Abstract
Objective:
To determine the effect of dietary weight loss on neuropathy outcomes in the severely obese.
Methods:
We followed a prospective, cohort study of participants attending a medical weight management program. Weight loss was achieved with meal replacement of 800 kcal/day for 12 weeks, then transitioning to 1200–1500 kcal/day. The co-primary outcomes were change in intraepidermal nerve fiber density (IENFD) at the distal leg and proximal thigh. Secondary outcomes included nerve conduction studies, Michigan Neuropathy Screening Instrument (MNSI) questionnaire and exam, Neuro-QOL, quantitative sensory testing (QST).
Results:
Among 131 baseline participants, 72 (mean (SD) age: 50.1 (10.5), 51.4% females) completed 2 years of follow-up. Participants lost 12.4 kg (11.8). All metabolic syndrome components improved with the exception of blood pressure. IENFD in the distal leg (0.4 (3.3), p=0.29), and proximal thigh (0.3 (6.3), p=0.74) did not significantly change. Improvements were observed on the MNSI Questionnaire, two NeuroQoL subdomains, and QST cold.
Conclusions:
Dietary weight loss was associated with improvements in all metabolic parameters except blood pressure, and both IENFD outcomes remained stable after 2 years. Given that natural history studies reveal decreases in IENFD over time, dietary weight loss may halt this progression, but randomized controlled trials are needed.
Introduction
Neuropathy is a highly prevalent condition that results in pain, falls, and lower quality of life.1 While diabetes has long been known to be the leading cause of neuropathy,2–4 obesity has recently emerged as an important risk factor.5–14 Furthermore, obesity is likely sufficient to cause neuropathy even in those with normal glucose control.7,9 In addition to hyperglycemia and obesity, other individual components of metabolic syndrome (hypertension, hypertriglyceridemia, and low high-density lipoprotein (HDL) cholesterol) have also been shown to be associated with neuropathy.13 Unfortunately, despite multiple potentially modifiable risk factors, the only established disease modifying therapy for neuropathy is glycemic control, which prevents neuropathy to a much larger degree in type 1 than in type 2 diabetes.14 We contend that newer interventions are needed to treat and prevent neuropathy.
Few studies have evaluated the effects of weight loss on neuropathy. Two uncontrolled studies have shown the potential for lifestyle interventions to improve neuropathy, but both primarily focused on exercise with only minimal weight loss.15,16 The most rigorous investigation to date, the Look AHEAD study, randomized 5,145 participants with diabetes to 9–11 years of a lifestyle intervention designed to achieve and maintain weight loss compared to a diabetes support group.17 They found that the MNSI questionnaire, but not the examination score, improved in those in the lifestyle intervention group and that changes in weight, HbA1C, HDL, and triglycerides were associated with changes in the MNSI questionnaire. No studies have investigated the effects of significant dietary weight loss on neuropathy outcomes in populations without diabetes or utilizing the best quantitative measure of small fiber nerve injury, intraepidermal nerve fiber density (IENFD), as the primary outcome.
In an obese population with and without diabetes, we aimed to determine the effects of two years of a dietary weight loss intervention on extensive neuropathy outcomes with the co-primary outcomes defined as IENFD at the distal leg and proximal thigh.
Methods
Population
From November 2010 to December 2014, we recruited obese participants attending the University of Michigan Weight Management Program and followed them for two years after starting a dietary weight loss intervention. Inclusion criteria included age 18 years or older and a body mass index (BMI) ≥ 35 kg/m^2 or ≥ 32 kg/m^2 if they had one or more comorbidity.18 The intervention consisted of a very-low energy diet (VLED) in the form of liquid meal replacement plus 2 cups of non-starchy vegetables (~800 kcal/day) for approximately 12 weeks to promote a 15% weight reduction from pre dietary intervention weight. Participants were then slowly transitioned to a 1000–1200 kcal/day partial meal replacement diet until the participants desired weight loss was achieved. This consisted of three replacement products and 400 kcal of conventional food, consisting of half a plate of non-starchy vegetables, 3 – 4 ounces of lean protein, and ½ cup of whole grain or fruit. Participants were counseled to perform 40 min/day of moderate activity including cardio and light strength training during the initial intensive dietary phase and then 60 min/day during the weight loss maintenance phase.
This study was approved by the University of Michigan Institutional Review Board, registered on ClinicalTrials.gov (NCT02043457), and all participants signed informed consent documents.
Metabolic phenotyping
Participants underwent glucose tolerance testing (except those with a previous diagnosis of diabetes), and a fasting lipid panel at baseline and after 2 years. Participants also had blood pressure, height, weight, waist circumference, and BMI measurements monthly throughout the study. Participants with diabetes also had a HbA1C test. Diabetes and pre-diabetes were defined at baseline and after 2 years using HbA1c and glucose tolerance testing, according to the Expert Committee on the diagnosis and classification of diabetes mellitus.19
Polyneuropathy definition (primary outcome)
Our co-primary outcome measures were the IENFD measured at the distal leg and proximal thigh. IENFD was evaluated using brightfield immunohistochemistry using an established protocol.20
Secondary neuropathy outcomes
Our secondary outcome measures included 17 nerve conduction study parameters from 6 different nerves (sural sensory, median sensory, ulnar sensory, peroneal motor, tibial motor, and median motor). Nerve conduction studies were performed using the CareFusion’s Viking on Nicolet EDX electrodiagnostic system. The Michigan Neuropathy Screening Instrument (MNSI) questionnaire and examination (performed by a neuromuscular specialist) were completed as previously described.21 Quantitative sensory testing (QST) measurements of vibration and cold detection thresholds were performed using the WR Medical Electronics Co. Computer Aided Sensory Evaluator (CASE) IV. Quantitative sudomotor axon reflex testing (QSART) measurements were performed at the foot, distal leg, proximal leg, and arm using the WR Medical Electronics Co. Q-Sweat, Quantitative Sweat Measurement System. Monofilament testing was performed with a Semmes Weinstein 5.07/10-g monofilament on the dorsum of the dominant great toe. Monofilament testing was normal if the participant felt 8 or more out of 10 responses, reduced for 1–7 responses, and absent for zero responses. Clinical neuropathy was defined using the Toronto consensus definition of probable polyneuropathy, which requires 2 or more of the following: neuropathy symptoms, abnormal sensory examination, and abnormal reflexes as determined by one of 4 neuromuscular specialists22.
Patient oriented neuropathy outcomes
The validated Neuro-QOL instrument was utilized to measure neuropathy specific quality of life with higher numbers reflecting a worse quality of life.23 The validated short form McGill Pain questionnaire was employed to measure pain with a visual analogue scale (VAS), a 6 point rating scale of Present Pain Intensity (PPI) score, and 4 point rating scale of 15 different neuropathic pain descriptors (McGill pain score).24
Cardiovascular autonomic neuropathy outcomes
All cardiovascular autonomic tests were performed using the ANX 3.0 device (Ansar Group, Inc). Outcomes included three cardiovascular reflex tests (expiration to inspiration (E:I) ratio, 30:15 ratio, and the average of two Valsalva ratios), which are associated with mortality25 and are considered the gold standard tests for autonomic neuropathy.26 Other measurements that were recorded include the resting median heart rate, frequency-domain measures (low frequency area (LFA, measure of sympathetic activity), respiratory frequency area (RFA, measure of parasympathetic activity), and LFA/RFA (measure of sympathovagal balance), and time-domain measures (standard deviation of the normal to normal interval (sdNN) and root mean square of successive differences of the normal to normal interval (rmsSD).
Statistical Analysis
Descriptive statistics were used to characterize participants in terms of demographics, metabolic phenotyping, and neuropathy outcomes at baseline and after 2 years of follow-up. For continuous measurements, we determined the within-participant change during the study, by subtracting baseline measurements from measurements taken after 2 years of follow-up.
We compared demographic information between participants who competed follow-up and those who did not, using 2-sample t-tests for continuous covariates and Pearson’s Chi-Square tests or Fisher’s Exact tests for categorical covariates. Paired t-tests were used to compare within-patient differences in continuous metabolic factors and all outcomes during follow-up. For ordinal outcomes, the Wilcoxon Signed-Rank Test was used to determine within-patient change during follow-up.
All analyses were completed using R.v.3.4.2
Results
Population
During recruitment, the University of Michigan Weight Management Program enrolled 532 participants, 394 consented to be contacted about research studies, and 131 consented to our study. Of the 131 participants that completed baseline assessments, 72 completed assessments at 2 years. Reasons for attrition included the following: 16 participants decided to opt out of the study, 2 moved out of state, 1 died, 30 did not respond to multiple contacts, and 10 stopped participation for unclear reasons. Of the 59 that did not complete the neuropathy outcomes, twelve completed 2 years of follow up with the weight management program, but did not want to complete the neuropathy outcomes. Of the remainder of patients, median (IQR) follow-up in the weight management clinic was 370 days (179–528 days).
Several outcome variables had missing information at baseline (V1) or at 2 years (V2): IENFD leg (V1:1,V2:6), IENFD thigh (V1:1,V2:8), NCS parameters including sural (V1:1), peroneal F (V2:1), median motor F (V2:2), median motor CV (V2:1), QST cold (V1:2, V2:2), QST vibration (V1:1,V2:1), QSART parameters including arm (V1:2,V2:3), proximal leg (V1:1,V2:7), distal leg, (V1:1,V2:2) proximal foot (V1:3,V2:4), MNSI questionnaire (V2:1), monofilament (V2:1), Neuro-QOL parameters including social (V2:1), emotional (V2:1) and total (V2:2), CAN measures including, E:I ratio (V2:2), 30:15 ratio (V1:1,V2:4), Valsalva ratio (V1:1,V2:3), RFA (V2:2), LFA (V2:2), sdNN (V2:2), rmsSD (V2:2), mHR (V2:2), waist circumference (V2:7), triglycerides (V1:1,V2:7), HDL (V1:1, V2:7), LDL (V1:2,V2:7) fasting glucose (V1:12,V2:8). All patients had at least one measure of glycemic status at baseline, but 3 patients had no measure of glycemic status at 2 years.
Among those with complete follow-up, 19 (26.4%) had clinical neuropathy at baseline, and 14 (19.4%) had clinical neuropathy after 2 years. No difference in the dropout rate between those with and without neuropathy at baseline was observed (p=0.4).
Demographics
At baseline, the mean (SD) age was 49.1 years (10.6) and 55.0% of participants were female (Table 1). No significant demographic differences were observed between those who completed follow compared to those who did not with the exception of employment status (69.4% vs. 87.9%, p=0.03).
Table 1:
Demographics of primary cohort and those lost during follow-up
| Variable | All participants (n=131) |
Completed follow-up (N=72) |
Lost to Follow-up (n=59) |
P-Value |
|---|---|---|---|---|
| Age, mean (SD) | 49.1 (10.6) | 50.2 (10.2) | 47.8 (11.0) | 0.19 |
| Sex, N (%) Female | 72 (55.0%) | 37 (51.4%) | 35 (59.3%) | 0.47 |
|
Race, N (%) Asian Black White Unknown |
1 (0.8%) 10 (7.6%) 118 (90.1%) 2 (1.5%) |
0 (0.0%) 7 (9.7%) 64 (88.9%) 1 (1.4%) |
1 (1.7%) 3 (5.1%) 54 (91.5%) 1 (1.7%) |
0.59 |
|
Ethnicity, N (%) Hispanic/Latino |
2 (1.5%) |
1 (1.4%) |
1 (1.7%) |
1.0 |
|
Smoking Status, N (%) Current Smoker Ex-Smoker Never Smoker |
3 (2.3%) 42 (32.6%) 84 (65.1%) |
3 (4.2%) 22 (31.0%) 46 (64.8%) |
0 (0.0%) 20 (34.5%) 38 (65.5%) |
0.39 |
|
Marital Status, N (%) Divorced Married Single Separated Widowed |
7 (5.6%) 94 (75.2%) 21 (16.8%) 2 (1.6%) 1 (0.8%) |
4 (5.9%) 53 (77.9%) 11 (16.2%) 0 (0.0%) 0 (0.0%) |
3 (5.3%) 41 (71.9%) 10 (17.5%) 2 (3.5%) 1 (1.8%) |
0.52 |
|
Education, N (%) Professional or Graduate Degree College Degree Some College or Vocational College High School or Less |
51 (39.2%) 54 (41.5%) 23 (17.7%) 2 (1.5%) |
33 (45.8%) 25 (34.7%) 14 (19.4%) 0 (0.0%) |
18 (31.0%) 29 (50.0%) 9 (15.5%) 2 (3.4%) |
0.09 |
|
Employment status, N (%) Employed Retired Seeking Work Keeping House Other |
101 (77.7%) 19 (14.6%) 2 (1.5%) 4 (3.1%) 4 (3.1%) |
50 (69.4%) 16 (22.2%) 1 (1.4%) 2 (2.8%) 3 (4.2%) |
51 (87.9%) 3 (5.2%) 1 (1.7%) 2 (3.5%) 1 (1.7%) |
0.03 |
|
Insurance, N (%) Blue Care Network (HMO) Other |
77 (59.7%) 52 (40.3%) |
41 (56.9%) 31 (43.1%) |
36 (63.2%) 21 (36.8%) |
0.59 |
HMO=health maintenance organization
Change in metabolic risk factors
With the exception of systolic blood pressure and LDL cholesterol, all metabolic parameters significantly changed after 2 years (Table 2). Among those with complete follow-up, 22.2% had diabetes, 37.5% pre-diabetes, and 40.3% normoglycemia at baseline. After 2 years, 14.7% had diabetes, 27.9% pre-diabetes, and 57.4% normoglycemia (p<0.01). The median (IQR) weight loss comparing baseline to the end of the study was 5.5% (5.0–14.7%). At maximum weight loss, participants had lost 16.4% (13.0–22.4%) of their weight. Comparing minimum weight to the end of the study, participants regained 8.5% (5.4–13.9%) of their weight.
Table 2:
Change in metabolic factors after dietary weight loss intervention
| Variable | Baseline Mean (SD) |
2 Year Follow-up Mean (SD) |
Change Mean (SD) |
P-Value (Paired T-Test) |
|---|---|---|---|---|
| Weight (kg) | 120.7 (23.0) | 108.3 (22.3) | −12.4 (11.8) | <0.01 |
| Height (cm) | 171.7 (10.3) | 171.8 (10.4) | 0.1 (3.1) | 0.86 |
| BMI | 40.8 (6.0) | 36.5 (5.8) | −4.3 (3.8) | <0.01 |
| Waist Circumference (cm) | 123.1 (15.0) | 114.7 (15.7) | −9.0 (9.7) | <0.01 |
| Systolic Blood Pressure (mmHg) | 126.4 (11.0) | 126.9 (13.9) | 0.5 (12.5) | 0.74 |
| Diastolic Blood Pressure (mmHg) | 64.4 (7.3) | 68.2 (9.0) | 3.8 (9.0) | <0.01 |
| Triglycerides, (mg/dL) | 153.4 (78.3) | 127.8 (65.1) | −27.1 (55.6) | <0.01 |
| HDL, (mg/dL) | 45.3 (11.1) | 51.0 (11.5) | 5.2 (7.9) | <0.01 |
| LDL, (mg/dL) | 97.5 (23.6) | 98.1 (28.0) | 1.1 (18.4) | 0.64 |
| Cholesterol (mg/dL) | 172.2 (30.0) | 174.6 (33.9) | 0.7 (25.1) | 0.83 |
| Fasting Glucose, (mg/dL) | 101.7 (23.9) | 99.3 (23.6) | −7.5 (22.9) | 0.02 |
| 2 Hour Glucose, (mg/dL) | 134.8 (57.3) | 110.9 (34.4) | −21.8 (43.5) | <0.01 |
| HbA1C (%) | 6.0 (0.9) | 5.8 (0.7) | −0.3 (0.6) | 0.01 |
HDL=high density lipoproteins, LDL=low density lipoproteins, HbA1C=hemoglobin A1C
Change in co-primary outcomes
IENFD did not change significantly in the distal leg (0.4 fiber/mm, (3.3), p=0.29) or proximal thigh (0.3 fibers/mm, (6.3), p=0.74) after 2 years (Figure 2).
Figure 2. Change in IENFD (primary outcome) over 2 years.
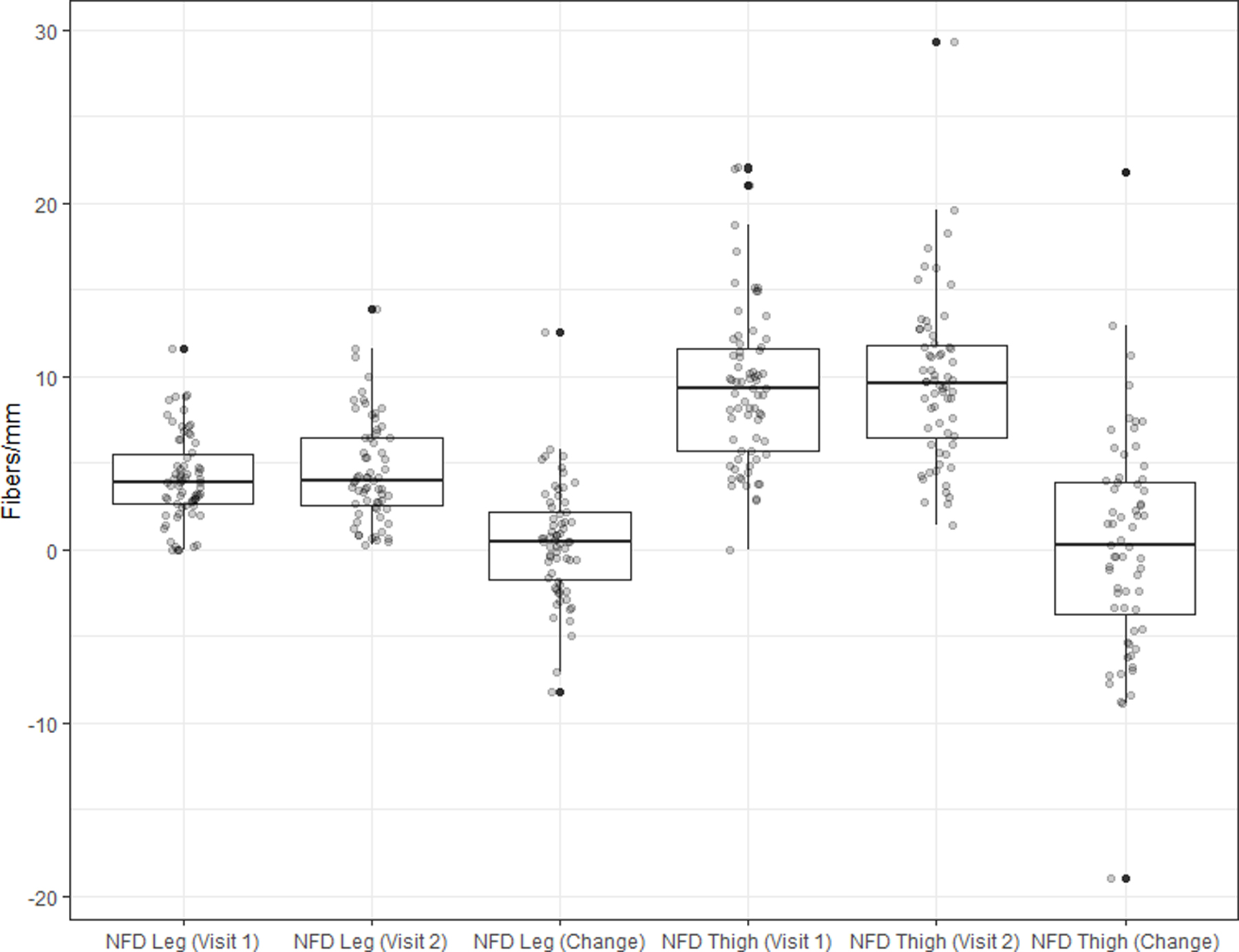
Change in IENFD of the distal leg and proximal thigh after 2 years of a dietary weight loss intervention
Change in secondary neuropathy outcomes
Of the 17 NCS parameters studies, significant changes were only observed in the ulnar sensory peak latency (0.1 ms, (0.4), p<0.01) and median sensory peak latency (0.2 ms, (0.4), p<0.01), which both worsened after 2 years (Table 3). The MNSI Questionnaire (−0.6, (1.4), p<0.01) improved, but there was no significant change in the MNSI Examination (0.04, (1.2), p=0.76). The QST cold threshold (−2.0 JND, (4.9), p<0.01) improved, but there was no change in the QST vibration threshold (0.2 JND, (4.2), p=0.77). Monofilament and QSART measures were unchanged.
Table 3:
outcome changes following weight loss
| Variable | Baseline Mean (SD) |
2 Year Follow-up Mean (SD) |
Change Mean (SD) |
P-Value (Paired T-Test) |
|---|---|---|---|---|
| Neuropathy Outcomes | ||||
| IENFD Leg, fibers/mm | 4.1 (2.5) | 4.6 (3.0) | 0.4 (3.3) | 0.29 |
| IENFD Thigh, fibers/mm | 9.4 (4.6) | 9.7 (4.8) | 0.3 (6.3) | 0.74 |
| Nerve Conduction Study Outcomes | ||||
| Ulnar Peak Latency, ms | 3.4 (0.4) | 3.5 (0.4) | 0.1 (0.4) | <0.01 |
| Ulnar Amplitude, uV | 28.0 (13.9) | 25.0 (12.7) | −3.0 (12.5) | 0.05 |
| Peroneal Distal Motor Latency, ms | 4.9 (0.9) | 5.0 (0.7) | 0.1 (0.8) | 0.41 |
| Peroneal Amplitude, uV | 5.4 (2.6) | 5.2 (2.9) | −0.2 (2.2) | 0.38 |
| Peroneal F, ms | 50.6 (5.8) | 50.9 (5.7) | 0.1 (5.9) | 0.85 |
| Peroneal CV, m/s | 44.7 (5.2) | 44.0 (5.3) | −0.9 (5.7) | 0.21 |
| Sural Peak Latency, ms | 3.8 (0.4) | 3.9 (0.4) | 0.1 (0.4) | 0.08 |
| Sural Amplitude, uV | 12.3 (6.9) | 13.1 (8.8) | 0.9 (7.9) | 0.38 |
| Tibial Distal Motor Latency, ms | 4.8 (0.9) | 5.1 (0.8) | 0.2 (1.0) | 0.08 |
| Tibial Amplitude, uV | 9.1 (5.3) | 8.5 (4.5) | −0.5 (4.2) | 0.30 |
| Tibial F, ms | 53.1 (6.5) | 52.3 (6.0) | −0.4 (6.6) | 0.65 |
| Median Motor Distal Motor Latency, ms | 3.9 (0.7) | 3.9 (0.8) | 0.001 (0.5) | 0.98 |
| Median Motor Amplitude, uV | 7.7 (3.3) | 7.9 (3.3) | 0.2 (3.6) | 0.60 |
| Median Motor F, ms | 29.0 (2.7) | 29.0 (3.6) | 0.2 (2.8) | 0.56 |
| Median Motor CV, m/s | 52.6 (5.2) | 53.2 (6.3) | 0.5 (6.2) | 0.47 |
| Median Sensory Peak Latency, ms | 3.8 (0.6) | 3.9 (0.6) | 0.2 (0.4) | <0.01 |
| Median Sensory Amplitude, uV | 28.8 (15.4) | 28.0 (16.1) | −0.7 (5.8) | 0.29 |
| QST | ||||
| QST Cold | 13.1 (4.8) | 10.8 (4.4) | −2.0 (4.9) | <0.01 |
| QST Vibration | 17.8 (3.7) | 17.9 (4.2) | 0.2 (4.2) | 0.77 |
| QSART | ||||
| QSART Arm | 1.2 (1.1) | 1.9 (6.7) | 0.7 (6.9) | 0.43 |
| QSART Proximal Leg | 0.5 (0.5) | 1.4 (9.3) | 1.0 (9.4) | 0.41 |
| QSART Distal Leg | 0.5 (0.4) | 1.3 (6.9) | 0.9 (7.0) | 0.31 |
| QSART Proximal Foot | 0.5 (0.5) | 0.9 (4.7) | 0.5 (4.8) | 0.45 |
| Michigan Neuropathy Screening Instrument | ||||
| MNSI Questionnaire | 2.8 (2.5) | 2.2 (2.2) | −0.6 (1.4) | <0.01 |
| MNSI Exam | 1.0 (1.5) | 1.1 (1.6) | 0.04 (1.2) | 0.76 |
| Monofilament | ||||
| Normal Reduced Absent |
65 (90.3%) 5 (6.9%) 2 (2.8%) |
66 (93.0%) 2 (2.8%) 3 (4.2%) |
Worsened: 2 (2.8%) Stable: 65 (91.6%) Improved: 4 (5.6%) |
1.00# |
| Patient Oriented Outcomes | ||||
| McGill Pain Scale | ||||
| McGill Total | 4.7 (6.0) | 3.9 (5.7) | −0.8 (4.7) | 0.15 |
| McGill Sensory | 4.0 (5.0) | 3.3 (4.6) | −0.7 (4.0) | 0.12 |
| McGill Affective | 0.6 (1.3) | 0.5 (1.4) | −0.1 (1.3) | 0.58 |
| VAS Total | 19.6 (24.6) | 17.8 (22.8) | −1.8 (29.3) | 0.60 |
| PPI | ||||
| No Pain Mild Discomforting |
54 (77.1%) 9 (12.9%) 7 (10.0%) |
52 (72.2%) 14 (19.4%) 6 (8.3%) |
Worsened: 12 (17.1%) Stable: 49 (70.0%) Improved: 9 (12.9%) |
0.75# |
| Neuro-QOL | ||||
| Neuro-QOL Total | 2.3 (1.8) | 2.0 (1.1) | −0.3 (1.4) | 0.06 |
| Neuro-QOL Pain | 2.3 (1.8) | 2.0 (1.5) | −0.4 (1.1) | 0.01 |
| Neuro-QOL Reduced Sensation | 2.1 (2.3) | 2.0 (2.4) | −0.1 (1.9) | 0.65 |
| Neuro-QOL Sensory Motor | 1.9 (1.6) | 2.0 (1.8) | 0.04 (1.3) | 0.80 |
| Neuro-QOL Social | 2.1 (1.6) | 2.0 (1.0) | −0.03 (1.9) | 0.90 |
| Neuro-QOL Emotional | 2.4 (2.6) | 1.7 (1.0) | −0.7 (2.2) | 0.01 |
| Neuro-QOL Activities of Daily Living | 3.2 (3.0) | 2.9 (2.1) | −0.3 (2.7) | 0.34 |
| CAN Outcomes | ||||
| E:I Ratio | 1.13 (0.08) | 1.19 (0.40) | 0.07 (0.41) | 0.17 |
| 30:15 Ratio | 1.40 (0.64) | 1.38 (0.53) | −0.02 (0.46) | 0.71 |
| Valsalva Ratio | 1.48 (0.34) | 1.54 (0.51) | 0.07 (0.60) | 0.35 |
| RFA | 10.5 (65.5) | 26.5 (196.2) | 15.8 (208.4) | 0.53 |
| LFA | 7.5 (45.1) | 57.9 (446.0) | 50.2 (449.2) | 0.35 |
| LFA/RFA | 3.1 (4.1) | 5.4 (17.5) | 2.2 (18.0) | 0.31 |
| sdNN | 53.9 (31.1) | 54.1 (40.2) | 0.5 (48.7) | 0.93 |
| rmsSD | 35.3 (32.7) | 38.7 (49.4) | 3.9 (59.5) | 0.58 |
| Median heart rate | 66.8 (11.9) | 67.6 (13.9) | 0.5 (16.6) | 0.80 |
P-Value represents results from Wilcoxon Signed-Rank Test
IENFD=intraepidermal nerve fiber density, CV=conduction velocity, QST=quantitative sensory testing, QSART=quantitative sudomotor axon reflex testing, PPI=present pain intensity, QOL=quality of life, CAN=cardiovascular autonomic neuropathy, RFA=respiratory frequency area, measure of parasympathetic activity, LFA=low frequency area, measure of sympathetic activity, LFA/RFA=low frequency area/ respiratory frequency area, measure of sympathovagal balance, sdNN=standard deviation of the normal to normal interval, rmdSD=root mean square of successive differences of the normal to normal interval
Change in patient-oriented neuropathy outcomes
The Neuro-QOL (−0.3, (1.4), p=0.06), the VAS pain scores (−1.8 mm, (29.3), p=0.60), McGill pain scores (−0.8, (4.7), p=0.15). and PPI (17.1% worsened, 12.9% improved, p=0.75) were unchanged after 2 years (Table 3). However, the Neuro-QOL subdomains of pain (−0.4, (1.1), p=0.01) and emotion (−0.7, (2.2), p=0.01) were improved.
Change in CAN outcomes
No significant changes were seen in any of the CAN outcomes including E/I ratio, 30:15 ratio, Valsalva ratio, RFA, LFA, RFA/LFA ratio, sdNN, rmsSD, or mHR (Table 3).
Discussion
A successful dietary weight loss intervention in the severely obese was associated with no change in our co-primary neuropathy outcomes (IENFD of the distal leg and proximal thigh). This sharply contrasts to the natural history of IENFD decline in those with small fiber neuropathy of any cause including prediabetes and diabetes,27 but is not as impressive as the improvements that have been reported with exercise interventions studies.15,16,28,29 Importantly, the natural history of IENFD decline in obese populations is unknown. Some secondary outcomes revealed improvements, specifically MNSI questionnaire, QST cold, and 2 subdomains of the Neuro-QOL, but nerve conduction study parameters and other secondary outcomes remained unchanged. Similar to neuropathy outcomes, CAN measures were also stable after 2 years.
This study is the second to evaluate the effects of a lifestyle intervention that was focused on dietary weight loss on neuropathy outcomes. The Look AHEAD study randomized over 5,000 participants who had type 2 diabetes and were overweight or obese to a dietary weight loss intervention for 9–11 years.17 Our study is complementary in that we were able to study an obese population that included those with normoglycemia and pre-diabetes in addition to those with diabetes. We also performed much more detailed neuropathy phenotyping. Both Look AHEAD and our study found that dietary weight loss was associated with improvements on the MNSI questionnaire, but not the MNSI examination. The consistency of these results provides more evidence for the benefits of dietary weight loss for peripheral nerves, but also highlights the limitations of this intervention. Subjective measures such as the MNSI questionnaire improved, which we also observed for two Neuro-QOL subdomains. In contrast, objective measures of neuropathy such as the MNSI examination were largely stable. Our study included IENFD and nerve conduction study parameters that also demonstrated stability. Taken together, these studies indicate that dietary weight loss can halt the progression of neuropathy, if not lead to mild improvements, but that different interventions are likely needed if more dramatic improvement is the goal. Importantly, the natural history of small fiber neuropathy, regardless of cause, is to decline at a predictable rate;27 therefore, any intervention that leads to stability should be considered a success.
Other potential interventions to improve multiple metabolic risk factors and neuropathy outcomes include surgical weight loss, medication induced weight loss, and exercise. Surgical weight loss has only been evaluated in one small study of 12 participants before and after Rouy-en-Y gastric bypass with a hint of efficacy.30 Medication induced weight loss has not been studied. In contrast, exercise has been the most studied of these interventions, including 3 uncontrolled and 1 randomized study.15,16,28,29 Two of these studies also had a dietary component, but weight loss was minimal: 0.1 kg/m2 and 1.1 kg.m2 decrease in BMI compared with 4.3 kg/m2 in our study.15,16 Three of these exercise studies demonstrated improvements in IENFD outcomes, and the randomized trial revealed improvements in nerve conductions studies and vibration thresholds. Taken together, the previous exercise studies indicate an improvement in neuropathy outcomes whereas dietary weight loss demonstrates stability or mild improvement in subjective outcomes. However, more definitive studies comparing the effects of exercise and different weight loss strategies (dietary, surgical, and medication induced) are needed before strong clinical recommendations can be made favoring one of these interventions. Given the modest effect size and adherence issues with dietary weight loss, future interventions may need to combine exercise and dietary weight loss or include dietary adjustments designed to improve adherence and/or to improve neuropathy through limiting certain metabolites that may lead to nerve injury.
While IENFD and nerve conduction study parameters did not change after dietary weight loss, the MNSI questionnaire, 2 Neuro-QOL subdomains, and the QST cold all did demonstrate improvement after 2 years. This is an important finding because utilizing more sensitive measures of neuropathy improvement has the potential to lead to more efficient clinical trials. We looked at several secondary outcomes measures; therefore, these results should be considered hypothesis generating rather than definitive. On the other hand, the Look AHEAD study also demonstrated improvement in MNSI questionnaire over the first couple years in addition to after 9–11 years of dietary weight loss.17 These results provide stronger justification for using the MNSI questionnaire as a sensitive measure of neuropathy improvement. Interestingly, the Neuro-QOL may also be a sensitive indicator of improvement, which could be important as this is also a patient-oriented outcome. Future studies will be needed to determine if the Neuro-QOL demonstrated sensitivity to neuropathy improvement in future studies. Despite these encouraging results, more sensitive biomarkers are needed to detect earlier changes in patients with neuropathy, which would enable more efficient clinical trials. Furthermore, the MNSI questionnaire and Neuro-QOL are more subjective neuropathy measures when compared to IENFD and NCS, which may account for the differences observed with these outcomes.
Similar to neuropathy, CAN also demonstrated stability 2 years after dietary weight loss on all 9 measures. Only one randomized trial has investigated the effects of dietary weight loss, in combination with exercise, on CAN outcomes, and this was in a population of individuals with type 2 diabetes.31 The investigators found no improvement on the E:I ratio in the overall population, although diet and exercise did lead to improvement in women. Analogously to neuropathy, the natural history of CAN is to worsen over a 2 year time period in those with diabetes.32 Therefore, the stability in CAN measures after dietary weight loss in both this study and our current study provides supporting evidence for a positive effect, but future randomized studies are needed especially since the natural history of CAN in obese populations is unknown. Comparable to neuropathy, most of the interventions studies have focused on the effects of exercise on CAN.33 These studies have generally showed improvement in CAN outcomes, but they are uniformly small, with varying outcomes and exercise regimens, which limits interpretability.28,34–38 Just like with neuropathy, studies that compare the effects of exercise to different weight loss strategies are needed to guide clinical recommendations.
Interestingly, the improvement in neuropathy outcomes that we observe in obese humans after a dietary intervention has also been observed in obese mice. Mice on a high-fat diet develop neuropathy that is completely normalized after dietary reversal39. While we did not observe such robust results in humans, the dietary reversal is not nearly as complete as in the murine models and the metabolic impairments have been present for far longer in humans. Importantly, lipidomic analyses have shed light on potential biologic mechanisms of obesity-related neuropathy. Nerves from high-fat fed mice with neuropathy contain an increase in triglycerides containing saturated fatty acids compared to nerves from control mice40. Mice fed a high-fat diet consisting of saturated fatty acids develop neuropathy that is completely reversed by switching to a high-fat diet consisting of monounsaturated fatty acids41. The monounsaturated fatty acid oleate also prevents defects in axonal mitochondrial transport and membrane potential that are present in sensory neurons treated with the saturated fatty acid palmitate. These results indicate that nerve-lipid signaling is an important factor in peripheral nerve injury42.
Limitations include the small sample size, which limits our power to detect small changes. However, we did observe significant changes in multiple secondary outcomes. We also had significant loss to follow-up, but only employment status was significantly different between the whole cohort and those who followed up after 2 years. We were unable to investigate longer term effects of dietary weight loss on outcomes after 2 years, but our results are consistent with the Look AHEAD study, which followed participants for 9–11 years.17 Our pre-post intervention design does not allow for causal inferences. Generalizability to other populations, particularly those with different race/ethnicity and educational status, is unclear. Strengths include the comprehensive metabolic and neuropathy phenotyping before and after a successful dietary weight loss intervention.
After a dietary weight loss intervention, severely obese participants had large improvements in multiple metabolic risk factors. Neuropathy, as measured by IENFD, and CAN were stable after 2 years, which is an improvement from the known natural history of decline in those with small fiber neuropathy from any cause.28 Randomized trials are needed to definitely address the effects of dietary weight loss on neuropathy and compare and contrast to other weight loss measures and/or exercise.
Figure 1. Change in obesity measures over 2 years.
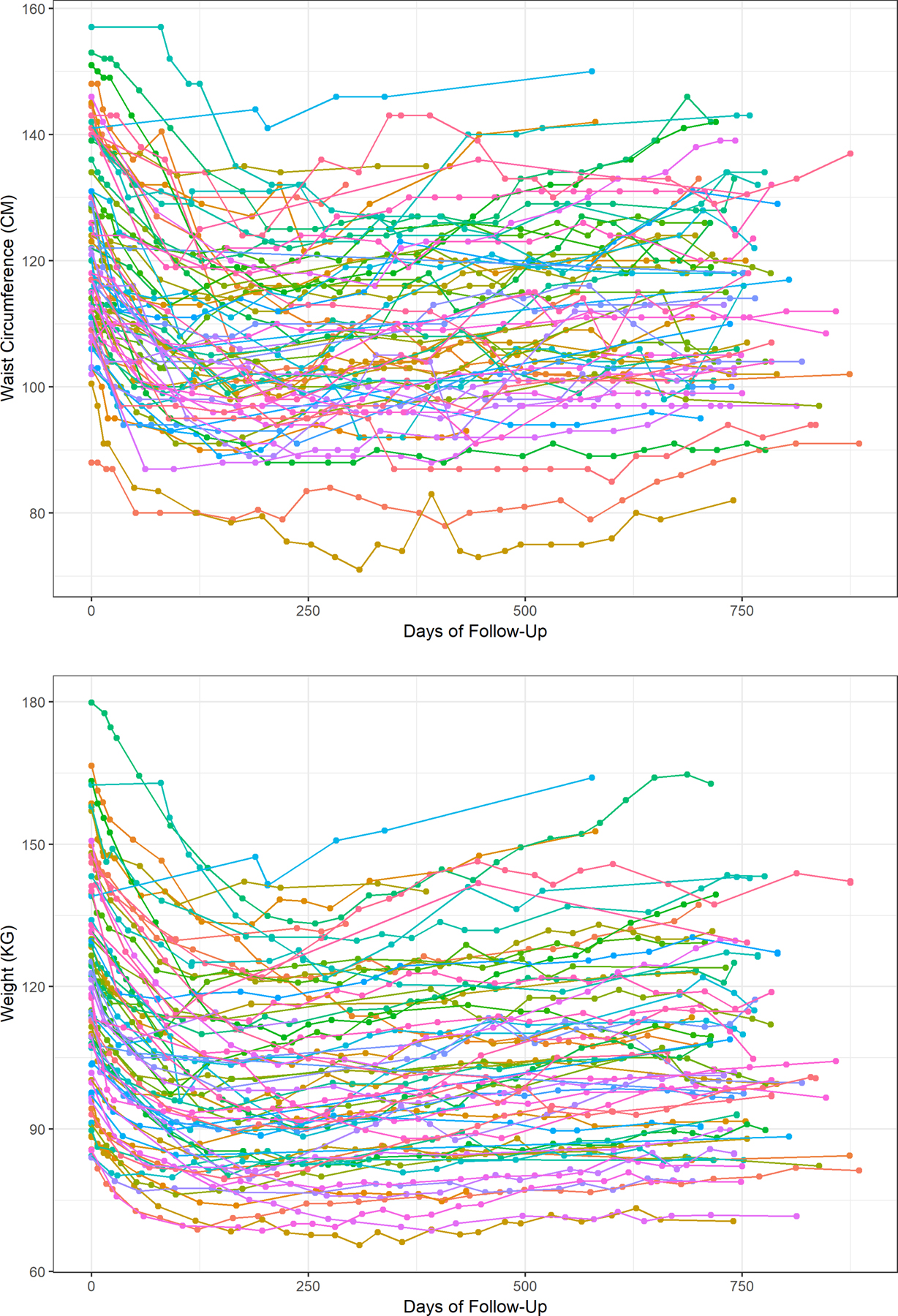
Longitudinal measures of waist circumference (1A) and weight (1B) during 2 years of follow-up after a dietary weight loss intervention
What is already known about this subject?
Obesity is a consistent risk factor for neuropathy across many studies in different populations across the world
The metabolic syndrome and its individual components are also associated with neuropathy
Dietary weight loss has been demonstrated to improve questionnaire assessments of neuropathy in patients with diabetes, but not in patients without diabetes and no studies have utilized more comprehensive neuropathy phenotyping
What are the new findings in your manuscript?
After 2 years, successful dietary weight loss in the severely obese leads to stable neuropathy as measured by our primary outcome, intraepidermal nerve fiber density
Successful dietary weight loss leads to improvements in secondary outcomes such as the MNSI Questionnaire, two NeuroQoL subdomains, and QST cold
Dietary weight loss also leads to stable cardiovascular autonomic neuropathy
How might your results change the direction of research or the focus of clinical practice?
Future randomized clinical trials are needed to confirm that dietary weight loss can stabilize neuropathy.
If successful, dietary weight loss would become the second disease modifying therapy for neuropathy along with glycemic control.
Furthermore, studies are needed to compare the effectiveness of dietary weight loss, surgical weight loss, and exercise to allow clinicians to focus on the best intervention to prevent neuropathy.
Study Funding:
The project described was supported by a NIH K23 grant (NS079417). Dr. Callaghan is currently funded by a NIH NIDDK R-01 award (DK115687). Dr. Reynolds is supported by is supported by NIH T32 (NS0007222). Dr. Feldman was supported by an NIH NIDDK DP3 award (DK094292) and is currently funded by NIH NIDDK (R24082841 and R21 NS102924) and the Novo Nordisk Foundation Center for Basic Metabolic Research (NNF14°C0011633). Drs. Callaghan, Reynolds, and Feldman receive support from the NeuroNetwork for Emerging Therapies and the A. Alfred Taubman Research Institute.
Footnotes
Disclosures:
Dr. Callaghan consults for DynaMed, performs medical legal consultations including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Rothberg consults for Nestle, Rhythm Pharma, and REWIND, Inc. Drs. Reynolds, Banerjee, Akinci, Burant, and Feldman report no disclosures. Mrs. Villegas-Umana and Ms. Chant report no disclosures.
References
- 1.Callaghan B, Kerber K, Langa KM, et al. Longitudinal patient-oriented outcomes in neuropathy: Importance of early detection and falls. Neurology. 2015;85(1):71–79. doi: 10.1212/WNL.0000000000001714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaghan BC, Kerber KA, Lisabeth LL, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol. 2014;71(9):1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johannsen L, Smith T, Havsager A-M, et al. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J Clin Neuromuscul Dis. 2001;3(2):47–52. [DOI] [PubMed] [Google Scholar]
- 4.Lubec D, Müllbacher W, Finsterer J, Mamoli B. Diagnostic work-up in peripheral neuropathy: an analysis of 171 cases. Postgrad Med J. 1999;75(890):723–727. doi: 10.1136/pgmj.75.890.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen ST, Witte DR, Dalsgaard E-M, et al. Risk Factors for Incident Diabetic Polyneuropathy in a Cohort With Screen-Detected Type 2 Diabetes Followed for 13 Years: ADDITION-Denmark. Diabetes Care. 2018;41(5):1068–1075. doi: 10.2337/dc17-2062 [DOI] [PubMed] [Google Scholar]
- 6.Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397–405. doi: 10.1002/acn3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Central Obesity is Associated With Neuropathy in the Severely Obese. Mayo Clin Proc. 2020;95(7):1342–1353. doi: 10.1016/j.mayocp.2020.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaghan BC, Xia R, Banerjee M, et al. Metabolic Syndrome Components Are Associated With Symptomatic Polyneuropathy Independent of Glycemic Status. Diabetes Care. 2016;39(5):801–807. doi: 10.2337/dc16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaghan BC, Xia R, Reynolds E, et al. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol. 2016;73(12):1468–1476. doi: 10.1001/jamaneurol.2016.3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanewinckel R, Drenthen J, Ligthart S, et al. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population-based cohort study. J Neurol Neurosurg Psychiatry. 2016;87(12):1336–1342. doi: 10.1136/jnnp-2016-314171 [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Hu J, Wen J, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes - ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PloS One. 2013;8(4):e61053. doi: 10.1371/journal.pone.0061053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlesinger S, Herder C, Kannenberg JM, et al. General and Abdominal Obesity and Incident Distal Sensorimotor Polyneuropathy: Insights Into Inflammatory Biomarkers as Potential Mediators in the KORA F4/FF4 Cohort. Diabetes Care. 2019;42(2):240–247. doi: 10.2337/dc18-1842 [DOI] [PubMed] [Google Scholar]
- 13.Callaghan B, Feldman E. The metabolic syndrome and neuropathy: Therapeutic challenges and opportunities: Metabolic Syndrome. Ann Neurol. 2013;74(3):397–403. doi: 10.1002/ana.23986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaghan BC, Little AA, Feldman EL, Hughes RAC. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;(6):CD007543. doi: 10.1002/14651858.CD007543.pub2 [DOI] [PMC free article] [PubMed]
- 15.Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. 2015;77(1):146–153. doi: 10.1002/ana.24310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294–1299. doi: 10.2337/dc06-0224 [DOI] [PubMed] [Google Scholar]
- 17.Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia. 2017;60(6):980–988. doi: 10.1007/s00125-017-4253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothberg AE, McEwen LN, Kraftson AT, et al. Factors associated with participant retention in a clinical, intensive, behavioral weight management program. BMC Obes. 2015;2:11. doi: 10.1186/s40608-015-0041-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 20.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Fe-deration of Neurological Societies and the Peripheral Ne: EFNS/PNS guideline on skin biopsy. Eur J Neurol. 2010;17(7):903–e49. doi: 10.1111/j.1468-1331.2010.03023.x [DOI] [PubMed] [Google Scholar]
- 21.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281 [DOI] [PubMed] [Google Scholar]
- 22.Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vileikyte L, Peyrot M, Bundy C, et al. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–2555. doi: 10.2337/diacare.26.9.2549 [DOI] [PubMed] [Google Scholar]
- 24.Grafton KV, Foster NE, Wright CC. Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. Clin J Pain. 2005;21(1):73–82. [DOI] [PubMed] [Google Scholar]
- 25.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895 [DOI] [PubMed] [Google Scholar]
- 26.Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management: Diabetic Cardiovascular Autonomic Neuropathy in Clinical Practice. Diabetes Metab Res Rev. 2011;27(7):639–653. doi: 10.1002/dmrr.1239 [DOI] [PubMed] [Google Scholar]
- 27.Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal Assessment of Small Fiber Neuropathy: Evidence of a Non-Length-Dependent Distal Axonopathy. JAMA Neurol. 2016;73(6):684–690. doi: 10.1001/jamaneurol.2016.0057 [DOI] [PubMed] [Google Scholar]
- 28.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 29.Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller-Stich BP, Fischer L, Kenngott HG, et al. Gastric bypass leads to improvement of diabetic neuropathy independent of glucose normalization--results of a prospective cohort study (DiaSurg 1 study). Ann Surg. 2013;258(5):760–765; discussion 765–766. doi: 10.1097/SLA.0b013e3182a618b2 [DOI] [PubMed] [Google Scholar]
- 31.Vanninen E, Uusitupa M, Länsimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed type 2 diabetes. Diabet Med J Br Diabet Assoc. 1993;10(1):66–73. doi: 10.1111/j.1464-5491.1993.tb01999.x [DOI] [PubMed] [Google Scholar]
- 32.Karamitsos DT, Didangelos TP, Athyros VG, Kontopoulos AG. The natural history of recently diagnosed autonomic neuropathy over a period of 2 years. Diabetes Res Clin Pract. 1998;42(1):55–63. doi: 10.1016/s0168-8227(98)00089-8 [DOI] [PubMed] [Google Scholar]
- 33.Zilliox LA, Russell JW. Physical activity and dietary interventions in diabetic neuropathy: a systematic review. Clin Auton Res Off J Clin Auton Res Soc. 2019;29(4):443–455. doi: 10.1007/s10286-019-00607-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhagyalakshmi S, Nagaraja H, Anupama B, et al. Effect of supervised integrated exercise on heart rate variability in type 2 diabetes mellitus. Kardiol Pol. 2007;65(4):363–368; discussion 369. [PubMed] [Google Scholar]
- 35.Goit RK, Pant BN, Shrewastwa MK. Moderate intensity exercise improves heart rate variability in obese adults with type 2 diabetes. Indian Heart J. 2018;70(4):486–491. doi: 10.1016/j.ihj.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howorka K, Pumprla J, Haber P, Koller-Strametz J, Mondrzyk J, Schabmann A. Effects of physical training on heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Cardiovasc Res. 1997;34(1):206–214. doi: 10.1016/s0008-6363(97)00040-0 [DOI] [PubMed] [Google Scholar]
- 37.Pagkalos M, Koutlianos N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med. 2008;42(1):47–54. doi: 10.1136/bjsm.2007.035303 [DOI] [PubMed] [Google Scholar]
- 38.Zoppini G, Cacciatori V, Gemma ML, et al. Effect of moderate aerobic exercise on sympatho-vagal balance in Type 2 diabetic patients. Diabet Med J Br Diabet Assoc. 2007;24(4):370–376. doi: 10.1111/j.1464-5491.2007.02076.x [DOI] [PubMed] [Google Scholar]
- 39.Hinder LM, O’Brien PD, Hayes JM, et al. Dietary reversal of neuropathy in a murine model of prediabetes and metabolic syndrome. Dis Model Mech. 2017;10(6):717–725. doi: 10.1242/dmm.028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien PD, Guo K, Eid SA, et al. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Model Mech. 2020;13(2). doi: 10.1242/dmm.042101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rumora AE, LoGrasso G, Hayes JM, et al. The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. J Neurosci Off J Soc Neurosci. 2019;39(19):3770–3781. doi: 10.1523/JNEUROSCI.3173-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savelieff MG, Callaghan BC, Feldman EL. The emerging role of dyslipidemia in diabetic microvascular complications. Curr Opin Endocrinol Diabetes Obes. 2020;27(2):115–123. doi: 10.1097/MED.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]


