Abstract
Objectives
The aim of this study was to compare ultrasound (US) grading and laboratory measures in patients with rheumatoid arthritis.
Methods
Two-hundred four patients with rheumatoid arthritis who received US evaluation for synovitis were included after excluding those using tocilizumab. Ultrasound grading of synovial hypertrophy (SH) and power Doppler (PD) at the most severe site were recorded. An assessment of the correlation of laboratory measures and US grading was conducted by reviewing the electronic medical records.
Results
High-titer anti–cyclic citrullinated peptide (anti-CCP) antibodies positivity was associated with SH grade ≥2 (odds ratio [OR], 6.00; 95% confidence interval [CI], 1.78–20.2) and PD grade ≥2 (OR, 5.56; 95% CI, 1.82–16.9). Recent C-reactive protein (CRP) levels ≥0.3 mg/dL were associated with SH grade ≥2 (OR, 3.13; 95% CI, 1.38–7.10) and PD grade ≥2 (OR, 2.38; 95% CI, 1.31–4.31). Anti-CCP antibody levels correlated with US scores better than the levels of CRP with higher Spearman ρ correlation coefficients. Most of the patients with recent CRP levels <0.3 mg/dL had US synovitis. In logistic regression, high levels of anti-CCP antibodies and CRP were both independently associated with SH grade ≥2 and PD grade ≥2.
Conclusions
Higher levels of anti-CCP antibodies and CRP may predict synovitis on US, whereas discrepancies existed between inflammatory markers and US grading. These findings suggest that US has a role in the comprehensive assessment of disease activity, especially for patients with high-titer positive anti-CCP antibodies.
Key Words: laboratory measures, power Doppler, rheumatoid arthritis, synovial hypertrophy, ultrasound grading
Ultrasound (US) has been widely applied in rheumatoid arthritis (RA) in recent decades as a reliable imaging technique that detects more erosions than conventional radiography, especially in early RA.1,2 When there is diagnostic doubt, US can be used to improve the certainty of a diagnosis of RA.2 Ultrasound can detect synovial proliferation, joint effusion, tendinitis, and bone erosion in RA; therefore, it is also used to assess treatment response, monitor disease activity, evaluate remission, and predict erosion progression.3–5 The ability of US to evaluate inflammation and structural damage in RA has been validated with magnetic resonance imaging.6 In addition, US has a role in guiding treatment for treat-to-target in RA.7 However, the optimal utilization of US in routine clinical practice for patients with established RA remains uncertain.3,8,9
The objective assessment of disease activity in RA includes erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), the markers of inflammation. In addition, rheumatoid factor (RF) and anti–cyclic citrullinated peptide (anti-CCP) antibodies are not only indicators for the diagnosis, but also predictors of disease outcome.10,11 Higher levels of ESR, anti-CCP antibody positivity were reported to be associated with more adverse clinical consequences.12,13 The absence of US power Doppler (PD) synovitis was associated with the achievement of complete remission, lower median CRP values, and fewer swollen joints.14 Power Doppler was an effective tool in assessing response to treatment.3,15 Synovial hypertrophy (SH) without PD activity was also reported to reflect active disease.16 The US score for patients with RA was developed.17,18 The European League Against Rheumatism (EULAR) Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) score demonstrated moderate to good reliability in metacarpophalangeal (MCP) and non-MCP joints using a standardized scan.19,20 A comparison of the US grading system and clinical and laboratory parameters in patients with RA in real-world database is required.
In this study, we focus on patients with RA who received US scans for assessing synovitis severity and investigated the relation of the US score and the clinical and laboratory parameters in a tertiary referral center.
MATERIALS AND METHODS
Patient Selection
The patients who had been adequately followed and registered for catastrophic illnesses for RA in National Taiwan University Hospital, Taipei, Taiwan, were screened for eligibility in this study. The registry of RA was based on the 1987 American College of Rheumatology classification criteria for RA21 and/or the 2010 American College of Rheumatology/EULAR classification criteria.22 According to the Taiwan National Health Insurance system, people who have been diagnosed with catastrophic illness (including RA) are eligible to apply for a medical certificate that confirms the diagnosis and allows an exemption from National Health Insurance copayment. Because all applications must be carefully reviewed by medical experts, the classification of catastrophic illness is considered accurate and therefore reliable. Patients who received US scans for the evaluation of their disease activity from June 2013 to June 2018 in the rheumatology department were reviewed. We included the patients who underwent US examinations for the assessment of active synovitis in the elbows, wrists, MCP joints, proximal interphalangeal (PIP) joints, knees, ankles, and/or metatarsophalangeal (MTP) joints where the validated scoring system was applicable.18 The exclusion criteria were as follows: age younger than 20 years, a history of trauma at the examined joint, or active infection of the examined joint. Data from patients using tocilizumab were also excluded because of effects on CRP levels.
Clinical Data Collection
The baseline characteristics and disease duration at the time of US examinations were obtained by reviewing the electronic medical records. A duration of fewer than 6 months of symptoms of disease was defined as early RA.23 Laboratory measures related to RA were collected for analysis. The median levels of autoantibodies within 12 months from US examinations, including RF and anti-CCP antibodies, were used for the analysis. A high-positive RF (by nephelometry) and anti-CCP antibodies refer to international units (IU) values that were more than 3 times the upper limit of normal for the laboratory and assay (anti-CCP antibody level >30 U/mL or RF level >47.7 IU/mL). For inflammatory markers, including CRP (reference value, <0.3 mg/dL) and ESR (reference value, <15 mm/h), the levels closest to the day of US examinations within 30 days were included in the analysis.
US Assessment and Scoring
Ultrasound examinations were performed with a Toshiba Xario XG US system (Toshiba Medical Systems Corporation, Tochigi, Japan) using a broadband 7.2- to 14-MHz linear array transducer set to muscle-skeleton. The operators were rheumatologists who had been certified by the Chinese Taipei Society of Ultrasound in Medicine after independently scanning more than 150 patients. The US scans used in this study were obtained by a certified sonographer with more than 3 years of experience. Both gray-scale and PD ultrasonography of the joints were performed following the guidelines for musculoskeletal US in rheumatology.24 The definition of sonographic findings was based on the OMERACT US consensus. The grading of SH and PD was according to the definition developed by the OMERACT US Task Force, which divides the severity of SH and the intensity of PD from normal (grade 0) to the most severe (grade 3),18 and the EULAR-OMERACT combined score was calculated.19,20 If multiple joints in a patient were examined at the same time, only the US grading of the joint that displayed the highest EULAR-OMERACT combined score was included for analysis. The determination of bone erosion, if it occurred at the examined joints, was based on the OMERACT definition.18 If a patient received US examinations more than once during the eligible period, the earlier one would be included for analysis.
Ethics Statement
The study protocol was approved by the institutional review board and ethical committee of National Taiwan University Hospital, Taipei, Taiwan (201811095RIND).
Statistical Analysis
Quantitative and continuous variables were examined using the Mann-Whitney U test. Relationships among categorical data were assessed with Fisher exact test. Spearman rank correlation analysis was used to evaluate the correlation between the grade of SH and PD, combined score, and laboratory parameters. Spearman ρ ≥ 0.3 indicated a fair to strong positive correlation.25 Association with p < 0.05 was considered statistically significant. Statistical analyses were performed using R version 3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Clinical Features of Patients
In the 2364 patients registered for catastrophic illnesses for RA, 289 patients received US examinations during the eligible period, and 204 of them met the eligibility criteria for this study. Their baseline clinical features are summarized in Table 1. The duration of disease (median and interquartile range) was 51.8 months (23.0–94.8 months), and patients with early RA accounted for only 9.3% of the included cases. The most recent levels of CRP and ESR within 30 days and the median levels of RF and anti-CCP antibodies within 12 months from the day of US examinations were available in 183 (89.7%), 178 (87.3%), 141 (69.1%), and 66 (32.4%) patients, respectively. The median number (interquartile range) of joints with combined score grade ≥1 in the US examination was 2 (1 to 4). The hand (wrist, MCP, and PIP joints) was the site that displayed the most active synovitis in the majority of the patients (61.8%). In the most severely inflamed joint, SH grade ≥2 and PD grade ≥2 were found in 160 (78.4%) and 96 (47.1%) patients, respectively.
TABLE 1.
Clinical Features of Patients (n = 204)
| Sex, male/female (male %) | 32/172 (15.7) |
| Age, mean ± SD, y | 56.2 ± 12.8 |
| The duration of disease, median (IQR), mo | 51.8 (23.0–94.8) |
| Early RA (duration ≤6 mo), n (%) | 19 (9.3) |
| Laboratory measures | |
| Median levels of RF (n = 141), median (IQR), IU/mL | 33.4 (5.5–125) |
| Median levels of anti-CCP antibodies (n = 66), median (IQR), IU/mL | 66.8 (1.3–280.8) |
| High-titera RF positivity, n/available (%) | 62/141 (44.0) |
| High-titera anti-CCP antibody positivity, n/available (%) | 40/66 (60.6) |
| The most recent CRP level (n = 183), median (IQR), mg/dL | 0.25 (0.08–1.02) |
| The most recent ESR (n = 178), median (IQR), mm/h | 16 (10–29) |
| No. inflamed joints in the US examination | |
| Joints with combined score grade >0, median (IQR) | 2 (1–4) |
| Joints with combined score grade ≥2, median (IQR) | 1 (1–2) |
| Sites displayed the most severe synovitis | |
| Elbow, n (%) | 22 (10.8) |
| Wrist/MCP/PIP, n (%) | 126 (61.8) |
| Knee, n (%) | 38 (18.6) |
| Ankle/MTP, n (%) | 18 (8.8) |
| US score of the most severely inflamed joint | |
| Grade of SH, median (IQR) | 3 (2–3) |
| SH grade ≥2, n (%) | 160 (78.4) |
| Grade of PD, median (IQR) | 1 (1–2) |
| PD grade ≥2, n (%) | 96 (47.1) |
| Combined score, median (IQR) | 3 (2–3) |
| Combined score grade ≥2, n (%) | 40 (80.4) |
| Associated US findings | |
| Any bone erosion, n (%) | 96 (47.1) |
| Joint injection at the examination, n (%) | 30 (14.7) |
| Concurrent medical therapies | |
| NSAIDs, n (%) | 154 (75.5) |
| Glucocorticoids, n (%) | 91 (44.6) |
| MTX, n (%) | 126 (61.8) |
| Conventional DMARDs other than MTX, n (%) | 177 (86.8) |
| biologic DMARDs, n (%)b | 50 (24.5) |
aHigh-positive refers to IU values that are >3 times the upper limit of normal for the assay.
bData from patients using tocilizumab were excluded in this study.
DMARDs, disease-modifying antirheumatic drugs; IQR, interquartile range; MTP, metatarsophalangeal; MTX, methotrexate; NSAIDs, nonsteroidal anti-inflammatory drugs.
Analysis of the Factors Associated With Positive US Findings
The associations between the positive US findings and clinical and laboratory parameters are summarized in Table 2. Male sex tended to be associated with PD grade ≥2 (odds ratio [OR], 2.11; 95% confidence interval [CI], 0.97–4.58). A high-titer anti-CCP antibody positivity was associated with SH grade ≥2 (OR, 6.00; 95% CI, 1.78–20.2), PD grade ≥2 (OR, 5.56; 95% CI, 1.82–16.9), and the presence of bone erosions (OR, 4.51; 95% CI, 1.49–13.6). The most recent CRP level ≥0.3 mg/dL was associated with SH grade ≥2 (OR, 3.13; 95% CI, 1.38–7.10) and PD grade ≥2 (OR, 2.38; 95% CI, 1.31–4.31). No significant association could be found between positive US findings and the duration of disease, recent ESR ≥15 mm/h, or high-titer RF positivity.
TABLE 2.
Association Between the Positive US Finding and Clinical and Laboratory Parameters (n = 204)
| SH Grade ≥ 2 | PD Grade ≥ 2 | Bone Erosion | ||||
|---|---|---|---|---|---|---|
| p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | |
| Sex (male) | 0.242 | 2.12 (0.70–6.41) | 0.082 | 2.11 (0.97–4.58) | 0.563 | 1.33 (0.63–2.84) |
| Age | 0.415 | n/a | 0.214 | n/a | 0.618 | n/a |
| Duration of disease | 0.574 | n/a | 0.102 | n/a | 0.238 | n/a |
| Early RA | 0.770 | 1.52 (0.42–5.47) | 0.637 | 1.28 (0.50–3.29) | 1.000 | 1.01 (0.39–2.61) |
| High-titera RF positivity (n = 141) | 0.835 | 1.14 (0.50–2.61) | 0.235 | 1.57 (0.80–3.07) | 0.309 | 1.47 (0.75–2.87) |
| High-titera anti-CCP antibody positivity (n = 66) | 0.004b | 6.00 (1.78–20.2) | 0.002b | 5.56 (1.82–16.9) | 0.010b | 4.51 (1.49–13.6) |
| Recent CRP level ≥0.3 mg/dL (n = 183) | 0.005b | 3.13 (1.38–7.10) | 0.005b | 2.38 (1.31–4.31) | 0.301 | 1.38 (0.77–2.48) |
| Recent ESR ≥15 mm/h (n = 178) | 0.120 | 1.92 (0.89–4.13) | 0.451 | 1.31 (0.72–2.37) | 0.451 | 0.77 (0.43–1.40) |
aHigh-positive refers to IU values that are >3 times the upper limit of normal for the assay.
bp < 0.05.
Correlation Between US Grading and Clinical and Laboratory Parameters
The most recent CRP level showed a fair correlation with PD grade (ρ = 0.30, p < 0.001), as summarized in Table 3. The median anti-CCP antibody levels within 1 year showed a fair correlation with US scores including SH grade (ρ = 0.38, p = 0.002), PD grade (ρ = 0.46, p < 0.001), and the combined score (ρ = 0.39, p = 0.001). The most recent ESR poorly correlated with US grading. The median RF level poorly correlated with the PD grade. No significant correlation was found between US grading and the duration of disease.
TABLE 3.
Correlation Between the Indexes of US Grading and Clinical and Laboratory Parameters
| Laboratory Parameters | Grade of SH | Grade of PD | Combined Score | |||
|---|---|---|---|---|---|---|
| ρ | p value | ρ | p value | ρ | p value | |
| Duration of disease | 0.09 | 0.207 | −0.05 | 0.437 | 0.07 | 0.296 |
| Median RF level (n = 141) | 0.07 | 0.397 | 0.18 | 0.036a | 0.08 | 0.333 |
| Median anti-CCP antibody level (n = 66) | 0.38b | 0.002a | 0.46b | <0.001a | 0.39b | 0.001a |
| Recent CRP level (n = 183) | 0.17 | 0.019a | 0.30b | <0.001a | 0.19 | 0.011a |
| Recent ESR (n = 178) | 0.16 | 0.029a | 0.18 | 0.018a | 0.16 | 0.031a |
ap < 0.05.
bρ ≥ 0.3.
Ultrasound Grading in Patients With a Low CRP Level <0.3 mg/dL
In patients with the most recent CRP levels <0.3 mg/dL (n = 99), there was considerable proportion of patients who had grade 3 SH (48.5%) and grade 3 PD (12.1%). Compared with the patients with the most recent CRP levels ≥0.3 mg/dL (n = 84), differences were found in the frequencies of grade 1 SH, grade 0 PD, and grade 2 PD (Fig.). No significant difference could be found in the frequency of grade 3 SH (p = 0.141) or grade 3 PD (p = 0.220). In the patients with available anti-CCP antibody levels (n = 66), 12 patients had the most recent CRP level <0.3 mg/dL and a combined score of 3, and 10 (83.3%) of them presented with high-titer anti-CCP antibody positivity.
FIGURE.
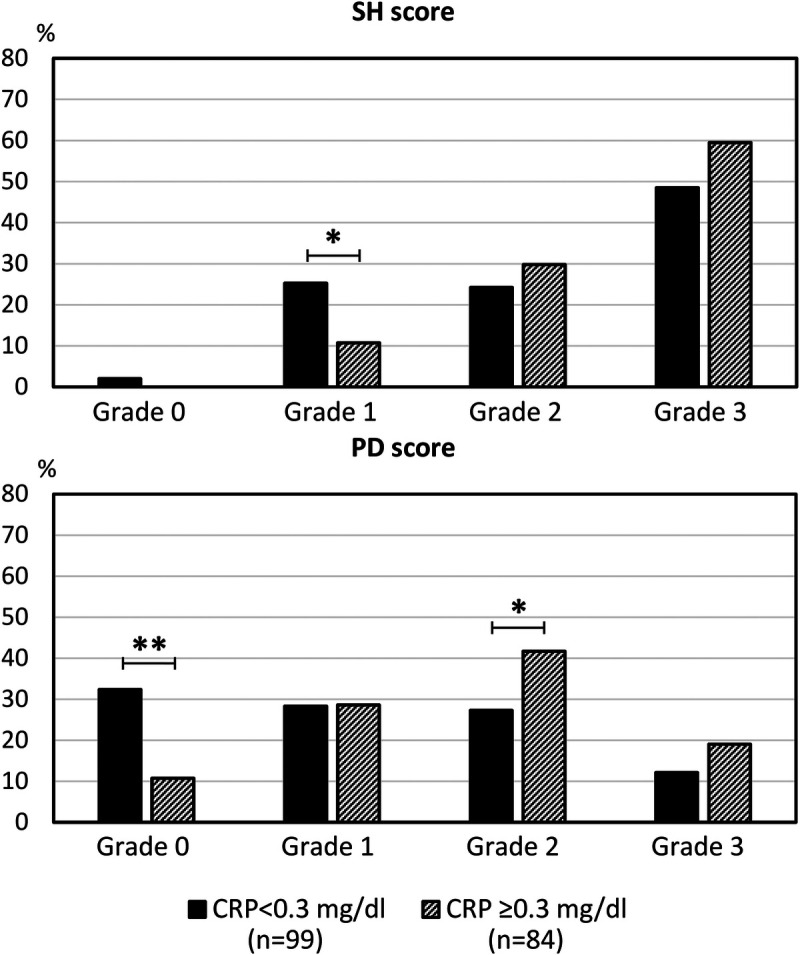
Comparison of the frequencies of grading of SH and PD in patients with a recent CRP level ≥0.3 or <0.3 mg/dL. A significant difference was not found in the frequency of grade 3 SH or PD. *p < 0.05 and **p < 0.01.
Subgroup Analysis of the Patients Whose Anti-CCP Antibody and CRP Levels Were Both Available
In the 61 patients whose anti-CCP antibody and CRP levels were both available, the duration of disease was 25 months (7.3–65.7 months). Synovial hypertrophy grade ≥2 and PD grade ≥2 were found in 47 (77.0%) and 30 (49.2%) of the patients, respectively. The best correlation to SH grade, PD grade, and the combined score was still found with the median anti-CCP antibody levels (ρ = 0.41, p = 0.001 for SH grade; ρ = 0.45, p < 0.001 for PD grade; ρ = 0.41, p = 0.001 for the combined score). For CRP levels, positive correlation to US grading was also demonstrated (ρ = 0.22, p = 0.082 for SH grade; ρ = 0.41, p < 0.001 for PD grade; ρ = 0.26, p = 0.047 for the combined score). In multivariate logistic regression analysis, high-titer anti-CCP antibody positivity and the most recent CRP level ≥0.3 mg/dL were both independently associated with SH grade ≥2 (OR, 5.60; 95% CI, 1.48–24.8 for anti-CCP antibodies; and OR, 4.98; 95% CI, 1.24–25.8 for CRP) and PD grade ≥2 (OR, 4.23; 95% CI, 1.33–14.9 for anti-CCP antibodies; OR, 3.69; 95% CI, 1.22–11.9 for CRP).
DISCUSSION
In the presented study, we examined the association between inflammatory markers and US findings in patients with RA using the grading system developed by the EULAR-OMERACT US task force. Patients with early RA consisted of only 9.3% of the included patients. The duration of disease was not associated with bone erosion, probably due to the drug treatment including the use of biological agents in 24.5% of the patients before the US examinations. Unlike many clinical studies that focused on early RA,6,13,26 our study could address real-world evidence about established RA.
In addition to the role it plays in the diagnosis of RA, anti-CCP antibody is well-known to predict outcomes in RA.27–29 The results of our study also emphasize the value of anti-CCP antibody level in predicting active synovitis and structural changes. More severe synovitis and more frequent bone erosions were demonstrated in patients with high-titer anti-CCP antibodies. Besides, the levels of anti-CCP antibodies were correlated with the grade of SH and PD and the EULAR-OMERACT combined score.
The more aggressive disease was reported in men than in women,30 and our study also revealed that more male patients tended to have a PD grade ≥2. The role of PD grade in RA synovitis for evaluating the activity and predicting outcomes was reported in many studies.6,26,31–33 The anti-CCP antibodies had a reasonably significant correlation with the grade of PD in the presented study (ρ = 0.46, p < 0.001). We also performed exploratory analysis for the relationship between the laboratory measures and the number of inflamed joints. The only fairly positive correlation was found between the anti-CCP antibody levels and the number of joints with PD grade ≥2 (ρ = 0.35, p = 0.004). On the other hand, the correlation of the most recent ESR with US scores was poor. A fairly positive correlation was still found between the most recent CRP levels and PD grade (ρ = 0.30, p < 0.001), although the correlation was not as high as that between anti-CCP antibodies and PD grade. Most of the patients (n = 168) had the ESR and CRP levels measured on the same day. However, many factors could result in a falsely high or low ESR. The findings in our study suggest that the CRP level might be more reliable than ESR to measure the activity of synovitis in daily clinical practice.
Although the recent CRP level showed a fair correlation to the grade of PD, a considerable number of patients with low CRP levels had grade 3 PD, and the frequency of high-titer positive anti-CCP antibodies among these patients was high. The lack of high CRP levels in patients with active RA has been reported,34 and there might be altered immunological mechanisms.35 Because PD predicts treatment response and disease flares,31–33 the objective evaluation of the disease activity of RA should not be completely dependent on laboratory measures. A US examination, including PD, may help us to monitor synovitis and guide therapeutic decisions, especially for the patients with high-titer positive anti-CCP antibodies, even if the CRP levels are low.
Given the nature of the study, our investigation was limited by the heterogeneity of the patients. Only the joint displaying the highest combined score could be used for analysis, and the small case number restricted performing additional subgroup analysis. The dates of the CRP and ESR tested in this study had a median of 10 days from the day of US examinations and were not the same day. Anti-CCP antibody levels within 1 year were available in only 66 patients (32.4%). Besides, the frequently used disease activity indexes such as the Disease Activity Score in 28 joints, the Clinical Disease Activity Index, and the Simplified Disease Activity Index were not available in the electronic medical records. However, the patients were selected from a database that consisted of more than 2000 RA patients. We excluded patients using tocilizumab because of its effects on CRP levels. By the adoption of commonly used laboratory indices related to RA, we could still analyze and compare the performance of autoantibodies, ESR and CRP, and US grading in real-world practice. Moreover, the correlation between the laboratory measures and the US grading in the subgroup with complete data was very similar to the whole group. Further longitudinal observational studies are warranted.
In conclusion, this study demonstrated that higher levels of anti-CCP antibodies and CRP might predict synovitis on US and higher grades of PD in patients with RA. Anti-CCP antibodies correlate to US synovitis better than CRP. In addition, discrepancies existed between inflammatory markers and the grading of synovitis. In real-world practice settings, our findings suggest that US should be included in the comprehensive assessment of disease activity in RA.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Cheng-Hsun Lu, Email: b89401085@ntu.edu.tw.
Lung-Fang Chen, Email: lungfangchen@gmail.com.
Yi-Min Huang, Email: zeamayschang@gmail.com.
Chiao-Feng Cheng, Email: chiaofengcheng@gmail.com.
Song-Chou Hsieh, Email: hsiehsc@ntu.edu.tw.
REFERENCES
- 1.Wakefield RJ Gibbon WW Conaghan PG, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43:2762–2770. [DOI] [PubMed] [Google Scholar]
- 2.Colebatch AN Edwards CJ Ostergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–814. [DOI] [PubMed] [Google Scholar]
- 3.D'Agostino MA Terslev L Wakefield R, et al. Novel algorithms for the pragmatic use of ultrasound in the management of patients with rheumatoid arthritis: from diagnosis to remission. Ann Rheum Dis. 2016;75:1902–1908. [DOI] [PubMed] [Google Scholar]
- 4.Forien M, Ottaviani S. Ultrasound and follow-up of rheumatoid arthritis. Joint Bone Spine. 2017;84:531–536. [DOI] [PubMed] [Google Scholar]
- 5.Tan YK, Østergaard M, Conaghan PG. Imaging tools in rheumatoid arthritis: ultrasound vs magnetic resonance imaging. Rheumatology (Oxford). 2012;51(suppl 7):vii36–vii42. [DOI] [PubMed] [Google Scholar]
- 6.Xu H Zhang Y Zhang H, et al. Comparison of the clinical effectiveness of US grading scoring system vs MRI in the diagnosis of early rheumatoid arthritis (RA). J Orthop Surg Res. 2017;12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandl P, Aletaha D. The role of ultrasound and magnetic resonance imaging for treat to target in rheumatoid arthritis and psoriatic arthritis. Rheumatology (Oxford). 2019;58:2091–2098. [DOI] [PubMed] [Google Scholar]
- 8.Dale J Stirling A Zhang R, et al. Targeting ultrasound remission in early rheumatoid arthritis: the results of the TaSER study, a randomised clinical trial. Ann Rheum Dis. 2016;75:1043–1050. [DOI] [PubMed] [Google Scholar]
- 9.Nessrine A Siham D Meryem B, et al. Should the ultrasound of hands be a component of rheumatoid arthritis remission criteria? Curr Rheumatol Rev. 2018;15:312–315. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi T Miyasaka N Inui T, et al. High titers of both rheumatoid factor and anti-CCP antibodies at baseline in patients with rheumatoid arthritis are associated with increased circulating baseline TNF level, low drug levels, and reduced clinical responses: a post hoc analysis of the RISING study. Arthritis Res Ther. 2017;19:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alten R Mariette X Lorenz HM, et al. Predictors of abatacept retention over 2 years in patients with rheumatoid arthritis: results from the real-world ACTION study. Clin Rheumatol. 2019;38:1413–1424. [DOI] [PubMed] [Google Scholar]
- 12.Alemao E Guo Z Frits ML, et al. Association of anti–cyclic citrullinated protein antibodies, erosions, and rheumatoid factor with disease activity and work productivity: a patient registry study. Semin Arthritis Rheum. 2018;47:630–638. [DOI] [PubMed] [Google Scholar]
- 13.Quintana-Duque MA Rondon-Herrera F Mantilla RD, et al. Predictors of remission, erosive disease and radiographic progression in a Colombian cohort of early onset rheumatoid arthritis: a 3-year follow-up study. Clin Rheumatol. 2016;35:1463–1473. [DOI] [PubMed] [Google Scholar]
- 14.Gul HL Eugenio G Rabin T, et al. Defining remission in rheumatoid arthritis: does it matter to the patient? A comparison of multi-dimensional remission criteria and patient reported outcomes. Rheumatology (Oxford). 2020;59:613–621. [DOI] [PubMed] [Google Scholar]
- 15.Ellegaard K Christensen R Torp-Pedersen S, et al. Ultrasound Doppler measurements predict success of treatment with anti–TNF-α drug in patients with rheumatoid arthritis: a prospective cohort study. Rheumatology (Oxford). 2011;50:506–512. [DOI] [PubMed] [Google Scholar]
- 16.Terslev L Ostergaard M Sexton J, et al. Is synovial hypertrophy without Doppler activity sensitive to change? Post-hoc analysis from a rheumatoid arthritis ultrasound study. Arthritis Res Ther. 2018;20:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szkudlarek M Court-Payen M Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–962. [DOI] [PubMed] [Google Scholar]
- 18.Ohrndorf S, Backhaus M. Advances in sonographic scoring of rheumatoid arthritis. Ann Rheum Dis. 2013;72(suppl 2):ii69–ii75. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino MA Terslev L Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT Ultrasound Taskforce—part 1: definition and development of a standardised, consensus-based scoring system. RMD Open. 2017;3:e000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terslev L Naredo E Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT Ultrasound Taskforce—part 2: reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open. 2017;3:e000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett FC Edworthy SM Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D Neogi T Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. [DOI] [PubMed] [Google Scholar]
- 23.Singh JA Saag KG Bridges SL Jr., et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 24.Backhaus M Burmester GR Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. 2018;18:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foltz V Gandjbakhch F Etchepare F, et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis Rheum. 2012;64:67–76. [DOI] [PubMed] [Google Scholar]
- 27.Kastbom A Strandberg G Lindroos A, et al. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis. 2004;63:1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hejblum BP Cui J Lahey LJ, et al. Association between anti–citrullinated fibrinogen antibodies and coronary artery disease in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2018;70:1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetland ML Østergaard M Stengaard-Pedersen K, et al. Anti–cyclic citrullinated peptide antibodies, 28-joint Disease Activity Score, and magnetic resonance imaging bone oedema at baseline predict 11 years’ functional and radiographic outcome in early rheumatoid arthritis. Scand J Rheumatol. 2019;48:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Weyand CM Schmidt D Wagner U, et al. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41:817–822. [DOI] [PubMed] [Google Scholar]
- 31.Naredo E Valor L de la Torre I, et al. Predictive value of Doppler ultrasound–detected synovitis in relation to failed tapering of biologic therapy in patients with rheumatoid arthritis. Rheumatology (Oxford). 2015;54:1408–1414. [DOI] [PubMed] [Google Scholar]
- 32.Filippou G Sakellariou G Scire CA, et al. The predictive role of ultrasound-detected tenosynovitis and joint synovitis for flare in patients with rheumatoid arthritis in stable remission. Results of an Italian multicentre study of the Italian Society for Rheumatology Group for ultrasound: the STARTER study. Ann Rheum Dis. 2018;77:1283–1289. [DOI] [PubMed] [Google Scholar]
- 33.Nordberg LB Lillegraven S Aga AB, et al. The impact of ultrasound on the use and efficacy of intraarticular glucocorticoid injections in early rheumatoid arthritis: secondary analyses from a randomized trial examining the benefit of ultrasound in a clinical tight control regimen. Arthritis & rheumatology (Hoboken, NJ). 2018;70:1192–1199. [DOI] [PubMed] [Google Scholar]
- 34.Orr CK Najm A Young F, et al. The utility and limitations of CRP, ESR and DAS28-CRP in appraising disease activity in rheumatoid arthritis. Front Med (Lausanne). 2018;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradford CM Mak HYJ Kidd L, et al. Lack of CRP response in patients with active rheumatoid arthritis—what are the immunological causes, and how can we harness this data to improve disease outcomes. Ann Rheum Dis. 2016;75:180.4–180.1. [Google Scholar]


