Li Y et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 20, 385–396.e383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH & Reiner O Human brain organoids on a chip reveal the physics of folding. Nat. Phys 14, 515–522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study induces surface-level wrinkling of neural organoids encapsulated in Matrigel within a physically confining microfluidic device.
- 95.Iefremova V et al. An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller-Dieker syndrome. Cell Rep. 19, 50–59 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Borrell V How cells fold the cerebral cortex. J. Neurosci 38, 776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qian X, Song H & Ming GL Brain organoids: advances, applications and challenges. Development 146, dev166074 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakanishi S Extracellular matrix during laminar pattern formation of neocortex in normal and reeler mutant mice. Dev. Biol 95, 305–316 (1983). [DOI] [PubMed] [Google Scholar]
- 99.Bignami A & Delpech B Extracellular matrix glycoprotein (hyaluronectin) in early cerebral development: immunofluorescence study of the rat embryo. Int. J. Dev. Neurosci 3, 301–307 (1985). [DOI] [PubMed] [Google Scholar]
- 100.Stewart GR & Pearlman AL Fibronectin-like immunoreactivity in the developing cerebral cortex. J. Neurosci 7, 3325–3333 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hunter DD, Llinas R, Ard M, Merlie JP & Sanes JR Expression of s-laminin and laminin in the developing rat central nervous system. J. Comp. Neurol 323, 238–251 (1992). [DOI] [PubMed] [Google Scholar]
- 102.Kostović I, Judas M, Rados M & Hrabac P Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb. Cortex 12, 536–544 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Kostović I, Išasegi IŽ & Krsnik Ž Sublaminar organization of the human subplate: developmental changes in the distribution of neurons, glia, growing axons and extracellular matrix. J. Anat 235, 481–506 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhaduri A et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 578, 142–148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams DF On the nature of biomaterials. Biomaterials 30, 5897–5909 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Zimmermann DR & Dours-Zimmermann MT Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol 130, 635–653 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Barros CS, Franco SJ & Müller U Extracellular matrix: functions in the nervous system. Cold Spring Harb. Perspect. Biol 3, a005108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Long KR & Huttner WB How the extracellular matrix shapes neural development. Open. Biol 9, 180216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mouw JK, Ou G & Weaver VM Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol 15, 771–785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dauth S et al. Extracellular matrix protein expression is brain region dependent. J. Comp. Neurol 524, 1309–1336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicholson C & Syková E Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215 (1998). [DOI] [PubMed] [Google Scholar]
- 112.Margolis RU, Margolis RK, Chang LB & Preti C Glycosaminoglycans of brain during development. Biochemistry 14, 85–88 (1975). [DOI] [PubMed] [Google Scholar]
- 113.Okun E et al. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J. Neurochem 114, 462–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Struve J et al. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia 52, 16–24 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Khaing ZZ et al. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng 8, 046033 (2011). [DOI] [PubMed] [Google Scholar]
- 116.Sirko S, von Holst A, Wizenmann A, Götz M & Faissner A Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 134, 2727 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Tham M et al. CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS ONE 5, e15341 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo J-M & Alvarez-Buylla A EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36, 1021–1034 (2002). [DOI] [PubMed] [Google Scholar]
- 119.Drago J, Nurcombe V & Bartlett PF Laminin through its long arm E8 fragment promotes the proliferation and differentiation of murine neuroepithelial cells in vitro. Exp. Cell Res 192, 256–265 (1991). [DOI] [PubMed] [Google Scholar]
- 120.Hall PE, Lathia JD, Caldwell MA & Ffrench-Constant C Laminin enhances the growth of human neural stem cells in defined culture media. BMC Neurosci. 9, 71 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kerever A et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25, 2146–2157 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Stenzel D, Wilsch-Bräuninger M, Wong FK, Heuer H & Huttner WB Integrin αβ3 and thyroid hormones promote expansion of progenitors in embryonic neocortex. Development 141, 795–806 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Liao H et al. beta1 integrin-mediated effects of tenascin-r domains EGFL and FN6–8 on neural stem/progenitor cell proliferation and differentiation in vitro. J. Biol. Chem 283, 27927–27936 (2008). [DOI] [PubMed] [Google Scholar]
- 124.Pesheva P, Gloor S, Schachner M & Probstmeier R Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J. Neurosci 17, 4642 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garwood J et al. The extracellular matrix glycoprotein tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. Eur. J. Neurosci 20, 2524–2540 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Czopka T, Von Holst A, Schmidt G, Ffrench-Constant C & Faissner A Tenascin C and tenascin R similarly prevent the formation of myelin membranes in a RhoA-dependent manner, but antagonistically regulate the expression of myelin basic protein via a separate pathway. Glia 57, 1790–1801 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Long K, Moss L, Laursen L, Boulter L & Ffrench-Constant C Integrin signalling regulates the expansion of neuroepithelial progenitors and neurogenesis via Wnt7a and decorin. Nat. Commun 7, 10354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Summers DDB Degeneration and regeneration of the nervous system. Nature 125, 230–231 (1930). [Google Scholar]
- 129.Nagy JI, Hacking J, Frankenstein UN & Turley EA Requirement of the hyaluronan receptor RHAMM in neurite extension and motility as demonstrated in primary neurons and neuronal cell lines. J. Neurosci 15, 241 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Z-L, Haegeli V, Yu H & Strickland S Cortical deficiency of laminin gamma1 impairs the AKT/GSK-3beta signaling pathway and leads to defects in neurite outgrowth and neuronal migration. Dev. Biol 327, 158–168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith-Thomas LC et al. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J. Cell Sci 108, 1307 (1995). [DOI] [PubMed] [Google Scholar]
- 132.Moon LDF, Asher RA, Rhodes KE & Fawcett JW Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci 4, 465–466 (2001). [DOI] [PubMed] [Google Scholar]
- 133.Bradbury EJ et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002). [DOI] [PubMed] [Google Scholar]
- 134.Wang H et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J. Cell Sci 121, 3083–3091 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szabó A et al. In vivo confinement promotes collective migration of neural crest cells. J. Cell Biol 213, 543–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luckenbill-Edds L & Carrington JL Effect of hyaluronic acid on the emergence of neural crest cells from the neural tube of the quail, Coturnix coturnix japonica. Cell Tissue Res. 252, 573–579 (1988). [DOI] [PubMed] [Google Scholar]
- 137.Lindwall C, Olsson M, Osman AM, Kuhn HG & Curtis MA Selective expression of hyaluronan and receptor for hyaluronan mediated motility (Rhamm) in the adult mouse subventricular zone and rostral migratory stream and in ischemic cortex. Brain Res. 1503, 62–77 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Saghatelyan A, de Chevigny A, Schachner M & Lledo P-M Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat. Neurosci 7, 347–356 (2004). [DOI] [PubMed] [Google Scholar]
- 139.Tsuda S et al. FAK-mediated extracellular signals are essential for interkinetic nuclear migration and planar divisions in the neuroepithelium. J. Cell Sci 123, 484 (2010). [DOI] [PubMed] [Google Scholar]
- 140.Relvas JB et al. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr. Biol 11, 1039–1043 (2001). [DOI] [PubMed] [Google Scholar]
- 141.Chun SJ, Rasband MN, Sidman RL, Habib AA & Vartanian T Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J. Cell Biol 163, 397–408 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barros CS et al. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 136, 2717–2724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garcion E, Halilagic A, Faissner A & ffrench-Constant C Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 131, 3423 (2004). [DOI] [PubMed] [Google Scholar]
- 144.Hockfield S, Kalb RG, Zaremba S & Fryer H Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb. Symp. Quant. Biol 55, 505–514 (1990). [DOI] [PubMed] [Google Scholar]
- 145.Yamaguchi Y Lecticans: organizers of the brain extracellular matrix. Cell. Mol. Life Sci 57, 276–289 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rauch U Extracellular matrix components associated with remodeling processes in brain. Cell. Mol. Life Sci 61, 2031–2045 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galtrey CM & Fawcett JW The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev 54, 1–18 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Barritt AW et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci 26, 10856 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ranga A et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl Acad. Sci 113, E6831 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses synthetic biomaterials to systematically characterize the effects of distinct ECM-derived signalling cues on 3D neuroepithelial cysts.
- 150.Simão D et al. Recapitulation of human neural microenvironment signatures in iPSC-derived NPC 3D differentiation. Stem Cell Rep. 11, 552–564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kratochvil MJ et al. Engineered materials for organoid systems. Nat. Rev. Mater 4, 606–622 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luo C et al. Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep. 17, 3369–3384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bian S et al. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 15, 631–639 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bandtlow CE & Zimmermann DR Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiological. Rev 80, 1267–1290 (2000). [DOI] [PubMed] [Google Scholar]
- 155.Hughes CS, Postovit LM & Lajoie GA Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010). [DOI] [PubMed] [Google Scholar]
- 156.Miyata S & Kitagawa H Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta Gen. Subj 1861, 2420–2434 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Vukicevic S et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res 202, 1–8 (1992). [DOI] [PubMed] [Google Scholar]
- 158.Ma W et al. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev. Biol 8, 90 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kothapalli CR & Kamm RD 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials 34, 5995–6007 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Baron-Van Evercooren A et al. Nerve growth factor, laminin, and fibronectin promote neurite growth in human fetal sensory ganglia cultures. J. Neurosci. Res 8, 179–193 (1982). [DOI] [PubMed] [Google Scholar]
- 161.Rogers SL, Letourneau PC, Palm SL, McCarthy J & Furcht LT Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev. Biol 98, 212–220 (1983). [DOI] [PubMed] [Google Scholar]
- 162.Manthorpe M et al. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J. Cell Biol 97, 1882–1890 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nasu M et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS ONE 7, e53024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors add exogenous laminin to 3D cortical epithelium to induce the formation of a polarized neuroepithelium.
- 164.Bozza A et al. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials 35, 4636–4645 (2014). [DOI] [PubMed] [Google Scholar]
- 165.Lindborg BA et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cell Transl. Med 5, 970–979 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sood D et al. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep 9, 17874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Z-N et al. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc. Natl Acad. Sci. USA 113, 3185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Agmon G & Christman KL Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid State Mater. Sci 20, 193–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lam J, Carmichael ST, Lowry WE & Segura T Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater 4, 534–539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors leverage statistics-guided experimental design to efficiently screen the effects of adhesive ligands on NPCs.
- 170.Ranga A et al. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun 5, 4324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Freeman R et al. Instructing cells with programmable peptide DNA hybrids. Nat. Commun 8, 15982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates a biomaterial approach to achieving temporally dynamic presentation of ECM-derived biochemical signals.
- 172.Galarza S, Crosby AJ, Pak C & Peyton SR Control of astrocyte quiescence and activation in a synthetic brain hydrogel. Adv. Healthc. Mater 9, 1901419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lutolf MP & Hubbell JA Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 23, 47–55 (2005). [DOI] [PubMed] [Google Scholar]
- 174.Aisenbrey EA & Murphy WL Synthetic alternatives to Matrigel. Nat. Rev. Mater 5, 539–551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loebel C, Mauck RL & Burdick JA Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater 18, 883–891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use bio-orthogonal chemistry to identify nascent ECM protein secretion.
- 176.Tekin H et al. Effects of 3D culturing conditions on the transcriptomic profile of stem-cell-derived neurons. Nat. Biomed. Eng 2, 540–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Co JY et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep. 26, 2509–2520.e2504 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brandenberg N et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng 4, 863–874 (2020). [DOI] [PubMed] [Google Scholar]
- 179.Sengupta D & Heilshorn SC Protein-engineered biomaterials: highly tunable tissue engineering scaffolds. Tissue Eng. Part. B: Rev 16, 285–293 (2010). [DOI] [PubMed] [Google Scholar]
- 180.Cai L & Heilshorn SC Designing ECM-mimetic materials using protein engineering. Acta Biomater. 10, 1751–1760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chighizola M et al. Mechanotransduction in neuronal cell development and functioning. Biophys. Rev 11, 701–720 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barnes JM, Przybyla L & Weaver VM Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci 130, 71–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Javier-Torrent M, Zimmer-Bensch G & Nguyen L Mechanical forces orchestrate brain development. Trends Neurosci. 44, 110–121 (2021). [DOI] [PubMed] [Google Scholar]
- 184.Elkin BS, Azeloglu EU, Costa KD & Morrison B 3rd. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma 24, 812–822 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 186.Seidlits SK et al. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 31, 3930–3940 (2010). [DOI] [PubMed] [Google Scholar]
- 187.Xu Z, Chen Y & Chen Y Spatiotemporal regulation of Rho GTPases in neuronal migration. Cells 8, 568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Campos LS et al. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131, 3433–3444 (2004). [DOI] [PubMed] [Google Scholar]
- 189.Koser DE et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci 19, 1592–1598 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kilinc D The emerging role of mechanics in synapse formation and plasticity. Front. Cell Neurosci 12, 483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gefen A & Margulies SS Are in vivo and in situ brain tissues mechanically similar? J. Biomech 37, 1339–1352 (2004). [DOI] [PubMed] [Google Scholar]
- 192.Budday S et al. Rheological characterization of human brain tissue. Acta Biomater. 60, 315–329 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Saha K et al. Substrate modulus directs neural stem cell behavior. Biophys. J 95, 4426–4438 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Butcher DT, Alliston T & Weaver VM A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Musah S et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc. Natl Acad. Sci. USA 111, 13805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that material stiffness can bias differentiation of human ES cells into a neural cell fate.
- 196.Rammensee S, Kang MS, Georgiou K, Kumar S & Schaffer DV Dynamics of mechanosensitive neural stem cell differentiation. Stem Cell 35, 497–506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies a temporal window during which variations in ECM stiffness affected neural lineage commitment.
- 197.Dupont S et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [DOI] [PubMed] [Google Scholar]
- 198.Brusatin G, Panciera T, Gandin A, Citron A & Piccolo S Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat. Mater 17, 1063–1075 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Banerjee A et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 30, 4695–4699 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Keung AJ, de Juan-Pardo EM, Schaffer DV & Kumar S Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29, 1886–1897 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kang PH, Schaffer DV & Kumar S Angiomotin links ROCK and YAP signaling in mechanosensitive differentiation of neural stem cells. Mol. Biol. Cell 31, 386–396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Green MA, Bilston LE & Sinkus R In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 21, 755–764 (2008). [DOI] [PubMed] [Google Scholar]
- 203.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ & Shenoy VB Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao X, Huebsch N, Mooney DJ & Suo Z Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys 107, 063509 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chaudhuri O Viscoelastic hydrogels for 3D cell culture. Biomater. Sci 5, 1480–1490 (2017). [DOI] [PubMed] [Google Scholar]
- 206.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater 15, 326–334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sack I et al. The impact of aging and gender on brain viscoelasticity. NeuroImage 46, 652–657 (2009). [DOI] [PubMed] [Google Scholar]
- 208.Streitberger K-J et al. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PLoS ONE 7, e29888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Darnell M et al. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl Acad. Sci. USA 115, E8368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that culturing human NPCs in 3D biomaterials with different stress relaxation rates yields a high number of differentially expressed genes, highlighting the potential role of stress relaxation in NPC mechanosignalling.
- 210.Baker BM & Chen CS Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell Sci 125, 3015–3024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Madl CM et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater 16, 1233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies cell-mediated biomaterial degradation and the resultant increase in cell–cell contact as critical for the maintenance of stemness in mouse NSCs.
- 212.Madl CM, LeSavage BL, Dewi RE, Lampe KJ & Heilshorn SC Matrix remodeling enhances the differentiation capacity of neural progenitor cells in 3D hydrogels. Adv. Sci 6, 1801716 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peerani R et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 26, 4744–4755 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bauwens CL et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cell 26, 2300–2310 (2008). [DOI] [PubMed] [Google Scholar]
- 215.Sakai Y, Yoshiura Y & Nakazawa K Embryoid body culture of mouse embryonic stem cells using microwell and micropatterned chips. J. Biosci. Bioeng 111, 85–91 (2011). [DOI] [PubMed] [Google Scholar]
- 216.Warmflash A, Sorre B, Etoc F, Siggia ED & Brivanlou AH A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods 11, 847–854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Etoc F et al. A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev. Cell 39, 302–315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.His W Unsere Körperform und das physiologische Problem ihrer Enstehung: Briefe an einen Befreundeten Naturforscher (F.C.W.Vogel, 1874). [Google Scholar]
- 219.Vijayraghavan DS & Davidson LA Mechanics of neurulation: from classical to current perspectives on the physical mechanics that shape, fold, and form the neural tube. Birth Defects Res. 109, 153–168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xue X et al. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater 17, 633–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Britton G, Heemskerk I, Hodge R, Qutub AA & Warmflash A A novel self-organizing embryonic stem cell system reveals signaling logic underlying the patterning of human ectoderm. Development 146, dev179093 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Knight GT et al. Engineering induction of singular neural rosette emergence within hPSC-derived tissues. eLife 7, e37549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sha J, Lippmann ES, McNulty J, Ma Y & Ashton RS Sequential nucleophilic substitutions permit orthogonal click functionalization of multicomponent PEG brushes. Biomacromolecules 14, 3294–3303 (2013). [DOI] [PubMed] [Google Scholar]
- 224.Knight GT, Klann T, McNulty JD & Ashton RS Fabricating complex culture substrates using robotic microcontact printing (R-μCP) and sequential nucleophilic substitution. J. Vis. Exp 92, e52186 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lancaster MA et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol 35, 659–666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Iwashita M, Kataoka N, Toida K & Kosodo Y Systematic profiling of spatiotemporal tissue and cellular stiffness in the developing brain. Development 141, 3793 (2014). [DOI] [PubMed] [Google Scholar]; The authors use atomic force microscopy to analyse the mechanical properties of the mouse embryonic cerebral cortex and find that stiffness changes over the course of development and across different subregions.
- 227.Ondeck MG et al. Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling. Proc. Natl Acad. Sci. USA 116, 3502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu H-Y et al. Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells. Acta Biomater. 48, 258–269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Giachini PAGS et al. Additive manufacturing of cellulose-based materials with continuous, multidirectional stiffness gradients. Sci. Adv 6, eaay0929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Major LG et al. Volume adaptation controls stem cell mechanotransduction. ACS Appl. Mater. Interfaces 11, 45520–45530 (2019). [DOI] [PubMed] [Google Scholar]
- 231.Dou J, Mao S, Li H & Lin J-M Combination stiffness gradient with chemical stimulation directs glioma cell migration on a microfluidic chip. Anal. Chem 92, 892–898 (2020). [DOI] [PubMed] [Google Scholar]
- 232.Kim TH et al. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing–thawing method to investigate stem cell differentiation behaviors. Biomaterials 40, 51–60 (2015). [DOI] [PubMed] [Google Scholar]
- 233.Stukel JM & Willits RK Mechanotransduction of neural cells through cell–substrate interactions. Tissue Eng. B: Rev 22, 173–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zheng J et al. Tensile regulation of axonal elongation and initiation. J. Neurosci 11, 1117–1125 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bard L et al. A molecular clutch between the actin flow and n-cadherin adhesions drives growth cone migration. J. Neurosci 28, 5879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Arulmoli J et al. Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner. Sci. Rep 5, 8499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sawamoto K et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629 (2006). [DOI] [PubMed] [Google Scholar]
- 238.Guirao B et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol 12, 341–350 (2010). [DOI] [PubMed] [Google Scholar]
- 239.Maoz BM et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol 36, 865–874 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Herland A et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 11, e0150360 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Linville RM et al. Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials 190–191, 24–37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Llinares-Benadero C & Borrell V Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat. Rev. Neurosci 20, 161–176 (2019). [DOI] [PubMed] [Google Scholar]
- 243.Mota B & Herculano-Houzel S Cortical folding scales universally with surface area and thickness, not number of neurons. Science 349, 74 (2015). [DOI] [PubMed] [Google Scholar]
- 244.Kroenke CD & Bayly PV How forces fold the cerebral cortex. J. Neurosci 38, 767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Long KR et al. Extracellular matrix components HAPLN1, lumican, and collagen I cause hyaluronic acid-dependent folding of the developing human neocortex. Neuron 99, 702–719.e706 (2018). [DOI] [PubMed] [Google Scholar]; This study identifies neural ECM components of the HLC complex in the process of cortical folding.
- 246.Sagner A & Briscoe J Morphogen interpretation: concentration, time, competence, and signaling dynamics. WIREs Dev. Biol 6, e271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cadwell CR, Bhaduri A, Mostajo-Radji MA, Keefe MG & Nowakowski TJ Development and arealization of the cerebral cortex. Neuron 103, 980–1004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Belair DG, Le NN & Murphy WL Design of growth factor sequestering biomaterials. Chem. Commun 50, 15651–15668 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Brudno Y & Mooney DJ On-demand drug delivery from local depots. J. Control. Release 219, 8–17 (2015). [DOI] [PubMed] [Google Scholar]
- 250.Rambhia KJ & Ma PX Controlled drug release for tissue engineering. J. Control. Release 219, 119–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lotz S et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 8, e56289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Agbay A, De La Vega L, Nixon G & Willerth S Guggulsterone-releasing microspheres direct the differentiation of human induced pluripotent stem cells into neural phenotypes. Biomed. Mater 13, 034104 (2018). [DOI] [PubMed] [Google Scholar]
- 253.Nguyen EH, Schwartz MP & Murphy WL Biomimetic approaches to control soluble concentration gradients in biomaterials. Macromol. Biosci 11, 483–492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Toh AGG, Wang ZP, Yang C & Nguyen N-T Engineering microfluidic concentration gradient generators for biological applications. Microfluid. Nanofluidics 16, 1–18 (2014). [Google Scholar]
- 255.Yang K et al. Recapitulation of in vivo-like paracrine signals of human mesenchymal stem cells for functional neuronal differentiation of human neural stem cells in a 3D microfluidic system. Biomaterials 63, 177–188 (2015). [DOI] [PubMed] [Google Scholar]
- 256.Romano NH, Lampe KJ, Xu H, Ferreira MM & Heilshorn SC Microfluidic gradients reveal enhanced neurite outgrowth but impaired guidance within 3D matrices with high integrin ligand densities. Small 11, 722–730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Park SE, Georgescu A & Huh D Organoids-on-a-chip. Science 364, 960 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hofer M & Lutolf MP Engineering organoids. Nat. Rev. Mater 6, 402–420 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Garreta E et al. Rethinking organoid technology through bioengineering. Nat. Mater 20, 145–155 (2021). [DOI] [PubMed] [Google Scholar]
- 260.Leijten J et al. Spatially and temporally controlled hydrogels for tissue engineering. Mater. Sci. Eng. R: Rep 119, 1–35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ruskowitz ER & DeForest CA Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat. Rev. Mater 3, 17087 (2018). [Google Scholar]
- 262.Leipzig ND, Wylie RG, Kim H & Shoichet MS Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials 32, 57–64 (2011). [DOI] [PubMed] [Google Scholar]
- 263.Ham TR, Cox DG & Leipzig ND Concurrent delivery of soluble and immobilized proteins to recruit and differentiate neural stem cells. Biomacromolecules 20, 3445–3452 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wylie RG et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater 10, 799–806 (2011). [DOI] [PubMed] [Google Scholar]
- 265.Broguiere N et al. Morphogenesis guided by 3D patterning of growth factors in biological matrices. Adv. Mater 10.1002/adma.201908299 (2020). [DOI] [PubMed] [Google Scholar]
- 266.Joung D et al. 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Adv. Funct. Mater 28, 1801850 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hull SM et al. 3D bioprinting using UNIversal orthogonal network (UNION) bioinks. Adv. Funct. Mater 10.1002/adfm.202007983 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lindsay CD, Roth JG, LeSavage BL & Heilshorn SC Bioprinting of stem cell expansion lattices. Acta Biomater. 95, 225–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nunamaker EA, Purcell EK & Kipke DR In vivo stability and biocompatibility of implanted calcium alginate disks. J. Biomed. Mater. Res. A 83A, 1128–1137 (2007). [DOI] [PubMed] [Google Scholar]
- 270.Gu Q, Tomaskovic-Crook E, Wallace GG & Crook JM 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater 6, 1700175 (2017). [DOI] [PubMed] [Google Scholar]
- 271.Sharma R, Smits IPM, De La Vega L, Lee C & Willerth SM 3D bioprinting pluripotent stem cell derived neural tissues using a novel fibrin bioink containing drug releasing microspheres. Front. Bioeng. Biotechnol 10.3389/fbioe.2020.00057 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Marin-Padilla M Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J. Comp. Neurol 241, 237–249 (1985). [DOI] [PubMed] [Google Scholar]
- 273.Tata M, Ruhrberg C & Fantin A Vascularisation of the central nervous system. Mech. Dev 138, 26–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bjornsson CS, Apostolopoulou M, Tian Y & Temple S It takes a village: constructing the neurogenic niche. Dev. Cell 32, 435–446 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Quiñones-Hinojosa A et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J. Comp. Neurol 494, 415–434 (2006). [DOI] [PubMed] [Google Scholar]
- 276.Leventhal C, Rafii S, Rafii D, Shahar A & Goldman SA Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell. Neurosci 13, 450–464 (1999). [DOI] [PubMed] [Google Scholar]
- 277.Bayas A et al. Human cerebral endothelial cells are a potential source for bioactive BDNF. Cytokine 19, 55–58 (2002). [DOI] [PubMed] [Google Scholar]
- 278.Palmer TD, Willhoite AR & Gage FH Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol 425, 479–494 (2000). [DOI] [PubMed] [Google Scholar]
- 279.Schänzer A et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 14, 237–248 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mosher KI et al. Neural progenitor cells regulate microglia functions and activity. Nat. Neurosci 15, 1485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ottone C et al. Direct cell–cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol 16, 1045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Novosel EC, Kleinhans C & Kluger PJ Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev 63, 300–311 (2011). [DOI] [PubMed] [Google Scholar]
- 283.Santos MI & Reis RL Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol. Biosci 10, 12–27 (2010). [DOI] [PubMed] [Google Scholar]
- 284.Shen Q et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304, 1338–1340 (2004). [DOI] [PubMed] [Google Scholar]
- 285.Kuhnert F et al. Essential regulation of CNS angiogenesis by the orphan G protein–coupled receptor GPR124. Science 330, 985–989 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Boutin ME et al. A three-dimensional neural spheroid model for capillary-like network formation. J. Neurosci. Methods 299, 55–63 (2018). [DOI] [PubMed] [Google Scholar]
- 287.Giandomenico SL et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci 22, 669–679 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Qian X et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 26, 766–781.e769 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Osaki T, Sivathanu V & Kamm RD Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci. Rep 8, 5168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ronaldson-Bouchard K & Vunjak-Novakovic G Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell 22, 310–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Cederquist GY et al. Specification of positional identity in forebrain organoids. Nat. Biotechnol 37, 436–444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Uzel SGM et al. Simultaneous or sequential orthogonal gradient formation in a 3D cell culture microfluidic platform. Small 12, 612–622 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.DeForest CA & Tirrell DA A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater 14, 523–531 (2015). [DOI] [PubMed] [Google Scholar]
- 294.Badeau BA, Comerford MP, Arakawa CK, Shadish JA & DeForest CA Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem 10, 251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shadish JA, Benuska GM & DeForest CA Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater 18, 1005–1014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Stejskalová A, Oliva N, England FJ & Almquist BD Biologically inspired, cell-selective release of aptamer-trapped growth factors by traction forces. Adv. Mater 31, 1806380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Homan KA et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Song KH, Highley CB, Rouff A & Burdick JA Complex 3D-printed microchannels within cell-degradable hydrogels. Adv. Funct. Mater 28, 1801331 (2018). [Google Scholar]
- 299.Li S et al. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater 16, 953–961 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Skylar-Scott MA et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv 5, eaaw2459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pai VP et al. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via notch signaling and regulation of proliferation. J. Neurosci 35, 4366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Vitali I et al. Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell 174, 1264–1276.e1215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Herrera-Rincon C, Pai VP, Moran KM, Lemire JM & Levin M The brain is required for normal muscle and nerve patterning during early Xenopus development. Nat. Commun 8, 587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Voge CM & Stegemann JP Carbon nanotubes in neural interfacing applications. J. Neural Eng 8, 011001 (2011). [DOI] [PubMed] [Google Scholar]
- 305.Vashist A et al. Advances in carbon nanotubes–hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthc. Mater 7, 1701213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hu L, Hecht DS & Grüner G Carbon nanotube thin films: fabrication, properties, and applications. Chem. Rev 110, 5790–5844 (2010). [DOI] [PubMed] [Google Scholar]
- 307.Park S, Vosguerichian M & Bao Z A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 5, 1727–1752 (2013). [DOI] [PubMed] [Google Scholar]
- 308.Mattson MP, Haddon RC & Rao AM Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci 14, 175–182 (2000). [DOI] [PubMed] [Google Scholar]
- 309.Hu H, Ni Y, Montana V, Haddon RC & Parpura V Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 4, 507–511 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cellot G et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol 4, 126–133 (2009). [DOI] [PubMed] [Google Scholar]
- 311.Landers J et al. Carbon nanotube composites as multifunctional substrates for in situ actuation of differentiation of human neural stem cells. Adv. Healthc. Mater 3, 1745–1752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Shao H et al. Carbon nanotube multilayered nanocomposites as multifunctional substrates for actuating neuronal differentiation and functions of neural stem cells. Biomaterials 175, 93–109 (2018). [DOI] [PubMed] [Google Scholar]
- 313.Hansen SF & Lennquist A Carbon nanotubes added to the SIN List as a nanomaterial of very high concern. Nat. Nanotechnol 15, 3–4 (2020). [DOI] [PubMed] [Google Scholar]
- 314.Heller DA et al. Banning carbon nanotubes would be scientifically unjustified and damaging to innovation. Nat. Nanotechnol 15, 164–166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Fadeel B & Kostarelos K Grouping all carbon nanotubes into a single substance category is scientifically unjustified. Nat. Nanotechnol 15, 164–164 (2020). [DOI] [PubMed] [Google Scholar]
- 316.Shin SR et al. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev 105, 255–274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lalwani G, D’Agati M, Khan AM & Sitharaman B Toxicology of graphene-based nanomaterials. Adv. Drug Deliv. Rev 105, 109–144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Akhavan O, Ghaderi E, Shirazian SA & Rahighi R Rolled graphene oxide foams as three-dimensional scaffolds for growth of neural fibers using electrical stimulation of stem cells. Carbon 97, 71–77 (2016). [Google Scholar]
- 319.Tang M et al. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 34, 6402–6411 (2013). [DOI] [PubMed] [Google Scholar]
- 320.Guo B & Ma PX Conducting polymers for tissue engineering. Biomacromolecules 19, 1764–1782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Farokhi M et al. Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol. Biosci 21, 2000123 (2021). [DOI] [PubMed] [Google Scholar]
- 322.Pires F, Ferreira Q, Rodrigues CA, Morgado J & Ferreira FC Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta 1850, 1158–1168 (2015). [DOI] [PubMed] [Google Scholar]
- 323.Stewart E et al. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: a biocompatible platform for translational neural tissue engineering. Tissue Eng. C. Methods 21, 385–393 (2015). [DOI] [PubMed] [Google Scholar]
- 324.Liu J et al. Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals. Science 367, 1372 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors induce the polymerization of a CP on neural cell surfaces via genetic modification.
- 325.Song S et al. Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci. Rep 9, 19565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang S et al. Neural stem cell proliferation and differentiation in the conductive PEDOT-HA/Cs/Gel scaffold for neural tissue engineering. Biomater. Sci 5, 2024–2034 (2017). [DOI] [PubMed] [Google Scholar]
- 327.Wang S et al. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater. Sci. Eng. C. Mater Biol. Appl 93, 890–901 (2018). [DOI] [PubMed] [Google Scholar]
- 328.Feig VR et al. Conducting polymer-based granular hydrogels for injectable 3D cell scaffolds. Adv. Mater. Technol 10.1002/admt.202100162 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Noshadi I et al. Engineering biodegradable and biocompatible bio-ionic liquid conjugated hydrogels with tunable conductivity and mechanical properties. Sci. Rep 7, 4345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Feig VR, Tran H, Lee M & Bao Z Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun 9, 2740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors create mechanically tunable interpenetrating hydrogel networks with high conductivity.
- 331.Trujillo CA et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, P558–569.E7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Fattahi P, Yang G, Kim G & Abidian MR A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater 26, 1846–1885 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rivnay J, Wang H, Fenno L, Deisseroth K & Malliaras GG Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv 3, e1601649 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Song E, Li J, Won SM, Bai W & Rogers JA Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater 19, 590–603 (2020). [DOI] [PubMed] [Google Scholar]
- 335.Stiles J & Jernigan TL The basics of brain development. Neuropsychol. Rev 20, 327–348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lui Jan H., Hansen David, V. & Kriegstein Arnold, R. Development and evolution of the human neocortex. Cell 146, 18–36 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hébert JM & Fishell G The genetics of early telencephalon patterning: some assembly required. Nat. Rev. Neurosci 9, 678–685 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kolodkin AL & Tessier-Lavigne M Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb. Perspect Biol 10.1101/cshperspect.a001727 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Chung K et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yang B et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945–958 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Treweek JB et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc 10, 1860–1896 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lee E et al. ACT-PRESTO: rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci. Rep 6, 18631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ku T et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat. Biotechnol 34, 973–981 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Chen F, Tillberg PW & Boyden ES Expansion microscopy. Science 347, 543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Murray E et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell 163, 1500–1514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wang X et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Xia C, Fan J, Emanuel G, Hao J & Zhuang X Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc. Natl Acad. Sci. USA 116, 19490 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Farahany NA et al. The ethics of experimenting with human brain tissue. Nature 556, 429–432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Greely HT Human brain surrogates research: the onrushing ethical dilemma. Am. J. Bioeth 21, 34–45 (2018). [DOI] [PubMed] [Google Scholar]
- 350.Xiao D et al. Generation of self-organized sensory ganglion organoids and retinal ganglion cells from fibroblasts. Sci. Adv 6, eaaz5858 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ohayon EL & Lam TPW in Society for Neuroscience 2019 Annual Meeting https://www.abstractsonline.com/pp8/#!/7883/presentation/58037 (Society for Neuroscience, 2019). [Google Scholar]
- 352.National Academies of Sciences, Engineering, and Medicine. The Emerging Field of Human Neural Organoids, Transplants, and Chimeras: Science, Ethics and Governance (The National Academies Press, 2021). [PubMed] [Google Scholar]


