Abstract
The invasive, locally aggressive nature of feline injection-site sarcomas (FISS) pose a unique challenge for surgeons to obtain complete margins with surgical excision. Optical coherence tomography (OCT), an imaging technology that uses light waves to generate real-time views of tissue architecture, provides an emerging solution to this dilemma by allowing fast, high-resolution scanning of surgical margins. The purpose of this study was to use OCT to assess surgical margins of FISS and to evaluate the diagnostic accuracy of OCT for detecting residual cancer using six evaluators of varying experience. Five FISS were imaged with OCT to create a training set of OCT images that were compared with histopathology. Next, 25 FISS were imaged with OCT prior to histopathology. Six evaluators of varying experience participated in a training session on OCT imaging after which each of the evaluators was given a dataset that included OCT images and videos to score on a scale from cancerous to non-cancerous. Diagnostic accuracy statistics were calculated. The overall sensitivity and specificity for classification of OCT images by evaluators was 78.9% and 77.6%, respectively. Correct classification rate of OCT images was associated with experience, while individual sensitivities and specificities had more variation between experience groups. This study demonstrates the ability of evaluators to correctly classify OCT images with overall low levels of experience and training, and also illustrates areas where increased training can improve accuracy of evaluators in interpretation of OCT surgical margin images.
Keywords: Cats, Margins of Excision, Tomography, Optical Coherence, Sarcomas, Soft Tissue Neoplasms
Introduction
Feline injection-site sarcomas (FISS) are a subset of soft-tissue sarcomas (STS) that are recognized for displaying significant locally invasive biologic behavior.1 It has been shown that aggressive surgical management of these tumors with 5 cm lateral margins and 2 deep fascial margins leads to good outcomes in affected cats.2,3 Cats with FISS that are incompletely excised during the initial surgery have increased rates of morbidity, tumor recurrence, and overall decreased disease-free interval and survival times.4,5 Tumor recurrence has also been reported to occur in 14–42% of cases despite wide or radical excision with complete margins.3–7 The disparity between histopathologic interpretation and biologic outcome remains a clinical dilemma when treating FISS. Several factors may contribute to this discrepancy. Sarcomas locally invade tissues, with the potential for more aggressive tumors to grow in an asymmetric infiltrative manner. Furthermore, there are some limitations to surgical margin assessment in veterinary medicine.8 Firstly, there is little standardization in how margins are assessed by pathologists.9 Secondly, only a small portion of the surgical margins (<1%) is sampled for histopathologic evaluation when radial tissue trimming is used for assessment of small or medium sized specimens.10–11 An evaluation of the totality of surgical margins of a given specimen is not feasible from either a cost or a time perspective with current methods, which is unlikely to change without new developments in veterinary pathology.
As a result of these inherent limitations with margin evaluation, other technologies may be the key to providing ways to assess residual microscopic tumor cells at surgical margins. Optical coherence tomography (OCT) is an optical imaging technology that uses light-waves to generate images of microscopic tissues in real-time. OCT could allow for complete, fast, and high-resolution imaging of surgical margins.12 In human oncology, OCT has been used in surgical margin assessment and lymph node evaluation of human breast carcinomas post-lumpectomy.13,14 It has also been investigated for evaluation of oral squamous cell carcinoma surgical margins in human patients.15,16 Comparatively, little research has been done specifically validating OCT to assess human STS surgical margins. However, the use of OCT to discern normal adipose from both well-differentiated and de-differentiated retroperitoneal liposarcomas has been reported.17 Another study also evaluated the use of three-dimensional computation analysis to assess OCT images of STS.18 In veterinary medicine, one study described OCT characteristics of tissues at surgical margins in canine STS.22
The aims of this study were to compare normal and abnormal histologic features with OCT images for surgical margins from resected FISS (Aim 1), and to determine the diagnostic accuracy of OCT imaging for detection of incomplete margins after surgical excision of FISS (Aim 2). We hypothesized that OCT imaging would correspond well with histopathology and have high sensitivity for detection of residual cancerous margins in cats with FISS.
Methods
Part of this study was performed at the University of Illinois at Urbana-Champaign Veterinary Teaching Hospital (UI VTH) and part at the Ohio State University Veterinary Medical Center (OSU VMC). Cases were also contributed from multiple institutions across the USA. The protocol for this study was approved by the Institutional Animal Care and Use Committees at UI VTH and OSU VMC. Prior to case enrollment at any institution, owner written and informed consent was obtained.
Aim 1: Comparisons of OCT images with histopathology
Cats were included that were undergoing surgical excision of STS or FISS at UI VTH. Cytologic or histopathologic confirmation of STS or FISS was required before enrollment. Cats were excluded if the final histopathology report revealed the tumor was not a STS or FISS.
An American College of Veterinary Surgeons (ACVS) board-certified surgeon or supervised trainee resected the sarcomas. Surgical doses for tumor removal varied from marginal to radical excisions, the surgical dose was determined by the primary surgeon. After removal, the specimens were wrapped in saline-soaked sponges until OCT imaging could be performed, usually within 2–3 hours. Before imaging, up to four suspicious areas (8mm by 8mm) of possible tumor infiltrated margins were identified either by visual inspection or by palpation by the investigator imaging the specimen (LES). If no specific areas were identified as suspicious, representative areas were chosen for evaluation. Imaging of these areas was performed using a commercial spectral-domain OCT system with a wavelength of 1310 nm and an incident illumination power of 5 mW (Envisu C2300; Bioptigen Inc., Durham, North Carolina). The OCT system could image to a depth of 1–2 mm, with variability primarily due to the type of tissue being imaged. Images produced by this system had a resolution of 8 μm axially and 10 μm laterally. After imaging, surgical ink was used to mark each area that was imaged, so that sections could be taken for histopathologic comparisons. These specific areas of margin were used to draw comparisons between OCT and histopathology. (The Davidson Marking System, Bradley Products, Inc., Bloomington, MN). Samples were then placed in 10% neutral buffered formalin. An American College of Veterinary Pathologists (ACVP) board-certified pathologist (JS) performed tissue trimming for histopathologic tumor and surgical margin assessment. A cross-section of each inked area was taken to allow comparison with OCT images. All tissue sections were paraffin embedded, slides were created and stained with haematoxylin and eosin (H&E).
A software package was used to obtain OCT images in a raw format and view them in real-time (InVivoVue 1.7; Bioptigen Inc., Durham, NC). Investigators present at the time of real-time imaging (LES & PC) ensured that images collected were of a sufficient diagnostic quality. Six hundred consecutive OCT images were generated for each area. A different software package was used to convert raw files into TIFF images for viewing (Matlab; MathWorks, Inc., Natick, MA). Representative OCT images from each of the inked sections from every sample were selected for comparison with histopathology images. Histopathology H&E stained slides were scanned, and converted to digital files that could then be viewed with specialized viewing software (NDP.view2; Hamamatsu Photonics, Shizuoka, Japan). The digital histopathology sections were assessed to identify inked areas then positioned to acquire an image with size of 8mm in length and a 2mm depth to be consistent with the OCT images generated. When viewing the OCT images created, several characteristics are identified to determine tissue type. These characteristics include depth of penetration of light waves, amount of light wave scatter, and organization of tissues. This information considered together is used to determine whether or not neoplasia is found in an area scanned with OCT.
Aim 2: Assessment of diagnostic accuracy
Cats were included in Aim 2 if there was a preoperative cytologic or histopathologic diagnosis of STS or FISS, and the cat was undergoing surgical resection at UI VTH or OSU VMC or one of the participating institutions. Initial and recurrent tumors were included. At time of enrollment, signalment information was collected including age, breed, sex, and neuter status. Information about the tumor and surgery was also recorded including the anatomic location and size of the tumor, and nature of the surgical dose (marginal, wide, or radical excision) and surgical margins (measured at surgery).19
Board-certified surgeons or supervised trainees resected the tumors. Samples were wrapped in saline-soaked sponges and were shipped overnight on ice if from outside institutions. OCT imaging of the specimens occurred using a laboratory-built spectral-domain OCT system with a custom-built handheld OCT probe at UI VTH (Custom OCT system, University of Illinois Urbana-Champaign; and Diagnostic Photonics, Chicago, IL), or a commercial spectral-domain OCT system with a hand-held probe at OSU VMC (TELESTO, 1300 nm, 76 kHz, Thorlab, Lubeck, Germany). All margins were systematically imaged using the hand-held probe. Images were viewed in real-time and evaluated by an investigator (PC and/or LES) to ensure quality. Specimens were fixed in formalin. An ACVP board-certified pathologist (UI VTH: JS or OSU VMC: RJ) performed tissue trimming of the specimen. Radial sections were trimmed for standard histopathology and margin assessment (Figure 2). The specimen was then trimmed using a tangential trimming technique; creating 2–3 mm thickness tangential sections covering the surface area of the specimen. The sections were paraffin embedded and H&E slides were created. The blinded pathologist established tumor type and determined whether histopathologic margins were complete. Complete margins were defined as tumor cells greater than 2 mm away from the surgical margins.
Figure 2:
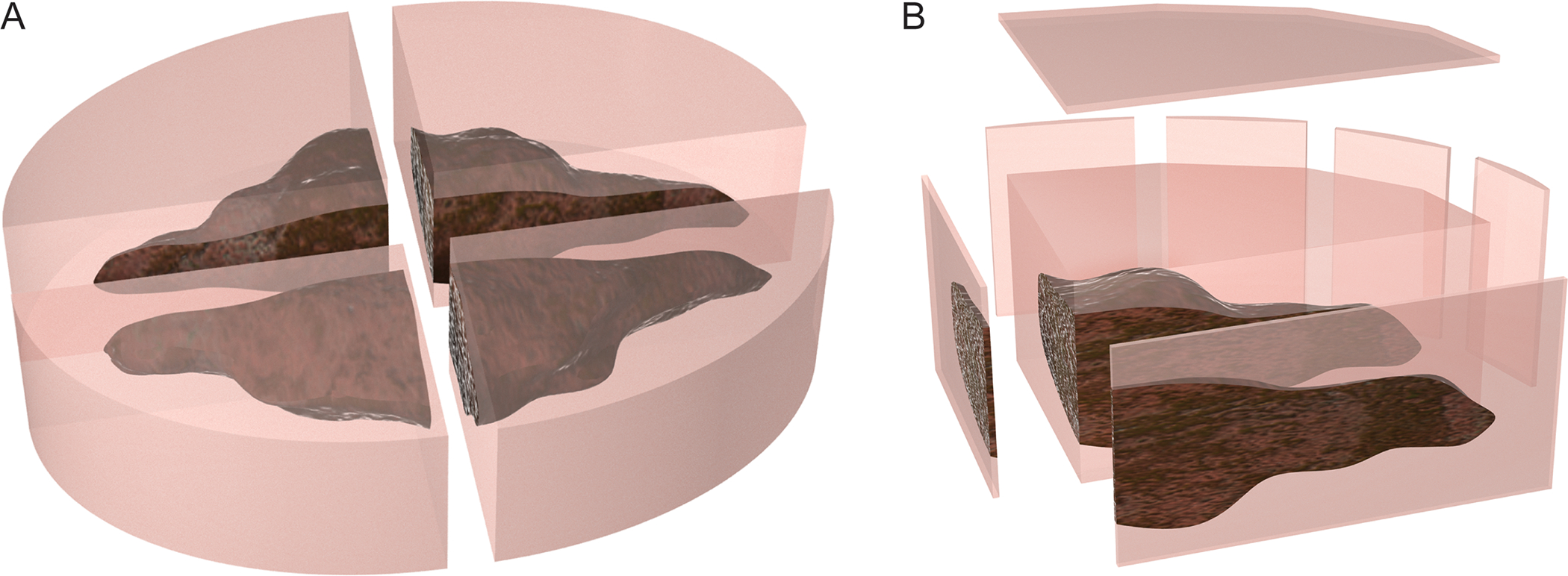
Figure 2a demonstrates section of tissue using the radial method. Figure 2b demonstrates tangential tissue sectioning, which allows for evaluation of a larger sample of the tissue.
After data collection, representative images collected from Aim 1 cases were selected and compiled into a presentation (PowerPoint, Microsoft) that was used to train evaluators. Specific features of the various tissue types were identified and described in the observer training. Images from both Aim 1 and Aim 2 were used to create test and data set slides decks. Six blinded evaluators were selected to independently assess the images and videos in the presentations. Evaluators had a variety of experience ranging from two evaluators with no experience at all, to two evaluators that had experience with prior studies that involved evaluation of OCT images, as well as two evaluators that had prior experience evaluating OCT images in real-time. Regardless experience level, all evaluators underwent the same one-hour training session on OCT image evaluation prior to being given study images to assess. Training was completed via video conference with participating evaluators and included an overview of OCT, followed by image examples of various tissue types and common artifacts seen. Following training, a practice test set of 10 OCT images and 2 videos was given to the evaluators to allow application of knowledge and practice. The evaluators were given standardized feedback on the test set results before receiving the study image set. The study set included 50 images and 8 videos for the evaluators to assess with half of the images being mirrors of an original. Evaluators were instructed to assess all images and videos using a 4-point scoring system (Table 1). All six evaluators were given a maximum time-frame of fourteen days from the training to evaluate both the test set and the dataset presentations, to ensure a standard period between training and application.
Table 1:
Each evaluator scored images on a 1–4 scale based on their own interpretation of cancerous vs. non-cancerous.
| OCT Grade | Grade Key |
|---|---|
| 1 | No cancer seen in image or video |
| 2 | No cancer seen in image or video, with less certainty |
| 3 | Cancer seen in image or video, with less certainty |
| 4 | Cancer seen in image of video |
Statistical analysis
A sample size of 5 client owned cats with 4 imaged areas per excised tumor was used for Aim 1 to create a training set of OCT images of surgical margin tissues to compare to histopathology. This number of cases was determined to be adequate based on a previous publication in intraoperative OCT imaging in human breast cancer.14 For Aim 2, a sample size calculation was performed based on methods we described in a prior manuscript based on those reported by Flahault et al. and Zhou et al.20–21 The estimated number of cats needed with incomplete margins was 8, using a confidence interval halfwidth of 0.3, predicted sensitivity of 90%, power of 80% and alpha of 0.05. We used a predicted sensitivity of 90% due to the high sensitivity of detection of incomplete surgical margin that has been reported in a recent human breast cancer clinical trial.14 We utilized an estimated prevalence of incomplete margins of 0.33, as this reported prevalence of incomplete margins following surgical resection of FISS in a recent study.23 With 8 cases of cats with incomplete margins and an estimated prevalence of 0.33 (proportion of cats with incomplete margins of 33%), 17 cats with complete margins were needed. A total of 25 cats were needed for Aim 2.
In Aim 2, diagnostic accuracy statistics were used to assess performance in scoring images correctly. Statistics included the sensitivity (true positive rate), specificity (true negative rate), and the overall correct classification rate. These statistics and their corresponding confidence intervals were calculated from marginal logistic regression models accounting for between-subject effects of each evaluator and within-subject effects of images coming from the same cat (to account for how each image is shown once in its original orientation, and again in the reversed orientation). Models included no covariates for the overall statistics and experience level for the group comparisons. Pairwise comparisons between groups were made using Holm’s adjustment on the p-values. For each evaluator, their performance on pairs of the same image and mirror was assessed to determine how often they gave different scores to the same image. The images that were most often scored incorrectly were also determined. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Cell line validation statement:
Cell lines were not used in this study.
Results
Aim 1: Comparisons of OCT with histopathology
Six client-owned cats were enrolled. One cat was excluded after histopathology determined the tumor was not a STS, leaving a total of five cats included. The tumors varied in size and were excised from different locations; the surgical margins used also varied with each animal (Table 2).
Table 2:
Signalment, tumor location, and margin information was recorded for all cats in both Aim 1 and Aim 2 of the study.
| Patient # | Study Aim | Age (yrs) | Breed | Sarcoma Location | Margins |
|---|---|---|---|---|---|
| 1 | 1 | 12 | DLH | Dorsal epaxial muscles | Marginal |
| 2 | 1 | 6 | DSH | Interscapular | Wide |
| 3 | 1 | 14 | DMH | Left elbow | Radical |
| 4 | 1 | 4 | Maine Coone | Right Proximal Thigh | Wide |
| 5 | 1 | 8 | DSH | Right Perineal Area | Marginal |
| 6 | 2 | 6 | DSH | Right proximal thigh | Wide |
| 7 | 2 | 3 | DSH | Right dorsal flank | Radical |
| 8 | 2 | 13 | DSH | Left thoracic paraspinal region | Marginal |
| 9 | 2 | 7 | DLH | Left abdominal wall | Wide |
| 10 | 2 | 14 | DSH | Craniodorsal left scapula | Marginal |
| 11 | 2 | 8 | DSH | Ventral left hindleg | Wide |
| 12 | 2 | 11 | DSH | Right cranioproximal thigh | Wide |
| 13 | 2 | 13 | DSH | Right elbow | Wide |
| 14 | 2 | 14 | DSH | Right proximal thigh | Wide |
| 15 | 2 | 14 | DSH | Left hock | Radical |
| 16 | 2 | 10 | DSH | Left thoracic wall | Wide |
| 17 | 2 | 10 | DSH | Right thigh | Radical |
| 18 | 2 | 14 | DSH | Right distal thigh | Radical |
| 19 | 2 | 8 | DMH | Right forelimb | Radical |
| 20 | 2 | 4 | DSH | Left hindlimb | Radical |
| 21 | 2 | 5 | DSH | Left hindlimb | Radical |
| 22 | 2 | 5 | DSH | Left abdominal wall | Wide |
| 23 | 2 | 9 | DSH | Left flank | Wide |
| 24 | 2 | 14 | DSH | Interscapular | Wide |
| 25 | 2 | 9 | DSH | Right thorax/flank | Wide |
| 26 | 2 | 9 | Angora | Right latismus dorsi | Wide |
| 27 | 2 | 10 | DSH | Left hindlimb | Wide |
| 28 | 2 | 15 | DMH | Right hind digit | Radical |
| 29 | 2 | 18 | DSH | Left forepaw | Radical |
| 30 | 2 | 13 | DSH | Left lateral thigh | Radical |
When assessing OCT imaging and histopathology sections, five tissue types were present at the surgical margins: adipose, skin, fascia, muscle, and sarcoma (Figure 1, Table 3). Adipose was the lowest-scattering tissue, with white higher scattering outlines of adipocytes resulting in a honeycomb appearance. Skin was intermediate scattering. Fascia often appeared as bright, linear striations throughout OCT images. In some areas, fascia had a distinct high-scattering appearance with a low-scattering center. Muscle was high-scattering, with an organized microstructure, and demonstrated a greater depth of image due to increased light penetration compared to sarcoma. Often muscle bundles could be identified in OCT images characteristically surrounded by fascia which appears as linear white striations. Comparatively, sarcoma had a highly-scattering, non-uniform appearance, and had low imaging depth on OCT.
Figure 1:
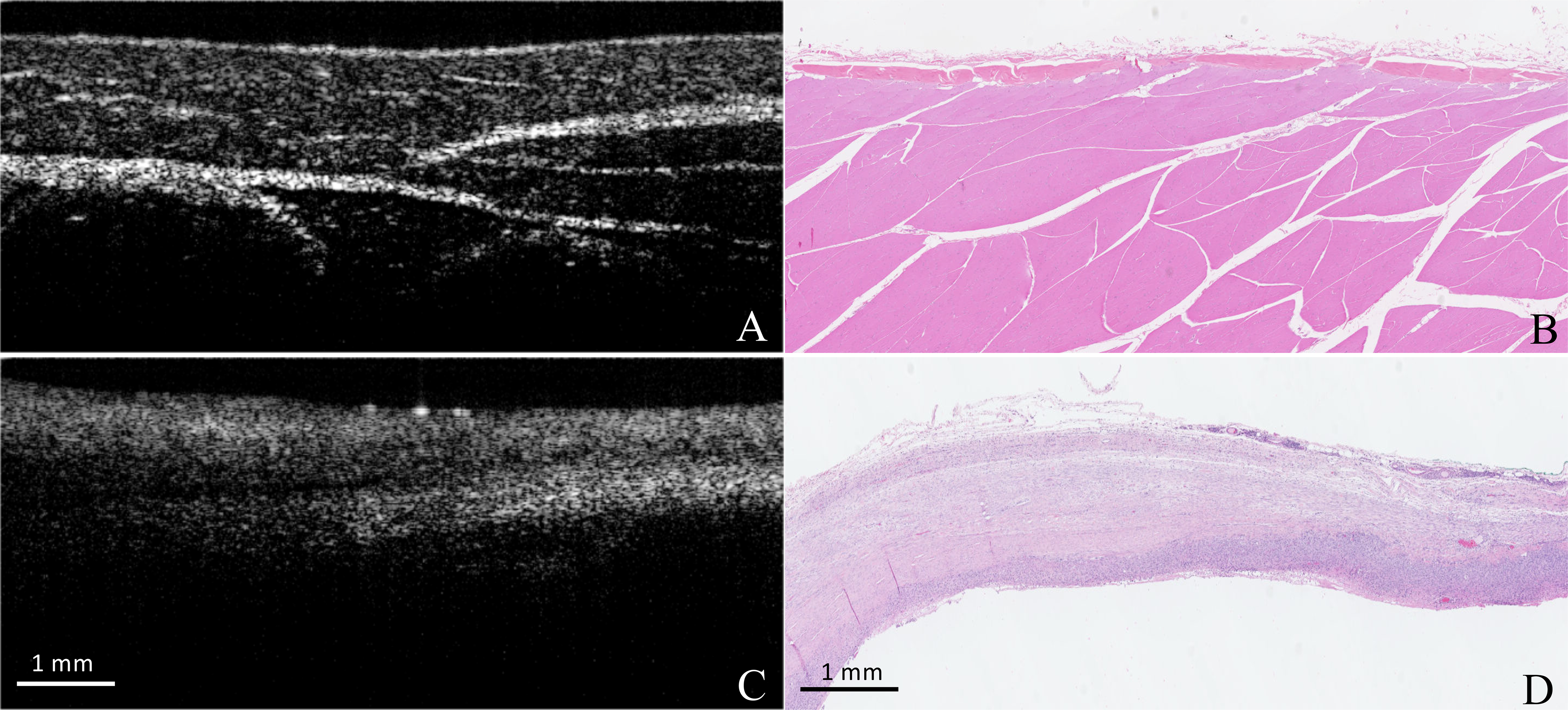
A comparison of OCT and Histopathologic appearance of tissues from cats in Aim 1. Image A demonstrates the normal organized, high-scatter appearance of muscle on OCT. Image B is corresponding histopathology image. Image C demonstrated the high-scattering, disorganized, appearance of sarcoma. Corresponding histopathology is seen in image D.
Table 3:
Results of histopathology from each area of the four sampled for Aim 1 cats.
| Patient Number | Species | Age (y) | Sarcoma Location | Histopathologic margins | Area 1 | Area 2 | Area 3 | Area 4 |
|---|---|---|---|---|---|---|---|---|
| 1 | Feline | 12 | Dorsal thoracic epaxial muscles | Lateral margins > 2cm Deep margin incomplete | skeletal muscle and bone, with sarcoma effacing 75% of tissue section | skeletal muscle and adipose tissue | sarcoma effaces 70% of tissue section and extends to deep margin, skeletal muscle also lines deep margin | haired skin, subcutaneous tissue, and a small amount of skeletal muscle. Deep margin lined by adipose. |
| 2 | Feline | 6 | Interscapular | Lateral margins 1.5 cm Deep margin 0.5 cm | haired skin, subcutis, and skeletal muscle | haired skin, subcutis, and skeletal muscle | haired skin, subcutis, and skeletal muscle | haired skin, subcutis and skeletal muscle, neoplasm compromises 15% of section |
| 3 | Feline | 14 | Left elbow | Ampuations; narrowest margin was 1.0 cm from the sarcoma | subcutis and STS effacing 80% of tissue section. Extends less than 0.1 cm from deep margin | skeletal muscle, fibrovascular connective tissue, and adipose | subcutis and sts effacing 70% of tissue section. Extends 0.1 cm from deep margin | skeletal muscle, fibrovascular connective tissue, and adipose |
| 4 | Feline | 4 | Right proximal thigh | Amputation; narrowest margin was 0.2 cm | skeletal muscle, fibrovascular connective tissue, and adipose tissue | skeletal muscle, fibrovascular connective tissue, nerve fibers, and a STS effacing 60% of tissue section. STS extends < 0.1 cm from deep margin. | skeletal muscle, fibrovascular connective tissue, and adipose tissue | adipose tissue, fibrovascualr connective tissue, and some skeletal muscle |
| 5 | Feline | 8 | Right perineal area | narrowest margin 1.0 mm | majority of section effaced by sarcoma; neoplasm <1.0 mm from surgical margin | majority of section effaced by sarcoma; neoplasm <1.0 mm from margin | skeletal muscle, adipose tissue, with 90% of section sarcoma; neopalsm 1.0 mm from surgical margin | adipose tissue and fibrous connective tissue, majority of section is soft tissue sarcoma; neoplasm is 1.0 mm from surgical margin |
Aim 2: Assessment of diagnostic accuracy
Twenty-six cats diagnosed with a FISS were enrolled in Aim 2 of this study. The sensitivity, specificity, and correct classification rate for the scored feline tumor images, overall and by experience level were evaluated (Table 4). Sensitivity was highest in those evaluators with no prior experience. For those two evaluators, one scored all 19 cancer positive images and videos correctly, while the other only had one incorrect. For the evaluators with real-time experience, the individual sensitivities were 84.2% and 68.4%. For the evaluators with OCT image interpretation experience but no real-time experience, the individual sensitivities were 84.2% and 42.1%. The differences between evaluators with real-time experience and those with only OCT image interpretation experience were insignificant (p=0.37). The sensitivities for evaluators with no prior experience were significantly higher compared to those with real-time experience (p=0.005) and those with only OCT image interpretation experience (p=0.005).
Table 4:
Sensitivity, specificity, and overall accuracy for all evaluators.
| Number | Sensitivity (95% CI) | Specificity (95% CI) | Correct Classification rate (95% CI) | |
|---|---|---|---|---|
| Overall | 6 | 78.9% (59.8–90.4%) | 76.9% (58.3–88.8%) | 77.6% (69.8–83.8%) |
| Experience Level | ||||
| Real-time imaging experience | 2 | 76.3% (63.8–85.5%) | 92.3% (81.5–97.0%) | 87.1% (85.8–88.2%) |
| OCT imagine but no real-time experience | 2 | 63.2% (32.9–85.7%) | 87.2% (72.4–94.6%) | 79.3% (76.8–81.6%) |
| No prior experience | 2 | 97.4% (89.9–99.4%) | 51.3% (44.2–58.3%) | 66.4% (62.7–69.9%) |
Thirty-nine of the images and videos had no evidence of cancer. Specificity was highest in the evaluators with real-time experience, and lowest in the evaluators without experience. The individual specificities for the evaluators with real-time experience were 87.2% and 97.4%. For those with only OCT image interpretation experience, the specificities were 79.5% and 94.9%. For those with no prior experience, the specificities were 56.4% and 46.2%. The differences in specificities between evaluators with real-time experience and those with only OCT image interpretation experience were insignificant (p=0.42). The specificities for evaluators with no prior experience were significantly lower compared to those with real-time experience (p<0.0001) and those with only OCT image interpretation experience (p=0.0004).
The overall correct classification rate was 77.6%. Given that there were more images without cancer cells present, evaluators with real-time experience had the best correct classification rate, and the worst rate was for the evaluators with no experience. The individual correct classification rates for the two evaluators with real-time experience were 86.2% and 87.9%. For those with only OCT image interpretation experience, the rates were 77.6% and 81.0%. For those with no prior experience, the rates were 69.0% and 63.8%. The correct classification rate was significantly higher for evaluators with real-time imaging experience compared to those with only OCT image interpretation experience (p<0.0001) and those with no prior experience (p<0.0001). Correct classification rate was also significantly higher for evaluators with OCT image interpretation experience compared to those with no experience (p<0.0001). For the most part, scorers responded the same way to each mirrored image pair. Of the 25 image pairs viewed by the six scorers, there were only five instances where a scorer gave different interpretations to the images within a pair. All five of these instances came in pairs of non-cancer images. Scorers always responded the same way for both images in the pairs of cancer images.
Discussion
This is the first study reporting the ex vivo use of OCT to evaluate surgical margins of FISS. We compared characteristics of ex vivo tissues imaged with OCT to histopathology sections and identified distinct scattering characteristics and microstructural features of different normal feline tissues that can be used to distinguish these tissues from FISS. Furthermore, we showed that it is possible to train clinicians to recognize these cancerous and non-cancerous OCT tissue features and reported the diagnostic accuracy of clinicians with varied experience for diagnosing residual FISS in surgical margins.
The six evaluators were divided into three groups depending on prior experience with OCT: overall correct classification rate increased with experience, but individual sensitivity and specificity showed more variation between groups. Evaluators were specifically chosen with varying experience to more appropriately mimic real-world scenarios, where it is likely that individuals assessing images will have a range of experience. Our most experienced evaluators were the two evaluators with real-time OCT experience that demonstrated the highest overall correct classification rate and the highest specificity. In an interesting contrast, the overall sensitivity of correct image identification in this group fell below those in the group without any prior OCT experience. The evaluators with prior experience were consistently identifying normal tissues correctly but inconsistently identifying FISS images correctly. Both evaluators had some real-time experience in canine skin and subcutaneous tumor ex vivo tissue imaging and imaged one or two samples every week for < 6 months. The high specificity is likely due to the fact a majority of the images presented to evaluators were non-cancerous, with a much smaller percentage being cancer. It is possible that the lower sensitivity in this group is actually a result of their experience; evaluators with more experience are likely to be more aware of artifacts associated with OCT. It is also possible that they relied on previous experience and spent less time focusing on feline-specific images that were provided to all evaluators in training. Within this group, when cancerous images were incorrectly labeled as non-cancerous both the image and the mirror image were incorrectly identified. The two evaluators with OCT image interpretation experience but without real-time experience performed better overall than those with no OCT experience, but did not perform as well as those with real-time experience. For reasons that are unclear, this group had the lowest sensitivity when identifying FISS images. Considering that these evaluators had some experience with OCT images, primarily canine STS, it is possible that there is more variation in the appearance of FISS compared to canine STS that made identification of FISS more challenging for this group. It is notable that it had been over a year since this group last had experience with OCT image interpretation, which may have been enough time for evaluators to forget details of previous training. All mis-identified images within this group were also incorrectly identified when mirrored, which signifies some consistency in how these images were identified, albeit incorrectly. The only two evaluators to correctly identify all cancerous images were both evaluators with no prior OCT experience. Interesting, this same group had the lowest specificity of identifying non-cancerous images. Considering this information together, it is possible to conclude that this lower specificity in an evaluator group with no prior OCT experiencee may demonstrate a tendency to over-interpret non-cancerous OCT images. Inexperienced evaluators have not only seen the least number of images to begin with, but also may misinterpret specific features of OCT due to not wanting to miss a cancerous image.
The overall sensitivity and specificity (78.9%, 76.9% respectively) of OCT image classification in this study, while good, was lower when comparing results to previous studies performed in people with breast cancer. Several factors may contribute to this difference. Previous studies evaluating surgical breast tumor margins with OCT found a sensitivity of 91.7–100% and a specificity of 82–92.1%; however, some differences must be noted, as one of these human studies used an experienced researcher with extensive training in OCT image interpretation to calculate the sensitivity and specificity of OCT imaging.14 Another study had more variation in experience, but compensated for this by calculating overall sensitivity and specificity using a majority.24 Comparatively, our most-experienced evaluators with real-time OCT experience had a maximum of 6 months of prior experience. These evaluators participated in only two studies previously. Despite participating in these studies, overall, their experience was considerably lower, especially compared to OCT studies done in humans. Furthermore, no evaluators with OCT experience had specific experience with FISS. Dense, high-scatter tissues of both cancerous and non-cancerous origin may appear the most similar and be the most challenging for the less-experienced evaluator; this is where increased training and exposure can improve the ability to differentiate between them. One group demonstrated a potential method to improve evaluator understanding.13 They used a method similar to our design with both a test set and a dataset of images; however, if evaluators in their study evaluated less than 70% of the images correctly in their test set, they provided more training prior to giving the data set images to an individual for evaluation. This extra step of setting a cut-off for test set performance could help improve sensitivity and specificity of the evaluators and help to identify the evaluators who may need additional training to improve understanding.
Videos also proved more difficult to interpret for all evaluators. None of the six evaluators correctly interpreted all videos. Experience also appeared to play a role in ability to correctly interpret OCT videos; evaluators with experience scanning tissues in real-time performed better. The increased level of difficulty for evaluators viewing OCT videos is likely due to the increased amount of images viewed in each video, and the increased number of tissue types seen; each video was comprised of approximately 100–200 OCT static slices and was an average of 10–30 seconds in length. No videos of OCT were shown in the one-hour training that was completed by all evaluators.
Overall, OCT has the potential to provide an effective and complete assessment of surgical margins after FISS surgery. OCT can assess the entire surgical margin within a short period of time. Evaluating the entire surgical margin with histopathology may be impractical and cost-prohibitive for routine use. While histopathology provides a higher level of detail at the microscopic level, there is no question that the amount of margin assessed is substantially greater with OCT. With a short training session, individuals can learn to interpret OCT images and determine whether or not there is cancer present in surgical margin images. When evaluating histopathology sections, the hope is that the sections you are viewing are representative of the entire margin. In contrast, with OCT, we know that the entire margin is being evaluated. This ultimately allows for more precision and less guess-work or assumptions being made about the biological behavior of an individual tumor. Furthermore, the use of OCT includes the benefit of being able to assess surgical margin both in and out of the operating room. Margins can be assessed intraoperatively or after surgery on samples that have not yet been formalin fixed. OCT has the real-time advantage compared to histopathology which is limited to margins in a post-operative setting in veterinary medicine, which ultimately has the benefit of potentially altering and improving intraoperative decision making.
One limitation of this study included use of multiple OCT imaging systems to collect the imaging data. This occurred due to an institutional move of the principal investigator during this project. Use of multiple systems resulted in some variability in image appearance and resolution between systems used. As a result, evaluators were trained using a combination of images generated by all OCT systems, but differences in image appearance based on the OCT system used could have had an impact on image interpretation by evaluators in this study.
For future studies, multiple improvements could help to increase evaluator accuracy. The creation of an image library including multiple tissues types would allow evaluators to be exposed to a larger degree of variation in OCT image appearance among. This image library could be specific to FISS, as well as other soft-tissue neoplasms. The inclusion of videos in this library, as well as in the training given to evaluators could also be of added benefit to future studies. Additionally, the use of artificial intelligence systems such as Deep Learning (DL), may increase accuracy of OCT, as well as the clinical practicality. Early development of a DL system to evaluate human colorectal tumors ex-vivo with OCT has been described.25 Deep learning-based software could be used real-time to flag abnormal tissues real-time to aid evaluator interpretation and is a promising area of future study.
Acknowledgments:
This project was supported by Morris Animal Foundation (grant no. D16FE-034) and the National Institutes of Health (Grant R01CA213149). The views expressed in this publication have not been reviewed or endorsed by Morris Animal Foundation, and as such do not necessarily reflect the views of the Foundation, its officers, directors, affiliates or agents. The authors would like to acknowledge Jennifer Reagan and Rachel Tulipan for contributing cases to this study, and Kelly Mesa for her help with optical coherence tomography imaging of initial specimens.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest related to this report.
References
- 1.Shaw SC, Kent MS, Gordon IK, et al. Temporal changes in characteristics of injection-site sarcomas in cats: 392 cases (1990–2006). J Am Vet Med Assoc. 2009;234(3):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershey AE, Sorenmo KU, Hendrick MJ, Shofer FS, Vail DM. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996). J Am Vet Med Assoc. 2000;216(1):58–61. [DOI] [PubMed] [Google Scholar]
- 3.Phelps HA, Kuntz CA, Milner RJ, Powers BE, Bacon NJ. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998–2002). J Am Vet Med Assoc 2011;239:97–106. [DOI] [PubMed] [Google Scholar]
- 4.Davidson EB, Gregory CR, Kass PH. Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg 1997;26:265–269. [DOI] [PubMed] [Google Scholar]
- 5.Müller N, Kessler M. Curative-intent radical en bloc resection using a minimum of a 3 cm margin in feline injection-site sarcomas: a retrospective analysis of 131 cases. J Feline Med Surg 2018;20:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giudice C, Stefanello D, Sala M, et al. Feline injection-site sarcoma: Recurrence, tumour grading and surgical margin status evaluated using the three-dimensional histological technique. Vet J 2010;186:84–88. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Hauck ML, Dodge R, et al. Preoperative radiotherapy for vaccine associated sarcoma in 92 cats. Vet Radiol Ultrasound 2002;43:473–479. [DOI] [PubMed] [Google Scholar]
- 8.Stromberg PC, Meuten D Trimming Tumors for Diagnosis and Prognosis. In: Tumors in Domestic Animals. John Wiley & Sons, Ltd; 2016:27–43. [Google Scholar]
- 9.Kamstock DA, Ehrhart EJ, Getzy DM, et al. Recommended for submission, trimming, margin evaluation, and reporting of tumor biopsy guidelines specimens in veterinary surgical pathology. Vet Pathol 2011;48:19–31. [DOI] [PubMed] [Google Scholar]
- 10.Milovancev M, Russell DS. Surgical margins in the veterinary cancer patient. Vet Comp Oncol 2017;15(4):1136–1157. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein JA, Hodgin EC, Holloway HW, Hedlund CS, Storey ES, Hubert JD. Mohs micrographic surgery: a technique for total margin assessment in veterinary cutaneous oncologic surgery. Vet Comp Oncol 2006;4:151–160. [DOI] [PubMed] [Google Scholar]
- 12.Holt D, Singhal S, Selmic LE. Near-infrared imaging and optical coherence tomography for intraoperative visualization of tumors. Vet Surg 2020;49:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha R, Friedlander LC, Hibshoosh H, et al. Optical coherence tomography: A novel imaging method for post-lumpectomy breast margin assessment-a multi-reader study. Acad Radiol 2018;25:279–287. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen FT, Zysk AM, Chaney EJ, et al. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res 2009;69:8790–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C. Optical coherence tomography in the assessment of oral squamous cell carcinoma resection margins. Photodiagnosis Photodyn Ther 2016;13:211–217. [DOI] [PubMed] [Google Scholar]
- 16.Sunny SP, Agarwal S, James BL, et al. Intra-operative point-of-procedure delineation of oral cancer margins using optical coherence tomography. Oral Oncol 2019;92:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbajal EF, Baranov SA, Manne VGR, et al. Revealing retroperitoneal liposarcoma morphology using optical coherence tomography. J Biomed Opt 2011;16:020502. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Liu C-H, Zakharov VP, Lazar AJ, Pollock RE, Larin KV. Three-dimensional computational analysis of optical coherence tomography images for the detection of soft tissue sarcomas. J Biomed Opt 2014;19:21102. [DOI] [PubMed] [Google Scholar]
- 19.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980;153:106–120. [PubMed] [Google Scholar]
- 20.Zhou X, Obuchowski NA, McClish DK. Sample Size Calculations. Statistical Methods in Diagnostic Medicine. 2nd ed: Wiley, 2011; 193–229. [Google Scholar]
- 21.Flahault A, Cadilhac M, Thomas G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol 2005;58:859–862. [DOI] [PubMed] [Google Scholar]
- 22.Selmic LE, Samuelson J, Reagan JK, Mesa KJ et al. Intra-operative imaging of surgical margins of canine soft tissue sarcoma using optical coherence tomography. Vet Comp Oncol 2019; 17: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giudice C, Stefanello D, Sala M, et al. Feline injection-site sarcoma: recurrence, tumour grading and surgical margin status evaluated using the three-dimensional histological technique. Vet J 2010;186:84–88. [DOI] [PubMed] [Google Scholar]
- 24.Erickson-Bhatt SJ, Nolan RM, Shemonski ND, et al. Real-time imaging of the resection bed using a handheld probe to reduce incidence of microscopic positive margins in cancer surgery. Cancer Res 2015;75:3706–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Xu S, Chapman WC, et al. Real-time colorectal cancer diagnosis using PR-OCT with deep learning. Theranostics 2020;10:2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]


