Abstract
Objectives
The primary study objective was to investigate the impact of surveillance monitoring (i.e., continuous monitoring optimized for deterioration detection) on mortality and severe morbidity associated with administration of sedative/analgesic medications in the general care setting. A second objective was consideration of the results in the context of previous investigations to establish practice recommendations for this approach to patient safety.
Methods
Retrospective review of available rescue event and patient safety data from a tertiary care hospital in a rural setting was performed for a 10-year period. Systematic analysis of all adult general care inpatient data followed by chart review for individual patients was used to identify patient death or permanent harm (i.e., ventilator dependency, hypoxic encephalopathy) related to administration of sedative/analgesics.
Results
Of 111,488 patients in units with surveillance monitoring available, none died or were harmed by opioid-induced respiratory depression when surveillance monitoring was in use. One patient died from opioid-induced respiratory depression in a unit where surveillance monitoring was available; however, the patient was not monitored at the time of the adverse event. In unmonitored units (15,209 patients during 29 months of incremental implementation), three patients died from opioid overdose (19.73 deaths per 100,000 at risk patients). The reduced death rate when surveillance monitoring was available (0.0009%) versus not available (0.02%) was significant (P = 0.03).
Conclusions
For a 10-year period, the rescue system with continuous surveillance monitoring had a profound effect on death from sedative/analgesic administration in the general care setting. This approach to patient safety can help address the risk of sedative/analgesic-related respiratory arrests in hospitals.
Key Words: opioid-induced respiratory depression, surveillance monitoring, sedative and analgesic overdose, continuous monitoring
Despite the trend toward multimodal pain management, opioid analgesics remain the most used pain medication in the inpatient general care setting and are frequently used in combination with sedatives such as antiemetics and benzodiazepines. Though effective, sedative/analgesic medications have unintended adverse effects ranging from negligible to complete obtundation with respiratory compromise and/or arrest.1 Inpatients are at particularly high risk for opioid-related adverse events given the many painful scenarios they experience (i.e., postsurgical, postprocedural, etc.) requiring treatment with opioids.
To understand the patient safety impact of sedatives/analgesics in the inpatient setting, consider that there are more than 36 million annual acute care hospitalizations in the United States,2 with reported inpatient exposure to opioids between 50% and 88%,3,4 and opioid-related serious adverse events of nearly 1% in exposed populations.4 These rates translate into a conservative estimate of 180,000 patients experiencing opioid-related serious adverse events annually. Such statistics indicate that sedative/analgesic-related adverse events pose a significant clinical and financial burden and should be a prime target for improving safety in hospitals.5
There is increasing evidence that detectable physiologic instability precedes adverse events,6,7 with improper monitoring implicated as one of the major causes of morbidity, second only to wrong dose medication errors.8 To address the risk of medication-induced respiratory depression/arrest, continuous physiologic monitoring has been advocated9 but is not in widespread use. In contrast, continuous patient monitoring for other conditions has been widely adopted and shown to be effective in addressing adverse outcomes. For instance, Brady et al10 reported that patients with cardiopulmonary arrest that was witnessed or monitored (e.g., using cardiotelemetry) had twofold better odds of surviving to discharge compared with patients with unwitnessed or unmonitored cardiopulmonary arrest. Notably, the causes of cardiac arrest are less treatable and reversible than causes of respiratory arrest. Thus, one would expect the impact of early recognition prompted with continuous monitoring to have an even greater survival impact in patients at risk of respiratory arrest, including instances associated with analgesic/sedative administration.
Since 2007, the study institution has used pulse oximetry–based continuous monitoring in general care inpatient units (known as surveillance monitoring) in an effort to prevent “failure to rescue” events.11 Significant reductions in rescue events and care escalations were demonstrated with the system in place, and the application of human factors design techniques to alarm design was found to be effective.12 Subsequent studies have confirmed the positive effect of surveillance monitoring13,14 and also addressed implementation and adoption issues including population selection and cost-effectiveness.11,15,16 A positive effect on opioid-related harm and death 5 years after implementation was also described.16
The primary objective of this study is to investigate the long-term impact of surveillance monitoring on deaths and/or serious permanent harm (e.g., ventilator dependency, hypoxic encephalopathy), looking specifically at sedative/analgesic (i.e., opioids, benzodiazepines) medication-related respiratory arrest. The study focuses on general care inpatient units at a tertiary medical center where surveillance monitoring has been sustained for over a decade since it was first introduced. In a healthcare environment where opioid use is prevalent, the measured impact of the surveillance system over a long-term period has wide-ranging implications for quality improvement and safety.
METHODS
Setting
This retrospective study included adult patients admitted to the study institution over a 10-year period (December 2007–November 2017) and was approved by the institutional committee for the protection of human subjects. The study hospital is a 425-bed tertiary/quaternary care medical center located in a rural setting.
Patient Surveillance and Rescue Response Systems
Continuous monitoring with pulse oximetry is standard for all patients residing in general care medical and surgical units, except when ambulating, in the presence of a caregiver, contraindicated (e.g., confused patients at risk for cable entanglement), or refused by the patient. The monitoring system (Masimo Root and Radical 87 with Patient Safety Net; Masimo Corp, Irvine, CA) consists of bedside monitors, centralized data viewing stations, and nurse pager notification for alarm escalation as shown in Figure 1. Pulse oximetry–derived data include peripheral oxygen saturation (Spo2, percent saturation) and pulse rate (PR, beats per minute), calculated every second. Default threshold-based alarms for adults are Spo2 < 80% and 50 > PR >140 beats per minute.11 To minimize impact of nuisance alarms and alarm fatigue, parameter averaging is applied with alarm delay of 15 seconds for audible Spo2 alarms (PR alarms cannot be delayed), pager notifications at 30 seconds, and pager repeat/escalation at 1 minute. Alarms bring nursing resources to the bedside and appropriate rescue teams are then activated, if necessary. Details of system configuration, operation, and appropriate application are documented in policies and procedures approved through an institutional-level governance structure. Primary surveillance system application and operation characteristics, including default alarm thresholds, clinician notification methods, and escalation procedures remained unchanged during the study period. Surveillance monitoring was implemented first in a 36-bed surgical unit and then deployed to a total of 231 beds over a 29-month period as shown in Table 1.
FIGURE 1.
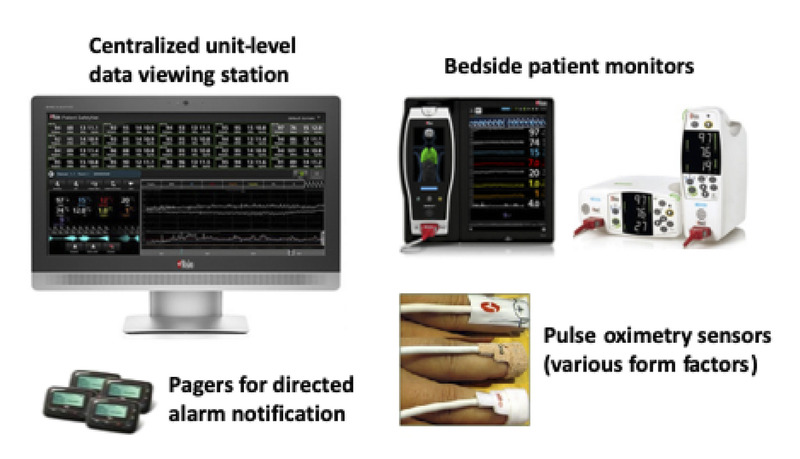
Inpatient surveillance monitoring system. The system consists of continuous pulse oximetry–based bedside monitors, centralized data viewing stations, and nurse pager notification for alarm escalation.
TABLE 1.
Surveillance System Deployment Timeline
| Date | Unit Type | Beds, n |
|---|---|---|
| December 2007 | Pilot surgical unit (primarily orthopedics) | 36 |
| February 2009 | Remainder of surgical units | 101 |
| April 2010 | All medicine units | 94 |
| Total adult inpatient surveillance monitoring beds | 231 | |
Implementation was initiated in a 36-bed surgical unit and progressed to all surgical and medicine units over a 29-month period.
The rescue event response structure is a core component of the Life Safety program at the study institution, supported by policies and procedures defining rescue hierarchy, activation guidelines, and interventions. The structure, which was in place and stable during the study period, is based on tiered response for managing events and includes consultation, rapid response, stat airway, and code blue activations.
Analysis Approach
Figure 2 depicts the general systematic approach taken to identify patients who may have experienced sedative/analgesic-related harm or death. The organization adheres to all state and federal patient safety reporting requirements, using evidence-based practices and procedures to track harm and adverse events.17 Event reporting occurs through a variety of avenues and data are reviewed and annotated by clinicians trained to recognize, describe, and categorize events using accepted causal analysis methods.18 These practices provide a high level of confidence that the study population included all patients who could have experienced serious harm or death related to sedative/analgesic administration. Several primary institutional sources were used in this review including a serious adverse event database, rescue activity database, mortality review reports, and medication reversal administration lists. Table 2 describes the data sources and specific analytic processes.
FIGURE 2.
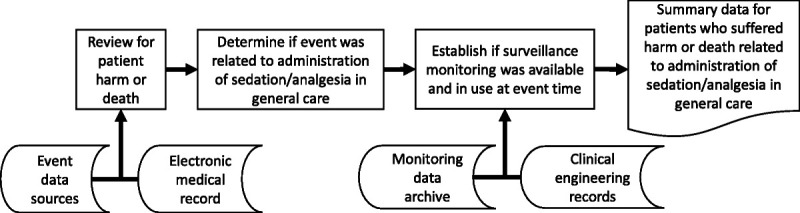
Analysis approach. A structured approach was to identify patients who died or were harmed by postoperative administration of sedative/analgesic medications in general care units. Various data sources, including the electronic medical record, event databases (e.g., rescues, reversals), clinical engineering equipment records, and archived physiologic data were accessed as shown during the review process.
TABLE 2.
Data Sources and Review Processes
| Source | Description | Review Process |
|---|---|---|
| Serious safety events | Cases meeting SSE definitions from: adverse events reported through institutional event reporting system, direct reports to quality assurance or risk management, and 100% mortality review. | All cases involving adults (n = 575) were reviewed for signs of deterioration in hospital (n = 103) of respiratory nature, and or where opioids or other sedating medications were involved. Association of the event to the administration of sedative/analgesic medication (n = 4) was assessed by detailed chart review. |
| Reversal administrations | List with documentation on all medication reversals with naloxone or flumazenil. Detailed and standardized harm classifications determined via quality assurance case review. | All general care patients administered a reversal agent with indicator of possible harm or death were selected for review (n = 920). Severe harm was identified by a diagnosis of anoxic brain injury or tracheostomy. Association of death or serious harm and the administration of sedative/analgesic medication (n = 33) was assessed by detailed chart review. |
| Rescue events | Institutional database with detailed documentation of rescue activations including consultations, rapid response team activations, code blue, and stat airways. | Patients discharged deceased after rescue in the general care setting were reviewed as part of the serious safety event process (n = 657). Patients transferred after rescue to skilled nursing facility or hospice (n = 170) had further review for serious harm identified by diagnosis of anoxic brain injury or tracheostomy (n = 8). Association of the rescue to the administration of sedative/analgesic medication was assessed by detailed chart review. |
Primary sources used to identify patients who died or were harmed by administration of sedative/analgesic medication are described. Data for each source are collected and maintained per standardized institutional processes.
Multiple patient care trajectories were considered. All patients who had rescue events in general care units and were ultimately discharged deceased, discharged to a skilled nursing facility, discharged to hospice, or had a discharge diagnosis of a tracheostomy or anoxic brain injury were selected for case review. Individual case review was performed by a critical care research nurse (RN) and by the organizational chief quality officer, also a practicing anesthesiologist (MD), to determine if the death or harm was related to administration of sedatives/analgesics. The review process included deliberate search for comments describing opioid administration, presence of respiratory depression, and evidence of respiratory depression being related to the administration of the opioid, e.g., “… patient was receiving hydromorphone and oxycodone for post-op pain and on reassessment found somnolent and hypoventilating…. The most likely causes for deterioration include opioid effect....”
The RN and MD reviewers used a 6-point scale to grade confidence that an adverse event was due to sedative analgesic medications, and not a disease process. The scale applied was described by Thomas et al,19 modified from assessing for adverse events due to medical management to specifically assess for adverse events due to sedative/analgesic management. The confidence scale was applied as follows: 1 = little or no evidence that sedative/analgesic management caused the event (e.g., patients who did not receive sedative/analgesics); 2 = slight evidence (e.g., patients who received sedative/analgesics but not in proximity to an event); 3 = not quite likely i.e., less than 50:50, but a close call (e.g., patients who received a sedative/analgesic and showed proximal signs of decline, but whose decline could not be unequivocally correlated with sedative/analgesic administration); 4 = more likely than not i.e., more than 50:50 but a close call (e.g., patients who received a sedative/analgesic with permanent harm or death, where deterioration was more likely than not related to sedative/analgesic), 5 = strong evidence (e.g., same as patients in category 4 but with strong evidence for relation to sedative/analgesic), and 6 = virtually certain evidence (e.g, same as patients in category 4 but where evidence was nearly certain for relation to sedative/analgesic). The two reviewers were trained in the use of the scale using cases from the seminal adverse event study of hospitalized patients described by Brennan et al.20 Patients determined to have experienced sedative/analgesic caused respiratory depression and/or arrest had a confidence scale equal to or greater than 4.
Measures
Counts of patient death and harm were calculated for three different circumstances: when surveillance was available and in use during the period preceding the event leading to death or harm; when surveillance was available but not in use; and when surveillance was not available in the unit where the patient was admitted. Counts were normalized using discharge data obtained via institutional records. Fisher’s exact test was used to compare the rates of death between the intent-to-treat scenario (i.e., deaths in units where surveillance was available, whether or not it was in use) and the no-treatment rate when monitoring was unavailable.
RESULTS
As shown in Table 3, there were 126,697 general care unit discharges, with 111,488 discharges in units with surveillance monitoring in place and 15,209 discharges in unmonitored units during the 10-year period of review. Overall, there were more discharges from surgical units than medicine units. There were more unmonitored medical unit discharges than surgical unit discharges driven largely by the progression of system implementation from surgical to medical units. There was one death due to sedative/analgesic medication administration, when surveillance monitoring was available (0.9/100,000 discharges). There were three deaths related to sedative/analgesic medication administration in units without surveillance monitoring available (19.7/100,000 discharges). These deaths all occurred during the 29-month period during which surveillance monitoring was being implemented throughout the institution. No patients experienced permanent harm due to sedative/analgesic medication administration during the review period. The reduced death rate when surveillance monitoring was available (0.0009%) versus not available (0.02%) is significant (P = 0.03).
TABLE 3.
Medical and Surgical Unit Discharge Data
| Surveillance Status | Discharges From General Care | Discharges From Medical Units | Discharges From Surgical Units |
|---|---|---|---|
| Surveillance available | 111,488 | 40% | 60% |
| Surveillance unavailable | 15,209 | 78% | 22% |
| Total | 126,697 | 45% | 55% |
Total discharges and percentage of total discharges in medical and surgical units for the 10-year study period are shown. Data are segmented by availability of surveillance monitoring.
All four of the cases of death scored higher than 4 on the confidence scale rating, indicating that there was strong or virtually certain evidence that sedative/analgesic management caused the event. Of the four patients who experienced death deemed due to sedative/analgesic management, there were two septuagenarians and one nonagenarian. These three patients had multiple comorbidities including hypertension, cardiomyopathy, obesity, and hypothyroidism. The fourth patient was in their 40s with several comorbidities including diabetes mellitus type 1, atherosclerotic cardiovascular disease, pulmonary hypertension, hypertension, and severe peripheral vascular disease. Two patients had surgical (orthopedic and abdominal) procedures during their admissions and two were admitted for medical management of nonsurgical orthopedic injuries (hip and spine) experienced before admission. All four patients were receiving opioids. Three of the four patients were receiving multiple opioids and/or other sedative analgesics (one patient receiving a second opioid, one receiving a benzodiazepine, and one receiving an antiemetic). The patient in their 40s was receiving opioids, despite a history of sensitivity to opioid adverse effects, and was not being monitored on the surveillance system during the time of the event.
DISCUSSION
This 10-year retrospective study demonstrated that continuous surveillance monitoring system in the general care setting had a profound effect on death from sedative/analgesic management. This result could be expected given the treatable nature of drug-induced respiratory depression. The surveillance and response system described was designed by applying the fundamental knowledge that (a) sedative/analgesic medications can cause unexpected severe respiratory depression and apnea/arrest1,5,21; (b) unrecognized and untreated respiratory arrest can progress to severe hypoxemia, cardiac arrest, hypoxic encephalopathy, and death in as little as 5 minutes; and (c) most importantly, respiratory arrest due to sedative/analgesics is readily treatable with airway support, oxygen, positive-pressure ventilation, and reversal agents (e.g., Narcan, Flumazenil). Therefore, a system designed to detect severe hypoxemia and reliably mobilize treatment in 3 minutes or less is highly likely to avert anoxic brain injury and/or death from this known complication of sedative/analgesic medications. Multiple previous studies have demonstrated that surveillance monitoring can reliably meet these requirements.9,10,22 Now, this retrospective review confirms that no monitored patient died because of sedative/analgesic-related respiratory depression during a 10-year period when pulse oximetry–based surveillance monitoring was in use. The fact that one patient with known risk for opioid sensitivity died while in a unit where monitoring was available but not in use highlights the importance of system adoption and adherence to standards of care.
Medical record review played a key role in study analysis, especially with regard to the trajectory of decompensation and impact of patient health issues, such as comorbidities. Otherwise healthy individuals are still vulnerable to unexpected sedative/analgesic-induced respiratory depression leading to death. For patients with comorbidities, which are common in a tertiary hospital patient population, this vulnerability is greatly increased and can lead to a cascade of other complications resulting in death.23 For example, a patient with pre-existing lung disease will experience hypoxia sooner when faced with opioid-induced respiratory depression. When combined with a history of coronary artery disease, the threshold for myocardial injury secondary to ischemia is lowered, increasing the risk of hemodynamic compromise, and so on. Although each of the deaths reported involved acutely ill patients who had reason to be hospitalized, the administration of an analgesic/sedative with associated respiratory depression was deemed through medical record review to be a pivotal upstream event triggering a cascade leading to death. As noted by Wong et al.24 regarding failure to rescue in the surgical oncology inpatient population, “Hospitals with low failure to rescue rates may be more effective in the early stages of diagnosis and management of seminal complications, that is, the first “dominos” in the series of adverse outcomes that frequently occur in patients dying after surgery.”
One study limitation is that comparison using study hospital baseline rates of death and harm before surveillance system implementation commenced in 2007 is not possible, as most relevant data sources were developed in conjunction with the surveillance monitoring system to better understand patient deterioration and rescue in the general care setting. Such a comparison may have provided a more comprehensive view of the before and after impact of the intervention. However, given the relatively brief time required for implementation across the various inpatient units, the monitored and unmonitored patients existed simultaneously and can be compared with respect to pre- and post-intervention. During this period, there were no significant differences in patient population or case mix index between the monitored and unmonitored patients.
Another limitation is that the methods may not yield the same results in other hospitals given possible differences in patient populations and sedative/analgesic utilization policies. For the last 10 years, the study institution has moved toward increased multimodal pain control methods and opiate reduction techniques in the postoperative population, similar to the rest of the nation. In addition, it should not be assumed that monitoring alone will yield similar results elsewhere. Implementation of surveillance or condition monitoring systems must use a robust systems engineering approach to ensure that alarms are well managed and components of the rescue system (rapid response team, etc.) are highly reliable.11,25 Finally, although cost is often raised as a barrier to implementation, a previously performed financial analysis demonstrated cost-effectiveness of surveillance monitoring due primarily to intensive care unit patient days avoided when early detection of patient deterioration occurs.16
CONCLUSIONS
This study confirms that surveillance monitoring for pharmacologically induced respiratory arrest in hospitalized patients can virtually eliminate deaths due to this serious but treatable complication. In other high-risk, safety-focused industries, the level of evidence26 that currently exists for continuous surveillance monitoring to mitigate the risk of accidental sedative/analgesic overdose would likely prompt immediate calls for widespread implementation of safety interventions. Consider, for example, that the automobile industry did not await results from multiyear, multisite randomized control trials before saving lives by designing and implementing seatbelt restraint systems as a car accident safety measure. Such is also the case in the nuclear power and aviation industries, where widespread safety interventions have also been implemented based on effective application of engineering practices and principles rather than experimental constructs created primarily for purposes other than systems design.27,28 Thus, although further study across inpatient populations and more hospitals could potentially provide a more accurate estimate of the broader impact of surveillance monitoring, considerable risk to an estimated 180,000 patients per annum remains. By applying engineering design principles and using systems and tools already available to us, it is time to eliminate deaths due to sedative/analgesic medications in hospitalized patients.
ACKNOWLEDGMENTS
The authors thank Jennifer Snide, Steve Houston, Carol Feloni, and Karen Chandler for compiling data analyzed in this study as well as Todd Mackenzie and Patrick McGrath for reviewing the methods and manuscript.
Footnotes
This study was supported by Grant Number P30HS024403 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
S.P.M. declares a relationship with Masimo limited to providing educational presentations on the topic of surveillance monitoring to clinical audiences, as of April, 2017. There was no direct or or indirect support from Masimo related to this study.
The other authors disclose no potential conflict of interest.
Contributor Information
Krystal M. McGovern, Email: krystal.m.mcgovern@hitchcock.org.
Irina M. Perreard, Email: irina.m.perreard@hitchcock.org.
Viola Huang, Email: viola.tiger.huang@gmail.com.
Linzi B. Moss, Email: linzi.b.moss@hitchcock.org.
George T. Blike, Email: george.t.blike@hitchcock.org.
REFERENCES
- 1.Macintyre PE, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care. 2011;39:545–558. [DOI] [PubMed] [Google Scholar]
- 2.Freeman WJ, Weiss AJ, Heslin KC. Overview of U.S. Hospital Stays in 2016: Variation by Geographic Region. HCUP Statistical Brief #246. [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2018. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb246-Geographic-Variation-Hospital-Stays.pdf. Accessed February 28, 2020. [PubMed] [Google Scholar]
- 3.Herzig SJ Rothberg MB Cheung M, et al. Opioid utilization and opioid-related adverse events in non-surgical patients in U.S. hospitals. J Hosp Med. 2014;9:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafi S Collinsworth AW Copeland LA, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018;153:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasero C. Opioid-induced sedation and respiratory depression: evidence-based monitoring guidelines. J Perianesth Nurs. 2012;27:208–211. [DOI] [PubMed] [Google Scholar]
- 6.Buist M Bernard S Nguyen TV, et al. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62:137–141. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandran SK Haider N Saran KA, et al. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth. 2011;23:207–213. [DOI] [PubMed] [Google Scholar]
- 8.Weinger MB, Lee LA. No patient shall be harmed by opioid-induced respiratory depression. The official journal of the Anesthesia Patient Safety Foundation, proceedings of “essential monitoring strategies to detect clinically significant drug-induced respiratory depression in the postoperative period”. Conference. 2011;26:20. [Google Scholar]
- 9.Weinger MB, Lee LA. No patient shall be harmed by opioid-induced respiratory depression. [Proceedings of “Essential Monitoring Strategies to Detect Clinically Significant Drug-Induced Respiratory Depression in the Postoperative Period” Conference]. APSF Newsletter. 2011;26:21, 26–28. [Google Scholar]
- 10.Brady WJ Gurka KK Mehring B, et al. In-hospital cardiac arrest: impact of monitoring and witnessed event on patient survival and neurologic status at hospital discharge. Resuscitation. 2011;82:845–852. [DOI] [PubMed] [Google Scholar]
- 11.McGrath SP Taenzer AH Karon N, et al. Surveillance monitoring management for general care units: strategy, design, and implementation. Jt Comm J Qual Patient Saf. 2016;42:293–302. [DOI] [PubMed] [Google Scholar]
- 12.Taenzer AH Pyke JB McGrath SP, et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010;112:282–287. [DOI] [PubMed] [Google Scholar]
- 13.Brown H Terrence J Vasquez P, et al. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med. 2014;127:226–232. [DOI] [PubMed] [Google Scholar]
- 14.Lam T Nagappa M Wong J, et al. Continuous pulse oximetry and capnography monitoring for postoperative respiratory depression and adverse events: a systematic review and meta-analysis. Anesth Analg. 2017;125:2019–2029. [DOI] [PubMed] [Google Scholar]
- 15.McGrath SP, Pyke J, Taenzer AH. Assessment of continuous acoustic respiratory rate monitoring as an addition to a pulse oximetry-based patient surveillance system. J Clin Monit Comput. 2017;31:561–569. [DOI] [PubMed] [Google Scholar]
- 16.Taenzer AH, Blike GT. Postoperative monitoring - the Dartmouth experience. ASPF Newsletter. 2012;27(1):1, 3–4, 21 (Spring-Summer 2012). [Google Scholar]
- 17.Henriksen K Battles JB Marks ES, et al., eds. Advances in Patient Safety: From Research to Implementation (Volume 4: Programs, Tools, and Products) [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. (Advances in Patient Safety). Available at: http://www.ncbi.nlm.nih.gov/books/NBK20594/. Accessed December 17, 2019. [PubMed] [Google Scholar]
- 18.Browne AM Mullen R Teets J, et al. Common cause analysis: focus on institutional change. In: Henriksen K Battles JB Keyes MA, et al., eds. Advances in Patient Safety: New Directions and Alternative Approaches (Vol 1: Assessment) [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2008. (Advances in Patient Safety). Available at: http://www.ncbi.nlm.nih.gov/books/NBK43639/. Accessed December 17, 2019. [PubMed] [Google Scholar]
- 19.Thomas EJ Studdert DM Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–271. [DOI] [PubMed] [Google Scholar]
- 20.Brennan TA Leape LL Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324:370–376. [DOI] [PubMed] [Google Scholar]
- 21.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–223. [DOI] [PubMed] [Google Scholar]
- 22.Pyke J Taenzer AH Renaud CE, et al. Developing a continuous monitoring infrastructure for detection of inpatient deterioration. Jt Comm J Qual Patient Saf. 2012;38:428–31, 385. [DOI] [PubMed] [Google Scholar]
- 23.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009;250:1029–1034. [DOI] [PubMed] [Google Scholar]
- 24.Wong SL Revels SL Yin H, et al. Variation in hospital mortality rates with inpatient cancer surgery. Ann Surg. 2015;261:632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath SP Ryan KL Wendelken SM, et al. Pulse oximeter plethysmographic waveform changes in awake, spontaneously breathing, hypovolemic volunteers. Anesth Analg. 2011;112:368–374. [DOI] [PubMed] [Google Scholar]
- 26.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berwick DM. The science of improvement. JAMA. 2008;299:1182–1184. [DOI] [PubMed] [Google Scholar]
- 28.Pronovost PJ, Bo-Linn GW. Preventing patient harms through systems of care. JAMA. 2012;308:769–770. [DOI] [PubMed] [Google Scholar]


