Supplemental digital content is available in the text.
Key Words: ankylosing spondylitis, fatigue, pain, quality of life, tumor necrosis factor inhibitors
Abstract
Background/Objective
Patients with ankylosing spondylitis (AS) experience symptoms and comorbidities that impact their health-related quality of life (HRQoL) and ability to work. This real-world, global survey was conducted among AS patients receiving tumor necrosis factor inhibitors (TNFis) to evaluate both the frequency and severity of persistent symptoms, and the impact of pain and fatigue on HRQoL, employment status, and work activity.
Methods
Patients with AS and their treating physicians from 13 countries across 5 continents completed questionnaires capturing demographics, patient symptoms, current disease status, HRQoL, current therapy, employment status, and Work Productivity and Activity Impairment.
Results
Seven hundred five patients who had been receiving a TNFi for 3 months or more and completed both Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) pain and fatigue domains were included in the analysis; of these, 37.6% reported high BASDAI pain scores and 41.3% high BASDAI fatigue scores. Medical Outcomes Study–Short Form, 36-item version 2 domain, 5-dimensional EuroQoL Questionnaire, and 5-dimensional EuroQoL visual analog scale scores were significantly lower (p < 0.0001), and Work Productivity and Activity Impairment scores significantly higher (p < 0.0001), in patients with high levels of pain or fatigue than low levels.
Conclusions
Globally, levels of pain and fatigue remained high in AS patients receiving TNFi treatment, which were significantly associated with reduced HRQoL and work productivity. Such persistent symptoms in usual care suggest a substantial unmet need in AS pharmacologic and nonpharmacologic therapeutic pathways.
The prevalence of ankylosing spondylitis (AS) varies greatly across the world, reportedly ranging from 6.5/100,000 in Japan, 100/100,000 in Mexico, 120/100,000 in Taiwan, 130/100,000 in Spain, 213/100,000 in Canada, to 540/100,000 in Turkey.1 Ankylosing spondylitis is characterized by inflammatory back pain and sacroiliac joint structural damage with or without peripheral arthritis and fatigue. It can lead to spinal stiffness or immobility from vertebral fusions.2–4 Back pain, stiffness, fatigue, peripheral arthritis, and comorbidities, such as uveitis, inflammatory bowel disease, psoriasis, and osteoporotic fractures pose a significant burden, and AS patients, report impaired health-related quality of life (HRQoL) and ability to work.3–5
Inflammatory back pain is a common complaint in AS patients and a component of the clinical criteria for the classification of AS, prevalent in up to 45% of AS patients.6–9 Fatigue, also common in AS patients, strongly correlates with pain and can be as difficult to manage, with a reported global prevalence of 45% based on a study of 6120 patients with rheumatic diseases from the Netherlands, Belgium, United Kingdom, United States, Canada, Germany, France, Spain, Argentina, Mexico, Chile, and Portugal.10–12 Elevated levels of the proinflammatory cytokines tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), IL-23, and IL-17 are present in the serum of patients with AS13 and implicated in mediation of inflammatory pain, fatigue, and depression.14–19 Until recently, TNF inhibitors (TNFis) were the main biologic disease-modifying antirheumatic drugs for treatment of patients with persistently high disease activity despite regular use of nonsteroidal anti-inflammatory drugs (NSAIDs).20
The purpose of this global real-world study in AS patients was to evaluate the frequency and severity of symptoms despite treatment with TNFis and the relationship of pain and fatigue with important patient-centric outcomes including HRQoL, employment status and work activity, and impairment.
METHODS
Data Source
This analysis of data from the Adelphi Spondyloarthritis AS Disease Specific Programme (DSP) was conducted in 13 countries between 2015 and 2016 in North America (N America covering the United States and Mexico), Europe (EU5, covering France, Germany, Italy, Spain, the United Kingdom), Asia Pacific (APAC, covering Japan, South Korea, Taiwan, Australia), and Turkey and Middle East (T&ME, covering Turkey and the United Arab Emirates).21 DSPs are large, multinational surveys designed to identify current disease management and patient- and physician-reported disease impact. They are point-in-time surveys conducted in real-world clinical practice.
Physicians included in the survey completed a prespecified form for the next 1 to 8 (variable by country) consecutive patients with AS seen for diagnosis or routine care. Physician-reported forms included detailed questions querying patient demographics, clinical assessments, concomitant conditions (including fibromyalgia), medication use, and treatment history. Each patient for whom a form was completed by the physician was invited to complete a voluntary patient-reported form, providing informed consent to participate.
Patient-reported forms included the 3-level 5-dimension EuroQoL questionnaire (EQ-5D-3L) deriving both the EQ-5D health utility and a visual analog scale (EQ-VAS), recording general health status,22 Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),23 Medical Outcomes Study–Short Form (36-item) Health Survey version 2 (SF-36v2),24 and Work Productivity and Activity Impairment General Health Questionnaire (WPAI).25 Patients completed forms independently from physicians and returned them in sealed envelopes to ensure confidentiality.
The DSP collected retrospective data using a noninterventional market research approach; no identifiable protected health information was collected. The DSP was conducted in accordance with the relevant legislation at time of data collection including the US Health Insurance Portability and Accountability Act 199626 and Health Information Technology for Economic and Clinical Health Act legislation.27 As this market research was run in accordance with the European Pharmaceutical Marketing Research Association guidelines, it did not require ethics committee approvals.28
Participating Physicians and Patients
Rheumatologists (and orthopedists and internists in Japan) were eligible to participate in the DSP if they had worked for 3 years or more as a physician, had qualified between 1979 and 2012, and were responsible for treatment decisions and management of AS patients.
Patients were eligible for inclusion in the AS DSP if 18 years or older with a physician-confirmed diagnosis of AS and not currently involved in a clinical trial. There were no exclusion criteria for the DSP. Patients were included in the analysis if receiving TNFis for 3 months or more and had completed both BASDAI pain and fatigue domains. Of 1508 eligible patients, 705 patients completed the BASDAI pain and fatigue domains and could be included in the analysis. However, demographic characteristics of 1508 eligible patients have been similar to the final 705 patients included in the analysis.
Study Variables
The BASDAI pain scores from BASDAI question 2 (overall level of neck, back, or hip pain in past 7 days) were stratified into low pain (<5) and high pain (5–10) to define patient groups.23
Fatigue, assessed using the first question of BASDAI, has been validated as an effective measure.29 The fatigue scale assesses levels of fatigue by asking “How would you describe the overall level of fatigue/tiredness you have experienced in the last week?” scored on a 10-point scale from 1 “none” to 10 “worst.” Fatigue was arbitrarily divided into low scores (<5) and high scores (5–10).
Patient characteristics were provided by physicians and included demographics, comorbidities, disease status including duration of disease, disease activity (judged by the physician and measured by physician-reported BASDAI if available), presence of inflammation indicated by elevated erythrocyte sedimentation rate (ESR), and/or C-reactive protein (CRP), and treatment details including type of TNFi therapy and use of prescribed and nonprescribed pain medications. The BASDAI pain domains, EQ-5D-3L, SF-36v2 domains, employment status, and WPAI also assessed patient outcomes.25,30 The SF-36v2 age and sex normative data were generated based on US norms published in SF-36 manuals and updates.31 Patient-reported happiness was assessed using SF-36v2 question 9, “How much of the time during the past week have you been happy?” with possible responses: all of the time, most of the time, some of the time, a little of the time, and none of the time.
Statistical Analyses
Categorical variables were described by counts and proportion of respondents, and continuous numerical variables were described by their means and standard deviations. Descriptive analyses were conducted at a global level and regional levels (N America, EU5, APAC, T&ME).
At a global level, pain and fatigue cohorts were compared against each other to assess whether high pain or high fatigue was associated with differences in other study measures. Statistical differences by levels of pain and fatigue were assessed using Kruskal-Wallis tests for continuous or ordinal data and χ2 tests for categorical data. A significance level of 95% was used throughout.
All analyses used Stata Statistical Software: Release 15 (StataCorp LP, College Station, TX).
RESULTS
Patients and Physicians
Data from a total of 2887 AS patients were collected in the DSP. One thousand five hundred eight patients (52.2%) had been receiving a TNFi for 3 months or more at the time of data collection, of whom 705 (46.8%), who had completed both BASDAI pain and fatigue domains, were included in this analysis (N America, n = 253; EU5, n = 328; APAC, n = 88; T&ME, n = 36). The demographics of included patients across regions were as follows: males from 75.5% in North America to 100% in T&ME, mean age in years from 36.1 in T&ME to 45.1 in EU5, mean years of disease duration from 1.3 in T&ME to 8.4 in EU5, patients who had ever received only 1 TNFi from 83.8% in N America to 97.2% in T&ME, physician-reported disease severity as “mild” from 60.5% in N America to 86.1% in T&ME, and physician-reported comorbid fibromyalgia from 2.7% in EU5 to 8.0% in APAC (Supplementary Table 1, http://links.lww.com/RHU/A207). The included patients were treated by a total of 307 physicians from 13 countries (N America, n = 85; EU5, n = 145; APAC, n = 52; T&ME, n = 25).
Of 705 patients included in the analysis, 705 patients (100%) completed SF-36v2, 694 patients (98.4%) completed EQ-5D-3L, and 685 patients (97.2%) completed WPAI questionnaires, with 525 patients (74.5%) employed at the time of questionnaire completion.
Levels of Pain and Fatigue
Stratification of patients based on pain levels resulted in 2 groups: one with low BASDAI pain scores: less than 5, n = 440 (62.4%); and the other with high BASDAI pain: 5 to 10, n = 265 (37.6%). Stratification of patients based on fatigue levels resulted in 2 groups: low BASDAI fatigue scores: less than 5, n = 414 (58.7%); and the other with high BASDAI fatigue: 5 to 10, n = 291 (41.2%). Rates of high pain and fatigue were similar for N America, EU5 and APAC regions, but not for T&ME. Although T&ME patients reported the greatest levels of high pain and fatigue (86.1% and 88.9%, respectively), this must be interpreted with caution due to the small sample size (n = 36) (Fig. 1).
FIGURE 1.
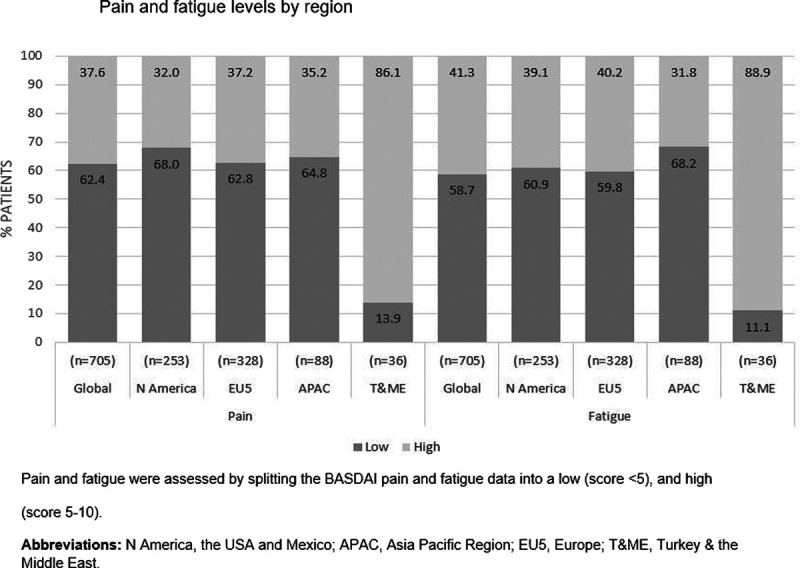
Pain and fatigue levels by region. Pain and fatigue were assessed by splitting the BASDAI pain and fatigue data into a low (score <5) and high (score 5–10). N America, the United States and Mexico; APAC, Asia Pacific Region; EU5, Europe; T&ME, Turkey and the Middle East.
The BASDAI pain and fatigue domains used to stratify these cohorts were highly correlated with both the pain dimension of EQ-5D-3L and the SF-36v2 vitality (VT) domains (BASDAI pain and EQ-5D-3L pain, Spearman correlation coefficient = 0.5391; BASDAI fatigue and SF-36v2 VT, Spearman correlation coefficient = 0.6099). The BASDAI pain domain was also highly correlated with the BASDAI fatigue domain (Spearman correlation coefficient = 0.8400).
Patient Demographics and Clinical Characteristics by Pain and Fatigue Levels
Patient characteristics including body mass index, time since symptom onset and diagnosis, and human leukocyte antigen B27 status were similar between patients with low or high pain and/or fatigue. Patients who reported high pain levels tended to be slightly older compared with those with low levels (45.8 vs. 42.2 years). Comorbid fibromyalgia was reported in 7.9% and 7.6% of patients with high levels of pain and fatigue, respectively, and 2.3% and 2.2% in patients with low pain and fatigue levels, respectively (Table 1).
TABLE 1.
Patient Demographics and Clinical Characteristics by Pain and Fatigue Levels
| Characteristics | Pain | Fatigue | |||
|---|---|---|---|---|---|
| Overall (n = 705) |
Low (Score <5) (n = 440) |
High (Score 5–10) (n = 265) |
Low (Score <5) (n = 414) |
High (Score 5–10) (n = 291) |
|
| Male, n (%) | 549 (77.9) | 349 (79.3) | 200 (75.5) | 331 (80.0) | 218 (74.9) |
| BMI, mean (SD), kg/m2 | 25.7 (3.8) | 25.5 (3.7) | 26.0 (3.9) | 25.4 (3.6) | 26.0 (4.0) |
| Age, y | |||||
| Mean (SD) | 43.6 (12.2) | 42.2 (12.0) | 45.8 (12.4) | 42.1 (12.0) | 45.7 (12.2) |
| <65 y, n (%) | 666 (94.5) | 424 (96.4) | 242 (91.3) | 399 (96.4) | 267 (91.8) |
| Symptom duration, y | (n = 623) | (n = 390) | (n = 233) | (n = 367) | (n = 256) |
| Mean (SD) | 10.5 (9.4) | 10.4 (9.0) | 10.8 (10.1) | 10.9 (9.0) | 10.0 (9.9) |
| Disease diagnosis duration, y | (n = 648) | (n = 410) | (n = 238) | (n = 381) | (n = 267) |
| Mean (SD) | 7.3 (7.8) | 7.1 (6.9) | 7.8 (9.2) | 7.4 (7.1) | 7.2 (8.8) |
| HLA-B27 status, n (%) | (n = 487) | (n = 324) | (n = 163) | (n = 313) | (n = 174) |
| + ve, n (%) | 434 (89.1) | 290 (89.5) | 144 (88.3) | 282 (90.1) | 152 (87.4) |
| Peripheral joint involvement, n (%) | 335 (47.5) | 193 (43.9) | 142 (53.6) | 184 (44.4) | 151 (51.9) |
| Comorbid fibromyalgia, n (%) | 31 (4.4) | 10 (2.3) | 21 (7.9) | 9 (2.2) | 22 (7.6) |
| No. TNFis ever received, n (%) | |||||
| 1 | 610 (86.5) | 384 (87.3) | 226 (85.3) | 366 (88.4) | 244 (83.8) |
| 2 | 63 (8.9) | 40 (9.1) | 23 (8.7) | 35 (8.5) | 28 (9.6) |
| ≥3 | 16 (2.3) | 9 (2.0) | 7 (2.6) | 6 (1.4) | 10 (3.4) |
| Unknown | 16 (2.3) | 7 (1.6) | 9 (3.4) | 7 (1.7) | 9 (3.1) |
The bold entries indicates the n values differ from the overall n value provided in the column header.
BMI, body mass index; HLA-B27, human leukocyte antigen B27.
Significant differences in disease severity and disease status were observed in patients reporting high pain (both p < 0.0001) and/or fatigue (p < 0.0001 and p = 0.0005, respectively) than in those reporting low pain and/or fatigue. A higher proportion of patients with high pain and/or fatigue were reported to have disease rated as “severe” (from mild/moderate/severe) by their physician than those with low pain and/or fatigue (high vs. low pain; 9.1% vs. 2.3%; high vs. low fatigue: 8.2% vs. 2.4%) (Table 2). A higher proportion of patients with high pain and/or fatigue levels were reported to have physician-rated disease status as “unstable” or “deteriorating” than those with low pain and/or fatigue levels (high vs. low pain: 14.4% vs. 2.5%; high vs. low fatigue: 14.1% vs. 1.9%). Significant differences were observed in current flare status between patients reporting high pain and/or fatigue (both p < 0.0001). Current flares were more frequently reported in patients with high rather than low pain levels (11.4% vs. 3.2%), as well as those with high versus low fatigue levels (11% vs. 2.9%). However, a notable proportion of patients with high pain (39.6%) and/or fatigue (40.2%) were deemed to be in remission, whereas 85.6% and 85.9% of patients with high levels of pain and/or fatigue, respectively, were considered to have stable or improving disease by their treating physician, indicating discordance between patient-reported and physicians' assessment of disease. Patients with high pain and/or fatigue levels had significantly higher levels of the inflammatory markers CRP and ESR than those with low pain and/or fatigue levels. C-reactive protein levels in patients reporting high versus low pain were 6.3 versus 4.3 mg/L, respectively (p = 0.0006), and 5.8 versus 4.5 mg/L, respectively (p = 0.0243), in patients reporting high versus low fatigue. Erythrocyte sedimentation rate levels in patients reporting high versus low pain were 18.3 versus 14.1 mm/h, respectively (p = 0.003), and 18.2 versus 13.9 mm/h, respectively (p = 0.0005), in patients reporting high versus low fatigue.
TABLE 2.
Physician-Reported Disease Characteristics by Pain and Fatigue Levels
| Pain | Fatigue | ||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 705) |
Low (Score <5) (n = 440) |
High (Score 5–10) (n = 265) |
p value | Low (Score <5) (n = 414) |
High (Score 5–10) (n = 291) |
p value | |
| Current severity,a n (%) | |||||||
| Mild | 460 (65.2) | 346 (78.6) | 114 (43.0) | <0.0001 | 324 (78.3) | 136 (46.7) | <0.0001 |
| Moderate | 211 (29.9) | 84 (19.1) | 127 (47.9) | 80 (19.3) | 131 (45.0) | ||
| Severe | 34 (4.8) | 10 (2.3) | 24 (9.1) | 10 (2.4) | 24 (8.2) | ||
| In remission, n (%) | 382 (54.2) | 277 (63.0) | 105 (39.6) | <0.0001 | 265 (64.0) | 117 (40.2) | <0.0001 |
| Disease status, n (%) | (n = 704) | (n = 440) | (n = 264) | <0.0001 | (n = 414) | (n = 290) | |
| Stable/improving | 655 (93.0) | 429 (97.5) | 226 (85.6) | 406 (98.1) | 249 (85.9) | 0.0005 | |
| Unstable/deteriorating | 49 (7.0) | 11 (2.5) | 38 (14.4) | 8 (2.0) | 41 (14.1) | ||
| Current flare status, n (%) | (n = 704) | (n = 440) | (n = 264) | <0.0001 | (n = 414) | (n = 290) | <0.0001 |
| Never flared | 389 (55.3) | 280 (63.6) | 109 (41.3) | 256 (61.8) | 133 (45.9) | ||
| Flares, not current | 271 (38.5) | 146 (33.2) | 125 (47.3) | 146 (35.3) | 125 (43.1) | ||
| Current flares | 44 (6.3) | 14 (3.2) | 30 (11.4) | 12 (2.9) | 32 (11.0) | ||
| ESR, mm/h | (n = 409) | (n = 252) | (n = 157) | (n = 238) | (n = 171) | ||
| Mean (SD) | 15.7 (13.1) | 14.1 (12.3) | 18.3 (13.9) | 0.0003 | 13.9 (12.1) | 18.2 (13.9) | 0.0005 |
| CRP, mg/L | (n = 386) | (n = 242) | (n = 144) | (n = 224) | (n = 162) | ||
| Mean (SD) | 5.0 (7.6) | 4.3 (6.2) | 6.3 (9.3) | 0.0006 | 4.5 (6.4) | 5.8 (8.9) | 0.0243 |
| BASDAI score | (n = 253) | (n = 157) | (n = 96) | (n = 150) | (n = 103) | ||
| Mean (SD) | 3.0 (2.2) | 2.7 (2.2) | 3.6 (2.1) | <0.0001 | 2.8 (2.2) | 3.4 (2.1) | 0.0041 |
aPhysician-reported.
HRQoL by Pain and Fatigue Levels
The SF-36v2 scores were compared between patients reporting low or high pain and fatigue levels and against age- and sex-matched normative data (Fig. 2). Patients with low pain and fatigue reported scores that approximated norms across most SF-36v2 domains; however, among patients with high pain and fatigue, scores across all SF-36v2 domains were significantly lower versus patients with low pain and fatigue (both p < 0.0001).
FIGURE 2.
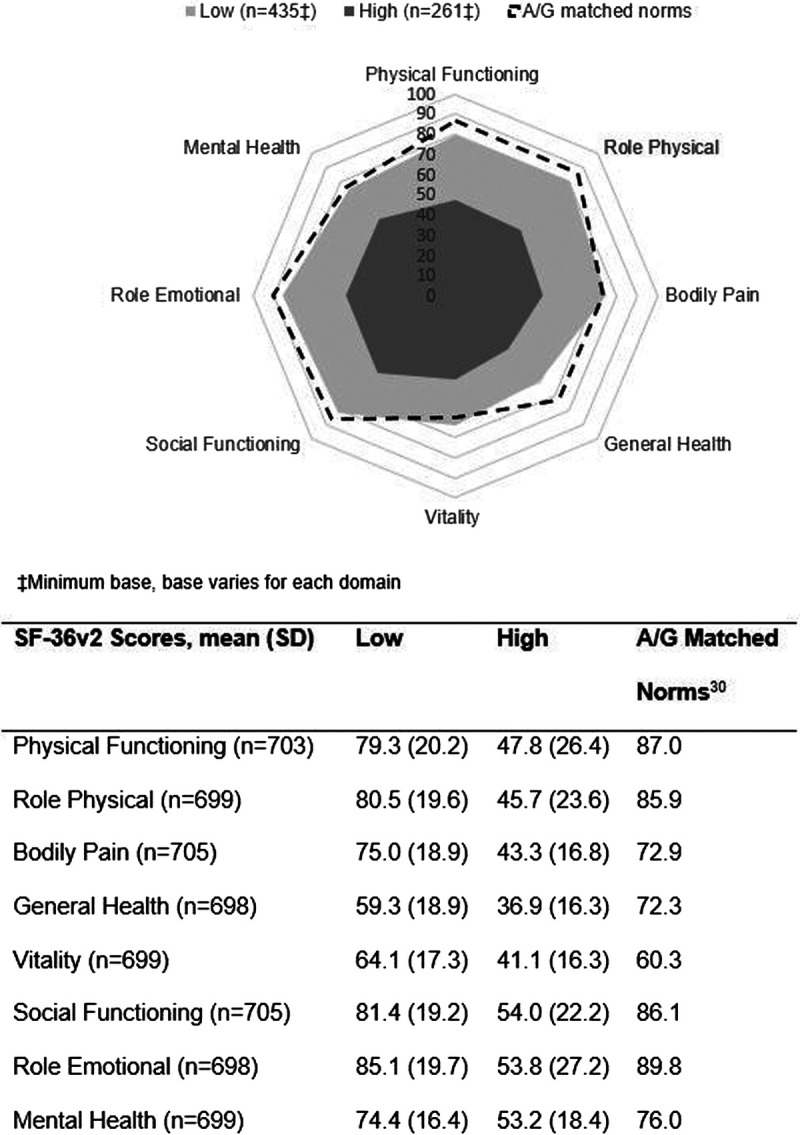
Spydergram of SF-36v2 domain scores by pain (A) and fatigue (B) levels. A/G, age/sex. All p < 0.0001.
More patients reporting high pain and/or fatigue reported less happiness in the past week by SF-36v2 compared with low pain and/or fatigue; 29.5% of patients with high pain and 30.3% with high fatigue reported feeling happy a “little or none of the time” versus 12.0% of patients with low pain and 10.3% of patients with low fatigue (p < 0.0001) (Table 3).
TABLE 3.
Patient-Reported Quality of Life by Pain and Fatigue Levels
| Pain | Fatigue | ||||
|---|---|---|---|---|---|
| Overall | Low (Score <5) |
High (Score 5–10) |
Low (Score <5) |
High (Score 5–10) |
|
| BASDAI, mean (SD)a | (n = 702) | (n = 439) | (n = 263) | (n = 413) | (n = 289) |
| Fatigue/tiredness level (reference) | 3.9 (2.7) | 2.4 (1.8) | 6.5 (1.7) | 1.9 (1.2) | 6.8 (1.3) |
| Neck, back or hip pain (reference) | 3.7 (2.7) | 1.9 (1.3) | 6.7 (1.3) | 2.0 (1.6) | 6.1 (2.0) |
| Pain, swelling in other joints | 3.0 (2.7) | 1.5 (1.5) | 5.4 (2.4) | 1.5 (1.4) | 5.2 (2.5) |
| Discomfortb | 3.2 (2.7) | 1.7 (1.5) | 5.7 (2.3) | 1.6 (1.4) | 5.4 (2.4) |
| Morning stiffness severity | 3.4 (2.6) | 2.0 (1.5) | 5.8 (2.2) | 1.9 (1.4) | 5.6 (2.3) |
| Morning stiffness duration | 3.2 (2.5) | 2.0 (1.7) | 5.0 (2.6) | 2.0 (1.7) | 4.8 (2.6) |
| EQ-5D-3L | (n = 694) | (n = 434) | (n = 260) | (n = 408) | (n = 286) |
| Mean (SD) | 0.755 (0.279) | 0.877 (0.149) | 0.550 (0.321) | 0.889 (0.134) | 0.563 (0.317) |
| EQ-VAS | (n = 704) | (n = 440) | (n = 264) | (n = 414) | (n = 290) |
| Mean (SD) | 70.2 (21.5) | 78.3 (18.5) | 56.7 (19.4) | 78.2 (19.1) | 58.6 (19.5) |
| SF-36v2 Q9; happy, n (%) | (n = 699) | (n = 435) | (n = 264) | (n = 409) | (n = 290) |
| All of the time/most of the time | 336 (48.1) | 265 (60.9) | 71 (26.9) | 261 (63.8) | 75 (25.9) |
| Some of the time | 233 (33.3) | 118 (27.1) | 115 (43.6) | 106 (25.9) | 127 (43.8) |
| A little of the time/none of the time | 130 (18.6) | 52 (12.0) | 78 (29.5) | 42 (10.3) | 88 (30.3) |
All p < 0.0001.
aBase varies by domain, minimum base shown.
bOverall discomfort from areas tender to touch or pressure.
Regional SF-36v2 mean domain scores can be found in Supplementary Table 2, http://links.lww.com/RHU/A207. Patients from T&ME had lower scores across all domains compared with other regions. Physical functioning domain mean scores ranged from 32.8 in T&ME to 71.4 in EU5. This wide range in scores between T&ME and other regions was observed for all domains with the exception of the General Health Questionnaire and VT domains, in which mean scores ranged from 44.9 in T&ME to 55.0 in N America and from 48.8 in T&ME to 57.4 in N America and APAC.
A significantly higher percentage of patients reporting high pain or fatigue reported “some” or “extreme” problems with mobility, self-care, usual activities, pain, and anxiety/depression as measured by EQ-5D-3L compared with those reporting low pain and fatigue (p < 0.0001) (Fig. 3). Regional EQ-5D-3L health utility and EQ-VAS scores (Table 3) were significantly lower in patients reporting high pain and/or fatigue levels compared with low pain and/or fatigue (all p < 0.0001).
FIGURE 3.
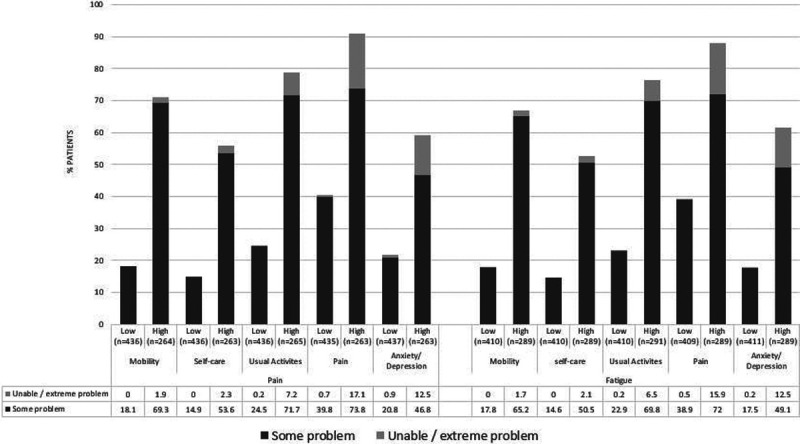
The 3-level 5-dimension EuroQoL questionnaire by pain and fatigue levels. Categories of EQ-5D-3L response are percentage of patients with (A) no problems, (B) some problems, (C) confined to bed/unable to perform task/extreme problem; (B) and (C) are shown on graph. p values across (A–C) response categories of EQ-5D-3L for each level of pain and fatigue are all p < 0.0001.
Regional mean EQ-5D-3L and EQ-VAS scores can be found in Supplementary Table 2, http://links.lww.com/RHU/A207. The EQ-5D-3L scores were similar between N America, EU5, and APAC ranging from 0.773 in EU5 to 0.808 in APAC; however, the T&ME EQ-5D-3L score was low at 0.149. The EQ-VAS scores ranged from 56.7 in T&ME to 77.4 in N America.
All nonreference BASDAI domains were significantly higher in patients with high pain and/or fatigue levels. Patients with high pain and/or fatigue levels also reported significantly higher BASDAI scores for joint pain and swelling (other than neck, back, or hip), discomfort, and severity and duration of morning stiffness (p < 0.0001) compared with patients reporting low pain and/or fatigue (Table 3). Regional mean BASDAI scores can be found in Supplementary Table 2, http://links.lww.com/RHU/A207. Regional mean scores for N America, EU5, and APAC across all BASDAI domains ranged from 2.6 in APAC (for “pain, swelling in other joints”) to 3.9 in EU5 (for “fatigue/tiredness level”). Mean BASDAI scores for T&ME were greater than 6.0 for all domains apart from morning stiffness duration, which had a score of 4.6.
Societal Burden by Pain and Fatigue Levels
Of 631 patients who provided employment information and were of working age (<65 years), 525 (83.2%) were employed, defined as employed, a student, or a homemaker (Table 4). Employment status differed significantly: patients reporting high pain (n = 164, 71.6%) had lower employment status compared with those reporting low pain (n = 361, 89.8%; p < 0.0001), and there was higher retirement or unemployment due to AS in patients reporting high pain (n = 25, 10.9%) compared with low pain (n = 30, 7.5%; p < 0.0001). Similar results were evident with fatigue: there were lower employment rates in patients reporting high fatigue (n = 188, 74.3%) compared with low fatigue (n = 337, 89.2%; p < 0.0001) and higher retirement or unemployment due to AS in patients reporting high fatigue (n = 29, 11.5%) compared with low fatigue (n = 26, 6.9%; p < 0.0001).
TABLE 4.
Societal Burden by Pain and Fatigue Levels
| Overall | Pain | Fatigue | |||
|---|---|---|---|---|---|
| Low (Score <5) |
High (Score 5–10) |
Low (Score <5) |
High (Score 5–10) |
||
| Current employment status among patients of working age, n (%) | (n = 631) | (n = 402) | (n = 229) | (n = 378) | (n = 253) |
| Employed/student/homemaker | 525 (83.2) | 361 (89.8) | 164 (71.6) | 337 (89.2) | 188 (74.3) |
| Unemployed/retired due to condition | 51 (8.1) | 11 (2.7) | 40 (17.5) | 15 (4.0) | 36 (14.2) |
| Unemployed/retired not due to condition or unspecified | 55 (8.7) | 30 (7.5) | 25 (10.9) | 26 (6.9) | 29 (11.5) |
| WPAI: % overall work impairment | (n = 415) | (n = 280) | (n = 135) | (n = 259) | (n = 156) |
| Mean (SD) | 27.6 (25.7) | 15.9 (15.7) | 51.7 (25.5) | 14.7 (14.5) | 48.9 (25.9) |
| WPAI: % presenteeism | (n = 427) | (n = 289) | (n = 138) | (n = 268) | (n = 159) |
| Mean (SD) | 24.8 (23.9) | 13.6 (13.1) | 48.3 (24.3) | 12.6 (12.2) | 45.3 (24.7) |
| WPAI: % absenteeism | (n = 422) | (n = 283) | (n = 139) | (n = 262) | (n = 160) |
| Mean (SD) | 6.4 (15.0) | 3.7 (11.9) | 11.9 (18.9) | 3.2 (11.5) | 11.5 (18.4) |
| WPAI: % activity Impairment | (n = 685) | (n = 428) | (n = 257) | (n = 406) | (n = 279) |
| Mean (SD) | 32.2 (26.6) | 17.7 (15.5) | 56.2 (23.7) | 16.5 (14.5) | 54.9 (23.6) |
All p < 0.0001.
The mean percentage (SD) of overall work impairment among all included patients was 27.6% (25.7%). All WPAI measures were significantly higher in patients with high pain and/or fatigue levels compared with low pain and/or fatigue (p < 0.0001), indicating that high pain and/or fatigue are associated with more work impairment, work time missed, impairment while working, and impairment in daily activities (Table 4).
Societal burden by region is shown in Supplementary Table 2, http://links.lww.com/RHU/A207. More than 80% of patients in all regions were employed. Overall work impairment and presenteeism were less than 25% in N America, EU5, and APAC, but greater than 60% in T&ME. Absenteeism was also highest in T&ME at 16.4% compared with 2.7%, 4.9%, and 6.9% in APAC, N America, and EU5, respectively.
Use of Pain Medication by Pain Levels
Globally, more than twice the number of patients reporting high pain levels reported using nonprescription pain medications than those with low pain (27.9% vs. 10.8%; p < 0.0001). Prescription pain medications included NSAIDs (including cyclooxygenase 2 inhibitors), nonopioids, and opioids. Significantly more patients reporting high pain levels received NSAIDs compared with patients reporting low pain levels (52.8% vs. 39.3%; p = 0.0006). Use of nonopioid prescription medications was similar in patients reporting high and low pain levels (Fig. 4).
FIGURE 4.
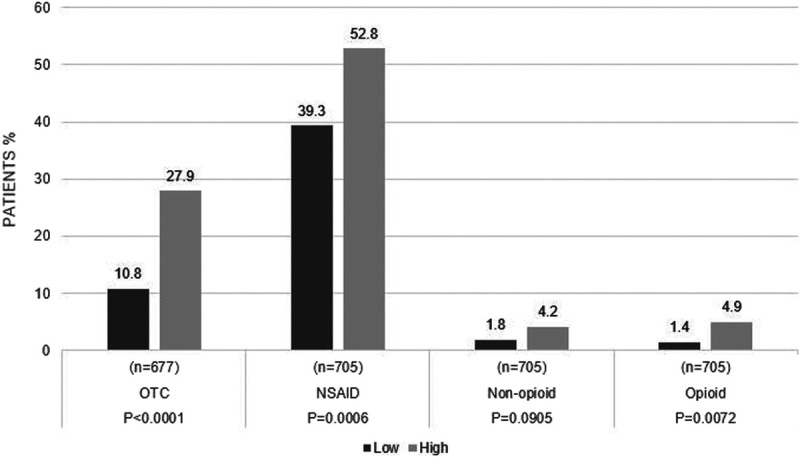
Use of pain medication by pain levels. OTC, over the counter.
DISCUSSION
The present analysis of real-world patient- and physician-reported data is the first global study examining the association between pain and/or fatigue with HRQoL and WPAI in AS patients treated with TNFis. We have demonstrated that increasing severity of pain and/or fatigue are associated with impaired HRQoL and mental wellness, as well as reduced physical functioning, engagement in activities, and work productivity.
Managing pain is an important treatment goal in patients with AS, however, pain is often underestimated by clinical disease activity scoring tools, and 20% to 30% of patients report pain despite treatment with TNFis.6,32,33 We observed increased levels of inflammatory markers with increasing severity of pain, indicating that patients may not have been adequately responding to therapy or were poorly adherent with their TNFis. The former concept is supported by previous work demonstrating that switching TNFi is often delayed in patients with AS despite lack of efficacy.12,34 Inflammatory mediators in AS can directly and indirectly influence pain, with animal studies demonstrating that IL-17 can contribute to neuroinflammatory responses and pain hypersensitivity following neuropathic injury.14 However, pain in AS is not only inflammatory, but also may involve soft tissue causes associated with muscle deconditioning, as well as a neuropathic component; therefore, strategies to treat chronic pain might differ from those that target inflammation.35
We observed a strong correlation between pain and fatigue levels, consistent with a UK study where fatigue was most strongly associated with pain in patients with AS compared with all other factors explored including age, depression, motivation, anxiety, physical activity, and sleep.12 The pathology of fatigue is unknown; however, the high prevalence of fatigue in rheumatic diseases and significantly increased levels of inflammatory markers observed in AS patients with severe fatigue suggest that inflammation is a likely contributing factor.10 This is consistent with several studies establishing that elevated levels of proinflammatory cytokines are associated with debilitating fatigue in autoimmune and inflammatory disorders.36 This also suggests that patients with high levels of proinflammatory cytokines may have persistent inflammatory disease.37,38
Consistent with a Scottish registry study,6 our global study demonstrates that pain and/or fatigue are common complaints in AS patients treated with TNFi and are associated with reduced HRQoL and that fatigue is not reliably controlled by pain management. Severe fatigue is described as overwhelming, unlike normal tiredness, permeating every aspect of life and difficult to self-manage with little outside support,39 and inadequate relief of both pain and/or fatigue have been shown to contribute to reduced HRQoL.40,41
We also observed discordance between patient-reported symptoms and physicians' assessments of disease in our global sample of AS patients, where some patients reporting high levels of pain and/or fatigue were deemed to be in remission or have stable or improving disease by their physician. Patient-physician discordance is well documented in rheumatology, and our data agree with other studies demonstrating discordance between patient and physician perceptions of AS, highlighting the importance of appropriate evaluation and management of pain and fatigue in the management of AS.42–45
We observed that globally, work impairment including absenteeism and presenteeism affected 27.6% of AS patients, consistent with previous studies.46 Our study also demonstrated that patients with severe pain and/or fatigue report an impaired ability to work and high rates of unemployment and retirement. This is consistent with reports that impaired work productivity is associated with fatigue in patients with axial spondyloarthritis,46 and overall employment rates are significantly lower in AS patients than in the general population.47 An impaired ability to work impacts HRQoL and is costly to society as a whole.48,49 In the NOR-DMARD study, the cost of absenteeism in AS patients taking biologic disease-modifying antirheumatic drugs over a 2-year period was €115,319.49
A major strength of this study is that it presents real-world data in AS patients treated with TNFi around the world and the negative impact of pain and/or fatigue on HRQoL and WPAI. Real-world studies play an important part in highlighting areas of concern that are not addressed in clinical trials. Patients included in clinical trials represent a small proportion of the consulting population as a result of age restrictions and stringent eligibility criteria, which typically exclude many comorbidities.50 Patients treated in the real-world setting may be less likely to be adherent to medication than those included in clinical trials.51 As a result, data from real-world studies can complement clinical trials and provide insight into the efficacy of interventions in patients commonly seen in clinical practice. Although the DSPs are exploratory studies that complement rather than replace larger studies, advantages include the ability to rapidly perform studies in relatively small populations that nonetheless provide insights into diseases, attitudes, and outcomes that might otherwise be difficult to obtain in such a timely manner and at in-depth patient level. A consistent methodology is used for DSPs across countries and economic environments, enabling cross-country comparisons, in comparison with registries or databases specific for a particular country or region. DSPs can include elements related to patient-reported outcomes and impacts on usual activities, providing insights into aspects not routinely assessed in randomized clinical trials. DSPs can be used to complement data from clinical trials performed in well-defined but potentially unrepresentative populations to provide an update on data otherwise obtained from large-scale but costly and time-consuming epidemiological studies. A recent DSP validity article has been published.52
Several potential limitations associated with data derived from this cross-sectional, real-world study should be considered. Cross-sectional studies are limited in their selection of patients, sample sizes, and data collection. A primary limitation of this study was that working age was set to those younger than 65 years; however, working age varies by country. The diagnosis of AS was based primarily on the judgment and diagnostic skills of the respondent physician, and a formalized diagnostic checklist was not mandated. However, this is consistent with the diagnostic decisions made by physicians in routine clinical practice and therefore reflects real-world practice. In contrast to a clinical trial where disease activity is assessed by validated measures, physician's rating of disease activity, including remission status, in our study may be considered subjective and hence represents a limitation. The sample collected was not a random sample of AS patients as the DSP methodology states that the next “n” consulting patients meeting the inclusion criteria are included. Therefore, they were not fully representative of the overall population of AS patients as patients who consult frequently were more likely to be included in the sample. The DSP systematic approach to recruitment nevertheless reduces selection bias. Although physicians are requested to collect data on a series of consecutive patients to avoid selection bias, in the absence of randomization this is contingent on the integrity of the participating physician rather than formalized source verification procedures. Additionally, because of the cross-sectional nature of our study, levels of pain and/or fatigue were captured only at the time of data collection. Therefore, a change in levels of pain and/or fatigue while on TNFi treatment could not be assessed, and there were no data collected on levels of pain and/or fatigue before starting such therapy. Limited evidence exists to determine appropriate cutoff points for pain and fatigue, so midpoints of 5 were used to allow consistent proportions and comparisons across all countries included in the study. Also, data for our study were obtained in 2015–2016; therefore, limited data were available for biologic treatments other than TNFis. We observed that overall physician-reported comorbid fibromyalgia levels were low but increased in the patient groups with high pain and/or fatigue; however, we are aware that variability in reporting fibromyalgia regionally may be due to different levels of sensitivity and instruments used, and we cannot determine the impact of undiagnosed fibromyalgia on our results. Another limitation is that although Ankylosing Spondylitis Disease Activity Score is a key measure for disease activity in AS, there was low utilization of Ankylosing Spondylitis Disease Activity Score in our study. However, our study reflects how physicians practice in a real-world clinical setting where assessments may be more holistic rather than focusing solely on disease activity. Finally, although recall bias is a common limitation of surveys, data were collected at the time of patients' appointments, reducing the likelihood of recall bias.
CONCLUSIONS
This real-world study agrees with previous reports that AS patients commonly report pain despite apparent control of disease with commonly available biologic therapies such as TNFis.35 These persistent high pain and/or fatigue levels place a high burden on patients in terms of HRQoL, reducing their ability to contribute to society as part of the workforce. Improved treatment options (in addition to TNFis) in AS are urgently needed, and appropriate evaluation and management of pain and/or fatigue should be a focus of outcome measures in AS clinical trials. Our observations indicate the importance of appropriately treating AS to minimize the impact on patients' social lives, work participation, and economic and health burdens. Lastly, our results highlight the need to explore other therapeutic approaches including nonpharmacologic interventions for the management of AS.
KEY POINTS
This is the first global study examining the association between pain and/or fatigue with HRQoL and work productivity in AS patients treated with TNFis.
We have demonstrated that increasing severity of pain and/or fatigue are associated with impaired HRQoL and mental wellness, as well as reduced physical functioning, engagement in activities, and work productivity.
Our conclusions agree with previous reports that AS patients commonly report pain despite apparent control of disease with commonly available biologic therapies such as TNFis.
ACKNOWLEDGMENT
The authors and Novartis thank all patients and physicians who participated in this study.
Footnotes
V.S. has received consulting fees from AbbVie, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Celltrion, Corrona LLC, Crescendo Bioscience, EMD Serono, F. Hoffmann-La Roche Ltd/Genentech, Inc., GSK, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi, and UCB and has served on advisory boards for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Celltrion, Crescendo/Myriad Genetics, EMDSerono, GSK, Novartis, Pfizer, Samsung, Sandoz, and Sanofi. A.D. has received grants or research support from Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer and UCB Pharma, and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB Pharma. R.A. has received grants or research support from Bristol-Myers Squibb and consulting fees from Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Eli Lilly. P.G.C. has received speakers' bureau or consulting fees from Abbvie, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Eli Lilly and is supported in part by the UK NIHR Leeds Biomedical Research Centre. E.S. and S.B. are employees of Adelphi Real World. H.T. and K.K.G. are shareholders and employees of Novartis. S.M.J. is a shareholder and employee of Novartis Pharma AG.
This study was supported by Novartis Pharma AG, Switzerland. Medical writing support was provided by Kate Revill of Adelphi Real World Ltd., funded by Novartis Pharma AG. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.jclinrheum.com).
Contributor Information
Vibeke Strand, Email: vibekestrand@me.com.
Atul Deodhar, Email: deodhara@ohsu.edu.
Rieke Alten, Email: Rieke.Alten@schlosspark-klinik.de.
Stuart Blackburn, Email: s.blackburn1993@gmail.com.
Haijun Tian, Email: haijun.tian@novartis.com.
Kunal K. Gandhi, Email: kunal.gandhi@novartis.com.
Steffen M. Jugl, Email: steffen.jugl@novartis.com.
Philip G. Conaghan, Email: P.Conaghan@leeds.ac.uk.
REFERENCES
- 1.Bohn R Cooney M Deodhar A, et al. Incidence and prevalence of axial spondyloarthritis: methodologic challenges and gaps in the literature. Clin Exp Rheumatol. 2017. [PubMed] [Google Scholar]
- 2.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. [DOI] [PubMed] [Google Scholar]
- 3.Dean LE Jones GT MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53:650–657. [DOI] [PubMed] [Google Scholar]
- 4.Salaffi F Carotti M Gasparini S, et al. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boonen A Boone C Albert A, et al. Understanding limitations in at-work productivity in patients with active ankylosing spondylitis: the role of work-related contextual factors. J Rheumatol. 2015;42:93–100. [DOI] [PubMed] [Google Scholar]
- 6.Dean LE, Macfarlane GJ, Jones GT. Five potentially modifiable factors predict poor quality of life in Ankylosing spondylitis: results from the Scotland registry for Ankylosing spondylitis. J Rheumatol. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374:2563–2574. [DOI] [PubMed] [Google Scholar]
- 8.Nhan DT, Caplan L. Patient-reported outcomes in axial spondyloarthritis. Rheum Dis Clin North Am. 2016;42:285–299. [DOI] [PubMed] [Google Scholar]
- 9.Mogard E Bremander A Lindqvist E, et al. Prevalence of chronic widespread pain in a population-based cohort of patients with spondyloarthritis—a cross-sectional study. BMC Rheumatol. 2018;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overman CL Kool MB Da Silva JA, et al. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin Rheumatol. 2016;35:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh JA McFadden ML Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670–1674. [DOI] [PubMed] [Google Scholar]
- 12.Brophy S Davies H Dennis MS, et al. Fatigue in ankylosing spondylitis: treatment should focus on pain management. Semin Arthritis Rheum. 2013;42:361–367. [DOI] [PubMed] [Google Scholar]
- 13.Sveaas SH Berg IJ Provan SA, et al. Circulating levels of inflammatory cytokines and cytokine receptors in patients with ankylosing spondylitis: a cross-sectional comparative study. Scand J Rheumatol. 2015;44:118–124. [DOI] [PubMed] [Google Scholar]
- 14.Kim CF, Moalem-Taylor G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain. 2011;12:370–383. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louati K, Berenbaum F. Fatigue in chronic inflammation—a link to pain pathways. Arthritis Res Ther. 2015;17:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen T Rasmussen TK Hvid M, et al. Increased plasma levels of IL-21 and IL-23 in spondyloarthritis are not associated with clinical and MRI findings. Rheumatol Int. 2012;32:387–393. [DOI] [PubMed] [Google Scholar]
- 18.Mei Y Pan F Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–273. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Sanchez C Jaimes DA Londono J, et al. Association between Th-17 cytokine profile and clinical features in patients with spondyloarthritis. Clin Exp Rheumatol. 2011;29:828–834. [PubMed] [Google Scholar]
- 20.van der Heijde D Ramiro S Landewe R, et al. 2016 Update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Anderson P Benford M Harris N, et al. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24:3063–3072. [DOI] [PubMed] [Google Scholar]
- 22.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 23.Garrett S Jenkinson T Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–2291. [PubMed] [Google Scholar]
- 24.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 25.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a Work Productivity and Activity Impairment instrument. Pharmacoeconomics. 1993;4:353–365. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services . Summary of the HIPAA Privacy Rule. Available at: http://www.hhs.gov/sites/default/files/privacysummary.pdf. Published 2003. Accessed May 11, 2017.
- 27.Health Information Technology Act. Available at: https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf. Accessed May 11, 2017.
- 28.Association EPMR . European Pharmaceutical Market Research Association (EphMRA) code of conduct. Available at: http://www.ephmra.org/Code-of-Conduct-Support. Published 2017. Updated January 2017. Accessed May 11, 2017.
- 29.van Tubergen A Coenen J Landewe R, et al. Assessment of fatigue in patients with ankylosing spondylitis: a psychometric analysis. Arthritis Rheum. 2002;47:8–16. [DOI] [PubMed] [Google Scholar]
- 30.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE Kosinski M Bjorner JB, et al. User's Manual for the SF-36v2® Health Survey (2nd ed.). Lincoln, RI: Quality Metric Incorporated; 2007. [Google Scholar]
- 32.Fischer BD Adeyemo A O'Leary ME, et al. Animal models of rheumatoid pain: experimental systems and insights. Arthritis Res Ther. 2017;19:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrido-Cumbrera M Hillmann O Mahapatra R, et al. Improving the management of psoriatic arthritis and axial spondyloarthritis: roundtable discussions with healthcare professionals and patients. Rheumatol Ther. 2017;4:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradeep DJ Keat AC Gaffney K, et al. Switching anti-TNF therapy in ankylosing spondylitis. Rheumatology (Oxford). 2008;47:1726–1727. [DOI] [PubMed] [Google Scholar]
- 35.Bidad K Gracey E Hemington KS, et al. Pain in ankylosing spondylitis: a neuro-immune collaboration. Nat Rev Rheumatol. 2017;13:410–420. [DOI] [PubMed] [Google Scholar]
- 36.Morris G Berk M Walder K, et al. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo A Guggino G Ferrante A, et al. Role of subclinical gut inflammation in the pathogenesis of spondyloarthritis. Front Med (Lausanne). 2018;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valente RL Valente JM de Castro GR, et al. Subclinical atherosclerosis in ankylosing spondylitis: is there a role for inflammation? Rev Bras Reumatol. 2013;53:377–381. [PubMed] [Google Scholar]
- 39.Kirwan JR, Hewlett S. Patient perspective: reasons and methods for measuring fatigue in rheumatoid arthritis. J Rheumatol. 2007;34:1171–1173. [PubMed] [Google Scholar]
- 40.Dernis-Labous E, Messow M, Dougados M. Assessment of fatigue in the management of patients with ankylosing spondylitis. Rheumatology (Oxford). 2003;42:1523–1528. [DOI] [PubMed] [Google Scholar]
- 41.Wu Q, Inman RD, Davis KD. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain. 2015;156:297–304. [DOI] [PubMed] [Google Scholar]
- 42.Lindstrom Egholm C Krogh NS Pincus T, et al. Discordance of global assessments by patient and physician is higher in female than in male patients regardless of the physician's sex: data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO registry. J Rheumatol. 2015;42:1781–1785. [DOI] [PubMed] [Google Scholar]
- 43.Desthieux C Molto A Granger B, et al. Patient-physician discordance in global assessment in early spondyloarthritis and its change over time: the DESIR cohort. Ann Rheum Dis. 2016;75:1661–1666. [DOI] [PubMed] [Google Scholar]
- 44.Wang CTM Fong W Kwan YH, et al. A cross-sectional study on factors associated with patient-physician discordance in global assessment of patients with axial spondyloarthritis: an Asian perspective. Int J Rheum Dis. 2018;21:1436–1442. [DOI] [PubMed] [Google Scholar]
- 45.Khan NA Spencer HJ Abda E, et al. Determinants of discordance in patients' and physicians' rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). 2012;64:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espahbodi S Bassett P Cavill C, et al. Fatigue contributes to work productivity impairment in patients with axial spondyloarthritis: a cross-sectional UK study. Clin Exp Rheumatol. 2017;35:571–578. [PubMed] [Google Scholar]
- 47.Mau W Listing J Huscher D, et al. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol. 2005;32:721–728. [PubMed] [Google Scholar]
- 48.Williams EM Walker RJ Faith T, et al. The impact of arthritis and joint pain on individual healthcare expenditures: findings from the Medical Expenditure Panel Survey (MEPS), 2011. Arthritis Res Ther. 2017;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kvamme MK Lie E Kvien TK, et al. Two-year direct and indirect costs for patients with inflammatory rheumatic joint diseases: data from real-life follow-up of patients in the NOR-DMARD registry. Rheumatology (Oxford). 2012;51:1618–1627. [DOI] [PubMed] [Google Scholar]
- 50.Saunders C Byrne CD Guthrie B, et al. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med. 2012;30:300–308. [DOI] [PubMed] [Google Scholar]
- 51.Hubbard TE Paradis R, NEHI . Real World Evidence: A New Era for Health Care Innovation. Available at: https://www.nehi.net/writable/publication_files/file/rwe_issue_brief_final.pdf. Published 2015. Accessed May 11, 2020.
- 52.Babineaux SM Curtis B Holbrook T, et al. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific programme. BMJ Open. 2016;6:e010352. [DOI] [PMC free article] [PubMed] [Google Scholar]


