Abstract
Background
Axial spondyloarthritis (axSpA) is a chronic, rheumatic disease characterized by inflammation of the sacroiliac joint, spine, and entheses. Axial spondyloarthritis affects up to 1.4% of adults in the United States and is associated with decreased quality of life, increased mortality, and substantial health care–related costs, imposing a high burden on patients, their caregivers, and society.
Summary of Work
Diagnosing axSpA can be difficult. In this review, we seek to help rheumatologists in recognizing and diagnosing axSpA.
Major Conclusions
A discussion of challenges associated with diagnosis is presented, including use and interpretation of imaging, reasons for diagnostic delays, differences in disease presentation by sex, and differential diagnoses of axSpA.
Future Research Directions
The early diagnosis of axSpA and advances in available therapeutic options have improved patient care and disease management, but delays in diagnosis and treatment remain common. Additional research and education are critical for recognizing diverse axSpA presentations and optimizing management early in the course of disease.
Key Words: ankylosing spondylitis, axial spondyloarthritis, diagnosis, inflammatory back pain, primary care physicians
Spondyloarthritis (SpA) encompasses a group of inflammatory diseases that may be referred to as ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis, juvenile SpA, SpA associated with inflammatory bowel disease, undifferentiated SpA, peripheral SpA, axial SpA (axSpA), nonradiographic axSpA (nr-axSpA), or radiographic axSpA.1,2 Spondyloarthritis with predominantly axial or peripheral involvement is termed axSpA (including nonradiographic and radiographic axSpA) or peripheral SpA, respectively.1 Axial SpA is a chronic disease that mainly involves the sacroiliac joints (SIJs) and spine.3 The National Health and Nutrition Examination Survey conducted in 2009–2010 estimated that the prevalence of axSpA ranges from 0.9% to 1.4% in the adult population in the United States.4 However, the true prevalence is unknown because of the significant delay in diagnosis, underrecognition of the disease, and challenges regarding case ascertainment in epidemiological data sets.5
Patients with axSpA commonly present with back pain that starts before 45 years of age.6 The characteristic features of the back pain include chronicity (>3 months), insidious onset, improvement with exercise, occurrence at night with improvement upon waking, and no improvement with rest (Fig. 1).7–11 Inflammatory back pain (IBP) criteria are important in screening for axSpA. The relatively high sensitivity of IBP (approximately 70%–95%) for axSpA among at-risk patients (back pain >3 months with onset age <45 years) renders it useful in axSpA screening.11–13 However, studies evaluating strategies for referral to a rheumatologist showed that only 17% to 33% of at-risk patients with IBP received an axSpA diagnosis, demonstrating that IBP criteria alone are not specific for diagnosis of axSpA.11,12,14,15
FIGURE 1.
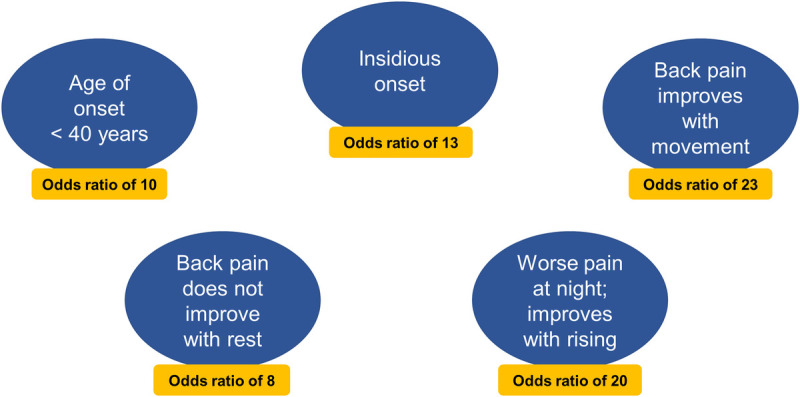
The 5 parameters that independently contribute to inflammatory back pain based on the ASAS criteria; inflammatory back pain requiring further investigation is usually indicated if 4 or more parameters are positive.10
The average symptom duration before diagnosis of axSpA has been reported to be as long as 13 years.16 The early diagnosis and subsequent treatment of axSpA may substantially decrease the burden of disease and increase quality of life; however, misdiagnoses, diagnostic delays, and underdiagnosis remain challenges.5,17 Additionally, sex differences have been described, with women experiencing longer delays in diagnosis,18,19 even though the age at disease onset is similar for men and women or even slightly earlier in women.20,21 In this narrative review, we seek to help rheumatologists in diagnosing axSpA. We also discuss current challenges with axSpA diagnosis, including use and interpretation of imaging, reasons for diagnostic delays, differences in disease presentation by sex, and differential diagnoses of axSpA.
Concepts of axSpA and Common Disease Features
The Assessment of SpondyloArthritis international Society (ASAS) have developed classification criteria for axSpA,6,22 as well as peripheral SpA or SpA in general.23 The criteria for axSpA are based on the presence of sacroiliitis on imaging or human leukocyte antigen B27 (HLA-B27) positivity in the presence of other SpA features (Fig. 2A).6,22 Axial SpA with radiographic sacroiliitis is designated as radiographic axSpA, which overlaps with the definition of AS based on the 1984 modified New York criteria (Fig. 2B).6,24 Radiographic sacroiliitis is the hallmark of AS25; however, AS symptoms such as back pain, difficulty sleeping, and fatigue may be present for several years before radiographic changes are detected. Axial SpA without definitive radiographic sacroiliitis is termed nr-axSpA. Patients with nr-axSpA may or may not have sacroiliitis on magnetic resonance imaging (MRI).26
FIGURE 2.
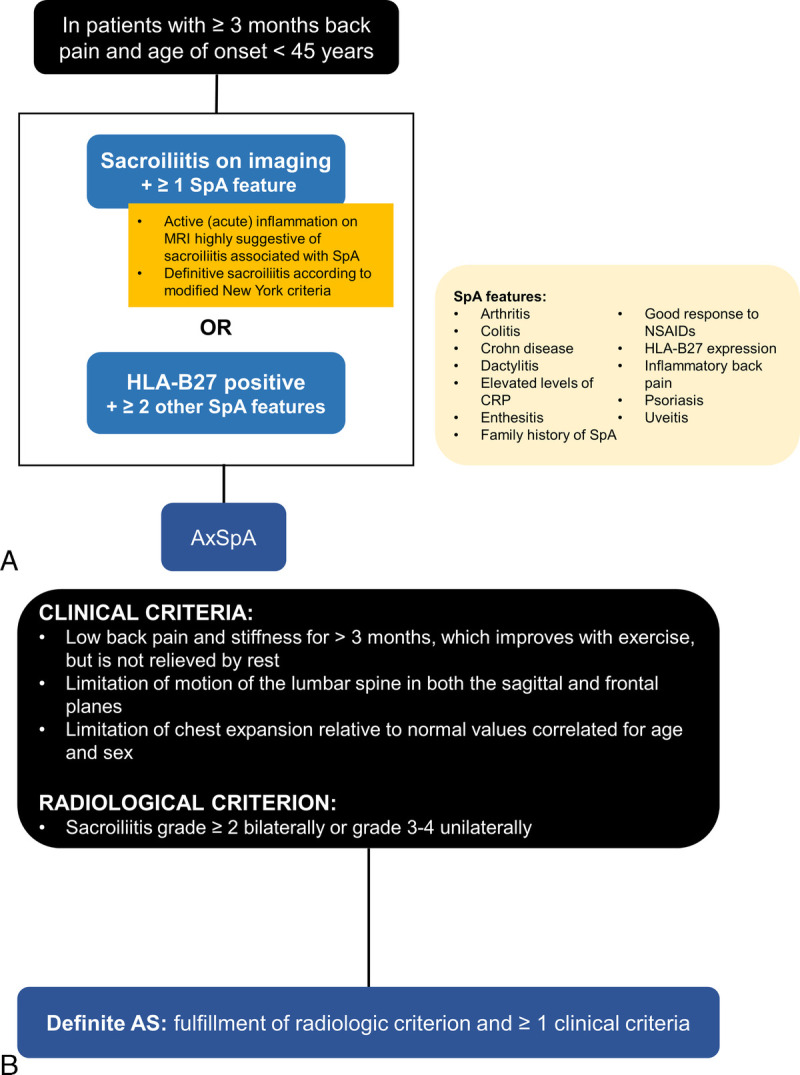
A summary of the (A) 2009 ASAS classification criteria for axSpA in patients presenting with chronic back pain lasting 3 months or more and age at onset of younger than 45 years6,22 and (B) 1984 modified New York criteria for AS. The ASAS criteria account for patients with and without radiographic sacroiliitis. Furthermore, patients meeting both the ASAS and modified New York criteria are classified as having AS.6,24
The conceptual paradigm of axSpA has been evolving. Nonradiographic axSpA and radiographic axSpA may be considered as 2 distinct but overlapping subtypes of axSpA. Axial SpA may also be conceptualized along a continuum of structural damage, with nonradiographic disease representing little or no structural damage and radiographic axSpA damage representing more advanced structural damage to the SIJs.27,28 Some patients initially classified with nr-axSpA will progress along the damage continuum to later fulfill criteria for radiographic axSpA29,30; studies have shown that 5% to 25% of patients with nr-axSpA progressed to radiographic axSpA within 2 to 8 years,31–33 and up to 28% progressed 10 years or more after diagnosis.34 Regardless, the overall burden of disease, as determined by disease activity, quality-of-life measures, and responses to treatment, is similar in patients with AS and those with nr-axSpA.27,35–38
In general, the diagnosis of axSpA is a clinical judgment based on features that, taken together, are characteristic of the disease.39 These features include diverse combinations of historical manifestations, physical findings, laboratory results, and imaging data. Common SpA features are listed in Table 1.7–9 Likelihood ratio estimates are also included for conceptualizing the relative importance of various SpA features for detecting axSpA (Table 1).7–9
TABLE 1.
| SpA Features | LR |
|---|---|
| Psoriasis | 2.5 |
| Increased levels of acute-phase reactantsa | 2.5 |
| Inflammatory back pain | 3.1 |
| Heel pain (enthesitis) | 3.4 |
| Inflammatory bowel diseaseb | 4.0 |
| Peripheral arthritis | 4.0 |
| Dactylitis | 4.5 |
| Good response to NSAIDs | 5.1 |
| Family history of axSpA, reactive arthritis, inflammatory bowel disease, psoriasis, or uveitis | 6.4 |
| Iritis or anterior uveitis | 7.3 |
| HLA-B27 expression | 9.0 |
| Sacroiliitis by MRI | 9.0 |
aAcute-phase reactants include erythrocyte sedimentation rate and CRP.
bInflammatory bowel disease includes Crohn disease and ulcerative colitis.
Extra-articular manifestations such as uveitis, inflammatory bowel disease (including Crohn disease and ulcerative colitis), enthesitis, and psoriasis are relatively common in SpA diseases.40 Reports of the prevalence of uveitis among patients with axSpA range from 6% to greater than 30%.28,41–45 Uveitis in axSpA is an HLA-B27–associated disease characterized by episodes of acute, painful inflammation in the anterior chamber of the eye; it is an indicator of disease severity in axSpA and has been linked to worse physical function.46 The prevalence of inflammatory bowel disease is approximately 4% to 6% among patients with axSpA.40 Minimally symptomatic gut inflammation occurs in up to 60% of patients with AS, and up to 20% of these patients may develop Crohn disease within 5 years.47–50 Enthesitis refers to inflammation at the insertion sites of tendons, ligaments, or joint capsule fibers into bone,51 with a prevalence of approximately 35% to 60% in axSpA.40,52,53
Psoriasis affects approximately 10% of patients with axSpA.28,40 Some patients with psoriatic disease and axial involvement (axial psoriatic arthritis [axPsA]) may present differently than patients with other forms of axSpA. Age at onset may be older than 45 years in some patients, and IBP has been reported in approximately half of patients with axPsA.54 Sacroiliitis may be less symmetrical and of a lower grade with axPsA than AS, and syndesmophytes are more frequently paramarginal and bulky in axPsA.55 Furthermore, in AS, vertebral involvement typically begins in the lumbar spine or thoracolumbar junction and progresses caudally along contiguous vertebrae,25 whereas in axPsA, noncontiguous vertebrae may be affected, and isolated cervical involvement may occur.55 Importantly, up to one-third of patients with axPsA have spondylitis without sacroiliitis, whereas sacroiliitis is believed to occur before or in conjunction with spondylitis in patients with AS.55,56 Patients with axPsA have lower rates of HLA-B27 positivity, particularly patients with manifestations that differ from classic AS-like presentations.55,56 The relative impact of various axPsA subphenotypes is largely unknown, but the burden of axPsA (all subphenotypes) has been reported to be comparable to that of AS with respect to disease activity, function, and quality of life.55–59 Although additional research is required to better understand the spectrum of axPsA, improved awareness of the diversity of axPsA may influence recognition of axial involvement, particularly in patients who lack classic IBP or involvement of the SIJs.
Diagnostic Considerations
Diagnostic algorithms have been designed to guide rheumatologists in assessing clinical SpA features, imaging characteristics, and laboratory results. The algorithm shown in Figure 3 was tested in populations with chronic back pain (lasting >3 months) at onset age of younger than 45 years; using diagnosis by a rheumatologist as the external standard, the ASAS modifications of the original Berlin algorithm had sensitivities of 77.9% to 79.7% and specificities of 78.3% to 80.4%.60 Although the overall performance of this algorithm was favorable, approximately 20% of patients with axSpA were misdiagnosed as not having axSpA. For patients with a high suspicion of axSpA, despite a negative diagnosis per the algorithm, additional imaging may be considered. For example, SIJ MRI may be appropriate in some patients who are x-ray and HLA-B27 negative but have 2 to 3 SpA features, and spondylitis may be considered in symptomatic psoriatic patients without sacroiliitis. Symptom onset later in life would be unusual, although some patients with axSpA may present with loss of spinal mobility rather than back pain as the primary symptom, and more research about onset age is required for axSpA subsets such as axPsA.
FIGURE 3.
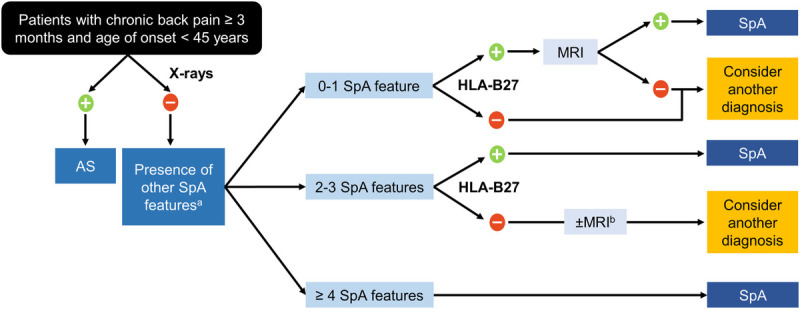
A diagnostic guide for axSpA among patients with chronic low back pain lasting 3 months or more and age at onset of younger than 45 years.60 A, Spondyloarthritis features include alternating buttock pain, dactylitis, asymmetrical arthritis, elevated acute-phase reactants (e.g., CRP or erythrocyte sedimentation rate), enthesitis, inflammatory back pain, inflammatory bowel disease, family history of SpA in a first- or second-degree relative, marked response to NSAIDs, psoriasis, and uveitis. B, The figure was modified from its original version to suggest that MRI of the sacroiliac joints may be appropriate in some patients who are x-ray– and HLA-B27–negative but who have 2 to 3 SpA features.
Clinical SpA Feature Evaluation: History and Physical Examination
Clinicians should inquire about SpA features (e.g., IBP, peripheral joint inflammation, enthesitis, uveitis, or response to nonsteroidal anti-inflammatory drugs [NSAIDs]) and arrange for referrals to collaborating specialists, when appropriate, for diagnostic confirmation of suspected uveitis, inflammatory bowel disease, and psoriasis.61 Family histories of first- and second-degree relatives with axSpA, psoriatic disease, inflammatory bowel disease, and uveitis are also useful in assessing axSpA risk. A physical examination of patients with axSpA may be completely unremarkable, but clinicians should examine patients for SpA features (e.g., tender and swollen joints, enthesitis, dactylitis, cutaneous psoriasis, psoriatic nail changes) and alternative explanations of back pain (e.g., disk disease, scoliosis). Measurements of spinal mobility, such as the Schober test, are highly variable in healthy individuals and may not be particularly helpful in the diagnosis of axSpA.62 Physical examinations for sacroiliitis have low sensitivity and specificity.63
Laboratory Tests
Although no laboratory tests are diagnostic of axSpA, testing for HLA-B27 may be useful in diagnostic assessments (Table 1). Human leukocyte antigen B27 positivity occurs in 70% to 90% of White patients with AS but in less than 10% of the general population64–66; however, the prevalence of AS in the White population with HLA-B27 positivity is approximately only 5%.66 Studies in patients with nr-axSpA have reported the prevalence of HLA-B27 positivity at 74% to 86%, but these estimates should be interpreted cautiously, because HLA-B27 positivity was used as a criterion for identifying these patients.29 Hence, HLA-B27 positivity alone is not diagnostic for axSpA, and a lack of a positive HLA-B27 test does not exclude the diagnosis.67 The diagnostic utility of markers of systemic inflammation, such as elevated erythrocyte sedimentation rate or C-reactive protein (CRP), is low; less than 50% of patients with AS present with elevated inflammatory markers at initial presentation, and many other conditions also cause elevations in these inflammatory markers.68
Imaging
For radiographic imaging of patients with chronic low back pain and suspected axSpA, a single, anteroposterior view of the pelvis or a Ferguson view is commonly taken to evaluate for sacroiliitis. Oblique views may arguably provide additional useful information but require slightly higher radiation levels. Changes suggestive of sacroiliitis include sclerosis, erosions, changes in joint width, and ankylosis. Interpretation of sacroiliitis on radiographs can be challenging, and agreement among expert readers is lower when changes are limited to sclerosis or small, localized erosions without changes in joint width or ankylosis.
If the pelvis radiograph of the SIJ is unremarkable or equivocal for sacroiliitis and there is ongoing clinical suspicion for axSpA, a pelvic MRI should be considered. In contrast to plain radiographs or computed tomography (CT), MRI can detect active inflammatory lesions, regardless of structural lesions, and is more sensitive for detecting active sacroiliitis.39,69 Currently, the recommended MRI studies include axial and semicoronal sections of the SIJs, T1 sequence (for structural evaluation), and a water-sensitive sequence (to detect inflammation), such as short tau inversion recovery (STIR) or T2-weighted sequence with fat suppression.
The ASAS definition of active sacroiliitis was updated in 2016 to state that active inflammatory lesions of the SIJs should appear as osteitis or bone marrow edema on STIR or T2-weighted images with fat suppression and be clearly present in typical locations, such as the subchondral or periarticular bone marrow.70 A positive MRI should show 2 or more bone marrow edema lesions on the same slice or 1 lesion in the same quadrant on 2 or more consecutive slices.70 Structural changes may be seen on T1 images, including erosions, partial or complete ankylosis of the SIJs, sclerosis, and fat metaplasia of the subchondral bone.71
Although the site of pain is associated with the site of MRI inflammation overall, a limitation of SIJ MRI is that the axial site with the most bone marrow edema may not be the site with the most pain72; thus, clinical activity may not consistently correlate with MRI activity.73 Also, MRI activity may change over time.74 Magnetic resonance imaging reading and interpretation by radiologists and/or rheumatologists are inherently subjective and can vary even among experienced evaluators.75 No objective reference could be established during the development of the ASAS criteria.76–78 The presence of bone marrow edema on MRI is not specific for axSpA, as it has been observed in patients with mechanical back pain and healthy volunteers.79–81 Bone marrow edema fulfilling ASAS criteria was observed among recreational runners, elite ice hockey players, and military recruits before and 6 weeks after intensive physical training82,83 and in women with postpartum back pain.84 In the SPondyloArthritis Caught Early (SPACE) cohort, a study examining patients with unexplained chronic back pain, 11 of 47 healthy volunteers (23.4%) and 43 of 47 patients with axSpA (91.5%) had MRI lesions consistent with sacroiliitis.84 Additional research with MRI and other imaging techniques, such as low-dose CT, may lead to improvements in the diagnostic assessment of axSpA. Low-dose CT shows promise for the evaluation of SIJs, because it demonstrates subtle bony changes better than radiographs, and the radiation risk is low (albeit slightly higher than with radiographs). At the time of writing, the role of low-dose CT in clinical practice remains unclear because of limited availability and uncertain cost.
Spinal imaging is not recommended for axSpA diagnosis in most cases, but spine images may identify alternative causes of back pain, provide prognostic information, and help identify subsets of patients with less-typical axial presentations (e.g., axPsA). If a spinal MRI is deemed appropriate, the sequences should include T1 and water-sensitive sequences (STIR or T2 fat suppressed).
Underdiagnosis and Diagnostic Delay
Early diagnosis of axSpA is important to minimize disease burden. Importantly, disease activity as measured by the Bath Ankylosing Spondylitis Disease Activity Index and patient-reported pain is high in the early stages of the disease, independent of radiographic changes.85 Treatment improves symptoms and physical function,86 and patients with AS with a shorter disease duration have been shown to have better response rates than patients with a longer disease duration.87 Thus, delayed diagnosis and treatment contribute to the substantial burden of disease on patients.88
Although the magnitude of underdiagnosis in axSpA is difficult to quantify, the gap between axSpA prevalence estimates from epidemiological screening research4 and health care record data suggests that underdiagnosis is common.89 In a National Health and Nutrition Examination Survey, adults representative of the general population of the United States were prospectively screened for axSpA with face-to-face evaluations, and the axSpA prevalence was estimated at 0.9% to 1.4%.4 In contrast, chart review studies reported an axSpA prevalence ranging from 0.2% to 0.7%.89 This difference in prevalence estimates suggests that axSpA is widely underdiagnosed and underaddressed in our current health care systems. Diagnostic delays are also common. Feldtkeller and colleagues21 published a seminal report in 2003 describing an average diagnosis delay from symptom onset of 8.5 to 11.4 years in patients with AS, with a longer delay in patients lacking HLA-B27 positivity. More recently, the estimated delay between AS symptom onset and diagnosis in the United States has been reported to be 14 years.16
Reasons for underdiagnosis and diagnostic delays are multifactorial. Patients may be slow to seek care because of the insidious, intermittent nature of symptoms or because of logistical considerations that disproportionately affect young adults (e.g., inadequate insurance, limited flexibility with work schedules). Also, providers may not consider axSpA in patients with back pain, because a relatively small percentage of patients with back pain has axSpA.9,90,91 Additionally, the absence of remarkable physical findings or unique biomarkers and the frequent lack of extra-articular manifestations make early diagnosis challenging.92 Strategies for screening and referring patients with suspected axSpA to a rheumatologist may be helpful, but they are not practical for many clinical settings in the United States and are infrequently implemented. Thus, processes for identifying and prioritizing appropriate patients for rheumatologist evaluations are often inconsistent or inefficient.93,94
Misdiagnoses are also common contributors to diagnostic delays and underdiagnosis among patients with axSpA.17 Prior to receiving their axSpA diagnoses, patients often consult with various types of health care providers for unrecognized axSpA symptoms,17 thus experiencing unnecessary diagnostic workups and interventions that may delay appropriate evaluations and referrals.17,95 Interventions to alleviate pain symptoms may also delay diagnosis.16 Importantly, limited accessibility to a rheumatologist leads to diagnostic delays in many communities.96,97
Sex Differences in AxSpA Diagnosis
Women experience longer delays in receiving a diagnosis of axSpA,18,19,98 even though studies show that the age at onset does not vary between men and women or that women have a slightly younger age at onset of AS20,21; this may reflect erroneous assumptions that women rarely have AS and insufficient awareness of the differences in disease presentations between women and men.88 Specifically, enthesitis, patient-reported disease activity, and quality of life were significantly worse in women than in men with AS in several studies.99–105 In a study, women reported more pelvic, heel, and widespread pain than men during the course of the disease.106 Women may also be more likely than men to receive misdiagnoses of fibromyalgia and psychosomatic disorders.17
Women and men also differ with regard to structural changes in the spine and SIJs. Male sex has been associated with more severe radiological progression.107,108 Men had more limited chest expansion and increased occiput-to-wall distance than women.106 In a 5-year, prospective study of spinal radiographic progression in AS, elevated CRP levels and increased smoking were reported as predictors of structural progression in men but not in women.109
Much is to be learned about the reasons that axSpA differs between men and women. Several studies have demonstrated that women and men with AS have different gene expression110 and immunologic processes.111,112 Despite equivalent interferon γ levels among men and women with AS, higher levels of TH17 cells and cellular responses were observed in men.111 Additionally, levels of inflammatory molecules, such as vascular endothelial growth factor, interleukin 18, and tumor necrosis factor α, were significantly higher in men with AS than in women in 1 study, which led investigators to believe that men may more frequently experience irregular bone metabolism, leading to osteoproliferation and syndesmophyte formation.112 Genetic studies reported a modest to strong association of the ANKH haplotype with AS, with more frequent ANKH variants on the 3′ end in men and 5′ end in women.110 The murine homolog of the ANKH protein, Ank, regulates normal osteogenesis; mutant mice expressing a premature stop codon at the 3′ end of the Ank gene develop severe ankylosis.113 Understanding sex differences in axSpA is important for decreasing diagnostic delays and misdiagnoses, particularly among women.
Differential Diagnosis
The differential diagnosis of axSpA is broad; details are discussed below, and a summary is provided in Table 2. Diseases that may be particularly challenging to differentiate from axSpA include causes of chronic back pain beginning in adolescence or early adulthood and diseases that mimic axSpA changes on imaging of the spine or SIJs.
TABLE 2.
Summary of the Differential Diagnosis of AxSpA
| Chronic Back Pain With Onset in Adolescence or Early Adulthood | Distinguishing Features |
|---|---|
| Degenerative disk disease114–119 | • MBP exacerbated by flexion ± radicular pain radiating below the knee • Risk factors: obesity, genetics, repetitive microtrauma, other injury • Imaging: disk space narrowing. Disk herniation may be visible on MRI |
| Fibromyalgia120–123 | • MBP with widespread pain in other areas of the body, fatigue, and sleep disturbances. Minimal relief with NSAIDs • Risk factors: female sex, obesity, ± adverse childhood experiences • Imaging: no evidence of inflammatory disease or other etiology |
| Spondylolysis and spondylolisthesis124–129 | • MBP exacerbated by hyperextension that may extend into buttocks or posterior thighs • Risk factors: activities with repetitive flexion and extension of the lower back (gymnastics, football, ice skating, weightlifting, etc.) • Imaging: with spondylolysis, separation or fracture of the pars interarticularis (pars defect). With bilateral pars defects, the injured vertebra may be shifted forward relative to the vertebra directly below it (spondylolisthesis) |
| Scheuermann disease130–132 | • MBP occurring in early adolescence without precipitating trauma • Risk factors: male sex, more severe with tall height • Imaging: vertebral wedging (wider posterior vs. anterior angle) and possible Schmorl nodes (protrusion of disk material into the vertebrae) |
| Astrocytomas133,134 | • Gradual or subacute MBP onset, often with subsequent development of sensory or motor dysfunction • Risk factors: genetics, ionizing radiation • Imaging: asymmetrical spinal cord expansion on MRI |
| Mimics of AxSpA on Imaging | Distinguishing Features |
| Osteitis condensans ilii135–138 | • Asymptomatic or nonradicular low back pain that can extend to the posterior thighs • Risk factors: multiparity, other mechanical stress • Imaging: SIJ sclerosis of the iliac side without erosions or fusion |
| DISH139–145 | • Asymptomatic or MBP • Risk factors: male gender, age >50 y, diabetes, obesity • Imaging: flowing bone along the anterolateral vertebral bodies and across the disk space in the thoracic spine with or without lumbar and cervical spine involvement. A lucent area may be seen between the anterior longitudinal ligament and midportion of the vertebral body. SIJs are often unaffected, but the superior aspect of the SIJ may appear fused. Peripheral calcific enthesopathy may occur |
| Infectious sacroiliitis146–151 | • Subacute onset of unilateral buttock or back pain with elevated CRP. Fever is usually absent or low grade • Risk factors: IV drug use, pelvic trauma, infectious endocarditis, immunosuppression, cutaneous or genitourinary infection • Imaging: on MRI, unilateral periarticular muscle edema, thick capsulitis, and extracapsular fluid collection may be useful in differentiating infectious sacroiliitis from sacroiliitis due to axSpA |
| Whipple disease152–161 | • Large joint migratory arthralgias, abdominal pain, weight loss, and diarrhea, with or without IBP • Risk factors: occupational exposure to soil or animals • Imaging: sacroiliitis and spondylitis indistinguishable from axSpA |
| Familial Mediterranean fever143,162–164 | • Intermittent fevers, abdominal pain, large joint arthritis, enthesitis, IBP. Childhood or adolescent onset is typical, but may occur in adulthood • Risk factors: genetics (MEFV gene mutations); Turkish, Armenian, North African, Jewish, and Arab descent • Imaging: sacroiliitis indistinguishable from axSpA |
| Sarcoidosis149,165–170 | • IBP • Risk factors: sacroiliitis may occur most frequently in sarcoidosis limited to the thorax (thoracic lymph nodes and lungs) • Imaging: sacroiliitis indistinguishable from axSpA |
| Spinal calcium pyrophosphate deposition disease171–173 | • Periodic IBP with elevated inflammatory markers • Risk factors: widespread peripheral chondrocalcinosis • Imaging: linear calcium deposition in intervertebral disks, SIJs, and/or peripheral joints |
| Idiopathic hypoparathyroidism174–178 | • Hypocalcemia presentation ± back pain • Risk factors: long-standing hypoparathyroidism • Imaging: syndesmophytes. SIJ usually not involved but subchondral bone resorption or sclerosis may occur |
| Behçet disease179–188 | • Recurrent mucocutaneous ulcers, ocular inflammation, peripheral arthritis, ± entheseal inflammation • Risk factors: HLA-B51 gene; Turkish Japanese, Korean, Chinese, Iranian, Iraqi, Saudi Arabia descent • Imaging: sacroiliitis indistinguishable from axSpA |
| Hereditary hypophosphatemic rickets189–191 | • Growth limitations, osteoarthritis, back pain, enthesopathies • Risk factors: genetics (autosomal recessive) • Imaging: syndesmophytes without inflammatory spinal lesions beginning in the second or third decade of life. SIJs typically spared |
| Ochronosis192–195 | • Hyperpigmentation of skin or sclera, back pain, range-of-motion limitations, and kyphosis beginning in the third decade of life • Risk factors: genetics (autosomal recessive) or exposure to hydroquinone or phenols • Imaging: affects the intervertebral disks and large joints. Ankylosis in the lumbar or thoracic spine may develop later in the disease course. SIJs usually spared |
IV, intravenous; MBP, mechanical back pain.
Causes of Chronic Back Pain With Onset in Adolescence or Early Adulthood
Degenerative Changes
Spinal degenerative changes are common in young adults. In a study of 20- to 22-year-old patients, 47% had degenerative changes on lumbar MRI, and 52% reported low back pain.114 In a population of patients with low back pain younger than 40 years, 38% had degenerative disk changes, including 13% with vertebral end plate spinal changes.115 Differentiating degenerative changes from axSpA changes can be challenging.116,117 For instance, degenerative changes on end plates may be mistaken for inflammatory lesions, as both are associated with bone marrow edema.118 In the Dutch and French early axSpA study populations, the prevalence of spinal degenerative changes on MRI was high and similar in patients with and without axSpA.118,119 Despite the high prevalence of degenerative changes and overlapping features of inflammatory and degenerative changes on MRI of the spine, several features can be used to distinguish between degenerative and inflammatory spine disease, including the nature of the back pain, MRI findings (e.g., location of bone marrow edema in the anterior corners of the vertebrae, fatty lesions at the anterior vertebral corners), sacroiliitis, and extra-axial inflammatory arthritis features (e.g., peripheral arthritis, enthesitis, uveitis, HLA-B27 positivity).118,119
Fibromyalgia
Patients with fibromyalgia experience widespread pain, including chronic back pain and tenderness at multiple sites that may mimic enthesitis.120,121 However, back pain with fibromyalgia is usually mechanical in nature, rather than inflammatory.122 In contrast to patients with axSpA, those with fibromyalgia experience little or no pain relief with NSAIDs.123 Furthermore, the sites of maximal point tenderness with fibromyalgia are usually not located precisely at entheseal sites. Differentiation between fibromyalgia and axSpA can be challenging, particularly in patients without definitive imaging findings of axSpA. Fibromyalgia may co-occur with axSpA, with prevalence of fibromyalgia within populations of AS or axSpA ranging from 4% to 25%.120
Spondylolysis and Spondylolisthesis
Among pediatric patients and young adults, spondylolysis manifests as a fracture of the posterior arch in the lower lumbar spine due to overuse (i.e., hyperextension observed in particular athletes), and spondylolisthesis refers to the anterior displacement of a vertebral body due to bilateral defects of the posterior arch.124,125 Spondylolysis may progress to spondylolisthesis.124 For imaging, MRI is usually preferred due to the lack of radiation exposure. Magnetic resonance imaging detection of a typical spondylolytic lesion requires an edema-sensitive sequence with a STIR or fat-saturated T2 image; a cortex-sensitive image with a T1 or non–fat-saturated T2 sequence; and axial, sagittal, and coronal views.126 Magnetic resonance imaging may reveal increased metabolic activity and the presence of a fracture, although further radiographic evaluation may be needed.127–129
Scheuermann Disease
This condition of unknown etiology is characterized by uneven growth of the vertebrae with respect to the sagittal plane, with anterior compression of 5° or greater in 3 or more adjacent vertebral bodies and thoracic spine kyphosis greater than 40° or thoracolumbar spine kyphosis greater than 30°.130 Scheuermann disease usually presents in early adolescence and is associated with subacute pain without precipitating trauma.131 The pain improves with rest and is worse with activity and extension. Pain often improves with skeletal maturity in adulthood,131 but long-term follow-up of adolescents with Scheuermann disease indicates an increased prevalence of back pain in adulthood.132 Standing lateral spine radiographs are required for diagnosis, and Schmorl nodes, with protrusion of disk material into the vertebrae, may be observed.131
Primary Tumors of the Spine
Spinal tumors are rare but should be considered in adolescents and young adults with unexplained back pain. Astrocytomas represent the most common spinal cord tumors in both the pediatric and adult populations and typically present with gradual or subacute onset of regional back pain.133,134 Astrocytomas can be differentiated from axSpA by the noninflammatory nature of the back pain, sensory or motor dysfunction that may evolve over the course of months or years, and MRI lesions that typically appear as an asymmetrical spinal cord expansion.133
Diseases With Axial Imaging Features That May Mimic axSpA
Osteitis Condensans Ilii
Osteitis condensans ilii, characterized by benign sclerosis of the ilium adjacent to the SIJ, affects 0.9% to 2.5% of the general population.135 It is typically an incidental finding but it can cause nonradicular low back pain,135,136 especially in women who have given birth.135,137 No fusion or erosion is observed in the SIJ. Subchondral bone marrow edema may be present later in the disease stage.138
Diffuse Idiopathic Skeletal Hyperostosis
Diffuse idiopathic skeletal hyperostosis (DISH) is characterized by flowing hyperostosis at 4 or more contiguous vertebral bodies and the calcification and ossification of soft tissues, principally ligaments and entheses.139 Diffuse idiopathic skeletal hyperostosis is mostly seen in men older than 50 years.140 In a Hungarian study among those older than 50 years, the prevalence of DISH was 4.9% and 1.4% in men and women, respectively.141 Its etiology is unknown but has been linked to metabolic disorders, such as obesity and insulin-dependent diabetes mellitus.142 Although most patients are asymptomatic, some experience chronic back pain, stiffness, and limited mobility of the spine.143 This condition may affect the SIJ, leading to a suspicion of sacroiliitis, as seen in AS; however, several distinguishing factors may delineate the 2 diseases.139 Diffuse idiopathic skeletal hyperostosis tends to involve the superior portion of the SIJ, whereas AS involves the inferior portion. Erosions may be seen in the SIJs of patients with AS, but not in patients with DISH.139,144 Diffuse idiopathic skeletal hyperostosis tends to occur on the right side of the spine, and radiolucencies between the calcified anterior longitudinal ligament and the anterior vertebral body may be seen in DISH.145 Ankylosing spondylitis and DISH can occur concurrently, although this co-occurrence is infrequently observed.139
Infectious Sacroiliitis
Accounting for up to 4% of bone and joint infections,146,147 infectious sacroiliitis is usually seen in children and younger patients.146 The time to diagnosis of infectious sacroiliitis may be delayed (average of ≈45 days) because of a lack of specific symptoms; patients usually present with unilateral pain and elevated CRP but do not always have fever.148Staphylococcus aureus is the primary etiologic agent responsible for this infection; Streptococcus, Escherichia coli, and Salmonella have also been recovered from affected patients.147,148 The SIJ is the most commonly reported osteoarticular space for infections with Brucella, evidenced by its recovery from synovial fluid.149
Musculoskeletal symptoms occurring in infectious sacroiliitis are not easily distinguishable from other causes of sacroiliitis.149 Radiographic changes initially show widespread erosions and subsequent bony repair, possibly involving more than the anterior-inferior synovium of the joint; radiographic findings are usually delayed by approximately 2 weeks. Although the findings are not specific, MRI is the imaging technique of choice and will demonstrate intra-articular fluid, bone marrow edema, and periarticular involvement, particularly during the earlier stage of the infection, whereas subchondral sclerosis, erosions, and ankylosis will be apparent in the later stages of the infection.150,151
Whipple Disease
A rare, bacterial infection caused by the gram-positive bacillus Tropheryma whipplei, Whipple disease is marked by the lack of inflammatory response to or cytotoxic effects from the infection, suggesting host immune deficiency or immune system downregulation by the bacteria.152–156 This condition is commonly characterized by peripheral joint symptoms, which are present in up to 80% of patients.157,158 Inflammatory back pain, sacroiliitis, and spondylitis may occur with Whipple disease. Many patients are initially misdiagnosed with enteropathic arthritis or other forms of seronegative inflammatory arthritis, with at least 50% in 2 studies receiving immunomodulatory therapy, including tumor necrosis factor inhibition.159,160 A diagnosis of Whipple disease should be considered in patients presenting with the 4 principal manifestations of arthralgias, diarrhea, abdominal pain, and weight loss.161
Familial Mediterranean Fever
Patients with familial Mediterranean fever may have back pain, peripheral arthritis, enthesitis, and imaging changes typical of sacroiliitis.162–164 Sacroiliitis more commonly occurs in patients with familial Mediterranean fever with HLA-B27 positivity and/or M694V.143 A history of intermittent fevers and serositis may distinguish patients with familial Mediterranean fever from those with axSpA.143
Sarcoidosis
A systemic, chronic granulomatous disease, sarcoidosis commonly affects the skin, lungs, and musculoskeletal system149; the prevalence of radiographic sacroiliitis in sarcoidosis ranges from 6% to 14%.165–167 Sacroiliac joint involvement in sarcoidosis often occurs with a history of IBP but may present in patients with mechanical back pain.149,168 Radiographic evidence of sacroiliitis in sarcoidosis may be similar to that of AS; a biopsy of the SIJ may reveal noncaseating granulomata in the synovium, but SIJ biopsy is usually unnecessary if a sarcoidosis diagnosis can be made via clinical presentation or biopsy of extra-articular tissue.169 Sacroiliitis may occur most frequently in sarcoidosis limited to the thorax.170
Spinal Calcium Pyrophosphate Deposition Disease
Although deposition of calcium pyrophosphate dihydrate crystals into fibrocartilage usually occurs in peripheral joints, involvement of the cervical, thoracic, and lumbar spine has been documented.171 Patients may experience episodic IBP from acute sacroiliitis with elevated inflammatory markers.172,173 Asymptomatic changes to the SIJs that may resemble sacroiliitis from AS may also occur.171,173 Linear calcium deposition in the SIJs, intervertebral disks, and peripheral joints is useful in differentiating calcium pyrophosphate deposition disease from axSpA.171
Idiopathic Hypoparathyroidism
Patients with this condition present with various clinical manifestations, including morning stiffness, spinal pain, nail deformities, renal abnormalities, increased bone mineral density, and/or vertical syndesmophyte formation similar to axSpA.174,175 Syndesmophytes are associated with long-standing hypoparathyroidism. Sacroiliac joints are usually not involved, but there may be subchondral bone resorption of the SIJ with minimal cartilage irregularities.174,176,177 Hypoparathyroidism should be suspected in patients with low serum total calcium or low ionized calcium levels, especially those with a personal or family history of autoimmune diseases.176,178
Behçet Disease
Behçet disease is marked by recurrent mucocutaneous ulcers, ocular inflammation, and peripheral inflammatory arthritis; entheseal inflammation may also occur.179 Interestingly, an elevated prevalence of sacroiliitis has been reported in patients with Behçet disease; however, the prevalence has been highly variable, likely due to interobserver variability, and has been more recently shown to be comparable to healthy controls.180–184 On imaging, SIJ changes may be indistinguishable in patients with axSpA versus Behçet disease.181 Uveitis may occur with both axSpA and Behçet disease, but with axSpA, uveitis is typically limited to the anterior chamber of the eye, whereas uveitis with Behçet disease may also involve the posterior and intermediate ocular chambers.43,185,186 Like axSpA, arthritis in medium and large joints is common in Behçet disease, occurring in approximately one-half of patients.187 Behçet disease differs from axSpA through a strong association with the HLA-B51 but not HLA-B27 gene.188
Hereditary Hypophosphatemic Rickets
X-linked hypophosphatemia, due to a mutation in the phosphate-regulating endopeptidase on the X chromosome (Phex gene), is primarily characterized by musculoskeletal anomalies among children and osteoarthritis and enthesopathy among adults.189,190 In the late second or third decade of life, adults with hypophosphatemic rickets may experience new bone formation in the spine that resembles axSpA changes.190 In contrast to axSpA, patients with X-linked hypophosphatemia do not develop sacroiliitis or inflammatory lesions in the spine, such as erosions or bone marrow edema.191
Ochronosis
Ochronosis is a manifestation of alkaptonuria, a rare, autosomal recessive disorder that causes blue-black pigmentation in skin, sclera, and other connective tissues.192,193 Spinal ochronosis, a progressive condition, results from the deposition of the ochronotic pigment within articular cartilages, especially in the dorsolumbar spine, leading to decreased intervertebral disk spaces, disk calcification, and osteopenia; ankylosis may develop spontaneously later in the disease course that may mimic the spinal bony productive lesions of axSpA.194 In ochronosis, bamboo spine, annular ossification, joint erosion, and fusion of the SIJs do not occur.193,195
CONCLUSIONS
Over the past decade, the concept of axSpA has broadened to include AS and previously unrecognized axSpA without sacroiliitis on radiographs. This conceptual evolution has spurred research on the early detection and diagnosis of axSpA, revealing differences in disease presentation and severity between men and women, barriers to early diagnosis, and other axSpA-like conditions that may confound diagnosis. The early detection and diagnosis of axSpA, along with breakthroughs in available therapeutic options, have led to improvements in patient care and disease management, but delays in diagnosis and treatment remain common. Additional research and education are critical for recognizing diverse axSpA presentations and optimizing management early in the course of disease.
ACKNOWLEDGMENT
The authors thank Kheng Bekdache, PhD, and Eric Deutsch, PhD, CMPP, of Health Interactions, Inc., for providing medical writing and editorial support, which was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Footnotes
J.A.W. received research funding from AbbVie and Pfizer and served as a consultant for Amgen, Lilly, Novartis, and UCB. M.M. received research funding from AbbVie for clinical trials, served as a consultant for Janssen and Novartis, and was a member of advisory boards for Janssen, Novartis, and UCB.
REFERENCES
- 1.Sieper J Rudwaleit M Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(Suppl 2):ii1–ii44. [DOI] [PubMed] [Google Scholar]
- 2.Costantino F, Breban M, Garchon HJ. Genetics and functional genomics of spondyloarthritis. Front Immunol. 2018;9:2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol. 2019;38:625–634. [DOI] [PubMed] [Google Scholar]
- 4.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken). 2012;64:905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011;341:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudwaleit M van der Heijde D Landewe R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. [DOI] [PubMed] [Google Scholar]
- 7.Rudwaleit M van der Heijde D Khan MA, et al. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudwaleit M, Feldtkeller E, Sieper J. Easy assessment of axial spondyloarthritis (early ankylosing spondylitis) at the bedside. Ann Rheum Dis. 2006;65:1251–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol. 2012;8:262–268. [DOI] [PubMed] [Google Scholar]
- 10.Sieper J van der Heijde D Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784–788. [DOI] [PubMed] [Google Scholar]
- 11.Sieper J Srinivasan S Zamani O, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis. 2013;72:1621–1627. [DOI] [PubMed] [Google Scholar]
- 12.Solmaz D Akar S Soysal O, et al. Performance of different criteria sets for inflammatory back pain in patients with axial spondyloarthritis with and without radiographic sacroiliitis. Clin Rheumatol. 2014;33:1475–1479. [DOI] [PubMed] [Google Scholar]
- 13.Poddubnyy D Callhoff J Spiller I, et al. Diagnostic accuracy of inflammatory back pain for axial spondyloarthritis in rheumatological care. RMD Open. 2018;4:e000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermann J Giessauf H Schaffler G, et al. Early spondyloarthritis: usefulness of clinical screening. Rheumatology (Oxford). 2009;48:812–816. [DOI] [PubMed] [Google Scholar]
- 15.Poddubnyy D Vahldiek J Spiller I, et al. Evaluation of 2 screening strategies for early identification of patients with axial spondyloarthritis in primary care. J Rheumatol. 2011;38:2452–2460. [DOI] [PubMed] [Google Scholar]
- 16.Deodhar A Mittal M Reilly P, et al. Ankylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delay. Clin Rheumatol. 2016;35:1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogdie A Nowell WB Reynolds R, et al. Real-world patient experience on the path to diagnosis of ankylosing spondylitis. Rheumatol Ther. 2019;6:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calin A Elswood J Rigg S, et al. Ankylosing spondylitis—an analytical review of 1500 patients: the changing pattern of disease. J Rheumatol. 1988;15:1234–1238. [PubMed] [Google Scholar]
- 19.Jovaní V Blasco-Blasco M Ruiz-Cantero MT, et al. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: a systematic review and metaanalysis. J Rheumatol. 2017;44:174–183. [DOI] [PubMed] [Google Scholar]
- 20.Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol. 2000;12:239–247. [DOI] [PubMed] [Google Scholar]
- 21.Feldtkeller E Khan MA van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–66. [DOI] [PubMed] [Google Scholar]
- 22.Rudwaleit M Landewe R van der Heijde D, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68:770–776. [DOI] [PubMed] [Google Scholar]
- 23.Rudwaleit M van der Heijde D Landewe R, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. [DOI] [PubMed] [Google Scholar]
- 25.Ostergaard M, Lambert RG. Imaging in ankylosing spondylitis. Ther Adv Musculoskelet Dis. 2012;4:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maksymowych WP Wichuk S Dougados M, et al. MRI evidence of structural changes in the sacroiliac joints of patients with non-radiographic axial spondyloarthritis even in the absence of MRI inflammation. Arthritis Res Ther. 2017;19:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deodhar A Strand V Kay J, et al. The term ‘non-radiographic axial spondyloarthritis’ is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann Rheum Dis. 2016;75:791–794. [DOI] [PubMed] [Google Scholar]
- 28.Kiltz U Baraliakos X Karakostas P, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res (Hoboken). 2012;64:1415–1422. [DOI] [PubMed] [Google Scholar]
- 29.Sieper J, van der Heijde D. Review: nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum. 2013;65:543–551. [DOI] [PubMed] [Google Scholar]
- 30.van der Heijde D Ramiro S Landewe R, et al. 2016 Update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. [DOI] [PubMed] [Google Scholar]
- 31.Dougados M Sepriano A Molto A, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann Rheum Dis. 2017;76:1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poddubnyy D Rudwaleit M Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:1369–1374. [DOI] [PubMed] [Google Scholar]
- 33.Sampaio-Barros PD Bortoluzzo AB Conde RA, et al. Undifferentiated spondyloarthritis: a longterm followup. J Rheumatol. 2010;37:1195–1199. [DOI] [PubMed] [Google Scholar]
- 34.Ruderman E Strand V Joshi A, et al. Spondyloarthritis epidemiology and burden phase 2 [SPEED 2] study: disease progression in axial spondyloarthropathy (SpA). Arthritis Rheum. 2013;65:S1052–S1053. [Google Scholar]
- 35.Barkham N Keen HI Coates LC, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging–determined early sacroiliitis. Arthritis Rheum. 2009;60:946–954. [DOI] [PubMed] [Google Scholar]
- 36.Braun J, Baraliakos X, Kiltz U. Non-radiographic axial spondyloarthritis: a classification or a diagnosis? Clin Exp Rheumatol. 2016;34(1 Suppl 95):S5–S6. [PubMed] [Google Scholar]
- 37.Haibel H Rudwaleit M Listing J, et al. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum. 2008;58:1981–1991. [DOI] [PubMed] [Google Scholar]
- 38.Mease PJ Heijde DV Karki C, et al. Characterization of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis in the US-based Corrona Registry. Arthritis Care Res (Hoboken). 2018;70:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu DT, van Tubergen A. Diagnosis and differential diagnosis of axial spondyloarthritis (ankylosing spondylitis and nonradiographic axial spondyloarthritis) in adults. In: Sieper J, editor. UpToDate. Waltham, MA: UpToDate; 2018. p. Available at: https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-axial-spondyloarthritis-ankylosing-spondylitis-and-nonradiographic-axial-spondyloarthritis-in-adults. Accessed August 25, 2019. [Google Scholar]
- 40.de Winter JJ van Mens LJ van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dougados M d'Agostino MA Benessiano J, et al. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine. 2011;78:598–603. [DOI] [PubMed] [Google Scholar]
- 42.Deodhar AA Miceli-Richard C Baraliakos X, et al. Incidence of uveitis in secukinumab-treated patients with ankylosing spondylitis: pooled data analysis from three phase 3 studies [published online April 30, 2020]. ACR Open Rheumatol. 2020;2:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stolwijk C van Tubergen A Castillo-Ortiz JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:65–73. [DOI] [PubMed] [Google Scholar]
- 44.Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67:955–959. [DOI] [PubMed] [Google Scholar]
- 45.Rudwaleit M Rosenbaum JT Landewe R, et al. Observed incidence of uveitis following certolizumab pegol treatment in patients with axial spondyloarthritis. Arthritis Care Res (Hoboken). 2016;68:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canoui-Poitrine F Lekpa FK Farrenq V, et al. Prevalence and factors associated with uveitis in spondylarthritis patients in France: results from an observational survey. Arthritis Care Res (Hoboken). 2012;64:919–924. [DOI] [PubMed] [Google Scholar]
- 47.Cypers H Varkas G Beeckman S, et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann Rheum Dis. 2016;75:1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Praet L Van den Bosch FE Jacques P, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. 2013;72:414–417. [DOI] [PubMed] [Google Scholar]
- 49.Gilis E Mortier C Venken K, et al. The role of the microbiome in gut and joint inflammation in psoriatic arthritis and spondyloarthritis. J Rheumatol. 2018;94:36–39. [DOI] [PubMed] [Google Scholar]
- 50.De Vos M Mielants H Cuvelier C, et al. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology. 1996;110:1696–1703. [DOI] [PubMed] [Google Scholar]
- 51.Ritchlin CT. Therapies for psoriatic enthesopathy. A systematic review. J Rheumatol. 2006;33:1435–1438. [PubMed] [Google Scholar]
- 52.Ciurea A Scherer A Exer P, et al. Tumor necrosis factor alpha inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum. 2013;65:3096–3106. [DOI] [PubMed] [Google Scholar]
- 53.Vander Cruyssen B Ribbens C Boonen A, et al. The epidemiology of ankylosing spondylitis and the commencement of anti-TNF therapy in daily rheumatology practice. Ann Rheum Dis. 2007;66:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yap KS Ye JY Li S, et al. Back pain in psoriatic arthritis: defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann Rheum Dis. 2018;77:1573–1577. [DOI] [PubMed] [Google Scholar]
- 55.Feld J Chandran V Haroon N, et al. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol. 2018;14:363–371. [DOI] [PubMed] [Google Scholar]
- 56.Jadon DR Sengupta R Nightingale A, et al. Axial disease in psoriatic arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis. 2017;76:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aydin SZ Kucuksahin O Kilic L, et al. Axial psoriatic arthritis: the impact of underdiagnosed disease on outcomes in real life. Clin Rheumatol. 2018;37:3443–3448. [DOI] [PubMed] [Google Scholar]
- 58.Baraliakos X, Coates LC, Braun J. The involvement of the spine in psoriatic arthritis. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S31–S35. [PubMed] [Google Scholar]
- 59.Perez Alamino R Maldonado Cocco JA Citera G, et al. Differential features between primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease. J Rheumatol. 2011;38:1656–1660. [DOI] [PubMed] [Google Scholar]
- 60.van den Berg R de Hooge M Rudwaleit M, et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann Rheum Dis. 2013;72:1646–1653. [DOI] [PubMed] [Google Scholar]
- 61.Poddubnyy D van Tubergen A Landewe R, et al. Assessment of SpondyloArthritis international Society (ASAS) . Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483–1487. [DOI] [PubMed] [Google Scholar]
- 62.Marques ML Ramiro S Goupille P, et al. Measuring spinal mobility in early axial spondyloarthritis: does it matter? Rheumatology (Oxford). 2019;58:1597–1606. [DOI] [PubMed] [Google Scholar]
- 63.Slobodin G Hussein H Rosner I, et al. Sacroiliitis—early diagnosis is key. J Inflamm Res. 2018;11:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bakland G, Nossent HC. Epidemiology of spondyloarthritis: a review. Curr Rheumatol Rep. 2013;15:351. [DOI] [PubMed] [Google Scholar]
- 65.Stolwijk C Boonen A van Tubergen A, et al. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am. 2012;38:441–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci. 2013;345:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown MA. Human leucocyte antigen-B27 and ankylosing spondylitis. Intern Med J. 2007;37:739–740. [DOI] [PubMed] [Google Scholar]
- 68.Reveille JD. Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clin Rheumatol. 2015;34:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heuft-Dorenbosch L Landewe R Weijers R, et al. Combining information obtained from magnetic resonance imaging and conventional radiographs to detect sacroiliitis in patients with recent onset inflammatory back pain. Ann Rheum Dis. 2006;65:804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lambert RG Bakker PA van der Heijde D, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016;75:1958–1963. [DOI] [PubMed] [Google Scholar]
- 71.Weber U Zubler V Pedersen SJ, et al. Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis Care Res (Hoboken). 2013;65:977–985. [DOI] [PubMed] [Google Scholar]
- 72.Blachier M Coutanceau B Dougados M, et al. Does the site of magnetic resonance imaging abnormalities match the site of recent-onset inflammatory back pain? The DESIR cohort. Ann Rheum Dis. 2013;72:979–985. [DOI] [PubMed] [Google Scholar]
- 73.Machado P Landewe RB Braun J, et al. MRI inflammation and its relation with measures of clinical disease activity and different treatment responses in patients with ankylosing spondylitis treated with a tumour necrosis factor inhibitor. Ann Rheum Dis. 2012;71:2002–2005. [DOI] [PubMed] [Google Scholar]
- 74.Baraliakos X Sieper J Chen S, et al. Non-radiographic axial spondyloarthritis patients without initial evidence of inflammation may develop objective inflammation over time. Rheumatology (Oxford). 2017;56:1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Berg R Lenczner G Feydy A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol. 2014;66:2403–2411. [DOI] [PubMed] [Google Scholar]
- 76.Rudwaleit M Jurik AG Hermann KG, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–1527. [DOI] [PubMed] [Google Scholar]
- 77.van den Berg R de Hooge M van Gaalen F, et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the SPondyloArthritis Caught Early (SPACE) cohort. Rheumatology (Oxford). 2013;52:1492–1499. [DOI] [PubMed] [Google Scholar]
- 78.van den Berg R Lenczner G Thevenin F, et al. Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann Rheum Dis. 2015;74:2016–2021. [DOI] [PubMed] [Google Scholar]
- 79.Arnbak B Grethe Jurik A Horslev-Petersen K, et al. Associations between spondyloarthritis features and magnetic resonance imaging findings: a cross-sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol. 2016;68:892–900. [DOI] [PubMed] [Google Scholar]
- 80.Marzo-Ortega H McGonagle D O'Connor P, et al. Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann Rheum Dis. 2009;68:1721–1727. [DOI] [PubMed] [Google Scholar]
- 81.Weber U Lambert RG Ostergaard M, et al. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62:3048–3058. [DOI] [PubMed] [Google Scholar]
- 82.Varkas G de Hooge M Renson T, et al. Presence of bone marrow edema and structural lesions on magnetic resonance imaging of the sacroiliac joints in young military recruits before and after 6 weeks of intensive physical training. Arthritis Rheumatol. 2017;69(Suppl 10):363 [abstract 253]. [Google Scholar]
- 83.Weber U Jurik AG Zejden A, et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “background noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol. 2018;70:736–745. [DOI] [PubMed] [Google Scholar]
- 84.de Winter J de Hooge M van de Sande M, et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the Assessment of SpondyloArthritis international Society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 2018;70:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sieper J, Rudwaleit M. How early should ankylosing spondylitis be treated with tumour necrosis factor blockers? Ann Rheum Dis. 2005;64(Suppl 4):iv61–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strand V, Singh JA. Patient burden of axial spondyloarthritis. J Clin Rheumatol. 2017;23:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudwaleit M Listing J Brandt J, et al. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis. 2004;63:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan MA. Ankylosing spondylitis: introductory comments on its diagnosis and treatment. Ann Rheum Dis. 2002;61(Suppl 3):iii3–iii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Curtis JR Harrold LR Asgari MM, et al. Diagnostic prevalence of ankylosing spondylitis using computerized health care data, 1996 to 2009: underrecognition in a US health care setting. Perm J. 2016;20:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;52:1000–1008. [DOI] [PubMed] [Google Scholar]
- 91.Sieper J, Rudwaleit M. Early referral recommendations for ankylosing spondylitis (including pre-radiographic and radiographic forms) in primary care. Ann Rheum Dis. 2005;64:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poddubnyy D, Rudwaleit M. Early spondyloarthritis. Rheum Dis Clin North Am. 2012;38:387–403. [DOI] [PubMed] [Google Scholar]
- 93.Chou R Qaseem A Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American pain society. Ann Intern Med. 2007;147:478–491. [DOI] [PubMed] [Google Scholar]
- 94.Herndon CM, Zoberi KS, Gardner BJ. Common questions about chronic low back pain. Am Fam Physician. 2015;91:708–714. [PubMed] [Google Scholar]
- 95.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374:2563–2574. [DOI] [PubMed] [Google Scholar]
- 96.Magrey M Yi E Wolin D, et al. Delayed diagnosis of ankylosing spondylitis: results from a survey of 1690 US physicians from 10 specialties. Ann Rheum Dis. 2019;78(suppl 2):1247–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Magrey M Yi E Wolin D, et al. Recognition of inflammatory back pain by US healthcare providers and barriers to specialist referral. Arthritis Rheumatol. 2019;71(Suppl 10): [abstract 631]. [Google Scholar]
- 98.Redeker I Callhoff J Hoffmann F, et al. Which factors influence the diagnostic delay in patients with axial spondyloarthritis? Arthritis Rheumatol. 2018;70(Suppl 10): [abstract 1658]. [Google Scholar]
- 99.Glintborg B Ostergaard M Krogh NS, et al. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti–tumour necrosis factor: results from 8 years' surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis. 2010;69:2002–2008. [DOI] [PubMed] [Google Scholar]
- 100.Kristensen LE Karlsson JA Englund M, et al. Presence of peripheral arthritis and male sex predicting continuation of anti–tumor necrosis factor therapy in ankylosing spondylitis: an observational prospective cohort study from the south Swedish arthritis treatment group register. Arthritis Care Res (Hoboken). 2010;62:1362–1369. [DOI] [PubMed] [Google Scholar]
- 101.Landi M Maldonado-Ficco H Perez-Alamino R, et al. Gender differences among patients with primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease in an Iberoamerican spondyloarthritis cohort. Medicine (Baltimore). 2016;95:e5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lubrano E Perrotta FM Manara M, et al. The sex influence on response to tumor necrosis factor-α inhibitors and remission in axial spondyloarthritis. J Rheumatol. 2018;45:195–201. [DOI] [PubMed] [Google Scholar]
- 103.Shahlaee A Mahmoudi M Nicknam MH, et al. Gender differences in Iranian patients with ankylosing spondylitis. Clin Rheumatol. 2015;34:285–293. [DOI] [PubMed] [Google Scholar]
- 104.van der Horst-Bruinsma IE Zack DJ Szumski A, et al. Female patients with ankylosing spondylitis: analysis of the impact of gender across treatment studies. Ann Rheum Dis. 2013;72:1221–1224. [DOI] [PubMed] [Google Scholar]
- 105.Webers C Essers I Ramiro S, et al. Gender-attributable differences in outcome of ankylosing spondylitis: long-term results from the Outcome in Ankylosing Spondylitis International Study. Rheumatology (Oxford). 2016;55:419–428. [DOI] [PubMed] [Google Scholar]
- 106.Slobodin G Reyhan I Avshovich N, et al. Recently diagnosed axial spondyloarthritis: gender differences and factors related to delay in diagnosis. Clin Rheumatol. 2011;30:1075–1080. [DOI] [PubMed] [Google Scholar]
- 107.Ramiro S van der Heijde D van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis. 2014;73:1455–1461. [DOI] [PubMed] [Google Scholar]
- 108.Rudwaleit M Haibel H Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis inception cohort. Arthritis Rheum. 2009;60:717–727. [DOI] [PubMed] [Google Scholar]
- 109.Deminger A Klingberg E Geijer M, et al. A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther. 2018;20:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsui HW Inman RD Paterson AD, et al. ANKH variants associated with ankylosing spondylitis: gender differences. Arthritis Res Ther. 2005;7:R513–R525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gracey E Yao Y Green B, et al. Sexual dimorphism in the TH17 signature of ankylosing spondylitis. Arthritis Rheumatol. 2016;68:679–683. [DOI] [PubMed] [Google Scholar]
- 112.Huang WN Tso TK Kuo YC, et al. Distinct impacts of syndesmophyte formation on male and female patients with ankylosing spondylitis. Int J Rheum Dis. 2012;15:163–168. [DOI] [PubMed] [Google Scholar]
- 113.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. [DOI] [PubMed] [Google Scholar]
- 114.Takatalo J Karppinen J Niinimäki J, et al. Prevalence of degenerative imaging findings in lumbar magnetic resonance imaging among young adults. Spine (Phila Pa 1976). 2009;34:1716–1721. [DOI] [PubMed] [Google Scholar]
- 115.Martínez-Quiñones JV Aso-Escario J González-García L, et al. Are Modic changes able to help us in our clinical practice? A study of the Modic changes in young adults during working age. Clin Spine Surg. 2017;30:259–264. [DOI] [PubMed] [Google Scholar]
- 116.Aggarwal R, Malaviya AN. Diagnosis delay in patients with ankylosing spondylitis: factors and outcomes—an Indian perspective. Clin Rheumatol. 2009;28:327–331. [DOI] [PubMed] [Google Scholar]
- 117.Gerdan V Akar S Solmaz D, et al. Initial diagnosis of lumbar disc herniation is associated with a delay in diagnosis of ankylosing spondylitis. J Rheumatol. 2012;39:1996–1999. [DOI] [PubMed] [Google Scholar]
- 118.de Bruin F Treyvaud MO Feydy A, et al. Prevalence of degenerative changes and overlap with spondyloarthritis-associated lesions in the spine of patients from the DESIR cohort. RMD Open. 2018;4:e000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Bruin F ter Horst S Bloem HL, et al. Prevalence of degenerative changes of the spine on magnetic resonance images and radiographs in patients aged 16–45 years with chronic back pain of short duration in the SPondyloArthritis Caught Early (SPACE) cohort. Rheumatology (Oxford). 2016;55:56–65. [DOI] [PubMed] [Google Scholar]
- 120.Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol. 2017;29:304–310. [DOI] [PubMed] [Google Scholar]
- 121.Creed F. A review of the incidence and risk factors for fibromyalgia and chronic widespread pain in population-based studies [published online June 2020]. Pain. 2020;161:1169–1176. [DOI] [PubMed] [Google Scholar]
- 122.Staud R, Rodriguez ME. Mechanisms of disease: pain in fibromyalgia syndrome. Nat Clin Pract Rheumatol. 2006;2:90–98. [DOI] [PubMed] [Google Scholar]
- 123.Derry S Wiffen PJ Häuser W, et al. Oral nonsteroidal anti-inflammatory drugs for fibromyalgia in adults. Cochrane Database Syst Rev. 2017;3:CD012332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gagnet P Kern K Andrews K, et al. Spondylolysis and spondylolisthesis: a review of the literature. J Orthop. 2018;15:404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Syrmou E Tsitsopoulos PP Marinopoulos D, et al. Spondylolysis: a review and reappraisal. Hippokratia. 2010;14:17–21. [PMC free article] [PubMed] [Google Scholar]
- 126.Delavan JA Stence NV Mirsky DM, et al. Confidence in assessment of lumbar spondylolysis using three-dimensional volumetric T2-weighted MRI compared with limited field of view, decreased-dose CT. Sports Health. 2016;8:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamashita K Sakai T Takata Y, et al. Utility of STIR-MRI in detecting the pain generator in asymmetric bilateral pars fracture: a report of 5 cases. Neurol Med Chir (Tokyo). 2018;58:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masci L Pike J Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamaguchi KT Skaggs DL Acevedo DC, et al. Spondylolysis is frequently missed by MRI in adolescents with back pain. J Child Orthop. 2012;6:237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Summers BN, Singh JP, Manns RA. The radiological reporting of lumbar Scheuermann's disease: an unnecessary source of confusion amongst clinicians and patients. Br J Radiol. 2008;81:383–385. [DOI] [PubMed] [Google Scholar]
- 131.Mansfield JT, Bennett M. Scheuermann disease. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK499966/. Accessed September 21, 2020. [PubMed] [Google Scholar]
- 132.Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann kyphosis. J Bone Joint Surg Am. 1993;75:236–248. [DOI] [PubMed] [Google Scholar]
- 133.Huisman TA. Pediatric tumors of the spine. Cancer Imaging. 2009;(9 Spec No A):S45–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ogunlade J Wiginton JG Elia C, et al. Primary spinal astrocytomas: a literature review. Cureus. 2019;11:e5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mitra R. Osteitis condensans ilii. Rheumatol Int. 2010;30:293–296. [DOI] [PubMed] [Google Scholar]
- 136.Ayoub MA. Refractory osteitis condensans ilii: outcome of a novel mini-invasive surgical approach. Int Orthop. 2013;37:1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tuite MJ. Sacroiliac joint imaging. Semin Musculoskelet Radiol. 2008;12:72–82. [DOI] [PubMed] [Google Scholar]
- 138.Ma L Gao Z Zhong Y, et al. Osteitis condensans ilii may demonstrate bone marrow edema on sacroiliac joint magnetic resonance imaging. Int J Rheum Dis. 2018;21:299–307. [DOI] [PubMed] [Google Scholar]
- 139.Olivieri I D'Angelo S Palazzi C, et al. Diffuse idiopathic skeletal hyperostosis: differentiation from ankylosing spondylitis. Curr Rheumatol Rep. 2009;11:321–328. [DOI] [PubMed] [Google Scholar]
- 140.Westerveld LA van Ufford HM Verlaan JJ, et al. The prevalence of diffuse idiopathic skeletal hyperostosis in an outpatient population in the Netherlands. J Rheumatol. 2008;35:1635–1638. [PubMed] [Google Scholar]
- 141.Kiss C O'Neill TW Mituszova M, et al. The prevalence of diffuse idiopathic skeletal hyperostosis in a population-based study in Hungary. Scand J Rheumatol. 2002;31:226–229. [DOI] [PubMed] [Google Scholar]
- 142.Sarzi-Puttini P, Atzeni F. New developments in our understanding of DISH (diffuse idiopathic skeletal hyperostosis). Curr Opin Rheumatol. 2004;16:287–292. [DOI] [PubMed] [Google Scholar]
- 143.Merjanah S, Igoe A, Magrey M. Mimics of axial spondyloarthritis. Curr Opin Rheumatol. 2019;31:335–343. [DOI] [PubMed] [Google Scholar]
- 144.Imamura T Saiki K Okamoto K, et al. Characterization of individuals with sacroiliac joint bridging in a skeletal population: analysis of degenerative changes in spinal vertebrae. Biomed Res Int. 2014;2014:879645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mader R Verlaan JJ Eshed I, et al. Diffuse idiopathic skeletal hyperostosis (DISH): where we are now and where to go next. RMD Open. 2017;3:e000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abid H Chaabouni S Frikha F, et al. Contribution of imaging in the diagnosis of infectious sacroiliitis: about 19 cases. Pan Afr Med J. 2014;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu MS Chang SS Lee SH, et al. Pyogenic sacroiliitis—a comparison between paediatric and adult patients. Rheumatology (Oxford). 2007;46:1684–1687. [DOI] [PubMed] [Google Scholar]
- 148.Hermet M Minichiello E Flipo RM, et al. Infectious sacroiliitis: a retrospective, multicentre study of 39 adults. BMC Infect Dis. 2012;12:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Antonelli MJ, Magrey M. Sacroiliitis mimics: a case report and review of the literature. BMC Musculoskelet Disord. 2017;18:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bozgeyik Z Aglamis S Bozdag PG, et al. Magnetic resonance imaging findings of musculoskeletal brucellosis. Clin Imaging. 2014;38:719–723. [DOI] [PubMed] [Google Scholar]
- 151.Kang Y Hong SH Kim JY, et al. Unilateral sacroiliitis: differential diagnosis between infectious sacroiliitis and spondyloarthritis based on MRI findings. AJR Am J Roentgenol. 2015;205:1048–1055. [DOI] [PubMed] [Google Scholar]
- 152.Moos V Schmidt C Geelhaar A, et al. Impaired immune functions of monocytes and macrophages in Whipple's disease. Gastroenterology. 2010;138:210–220. [DOI] [PubMed] [Google Scholar]
- 153.Marth T Neurath M Cuccherini BA, et al. Defects of monocyte interleukin 12 production and humoral immunity in Whipple's disease. Gastroenterology. 1997;113:442–448. [DOI] [PubMed] [Google Scholar]
- 154.Marth T Roux M von Herbay A, et al. Persistent reduction of complement receptor 3 alpha-chain expressing mononuclear blood cells and transient inhibitory serum factors in Whipple's disease. Clin Immunol Immunopathol. 1994;72:217–226. [DOI] [PubMed] [Google Scholar]
- 155.Kalt A Schneider T Ring S, et al. Decreased levels of interleukin-12p40 in the serum of patients with Whipple's disease. Int J Colorectal Dis. 2006;21:114–120. [DOI] [PubMed] [Google Scholar]
- 156.Ectors N Geboes K De Vos R, et al. Whipple's disease: a histological, immunocytochemical and electronmicroscopic study of the immune response in the small intestinal mucosa. Histopathology. 1992;21:1–12. [DOI] [PubMed] [Google Scholar]
- 157.Moos V, Schneider T. Changing paradigms in Whipple's disease and infection with Tropheryma whipplei. Eur J Clin Microbiol Infect Dis. 2011;30:1151–1158. [DOI] [PubMed] [Google Scholar]
- 158.Schneider T Moos V Loddenkemper C, et al. Whipple's disease: new aspects of pathogenesis and treatment. Lancet Infect Dis. 2008;8:179–190. [DOI] [PubMed] [Google Scholar]
- 159.Lagier JC Lepidi H Raoult D, et al. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore). 2010;89:337–345. [DOI] [PubMed] [Google Scholar]
- 160.Meunier M Puechal X Hoppé E, et al. Rheumatic and musculoskeletal features of Whipple disease: a report of 29 cases. J Rheumatol. 2013;40:2061–2066. [DOI] [PubMed] [Google Scholar]
- 161.Apstein M, Schneider T. Whipple's disease. In: Bloom A, editor. UpToDate. Waltham, MA: UpToDate; 2019. p. Available at: https://www.uptodate.com/contents/whipples-disease. Accessed September 28, 2019. [Google Scholar]
- 162.Balaban B Yasar E Ozgul A, et al. Sacroiliitis in familial Mediterranean fever and seronegative spondyloarthropathy: importance of differential diagnosis. Rheumatol Int. 2005;25:641–644. [DOI] [PubMed] [Google Scholar]
- 163.Eshed I Rosman Y Livneh A, et al. Exertional leg pain in familial Mediterranean fever: a manifestation of an underlying enthesopathy and a marker of more severe disease. Arthritis Rheumatol. 2014;66:3221–3226. [DOI] [PubMed] [Google Scholar]
- 164.Langevitz P Livneh A Zemer D, et al. Seronegative spondyloarthropathy in familial Mediterranean fever. Semin Arthritis Rheum. 1997;27:67–72. [DOI] [PubMed] [Google Scholar]
- 165.Kobak S Sever F Ince O, et al. The prevalence of sacroiliitis and spondyloarthritis in patients with sarcoidosis. Int J Rheumatol. 2014;2014:289454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Erb N Cushley MJ Kassimos DG, et al. An assessment of back pain and the prevalence of sacroiliitis in sarcoidosis. Chest. 2005;127:192–196. [DOI] [PubMed] [Google Scholar]
- 167.Shariatmaghani S Salari R Sahebari M, et al. Musculoskeletal manifestations of sarcoidosis: a review article. Curr Rheumatol Rev. 2019;15:83–89. [DOI] [PubMed] [Google Scholar]
- 168.Binicier O Sari I Sen G, et al. Axial sarcoidosis mimicking radiographic sacroiliitis. Rheumatol Int. 2009;29:343–345. [DOI] [PubMed] [Google Scholar]
- 169.Briongos-Figuero LS, Ruiz-de-Temino A, Perez-Castrillon JL. Sarcoidosis and sacroiliitis, a case report. Rheumatol Int. 2012;32:2949–2950. [DOI] [PubMed] [Google Scholar]
- 170.Sigaux J Semerano L Nasrallah T, et al. High prevalence of spondyloarthritis in sarcoidosis patients with chronic back pain. Semin Arthritis Rheum. 2019;49:246–250. [DOI] [PubMed] [Google Scholar]
- 171.Moshrif A Laredo JD Bassiouni H, et al. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: a series of 37 patients. Semin Arthritis Rheum. 2019;48:1113–1126. [DOI] [PubMed] [Google Scholar]
- 172.Rosenthal AK, Ryan LM. Calcium pyrophosphate deposition disease. N Engl J Med. 2016;374:2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Francois S, Guaydier-Souquieres G, Marcelli C. Acute sacroiliitis as a manifestation of calcium pyrophosphate dihydrate crystal deposition disease. A report of two cases. Rev Rhum Engl Ed. 1997;64:508–512. [PubMed] [Google Scholar]
- 174.Goswami R Ray D Sharma R, et al. Presence of spondyloarthropathy and its clinical profile in patients with hypoparathyroidism. Clin Endocrinol (Oxf). 2008;68:258–263. [DOI] [PubMed] [Google Scholar]
- 175.Shoback DM Bilezikian JP Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab. 2016;101:2300–2312. [DOI] [PubMed] [Google Scholar]
- 176.Kajitani TR Silva RV Bonfá E, et al. Hypoparathyroidism mimicking ankylosing spondylitis and myopathy: a case report. Clinics (Sao Paulo). 2011;66:1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jakkani RK, Sureka J, Mathew J. Spondyloarthropathy occurring in long-standing idiopathic hypoparathyroidism. Radiol Case Rep. 2011;6:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Goltzman D. Hypoparathyroidism. In: Mulder JE, editor. UpToDate. Waltham, MA: UpToDate; 2019. p. Available at: https://www.uptodate.com/contents/hypoparathyroidism. Accessed September 9, 2019. [Google Scholar]
- 179.Nair JR, Moots RJ. Behcet's disease. Clin Med (Lond). 2017;17:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bicer A. Musculoskeletal findings in Behcet's disease. Patholog Res Int. 2012;2012:653806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chamberlain MA, Robertson RJ. A controlled study of sacroiliitis in Behçet's disease. Br J Rheumatol. 1993;32:693–698. [DOI] [PubMed] [Google Scholar]
- 182.Yazici H, Tuzlaci M, Yurdakul S. A controlled survey of sacroiliitis in Behcet's disease. Ann Rheum Dis. 1981;40:558–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Canella C Costa F Bacchiega AB, et al. Sacroiliitis in Behçet syndrome. J Clin Rheumatol. 2013;19:356. [DOI] [PubMed] [Google Scholar]
- 184.Cobankar V Topaloglu M Balkarli A, et al. Determining the prevalence of sacroiliitis in Behcet's disease by magnetic resonance imaging. Ann Rheum Dis. 2014;73:699–700. [Google Scholar]
- 185.Nussenblatt RB. Uveitis in Behçet's disease. Int Rev Immunol. 1997;14:67–79. [DOI] [PubMed] [Google Scholar]
- 186.Tugal-Tutkun I Onal S Altan-Yaycioglu R, et al. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–380. [DOI] [PubMed] [Google Scholar]
- 187.Kim HA, Choi KW, Song YW. Arthropathy in Behçet's disease. Scand J Rheumatol. 1997;26:125–129. [DOI] [PubMed] [Google Scholar]
- 188.Leccese P, Alpsoy E. Behçet's disease: an overview of etiopathogenesis. Front Immunol. 2019;10:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marie PJ, Glorieux FH. Relation between hypomineralized periosteocytic lesions and bone mineralization in vitamin D-resistant rickets. Calcif Tissue Int. 1983;35:443–448. [DOI] [PubMed] [Google Scholar]
- 190.Polisson RP Martinez S Khoury M, et al. Calcification of entheses associated with X-linked hypophosphatemic osteomalacia. N Engl J Med. 1985;313:1–6. [DOI] [PubMed] [Google Scholar]
- 191.Adams J. Chapter 49—radiology of rickets and osteomalacia. In: Feldman D, Pike J, Adams J, editors. Vitamin D. Cambridge, MA: Academic Press; 2011. p. 861–889. [Google Scholar]
- 192.Ranga U Aiyappan SK Shanmugam N, et al. Ochronotic spondyloarthropathy. J Clin Diagn Res. 2013;7:403–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Al-Mahfoudh R, Clark S, Buxton N. Alkaptonuria presenting with ochronotic spondyloarthropathy. Br J Neurosurg. 2008;22:805–807. [DOI] [PubMed] [Google Scholar]
- 194.Balaban B Taskaynatan M Yasar E, et al. Ochronotic spondyloarthropathy: spinal involvement resembling ankylosing spondylitis. Clin Rheumatol. 2006;25:598–601. [DOI] [PubMed] [Google Scholar]
- 195.Groseanu L Marinescu R Laptoiun D, et al. A late and difficult diagnosis of ochronosis. J Med Life. 2010;3:437–443. [PMC free article] [PubMed] [Google Scholar]


