Abstract
Background:
For patients with non-small cell lung cancer (NSCLC) receiving immune checkpoint inhibitors, PD-L1 tumor proportion score has been validated as a predictive biomarker for improved overall survival. However, its histology-specific predictive value in patients with advanced squamous versus nonsquamous cancers remains unclear.
Purpose:
To assess the differential value of PD-L1 tumor proportion score (TPS) as a predictive biomarker for overall survival after first-line pembrolizumab in patients with squamous versus nonsquamous NSCLC.
Basic procedures:
Retrospective, observational study of patients diagnosed with advanced NSCLC who were treated between October 2015 and April 2019 at community oncology clinics and academic medical centers in a de-identified EHR-derived database. Included patients were diagnosed with advanced or metastatic NSCLC, received treatment with first-line, single agent pembrolizumab, and had documentation of PD-L1 testing with a numeric result. Exclusion criteria included alterations in EGFR, ALK, and ROS1.The primary endpoint was overall survival from start of first-line pembrolizumab therapy by squamous or nonsquamous histology and PD-1 expression level measured by TPS (low, <50% or high, ≥50%).
Main findings:
The cohort of 1460 patients with NSCLC who received pembrolizumab as a first-line therapy had a mean age of 72 years. Histology was 28% squamous and 72% nonsquamous. PD-L1 expression was low in 13% and high in 87%. No meaningful differences in age, gender, or smoking history were observed by PD-L1 TPS or histology type. A generalized gamma model adjusting for gender and stage at diagnosis found that for patients with nonsquamous histology, high PD-L1 TPS was significantly associated with improved overall survival by a median OS difference of 8.4 months (p <0.001). In contrast, for patients with squamous histology, there was no evidence of association between PD-L1 expression level and overall survival (p = 0.283). PD-L1-related incremental differences in median OS between the patients with squamous and nonsquamous tumors were significantly different (p = 0.034).
Principal conclusions:
Among NSCLC patients treated with 1L pembrolizumab, high PD-L1 TPS is associated with OS among patients with nonsquamous NSCLC, but not among patients with squamous NSCLC.
Introduction
The predictive value of programmed death ligand-1 (PD-L1) expression on non-small cell lung cancer (NSCLC) tumor cells as a biomarker for improved overall survival (OS) after therapy with immune checkpoint inhibitors is well established.1 Whether this relationship is histology-specific remains unclear. In phase III studies of nivolumab compared to docetaxel in the second-line setting for patients with advanced NSCLC, PD-L1 tumor proportion score (TPS) was associated with improved survival in patients with nonsquamous histologies but not in those with squamous cell carcinoma.2,3 Phase III clinical trials of pembrolizumab alone or in combination with chemotherapy have demonstrated variability with respect to histology and predictive value of PD-L1 TPS.4–6
It is plausible that NSCLC histology affects the mechanism of action of anti-PD-L1 therapy resulting in differential treatment effectiveness. To assess this hypothesis, we conducted an observational study of real world patients with NSCLC who received pembrolizumab as first-line therapy, assessing the relationship between PD-L1 TPS and OS, separately for squamous and nonsquamous histologies, to gain insight into potential modification of treatment effectiveness by histology.
Methods
We performed a retrospective cohort study of patients diagnosed with advanced or metastatic NSCLC who received single-agent pembrolizumab as first-line therapy between October 1, 2015 and April 31, 2019 and had documentation of a numeric result from PD-L1 testing prior to the start of treatment. Patients were excluded if only a yes/no PD-L1 result was documented and if alterations in EGFR, ALK, or ROS1 were documented. The data source was the nationwide Flatiron Health electronic health record (EHR)-derived de-identified database of patients treated for cancer at community oncology clinics and at academic medical centers in the United States.7 During the study period, de-identified data originated from approximately 280 US cancer clinics (~800 sites of care). The longitudinal database, abstracted from electronic health records, comprises both patient-level structured and unstructured data curated via technology-enabled abstraction.7
The primary objective was to evaluate the effect modification of histology-dependent treatment effectiveness by PD-L1 TPS. Histology was defined as squamous or nonsquamous. PD-L1 expression was measured by TPS with ≥50% classified as high and <50% as low, based on prior evidence of decreased inter-pathologist concordance for cut points of TPS <50%.8 For patients with more than one PD-L1 result, the test closest to the pembrolizumab start date was chosen. Other variables of interest included age, gender, stage at initial diagnosis (I-III versus IV), and history of smoking. The primary endpoint was real-world overall survival (rwOS)9 from the first dose of pembrolizumab to death. The secondary endpoint was progression free survival (PFS) from the first dose of pembrolizumab. Patients were censored at their last activity date.
Statistical Analysis
Baseline characteristics of the patients were compared between groups using Pearson’s chi-square or Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. Tests were 2-sided with a significance level of 0.05. OS was estimated using the Kaplan–Meier method. Univariate Cox proportional hazards models were fit to identify significant baseline covariates for inclusion in multivariable modeling (i.e. p value <0.05). We fit a Cox proportional hazards model with PD-L1 expression level, histology, an interaction between PD-L1 and histology, and other covariates. The proportional-hazards assumption was examined using graphical methods and Schoenfeld residuals. In the case of non-proportional hazards, we constructed a generalized gamma model of OS using histology and PD-L1 expression subgroups (squamous/nonsquamous and high/low), adjusting for relevant covariates.10,11 The presence of histology-PD-L1 interaction was examined by comparing the median OS differences (PD-L1 high versus low) between patients with squamous and nonsquamous histologies. Bootstrapping procedures were used to estimate the 95% confidence intervals for median OS and PD-L1-related incremental differences in median OS.
Results
Of 16,690 patients diagnosed with advanced NSCLC, the study included 1460 patients who received pembrolizumab as first-line therapy; mean age was 72 years (Table 1 and Supplemental Figure 1). Histology was 28% squamous and 72% nonsquamous. PD-L1 TPS was negative or low in 13% and high in 87%. Only 17 patients, or 1.16% of the cohort, had a PD-L1 TPS of <1%. Compared to patients with nonsquamous NSCLC, patients with squamous NSCLC were significantly more likely to be males (64% versus 46%), have a history of smoking (96% versus 93%), and were less likely to have tumors with high PD-L1 TPS (81% versus 90%). Baseline characteristics for each histology group stratified by PD-L1 expression level revealed no meaningful differences in age, gender, or smoking history. Patients with stage I-III disease at diagnosis were more likely to have PD-L1 <50% (Supplemental Tables 1 and 2). However, 447 patients, or 73%, were treated with pembrolizumab ≤6 months after the date of PD-L1 TPS assessment.
Table 1.
Baseline Characteristics of patients with squamous and nonsquamous NSCLC
| Variable | Overall (N=1460) | Nonsquamous (N = 1055) | Squamous (N = 405) | p-value |
|---|---|---|---|---|
| Mean Age (SD), years | 72.1(9.6) | 72.0 (9.8) | 72.6 (9.1) | 0.286 |
| Gender, n (%) | <0.001 | |||
| Female | 718(49.2) | 570 (54.0) | 148 (36.5) | |
| Male | 742(50.8) | 485 (46.0) | 257 (63.5) | |
| Smoking History, n(%) | 0.012 | |||
| History of smoking | 1366(93.6) | 976 (92.5) | 390 (96.3) | |
| No history of smoking | 94(6.4) | 79 (7.5) | 15 (3.7) | |
| Stage at diagnosis, n (%) | 0.002 | |||
| I, II, or III | 407(28.4) | 271 (26.0) | 136 (34.5) | |
| IV | 1028(71.6) | 770 (74.0) | 258 (65.5) | |
| PD-L1 (%) | <0.001 | |||
| <50% | 190(13.0) | 111 (10.5) | 79 (19.5) | |
| ≥50% | 1270(87.0) | 944 (89.5) | 326 (80.5) |
Abbreviations: TPS, tumor proportion score
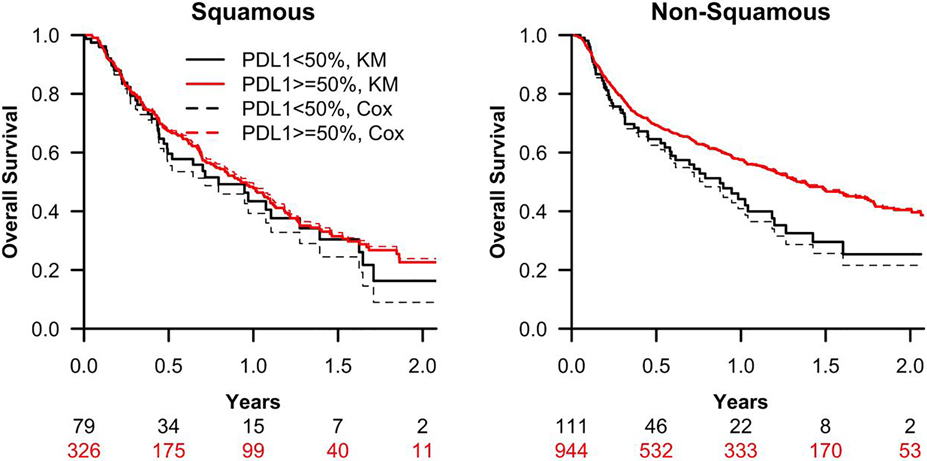
Red lines indicate high PD-L1 TPS (≥50%); black lines indicate low PD-L1 TPS (<50%). Overall survival is measured from the start date of first-line pembrolizumab. Solid lines represent estimates from the Kaplan-Meier (KM) analysis; dashed lines are estimates from a generalized gamma regression model adjusting for gender and stage of diagnosis. A generalized gamma model adjusting for gender and stage at diagnosis found that for patients with nonsquamous histology, high PD-L1 TPS was significantly associated with improved overall survival by a median OS difference of 8.4 months (p <0.001). In contrast, for patients with squamous histology, there was no evidence of association between PD-L1 expression level and overall survival (p = 0.283). PD-L1-related incremental differences in median OS between the patients with squamous and nonsquamous tumors were significantly different (p = 0.034). Abbreviations: KM, Kaplan-Meier; TPS, tumor proportion score
Kaplan-Meier estimates of median OS among patients with squamous PD-L1 low was 9.5 months (95% CI: 5.9 to 16.7); squamous PD-L1 high 11.1 months (95% CI: 9.3 to 13.2); nonsquamous PD-L1 low 10.5 months (95% CI: 7.1 to 14.4); and nonsquamous PD-L1 high 16.2 months (95% CI: 14.4 to 19.3). Univariate analysis (Supplemental Table 3) identified gender and stage at diagnosis as significantly associated with OS in the population. Due to the violation of the proportional hazards (PH) assumption based on a test of Schoenfeld residuals (P<0.001) and graphical methods (Supplemental Figure 2, Supplemental Table 4), the generalized gamma regression model estimation was employed for the primary analysis.
The median OS estimated using the generalized gamma model is shown in Figure 1. Among patients with squamous NSCLC, PD-L1 TPS was not meaningfully associated with OS (p = 0.28). In contrast, among patients with nonsquamous histology, PD-L1 TPS was significantly associated with OS (p < 0.001). Those patients with nonsquamous histology and high PD-L1 TPS had median OS 8.4 months longer (95% CI: 4.1 to 12.5) than patients with low PD-L1 TPS with similar histology, gender, and stage at diagnosis (Table 2). Testing our hypothesis of treatment effect modification, PD-L1-related incremental differences in median OS between the patients with squamous and nonsquamous tumors were significantly different (p = 0.034). For the secondary analysis of PFS using a Cox multivariable proportional hazards model, PD-L1-histology interaction was not significant (P=0.539) (Supplemental Figure 3, Supplemental Table 5).
Figure 1.
The observed (KM) and predicted (Cox Model) of overall survival of patients with squamous and nonsquamous NSCLC with high (≥50%) and low (<50%) PD-L1 tumor proportion score
Red lines indicate high PD-L1 TPS (≥50%); black lines indicate low PD-L1 TPS (<50%). Overall survival is measured from the start date of first-line pembrolizumab. Solid lines represent estimates from the Kaplan-Meier (KM) analysis; dashed lines are estimates from a generalized gamma regression model adjusting for gender and stage of diagnosis. A generalized gamma model adjusting for gender and stage at diagnosis found that for patients with nonsquamous histology, high PD-L1 TPS was significantly associated with improved overall survival by a median OS difference of 8.4 months (p <0.001). In contrast, for patients with squamous histology, there was no evidence of association between PD-L1 expression level and overall survival (p = 0.283). PD-L1-related incremental differences in median OS between the patients with squamous and nonsquamous tumors were significantly different (p = 0.034).
Abbreviations: KM, Kaplan-Meier; TPS, tumor proportion score
Table 2.
Overall survival by PD-L1 expression level and histology type, estimated using the generalized gamma model. Effect modification was examined by comparing the model predicted median OS differences (Median OS of PD-L1 high - Median OS of PD-L1 low) between patients with squamous and nonsquamous histologies. The 95% confidence intervals for median OS and the PD-L1 related incremental differences were estimated with the use of the bootstrap method. Bootstrapping procedures were also used to compute the p values associated with incremental difference and effect modification.
| Overall Survival, months (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Histology | PD-L1 low expression | PD-L1 high expression | Incremental Difference | Significance |
| Squamous | 9.3 (6.5 to 14.0) | 11.6 (9.6 to 13.6), | 2.2 (−2.6 to 5.9) | p = 0.283 |
| Nonsquamous | 8.7 (6.4 to 11.8) | 17.1 (14.5 to 20.3). | 8.4 (4.1 to 12.5) | p < 0.001 |
| Effect modification | 6.2 (0.6 to 12.1) | p = 0.034 | ||
Discussion
In this retrospective, observational study of patients with advanced NSCLC who received first-line pembrolizumab and had documented numeric value for PD-L1 testing results prior to treatment, we found that high PD-L1 TPS was predictive of improved OS for patients with nonsquamous NSCLC but not for patients with squamous histology.
These findings diverge from the histology-PD-L1 relationship observed in KEYNOTE-042. In this randomized study of first-line pembrolizumab versus chemotherapy, patients with advanced squamous NSCLC treated with first-line pembrolizumab appeared to benefit more with higher PD-L1 expression, while patients with nonsquamous histologies displayed no such trend.4 However, an unplanned subset analysis suggested that pembrolizumab did not appear superior to chemotherapy among patients with nonsquamous histologies, which might explain why high PD-L1 TPS did not predict improved OS in these patients.4
Our study has several limitations. Patients initially diagnosed with stage I-III disease likely received prior surgery, radiation, and/or chemotherapy, which may have impacted OS. The cohort had a relatively small number of patients with PD-L1 TPS <50% (13.0%). This is likely due to the fact that first-line pembrolizumab was only FDA-approved for patients whose tumors had a PD-L1 TPS of ≥50% during the study period.11 These patients may have received pembrolizumab between the presentation of KEYNOTE-042 results at ASCO in June 2018 and its FDA approval for patients with TPS 1–49% in April 2019. Another possibility is that patients determined to be unfit for platinum-based chemotherapy were treated off-label with pembrolizumab, thus biasing clinical outcomes in favor of patients with TPS ≥50%. Moreover, 22% of patients with early stage disease at diagnosis, who were more likely to have a TPS of <50%, were treated with pembrolizumab more than 6 months after the date of PD-L1 assessment which may not have accurately reflected TPS at the time of pembrolizumab monotherapy for metastatic disease. In addition, we note the dynamic and heterogeneous nature of PD-L1 as a biomarker.1
No difference in the predictive capacity of PD-L1 TPS by histology was observed with respect to PFS. However, the value of PFS as a meaningful endpoint for patients treated with immune checkpoint inhibitors may be limited by observer bias, imperfect correlation with OS, and pseudoprogression.2 Future studies could examine objective response rate as an alternative clinical endpoint. Finally, our ability to generalize these findings to other checkpoint inhibitors was limited by the sample size of patients in the database with PD-L1 low tumors who had received other PD-(L)1 inhibitors.
We conclude that high PD-L1 TPS may not be predictive of OS from first-line pembrolizumab in patients with squamous cancers. Further studies should include pre-planned analyses of PD-L1 TPS and clinical outcomes by histology.
Supplementary Material
Acknowledgments
The authors report the following conflicts of interest:
D. Doroshow: Consulting/Advisory role, Ipsen, Boehringer Ingelheim, Atheneum Partners, Boston Healthcare Associates, Dedham Group, Guidepoint Global Advisors, MJH Life Sciences; Travel/Accommodations/Expenses, Ipsen; Research funding, Janssen Oncology (inst.), Dendreon (inst.), Novartis (inst.), Bristol-Myers Squibb (inst.), Merck (inst.), AstraZeneca (inst.), Genentech/Roche (inst.)
J. Zugazagoitia: Consulting/Advisory role, Guardant Health; Speakers’ Bureau, Guardant Health, NanoString Technologies, Pfizer Pharmaceuticals Israel, Roche; Travel/Accommodations/Expenses, Roche
G. Gupta: Employment, H3 Biomedicine
B. Adamson: Employment, Flatiron Health; Stock and Other Ownership Interests, Roche; Research Funding, Flatiron Health; Patents, Royalties, Other Intellectual Property, Flatiron Health
D.L. Rimm: Stock and Other Ownership Interests, Pixel Gear; Honoraria, Amgen, Bristol-Myers Squibb, Ventana Medical Systems; Consulting/Advisory Role, Agendia, AstraZeneca, Bristol-Myers Squibb, Cell Signaling Technology, Cepheid; Daiichi Sankyo, GlaxoSmithKline, Konica Minolta, Merck, NanoString Technologies, NextCure, PAIGE.AI, Perkin Elmer, Roche, Sanofi, Ultivue; Research Funding, Amgen (Inst), AstraZeneca/MedImmune (Inst), Cepheid (Inst), Konica Minolta (Inst), Lilly (Inst), NanoString Technologies (Inst), Navigate Biopharma (Inst), NextCure (Inst), Perkin Elmer (Inst), Ultivue (Inst); Patents/Royalties/Other Intellectual Property: Quantitative Immunofluorescence (AQUA) (Inst), Rarecyte Circulating tumor cells; Travel/Accommodations/Expenses, Bristol-Myers Squibb, NextCure, Ventana Medical Systems
All other authors have declared no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res. 2019;25(15):4592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. New Engl J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. New Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. New Engl J Med. 2018;379(21):2040–51. [DOI] [PubMed] [Google Scholar]
- 7.Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. medRxiv 2020; doi: 10.1101/2020.03.16.20037143 [DOI] [Google Scholar]
- 8.Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017;3(8):1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis MD, Griffith SD, Tucker M, et al. Development and Validation of a High-Quality Composite Real-World Mortality Endpoint. Health Serv Res. 2018;53(6):4460–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prentice RL. A Log Gamma Model and Its Maximum Likelihood Estimation. Biometrika. 1974;61(3):539–44. [Google Scholar]
- 11.Jackson C flexsurv: A Platform for Parametric Survival Modeling in R. J Stat Softw. 2016;70(1):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai-Scherf L, Blumenthal GM, Li H, et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist. 2017;22(11):1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.