Abstract
Objectives
This study investigated severe medication errors (MEs) reported to the National Supervisory Authority for Welfare and Health (Valvira) in Finland and evaluated how the incident documentation applies to learning from errors.
Methods
This study was a retrospective document analysis consisting of medication-related complaints and authoritative statements investigated by Valvira in 2013 to 2017 (n = 58).
Results
Medication errors caused death or severe harm in 52% (n = 30) of the cases (n = 58). The majority (83%; n = 48) of the incidents concerned patients older than 60 years. Most likely, the errors occurred in prescribing (n = 38; 47%), followed by administration (n = 15; 19%) and monitoring (n = 14; 17%). The error process often included many failures (n = 24; 41%) or more than one health professional (n = 16; 28%). Antithrombotic agents (n = 17; 13%), opioids (n = 10; 8%), and antipsychotics (n = 10; 8%) were the therapeutic groups most commonly involved in the errors. Almost all error cases (91%; n = 53) were assessed as likely or potentially preventable. In 60% (n = 35) of the cases, the organization reported actions taken to improve medication safety after the occurrence of the investigated incident.
Conclusions
Medication errors reported to the national health care supervisory authority provide a valuable source of risk information and should be used for learning from severe errors at the level of health care systems. High age remains a key risk factor to severe MEs, which may be associated with a wide range of medications including those not typically perceived as high-alert medications or high-risk administration routes. Despite being complex processes, the severe MEs have a great potential to lead to developing systems, processes, resources, and competencies of health care organizations.
Key Words: patient safety, medication safety, severe medication error, health care supervisory authority
Medication errors (MEs) are one of the most prevalent types of errors in patient care.1,2 The reduction of severe MEs should be a primary target of medication risk management in health care systems.3 This concept is also promoted by current global actions, such as the World Health Organization third patient safety challenge aiming to reduce ME-associated deaths by 50% by the year 2022.4 The definitions of severe patient safety incidents vary greatly,5 and in this article, severe MEs refer to the errors that are causing severe harm or have the potential to cause severe harm.
Previous studies on severe MEs have demonstrated challenges in several phases of the medication process, especially in prescribing and follow-up, administration, and patient transitions.4,6–13 The patient groups most vulnerable to severe MEs and harm have been pediatric patients and patients with comorbidities, polypharmacy, and high age.6,7,13–15 Also, high-alert medications causing more likely severe harm to patients, if associated with the error, have been identified and recommended to be prioritized in the development of medication safety.3,6,7,13,15–19
Medication error reporting systems (MERs) are among the recommended actions to learn from errors and near misses in health care.1,20–23 However, comprehensive national or even local MER systems are not established in many countries.22,24 There are also other challenges associated with MER systems, such as underreporting and the quality of retrospective data.21,23 Furthermore, these systems rarely capture severe, fatal errors.25 Therefore, MER systems should be complemented with other data sources and methods for medication risk management.21 Health care authority or patient insurance-based safety incident databases could serve as valuable data sources in this respect.26 However, these databases have been underused, although some evidence exists on their successful use in medication risk management research.9,26–30
Finnish health care system is based on public services available for all citizens. Municipalities are responsible for organizing and financing the services. The care is arranged in primary care (e.g., hospitals and public care centers), secondary and tertiary care (central and university hospitals), and social care units (e.g., assisted living facilities and home care for elderly or disabled people). Private sector produces only a quarter of the health care services. In Finland, systematic patient safety work has been conducted since 2005. However, a national MER system is still lacking. The Finnish National Supervisory Authority for Welfare and Health (Valvira) is the national authority that investigates patient safety incidents that have led to severe harm or death because of inappropriate care.31 Valvira’s legal mandate is to improve the quality and safety of health care services through the guidance and supervision of registered health care professionals. Valvira undertakes the legitimation and registration of health care professionals and supervises their professional performance after the registration. The postregistration supervision is based mainly on complaints from patients and/or their relatives and notifications from, for example, employer or the police. This study aimed to analyze severe MEs reported to the Valvira in Finland and to evaluate how the documentation of incidents in such a data source applies to learning from errors at the national level.
METHODS
Study Material
This study was a retrospective document analysis.32 The material consisted of (1) medication-related complaints that Valvira had investigated and closed, and (2) medication-related authoritative statements that Valvira had made for the police of Finland during the period 2013–2017. In the authoritative statements, Valvira assesses the appropriateness of the provided care in cases under inspection by the police to assist in determining whether criminal proceedings should take place.
The medication-related complaints and statements fulfilling the following inclusion criteria were included in the study: the primary cause was classified as “pharmacotherapy” by Valvira, the case was closed, and Valvira assessed the case to include inappropriate patient care (Fig. 1). The data search was done first in Valvira’s electronic database using the automated search tool and then finalized manually by a researcher (A.T.; Fig. 1). The cases were not included or excluded based on the severity of the outcome to the patient; instead, both cases with actual harm or near miss (the error was noticed and corrected before it reached the patient) were included in the study material. The documentation of the complaints and statements was included in most of the cases: (1) a copy of the patient records and other documents needed for incident evaluation, (2) responses from the professionals involved and/or managers of the health care organization, (3) an external expert (physicians or other specialists) opinion, and (4) the incident report written by the Valvira’s senior medical or legal officer. This incident documentation was qualitative narrative data in nature, and it described the incident and its circumstances, as well as the conclusion of the case. The total material per case varied between 20 and 150 pages.
FIGURE 1.
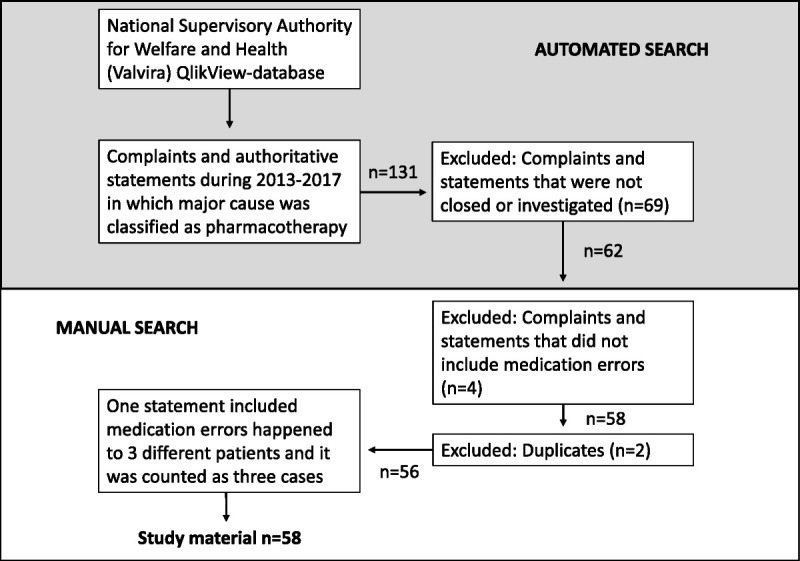
Data inclusion and collection protocol for MEs investigated by the National Supervisory Authority for Welfare and Health (Valvira) in Finland in 2013 to 2017.
Theoretical Framework
The theoretical framework for the study was the system approach to human error and error management to understand the processes leading to severe MEs and harm.33,34 This study applied the comprehensive ME definition by the National Coordinating Council for Medication Error Reporting and Prevention, which is also the widely used definition in previous studies.35,36
Data Collection
For collecting the data, a structured data collection form was developed by the research group based on their previous study.37 Data from the complaints and statements were collected by a researcher (A.T.) using the data collection form. The data collection form was anonymous and therefore did not include any information on the patients, professionals, or organizations involved in the error cases. The data collection form recorded the following information: (1) patient background information (age, sex), (2) medicines involved in the error, (3) step(s) of the medication process where the error happened, (4) setting (e.g., hospital) where the error happened, (5) professional group(s) involved, (6) harm to the patient, (7) researcher’s assessment of preventability of the error.
Data Analysis
Harm for the patient in the cases was assessed with 4 categories: death, severe harm, nonsevere harm, and no harm. Harm for the patient was defined as severe when the error had been life-threatening, led to hospitalization or prolonged hospitalization, or caused permanent or significant injury with incapacity.38 Medicines were regarded as high-alert medicines if they were present in the lists of the Institute for Safe Medication Practices (ISMP).17–19 The preventability of the errors in this study was defined according to the systems approach to human error33 and by modifying the definitions used in previous studies37,39 (Table 1). Those MEs that had the potential to cause harm for the patients but were noticed before reaching the patient were categorized as prevented.
TABLE 1.
Definition of the ME Preventability Used in the Study (Modified According to Hallas et al39 and Linden-Lahti et al37
| ME Preventability | Description |
|---|---|
| Prevented | The error was prevented before it reached the patient. |
| Likely preventable | There was an existing procedure, operating model, or a guideline, and the error would have been prevented when acting according to it, but it was not followed. |
| Potentially preventable | There was no existing procedure, operating model, or guideline, but the error could have potentially been prevented from reoccurring with some medication safety development actions. |
| Unlikely preventable | Error or adverse drug event that was unlikely to be anticipated and would be difficult to prevent to reoccur even with new systemic defenses or other prospective medication risk management actions. |
Although the qualitative data documented in the incident reports were carefully read by case, the information of interest was recorded in a structured data collection forms. In cases with difficulties categorizing the data, discrepancies were solved as the consensus of 2 researchers (A.T. and C.L.-L.). The quantified, structured, and categorized data were analyzed using descriptive statistics (frequencies and percentages; Microsoft Excel; Microsoft Corporation, Redmond, Washington).40
Cases that included information on the organizations’ actions to prevent reoccurrence of such MEs and improve medication safety were further analyzed. Those medication safety actions were identified, analyzed, and categorized using the Institute for Healthcare Improvement (IHI) Action Hierarchy Template.41 According to the action hierarchy, the stronger the preventive action is, the less it is based on human performance.41,42
Research Ethics
The study was conducted with the research permit and supervision of Valvira. Guidelines for research ethics and data protection were followed throughout the research process.43
RESULTS
Characteristics of MEs
During the period 2013–2017, Valvira received a total of 1654 complaints and statement requests.44 In this 5-year study period, 58 cases with MEs fulfilling the study criteria were found from Valvira’s database (Table 2). In the medication process, errors were fatal in 21 cases (36%) and caused severe harm in 9 cases (16%). Nonsevere harm was the end-result of an error in 19 cases (33%). In 3 cases (5%), the error was detected before it reached the patient, and in 6 cases (10%), the researcher was not able to assess the harm level because of the lack of information in the case reports.
TABLE 2.
Characteristics of MEs (n = 58) Investigated by Valvira During the Period 2013–2017
| Characteristic | n | % |
|---|---|---|
| Patient sex | 58 | |
| Female | 34 | 59 |
| Male | 24 | 41 |
| Patient age, y | 58 | |
| 0–19 | 0 | 0 |
| 20–39 | 4 | 7 |
| 40–59 | 6 | 10 |
| 60–79 | 15 | 26 |
| 80–99 | 33 | 57 |
| Severity of harm | 58 | |
| Death | 21 | 36 |
| Severe harm | 9 | 16 |
| Nonsevere harm | 19 | 33 |
| No harm | 3 | 5 |
| Not able to assess | 6 | 10 |
| Preventability | 58 | |
| Likely preventable | 39 | 67 |
| Potentially preventable | 14 | 24 |
| Unlikely preventable | 2 | 4 |
| Prevented | 3 | 5 |
| Error setting* | 64 | |
| Assisted living facility | 16 | 25 |
| University hospital | 10 | 16 |
| Primary care ward outside hospital | 10 | 16 |
| Central hospital | 10 | 16 |
| Primary care hospitals | 9 | 14 |
| Public health center | 4 | 6 |
| Home care | 3 | 5 |
| Pharmacy | 1 | 2 |
| Private medical center | 1 | 2 |
| Health care professional involved* | 74 | |
| Physician | 37 | 50 |
| Practical nurse | 17 | 23 |
| Nurse | 13 | 18 |
| Student | 5 | 7 |
| Pharmacist | 2 | 3 |
| Medication process phase* | 81 | |
| Prescribing | 38 | 47 |
| Administration | 15 | 19 |
| Monitoring | 14 | 17 |
| Dispensing | 6 | 7 |
| Documentation | 5 | 6 |
| Use of medicine by the patient | 1 | 1 |
| Distribution from pharmacy | 1 | 1 |
| Ordering medication from the pharmacy | 1 | 1 |
*One error process can include several settings, professionals involved, or failures.
Of the patients who had suffered from ME, 59% (n = 34) were female and 41% (n = 24) were male (Table 2). The average age of the patients was 74 years, with a range of 25 to 99 years. A majority (83%; n = 48) of the patients were older than 60 years. According to these data, the ME victim was most likely a woman older than 80 years (n = 25; 43%). In total, 91% (n = 53) of the errors were assessed as likely or potentially preventable, with only 2 cases (3%) resulting in patient death assessed to be unlikely preventable.
A typical setting for an ME was a hospital (in secondary or tertiary care n = 20; in primary care, n = 9; total, n = 29 [45%]), but also settings where older people are mostly cared (e.g., primary care wards outside hospital, assisted living facilities, home care), were highly represented (n = 29; 45%). In 6 cases (10%), 2 different organizations were involved in the ME process, whereas most of the cases (n = 52; 90%) were associated with one organization.
Physicians (n = 37; 50%) were the health care professionals most commonly involved in the investigated ME incidents, followed by practical nurses (n = 17; 23%) and nurses (n = 13; 18%) (Table 2). In 28% (n = 16) of the cases, more than 1 health care professional was involved in the ME process. In 7 cases (12%), Valvira had concluded that the ME was caused by process deficiencies in the organization, not by individual health care professionals’ inappropriate performance. Most of the errors occurred in prescribing (n = 38; 47%), administration (n = 15; 19%), and monitoring (n = 14; 17%) phases of the medication process. In 41% (n = 24) of the cases, the same ME was observed in several phases of the medication use process.
The Medicines Involved in the Errors
The total number of medicines involved in all error cases (n = 58) was 131, representing 77 different effective substances (Table 3). In these cases, specific effective substances were identified for 126 medicines, whereas for 5 medicines, only the therapeutic ATC group (level 2–3 code) was known.45 Nearly half of the cases (n = 26; 45%) included more than 1 effective substance. The top 5 therapeutic groups (ATC levels 2–3) most frequently involved in the errors (n = 58) were antithrombotic agents (n = 17; 13%), opioids (n = 10; 8%), antipsychotics (n = 10; 8%), drugs used in diabetes (n = 8; 6%), and drugs for cardiac therapy (n = 8; 6%). Oxycodone and enoxaparin were found to be the most common specific effective substances associated with the MEs. Both medicines were reported in 7 of 58 cases (Table 3). Errors with enoxaparin were associated with prescribing too high doses, insufficient therapeutic monitoring, or treatment duration. Problems with oxycodone were typically exceeding the prescribed dose when using oral suspension, giving medicine to a wrong patient, wrong administration route, or failure in adjusting the dose according to changes in patient condition.
TABLE 3.
Medicines (n = 131) Involved in All Error Cases (n = 58) According to Level 2–3 ATC Codes45
| ATC Group | n (%) | Specific Effective Substance Mentioned in >1 Error Case (n of the Cases) |
|---|---|---|
| B01 Antithrombotic agents | 17 (13.0) | Enoxaparin (7), warfarin (4), acetylsalicylic acid (3) |
| N02A Opioids | 10 (7.6) | Oxycodone (7), fentanyl (2) |
| N05A Antipsychotics | 10 (7.6) | Quetiapine (4), haloperidol (3) |
| A10 Drugs used in diabetes | 8 (6.1) | Metformin (3) |
| C01 Cardiac therapy | 8 (6.1) | Isosorbide mononitrate (2), isosorbide dinitrate (2), digoxin (2) |
| N05C Hypnotics and sedatives | 7 (5.3) | Temazepam (4) |
| N05B Anxiolytics | 6 (4.6) | Diazepam (3), lorazepam (2) |
| C03 Diuretics | 5 (3.8) | Furosemide (5) |
| C07 β-blocking agents | 5 (3.8) | Metoprolol (3), bisoprolol (2) |
| N03 Antiepileptics | 5 (3.8) | — |
| A12AX Calcium, combinations | 4 (3.1) | Calcium and vitamin D combination (4) |
| J01 Antibacterials for systemic use | 4 (3.1) | — |
| R03 Drugs for obstructive airway diseases | 4 (3.1) | — |
| H03 Thyroid therapy | 3 (2.3) | Levothyroxine (3) |
| A06 Drugs for constipation | 3 (2.3) | — |
| N01 Anesthetics | 3 (2.3) | — |
| N06D Antidementia drugs | 3 (2.3) | — |
| V03 All other therapeutic subgroups | 3 (2.3) | Naloxone (3) |
| A02 Drugs for acid related disorders | 2 (1.5) | — |
| A07 Antidiarrheals, intestinal anti-inflammatory/anti-infective agents | 2 (1.5) | — |
| H02 Corticosteroids for systemic use | 2 (1.5) | — |
| N02B Other analgesics and antipyretics | 2 (1.5) | Paracetamol (2) |
| N06A Antidepressants | 2 (1.5) | — |
| S01 Ophthalmologicals | 2 (1.5) | — |
| Other | 11 (8.4) | — |
| Total | 131 |
Only medicines mentioned in >1 error cases are presented according to specific effective substances.
In total, 36% (n = 47) of the effective substances in MEs were identified as high-alert medicines.17–19 Effective substances involved in the MEs resulting in severe harm or death of a patient (n = 30) are described in Table 4. Many high-alert medicines are found at the top of the severe ME list (enoxaparin, oxycodone, warfarin), but also other medicines were associated with severe harm or death of the patient.
TABLE 4.
Effective Substances (n = 78) Involved in MEs That Caused Severe Harm or Death of a Patient (n = 30)
| Medicine (n = 78) | n (%) |
|---|---|
| Enoxaparin* | 5 (6.4) |
| Furosemide | 4 (5.1) |
| Oxycodone* | 4 (5.1) |
| Warfarin* | 3 (3.8) |
| Naloxone | 3 (3.8) |
| Quetiapine | 3 (3.8) |
| Metoprolol | 3 (3.8) |
| Bisoprolol | 2 (2.6) |
| Isosorbide mononitrate | 2 (2.6) |
| Metformin* | 2 (2.6) |
| Digoxin* | 2 (2.6) |
| Diazepam | 2 (2.6) |
| Other | 43 (55.1) |
| Total | 78 |
The administration route for 130 medicines involved in all errors (n = 58) was identified. The medicines were administered typically orally, intravenously, or subcutaneously (Table 5). Most of the medicines in severe MEs were administered per oral (72%).
TABLE 5.
Administration Routes of the Medicines Involved in All MEs (n = 58) and MEs That Caused Severe Harm or Death of a Patient (n = 30)
| Administration Route of the Medicine | Medicines in All MEs (n = 130), n (%) | Medicines in MEs Causing Severe Harm or Death (n = 81), n (%) |
|---|---|---|
| Per oral | 89 (69) | 58 (72) |
| Intravenous | 15 (12) | 7 (9) |
| Subcutan | 13 (10) | 10 (12) |
| Epidural | 4 (3) | 4 (5) |
| Inhalation | 4 (3) | — |
| Intramuscular | 2 (2) | 2 (3) |
| Ocular | 2 (2) | — |
| Transdermal | 1 (1) | — |
Actions Taken in the Organizations After the ME Had Occurred
In 60% (n = 35) of the cases (n = 58), the documents available in Valvira included a description of the organization’s changes to their medication processes to prevent the reoccurrence of the errors. Reported organizational changes and/or actions to improve medication safety were multiple, ranging from staff training to introducing technology-based systemic defenses to the processes. A summary of different actions and their level of strength based on the IHI Action Hierarchy41 is presented in Figure 2.
FIGURE 2.
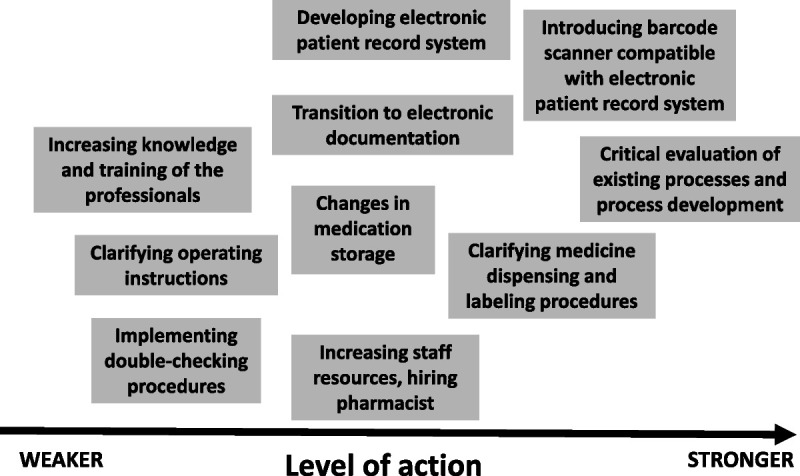
Reported actions taken by health care organizations to improve medication safety after the ME had occurred. The strength of the actions classified according to the IHI Action Hierarchy.41
DISCUSSION
Health Care Authority Data as a Source of Medication Safety Information
This register-based retrospective document analysis was intended to explore MEs reported to a national health care authority Valvira in Finland. Based on our study, the health care authority data proved to be a rich and multidimensional source of medication safety information. A primary reason for its uniqueness is that it provides information on the severe errors that are rare46 and may not be reported to other MER systems.23,25 Because the authority documentation is descriptive and qualitative, it provides a detailed picture of “what went wrong” and in which phases of the medication process, causing a severe incident. Furthermore, the incident reporting to Valvira is patient-driven; a central patient expectation of a complaint process is that a similar error would not happen to anyone else.29 Therefore, our findings complemented with the previous studies recommend using the authority documentation as a source of medication safety information. In countries with well-established MER systems providing structured information, authority documentation could perform as a supplementary data source on severe MEs. The national supervisory authorities’ central role as a provider of medication safety information should also be recognized and established in national and international patient safety improvement policies.
What Can We Learn From Severe MEs?
Our study revealed that elderly people, particularly those older than 80 years were the most vulnerable to severe MEs investigated by Valvira. In the Finnish population, the proportion of the age group of 60 years or older is approximately 29%.47 Although medication treatment in this population is more common, they presented 83% of the patients involved in the severe MEs of this study. Previous studies have reported similar findings, indicating that the effects of MEs are likely to be more harmful to the elderly population with reduced physiological and cognitive functions.6,7,14,48 Because the elderly people were prevalent in the Valvira’s data, the medicines involved in the errors represent medicines typically used in the care of this particular age group. However, many of those medicines are also categorized as potentially inappropriate medication used in elderly people.49
Many of the top 10 medicines associated with severe errors in this study, such as antithrombotic agents and opioids, have been reported as high-alert medications by the previous studies and the ISMP.3,6,13,17–19,50 This finding strengthens the need to adopt effective, evidence-based error safeguarding interventions to different stages of these medicines’ medication process. However, severe errors also occurred with medicines that are not regarded as high-alert medications. This finding may indicate that the severity of the error may be cause of not only the medication itself but also the health status, multimorbidities, and age of the patient, and other systemic contributing factors.6,15 Although many studies have emphasized, for example, intravenous administration as a high-risk administration route,51 our study also highlights the risk associated with the oral treatment.
The present study demonstrates that severe MEs are a challenge to safe care in all health care settings where medicines are used. To our knowledge, there are only few studies on severe MEs that have included all patient care settings. Our study revealed that assisted living facilities but also primary care wards outside the hospitals and home care were equally prone to severe MEs than hospitals. Those settings are often environments where the elderly population is treated and may lack established safe and high-quality medication processes and even health care staff competency.52 This study indicates that preventive medication safety risk management actions in the care of elderly people, and possibly other high-risk populations such as children, should be a high priority for health care settings. In Finland, patient safety challenges in assisted living facilities have been a national crisis reflecting deficiencies in several key areas of safe medication care, such as lack of staff competencies and recourses allocated to elderly care, and often occurring absence of a physician in charge of the entire medication treatment of an individual patient.
This study is in line with the previous studies demonstrating that most of the MEs take place in prescribing and administration stages of the medication process.7,8,10,13,48 According to our study supported by similar findings by Panesar et al,10 monitoring medication use represents a phase of the medication process that defensive actions should strengthen. The severe incidents in this study also typically included more than 1 ME, many organizations, and several medicines. Like the Human Error model by Reason33,34 suggests, severe MEs are often complex processes including many errors and professionals failing to block the error before it causes harm for the patient. There is a need for more qualitative studies to understand the whole process chain behind the severe errors, instead of simple calculations and classifications of the error types to learn effectively from MEs.
Preventing Severe MEs
As suggested by previous studies, most of the severe MEs in this study were assessed as likely or potentially preventable, providing the health care organizations an opportunity to reduce the reoccurrence of these errors by systems-based defences.13,53 More than half of the errors had already led to developing such systems, processes, resources, and competencies. Indeed, it is an encouraging finding that the current severe ME prevention measures seemed to focus on a systems perspective, understanding human errors, and applying medication safety interventions with varying levels of strength, such as adding pharmacist resources or technical solutions.41,42 When developing medication safety, it is important to develop medication processes with varying actions and always, when possible, to select the strongest possible defenses.
Limitations
In this retrospective document analysis, the researchers could not contact the professionals or organizations involved in the errors. Therefore, the possibility existed to misinterpret the free-text data, and all the information to determine comprehensively why the errors happened was not available. The preventability of the errors was especially challenging to evaluate, and the information on the conducted safety development actions in the organizations was, in many cases, missing or incomplete. Also, determining whether one specific ME caused the harm or death of a fragile patient with comorbidity was not always a clear cause-effect relationship. These findings represent key development targets for countries willing to develop the quality and use of their similar data sources for learning from severe MEs. Further research is also needed on the organizational development actions after severe MEs to widen the learning opportunities nationally and internationally.
CONCLUSIONS
Medication errors reported to a national health care supervisory authority are a valuable and unique information source of severe errors, and these data should be regarded as a part of national incident reporting and learning systems in different countries. High age remains a key risk factor to severe MEs, which may be associated with a wide range of medications including those not typically perceived as high-alert medications or high-risk administration routes. Ensuring comprehensive medication safety of elderly and fragile patients should be a primary focus of all care settings, with an emphasis in primary care and long-term care facilities. Despite being complex processes, severe MEs have a great potential to lead to developing systems, processes, resources, and competencies of health care organizations. In conclusion, learning from severe MEs and finding the most effective process defenses to combat their occurrence remain the challenge of health care systems, nationally and globally.
ACKNOWLEDGMENTS
We want to thank Valvira and especially Unit Manager Helena Mönttinen for their valuable contributions to designing and carrying out the study.
Footnotes
This research was funded by the Helsinki University Pharmacy and Finnish Cultural Foundation.
The authors disclose no conflict of interest.
Contributor Information
Anna Takala, Email: annakatariinatakala@gmail.com.
Anna-Riia Holmström, Email: anna-riia.holmstrom@helsinki.fi.
Marja Airaksinen, Email: marjaairaksinen@gmail.com.
REFERENCES
- 1.Institute of Medicine (IOM) . Preventing Medication Errors: Quality Chasm Series. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 2.Elliott RA Camacho E Jankovic D, et al. Economic analysis of the prevalence and clinical and economic burden of medication error in England [published online June 11, 2020]. BMJ Qual Saf. doi: 10.1136/bmjqs-2019-010206. [DOI] [PubMed] [Google Scholar]
- 3.Saedder EA Brock B Nielsen LP, et al. Identifying high-risk medication: a systematic literature review. Eur J Clin Pharmacol. 2014;70:637–645. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) . The third WHO Global Patient Safety Challenge: Medication Without Harm. Launched March 29, 2017. Available at: https://www.who.int/patientsafety/medication-safety/en/. Accessed January 18, 2021.
- 5.Hegarty J Flaherty SJ Saab MM, et al. An international perspective on definitions and terminology used to describe serious reportable patient safety incidents: a systematic review [published online April 6, 2020]. J Patient Saf. doi: 10.1097/PTS.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buajordet I Ebbesen J Erikssen J, et al. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250:327–341. [DOI] [PubMed] [Google Scholar]
- 7.Phillips J Beam S Brinker A, et al. Retrospective analysis of mortalities associated with medication errors. Am J Health Syst Pharm. 2001;58:1835–1841. [DOI] [PubMed] [Google Scholar]
- 8.Lewis PJ Dornan T Taylor D, et al. Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32:379–389. [DOI] [PubMed] [Google Scholar]
- 9.Björkstén KS Bergqvist M Andersén-Karlsson E, et al. Medication errors as malpractice-a qualitative content analysis of 585 medication errors by nurses in Sweden. BMC Health Serv Res. 2016;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panesar SS deSilva D Carson-Stevens A, et al. How safe is primary care? A systematic review. BMJ Qual Saf. 2015;25:477–479. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen JN Nielsen LP Hellebek A, et al. Medication errors involving anticoagulants: data from the Danish patient safety database. Pharmacol Res Perspect. 2017;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yardley I Yardley S Williams H, et al. Patient safety in palliative care: a mixed-methods study of reports to a national database of serious incidents. Palliat Med. 2018;32:1353–1362. [DOI] [PubMed] [Google Scholar]
- 13.Mulac A Taxis K Hagesaether E, et al. Severe and fatal medication errors in hospitals: findings from the Norwegian Incident Reporting System [published online June 23, 2020]. Eur J Hosp Pharm. doi: 10.1136/ejhpharm-2020-002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saedder EA Lisby M Nielsen LP, et al. Number of drugs most frequently found to be independent risk factors for serious adverse reactions: a systematic literature review. Br J Clin Pharmacol. 2015;80:808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) . Medication Safety in High-risk Situations: Technical report. Published 2019. Available at: https://apps.who.int/iris/bitstream/handle/10665/325131/WHO-UHC-SDS-2019.10-eng.pdf?sequence=1&isAllowed=y. Accessed January 18, 2021.
- 16.Thomas AN, MacDonald JJ. A review of patient safety incidents reported as ‘severe’ or ‘death’ from critical care units in England and Wales between 2004 and 2014. Anaesthesia. 2016;71:1013–1023. [DOI] [PubMed] [Google Scholar]
- 17.Institute for Safe Medication Practices (ISMP) . High-Alert Medications in Acute Care Settings. August 23, 2018. Available at: https://www.ismp.org/recommendations/high-alert-medications-acute-list. Accessed January 18, 2021.
- 18.Institute for Safe Medication Practices (ISMP) . High-Alert Medications in Community/Ambulatory Settings. January 31, 2011. Available at: https://www.ismp.org/recommendations/high-alert-medications-community-ambulatory-list. Accessed January 18, 2021.
- 19.Institute for Safe Medication Practices (ISMP) . High-Alert Medications in Long-Term Care (LTC) Settings. November 20, 2017. Available at: https://www.ismp.org/recommendations/high-alert-medications-long-term-care-list. Accessed January 18, 2021.
- 20.Council of Europe . Creation of a better medication safety culture in Europe: building up safe medication practices. 2006. Expert Group on Safe Medication Practices. Available at: http://www.edqm.eu/medias/fichiers/Report_2006.pdf. Accessed January 18, 2021.
- 21.World Health Organization (WHO) . Reporting and learning systems for medication errors: the role of pharmacovigilance centres. Published 2014. Available at: https://apps.who.int/iris/bitstream/handle/10665/137036/9789241507943_eng.pdf. Accessed January 18, 2021.
- 22.Holmström AR. Learning from Medication Errors in Healthcare: How to Make Medication Error Reporting Systems Work? PhD Thesis March 28, 2017. Available at: https://helda.helsinki.fi/handle/10138/179230. Accessed January 18, 2021.
- 23.Holmström AR Järvinen R Laaksonen R, et al. Inter-rater reliability of medication error classification in a voluntary patient safety incident reporting system HaiPro in Finland. Res Social Adm Pharm. 2019;15:864–872. [DOI] [PubMed] [Google Scholar]
- 24.Holmström AR Airaksinen M Weiss M, et al. National and local medication error reporting systems—a survey of practices in 16 countries. J Patient Saf. 2012;8:165–176. [DOI] [PubMed] [Google Scholar]
- 25.Cheung K van den Bemt PMLA Bouvy ML, et al. A nationwide medication incidents reporting system in the Netherlands. J Am Med Inform Assoc. 2011;18:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent C Walshe K Davy C, et al. Learning from litigation—the role of claims analysis in patient safety. In: Walshe K, Boaden R, eds. Patient Safety: Research Into Practice. Berkshire, United Kingdom: Open University Press; 2006:144–160. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson PM, Ovretveit J. Patient claims and complaints data for improving patient safety. Int J Health Care Qual Assur. 2008;21:60–74. [DOI] [PubMed] [Google Scholar]
- 28.van Noord I Eikens MP Hamersma AM, et al. Application of root cause analysis on malpractice claim files related to diagnostic failures. Qual Saf Health Care. 2010;19:e21. [DOI] [PubMed] [Google Scholar]
- 29.Bismark MM Spittal MJ Gogos AJ, et al. Remedies sought and obtained in healthcare complaints. BMJ Qual Saf. 2011;20:806–810. [DOI] [PubMed] [Google Scholar]
- 30.Jylhä V, Saranto K, Bates DW. Preventable adverse drug events and their causes and contributing factors: the analysis of register data. Int J Qual Health Care. 2011;23:187–197. [DOI] [PubMed] [Google Scholar]
- 31.Finnish National Supervisory Authority for Welfare and Health (Valvira) . Available at: https://www.valvira.fi/web/en. Accessed January 7, 2021.
- 32.Weinger MB Slagle J Jain S, et al. Retrospective data collection and analytical techniques for patient safety studies. J Biomed Inform. 2003;36:106–119. [DOI] [PubMed] [Google Scholar]
- 33.Reason J. Human Error. Cambridge, United Kingdom: Cambridge University Press; 1990. [Google Scholar]
- 34.Reason J. Human error: models and management. BMJ. 2000;320:768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) . What is a Medication Error? Available at: https://www.nccmerp.org/about-medication-errors. Accessed January 18, 2021. [DOI] [PubMed]
- 36.Lisby M Nielsen LP Brock B, et al. How are medication errors defined? A systematic literature review of definitions and characteristics. Int J Qual Health Care. 2010;22:507–518. [DOI] [PubMed] [Google Scholar]
- 37.Linden-Lahti C Airaksinen M Pennanen P, et al. Severe medication errors—challenge for patient safety (summary in English). Suom Laakaril. 2009;41:3429–3434. [Google Scholar]
- 38.Gates PJ Baysari MT Mumford V, et al. Standardising the classification of harm associated with medication errors: the Harm Associated with Medication Error Classification (HAMEC). Drug Saf. 2019;42:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallas J Harvald B Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228:83–90. [DOI] [PubMed] [Google Scholar]
- 40.Howell DC. Statistical Methods for Psychology. Belmont, CA: Cengage Learning; 2012. [Google Scholar]
- 41.Institute for Healthcare Improvement (IHI) . Patient Safety Essential Toolkit: Action Hierarchy (part of RCA2). Published 2019. Available at: https://www.health.state.mn.us/facilities/patientsafety/adverseevents/toolkit/docs/safetytoolkit_actionhierarchy.pdf. Accessed January 18, 2021.
- 42.VHA National Center for Patient Safety (NCPS) . Root Cause Analysis Tools. Revised October 10, 2020. Available at: https://www.patientsafety.va.gov/docs/RCA_Guidebook_10212020.pdf. Accessed January 18, 2021.
- 43.All European Academies (ALLEA) . The European Code of Conduct for Research Integrity. Revised Edition. 2017. Available at: https://www.allea.org/wp-content/uploads/2017/05/ALLEA-European-Code-of-Conduct-for-Research-Integrity-2017.pdf. Accessed January 7, 2021.
- 44.Finnish National Supervisory Authority for Welfare and Health (Valvira) . Healthcare supervision statistics (in Finnish). Available at: https://www.valvira.fi/terveydenhuolto/tilastot. Accessed January 7, 2021.
- 45.Finnish Medicines Agency (FIMEA) . ATC codes. Available at: https://www.fimea.fi/web/en/databases_and_registers/atc-codes. Accessed January 7, 2021.
- 46.Ferrah N, Lovell JJ, Ibrahim JE. Systematic review of the prevalence of medication errors resulting in hospitalization and death of nursing home residents. J Am Geriatr Soc. 2017;65:433–442. [DOI] [PubMed] [Google Scholar]
- 47.Statistics Finland . Age structure of the population. Available at: https://findikaattori.fi/fi/14. Updated March 31, 2021. Accessed July 7, 2021.
- 48.Salmasi S Wimmer BC Khan TM, et al. Quantitative exploration of medication errors among older people: a systematic review. Drugs Ther Perspect. 2018;34:129–137. [Google Scholar]
- 49.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel . American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67:674–694. [DOI] [PubMed] [Google Scholar]
- 50.Schepel L Lehtonen L Airaksinen M, et al. How to identify organizational high-alert medications [published online July 7, 2018]. J Patient Saf. doi: 10.1097/PTS.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 51.Kuitunen S Niittynen I Airaksinen M, et al. Systemic causes of in-hospital intravenous medication errors: a systematic review [published online January 31, 2020]. J Patient Saf. doi: 10.1097/PTS.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mononen N Pohjanoksa-Mäntylä M Airaksinen MS, et al. How far are we from a medication use process aiming at well-informed adherent patients with long-term medications in Finland? Qualitative study. BMJ Open. 2020;10:e036526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurwitz JH Field TS Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109:87–94. [DOI] [PubMed] [Google Scholar]


