Summary
Background
Antimicrobial resistance is a major global health concern, driven by overuse of antibiotics. We aimed to assess the effectiveness of a national antimicrobial stewardship intervention, the National Health Service (NHS) England Quality Premium implemented in 2015–16, on broad-spectrum antibiotic prescribing and Escherichia coli bacteraemia resistance to broad-spectrum antibiotics in England.
Methods
In this quasi-experimental, ecological, data linkage study, we used longitudinal data on bacteraemia for patients registered with a general practitioner in the English National Health Service and patients with E coli bacteraemia notified to the national mandatory surveillance programme between Jan 1, 2013, and Dec 31, 2018. We linked these data to data on antimicrobial susceptibility testing of E coli from Public Health England's Second-Generation Surveillance System. We did an ecological analysis using interrupted time-series analyses and generalised estimating equations to estimate the change in broad-spectrum antibiotics prescribing over time and the change in the proportion of E coli bacteraemia cases for which the causative bacteria were resistant to each antibiotic individually or to at least one of five broad-spectrum antibiotics (co-amoxiclav, ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin), after implementation of the NHS England Quality Premium intervention in April, 2015.
Findings
Before implementation of the Quality Premium, the rate of antibiotic prescribing for all five broad-spectrum antibiotics was increasing at rate of 0·2% per month (incidence rate ratio [IRR] 1·002 [95% CI 1·000–1·004], p=0·046). After implementation of the Quality Premium, an immediate reduction in total broad-spectrum antibiotic prescribing rate was observed (IRR 0·867 [95% CI 0·837–0·898], p<0·0001). This effect was sustained until the end of the study period; a 57% reduction in rate of antibiotic prescribing was observed compared with the counterfactual situation (ie, had the Quality Premium not been implemented). In the same period, the rate of resistance to at least one broad-spectrum antibiotic increased at rate of 0·1% per month (IRR 1·001 [95% CI 0·999–1·003], p=0·346). On implementation of the Quality Premium, an immediate reduction in resistance rate to at least one broad-spectrum antibiotic was observed (IRR 0·947 [95% CI 0·918–0·977], p=0·0007). Although this effect was also sustained until the end of the study period, with a 12·03% reduction in resistance rate compared with the counterfactual situation, the overall trend remained on an upward trajectory. On examination of the long-term effect following implementation of the Quality Premium, there was an increase in the number of isolates resistant to at least one of the five broad-spectrum antibiotics tested (IRR 1·002 [1·000–1·003]; p=0·047).
Interpretation
Although interventions targeting antibiotic use can result in changes in resistance over a short period, they might be insufficient alone to curtail antimicrobial resistance.
Funding
National Institute for Health Research, Economic and Social Research Council, Rosetrees Trust, and The Stoneygate Trust.
Introduction
Antibiotic resistance is a persistent health issue, with approximately 700 000 deaths due to antibiotic-resistant bacteria reported per year globally.1 Escherichia coli is a frequent cause of bacteraemia, which has accounted for 55% of all Gram-negative bacteraemia since 2017 in England.2 The organism has become a particular concern because of its widespread antibiotic resistance.3 Inappropriate use of antibiotics is associated with the development and spread of antibiotic-resistant bacteria through promoting the selection of antibiotic-resistant strains.4, 5, 6, 7 In England, more than 70% of antibiotics are prescribed in primary care, many of which are deemed inappropriate.8 To control antibiotic resistance, antimicrobial stewardship interventions targeting antibiotic prescribing in primary care settings have been introduced.9
Research in context.
Evidence before this study
We searched PubMed for studies published in English between Jan 1, 2000, and Jan 1, 2021, using the search terms: (“antimicrobial stewardship” OR “antibiotic stewardship” OR “quality premium”) AND (“primary care”) AND (“antimicrobial resistance” OR “antibiotic resistance”). We reviewed the studies to identify relevant literature on the effect of antimicrobial stewardship interventions in primary care on antimicrobial resistance. Our search yielded 107 studies. Studies investigating the impact of the Quality Premium stewardship reward on antibiotic prescribing have shown reductions in antibiotic prescribing in primary care. One evaluation of the Quality Premium identified an 8·2% decrease in the total number of antibiotics prescribed since implementation. Another study reported a 3% reduction in number of antibiotics prescribed for respiratory tract infections. An additional study reported that the reduction in antibiotic prescribing after implementation of the Quality Premium was significantly higher among the top 20% of prescribers in England. However, none of these evaluations included an assessment of the effect of the intervention on antibiotic resistance. Most evaluations of the effect of antimicrobial stewardship interventions in England have focused on antibiotic prescribing rate as an outcome, with little evidence on their effect on antibiotic resistance, which is a growing threat to global health security. Understanding the effect of such national interventions targeting antibiotic prescribing on resistance is important to quantify the contributions of reduced prescribing to resistance patterns, an area for which evidence is scarce. A population-based study done in Scotland is one of the few analyses of the effect of antimicrobial stewardship interventions on antibiotic resistance in the community. The study reported moderate reductions in resistance to three broad-spectrum antibiotics classes (fluoroquinolones, cephalosporins, and penicillins [co-amoxiclav]) among coliform bacteraemia. Another study done in Spain reported that implementation of antimicrobial stewardship interventions in primary care improved appropriate antibiotic usage, reducing incidence of infections due to extended-spectrum β-lactamase-producing Escherichia coli in the community.
Added value of this study
The study findings suggest that broad-spectrum antimicrobial prescribing in primary care was substantially reduced after implementation of the 2015–16 Quality Premium antimicrobial stewardship intervention. Corresponding changes in antimicrobial resistance in E coli strains causing bacteraemia, to all antibiotics individually and in combination, were more modest after adjusting for confounding variables, including variations in general practitioner antibiotic prescribing. The present study also showed that although the Quality Premium intervention has succeeded in reducing antibiotic usage, the corresponding resistance in E coli causing bacteraemia, although attenuated after the implementation of the Quality Premium, remains on an upward trajectory.
Implications of all the available evidence
Strategies to reduce inappropriate antibiotic prescribing can result in short-term reductions in resistance; however, these strategies alone might be insufficient to prevent the increase in antibiotic resistance. Antibiotic prescribing in health care has been the mainstay of antibiotic stewardship, but this is only one component of the stewardship landscape. It is becoming clearer that a leading cause of the unhindered spread of resistance, despite decreases in antibiotic prescribing, is transmission of antibiotic resistance genes in the environment. It is therefore important to consider the impact of resistance genes and the ways in which they are introduced into the population. Thus, it is discernible that a more radical, multi-sectorial approach, such as surveillance of resistance genes, is urgently needed to tackle the growing threat of antibiotic resistance. Identification of the distribution of genes that drive antibiotic resistance, and investigation of how the bacterial population evolve and adapt to changing pressures of antibiotics, will enable the development of targeted measures, diagnostics, and treatments to prevent and control bacteraemia caused by resistant E coli.
The Quality Premium is a National Health Service (NHS) England intervention that provided performance-related financial rewards to clinically led statutory bodies, known as Clinical Commissioning Groups, who are responsible for planning and commissioning local health-care services.9 The scheme rewards Clinical Commissioning Groups for meeting annual targets. Antibiotic optimisation as an antibiotic resistance indicator was included in 2015–16 guidance on the Quality Premium, in addition to targets for Clinical Commissioning Groups to reduce both total antibiotic prescribing and broad-spectrum antibiotic prescribing (co-amoxiclav, cephalosporins, and quinolone items) each financial year.10
Studies assessing the effect of antimicrobial stewardship interventions, such as the Quality Premium, have focused on changes in antibiotic prescribing rate, with little evidence on the subsequent effect of these changes on antibiotic resistance.11 Understanding the effect of such national interventions on antibiotic resistance is important to quantify the contributions of reduced prescribing to resistance trends. A population-based study done in Scotland is one of the few evaluations of the effect of antimicrobial stewardship interventions on antibiotic resistance in the community; however, the analysis did not control for the possible effect of prescribing of antibiotics on antibiotic resistance.11
Five broad-spectrum antibiotics used to treat common infections in community practice as per the National Institute of Clinical Excellence guidelines were investigated in this analysis (co-amoxiclav, levofloxacin, ciprofloxacin, moxifloxacin, and ofloxacin).12 In this study, we used longitudinal data on prescribing and bacteraemia-causing E coli resistance to assess the effectiveness of the Quality Premium antimicrobial stewardship intervention on broad-spectrum prescribing and resistance to broad-spectrum antibiotics, with adjustment for prescribing in primary care practices in England.
Methods
Study design and setting
We did a quasi-experimental ecological study using monthly prescribing data from 6882 general practitioner (GP) practices in England for the period Jan 1, 2013, to Dec 31, 2018. We selected this time period because it included a 27-month pre-implementation period and a 45-month post-implementation period for the Quality Premium intervention.
Data sources and linkage
We used data on antimicrobial susceptibility testing of E coli bacteraemia from the Communicable Disease Report module of the Second-Generation Surveillance System (SGSS), a national microbiology surveillance database maintained by Public Health England (PHE). SGSS contains data on patients' GP practices by linkage to data included in the NHS Spine, a central repository containing information on patient demographics, which occurs on the day each specimen is reported to SGSS. For all analyses, we classified intermediate antimicrobial susceptibility testing results as susceptible to reflect the latest European Committee on Antimicrobial Susceptibility (EUCAST) definitions for antimicrobial susceptibility testing.13 We linked the SGSS data to data from the mandatory surveillance scheme, a data reporting and analysis system for surveillance of health-care-associated infections at PHE, to confirm the onset location of each episode of bacteraemia using an apportionment algorithm.2 The E coli bacteraemia antimicrobial susceptibility testing results were clustered at the unit of GP practice level using the GP practice code for each patient. We obtained monthly practice-level antibiotic prescribing data from OpenPrescribing, an evidence-based medicine DataLab project at the University of Oxford (Oxford, UK),14 and subsequently linked patient data to the SGSS data using the GP practice code. Data from OpenPrescribing were obtained from the NHS Business Services Authority prescribing and dispensing information systems.
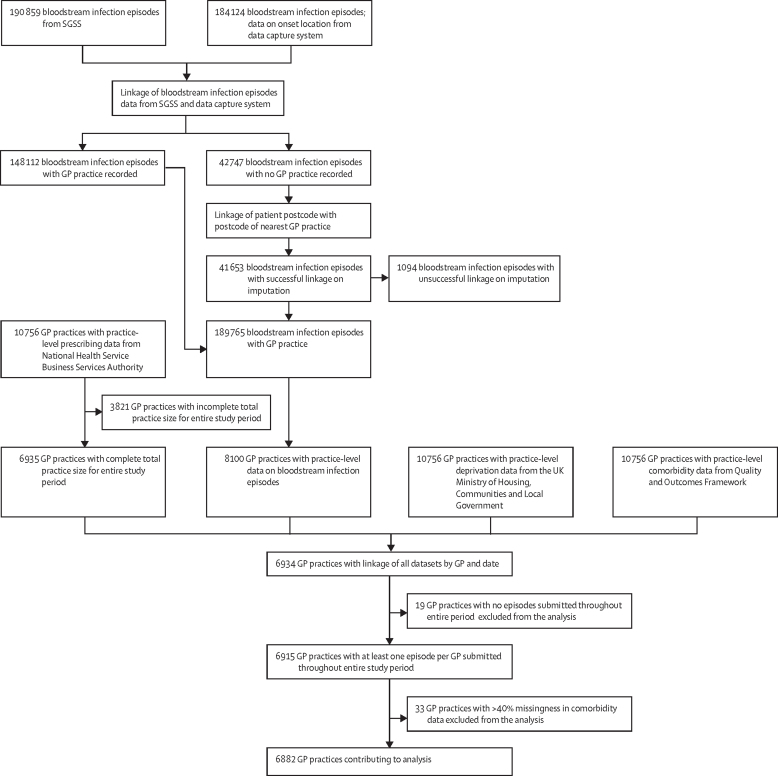
Based on previous ecological studies that have examined population-level changes in antibiotic prescribing and resulting resistance, changes in antibiotic prescribing at the GP practice level were assumed to have an effect on antimicrobial resistance associated with E coli bacteraemia within 6 months.4 A 6-month lag period was therefore computed when linking the prescribing dataset and the E coli bacteraemia antimicrobial susceptibility testing results to account for this delayed effect. We included practices that had complete observations for the variable on the number of patients in the practice for the 72 months covered in this study. The final linked dataset included monthly counts of resistant isolates for each antibiotic and the monthly count of usage of each antibiotic per practice (figure 1).
Figure 1.
Study flowchart for dataset creation and linkage
SGSS=Second-Generation Surveillance System. GP=general practitioner.
Model generation and outcomes
We assessed prescribing patterns of five broad-spectrum antibiotics (co-amoxiclav, ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin) individually and as an aggregate. We examined resistance patterns in E coli isolates causing bacteraemia following the implementation of the Quality Premium. A binary variable was created to denote resistance to at least one of the five commonly prescribed broad-spectrum antibiotics. All analyses were done at the GP practice level. The main predictor was a binary variable indicating the implementation of the 2015–16 Quality Premium. The intervention period was defined as April 1, 2015, to Dec 31, 2018, with the pre-intervention period used as the control. The number of months since implementation of the 2015–16 Quality Premium was used to examine long-term changes in antibiotic prescribing and antimicrobial resistance patterns after the intervention until the end of the study period.
Confounders and effect modifiers included the Index of Multiple Deprivation (IMD) from the English Indices of Deprivation 2015, based on GP practice postcodes.15, 16 The IMD is a numeric relative measure of deprivation based on information from seven domains: income, employment, education, skills and training, health and disability, crime, barriers to housing services, and living environment.16 The geographical region of each practice was also included as a potential confounder, defined by PHE centre. These centres were North East, North West, Yorkshire and the Humber, East Midlands, East of England, West Midlands, South East, South West, and London.17 Additionally, prevalence of comorbidities and age distribution will affect the rate of antibiotic prescribing and will vary across practices;18 therefore, we adjusted models for the proportion of patients aged between 0 and 14 years and patients aged 65 years and older,19 and the annual prevalence of asthma, chronic obstructive pulmonary disease, diabetes, cancer, and chronic kidney disease per 100 patients.20 We linked these data to the prescribing and E coli bacteraemia antimicrobial susceptibility testing results using a unique identification code for each GP practice.
Statistical analysis
We used interrupted time-series analysis to estimate changes in the rate of antibiotic prescribing and E coli resistance to antibiotics over time after the implementation of the 2015–16 Quality Premium. We examined changes in patterns of E coli resistance through the assessment of change in number of resistant community-onset E coli bacteraemia isolates per 1000 isolates tested per month. For this outcome, the total number of isolates that were tested against the antibiotic during antimicrobial susceptibility testing was used as the offset, which was used in the regression model to account for the varying number of isolates submitted over the study period. We assessed changes in patterns of prescribing of antibiotics through the assessment of change in number of antibiotic prescriptions per 1000 patients per month. The offset for this outcome was total number of patients registered at the GP practice. We assessed outcomes using generalised linear regression analysis of the time series, aggregating the outcomes for each month with inclusion of the offset. We assessed these outcomes immediately after the intervention, which was defined as the month in which the Quality Premium intervention was introduced. We also assessed changes in trend for the pre-intervention and post-intervention period. We exponentiated the regression coefficients obtained from interrupted time-series models to calculate incidence rate ratios (IRRs). A trend line denoting the counterfactual scenario was generated, which was the predicted trend that would have been expected had the Quality Premium not been implemented. We compared this trend line with the observed post-intervention trend line. Absolute changes were assessed by calculating the difference between the predicted pre-intervention trend of the outcomes and the actual trend at the end of the study period. We assessed the relative change by calculating the absolute change as a relative proportion.
We tested the data for overdispersion by comparing the Akaike Information Criterion of the negative binomial and Poisson regressions. Models were selected where the model fit improved, denoted by a lower Akaike Information Criterion. All models were a priori adjusted for season by adding month as a variable on the basis of the accepted assumption that antimicrobial prescribing and resistance differ by season.21, 22 To assess autocorrelation, we inspected the residual, autocorrelation, and partial autocorrelation function plots (appendix pp 3–6). We also used the Durbin–Watson test to examine first-order autocorrelation. For cases in which autocorrelation was identified, we added lag terms to the models. Since the data were at the monthly level, any significant lags up until 12th order were chosen for further analyses. To assess whether the lag term should be included in the final model, we assessed whether the addition of the lag term: improved the Durbin–Watson statistic; was statistically significant in the model; improved model fit when comparing the Akaike Information Criterion of the models and using likelihood ratio tests; or indicated residual autocorrelation when implementing the Breusch–Godfrey test.
We used generalised estimating equations (GEE) with ecological resistance data at the GP practice level clustered by time (month) to predict the impact of Quality Premium on antibiotic resistance. The Poisson variance was used for GEE models. The GEE method accounts for overdispersion because of its basis in quasi-likelihood.23 Models with resistance as the outcome used total number of isolates submitted per month as an offset. A continuity correction of 0·5 was added across all variables associated with resistance to enable total isolates in the offset. Models with antibiotic usage as the outcome used total number of patients per month as an offset. A continuity correction of 0·5 was also added to variables associated with usage. The correlation structure was assessed using the Quasi-Information Criterion and the model with the lowest Quasi-Information Criterion was selected. We adjusted the GEE models to account for prescribing rates of included antibiotics (defined as the number of prescriptions per month since the start of the study period), the proportion of patients aged 65 years or older and aged 0–14 years at each practice, PHE region, season, deprivation level, and prevalence of comorbidities. The regression coefficients obtained from GEE models were exponentiated to obtain IRRs.
We used R Studio (vesion 1.2.1335) for data management and interrupted time-series analysis using the glm and glm.nb functions of the MASS statistical package. We used Stata (version 14.0) for modelling GEE using the xtgee function.
GP practices that did not meet analysis criteria were excluded (figure 1) and imputation methods were used to address missingness. Where comorbidity data were missing for individual months, we used the last observation carried forward method.15 We manually imputed missing GP practice IMD scores using the practice postcode (n=24). We used the patients' registered GP practice for most practices. Where GP practice code was missing, we imputed GP practice from those closest to patient address using the first three letters of the patient postcode. We did three sensitivity analyses. First, we did a sensitivity analysis using GEE, using the dataset with only complete GP practice codes and compared effect sizes across models with imputed versus fully observed datasets. Second, we did a sensitivity analysis using GEE to compare the effect of the Quality Premium in different GP practice types. A binary variable was created to denote the top 20% of GP practices that observed the largest decreases in prescribing following the implementation of the Quality Premium, with the remaining 80% of GP practices used as the baseline of this variable. The variable was then added as an interaction term in the GEE model comparing effect sizes across the practice types. Third, we did a sensitivity analysis to investigate the prevalence of extended-spectrum β-lactamase (ESBL)-producing isolates.24 We therefore did a separate interrupted time-series analysis for the third-generation cephalosporin ceftriaxone as ceftriaxone-resistant E coli are a known proxy for identifying ESBL-producing isolates.24 Ceftriaxone antimicrobial susceptibility testing data was obtained from the Communicable Disease Report module of the SGSS.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Jan 1, 2013, and Dec 31, 2018, the total number of prescriptions issued for all five antibiotics was 7 002 756 (ciprofloxacin [n=760 497], co-amoxiclav [n=6 068 217], levofloxacin [n=82 500], moxifloxacin [n=29 953], and ofloxacin [n=61 589]). During the study period, the mean number of antibiotics prescribed per GP practice was 1017·55 (SD 816·35; 95% CI 998·26–1036·84; table 1). The mean number of prescriptions for the pre-intervention and post-intervention periods are shown in the appendix (p 1). For each GP practice, the mean number of total antibiotics prescribed was 423·58 (SD 364·19) in the 27-month pre-intervention period and 593·97 (483·84) in the 45-month post-intervention period.
Table 1.
GP practice antibiotic prescribing for community-onset Escherichia coli bacteraemia isolates
|
Number of antibiotics prescribed during study period |
Number of antibiotics prescribed in the 6-month period before implementation of the Quality Premium |
Number of antibiotics prescribed in the 6-month period after implementation of the Quality Premium |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| Total | 1017·55 (816·35) | 998·26–1036·84 | 99·35 (88·15) | 97·27–101·43 | 84·79 (74·35) | 83·04–86·55 |
| Ciprofloxacin | 110·51 (116·46) | 107·75–113·26 | 10·79 (13·22) | 10·48–11·10 | 9·38 (11·34) | 9·11–9·64 |
| Co-amoxiclav | 881·75 (737·71) | 864·32–899·18 | 86·59 (81·30) | 84·67–88·51 | 73·63 (67·79) | 72·03–75·24 |
| Levofloxacin | 11·99 (36·71) | 11·12–12·86 | 0·99 (3·75) | 0·90–1·08 | 0·86 (3·33) | 0·78–0·94 |
| Moxifloxacin | 4·35 (12·53) | 4·06 −4·65 | 0·39 (1·61) | 0·35–0·43 | 0·34 (1·43) | 0·30–0·37 |
| Ofloxacin | 8·95 (15·94) | 8·57–9·33 | 0·59 (1·56) | 0·56–0·63 | 0·59 (1·55) | 0·55–0·62 |
Data are reported per GP practice (n=6882).
In the final analysis, we included data on 6882 (99·3%) of all 6929 GP practices in England as of December, 2018 (the last month of observation in our dataset).19 The remaining 52 (0·7%) practices did not have complete observations for preceding months in our study (eg, practices that opened or closed during the observation period).
A total of 138 787 E coli bacteraemia isolates were recorded in the study period (appendix p 2). Of these, 84 078 (60·6%) E coli isolates were susceptible to all five antibiotics included in the study, and 54 709 (39·4%) were resistant to at least one of the five antibiotics. Of 138 787 isolates, 74 519 (53·7%) were obtained from female patients. The highest number of isolates were obtained from individuals aged 65 years and older (100 665 [72·5%] of 138 787 isolates). The South East region of England had the highest number of isolates submitted (21 606 [15·6%] of 138 787 isolates), and the North East region contributed the lowest number of isolates (9068 [6·5%] of 138 787 isolates). 119 140 isolates were tested for resistance to co-amoxiclav, whereas only 112 were tested against ofloxacin (appendix p 2).
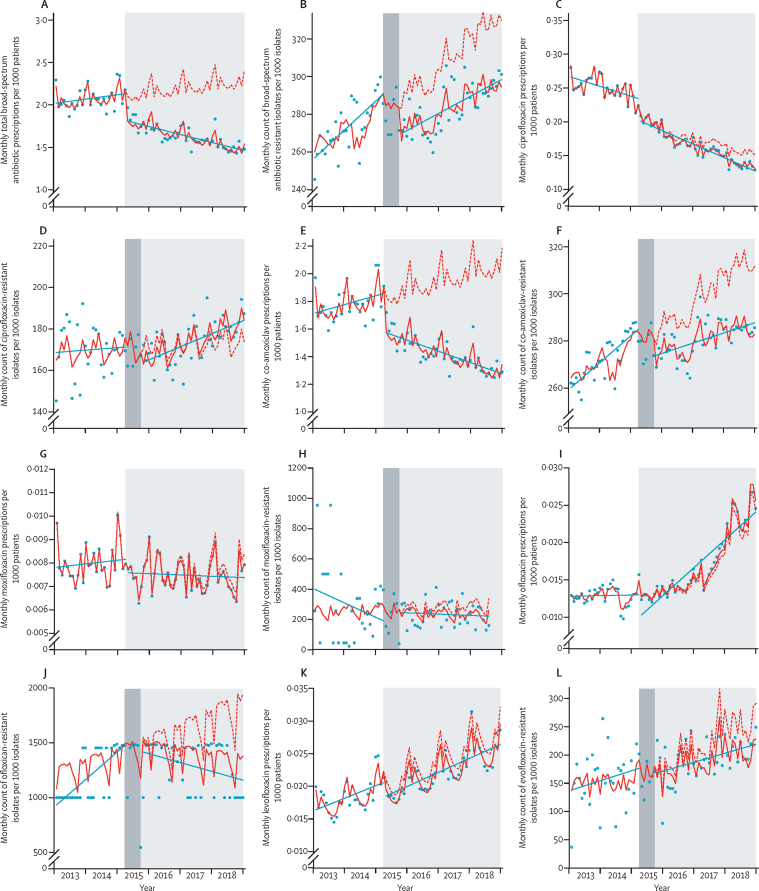
A time-series plot showed an overall reduction in the monthly number of antibiotic prescriptions for all five broad-spectrum antibiotics over the study period. The interrupted time-series analysis showed an immediate downward step-change in the prescribing rate of the broad-spectrum antibiotics after the implementation of the Quality Premium (table 2). Before implementation of the Quality Premium, the rate of antibiotic prescribing was increasing at a rate of 0·2% per month (IRR 1·002 [1·000–1·004], p=0·046; figure 2). However, after implementation of the Quality Premium, an immediate reduction in the total broad-spectrum antibiotic prescribing rate was observed (IRR 0·867 [95% CI 0·837–0·898], p<0·0001; figure 2). This effect was sustained until the end of the study period with a 57% reduction in rate of antibiotic prescribing observed, compared with the counterfactual situation (ie, had the Quality Premium not been implemented); figure 2). Similar trends and changes in trends were observed for most of the antibiotics when plotted individually. In the pre-intervention period, resistance to broad-spectrum antibiotics increased, which was attenuated following the implementation of the Quality Premium. Interrupted time-series analysis showed there was an immediate downward step-change in the rate of resistance after the implementation of the Quality Premium. In the same period, the rate of resistance to at least one broad-spectrum antibiotic before the implementation of the Quality Premium was increasing at rate of 0·1% per month (IRR 1·001 [0·999–1·003], p=0·346; figure 2). On implementation of the Quality Premium, an immediate reduction in resistance rate to at least one broad-spectrum antibiotic was observed (IRR 0·947 [0·918–0·977], p=0·0007; figure 2). By the end of the study period, a 12·03% reduction in resistance rate was observed compared with the counterfactual situation (ie, had the Quality Premium not been implemented) (figure 2).
Table 2.
Interrupted time-series analysis of changes in trends for antibiotic usage and antimicrobial resistance
| Regression intercept, IRR (95% CI) | Pre-intervention trend, IRR (95% CI) | Immediate change after implementation of the Quality Premium, IRR (95% CI) | Change in trend over study period, IRR (95% CI) | Absolute change | Relative change (%) | |
|---|---|---|---|---|---|---|
| Antibiotic usage* | ||||||
| Total | 0·002 (0·002–0·002) | 1·002 (1·000–1·004) | 0·867 (0·837–0·898) | 0·993 (0·991–0·995) | 0·870 | −56·50 |
| Co-amoxiclav | 0·002 (0·002–0·002 | 1·003 (1·001–1·005) | 0·866 (0·835–0·897) | 0·992 (0·990–0·994) | 0·842 | −62·68 |
| Ciprofloxacin | 0·000 (0·000–0·000) | 0·999 (0·999–1·000) | 1·009 (0·996–0·914) | 0·997 (0·996–0·997) | 0·020 | −15·26 |
| Levofloxacin | 0·000 (0·000–0·000) | 1·008 (1·006–1·010) | 0·928 (0·890–0·968) | 1·000 (0·997–1·002) | 0·003 | −8·71 |
| Ofloxacin | 0·000 (0·000–0·000) | 0·999 (0·998–1·001) | 1·008 (0·969–1·050) | 1·001 (0·998–1·004) | 0·001 | 5·30 |
| Moxifloxacin | 0·000 (0·000–0·000) | 1·001 (0·998–1·004) | 0·951 (0·899–1·005) | 0·998 (0·995–1·001) | 0·000 | −5·68 |
| Antimicrobial resistance† | ||||||
| Total | 0·226 (0·207–0·246) | 1·001 (0·999–1·003) | 0·947 (0·918–0·977) | 0·999 (0·997–1·000) | 35·44 | −12·03 |
| Co-amoxiclav | 0·356 (0·341–0·373) | 1·004 (1·003–1·006) | 0·942 (0·908–0·978) | 0·998 (0·996–1·000) | 72·25 | −17·56 |
| Ciprofloxacin | 0·170 (0·159–0·181) | 1·000 (0·998–1·003) | 0·939 (0·887–0·993) | 1·003 (1·000–1·006) | 10·43 | 5·66 |
| Levofloxacin | 0·134 (0·102–0·178) | 0·999 (0·988–1·009) | 0·994 (0·813–1·241) | 0·995 (0·983–1·006) | 63·01 | −27·63 |
| Ofloxacin | 0·777 (0·147–4·123) | 1·009 (0·952–1·070) | 1·062 (0·503–2·244) | 0·988 (0·928–1·051) | 566·03 | −64·29 |
| Moxifloxacin | 0·231 (0·100–0·534) | 1·003 (0·974–1·032) | 0·942 (0·663–1·339) | 0·995 (0·965–1·026) | 77·59 | −29·81 |
IRRs were calculated using Poisson or negative binomial regression analyses. IRR=incidence rate ratio.
Change in rate of antibiotics prescribed per 1000 patients in general practitioner practices.
Change in rate of resistant isolates per 1000 isolates submitted to Public Health England.
Figure 2.
Community antimicrobial exposure and GP practice level rates of resistance in E coli community-onset E coli bacteraemia isolates in England (2013–18)
(A) Total broad-spectrum antibiotic prescribing. (B) Total broad-spectrum antibiotic resistance. (C) Ciprofloxacin prescribing. (D) Ciprofloxacin resistance. (E) Co-amoxiclav prescribing. (F) Co-amoxiclav resistance. (G) Moxifloxacin prescribing. (H) Moxifloxacin resistance. (I) Ofloxacin prescribing. (J) Ofloxacin resistance. (K) Levofloxacin prescribing. (L) Levofloxacin resistance. Light grey shaded areas show the post-intervention period for the Quality Premium antimicrobial stewardship intervention and dark grey shaded areas show the 6-month lag period. Red lines show actual trend and red dots show the predicted trend had the Quality Premium not been implemented. GP=general practitioner.
In interrupted time-series analysis, no change in the rate of ciprofloxacin prescribing was identified after implementation of the Quality Premium (IRR 1·009 [95% CI 0·996–1·022], p=0·198); however, a downward step-change in co-amoxiclav prescribing was identified (0·866 [0·835–0·897], p<0·0001). The increase in co-amoxiclav resistance observed during the study period was attenuated after implementation of the Quality Premium. Interrupted time-series analysis showed an immediate downward step-change in resistance rates for both antibiotics after Quality Premium implementation (0·939 [0·887–0·993], p=0·028 for ciprofloxacin; 0·942 [0·908–0·978], p=0·039 for co-amoxiclav).
No change in moxifloxacin prescribing or resistance was observed after implementation of the Quality Premium (IRR 0·951 [95% CI 0·899–1·005], p=0·860 for prescribing; 0·942 [0·887–1·339], p=0·740 for resistance).
During the study period, an increase in levofloxacin prescribing was observed. An attenuation of the increase in levofloxacin prescribing was observed after Quality Premium implementation (IRR 0·928 [95% CI 0·890–0·968]; p=0·0004). No change in ofloxacin prescribing was observed after Quality Premium implementation (1·008 [0·969–1·050]; p=0·680). No significant differences in antibiotic resistance to levofloxacin and ofloxacin were identified after implementation of the Quality Premium, although few isolates were reported for these antibiotics and in some months no isolates were reported (table 1). All interrupted time-series coefficients are shown in table 2 and corresponding plots are shown in figure 2. Residual diagnostics are in the appendix (pp 3–6).
The GEE model showed that no changes in resistance to at least one of the five broad-spectrum antibiotics tested were observed after implementation of the Quality Premium (IRR 0·996 [95% CI 0·986–1·003]; p=0·207). After adjusting for prescribing, comorbidities, age, deprivation index, and geographical region, the effect of the Quality Premium on total resistance to broad-spectrum antibiotics did not change (0·996 [0·987–1·005]; p=0·410). The GEE model showed that in the long term, there was a sustained increase in the number of E coli isolates resistant to at least one of the broad-spectrum antibiotics tested after implementation of the Quality Premium (IRR 1·002 [1·000–1·003]; p=0·047; table 3).
Table 3.
Immediate and long-term effect of the Quality Premium intervention on the number of community-onset Escherichia coli bacteraemia isolates resistant to at least one of five antibiotics used for common infections
| IRR (95% CI) | p value | |
|---|---|---|
| Unadjusted model | ||
| Immediate effect after implementation of the Quality Premium | 0·9958 (0·9862–1·0030) | 0·207 |
| Long-term effect 39 months after implementation of the Quality Premium | 1·0012 (0·9999–1·0030) | 0·080 |
| Adjusted model* | ||
| Immediate effect after implementation of the Quality Premium | 0·9962 (0·9872–1·0053) | 0·410 |
| Long-term effect 39 months after implementation of the Quality Premium | 1·0024 (1·0000–1·0030) | 0·047 |
IRR=incidence rate ratio. GP=general practitioner.
Adjusted for prescribing for all five antibiotics per GP practice, the proportion of patients per GP practice with chronic obstructive pulmonary disorder, chronic kidney disease, asthma, cancer, and diabetes, the proportion of patients in a GP practice aged between 0–14 years, the proportion of patients in a GP practice aged older than 65 years, GP practice deprivation index, Public Health England region, and season.
We did a sensitivity analysis focusing on only community-onset E coli bacteraemia isolates that had a GP practice code recorded (n=138 576) compared with the dataset including imputed GP practice codes. Descriptive analyses for practice-level and patient-level data for the fully observed dataset showed similar patterns of antibiotic usage and antimicrobial resistance at the geographical level (appendix pp 11–12). The adjusted GEE model estimates on the effect of Quality Premium on resistance to these five antibiotics was similar to the main model for each dataset with imputed data showing a conservative estimate (IRR 0·993 [95% CI 0·981–0·999], p=0·036, for the fully observed dataset vs IRR 0·996 [0·987–1·005], p=0·410, for the imputed dataset). An additional sensitivity analysis was done to assess the effect of the Quality Premium in GP practices that had the largest decreases in prescribing after implementation of the Quality Premium. The adjusted GEE model estimates on the effect of Quality Premium on resistance to tested antibiotics was the same for the main dataset and the model with the interaction term (IRR 0·993 [0·983–1·003], p=0·152, for the model with the interaction term vs 0·996 [0·987–1·005], p=0·410, for the imputed data). An additional sensitivity analysis was done using interrupted time-series analysis to assess the effect of the Quality Premium on ceftriaxone prescribing and resistance. No differences in ceftriaxone prescribing (IRR 0·929 [95% CI 0·817–1·057], p=0·955) or ceftriaxone resistance (1·043 [0·905–1·201], p=0·562) were identified after implementation of the Quality Premium (appendix pp 7–8).
Discussion
This study provides information on patterns in broad-spectrum prescribing and antibiotic resistance in E coli bacteraemia isolates before and after the implementation of a national financial reward programme in 2015–16. The study findings demonstrate attainment of the Quality Premium objective of reducing the prescribing rate of co-amoxiclav and levofloxacin, but not of ciprofloxacin, ofloxacin, or levofloxacin. We observed a step-change in resistance to all antibiotics individually and in combination following implementation of the Quality Premium. However, the overall pattern was one of attenuation rather than a reversal of previously rising rates of antibiotic-resistant E coli isolates, with the pre-intervention increase in rates of antibiotic resistance persisting in the long term.
This analysis was strengthened by adjusting for antibiotic prescribing data in the analysis, which similar studies have not controlled for.11 Our previous work shows strong evidence of an association between antimicrobial prescribing and development of resistance.5, 25 To advance understanding of the effect of antimicrobial stewardship interventions on antibiotic resistance and the resulting implications for policy and practice, assessment of both antimicrobial prescribing and antibiotic resistance data is necessary. To our knowledge, this is the first longitudinal study to assess data representative of English primary care practices.
This study has some limitations. Antibiotic susceptibility testing reports made to the SGSS occur voluntarily, which might result in incomplete data collection. However, the incidence of bacteraemia from laboratories in the voluntary laboratory surveillance scheme were similar to those reported to the mandatory surveillance scheme.3 Another possible limitation is the variation in definitions of clinical breakpoints. Most laboratories use EUCAST definitions of susceptibility to determine minimum inhibitory concentrations.26 However, a small subset of laboratories use Clinical and Laboratory Standards Institute definitions. This difference might lead to discrepancies in the interpretation of antimicrobial susceptibility testing results for specific bacteria, which could affect the overall results of this study. This difference, in addition to the potential usage of either of these guidelines at different timepoints during the study period, might lead to discepancies in interpretation of breakpoints. Additionally, other factors might explain the variability in isolate submission, such as local policies around screening for serious infections and infection outcomes such as sepsis (eg, Commissioning for Quality and Innovation).27 Previous literature has cited increased detection of bacteraemia as an unintended consequence of policies that increase awareness of sepsis.28 Moreover, cephalosporins were not included in the main analysis since the majority of cephalosporins in primary care are prescribed as cefalexin, a first-generation cephalosporin.12, 29 E coli bacteraemia isolate resistance to first-generation cephalosporins is not commonly tested as a key drug–bacteria combination30 and was thus unavailable in the reported data. Furthermore, second-generation and third-generation cephalosporins, are rarely prescribed in the community setting for the management of common infections.12 These cephalosporins are prescribed in specialist cases and do not accurately reflect cephalosporin use in primary care.12 Additionally, since this was an ecological study with data aggregated at the group level, this might lead to bias because of ecological fallacy (ie, inappropriately attributing population-level characteristics to an individual). Caution must be taken to avoid making inferences about antibiotic-resistant E coli isolates from individual patients on the basis of GP practice-level data. Since this study originates in a high-income country, the generalisability of the study findings beyond other similar high-income settings is poor. Therefore, future work on addressing this gap is necessary.
Although the current study identified a reduction in prescribing following the implementation of a financial reward, which is consistent with previous studies,31, 32 it has also been shown that such interventions can have little effect after implementation.33 The reduction in total and individual prescribing in this study was consistent with that identified in previous studies.3, 18, 31 The 2019 English Surveillance Programme for Antimicrobial Usage and Resistance (ESPAUR) at PHE reported a decrease in the total prescribing of antibiotics in primary care practices between April, 2015, and March, 2019. Decreases were also reported for co-amoxiclav and some quinolones.3 Our study found an increase in levofloxacin prescribing, consistent with ESPAUR, which noted a steady increase from 2014 to 2018.3 Other studies on the effect of the Quality Premium on antibiotic prescribing also identified a reduction in antibiotic prescribing in primary care.18, 31
The association between antibiotic prescribing and antibiotic resistance has been established by several studies.4, 5, 6, 7 Although some evidence suggests that reduction and restriction of antibiotic prescribing corresponds to a decrease in antibiotic resistance in other similar high-income settings,34, 35 previous research has identified persistent antibiotic resistance despite decreasing prescribing.11, 36, 37, 38 Several mechanisms might contribute to this persistence. First, although the use of penicillins, such as co-amoxiclav, has been shown to decrease in primary care, the use of penicillins in hospital inpatients has steadily increased since 2015.3 This increase in use among hospital patients might lead to increased E coli bacteraemia resistance because it could promote the selection of antibiotic-resistant strains of organisms within the whole health-care economy (ie, inter-related production and consumption activities in health-care settings that determine the overall health of the population).5 Additionally, a study by Vihta and colleagues found that GP practices that prescribed more co-amoxiclav in the previous year were more likely to see more patients with urinary tract infections caused by co-amoxiclav-resistant E coli.39 Since E coli bacteraemia is frequently known to be preceded by an underlying urinary tract infection, these urinary tract infections might have then progressed to bacteraemia. Additionally, studies have reported that a decrease in prescribing might require many years, perhaps up to a decade, to reduce corresponding resistance, which might suggest that effects of the Quality Premium intervention might take more time to manifest.36 One study by Pouwels and colleagues noted that the use of specific antibiotics, such as levofloxacin, for E coli urinary tract infections is associated with resistance to other antibiotics used for other indications, such as ciprofloxacin, leading to co-selection of antibiotic resistance E coli isolates.40 One of the most likely reasons for the unhindered resistance despite decreases in antibiotic prescribing is the accumulation of mutations conferring resistance to antibiotics and acquired antibiotic resistance genes within the bacterial populations.41 Collignon and colleagues suggested that transmission of antibiotic-resistant genes was likely to be the most dominant contributor to antibiotic resistance, implying that resistance might continue despite a restriction of prescribing.41 Transmission of antibiotic resistant genes is of particular concern since the acquired antibiotic resistance genes are usually present on mobile genetic elements (such as plasmids and transposons), which can be easily transmitted from one bacterial cell to another within the same species and between different bacterial species.42 These mobile genetic elements can carry more than one antibiotic resistance gene conferring resistance to bacterial isolates against multiple antibiotics.42 One example is the widely disseminated CTX-M enzyme, which has become widespread globally and is responsible for most common antimicrobial resistance in E coli in the UK and the rest of Europe.43 Furthermore, anthropogenic activities that rely heavily on antibiotic use such as animal rearing and wastewater treatment become reservoirs that promote transmission of antimicrobial resistance genes.43 These genes can then be transmitted to humans from the environment or through the food chain.43
The present study has shown that although the Quality Premium intervention has succeeded in reducing antibiotic prescribing, resistance among E coli causing bacteraemia, although attenuated after the implementation of the Quality Premium, remains on an upward trajectory. This study suggests that reducing prescribing might be insufficient as a standalone strategy to curtail antimicrobial resistance in the primary care setting, although it is effective in attenuating trends in resistance. Antibiotic resistance is a complex phenomenon that requires a collaborative effort across multiple sectors. We recommend surveillance of resistance genes since inappropriate antimicrobial use could have irreversible genetic consequences. The ability to identify and map the distribution of genes that drive antibiotic resistance and how the bacterial population evolve and adapt to changing pressures of antibiotics in use is important for the rational development of targeted measures, diagnostics, and treatments to prevent and control bacteraemia caused by resistant E coli.
Our findings suggest that focusing on reducing antibiotic use in primary care setting, a target for UK antibiotic resistance strategy for many years, might be insufficient alone to counter resistance that has become established in the bacteraemia causing E coli population.
Data sharing
The data that support the findings of this study are available from Public Health England (PHE). The data are compliant with the Data Protection Act (1998). Restrictions apply to the availability of these data. SA has an honorary contract with PHE for data access under a fellowship to access this data (grant 2016-10-95 awarded to CC). Data are available with the permission of PHE with investigator support, after approval of a proposal, with a signed data access agreement.
For more on the Clinical and Laboratory Standards Institute see https://clsi.org/
Declaration of interests
APRW was a member of the drug safety monitoring board for Roche and has given lectures at Merck Sharpe Dohme. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The authors are grateful for support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre funding scheme and the NIHR NW London Applied Research Collaboration. The views and opinions expressed here are those of the authors and do not necessarily reflect those of the NIHR or the Department of Health and Social Care. SA is funded as part of CC's NIHR Career Development Fellowship (grant 2016-10-95). PEA is funded by the Economic and Social Research Council (ESRC) STEP-UP project through the Antimicrobial Resistance Cross Council Initiative supported by the seven research councils in partnership with other funders (grant ES/P008232/1). The authors gratefully acknowledge the support of the ESRC. EJ is an Imperial College London Research Fellow jointly funded by Rosetrees Trust and The Stoneygate Trust 2017 (grant M683). EJ also acknowledges funding from the NIHR Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with PHE [HPRU-2012–10047]. CC is funded by a personal NIHR Career Development Fellowship (grant 2016-10-95). APRW is part supported by the NIHR University College London Hospitals Biomedical Research Centre. We gratefully acknowledge the support of Kate Honeyford at The Department of Primary Care and Public Health (Imperial College London, London, UK).
Contributors
SA contributed to design and data analysis, data cleaning, and interpretation of results. PEA was involved with study design, the data analysis plan, and oversaw statistical analysis and data interpretation. CC conceptualised the study, designed and led the study, designed the data analysis plan, and oversaw statistical analysis and data interpretation. All authors contributed to writing and revision of the manuscript. SA, PEA, CC, RH, and BMP had full access to and verified the data, and contributed to its preparation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.O'Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. The review on antimicrobial resistance. 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
- 2.Public Health England Annual epidemiological commentary: bacteraemia, MSSA bacteraemia and C. difficile infections, up to and including financial year April 2018 to March 2019. Dec 2, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/940716/Annual_epidemiology_commentary_April_2019_March_2020.pdf
- 3.Public Health England English surveillance programme for antimicrobial utilisation and resistance (ESPAUR). Report 2019 to 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/936199/ESPAUR_Report_2019-20.pdf
- 4.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340 doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. European Food Safety Authority. European Medicines Agency ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals: Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wojkowska-Mach J, Godman B, Glassman A, et al. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob Resist Infect Control. 2018;7:8–10. doi: 10.1186/s13756-018-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray J, Oppenheim B, Mahida N. Preventing healthcare-associated Gram-negative bloodstream infections. J Hosp Infect. 2018;98:225–227. doi: 10.1016/j.jhin.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 9.NHS England Quality Premium: guidance for 2017–19. Dep Heal, 2018: 1–32. https://www.england.nhs.uk/ccg-out-tool/qual-prem/
- 10.NHS England About CCGs. 2013. https://www.nhscc.org/ccgs/
- 11.Hernandez-Santiago V, Davey PG, Nathwani D, Marwick CA, Guthrie B. Changes in resistance among coliform bacteraemia associated with a primary care antimicrobial stewardship intervention: a population-based interrupted time series study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Health England Summary of antimicrobial prescribing guidance: managing common infections. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/994444/Common_Infect_PHE_context_references_and_rationale_May_2021_Bites_and_Eczema__1_.pdf
- 13.European Committee on Antimicrobial Susceptibility Testing New definitions of S, I and R from 2019. https://www.eucast.org/newsiandr/
- 14.Curtis HJ, Goldacre B. OpenPrescribing: normalised data and software tool to research trends in English NHS primary care prescribing 1998–2016. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salkind N. SAGE Publications; California (CA): 2012. Encyclopedia of research design. [Google Scholar]
- 16.UK Department for Communities and Local Government The English Indices of Deprivation 2015–Frequently Asked Questions (FAQs) 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/579151/English_Indices_of_Deprivation_2015_-_Frequently_Asked_Questions_Dec_2016.pdf
- 17.Public Health England . UK Government; London: 2014. Contacts: PHE regions and local centres.https://www.gov.uk/contacts-phe-regions-and-local-centres [Google Scholar]
- 18.Anyanwu PE, Pouwels K, Walker A, et al. Investigating the mechanism of impact and differential effect of the Quality Premium scheme on antibiotic prescribing in England: a longitudinal study. BJGP Open. 2020;4 doi: 10.3399/bjgpopen20X101052. bjgpopen20X101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NHS Digital Patients registered at a GP practice. December 2019. https://digital.nhs.uk/data-and-information/publications/statistical/patients-registered-at-a-gp-practice/december-2019
- 20.NHS Digital Quality and Outcomes Framework, Achievement, prevalence and exceptions data 2018–19 [PAS] https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2018-19-pas
- 21.Ramsey EG, Royer J, Bookstaver PB, et al. Seasonal variation in antimicrobial resistance rates of community-acquired Escherichia coli bloodstream isolates. Int J Antimicrob Agents. 2019;54:1–7. doi: 10.1016/j.ijantimicag.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Deeny SR, van Kleef E, Bou-Antoun S, Hope RJ, Robotham JV. Seasonal changes in the incidence of Escherichia coli bloodstream infection: variation with region and place of onset. Clin Microbiol Infect. 2015;21:924–929. doi: 10.1016/j.cmi.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7:127–150. [Google Scholar]
- 24.Huang Y, Carroll KC, Cosgrove SE, Tamma PD. Determining the optimal ceftriaxone MIC for triggering extended-spectrum β-lactamase confirmatory testing. J Clin Microbiol. 2014;52:2228–2230. doi: 10.1128/JCM.00716-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ. 2016;352:i939. doi: 10.1136/bmj.i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kassim A, Omuse G, Premji Z, Revathi G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: a cross-sectional study. Ann Clin Microbiol Antimicrob. 2016;15:21. doi: 10.1186/s12941-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NHS Improvement Reducing the impact of serious infections CQUIN 2017/18. https://webarchive.nationalarchives.gov.uk/20200501110547/https://improvement.nhs.uk/resources/reducing-impact-serious-infections-antimicrobial-resistance-and-sepsis-cquin/
- 28.Simmons MD, Daniel S, Temple M. Sepsis programme successes are responsible for the increased detection of bacteraemia. J Hosp Infect. 2019;101:93–99. doi: 10.1016/j.jhin.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Dolk FCK, Pouwels KB, Smith DRM, Robotham JV, Smieszek T. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018;73(suppl 2):ii2–i10. doi: 10.1093/jac/dkx504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace J. Public Health England; London: February 2012–March 2013. Advisory Committee on antimicrobial resistance and healthcare associated infection (ARHAI). 4th Annual Report.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/256479/ARHAI_Annual_Report_2012-2013.pdf [Google Scholar]
- 31.Bou-Antoun S, Costelloe C, Honeyford K, et al. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotics in the community: an interrupted time series analysis. J Antimicrob Chemother. 2018;73:2883–2892. doi: 10.1093/jac/dky237. [DOI] [PubMed] [Google Scholar]
- 32.Balinskaite V, Johnson AP, Holmes A, Aylin P. The impact of a national antimicrobial stewardship program on antibiotic prescribing in primary care: an interrupted time series analysis. Clin Infect Dis. 2019;69:227–232. doi: 10.1093/cid/ciy902. [DOI] [PubMed] [Google Scholar]
- 33.MacBride-Stewart SP, Elton R, Walley T. Do quality incentives change prescribing patterns in primary care? An observational study in Scotland. Fam Pract. 2008;25:27–32. doi: 10.1093/fampra/cmm074. [DOI] [PubMed] [Google Scholar]
- 34.Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J Antimicrob Chemother. 2013;68:2667–2674. doi: 10.1093/jac/dkt212. [DOI] [PubMed] [Google Scholar]
- 35.Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin G-nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930–938. doi: 10.1086/432721. [DOI] [PubMed] [Google Scholar]
- 36.Enne VI, Livermore DM, Stephens P, Hall LMC. Persistence of sulphonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet. 2001;357:1325–1328. doi: 10.1016/S0140-6736(00)04519-0. [DOI] [PubMed] [Google Scholar]
- 37.Peñalva G, Fernández-Urrusuno R, Turmo JM, et al. Long-term impact of an educational antimicrobial stewardship programme in primary care on infections caused by extended-spectrum β-lactamase-producing Escherichia coli in the community: an interrupted time-series analysis. Lancet Infect Dis. 2020;20:199–207. doi: 10.1016/S1473-3099(19)30573-0. [DOI] [PubMed] [Google Scholar]
- 38.Sundqvist M, Geli P, Andersson DI, et al. Little evidence for reversibility of trimethoprim resistance after a drastic reduction in trimethoprim use. J Antimicrob Chemother. 2010;65:350–360. doi: 10.1093/jac/dkp387. [DOI] [PubMed] [Google Scholar]
- 39.Vihta KD, Stoesser N, Llewelyn MJ, et al. Trends over time in Escherichia coli bloodstream infections, urinary tract infections, and antibiotic susceptibilities in Oxfordshire, UK, 1998–2016: a study of electronic health records. Lancet Infect Dis. 2018;18:1138–1149. doi: 10.1016/S1473-3099(18)30353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pouwels KB, Muller-Pebody B, Smieszek T, Hopkins S, Robotham JV. Selection and co-selection of antibiotic resistances among Escherichia coli by antibiotic use in primary care: an ecological analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2:e398–e405. doi: 10.1016/S2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 42.Hughes D, Andersson DI. Evolutionary trajectories to antibiotic resistance. Annu Rev Microbiol. 2017;71:579–596. doi: 10.1146/annurev-micro-090816-093813. [DOI] [PubMed] [Google Scholar]
- 43.Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72:2145–2155. doi: 10.1093/jac/dkx146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Public Health England (PHE). The data are compliant with the Data Protection Act (1998). Restrictions apply to the availability of these data. SA has an honorary contract with PHE for data access under a fellowship to access this data (grant 2016-10-95 awarded to CC). Data are available with the permission of PHE with investigator support, after approval of a proposal, with a signed data access agreement.
For more on the Clinical and Laboratory Standards Institute see https://clsi.org/