Abstract
Objectives
This study aimed to evaluate the effectiveness of using 1 to 4 mobile or fixed automated video monitoring systems (AVMSs) to decrease the risk of unattended bed exits (UBEs) as antecedents to unassisted falls among patients at high risk for falls and fall-related injuries in 15 small rural hospitals.
Methods
We compared UBE rates and fall rates during baseline (5 months in which patient movement was recorded but nurses did not receive alerts) and intervention phases (2 months in which nurses received alerts). We determined lead time (seconds elapsed from the first alert because of patient movement until 3 seconds after an UBE) during baseline and positive predictive value and sensitivity during intervention.
Results
Age and fall risk were negatively associated with the baseline patient rate of UBEs/day. From baseline to intervention: in 9 hospitals primarily using mobile systems, UBEs/day decreased from 0.84 to 0.09 (89%); in 5 hospitals primarily using fixed systems, UBEs/day increased from 0.43 to 3.18 (649%) as patients at low risk for falls were observed safely exiting the bed; and among 13 hospitals with complete data, total falls/1000 admissions decreased from 8.83 to 5.53 (37%), and injurious falls/1000 admissions decreased from 2.52 to 0.55 (78%). The median lead time of the AVMS was 28.5 seconds, positive predictive value was nearly 60%, and sensitivity was 97.4%.
Conclusions
Use of relatively few AVMSs may allow nurses to adaptively manage UBEs as antecedents to unassisted falls and fall-related injuries in small rural hospitals. Additional research is needed in larger hospitals to better understand the effectiveness of AVMSs.
Key Words: falls, automated video monitoring, artificial intelligence, critical access hospitals, patient safety
Approximately 2% to 4% of hospitalized patients fall annually,1–4 and up to one-third of these falls result in injury.5,6 Estimates of excess costs due to serious fall-related injuries vary from $70007 to $13,0003,8 per injury. Costs associated with noninjurious falls include increased monitoring, length of stay,3 and imaging to rule out injury.9 For patients, noninjurious falls may increase fear of falling that limits mobility and accelerates functional decline.10 Approximately 85% of falls are unassisted.11 An unassisted fall is a “sudden, unintended, uncontrolled, downward displacement of a patient’s body to the ground or other object,“12 which is a system failure.13,14 An assisted fall occurs when staff lower a patient to the ground. Assisted falls are significantly less likely to result in injury than unassisted falls6,13 and are a system success in the context of early mobilization to prevent secondary functional decline.15 Using gait belts to assist mobility is associated with decreased odds of unassisted falls and decreased odds that assisted falls result in injury.6
Since 2008, the Centers for Medicare & Medicaid Services have included serious fall-related injuries in its list of 14 preventable hospital-acquired conditions for which it no longer reimburses hospitals.16 From 2014 to 2017, this pay-for-performance strategy resulted in a 13% reduction in all hospital-acquired conditions, but just a 5% reduction in serious fall-related injuries.17 Two factors may account for this limited progress. First, falls are an outcome of the interaction between patient (e.g., age, muscle weakness, and impaired cognition),1,6,18–20 environmental (e.g., unit/room design and clutter/tripping hazards),1,21–23 and system risk factors (e.g., poor teamwork, the attitude that falls are inevitable, and gait belt usage).6,24–26 Second, too few studies evaluate system interventions that improve nurses’ ability to adaptively manage fall risk as a complex problem with a multifactorial etiology.27,28
Because of the prevalence of unassisted falls, many fall risk reduction interventions are intended to prevent unattended bed exits (UBEs) as antecedents to falls without restraining patients. Social interventions to prevent UBEs include hourly rounding and sitters. Hourly rounding has been associated with decreased risk of falls,29,30 whereas use of sitters has had conflicting results.31–34 Technical interventions to prevent UBEs include bed pressure-sensor alarms and central video monitoring (CVM). Randomized controlled trials of bed pressure-sensor alarms35,36 revealed that their use did not significantly decrease fall rates35,36 owing to the prevalence of false alarms.36,37
Hospitals began using CVM in 201238 as a lower cost alternative to sitters.38–41 Central video monitoring uses unlicensed38–41 or licensed personnel42 to continuously observe up to 16 patients38,40–42 on video monitors from a central location. To protect privacy, CVM uses live video and does not record.39,40 Upon observing unsafe patient behavior, a monitoring technician may communicate directly with the patient via intercom, call the assigned nurse, activate the patient call system or an alarm, or use an overhead paging system.40,41 Of 6 evaluations of CVM, 3 reported decreases in total fall rates of 20% to 29% and decreases in sitter-related costs,38,40,43 2 were underpowered because of too few patients42 or cameras,44 and 1 was descriptive without pre-post comparisons.41 Limitations to CVM include:
potential for human error by monitoring technicians,40
response delays due to hand-offs between monitoring technicians and nurses,39,40 and
privacy concerns of patients and staff.42
Automated video monitoring systems (AVMSs) use video streams to find the floor and bed and machine learning to detect body segments and then predict the likelihood that a patient will exit the bed.45 At a threshold expectation of an UBE, the AVMS sends a predictive alert (i.e., before an UBE) and real-time video to nursing staff via a mobile device or central monitor.45 The goal is to provide a nurse the time and information needed to assess a patient’s behavior in clinical and environmental context (i.e., adaptively manage fall risk), respond to meet the patient’s needs, and prevent an UBE.
An AVMS may mitigate limitations associated with CVM. First, an AVMS eliminates monitoring technicians, their associated costs, delayed response, and their potential for human error, misinterpretation of behavior, and miscommunication. Second, an AVMS may use 3-dimensional (3D) images that appear as grayed-out shapes (Fig. 1) rather than photographic images to mitigate privacy concerns. To our knowledge, the evidence needed to transfer AVM technology from pilot testing to standard of care does not exist. The purpose of this study was to evaluate the feasibility and effectiveness of using a prototypical AVMS from Ocuvera, LLC (Lincoln, Nebraska)45 to decrease the risk of UBEs as antecedents to unassisted falls among patients at high risk for falls and fall-related injuries in small rural hospitals (SRHs). These hospitals may have higher fall rates than larger, urban hospitals26,46 due to limited resources and a higher prevalence of older adults who are at high risk for falls and fall-related injuries.26 This study was approved by the institutional review board of the University of Nebraska Medical Center.
FIGURE 1.

Simulated 3D grayed-out shapes produced by Ocuvera AVMS.
METHODS
Setting, Design, Participants, and Procedures
From April 2017 to May 2018, we recruited 15 hospitals in a Midwestern state to participate in this no-cost study. We used a 1-group pretest-posttest design to compare patient and hospital rates of UBEs during baseline and intervention phases, which lasted approximately 5 and 2 months, respectively, within each hospital. To determine baseline rates of UBEs, the AVMS recorded patient movement during baseline but did not send predictive alerts to nurses. During intervention, nurses received alerts on mobile devices and a central monitor. Of the 15 hospitals, 13 were critical access hospitals licensed for 25 beds or less (Table 1). Patients admitted for acute care, skilled rehabilitation, observation, or hospice and deemed to be at high risk for falls or fall-related injuries were eligible to participate.
TABLE 1.
Hospital Characteristics and Study Enrollment
| Hospital | Bed Size | Camera No. and Type | Admissions During Baseline (n = 4680), n (%) | Admissions During Intervention (n = 3771), n (%) | Baseline Patients (n = 221), n (%) | Intervention Patients (n = 151), n (%) |
|---|---|---|---|---|---|---|
| A | 100–200 | 4 fixed | 1318 (28.2) | 2440 (64.7) | 21 (9.5) | 15 (9.9) |
| B | ≤25 | 1 mobile | 171 (3.7) | 126 (3.3) | 8 (3.6) | 8 (5.3) |
| C | ≤25 | 1–2 mobile* | 151 (3.2) | 32 (0.8) | 12 (5.4) | 5 (3.3) |
| D | 26–99 | 4 fixed | — | — | 17 (7.7) | 13 (8.6) |
| E | ≤25 | 1–2 mobile* | 185 (4.0) | 45 (1.2) | 3 (1.4) | 7 (4.6) |
| F | ≤25 | 1–2 mobile* | 714 (15.3) | 153 (4.1) | 11 (5.0) | 8 (5.3) |
| G | ≤25 | 1–2 mobile* | 472 (10.1) | 86 (2.3) | 13 (5.9) | 5 (3.3) |
| H | ≤25 | 1–2 mobile* | 473 (10.1) | 89 (2.4) | 4 (1.8) | 0 (0.0) |
| I | ≤25 | 4 fixed | 282 (6.0) | 222 (5.9) | 28 (12.7) | 50 (33.1) |
| J | ≤25 | 4 fixed | 214 (4.6) | 220 (5.8) | 22 (10.0) | 16 (10.6) |
| K | ≤25 | 2 mobile | 158 (3.4) | 131 (3.5) | 13 (5.9) | 4 (2.6) |
| L | ≤25 | 1 mobile | 100 (2.1) | 42 (1.1) | 18 (8.1) | 4 (2.6) |
| M | ≤25 | 4 fixed | 312 (6.7) | 71 (1.9) | 21 (9.5) | 8 (5.3) |
| N | ≤25 | 2 mobile | 97 (2.1) | 96 (2.5) | 16 (7.2) | 5 (3.3) |
| O | ≤25 | 1 mobile | 33 (0.7) | 18 (0.5) | 14 (6.3) | 3 (2.0) |
*These hospitals received 1 mobile camera during baseline and 2 mobile cameras during intervention.
Procedures included installing the AVMS, training nurses to consent patients and use the system, and collecting and analyzing patient demographic data, fall event data, and video data. We requested that hospitals report fall event data during the study using a secure online system developed for previous studies.6,14 The AVMS consisted of a room sensor, mobile devices for nursing staff, and a central monitor at the nurse’s station (Fig. 2). The room sensor included a Microsoft Kinect (Microsoft Corporation, Redmond, WA) for Xbox One depth camera (field of view, 70.6 degrees wide by 60 degrees tall), a touchscreen, and an internal computer to analyze patient movement and send predictive alerts when movement exceeded the threshold expectation of an UBE. The camera used an infrared signal for night vision and provided 3D images of grayed-out shapes (Fig. 1) by measuring the depth for each pixel as its distance to the camera’s imaging plane.45 Cameras were installed as mobile units on a cart or fixed units on a wall to accommodate the needs/environment of each hospital. Cameras were 3 to 6 ft from the foot of the bed and 6.5 to 7 ft above the floor. We provided onsite training for nurses with supporting documentation about the AVMS, starting/stopping patients, and consenting patients. We emphasized 2 topics:
FIGURE 2.
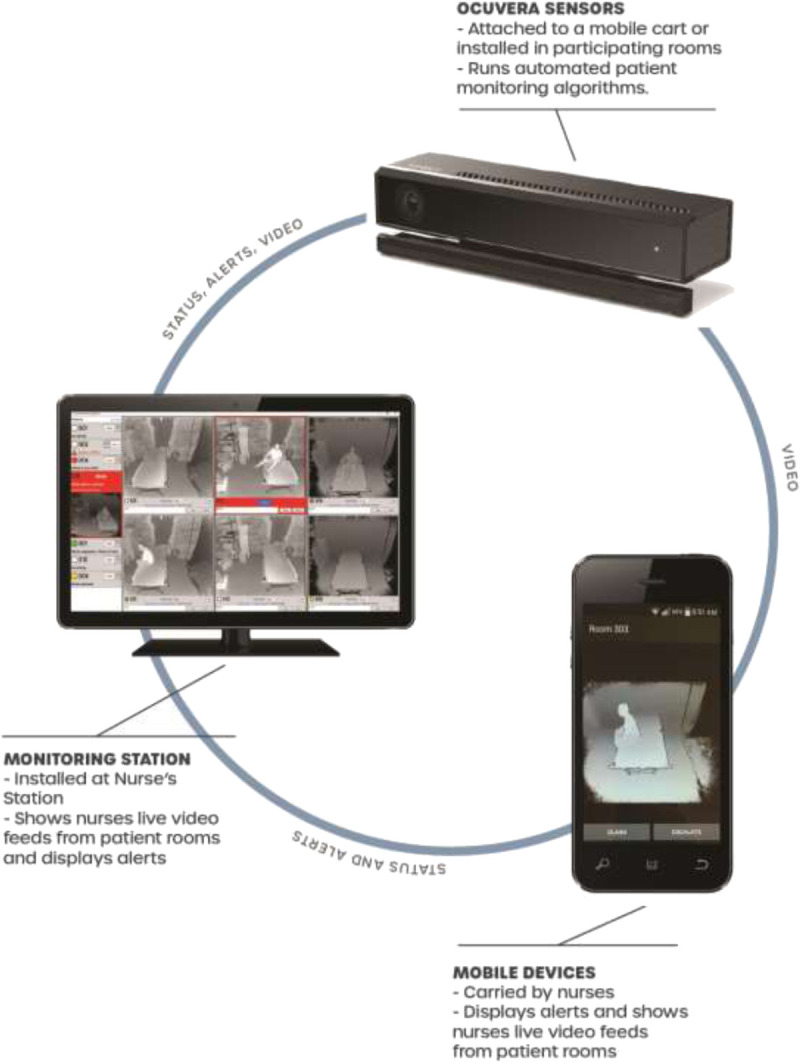
Ocuvera AVMS.
Point the camera at the foot of the bed to improve accuracy of predictive algorithms.
Press the “Privacy Mode” button during sensitive patient care to stop the camera for 15-minute increments.
We regularly exchanged a hard drive within each room sensor, transported it to our office, downloaded system performance data and video to a restricted-access hard drive, and converted the video into numeric data for analysis. We manually identified UBEs in the video by viewing every sixth frame at high speed and then determining whether there was an associated alert for each identified UBE. To adjust for errors associated with this manual process, we randomly sampled for missed UBEs. To account for UBEs found by random sampling, we weighted the number of manually found UBEs using the ratio: (total time for given patient not within 7 minutes before and 2 minutes after a manually found UBE)/(total time not within 7 minutes before and 2 minutes after a randomly found UBE). The average weight for a randomly found UBE was approximately 50.
Measures and Analyses
Measures included hospital characteristics (Table 1), patient demographics (Table 2), hospital admissions and fall event information, hours of video recorded, UBEs per patient, and lead time for baseline UBEs. We calculated 2 UBE rates:
TABLE 2.
Patient Demographics
| Demographics | Aggregate | Baseline | Intervention | P* |
|---|---|---|---|---|
| Age category | n = 358, n (%) | n = 216, n (%) | n = 142, n (%) | 0.687 |
| <65 | 29 (8.1) | 17 (7.9) | 12 (8.5) | |
| 65–84 | 150 (41.9) | 87 (40.3) | 63 (44.4) | |
| 85+ | 179 (50.0) | 112 (51.9) | 67 (47.2) | |
| Sex | n = 350, n (%) | n = 211, n (%) | n = 139, n (%) | 0.826 |
| Female | 195 (55.7) | 119 (56.4) | 76 (54.7) | |
| Male | 155 (44.3) | 92 (43.6) | 63 (45.3) | |
| Diagnoses† | n = 354, n (%) | n = 217, n (%) | n = 137, n (%) | 0.549 |
| Cardiovascular | 47 (13.3) | 32 (14.7) | 15 (10.9) | |
| Fall history | 24 (6.8) | 14 (6.5) | 10 (7.3) | |
| Gastrointestinal | 19 (5.4) | 12 (5.5) | 7 (5.1) | |
| Infection | 31 (8.8) | 16 (7.4) | 15 (10.9) | |
| Mental status change | 22 (6.2) | 9 (4.1) | 13 (9.5) | |
| Neurological | 23 (6.5) | 13 (6.0) | 10 (7.3) | |
| Orthopedic | 47 (13.3) | 29 (13.4) | 18 (13.1) | |
| Renal\Urinary | 22 (6.2) | 16 (7.4) | 6 (4.4) | |
| Respiratory | 59 (16.7) | 37 (17.1) | 22 (16.1) | |
| Weak | 19 (5.4) | 14 (6.5) | 5 (3.6) | |
| Other | 41 (11.6) | 25 (11.5) | 16 (11.7) | |
| Fall risk category‡ | n = 326, n (%) | n = 196, n (%) | n = 130, n (%) | 0.459 |
| Low | 23 (7.1) | 13 (6.6) | 10 (7.7) | |
| High | 116 (35.6) | 75 (38.3) | 41 (31.5) | |
| Very high | 187 (57.4) | 108 (55.1) | 79 (60.8) |
*Differences between baseline and intervention phases calculated using the Pearson χ2 test or Fisher exact test.
†Admitting diagnoses were sorted into categories consistent with those used by Morse et al.47
patient rate of UBEs per day = (total number of UBEs per patient/total hours of video per patient) × (24 hours per day) and
hospital rate of UBEs per day = (total number of UBEs per hospital/total hours of video per hospital) × (24 hours per day).
To determine the potential prospective advantage of the AVMS, we measured the lead time for UBEs that occurred during baseline when nurses did not receive alerts. Lead time was the number of seconds that elapsed from the first alert generated by the system due to patient movement until 3 seconds after the UBE. If the system did not generate an alert until after the UBE, then lead time was negative. We calculated lead time if the patient movement that precipitated the alert began when the patient was lying in bed and was alone from the time movement began until 3 seconds after the UBE. We used descriptive statistics and hypothesis tests appropriate for the distribution and sample size of the measure to compare differences between baseline and intervention values. We attempted a mixed linear model to predict median lead time using patient as a random effect, but this model did not converge because of limited sample size. We report the specific tests used in the notes section of our tables and within figure titles. We considered P values less than 0.05 to be statistically significant and those less than 0.10 to be marginally significant (of interest) given our sample size of 15 hospitals and the value of identifying potentially promising evidence.52,53 We used IBM SPSS Statistics version 25 to conduct all analyses (IBM, Armonk, New York).
We determined the positive predictive value (PPV) and sensitivity of alerts received by nurses during intervention. To determine PPV, 1 or 2 nurses from hospitals not participating in the study independently reviewed video of patient movement that led to alerts during intervention. Nurses classified an alert as “true positive” if they judged that patient behavior in the video warranted assessment or “false positive” if they believed that patient behavior did not warrant assessment. Disagreements were resolved by consensus when possible. To determine the sensitivity of the alerts, we calculated the proportion of UBEs during intervention that were preceded by an alert.
RESULTS
During the 13 months of the study, 408 patients consented to participate. We analyzed video from 372 (91%) patients (Table 1). Video was not analyzed when a camera was improperly positioned such that the bed was not fully visible for more than half of the admission, which typically occurred with mobile cameras during baseline. Five hospitals used fixed cameras, and 10 used mobile cameras. Approximately 4% of hospital admissions participated in the study.
Patient Demographics
The characteristics of patients did not vary significantly from baseline to intervention (Table 2). More than 90% of patients were 65 years and older, and half were 85 years and older. Most were female; respiratory, cardiovascular, and orthopedic conditions were the most prevalent diagnoses. Approximately 93% of patients were at high or very high risk for falls.
Unattended Bed Exits
Of 221 patients in the baseline phase, 71 (32%) exited the bed unattended 507 times (mean, 7.14; median, 3.00; range, 1–66). Of 151 patients in the intervention phase, 47 (31%) exited the bed unattended 815 times (mean, 17.35; median, 6.00; range, 1–203). Weights were applied to the UBE count for 1 baseline patient (79-year-old man at high risk for falls whose UBE count increased from 22 to 66) and 1 intervention patient (79-year-old woman at low risk for falls, whose UBE count increased from 52 to 203). Age and fall risk were significantly associated with the rate of UBEs/day during baseline (Table 3). Specifically, patients younger than 65 years and those at low risk for falls had significantly greater median rates of UBEs/day than did older patients and those at high/very high risk for falls. Only age was significantly associated with the rate of UBEs/day during intervention; patients younger than 65 years had greater rates of UBEs/day than did older patients.
TABLE 3.
Patient Rate of UBEs/Day During Baseline and Intervention by Patient Demographics
| Category (n Baseline, n Intervention) | Patient Rate of UBEs/Day in Baseline, Median (Range) | P* | Patient Rate of UBEs/Day in Intervention, Median (Range) | P* |
|---|---|---|---|---|
| Sex | 0.932 | 0.267 | ||
| Female | 0.00 (0.00–16.75) | 0.00 (0.00–73.06) | ||
| Male | 0.00 (0.00–40.39) | 0.00 (0.00–17.94) | ||
| Age | <0.001 | 0.001 | ||
| <65 y (17, 12) | 0.78 (0.00–16.75) | 2.14 (0.00–17.18) | ||
| 65–84 y (87, 63) | 0.00 (0.00–40.39) | 0.00 (0.00–73.06) | ||
| 85+ y (112, 67) | 0.00 (0.00–4.26) | 0.00 (0.00–13.42) | ||
| Fall risk | 0.015 | 0.130 | ||
| Low (13, 10) | 3.41 (0.00–15.75) | 0.80 (0.00–73.06) | ||
| High (75, 41) | 0.00 (0.00–22.74) | 0.00 (0.00–17.94) | ||
| Very high (108, 79) | 0.00 (0.00–40.39) | 0.00 (0.00–17.18) |
*Nonparametric independent-samples median test.
The hospital rate of UBEs/day ranged from 0 to 1.68 during baseline and from 0 to 5.16 during intervention (Table 4). Thus, it seemed that hospitals used the system for 2 different purposes during intervention: to intervene and prevent UBEs or to monitor patients as they exited the bed unattended. Specifically, the rate of UBEs/day increased from baseline to intervention in 5 hospitals, and it remained 0 or decreased from baseline to intervention in 9 hospitals. (One hospital did not participate in the intervention). Among the 5 monitoring hospitals, the aggregate rate of UBEs/day increased significantly from 0.43 during baseline to 3.18 (649%) during intervention; 3 of these 5 had fixed cameras. Nurses reported that when census was high, patients at low risk for falls were often admitted to rooms with fixed cameras and were not moved because of the cost of cleaning rooms. Conversely, among the 9 intervening hospitals, the aggregate rate of UBEs/day decreased significantly from 0.84 during baseline to 0.09 (89%) during intervention; 8 of these 9 had mobile cameras.
TABLE 4.
Hospital Use of AVM System During Intervention and Comparison of Rate of UBEs Per Day by Study Phase
| Hospital (Intervention Cameras) | Hospital Admissions* in Intervention, % | Patients at High/Very High Risk for Falls in Intervention, % | UBEs/Day | |||
|---|---|---|---|---|---|---|
| Baseline, n | Intervention, n | Difference | P † | |||
| Aggregate monitoring | 3.11 | 89.0 | 0.43 | 3.18 | 2.75 | 0.043 |
| A (4 fixed) | 0.6 | 60.0 | 1.29 | 5.16 | 3.87 | |
| D‡ (4 fixed) | — | 91.7 | 0.15 | 0.18 | 0.03 | |
| I (4 fixed) | 22.5 | 97.3 | 0.40 | 3.60 | 3.20 | |
| L (1 mobile) | 9.5 | 100.0 | 0.03 | 1.14 | 1.11 | |
| N (2 mobile) | 5.2 | 100.0 | 0.12 | 0.24 | 0.12 | |
| Aggregate intervening | 6.59 | 96.5 | 0.84 | 0.09 | −0.75 | 0.018 |
| B (1 mobile) | 6.3 | 100.0 | 0.49 | 0.00 | −0.49 | |
| C (2 mobile) | 15.6 | 100.0 | 0.00 | 0.00 | 0.00 | |
| E (2 mobile) | 15.6 | 71.4 | 0.00 | 0.00 | 0.00 | |
| F (2 mobile) | 5.2 | 100.0 | 0.77 | 0.36 | −0.41 | |
| G (2 mobile) | 5.8 | 100.0 | 1.44 | 0.20 | −1.24 | |
| H§ (2 mobile) | 0.0 | — | 0.00 | — | — | |
| J (4 fixed) | 7.3 | 100.0 | 0.66 | 0.02 | −0.64 | |
| K (2 mobile) | 3.1 | — | 0.98 | 0.04 | −0.94 | |
| M (2 mobile) | 11.3 | 100.0 | 1.68 | 0.28 | −1.40 | |
| O (1 mobile) | 16.7 | 100.0 | 0.62 | 0.00 | −0.62 | |
*Fourteen hospitals reported admissions by month during the study for all patients admitted to acute, skilled rehabilitation, observation, or hospice beds.
†Differences between baseline and intervention phases calculated using the related-samples Wilcoxon signed ranks test.
‡Hospital D did not report admissions data.
§Hospital H did not contribute any patients to the intervention phase of the study.
Among the 9 intervening hospitals during baseline, age was significantly and negatively associated with the rate of UBEs/day. Among 118 patients (Fig. 3A),
FIGURE 3.
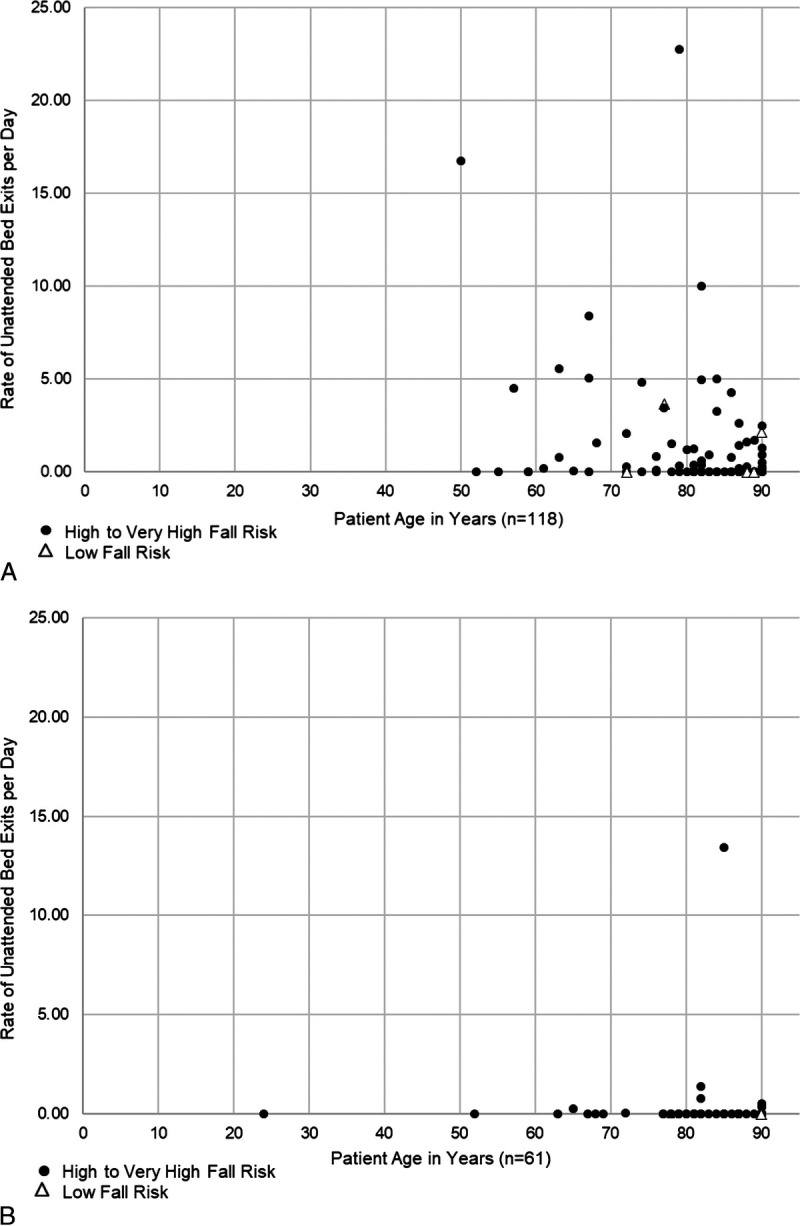
A, Baseline: age is significantly associated with rate of UBEs per day for 9 sites that used AVM system to intervene and prevent UBEs (Spearman ρ = −0.333, P < 0.001). B, Intervention: age is not associated with rate of UBEs per day for 9 sites that used AVM system to intervene and prevent UBEs (Spearman ρ = −0.075, P = 0.567).
71 (60%) had “0” UBEs/day and
47 (40%) had a median rate of 1.31 UBEs/day (range, 0.04–22.74 UBEs/day).
Among these 9 during intervention, age was not significantly associated with the rate of UBEs/day. Among 61 patients (Fig. 3B),
54 (89%) had “0” UBEs/day and
7 (11%) had a median rate of 0.54 UBEs/day (range, 0.054–13.42 UBEs/day).
Among the 5 monitoring hospitals during baseline, age was significantly and negatively associated with the rate of UBEs/day. Among 98 patients (Fig. 4A),
FIGURE 4.
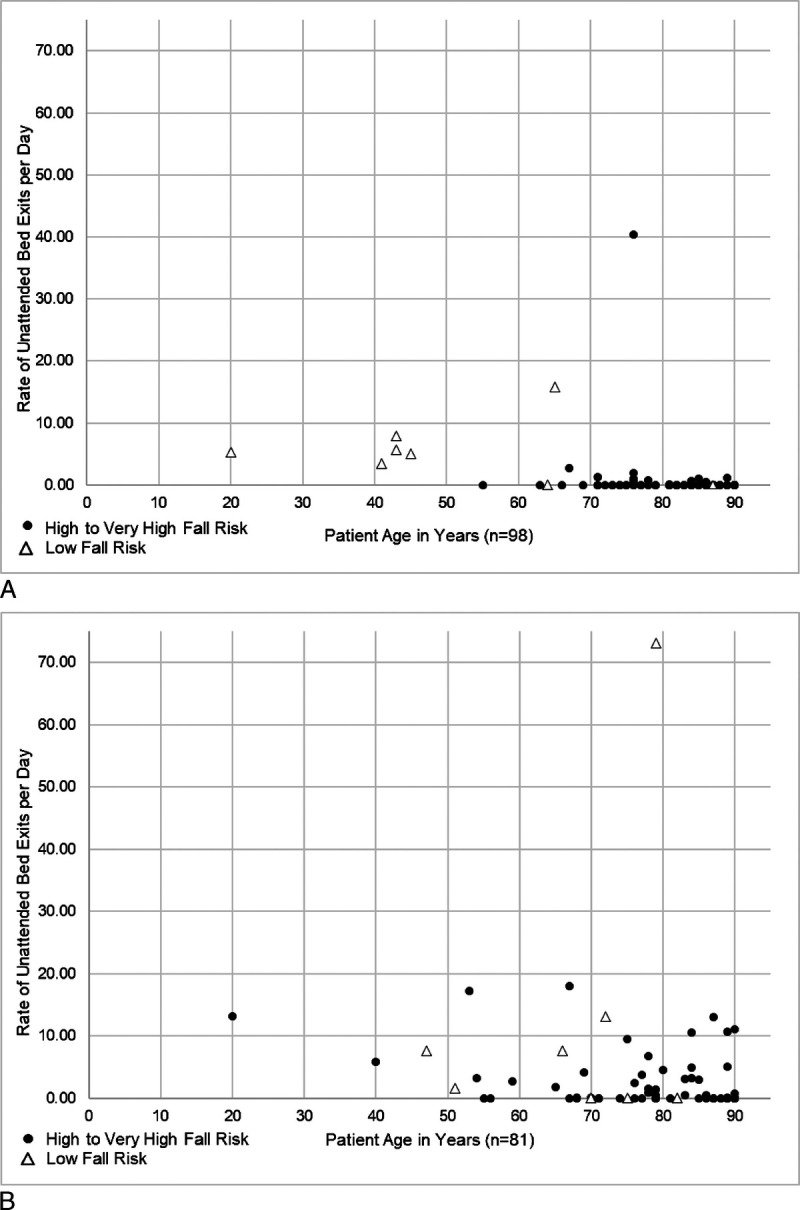
A, Baseline: age is significantly associated with rate of UBEs per day for 5 sites that used AVM system to monitor patients as they exited the bed unattended (Spearman ρ = −0.412, P < 0.001). B, Intervention stage: age is significantly associated with rate of UBEs per day for 5 sites that used AVM system to monitor patients as they exited the bed unattended (Spearman ρ = −0.361, P = 0.001).
77 (79%) had “0” UBEs/day and
21 (21%) had a median rate of 1.11 UBEs/day (range, 0.07–40.39 UBEs/day).
Among these 5 during intervention, age was significantly and negatively associated with the rate of UBEs/day. Among 81 patients (Fig. 4B),
44 (54%) had “0” UBEs/day and
37 (46%) had a median rate of 3.72 UBEs/day (range, 0.06–73.06 UBEs/day).
Lead Time
We calculated the median lead time in seconds for the 318 UBEs associated with 64 of the 71 patients who exited the bed during baseline. The distribution of these medians was right-skewed (mean [SD], 53.61 [71.05]; median, 28.00; range, 0–396). To summarize these skewed data, we identified the 95% central range of the 64 medians54 (mean [SD], 50.58 [56.99]; median, 28.50; range, 1–248). Among the latter 60 observations in the 95% central range, age was positively and significantly associated with median lead time (Fig. 5). Sex and fall-risk category were not significantly associated with median lead time.
FIGURE 5.
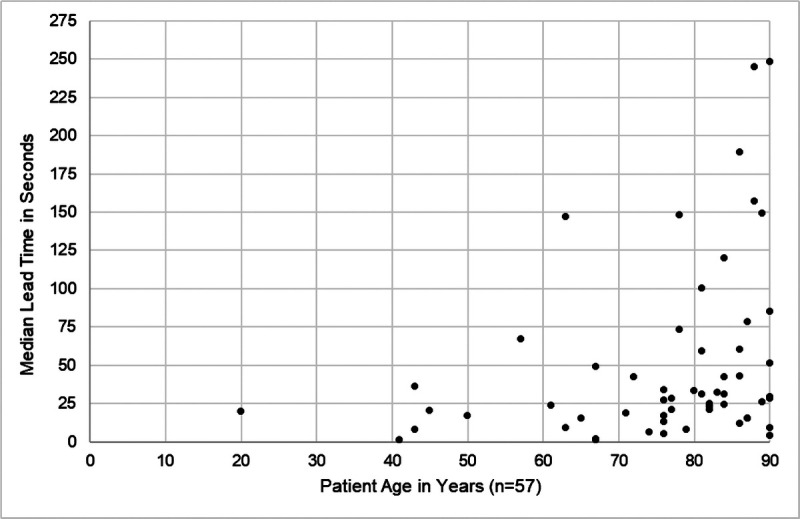
Baseline: age is significantly associated with median lead time (Spearman ρ = 0.359, P = 0.006).
PPV and Sensitivity
The denominator for calculating the PPV of the AVMS was the 4190 alerts associated with patient movement generated during intervention. Because 2 nurses reviewing alerts were not consistently available to independently review and concurrently resolve disagreements, we calculated a conservative and an optimistic estimate of PPV. The numerator for the conservative estimate was the 2362 alerts that 2 nurses agreed were true positives, or if there was only one review, it was also true positive. The numerator for the optimistic estimate was the 2487 alerts that 2 nurses agreed were true positives, and if there was a disagreement, at least one nurse identified the alert as true positive, or if there was only one review, it was also true positive. Thus, the conservative PPV was 56.4% and the optimistic PPV was 59.4%. Forty-seven patients exited the bed unattended 815 times during intervention. Of these 815 UBEs, 794 were preceded by an alert. Thus, the sensitivity of the AVMS to detect UBEs was 97.4%.
Fall Events
From baseline to intervention among the 13 hospitals with complete admissions and fall event data, total falls/1000 admissions decreased from 8.83 to 5.53 (37%), and injurious falls/1000 admissions decreased significantly from 2.52 to 0.55 (78%; Table 5). Approximately 41% of patient falls during the study occurred at bedside. From baseline to intervention among the 5 monitoring hospitals, no study patients fell at the bedside, and the proportions of:
TABLE 5.
Fall Event Characteristics and Falls Per 1000 Admissions
| Hospital Used AVM to Monitor | Hospital Used AVM to Intervene | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fall Event Characteristics | All Falls During Study | Baseline | Intervention | P* | Baseline | Intervention | P* | ||
| Fall location, n = 66, n (%) | 0.729 | 0.271 | |||||||
| Bedside | 27 (40.9) | 6 (40.0) | 9 (50.0)† | 12 (41.4) | 0 (0.00) | ||||
| Not bedside | 39 (59.1) | 9 (60.0) | 9 (50.0) | 17 (58.6) | 4 (100.0) | ||||
| Bathroom | 24 (36.4) | ||||||||
| Chairside | 13 (19.7) | ||||||||
| Hallway | 1 (1.5) | ||||||||
| Room | 1 (1.5) | ||||||||
| Fall assistance, n = 65, n (%) | 0.004 | 1.00 | |||||||
| Unassisted | 47 (72.3) | 14 (100.0) | 10 (55.6) | 20 (69.0) | 3 (75.0) | ||||
| Assisted | 18 (27.7) | 0 (0.00) | 8 (44.4) | 9 (31.0) | 1 (25.0) | ||||
| Extent of harm, n = 66, n (%) | 0.070 | 0.241 | |||||||
| No harm | 52 (78.8) | 10 (66.7) | 17 (94.4) | 23 (79.3) | 2 (50.0) | ||||
| Harm | 14 (21.2) | 5 (33.3) | 1 (5.6) | 6 (20.7) | 2 (50.0) | ||||
| Actions taken due to fall, n = 66, n (%) | |||||||||
| Increased observation | 11 (16.7) | 5 (33.3) | 1 (5.6) | 0.070 | 4 (13.8) | 1 (25.0) | 0.500 | ||
| Imaging | 9 (13.6) | 3 (20.0) | 2 (11.1) | 0.639 | 4 (13.8) | 0 (0.00) | 1.00 | ||
| Medication change | 3 (4.5) | 0 | 0 | NA | 3 (10.3) | 0 (0.00) | 1.00 | ||
| Surgical procedure | 1 (1.5) | 0 | 0 | NA | 1 (3.4) | 0 (0.00) | 1.00 | ||
| Falls/1000 Admissions‡ | Baseline | Intervention | P § | P § | P § | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total falls/1000 admissions | 8.83 | 5.53 | 0.136 | 3.34 | 6.07 | 0.109 | 13.37 | 3.67 | 0.028 | |
| Unassisted falls/1000 admissions | 6.56 | 3.04 | 0.214 | 3.34 | 3.21 | 0.593 | 9.22 | 2.44 | 0.173 | |
| Assisted falls/1000 admissions | 2.27 | 2.49 | 0.285 | 0.00 | 2.86 | 0.109 | 4.15 | 1.22 | 0.028 | |
| Injurious falls/1000 admissions | 2.52 | 0.55 | 0.025 | 2.23 | 0.36 | 0.109 | 2.77 | 1.22 | 0.080 |
*Differences between baseline and intervention phases calculated using the Fisher exact test.
†None of these bedside falls occurred among study patients.
‡Two hospitals either did not report admissions or did not report fall event data and were removed from this analysis.
§Differences between baseline and intervention phases calculated using the related-samples Wilcoxon signed ranks test.
assisted falls increased significantly from 0% to 44%,
injurious falls decreased marginally from 33% to 6%, and
patients requiring increased postfall observation decreased marginally from 33% to 6%.
From baseline to intervention among the 9 intervening hospitals:
the proportion of falls that occurred at the bedside decreased from 41% to 0%,
total falls/1000 admissions decreased significantly from 13.37 to 3.67 (72.6%), and
injurious falls/1000 admissions decreased marginally from 2.77 to 1.22 (56%).
Although not statistically significant, the proportions of patients requiring postfall observation and imaging decreased among all hospitals.
DISCUSSION
We sought to evaluate the feasibility and effectiveness of using a prototypical AVMS to decrease the risk of UBEs as antecedents to unassisted falls among patients at high risk for falls and fall-related injuries in SRHs. Our results demonstrate that the high sensitivity and median lead time of 28 seconds make it feasible to significantly decrease the median rate of UBEs/day in SRHs by 89% with 1 to 2 mobile cameras (Table 4, Fig. 3). Surprisingly, we found that the effectiveness of the AVMS to decrease rates of UBEs may be associated with camera type and hospital usage (Table 4, Fig. 4). Specifically, when census is high, hospitals using few fixed cameras may admit patients at low risk for falls to rooms with the AVMS and then use it to observe these patients as they exit the bed.
Regardless of camera type and hospital usage, when evaluated using the intention-to-treat principle,55 the AVMS may have been effective in preventing bedside falls and decreasing total falls/1000 admissions by 37% and injurious falls by 78% (Table 5). In contrast, CVM decreased total fall rates by 20% to 29%.38,40,43 Thus, nurses may use the sensitivity, lead time, and information generated by the AVMS to adaptively manage fall risk (e.g., apply and use a gait belt and assistive device) and decrease the risk of UBEs as antecedents to unassisted falls and fall-related injuries and not to limit safe mobility. This interpretation is consistent with a PPV value of nearly 60% and is in contrast to the alarm fatigue associated with bed pressure-sensor alarms.36,37
This may be the first study to report the incidence of UBEs among hospitalized patients and to report that age and fall risk are associated with a patient’s rate of UBEs/day. Specifically, younger adults and those at low risk for falls may exit the bed unattended more frequently than older adults and those at high risk for falls (Table 3). However, the older a patient, the longer is the lead time provided by an alert so that older patients, who are at highest risk for fall-related injuries,6,56 also have the greatest lead time before an UBE (Fig. 5). These findings are consistent with normative studies that have documented the negative correlation between age and physical function.57–59
Limitations and Future Research
Limitations to this study include missing data that decreased sample size, using admissions rather than patient days as the denominator to calculate fall rates, and using few cameras per hospital, some of which were fixed. Nurses failed to submit complete demographics for nearly 10% of patients, and we could not calculate fall rates for 2 of 15 hospitals because of missing admissions and fall event data. Using few cameras per hospital led to collecting admissions rather than patient days because we anticipated the need to explain the lack of an impact on fall rates of an intervention applied to 4% of admissions. Using few cameras may have led to “rationing” of the AVMS to patients at highest risk for falls, and using fixed cameras may have led nurses to use the AVMS for some patients at low risk for falls and to not use it for some at high risk for falls and fall-related injuries. As rare events, the rate of falls may vary considerably over a few months.60 Thus, changes in falls/1000 admissions from baseline to intervention should be interpreted as indicating potential promising evidence rather than proof of causation. Finally, this study took place in SRHs in which half of patients were 85 years and older and should not be generalized to larger hospitals. Additional research is needed to better understand the effect of the AVMS on fall rates and the costs of post-fall assessment and treatment. These studies should be conducted in larger hospitals in which every patient at high risk for falls within a unit has access to an AVMS. These studies should compare fall rates and postfall costs between study and control units for up to 1 year before and after intervention.
CONCLUSIONS
Nurses used the high sensitivity and lead time provided by this prototypical AVMS to adaptively manage UBEs as antecedents to unassisted falls and fall-related injuries. Because of the low census in SRHs, just 1 to 2 mobile cameras may improve patient safety in these hospitals with limited resources and high proportions of older adults who are at high risk for falls and fall-related injuries. Additional research is needed to better understand the effect of an AVMS on fall rates and the costs of postfall assessment and treatment.
Footnotes
This project was supported by the U.S. Department of Agriculture National Institute of Food and Agriculture Small Business Innovation Research Phase I (project 1012701), the Nebraska Department of Economic Development, and Ocuvera LLC, Lincoln, Nebraska. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Food and Agriculture, U.S. Department of Agriculture.
K.J. was an associate professor in the College of Allied Health Professions at the University of Nebraska Medical Center until June 2018, when she retired. She completed this article as an independent contractor. L.S. is employed by Ocuvera, LLC, and has an ownership interest in the company; he did not participate in data analysis. G.H. has no conflicts of interest to declare.
Contributor Information
Gleb Haynatzki, Email: ghaynatzki@unmc.edu.
Lucas Sabalka, Email: lsabalka@ocuvera.com.
REFERENCES
- 1.Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26:645–692. [DOI] [PubMed] [Google Scholar]
- 2.Weiss AJ, Elixhauser A, eds. Overview of Hospital Stays in the United States, 2012. Rockville, MD: Agency for Healthcare Research and Quality; October 2014 HCUP Statistical Brief; No. 180. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed February 26, 2020. [PubMed] [Google Scholar]
- 3.Morello RT Barker AL Watts JJ, et al. The extra resource burden of in-hospital falls: a cost of falls study. Med J Aust. 2015;203:367. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141–158. [DOI] [PubMed] [Google Scholar]
- 5.Bouldin EL Andresen EM Dunton NE, et al. Falls among adult patients hospitalized in the United States: prevalence and trends. J Patient Saf. 2013;9:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venema DM Skinner AM Nailon R, et al. Patient and system factors associated with unassisted and injurious falls in hospitals: an observational study. BMC Geriatr. 2019;19:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NORC at the University of Chicago . Estimating the additional hospital inpatient cost and mortality associated with selected hospital-acquired conditions. Agency for Healthcare Research and Quality Web site. Available at: https://www.ahrq.gov/hai/pfp/haccost2017.html. Published November 2017. Updated 2017. Accessed February 29, 2020.
- 8.Wong CA Recktenwald AJ Jones ML, et al. The cost of serious fall-related injuries at three midwestern hospitals. Jt Comm J Qual Patient Saf. 2011;37:81–87. [DOI] [PubMed] [Google Scholar]
- 9.Fields J Alturkistani T Kumar N, et al. Prevalence and cost of imaging in inpatient falls: the rising cost of falling. Clinicoecon Outcomes Res. 2015;7:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande N Metter EJ Lauretani F, et al. Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc. 2008;56:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staggs VS, Dunton N. Associations between rates of unassisted inpatient falls and levels of registered and non-registered nurse staffing. Int J Qual Health Care. 2014;26:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality . Supporting documents—common formats—hospital version 1.2. Available at: https://www.psoppc.org/psoppc_web/publicpages/supportingDocsV1.2. Accessed June 13, 2018.
- 13.Staggs VS, Mion LC, Shorr RI. Assisted and unassisted falls: different events, different outcomes, different implications for quality of hospital care. Jt Comm J Qual Patient Saf. 2014;40:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KJ Skinner A Venema D, et al. Evaluating the use of multiteam systems to manage the complexity of inpatient falls in rural hospitals. Health Serv Res. 2019;54:994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet A DeJuilio P Harkless S, et al. Move to improve: the feasibility of using an early mobility protocol to increase ambulation in the intensive and intermediate care settings. Phys Ther. 2013;93:197–207. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services . Hospital-acquired conditions. CMS.gov Web site. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Updated 2020. Accessed March 28, 2020.
- 17.Agency for Healthcare Research and Quality . AHRQ national scorecard on hospital-acquired conditions updated baseline rates and preliminary results 2014–2017. AHRQ National Scorecard on Hospital-Acquired Conditions Web site. Available at: https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/pfp/hacreport-2019.pdf. Published January 2019. Updated 2019. Accessed February 26, 2020.
- 18.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 19.Evans D Hodgkinson B Lambert L, et al. Fall risk factors in the hospital setting: a systematic review. IJNP. 2001;7:38–45. [DOI] [PubMed] [Google Scholar]
- 20.Oliver D Daly F Martin FC, et al. Risk factors and risk assessment tools for falls in hospital in-patients: a systematic review. Age Ageing. 2004;33:122–130. [DOI] [PubMed] [Google Scholar]
- 21.Tzeng HM, Yin CY. The extrinsic risk factors for inpatient falls in hospital patient rooms. J Nurs Care Qual. 2008;23:233–241. [DOI] [PubMed] [Google Scholar]
- 22.Brewer BB Carley KM Benham-Hutchins M, et al. Nursing unit design, nursing staff communication networks, and patient falls: are they related? HERD. 2018;11:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Real K, Bardach SH, Bardach DR. The role of the built environment: how decentralized nurse stations shape communication, patient care processes, and patient outcomes. Health Commun. 2017;32:1557–1570. [DOI] [PubMed] [Google Scholar]
- 24.Miake-Lye IM Hempel S Ganz DA, et al. Inpatient fall prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):390–396. [DOI] [PubMed] [Google Scholar]
- 25.Kalisch BJ, Lee KH. The impact of teamwork on missed nursing care. Nurs Outlook. 2010;58:233–241. [DOI] [PubMed] [Google Scholar]
- 26.Jones KJ Venema DM Nailon R, et al. Shifting the paradigm: an assessment of the quality of fall risk reduction in Nebraska hospitals. J Rural Health. 2015;31:135–145. [DOI] [PubMed] [Google Scholar]
- 27.Healey F. Preventing falls in hospitals. BMJ. 2016;532:i251. [DOI] [PubMed] [Google Scholar]
- 28.Vincent C, Amalberti R. Safety in healthcare is a moving target. BMJ Qual Saf. 2015;24:539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell MD Lavenberg JG Trotta RL, et al. Hourly rounding to improve nursing responsiveness: a systematic review. J Nurs Adm. 2014;44:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldsack J Bergey M Mascioli S, et al. Hourly rounding and patient falls: what factors boost success? Nursing. 2015;45:25–30. [DOI] [PubMed] [Google Scholar]
- 31.Lang CE. Do sitters prevent falls? A review of the literature. J Gerontol Nurs. 2014;40:24–33; quiz 34-5. [DOI] [PubMed] [Google Scholar]
- 32.Boswell DJ Ramsey J Smith MA, et al. The cost-effectiveness of a patient-sitter program in an acute care hospital: a test of the impact of sitters on the incidence of falls and patient satisfaction. Qual Manag Health Care. 2001;10:10–16. [PubMed] [Google Scholar]
- 33.Tzeng HM, Yin CY, Grunawalt J. Effective assessment of use of sitters by nurses in inpatient care settings. J Adv Nurs. 2008;64:176–183. [DOI] [PubMed] [Google Scholar]
- 34.Donoghue J Graham J Mitten-Lewis S, et al. A volunteer companion-observer intervention reduces falls on an acute aged care ward. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18:24–31. [DOI] [PubMed] [Google Scholar]
- 35.Sahota O Drummond A Kendrick D, et al. REFINE (REducing falls in in-patieNt elderly) using bed and bedside chair pressure sensors linked to radio-pagers in acute hospital care: a randomised controlled trial. Age Ageing. 2014;43:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shorr RI Chandler AM Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med. 2012;157:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels K. Fighting bed alarm fatigue in orthopedic units. Nursing. 2014;44:66–67. [DOI] [PubMed] [Google Scholar]
- 38.Cournan M, Fusco-Gessick B, Wright L. Improving patient safety through video monitoring. Rehabil Nurs. 2018;43:111–115. [DOI] [PubMed] [Google Scholar]
- 39.Bradley K. Remote video monitoring: a novel approach in fall prevention. J Contin Educ Nurs. 2016;47:484–486. [DOI] [PubMed] [Google Scholar]
- 40.Sand-Jecklin K, Johnson JR, Tylka S. Protecting patient safety: can video monitoring prevent falls in high-risk patient populations? J Nurs Care Qual. 2016;31:131–138. [DOI] [PubMed] [Google Scholar]
- 41.Quigley PA, Votruba L, Kaminski J. Outcomes of patient-engaged video surveillance on falls and other adverse events. Clin Geriatr Med. 2019;35:253–263. [DOI] [PubMed] [Google Scholar]
- 42.Hardin SR Dienemann J Rudisill P, et al. Inpatient fall prevention: use of in-room webcams. J Patient Saf. 2013;9:29–35. [DOI] [PubMed] [Google Scholar]
- 43.Jeffers S Searcey P Boyle K, et al. Centralized video monitoring for patient safety: a Denver health lean journey. Nurs Econ. 2013;31:298–306. [PubMed] [Google Scholar]
- 44.Goodlett D Robinson C Carson P, et al. Focusing on video surveillance to reduce falls. Nursing. 2009;39:20–21. [DOI] [PubMed] [Google Scholar]
- 45.Bauer P Kramer JB Rush B, et al. Modeling bed exit likelihood in a camera-based automated video monitoring application. 2017 IEEE International Conference on Electro Information. 2017;56–61. [Google Scholar]
- 46.Baernholdt M Hinton ID Yan G, et al. Fall rates in urban and rural nursing units: does location matter? J Nurs Care Qual. 2018;33:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morse JM, Tylko SJ, Dixon HA. Characteristics of the fall-prone patient. Gerontologist. 1987;27:516–522. [DOI] [PubMed] [Google Scholar]
- 48.Morse JM, Tylko SJ, Dixon HA. The patient who falls—and falls again: defining the aged at risk. J Gerontol Nurs. 1985;11:15–18. [DOI] [PubMed] [Google Scholar]
- 49.Schmid NA. 1989 Federal Nursing Service Award Winner. Reducing patient falls: a research-based comprehensive fall prevention program. Mil Med. 1990;155:202–207. [PubMed] [Google Scholar]
- 50.Victoria Department of Human Services Metropolitan Health and Aged Care Division . Minimising the Risk of Falls and Fall-Related Injuries: Guidelines for Acute, Sub-acute and Residential Care Settings. Melbourne, Australia: Victorian Government Department of Human Services; 2004. [Google Scholar]
- 51.Poe SS Cvach M Dawson PB, et al. The Johns Hopkins fall risk assessment tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293–298. [DOI] [PubMed] [Google Scholar]
- 52.Berwick DM. The science of improvement. JAMA. 2008;299:1182–1184. [DOI] [PubMed] [Google Scholar]
- 53.Kirk RE. Practical significance: a concept whose time has come. Educ Psychol Meas. 1996;56:746–759. [Google Scholar]
- 54.Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1999. [Google Scholar]
- 55.Detry MA, Lewis RJ. The intention-to-treat principle: how to assess the true effect of choosing a medical treatment. JAMA. 2014;312:85–86. [DOI] [PubMed] [Google Scholar]
- 56.Anderson C Dolansky M Damato EG, et al. Predictors of serious fall injury in hospitalized patients. Clin Nurs Res. 2015;24:269–283. [DOI] [PubMed] [Google Scholar]
- 57.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- 58.Chen HT, Lin CH, Yu LH. Normative physical fitness scores for community-dwelling older adults. J Nurs Res. 2009;17:30–41. [DOI] [PubMed] [Google Scholar]
- 59.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–267. [DOI] [PubMed] [Google Scholar]
- 60.He J, Dunton N, Staggs V. Unit-level time trends in inpatient fall rates of US hospitals. Med Care. 2012;50:801–807. [DOI] [PubMed] [Google Scholar]


